Abstract
The purpose of this study was to mask the bitter taste of nizatidine (NZD) using cation-exchange resins. Amberlite IRP-69 and Dowex-50 containing cross-linked polystyrene backbones were used. The drug resin complexes were prepared by batch process using drug: resin ratios of 1:1, 1:3, and 1:5. The optimum drug: resin ratio and the time required for maximum percentage drug loading into the complexes were determined. The selected drug-resin complexes were evaluated for morphology, drug release, and taste. The NZD-Dowex complex was obtained at a drug: resin ratio of 1:5 using a stirring time of 1 h in order to get 100% loading of NZD. The NZD-Dowex complex had a spherical shape and smooth texture similar to Dowex resin. The NZD-Dowex complex with a ratio of 1:5 showed that in vitro drug release of 4.27% at 5 min in simulated salivary fluid of pH 6.8 and 99.67% at 1 h in simulated gastric fluid of pH 1.2. NZD’s bitter taste was effectively masked when it formed a complex with Dowex at a ratio of 1:5. This was proved by an electronic tongue and human test panel.
1. Introduction
The oral administration of several important pharmaceutical formulations is often hampered by their unpleasant bitter taste, especially for children and elderly patients, leading to noncompliance and hindering therapeutic management. Therefore, taste-masking technologies are crucial factors for better patient compliance and therapeutic value [1,2]. It is a challenge to mask the bitter taste of drugs in the development of most oral formulations. It is also necessary to choose the masking process which involves the minimum number of excipients, types of equipment, processing steps, is rapid and easy to prepare, can be carried out at room temperature, is low cost, and is environmentally friendly [3]. Various methods have been reported to achieve taste masking of bitter drugs, i.e., polymer coating, ion-exchange resins, spray-or freeze-drying, complexation, congealing with lipids, making multiple emulsions, liposomes, microcapsules, or polymeric membranes [1]. Ion-exchange resins (IERs) are cross-linked, water-insoluble, high molecular-weight polyelectrolytes that can reversibly exchange their mobile ions of equal charge with the surrounding medium stochiometrically [4]. Since most drugs have ionic sites in their molecule, the resin’s charge provides a means to loosely bind such drugs. This complex prevents the drug release in the saliva, thus resulting in taste masking; however, it is weak enough to be broken down by hydrochloric acid present in the stomach. Thus, the drug resin complex is absolutely tasteless, and its bioavailability remains unaffected [5]. The various ion exchange materials available can be classified based on the basis of nature of structural and functional components. IERs contain positively or negatively charged sites are accordingly classified as either cation or anion exchanger. For example, strong cation exchangers such as Amberlite IRP-69 and Dowex 50 contain sulfuric acid sites whereas weak cation exchangers (Amberlite IRC-50, Indion 204) are based on carboxylic acid moieties. The strong anion exchange resins such as Dowex-1 and Amberlite IR-400 have quaternary amine ionic sites, whereas weak anion exchanger (Amberlite IR-4B) has predominantly tertiary amine substituents [6]. The selection of IER for taste masking applications is primarily governed by the functional group properties of the IER and the nature of drug and site of drug delivery [7]. Both weak and strong cation and anion resins are being used to mask the bitter taste of drugs. Some ion IERs used extensively for taste and flavor-masking in industries are Amberlite IRP-64, Amberlite IRP-69, Indion 204, Indion 214, Kyron T-114 and Kyron T-104 [8].
Nizatidine (NZD) (Figure 1) is a histamine type 2 receptor antagonist (H2 blocker) that inhibits gastric acid secretion. Its dosage in adults is 150 mg twice daily or 300 mg as a single dose for the treatment of active duodenal ulcers, benign gastric ulcers, and gastroesophageal reflux disease [9,10]. This drug has a bitter taste and mild sulfuric odor; therefore, it serves as a model drug to evaluate taste-masking efficiency for several types of oral formulations. Recently, NZD-Eudragit E100 complexes prepared by various methods including solvent evaporation, spray-drying, and mass extrusion have been developed in order to mask the bitter taste of NZD and formulate it as a fast-dissolving tablet [11,12]. There are limited approaches available to improve the taste of NZD by the ion-exchange resin method. We could find only one scientific paper about taste masking of NZD using Kyron T134 in the literature [13]. That study aimed to mask the bitter taste of NZD and formulate as NZD orodispersible tablets. The researchers mainly focused on the evaluation of the prepared tablets, such as Carr’s index, angle of repose, hardness, friability, wetting time and in vitro disintegration time. There were no studied about optimization of drug and resin ratio, NZD release from drug-resin complex in salivary fluid or bitter taste evaluation. In the present study, our goal was to assess the possibility of an NZD-resinate complex formation with two strong cation-exchange resins, which are Amberlite IRP-69 and Dowex 50. The influences of the resins on the equilibrium time of the NZD-resinate complex and interaction between the resin and drug by Fourier-transform infrared spectroscopy (FTIR) were evaluated. Then, morphology, in vitro NZD-release behavior, and taste-masking assessment performed by electronic tongue and human test panel of the selected NZD-resinate complexes were evaluated.
