Reaction Kinetics of Carbon Dioxide in Aqueous Blends of N-Methyldiethanolamine and L-Arginine Using the Stopped-Flow Technique
Abstract
:1. Introduction
2. Reaction Models
2.1. Reaction of CO2 with MDEA
2.2. Reaction of CO2 with Amino Acid
2.2.1. Zwitterion Mechanism
2.2.2. Termolecular Mechanism
2.3. Reaction of CO2 with Mixtures of MDEA and L-Arginine
3. Materials and Methods
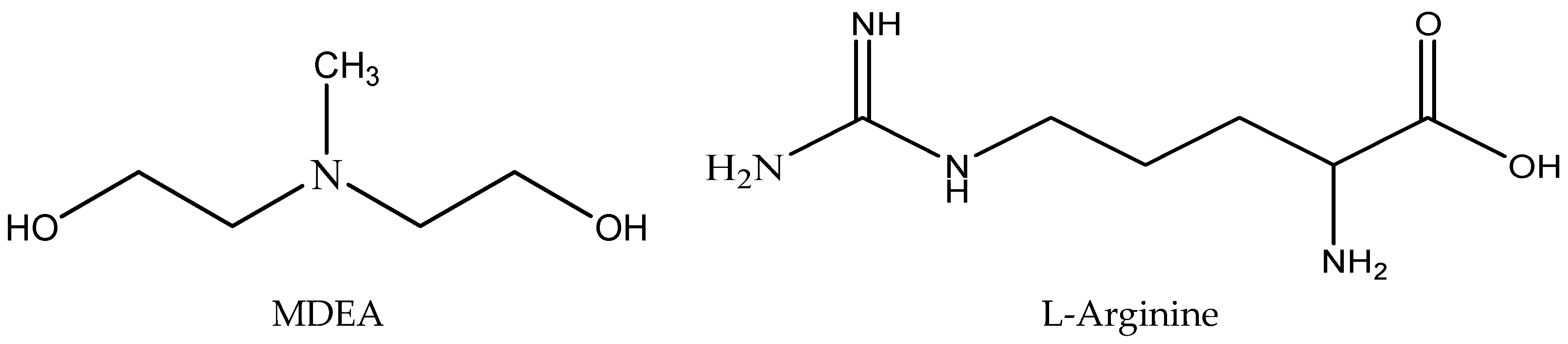
3.1. Materials
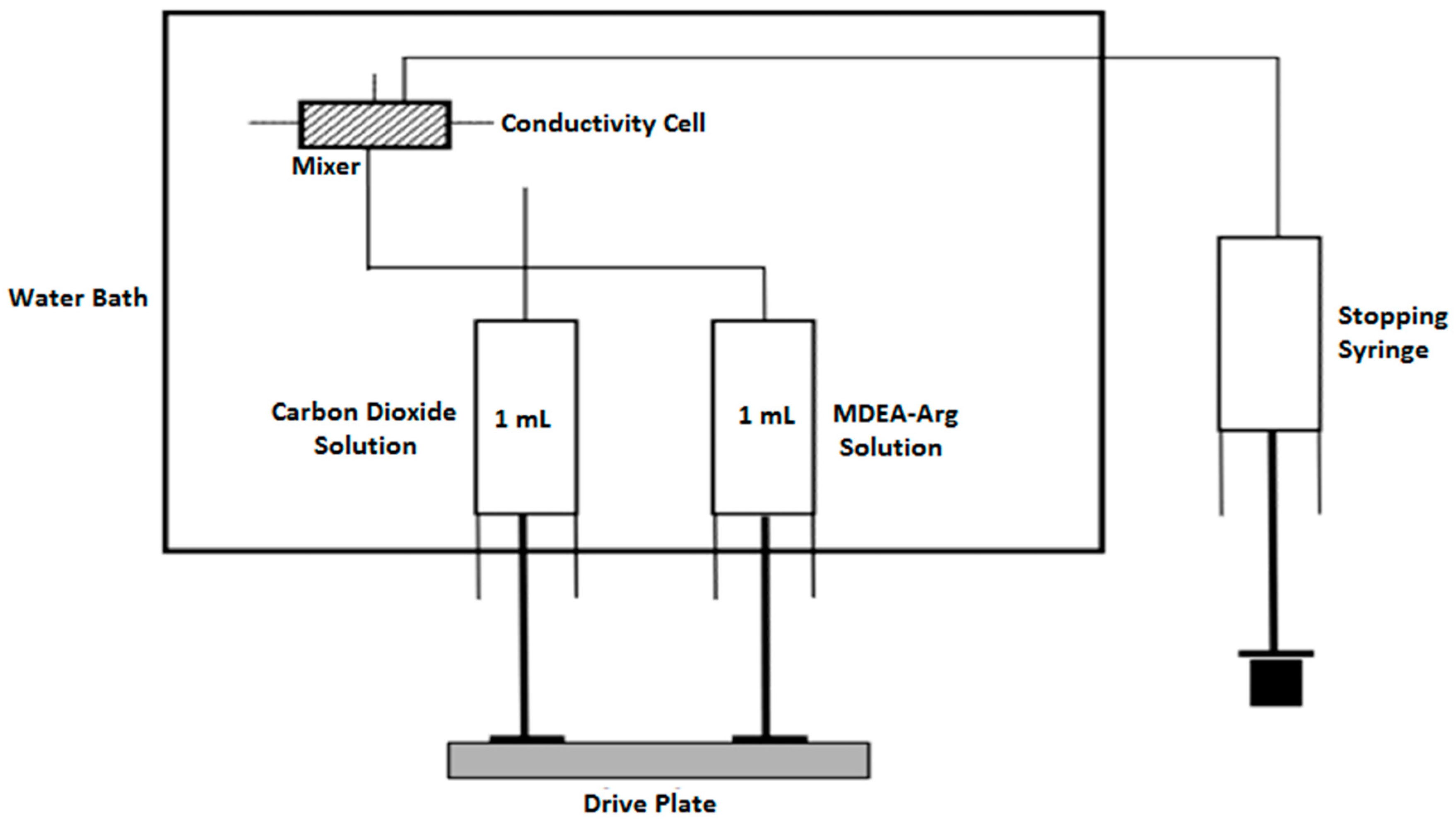
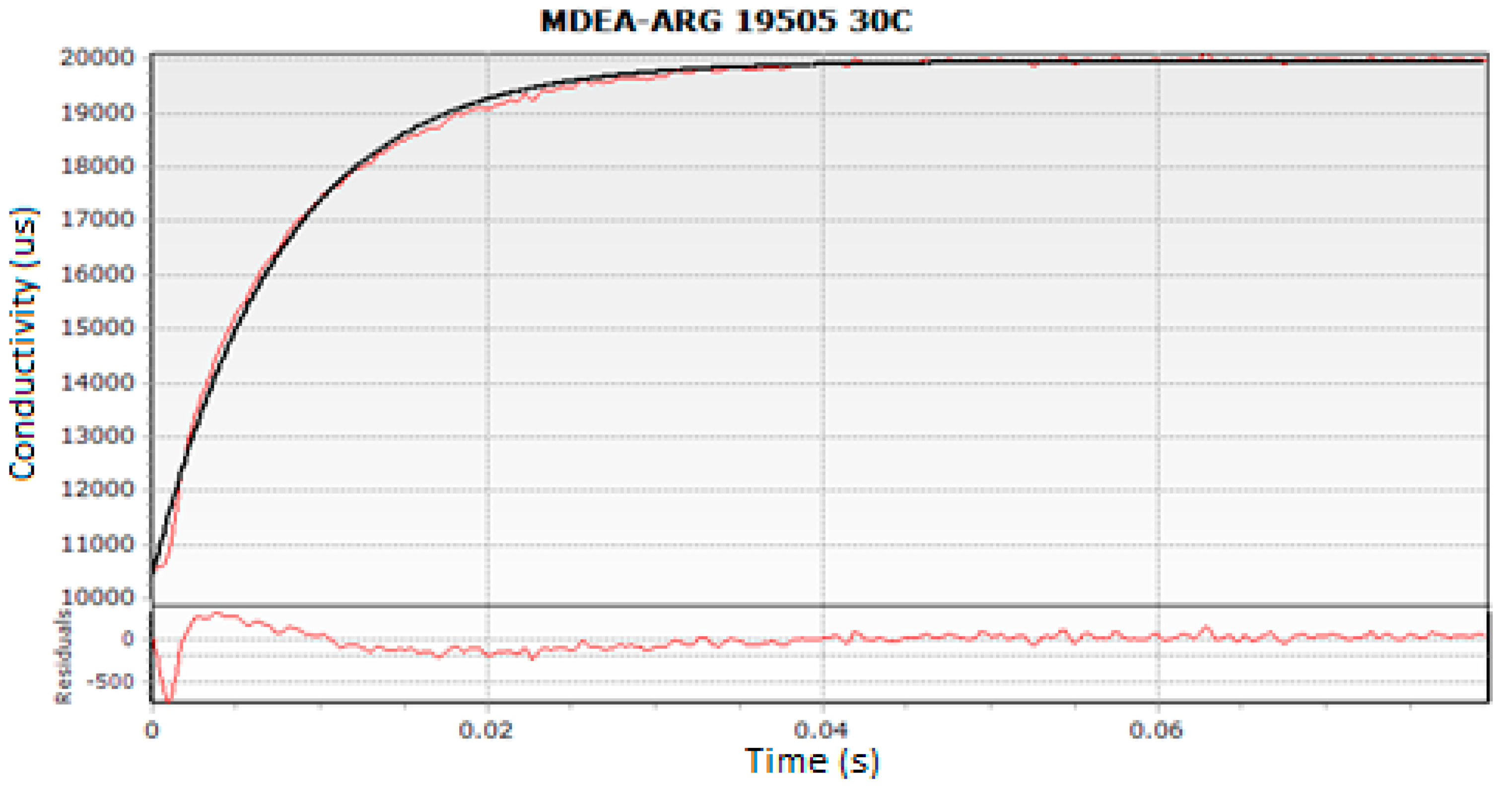
3.2. Methods
4. Results and Discussion
4.1. Reaction of CO2 with MDEA and L-Arginine
4.2. Zwitterion Mechanism
4.3. Termolecular Mechanism
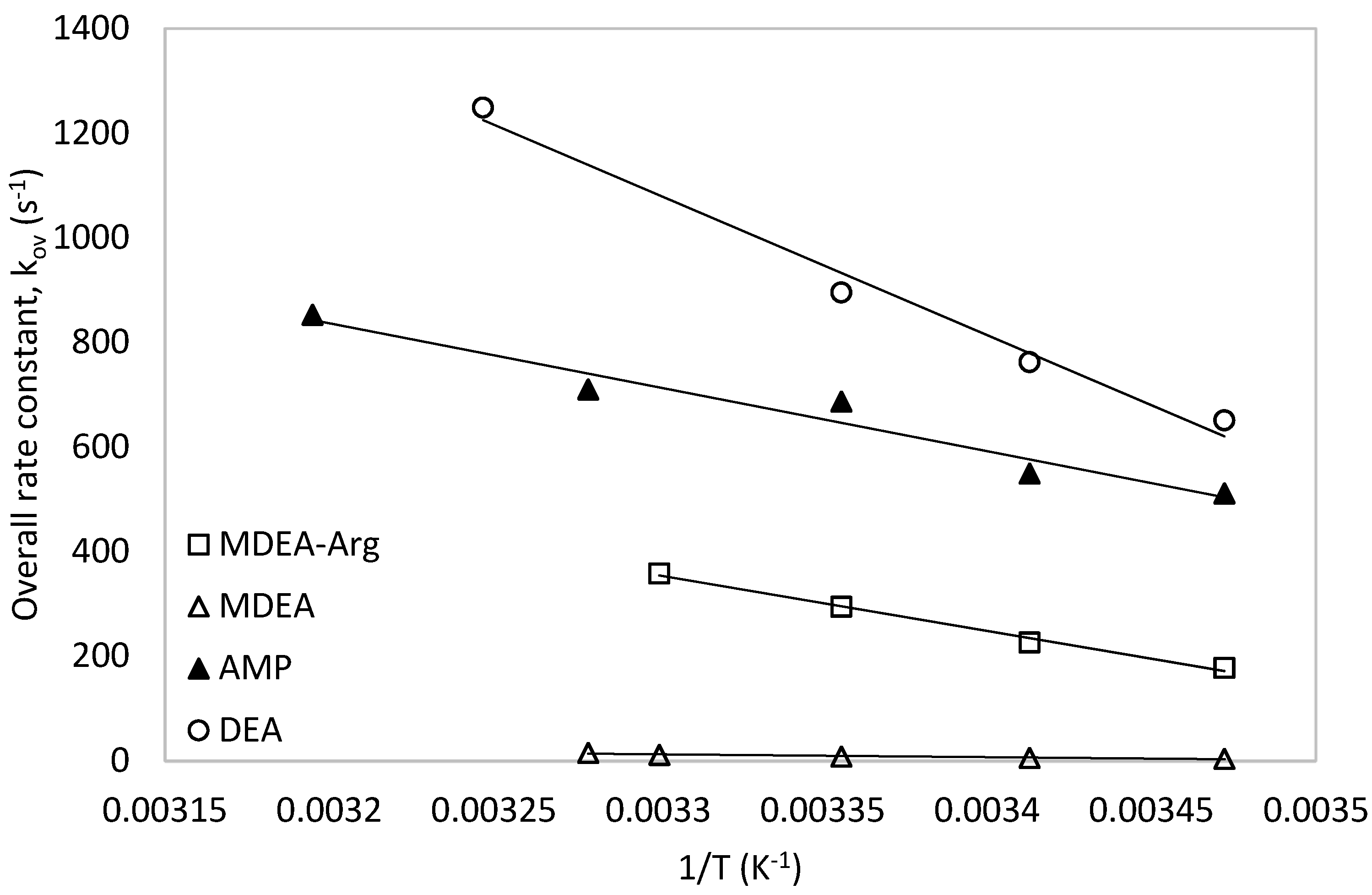
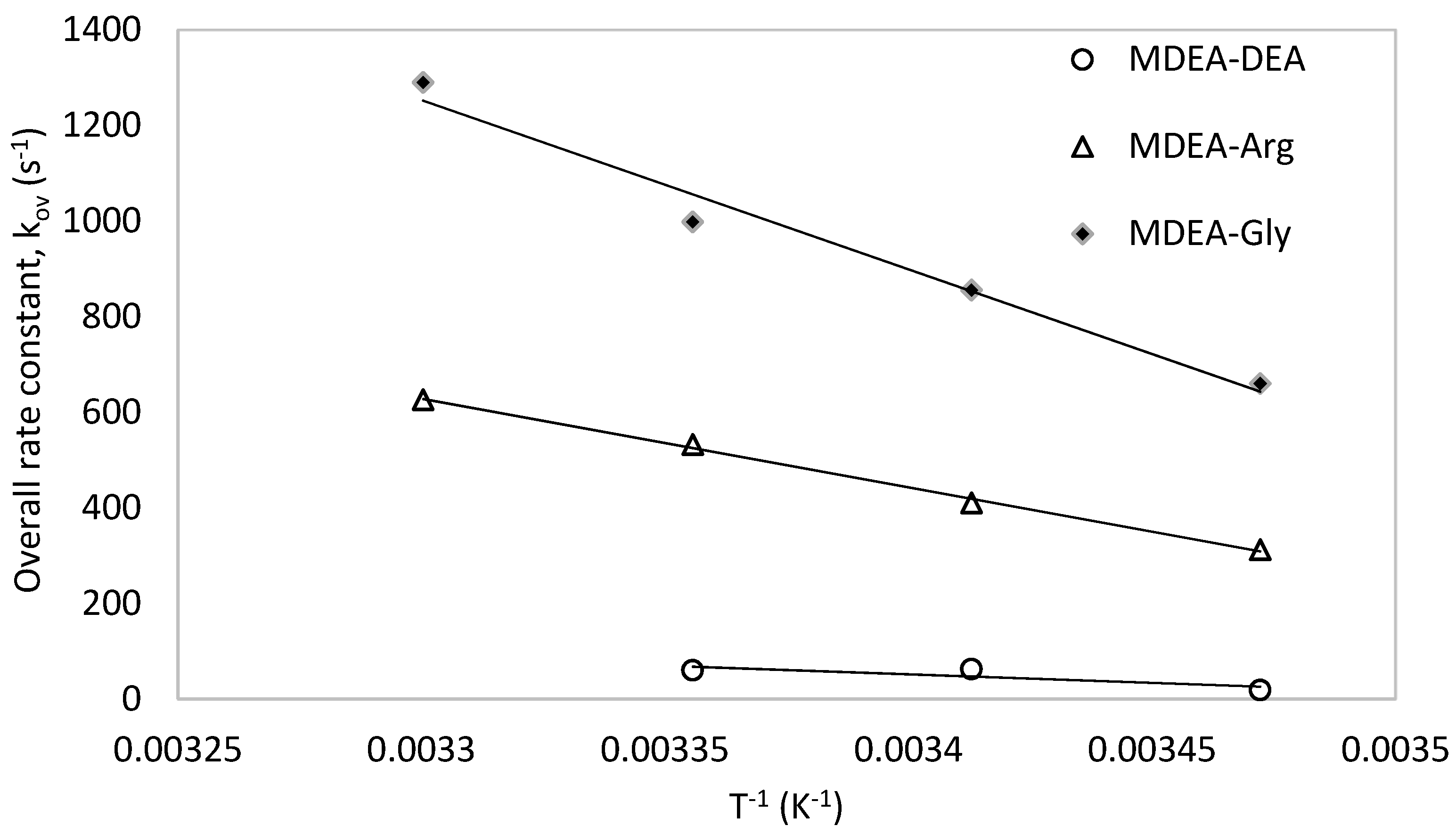
5. Comparison with Other Amine Systems
5.1. Comparison with Secondary, Tertiary and Sterically Hindered Amine
5.2. Comparison of the Promoting Effect of L-Arginine
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| MDEA | N-methyldiethanolamine |
| Arg | L-Arginine |
| Gly | Glycine |
| MEA | Monoethanolamine |
| DEA | Diethanolamine |
| AMP | 2-Amino-2-Methyl-1-Propanol |
| rCO2-MDEA (l mol−1 s−1) | Reaction rate of CO2 with MDEA |
| rCO2-OH (l mol−1 s−1) | Reaction rate of CO2 with hydroxyl ions |
| rCO2-OH (l mol−1 s−1) | Reaction rate of CO2 with L-Arginine |
| rCO2 (l mol−1 s−1) | Reaction rate of CO2 with MDEA-Arginine |
| (s−1) | Overall reaction rate of CO2 and MDEA |
| (s−1) | Overall reaction rate of CO2 and Hydroxyl ion |
| (s−1) | Overall reaction rate of CO2 and L-Arginine |
| kov (s−1) | Overall reaction rate with CO2 with MDEA and L-Arginine |
| k1 (m3 kmol−1 s−1) | Reaction rate constant of the formation of the intermediate Zwitterion |
| KMDEA (m3 kmol−1 s−1) | Reaction rate constant of CO2 and MDEA reaction. |
| KOH (m3 kmol−1 s−1) | Reaction rate constant of CO2 and hydroxyl ion reaction. |
| k−1 (s−1) | Reaction rate constant of the consumption of the intermediate Zwitterion |
| kb,I (s−1) | Individual reaction rate constants according to zwitterion mechanism |
| T (K) | Temperature |
| t (s) | Time |
| Kw (mol l−1) | Water dissociation constant |
| (mol l−1) | Protonation constant for MDEA and L-Arginine |
| kArg-exp (s−1) | Experimental apparent rate constant of CO2 and L-Arginine. |
| kArg-pred (s−1) | Predicted apparent rate constant of CO2 and L-Arginine. |
| Ea (kJ mol−1) | Activation energy |
| EaZ (kJ mol−1) | Activation energy obtained in zwitterion mechanism |
| EaT (kJ mol−1) | Activation energy obtained in termolecular mechanism |
| ka (m6 kmol−2 s−1) | Catalytic contribution of L-Arginine in the reaction rate according to zwitterion mechanism |
| khyd (m6 kmol−2 s−1) | Catalytic contribution of hydroxyl ion in the reaction rate according to zwitterion mechanism |
| kb (m6 kmol−2 s−1) | Catalytic contribution of MDEA in the reaction rate according to zwitterion mechanism |
