Photoactive Nanomaterials Containing Metals for Biomedical Applications: A Comprehensive Literature Review
Abstract
1. Introduction
2. Physical Principles and Photothermal Mechanisms
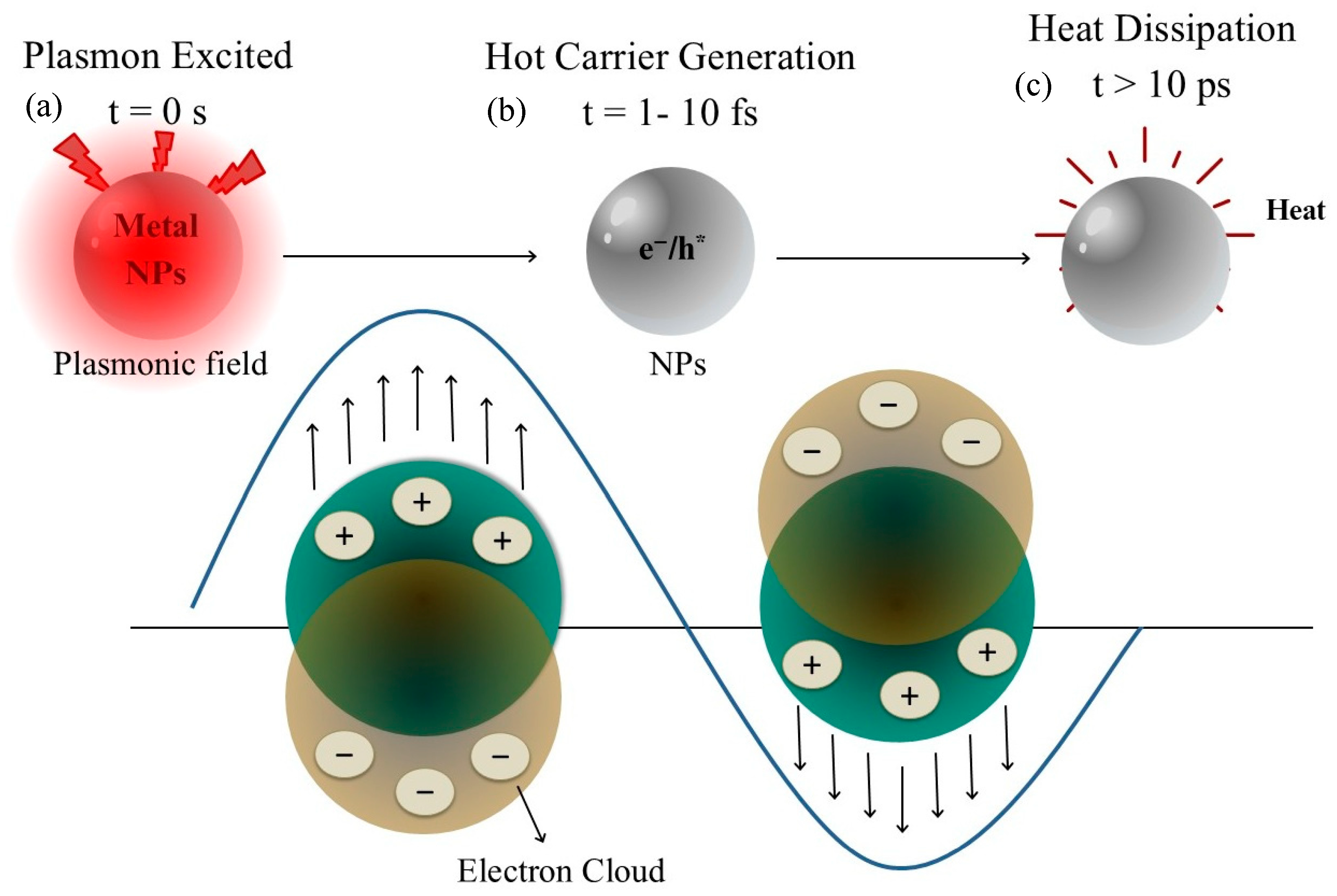
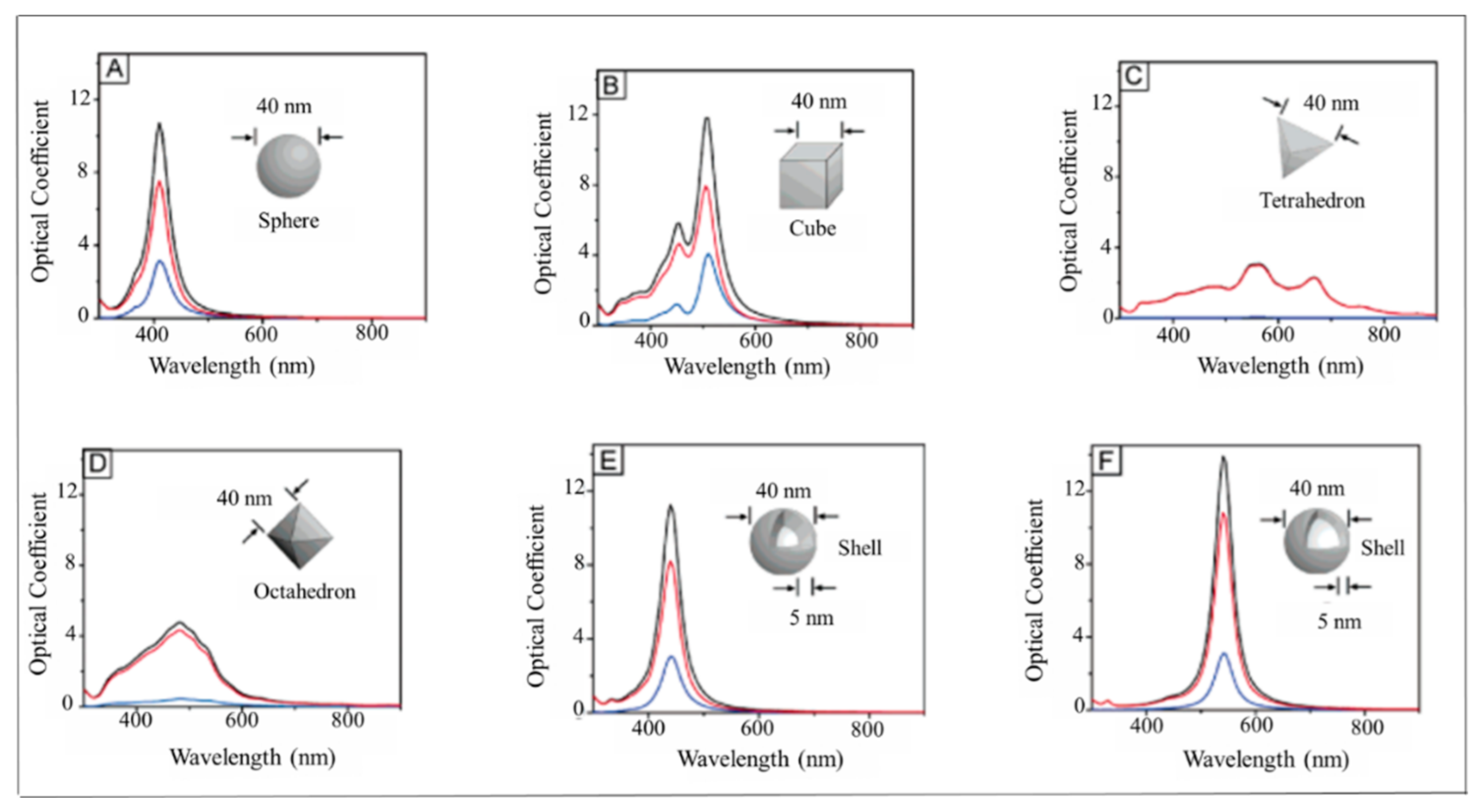
2.1. Localized Surface Plasmon Resonance (LSPR) Fundamentals
2.2. Light-to-Heat Conversion and Tumor Ablation
2.3. Mechanism of Cell Death Induced by Photothermal Therapy
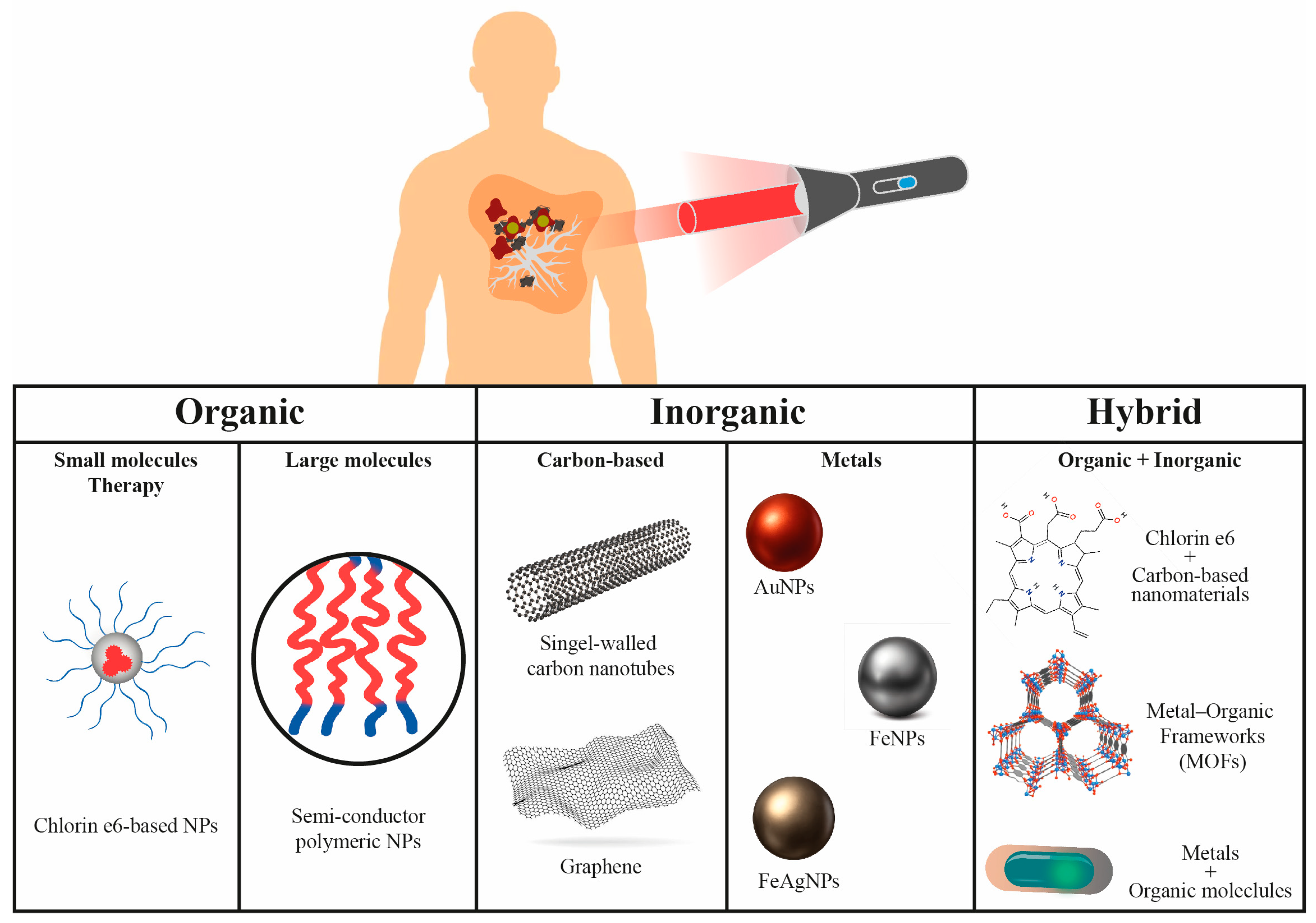
3. Types of Photoactive Nanomaterials
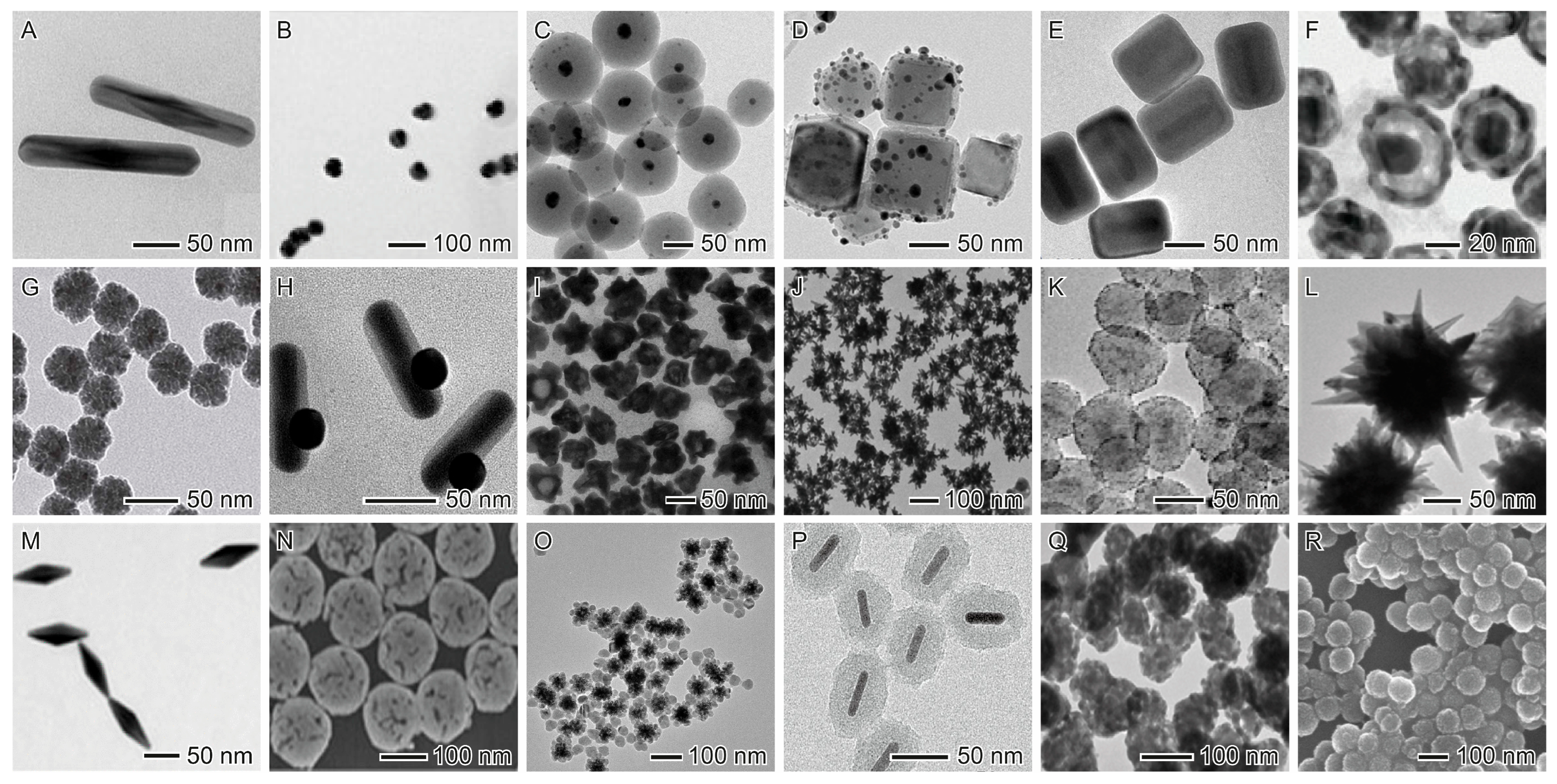
3.1. Noble Metal Nanoparticles (Au and Ag)
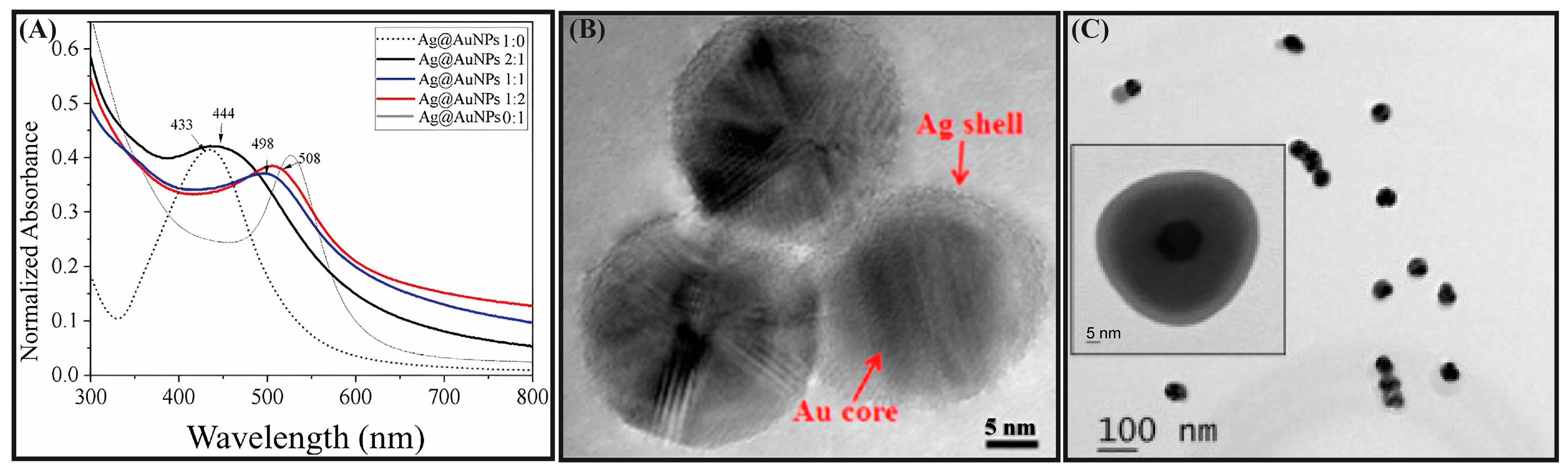
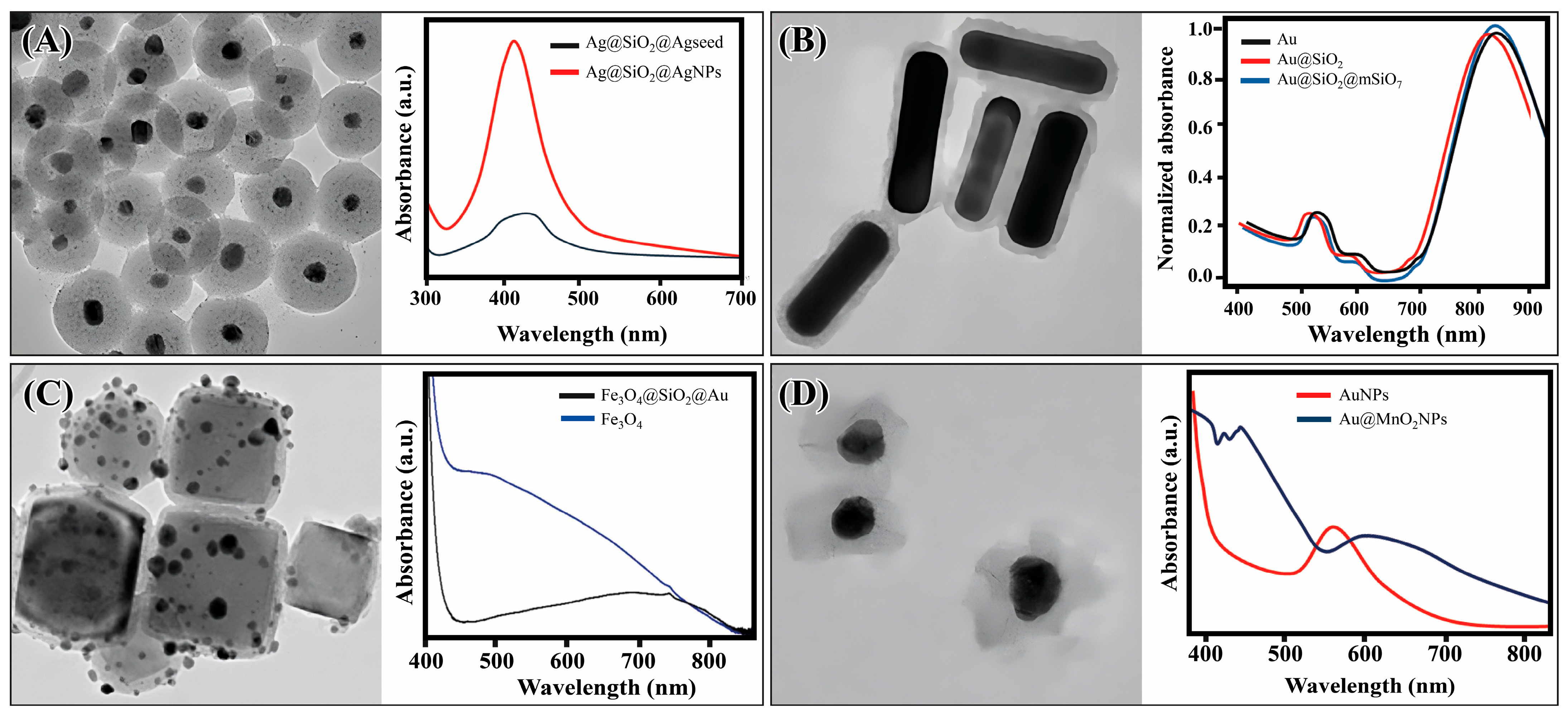
3.2. Core@Shell Nanostructures
3.3. Metal–Organic Frameworks (MOFs)
3.4. Carbon-Based Nanomaterials (CNTs, Graphene)
3.5. Other Inorganic and Hybrid Nanomaterials
4. Functional Modifications and Dye Encapsulation
4.1. Incorporation of NIR-Sensitive Dyes (e.g., ICG, IR780)
4.2. Polymer Coatings and Surface Functionalization
4.3. Optimization of Size, Shape, and Surface Charge
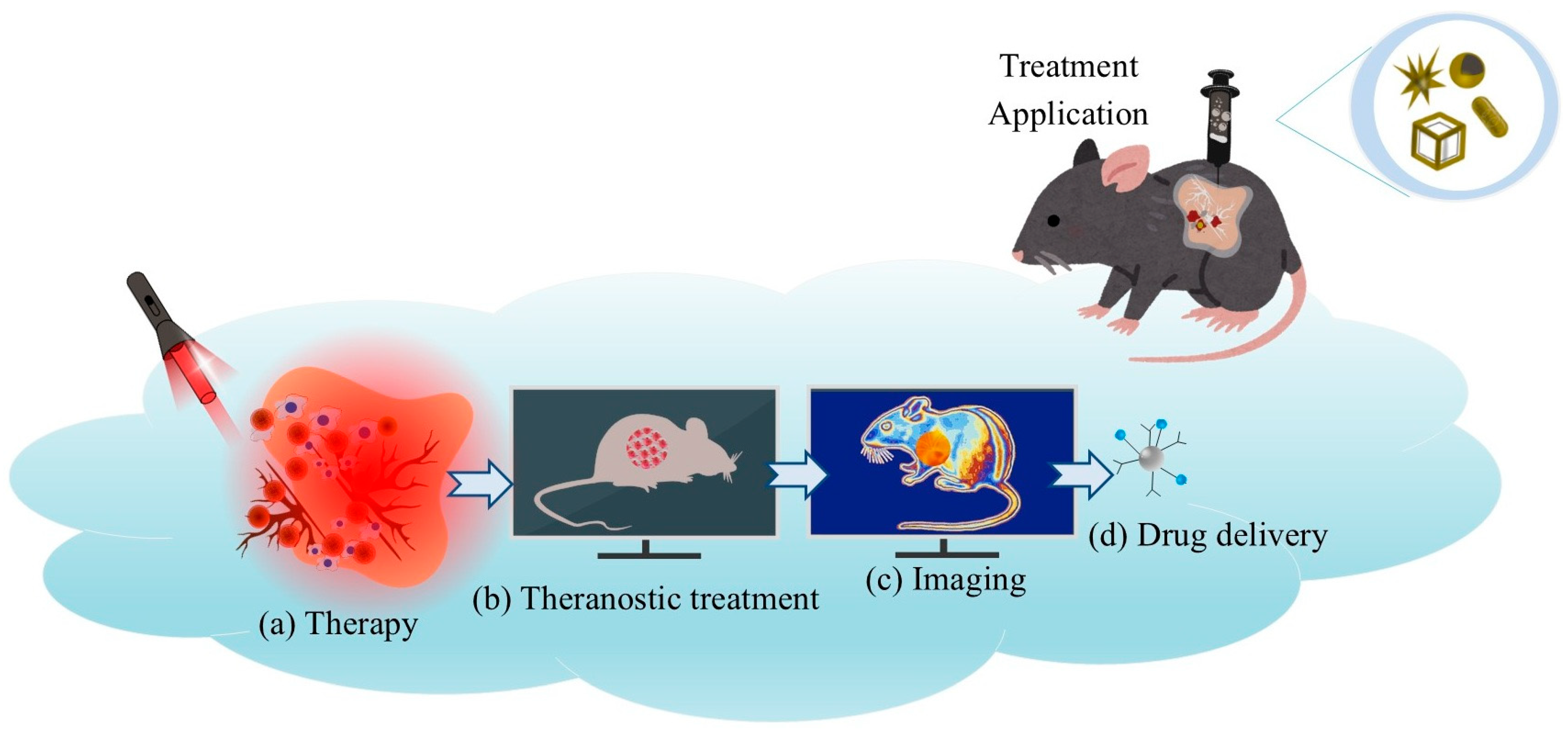
5. Biomedical Applications
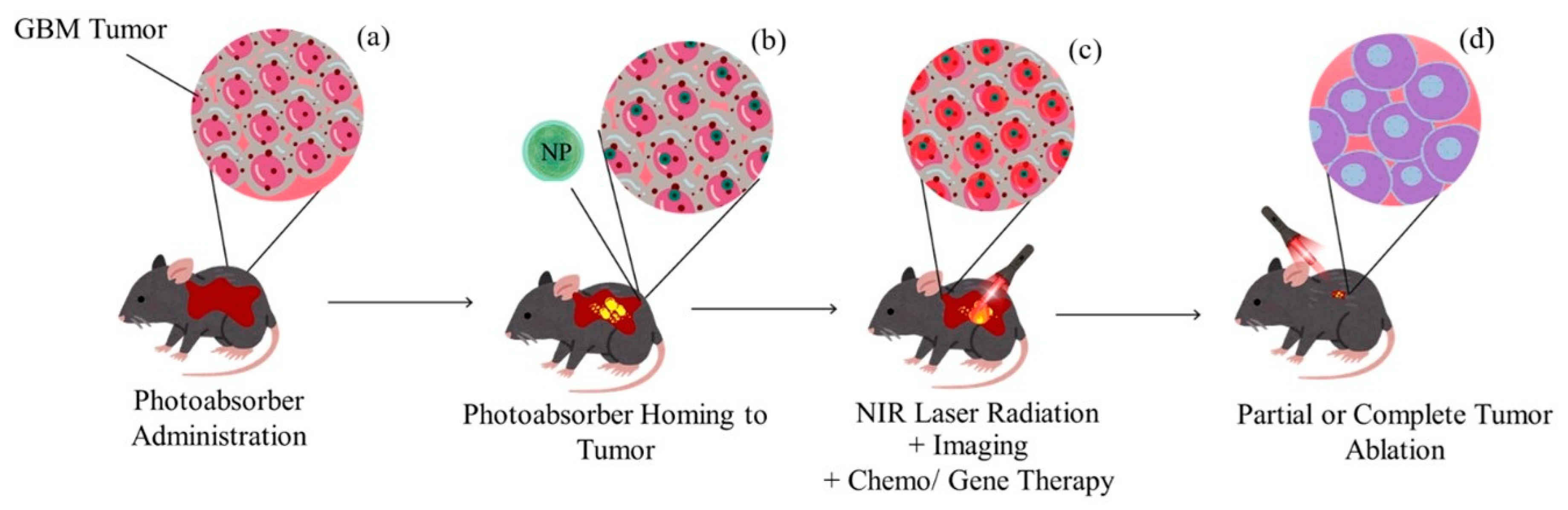
5.1. Photothermal Therapy (PTT) in Cancer Treatment
5.2. Photodynamic Therapy (PDT): Mechanisms and Limitations
5.3. Combined Therapies: PTT with Chemotherapy, Immunotherapy
5.4. Theranostics: Integration of Diagnosis and Therapy
6. Optical Detections and Tumor Theranostics
6.1. Optical and Spectroscopic Diagnostic Techniques
6.2. Fluorescence-Guided Imaging and PTT (NIR-II)
6.3. Nanoplatforms for Dual Imaging and Treatment
7. Computational Tools for Nanomaterials Desing
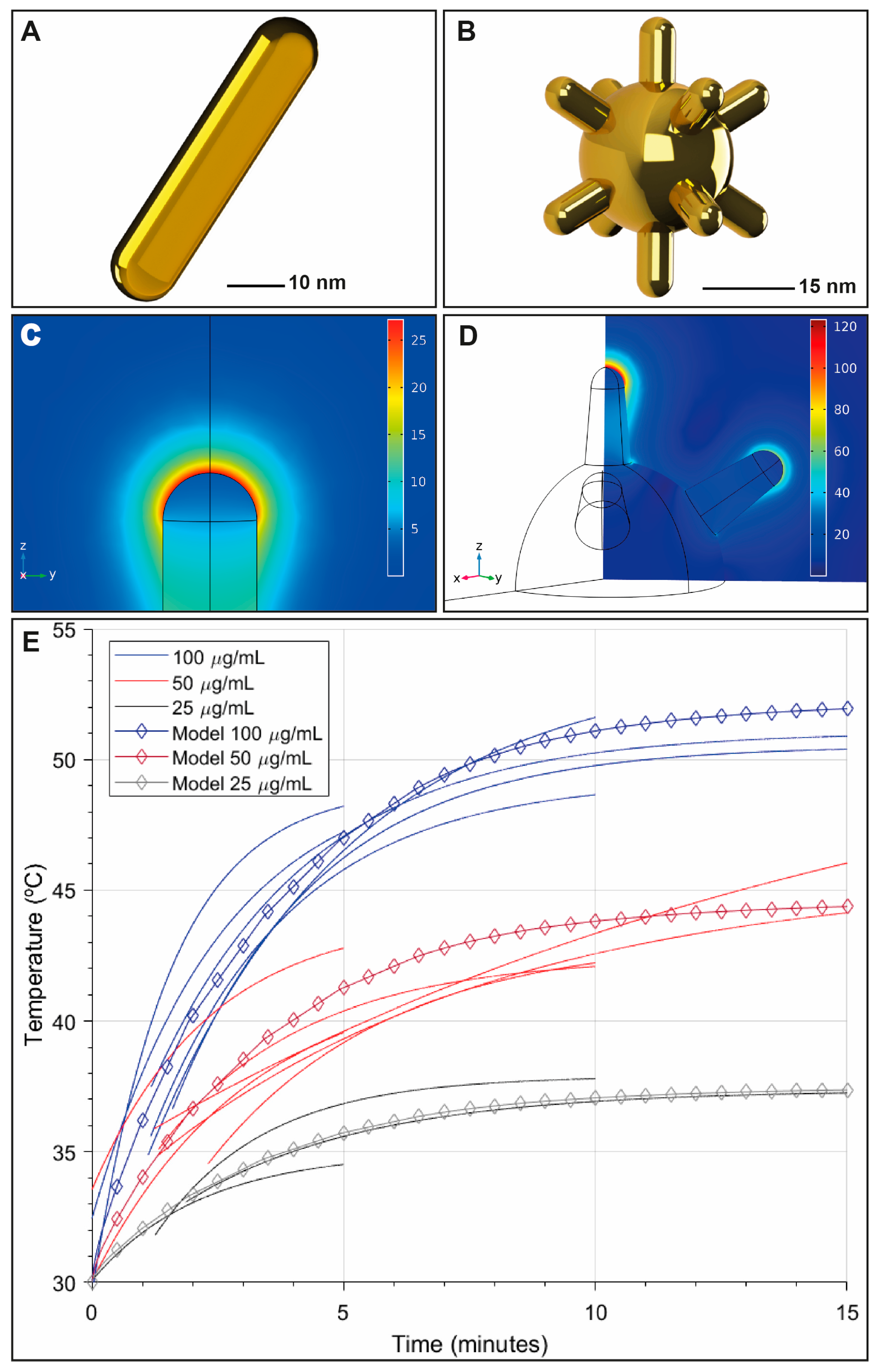
7.1. Numerical Simulations (FEM, FDTD)
7.2. Light–Matter Interaction Modeling
7.3. Experimental Validation and Multiphysics Integration
7.4. AI and Multiscale Modeling for Predictive Desing
8. Comparative Analysis of Photoactive Nanomaterials
8.1. Key Optical Properties and Action Mechanisms
8.2. Advantages and Drawbacks of Each Nanomaterial
8.3. Clinical Potential and Translational Readiness
9. Advances and Future Perspectives
9.1. Addressing Toxicity and Stability Challenges
9.2. Optimization for Tumor Targeting, Excretion, and Selectivity
9.3. Ethical Considerations and Scalability
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 188Re | Rhenium-188 |
| AIEgens | Aggregation-induced emission luminogens |
| AI | Artificial intelligence |
| AO | Acridine orange |
| Au-MSS/AuMSS | Gold core–mesoporous silica shell nanorods |
| AuNP(s) | Gold nanoparticle(s) |
| AuNR(s) | Gold nanorod(s) |
| BBTD | Benzobisthiadiazole |
| BSA | Bovine serum albumin |
| CT | Computed tomography |
| CTAB | Cetyltrimethylammonium bromide |
| DDA | Discrete dipole approximation |
| DOX | Doxorubicin |
| EPR | Enhanced permeability and retention |
| FA | Folic acid |
| FDTD | Finite-difference time-domain |
| FEM | Finite element method |
| FTIR | Fourier transform infrared spectroscopy |
| AuBPs | Gold bipyramids |
| AuNCs | Gold nanocages |
| AuNSts | Gold nanostars |
| H2O2 | Hydrogen peroxide |
| IR-780 | Near-infrared dye IR-780 |
| IR-825 | Near-infrared dye IR-825 |
| LSPR | Localized surface plasmon resonance |
| ML | Machine learning |
| MRI | Magnetic resonance imaging |
| MSN(s) | Mesoporous silica nanoparticle(s) |
| NIR | Near-infrared |
| NIR-I | First near-infrared window |
| NIR-II | Second near-infrared window |
| NP(s) | Nanoparticle(s) |
| PDA | Polydopamine |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PEI | Polyethylenimine |
| PIT | Photoimmunotherapy |
| PLGA | Poly(lactic-co-glycolic acid) |
| PTA(s) | Photothermal agent(s) |
| PTT | Photothermal therapy |
| PVP | Polyvinylpyrrolidone |
| RBC | Red blood cell |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscopy |
| SWCNT(s) | Single-walled carbon nanotube(s) |
| TEM | Transmission electron microscopy |
| TESPA | 3-Triethoxysilylpropylamine |
| TPGS | D-α-Tocopheryl polyethylene glycol 1000 succinate |
| UCNP(s) | Upconversion nanoparticle(s) |
| UV–Vis | Ultraviolet–visible spectroscopy |
References
- Ripoll-Viladomiu, I.; Prina-Mello, A.; Movia, D.; Marignol, L. Extracellular Vesicles and the “Six Rs” in Radiotherapy. Cancer Treat. Rev. 2024, 129, 102799. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Tafech, A.; Stéphanou, A. On the Importance of Acidity in Cancer Cells and Therapy. Biology 2024, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Cao, Y.; He, L.; Ding, S.; Bian, X.W.; Tian, G. Metal-Ligand Coordination Nanomaterials for Radiotherapy: Emerging Synergistic Cancer Therapy. J. Mater. Chem. B 2021, 9, 208–227. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Fan, G.; Zhang, B.; Ma, G.; Xiao, H.; Wang, L. Daptomycin-Biomineralized Silver Nanoparticles for Enhanced Photothermal Therapy with Anti-Tumor Effect. Polymers 2022, 14, 2787. [Google Scholar] [CrossRef]
- Zou, J.; Li, L.; Yang, Z.; Chen, X. Phototherapy Meets Immunotherapy: A Win-Win Strategy to Fight against Cancer. Nanophotonics 2021, 10, 3229–3245. [Google Scholar] [CrossRef]
- Amaral, M.N.; Kumar, P.; Faísca, P.; Ferreira, H.A.; Coelho, J.M.P.; Gaspar, M.M.; Reis, C.P. Gold Nanoparticle-Mediated Photothermal Therapy: Expanding the Frontiers of Cancer Treatment and Theragnostics. Biomed. Pharmacother. 2025, 190, 118399. [Google Scholar] [CrossRef]
- Atkinson, R.L.; Zhang, M.; Diagaradjane, P.; Peddibhotla, S.; Contreras, A.; Hilsenbeck, S.G.; Woodward, W.A.; Krishnan, S.; Chang, J.C.; Rosen, J.M. Thermal Enhancement with Optically Activated Gold Nanoshells Sensitizes Breast Cancer Stem Cells to Radiation Therapy. Sci. Transl. Med. 2010, 2, 55ra79. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Wu, Y.; Tang, Y.; Xiao, H.; Chen, K.; Han, T.; Fang, N.; Wu, R.; El-Sayed, M.A. Targeting Cancer Cell Integrins Using Gold Nanorods in Photothermal Therapy Inhibits Migration through Affecting Cytoskeletal Proteins. Proc. Natl. Acad. Sci. USA 2017, 114, E5655–E5663. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Ganjali, F.; Zarei-Shokat, S.; Dinmohammadi, R.; Asl, F.R.; Emami, A.; Mojtabapour, Z.S.; Rashvandi, Z.; Kashtiaray, A.; Jalali, F.; et al. Plasmonic Porous Micro- and Nano-Materials Based on Au/Ag Nanostructures Developed for Photothermal Cancer Therapy: Challenges in Clinicalization. Nanoscale Adv. 2023, 5, 6768–6786. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent Advances in Selective Photothermal Therapy of Tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Hu, Y.; Zhao, Y.; Tang, K.; Zhang, Z.; Liu, Z.; Wang, Y.; Guo, H.; Miao, Y.; Du, H.; et al. Nanomaterials for Photothermal Cancer Therapy. RSC Adv. 2023, 13, 14443–14460. [Google Scholar] [CrossRef] [PubMed]