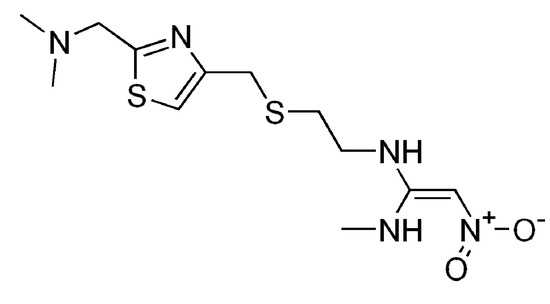
Figure 1.
Structural formula of nizatidine.
2. Materials and Methods
2.1. Materials
Nizatidine (NZD) was kindly supplied by KyungDong Pharm Co., Ltd. (Seoul, South Korea). AmberliteTM IRP-69 (sodium polystyrene sulfonate, gel resin, 100–500 wet mesh; 8% degree of cross-linking) was purchased from Colorcon, Inc. (Harleysville, PA, USA). DowexTM-50w (sulfonated polymer of styrene, ethylstyrene and divinylbenzene in the hydrogen form, nonmacroporous gelform bead resin, 200–400 mesh; 4% degree of cross-linking) was purchased from DOW Chemical (Midland, Michigan, USA). Hydrochloric acid (HCl), potassium chloride (KCl), potassium phosphate monobasic (KH2PO4), sodium phosphate dibasic (Na2HPO4), sodium chloride (NaCl), and sodium hydroxide (NaOH) were purchased from Samchun Pure Chemical Co., Ltd. (Gyeonggi-do, South Korea).
2.2. Purification and Activation of Ion-Exchange Resins
Amberlite IRP-69 and Dowex-50 were purified [14]. Briefly, each resin (5 g) was washed successively with distilled water, ethanol, and several times with distilled water to eliminate the solvent and color impurities. Strong cation exchanger can be in several forms. It can be in the protonated or hydrogen form, or the salt form with the hydrogen replaced by a cation such as sodium or ammonium. So, the resins were activated by washing the resins with acid in order to be in the hydrogen form. For acid-activated resins, each wet resin was placed on filter paper in a funnel, then it was washed with distilled water, then with 1 M HCl (100 mL), and then washed several times with distilled water until a neutral pH was reached. Finally, the resin was dried overnight in a hot air oven at 50 °C and kept in an amber glass vial.
2.3. Preparation of Taste-Masked Spheres
Drug-resinate complexes were prepared by a single-batch process. The time required for a constant amount of the drug to react with the resin was taken as the equilibrium time. This was achieved by assaying the supernatant during preparation of the resinate complex. Inactivated and activated Amberlite IRP-69 and Dowex-50 (150, 450, and 750 mg) were soaked overnight in a beaker and stirred with a magnetic stirrer (Sigma-Aldrich®, St. Louis, Missouri, USA) to facilitate swelling of the resins. Strong cation exchanger such as Amberlite IRP-69 and Dowex-50 can be in several forms. NZD (150 mg) was added to each beaker containing inactivated and activated Amberlite IRP-69 and Dowex-50 to prepare complexes with 1:1, 1:3, and 1:5 drug-to-resin ratios by weight. The resultant solutions were stirred at room temperature for 24 h. The NZD-resinate complexes were separated by decantation and washed two times with deionized water to remove unassociated drugs and other ions. The complexes were then dried in a hot air oven (JEIO Tech Co., Ltd., Seoul, South Korea) for 5 h at 45 °C to a constant weight and stored in a tight glass vial.
In order to investigate how quickly equilibrium could be reached, 40 µL of supernatant was collected at predetermined intervals during complex formation at room temperature. The supernatant was filtered through a 0.45 µm membrane filter to obtain a clear solution and diluted 300 times with HCl (pH 4.0) for the activated resins or diluted with distilled water for the non-activated one, then measured by UV spectrophotometer (UVmini-1240, Shimadzu, Kyoto, Japan) at 314 nm. The NZD contents in the supernatant were determined from the standard curve of standard NZD in HCl (activated resins) and distilled water (non-activated resins), which demonstrated linearity with high correlations (r2 = 0.9997 and 0.9999, respectively). The amount of NZD in the resin was calculated [amount of NZD in resin (%) = [(total amount of NZD − amount of NZD in supernatant)/total amount of NZD] × 100]. The experiment was done in triplicate.
2.4. Scanning Electron Microscopy
The surface morphology and the shapes of NZD-resinate taste-masked spheres were obtained by scanning electron microscopy (SEM, JSM-5410LV, JEOL Ltd., Peabody, MA, USA). The taste-masked spheres were mounted on double-sided adhesive tape, spattered with platinum, and scanned at 10 kV voltage. The micrographs were examined at magnification ratios of 200× and 500.
2.5. Fourier Transform Infrared Spectroscopy
Infrared spectroscopy was conducted using a Fourier transform IR spectrophotometer (FTIR, Thermo Fisher Scientific Inc., Massachusetts, USA). The sample was mixed with KBr and molded into a disc. The spectrum was recorded within the region of 400–4000 cm−1 for NZD powder, Amberlite IRP-69, Dowex 50, their corresponding physical mixtures, and dry NZD-resinate complexed in 3 different ratios.
