Study on Mass Transfer Kinetics of Sugar Extraction from Sweet Sorghum Biomass via Diffusion Process and Ethanol Yield Using SSF
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Content and Moisture Content Analysis
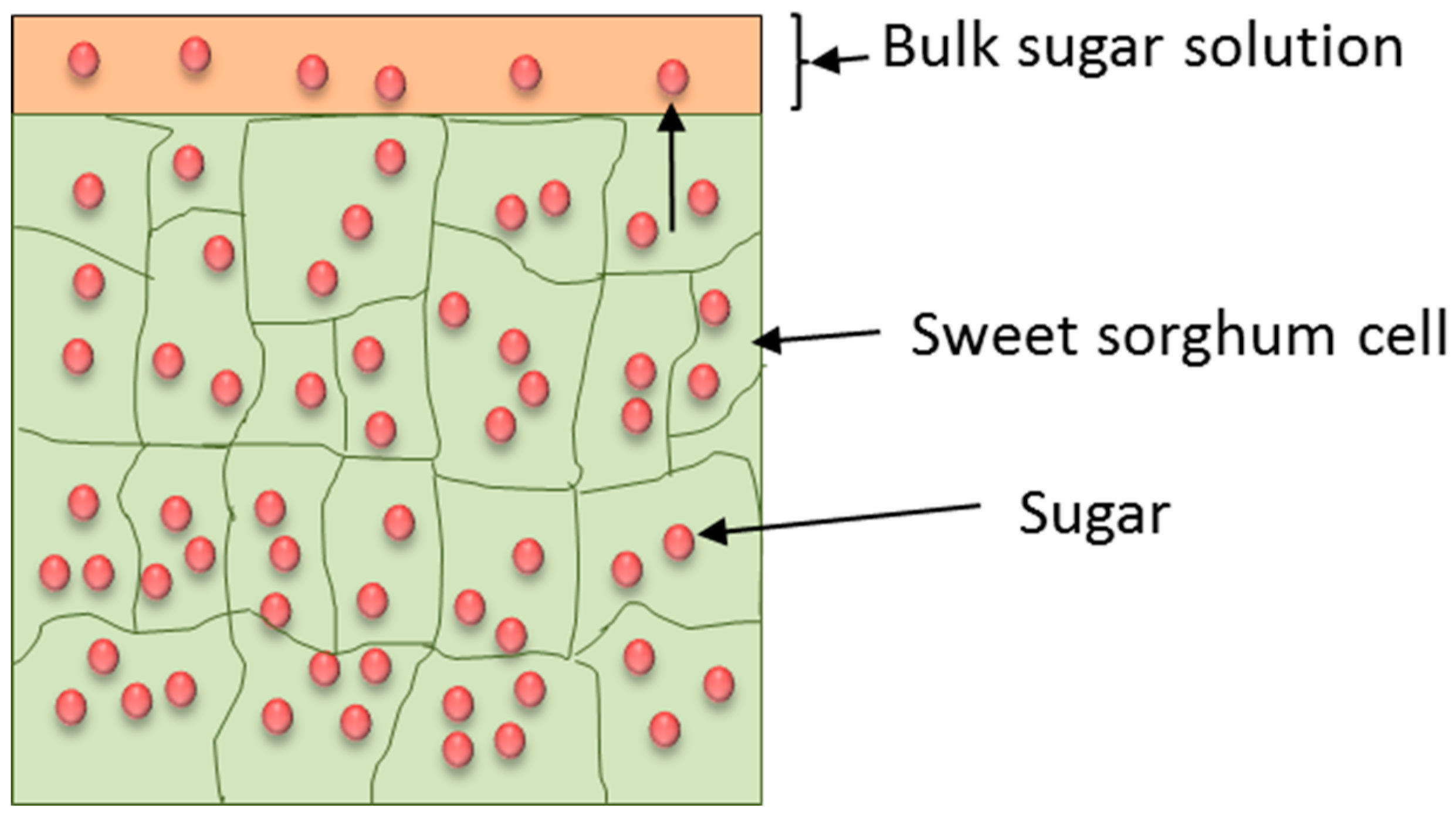
2.3. Kinetic Model Study
2.4. Sugar Extraction
2.5. Sugar and Ethanol Analysis
2.6. Inoculum Preparation
2.7. Ethanol Fermentation
2.8. Statistical Analysis
3. Results and Discussion
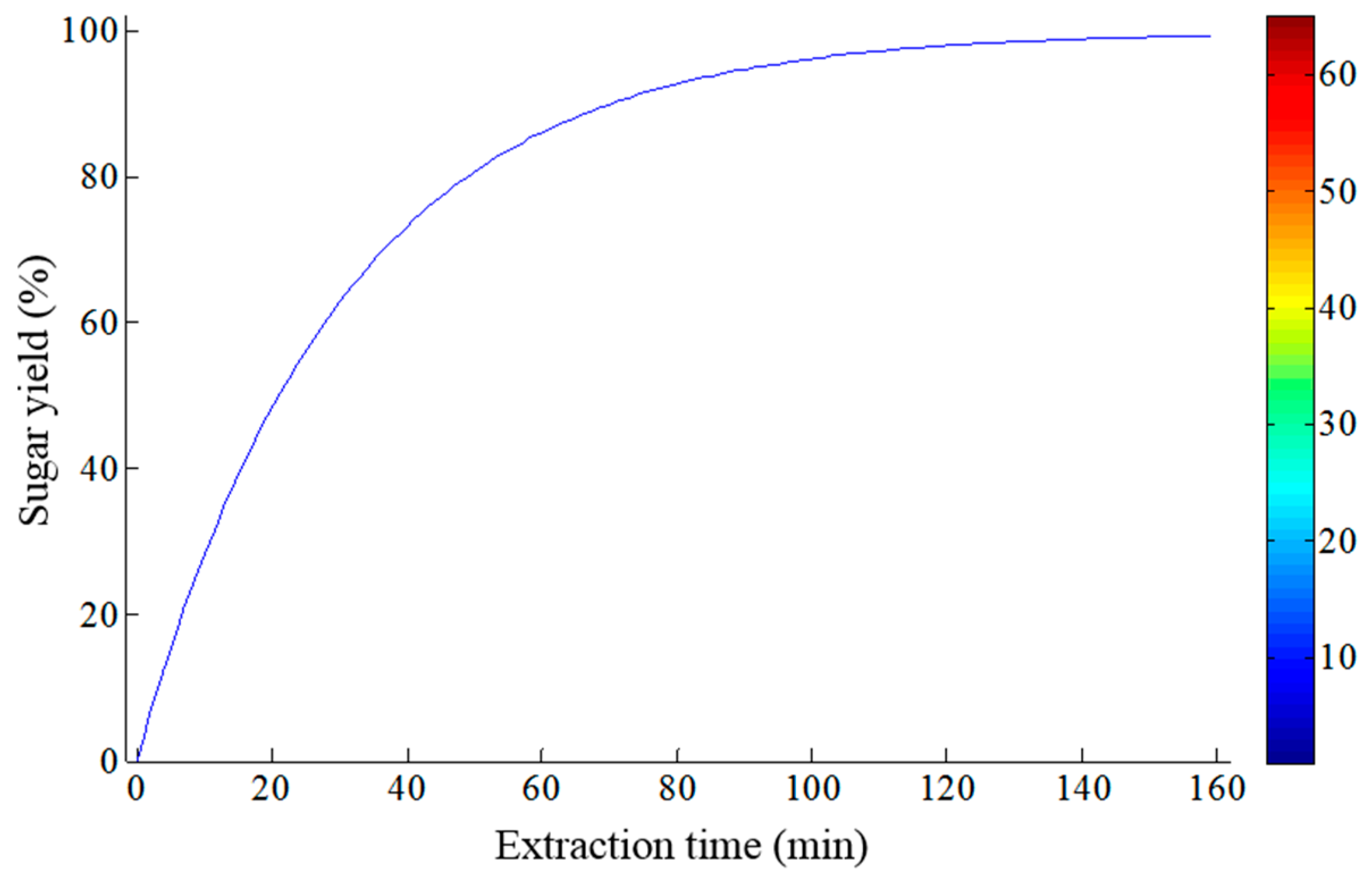
3.1. Kinetic Study of Sugar Transfer from Sweet Sorghum Biomass via Diffusion
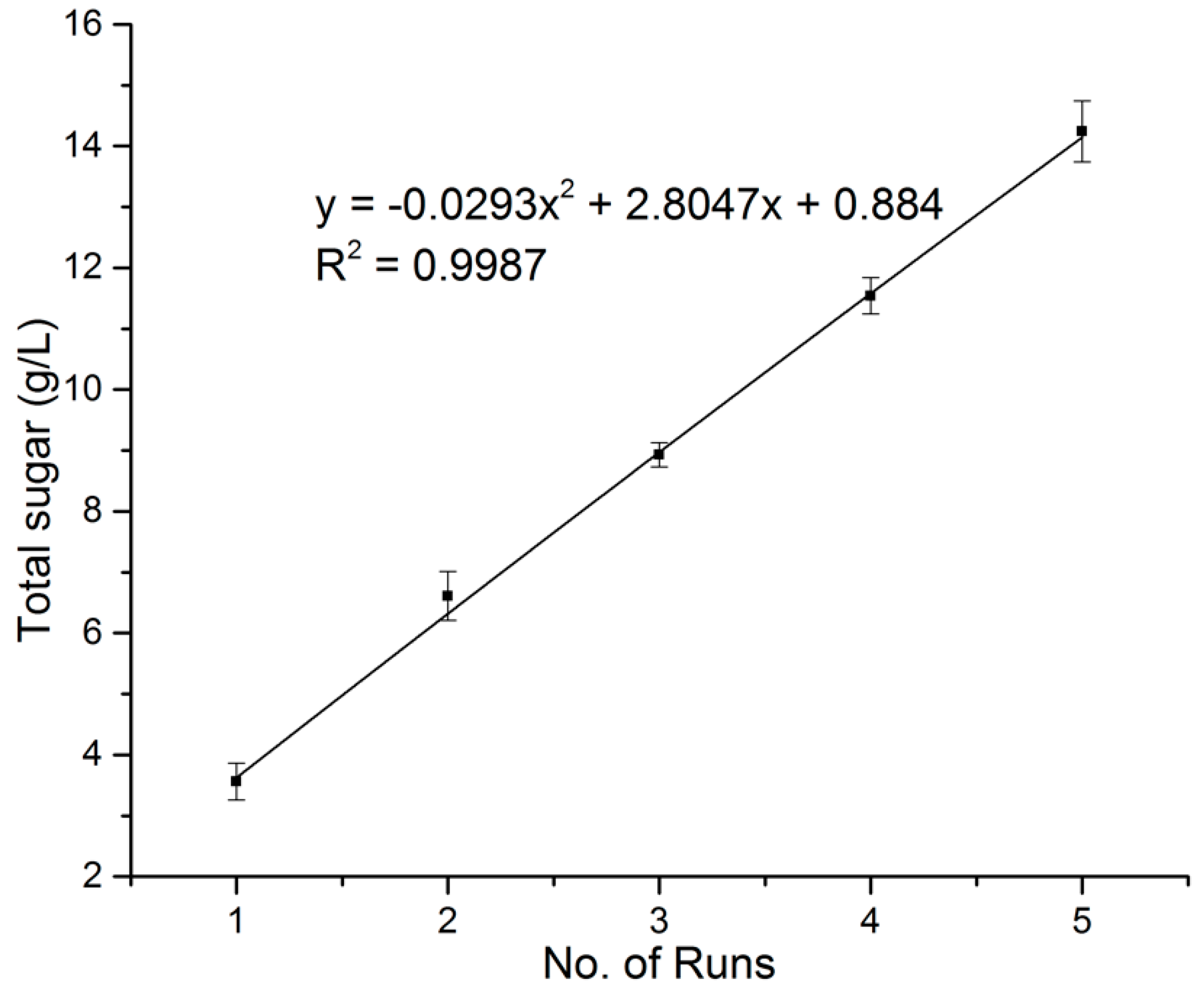
3.2. Sugar Yields
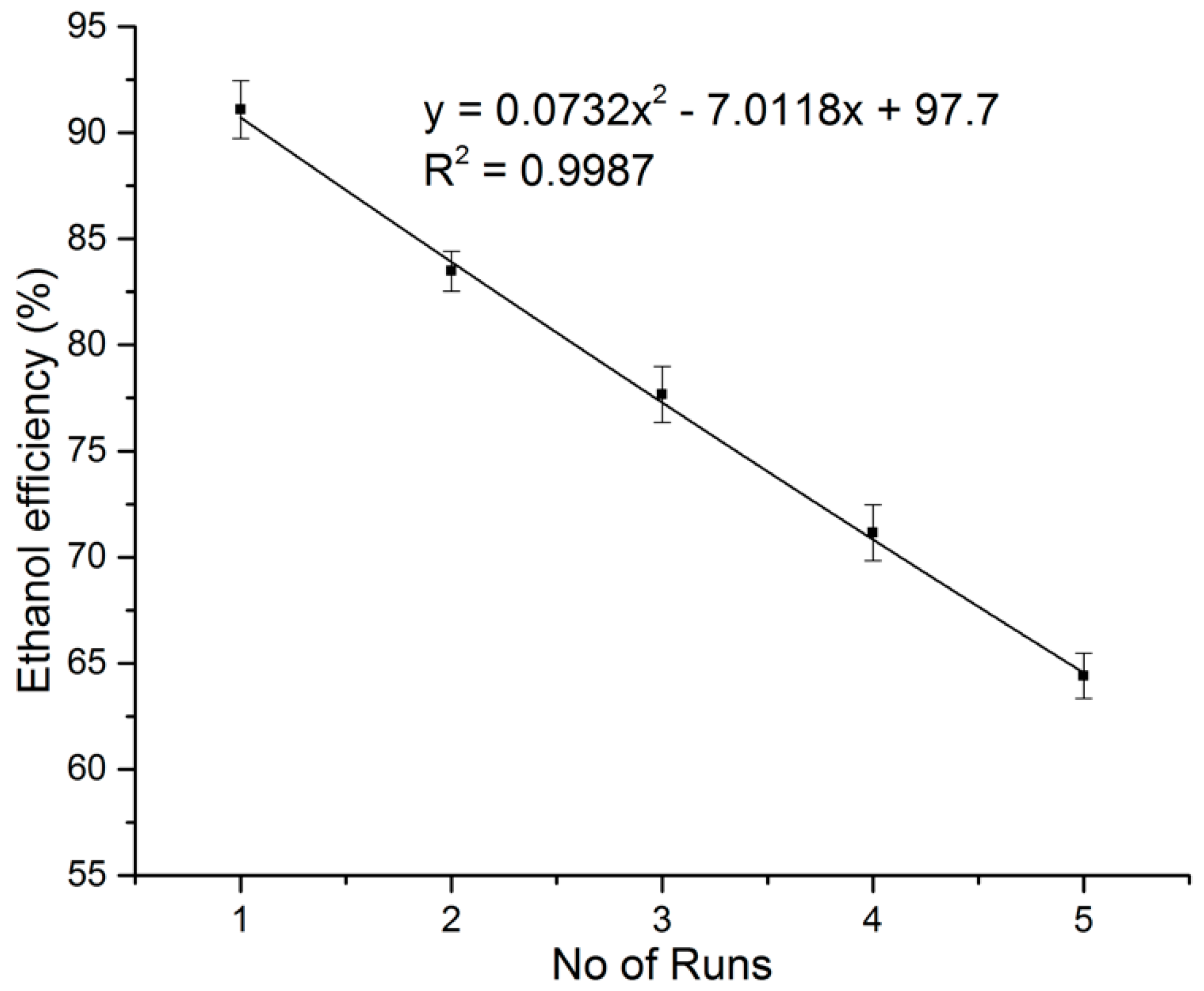
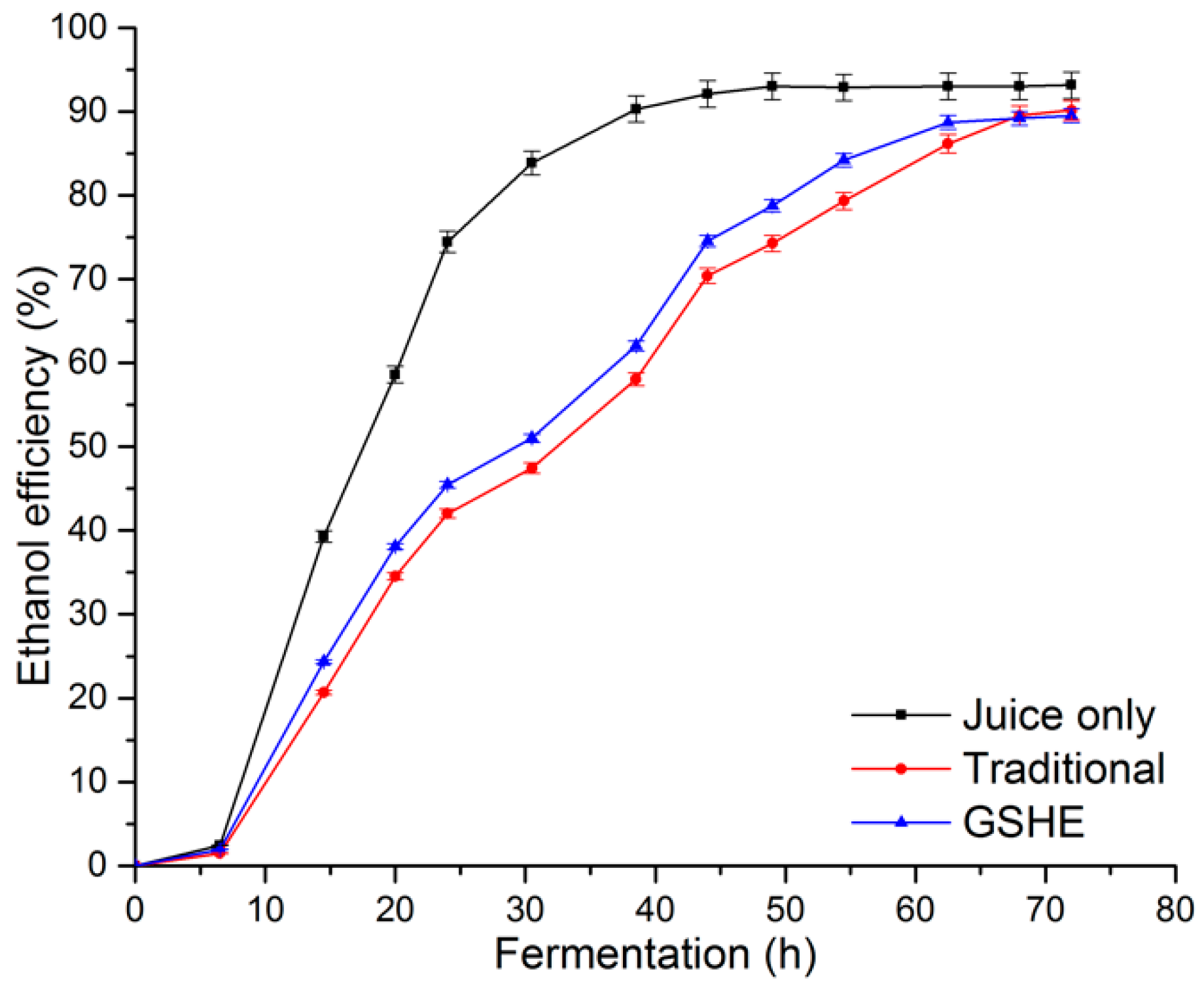
3.3. Ethanol Fermentation Yield and Efficiencies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du, R.; Yan, J.; Feng, Q.; Li, P.; Zhang, L.; Chang, S.; Li, S. A novel wild-type Saccharomyces cerevisiae strain TSH1 in scaling-up of solid-state fermentation of ethanol from sweet sorghum stalks. PLoS ONE 2014, 9, e94480. [Google Scholar] [CrossRef] [PubMed]