- Frusca, V.; Cavallini, C.; Zamborlin, A.; Drava, G.; Barone, V.; Gherardini, L.; Chiariello, M.; Armanetti, P.; Ermini, M.L.; Menichetti, L.; et al. In Vivo Combined Photoacoustic Imaging and Photothermal Treatment of HPV-Negative Head and Neck Carcinoma with NIR-Responsive Non-Persistent Plasmon Nano-Architectures (Adv. Therap. 10/2024). Adv. Ther. 2024, 7, 2470023. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Martínez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for Photothermal Therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Frey, W.; Kim, S.; Homan, K.; Kruizinga, P.; Sokolov, K.; Emelianov, S. Enhanced Thermal Stability of Silica-Coated Gold Nanorods for Photoacoustic Imaging and Image-Guided Therapy. Opt. Express 2010, 18, 8867. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Tian, Q.; Hu, J.; Zhu, Y.; Zou, R.; Chen, Z.; Yang, S.; Li, R.; Su, Q.; Han, Y.; Liu, X. Sub-10 Nm Fe3O4 @Cu2–x S Core–Shell Nanoparticles for Dual-Modal Imaging and Photothermal Therapy. J. Am. Chem. Soc. 2013, 135, 8571–8577. [Google Scholar] [CrossRef]
- Chang, C.; Wang, C.; Zhang, C.; Li, L.; Zhang, Q.; Huang, Q. Albumin-Encapsulated Platinum Nanoparticles for Targeted Photothermal Treatment of Glioma. J. Biomed. Nanotechnol. 2019, 15, 1744–1753. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Chen, M.; Chen, X.; Zheng, N. Palladium-Based Nanomaterials for Cancer Imaging and Therapy. Theranostics 2020, 10, 10057–10074. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Abed, A.; Derakhshan, M.; Karimi, M.; Shirazinia, M.; Mahjoubin-Tehran, M.; Homayonfal, M.; Hamblin, M.R.; Mirzaei, S.A.; Soleimanpour, H.; Dehghani, S.; et al. Platinum Nanoparticles in Biomedicine: Preparation, Anti-Cancer Activity, and Drug Delivery Vehicles. Front. Pharmacol. 2022, 13, 797804. [Google Scholar] [CrossRef]
- Geonmonond, R.S.; da Silva, A.G.M.; Rodrigues, T.S.; de Freitas, I.C.; Ando, R.A.; Alves, T.V.; Camargo, P.H.C. Addressing the Effects of Size-Dependent Absorption, Scattering, and Near-Field Enhancements in Plasmonic Catalysis. ChemCatChem 2018, 10, 3447–3452. [Google Scholar] [CrossRef]
- da Silva, A.G.M.; Rodrigues, T.S.; Wang, J.; Camargo, P.H.C. Plasmonic Catalysis with Designer Nanoparticles. Chem. Commun. 2022, 58, 2055–2074. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.G.M.; Rodrigues, T.S.; Wang, J.; Yamada, L.K.; Alves, T.V.; Ornellas, F.R.; Ando, R.A.; Camargo, P.H.C. The Fault in Their Shapes: Investigating the Surface-Plasmon-Resonance-Mediated Catalytic Activities of Silver Quasi-Spheres, Cubes, Triangular Prisms, and Wires. Langmuir 2015, 31, 10272–10278. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.G.M.; Rodrigues, T.S.; Macedo, A.; Da Silva, R.T.P.; Camargo, P.H.C. An Undergraduate Level Experiment on the Synthesis of Au Nanoparticles and Their Size-Dependent Optical and Catalytic Properties. Quim. Nova 2014, 37, 1716–1720. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Fernandes, N.; de Melo-Diogo, D.; Correia, I.J.; Moreira, A.F. Cell-Derived Vesicles for Nanoparticles’ Coating: Biomimetic Approaches for Enhanced Blood Circulation and Cancer Therapy. Adv. Healthc. Mater. 2022, 11, e2201214. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem.-Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Thakor, A.S.; Jokerst, J.; Zavaleta, C.; Massoud, T.F.; Gambhir, S.S. Gold Nanoparticles: A Revival in Precious Metal Administration to Patients. Nano Lett. 2011, 11, 4029–4036. [Google Scholar] [CrossRef]
- Ma, X.; Tian, Y.; Yang, R.; Wang, H.; Allahou, L.W.; Chang, J.; Williams, G.; Knowles, J.C.; Poma, A. Nanotechnology in Healthcare, and Its Safety and Environmental Risks. J. Nanobiotechnol. 2024, 22, 715. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Kumar, V.; Sharma, N.; John, N.; Umesh, M.; Kumar Dasarahally Huligowda, L.; Kaur, K.; Utreja, D. Recent Approaches in Nanotoxicity Assessment for Drug Delivery Applications: Challenges and Prospects. Med. Drug Discov. 2025, 25, 100204. [Google Scholar] [CrossRef]
- Moustaoui, H.; Saber, J.; Djeddi, I.; Liu, Q.; Diallo, A.T.; Spadavecchia, J.; Lamy De La Chapelle, M.; Djaker, N. Shape and Size Effect on Photothermal Heat Elevation of Gold Nanoparticles: Absorption Coefficient Experimental Measurement of Spherical and Urchin-Shaped Gold Nanoparticles. J. Phys. Chem. C 2019, 123, 17548–17554. [Google Scholar] [CrossRef]
- Taylor, M.L.; Wilson, R.E.; Amrhein, K.D.; Huang, X. Gold Nanorod-Assisted Photothermal Therapy and Improvement Strategies. Bioengineering 2022, 9, 200. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Yu, B.; Cong, H. Emerging Advanced Nanomaterials for Cancer Photothermal Therapy. Rev. Adv. Mater. Sci. 2018, 53, 131–146. [Google Scholar] [CrossRef]
- He, Z.; Zhang, C.Y.; Lei, Y.; Song, G.; Yao, Y. Plasmonic Nanomaterials: A Versatile Phototheranostic Platform of Cancers. Mater. Today 2023, 62, 168–189. [Google Scholar] [CrossRef]
- Gonçalves, A.S.C.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Strategies to Improve the Photothermal Capacity of Gold-Based Nanomedicines. Acta Biomater. 2020, 116, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Tan, H.; Peng, Q.; Chen, H.; Ma, H.; Wang, L.; Peng, A.; Shi, Q.; Cai, X.; Huang, H. Achieving Efficient NIR-II Type-I Photosensitizers for Photodynamic/Photothermal Therapy upon Regulating Chalcogen Elements. Adv. Mater. 2022, 34, 2108146. [Google Scholar] [CrossRef]
- Shiju, E.; Abhijith, T.B.; Narayana Rao, D.; Chandrasekharan, K. Nonlinear Optical Behavior of Au@Ag Core-Shell Nanostructures. J. Mol. Liq. 2021, 333, 115935. [Google Scholar] [CrossRef]