2.6. In vitro Release Study
The in vitro release test of taste-masked spheres was conducted using a USP dissolution tester apparatus type II (Vision Elite 8, Hanson Research Corp., Chatsworth, CA, USA). The taste-masked spheres (equivalent to 150 mg of NZD) were placed in test media (n = 6 per formulation). Test media were 900 mL of simulated salivary fluid with pH of 6.8 and gastric fluid of pH 1.2 without enzymes; these were prepared according to Guhmann et al. (2012) [15]. The temperature was maintained at 37 ± 0.5 °C with a paddle rotation speed of 50 rpm. For the in vitro release test in simulated salivary fluid, the sample was withdrawn at 1, 3, 5, 10 and 15 min. While, the in vitro release test in simulated gastric fluid, the sample was withdrawn at 1, 3, 5, 10, 15, 30, 45, and 60 min. At predetermined intervals, 3 mL of the dissolution medium were taken and replaced with an equal volume of fresh dissolution medium in order to maintain sink conditions throughout the experiment. Withdrawn samples were filtered, diluted, and analyzed by UV spectrophotometer (UVmini-1240, Shimadzu, Kyoto, Japan) at 314 nm. The average percentage released was calculated at each time interval. Blank experiments were also performed from resin without NZD to assess whether other compounds absorb at 314 nm.
2.7. In Vitro Taste-Masking Efficiency Study
In vitro taste-masking efficiency of taste-masked spheres was evaluated using the electronic tongue system (E-tongue): α–Astree electronic tongue (Alpha M.O.S, Toulouse, France). NZD powder and NZD-Dowex 1:3 and 1:5 spheres were used as samples. NZD-Dowex spheres (equivalent to 150 mg of NZD) were prepared by dissolving in 100 mL pure water (Daihan Pharm Co., Seoul, Korea) using magnetic stirrer at 37 °C for 5 min. Because, resins are insoluble in water, then they were separated by filtration using 0.45 µm nylon membrane filters (GE Healthcare WhatmanTM, Maidstone, Kent, UK). Only dissolved NZD in pure water was measured by E-tongue. Moreover, five serial concentrations (0.0938–1.5 mg/mL) of NZD solutions were prepared. Then, the solution was filtered through 0.45 µm nylon membrane filters. All of the samples were measured 8 times, and the last 4 datasets were used for analysis. Sensors were pre-tested by running, conditioning, calibration, and diagnostic processes before the analysis. Acquisition time was fixed at 120 s. The sensor was cleaned with pure water between acquisitions of each sample. After the analysis, data were analyzed and processed statistically by Alpha Soft V12.3 software (Alpha M.O.S, Toulouse, France).
2.8. Human Taste Panel
In vivo taste-masking efficiency in healthy volunteers was evaluated. The study protocol was approved by the Research Ethics Committee at Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand, approval number 13/2562. The taste of and NZD-Dowex 1:3 and 1:5 spheres was performed by 6 healthy adult volunteers (3 males and 3 females, with a mean age of 25.7 years), from whom informed consent was first obtained. Prior to the tasting, the volunteers were informed precisely about the purpose of the tasting and the possible about the adverse effects of NZD. They were also instructed not to swallow the spheres. They rinsed their mouths thoroughly after that each sample (equivalent to 150 mg of NZD) was held in the volunteer’s mouths for 30 s and then expectorated, and gargle their mouth thoroughly again with distilled water. The taste was evaluated and assigned a numerical value according to the following scale: 0, pleasant/not bitter; 1, slightly pleasant/slightly bitter; and 2, unpleasant/bitter. The scores given by all individuals were computed and presented as median taste scores. The median taste scores between the NZD–Dowex 1:3 spheres and NZD–Dowex 1:5 spheres were compared by using the Kruskal-Wallis test. A lower score indicated a greater masking effect.
2.9. Statistical Analysis
All the data were presented as mean ± SD. One-way ANOVA was used to evaluate the significance of differences at the significance level of p-value < 0.05. Statistical analysis was performed using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA).
3. Results and Discussion
3.1. Optimization of Drug: Resin Ratio
In the present study, Amberlite IRP-69 and Dowex-50 which are strong cation exchangers were used. Amberlite IRP-69 is a cross-linked sodium polystyrene polymer which has −SO3Na as the exchange species, whereas Dowex-50 is a cross-linked polystyrene divinylbenzene polymer which has −SO3H as the exchange species. Their exchange reaction occurred when placed in contact with a solution of cations. NZD which is amine drug (ionization constants in aqueous media were 2.1, 6.8 at 25 °C) provided the counter-cations (N+). Then, soluble anions removed from resins through the exchange with the counter-cations of NZD as in the exchange reactions scheme below:
Amberlite resin;
Resin-SO3− Na+ + NZD+  Resin-SO3− NZD+ + Na+
Resin-SO3− NZD+ + Na+
 Resin-SO3− NZD+ + Na+
Resin-SO3− NZD+ + Na+Dowex resin;
Resin-SO3− H+ + NZD+ Resin-SO3− NZD+ + H+
Resin-SO3− NZD+ + H+
 Resin-SO3− NZD+ + H+
Resin-SO3− NZD+ + H+OPTIMIZATION of drug: resin ratios was done by taking inactivated (NA) and activated (AC) resin from 0 to 24 h for Amberlite IRP-69 and 3 h for Dowex-50 (Figure 2). As the stirring times increase from 1 to 3 or 24 h, the free drug concentration in the supernatant decreases and drug-loading content in the complex increased, thereby increasing the taste-masking effect. In this study, NZD-loading contents in all NZD-Amberlite IRP-69 ratios were lower than those of Dowex-50. For activated (AC) resins, the percentage of drug loading in the NZD-Amberlite complex at ratios of 1:1, 1:3, and 1:5 were found to be 26.77 ± 1.22%, 28.58 ± 0.81% and 30.37 ± 0.78%, respectively at 24 h (Figure 2a). Also, NZD-activated Dowex complexes at 1:1, 1:3, and 1:5 ratios exhibited maximum drug loading of 64.35 ± 2.26%, 99.17 ± 1.35%, and 99.35 ± 1.22%, respectively at 3 h (Figure 2b). For NA resins, the percentages of drug loading in the NZD-Amberlite complexes at 1:1, 1:3, and 1:5 ratios were found to be 12.24 ± 0.18%, 13.36 ± 0.46%, and 18.27 ± 1.71%, respectively at 24 h (Figure 2c). For the NZD-inactivated Dowex complex at a 1:1 ratio, the maximum drug loading in the complex was found to be 60.22 ± 3.04% at 3 h. However, the maximum drug loading in NZD-inactivated Dowex complexes at ratios of 1:3 and 1:5 were found to be 100% within 1 h (Figure 2d).
