Upscaling of Toluene Oxidation Using Water-Sprinkled Pulsed Corona Discharge and Photocatalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
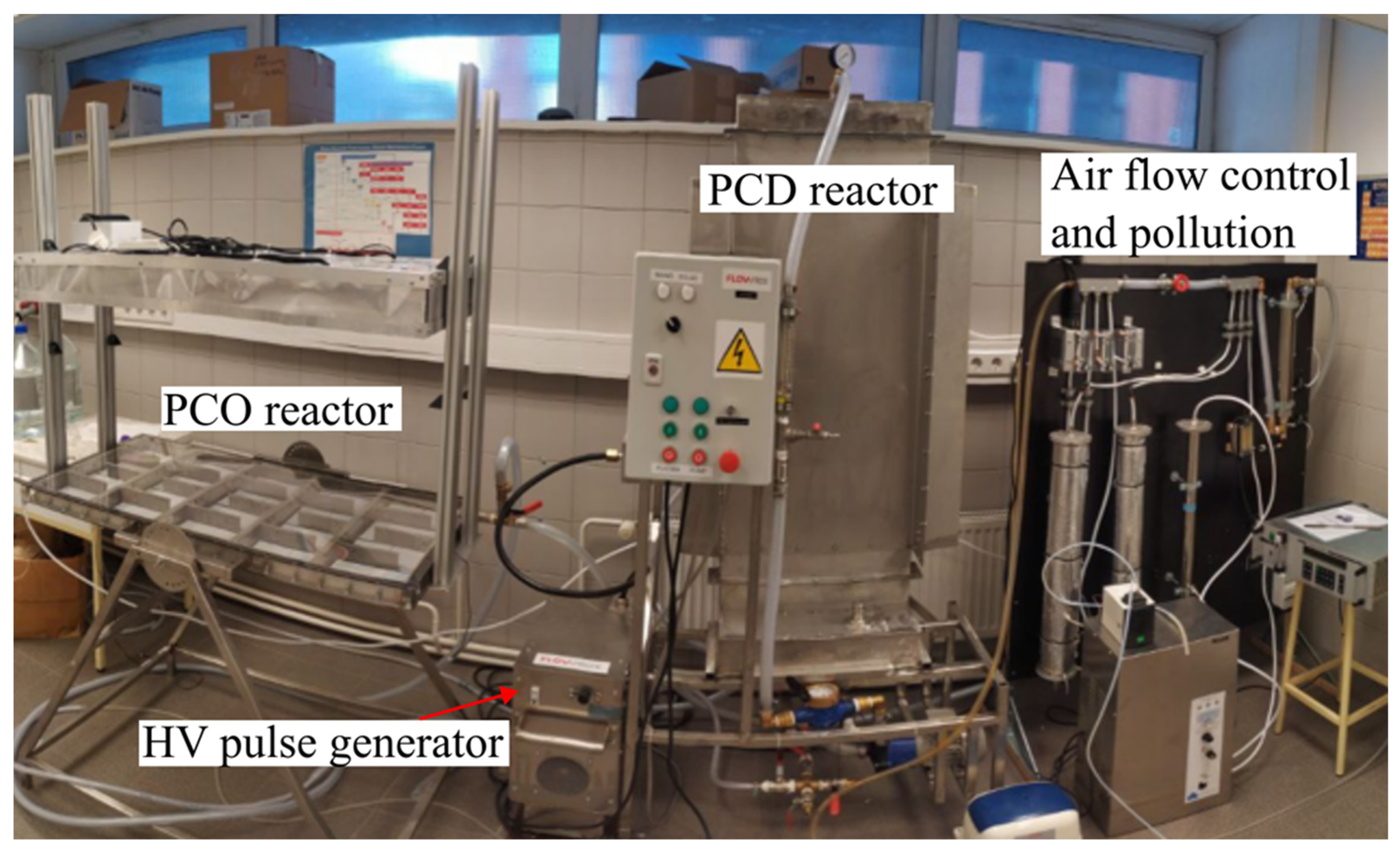
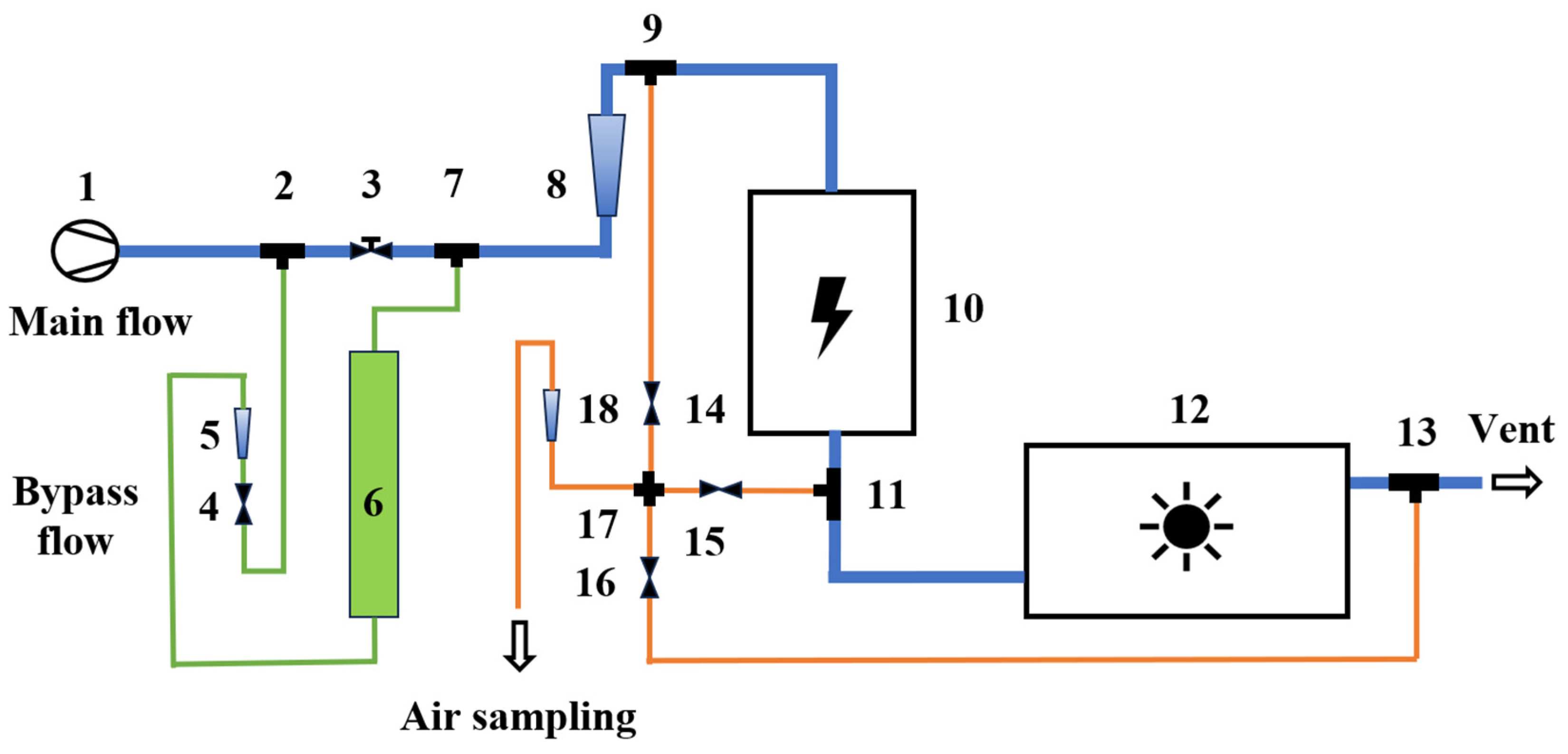
2.2. Experimental Device
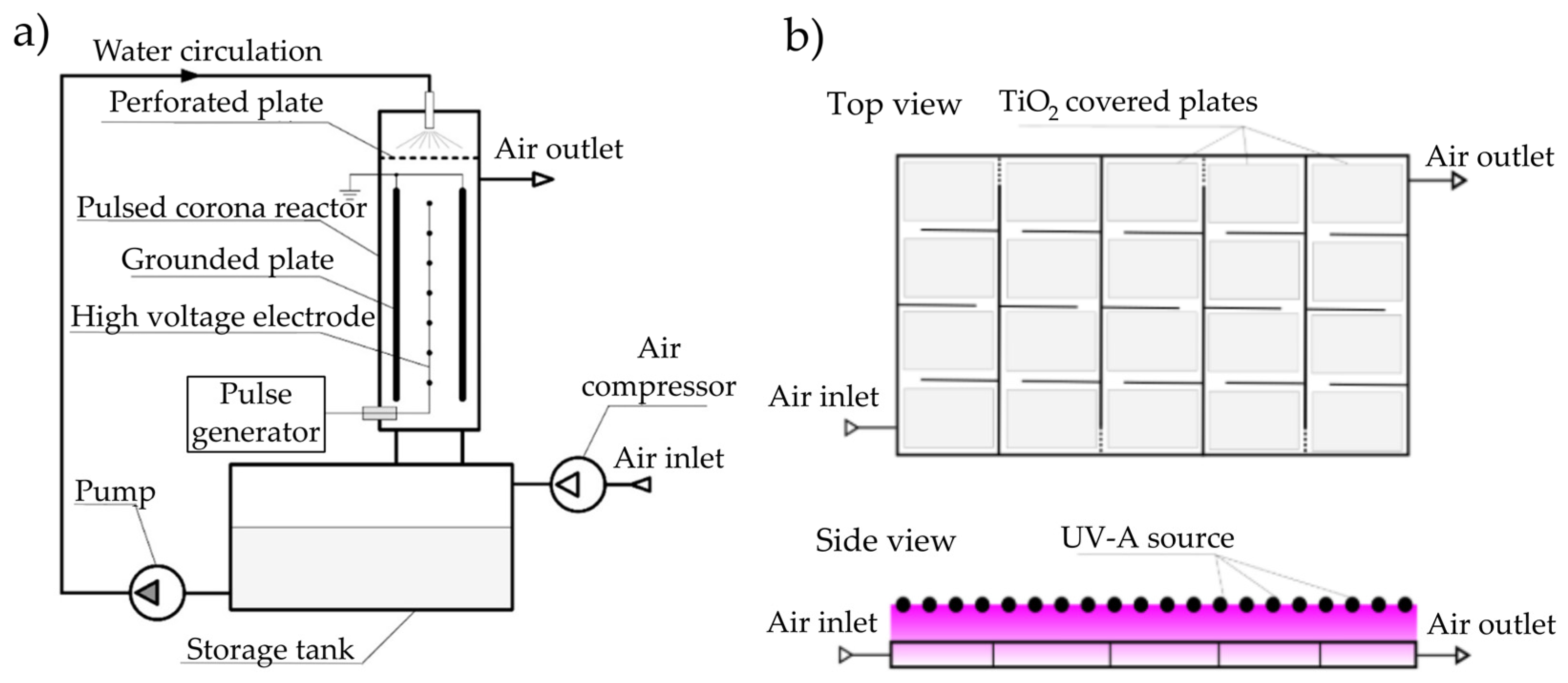
2.3. Reactors
2.4. Analyses
3. Results and Discussion