References
- Tontiwachwuthikul, P.; Idem, R.; Gelowitz, D.; Liang, Z.H.; Supap, T.; Chan, C.W.; Sanpasertparnich, T.; Saiwan, C.; Smithson, H. Recent progress and new development of post-combustion carbon-capture technology using reactive solvents. Carbon Manag. 2011, 2, 261–263. [Google Scholar] [CrossRef]
- Zhou, Q.; Chan, C.W.; Tontiwachiwuthikul, P.; Idem, R.; Gelowitz, D. A statistical analysis of the carbon dioxide capture process. Int. J. Greenh. Gas Control 2009, 3, 535–544. [Google Scholar] [CrossRef]
- Rubin, E.S.; Mantripragada, H.; Marks, A.; Versteeg, P.; Kitchin, J. The outlook for improved carbon capture technology. Prog. Energy Combust. Sci. 2012, 38, 630–671. [Google Scholar] [CrossRef]
- Lee, A.S.; Kitchin, J.R. Chemical and Molecular Descriptors for the Reactivity of Amines with CO2. Ind. Eng. Chem. Res. 2012, 51, 13609–13618. [Google Scholar] [CrossRef]
- Zhang, Z.; Borhani, T.N.G.; El-Naas, M.H. Chapter 4.5—Carbon Capture. In Exergetic, Energetic and Environmental Dimensions; Dincer, I., Colpan, C.O., Kizilkan, O., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 997–1016. ISBN 978-0-12-813734-5. [Google Scholar]
- Zhang, Z.; Yan, Y.; Chen, Y.; Zhang, L. Investigation of CO2 absorption in methyldiethanolamine and 2-(1-piperazinyl)-ethylamine using hollow fiber membrane contactors: Part, C. Effect of operating variables. J. Nat. Gas Sci. Eng. 2014, 20, 58–66. [Google Scholar] [CrossRef]
- Chakravarty, T.; Phukan, U.K.; Weiland, R.H. Reaction of Acid Gases with mixtures of amines. Chem. Eng. Prog. 1985, 81, 32–36. [Google Scholar]
- Liang, Z.; Rongwong, W.; Liu, H.; Fu, K.; Gao, H.; Cao, F.; Zhang, R.; Sema, T.; Henni, A.; Sumon, K.; et al. Recent progress and new developments in post-combustion carbon-capture technology with amine based solvents. Int. J. Greenh. Gas Control 2015, 40, 26–54. [Google Scholar] [CrossRef]
- Benamor, A.; Ali, B.S.; Aroua, M.K. Kinetic of CO2 absorption and carbamate formation in aqueous solutions of diethanolamine. Korean J. Chem. Eng. 2008, 25, 451–460. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, Q.; Wang, B.; Yan, X.; Singh, S.; Zhang, F.; Gao, X.; Li, Y. Recent progress of amine modified sorbents for capturing CO2 from flue gas. Chin. J. Chem. Eng. 2018, 26, 2292–2302. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Munoz, D.M.; Portugal, A.F.; Lozano, A.E.; de la Campa, J.G.; de Abajo, J. New liquid absorbents for the removal of CO2 from gas mixtures. Energy Environ. Sci. 2009, 2, 883–891. [Google Scholar] [CrossRef]
- Brœder, P.; Svendsen, H.F. Capacity and Kinetics of Solvents for Post-Combustion CO2 Capture. Energy Procedia 2012, 23, 45–54. [Google Scholar] [CrossRef]
