Abstract
Photocatalysts for applications in different sectors, e.g., civil and environmental, are already developed to a mature extent and allow, for example, the purification of gaseous and liquid streams or the self−cleaning surfaces. The application of photocatalysts in the industrial sector is, however, quite limited. The review addresses the specific topic of the photocatalytic reforming of methane and biomass derivates. In this regard, recent advances in materials science are reported and discussed, in particular regarding doped and modified oxides (TiO2 and ZrO2) or non−oxidic ceramics. Concerning process integration, a comparison between traditional two−dimensional photoreactors and fluidized bed systems is proposed and design guidelines are drawn, with indications of the possible benefits. Photocatalytic fluidized beds appear more suitable for small− and medium−scale integrated processes of reforming, operating at lower temperatures than traditional ones for distributed hydrogen generation.
1. Introduction
As the principal pathway for hydrogen and syngas production, hydrocarbon reforming is one of the most important processes of the chemical industry. More than 40% of the world’s hydrogen production is obtained through steam reforming of natural gas, and the hydrogen and syngas demands are expected to be ever increasing in the next future [1]. However, the conventional steam reforming reaction, typically performed on methane (R1), is highly endothermic and requires the use of a catalyst, high temperatures, and high amounts of excess water compared to stoichiometry to obtain suitable yields and limit side reactions, such as methane cracking (R2), which result in coke deposition on the catalyst’s surface [2,3].
| ΔH° = 204 kJ/mol | (R1) | |
| ΔH° = 74 kJ/mol | (R2) |
The high reaction temperature required results in a very energy intensive process, and while noble metal−based catalysts would be able to provide very good yields and high reaction selectivity towards syngas production with limited coke formation, they are too expensive to be commercially used, with nickel−based catalysts being the industry standard in modern processes instead [4,5]. However, nickel is a toxic metal [6], leading to safety problems when handling catalysts and during disposal. Coke formation and sulfur poisoning become particularly relevant problems when considering biogas dry reforming, which would otherwise be a much more sustainable process compared to steam reforming of natural gas, being potentially able to achieve negative carbon dioxide emissions [7]. Biogas usually contains high concentrations of carbon dioxide in addition to methane; therefore, the direct application of a dry reforming reaction (R3) would allow methane conversion without expensive separation pre−treatments [8]. Unfortunately, dry reforming is an even more endothermic reaction which is also much more affected by coke deposition compared to steam reforming, as CO2 is a weaker oxidant, with the Boudouard reaction (R4) and reverse water–gas shift reactions (R5) negatively affecting yield and H2/CO ratio [9,10].
| ΔH° = 247 kJ/mol | (R3) | |
| ΔH° = 172 kJ/mol | (R4) | |
| ΔH° = 41 kJ/mol | (R5) |
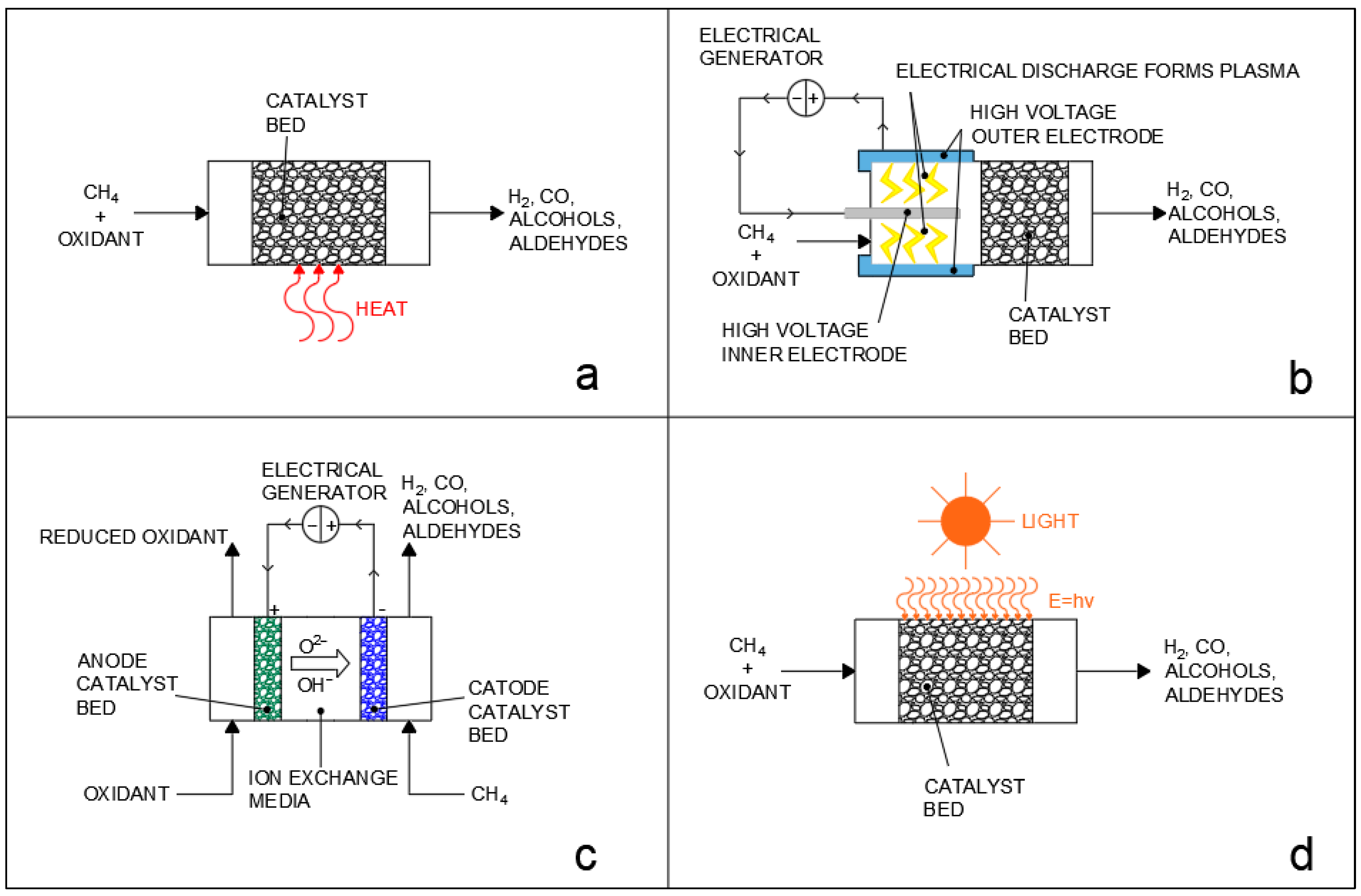
It should be noted that compared to the steam reforming reaction, dry reforming also has the distinction of obtaining a lower H2 to CO ratio, which is useful for obtaining syngas, which can be directly used in chemical synthesis without post−treatments. The steam and dry reforming reactions can finally also be applied to higher hydrocarbons and are, for example, also utilized for the abatement of tar from biomass gasification [11,12,13]; however, the high temperature requirements of the reaction and the subsequent deactivation of catalysts due to sintering and coke deposition remain great issues in the implementation of the process. Several alternatives in terms of catalyst support, dopants, promoters, and structure have, thus, been investigated to improve reactant conversion and limit poisoning [14,15,16,17,18,19,20,21,22]. In recent years, interest in alternatives to conventional thermochemical processes, such as plasma reforming [23], electrochemical reforming [24], and photocatalytic reforming [25], has also significantly grown, as briefly reported in Figure 1. In conventional thermochemical processes (Figure 1a), the energy required for the reaction is provided in the form of heat by operating at a high reaction temperature. On the other hand, the alternative processes employ a variety of strategies to decrease the temperature of operation: plasma reforming (Figure 1b) utilizes high−voltage electrodes integrated in the reactor design to produce an electrical discharge in the gas phase that causes the formation of plasma with a high electron temperature, which then activates methane and oxidant molecules by forming radical and ionized species which then interact over the catalyst surface [26,27]. Electrochemical processes (Figure 1c) also use electrical current and difference in electrical potential to provide the oxidation of methane and the reduction of the oxidant over the electrodes of a liquid phase or solid phase fuel cell, but they do not involve the formation of plasma and the charge is instead transferred through the diffusion of ions in a liquid or solid media between electrodes. Finally, photochemical processes (Figure 1d) make use of light to provide the energy required for reactant activation. Photocatalytic processes, in particular, can provide very eco−friendly pathways for chemical conversion, especially if directly driven by sunlight [28], and, in recent years, have been studied for a vast number of applications, ranging from wastewater treatment [29] to chemical synthesis [30]. The use of photocatalysis can enable forming reactions in much milder conditions compared to conventional thermochemical processes, lowering CO2 emissions and limiting problems, such as catalyst sintering [31]. Compared to conventional thermal processes and to electrochemical processes, the photocatalytic approach has the fundamental advantage that, through the activation of chemical bonds by photoexcited electrons, it is possible to drive uphill reactions against thermodynamic restrictions, as the mechanism of reaction is completely changed, allowing researchers to potentially bypass thermodynamic equilibriums [28,32,33]. This advantage is shared with the plasma−reforming processes, where the excited electrons of the non−thermal plasma can also activate chemical bonds; however, photocatalysis has the advantage of not requiring plasma generation, which is an energy intensive process [34,35,36].
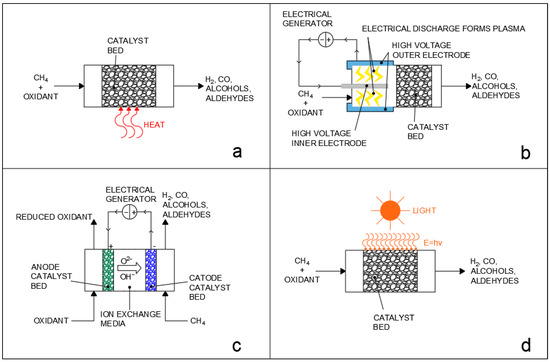
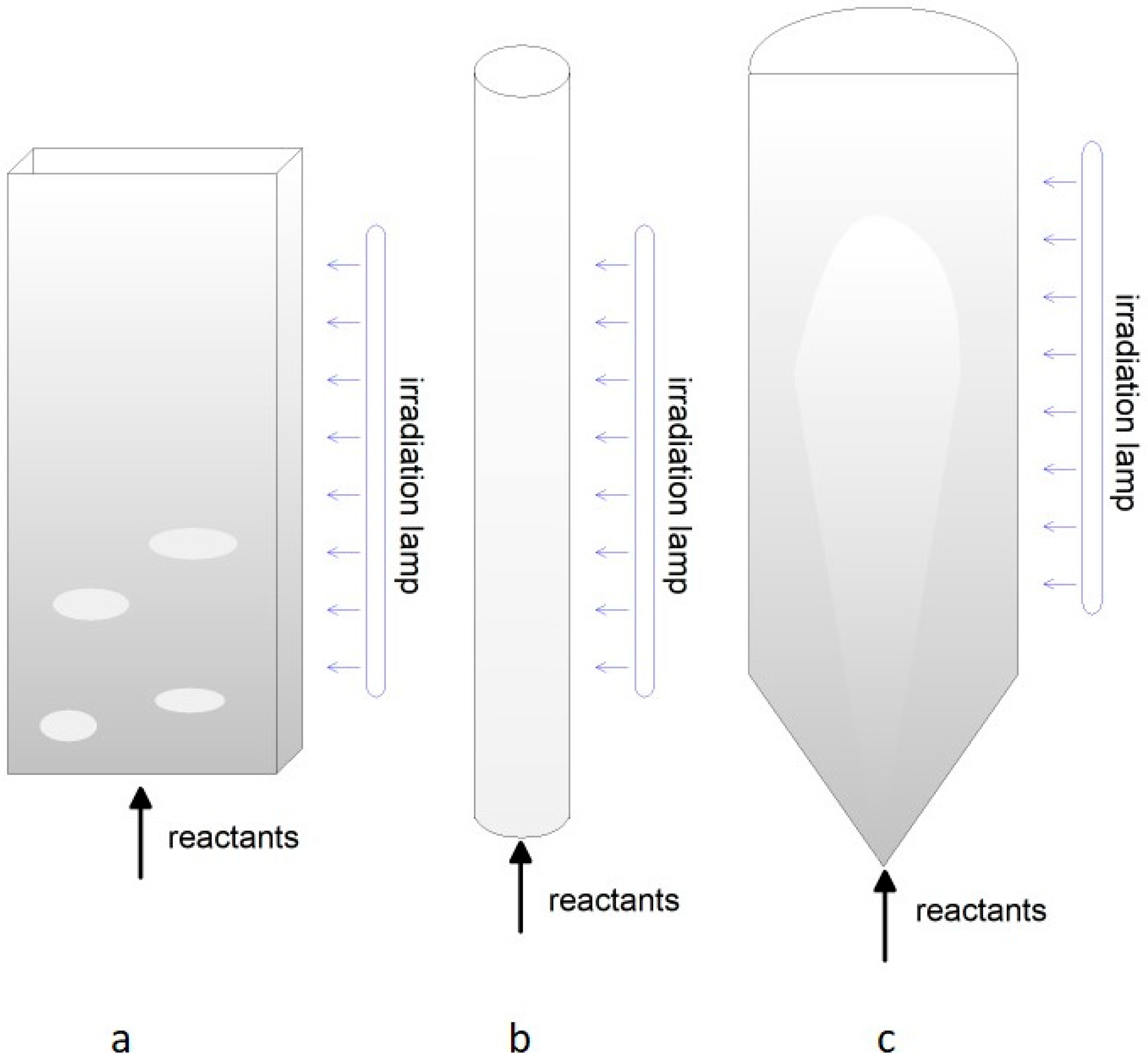
Figure 1.
Different approaches to methane/hydrocarbon reforming: (a) thermocatalytic reforming; (b) plasmacatalytic reforming; (c) electrocatalytic reforming; (d) photocatalytic reforming. Oxidants may be either oxygen (partial oxidation), steam (steam reforming), or carbon dioxide (dry reforming).
One of the most crucial aspects of photocatalysis is, thus, the selection of photocatalysts, which determines the effectiveness of light (LT) utilization through the induction of electrons and charge transfers when exposed to light [37,38]. In the last few years, significant effort has been dedicated to the enhancement of activity of semiconductor photocatalysts for photo−reforming processes by surface and nanostructure engineering, doping, and the utilization of cocatalysts. Previous reviews have been published on the state of the art of the photo−reforming process: Li et al. 2021 [39] and Song and Ye 2022 [40] both reviewed the state of the art of photocatalytic methane conversion, pointing out that the selection of the photocatalyst and the determination of the photocatalytic mechanism are the current bottlenecks of further process development. Recently, Cho et al. [41] provided a detailed review of photocatalysis for methane partial oxidation, steam, and dry reforming, pointing out specifics about reaction mechanisms and requirements for methane activation. Kulandaivalu et al. [42] instead reviewed in greater detail the photocatalytic dry reforming of methane: they noted that for the proper advancement of the process, there is a need to produce novel catalysts able to activate methane as well as carbon dioxide, which are currently lacking in the literature. It should be noted that several of the studied photocatalyst involve the use of expensive noble metal photocatalysts, which would greatly limit the viability of such processes on a large−scale application. Reviews by Liu et al. [25] and Wang et al. [43] discussed the state of the art of semiconductor−based photocatalysts, highlighting the main pathways available for catalyst improvement, such as surface engineering and use of dopants, electron scavengers, and cocatalysts: so far, modified TiO2, ZrO2, WO3, and C3N4 have been the most tested photocatalysts. The most recent review by Lei et al. [44] highlights that the study and development of catalysts with a ferroelectric effect may allow researchers to increase catalytic performances. As per the photocatalytic reforming of biomass, recent reviews were provided by Xu et al. [45] and Pan et al. [46]. On the other hand, to the authors’ knowledge, no comprehensive reviews have been published on the use of photocatalysis for tar reforming and abatement. As an alternative to conventional semiconductors, novel covalent organic framework (COF) catalysts have also been proposed [47] but are still in their infancy and suffer from slow preparation, limited versatility in structure modification, and limited efficiency. On the other hand, the development of quantum dots [48,49] and heterojunction−based semiconductor composites [50] could offer great promise in advancing conventional semiconductor materials.
In addition to photocatalyst selection, reaction conditions, such as the selected light source, reaction media, and temperature, as well as reactor design, also play a crucial role in determining process performance. In their reviews, Liu et al. [25] and Wang et al. [43] also briefly discussed the current state of the art of reactor designs for aqueous phase methane conversion. A more extensive discussion on batch and flow reactor designs was presented by Jiang et al. [51], who provided a selection of guidelines for homogenizing experimental practices and comparison of data results to focus on the most promising technologies. Compared to conventional thermal reactors, photoreactors generally face less extreme conditions in terms of reaction temperature and pressure, with their main design challenges instead being the need to properly design the fluid dynamics and the light generation and distribution system [52,53], which are essential to ensure optimal mass transfer and effective light utilization. In the case of aqueous phase reactions, pH and corrosion need to be also accounted for [54,55], as the photocatalytic reaction may require alkaline or acidic environments. The optimization of photocatalytic reactors is, thus, still an open field of investigation.
Finally, the coupling of thermocatalytic and photocatalytic processes, as well as coupled plasma–photocatalytic processes, has also been reported as another interesting perspective to enhance productivity for reforming reactions [56,57].
Despite the increasing effort that photocatalytic reforming has been receiving in the literature, an optimized photocatalytic reforming process is still lacking thus far. Great effort has been dedicated to the reforming of methanol and aqueous organic species, with methane photocatalytic reforming receiving more limited studies, with a great number of available works involving the use of expensive noble metals or toxic species, such as nickel. While solar photocatalytic reforming could allow for substantial reduction in the environmental impact, further improvements in reactor design and catalytic activity are still required [58]. Since photocatalysis is a rapidly growing field, there is a need for frequent reporting of up−to−date information. Thus, this review expands on the previously published literature, with the goal of highlighting new technological developments and strategies to design intensified photocatalytic processes, in particular by adopting fluidized bed (FB) technology.