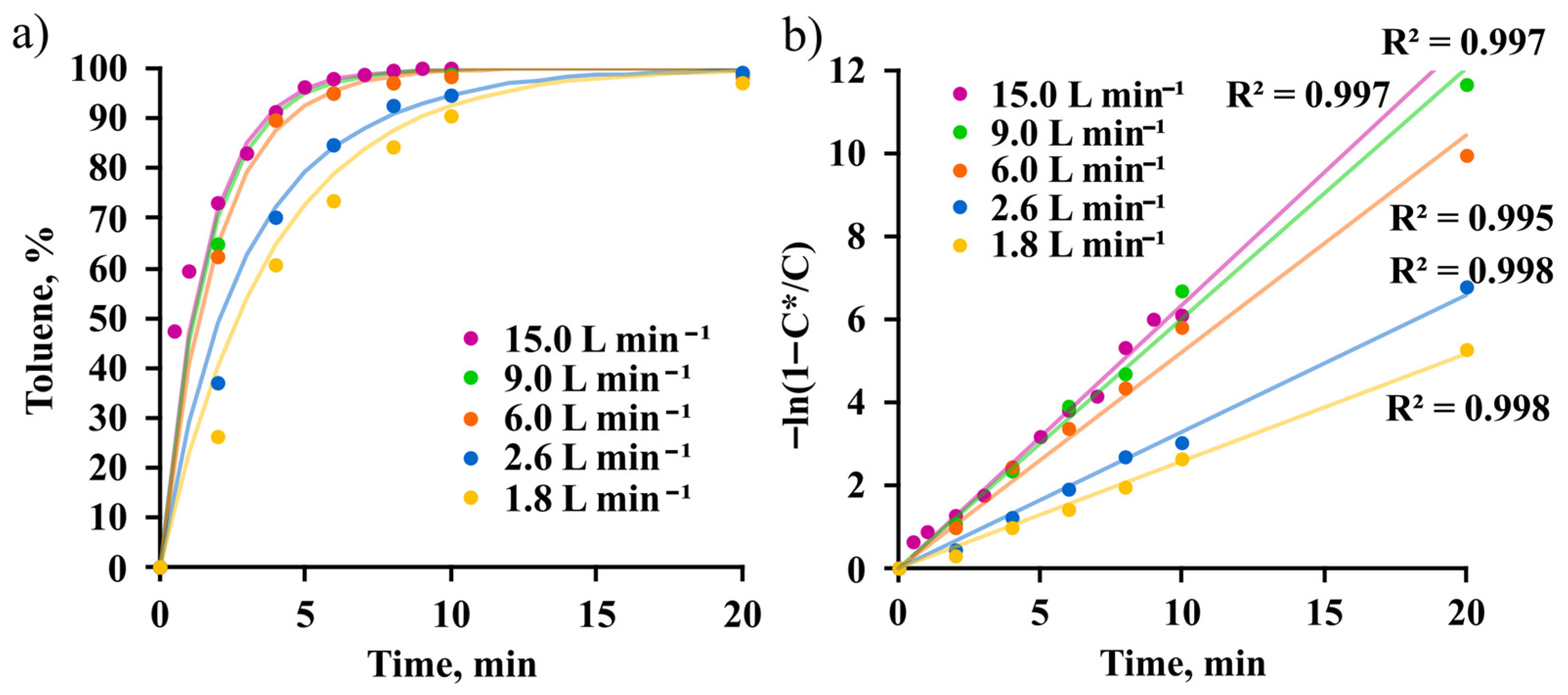
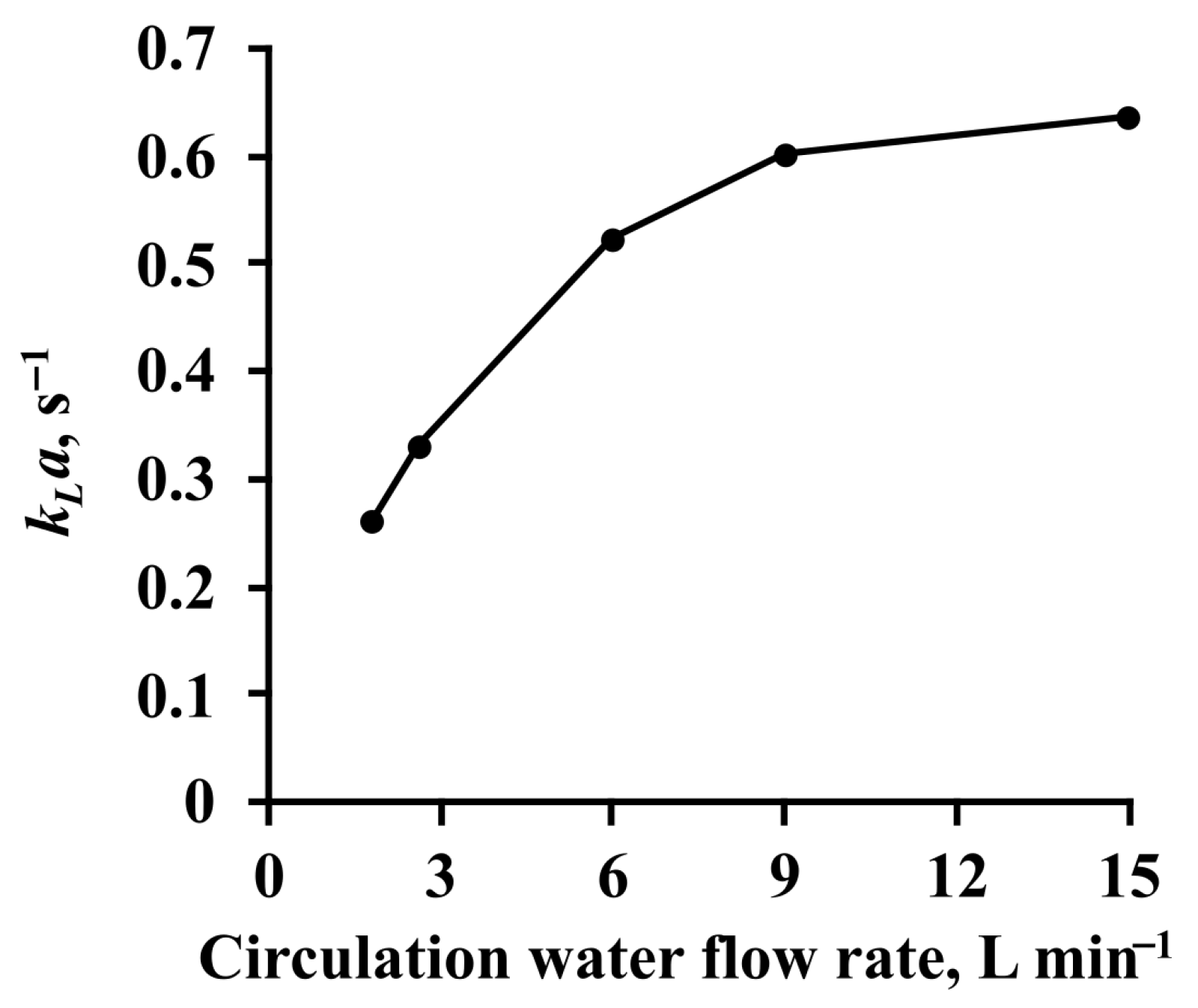
3.1. Toluene Absorption
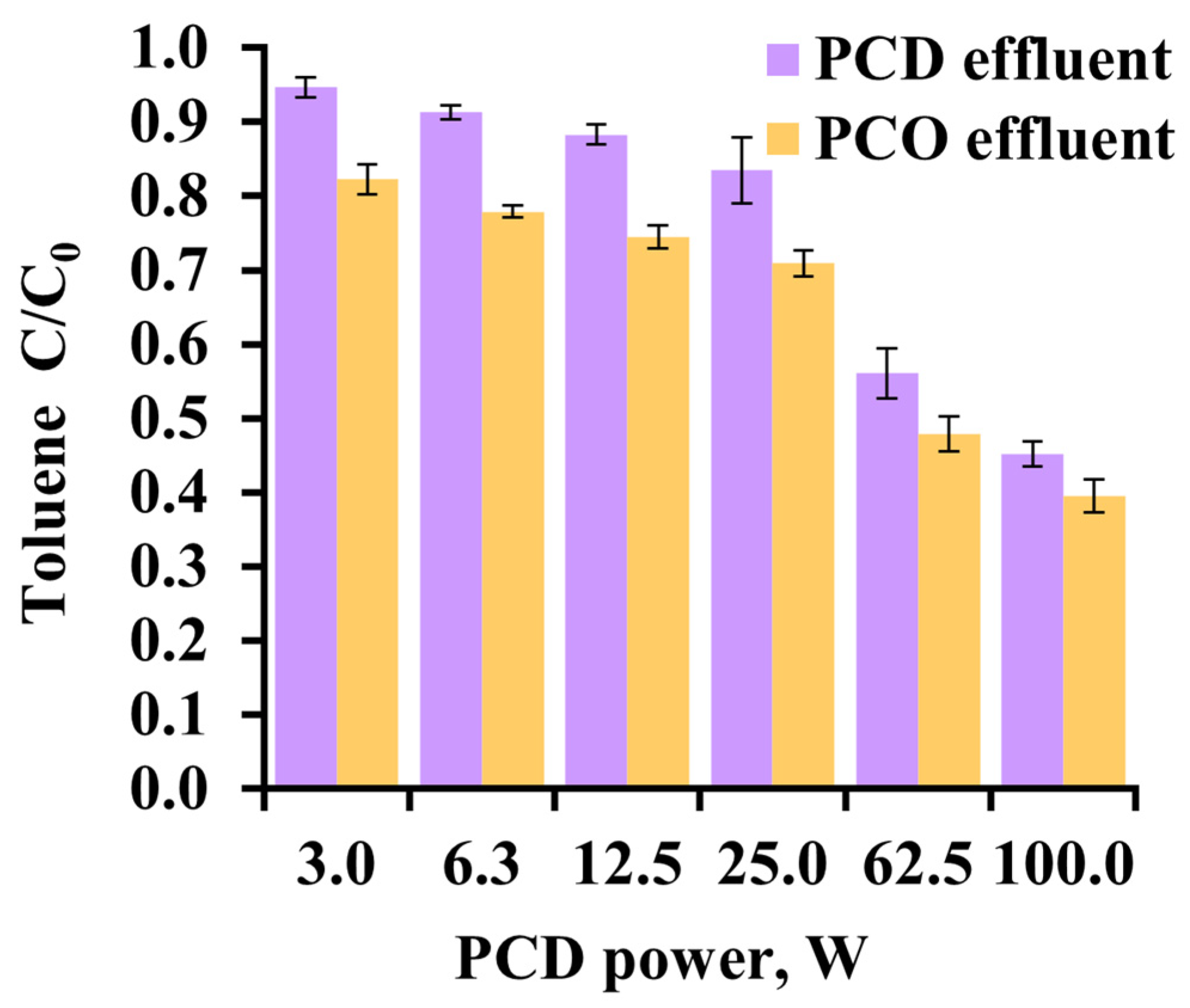
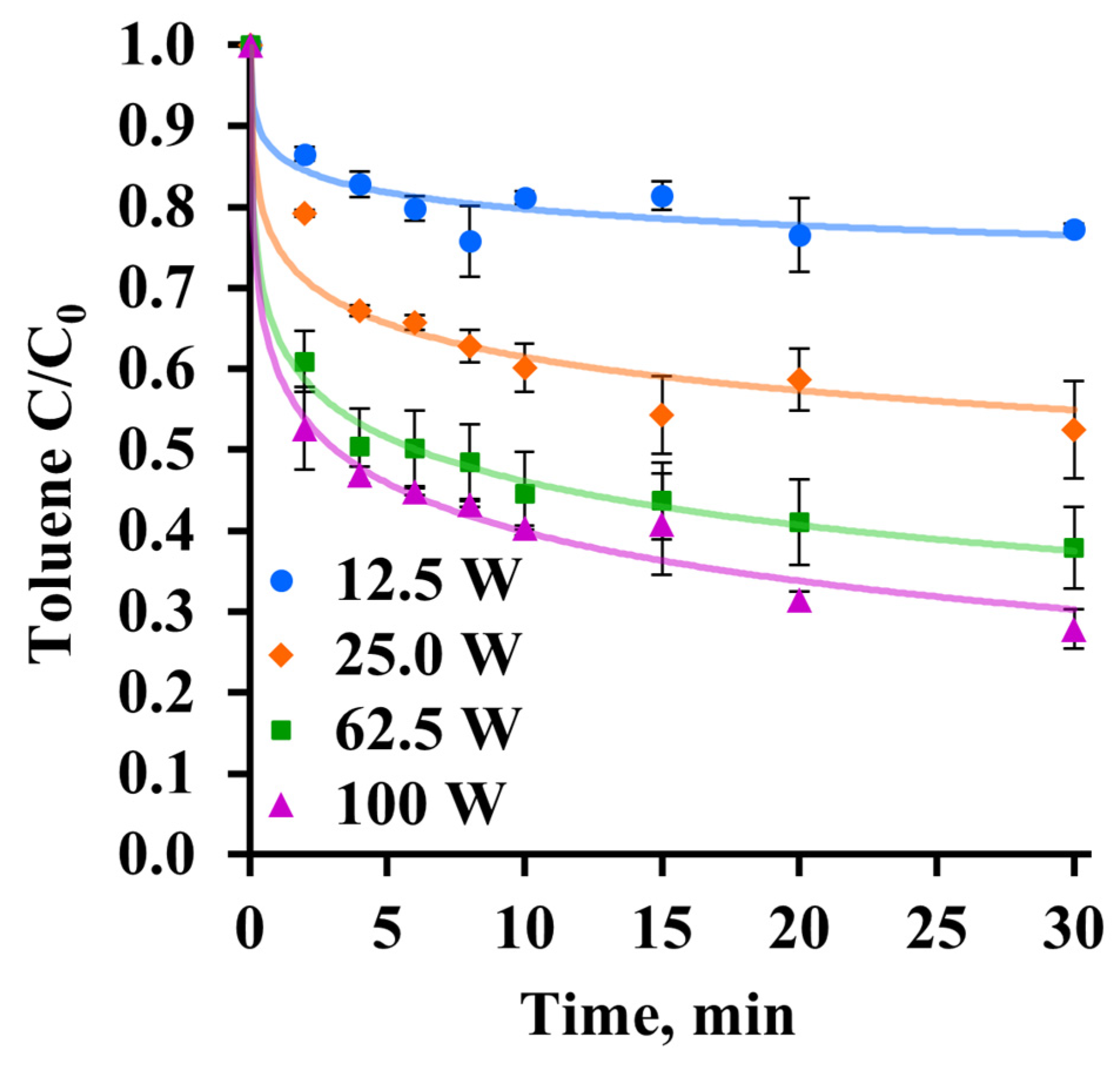
3.2. Toluene Oxidation in Gaseous Phase
3.3. Aqueous Toluene Oxidation
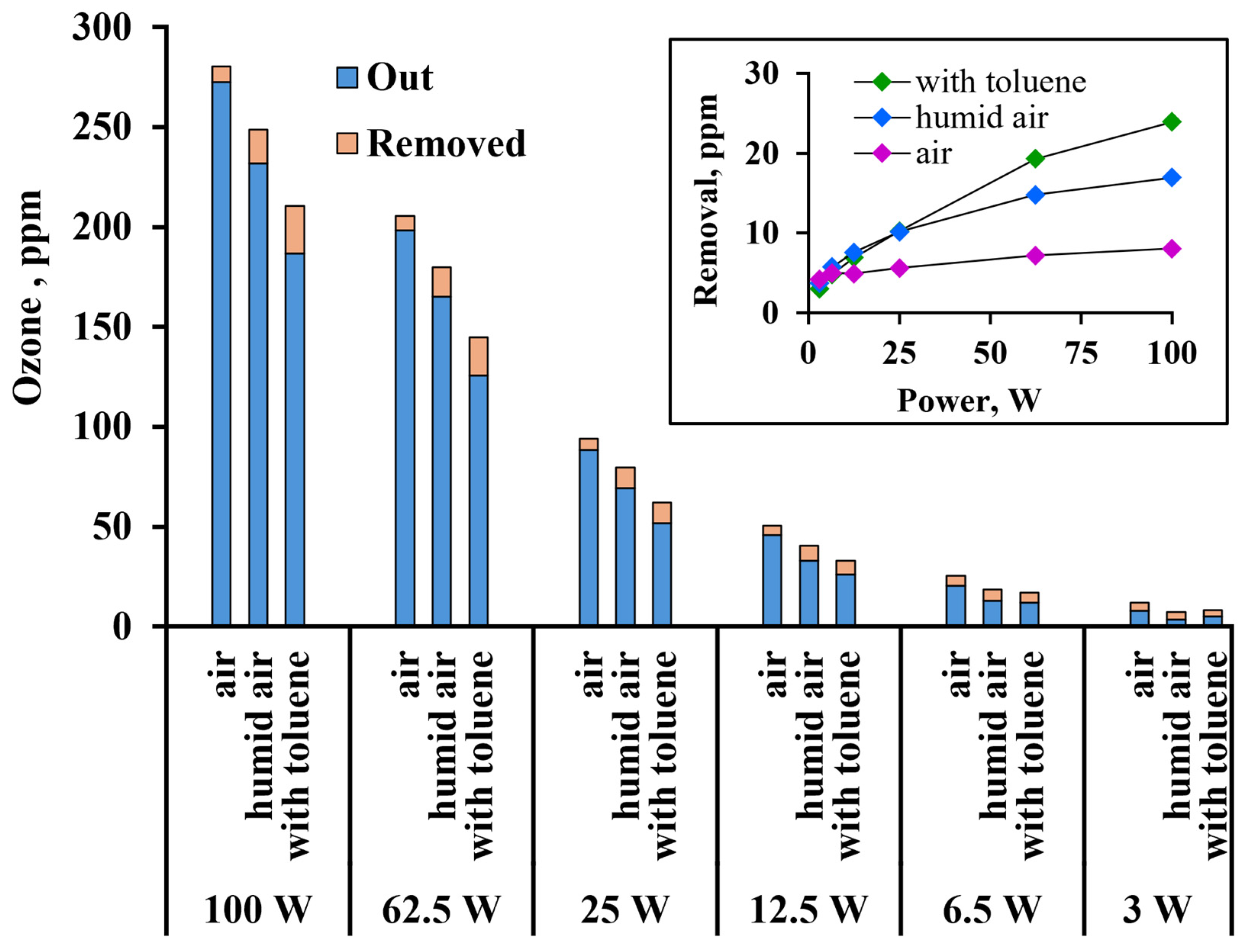
3.4. Ozone Removal in Photocatalytic Reactor
3.5. Phenols in Circulation Water
