Fluorescence Spectroscopy Applied to Thermal Conversion of Bitumen
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Protocol
2.3. Analyses
3. Results
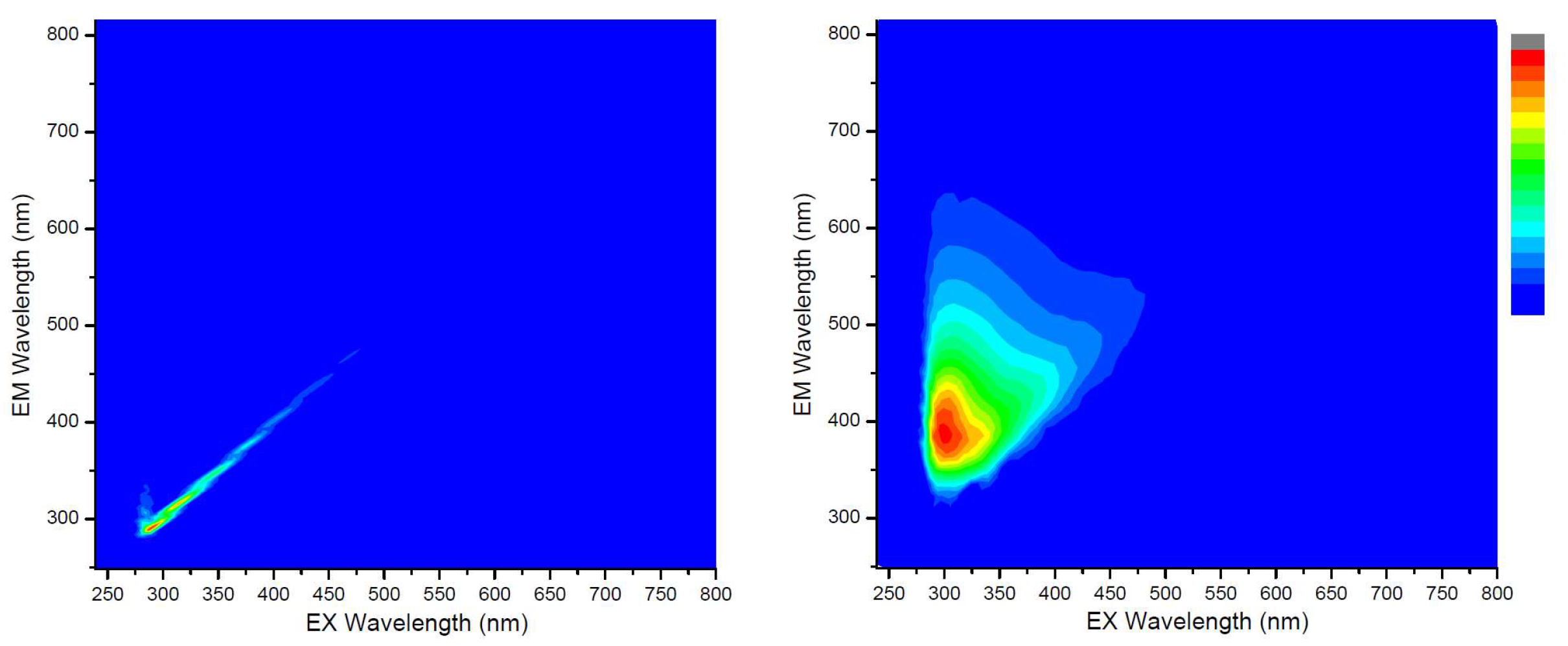