2. Catalysts for Photoconversion
2.1. General Mechanism of Photocatalysis and Photocatalyst Engineering
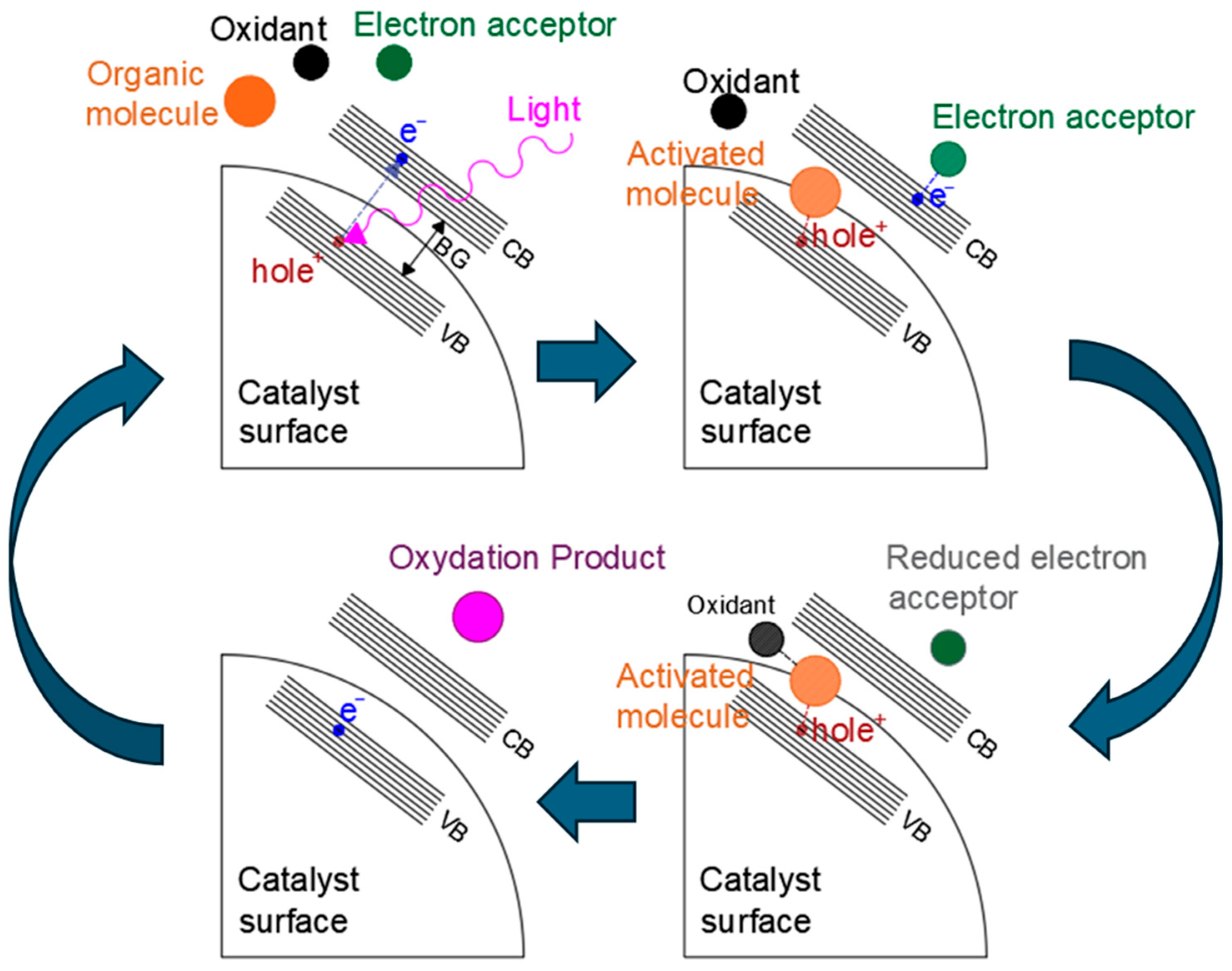
An example of a typical photocatalytic mechanism [59,60] for organic compound photooxidation on a semiconductor catalyst is shown in Figure 2.
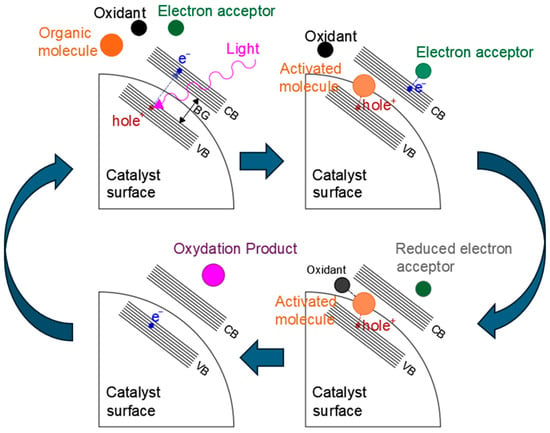
Figure 2.
Example of a photocatalytic reaction mechanism. VB = valence band, CB = conduction band, and BG = band gap.
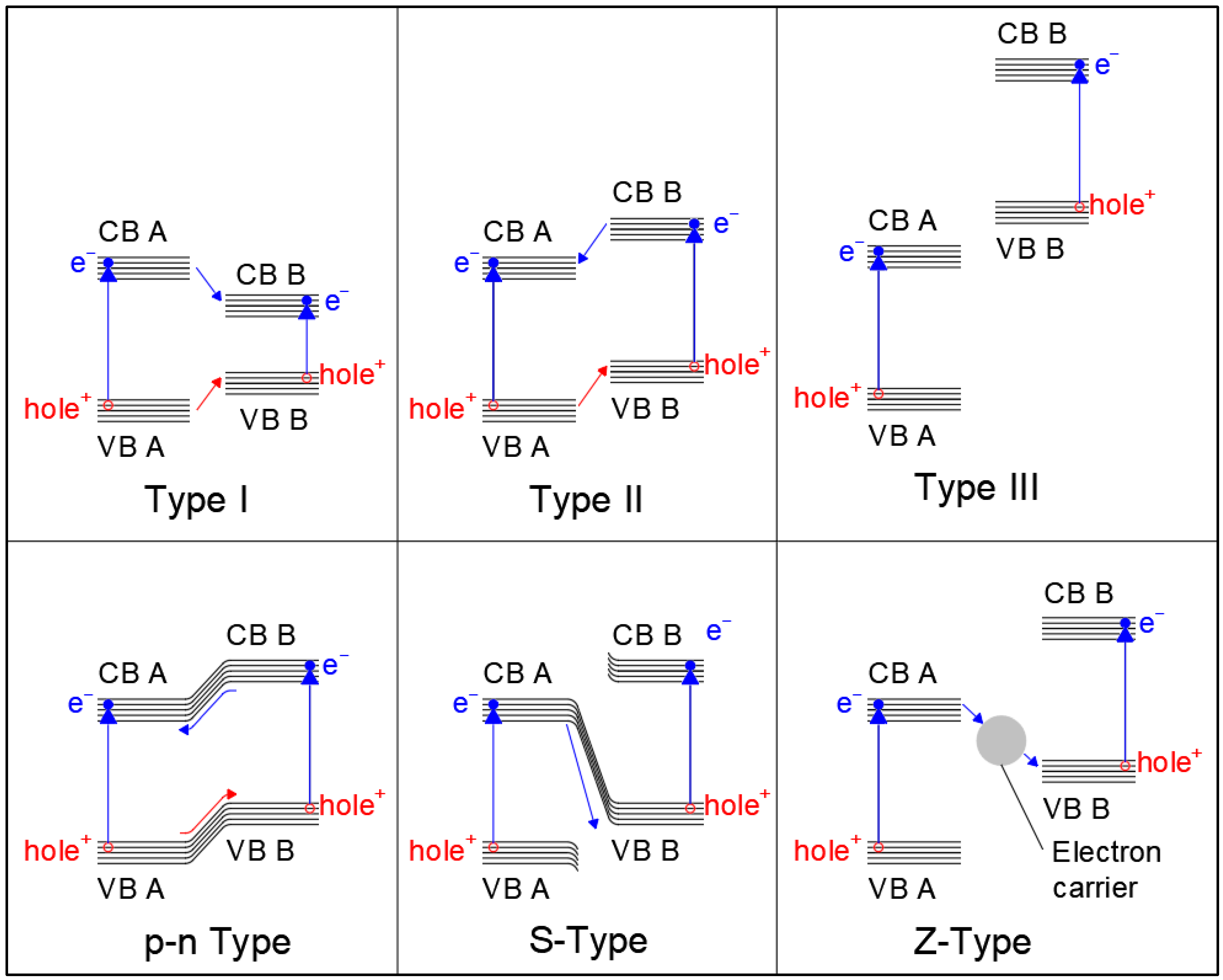
Generally speaking, the reaction starts with the light−induced excitation of an electron in the valence band of the photocatalyst. The excited electron is promoted to the valence band, leaving a positively charged hole in the conduction band. At this point, the electron can either recombine with the hole and re−emit the captured photon, thus resulting in a zero net−reaction, or it can be transported through the valence band, where it is then captured by an electron acceptor found in the reaction media. At the same time, the valence band hole interacts with the organic molecule, intercepting the electrons of its orbitals (for example the electrons of C−H bonds). The organic molecule is, thus, activated, allowing its reaction with the oxidant and leading to the formation of products and the restoration of the catalyst valence band by the filling of the electron hole due to electron donation, which can be followed by further electron excitation as a new photon is captured, starting the cycle again. Optimization of the band gap energy [61], inhibition of excessive hole–electron recombination [62], distribution and interaction of valence band holes, and conduction bands electrons with organic donors and acceptors [63,64] are, thus, essential aspects of photocatalyst design. In recent years, different approaches have been proposed to optimize activity of semiconductor heterogeneous catalysts. For example, the inclusion of metal and non−metal dopant atoms can induce the formation of localized intermediate energy levels between the conduction and valence bands of the photocatalyst [65,66], which lowers the energy required for electron transitions and widens the radiation spectra that is usable by the material, expanding it to higher wavelengths. Similar effects may also be obtained by defect and surface structure engineering [67,68], and particularly by producing nano−structured photocatalysts [69,70,71] (i.e., nanospheres, nanorods, 2D nanosheets, nanopores, nanotubes, etc.), where the incomplete coordination of atoms in surface and defect sites also causes the formation of energy levels that can act as charge separators, electron traps, or charge recombination sites. Loaded metal species on the catalyst surface demonstrate effective improvement of photocatalyst activity by capturing electrons and reducing charge recombination, as well as improving charge transfer [72,73]. Finally, in the last years, the study of more complex structures, such as quantum dots and heterojunction structures, has also shown promising results. Heterojunctions are formed at the interface by the mixture of different semiconductor materials with an unequal band structure, or at the interface between semiconductors and metals [74]: in these areas, the interaction of the two phases can induce alteration of the energy levels and create an electrical field that enhances charge separation. Heterojunctions between semiconductor surfaces can be distinguished as being type I, type II, type III, S−scheme, or Z−scheme [75], as shown in Figure 3. In type I heterojunctions, also called a “straddling gap heterojunction”, the valence and conduction bands of one of the semiconductors are located in between those of the other. In type 2 heterojunctions, called also “staggered gap” heterojunctions, the VB and CB of one of the catalysts are both higher than the respective bands of the other. Type 3 heterojunctions (“broken gap”), on the other hand, have one of the photocatalyst possess both a VB and CB higher than the other catalyst’s CB. Heterojunctions of the p−n type form at the interface between n− and p−types semiconductors, where the diffusion of electrons from the p−type semiconductor to the n−type one and the counter diffusion of holes from n−type to p−type occurs even before light exposure, leading to the formation of an internal electrical field at the interface that favors electron–hole separation once the material is excited by light. S−scheme heterojunctions (sometimes indicated as direct Z−scheme heterojunctions) are similar to type II, presenting a staggered band gap, but the electron transfer happens between the conduction band of a catalyst to the valence band of the other [76]. Finally, in Z−scheme heterojunctions, the electron transfer is mediated by an intermediate species that acts as an electron carrier [77]. Z−scheme heterojunctions may involve dissolved species in a solution as the electron mediator, or they can involve solid phase mediators. Another type of heterojunction, called a Schottky barrier heterojunction [78], is formed at the interface between a semiconductor and a conductor material, such as a metal, with electrons migrating towards the more conductive material and forming a potential energy barrier that inhibits their back diffusion, thus trapping them on the conductive species.
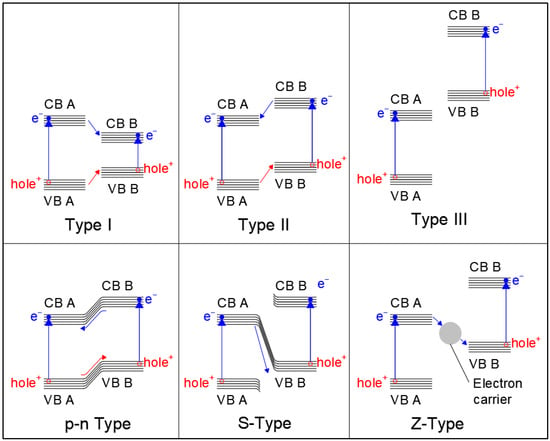
Figure 3.
Types of heterojunction structures. Blue lines indicate electron transfer; red lines indicate hole migration.
The type of heterojunction strongly affects the activity of the photocatalyst: type 1 and 3 heterojunctions, for example, cannot inhibit electron–hole recombination, since in the former, both electron and holes tend to migrate in the same direction, while in the latter, diffusion of both electrons and holes is inhibited by the band gap incompatibility of the semiconductors. Thus, type 2, S−scheme, Z−scheme, and p−n type band gaps are the most relevant for photocatalytic applications, with S−scheme and Z−scheme materials attracting much interest due to their superior charge separation ability.
Finally, quantum dots are formed when materials are deposited in near zero−dimensional nanostructures with a radius inferior to the radius of the material’s excitons (excited hole–electron pairs) [79,80]; in these conditions, the band gap of the material becomes size−dependent, offering great tunability of light absorption and activity under visible light, and, due to quantum confinement effects, the number of excitons generated is increased compared to bulk materials.
2.2. TiO2−Based Catalysts
Among various materials, titanium oxide is a typical choice for photocatalytic processes due to its high activity, good thermal and chemical stability, low cost, and non−toxicity [81]. Unfortunately, titanium oxide on its own is only active under ultraviolet (UV) radiation [82,83], which is only a small fraction of sunlight (3–5%) [60], thus leaving most of the solar spectrum unused and limiting process efficiency. Conventional mercury−based UV lamps pose safety and environmental problems due to the high toxicity of mercury, while they provide a limited light production efficiency with a short lifespan [84]. On the other hand, it should be noted that while the direct use of sunlight is a most desirable outcome, in the last few years, UV technology has also made substantial progress, which can offer the opportunity of efficiently employing UV in photocatalytic processes, thanks to the replacement of conventional mercury lamps with more efficient systems, such as excimer lamps or light−emission diodes (LEDs) [84,85,86,87]. Nevertheless, substantial effort has been dedicated in the last few years to expand TiO2 photocatalytic activity to the vs., including via surface treatments and functionalization, formation processes, inclusion in composite materials, use of organic and inorganic promoters, and doping of the titania structure with metals and non−metals [88,89,90,91,92,93,94]. In particular, doping titanium oxide and the subsequent formation of lattice defects can introduce intermediate electron levels between the valence and conduction bands and widen the respective bands themselves, thus lowering the band gap and allowing electron excitation and conduction even under the longer wavelengths of visible (VS) and infrared light (IR) [95]. When considering metal promoters and dopants, the addition of noble metals has been shown to significantly enhance TiO2 photocatalytic activity [96,97]; however, their high cost makes their use unfeasible for large scale operation. Among non−noble metals, both transition metals, such as Ni [98,99], Fe [100,101], Cu [102,103], Co [104], and Mn [105,106], and rare earths, such as Ce [107] and La [108], have all been found to effectively improve photocatalytic activity when used as dopants. While a significant number of the studies on TiO2 photocatalysis focus on the degradation of organic pollutants, typically in an aqueous environment, photocatalytic reforming and hydrogen production from methane and biomass derivatives have also attracted interest.
Noble metals, and in particular Pt, are able to increase TiO2 activity for the reforming of methane, biomass derivates, and tar compounds: Yamaguchi et al. [109] observed enhanced performance for steam methane reforming using TiO2 hollow spheres with spatially separated Pt−Rh noble metal cocatalysts: they proposed that in their hollow TiO2 sphere structure the excited electrons of the TiO2 could be transferred to Pt particles loaded in their internal surface, where they then produced hydrogen by water reduction, while the electron holes migrated to the Rh2O3 oxide layer deposited on the external surface, where they oxidized methane. This mechanism provided an effective charge separation not only by band gap energy, but also by spatial separation of the electron sink and hole sink materials. On the other hand, Al−Madanat et al. [110] investigated the mechanism of naphthalene aqueous phase reforming for hydrogen formation over Pt−loaded TiO2, concluding that water is the source of both hydrogen and oxygen. During naphthalene mineralization to CO2 Pt atoms again act as an electron sink and site for the reduction. Naphthalene is primarily activated through the formation of naphthalene cation radicals. The use of a Pt cocatalyst in conjunction with hydrothermally prepared Nb2O5−TiO2 photocatalysts proved effective for the photoreforming of ethanol aqueous solutions to hydrogen [111], obtaining an apparent quantum yield of 85% under simulated sunlight; the best results were observed for a composite that underwent 3 days of hydrothermal treatment, which outperformed pure P25 TiO2 catalyst, but in this case no direct investigation of the mechanism was performed, though the authors proposed that the formation of favorable type II and S−scheme heterojunctions is the cause of improved performance for this composite.
The use of nickel has been commonly investigated as an alternative to noble metals. Glycerol photoreforming was investigated by Eisapour et al. [112], who obtained a remarkable 58% glycerol conversion to hydrogen and value−added glyceraldehyde and dihydroxyacetone using a NiO−Ni−TiO2 sandwich−like heterojunction catalyst, bypassing the limitations of single p−n heterojunction catalysts. Glycerol was converted to glycerate through the formation of glyceraldehyde and to ketomalonate through the formation of hydroxyacetone and hydroxypiruvate. They proposed that the p−n heterojunction formed between NiO and TiO2 interfaces favored the initial separation of electron–hole pairs, and then the metallic Ni species further captured electrons from TiO2 by forming a Schottky heterojunction, thus acting as a barrier for charge recombination and functioning as a center for hydrogen production, while electron holes were transferred to the NiO surface and oxidized the organic substrate. On the other hand, Liu et al. [113] obtained a 96.1% selectivity towards syngas formation from glycerol by engineering Ni−O−Ni dimer sites in a TiO2−derived metal–organic framework (MOF) structure. Single Ni atom deposition reduces the contact angle between TiO2 and substrate species, favoring their adsorption on the catalyst surface. The electrons of NiO2 dimers are rapidly redistributed on the TiO2 surface, where they are used to produce hydrogen, and the formed holes in dimer sites adsorb and activate glycerol, oxidizing it through the formation of formaldehyde and formic acid intermediates. The porosity and structure of the TiO2 support also contribute to catalytic activity: Xie et al. [114] obtained improved performances for methane dry reforming by depositing Ni on mesoporous TiO2, while He et al. [115] observed the remarkable effects of the TiO2 crystal phase for Ni/TiO2 photocatalysts, with an optimized rutile to anatase ratio leading to better exposed Ni species and remarkable H2 and CO production rates reaching 87.4 and 220.2 mmol/(g·h), respectively. While the use of nickel is problematic from an environmental perspective, these studies still demonstrate the remarkable effect of catalyst structure engineering on activity and selectivity.