- Puxty, G.; Rowland, R.; Allport, A.; Yang, Q.; Bown, M.; Burns, R.; Maeder, M.; Attalla, M. Carbon Dioxide Postcombustion Capture: A Novel Screening Study of the Carbon Dioxide Absorption Performance of 76 Amines. Environ. Sci. Technol. 2009, 43, 6427–6433. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zhang, W.; Wang, J.; Soltanian, M.R.; Olabi, A.G. Effectiveness of amino acid salt solutions in capturing CO2: A review. Renew. Sustain. Energy Rev. 2018, 98, 179–188. [Google Scholar] [CrossRef]
- Lerche, B.M. CO2 Capture from Flue Gas Using Amino Acid Salt Solutions; Department of Chemical and Biochemical Engineering, Technical University of Denmark: Kongens Lyngby, Denmark, 2012. [Google Scholar]
- Goetheer, E.; Nell, L. First pilot results from TNO’s solvent development workflow. Carbon Capture J. 2009, 8, 2–3. [Google Scholar]
- Eide-Haugmo, I.; Brakstad, O.G.; Hoff, K.A.; da Silva, E.F.; Svendsen, H.F. Marine biodegradability and ecotoxicity of solvents for CO 2-capture of natural gas. Int. J. Greenh. Gas Control 2012, 9, 184–192. [Google Scholar] [CrossRef]
- Aronu, U.E.; Svendsen, H.F.; Hoff, K.A. Investigation of amine amino acid salts for carbon dioxide absorption. Int. J. Greenh. Gas Control 2010, 4, 771–775. [Google Scholar] [CrossRef]
- Kumar, P.; Hogendoorn, J.; Versteeg, G.; Feron, P. Kinetics of the reaction of CO2 with aqueous potassium salt of taurine and glycine. AIChE J. 2003, 49, 203–213. [Google Scholar] [CrossRef]
- Kumar, P.; Hogendoorn, J.; Feron, P.; Versteeg, G. Equilibrium solubility of CO2 in aqueous potassium taurate solutions: Part 1. Crystallization in carbon dioxide loaded aqueous salt solutions of amino acids. Ind. Eng. Chem. Res. 2003, 42, 2832–2840. [Google Scholar] [CrossRef]
- Aronu, U.E.; Hessen, E.T.; Haug-Warberg, T.; Hoff, K.A.; Svendsen, H.F. Vapor–liquid equilibrium in amino acid salt system: Experiments and modeling. Chem. Eng. Sci. 2011, 66, 2191–2198. [Google Scholar] [CrossRef]
- Kumar, P.; Hogendoorn, J.; Timmer, S.; Feron, P.; Versteeg, G. Equilibrium solubility of CO2 in aqueous potassium taurate solutions: Part 2. Experimental VLE data and model. Ind. Eng. Chem. Res. 2003, 42, 2841–2852. [Google Scholar] [CrossRef]
- Majchrowicz, M.; Brilman, D. Solubility of CO2 in aqueous potassium l-prolinate solutions—Absorber conditions. Chem. Eng. Sci. 2012, 72, 35–44. [Google Scholar] [CrossRef]
- Portugal, A.F.; Sousa, J.M.; Magalhães, F.D.; Mendes, A. Solubility of carbon dioxide in aqueous solutions of amino acid salts. Chem. Eng. Sci. 2009, 64, 1993–2002. [Google Scholar] [CrossRef]
- Schneider, R.; Schramm, H. Environmentally friendly and economic carbon capture from power plant flue gases. In Proceedings of the 1st Post Combustion Capture Conference, Abu Dhabi, UAE, 17–19 May 2011. [Google Scholar]
- Park, S.-W.; Son, Y.-S.; Park, D.-W.; Oh, K.-J. Absorption of carbon dioxide into aqueous solution of sodium glycinate. Sep. Sci. Technol. 2008, 43, 3003–3019. [Google Scholar] [CrossRef]
