New Approaches in Modeling and Simulation of CO2 Absorption Reactor by Activated Potassium Carbonate Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Mathematical Modeling of the Packed Reactor
- expression of chemisorption rate;
- characterization of phases circulation regime;
- characterization of thermal regime.
- stationary operating regime;
- ideal plug flow phase in the reactor;
- isothermal regime;
- isobar conditions;
- the gas phase contains one component soluble in the liquid phase (CO2).
- K2CO3 reacts with the soluble gas component;
- K2CO3 component does not undergo transformations at the reaction temperature;
- the solvent evaporates in the first part, while condensation occurs in the second part of the reactor;
- the reaction is irreversible;
- specific heat is considered to be constant.
3.1. Mass Balance
3.2. Heat Balance
3.3. Impulse Balance
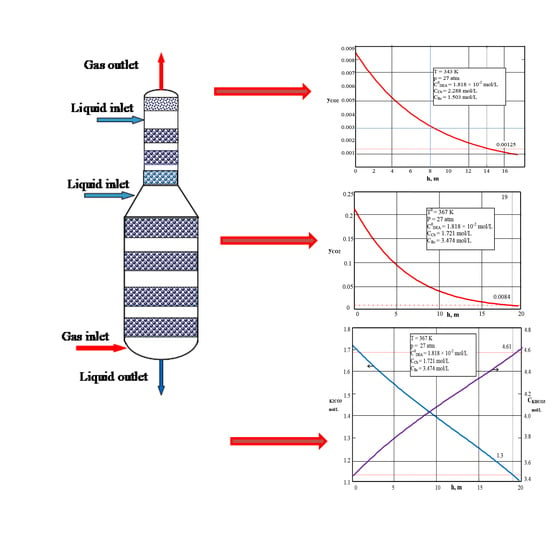
4. Testing the Proposed Mathematical Model
4.1. Testing the Mathematical Model of the Reactor under Isobar–Isotherm Conditions
4.2. Testing the Mathematical Model of the Reactor under Adiabatic Conditions
4.3. Testing the Impulse Transfer Equations
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviation
| A | column section, (m2) |
| CCb, CBc | molar concentration of K2CO3 and KHCO3, (mol/L) |
| CO2 concentration in the liquid phase, (mol/L) | |
| molar concentration of DEA, (mol/L) | |
| CpL, Cpg | molar heat capacity at constant pressure for liquid and gas (J/mol grad) |
| dech | equivalent diameter, (m) |
| dz | elemental thickness/height of differential section element, (m) |
| d | diameter of the flow section, in Equations (40) and (41), (m) |
| f | friction factor, dimensionless |
| G | mass gas flow, (kg/m2s) |
| g | gravitational acceleration, (m2/s) |
| h | height, (m) |
| hg, hL | enthalpy of gas and liquid, (J/mol) |
| i | interface |
| L | the liquid flow, (kg/m2s) |
| Mg, ML | molar mass of gas and liquid, (g/mol) |
| molar ratio | |
| nA” | inert gas flow, (mol/s) |
| ng, nL | molar flux of gas and liquid, (mol/s) |
| molar debit of H2O, (mol/s) | |
| p | pressure, (atm) |
| Sv | gas-liquid interfacial area per unit of liquid volume (m2/m3) |
| Tg, TL | temperature of gas and liquid, (K) |
| wg | gas velocity, (m/s) |
| CO2 molar fraction | |
| H2O molar fraction | |
| z | elemental length/height of the reactor element, (m) |
| αL, αg | heat transfer coefficients for liquid and gas, (W/m2grad) |
| ε | porosity |
| , | thermal reaction effect, thermal dissolution effect, (J/mol) |
| λL, λg | thermal conductivity for liquid and gas, (W/m grad) |
| ρL, ρg | density of liquid and gas phase, (kg/m3) |
| reaction rate component CO2 per unit of liquid volume, (mol/m3s) | |
| 0 | superscript, initial |
References
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Harja, M.; Ciobanu, G.; Rusu, L.; Lazar, L. The effectiveness factor approach for chemical absorption process. Environ. Eng. Manag. J. 2018, 17, 813–820. [Google Scholar]
- Soreanu, G.; Tomaszewicz, M.; Fernandez-Lopez, M.; Valverde, J.L.; Zuwała, J.; Sanchez-Silva, L. CO2 gasification process performance for energetic valorization of microalgae. Energy 2017, 119, 37–43. [Google Scholar] [CrossRef]
- Harja, M.; Rusu, L.; Barbuta, M. The viscosity and density of the aqueous solutions used for the absorption accompanied by chemical reaction. Bull. Pol. Inst. Iasi. Chem. Chem. Eng. 2008, 9, 55–62. [Google Scholar]
- Koronaki, I.P.; Prentza, L.; Papaefthimiou, V. Modeling of CO2 capture via chemical absorption processes —An extensive literature review. Renew. Sustain. Energy Rev. 2015, 50, 547–566. [Google Scholar] [CrossRef]
- Isa, F.; Suleman, H.; Zabiri, H.; Maulud, A.S.; Ramasamy, M.; Tufa, L.D.; Shariff, A.M. An overview on CO2 removal via absorption: Effect of elevated pressures in counter-current packed column. J. Nat. Gas Sci. Eng. 2016, 33, 666–677. [Google Scholar] [CrossRef]
- Tollkötter, A.; Kockmann, N. Absorption and Chemisorption of Small Levitated Single Bubbles in Aqueous Solutions. Processes 2014, 2, 200–215. [Google Scholar] [CrossRef]
- Omar, H.M.; Rohani, S. Removal of CO2 from landfill gas with landfill leachate using absorption process. Int. J. Greenh. Gas Control 2017, 58, 159–168. [Google Scholar] [CrossRef]
- Fernández, J.; Sotenko, M.; Derevschikov, V.; Lysikov, A.; Rebrov, E.V. A radiofrequency heated reactor system for post-combustion carbon capture. Chem. Eng. Process. 2016, 108, 17–26. [Google Scholar] [CrossRef]
- Sotenko, M.; Fernández, J.; Hu, G.; Derevschikov, V.; Lysikov, A.; Parkhomchuk, E.; Rebrov, E.V. Performance of novel CaO-based sorbents in high temperature CO2 capture under RF heating. Chem. Eng. Process. 2017, 122, 487–492. [Google Scholar] [CrossRef]
- Onda, K.; Sada, E.; Takeuchi, H. Gas absorption with chemical reaction in packed columns. J. Chem. Eng. Jpn. 1968, 1, 62–66. [Google Scholar] [CrossRef]
- Tait, P.; Buschle, B.; Milkowski, K.; Akram, M.; Pourkashanian, M.; Lucquiaud, M. Flexible operation of post-combustion CO2 capture at pilot scale with demonstration of capture-efficiency control using online solvent measurements. Int. J. Greenh. Gas Control 2018, 71, 253–277. [Google Scholar] [CrossRef]
- Tan, L.S.; Shariff, A.M.; Lau, K.K.; Bustam, M.A. Factors affecting CO2 absorption efficiency in packed column: A review. J. Ind. Eng. Chem. 2012, 18, 1874–1883. [Google Scholar] [CrossRef]
- Petrescu, S.; Harja, M. Chemical Reactor for Heterogen Systems; Venus Publishing House: Iasi, Romania, 2006. [Google Scholar]
- Binczak, G.; Pohorecki, R.; Moniuk, W.; Jakubowska-Mordecka, Z. Studies on carbon dioxide absorption rate into solutions of K2CO3/KHCO3 activated with amines. Industrial application of research results. Przem. Chem. 2017, 96, 681–684. [Google Scholar]
- Wu, Y.; Mirza, N.R.; Hu, G.; Smith, K.H.; Stevens, G.W.; Mumford, K.A. Precipitating characteristics of potassium bicarbonate using concentrated potassium carbonate solvent for carbon dioxide capture. Part I: Nucleation. Ind. Eng. Chem. Res. 2017, 56, 6764–6774. [Google Scholar] [CrossRef]
- Petrescu, S.; Mămăligă, I.; Ivaniciuc, M. Enhancement factor of the mass transfer for chemisorption process. Rev. Chim. 1998, 1, 64–67. [Google Scholar]
- Wang, F.; Zhao, X.; Liang, Y.; Li, X.; Chen, Y. Calculation Model and Rapid Estimation Method for Coal Seam Gas Content. Processes 2018, 6, 223. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Hu, C.; Wang, Y.; Shen, A.; Liang, S. Experimental Study on Feasibility of Enhanced Gas Recovery through CO2 Flooding in Tight Sandstone Gas Reservoirs. Processes 2018, 6, 224. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, Y.E.; Nam, S.C.; Park, S.Y.; Chun, I.S.; Yoon, Y.I.; Lee, J.H. Promoter Characteristic Study on the K2CO3 Absorbents for CO2 Capture: Mass Transfer According to Functional Group and Chain Length of Promoter. Energy Procedia 2017, 114, 898–905. [Google Scholar] [CrossRef]
- Devries, N.P. CO2 Absorption into Concentrated Carbonate Solutions with Promoters at Elevated Temperatures. Master’s Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2014. [Google Scholar]
- Khan, A.A.; Halder, G.N.; Saha, A.K. Experimental investigation on efficient carbon dioxide capture using piperazine (PZ) activated aqueous methyldiethanolamine (MDEA) solution in a packed column. Int. J. Greenh. Gas Control 2017, 64, 163–173. [Google Scholar] [CrossRef]
- Harja, M.; Siminicanu, I. Modelling and simulation of the industrial reactor for carbon dioxideabsorption into activated potassium carbonate aqueous solution. Ovidius Univ. Ann. Chem. 2000, XI, 135–139. [Google Scholar]
- Cowan, R.M.; Jensen, M.D.; Pei, P.; Steadman, E.N.; Harju, J.A. Current Status of CO2 Capture Technology Development and Application; National Energy Technology Laboratory, US Department of Energy: Morgantown, WV, USA, 2011.