- Phutela, U.G.; Kaur, J. Process Optimization for Ethanol Production from Sweet Sorghum Juice Using Saccharomyces cerevisiae Strain NRRL Y-2034 by Response Surface Methodology. Sugar Tech 2014, 16, 411–421. [Google Scholar] [CrossRef]
- Wu, X.; Staggenborg, S.; Propheter, J.L.; Rooney, W.L.; Yu, J.; Wang, D. Features of sweet sorghum juice and their performance in ethanol fermentation. Ind. Crop. Prod. 2011, 31, 164–170. [Google Scholar] [CrossRef]
- Wu, X.; Staggenborg, S.; Wang, D. Stabilization of sweet sorghum juice for long-term storage. Trans. ASABE 2015, 58, 169–175. [Google Scholar]
- Hetényi, K.; Gál, K.; Németh, Á.; Sevella, B. Use of sweet sorghum juice for lactic acid fermentation: Preliminary steps in a process optimization. J. Chem. Technol. Biotechnol. 2010, 85, 872–877. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, S.; Huang, J.; Zhang, Q.; Wang, X. Production of acetone and butanol by fermentation of sweet sorghum stalk juice. Trans. CSAE 2008, 24, 177–180. [Google Scholar]
- Whitfield, M.B.; Chinn, M.S.; Veal, M.W. Processing of materials derived from sweet sorghum for biobased products. Ind. Crop. Prod. 2012, 37, 362–375. [Google Scholar] [CrossRef]
- Appiah-Nkansah, N.B.; Saul, K.; Rooney, W.; Wang, D. Adding sweetsorghum juice into the current dry-grind ethanol process for improving ethanol yields and water efficiency. Int. J. Agric. Biol. Eng. 2015, 8, 97–103. [Google Scholar]
- Serna-Saldívar, S.O.; Chuck-Hernández, C.; Pérez-Carrillo, E.; Heredia-Olea, E. Sorghum as a Multifunctional Crop for the Production of Fuel Ethanol: Current Status and Future Trends; IntechOpen Limited: London, UK, 2012. [Google Scholar]