- Lee, S.; Song, H.-J.; Maken, S.; Park, J.-W. Kinetics of CO2 absorption in aqueous sodium glycinate solutions. Ind. Eng. Chem. Res. 2007, 46, 1578–1583. [Google Scholar] [CrossRef]
- Shen, S.; Yang, Y.-N.; Bian, Y.; Zhao, Y. Kinetics of CO2 Absorption into Aqueous Basic Amino Acid Salt: Potassium Salt of Lysine Solution. Environ. Sci. Technol. 2016, 50, 2054–2063. [Google Scholar] [CrossRef]
- Shen, S.; Yang, Y.-n.; Zhao, Y.; Bian, Y. Reaction kinetics of carbon dioxide absorption into aqueous potassium salt of histidine. Chem. Eng. Sci. 2016, 146, 76–87. [Google Scholar] [CrossRef]
- Portugal, A.; Derks, P.; Versteeg, G.; Magalhaes, F.; Mendes, A. Characterization of potassium glycinate for carbon dioxide absorption purposes. Chem. Eng. Sci. 2007, 62, 6534–6547. [Google Scholar] [CrossRef]
- Portugal, A.; Magalhaes, F.; Mendes, A. Carbon dioxide absorption kinetics in potassium threonate. Chem. Eng. Sci. 2008, 63, 3493–3503. [Google Scholar] [CrossRef]
- Hwang, K.-S.; Park, D.-W.; Oh, K.-J.; Kim, S.-S.; Park, S.-W. Chemical Absorption of Carbon Dioxide into Aqueous Solution of Potassium Threonate. Sep. Sci. Technol. 2010, 45, 497–507. [Google Scholar] [CrossRef]
- Wei, C.-C.; Puxty, G.; Feron, P. Amino acid salts for CO2 capture at flue gas temperatures. Chem. Eng. Sci. 2014, 107, 218–226. [Google Scholar] [CrossRef]
- Aronu, U.E.; Hartono, A.; Svendsen, H.F. Density, viscosity and N2O solubility of aqueous amino acid salt and amine amino acid salt solutions. J. Chem. Thermodyn. 2012, 45, 90–99. [Google Scholar] [CrossRef]
- Kumar, P.S.; Hogendoorn, J.; Feron, P.; Versteeg, G. Density, viscosity, solubility and diffusivity of N2O in aqueous amino acid salt solutions. J. Chem. Eng. Data 2001, 46, 1357–1361. [Google Scholar] [CrossRef]
- Hamborg, E.S.; Niederer, J.P.; Versteeg, G.F. Dissociation constants and thermodynamic properties of amino acids used in CO2 absorption from (293 to 353) K. J. Chem. Eng. Data 2007, 52, 2491–2502. [Google Scholar] [CrossRef]
- Hamborg, E.S.; van Swaaij, W.P.; Versteeg, G.F. Diffusivities in aqueous solutions of the potassium salt of amino acids. J. Chem. Eng. Data 2008, 53, 1141–1145. [Google Scholar] [CrossRef]
- Van Holst, J.; Versteeg, G.; Brilman, D.; Hogendoorn, J. Kinetic study of CO2 with various amino acid salts in aqueous solution. Chem. Eng. Sci. 2009, 64, 59–68. [Google Scholar] [CrossRef]
- Dalton, J.B.; Schmidt, C.L. The solubilities of certain amino acids in water, the densities of their solutions at twenty-five degrees and the calculated heats of solution and partial molal volumes. J. Biol. Chem. 1933, 103, 549–578. [Google Scholar]
- Mahmud, N.; Benamor, A.; Nasser, M.S.; Al-Marri, M.J.; Qiblawey, H.; Tontiwachwuthikul, P. Reaction kinetics of carbon dioxide with aqueous solutions of l-Arginine, Glycine & Sarcosine using the stopped flow technique. Int. J. Greenh. Gas Control 2017, 63, 47–58. [Google Scholar] [CrossRef]