- Hilliard, M.D. A Predictive Thermodynamic Model for an Aqueous Blend of Potassium Carbonate, Piperazine, and Monoethanolamine for Carbon Dioxide Capture from Flue Gas. Ph.D. Thesis, University of Texas at Austin, Austin, TX, USA, 2008. [Google Scholar]
- Sivtsova, O.N.; Eremenko, S.I.; Derevschikov, V.S.; Veselovskaya, J.V. Kinetics of carbon dioxide absorption from air in a flow reactor with a fixed bed of K2CO3-based sorbent. Russ. J. Phys. Chem. A 2017, 91, 850–855. [Google Scholar] [CrossRef]
- Tay, W.H.; Lau, K.K.; Shariff, A.M. High performance promoter-free CO2 absorption using potassium carbonate solution in an ultrasonic irradiation system. J. CO2 Util. 2017, 21, 383–394. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y. Surfactants facilitating carbonic anhydrase enzyme-mediated CO2 absorption into a carbonate solution. Environ. Sci. Technol. 2017, 51, 8537–8543. [Google Scholar] [CrossRef] [PubMed]
- Hagiu, C.; Ivaniciuc, M. Determination of the enhancement factor for CO2 absorption in activated potassium carbonate solution. Rev. Chim. 1997, 10–11, 856–862. [Google Scholar]