- Nghiem, N.P.; Montanti, J.; Johnston, D.B. Sorghum as a renewable feedstock for production of fuels and industrial chemicals. Curr. Biochem. Eng. 2016, 3, 75–91. [Google Scholar] [CrossRef]
- Eggleston, G.; Cole, M.; Andrzejewski, B. New commercially viable processing technologies for the production of sugar feedstocks from sweet sorghum (Sorghum bicolor L. Moench) for manufacture of biofuels and bioproducts. Sugar Tech 2013, 15, 232–249. [Google Scholar] [CrossRef]
- Rañola, R.F.; Layaoen, H.L.; Costales, C.; Halos, A.L.; Baracol, L.A. Feasibility Study for an Integrated Anhydrous Alcohol Production Plant Using Sweet Sorghum as Feedstock; International Society for Southeast Asian Agricultural Sciences: Los Baños, The Philippines, 2007. [Google Scholar]
- Rao, S.S.; Patil, J.V.; Chandrasekara Reddy, D.; Vijay Kumar, B.S.; Srinivasa Rao, P.; Gadakh, S.R. Effect of different crushing treatments on sweet sorghum juice extraction and sugar quality traits in different seasons. Sugar Tech 2013, 15, 311–315. [Google Scholar] [CrossRef]
- Regassa, T.H.; Wortmann, C.S. Sweet sorghum as a bioenergy crop: Literature review. Biomass Bioenergy 2014, 64, 348–355. [Google Scholar] [CrossRef]
- Reidenbach, V.G.; Coble, C.G. Sugarcane or sweet sorghum processing techniques for ethanol production. Trans. ASAE 1985, 28, 571–575. [Google Scholar] [CrossRef]
- Rein, P. Sugarcane Engineering, 2nd ed.; Verlag Dr. Albert Bartens KG.: Berlin, Germany, 2007. [Google Scholar]
- Appiah-Nkansah, N.B.; Zhang, K.; Rooney, W.; Wang, D. Model study on extraction of fermentable sugars and nonstructural carbohydrate from sweet sorghum using diffusion process. Ind. Crop. Prod. 2016, 83, 654–662. [Google Scholar] [CrossRef]
- Crank, J.; McFarlane, N.R.; Paterson, G.D.; Pedley, J.B. Diffusion Processes in Environmental Systems; Macmillan: London, UK, 1981. [Google Scholar]
- Rein, P.W. A comparison of cane diffusion and milling. Proc. S. Afr. Sugar Technol. Assoc. 1995, 69, 196–200. [Google Scholar]