- Benamor, A.; Al-Marri, M.J.; Khraisheh, M.; Nasser, M.S.; Tontiwachwuthikul, P. Reaction kinetics of carbon dioxide in aqueous blends of N-methyldiethanolamine and glycine using the stopped flow technique. J. Nat. Gas Sci. Eng. 2016, 33, 186–195. [Google Scholar] [CrossRef]
- Pinsent, B.R.W.; Pearson, L.; Roughton, F.J.W. The kinetics of combination of carbon dioxide with hydroxide ions. Trans. Faraday Soc. 1956, 52, 1512–1520. [Google Scholar] [CrossRef]
- Caplow, M. Kinetics of carbamate formation and breakdown. J. Am. Chem. Soc. 1968, 90, 6795–6803. [Google Scholar] [CrossRef]
- Crooks, J.E.; Donnellan, J.P. Kinetics and mechanism of the reaction between carbon dioxide and amines in aqueous solution. J. Chem. Soc. Perkin Trans. 1989, 2, 331–333. [Google Scholar] [CrossRef]
- Da Silva, E.F.; Svendsen, H.F. Computational chemistry study of reactions, equilibrium and kinetics of chemical CO2 absorption. Int. J. Greenh. Gas Control 2007, 1, 151–157. [Google Scholar] [CrossRef]
- Ali, S.H.; Merchant, S.Q.; Fahim, M.A. Kinetic study of reactive absorption of some primary amines with carbon dioxide in ethanol solution. Sep. Purifi. Technol. 2000, 18, 163–175. [Google Scholar] [CrossRef]
- Knipe, A.C.; McLean, D.; Tranter, R.L. A fast response conductivity amplifier for chemical kinetics. J. Phys. E Sci. Instrum. 1974, 7, 586–590. [Google Scholar] [CrossRef]
- Alper, E.; Bouhamra, W. Kinetics and mechanisms of reaction between carbon disulphide and morpholine in aqueous solutions. Chem. Eng. Technol. 1994, 17, 138–140. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Kenig, E.Y. Termolecular Kinetic Model for CO2-Alkanolamine Reactions: An Overview. Chem. Eng. Technol. 2010, 33, 1577–1581. [Google Scholar] [CrossRef]
- Peters, L.; Hussain, A.; Follmann, M.; Melin, T.; Hägg, M.B. CO2 removal from natural gas by employing amine absorption and membrane technology—A technical and economical analysis. Chem. Eng. J. 2011, 172, 952–960. [Google Scholar] [CrossRef]
- Astaria, G.; Savage, D.W.; Bisio, A. Gas Treating with Chemical Solvents; John Wiley & Sons Inc.: New York, NY, USA, 1983; ISBN 978-0471057680. [Google Scholar]
- Littel, R.J.; Bos, M.; Knoop, G.J. Dissociation constants of some alkanolamines at 293, 303, 318 and 333 K. J. Chem. Eng. Data 1990, 35, 276–277. [Google Scholar] [CrossRef]
- Edwards, T.; Maurer, G.; Newman, J.; Prausnitz, J. Vapor-liquid equilibria in multicomponent aqueous solutions of volatile weak electrolytes. AIChE J. 1978, 24, 966–976. [Google Scholar] [CrossRef]
- Guo, D.; Thee, H.; Tan, C.Y.; Chen, J.; Fei, W.; Kentish, S.; Stevens, G.W.; da Silva, G. Amino Acids as Carbon Capture Solvents: Chemical Kinetics and Mechanism of the Glycine + CO2 Reaction. Energy Fuels 2013, 27, 3898–3904. [Google Scholar] [CrossRef]