- Hu, G.; Smith, K.; Wu, Y.; Kentish, S.; Stevens, G. Recent progress on the performance of different rate promoters in potassium carbonate solvents for CO2 capture. Energy Procedia 2017, 114, 2279–2286. [Google Scholar] [CrossRef]
- Bui, M.; Gunawan, I.; Verheyen, V.; Feron, P.; Meuleman, E.; Adeloju, S. Dynamic modelling and optimisation of flexible operation in post-combustion CO2 capture plants: A review. Comput. Chem. Eng. 2014, 61, 245–265. [Google Scholar] [CrossRef]
- Saidi, M. Mathematical modeling of CO2 absorption into reactive DEAB solution in packed columns using surface-renewal penetration theory. J. Taiwan Inst. Chem. E 2017, 80, 301–313. [Google Scholar] [CrossRef]
- Altway, A.; Susianto, S.; Suprapto, S.; Nurkhamidah, S.; Nisa, N.I.F.; Hardiyanto, F.; Altway, S. Modeling and Simulation of CO2 Absorption into Promoted Aqueous Potassium Carbonate Solution in Industrial Scale Packed Column. Bull. Chem. React. Eng. Catal. 2015, 10, 111–124. [Google Scholar] [CrossRef]
- Xu, Z.; Afacan, A.; Chuang, K. Predicting mass transfer in packed columns containing structured packings. Chem. Eng. Res. Des. 2000, 78, 91–98. [Google Scholar] [CrossRef]