- Viator, H.P.; Alison, M.; Gravois, K.; Han, K.J.; Harrell, D.; Hogan, A.; Pittman, W.; Salassi, M.; Whatley, J. Sweet sorghum for biofuel production in Louisiana; Louisiana Agriculture: Baton Rouge, LA, USA, 2009. [Google Scholar]
- Kim, M.; Day, D.F. Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microb. Biotechnol. 2011, 38, 803–807. [Google Scholar] [CrossRef] [PubMed]
- El Belghiti, K.; Vorobiev, E. Mass transfer of sugar from beets enhanced by pulsed electric field. Food Bioprod. Process. 2004, 82, 226–230. [Google Scholar] [CrossRef]
- Jemai, A.B.; Vorobiev, E. Effect of moderate electric field pulses on the diffusion coefficient of soluble substances from apple slices. Int. J. Food Sci. Technol. 2002, 37, 73–86. [Google Scholar] [CrossRef]
- Mao, Y.; Li, J.; Li, S.; Chang, S.; Zhao, G. The mass transfer of sugar in sweet sorghum stalks for solid-state fermentation process. Fuel 2015, 144, 90–95. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Li, Z.; Cai, L.; Gu, Z.; Shi, Y.C. Effects of granule swelling on starch saccharification by granular starch hydrolyzing enzyme. J. Agric. Food Chem. 2014, 62, 8114–8119. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Maier, A.; Klocke, N.; Yan, S.; Rogers, D.; Tesso, T.; Wang, D. Impact of deficit irrigation on sorghum physical and chemical properties and ethanol yield. Trans. ASABE 2013, 56, 1541–1549. [Google Scholar]
- AOAC Method 925.10. Solids (Total) and Moisture in Flour; AOAC International: Gaithersburg, MD, USA, 2000.
- AACC Method 55-10: Test Weight per Bushel; AACC Int.: St. Paul, MN, USA, 2000.
- AACC Method 76-13: Total Starch Assay Procedure; AACC Int.: St. Paul, MN, USA, 2000.
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; Laboratory Analytical Procedure; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008.