- Littel, R.J.; Versteeg, G.F.; Van Swaaij, W.P.M. Kinetics of CO2 with primary and secondary amines in aqueous solutions—II. Influence of temperature on zwitterion formation and deprotonation rates. Chem. Eng. Sci. 1992, 47, 2037–2045. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.-W.; Otto, F.D.; Mather, A.E. Kinetics of the reaction of carbon dioxide with 2-amino-2-methyl-1-propanol solutions. Chem. Eng. Sci. 1996, 51, 841–850. [Google Scholar] [CrossRef]
- Benamor, A.; Al-Marri, M.J. Reactive Absorption of CO2 into Aqueous Mixtures of Methyldiethanolamine and Diethanolamine. Int. J. Chem. Eng. Appl. 2014, 5, 291–297. [Google Scholar] [CrossRef]
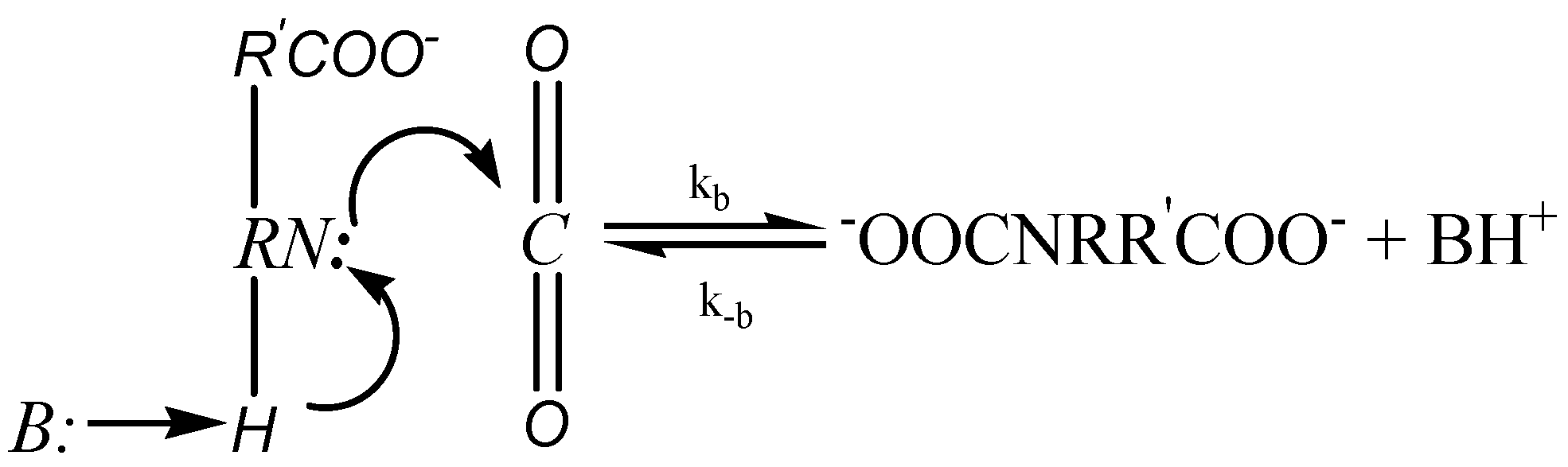
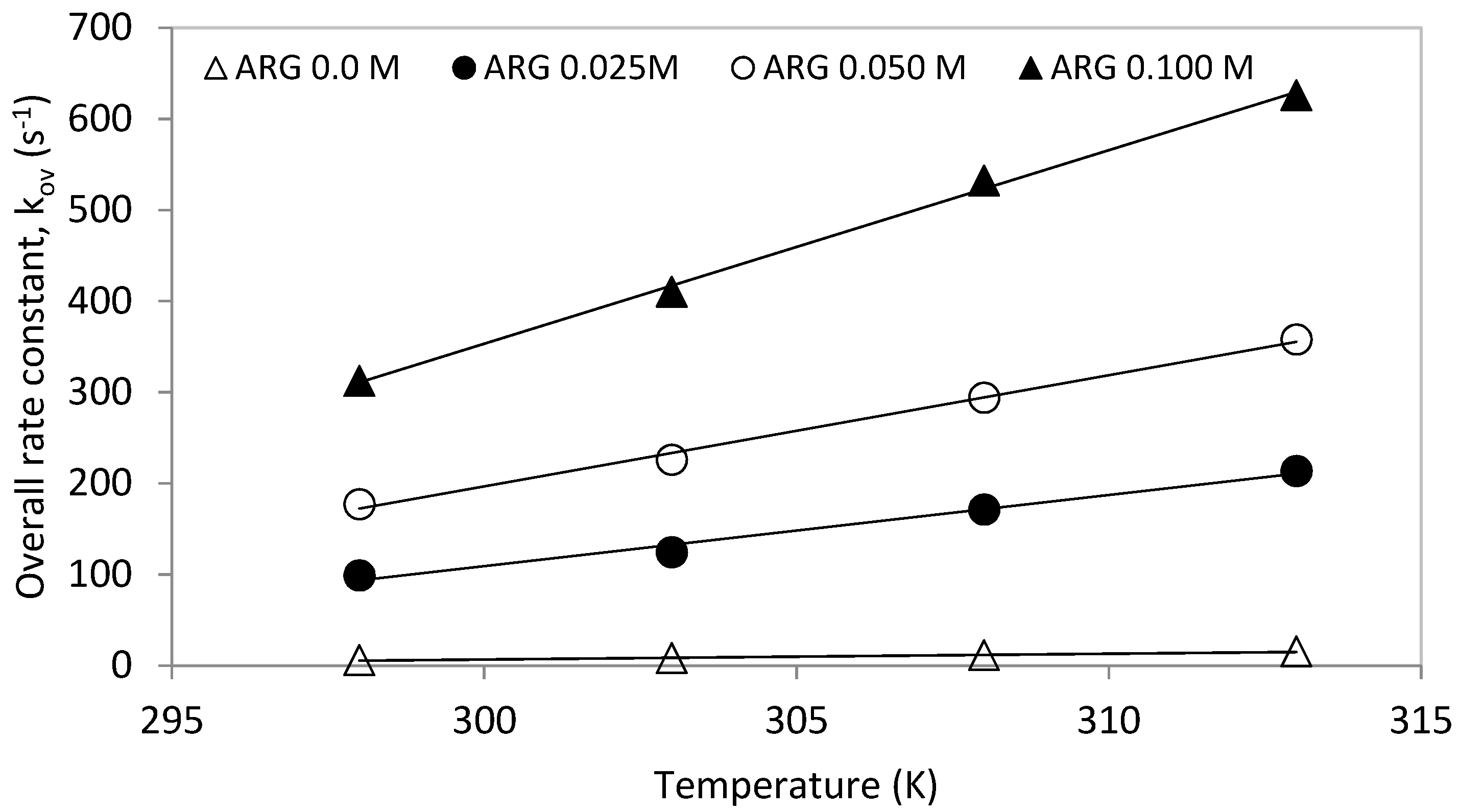
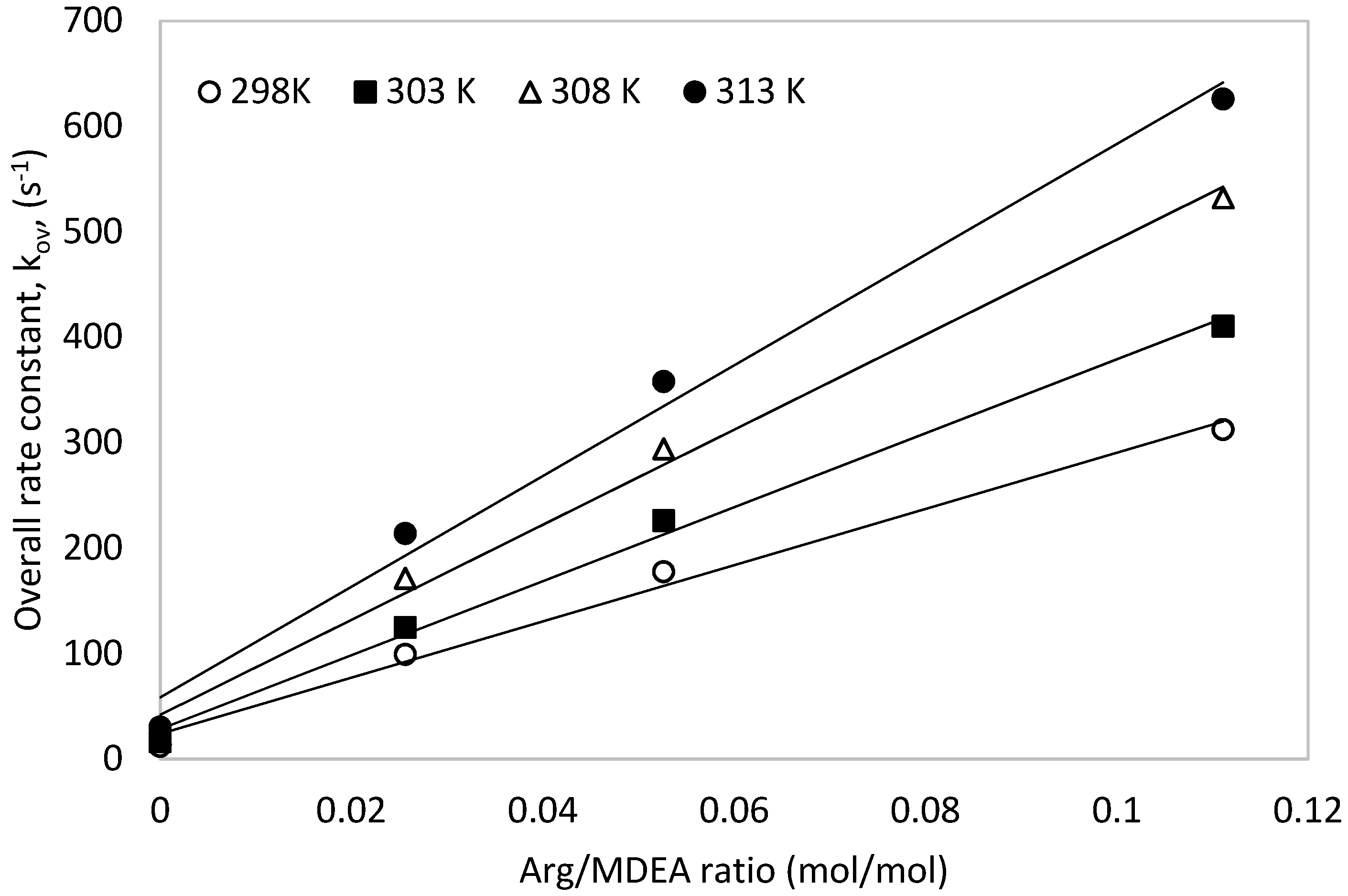
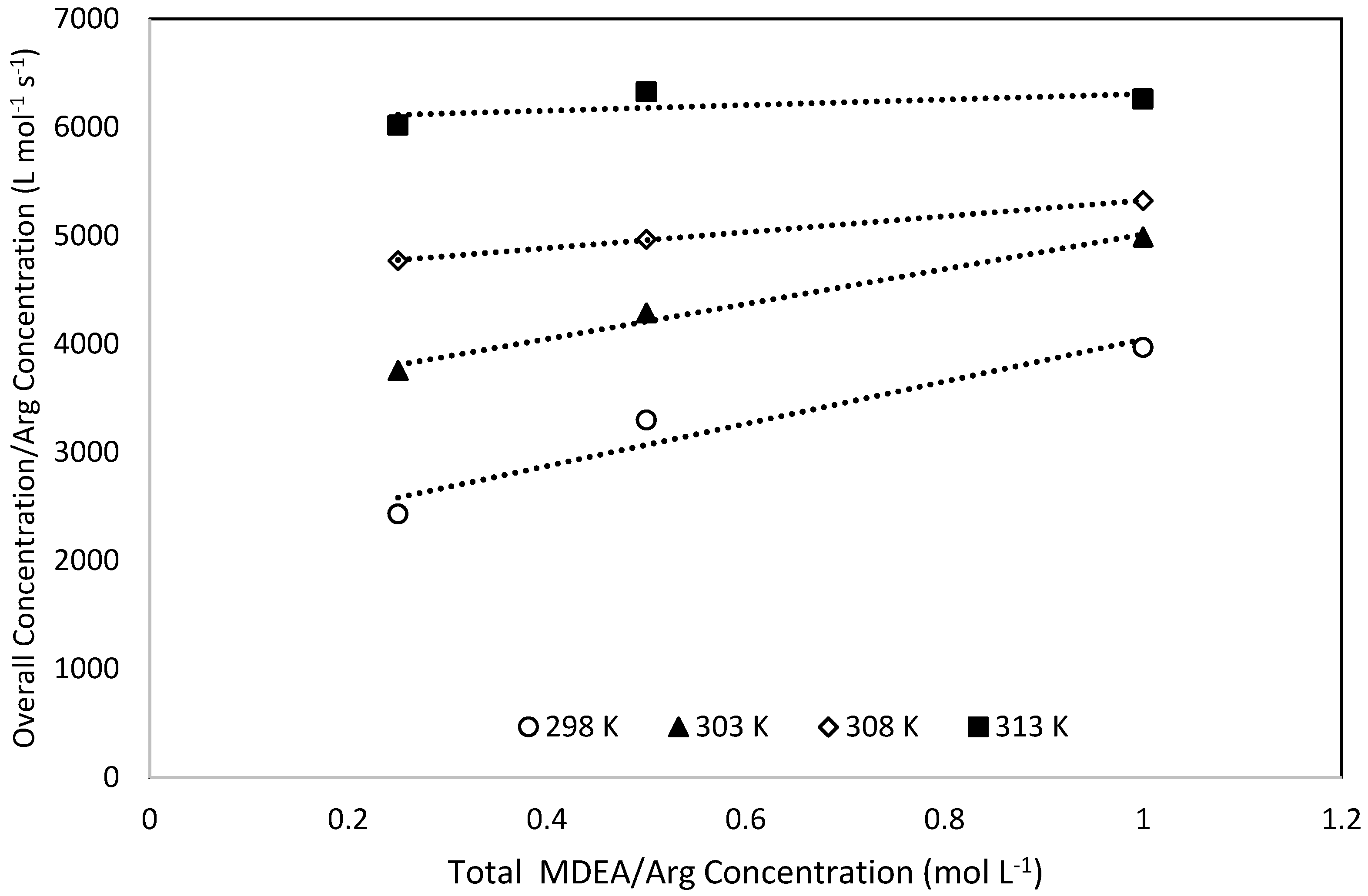
| ARG | MDEA | OH × 103 | H2O | kov | kArg-exp | kArg-pred | AAD% |
|---|---|---|---|---|---|---|---|
| mol L−1 | mol L−1 | mol L−1 | mol L−1 | s−1 | s−1 | s−1 | |
| 298 K | |||||||
| 0.025 | 0.975 | 2.07 | 49.16 | 99.17 | 93.30 | 80.50 | |
| 0.050 | 0.950 | 2.07 | 49.14 | 177.47 | 171.70 | 166.90 | |
| 0.100 | 0.900 | 2.07 | 49.08 | 312.35 | 306.90 | 356.00 | |
| 0.025 | 0.475 | 1.46 | 52.35 | 82.40 | 79.50 | 70.60 | 7.60 |
| 0.050 | 0.450 | 1.46 | 52.32 | 152.55 | 149.80 | 148.10 | |
| 0.100 | 0.400 | 1.46 | 52.26 | 320.43 | 318.00 | 321.80 | |
| 0.025 | 0.225 | 1.03 | 53.94 | 60.75 | 59.40 | 65.00 | |
| 0.050 | 0.200 | 1.03 | 53.91 | 125.47 | 124.30 | 137.50 | |
| 0.075 | 0.175 | 1.03 | 53.88 | 214.23 | 213.20 | 216.90 | |
| 303 K | |||||||
| 0.025 | 0.975 | 2.21 | 49.13 | 124.65 | 116.50 | 104.30 | |
| 0.050 | 0.950 | 2.21 | 49.11 | 226.15 | 218.20 | 216.20 | |
| 0.100 | 0.900 | 2.21 | 49.05 | 410.33 | 402.80 | 460.40 | |
| 0.025 | 0.475 | 1.56 | 52.33 | 107.15 | 103.20 | 91.00 | 6.30 |
| 0.050 | 0.450 | 1.56 | 52.31 | 195.64 | 191.90 | 190.80 | |
| 0.100 | 0.400 | 1.56 | 52.25 | 423.84 | 420.50 | 414.30 | |
| 0.025 | 0.225 | 1.10 | 53.93 | 78.32 | 76.40 | 83.50 | |
| 0.050 | 0.200 | 1.10 | 53.90 | 165.36 | 163.70 | 176.50 | |
| 0.075 | 0.175 | 1.10 | 53.88 | 281.52 | 280.00 | 278.40 | |
| 308 K | |||||||
| 0.025 | 0.975 | 2.35 | 49.11 | 171.38 | 160.20 | 136.10 | |
| 0.050 | 0.950 | 2.35 | 49.08 | 294.32 | 283.40 | 281.40 | |
| 0.100 | 0.900 | 2.35 | 49.03 | 532.24 | 521.90 | 596.90 | |
| 0.025 | 0.475 | 1.66 | 52.32 | 149.90 | 144.40 | 117.60 | 8.20 |
| 0.050 | 0.450 | 1.66 | 52.29 | 234.18 | 229.00 | 246.00 | |
| 0.100 | 0.400 | 1.66 | 52.24 | 496.55 | 491.90 | 532.50 | |
| 0.025 | 0.225 | 1.17 | 53.92 | 101.41 | 98.80 | 106.90 | |
| 0.050 | 0.200 | 1.17 | 53.90 | 231.21 | 228.90 | 225.80 | |
| 0.075 | 0.175 | 1.17 | 53.87 | 357.73 | 355.70 | 355.80 | |
| 313 K | |||||||
| 0.025 | 0.975 | 2.49 | 49.09 | 213.71 | 198.40 | 167.80 | |
| 0.050 | 0.950 | 2.49 | 49.07 | 357.97 | 343.10 | 347.80 | |
| 0.100 | 0.900 | 2.49 | 49.02 | 625.84 | 611.70 | 740.80 | |
| 0.025 | 0.475 | 1.76 | 52.31 | 188.23 | 180.80 | 145.90 | 8.40 |