- Manivannan, K.; Cheng, C.C.; Anbazhagan, R.; Tsai, H.C.; Chen, J.K. Fabrication of Silver Seeds and Nanoparticle on Core-Shell Ag@SiO2 Nanohybrids for Combined Photothermal Therapy and Bioimaging. J. Colloid Interface Sci. 2019, 537, 604–614. [Google Scholar] [CrossRef]
- Duan, Q.; Wang, J.; Zhang, B.; Wang, X.; Xue, J.; Zhang, W.; Sang, S. Polydopamine Coated Au-Pt Nanorods: Enhanced Photothermal Properties and Efficient Reactive Oxygen Scavengers. Colloids Surf. B Biointerfaces 2022, 210, 112247. [Google Scholar] [CrossRef]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef]
- Xu, C.; Pu, K. Second Near-Infrared Photothermal Materials for Combinational Nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef]
- Luo, H.; Gao, S. Recent Advances in Fluorescence Imaging-Guided Photothermal Therapy and Photodynamic Therapy for Cancer: From near-Infrared-I to near-Infrared-II. J. Control. Release 2023, 362, 425–445. [Google Scholar] [CrossRef]
- Jia, K.; Wang, P.; Yuan, L.; Zhou, X.; Chen, W.; Liu, X. Facile Synthesis of Luminescent Silver Nanoparticles and Fluorescence Interactions with Blue-Emitting Polyarylene Ether Nitrile. J. Mater. Chem. C Mater. 2015, 3, 3522–3529. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Leporatti, S. Silver Nanoparticles: Synthetic Routes, in Vitro Toxicity and Theranostic Applications for Cancer Disease. Nanomaterials 2018, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.N.; Nunes, D.; Fortunato, E.; Martins, R.; Rodrigues, C.; Faísca, P.; Ferreira, H.A.; Coelho, J.M.P.; Gaspar, M.M.; Reis, C.P. Gold Nanoparticles for Photothermal Therapy–Influence of Experimental Conditions on the Properties of Resulting AuNPs. J. Drug Deliv. Sci. Technol. 2024, 101, 106215. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Malviya, R. Understanding and Advancement in Gold Nanoparticle Targeted Photothermal Therapy of Cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188532. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Maheshwari, N.; Soni, N.; Singhai, N.J.; Sharma, M.C.; Prajapati, B.; Yele, S.; Maheshwari, R. Metallic Nanoparticles in Cancer: Types, Green Synthesis, Applications, Tumor Microenvironment and Toxicity Considerations. J. Drug Deliv. Sci. Technol. 2024, 92, 105307. [Google Scholar] [CrossRef]
- Cheng, Y.; Bao, D.; Chen, X.; Wu, Y.; Wei, Y.; Wu, Z.; Li, F.; Piao, J.G. Microwave-Triggered/HSP-Targeted Gold Nano-System for Triple-Negative Breast Cancer Photothermal Therapy. Int. J. Pharm. 2021, 593, 120162. [Google Scholar] [CrossRef]
- Cui, X.; Li, M.; Tong, L.; Li, M.; Tang, X.; Han, X. High Aspect Ratio Plasmonic Au/Ag Nanorods-Mediated NIR-II Photothermally Enhanced Nanozyme Catalytic Cancer Therapy. Colloids Surf. B Biointerfaces 2023, 223, 113168. [Google Scholar] [CrossRef]
- Kulpa-Greszta, M.; Tomaszewska, A.; Dziedzic, A.; Rzeszutek, I.; Pązik, R. Heat Generation on Fe3O4@SiO2@Au Core-Shell Structures Using the Synergy of an Alternating Magnetic Field and NIR Laser Light within Ist Biological Optical Window. Mater. Today Commun. 2023, 35, 105513. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, H.; Liu, J.; Liu, L.; Li, Y.; Xue, L.; Wu, Y.; Yang, R. Engineering of Multifunctional Carbon Nanodots-Decorated Plasmonic Au@Ag Nanoenzymes for Photoelectrochemical Biosensing of MicroRNA-155. Sens. Actuators B Chem. 2022, 360, 131653. [Google Scholar] [CrossRef]
- de Lima, S.L.S.; Miguel, V.M.; Rosado, T.F.; Petri, M.V.; Gardener, J.; Avillez, R.; Rodrigues, T.S.; de Torresi, S.I.C.; Solorzano, G.; da Silva, A.G.M. Sized-Controlled Pd Nanoflowers by a Non-Classical Growth Mechanism Combining the LaMer and DLVO Theories and Their Catalytic Activities. Mater. Today Commun. 2022, 33, 104397. [Google Scholar] [CrossRef]
- Dou, J.; Chen, B.; Liu, G.; Dong, X.; Yu, W.; Wang, J.; Zhang, Y.; Li, Z.; Zhu, J. Decorating Rare-Earth Fluoride Upconversion Nanoparticles on AuNRs@Ag Core–Shell Structure for NIR Light-Mediated Photothermal Therapy and Bioimaging. J. Rare Earths 2022, 40, 193–200. [Google Scholar] [CrossRef]
- Fu, Y.-F.; Zhu, J.; Li, X.; Weng, G.-J.; Li, J.-J.; Zhao, J.-W. Au-Ag Nano-Garlands as a Versatile SERS Substrate: Two-Step Synthesis Realizes the Growth of Petal-Shaped Branches on Hollow Au-Ag Nanoshells. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134541. [Google Scholar] [CrossRef]
- Holca, A.; Cucuiet, V.; Astilean, S.; Lamy De La Chapelle, M.; Focsan, M. Recent Advances in Gold Nanoparticle-Graphene Hybrid Nanoplatforms with Visible to near-Infrared Response for Photodynamic and Photothermal Therapy and Bioimaging. RSC Adv. 2025, 15, 11902–11922. [Google Scholar] [CrossRef]
- Xu, J.; Evers, K.; Lin, Y.C.; Tabish, T.A.; Zhang, Q.; Chung, R.J.; Ryan, M.P.; Xie, F. Near Infrared Window Active Magnetic Core Spiky Gold Nanostars for Dual Mode Imaging and Photothermal Therapy. Mater. Today Adv. 2025, 25, 100555. [Google Scholar] [CrossRef]
- Wang, H.; Han, J.; Lu, W.; Zhang, J.; Li, J.; Jiang, L. Facile Preparation of Gold Nanocages and Hollow Gold Nanospheres via Solvent Thermal Treatment and Their Surface Plasmon Resonance and Photothermal Properties. J. Colloid. Interface Sci. 2015, 440, 236–244. [Google Scholar] [CrossRef]
- Liang, J.; Yang, Y.; Luo, Y.; Huang, L.; Zhao, S. Mesoporous Platinum@copper Selenide-Based NIR-II Photothermal Agents with Photothermal Conversion Efficiency over 80% for Photoacoustic Imaging and Targeted Cancer Therapy. Chem. Eng. J. 2024, 496, 154172. [Google Scholar] [CrossRef]
- Zheng, D.; Zhao, X.; Wang, Y.; Dai, X.; Guo, K.; Xu, C.; Zhao, N.; Xu, F.J. Facile Construction of Polycation/Au@CuS Nanohybrids for Synergistic Gene/Photothermal Therapy. Chem. Eng. J. 2024, 486, 150271. [Google Scholar] [CrossRef]
- Chu, Z.; Tian, T.; Tao, Z.; Yang, J.; Chen, B.; Chen, H.; Wang, W.; Yin, P.; Xia, X.; Wang, H.; et al. Upconversion Nanoparticles@AgBiS2 Core-Shell Nanoparticles with Cancer-Cell-Specific Cytotoxicity for Combined Photothermal and Photodynamic Therapy of Cancers. Bioact. Mater. 2022, 17, 71–80. [Google Scholar] [CrossRef]