3.6. Toluene Oxidation Products
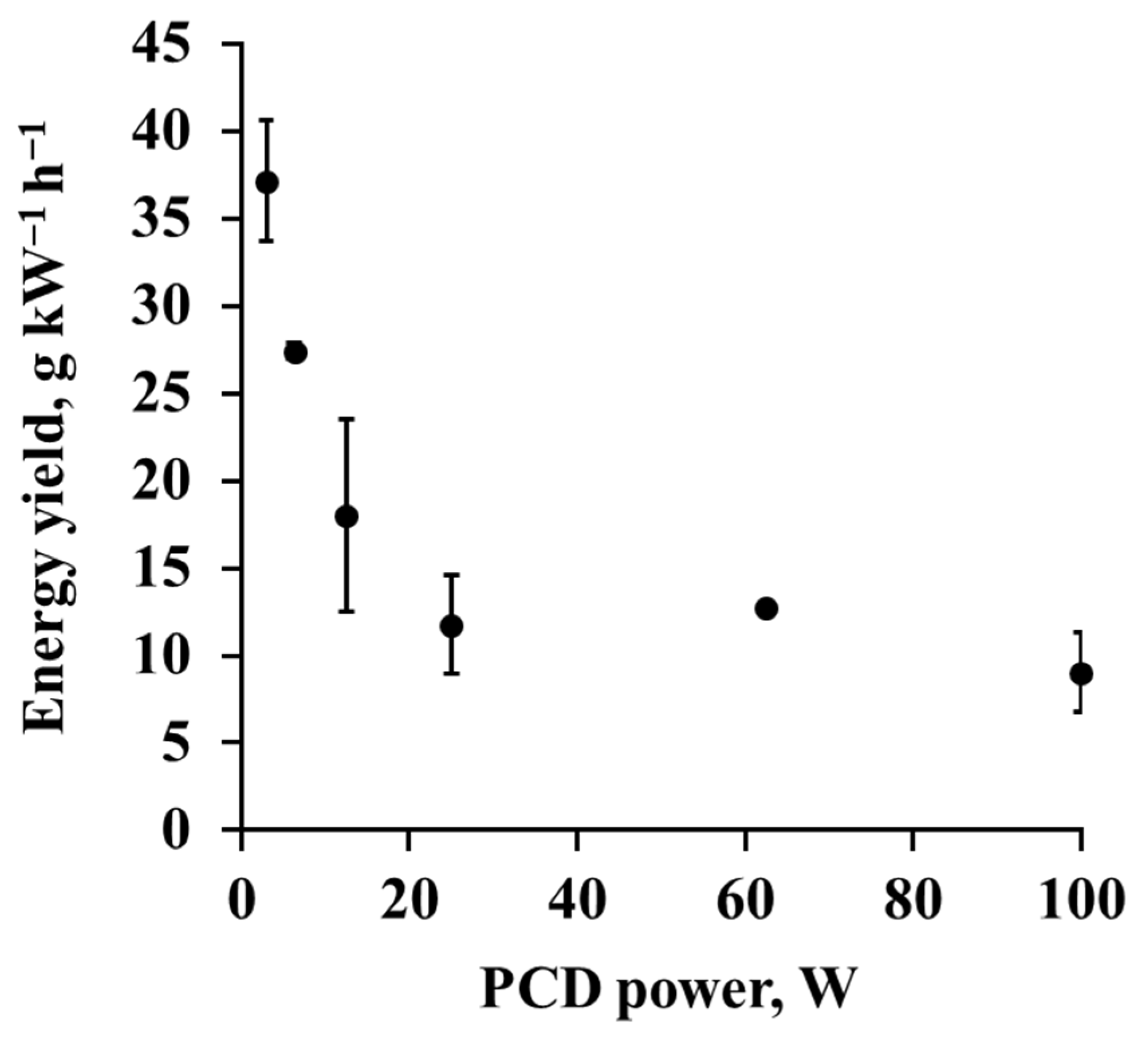
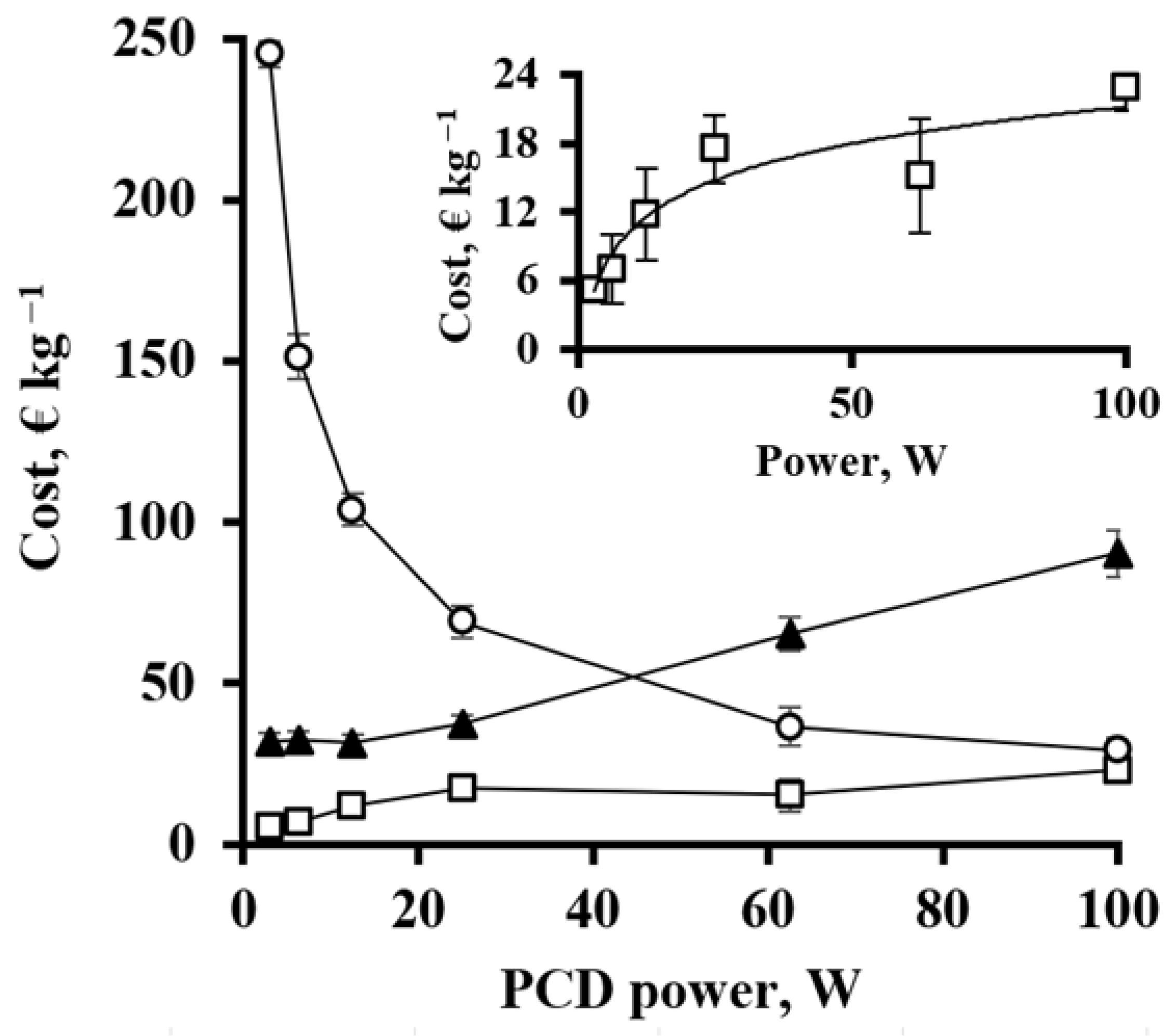
3.7. Energy and Cost Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOP | Advanced oxidation process |
| BTEX | Benzene, toluene, ethylbenzene, and xylene |
| CD | Corona discharge |
| DBD | Dielectric barrier discharge |
| DPBD | Dielectric packed-bed discharge plasma |
| FTIR | Fourier-transform infrared spectroscopy |
| GAD | Gliding arc discharge |
| GC-MS | Gas chromatograph–mass spectrometer |
| HPLC | High-performance liquid chromatography |
| PCD | Pulsed corona discharge |
| PCO | Photocatalytic oxidation |
| PDA | Photodiode array detector |
| PET | Polyethylene terephthalate |
| pm | Particulate matter |
| ppm | Parts per million |
| pps | Pulses per second |
| UV | Ultraviolet |
| VOC | Volatile organic compound |
Appendix A
References
- Council Directive 2016/2284/EU on the Reduction of National Emissions of Certain Atmospheric Pollutants. 2016. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02016L2284-20240206 (accessed on 6 October 2024).