| 0.050 | 0.450 | 1.76 | 52.29 | 310.72 | 303.70 | 306.10 | |
| 0.100 | 0.400 | 1.76 | 52.24 | 632.39 | 626.10 | 665.10 | |
| 0.025 | 0.225 | 1.24 | 53.92 | 130.31 | 126.80 | 133.50 | |
| 0.050 | 0.200 | 1.24 | 53.90 | 302.60 | 299.50 | 282.50 | |
| 0.075 | 0.175 | 1.24 | 53.87 | 451.29 | 448.50 | 445.90 | |
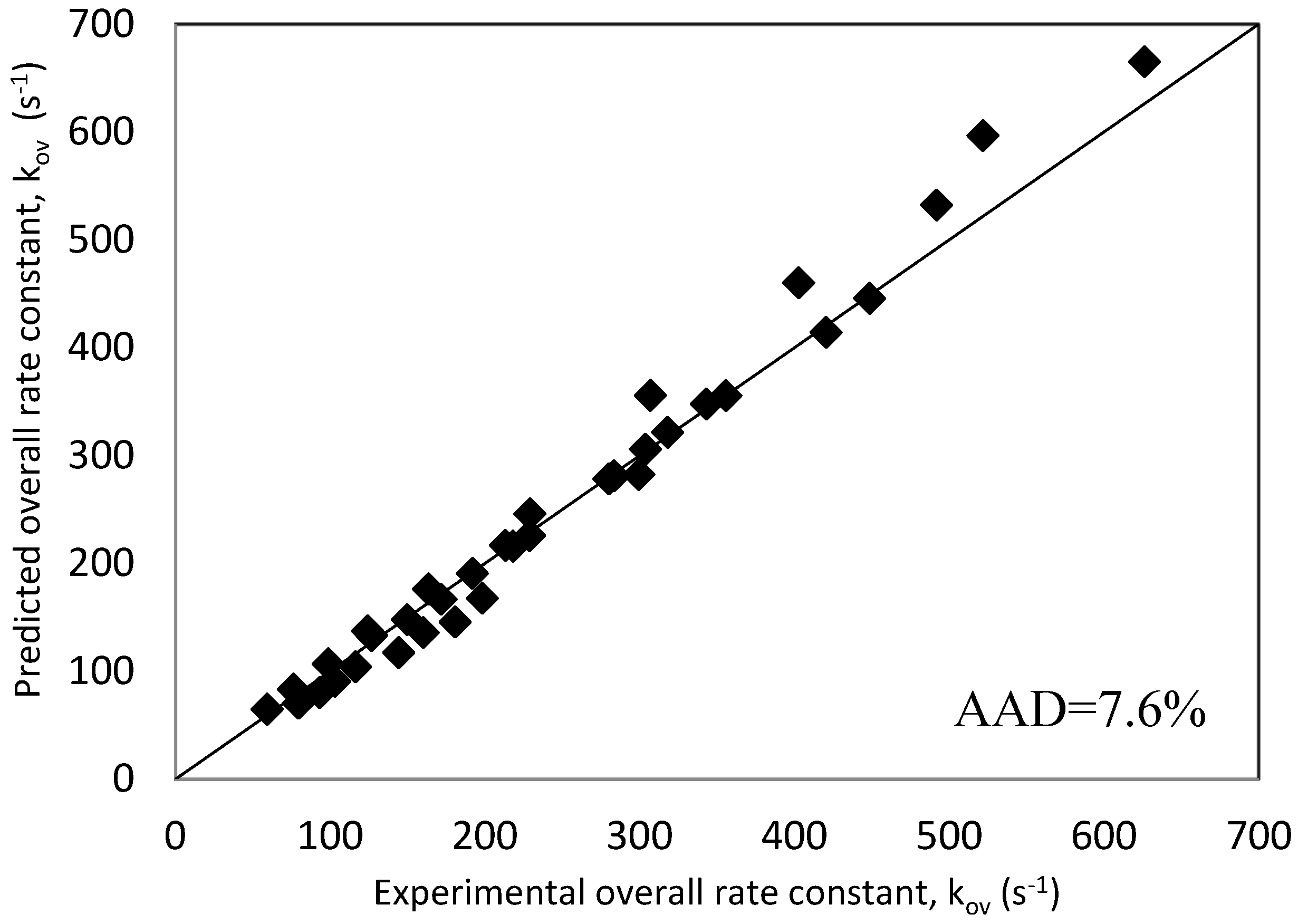
| AAD% | 7.60 | ||||||
| Rate | Equation | References |
|---|---|---|
| kMDEA (m3 kmol−2 s−1) | Benamor et al. [42] | |
| kOH (m3 kmol−2 s−1) | Pinsent et al. [43] | |
| k2 (m3 kmol−1 s−1) | Mahmud et al. [41] | |
| ka (m6 kmol−2 s−1) | Mahmud et al. [41] | |
| kw (m6 kmol−2 s−1) | Mahmud et al. [41] * |
| Parameter | ai | bi | ci | di | Validity Range | Source |
|---|---|---|---|---|---|---|
| Kp1(MDEA) | −8483.95 | −13.8328 | 0 | 87.39717 | 293–333 K | [53] |
| Kp2(Arginine) | −3268.3 | 0 | 0 | −9.9729 | 293–323 K | [41] |
| Kw | −13445.9 | −22.4773 | 0 | 140.932 | 273–498 K | [54] |
| Temperature | 298 K | 303 K | 308 K | 313 K |
|---|---|---|---|---|
| Rate constant, kb (m6 kmol−2 s−1) | 2321.0 | 3127.5 | 4400.5 | 5130 |
| Rate | References | |||
|---|---|---|---|---|
| Lnk0 | Ea (kJ mol−1) | Expression | ||
| kMDEA (m3 kmol−2 s−1) | 21.66 | 49.24 | Benamor et al. [42] | |
| kOH (m3 kmol−2 s−1) | 24.49 | 554.26 | Pinsent et al. [43] | |
| k2 (m3 kmol−2 s−1) | 24.06 | 37.27 | Mahmud et al. [41] | |
| ka (m6 kmol−2 s−1) | 25.10 | 38.28 | Mahmud et al. [41] | |
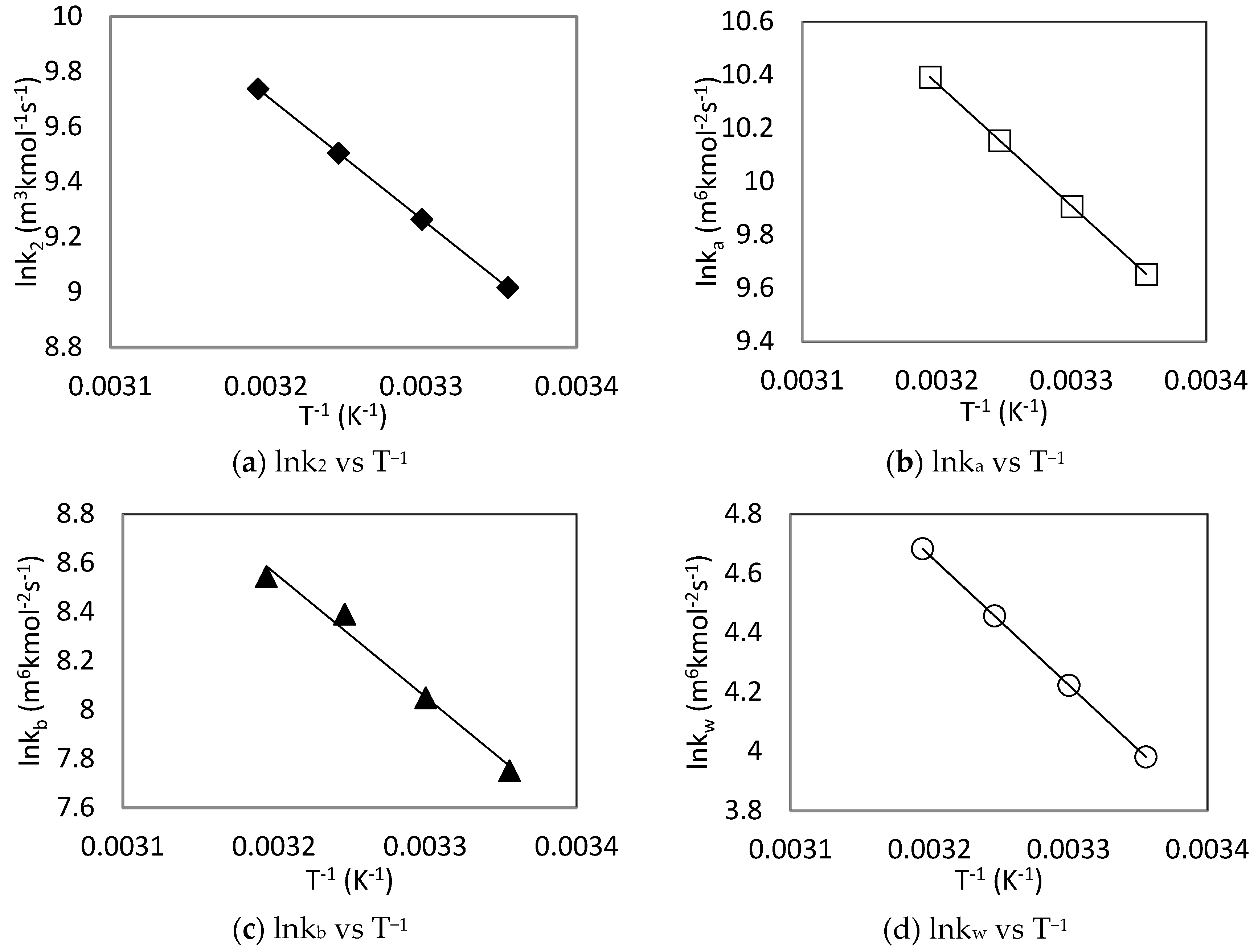
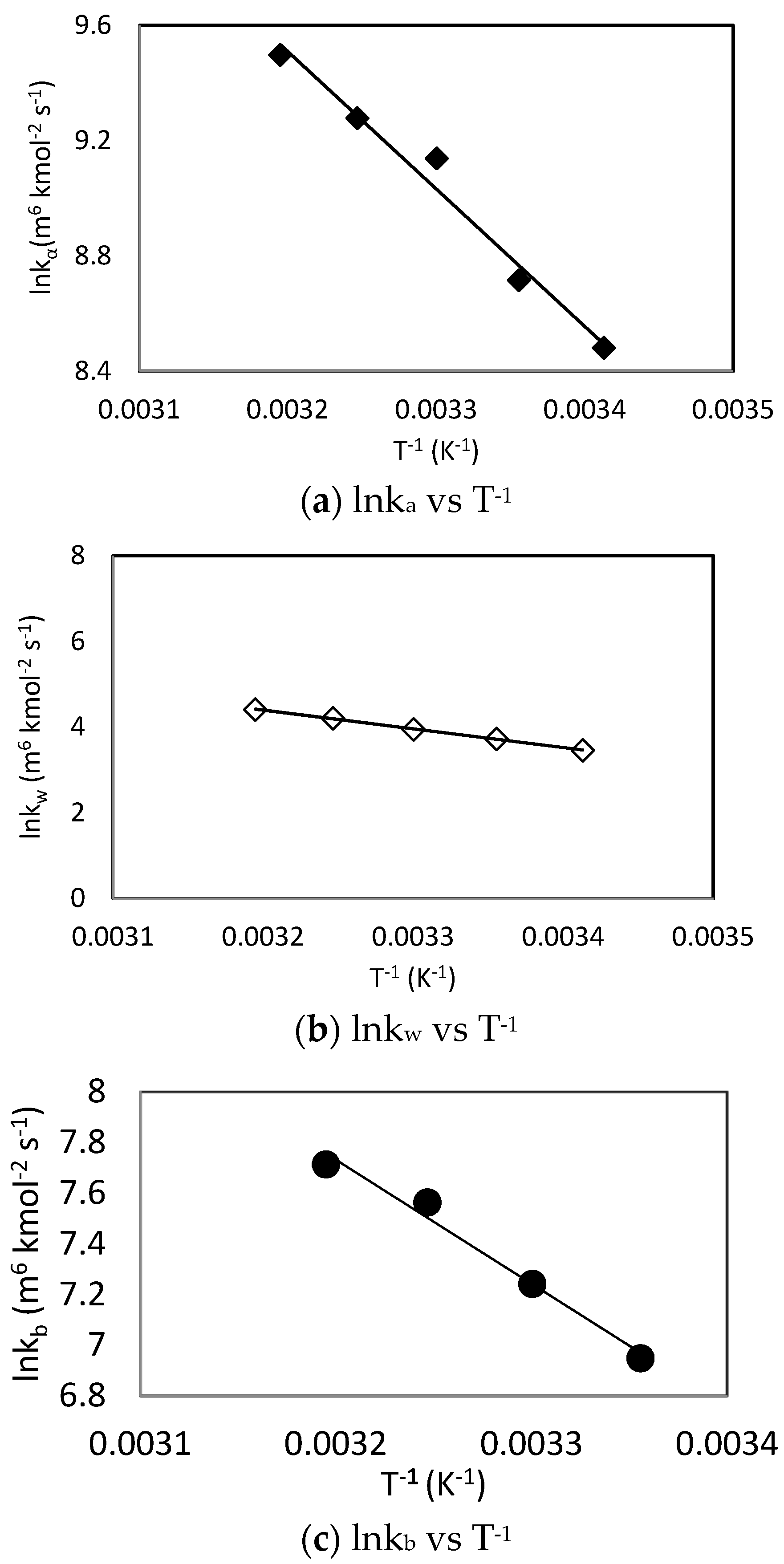
| kb (m6 kmol−2 s−1) | 24.83 | 42.27 | This Work | |
| kw (m6 kmol−2 s−1) | 18.63 | 36.29 | Mahmud et al. [41] * | |
| Rate | Equation | References |
|---|---|---|
| ka (m6 kmol−2 s−1) | Mahmud et al. [4] | |
| kw (m6 kmol−2 s−1) | Mahmud et al. [41] |
| ARG | MDEA | OH | H2O | kArg-exp | kArg-pred | AAD% |
|---|---|---|---|---|---|---|
| mol L−1 | mol L−1 | mol L−1 | mol L−1 | s−1 | s−1 | |
| 298 K | ||||||
| 0.025 | 0.975 | 3.2 × 10−4 | 49.16 | 93.3 | 79.7 | |
| 0.050 | 0.950 | 4.5 × 10−4 | 49.14 | 171.8 | 166.2 | |
| 0.100 | 0.900 | 6.2 × 10−4 | 49.08 | 306.9 | 358.9 | |
| 0.025 | 0.475 | 2.3 × 10−4 | 52.35 | 79.5 | 70.0 | 7.8 |
| 0.050 | 0.450 | 3.1 × 10−4 | 52.32 | 149.8 | 146.6 | |
| 0.100 | 0.400 | 4.1 × 10−4 | 52.26 | 318.0 | 319.9 | |
| 0.025 | 0.225 | 1.5 × 10−4 | 53.94 | 59.4 | 65.1 | |
| 0.050 | 0.200 | 2.1 × 10−4 | 53.91 | 124.3 | 136.8 | |
| 0.075 | 0.175 | 2.4 × 10−4 | 53.88 | 213.2 | 215.2 | |
| 303 K | ||||||
| 0.025 | 0.975 | 3.0 × 10−4 | 49.16 | 116.5 | 103.4 | |
| 0.050 | 0.950 | 4.2 × 10−4 | 49.14 | 218.2 | 215.3 | |
| 0.100 | 0.900 | 5.8 × 10−4 | 49.08 | 402.8 | 465.2 | |
| 0.025 | 0.475 | 2.1 × 10−4 | 52.35 | 103.2 | 90.0 | 6.9 |
| 0.050 | 0.450 | 2.9 × 10−4 | 52.32 | 191.9 | 188.7 | |
| 0.100 | 0.400 | 3.8 × 10−4 | 52.26 | 420.5 | 411.9 | |
| 0.025 | 0.225 | 1.4 × 10−4 | 53.94 | 76.4 | 83.4 | |
| 0.050 | 0.200 | 1.9 × 10−4 | 53.91 | 163.7 | 175.4 | |