- Harja, M. Contributions to the Modeling of Gas—Liquid Absorption with Chemical Reaction. Ph.D. Thesis, “Gheorghe Asachi” Technical University of Iasi, Iasi, Romania, 1999. [Google Scholar]
- Salvinder, K.M.S.; Zabiri, H.; Isa, F.; Taqvi, S.A.; Roslan, M.A.H.; Shariff, A.M. Dynamic modelling, simulation and basic control of CO2 absorption based on high pressure pilot plant for natural gas treatment. Int. J. Greenh. Gas Control 2018, 70, 164–177. [Google Scholar] [CrossRef]
- Tang, L.; Ji, J.; Cai, Y.; Qu, G. Study on Mass Transfer Rate of CO2 Absorption Process. Int. J. Dyn. Fluids 2017, 13, 335–344. [Google Scholar]
- Todinca, T.; Tănasie, C.; Pröll, T.; Căta, A. Absorption with chemical reaction: Evaluation of rate promoters effect on CO2 absorption in hot potassium carbonate solutions. Comput. Aided Chem. Eng. 2007, 24, 1065–1070. [Google Scholar]
- Wang, G.; Yuan, X.; Yu, K. Review of mass-transfer correlations for packed columns. Ind. Eng. Chem. Res. 2005, 44, 8715–8729. [Google Scholar] [CrossRef]
- Das, B.; Deogam, B.; Mandal, B. Experimental and theoretical studies on efficient carbon dioxide capture using novel bis (3-aminopropyl) amine (APA)-activated aqueous 2-amino-2-methyl-1-propanol (AMP) solutions. RSC Adv. 2017, 7, 21518–21530. [Google Scholar] [CrossRef]