- Tran, V.A.; Thi Vo, T.T.; Lee, S.W.; Tạ, N.D.; Vien, V.; Doan, V.D.; Thanh, N.C.; Le, V.T. Modulating Drug Retention and Release via Surface Anchors Using Hyperthermia and Photothermal Effects of Fe3O4-SiO2 Core-Shell Mesoporous Nanoparticles. J. Drug Deliv. Sci. Technol. 2024, 102, 106396. [Google Scholar] [CrossRef]
- Wiley, B.J.; Im, S.H.; Li, Z.Y.; McLellan, J.; Siekkinen, A.; Xia, Y. Maneuvering the Surface Plasmon Resonance of Silver Nanostructures through Shape-Controlled Synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; da Silva, A.G.M.; de Moura, A.B.L.; Freitas, I.G.; Camargo, P.H.C. Rational Design of Plasmonic Catalysts: Matching the Surface Plasmon Resonance with Lamp Emission Spectra for Improved Performance in AgAu Nanorings. RSC Adv. 2016, 6, 62286–62290. [Google Scholar] [CrossRef]
- Pakravan, A.; Salehi, R.; Mahkam, M. Comparison Study on the Effect of Gold Nanoparticles Shape in the Forms of Star, Hallow, Cage, Rods, and Si-Au and Fe-Au Core-Shell on Photothermal Cancer Treatment. Photodiagn. Photodyn. Ther. 2021, 33, 102144. [Google Scholar] [CrossRef]
- Yang, W.; Xia, B.; Wang, L.; Ma, S.; Liang, H.; Wang, D.; Huang, J. Shape Effects of Gold Nanoparticles in Photothermal Cancer Therapy. Mater. Today Sustain. 2021, 13, 100078. [Google Scholar] [CrossRef]
- Chen, X.; Wong, S.T.C. Cancer Theranostics: An Introduction. In Cancer Theranostics; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Gamal, H.; Tawfik, W.; El-Sayyad, H.I.; Emam, A.N.; Fahmy, H.M.; El-Ghaweet, H.A. A New Vision of Photothermal Therapy Assisted with Gold Nanorods for the Treatment of Mammary Cancers in Adult Female Rats. Nanoscale Adv. 2023, 6, 170–187. [Google Scholar] [CrossRef]
- Chegel, V.; Rachkov, O.; Lopatynskyi, A.; Ishihara, S.; Yanchuk, I.; Nemoto, Y.; Hill, J.P.; Ariga, K. Gold Nanoparticles Aggregation: Drastic Effect of Cooperative Functionalities in a Single Molecular Conjugate. J. Phys. Chem. C 2012, 116, 2683–2690. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Lim, S.C.; Lim, K.; Lee, E.S.; Oh, K.T.; Choi, H.G.; Youn, Y.S. Gold Nanocluster-Loaded Hybrid Albumin Nanoparticles with Fluorescence-Based Optical Visualization and Photothermal Conversion for Tumor Detection/Ablation. J. Control. Release 2019, 304, 7–18. [Google Scholar] [CrossRef]
- Iodice, C.; Cervadoro, A.; Palange, A.L.; Key, J.; Aryal, S.; Ramirez, M.R.; Mattu, C.; Ciardelli, G.; O’Neill, B.E.; Decuzzi, P. Enhancing Photothermal Cancer Therapy by Clustering Gold Nanoparticles into Spherical Polymeric Nanoconstructs. Opt. Lasers Eng. 2016, 76, 74–81. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Deng, Y.; Zeng, M.; Tang, Y.; Zhu, W.H.; Cheng, Y. Combination of Active Targeting, Enzyme-Triggered Release and Fluorescent Dye into Gold Nanoclusters for Endomicroscopy-Guided Photothermal/Photodynamic Therapy to Pancreatic Ductal Adenocarcinoma. Biomaterials 2017, 139, 30–38. [Google Scholar] [CrossRef]
- da Silva, A.G.M.; Rodrigues, T.S.; Haigh, S.J.; Camargo, P.H.C. Galvanic Replacement Reaction: Recent Developments for Engineering Metal Nanostructures towards Catalytic Applications. Chem. Commun. 2017, 53, 7135–7148. [Google Scholar] [CrossRef]
- Panfilova, E.; Shirokov, A.; Khlebtsov, B.; Matora, L.; Khlebtsov, N. Multiplexed Dot Immunoassay Using Ag Nanocubes, Au/Ag Alloy Nanoparticles, and Au/Ag Nanocages. Nano Res. 2012, 5, 124–134. [Google Scholar] [CrossRef]
- Cheng, H.; Huo, D.; Zhu, C.; Shen, S.; Wang, W.; Li, H.; Zhu, Z.; Xia, Y. Combination Cancer Treatment through Photothermally Controlled Release of Selenous Acid from Gold Nanocages. Biomaterials 2018, 178, 517–526. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Rodrigues, T.S.; da Silva, A.G.M.; Wang, J. Controlled Synthesis: Nucleation and Growth in Solution. In Metallic Nanostructures: From Controlled Synthesis to Applications; Xiong, Y., Lu, X., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 49–74. ISBN 978-3-319-11304-3. [Google Scholar]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold Nanostars: Surfactant-Free Synthesis, 3D Modelling, and Two-Photon Photoluminescence Imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Wen, S.; Song, Y.; Tang, Y.; Zhu, X.; Shen, M.; Mignani, S.; Majoral, J.P.; Zhao, Q.; et al. Construction of Polydopamine-Coated Gold Nanostars for CT Imaging and Enhanced Photothermal Therapy of Tumors: An Innovative Theranostic Strategy. J. Mater. Chem. B 2016, 4, 4216–4226. [Google Scholar] [CrossRef]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single Continuous Wave Laser Induced Photodynamic/Plasmonic Photothermal Therapy Using Photosensitizer-Functionalized Gold Nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-Photothermal Therapy Combination Elicits Anti-Tumor Immunity against Advanced Metastatic Cancer. Nat. Commun. 2018, 9, 1074. [Google Scholar] [CrossRef]
- Qiu, J.; Xie, M.; Wu, T.; Qin, D.; Xia, Y. Gold Nanocages for Effective Photothermal Conversion and Related Applications. Chem. Sci. 2020, 11, 12955–12973. [Google Scholar] [CrossRef]
- da Silva, A.G.M.; Rodrigues, T.S.; Slater, T.J.A.; Lewis, E.A.; Alves, R.S.; Fajardo, H.V.; Balzer, R.; da Silva, A.H.M.; de Freitas, I.C.; Oliveira, D.C.; et al. Controlling Size, Morphology, and Surface Composition of AgAu Nanodendrites in 15 s for Improved Environmental Catalysis under Low Metal Loadings. ACS Appl. Mater. Interfaces 2015, 7, 25624–25632. [Google Scholar] [CrossRef]
- da Silva, A.G.M.; Rodrigues, T.S.; Correia, V.G.; Alves, T.V.; Alves, R.S.; Ando, R.A.; Ornellas, F.R.; Wang, J.; Andrade, L.H.; Camargo, P.H.C. Plasmonic Nanorattles as Next-Generation Catalysts for Surface Plasmon Resonance-Mediated Oxidations Promoted by Activated Oxygen. Angew. Chem. Int. Ed. 2016, 55, 7111–7115. [Google Scholar] [CrossRef]
- Da Silva, A.G.M.; Lewis, E.A.; Rodrigues, T.S.; Slater, T.J.A.; Alves, R.S.; Haigh, S.J.; Camargo, P.H.C. Surface Segregated AgAu Tadpole-Shaped Nanoparticles Synthesized Via a Single Step Combined Galvanic and Citrate Reduction Reaction. Chem. A Eur. J. 2015, 21, 12314–12320. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Shaktawat, S.; Singh, K.R.; Thapa, S.; Verma, R.; Singh, J.; Singh, R.P. Optical Characteristics and Biosensing Application of Core@shell Nanomaterials. Mater. Lett. X 2023, 17, 100187. [Google Scholar] [CrossRef]