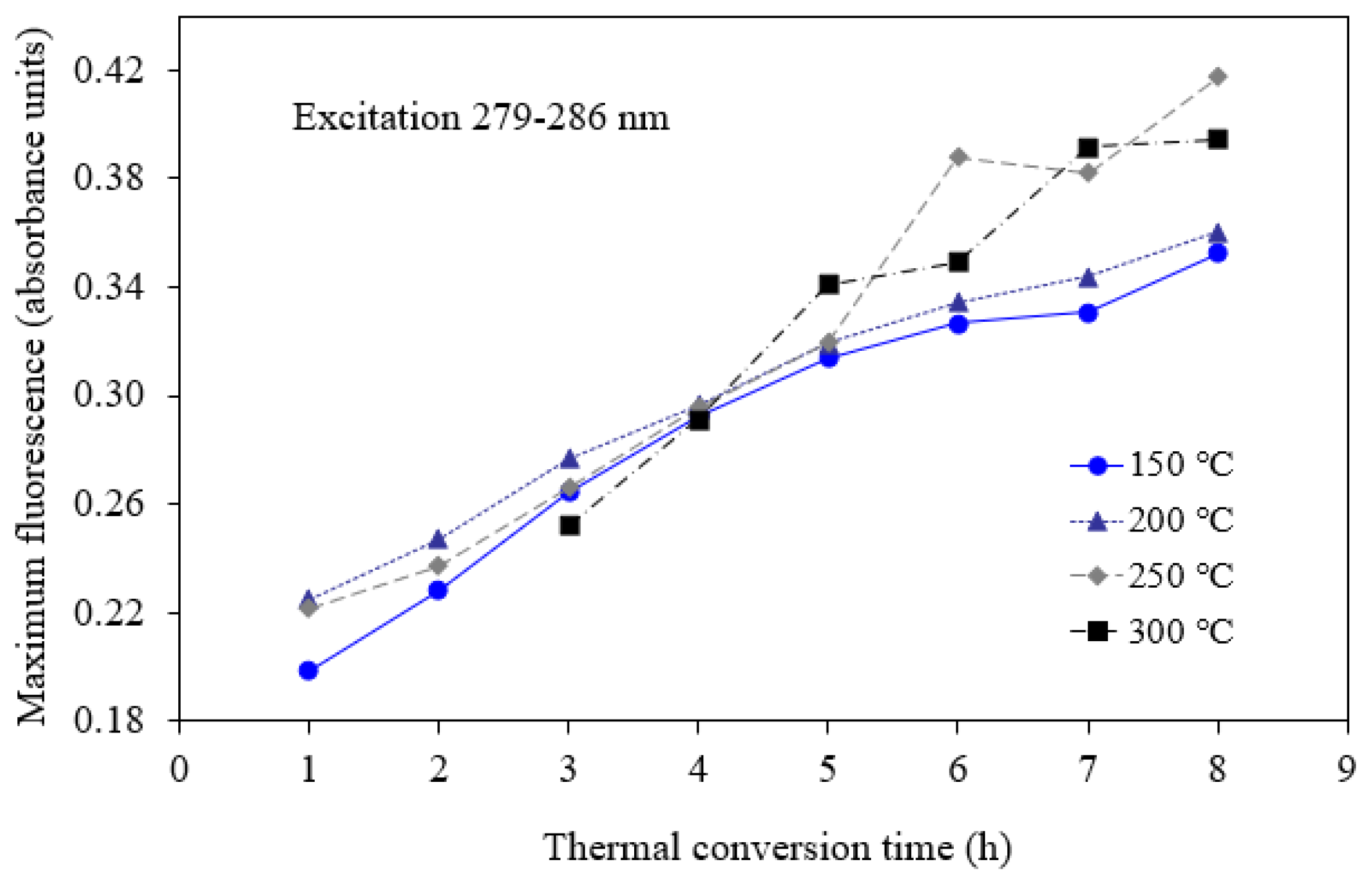
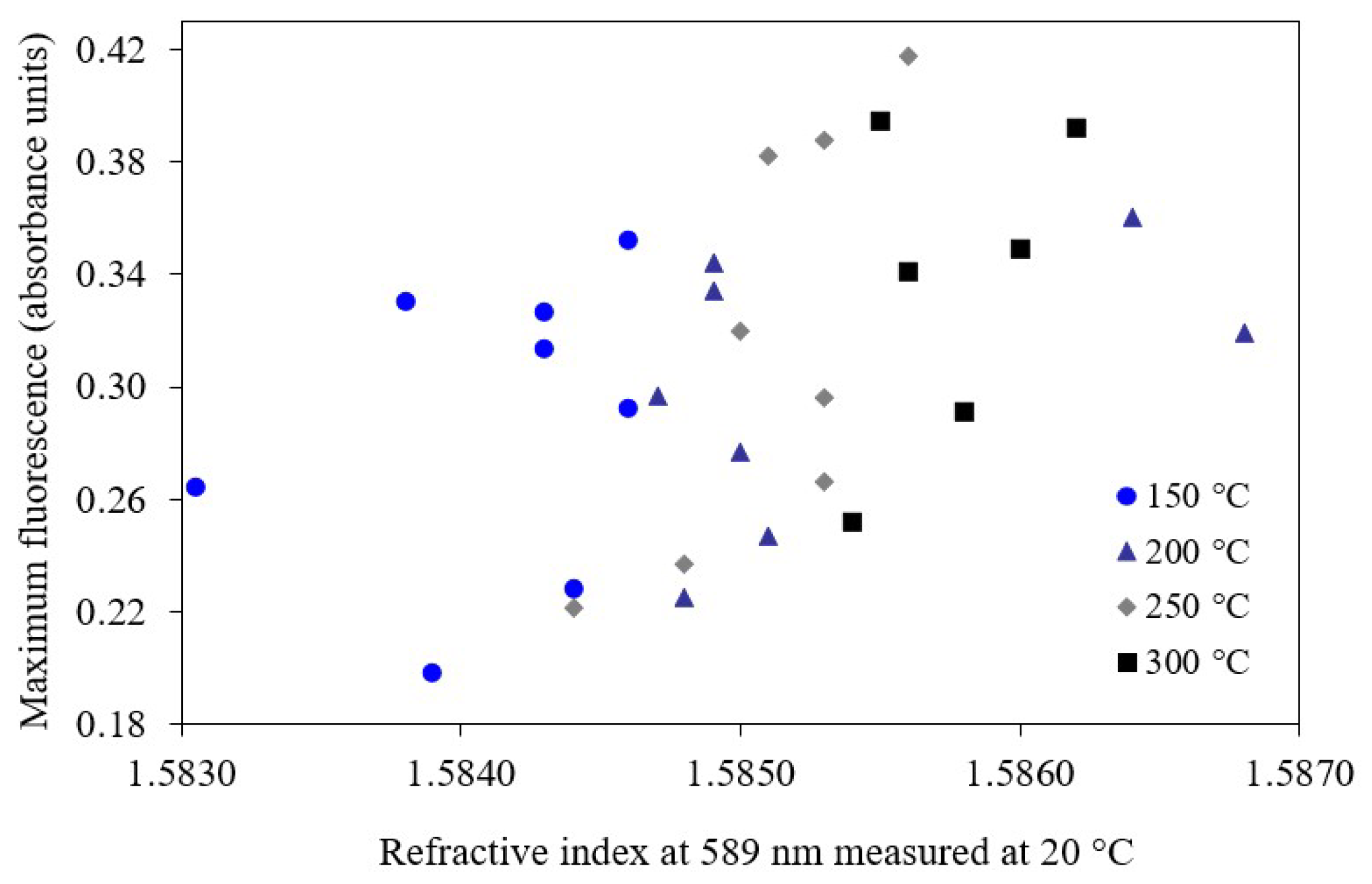
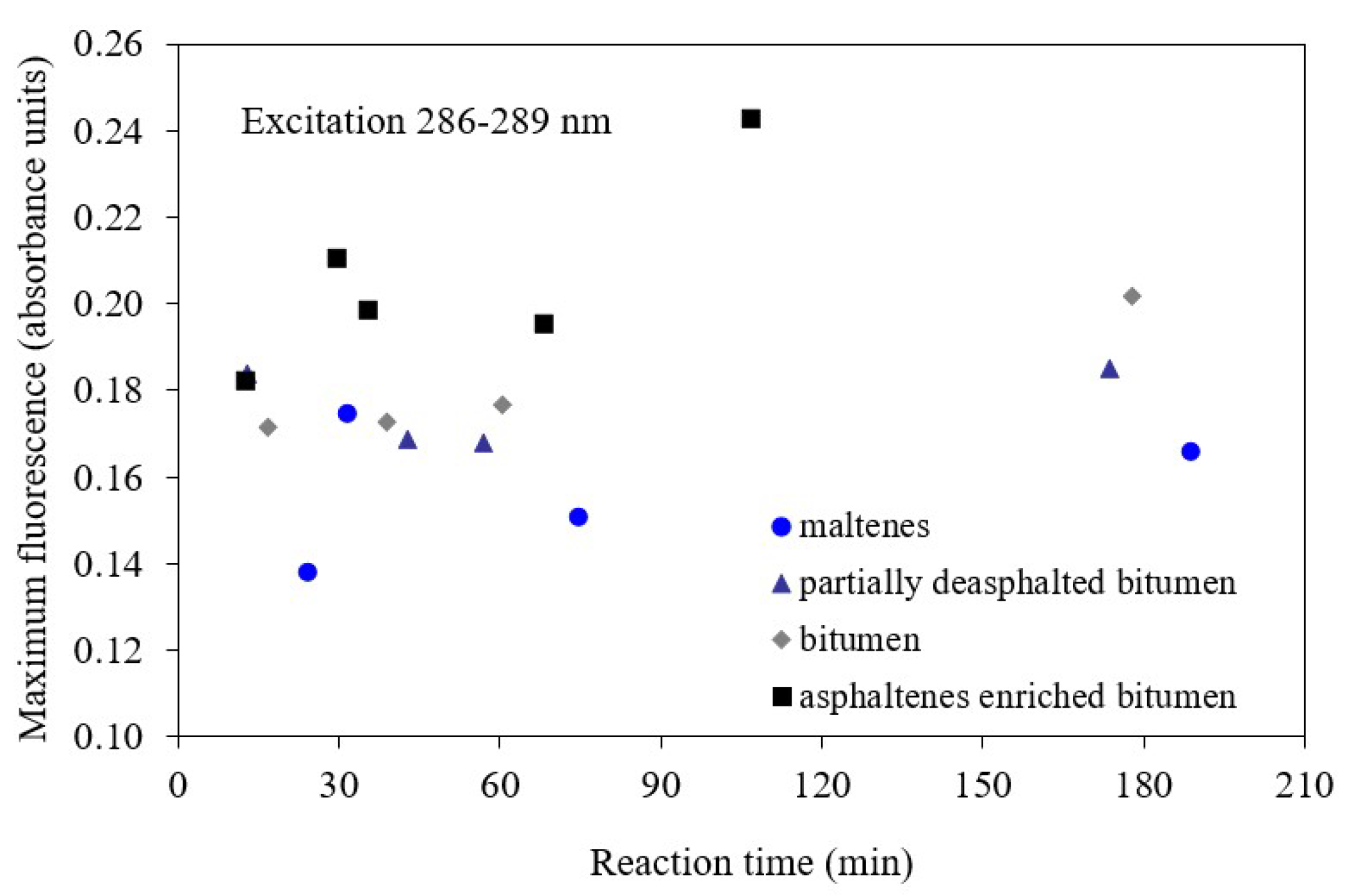
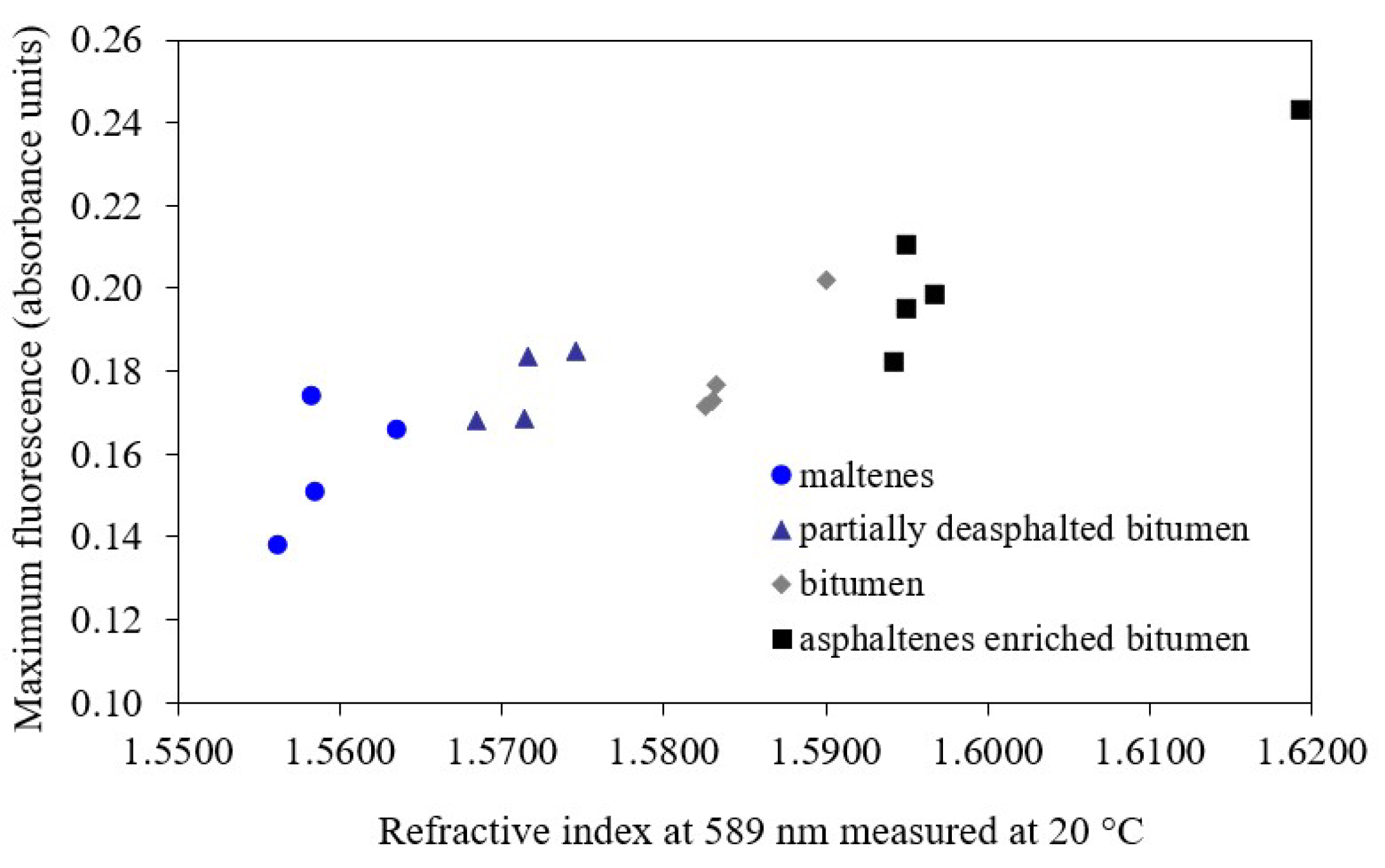
3.1. Cold Lake Bitumen Converted at 150–300 °C