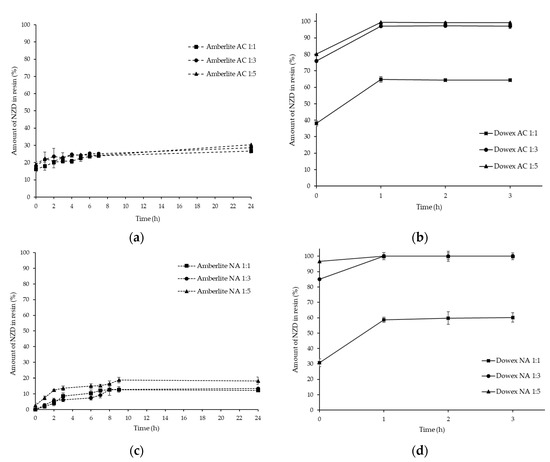
Figure 2.
Optimization of drug and resin at 1:1, 1:3, and 1:5 ratios of nizatidine (NZD)-activated Amberlite complex (a), NZD-activated Dowex complex (b), NZD-inactivated Amberlite complex (c), and NZD-inactivated Dowex complex (d).
As the drug to Amberlite IRP-69 resin ratio increased, the percentage of drug loading also increased. However, the percentage of drug loading in Dowex-50 was increased when compared with drug: resin ratios of 1:1 and 1:3, but the values of the percentage of drug loading did not indicate significant differences when complexed with Dowex-50 at ratios of 1:3 and 1:5. As compared to NA and AC resins, a slightly higher percentage of drug loading was found in the activated Amberlite IRP-69. However, there was no major effect found on the percentage of drug loading of Dowex-50, as the drug content values between NA and AC resins showed no significant differences. Sulfonated styrenedivinlybenzene copolymer products such as Amberlite IRP-69 and Dowex-50 can be used to mask the bitter taste of basic drugs as they function throughout the entire pH range [16,17]. The present study suggested that the taste of NZD, which is a cationic or amine-containing drug, can be masked by Dowex-50 resin prior to its formulation into suitable dosage forms. Thus, further experiments were made with inactivated Dowex-50 resin at 1:3 and 1:5 ratios using a stirring time of 1 h in order to get the maximum loading of NZD.
3.2. FTIR of NZD-Resinate Complex
FTIR studies were performed to detect the possible molecular interaction between NZD and the resins. The FTIR spectrum of NZD, Amberlite, Dowex, their physical mixture and NZD-resin complex at ratio of 1:1–1:5 are shown in Figure 3. The characteristic absorption bands of NZD were N–H stretching (~3210 cm−1) for the secondary amine group, NO2 stretching (~1380 cm−1) for the nitro group and C–N stretching (~1435 cm−1) for C–N amine of the drug. Other sharp absorption bands of NZD were C–H stretching (~2940 cm−1), CH2 stretching (~1440 cm−1), CH3 stretching (~2830 cm−1) and C=C stretching (~1520 cm−1) [18,19]. FTIR of the physical mixture of NZD and resins (NZD-Amberlite and NZD-Dowex physical mixtures) showed several sharp absorption bands of NZD and there is no significant change in the position of the characteristic absorption bands. This suggested no formation of the complexation between the resins and NZD in physical mixture. Drug molecules were external to the resin bead. However, when NZD formed a complex with Amberlite at 1:1–1:5 ratios, the spectra of NZD shifted to overlap with Amberlite absorption bands. The spectra of all NZD-Amberlite complex ratios were not significantly different (Figure 3a). For Dowex, the spectra gradually changed in the NZD-Dowex complex. The characteristic absorption bands of NZD at ~3210 cm−1 and ~1435 cm−1 region disappeared especially in NZD-Dowex at a ratio of 1:5 (Figure 3b), indicating the formation of a complex in these systems by interacting amine groups of NZD. This indicates that NZD-Dowex at a ratio of 1:5 could be used to construct a bitter taste-resistant barrier.