- Silva-Silva, T.P.; Silva, A.A.; Oliveira, M.C.D.; Souza, P.R.; Silva-Filho, E.C.; Garcia, H.A.; Costa, J.C.S.; Santos, F.E.P. Biosynthesis of Ag@Au Bimetallic Nanoparticles from Hymenaea courbaril extract (Jatobá) and Nonlinear Optics Properties. J. Mol. Liq. 2023, 389, 122641. [Google Scholar] [CrossRef]
- Zhang, W.S.; Cao, J.T.; Dong, Y.X.; Wang, H.; Ma, S.H.; Liu, Y.M. Enhanced Chemiluminescence by Au-Ag Core-Shell Nanoparticles: A General and Practical Biosensing Platform for Tumor Marker Detection. J. Lumin. 2018, 201, 163–169. [Google Scholar] [CrossRef]
- Villalobos-Noriega, J.M.A.; Rodríguez-León, E.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Plascencia-Jatomea, M.; Martínez-Higuera, A.; Acuña-Campa, H.; García-Galaz, A.; Mora-Monroy, R.; Alvarez-Cirerol, F.J.; et al. Au@Ag Core@Shell Nanoparticles Synthesized with Rumex Hymenosepalus as Antimicrobial Agent. Nanoscale Res. Lett. 2021, 16, 118. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Cruz-Romero, M.; Collins, T.; Cummins, E.; Kerry, J.P.; Morris, M.A. Synthesis of Monodisperse Chitosan Nanoparticles. Food Hydrocoll. 2018, 83, 355–364. [Google Scholar] [CrossRef]
- Botha, T.L.; Elemike, E.E.; Horn, S.; Onwudiwe, D.C.; Giesy, J.P.; Wepener, V. Cytotoxicity of Ag, Au and Ag-Au Bimetallic Nanoparticles Prepared Using Golden Rod (Solidago canadensis) Plant Extract. Sci. Rep. 2019, 9, 4169. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Ecofriendly Synthesis of Silver and Gold Nanoparticles by Euphrasia Officinalis Leaf Extract and Its Biomedical Applications. Artif. Cells Nanomed. Biotechnol. 2018, 46. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.R.S.S.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green Synthesis of Gold and Silver Nanoparticles from Cannabis sativa (Industrial Hemp) and Their Capacity for Biofilm Inhibition. Int. J. Nanomed. 2018, 13, 1163–1170. [Google Scholar] [CrossRef]
- Vazquez-Prada, K.X.; Moonshi, S.S.; Xu, Z.P.; Ta, H.T. Photothermal Nanomaterials for Theranostics of Atherosclerosis and Thrombosis. Appl. Mater. Today 2023, 35, 101967. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Qian, Y.; Hu, L.; Fang, J.; Tong, W.; Nie, R.; Chen, Q.; Wang, H. Magnetic-Induced Graphene Quantum Dots for Imaging-Guided Photothermal Therapy in the Second near-Infrared Window. Biomaterials 2020, 232, 119700. [Google Scholar] [CrossRef]
- Kabtamu, D.M.; Wu, Y.N.; Li, F. Hierarchically Porous Metal–Organic Frameworks: Synthesis Strategies, Structure(s), and Emerging Applications in Decontamination. J. Hazard. Mater. 2020, 397, 122765. [Google Scholar] [CrossRef]
- Fernández-López, C.; Mateo-Mateo, C.; Álvarez-Puebla, R.A.; Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Highly Controlled Silica Coating of PEG-Capped Metal Nanoparticles and Preparation of SERS-Encoded Particles. Langmuir 2009, 25, 13894–13899. [Google Scholar] [CrossRef]
- Liu, Y.; Mo, F.; Hu, J.; Jiang, Q.; Wang, X.; Zou, Z.; Zhang, X.Z.; Pang, D.W.; Liu, X. Precision Photothermal Therapy and Photoacoustic Imaging Byin Situactivatable Thermoplasmonics. Chem. Sci. 2021, 12, 10097–10105. [Google Scholar] [CrossRef]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Dumych, T.; Bilyy, R.; Turcheniuk, V.; Bouckaert, J.; Vovk, V.; Chopyak, V.; Zaitsev, V.; Mariot, P.; Prevarskaya, N.; et al. Plasmonic Photothermal Cancer Therapy with Gold Nanorods/Reduced Graphene Oxide Core/Shell Nanocomposites. RSC Adv. 2016, 6, 1600–1610. [Google Scholar] [CrossRef]
- Qiu, Z.; Hu, J.; Li, Z.; Yang, X.; Hu, J.; You, Q.; Bai, S.; Mao, Y.; Hua, D.; Yin, J. Graphene Oxide-Based Nanocomposite Enabled Highly Efficient Targeted Synergistic Therapy for Colorectal Cancer. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124585. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.P.; Ye, W.Q.; Xu, Z.R. Photothermal Visual Sensing of Alkaline Phosphatase Based on the Etching of Au@MnO2 Core-Shell Nanoparticles. J. Colloid Interface Sci. 2023, 641, 568–576. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, S.; Yi, G.; Wang, J.; Wang, M.; Li, S.; Huang, Q. Construction of Methylene Blue Embedded Core–Shell-Satellite Structured Au@Ag Nanorods@SiO2@Au Nanocomposites for Synergistic Therapy of Cancer. Mater. Des. 2024, 237, 112622. [Google Scholar] [CrossRef]
- Bai, L.Y.; Yang, X.Q.; An, J.; Zhang, L.; Zhao, K.; Qin, M.Y.; Fang, B.Y.; Li, C.; Xuan, Y.; Zhang, X.S.; et al. Multifunctional Magnetic-Hollow Gold Nanospheres for Bimodal Cancer Cell Imaging and Photothermal Therapy. Nanotechnology 2015, 26, 315701. [Google Scholar] [CrossRef]
- Liang, C.; Song, X.; Chen, Q.; Liu, T.; Song, G.; Peng, R.; Liu, Z. Magnetic Field-Enhanced Photothermal Ablation of Tumor Sentinel Lymph Nodes to Inhibit Cancer Metastasis. Small 2015, 11, 4856–4863. [Google Scholar] [CrossRef]
- Zhu, D.; Zhu, X.H.; Ren, S.Z.; Lu, Y.D.; Zhu, H.L. Manganese Dioxide (MnO2) Based Nanomaterials for Cancer Therapies and Theranostics. J. Drug Target. 2021, 29, 911–924. [Google Scholar] [CrossRef]
- Wang, J.; Sui, L.; Huang, J.; Miao, L.; Nie, Y.; Wang, K.; Yang, Z.; Huang, Q.; Gong, X.; Nan, Y.; et al. MoS2-Based Nanocomposites for Cancer Diagnosis and Therapy. Bioact. Mater. 2021, 6, 4209–4242. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, H.; Deng, Y.; Jiang, A.; Bao, X.; Guo, M.; Li, Z.; Wu, M.; Ji, X.; Zeng, X.; et al. Dual-Response Oxygen-Generating MnO2 Nanoparticles with Polydopamine Modification for Combined Photothermal-Photodynamic Therapy. Chem. Eng. J. 2020, 389, 124494. [Google Scholar] [CrossRef]
- Liu, J.; Feng, L.; Wu, Y. Enzymatically Synthesised MnO2 nanoparticles for Efficient Near-Infrared Photothermal Therapy and Dual-Responsive Magnetic Resonance Imaging. Nanoscale 2021, 13, 11093–11103. [Google Scholar] [CrossRef]