3.2. Athabasca Bitumen Derived Materials Converted at 380 °C
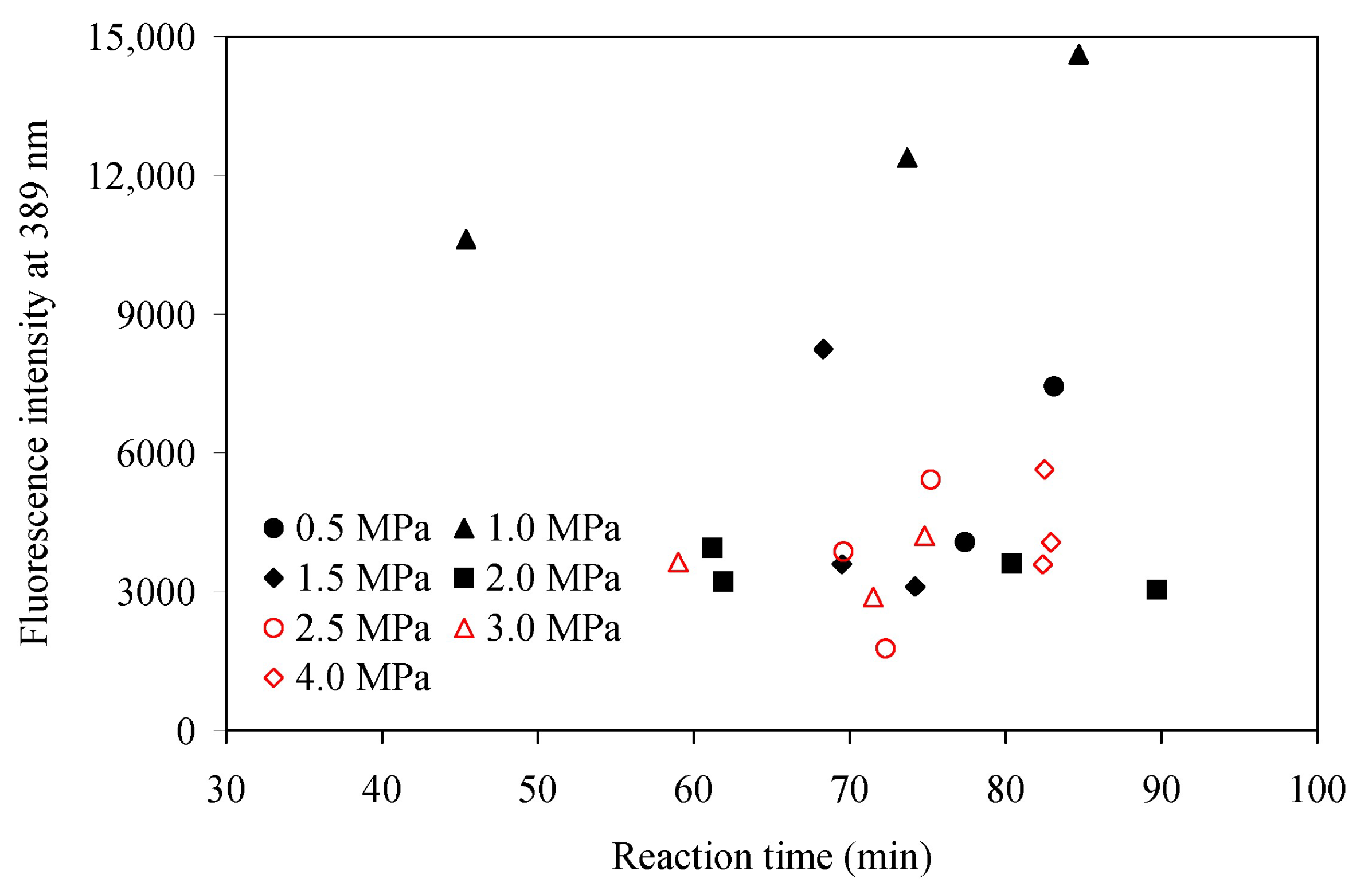
3.3. Athabasca Bitumen Converted at 400 °C and 0.5–4.0 MPa