Copper addition may provide an effective alternative to Ni in preparing more active photocatalysts. For example, the sequential deposition of NiCu catalyst over P25 TiO2 was observed to be effective for the conversion of cellulose to hydrogen and organic acids, with a H2 yield of 489 μmol/(g·h) for a 1% wt. CuNi−loaded catalyst [116]: sequential deposition of Ni followed by Cu led to a more active catalyst compared to a catalyst produced by simultaneous co−deposition, suggesting improved interaction between metal species, and a low loading was sufficient to enhance activity without incurring deactivation due to excessive TiO2 site covering observed at higher metal loading, at the same time allowing the reduction in Ni loading. Ni and Cu species were proposed to form Schottky heterojunctions with the TiO2 surface, acting as electron accumulation and hydrogen generation sites. Aside from the cleavage and oxidation of cellulose by electron holes on the catalyst surface, side reactions of transposition, hydrolysis, and decarboxylation are expected to occur, leading to the formation of subproducts, such as glucose, cellobiose, mannose, maltose, acetic and formic acids, and other. As per pure copper−modified TiO2−based catalysts, Clarizia et al. [103] provided a review on the aqueous reforming of organic species and observed a great scarcity of hydrogen production efficiency data for such processes. More recently, Muscetta et al. [117] observed a promising performance for aqueous phase reforming of biomass derivates using a ball−milling−prepared Cu2O−TiO2 photocatalyst: they observed that a 1% wt. addition of Cu2O resulted in nearly 60% of the hydrogen yield obtained under sunlight being derived from visible light absorption. The reaction rate could be described by the Langmuir–Hinshelwood model, and it was favored at alkaline pH and a temperature increase from 20 °C to 80 °C. Glycerol and ethylene glycol were the best substrates in terms of hydrogen yield, followed by methanol. Negligible yield was obtained from lactic and formic acid instead, attributed to limits in substrate adsorption on the catalyst surface and the reduced stability of copper species in acidic conditions. They proposed that the reaction mechanism involves the formation of a p−n heterojunction between Cu2O and TiO2 surfaces, where Cu2O can absorb visible sunlight and then transfer electrons to the TiO2 support. Umair et al. [118] tested a similar catalyst in a 10 L pilot−scale reactor for hydrogen production from biomass derivatives, such as glycerol, ethanol, and glucose, obtaining remarkable hydrogen yields for a 3% wt. Cu2O−loaded sample ball milled at 150 rpm for 2 h. The addition of Cu2O effectively shifted the catalyst band gap in the vs. light range, improving light utilization, and the best hydrogen yield was observed for degradation of glycerol. They also supported a mechanism involving the formation of a p−n heterojunction that favors electron–hole separation, where Cu2O acts as the species active to visible light absorption, with electrons being separated over TiO2. This represents one of the first efforts to implement photocatalytic hydrogen production on a pilot scale. Khan et al. [119] recently observed high activity for H2 production from methanol coupled with CO2 reduction on MOF−structured Cu−TiO2 composite catalysts, for the first time surpassing the performance of a reference 1% w, Pt−TiO2 composite after three photocatalytic reaction cycles: the MOF structure highly enhanced catalyst performance compared to simple Cu deposition at the same load, and hydrogen yield improved over subsequent reaction cycles, reaching 13.24 mmol/(g·h) after six cycles. Copper was initially present mainly in the +1 oxidation state in the catalyst, with some metallic Cu nanoclusters also being present, and was reduced to metallic Cu during the reaction. The formation of stable metallic Cu nanoclusters in the MOF structure was credited with the increased catalytic performance of the composite material. The MOF structure prevents aggregation of the nanoclusters and limits their back oxidation, improving catalyst stability. A Schottky heterojunction is formed between these metal nanoclusters and the TiO2 surface, acting as electron sink which prevents improved charge separation. Carboxylic groups are a fundamental intermediate in the reaction mechanism. The effects of Cu oxidation state on H2 production from methanol and CO2 reduction to methane were investigated by Qian et al. [120]: the bandgap of the copper–titania heterojunction catalysts narrowed and the vs. light absorption range significantly broadened with the transition of the Cu valence state from Cu(I) to Cu(0). The more reduced copper species were, thus, found to improve the charge separation of the catalyst and enhance its activity under vs. light. The surface area of the catalyst plays a very significant role in its activity: hydrothermally prepared Cu−TiO2 samples were found to outperform samples prepared by impregnation [121], even when their oxidation state was unchanged between the two different syntheses; the hydrothermally prepared sample’s increased activity was attributed to improved dispersion of the Cu species and the increased surface area, which favored substrate adsorption and oxidation and decreased the band gap of the photocatalyst due to the better interaction of copper with the TiO2 substrate. On the other hand, calcination was observed to negatively affect activity, as it reduced the availability of surface copper species. The addition of cerium dioxide together with copper was instead observed by Liu et al. [122], who concluded that a Ti/Ce ratio of 2:1 increases the performance of a Cu/TiO2−CeO2 photocatalyst for gas−phase methanol steam reforming, achieving up to 100% methanol conversion and high hydrogen selectivity.
Cobalt is another possible substitute for both Ni and noble metals. A high 95% conversion was observed for reforming glycerol to formic acid and formaldehyde on Co−doped TiO2 [123]: Co−doping induced lattice strain in TiO2 and increased the number of oxygen vacancies, which is further increased during light irradiation, providing numerous oxygen adsorption sites; the adsorbed oxygen was reduced to •O2− radicals by electrons concentrated on the Co specie, and these radicals then interacted with the glycerol molecules activated by electron holes, leading to C−C bond cleavage, with the primary oxidation pathway involving glyceraldehyde formation as a reaction intermediate. An S−shaped heterojunction−based 10% wt. −Co0.3Ni0.55Se/TiO2 catalyst achieved a remarkable hydrogen yield of 10,070.9 µmol/(g·h) when degrading 20% vol. triethanolamine (TEOA) aqueous solutions [124]: electrons were concentrated on the CoNiSe clusters and then used to generate hydrogen from H+ cations, while holes accumulated in the TiO2 support and oxidized the organic substrate.
Other metals could also provide useful improvements in photocatalyst activity. Ayodele et al. [125] optimized La−doped TiO2 through the use of response surface methodology and analysis of variance, obtaining a hydrogen yield of 2.43 μmol/min. In their optimization, they evaluated the effect of four different independent variables (irradiating time, metal loading, methane concentration, and steam concentration) on the rate of hydrogen production, obtaining optimum conditions of 146.15 min, 2.94%, 22.83%, and 1.24% for irradiation time, metal loading, methane concentration, and steam concentration, respectively. Unfortunately, they did not investigate the reaction mechanism and the effects of La addition on the band gap. On the other hand, Zhang et al. [126] tested W−doped TiO2 nanocrystals for the oxidation of ethylbenzene to acetophenone. The presence of the W atoms induces electron transfer from the TiO2 to W species, while at the same time inducing lattice distortion and the formation of numerous oxygen vacancies; W species then proceed to reduce oxygen to •O2− radicals, while electron holes activate ethylbenzene. The composite material obtained yields 16 times higher than pure TiO2 in the same reaction conditions. Finally, Hu et al. [127] demonstrated a 1653 μmol/(g·h) hydrogen yield from MoS2−modified TiO2 nanoparticles using agricultural biomass substrates. In this case, the conduction band of MoS2 acted as sink for the electrons generated by the photoexcitation of TiO2, which then converted H+ cations to hydrogen, while the holes in the titania valence band converted water to hydroxyl radicals that were then scavenged by the biomass substrate. No hole migration is expected to occur between TiO2 and MoS2 species, due to the wide difference in energy between the two materials’ valence bands.
Alternatives to metal doping have also been investigated: Falara et al. [128] demonstrated ethanol conversion and H2 generation on TiO2 modified by carbon dot deposition, studying quantum dots obtained from hydrothermal treatment of microwave irradiation. They observed a strong dependence of the carbon dots activity on the precursor and methodology, with microwave irradiation−prepared samples outperforming the ones produced by the hydrothermal method. Nitrogen presence in the quantum dots’ precursor is proposed to be important to ensure optimal charge separation efficiency, while excessive quantum dot deposition negatively impacts yield, which was attributed to such phenomena as light scattering recuing the absorption efficiency of light.
Finally, it should be noted that a simple change in catalyst morphology can lead to a very remarkable change in performance even without the presence of dopants or cocatalysts: Ubaidah et al. [129] observed that altering the TiO2 structure from commercial P25 powder to nanotubes caused the surface area to increase from 55.76 to 120.99 m2/g, while simultaneously reducing the band gap of the material from 3.32 to 3.23 eV and increasing the CH4 yield from 6560 to 17,020 µmol/(g·h) under UV−VS light. Compared to nanospheres, where electrons can move from the bulk to the surface of the material, in the nanotubes, the electrons are trapped in a new energy band with a lower energy level, and the alternative scenarios for electron and hole dynamic involve the movement of electrons and holes along the axis of the tube, thus making recombination less likely and improving the electron availability for reaction. Jiang et al. [130] also obtained a selective photocatalyst for the photooxidation of methane to formaldehyde by engineering a TiO2 crystal structure, demonstrating the possibility of controlling process selectivity through photocatalyst structure, and, in particular, they observed that a 90% anatase–10% rutile phase mixture of TiO2 was able to provide optimized activity towards methane activation through the formation of an heterojunction between the two different crystal phases, with electrons migrating to the anatase and holes migrating to the rutile. The hybrid crystal structure also demonstrated an improved lifetime. Finally, crystal−phase engineering was also demonstrated to be able to drastically enhance the performance of TiO2 for catalyzing non−oxidative methane coupling, with biphasic TiO2 (anatase 30% and rutile 70%) exhibiting the highest photocatalytic activity [131].
Morre novel approaches have also been proposed, such as the coupling of photocatalysis with plasma activation. For example, Sun et al. [132] obtained toluene conversion as high as 98% by coupling microwave plasma−catalyzed steam reforming with photocatalysis by anatase TiO2 at moderate humidities of 38%. Higher humidities were found to decrease conversion.
The results for TiO2 photocatalysts are reported in Table 1.

Table 1.
TiO2−based photocatalysts for reforming processes. A = aqueous phase, G = gas phase, AQY = apparent quantum yield, QE = quantum efficiency, and PE = photonic efficiency.
2.3. ZrO2−Based Catalysts
ZrO2−based photocatalysts are a common substitute for TiO2, sharing very similar properties in terms of their low cost and high thermal and chemical stability [133,134]. Unfortunately, they also share the problem of having a large band gap energy, thus being active only under UV light and needing modification to operate under vs. radiation. Metal doping of ZrO2 has, thus, been extensively investigated to enhance its photocatalytic activity [133]. Aside from noble metals, transition metals are the most studied for the enhancement of ZrO2’s performance, with a great number of recent applications using Ni deposition for enhancing the photoreforming activity of organics. For example, Tian et al. [135] observed enhanced performance of Ni/ZrO2 nanorods for ethanol photothermal dry reforming in a temperature range of 350–550 °C, with samples prepared by precipitation outperforming samples prepared by impregnation thanks to stronger NiO−ZrO2 interaction. Ni acted as the electron sink, capturing photoexcited electrons which were then transferred to CO2 for reduction to CO, while electron holes migrated to the ZrO2−Ni interface, were they activated ethanol for reaction with the O2− species produced by the previous reduction of CO2. Interestingly, the use of Ni and Ce as co−dopants for ZrO2 appears greatly favorable for the methane dry reforming reaction: Du et al. [136] observed that the addition of a small amount of CeO2 to a Ni/ZrO2 photocatalyst led to a dramatic increase in hydrogen and CO yields, going from a 542 mmol/(g·h) H2 yield and a 534 mmol/(g·h) CO yield for the Ce−free sample to a H2 yield of 713 mmol/(g·h) and CO yield of 693 mmol/(g·h) for a catalyst with 5% wt. Ce addition at 700 °C and a 30 suns irradiation intensity for methane reforming. The addition of cerium dioxide provided sites for oxygen vacancy formation and the activation of carbonated intermediates and contributed to reduced coke formation, with light increasing the generation of surface oxygen vacancies and, thus, increasing the number of active adsorption sites while also providing the active electrons. Similarly, Cheng et al. [137] observed high CH4 and CO2 conversions of 51.6% and 56.4%, respectively, for methane photothermal dry reforming at 600 °C over Ce−doped Ni−ZrO2 catalyst, with CO and H2 yields of mmol/(g·h) and 83.8 mmol/(g·h), respectively. The addtion of Ce led to a drop in band gap energy from 3.08 to 2.04 eV, and, at the same time, a charge redistribution occurred on material surface, leading electrons to migrate from Ce species to O atoms. An internal electric field is formed in the material, which enhances electron redistribution to Ni clusters during light exposure and enhances charge separation. The formation of oxygen vacancies activates CO2 and enables the avoidance of coke deposition. Tengfei et al. [138] enhanced the performance of Ni/CeO2−ZrO2 catalyst by encasing it in a core–shell structure using SiO2: the resulting catalyst proved stable over 60 h of prolonged reaction, providing CH4 and CO2 conversions as high as 63.5% and 55.9%, respectively, with H2 and CO yields of 137.0 mmol/(g·h) and 182.9 mmol/(g·h), respectively, at 600 °C. Light irradiation considerably lowers the activation energy of the reaction compared to pure thermal process. The addition of nickel–cerium and the core–shell structure were observed to greatly reduce the band gap energy compared to pure ZrO2. Most recently, Liu et al. [139] conducted a detailed investigation of tailoring of surface properties of a Ni/CeZrO2 photocatalyst through a MOF structure and achieved conversions of CH4 and CO2 as high as 63.9% and 71.1%, respectively, at 650 °C, with the catalyst remaining stable for 250 h of continued reaction. The MOF structure showed resistance to carbon deposition and improved charge separation capacity. The reaction mechanism involved the transfer of electrons to Ni clusters and CO2 activation to carboxylic intermediates, similar to previous studies.
The deposition of nickel nanoparticles combined with La2O3 and ZrO2 was tested by Meng et al. [140], where 10Ni/La2Zr2O7 demonstrated the highest catalytic activity, with production rates of H2 and CO of 4572 mmol/(g·h) and 5946 (g·h), respectively. Ni acted as the methane activation site, while oxygen vacancies on the La2O3 surface activated carbon dioxide, with light irradiation improving the stability of the vacancies. Light was also observed to inhibit reverse water–gas shift reaction.
As per nickel−free catalysts, Mendoza et al. [141] synthetized nanorods of Bi2S3 supported on ZrO2 and found them to be effective for the production of hydrogen from methanol in an aqueous solution: the H2 yield was 4440 μmol/(g·h) for 6% wt. Bi2S3/ZrO2 under UV light, six times the yield for bare ZrO2, and 1476 μmol/(g·h) under vs. light. The Bi2S3 nanorods acted as electron sinks for the photoexcited electrons of ZrO2. On the other hand, Yu et al. [142] obtained promising performance for a mixed Cu/Zn/Zr oxides catalyst for the reforming of aqueous−phase methanol at temperature as low as 130 °C under 16 suns of irradiation. ZrO2, ZnO, and CuO oxides are proposed to work as sequential type I heterojunctions, with both electrons and holes migrating to CuO from ZrO2 using ZnO as a carrier. At the same time, ZnO accepts hydrogen cations produced by the activation of methanol, and the photo−excited electrons accelerate the production of formate intermediate species.
Table 2 summarizes the ZrO2 catalysts herein discussed.

Table 2.
ZrO2−based photocatalysts for reforming processes. A = aqueous phase; G = gas phase.
2.4. C3N4−Based Catalysts
Graphitic carbon nitride (C3N4)−derived materials are another common choice for photocatalytic reforming applications [143,144]: compared to TiO2, C3N4 possesses a narrower band gap and is, thus, intrinsically more active under vs. light irradiation, while being metal free and chemically stable; however, it also generally suffers from a more complex preparation, which is an obstacle to scaling up the process, and still requires modification to achieve adequate performance under solar irradiation, particularly to avoid excessive charge recombination [145,146]. Again, noble metals are found to be effective cocatalysts for these applications. Speltini et al. [147] compared the performance of Pt and Cu−Ni cocatalysts supported on either TiO2 or C3N4 for aqueous−phase H2 production from biomass derivatives and observed overall lower performance for C3N4 photocatalysts; it should, however, be noted that C3N4 still managed to achieve H2 yields within 40 to 75% of those obtained on TiO2. In both cases, the metal cation acted as the hydrogen generation site, while electron holes on the semiconductor surface oxidized the organic substrate. Most recently, Bianchini et al. [148] reported successful pre−pilot scale generation of hydrogen from rice industry wastewater using C3N4 with H2PtCl6 used as a metal cocatalyst under natural sunlight. They reported an analysis of the process through a response surface model that was used to determine the optimal dilution of the waste to degrade, the optimal catalyst amount, and the optimal loading of the Pt cocatalyst, obtaining their best results with undiluted waste, a 0.5 g/L catalyst concentration, and a 35 wt. Pt loading. Considering the cost of noble metals, the optimization of their load is of great importance in their use as catalysts. While they did not investigate in detail the reaction mechanism, tests of the catalyst in pure water and in dark conditions produced a negligible hydrogen yield, confirming the crucial role that biomass plays as an electron donor and the fundamental role of light in promoting the reaction.