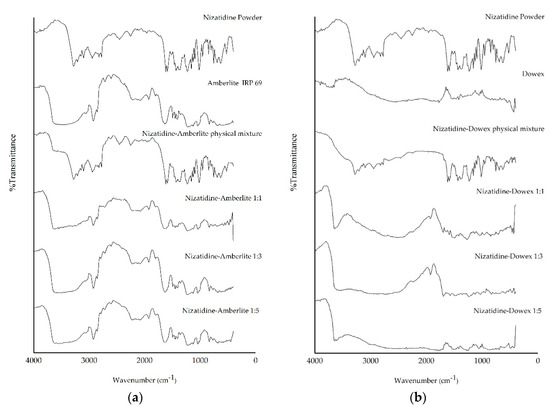
Figure 3.
Fourier-transform infrared spectroscopy (FTIR) spectra of NZD powder, Amberlite IRP-69 resin, a physical mixture of NZD and Amberlite and the NZD-Amberlite complex at ratios of 1:1, 1:3, and 1:5 (a) and NZD powder, Dowex 50w resin, a physical mixture of NZD and Dowex and the NZD-Dowex complex at ratios of 1:1, 1:3, and 1:5 (b).
3.3. NZD-Resinate Complex Morphology
The shape and surface of the NZD-Dowex complex at 1:3 and 1:5 ratios were observed at 200× and 500× magnifications and compared with Dowex resin (Figure 4). Both drug-resin ratios of the NZD-Dowex complex showed spherical shapes and smooth textures similar to Dowex resin. Dowex resins are produced through direct synthetic manufacture and then resulted in spherical resin beads. Dowex resins perform more reliably and consistently than ground resins which consist of irregular shaped particles and contain very fine particles. Dowex-50 is a non-macroporous gel-form bead resin prepared by the sulfonation of styrene-divenyl benzene copolymer beds with sulfuric acid [20]. The size of the NZD-resin complexes was found to be 70–120 µm.
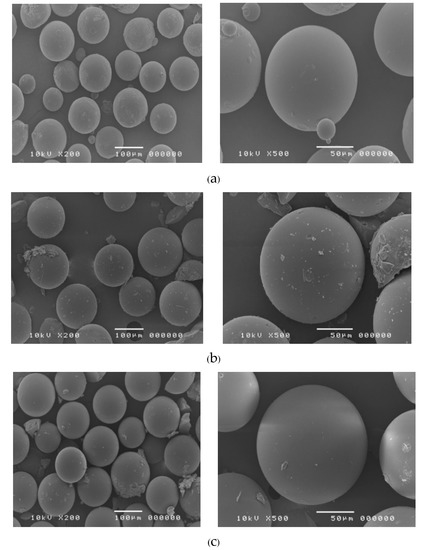
Figure 4.
Scanning electron microscopy of Dowex resin (a), NZD-resinate complex at ratios of 1:3 (b) and 1:5 (c) at magnification of 200× (left) and 500× (right).
3.4. In-Vitro NZD Release from Drug-Resin Complex
Drug-resinate system is a system using chemical principles which is formed by reacting a drug with an ion-exchange polymer to form drug-polymer complex. The means of drug release from chemically controlled drug-resinate systems is ion dissociation. When the drug-resinate reaches the target site, ions present in body fluid can exchange with the drug bound to the polymer matrix. In this study, in vitro release of NZD-Dowex complexes at 1:3 and 1:5 ratios were carried out in two different media, which were simulated salivary fluid of pH 6.8 and gastric fluid of pH 1.2, at different time intervals (Figure 5). Initially, the release was done in simulated salivary fluid of pH 6.8 for 15 min. In vitro release of NZD from the NZD-Dowex complex at a ratio of 1:3 at pH 6.8 was found to be 21.35% in 1 min and 45.25% in 15 min. These were significantly higher than were found in the NZD-Dowex complex at a ratio of 1:5 (3.32% and 10.19%, respectively). It was also found that only 4.27% of NZD was released from the NZD-Dowex complex at a ratio of 1:5 in 5 min. Furthermore, the drug release study was done in simulated gastric fluid media of pH 1.2 for 60 min. The release of NZD from the NZD-Dowex complex at a 1:3 ratio and pH 1.2 was found to be 89.40% in 30 min and 99.67% in 1 h, which were not significantly different from the NZD-Dowex complex at a 1:5 ratio (90.36% in 30 min and 99.67% in 1 h). In USP monograph of NZD dissolution, NZD should be dissolved not less than 75% (Q) within 30 min. Moreover, half an hour of NZD onset of action is reported in Nizac® product information. Thus, the percent releases of NZD in our investigation were in the appropriate range to obtain a proper NZD formulation. In addition, it is likely that the NZD-Dowex complexes remain for 1 h within the stomach. We suggest that the NZD-Dowex complexes should be administered as a sachet and taken within 1 h before eating or drinking anything that may cause the change in gastric pH which can influence the bioavailability of NZD.
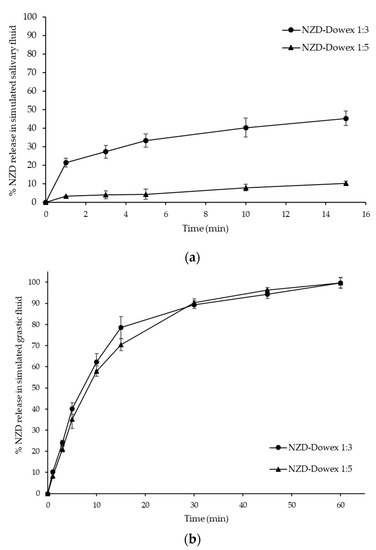
Figure 5.