- Shin, M.H.; Park, E.Y.; Han, S.; Jung, H.S.; Keum, D.H.; Lee, G.H.; Kim, T.; Kim, C.; Kim, K.S.; Yun, S.H.; et al. Multimodal Cancer Theranosis Using Hyaluronate-Conjugated Molybdenum Disulfide. Adv. Healthc. Mater. 2019, 8, e1801036. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H.; Liu, G.; Ma, L.; Wang, Z. The Controllable Growth of Ultrathin MnO2 on Polydopamine Nanospheres as a Single Nanoplatform for the MRI-Guided Synergistic Therapy of Tumors. J. Mater. Chem. B 2019, 7, 7152–7161. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, C.; Li, M.; Li, J.; Li, G.; Ma, D.; Li, Z.; Zou, D. Bottom-up Synthesis of Ultra-Small Molybdenum Disulfidepolyvinylpyrrolidone Nanosheets for Imaging-Guided Tumor Regression. Oncotarget 2017, 8, 106707. [Google Scholar] [CrossRef]
- Li, C.; Li, T.; Niu, K.; Xiao, Z.; Huang, J.; Pan, X.; Sun, Y.; Wang, Y.; Ma, D.; Xie, P.; et al. Mild Phototherapy Mediated by Manganese Dioxide-Loaded Mesoporous Polydopamine Enhances Immunotherapy against Colorectal Cancer. Biomater. Sci. 2022, 10, 3647–3656. [Google Scholar] [CrossRef]
- Slama, Y.; Arcambal, A.; Septembre-Malaterre, A.; Morel, A.L.; Pesnel, S.; Gasque, P. Evaluation of Core-Shell Fe3O4@Au Nanoparticles as Radioenhancer in A549 Cell Lung Cancer Model. Heliyon 2024, 10, e29297. [Google Scholar] [CrossRef]
- Leng, C.; Zhang, X.; Xu, F.; Yuan, Y.; Pei, H.; Sun, Z.; Li, L.; Bao, Z. Engineering Gold Nanorod–Copper Sulfide Heterostructures with Enhanced Photothermal Conversion Efficiency and Photostability. Small 2018, 14, e1703077. [Google Scholar] [CrossRef]
- Maji, S.K.; Yu, S.; Chung, K.; Sekkarapatti Ramasamy, M.; Lim, J.W.; Wang, J.; Lee, H.; Kim, D.H. Synergistic Nanozymetic Activity of Hybrid Gold Bipyramid-Molybdenum Disulfide Core@Shell Nanostructures for Two-Photon Imaging and Anticancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 42068–42076. [Google Scholar] [CrossRef]
- Frusca, V.; Cavallini, C.; Zamborlin, A.; Drava, G.; Barone, V.; Gherardini, L.; Chiariello, M.; Armanetti, P.; Ermini, M.L.; Menichetti, L.; et al. In Vivo Combined Photoacoustic Imaging and Photothermal Treatment of HPV-Negative Head and Neck Carcinoma with NIR-Responsive Non-Persistent Plasmon Nano-Architectures. Adv. Ther. 2024, 7, 2400110. [Google Scholar] [CrossRef]
- Farzam, O.R.; Mehran, N.; Bilan, F.; Aghajani, E.; Dabbaghipour, R.; Shahgoli, G.A.; Baradaran, B. Nanoparticles for Imaging-Guided Photothermal Therapy of Colorectal Cancer. Heliyon 2023, 9, e21334. [Google Scholar] [CrossRef]
- Xi, J.; Da, L.; Yang, C.; Chen, R.; Gao, L.; Fan, L.; Han, J. Mn2+-Coordinated PDA@DOX/PLGA Nanoparticles as a Smart Theranostic Agent for Synergistic Chemo-Photothermal Tumor Therapy. Int. J. Nanomed. 2017, 12, 3331–3345. [Google Scholar] [CrossRef]
- Mu, W.; Fang, W.; Yao, Y. Synthesis of Ag@Au Core–Shell NPs Loaded with Ciprofloxacin as Enhanced Antimicrobial Properties for the Treatment and Nursing Care of Escherichia coli Infection. Microb. Pathog. 2021, 150, 104619. [Google Scholar] [CrossRef]
- Li, D.; Zhao, J.; Ma, J.; Yang, H.; Zhang, X.; Cao, Y.; Liu, P. GMT8 Aptamer Conjugated PEGylated Ag@Au Core-Shell Nanoparticles as a Novel Radiosensitizer for Targeted Radiotherapy of Glioma. Colloids Surf. B Biointerfaces 2022, 211, 112330. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Tang, W.; Yang, W.; Liu, Y.; Li, R.; Liu, H. PEGylated Polypyrrole–Gold Nanocomplex as Enhanced Photothermal Agents against Tumor Cells. J. Mater. Sci. 2020, 55, 5587–5599. [Google Scholar] [CrossRef]
- Puleio, R.; Licciardi, M.; Varvarà, P.; Scialabba, C.; Cassata, G.; Cicero, L.; Cavallaro, G.; Giammona, G. Effect of Actively Targeted Copolymer Coating on Solid Tumors Eradication by Gold Nanorods-Induced Hyperthermia. Int. J. Pharm. 2020, 587, 119641. [Google Scholar] [CrossRef]
- Stern, J.M.; Kibanov Solomonov, V.V.; Sazykina, E.; Schwartz, J.A.; Gad, S.C.; Goodrich, G.P. Initial Evaluation of the Safety of Nanoshell-Directed Photothermal Therapy in the Treatment of Prostate Disease. Int. J. Toxicol. 2016, 35, 38–46. [Google Scholar] [CrossRef]
- Henderson, B.W.; Gollnick, S.O.; Snyder, J.W.; Busch, T.M.; Kousis, P.C.; Cheney, R.T.; Morgan, J. Choice of Oxygen-Conserving Treatment Regimen Determines the Inflammatory Response and Outcome of Photodynamic Therapy of Tumors. Cancer Res. 2004, 64, 2120–2126. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H. Oncologic Photodynamic Therapy Photosensitizers: A Clinical Review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Falk-Mahapatra, R.; Gollnick, S.O. Photodynamic Therapy and Immunity: An Update. Photochem. Photobiol. 2020, 96, 550–559. [Google Scholar] [CrossRef]
- Lim, C.K.; Heo, J.; Shin, S.; Jeong, K.; Seo, Y.H.; Jang, W.D.; Park, C.R.; Park, S.Y.; Kim, S.; Kwon, I.C. Nanophotosensitizers toward Advanced Photodynamic Therapy of Cancer. Cancer Lett. 2013, 334, 176–187. [Google Scholar] [CrossRef]
- Sternberg, E.D.; Dolphin, D.; Brückner, C. Porphyrin-Based Photosensitizers for Use in Photodynamic Therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar] [CrossRef]
- Mang, T.; Kost, J.; Sullivan, M.; Wilson, B.C. Autofluorescence and Photofrin-Induced Fluorescence Imaging and Spectroscopy in an Animal Model of Oral Cancer. Photodiagn. Photodyn. Ther. 2006, 3, 168–176. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alghamdi, M.S.; Alotaibi, A.S.; Alzhrani, R.; Alwuthaynani, F.; Althobaiti, Y.S.; Almalki, A.H.; Sau, S.; Iyer, A.K. Progress in Clinical Trials of Photodynamic Therapy for Solid Tumors and the Role of Nanomedicine. Cancers 2020, 12, 2793. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy: Review. Photodiagn. Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef]
- Wang, K.; Cai, Z.; Fan, R.; Yang, Q.; Zhu, T.; Jiang, Z.; Ma, Y. A Tumor-Microenvironment-Responsive Nanomaterial for Cancer Chemo-Photothermal Therapy. RSC Adv. 2020, 10, 22091–22101. [Google Scholar] [CrossRef]
- Mapanao, A.K.; Santi, M.; Voliani, V. Combined Chemo-Photothermal Treatment of Three-Dimensional Head and Neck Squamous Cell Carcinomas by Gold Nano-Architectures. J. Colloid Interface Sci. 2021, 582, 1003–1011. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Liu, Z.; Shi, P.; Dong, K.; Ju, E.; Ren, J.; Qu, X. A Multi-Stimuli Responsive Gold Nanocage-Hyaluronic Platform for Targeted Photothermal and Chemotherapy. Biomaterials 2014, 35, 9678–9688. [Google Scholar] [CrossRef]