Single−atom metal deposition may offer the possibility for preparing selective catalysts with a low metal load and, thus, reduce cost of noble metal catalyst use: Lazaar et al. [149] deposited highly dispersed Pt atoms on exfoliated C3N4, obtaining a maximized H2 production of 1660 mmol/(gPt·h) from 10% vol. aqueous triethanolamine solution with a total Pt loading of only 0.03% wt. A dark reactive deposition approach was used to obtain stable single−atom surface species chemically bound to carbon nitride, with Pt atoms being bound to N4 sites with high charge transfer ability. The high dispersion of Pt atoms increased their activity and prevented agglomeration, contributing to catalyst stability and allowing the catalyst to surpass the performance of other catalysts loaded with conventional techniques. The morphology of C3N4 also plays an important role in its activity: for example, Pt−loaded ultrathin C3N4 nanosheets were found to be more effective than Pt loaded on bulk C3N4 for the aqueous reforming of cellulose [150]: the nanosheet structure increased the oxidation potential of the carbon nitride, favoring the formation of hydroxyl radicals that could then react with biomass, while at the same time improving electrical conductivity, reducing band gap, and favoring electron–hole separation. The load of the cocatalyst was essential to optimize the yield, with a 2% wt. load providing optimum performance. On the other hand, the addition of other cocatalysts is also a potentially effective pathway. For example, Gao et al. [151] observed a 3.6−times increase in hydrogen production with a 5% wt. addition of SnS2, also obtaining an increase in degradation efficiency for methylene blue dye. A type II heterojunction was formed between carbon nitride and SnS2, with SnS2 acting as the electron sink. The addition of methyl viologen to the C3N4 structure was instead observed to enhance hydrogen production in the aqueous phase with the triethanolamine sacrificial agent [152], with methyl viologen species accumulating electrons formed by the photoexcitation of carbon nitride and accelerating their transfer to Pt cocatalyst, thus inhibiting charge recombination.
Other metal species have been tested as cheaper alternatives to noble metals. Similarly to the previously discussed investigation of Lazaar et al. for single Pt atom deposition, Li et al. [153] maximized the yield of direct methanol synthesis from CH4 by controlling the coordination number of single iron atom loading on carbon nitride surface, obtaining a remarkable methanol yield of 928.27 μmol/g for the Fe1/C3−xN4 with a Fe−N3 coordination. In these conditions, they observed that the reaction proceeded through a three−electron mechanism involving methane adsorption and activation as methyl surface group and the production of hydroxyl radicals from oxygen.
Hierarchical carbon nitride structure (H−C3N4) also demonstrated enhanced and stable activity for methane dry and bi−reforming, as well as methanol reforming [154], which was further enhanced by cobalt doping: under dry methane reforming with stoichiometric feed, an optimized 2% wt. Co/H−C3N4 achieved CO and H2 evolution rates of 555 and 41.2 μmol/(g·h), respectively, an increase of 18.28− and 1.74−fold than when using H−C3N4, itself possessing 1.85− and 1.81−fold higher yields than graphitic C3N4. The higher yields can be attributed to higher charge carrier separation induced by doping and structural modification. For photocatalytic bi−reforming of methane, the production of CO and H2 was reduced, whereas significantly higher CO and H2 evolved using the bi−reforming of methanol, achieving 10.77− and 1.39−fold more H2 and CO efficiency compared to dry reforming of methane in the latter case. In both cases, cobalt acted as the electron sink enhancing charge separation, while organizing the carbon nitride into layered hierarchical structure improved its own charge separation capacity by forming defects. CO and H2 were the main reaction products, produced from the transfer of two electrons.
The formation of heterojunctions of carbon nitride with other semiconductors was investigated by other authors. Khan et al. [155], for example, investigated the use of cobalt aluminum lanthanum−layered double hydroxide (LDH) as a support for a TiO2−Ti3C2 and C3N4 mixture in methane bi−reforming with water and carbon dioxide. The strong interaction between the three species allowed the researchers to reduce the charge carrier recombination, and the material reached maximum production of 24.35 μmol of production for CO at a catalyst loading of 0.13 g with a feed ratio of 1.67 at 4.47 h, while the maximum H2 production of 21.91 μmol was achieved at a catalyst loading of 0.14 g with the feed ratio of 1.41 at 4.93 h. A four−step heterojunction was formed between Ti3C2, TiO2, C3N4, and the cobalt−based LDH, with electrons concentrated on the carbide and LDH species. S−scheme heterojunctions formed between TiO2 and LDH and between TiO2 and carbon nitride, allowing for efficient charge transfer and separation. The good integration of all the catalyst species also allowed the enhancement of the surface area, thus favoring reactant adsorption. Tahir et al. observed an enhancement in reforming performance by addition of 15% wt. LaxCoyO3 perovskite for aqueous phase reforming of methanol [156], also forming an S−scheme heterojunction. Similarly promising results were also observed for S−scheme heterojunction composites prepared by the combination of C3N4 with MnMgPO4 [157]. S−scheme C3N4 heterojunctions have also received interest for the photodegradation of plastic waste, with Mohanty et al. [158] observing a hydrogen evolution rate of 12.6 ± 2 mmol/(g·h) for combined C3N4/α−MnO2 with the concurrent formation of value−added organic molecules (benzaldehyde, benzoic acid, toluene, benzene, and carbonic acid) from polystyrene reforming.
A metal−free modification of carbon nitride was instead attempted by Ikreedeegh and Tahir [159], who deposited graphene oxide (GO) nanosheets on a carbon nitride surface by a sonochemical thermal−assisted method and then tested the material for methane dry reforming under vs. light: they observed that a 0.5% wt. addition of graphene oxide increased the yield of CO up to 5 times compared to unmodified material, while a 0.25% wt. addition maximized H2 production. They also observed that dry reforming carried out with a stoichiometric CO2/CH4 ratio (1:1) performed better than both steam and bi−reforming on this catalyst; however, the quantum yields of the catalysts were still below 0.4% even for the optimized graphene oxide loading. The activity of the material in this case was enhanced by the graphene oxide acting as electron sink, similarly to what is commonly obtained through the loading of metal species. On the other hand, Xie et al. [160] demonstrated that carbon ring inclusion in C3N4 can potentially provide a pathway for photocatalyst activation under low−energy near−infrared light (NIR) for the degradation of lignocellulosic material coupled with H2 production. The inclusion of carbon graphitic rings in the catalyst structure introduced intermediate energy levels in the band gap structure, allowing the adsorption of near−infrared radiation, and created regions of low electron density, polarizing the surface and facilitating electron migration and charge separation.
Table 3 summarizes the literature reviewed for C3N4−based photocatalysts.

Table 3.
C3N4−based photocatalysts for reforming processes. A = aqueous phase, G = gas phase, AQY = apparent quantum yield, APE = apparent photon efficiency, GO = graphene oxide, LDH = layered double hydroxide, and NIR = near−infrared.
2.5. Other Catalysts
While the previously discussed TiO2, ZrO2, and C3N4 remain the most investigated materials for photocatalytic reforming processes, a number of other materials have also been tested as possible alternatives. Perovskite−based photocatalysts, for example, have been tested by Chung et al. [161] in an innovative combined plasma–photocatalytic process for methane dry reforming: synergistic effects between plasma− and photocatalysis contributed to increasing the H2 yield, and among LaFeO3, NiTiO3, and AgNbO3, the former displayed the highest performance in terms of both reactant conversion and energy efficiency. Not only are methane and CO2 pre−excited by exposure to the high electron temperature plasma, but the photocatalyst can be excited by plasma−produced photons, as well as by the capture of previously emitted free electrons. Plasma exposure can modify the photocatalyst surface structure and reduce particle aggregation, and the presence of a photocatalyst may enhance the power of plasma discharge thanks to photocatalyst polarization. In their further work [162], they concluded that LaFeO3 calcined at 600 °C demonstrates optimized CH4 and CO2 conversions and syngas generation efficiency of 53.6%, 40.0%, and 18.4 mol/kWh, respectively. On the other hand, LaFeO3 doped with 0.12% wt. Ni was observed to be active also for photoreforming of glucose to hydrogen in aqueous solution, with H2 yield of 2574 μmol/L after 4 h of UV irradiation [163]. Hydrogen was primarily produced from water reduction over Ni atom electron sinks, while glucose played the role of the hole scavenger. Hydroxyl radicals were identified as the main reactive species involved in the oxidative degradation process. Another perovskite, LaMnxNi1−xO3 (0 ≤ x ≤ 1), proved effective for the coupled thermo−photocatalytic conversion of toluene as a tar model compound [164], with 90% carbon conversion of toluene obtained on LaMn0.4Ni0.6O3 at 400 °C. The formation of oxygen vacancies and activated oxygen species under material irradiation was observed to play an important role in the adsorption and conversion of toluene. Overall, temperature and light displayed a synergistic effect on pollutant degradation.
Cerium dioxide also found interest in its use as a primary photocatalyst rather than only as an additive: Tavasoli et al. [165] investigated the use of Ni−supported catalysts on selectively phosphated CeO2 nanorods for the hybrid photothermal dry reforming of methane, obtaining results close to the ones obtained for noble metal catalysts. The phosphate groups allowed the researchers to tune the ceria surface alkalinity, and again temperature and light adsorption were observed to synergistically contribute to conversion. Light exposure enhances formation and promotes the reaction of surface carbonyl species, The combined catalyst proved to be active even under visible light−only monochromatic irradiation. Balsamo et al. [166] coupled cerium dioxide and graphene oxide to the use of an Au cocatalyst and obtained a 270 μmol/(g·h) H2 evolution rate from aqueous−phase 10% vol. glycerol solution under simulated sunlight. The material displayed stable operation, and hydrogen yield increased following the reduction of graphene oxide after five operation cycles. The graphene oxide acted as a mediator for the transfer of electrons from cerium to gold atoms. Iannaco et al. [167] performed photocatalytic hydrogen generation from lactic acid rich solutions, obtaining a hydrogen production of 3989 μmol/L for sample prepared with the supercritical antisolvent technique, higher than those obtained for commercial CeO2 powder (2519 μmol/L). The addition of an optimal CuO amount of 0.5% wt., determined an increase in hydrogen production of up to 9313 μmol/L after 4 h of UV irradiation time. They also confirmed that water acted as the hydrogen source, while lactic acid acted as sacrificial agent to refill the electron holes of the catalyst. The ceria and CuO interaction formed a type I heterojunctions, with electrons and holes being transferred to CuO. Zhang et al. [168] observed that the surface dispersion of Cu atoms over the CeO2 surface affected greatly the degradation of aqueous−phase toluene, with more even distribution of active sites Cu+−Ov−Ce3+ improving the adsorption and activation of reactants. On the other hand, surface lattice oxygen accelerates the rate−determining step of benzoate deep decomposition. The toluene degradation pathway was toluene → benzyl alcohol → benzaldehyde → benzoate →anhydride → CO2 and H2O. Aoun et al. [169] observed that a 15% wt. SrNiO3−CeO2 composite rapidly oxidated 2−propanol under both UV−LED and vs. light. A type I heterojunction was formed between ceria and the perovskite, with electrons and holes migrating to SrNiO3. Li et al. [170] synthetized a S−scheme heterojunction by depositing AgInS2 quantum dots on CeO2 surface and obtained the selective oxidation of xylose to xylonic acid and CO under vs. light (xylonic acid yield = 60.0%, CO evolution rate = 3689.9 μmol/(g·h)). On the other hand, Wang et al. [171] prepared a noble metal−free CeO2/CdS/NiS triple heterojunction with remarkable photocatalytic performance for the degradation of plastic waste, obtaining a hydrogen production rate of 32.40 mmol/(g·h) at the fifth hour, 29 times that of bare CdS and 463 times that of bare CeO2. In this case, Ni S was proposed to act as electron mediator between CdS and ceria conduction bands, with ceria acting as hydrogen production site and CdS electron holes carrying out the oxidation of waste.
WO3 is another material that has been tested for photocatalysis. Li et al. [172] reported the synthesis of a 2D CoP S−scheme heterojunction over a 0−dimensional WO3 support for H2 generation from a triethanolamine aqueous solution. CoP acted as the electron sink, while WO3 accumulated the electron holes. They obtained 218.63 mmol of hydrogen under vs. light exposure within 5 h of reaction, 287 times the amount of hydrogen formed for pure WO3 and 1.95 multiples the one produced from pure CoP. Similarly, the addition of CuO to WO3 increased the hydrogen yield from aqueous methanol solution by 64 times [173]. Surface oxygen vacancies capture the oxygen atom of CH3OH, while the Cu2+ cations activate the C−H bond. During the process, CH3OH accepts photogenerated electrons from WO3 and provides electrons to Cu2+. The addition of WO3 and graphene to TiO2 increased the catalytic activity for gaseous phase benzene degradation and hydrogen production [174]: the addition of WO3 only forms a type II heterojunction, improving charge separation, but reduces the activity compared to pure TiO2 due to the low potential of the photoexcited electrons. On the other hand, the addition of graphene not only facilitates electron transfer, but also offers a surface with a potential more suitable for hydrogen generation, thus compensating activity loss. However, despite the higher hydrogen production, the graphene−modified catalysts still display low oxidation capacity towards benzene. N−n heterojunction CuMn2O4/WO3 nanocomposites constructed by a sol–gel procedure were evaluated for H2 evolution from aqueous glycerol under vs. light by Albukhari et al. [175]. Their optimized 12% wt. CuMn2O4/WO3 nanocomposite exhibited a high maximal H2 evolution rate of 2856.6 μmol/(g·h). An S−scheme heterojunction transfers electrons from WO3 to CuMn2O4, which are then concentrated on Pt species.
Other catalytic systems were also investigated. Ye et al. [176], for example, investigated the use of 2D VxW1−xN1.5 bimetallic systems as cocatalysts coupled with CdS for the reforming of formic acid to syngas. A formulation of V0.1W0.9N1.5 boosted performance by over 60% compared to the unmodified W2N3 system. The VxW1−xN1.5 species acted as electron sinks for electrons of CdS. Zhou et al. [177] used Ni nanoparticles loaded on mesoporous silica for the photo−thermocatalytic steam reforming of cellulose under UV−VS light and obtained high production rates of H2 and CO (1966.2 and 1257.7 mmol/(g·h)) together with a light−to−fuel efficiency of 5.5%. On the other hand, Zhong et al. [178] performed the same reaction while using Ni nanoparticles loaded on θ−Al2O3, and they obtained significantly higher production rates of syngas (H2 3776.3 and CO 2028.1 mmol/(g·h)) and no deactivation after four cycles. In both cases, light and heat acted synergically to enhance reaction rate, and the degradation of cellulose followed a pyrolytic mechanism.
The literature on the catalysts discussed is summarized in Table 4.

Table 4.
Other photocatalysts for reforming processes. A = aqueous phase, G = gas phase, L = liquid phase, and AQY = apparent quantum yield.
3. Reactor and Process Design
Like photovoltaic generation, the requisite for the utilization of a photocatalyst in chemical reactors is the availability of a suitable light source and low attenuation of the irradiated power. Packed bed reactors, filled in with monoliths or granules, that are normally used for catalytic reactions even at high superficial velocity [179], are not usable because of the large attenuation of the irradiation in the bulk.
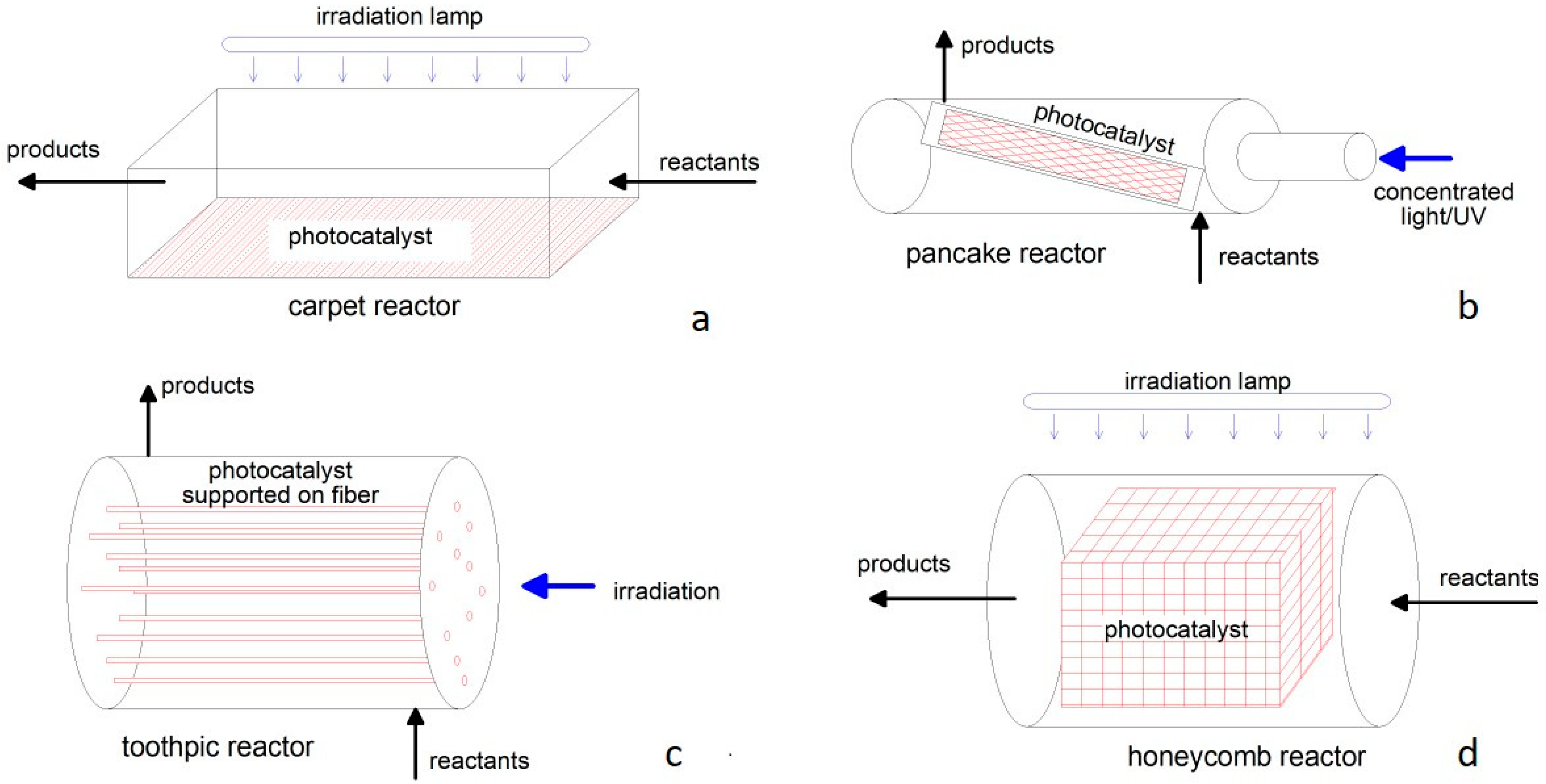
Conversely, a typical photoreactor has a planar or two−dimensional geometry, like in the “carpet”, “pancake”, “toothpick”, and “honeycomb” schemes reported in Figure 4 [180], with a transversal or longitudinal irradiation. The photocatalyst powder is dispersed and fixed over a suitable support that can be easily irradiated by natural or artificial light. The reactant is in contact with the catalyst mainly by a rather laminar flow, resulting in poor process intensification. Apart from reactions occurring in liquids, this configuration is suitable for the removal of pollutants from the environment, e.g., indoor air purification [181]. Photocatalytic CO2 reduction by steam has recently been addressed at laboratory−scale or modelled, demonstrating the possibility of distributed H2 production, even with solar irradiation [182,183].