Dissolution profiles of the NZD-Dowex complex at 1:3 and 1:5 ratios (n = 6) in simulated salivary fluid of pH 6.8 (a) and gastric fluid of pH 1.2 (b) at different time intervals.
As contact between the dissolved form of the drug and taste receptors can be prevented by suppressing drug release, the rate of dissolution is closely correlated with the efficiency of the taste masking [21]. The results indicate that the NZD-Dowex complex at a 1:5 ratio can suppress the drug release more efficiently than the NZD-Dowex complex at a 1:3 ratio at salivary pH (pH 6.8), hence suppressing the bitter taste at the time of administration until it is swallowed and transferred to the stomach. Release of API in the oral cavity is a critical factor for the taste of a drug. Taste-masking is achieved when NZD release at salivary pH is minimal and below the bitterness threshold in the oral cavity. There is no set pharmacologic release test for taste-masked particles. However, the Federation International Pharmaceutique/American Association of Pharmaceutical Scientists (FIP/AAPS) guideline recommends multi-point dissolution testing within early stages of analysis (e.g., ≤ 5 min) to address the taste-masking properties of a formulation [22]. This guideline recommends the use of a neutral medium where the drug should be typically less than or equal to 10% dissolved within 5 min to achieve sufficient taste masking. However, the acceptable amount of the drug released in the oral cavity depends on the bitterness of the API. Highly bitter molecules have lower acceptable limits of release and vice versa.
3.5. Taste Evaluation by Electronic Tongue and Human Taste Panel
The electronic tongue technology is becoming established as an alternative to the human sensory test [23]. It provides relative measurements of dissolved organic compounds that are responsible for taste sensation [24,25]. This technique reduces development time, development costs, subjectivity and bias [26]. The electronic tongue system was originally developed to evaluate the taste of food, wine, or beverages [27,28]. Nowadays, the electronic tongue system is used in the field of pharmaceuticals for the purpose of evaluating taste-masking and taste-assessment of drugs and excipients. The electronic tongue system is composed of sensors and is based on potentiometric measurements and measured data; these data are then transformed into electrical signals. Since the electrical signals are not straightforward to interpret, they are analyzed by multivariate statistical methods, including principal component analysis (PCA) and the masking efficiency method [21]. Euclidean distance from references can be obtained by using the masking efficiency method. The qualitative comparison of different drug-to-resin ratios and their pure drug solutions by PCA aims for the determination differences in the sensor response pattern indicating taste differences and taste masking efficiency due to the types of resins or drug-to-resin ratios of those samples. As explained in previous studies, the electronic tongue system detects the difference in taste by using potentiometric sensors, voltammetric sensors, impedimetric sensors, etc. [29,30]. This implies that the charged objects are more easily detected than the non-charged object. As it was unsure whether the nitro group of the NZD would function as a charged group, individual sensors were checked for the sensitivity for NZD. The adjusted correlation coefficient between the drug concentration (0.0938–0.15 mg/mL) and the sensors ZZ, AB, BB, CA, DA, and, JE were 0.7591, 0.3504, −0.0050, 0.8712, 0.2673, and −0.03716, respectively. Which indicates that only two out of six sensors could not detect the changes in NZD concentration. In particular, sensors ZZ and CA were highly sensitive, implying the suitability of the e-tongue system for detection of taste.
Electronic tongue data of NZD–Dowex 1:3 and 1:5 complexes are shown in Figure 6, Table 1 and Table 2. Since the PC1 and PC2 covered most of the variation in the dataset (approximately 96%), the PCA plot was regarded valid. The two principal components, PC1 and PC2, explains 96.037% of the total variance between the samples (PC1 67.825% and PC2 28.212%). Additionally, the increasing PC1 value accompanied by the increase in NZD concentration indicates that the sensors can precisely evaluate the bitterness of the samples, and the longer distance from NZD solution (e.g., from NZD 1.5 mg/mL) is considered to be a more taste-masked form. The longer distance from the 1.5 mg/mL NZD solution was observed in NZD–Dowex 1:5 complex (575.07) which was longer than NZD-Dowex 1:3 (323.87) of about 2 times. The longer distance indicated a low level of similarities and a high level of differences in the taste pattern compared to pure drug (NZD) solution and therewith good taste-masking capabilities. This result corresponds with an in vitro release test. As a result of in vitro release test in stimulated saliva fluid pH 6.8 and taste assessment, NZD-Dowex complex in a drug-resin ratio of 1:5 had the most taste-masking efficiency. NZD has been described as a bitter drug (FDA prescribing information). However, there was no literature review on the taste of NZD by e-Tongue. In 2006, Zheng and Keeney [24] evaluated drug substances known to be bitter at the same concentration by e-Tongue. Using multivariate statistical analysis, the group distance between a compound and water was calculated. One of them was ranitidine which is considered to be equipotent with NZD and differs by the substitution of a thiazole ring in place of the furan ring in ranitidine. The result from the e-Tongue indicates that the group distance was 804 for ranitidine HCl. For prednisolone Na and quinine HCl, which are known to have a very bitter taste at the tested concentration, the e-Tongue indicated the group distance was 695 for prednisolone and 686 for quinine. Based on the group distance, the relative ranking of bitterness for these compounds would be in the following order: ranitidine HCl > prednisolone Na > quinine HCl. Although the sensors of e-Tongue are mainly used for the determination of similarity between two solution formulations, it may still be possible to compare the qualitative bitterness evaluation of compounds.