- Aroonwilas, A.; Chakma, A.; Tontiwachwuthikul, P.; Veawab, A.; Austgen, D.M.; Rochelle, G.T.; Peng, X.; Chen, C.C. Mathematical modelling of mass transfer and hydrodynamics in CO2 absorbers packed with structured packings. Chem. Eng. Sci. 2003, 58, 4037–4053. [Google Scholar] [CrossRef]
- Benadda, B.; Kafoufi, K.; Monkam, P.; Otterbein, M. Hydrodynamics and mass transfer phenomena in counter-current packed column at elevated pressures. Chem. Eng. Sci. 2000, 55, 6251–6257. [Google Scholar] [CrossRef]
- Yancheshmeh, M.S.; Radfarnia, H.R.; Iliuta, M.C. High temperature CO2 sorbents and their application for hydrogen production by sorption enhanced steam reforming process. Chem. Eng. J. 2016, 283, 420–444. [Google Scholar] [CrossRef]
- Palys, M.; McCormick, A.; Cussler, E.; Daoutidis, P. Modeling and optimal design of absorbent enhanced ammonia synthesis. Processes 2018, 6, 91. [Google Scholar] [CrossRef]
- Razi, N.; Svendsen, H.F.; Bolland, O. Simulation of post-combustion CO2 capture system using new effective interfacial area correlation. Energy Procedia 2014, 51, 169–175. [Google Scholar] [CrossRef]