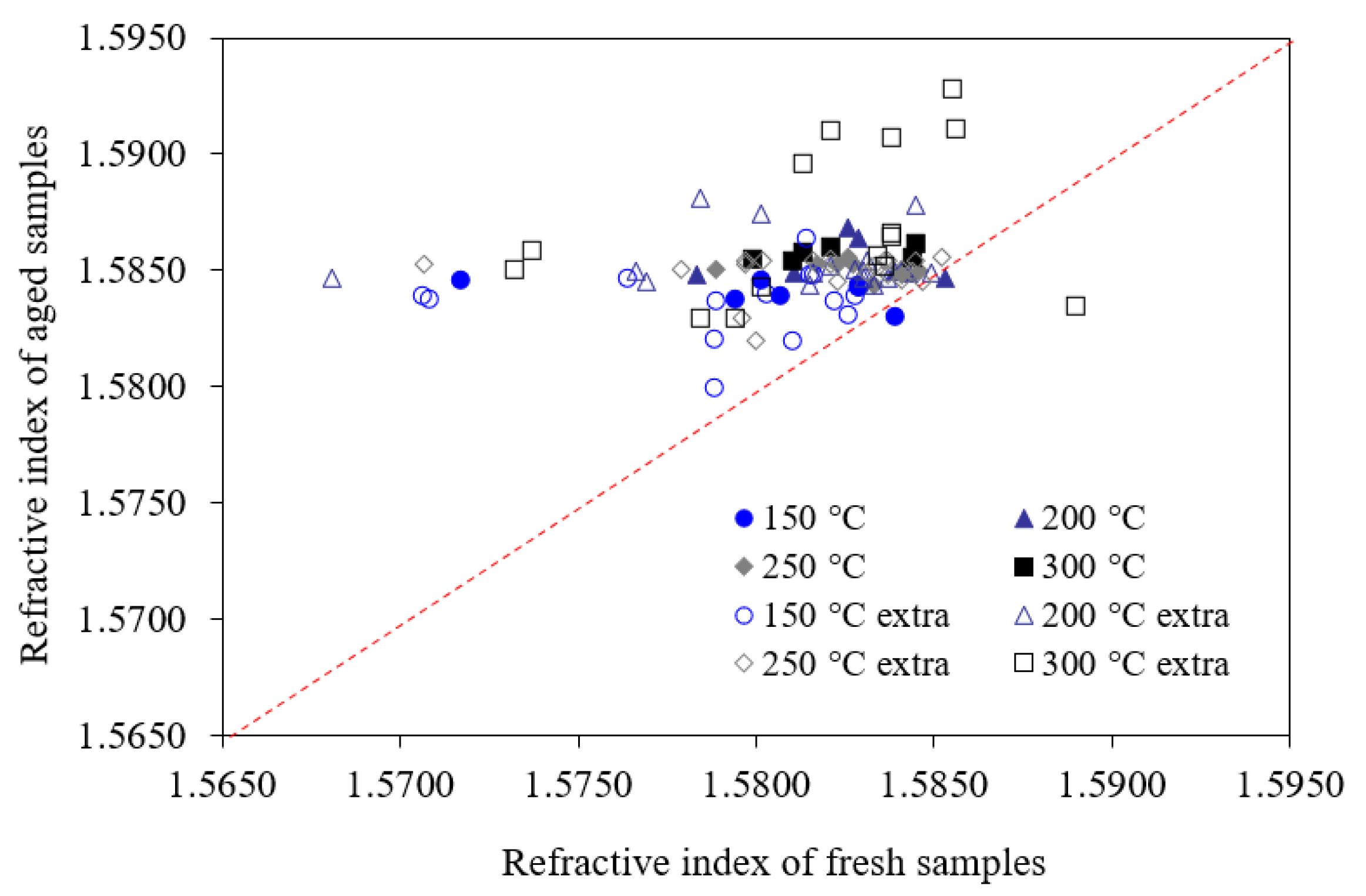
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mitkova, M.; Stratiev, D.; Shishkova, I.; Dobrev, D. Thermal and Thermo-Catalytic Processes for Heavy Oil Conversion (Bulgarian Academic Monograph 13); Bulgarian Academy of Sciences: Sofia, Bulgaria, 2017; ISBN 978-954-322-892-8. Available online: https://scholar.google.com/scholar_lookup?title=Thermal+and+Thermo-Catalytic+Processes+for+Heavy+Oil+Conversion&author=Mitkova,+M.&author=Stratiev,+D.&author=Shishkova,+I.&author=Dobrev,+D.&publication_year=2017 (accessed on 1 July 2025).