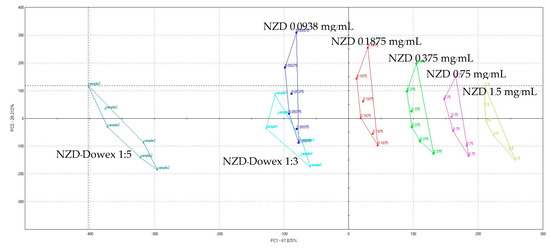
Figure 6.
Principal component analysis (PCA) map of the in vitro taste-masking efficiency test of NZD 0.0938–1.50 mg/mL and the NZD-Dowex 1:3 and 1:5 complexes.

Table 1.
Euclidean distance from reference samples of in vitro taste-masking efficiency test of NZD 1.50 mg/mL and NZD-Dowex 1:3 and 1:5 complexes.

Table 2.
Relative standard deviation (RSD) values (%, n = 3) for the e-tongue sensors in the intra-day repeatability study.
Obtained results from human taste panel have shown that all volunteers assessed designed formulations as pleasant or slightly pleasant tasting, no unpleasant or bitter taste was reported. The median taste scores for NZD-Dowex 1:3 spheres were 1.5. For NZD-Dowex 1:5 spheres, the median score was 0.5 which was significantly tasteless than NZD-Dowex 1:3 spheres (p < 0.05 in the Kruskal-Wallis test). Our human taste panel data confirms that NZD-Dowex 1:5 spheres effectively masked the taste of NZD.
4. Conclusions
Among many taste-masking techniques, the formation of a complex with an ion-exchange resin is simple and cost-effective. In the present study, the drug: resin ratio was optimized, and the morphology, in vitro drug release, and taste evaluation of the selected formulations were investigated. We found that Dowex-50 may be used for taste masking NZD. The tasteless NZD-Dowex complex was obtained at a drug: resin ratio of 1:5 using a stirring time of 1 h in order to get 100% loading of NZD. In the in vitro release study, NZD was released from the NZD-Dowex complex (1:5 ratio) of 4.27% at salivary pH in 5 min, and it was released 99.67% in 60 min at the stomach pH. The taste evaluation depicted the efficient taste masking of NZD, with the NZD-Dowex complex at a 1:5 ratio exhibiting a higher distance value than any other. Taste-masking assessment performed by e-tongue and human test panel methods revealed that Dowex is effective taste-masking carrier of NZD. The NZD-Dowex complex could be considered for future formulations as an orally disintegrating tablet (ODT) or granule (ODG). For an ODT, it will be necessary to produce a big tablet since a dosage unit of NZD is high. NZD in a dose of 150 mg and 300 mg with a ratio drug resin 1:5 require the total weight of tablet to be at least 750 mg and 1500 mg, respectively. In the market, there are some very high dose tablets in which the drug content is nearly 1000 mg, such as anti-HIV drugs, vitamin C and multi-vitamin supplements. However, we are concerned that a big size of tablet may affect patient compliance and acceptability of medication regimens or could lead to medication errors. Alternatively, in order to overcome the large size of tablet, NZD-Dowex complex could be formulated as an ODG.
Author Contributions
Conceptualization, P.J.; Methodology, P.P., K.B., K.-M.H., E.-S.P. and P.J.; Supervision, P.J.; Writing-original draft, K.-M.H., E.-S.P. and P.J.; Writing-review & editing, P.J.
Funding
This research received no external funding.