- Yasari, E. A green industrial scale di-methyl ether reactor with aiming to CO2 reduction: Staging and multi-objective optimization approach. J. Taiwan Inst. Chem. E 2017, 81, 110–118. [Google Scholar] [CrossRef]
- Erans, M.; Manovic, V.; Anthony, E.J. Calcium looping sorbents for CO2 capture. Appl. Energy 2016, 180, 722–742. [Google Scholar] [CrossRef]
- Diego, M.E.; Arias, B.; Méndez, A.; Lorenzo, M.; Díaz, L.; Sánchez-Biezma, A.; Abanades, J.C. Experimental testing of a sorbent reactivation process in La Pereda 1.7 MWth calcium looping pilot plant. Int. J. Greenh. Gas Control 2016, 50, 14–22. [Google Scholar] [CrossRef]
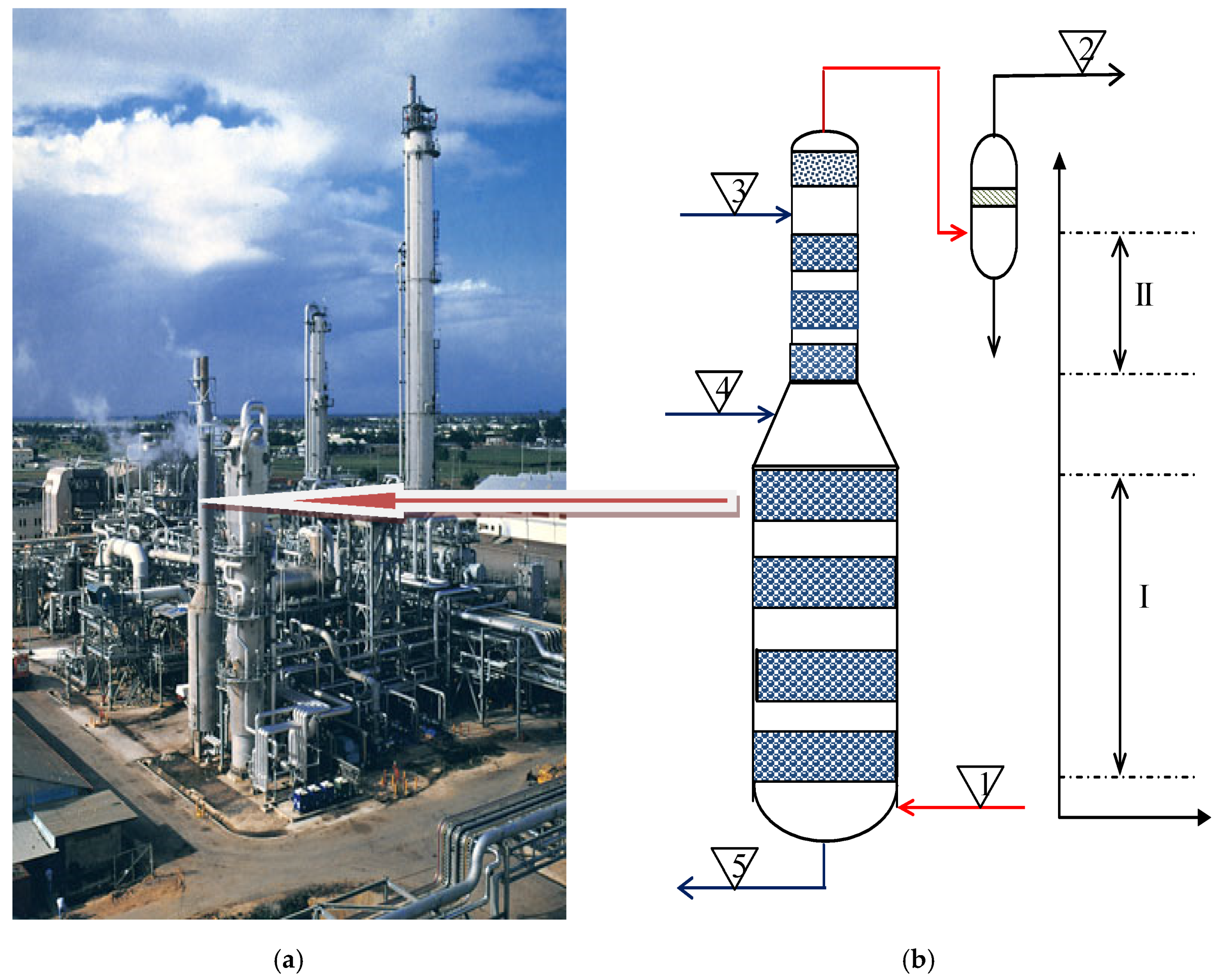


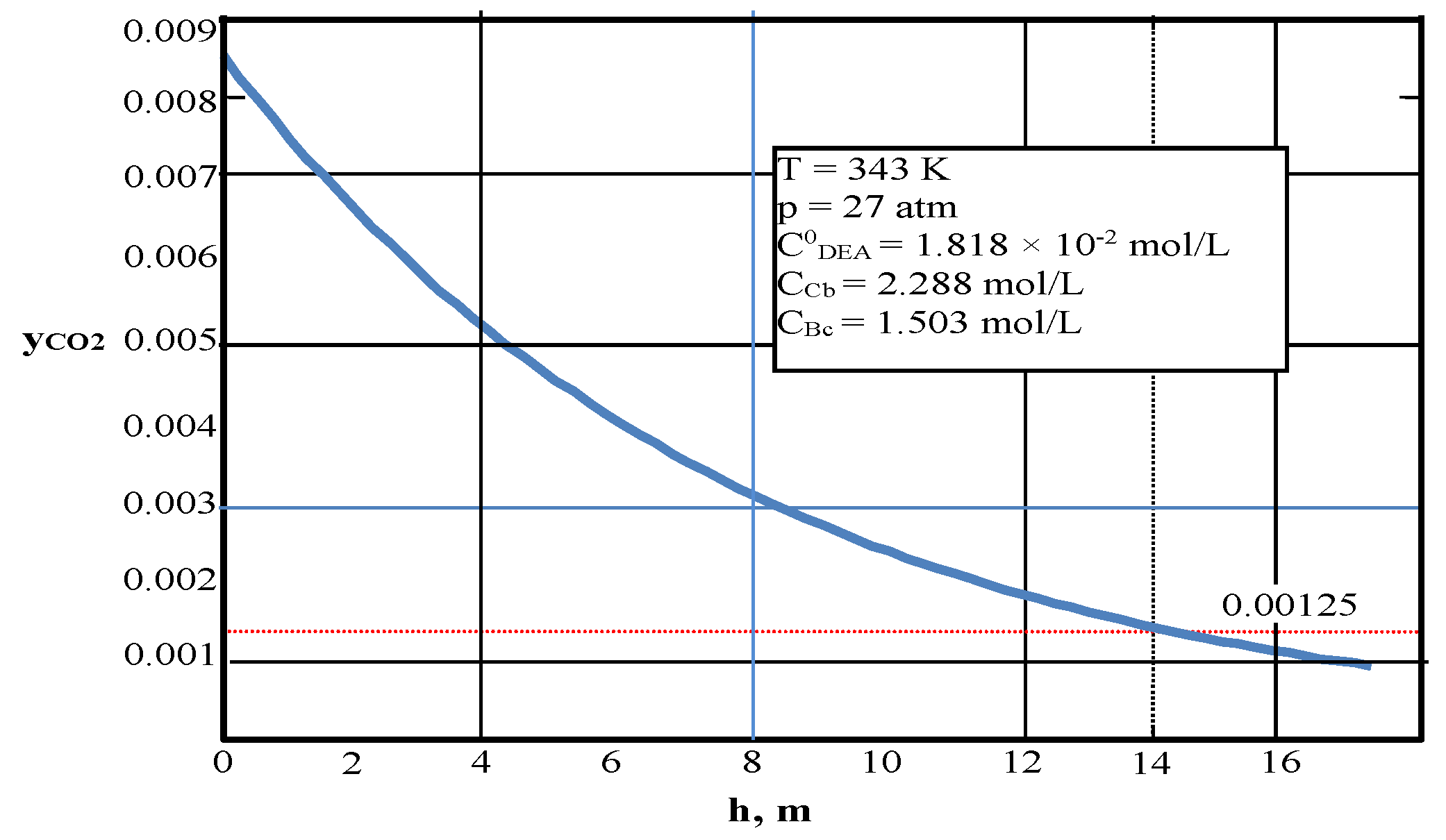
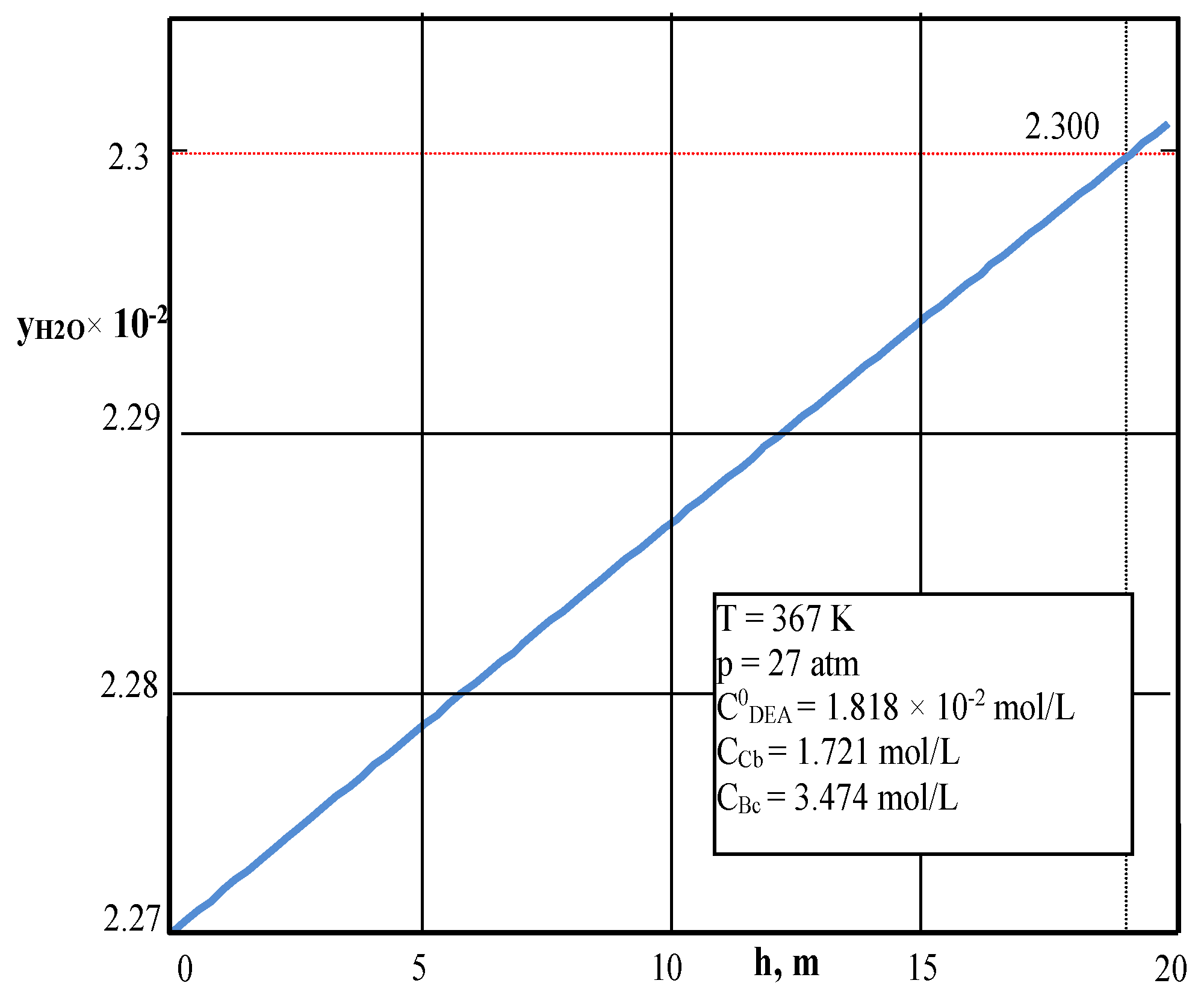
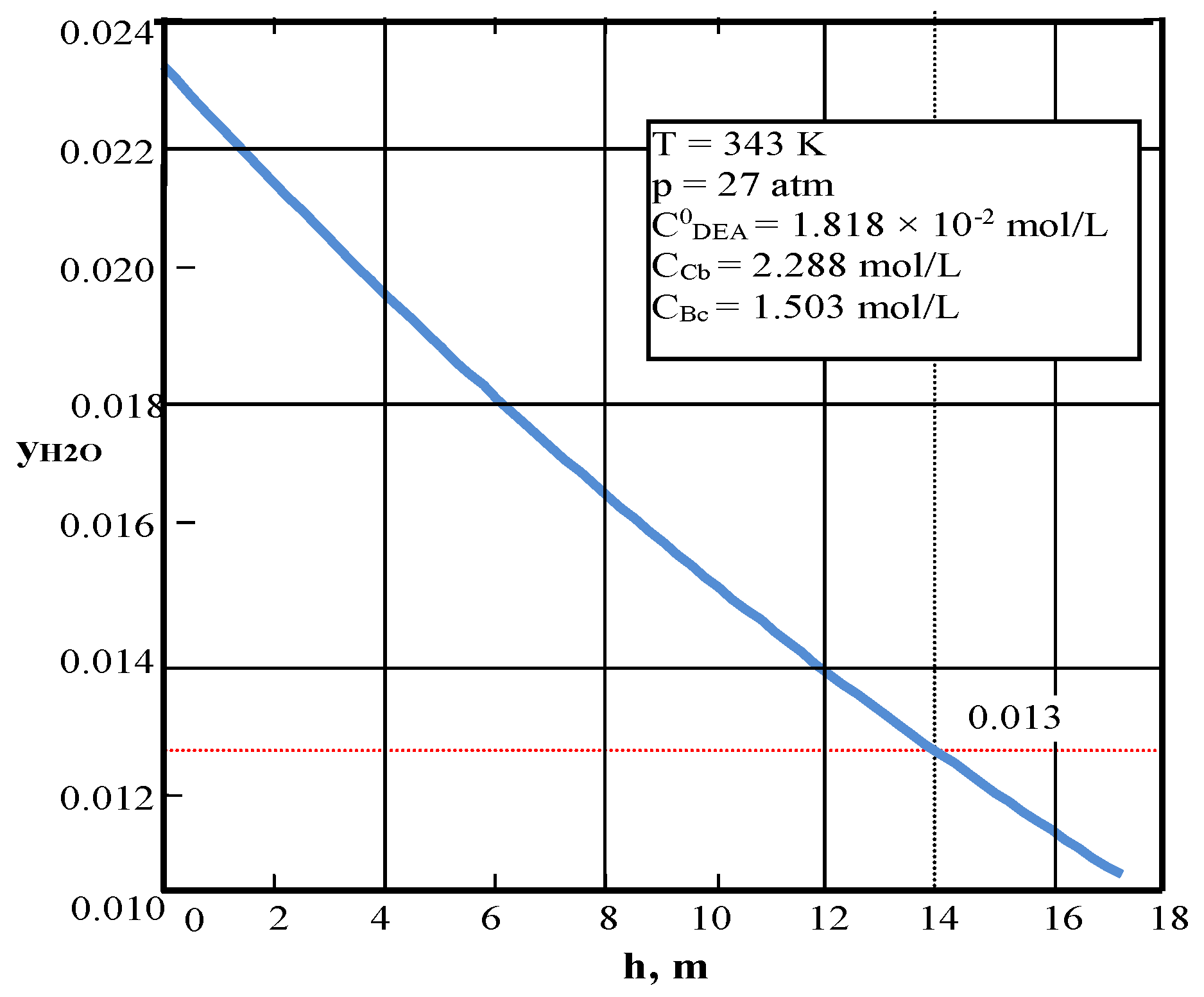
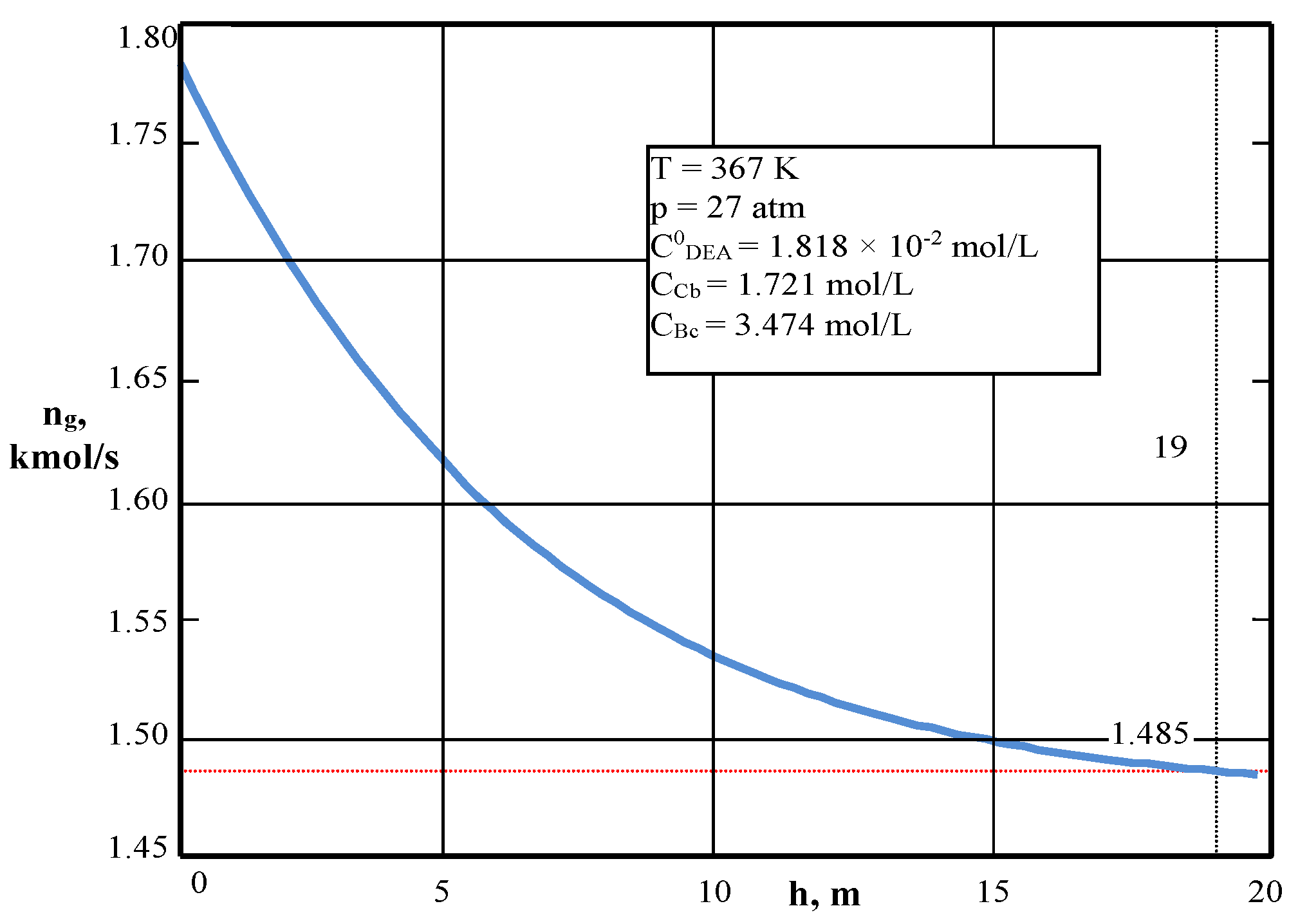
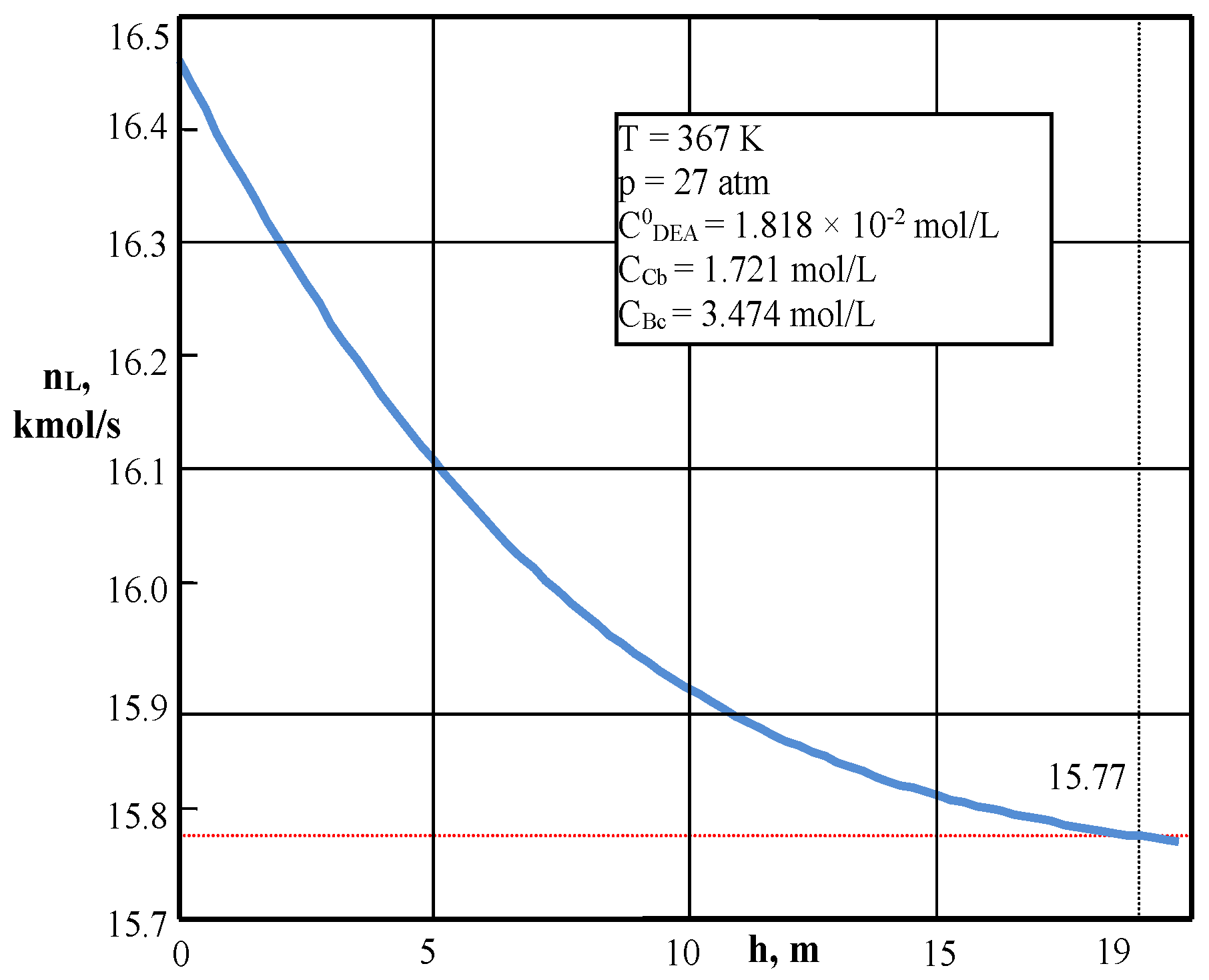
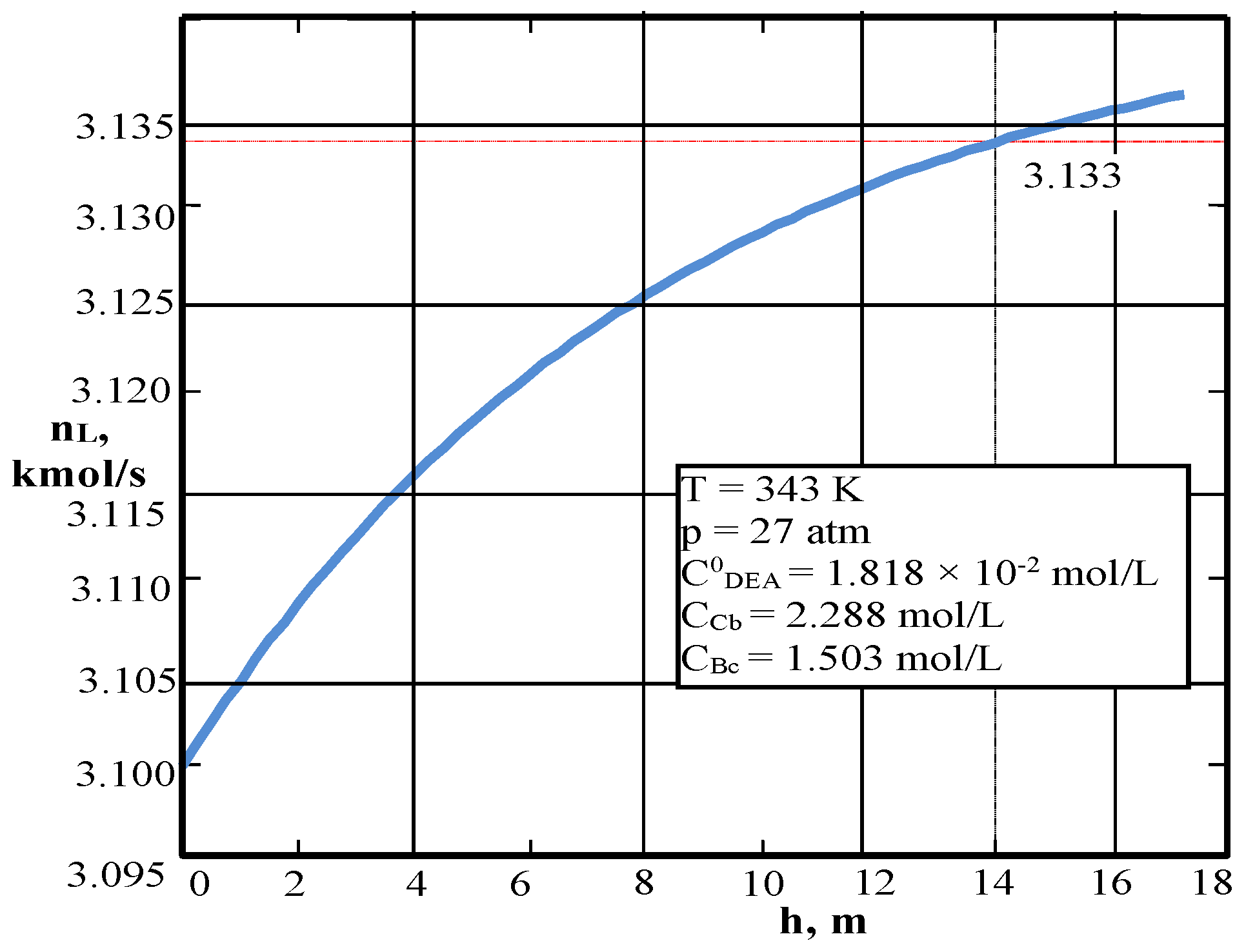
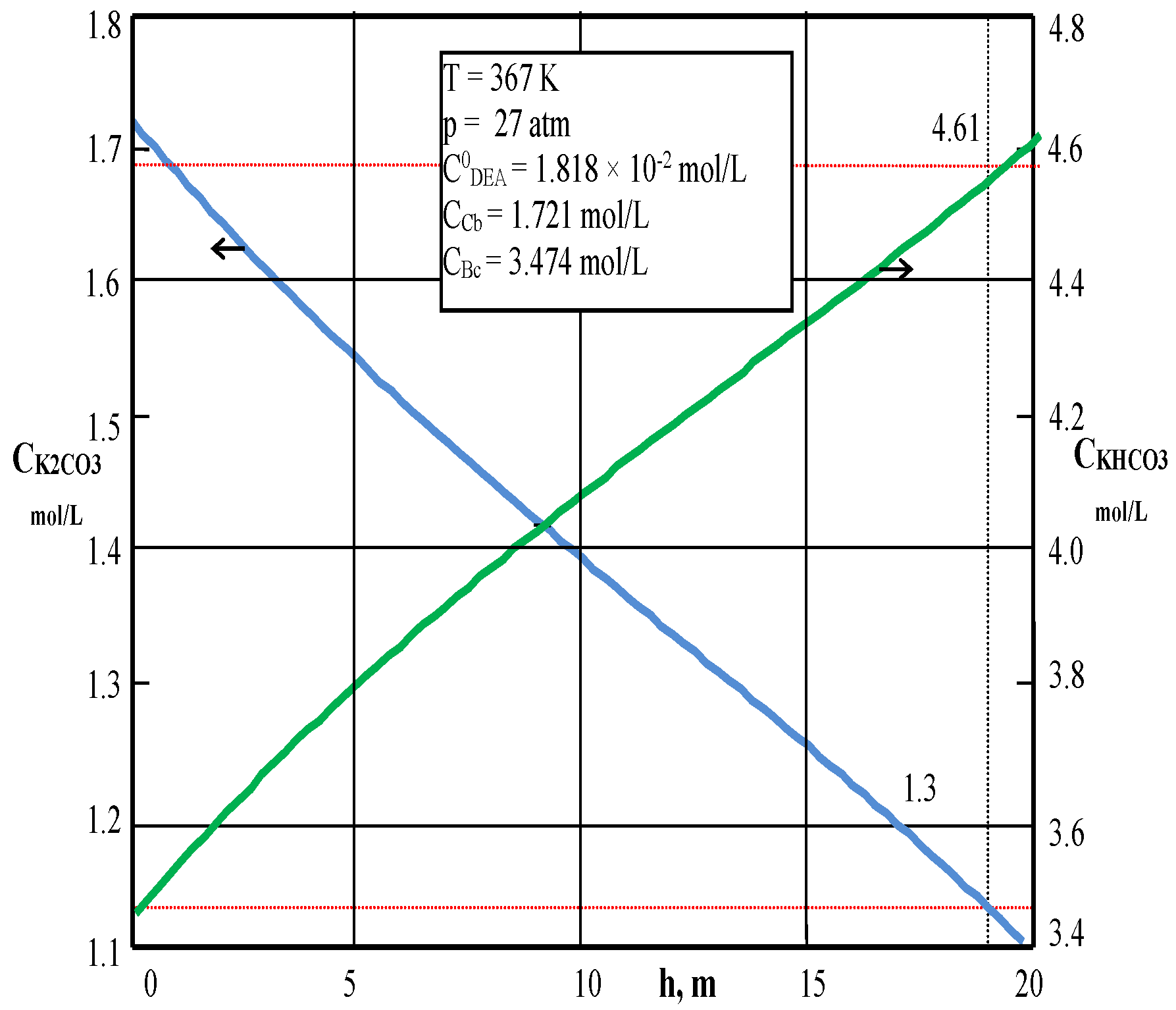
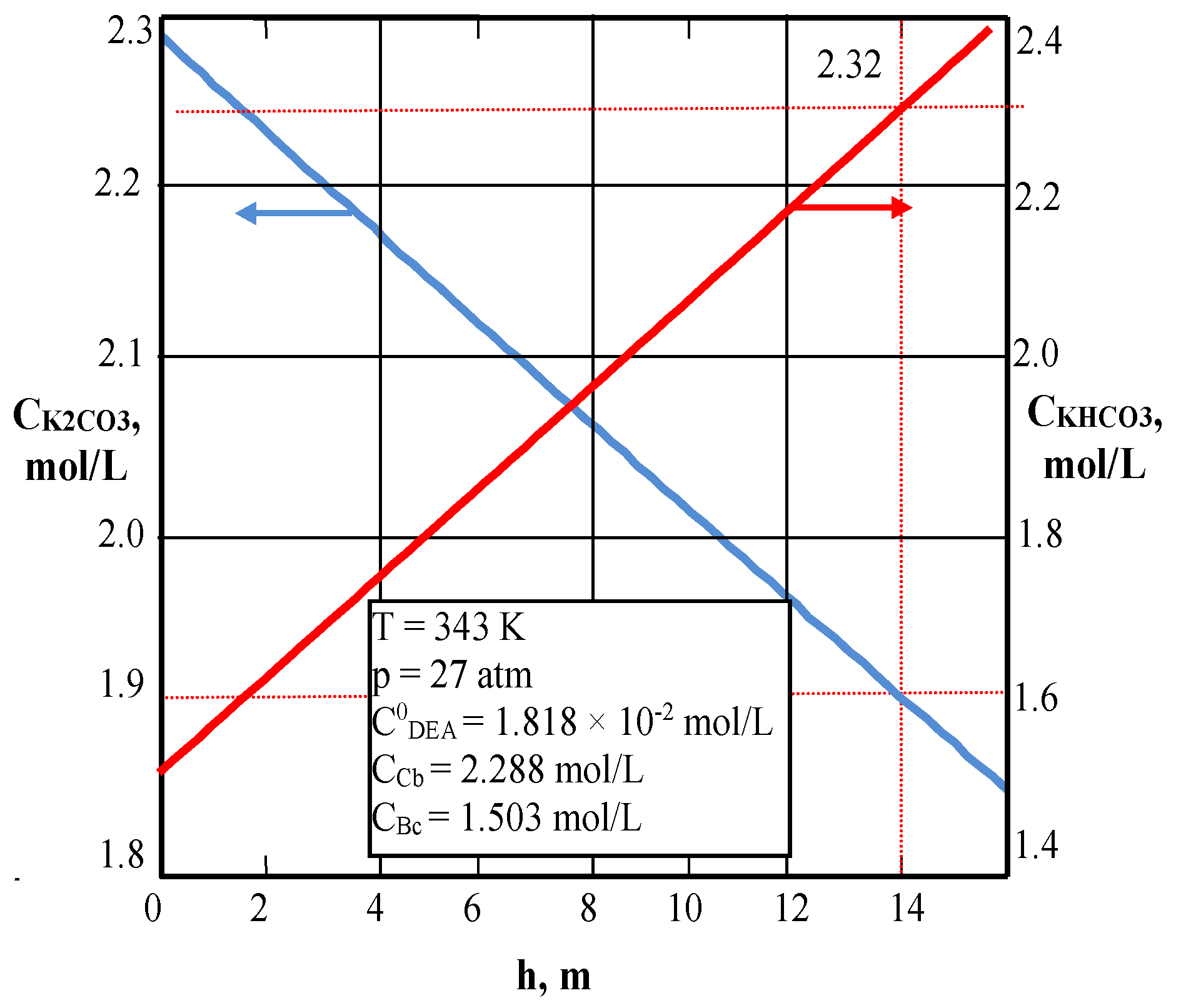
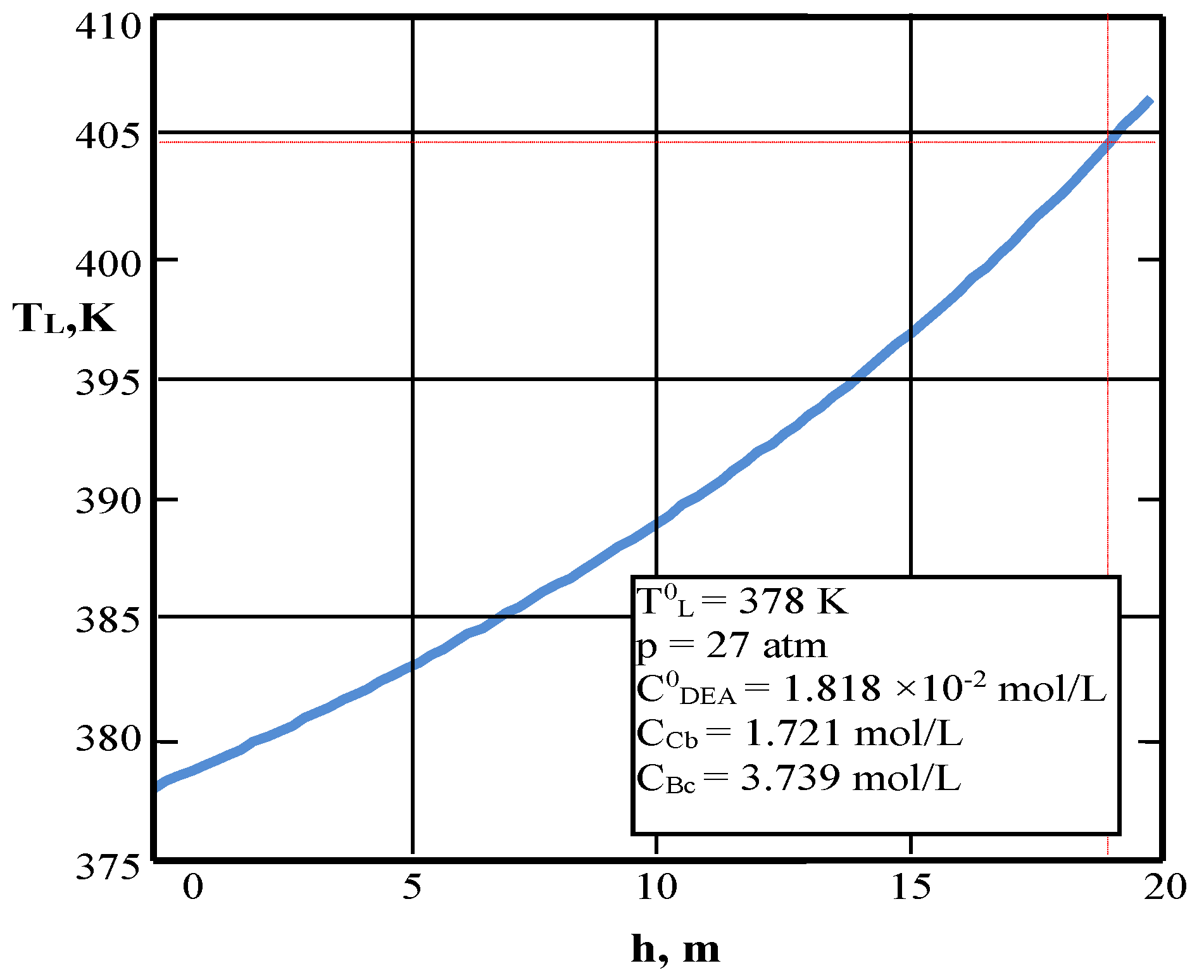
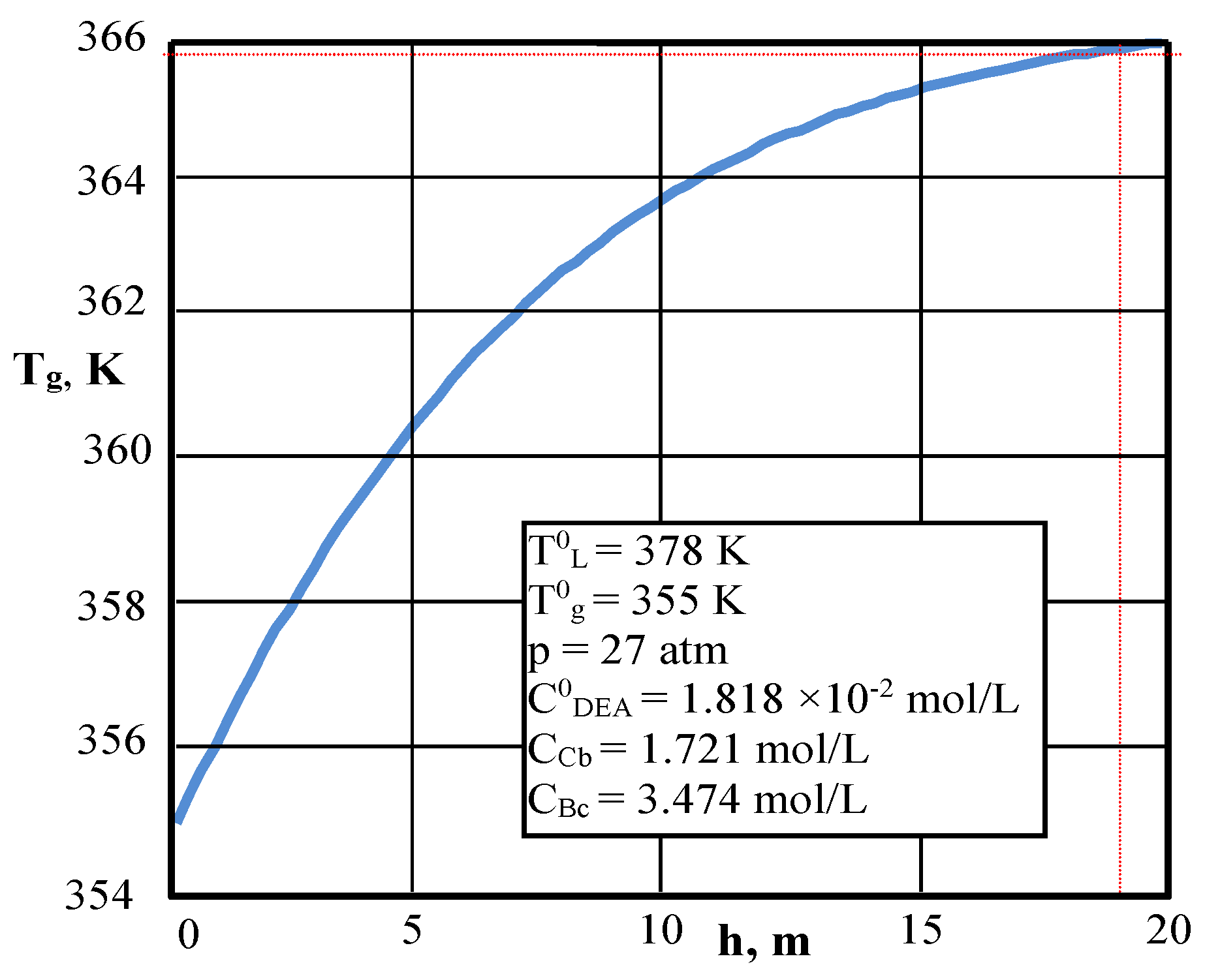