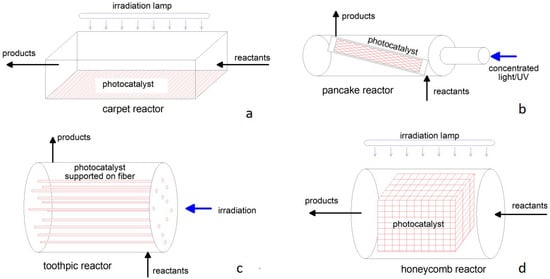
Figure 4.
Configurations of planar or two−dimensional photoreactors: (a) carpet; (b) pancake; (c) toothpick; (d) honeycomb.
Fluidized bed (FB) technology has been largely adopted for catalytic heterogeneous reactions thanks to its good mixing, excellent heat and mass transfer, and process reliability [184]. Normally, the FB reactor operates with a dense phase that is fluidized by a gas stream at velocities higher than the minimum for fluidization (Umf). Diluted fluidized beds are operated at a high gas velocity equal or higher than the terminal velocity Ut of the particle, thus allowing large plant potentiality. Therefore, FBs perform well when large diffusion coefficients and high heat transfer are needed for the catalytic process, for instance in the case of steam [185] and dry [186] reforming of hydrocarbons. They also allow easy regeneration of spent catalysts by looping with an external unit operated at different temperatures and atmosphere [187]. Nowadays, the utilization of photocatalytic fluidized beds is limited to only a few research activities dealing with pollutant conversion [188]. The photocatalytic elimination of phenol−derived compounds was studied in an FB reactor with a liquid/solid emulsion provided with a concentric UV light source [189]. The process efficiency increased with decreasing particle size, even for the same surface area concentration, with larger particles causing a blocking effect between the light source and the catalyst surface. Similarly, an excess of particle holdup caused light shielding, and an optimum bed concentration of 20 g/L of mixture was determined. Photocatalytic degradation of aromatics was successfully investigated in a FB photoreactor at lab−scale equipped with a UV source, taking advantage of the increased mass transfer [190]. For a non−purification−related application, Vaiano et al. [191] addressed the photocatalytic reduction of CO2 in FB via a steam reaction. They reported that an optimized formulation of the photocatalyst based on Ti and Pd, along with a UV−illuminated fluidized bed, can effectively allow the photocatalytic conversion of CO2, under mild reaction conditions.
The utilization of fine photocatalytic particles that are typically used over supports in other devices, e.g., self−cleaning TiO2 coating [192], is problematic in fluidized beds due to channeling and poor fluidization according to the Geldart classification of particulates [193]. In this respect, the synergy with other physical phenomena, like magnetism or sound [194,195], may significantly improve the quality of fluidization and the whole performance of the photocatalytic reactor. More frequently, the active phase is deposited over easily fluidizable and mechanically resistant particles, for instance γ−alumina, as reported for tar abetment in a FB gasifier [196]. In the case of photocatalytic reactions, the internal surface area could result inaccessible to irradiation, but inner sites may be suitable for combined steps, e.g., adsorption/release of CO2, in the whole process [197]. The use of two−dimensional or toroidal geometry systems favors uniform illumination of the catalyst but limits the potential of the system due to the smaller cross−section. Other FB configurations, e.g., conical geometry with an internal jet [198], could be particularly effective in gas–solid mixing and generation of diluted regions for vs. or UV irradiation. Figure 5 displays the different FB configurations.
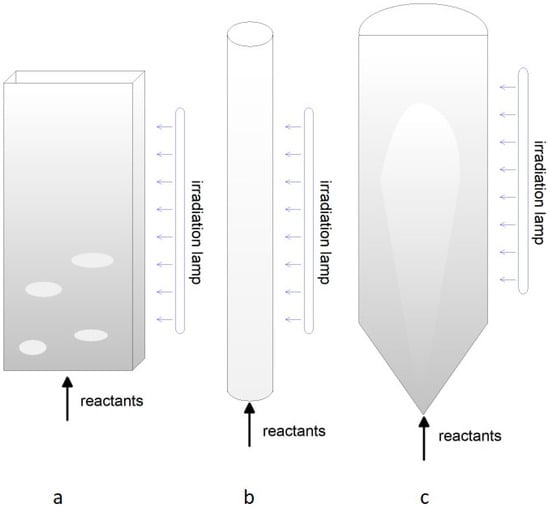
Figure 5.
Different designs of fluidized beds: (a) dense two−dimensional; (b) diluted; (c) conical with jet.
In a pioneering work [199], light transmission in a dense 2D fluidized bed was measured and correlated in regimes with up to 10 times Umf showing a rapid attenuation of the flux after a few centimeters. Thus, dense fluidized bed photoreactors can be limited to small−scale applications, as in the case of bidimensional devices, while large reaction chambers can effectively operate with a disperse particle phase [200] and a gas velocity approaching or exceeding Ut. In such transport regime conditions, the solid volume fraction can be limited to about 1%, despite having high solid flows, e.g., 20 kg m−2 s−1 [201].
Table 5 reports a short comparison between bidimensional and fluidized bed photocatalytic reactors. The 2D fixed configuration is more suitable for carrying out unitary operations, like purification, in easier, scalable, and less demanding equipment. In contrast, combined/intensified processes, requiring mass and heat transfer, are better conducted in FB configuration. The photocatalyst is more easily prepared in the form of fluidizable granules, for example by spray drying [202], rather than demanding two−dimensional devices, e.g., honeycomb or toothpick devices [180].

Table 5.
Comparison between different photoreactor configurations.
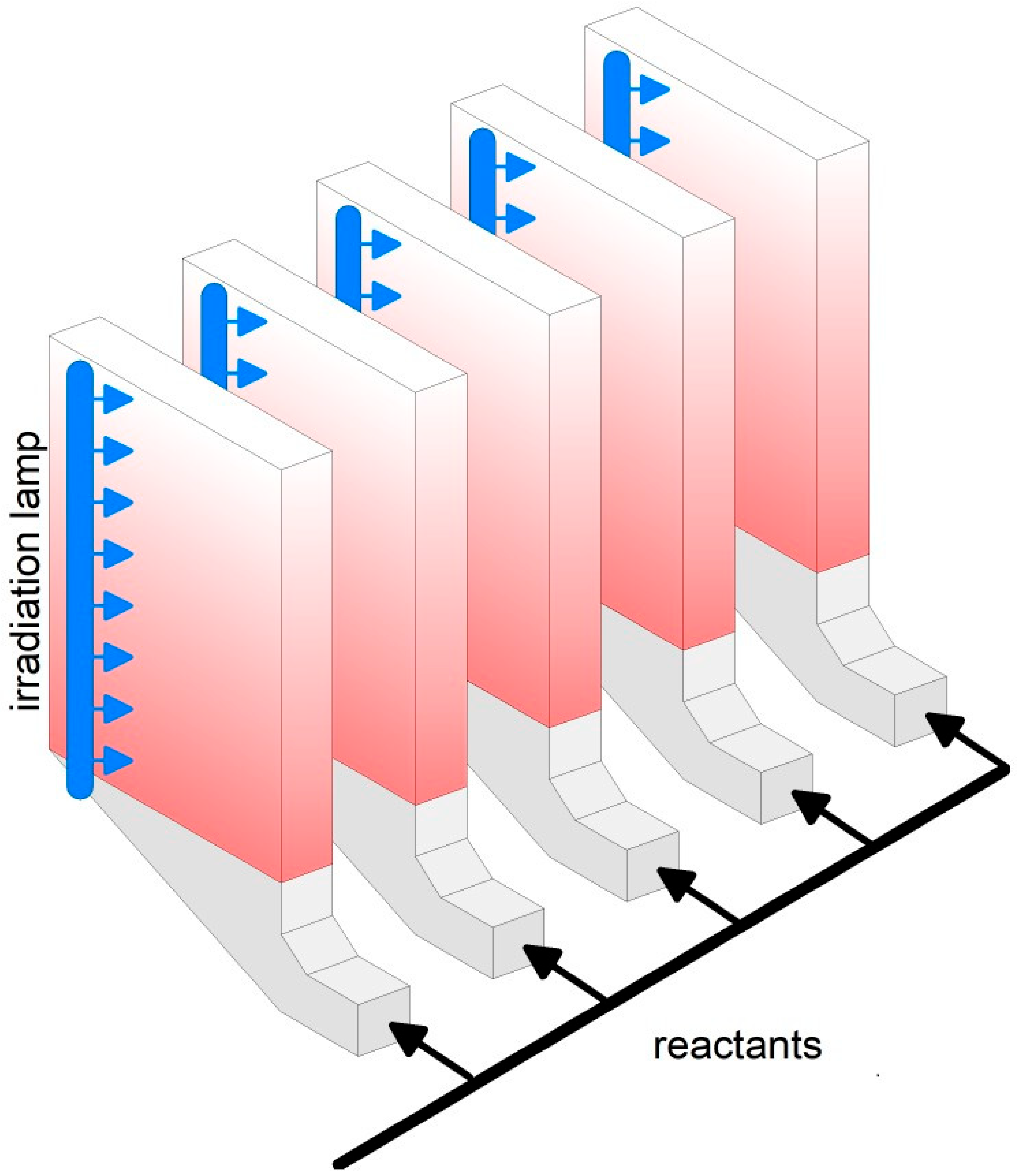
Large scale plants for steam or dry reforming may convert up to 300,000 Nm3/h of methane [203] in multiple channels catalytic reactors, for instance the “Steam reformer tube assembly and method of assembling or retrofitting same” [204]. Since the gas velocity in the packed bed is limited to a few cm/s because of the increased pressure drop at higher velocities, the transversal size of large−scale plants likely exceeds 10 m with a height of 2–3 m [203]. In comparison, the possibility to operate at a higher gas velocity, up to 1 m/s, in fluidized bed reactors [193], may largely reduce the volume of the catalytic reactor. In the case of photocatalysis, the need to provide effective irradiation calls for diluted FB systems (Figure 5b,c) or an array of two−dimensional fluidized beds (Figure 6). Thanks to the great flexibility of FB technology, the development of small−scale integrated systems, e.g., biomass gasification and photoreforming of the generated hydrocarbons, could enable distributed H2 production, with greater environmental sustainability [205].
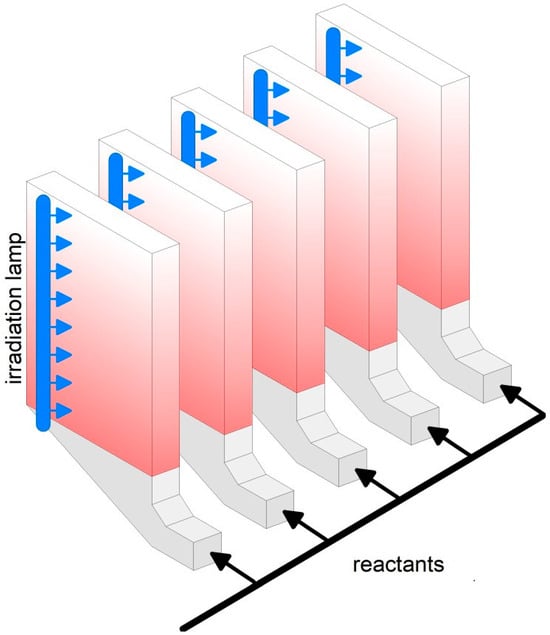
Figure 6.
Array of two−dimensional fluidized beds.
The development of the integrated process could benefit from the application of artificial intelligence (AI) and machine learning, both in the development of photocatalytic materials [206] and the optimization of the whole process [207], speeding up the technological progress thanks to increasingly precise predictive models.
4. Conclusions
Titanium oxide is the most diffused material used for photocatalytic processes due to its high activity, good thermal and chemical stability, low cost, and non−toxicity. In the last decades, a lot of research has been carried out to enhance the performance of TiO2 and to adapt it to different irradiation sources by doping it with other elements or producing nanostructured photocatalysts.
ZrO2−based photocatalysts possess similar properties as TiO2, in terms of low cost, high thermal and chemical stability, and mechanical resistance. They can be used under UV light and need modification to operate under VS radiation. Graphitic carbon nitride (C3N4) is non−oxidic material suitable for photocatalytic reforming applications, characterized by a narrower band gap and, thus, is intrinsically more active under vs. light irradiation. Conversely, it suffers from a more complex preparation method and related costs.
Overall, prior research has already developed photocatalysts with formulations based on a few elements that are relatively cheap, which allow the development of hydrocarbon reforming processes supported by VS− or UV light.
The application of photocatalysis in large−scale plants and the integration of more processes can be conceived with equipment from the process industry that has so far been scarcely applied in this sector, in particular through the use of fluidization technology. Although these devices may give rise to light attenuation, their superior performance in terms of mixing and energy transport can make up for the limitations of traditional photocatalysis technologies.
With reference to hydrocarbon reforming, the combination of photocatalytic devices and mature plant technologies, such as fluidization, can allow new strategies to obtain better performance and greater flexibility and efficiency, especially in relation to the lower temperature required for the process. Such integration could allow the development of more sustainable processes on a small scale, for example for the photoreforming of biomethane and bio−derivatives obtained from agro−industrial sector.
Although the proposed process integration is still at a low level of readiness, the development of research also supported by powerful artificial intelligence and machine learning tools could allow researchers to achieve pre−industrial solutions in less than 10 years and, therefore, pave the way to commercialization.