- European Environment Agency. Air pollution in Europe. In EEA Briefing; Publications Office: Luxembourg, 2024; Available online: https://data.europa.eu/doi/10.2800/019282 (accessed on 6 October 2024).
- Geiss, O.; Giannopoulos, G.; Tirendi, S.; Barrero-Moreno, J.; Larsen, B.R.; Kotzias, D. The AIRMEX study—VOC measurements in public buildings and schools/kindergartens in eleven European cities: Statistical analysis of the data. Atmos. Environ. 2011, 45, 3676–3684. [Google Scholar] [CrossRef]
- Bolden, A.L.; Kwiatkowski, C.F.; Colborn, T. New Look at BTEX: Are Ambient Levels a Problem? Environ. Sci. Technol. 2015, 49, 5261–5276. [Google Scholar] [CrossRef]
- Sharma, N.; Agarwal, A.K.; Eastwood, P.; Gupta, T.; Singh, A.P. (Eds.) Air Pollution and Control. In Energy, Environment, and Sustainability; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Khan, F.I.; Kr, A. Ghoshal, Removal of Volatile Organic Compounds from polluted air. J. Loss Prev. Process. Ind. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Schoofs, K.; Van Gastel, A.; Demuynck, M.; Baetens, D.; Vaes, K.; Denys, S. Closing the loop: A kinetic study on photocatalytic in situ regeneration of activated carbon filters for continuous indoor VOC removal. Chem. Eng. J. 2025, 518, 164590. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H.; Heynderickx, P.M.; Boukhvalov, D.W. Titanium carbide MXene/anatase titanium dioxide-supported gold catalysts for the low-temperature oxidation of benzene in indoor air. J. Colloid Interface Sci. 2025, 695, 137770. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, X.; Wang, J.; Liu, H.; Jin, H.; Zhang, J.; Li, G.; Tang, Y.; Ye, C. High-value utilization of agricultural waste: A study on the catalytic performance and deactivation characteristics of iron-nickel supported biochar-based catalysts in the catalytic cracking of toluene. Energy 2025, 323, 135806. [Google Scholar] [CrossRef]
- Fu, M.; Ma, Z.; Gao, C.; Ye, Y.; Li, W.; Hou, D.; Cao, Y. A Multi-Functional VOC Sensor Based on Cascaded Quartz Crystal Resonators. IEEE Electron Device Lett. 2025, 46, 476–479. [Google Scholar] [CrossRef]
- Kim, H. Nonthermal Plasma Processing for Air-Pollution Control: A Historical Review, Current Issues, and Future Prospects. Plasma Process. Polym. 2004, 1, 91–110. [Google Scholar] [CrossRef]
- Ono, R.; Oda, T. Dynamics of ozone and OH radicals generated by pulsed corona discharge in humid-air flow reactor measured by laser spectroscopy. J. Appl. Phys. 2003, 93, 5876–5882. [Google Scholar] [CrossRef]
- Wardman, P. Reduction Potentials of One-Electron Couples Involving Free Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef]
- Ajo, P.; Kornev, I.; Preis, S. Pulsed Corona Discharge Induced Hydroxyl Radical Transfer Through the Gas-Liquid Interface. Sci. Rep. 2017, 7, 16152. [Google Scholar] [CrossRef]
- Parvulescu, V.I.; Magureanu, M.; Lukes, P. (Eds.) Plasma Chemistry and Catalysis in Gases and Liquids, 1st ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- Feng, X.; Liu, H.; He, C.; Shen, Z.; Wang, T. Synergistic effects and mechanism of a non-thermal plasma catalysis system in volatile organic compound removal: A review. Catal. Sci. Technol. 2018, 8, 936–954. [Google Scholar] [CrossRef]
- Kask, M.; Bolobajev, J.; Krichevskaya, M. Gas-phase photocatalytic degradation of acetone and toluene, and their mixture in the presence of ozone in continuous multi-section reactor as possible air post-treatment for exhaust from pulsed corona discharge. Chem. Eng. J. 2020, 399, 125815. [Google Scholar] [CrossRef]
- Besley, L.M.; Bottomley, G.A. Vapor pressure of toluene from 273.15 to 298.15 K. J. Chem. Thermodyn. 1974, 6, 577–580. [Google Scholar] [CrossRef]
- Gan, E.V.; Haberman, H.F.; Menon, I.A. A simple and sensitive test for the determination of phenolic compounds in urine and its application to melanoma. J. Investig. Dermatol. 1975, 64, 139–144. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Leighton, D.T.; Calo, J.M. Distribution coefficients of chlorinated hydrocarbons in dilute air-water systems for groundwater contamination applications. J. Chem. Eng. Data 1981, 26, 382–385. [Google Scholar] [CrossRef]
- Altof, K.; Krichevskaya, M.; Preis, S.; Tähemaa, T.; Bolobajev, J. Ozone-assisted degradation of 2-methoxyethanol in a prototype plug flow photocatalytic reactor. Chem. Eng. J. 2024, 481, 148488. [Google Scholar] [CrossRef]
- Altof, K.; Krichevskaya, M.; Preis, S.; Bolobajev, J. Oxidation of Airborne m-Xylene in Pulsed Corona Discharge: Impact of Water Sprinkling. ChemEngineering 2024, 8, 99. [Google Scholar] [CrossRef]
- Lukes, P.; Clupek, M.; Babicky, V.; Janda, V.; Sunka, P. Generation of ozone by pulsed corona discharge over water surface in hybrid gas–liquid electrical discharge reactor. J. Phys. Appl. Phys. 2005, 38, 409–416. [Google Scholar] [CrossRef]
- Ng, N.L.; Brown, S.S.; Archibald, A.T.; Atlas, E.; Cohen, R.C.; Crowley, J.N.; Day, D.A.; Donahue, N.M.; Fry, J.L.; Fuchs, H.; et al. Nitrate radicals and biogenic volatile organic compounds: Oxidation, mechanisms, and organic aerosol. Atmos. Chem. Phys. 2017, 17, 2103–2162. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liao, X.; Fu, M.; Huang, H.; Ye, D. Toluene decomposition performance and NOx by-product formation during a DBD-catalyst process. J. Environ. Sci. 2015, 28, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Korzekwa, R.A.; Grothaus, M.G.; Hutcherson, R.K.; Roush, R.A.; Brown, R. Destruction of hazardous air pollutants using a fast rise time pulsed corona reactor. Rev. Sci. Instrum. 1998, 69, 1886–1892. [Google Scholar] [CrossRef]
- Byeon, J.H.; Park, J.H.; Jo, Y.S.; Yoon, K.Y.; Hwang, J. Removal of gaseous toluene and submicron aerosol particles using a dielectric barrier discharge reactor. J. Hazard. Mater. 2010, 175, 417–422. [Google Scholar] [CrossRef]
- Du, C.M.; Yan, J.H.; Cheron, B. Decomposition of toluene in a gliding arc discharge plasma reactor. Plasma Sources Sci. Technol. 2007, 16, 791–797. [Google Scholar] [CrossRef]
- Ondarts, M.; Hajji, W.; Outin, J.; Bejat, T.; Gonze, E. Non-Thermal Plasma for indoor air treatment: Toluene degradation in a corona discharge at ppbv levels. Chem. Eng. Res. Des. 2017, 118, 194–205. [Google Scholar] [CrossRef]
- Harling, A.M.; Glover, D.J.; Whitehead, J.C.; Zhang, K. Novel Method for Enhancing the Destruction of Environmental Pollutants by the Combination of Multiple Plasma Discharges. Environ. Sci. Technol. 2008, 42, 4546–4550. [Google Scholar] [CrossRef]
- Mista, W.; Kacprzyk, R. Decomposition of toluene using non-thermal plasma reactor at room temperature. Catal. Today 2008, 137, 345–349. [Google Scholar] [CrossRef]
- Preis, S.; Panorel, I.C.; Kornev, I.; Hatakka, H.; Kallas, J. Pulsed corona discharge: The role of ozone and hydroxyl radical in aqueous pollutants oxidation. Water Sci. Technol. 2013, 68, 1536–1542. [Google Scholar] [CrossRef]
- Eurostat. Electricity Prices for Non-Household Consumers—Bi-Annual Data (from 2007 Onwards). 2024. Available online: https://ec.europa.eu/eurostat/databrowser/product/page/NRG_PC_205 (accessed on 16 January 2020).