- Strong, L.E.; West, J.L. Hydrogel-Coated Near Infrared Absorbing Nanoshells as Light-Responsive Drug Delivery Vehicles. ACS Biomater. Sci. Eng. 2015, 1, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Nordquist, R.E.; Chen, W.R. Photonics Immunotherapy-A Novel Strategy for Cancer Treatment. J. Innov. Opt. Health Sci. 2016, 9, 1630001. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Bian, J.; Zhang, X.; Fu, Y.; Li, Z.; Wei, S.; Xu, Z.; Liu, X.; Liu, Z.; et al. Ag/Pd Bimetal Nanozyme with Enhanced Catalytic and Photothermal Effects for ROS/Hyperthermia/Chemotherapy Triple-Modality Antitumor Therapy. Chem. Eng. J. 2020, 397, 125438. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Reis, C.A.; Moreira, A.F.; Ferreira, P.; Correia, I.J. Optimization of Gold Core-Mesoporous Silica Shell Functionalization with TPGS and PEI for Cancer Therapy. Microporous Mesoporous Mater. 2019, 285, 1–12. [Google Scholar] [CrossRef]
- Wen, S.; Li, K.; Cai, H.; Chen, Q.; Shen, M.; Huang, Y.; Peng, C.; Hou, W.; Zhu, M.; Zhang, G.; et al. Multifunctional Dendrimer-Entrapped Gold Nanoparticles for Dual Mode CT/MR Imaging Applications. Biomaterials 2013, 34, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sultan, D.; Detering, L.; Cho, S.; Sun, G.; Pierce, R.; Wooley, K.L.; Liu, Y. Copper-64-Alloyed Gold Nanoparticles for Cancer Imaging: Improved Radiolabel Stability and Diagnostic Accuracy. Angew. Chem. Int. Ed. 2014, 53, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Dang, Y.; Liang, G.; Liu, G. Iodine-125-Labeled CRGD-Gold Nanoparticles as Tumor-Targeted Radiosensitizer and Imaging Agent. Nanoscale Res. Lett. 2015, 10, 160. [Google Scholar] [CrossRef]
- Hong, G.; Lee, J.C.; Robinson, J.T.; Raaz, U.; Xie, L.; Huang, N.F.; Cooke, J.P.; Dai, H. Multifunctional in Vivo Vascular Imaging Using Near-Infrared II Fluorescence. Nat. Med. 2012, 18, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.L.; Shih, Y.H.; Lee, P.C.; Hsieh, T.M.H.; Luo, T.Y.; Shieh, M.J. Multimodal Image-Guided Photothermal Therapy Mediated by 188Re-Labeled Micelles Containing a Cyanine-Type Photosensitizer. ACS Nano 2011, 5, 5594–5607. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Zhan, Z.; He, W.; Cheng, Z.; Li, Y.; Liu, Z. Near-Infrared Dye Bound Albumin with Separated Imaging and Therapy Wavelength Channels for Imaging-Guided Photothermal Therapy. Biomaterials 2014, 35, 8206–8214. [Google Scholar] [CrossRef]
- Hong, G.; Zou, Y.; Antaris, A.L.; Diao, S.; Wu, D.; Cheng, K.; Zhang, X.; Chen, C.; Liu, B.; He, Y.; et al. Ultrafast Fluorescence Imaging in Vivo with Conjugated Polymer Fluorophores in the Second Near-Infrared Window. Nat. Commun. 2014, 5, 4206. [Google Scholar] [CrossRef]
- Wang, L.; Qi, J.; Zhang, K.; Zhuang, Z.; Ding, K.; Chen, X.; Shan, H.; Ding, D.; Qin, A.; Tang, B.Z. Multifunctional Nanomicelles Constructed via an Aggregation and De-Aggregation Strategy for Magnetic Resonance/NIR II Fluorescence Imaging-Guided Type I Photodynamic Therapy. Mater. Chem. Front. 2023, 7, 3657–3667. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Z.; Kang, M.; Guo, H.; Li, Y.; Wen, H.; Lee, M.M.S.; Wang, Z.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Making the Best Use of Excited-State Energy: Multimodality Theranostic Systems Based on Second Near-Infrared (NIR-II) Aggregation-Induced Emission Luminogens (AIEgens). ACS Mater. Lett. 2020, 2, 1033–1040. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Correia, I.J.; Moreira, A.F. Red Blood Cell Membrane-Camouflaged Gold-Core Silica Shell Nanorods for Cancer Drug Delivery and Photothermal Therapy. Int. J. Pharm. 2024, 655, 124007. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Jacinto, T.A.; Moreira, A.F.; Costa, E.C.; Miguel, S.P.; Correia, I.J. Functionalization of AuMSS Nanorods towards More Effective Cancer Therapies. Nano Res. 2019, 12, 719–732. [Google Scholar] [CrossRef]
- He, J.; Wei, Q.; Wang, S.; Hua, S.; Zhou, M. Bioinspired Protein Corona Strategy Enhanced Biocompatibility of Ag-Hybrid Hollow Au Nanoshells for Surface-Enhanced Raman Scattering Imaging and on-Demand Activation Tumor-Phototherapy. Biomaterials 2021, 271, 120734. [Google Scholar] [CrossRef]
- Manrique-Bedoya, S.; Abdul-Moqueet, M.; Lopez, P.; Gray, T.; Disiena, M.; Locker, A.; Kwee, S.; Tang, L.; Hood, R.L.; Feng, Y.; et al. Multiphysics Modeling of Plasmonic Photothermal Heating Effects in Gold Nanoparticles and Nanoparticle Arrays. J. Phys. Chem. C 2020, 124, 17172–17182. [Google Scholar] [CrossRef]
- Chaparro, D.; Goudeli, E. Design of Engineered Nanoparticles for Biomedical Applications by Computational Modeling. Nanoscale 2025, 17, 9705–9737. [Google Scholar] [CrossRef]
- Tan, H.L.; Abdi, F.F.; Ng, Y.H. Heterogeneous Photocatalysts: An Overview of Classic and Modern Approaches for Optical, Electronic, and Charge Dynamics Evaluation. Chem. Soc. Rev. 2019, 48, 1255–1271. [Google Scholar] [CrossRef]
- Vahidzadeh, E.; Shankar, K. Artificial Neural Network-Based Prediction of the Optical Properties of Spherical Core–Shell Plasmonic Metastructures. Nanomaterials 2021, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Singh Verma, S. Plasmonic Effects in Noble Metal-Liquid Metal Based Nanoparticles. Biomed. Sci. 2019, 5, 27. [Google Scholar] [CrossRef]
- Hadded, M.; Hmima, A.; Maurer, T.; Chehaidar, A.; Plain, J. Theoretical Investigation of Optical Properties of Optimal Architectures of Magnetoplasmonic Nanoparticles in Human Tissue for Potential Applications in Photothermal Therapy. Opt. Mater. 2021, 114, 110946. [Google Scholar] [CrossRef]
- Tavangari, Z.; Asadi, M.; Irajirad, R.; Sarikhani, A.; Alamzadeh, Z.; Ghaznavi, H.; Khoei, S. 3D Modeling of in Vivo MRI-Guided Nano-Photothermal Therapy Mediated by Magneto-Plasmonic Nanohybrids. BioMed. Eng. Online 2023, 22, 77. [Google Scholar] [CrossRef]
- Adir, O.; Poley, M.; Chen, G.; Froim, S.; Krinsky, N.; Shklover, J.; Shainsky-Roitman, J.; Lammers, T.; Schroeder, A. Integrating Artificial Intelligence and Nanotechnology for Precision Cancer Medicine. Adv. Mater. 2020, 32, e1901989. [Google Scholar] [CrossRef]