Funding
This research was funded by the National Recovery and Resilience Plan (NRRP Italy), Mission 04 Component 2 Investment 1.5—NextGenerationEU, Call for tender no. 3277, dated 30 December 2021.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| A | Aqueous phase |
| AI | Artificial intelligence |
| APE | Apparent photon efficiency |
| AQY | Apparent quantum yield |
| BG | Band gap |
| CB | Conduction band |
| COF | Covalent organic framework |
| FB | Fluidized bed |
| G | Gas phase |
| GO | Graphene oxide |
| IR | Infrared |
| MOF | Metal–organic framework |
| NIR | Near−infrared |
| L | Liquid phase |
| LDH | Layered double hydroxide |
| LED | Light−emission diode |
| PE | Photonic efficiency |
| QE | Quantum efficiency |
| TEOA | Triethanolamine |
| Umf | Minimum fluidization velocity |
| Ut | Terminal velocity |
| UV | Ultraviolet |
| VB | Valence band |
| VS | Visible |
References
- Boscherini, M.; Storione, A.; Minelli, M.; Miccio, F.; Doghieri, F. New Perspectives on Catalytic Hydrogen Production by the Reforming, Partial Oxidation and Decomposition of Methane and Biogas. Energies 2023, 16, 6375. [Google Scholar] [CrossRef]
- Großmann, K.; Treiber, P.; Karl, J. Steam Methane Reforming at Low S/C Ratios for Power−to−Gas Applications. Int. J. Hydrogen Energy 2016, 41, 17784–17792. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam Reforming of Methane: Current States of Catalyst Design and Process Upgrading. Renew. Sust. Energy Rev. 2021, 149, 111330. [Google Scholar] [CrossRef]
- Wang, S.; Nabavi, S.A.; Clough, P.T. A Review on Bi/Polymetallic Catalysts for Steam Methane Reforming. Int. J. Hydrogen Energy 2023, 48, 15879–15893. [Google Scholar] [CrossRef]
- Lu, H.; Shi, X.; Costa, M.; Huang, C. Carcinogenic Effect of Nickel Compounds. Mol. Cell. Biochem. 2005, 279, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas Reforming to Syngas: A Review. iScience 2020, 23, 101082. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Moon, D.H.; Kim, K.H.; Kwon, E.E. Upgrading Biogas into Syngas through Dry Reforming. Renew. Sustain. Energy Rev. 2021, 143, 110949. [Google Scholar] [CrossRef]
- Minh, D.P.; Siang, T.J.; Vo, D.V.N.; Phan, T.S.; Ridart, C.; Nzihou, A.; Grouset, D. Hydrogen Production from Biogas Reforming: An Overview of Steam Reforming, Dry Reforming, Dual Reforming, and Tri−Reforming of Methane. In Hydrogen Supply Chain: Design, Deployment and Operation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–166. ISBN 9780128111970. [Google Scholar]
- Aramouni, N.A.K. Carbon Mitigation in the Dry Reforming of Methane. Ph.D. Thesis, University of Limerick, Limerick, Ireland, 2020. [Google Scholar]
- De Caprariis, B.; Bassano, C.; Deiana, P.; Palma, V.; Petrullo, A.; Scarsella, M.; De Filippis, P. Carbon Dioxide Reforming of Tar during Biomass Gasification. Chem. Eng. Trans. 2014, 37, 97–102. [Google Scholar] [CrossRef]
- Guan, G.; Kaewpanha, M.; Hao, X.; Abudula, A. Catalytic Steam Reforming of Biomass Tar: Prospects and Challenges. Renew. Sustain. Energy Rev. 2016, 58, 450–461. [Google Scholar] [CrossRef]
- Abdelaal, A.; Villot, A.; Patuzzi, F.; Baratieri, M.; Gerente, C. Steam Reforming of Main Tar Compounds over Industrial Gasification Char. Fuel 2025, 384, 133986. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Maneerung, T.; Kathiraser, Y.; Kawi, S. CO2 Dry-Reforming of Methane over La0.8Sr 0.2Ni0.8M0.2O3 Perovskite (M = Bi, Co, Cr, Cu, Fe): Roles of Lattice Oxygen on C-H Activation and Carbon Suppression. Int. J. Hydrogen Energy 2012, 37, 11195–11207. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. A Review on Coke Management during Dry Reforming of Methane. Int. J. Energy Res. 2015, 39, 1196–1216. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst Design for Dry Reforming of Methane: Analysis Review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A Review of Recent Developments in Hydrogen Production via Biogas Dry Reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Han, B.; Wang, F.; Zhang, L.; Wang, Y.; Fan, W.; Xu, L.; Yu, H.; Li, Z. Syngas Production from Methane Steam Reforming and Dry Reforming Reactions over Sintering−Resistant Ni@SiO2 Catalyst. Res. Chem. Intermed. 2020, 46, 1735–1748. [Google Scholar] [CrossRef]
- Manan, W.N.; Wan Isahak, W.N.R.; Yaakob, Z. CeO2−Based Heterogeneous Catalysts in Dry Reforming Methane and Steam Reforming Methane: A Short Review. Catalysts 2022, 12, 452. [Google Scholar] [CrossRef]
- Chaghouri, M.; Hany, S.; Cazier, F.; Tidahy, H.L.; Gennequin, C.; Abi−Aad, E. Impact of Impurities on Biogas Valorization through Dry Reforming of Methane Reaction. Int. J. Hydrogen Energy 2022, 47, 40415–40429. [Google Scholar] [CrossRef]
- Phan, T.S.; Pham Minh, D. New Performing Hydroxyapatite−Based Catalysts in Dry−Reforming of Methane. Int. J. Hydrogen Energy 2023, 48, 30770–30790. [Google Scholar] [CrossRef]
- Haug, L.; Thurner, C.; Bekheet, M.F.; Ploner, K.; Bischoff, B.; Gurlo, A.; Kunz, M.; Sartory, B.; Penner, S.; Klötzer, B. Pivotal Role of Ni/ZrO2 Phase Boundaries for Coke-Resistant Methane Dry Reforming Catalysts. Catalysts 2023, 13, 804. [Google Scholar] [CrossRef]
- Wang, K.; Ren, X.; Yin, G.; Hu, E.; Zhang, H. Recent Advances in Plasma−Based Methane Reforming for Syngas Production. Curr. Opin. Green Sustain. Chem. 2024, 50, 100981. [Google Scholar] [CrossRef]
- Fan, L.; Li, C.; van Biert, L.; Zhou, S.-H.; Tabish, A.N.; Mokhov, A.; Aravind, P.V.; Cai, W. Advances on Methane Reforming in Solid Oxide Fuel Cells. Renew. Sustain. Energy Rev. 2022, 166, 112646. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, B.; Jiang, Y.J.; Zhou, Y.; Sun, X.; Wang, Y.; Zhu, W. Photocatalytic Conversion of Methane: Current State of the Art, Challenges, and Future Perspectives. ACS Environ. Au 2023, 3, 252–276. [Google Scholar] [CrossRef]
- Ahasan, M.R.; Hossain, M.M.; Ding, X.; Wang, R. Non−Equilibrium Plasma−Assisted Dry Reforming of Methane over Shape−Controlled CeO2 Supported Ruthenium Catalysts. J. Mater. Chem. A 2023, 11, 10993–11009. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, N.; Lin, Y.; Mao, X.; Zhong, H.; Chang, Z.; Shneider, M.N.; Ju, Y. Enhancements of Electric Field and Afterglow of Non−Equilibrium Plasma by Pb(ZrxTi1−x)O3 Ferroelectric Electrode. Nat. Commun. 2024, 15, 3092. [Google Scholar] [CrossRef]
- Beil, S.B.; Bonnet, S.; Casadevall, C.; Detz, R.J.; Eisenreich, F.; Glover, S.D.; Kerzig, C.; Næsborg, L.; Pullen, S.; Storch, G.; et al. Challenges and Future Perspectives in Photocatalysis: Conclusions from an Interdisciplinary Workshop. JACS Au 2024, 4, 2746–2766. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Sundaram, B. A Review of the Photocatalysis Process Used for Wastewater Treatment. Mater. Today Proc. 2024, 102, 393–409. [Google Scholar] [CrossRef]
- Kobielusz, M.; Mikrut, P.; Macyk, W. Photocatalytic Synthesis of Chemicals. In Materials for Sustainable Energy. Advances in Inorganic Chemistry; van Eldik, R., Macyk, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 72, pp. 93–144. ISBN 978-0-12-815077-1. [Google Scholar]
- Yao, Y.; Gao, X.; Li, Z.; Meng, X. Photocatalytic Reforming for Hydrogen Evolution: A Review. Catalysts 2020, 10, 335. [Google Scholar] [CrossRef]
- Shoji, S.; Peng, X.; Yamaguchi, A.; Watanabe, R.; Fukuhara, C.; Cho, Y.; Yamamoto, T.; Matsumura, S.; Yu, M.W.; Ishii, S.; et al. Photocatalytic Uphill Conversion of Natural Gas beyond the Limitation of Thermal Reaction Systems. Nat. Catal. 2020, 3, 148–153. [Google Scholar] [CrossRef]
- Zhang, L.; Kuang, P.; Yu, J. Introductory Chapter: Fundamentals of Photocatalysis and Electrocatalysis. In Graphene Oxide−Metal Oxide and other Graphene Oxide−Based Composites in Photocatalysis and Electrocatalysis; Jiaguo, Y., Liuyang, Z., Panyong, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–30. [Google Scholar]
- Lian, H.-Y.; Li, X.-S.; Liu, J.-L.; Zhu, X.; Zhu, A.-M. Oxidative Pyrolysis Reforming of Methanol in Warm Plasma for an On−Board Hydrogen Production. Int. J. Hydrogen Energy 2017, 42, 13617–13624. [Google Scholar] [CrossRef]
- Cvetinović, D.; Erić, A.; Anđelković, J.; Ćetenović, N.; Jovanović, M.; Bakić, V. Economic Viability of Hydrogen Production via Plasma Thermal Degradation of Natural Gas. Processes 2025, 13, 1888. [Google Scholar] [CrossRef]
- Escribà−Gelonch, M.; Osorio−Tejada, J.; Yu, L.; Wanten, B.; Bogaerts, A.; Hessel, V. Techno−Economic and Life−Cycle Assessment for Syngas Production Using Sustainable Plasma−Assisted Methane Reforming Technologies. Energy Environ. Sci. 2025, 18, 6043–6062. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Benjamin, S.; Ameta, S.C. Photocatalysis. In Advanced Oxidation Processes for Waste Water Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–175. [Google Scholar]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Li, Z.; Yi, Z.; Li, Z.; Zou, Z. Photocatalytic and Thermocatalytic Conversion of Methane. Sol. RRL 2021, 5, 2000596. [Google Scholar] [CrossRef]
- Song, H.; Ye, J. Direct Photocatalytic Conversion of Methane to Value−Added Chemicals. Trends Chem. 2022, 4, 1094–1105. [Google Scholar] [CrossRef]
- Cho, Y.; Yamaguchi, A.; Miyauchi, M. Photocatalytic Methane Reforming: Recent Advances. Catalysts 2020, 11, 18. [Google Scholar] [CrossRef]
- Kulandaivalu, T.; Mohamed, A.R.; Ali, K.A.; Mohammadi, M. Photocatalytic Carbon Dioxide Reforming of Methane as an Alternative Approach for Solar Fuel Production−a Review. Renew. Sustain. Energy Rev. 2020, 134, 110363. [Google Scholar] [CrossRef]
- Wang, P.; Shi, R.; Zhao, J.; Zhang, T. Photodriven Methane Conversion on Transition Metal Oxide Catalyst: Recent Progress and Prospects. Adv. Sci. 2024, 11, e2305471. [Google Scholar] [CrossRef]
- Lei, Y.; Sala, X.; García−Antón, J.; Muñoz, J. A Review on Photocatalytic Methane Conversion Systems: From Fundamental Mechanisms to the Emerging Role of Ferroelectric Materials. J. Mater. Chem. A 2025, 13, 12712–12745. [Google Scholar] [CrossRef]
- Xu, S.; Huang, X.; Lu, H. Photocatalytic Reforming of Biomass for Hydrogen Production: A Comprehensive Overview. Fuel Process. Technol. 2024, 255, 108057. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Wang, Y.; Xia, Q.; Qiu, L.; Zhou, B. Solar−driven Biomass Reforming for Hydrogen Generation: Principles, Advances, and Challenges. Adv. Sci. 2024, 11, e2402651. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xu, Q.; Zeng, G. Developments of Photo−/Electro−catalysis Based on Covalent Organic Frameworks: A Review. Electron 2024, 2, e39. [Google Scholar] [CrossRef]
- Sahu, J.; Prusty, D.; Mansingh, S.; Parida, K. A Review on Alloyed Quantum Dots and Their Applications as Photocatalysts. Int. J. Hydrogen Energy 2023, 48, 29097–29118. [Google Scholar] [CrossRef]
- Luo, J.; Selopal, G.S.; Tong, X.; Wang, Z. Colloidal Quantum Dots and Two−dimensional Material Heterostructures for Photodetector Applications. Electron 2024, 2, e30. [Google Scholar] [CrossRef]
- Yang, H. A Short Review on Heterojunction Photocatalysts: Carrier Transfer Behavior and Photocatalytic Mechanisms. Mater. Res. Bull. 2021, 142, 111406. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Fan, Y.; Tang, Z. Best Practices for Experiments and Reports in Photocatalytic Methane Conversion. Angew. Chem. Int. Ed. 2024, 63, e202404658. [Google Scholar] [CrossRef]
- Khodadadian, F.; de Boer, M.W.; Poursaeidesfahani, A.; van Ommen, J.R.; Stankiewicz, A.I.; Lakerveld, R. Design, Characterization and Model Validation of a LED−Based Photocatalytic Reactor for Gas Phase Applications. Chem. Eng. J. 2018, 333, 456–466. [Google Scholar] [CrossRef]
- Wang, D.; Mueses, M.A.; Márquez, J.A.C.; Machuca−Martínez, F.; Grčić, I.; Peralta Muniz Moreira, R.; Li Puma, G. Engineering and Modeling Perspectives on Photocatalytic Reactors for Water Treatment. Water Res. 2021, 202, 117421. [Google Scholar] [CrossRef]
- Sundar, K.P.; Kanmani, S. Progression of Photocatalytic Reactors and It’s Comparison: A Review. Chem. Eng. Res. Des. 2020, 154, 135–150. [Google Scholar] [CrossRef]
- Binjhade, R.; Mondal, R.; Mondal, S. Continuous Photocatalytic Reactor: Critical Review on the Design and Performance. J. Environ. Chem. Eng. 2022, 10, 107746. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, W.; Zhang, Y.; Pan, C.; Xu, J.; Zhu, Y.; Lou, Y. Research Progress on Methane Conversion Coupling Photocatalysis and Thermocatalysis. Carbon Energy 2021, 3, 519–540. [Google Scholar] [CrossRef]
- Li, M.; Sun, Z.; Hu, Y.H. Thermo−Photo Coupled Catalytic CO2 Reforming of Methane: A Review. Chem. Eng. J. 2022, 428, 131222. [Google Scholar] [CrossRef]
- Tavasoli, A.V.; Preston, M.; Ozin, G. Photocatalytic Dry Reforming: What Is It Good For? Energy Environ. Sci. 2021, 14, 3098–3109. [Google Scholar] [CrossRef]
- Kisch, H. Semiconductor Photocatalysis—Mechanistic and Synthetic Aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef]
- Wang, L.; Yu, J. Chapter 1−Principles of Photocatalysis. In S−Scheme Heterojunction Photocatalysts: Fundamentals and Applications. Interface Science and Technology; Yu, J., Zhang, L., Wang, L., Zhu, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 35, pp. 1–52. [Google Scholar]
- Ayyub, M.M.; Rao, C.N.R. Design of Efficient Photocatalysts through Band Gap Engineering. In Nanostructured Photocatalysts−From Materials to Applications in Solar Fuels and Environmental Remediation; Boukherroub, R., Robertson, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–18. [Google Scholar]
- Tuama, A.N.; Alzubaidi, L.H.; Jameel, M.H.; Abass, K.H.; bin Mayzan, M.Z.H.; Salman, Z.N. Impact of Electron–Hole Recombination Mechanism on the Photocatalytic Performance of ZnO in Water Treatment: A Review. J. Sol.−Gel. Sci. Technol. 2024, 110, 792–806. [Google Scholar] [CrossRef]
- Yuan, J.; Li, H.; Wang, G.; Zhang, C.; Wang, Y.; Yang, L.; Li, M.; Lu, J. Adsorption, Isolated Electron/Hole Transport, and Confined Catalysis Coupling to Enhance the Photocatalytic Degradation Performance. Appl. Catal. B Environ. 2022, 303, 120892. [Google Scholar] [CrossRef]
- Tu, H.; Tian, B.; Chen, S.; Xu, J.; Yang, J.; Zhao, Z.; Chen, S.; Wu, J. Enhancing Photocatalytic Efficiency through Surface Modification to Manipulate Internal Electron−Hole Distribution. NPJ Clean Water 2025, 8, 48. [Google Scholar] [CrossRef]
- Akhter, P.; Arshad, A.; Saleem, A.; Hussain, M. Recent Development in Non−Metal−Doped Titanium Dioxide Photocatalysts for Different Dyes Degradation and the Study of Their Strategic Factors: A Review. Catalysts 2022, 12, 1331. [Google Scholar] [CrossRef]
- Fang, W.; Yan, J.; Wei, Z.; Liu, J.; Guo, W.; Jiang, Z.; Shangguan, W. Account of Doping Photocatalyst for Water Splitting. Chin. J. Catal. 2024, 60, 1–24. [Google Scholar] [CrossRef]
- Flores, N.M.; Pal, U.; Galeazzi, R.; Sandoval, A. Effects of Morphology, Surface Area, and Defect Content on the Photocatalytic Dye Degradation Performance of ZnO Nanostructures. RSC Adv. 2014, 4, 41099–41110. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, M.; You, J.; Liu, G.; Wang, L. Defect Engineering in Photocatalysts and Photoelectrodes: From Small to Big. Acc. Mater. Res. 2022, 3, 1127–1136. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, P.; Guo, L.; Chen, Z.; Wu, Q.; Ding, Y.; Zheng, W.; Cao, Y. The Design of TiO2 Nanostructures (Nanoparticle, Nanotube, and Nanosheet) and Their Photocatalytic Activity. J. Phys. Chem. C 2014, 118, 12727–12733. [Google Scholar] [CrossRef]
- Gao, P.; Li, A.; Sun, D.D.; Ng, W.J. Effects of Various TiO2 Nanostructures and Graphene Oxide on Photocatalytic Activity of TiO2. J. Hazard. Mater. 2014, 279, 96–104. [Google Scholar] [CrossRef]
- Lincho, J.; Zaleska−Medynska, A.; Martins, R.C.; Gomes, J. Nanostructured Photocatalysts for the Abatement of Contaminants by Photocatalysis and Photocatalytic Ozonation: An Overview. Sci. Total Environ. 2022, 837, 155776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, H.; Gao, H.; Li, A.; Li, T.; Cheng, S.; Wang, J.; Kasemchainan, J.; Yi, J.; Zhao, F.; et al. Recent Advances in Metal−Loaded MOFs Photocatalysts: From Single Atom, Cluster to Nanoparticle. Chin. Chem. Lett. 2024, 36, 110142. [Google Scholar] [CrossRef]
- Hamdan, S.; Wigglesworth, M.J.; Muscetta, M.; Ma, R.; Helal, M.I.; Martsinovich, N.; Palmisano, G.; Vernuccio, S. Unravelling the Photoactivity of Metal−Loaded TiO2 for Hydrogen Production: Insights from a Combined Experimental and Computational Analysis. Int. J. Hydrogen Energy 2025, 118, 394–406. [Google Scholar] [CrossRef]