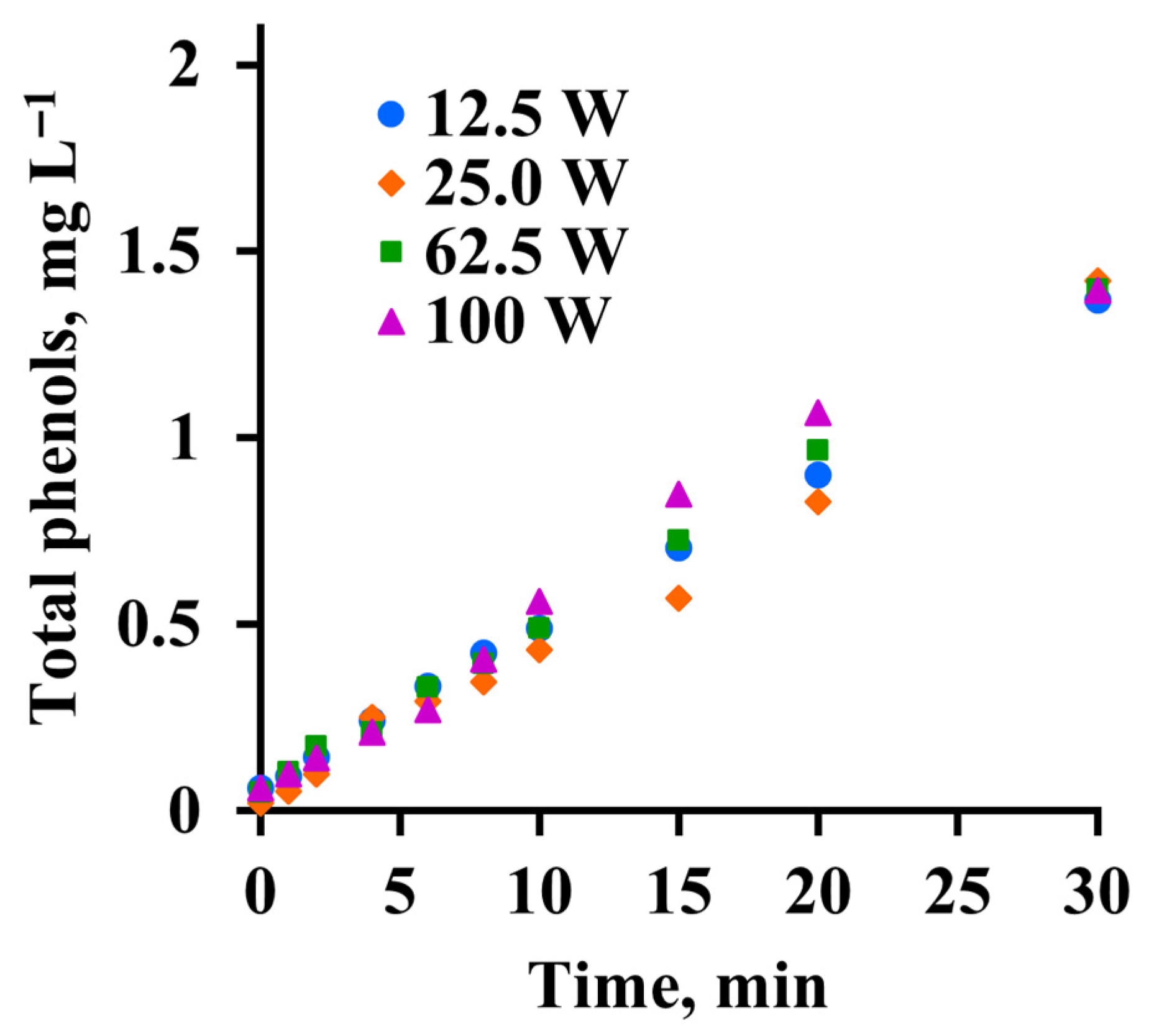
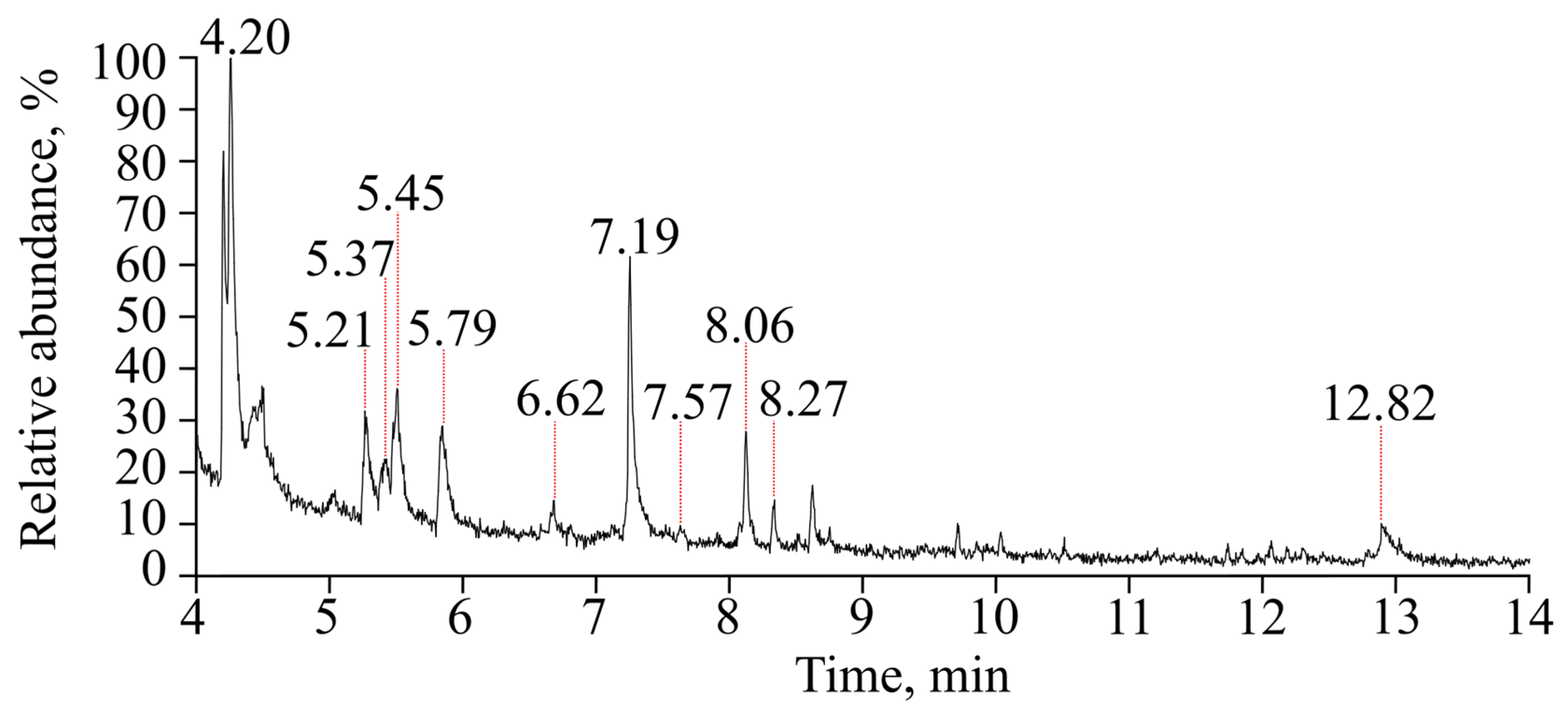
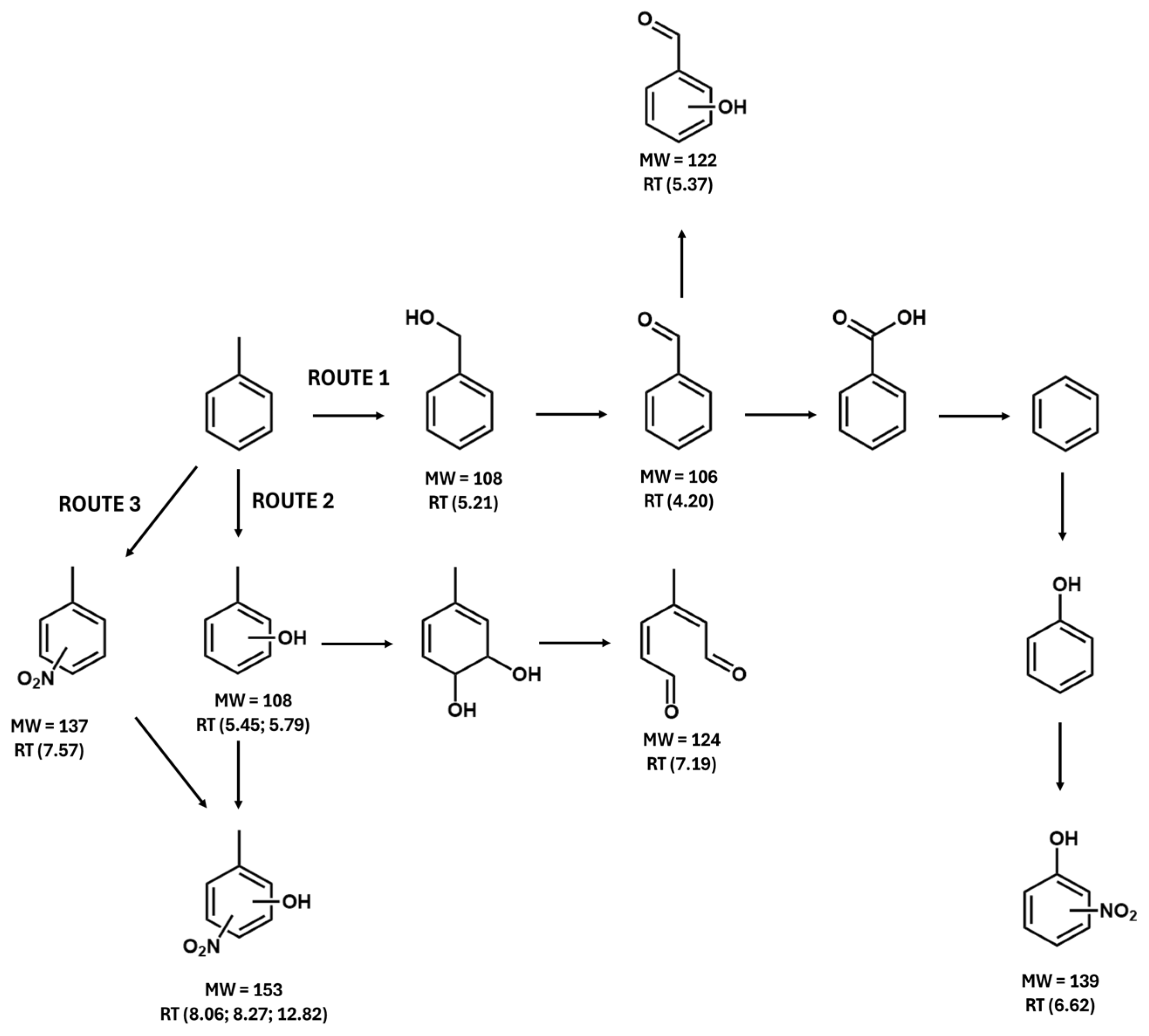
| Retention Time, min | Compound | Molecular Weight, g mol−1 |
|---|---|---|
| 4.20 | Benzaldehyde | 106 |
| 5.21 | Benzylalcohol | 108 |
| 5.37 | Hydroxybenzaldehyde | 122 |
| 5.45; 5.79 | Methylphenol | 108 |
| 6.62 | Nitrophenol | 139 |
| 7.19 | 3-Methylhexa-2,4-dienedial | 124 |
| 7.57 | Methylnitrobenzene | 137 |
| 8.06; 8.27; 12.82 | Methylnitrophenol | 153 |
| Plasma Type * | Gas Flow Rate, L min−1 | Specific Energy Density, J L−1 | Initial Toluene Concentration, ppm | Removal, % | Energy Yield, g kW−1h−1 | Source |
|---|---|---|---|---|---|---|
| PCD | 100 | 60 | 82 ± 14 | 5.3–54.8 | 9.0–37.1 | Ours |
| DBD | 1 | 2550 | 80 | 76 | 7.2 | [26] |
| PCD | 45 | 120 | 330 | 90 | 4.7 | [27] |
| DBD | 0.25 | 200 | 70 | 40 | 1.9 | [28] |
| GAD | 0.45 | 140 | 106 | 45 | 1.2 | [29] |
| CD | 150 | 6.8 | 0.5 | 86 | 1.0 | [30] |
| DPBD | 1 | 2502 | 110 | 72 | 0.6 | [31] |
| CD | 1 | 2064 | 100 | 51 | 0.3 | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teittinen, D.A.; Preis, S.; Bolobajev, J. Upscaling of Toluene Oxidation Using Water-Sprinkled Pulsed Corona Discharge and Photocatalysis. Processes 2025, 13, 2982. https://doi.org/10.3390/pr13092982
Teittinen DA, Preis S, Bolobajev J. Upscaling of Toluene Oxidation Using Water-Sprinkled Pulsed Corona Discharge and Photocatalysis. Processes. 2025; 13(9):2982. https://doi.org/10.3390/pr13092982
Chicago/Turabian StyleTeittinen, Daniel A., Sergei Preis, and Juri Bolobajev. 2025. "Upscaling of Toluene Oxidation Using Water-Sprinkled Pulsed Corona Discharge and Photocatalysis" Processes 13, no. 9: 2982. https://doi.org/10.3390/pr13092982
APA StyleTeittinen, D. A., Preis, S., & Bolobajev, J. (2025). Upscaling of Toluene Oxidation Using Water-Sprinkled Pulsed Corona Discharge and Photocatalysis. Processes, 13(9), 2982. https://doi.org/10.3390/pr13092982