- Wiehe, I.A. Self-incompatible crude oils and converted petroleum resids. J. Dispers. Sci. Technol. 2004, 25, 333–339. [Google Scholar] [CrossRef]
- Storm, D.A.; Decanio, S.J.; Edwards, J.C.; Sheu, E.Y. Sediment formation during heavy oil upgrading. Petrol. Sci. Technol. 1997, 15, 77–102. [Google Scholar] [CrossRef]
- Vezirov, R.R.; Gareev, R.G.; Obukhova, S.A.; Vezirova, N.R.; Khalikov, D.E. Problems in resid-feedstock heat exchange in visbreaking units. Chem. Technol. Fuels Oils 2010, 46, 37–42. [Google Scholar] [CrossRef]
- Wiehe, I.A. A phase-separation kinetic model for coke formation. Ind. Eng. Chem. Res. 1993, 32, 2447–2454. [Google Scholar] [CrossRef]
- Wiehe, I.A. A solvent-resid phase diagram for tracking resid conversion. Ind. Eng. Chem. Res. 1992, 31, 530–536. [Google Scholar] [CrossRef]
- Brauch, R.; Fainberg, V.; Kalchouck, H.; Hetsroni, G. Correlations between properties of various feedstocks and products of visbreaking. Fuel Sci. Technol. Int. 1996, 14, 753–765. [Google Scholar] [CrossRef]
- Rahimi, P.M.; Teclemariam, A.; Taylor, E.; De Bruijn, T.; Wiehe, I.A. Thermal processing limits of Athabasca bitumen during visbreaking using solubility parameters. ACS Symp. Ser. 2005, 895, 183–196. [Google Scholar] [CrossRef]
- Stratiev, D.; Kirilov, K.; Belchev, Z.; Petkov, P. How do feedstocks affect visbreaker operations? Hydrocarb. Process. 2008, 87, 105–112. Available online: https://www.researchgate.net/publication/274511312 (accessed on 1 July 2025).
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Geng, T.; Wang, Y.; Yin, X.-L.; Chen, W.; Gu, H.-W. A comprehensive review on the excitation-emission matrix fluorescence spectroscopic characterization of petroleum-containing substances: Principles, methods, and applications. Crit. Rev. Anal. Chem. 2024, 54, 2827–2849. [Google Scholar] [CrossRef]
- Ryder, A.G. Analysis of crude petroleum oils using fluorescence spectroscopy. In Reviews in Fluorescence 2005; Geddes, C.D., Lakowicz, J.R., Eds.; Springer: New York, NY, USA, 2005; pp. 169–198. [Google Scholar] [CrossRef]
- Ellingsen, G.; Fery-Forgues, S. Application de la spectroscopie de fluorescence à l’étude du pétrole: Le défi de la complexité (Engl. Transl. “Application of fluorescence spectroscopy to the study of petroleum: Challenging complexity”). Rev. IFP 1998, 53, 201–216. [Google Scholar] [CrossRef]
- Nandakumar, V.; Jayanthi, J.L. Hydrocarbon fluid inclusions, API gravity of oil, signature fluorescence emissions and emission ratios: An example from Mumbai offshore, India. Energy Fuels 2016, 30, 3776–3782. [Google Scholar] [CrossRef]
- El Hussein, A.; Marzouk, A. Characterization of petroleum crude oils using laser induced fluorescence. J. Petrol. Environ. Biotechnol. 2015, 6, 1000240. [Google Scholar] [CrossRef]
- Owens, P.; Ryder, A.G. Low temperature fluorescence studies of crude petroleum oils. Energy Fuels 2011, 25, 5022–5032. [Google Scholar] [CrossRef]
- Owens, P.; Ryder, A.G.; Blamey, N.J.F. Frequency domain fluorescence lifetime study of crude petroleum oils. J. Fluoresc. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Ryder, A.G. Time-resolved fluorescence spectroscopic study of crude petroleum oils: Influence of chemical composition. Appl. Spectrosc. 2004, 58, 613–623. Available online: https://opg.optica.org/as/abstract.cfm?URI=as-58-5-613 (accessed on 1 July 2025). [CrossRef] [PubMed]
- Ryder, A.G. Quantitative analysis of crude oils by fluorescence lifetime and steady state measurements using 380-nm excitation. Appl. Spectrosc. 2002, 56, 107–116. [Google Scholar] [CrossRef]