- Balapure, A.; Ray Dutta, J.; Ganesan, R. Recent Advances in Semiconductor Heterojunctions: A Detailed Review of the Fundamentals of Photocatalysis, Charge Transfer Mechanism and Materials. RSC Appl. Interfaces 2024, 1, 43–69. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al−Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Salazar−Marín, D.; Oza, G.; Real, J.A.D.; Cervantes−Uribe, A.; Pérez−Vidal, H.; Kesarla, M.K.; Torres, J.G.T.; Godavarthi, S. Distinguishing between Type II and S−Scheme Heterojunction Materials: A Comprehensive Review. Appl. Surf. Sci. Adv. 2024, 19, 100536. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.; Hong, L.; Ji, X.; Lin, Z.; Li, Z.; Pan, W. A Review of Metal Oxide−Based Z−Scheme Heterojunction Photocatalysts: Actualities and Developments. Mater. Today Energy 2021, 21, 100829. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Kong, L.; O’Dell, L.A.; Merenda, A.; Dumée, L.F. Engineering Schottky−like and Hetero−junction Materials for Enhanced Photocatalysis Performance—A Review. Mater Adv. 2022, 3, 2309–2323. [Google Scholar] [CrossRef]
- Rao, V.N.; Reddy, N.L.; Kumari, M.M.; Cheralathan, K.K.; Ravi, P.; Sathish, M.; Neppolian, B.; Reddy, K.R.; Shetti, N.P.; Prathap, P.; et al. Sustainable Hydrogen Production for the Greener Environment by Quantum Dots−Based Efficient Photocatalysts: A Review. J. Environ. Manag. 2019, 248, 109246. [Google Scholar] [CrossRef]
- Sun, P.; Xing, Z.; Li, Z.; Zhou, W. Recent Advances in Quantum Dots Photocatalysts. Chem. Eng. J. 2023, 458, 141399. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Dharma, H.N.C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Othman, M.H.D.; Rahman, M.A.; Jafri, N.N.M.; Suhaimin, N.S.; Nasir, A.M.; et al. A Review of Titanium Dioxide (TiO2)−Based Photocatalyst for Oilfield−Produced Water Treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and Application of Titanium Dioxide Photocatalysis for Energy, Decontamination and Viral Disinfection: A Review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef]
- Martín−Sómer, M.; Pablos, C.; van Grieken, R.; Marugán, J. Influence of Light Distribution on the Performance of Photocatalytic Reactors: LED vs. Mercury Lamps. Appl. Catal. B Environ. 2017, 215, 1–7. [Google Scholar] [CrossRef]
- Masoud, N.M.; Murnick, D.E. High Efficiency Fluorescent Excimer Lamps: An Alternative to Mercury Based UVC Lamps. Rev. Sci. Instrum. 2013, 84, 123108. [Google Scholar] [CrossRef]
- Hölz, K.; Lietard, J.; Somoza, M.M. High−Power 365 Nm UV LED Mercury Arc Lamp Replacement for Photochemistry and Chemical Photolithography. ACS Sustain. Chem. Eng. 2017, 5, 828–834. [Google Scholar] [CrossRef]
- Pelayo, D.; Rivero, M.J.; Santos, G.; Gómez, P.; Ortiz, I. Techno−Economic Evaluation of UV Light Technologies in Water Remediation. Sci. Total Environ. 2023, 868, 161376. [Google Scholar] [CrossRef]
- Rehman, S.; Ullah, R.; Butt, A.M.; Gohar, N.D. Strategies of Making TiO2 and ZnO Visible Light Active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Hashemizadeh, I.; Golovko, V.B.; Choi, J.; Tsang, D.C.W.; Yip, A.C.K. Photocatalytic Reduction of CO2 to Hydrocarbons Using Bio−Templated Porous TiO2 Architectures under UV and Visible Light. Chem. Eng. J. 2018, 347, 64–73. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Salih Al−Hamdani, A.A.; Sunghun, B.; Ko, Y.G. A Review on TiO2−Based Composites for Superior Photocatalytic Activity. Rev. Inorg. Chem. 2021, 41, 213–222. [Google Scholar] [CrossRef]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the Strategies for Enhancing the Photocatalytic Activity of TiO2: Conversion from UV−Light Active to Visible−Light Active Photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- Tinoco Navarro, L.K.; Jaroslav, C. Enhancing Photocatalytic Properties of TiO2 Photocatalyst and Heterojunctions: A Comprehensive Review of the Impact of Biphasic Systems in Aerogels and Xerogels Synthesis, Methods, and Mechanisms for Environmental Applications. Gels 2023, 9, 976. [Google Scholar] [CrossRef]
- Chauke, N.M.; Mohlala, R.L.; Ngqoloda, S.; Raphulu, M.C. Harnessing Visible Light: Enhancing TiO2 Photocatalysis with Photosensitizers for Sustainable and Efficient Environmental Solutions. Front. Chem. Eng. 2024, 6, 1356021. [Google Scholar] [CrossRef]
- Acharya, R.; Pani, P. Visible Light Susceptible Doped TiO2 Photocatalytic Systems: An Overview. Mater. Today Proc. 2022, 67, 1276–1282. [Google Scholar] [CrossRef]
- Prakash, J.; Sun, S.; Swart, H.C.; Gupta, R.K. Noble Metals−TiO2 Nanocomposites: From Fundamental Mechanisms to Photocatalysis, Surface Enhanced Raman Scattering and Antibacterial Applications. Appl. Mater. Today 2018, 11, 82–135. [Google Scholar] [CrossRef]
- Yadav, M.; Basheer, H.S.; Ágfalvi, Á.; Ábrahámné, K.B.; Kiss, J.; Halasi, G.; Sápi, A.; Kukovecz, Á.; Kónya, Z. Noble Metals−Deposited TiO2 Photocatalysts for Photoreduction of CO2: Exploration of Surface Chemistry and a Reflection on the Importance of Wavelength Dependence. Appl. Catal. A Gen. 2023, 668, 119434. [Google Scholar] [CrossRef]
- Blanco−Vega, M.P.; Guzmán−Mar, J.L.; Villanueva−Rodríguez, M.; Maya−Treviño, L.; Garza−Tovar, L.L.; Hernández−Ramírez, A.; Hinojosa−Reyes, L. Photocatalytic Elimination of Bisphenol A under Visible Light Using Ni−Doped TiO2 Synthesized by Microwave Assisted Sol−Gel Method. Mater. Sci. Semicond. Process. 2017, 71, 275–282. [Google Scholar] [CrossRef]
- Baruah, M.; Ezung, S.L.; Sharma, S.; Bora Sinha, U.; Sinha, D. Synthesis and Characterization of Ni−Doped TiO2 Activated Carbon Nanocomposite for the Photocatalytic Degradation of Anthracene. Inorg. Chem. Commun. 2022, 144, 109905. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. Highly Effective Fe−Doped TiO2 Nanoparticles Photocatalysts for Visible−Light Driven Photocatalytic Degradation of Toxic Organic Compounds. J. Colloid Interface Sci. 2015, 450, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Moradi, V.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Significant Improvement in Visible Light Photocatalytic Activity of Fe Doped TiO2 Using an Acid Treatment Process. Appl. Surf. Sci. 2018, 427, 791–799. [Google Scholar] [CrossRef]
- Khalid, N.R.; Ahmed, E.; Hong, Z.; Ahmad, M.; Zhang, Y.; Khalid, S. Cu−Doped TiO2 Nanoparticles/Graphene Composites for Efficient Visible−Light Photocatalysis. Ceram. Int. 2013, 39, 7107–7113. [Google Scholar] [CrossRef]
- Clarizia, L.; Spasiano, D.; Di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper Modified−TiO2 Catalysts for Hydrogen Generation through Photoreforming of Organics. A Short Review. Int. J. Hydrogen Energy 2014, 39, 16812–16831. [Google Scholar] [CrossRef]
- Iwasaki, M.; Hara, M.; Kawada, H.; Tada, H.; Ito, S. Cobalt Ion−Doped TiO2 Photocatalyst Response to Visible Light. J. Colloid Interface Sci. 2000, 224, 202–204. [Google Scholar] [CrossRef]
- Binas, V.D.; Sambani, K.; Maggos, T.; Katsanaki, A.; Kiriakidis, G. Synthesis and Photocatalytic Activity of Mn−Doped TiO2 Nanostructured Powders under UV and Visible Light. Appl. Catal. B Environ. 2012, 113–114, 79–86. [Google Scholar] [CrossRef]
- Sudrajat, H.; Babel, S.; Ta, A.T.; Nguyen, T.K. Mn−Doped TiO2 Photocatalysts: Role, Chemical Identity, and Local Structure of Dopant. J. Phys. Chem. Solids 2020, 144, 109517. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, D.; Chen, M.; Li, D.; Zhu, J.; Lü, X.; Yan, C. Preparation and Characterization of Monodisperse Ce−Doped TiO2 Microspheres with Visible Light Photocatalytic Activity. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 107–114. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Tailoring Performance of La−Modified TiO2 Nanocatalyst for Continuous Photocatalytic CO2 Reforming of CH4 to Fuels in the Presence of H2O. Energy Convers. Manag. 2018, 159, 284–298. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Kujirai, T.; Fujita, T.; Abe, H.; Miyauchi, M. Steam Reforming of Methane by Titanium Oxide Photocatalysts with Hollow Spheres. Sustain. Energy Fuels 2024, 8, 516–523. [Google Scholar] [CrossRef]
- Al−Madanat, O.; Alsalka, Y.; Curti, M.; Dillert, R.; Bahnemann, D.W. Mechanistic Insights into Hydrogen Evolution by Photocatalytic Reforming of Naphthalene. ACS Catal. 2020, 10, 7398–7412. [Google Scholar] [CrossRef]
- Genco, A.; García−López, E.I.; Megna, B.; Ania, C.; Marcì, G. Nb2O5 Based Photocatalysts for Efficient Generation of H2 by Photoreforming of Aqueous Solutions of Ethanol. Catal. Today 2025, 447, 115147. [Google Scholar] [CrossRef]
- Eisapour, M.; Huang, R.; Roostaei, T.; Zhao, H.; Hu, J.; Chen, Z. Sandwich−like Heterojunction of NiO−Ni−TiO2 for Simultaneous Production of Hydrogen and Value−Added Products from Glycerol Photoreforming. Environ. Surf. Interfaces 2025, 3, 46–54. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Fan, Y.; Sun, X.; Qiao, P.; Xiao, X.; Zhang, Q.; Tian, C.; Jiang, B. Regulating the Local Electronic Structure by Constructing Ni–O–Ni Sites Confined in TiO2 for Selective Photocatalytic Glycerol Reforming. J. Energy Chem. 2025, 108, 129–137. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, Z.-Y.; Zheng, H.-Y.; Xu, K.-D.; Hu, Z.; Lei, Y. Enhanced Photothermal Catalytic Performance of Dry Reforming of Methane over Ni/Mesoporous TiO2 Composite Catalyst. Chem. Eng. J. 2022, 429, 132507. [Google Scholar] [CrossRef]
- He, Z.; Gong, K.; Dai, Y.; Niu, Q.; Lin, T.; Zhong, L. Regulating Crystal Phase of TiO2 to Enhance Catalytic Activity of Ni/TiO2 for Solar−Driven Dry Reforming of Methane. J. Fuel Chem. Technol. 2024, 52, 1203–1213. [Google Scholar] [CrossRef]
- Belda−Marco, S.; Ayala, P.; Myakala, S.N.; Eder, D.; Lillo−Ródenas, M.Á.; Cherevan, A.; Román−Martínez, M.C. Production of H2 and Organic Acids by Cellulose Photo−Reforming with TiO2−Bimetallic (CuNi) Co−Catalysts: Metal Loading and Photodeposition Sequence Effects. Environ. Res. 2025, 271, 121141. [Google Scholar] [CrossRef]
- Muscetta, M.; Clarizia, L.; Race, M.; Andreozzi, R.; Marotta, R.; Di Somma, I. Visible−Light Driven Systems: Effect of the Parameters Affecting Hydrogen Production through Photoreforming of Organics in Presence of Cu2O/TiO2 Nanocomposite Photocatalyst. Appl. Sci. 2023, 13, 2337. [Google Scholar] [CrossRef]
- Umair, M.; Ruiz−Aguirre, A.; Berruti, I.; Rodríguez, S.M.; Palmisano, L.; Loddo, V.; Bellardita, M. Biomass Derivatives Photoreforming in Pilot Plant Scale to Obtain H2 under Green Conditions by Using Ball Milling Cu2O−TiO2 P25 Photocatalysts. Chem. Eng. J. 2025, 504, 158585. [Google Scholar] [CrossRef]
- Khan, A.; Le Pivert, M.; Ranjbari, A.; Dragoe, D.; Bahena−Uribe, D.; Colbeau−Justin, C.; Herrero, C.; Rutkowska−Zbik, D.; Deschamps, J.; Remita, H. Cu−Based MOF/TiO2 Composite Nanomaterials for Photocatalytic Hydrogen Generation and the Role of Copper. Adv. Funct. Mater. 2025, 2021. [Google Scholar] [CrossRef]
- Qian, H.; Yuan, B.; Liu, Y.; Wang, L.; Zhu, R.; Dong, P. Effect of Cu Valence States on Conduction Band Position and Reduction Selectivity of TiO2−Based Heterojunction Photocatalysts. iScience 2025, 28, 112697. [Google Scholar] [CrossRef]
- Belda−Marco, S.; Lillo−Ródenas, M.Á.; Román−Martínez, M.C. Optimization of Active Sites in TiO2−Cu Photocatalysts for H2 Generation via Cellulose Photo−Reforming. ChemCatChem 2025, 17, e202500250. [Google Scholar] [CrossRef]
- Liu, X.; Bao, C.; Zhu, Z.; Zheng, H.; Song, C.; Xu, Q. Thermo−Photo Synergic Effect on Methanol Steam Reforming over Mesoporous Cu/TiO2–CeO2 Catalysts. Int. J. Hydrogen Energy 2021, 46, 26741–26756. [Google Scholar] [CrossRef]
- Fan, H.; Su, J.; Zhao, E.; Zheng, Y.; Chen, Z. Photocatalytic Selective Oxidation of Glycerol to Formic Acid and Formaldehyde over Surface Cobalt−Doped Titanium Dioxide. J. Colloid Interface Sci. 2025, 684, 140–147. [Google Scholar] [CrossRef]
- Pei, M.; Zhang, M.; Tian, J.; Yang, Y.; Liu, E. Photocatalytic H2 Production over CoxNi0.85−xSe Decorated TiO2 S−Scheme Heterojunction. J. Alloys Compd. 2025, 1010, 177266. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Ghazali, A.A.; Mohd Yassin, M.Y.; Abdullah, S. Optimization of Hydrogen Production by Photocatalytic Steam Methane Reforming over Lanthanum Modified Titanium (IV) Oxide Using Response Surface Methodology. Int. J. Hydrogen Energy 2019, 44, 20700–20710. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Han, C.; Yan, M.; Ji, G.; Wang, W. Efficient Photo−Oxidation of C(Sp3)−H Bonds on Visible−Light−Responsive W−Doped TiO2 Nanocrystals Promoted by the Photochromic Effect. Inorg. Chem. Front. 2025. [Google Scholar] [CrossRef]
- Hu, Y.H.; Wang, J.H.; Chen, Y.; Tang, J.P.; Wang, Z.Y.; Yuan, Y.J. Solar Driven Conversion of Agricultural Biomass to H2 over Few−Layer MoS2 Modified Ultra−Small TiO2 Nanoparticle Photocatalysts. J. Mater. Chem. A 2025, 13, 13402–13409. [Google Scholar] [CrossRef]
- Falara, P.P.; Chatzikonstantinou, N.; Zourou, A.; Tsipas, P.; Sakellis, E.; Alexandratou, E.; Nasikas, N.K.; Kordatos, K.V.; Antoniadou, M. Optimizing Carbon Dot−TiO2 Nanohybrids for Enhanced Photocatalytic Hydrogen Evolution. Materials 2025, 18, 1023. [Google Scholar] [CrossRef] [PubMed]
- Udaibah, W.; Anggoro, D.D.; Prasetyaningrum, A.; Bafaqeer, A.; Amin, N.A.S. An Enhanced Production of Biomethane from Cellulose Photoreforming Driven by Titania Morphological Modification. React. Kinet. Mech. Catal. 2025, 138, 1–17. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, W.; Li, S.; Wang, S.; Fan, Y.; Wang, F.; Qiu, X.; Zhu, Y.; Zhang, Y.; Long, C.; et al. Elevating Photooxidation of Methane to Formaldehyde via TiO2 Crystal Phase Engineering. J. Am. Chem. Soc. 2022, 144, 15977–15987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J.; Che, L.; Shi, L. Crystal Phase Engineering of TiO2 for Efficient Photocatalytic Non−Oxidative Methane Coupling. Chem. Eur. J. 2025, 31, e202500216. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Q.; Wang, W.; Wang, K. Study on the Synergism of Steam Reforming and Photocatalysis for the Degradation of Toluene as a Tar Model Compound under Microwave−Metal Discharges. Energy 2018, 155, 815–823. [Google Scholar] [CrossRef]
- Aldeen, E.M.S.; Jalil, A.A.; Mim, R.S.; Alhebshi, A.; Hassan, N.S.; Saravanan, R. Altered Zirconium Dioxide Based Photocatalyst for Enhancement of Organic Pollutants Degradation: A Review. Chemosphere 2022, 304, 135349. [Google Scholar] [CrossRef]
- Rani, V.; Sharma, A.; Kumar, A.; Singh, P.; Thakur, S.; Singh, A.; Van Le, Q.; Nguyen, V.H.; Raizada, P. ZrO2−Based Photocatalysts for Wastewater Treatment: From Novel Modification Strategies to Mechanistic Insights. Catalysts 2022, 12, 1418. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Cao, D.; Liu, Q.; Cai, W. Photothermal Dry Reforming of Ethanol over Rod−like Ni/ZrO2 Catalysts. Mol. Catal. 2024, 564, 114308. [Google Scholar] [CrossRef]
- Du, Z.; Pan, F.; Yang, X.; Fang, L.; Gang, Y.; Fang, S.; Li, T.; Hu, Y.H.; Li, Y. Efficient Photothermochemical Dry Reforming of Methane over Ni Supported on ZrO2 with CeO2 Incorporation. Catal. Today 2023, 409, 31–41. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, X.; Li, T.; Li, D.; Jiang, Z.; Xu, D.; Patel, B.; Guo, Y. Ce Doping Boosted Photothermal Synergistic Catalytic Reforming of CH4 and CO2 into Syngas over Ni/ZrO2 at Medium−Low Temperature. Int. J. Hydrogen Energy 2024, 53, 1433–1444. [Google Scholar] [CrossRef]
- Tengfei, L.; Cheng, J.; Li, D.; Xu, D.; Patel, B.; Guo, Y. Syngas Production from Photothermal Synergistic Dry Reforming of CH4 over a Core−Shell Structured Ni/CeO2–ZrO2@SiO2 Catalyst. Int. J. Hydrogen Energy 2024, 67, 1194–1205. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Li, D.; Li, T.; Guo, Y. Tailored Interfaces Promoting Photo−Activation of Molecular Enclosed CO2 on MOF−Derived Ni/CeZrO2 for Photothermal Synergistic Dry Reforming of Methane. Chem. Eng. J. 2025, 512, 162499. [Google Scholar] [CrossRef]
- Meng, L.; Jia, Y.; Wu, S. Efficient Photothermal Catalytic Methane Dry Reforming over Rich Oxygen Vacancy Catalysts. ChemComm 2025, 61, 2301–2304. [Google Scholar] [CrossRef]