| 0.075 | 0.175 | 2.2 × 10−4 | 53.88 | 280.1 | 276.0 | |
| 308 K | ||||||
| 0.025 | 0.98 | 2.8 × 10−4 | 49.16 | 160.2 | 134.7 | |
| 0.050 | 0.95 | 3.9 × 10−4 | 49.14 | 283.4 | 280.3 | |
| 0.100 | 0.90 | 5.4 × 10−4 | 49.08 | 521.9 | 604.6 | |
| 0.025 | 0.48 | 2.0 × 10−4 | 52.35 | 144.5 | 115.8 | 8.6 |
| 0.050 | 0.45 | 2.7 × 10−4 | 52.32 | 229.0 | 242.6 | |
| 0.100 | 0.40 | 3.6 × 10−4 | 52.26 | 492.0 | 529.2 | |
| 0.025 | 0.23 | 1.3 × 10−4 | 53.94 | 98.8 | 106.4 | |
| 0.050 | 0.20 | 1.8 × 10−4 | 53.91 | 228.9 | 223.8 | |
| 0.075 | 0.18 | 2.1 × 10−4 | 53.88 | 355.7 | 352.2 | |
| 313 K | ||||||
| 0.025 | 0.98 | 2.6 × 10−4 | 49.16 | 198.5 | 164.8 | |
| 0.050 | 0.95 | 3.6 × 10−4 | 49.14 | 343.1 | 343.9 | |
| 0.100 | 0.90 | 5.0 × 10−4 | 49.08 | 611.8 | 745.2 | |
| 0.025 | 0.48 | 1.8 × 10−4 | 52.35 | 180.8 | 143.3 | 8.8 |
| 0.050 | 0.45 | 2.5 × 10−4 | 52.32 | 303.7 | 301.0 | |
| 0.100 | 0.40 | 3.3 × 10−4 | 52.26 | 626.1 | 659.5 | |
| 0.025 | 0.23 | 1.3 × 10−4 | 53.94 | 126.8 | 132.6 | |
| 0.050 | 0.20 | 1.7 × 10−4 | 53.91 | 299.5 | 279.6 | |
| 0.075 | 0.18 | 1.9 × 10−4 | 53.88 | 448.6 | 441.0 | |
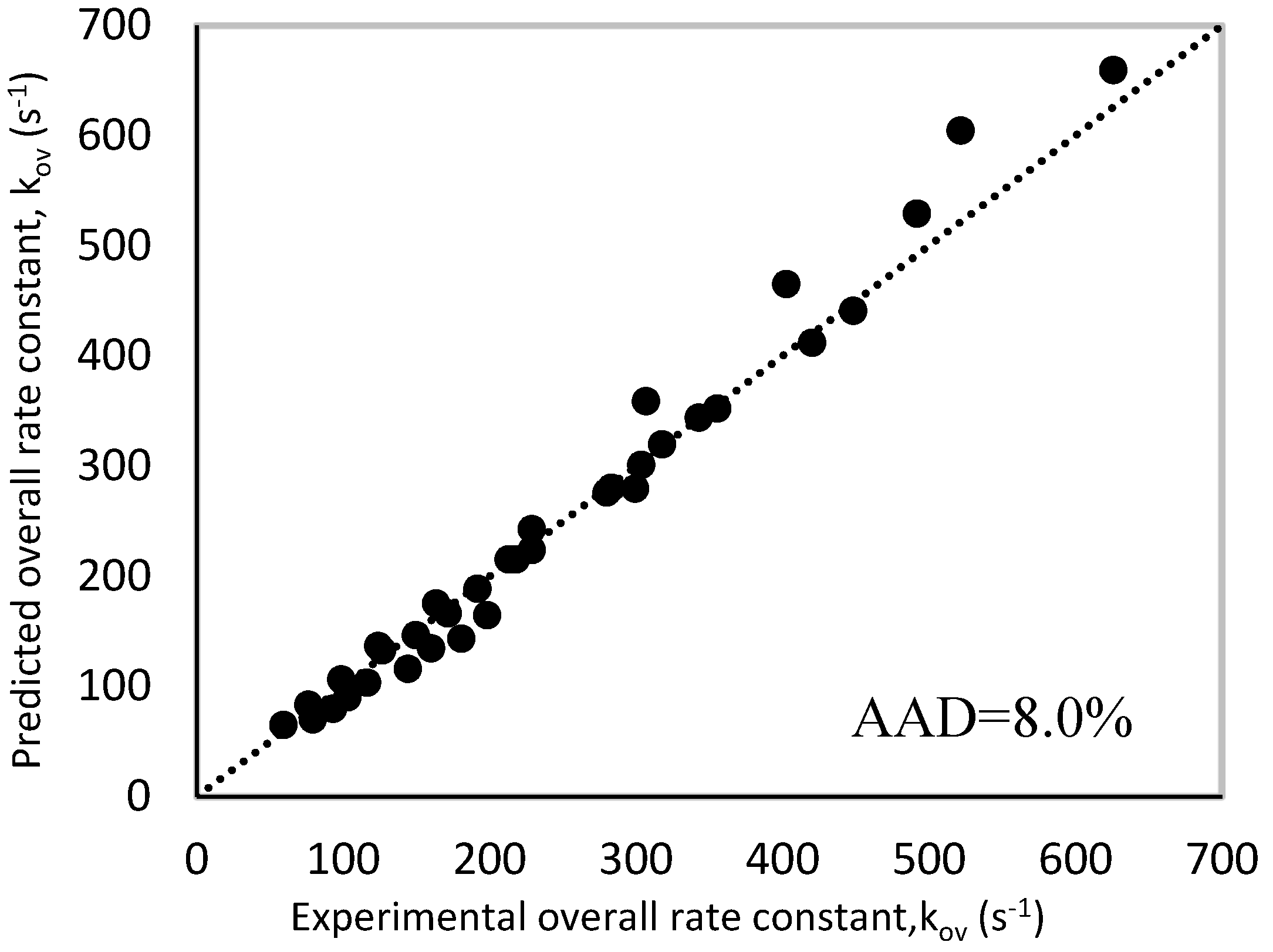
| Overall AAD% | 8.0 | |||||
| Rate Constant | 298 K | 303 K | 308 K | 313 K |
|---|---|---|---|---|
| kb (m6 kmol−2 s−1) | 1043.0 | 1397.0 | 1927.0 | 2240.0 |
| Rate | References | |||
|---|---|---|---|---|
| lnk0 | Ea (kJ mol−1) | Expression | ||
| kMDEA (m3 kmol−2 s−1) | 21.66 | 49.24 | Benamor et al. [42] | |
| kOH (m3 kmol−2 s−1) | 24.49 | 554.26 | Pinsent et al. [43] | |
| ka (m6 kmol−2 s−1) | 24.77 | 38.28 | Mahmud et al. [41] | |
| kb (m6 kmol−2 s−1) | 23.38 | 40.65 | This Work | |
| kw (m6 kmol−2 s−1) | 18.63 | 36.29 | Mahmud et al. [41] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmud, N.; Benamor, A.; Nasser, M.; El-Naas, M.H.; Tontiwachwuthikul, P. Reaction Kinetics of Carbon Dioxide in Aqueous Blends of N-Methyldiethanolamine and L-Arginine Using the Stopped-Flow Technique. Processes 2019, 7, 81. https://doi.org/10.3390/pr7020081
Mahmud N, Benamor A, Nasser M, El-Naas MH, Tontiwachwuthikul P. Reaction Kinetics of Carbon Dioxide in Aqueous Blends of N-Methyldiethanolamine and L-Arginine Using the Stopped-Flow Technique. Processes. 2019; 7(2):81. https://doi.org/10.3390/pr7020081
Chicago/Turabian StyleMahmud, Nafis, Abdelbaki Benamor, Mustafa Nasser, Muftah H. El-Naas, and Paitoon Tontiwachwuthikul. 2019. "Reaction Kinetics of Carbon Dioxide in Aqueous Blends of N-Methyldiethanolamine and L-Arginine Using the Stopped-Flow Technique" Processes 7, no. 2: 81. https://doi.org/10.3390/pr7020081
APA StyleMahmud, N., Benamor, A., Nasser, M., El-Naas, M. H., & Tontiwachwuthikul, P. (2019). Reaction Kinetics of Carbon Dioxide in Aqueous Blends of N-Methyldiethanolamine and L-Arginine Using the Stopped-Flow Technique. Processes, 7(2), 81. https://doi.org/10.3390/pr7020081