- Stillman, N.R.; Balaz, I.; Tsompanas, M.A.; Kovacevic, M.; Azimi, S.; Lafond, S.; Adamatzky, A.; Hauert, S. Evolutionary Computational Platform for the Automatic Discovery of Nanocarriers for Cancer Treatment. NPJ Comput. Mater. 2021, 7, 150. [Google Scholar] [CrossRef]
- Shimojo, Y.; Sudo, K.; Nishimura, T.; Ozawa, T.; Tsuruta, D.; Awazu, K. Transient Simulation of Laser Ablation Based on Monte Carlo Light Transport with Dynamic Optical Properties Model. Sci. Rep. 2023, 13, 11898. [Google Scholar] [CrossRef]
- Gheflati, B.; Naghavi, N. Computational Study of Nanoparticle Assisted Hyperthermia in Tumors Embedded with Large Blood Vessels. Int. J. Heat Mass Transf. 2020, 151, 119415. [Google Scholar] [CrossRef]
- Singh, A.V.; Rosenkranz, D.; Ansari, M.H.D.; Singh, R.; Kanase, A.; Singh, S.P.; Johnston, B.; Tentschert, J.; Laux, P.; Luch, A. Artificial Intelligence and Machine Learning Empower Advanced Biomedical Material Design to Toxicity Prediction. Adv. Intell. Syst. 2020, 2, 2000084. [Google Scholar] [CrossRef]
- Terrés-Haro, J.M.; Monreal-Trigo, J.; Hernández-Montoto, A.; Ibáñez-Civera, F.J.; Masot-Peris, R.; Martínez-Máñez, R. Finite Element Models of Gold Nanoparticles and Their Suspensions for Photothermal Effect Calculation. Bioengineering 2023, 10, 232. [Google Scholar] [CrossRef]
- Winkler, D.A. Role of Artificial Intelligence and Machine Learning in Nanosafety. Small 2020, 16, e2001883. [Google Scholar] [CrossRef]
- Hamilton, S.; Kingston, B.R. Applying Artificial Intelligence and Computational Modeling to Nanomedicine. Curr. Opin. Biotechnol. 2024, 85, 103043. [Google Scholar] [CrossRef]
- Manolis, G.D.; Dineva, P.S.; Rangelov, T.; Sfyris, D. Mechanical Models and Numerical Simulations in Nanomechanics: A Review across the Scales. Eng. Anal. Bound. Elem. 2021, 128, 149–170. [Google Scholar] [CrossRef]
- Xu, D.; Duan, Q.; Yu, H.; Dong, W. Photodynamic Therapy Based on Porphyrin-Based Metal–Organic Frameworks. J. Mater. Chem. B 2023, 11, 5976–5989. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, J.; Qu, Y.; Luo, X.; Wang, W.; Zheng, X. Evolution of NMOFs in Photodynamic Therapy: From Porphyrins to Chlorins and Bacteriochlorins for Better Efficacy. Front. Pharmacol. 2025, 16, 1533040. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, A.K.; Priyadarshini, N.; Priyadarshini, P.; Behera, G.C.; Parida, K. Recent Advancements in Metal Organic Framework-Modified Multifunctional Materials for Photodynamic Therapy. Mater. Adv. 2024, 5, 6030–6051. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Bremer-Hoffmann, S. Main Trends of Immune Effects Triggered by Nanomedicines in Preclinical Studies. Int. J. Nanomed. 2018, 13, 5419–5431. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Farcal, L.; Hardy, B.; Vázquez-Campos, S.; Hristozov, D.; Marcomini, A.; Lynch, I.; Valsami-Jones, E.; Alenius, H.; Savolainen, K. Advanced Tools for the Safety Assessment of Nanomaterials. Nat. Nanotechnol. 2018, 13, 537–543. [Google Scholar] [CrossRef] [PubMed]
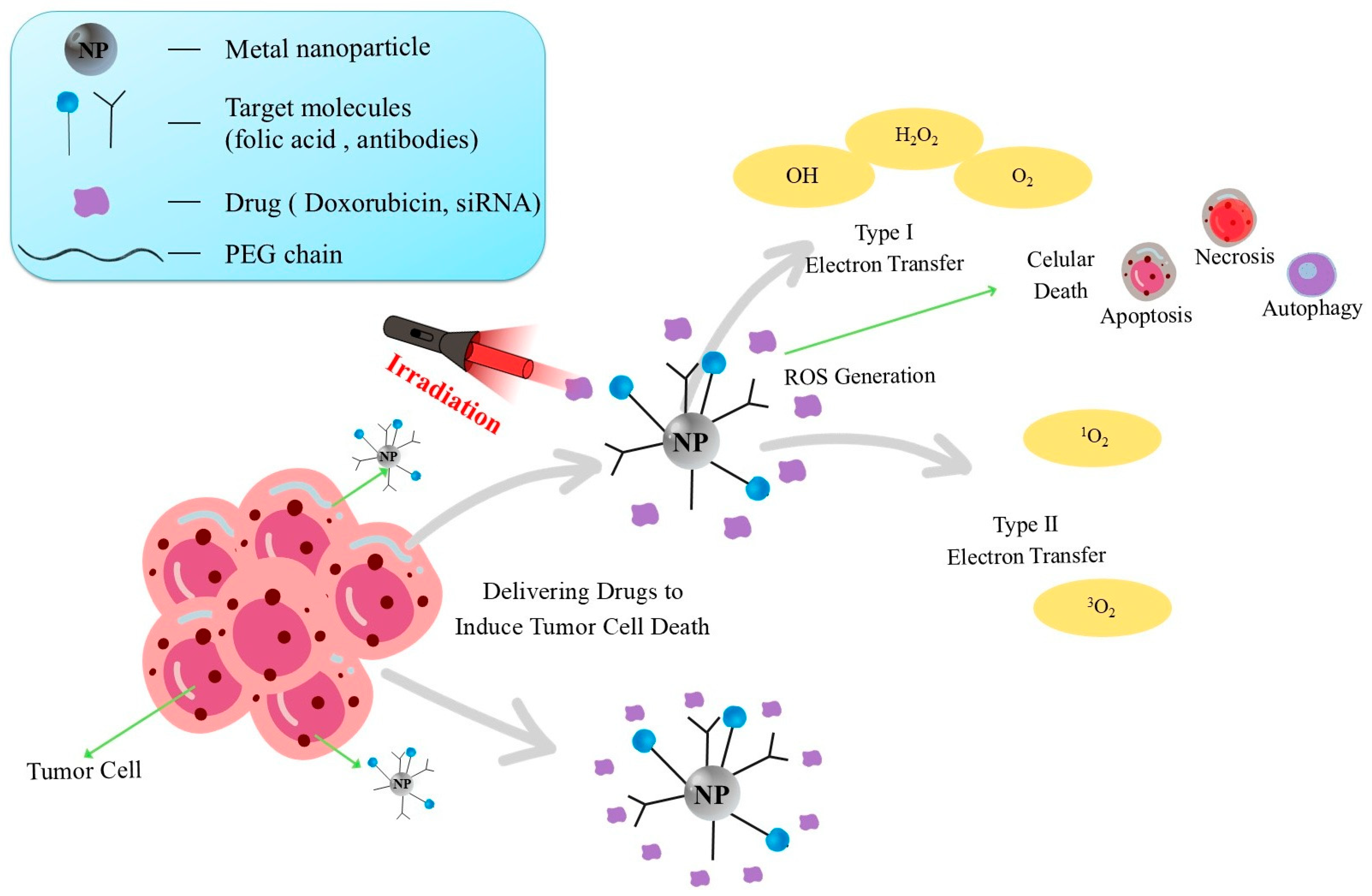
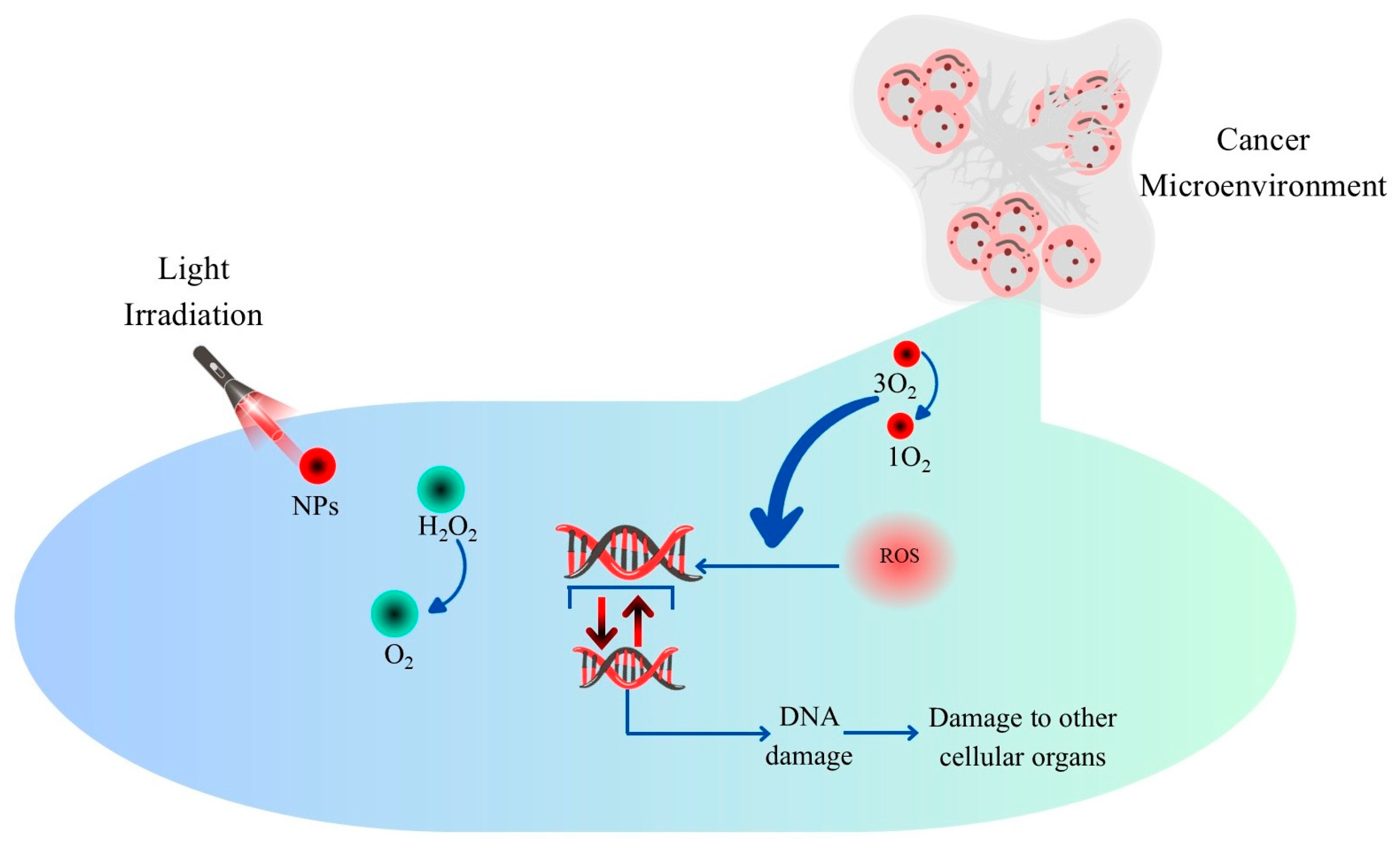
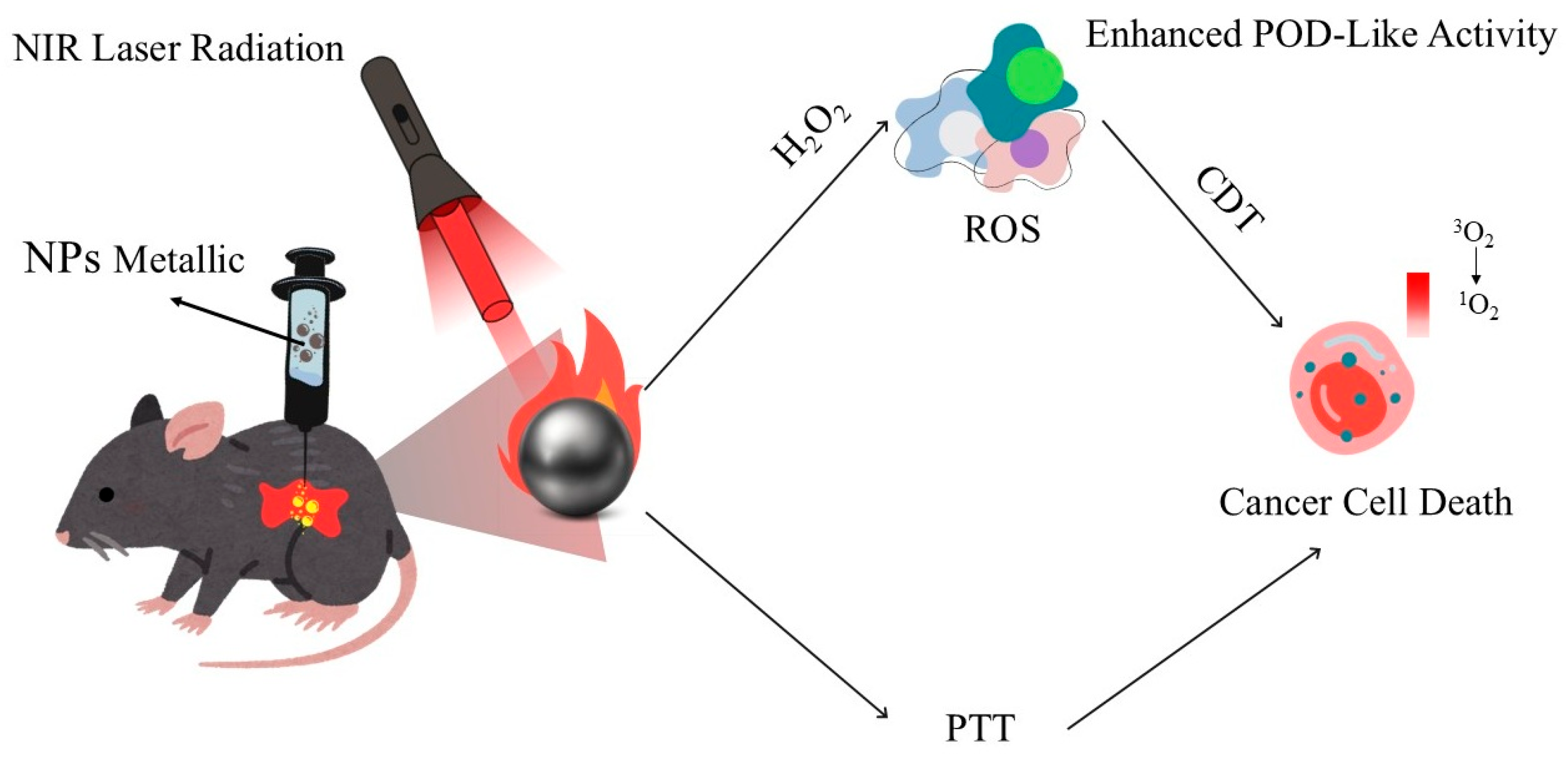
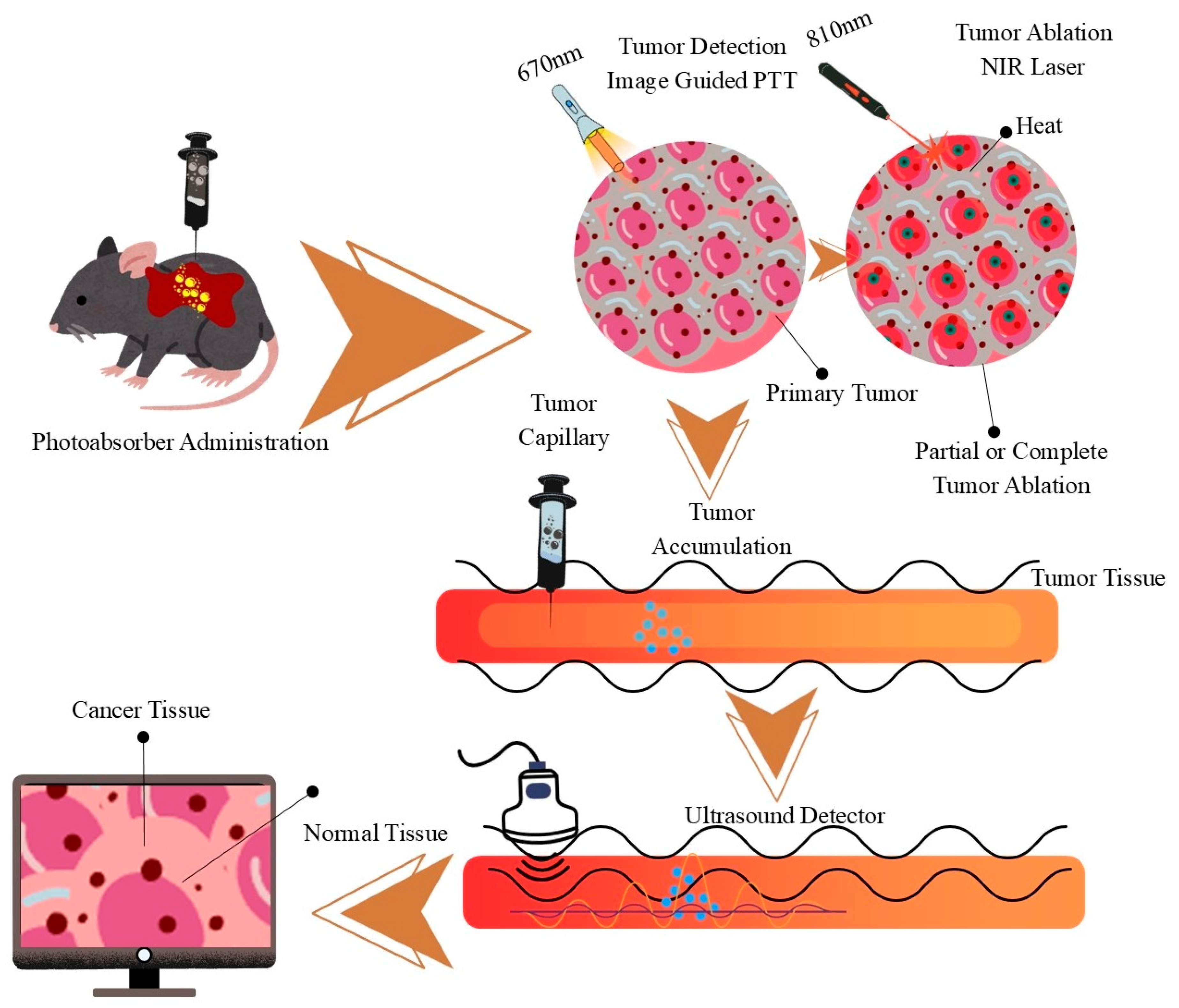
| Nanomaterial | Optical Properties | Mechanism of Action | Advantages | Limitations | Potential Clinical Applications | References |
|---|---|---|---|---|---|---|
| Gold (Au) | NIR absorption; tunable SPR | SPR-mediated heat generation; drug delivery | High biocompatibility; effective in imaging and PTT; simple and reproducible synthesis | High cost; cytotoxicity at high concentrations; risk of persistence and accumulation in the body | PTT, PDT, imaging diagnostic | [68,69] |
| Silver (Ag) | SPR in visible range; high conductivity | ROS generation; thermal conversion | Antibacterial properties; ease of synthesis | Cytotoxicity; reduced stability in biological environments | Combined PTT and PDT; antibacterial applications | [46,47] |
| Porous Silicon | Thermal stability; tunable porosity | Controlled drug release | High biocompatibility; integration with other materials | Altered optical properties upon functionalization | PTT, drug delivery systems | [38] |
| Iron Oxide (Fe3O4) | Magnetic and plasmonic properties | NIR-mediated heat conversion | Magnetic response; imaging properties | Requires high energy; potential cytotoxicity | PTT, magnetic hyperthermia; bioimaging | [54,107] |
| Manganese Oxide (MnO2) | High NIR absorption; paramagnetic | ROS generation; tumor microenvironment modulation | High photoconversion efficiency; low toxicity | Low efficacy at minimal concentrations | PTT, PDT, MRI diagnostic | [114,116] |
| Carbon Nanotubes (CNTs) | Broad NIR absorption; high conductivity | Thermal conversion for tumor ablation | Stability; functionalization for active targeting | Long-term toxicity concerns; synthesis challenges | Cancer therapy; drug delivery | [103,104] |
| MOFs | High porosity; therapeutic agent loading | Controlled drug release; plasmonic protection | Functional and tunable; combined therapies | Stability in biological environments | PTT, PDT, drug delivery | [11,98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinzón, D.L.S.; Lourenço, D.B.; Balbino, T.A.; Rodrigues, T.S. Photoactive Nanomaterials Containing Metals for Biomedical Applications: A Comprehensive Literature Review. Processes 2025, 13, 2978. https://doi.org/10.3390/pr13092978
Pinzón DLS, Lourenço DB, Balbino TA, Rodrigues TS. Photoactive Nanomaterials Containing Metals for Biomedical Applications: A Comprehensive Literature Review. Processes. 2025; 13(9):2978. https://doi.org/10.3390/pr13092978
Chicago/Turabian StylePinzón, Dayana Lizeth Sánchez, Daniel Bertolano Lourenço, Tiago Albertini Balbino, and Thenner Silva Rodrigues. 2025. "Photoactive Nanomaterials Containing Metals for Biomedical Applications: A Comprehensive Literature Review" Processes 13, no. 9: 2978. https://doi.org/10.3390/pr13092978
APA StylePinzón, D. L. S., Lourenço, D. B., Balbino, T. A., & Rodrigues, T. S. (2025). Photoactive Nanomaterials Containing Metals for Biomedical Applications: A Comprehensive Literature Review. Processes, 13(9), 2978. https://doi.org/10.3390/pr13092978