- García−Mendoza, C.; Oros−Ruiz, S.; Ramírez−Rave, S.; Morales−Mendoza, G.; López, R.; Gómez, R. Synthesis of Bi2S3 Nanorods Supported on ZrO2 Semiconductor as an Efficient Photocatalyst for Hydrogen Production under UV and Visible Light. J. Chem. Technol. Biotechnol. 2017, 92, 1503–1510. [Google Scholar] [CrossRef]
- Yu, X.; Yang, L.; Xuan, Y.; Liu, X.L.; Zhang, K. Solar−Driven Low−Temperature Reforming of Methanol into Hydrogen via Synergetic Photo− and Thermocatalysis. Nano Energy 2021, 84, 105953. [Google Scholar] [CrossRef]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Pisanu, A.; Malavasi, L.; Profumo, A. Improved Photocatalytic H2 Production Assisted by Aqueous Glucose Biomass by Oxidized G−C3N4. Int. J. Hydrogen Energy 2018, 43, 14925–14933. [Google Scholar] [CrossRef]
- García−López, E.I.; Palmisano, L.; Marcì, G. Overview on Photoreforming of Biomass Aqueous Solutions to Generate H2 in the Presence of G−C3N4−Based Materials. ChemEngineering 2023, 7, 11. [Google Scholar] [CrossRef]
- Zhu, G.; Shu, Y.; Zhou, M. Photocatalytic Conversion of Biomass over Modified Graphitic Carbon Nitride Catalysts for Environmental Sustainability—A Review. Glob. Environ. Sci. 2025, 1, 19–35. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, L.; Peng, J.; Gong, W.; Sun, W. Advance in the Modification of G−C3N4−Based Composite for Photocatalytic H2 Production. Carbon Lett. 2025, 35, 417–440. [Google Scholar] [CrossRef]
- Speltini, A.; Gualco, F.; Maraschi, F.; Sturini, M.; Dondi, D.; Malavasi, L.; Profumo, A. Photocatalytic Hydrogen Evolution Assisted by Aqueous (Waste)Biomass under Simulated Solar Light: Oxidized g−C3N4 vs. P25 Titanium Dioxide. Int. J. Hydrogen Energy 2019, 44, 4072–4078. [Google Scholar] [CrossRef]
- Bianchini, P.; Profumo, A.; Cerri, L.; Tedesco, C.; Malavasi, L.; Speltini, A. Exploiting Rice Industry Wastewater for More Sustainable Sunlight−Driven Photocatalytic Hydrogen Production Using a Graphitic Carbon Nitride Polymorph. RSC Sustain. 2025, 3, 1149–1156. [Google Scholar] [CrossRef]
- Lazaar, N.; Wu, S.; Qin, S.; Hamrouni, A.; Bikash Sarma, B.; Doronkin, D.E.; Denisov, N.; Lachheb, H.; Schmuki, P. Single−Atom Catalysts on C3N4: Minimizing Single Atom Pt Loading for Maximized Photocatalytic Hydrogen Production Efficiency. Angew. Chem. Int. Ed. 2025, 64, e202416453. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhang, X.; Lin, X.; Liu, E.; Wang, L.; Shi, W.; Duan, X. Ultrathin 2D G−C3N4 Nanosheets for Visible−Light Photocatalytic Reforming of Cellulose into H2 under Neutral Conditions. J. Chem. Technol. Biotechnol. 2022, 97, 1717–1725. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, M.; Yang, J.; Wang, Y.; Liu, B. Fabrication of SnS2/C3N5 Heterojunction Photocatalyst for Highly Efficient Hydrogen Production and Organic Pollutant Degradation. J. Fuel Chem. Technol. 2025, 53, 336–347. [Google Scholar] [CrossRef]
- Bu, F.; Yuan, R.; Zhang, Z.; Wang, J.; Liu, J.; Yong, Y.C. Viologen Doping Induced Charge Storage in Carbon Nitride for Enhanced Photocatalytic Hydrogen Production. Inorg. Chem. Front. 2024, 12, 801–811. [Google Scholar] [CrossRef]
- Li, L.; Shi, X.; Liu, L.; Tu, Y.; Liu, Y.; Zhang, Y.; Yang, H.B.; Dou, S.; Liu, B. Modulation of Single−Iron−Atom Coordination Environment toward Three−Electron Oxygen Reduction for Photocatalytic CH4 Conversion to CH3OH. Small 2025, 21, e2500835. [Google Scholar] [CrossRef]
- Tahir, M.; Ali Khan, A.; Bafaqeer, A.; Kumar, N.; Siraj, M.; Fatehmulla, A. Highly Stable Photocatalytic Dry and Bi−Reforming of Methane with the Role of a Hole Scavenger for Syngas Production over a Defective Co−Doped g−C3N4 Nanotexture. Catalysts 2023, 13, 1140. [Google Scholar] [CrossRef]
- Ali Khan, A.; Tahir, M.; Khan, N. Process Optimization and Kinetic Study for Solar−Driven Photocatalytic Methane Bi−Reforming over TiO2/Ti3C2 Supported CoAlLa−LDH−g−C3N4 Dual S−Scheme Nanocomposite. Energy Convers. Manag. 2023, 286, 117021. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Constructing LaxCoyO3 Perovskite Anchored 3D G−C3N4 Hollow Tube Heterojunction with Proficient Interface Charge Separation for Stimulating Photocatalytic H2 Production. Energy Fuels 2021, 35, 9727–9746. [Google Scholar] [CrossRef]
- Cheng, T.; Zhu, J.; Chen, C.; Hu, Y.; Wu, L.; Zhang, M.; Cui, L.; Dai, Y.; Zhang, X.; Tian, Y.; et al. Construction of Advanced S−Scheme Heterojunction Interface Composites of Bimetallic Phosphate MnMgPO4 with C3N4 Surface with Remarkable Performance in Photocatalytic Hydrogen Production and Pollutant Degradation. Coatings 2025, 15, 103. [Google Scholar] [CrossRef]
- Mohanty, C.; Samal, A.; Kumar, J.; Behera, A.K.; Das, R.; Das, N. Design and First−Principles Investigation of Step−Scheme (S−Scheme) g−C3N4/α−MnO2 Nanojunction for Polystyrene Photoreforming into Value−Added Chemicals and Hydrogen. Int. J. Hydrogen Energy 2025, 120, 628–641. [Google Scholar] [CrossRef]
- Ikreedeegh, R.R.; Tahir, M. Facile Fabrication of Well−Designed 2D/2D Porous g−C3N4–GO Nanocomposite for Photocatalytic Methane Reforming (DRM) with CO2 towards Enhanced Syngas Production under Visible Light. Fuel 2021, 305, 121558. [Google Scholar] [CrossRef]
- Xie, Z.K.; Jia, Y.J.; Huang, Y.Y.; Xu, D.B.; Wu, X.J.; Chen, M.; Shi, W.D. Near−Infrared Light−Driven Photocatalytic Reforming Lignocellulose into H2 and Chemicals over Heterogeneous Carbon Nitride. ACS Catal. 2023, 13, 13768–13776. [Google Scholar] [CrossRef]
- Chung, W.C.; Tsao, I.Y.; Chang, M.B. Novel Plasma Photocatalysis Process for Syngas Generation via Dry Reforming of Methane. Energy Convers. Manag. 2018, 164, 417–428. [Google Scholar] [CrossRef]
- Chung, W.C.; Lee, Y.E.; Chang, M.B. Syngas Production via Plasma Photocatalytic Reforming of Methane with Carbon Dioxide. Int. J. Hydrogen Energy 2019, 44, 19153–19161. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Sannino, D.; Puga, F.; Navío, J.A.; Hidalgo, M.C. LaFeO3 Modified with Ni for Hydrogen Evolution via Photocatalytic Glucose Reforming in Liquid Phase. Catalysts 2021, 11, 1558. [Google Scholar] [CrossRef]
- Dong, X.; Zhu, X.; Lin, F.; Yan, B.; Li, J.; Chen, G. UV–Vis−Enhanced Photothermal Catalytic Reforming of Biomass Model Tar via LaMnxNi1−xO3 Perovskite Catalyst. Chem. Eng. J. 2023, 457, 141112. [Google Scholar] [CrossRef]
- Tavasoli, A.; Gouda, A.; Zahringer, T.; Li, Y.F.; Quaid, H.; Viasus Perez, C.J.; Song, R.; Sain, M.; Ozin, G. Enhanced Hybrid Photocatalytic Dry Reforming Using a Phosphated Ni−CeO2 Nanorod Heterostructure. Nat. Commun. 2023, 14, 1435. [Google Scholar] [CrossRef]
- Balsamo, S.A.; La Greca, E.; Calà Pizzapilo, M.; Sciré, S.; Fiorenza, R. CeO2−RGO Composites for Photocatalytic H2 Evolution by Glycerol Photoreforming. Materials 2023, 16, 747. [Google Scholar] [CrossRef]
- Iannaco, M.C.; Mottola, S.; Vaiano, V.; Iervolino, G.; De Marco, I. CeO2−CuO Composites Prepared via Supercritical Antisolvent Precipitation for Photocatalytic Hydrogen Production from Lactic Acid Aqueous Solution. J. CO2 Util. 2024, 85, 102878. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Lv, X.; Chen, J.; Jia, H. Maximum Photoresponse in Enhancing Photothermocatalytic Toluene Oxidation: Modulation of Cu+−Ov−Ce3+ and Surface Lattice Oxygen via Cu/CeO2 Shape Effect. Appl. Catal. B Environ. 2025, 371, 125253. [Google Scholar] [CrossRef]
- Aoun, N.; García−López, E.I.; Boucheloukh, H.; Boulekroune, M.; Sehili, T.; Marcì, G. SrNiO3 Perovskite/CeO2 Composites as Heterogeneous Photocatalysts for the 2−Propanol Oxidation in Gas–Solid Regime. J. Photochem. Photobiol. A Chem. 2025, 458, 115930. [Google Scholar] [CrossRef]
- Li, A.; Ma, J.; Hong, M.; Sun, R. Enhanced CeO2 Oxygen Defects Decorated with AgInS2 Quantum Dots Form an S−Scheme Heterojunction for Efficient Photocatalytic Selective Oxidation of Xylose. Appl. Catal. B Environ. 2024, 348, 123834. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Yin, Z.; Zhang, M.; Yu, G.; Luo, Q.; Chen, H.; Tang, B.; Li, K.; Zhang, Z.; et al. Non−Noble CeO2/CdS/NiS Heterojunction for Photoredox−Catalyzed Plastic Waste Selective Synthesis and Hydrogen Production from Natural Seawater. Fuel 2025, 385, 134143. [Google Scholar] [CrossRef]
- Li, T.; Guo, X.; Zhang, L.; Yan, T.; Jin, Z. 2D CoP Supported 0D WO3 Constructed S−Scheme for Efficient Photocatalytic Hydrogen Evolution. Int. J. Hydrogen Energy 2021, 46, 20560–20572. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, M.; Wang, Z.; Chen, X.; Dai, W.; Fu, X. Visible Photocatalytic Hydrogen Production from CH3OH over CuO/WO3: The Effect of Electron Transfer Behavior of the Adsorbed CH3OH. Chem. Eng. J. 2023, 459, 141616. [Google Scholar] [CrossRef]
- Alaoui, C.; Karmaoui, M.; Bekka, A.; Edelmannova, M.F.; Gallardo, J.J.; Navas, J.; Touati, W.; Kadi Allah, I.; Figueiredo, B.; Labrincha, J.A.; et al. TiO2/WO3/Graphene for Photocatalytic H2 Generation and Benzene Removal: Widely Employed Still an Ambiguous System. J. Photochem. Photobiol. A Chem. 2023, 445, 115020. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Al−Hajji, L.A.; Ismail, A.A. Construction of N−n Heterojunction Copper Manganese Spinel/Mesoporous WO3 Photocatalyst for Efficient H2 Evolution Rate from Aqueous Glycerol. Renew. Energy 2024, 228, 120649. [Google Scholar] [CrossRef]
- Ye, X.; Dong, Y.; Zhang, Z.; Zeng, W.; Zhu, B.; Zhang, T.; Gao, Z.; Dai, A.; Guan, X. Syngas Production by Photoreforming of Formic Acid with 2D VxW1−xN1.5 Solid Solution as an Efficient Cocatalyst. Front. Energy 2024, 18, 640–649. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, J.; Li, Y.; Cao, H. Highly Efficient UV−Visible−Infrared Light−Driven Photothermocatalytic Steam Biomass Reforming to H2 on Ni Nanoparticles Loaded on Mesoporous Silica. Energy Environ. Sci. 2022, 15, 3041–3050. [Google Scholar] [CrossRef]
- Zhong, M.; Li, Y.; Wu, J.; Hu, Q.; Hu, Y.; Du, Q.; Ji, C.; Cao, H.; Ji, L. Light Promotion and Different Reaction Pathways Enhance Photothermocatalytic Syngas Production for Cellulose Steam Reforming over Ni/θ−Al2O3. Adv. Funct. Mater 2024, 34, 2405453. [Google Scholar] [CrossRef]
- Levenspiel, O. Chapter 19—The Packed Bed Catalytic Reactor. In Chemical Reaction Engineering; Levenspiel, O., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1998; Volume 1, ISBN 978-0-471-25424-9. [Google Scholar]
- Liu, L.; Li, Y.; He, J.; Wang, Q.; Deng, J.; Chen, X.; Yu, C. Progress on Gas−Solid Phase Photoreactor and Its Application in CO2 Reduction. Green Chem. Eng. 2024, 5, 290–306. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.; Park, H.-J.; Park, S.; Kim, J.Y.; Jeong, Y.W.; Yang, H.H.; Choi, Y.; Yeom, M.; Song, D.; et al. Practical Scale Evaluation of a Photocatalytic Air Purifier Equipped with a Titania−Zeolite Composite Bead Filter for VOC Removal and Viral Inactivation. Environ. Res. 2022, 204, 112036. [Google Scholar] [CrossRef]
- Nguyen, T.-V.; Wu, J.C.S. Photoreduction of CO2 to Fuels under Sunlight Using Optical−Fiber Reactor. Sol. Energy Mater. Sol. Cells 2008, 92, 864–872. [Google Scholar] [CrossRef]
- Tong, K.; Chen, L.; Yang, L.; Du, X.; Yang, Y. Energy Transport of Photocatalytic Carbon Dioxide Reduction in Optical Fiber Honeycomb Reactor Coupled with Trough Concentrated Solar Power. Catalysts 2021, 11, 829. [Google Scholar] [CrossRef]
- Kunii, D.; Levenspiel, O. Chapter 1—Introduction. In Fluidization Engineering; Butterworth−Heinemann; Elsevier: Newton, MA, USA, 1991; Volume 1, pp. 1–13. [Google Scholar]
- Lee, J.K.; Park, D. Hydrogen Production from Fluidized Bed Steam Reforming of Hydrocarbons. Korean J. Chem. Eng. 1998, 15, 658–662. [Google Scholar] [CrossRef]
- Zambrano, D.; Soler, J.; Herguido, J.; Menéndez, M. Conventional and Improved Fluidized Bed Reactors for Dry Reforming of Methane: Mathematical Models. Chem. Eng. J. 2020, 393, 124775. [Google Scholar] [CrossRef]
- Zheng, H.; Jiang, X.; Gao, Y.; Tong, A.; Zeng, L. Chemical Looping Reforming: Process Fundamentals and Oxygen Carriers. Discov. Chem. Eng. 2022, 2, 5. [Google Scholar] [CrossRef]
- Gusmão, C.; Palharim, P.H.; Diniz, L.A.; de Assis, G.C.; de Carvalho e Souza, T.; Ramos, B.; Teixeira, A.C.S.C. Advances in Fluidized Bed Photocatalysis: Bridging Gaps, Standardizing Metrics, and Shaping Sustainable Solutions for Environmental Challenges. Ind. Eng. Chem. Res. 2024, 63, 14967–14982. [Google Scholar] [CrossRef]
- Rincón, G.J.; La Motta, E.J. A Fluidized−Bed Reactor for the Photocatalytic Mineralization of Phenol on TiO2−Coated Silica Gel. Heliyon 2019, 5, e01966. [Google Scholar] [CrossRef]
- Prieto, O.; Fermoso, J.; Irusta, R. Photocatalytic Degradation of Toluene in Air Using a Fluidized Bed Photoreactor. Int. J. Photoenergy 2007, 2007, 032859. [Google Scholar] [CrossRef]
- Vaiano, V.; Sannino, D.; Ciambelli, P. Steam Reduction of CO2 in a Photocatalytic Fluidized Bed Reactor. Chem. Eng. Trans. 2015, 43, 1003–1008. [Google Scholar] [CrossRef]
- Costa, A.L.; Ortelli, S.; Blosi, M.; Albonetti, S.; Vaccari, A.; Dondi, M. TiO2 Based Photocatalytic Coatings: From Nanostructure to Functional Properties. Chem. Eng. J. 2013, 225, 880–886. [Google Scholar] [CrossRef]
- Kunii, D. Levenspiel Octave Chapter 3—Fluidization and Mapping of Regimes. In Fluidization Engineering; Butterworth−Heinemann; Elsevier: Newton, MA, USA, 1991; Volume 1, pp. 61–94. ISBN 978-0-08-050664-7. [Google Scholar]
- Ammendola, P.; Chirone, R. Aeration and Mixing Behaviours of Nano−Sized Powders under Sound Vibration. Powder Technol. 2010, 201, 49–56. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, W.; Cao, Y.; Gai, H.; Zhu, Q. Diverse Gas−Solid Magnetized Fluidized Beds with Different Magnetic Fields. Powder Technol. 2024, 433, 119241. [Google Scholar] [CrossRef]
- Ruoppolo, G.; Miccio, F.; Chirone, R. Fluidized Bed Cogasification of Wood and Coal Adopting Primary Catalytic Method for Tar Abatement. Energy Fuels 2010, 24, 2034–2041. [Google Scholar] [CrossRef]
- Xiong, H.; Dong, Y.; Liu, D.; Long, R.; Kong, T.; Xiong, Y. Recent Advances in Porous Materials for Photocatalytic CO2 Reduction. J. Phys. Chem. Lett. 2022, 13, 1272–1282. [Google Scholar] [CrossRef]
- Miccio, F.; Boscherini, M.; Minelli, M.; Doghieri, F. A Comparative Investigation on Mixing and Dispersion in Fluidized Bed Equipped with a Conical Distributor for Catalytic Processes. J. Ind. Eng. Chem. 2025; preprint. [Google Scholar] [CrossRef]
- Rizzuti, L.; Yue, P.L. The Measurement of Light Transmission through an Irradiated Fluidised Bed. Chem. Eng. Sci. 1983, 38, 1241–1249. [Google Scholar] [CrossRef]
- Braham, R.J.; Harris, A.T. A Complete Multi−Scale Simulation of Light Absorption within a Fluidized Bed Photoreactor Using Integrated Particle, Fluid and Photon Behaviour Models. Phys. Chem. Chem. Phys. 2013, 15, 12373. [Google Scholar] [CrossRef]
- Svoboda, K.; Kalisz, S.; Miccio, F.; Wieczorek, K.; Pohořelý, M. Simplified Modeling of Circulating Flow of Solids between a Fluidized Bed and a Vertical Pneumatic Transport Tube Reactor Connected by Orifices. Powder Technol. 2009, 192, 65–73. [Google Scholar] [CrossRef]
- Lolli, A.; Blosi, M.; Ortelli, S.; Costa, A.L.; Zanoni, I.; Bonincontro, D.; Carella, F.; Albonetti, S. Innovative Synthesis of Nanostructured Composite Materials by a Spray−Freeze Drying Process: Efficient Catalysts and Photocatalysts Preparation. Catal. Today 2019, 334, 193–202. [Google Scholar] [CrossRef]
- Tran, A.; Aguirre, A.; Durand, H.; Crose, M.; Christofides, P.D. CFD Modeling of a Industrial−Scale Steam Methane Reforming Furnace. Chem. Eng. Sci. 2017, 171, 576–598. [Google Scholar] [CrossRef]
- Garland, J.R.; van Helmond, P. Steam Reformer Tube Assembly and Method of Assembling or Retrofitting Same. U.S. Patent 8,776,344B2, 15 July 2014. [Google Scholar]
- Fonseca, J.D.; Camargo, M.; Commenge, J.-M.; Falk, L.; Gil, I.D. Trends in Design of Distributed Energy Systems Using Hydrogen as Energy Vector: A Systematic Literature Review. Int. J. Hydrogen Energy 2019, 44, 9486–9504. [Google Scholar] [CrossRef]
- Jaison, A.; Mohan, A.; Lee, Y.-C. Machine Learning−Enhanced Photocatalysis for Environmental Sustainability: Integration and Applications. Mater. Sci. Eng. R Rep. 2024, 161, 100880. [Google Scholar] [CrossRef]
- Mowbray, M.; Vallerio, M.; Perez−Galvan, C.; Zhang, D.; Del Rio Chanona, A.; Navarro−Brull, F.J. Industrial Data Science—A Review of Machine Learning Applications for Chemical and Process Industries. React. Chem. Eng. 2022, 7, 1471–1509. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).