- Brüniche-Olsen, H. Solid-Liquid Extraction with Particular Reference to Extraction of Sugar from Sugar-Beets: With a Danish Summary; Nytnordisk Forlag, Arnold Busck: Copenhagen, Denmark, 1962. [Google Scholar]
- Toda, T.A.; Sawada, M.M.; Rodrigues, C.E. Kinetics of soybean oil extraction using ethanol as solvent: Experimental data and modeling. Food Bioprod. Process. 2016, 98, 1–10. [Google Scholar] [CrossRef]
- Meziane, S.; Kadi, H. Kinetics and thermodynamics of oil extraction from olive cake. J. Am. Oil Chem. Soc. 2008, 85, 391–396. [Google Scholar] [CrossRef]
- Dagostin, J.L.A.; Carpiné, D.; Corazza, M.L. Extraction of soybean oil using ethanol and mixtures with alkyl esters (biodiesel) as co-solvent: Kinetics and thermodynamics. Ind. Crop. Prod. 2015, 74, 69–75. [Google Scholar] [CrossRef]
- Allawzi, M.A.; Abu-Arabi, M.K.; Al-Taher, F.A. Parametric study on the batch leaching process of Jojoba oil. Eur. J. Lipid Sci. Technol. 2005, 107, 469–475. [Google Scholar] [CrossRef]
- Baümler, E.R.; Crapiste, G.H.; Carelli, A.A. Solvent extraction: Kinetic study of major and minor compounds. J. Am. Oil Chem. Soc. 2010, 87, 1489–1495. [Google Scholar] [CrossRef]
- Fernández, M.B.; Perez, E.E.; Crapiste, G.H.; Nolasco, S.M. Kinetic study of canola oil and tocopherol extraction: Parameter comparison of nonlinear models. J. Food Eng. 2012, 111, 682–689. [Google Scholar] [CrossRef]
- Perez, E.E.; Carelli, A.A.; Crapiste, G.H. Temperature-dependent diffusion coefficient of oil from different sunflower seeds during extraction with hexane. J. Food Eng. 2011, 105, 180–185. [Google Scholar] [CrossRef]
- Doran, P.M. Bioprocess Engineering Principles, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Gohel, V.; Duan, G. No-cook process for ethanol production using Indian broken rice and pearl millet. Int. J. Microb. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Granular Starch Hydrolysis for Fuel Ethanol Production. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Urbana-Champaign, IL, USA, 2008. [Google Scholar]
- DuPont. Stargen 002. Available online: http://www.dupont.com/content/dam/dupont/products-and-services/industrial-biotechnology/documents/DuPont-STARGEN002-web-EN.pdf (accessed on 20 September 2018).


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baah Appiah-Nkansah, N.; Li, J.; Zhang, K.; Zhang, M.; Wang, D. Study on Mass Transfer Kinetics of Sugar Extraction from Sweet Sorghum Biomass via Diffusion Process and Ethanol Yield Using SSF. Processes 2019, 7, 137. https://doi.org/10.3390/pr7030137
Baah Appiah-Nkansah N, Li J, Zhang K, Zhang M, Wang D. Study on Mass Transfer Kinetics of Sugar Extraction from Sweet Sorghum Biomass via Diffusion Process and Ethanol Yield Using SSF. Processes. 2019; 7(3):137. https://doi.org/10.3390/pr7030137
Chicago/Turabian StyleBaah Appiah-Nkansah, Nana, Jun Li, Ke Zhang, Meng Zhang, and Donghai Wang. 2019. "Study on Mass Transfer Kinetics of Sugar Extraction from Sweet Sorghum Biomass via Diffusion Process and Ethanol Yield Using SSF" Processes 7, no. 3: 137. https://doi.org/10.3390/pr7030137
APA StyleBaah Appiah-Nkansah, N., Li, J., Zhang, K., Zhang, M., & Wang, D. (2019). Study on Mass Transfer Kinetics of Sugar Extraction from Sweet Sorghum Biomass via Diffusion Process and Ethanol Yield Using SSF. Processes, 7(3), 137. https://doi.org/10.3390/pr7030137