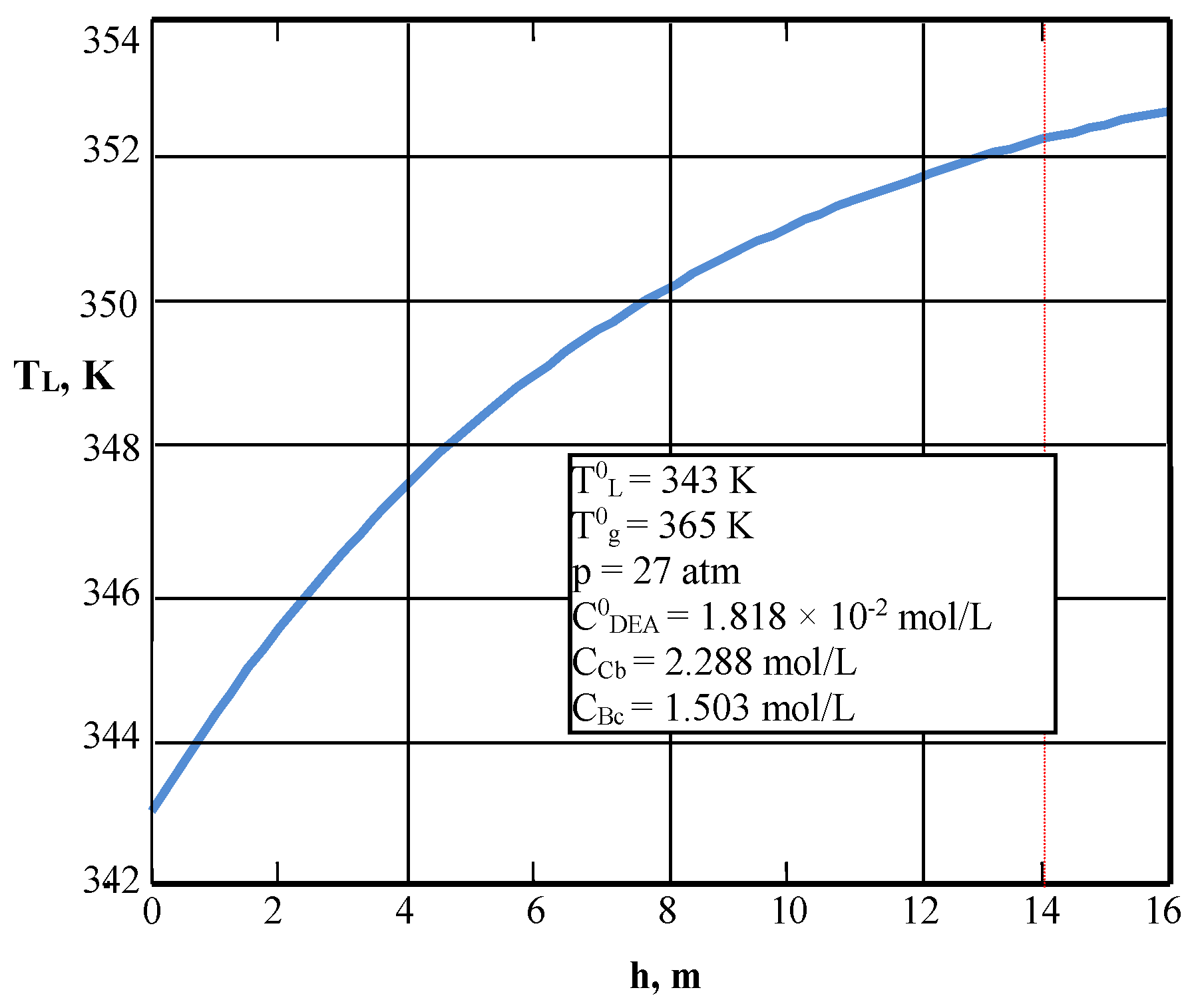
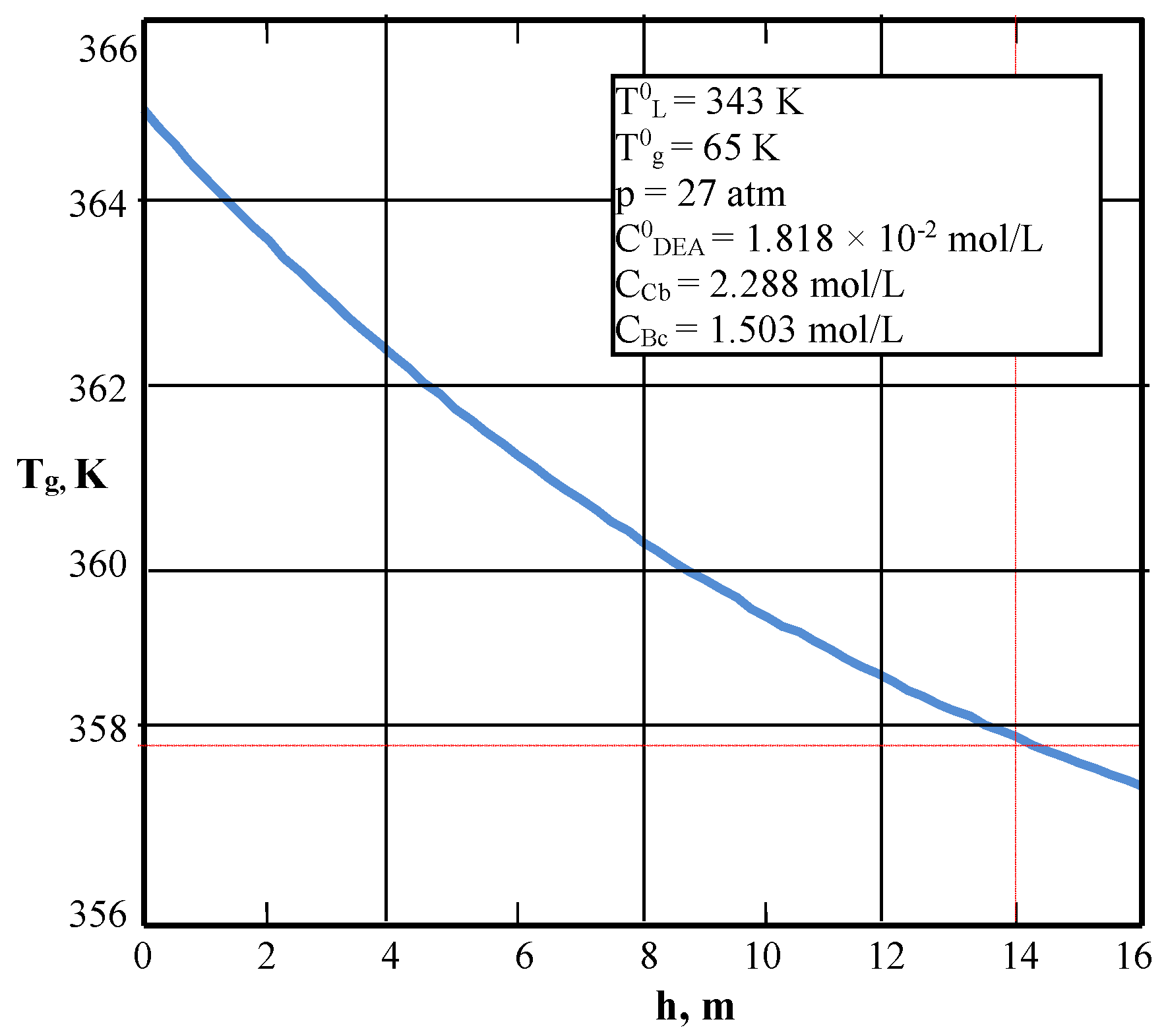

| Values | Part I | Part II |
|---|---|---|
| Inlet gas temperature, K (1) | 355 | – |
| Outlet gas temperature K (2) | – | 359 |
| Inlet liquid temperature, K (3) | 378 | 345 |
| Outlet liquid temperature, K (5) | 400 | – |
| Pressure, atm | 27 | 27 |
| Gas flow, kg/h | 95,657.00 | 45,877.00 |
| Inlet liquid flow, kg/h | 1,218,506 | 307,994 |
| Column diameter, m | 3.70 | 2.4 |
| % CO2 in gas (1) | 21.42 | – |
| % CO2 in gas (2) | – | 0.13 |
| % H2O in gas (1) | 2.27 | – |
| CCb, mol/L (3) | – | 2.2878 |
| CBc, mol/L (3) | – | 1.503 |
| CCb, mol/L (4) | 1.7156 | – |
| CBc, mol/L (4) | 3.438 | – |
| CCb, mol/L (5) | 1.1091 | – |
| CBc, mol/L (5) | 4.61 | – |
| CAm, mol/L | 0.01818 | 0.01818 |
| nL, kmol/s | 15.89 | 3.099 |
| ng, kmol/s | 1.750 | – |
| Values | Part I | Part II | ||
|---|---|---|---|---|
| Experimental | Theoretic | Experimental | Theoretic | |
| Outlet gas temperature K | 366 | 365 | 359 | 357.5 |
| Outlet liquid temperature, K | 400 | 405 | 345 | 353 |
| Pressure, atm | 28 | 27.45 | 28 | 27.1 |
| % CO2 in gas outlet | 0.8077 | 0.84 | 0.13 | 0.125 |