Acknowledgments
This research work was partially supported by Chiang Mai University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sohi, H.; Sultana, Y.; Khar, R.K. Taste masking technologies in oral pharmaceuticals: Recent developments and approaches. Drug Dev. Ind. Pharm. 2004, 30, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Ozer, A.Y.; Hincal, A.A. Studies on the masking of unpleasant taste of beclamide: Microencapsulation and tableting. J. Microencaosul. 1990, 7, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Pareek, A.; Bagdi, G.; Aahmad, D.; Ahmd, A. Taste masking methods for bitter drug—A review. Int. J. Pharm. Life Sci. 2010, 1, 336–339. [Google Scholar]
- Venkatesh, D.P.; Karki, R.; Goli, D.; Jha, S.K. Over view ion exchange resins in controlled drug delivery system. World J. Pharm. Pharm. Sci. 2013, 2, 4764–4777. [Google Scholar]
- Borodkin, S.; Yunker, M.H. Interaction of amine drugs with a polycarboxylic acid ion-exchange resin. J. Pharm. Sci. 1970, 59, 481–486. [Google Scholar] [CrossRef]
- Suhagiya, V.K.; Goyani, A.N.; Gupta, R.N. Taste masking by ion exchange resin and its new applications: A review. Int. J. Pharm. Sci. Res. 2010, 1, 22–37. [Google Scholar]
- Saunders, L. Ion-exchange resins in organic analysis. J. Pharm. Pharmacol. 1953, 5, 569–578. [Google Scholar] [CrossRef]
- Chauhan, R. Taste masking: A unique approach for bitter drugs. J. Stem Cell Biol. Transplant. 2017, 1, 12. [Google Scholar] [CrossRef][Green Version]
- Levine, L.R.; Cloud, M.L.; Enas, N.H. Nizatidine prevents peptic ulceration in high-risk patients taking nonsteroidal anti-inflammatory drugs. Arch. Intern. Med. 1993, 153, 2449–2454. [Google Scholar] [CrossRef]
- Abraham, N.S.; Hlatky, M.A.; Antman, E.M.; Bhatt, D.L.; Bjorkman, D.J.; Clark, C.B.; Furberg, C.D.; Johnson, D.A.; Kahi, C.J.; Laine, L.; et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: A focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am. J. Gastroenterol. 2010, 15, 2533–2549. [Google Scholar]
- Kumar, B.P.; Archana, G.; Swarupa, Y.; Devi, K.J. Formulation and evaluation of nizatidine fast dissolving tablets. World J. Pharm. Res. 2015, 4, 1358–1372. [Google Scholar]
- Rajan, R.P.; Makkena, P.; Thangavel, S. Formulation and evaluation of orodispersible tablets of taste masked nizatidine. Int. Res. J. Pharm. 2012, 3, 204–209. [Google Scholar]
- Mahal, R.H.; Samein, L.H.; Shehab, M.A. Formulation and in-vitro evaluation of orodispersible tablet. Int. J. Pharm. Sci. Res. 2015, 6, 689–696. [Google Scholar]
- Akkaramongakolporn, P.; Terada, K.; Yonemochi, E. Molecular properties of propranolol hydrochloride prepared as drug resin complexes. Drug Dev. Ind. Pharm. 2001, 27, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Guhmann, M.; Preis, M.; Gerber, F.; Pollinger, N.; Breikreutz, J.; Weitschies, W. Development of oral taste masked diclofenac formulations using a taste sensing system. Int. J. Pharm. 2012, 438, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Dureja, H. Ion exchange resin complexation technique for pharmaceutical taste masking: An overview. World J. Prep. Sch. 2015, 4, 600–614. [Google Scholar]
- Bhalekar, M.; Avari, J.G.; Jaiswal, S.B. Cation-exchanger in pharmaceutical formulation. Ind. J. Pharm. Sci. 2004, 38, 184–187. [Google Scholar]
- Motukuri, R.; Nagesh, P.; Venisetty, R.K. Development and evaluation of gastric retentive floating tablets of nizatidine. IJPRBS 2014, 3, 252–276. [Google Scholar]
- Urpayil, S.; Thayyil, M.S. Thermal and spectroscopic studies on nizatidine-PVP mixture. IOSR J. Pharm. 2012, 2, 17–23. [Google Scholar] [CrossRef]
- Magnotta, V.L.; Gates, B.C. Superacid polymers: Synthesis and analysis of AlCl3-sulfonic acid resin complexes. J. Polym. Sci. 1977, 15, 1341–1347. [Google Scholar] [CrossRef]
- Yi, E.; Kim, J.; Rhee, Y.; Kim, S.; Lee, H.; Park, C.; Park, E. Preparation of sildenafil citrate microcapsules and in vitro/in vivo evaluation of taste masking efficiency. Int. J. Pharm. 2014, 466, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Siewart, M.; Dressman, J.; Brown, C.K.; Shah, V.P.; Aiache, J.M.; Aoyagi, N.; Crison, J. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. Aaps Pharmscitech 2003, 4, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Rachid, O.; Simons, F.; Qualaji, M.; Simons, K. An electronic tongue: Evaluation of the masking efficacy of sweetening and/or flavoring agents on the bitter taste of epinephrine. Aaps Pharmscitech 2010, 11, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Y.; Keeney, M.P. Taste masking analysis in pharmaceutical formulation development using an electronic tongue. Int. J. Pharm. 2006, 310, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Legin, A.; Rudnitskaya, A.; Clapham, D.; Seleznev, B.; Lord, K.; Vlasov, Y. Electronic tongue for pharmaceutical analytics: Quantification of tastes and masking effects. Anal. Bioanal. Chem. 2004, 380, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Boateng, J.; Bonnefille, M.; Aranyos, A.; Mitchell, J.; Douroumis, D. Taste masking of paracetamol by hot-melt extrusion: An in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 80, 433–442. [Google Scholar] [CrossRef]
- Beullens, K.; Meszaros, P.; Vermeir, S.; Kirsanov, D.; Legin, A.; Buysens, S.; Cap, N.; Nicolai, B.; Lammertyn, J. Analysis of tomato taste using two types of electronic tongues. Sens. Actuators B 2008, 131, 10–17. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Vittayapadung, S. Identification of the green tea grade level using electronic tongue and pattern recognition. Food Res. Int. 2008, 41, 500–504. [Google Scholar] [CrossRef]
- del Valle, M. Electronic tongues employing electrochemical sensors. Electroanalysis 2010, 22, 1539–1555. [Google Scholar] [CrossRef]
- Münster, M.; Mohamed-Ahmed, A.H.A.; Immohr, L.I.; Schoch, C.; Schmidt, C.; Tuleu, C.; Breitkreutz, J. Comparative in vitro and in vivo taste assessment of liquid praziquantel formulations. Int. J. Pharm. 2017, 529, 310–318. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).