| % H2O in gas outlet | 2.27 | 2.30 | 1.31 | 1.30 |
| CCb, mol/L outlet | 1.1091 | 1.13 | 1.908 | 1.90 |
| CBc, mol/L | 4.61 | 4.616 | 2.321 | 2.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harja, M.; Ciobanu, G.; Juzsakova, T.; Cretescu, I. New Approaches in Modeling and Simulation of CO2 Absorption Reactor by Activated Potassium Carbonate Solution. Processes 2019, 7, 78. https://doi.org/10.3390/pr7020078
Harja M, Ciobanu G, Juzsakova T, Cretescu I. New Approaches in Modeling and Simulation of CO2 Absorption Reactor by Activated Potassium Carbonate Solution. Processes. 2019; 7(2):78. https://doi.org/10.3390/pr7020078
Chicago/Turabian StyleHarja, Maria, Gabriela Ciobanu, Tatjána Juzsakova, and Igor Cretescu. 2019. "New Approaches in Modeling and Simulation of CO2 Absorption Reactor by Activated Potassium Carbonate Solution" Processes 7, no. 2: 78. https://doi.org/10.3390/pr7020078
APA StyleHarja, M., Ciobanu, G., Juzsakova, T., & Cretescu, I. (2019). New Approaches in Modeling and Simulation of CO2 Absorption Reactor by Activated Potassium Carbonate Solution. Processes, 7(2), 78. https://doi.org/10.3390/pr7020078