- Cheng, P.; Ren, Y.; Yu, S. Determination of light and condensate oil categories in a complex petroleum system by fluorescence parameters: A case study on the Northern Tazhong uplift, Tarim basin, China. ACS Earth Space Chem. 2024, 8, 1997–2011. [Google Scholar] [CrossRef]
- Ryder, A.G. Assessing the maturity of crude petroleum oils using total synchronous fluorescence scan spectra. J. Fluoresc. 2004, 14, 99–104. [Google Scholar] [CrossRef]
- Ryder, A.G.; Glynn, T.J.; Feely, M.; Barwise, A.J.G. Characterization of crude oils using fluorescence lifetime data. Spectrochim. Acta A 2002, 58, 1025–1037. [Google Scholar] [CrossRef]
- Cheng, P.; Tian, H.; Xiao, X.; Gai, H.; Zhou, Q.; Zhou, L. Influence of thermal maturity on the time-resolved emission spectrum (TRES) fluorescence lifetime characteristics of crude oils: A preliminary study based on a thermal simulation experiment of crude oils. Energy Fuels 2023, 27, 5165–5178. [Google Scholar] [CrossRef]
- Cheng, P.; Liu, B.; Tian, H.; Xiao, X.; Gai, H.; Zhou, Q.; Li, T.; Liu, D. Fluorescence lifetime evolution of crude oils during thermal cracking: Implications from pyrolysis experiments in a closed system. Org. Geochem. 2021, 159, 104273. [Google Scholar] [CrossRef]
- Kershaw, J.R.; Fetzer, J.C. The room temperature fluorescence analysis of polycyclic aromatic compounds in petroleum and related materials. Polycylic Aromat. Compd. 1995, 7, 253–268. [Google Scholar] [CrossRef]
- Yañez Jaramillo, L.M.; De Klerk, A. Partial upgrading of bitumen by thermal conversion at 150–300 °C. Energy Fuels 2018, 32, 3299–3311. [Google Scholar] [CrossRef]
- Yañez Jaramillo, L.M.; De Klerk, A. Is solubility classification a meaningful measure in thermal conversion? Energy Fuels 2022, 36, 8649–8662. [Google Scholar] [CrossRef]
- Nascimento, P.T.H.; De Klerk, A. Effect of pressure on visbreaking product composition and properties. Energy Fuels 2024, 38, 12632–12644. [Google Scholar] [CrossRef]
- Jaffé, H.H.; Orchin, M. Theory and Applications of Ultraviolet Spectroscopy; John Wiley and Sons: New York, NY, USA, 1962; p. 253, LC: 62015181. [Google Scholar]
- Taylor, T.A.; Patterson, H.H. Excitation resolved synchronous fluorescence analysis of aromatic compounds and fuel oil. Anal. Chem. 1987, 59, 2180–2187. [Google Scholar] [CrossRef]
- Yan, Y.; Prado, G.H.C.; De Klerk, A. Storage stability of products from visbreaking of oilsands bitumen. Energy Fuels 2020, 34, 9585–9598. [Google Scholar] [CrossRef]
- Leprince, P. Visbreaking of residues. In Petroleum Refining. Vol. 3. Conversion Processes; Leprince, P., Ed.; Editions Technip: Paris, France, 2001; pp. 365–379. ISBN 2710807793. [Google Scholar]
- Raseev, S. Thermal and Catalytic Processes in Petroleum Refining; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Yañez Jaramillo, L.M.; Tannous, J.H.; De Klerk, A. Persistent free radicals in petroleum. Processes 2023, 11, 2067. [Google Scholar] [CrossRef]
- Wang, L.; Zachariah, A.; Yang, S.; Prasad, V.; De Klerk, A. Visbreaking oilsands-derived bitumen in the temperature range of 340–400 °C. Energy Fuels 2014, 28, 5014–5022. [Google Scholar] [CrossRef]
- Castillo, J.; De Klerk, A. Visbreaking of deasphalted oil from bitumen at 280–400 °C. Energy Fuels 2019, 33, 159–175. [Google Scholar] [CrossRef]
- Buckley, J.S. Predicting the onset of asphaltene precipitation from refractive index measurements. Energy Fuels 1999, 13, 328–332. [Google Scholar] [CrossRef]
- Jha, K.N.; Montgomery, D.S.; Strausz, O.P. Chemical composition of gases in Alberta bitumens and in low-temperature thermolysis of oil sand asphaltenes and maltenes. In Oil Sand and Oil Shale Chemistry; Strausz, O.P., Lown, E.M., Eds.; Verlag Chemie: New York, NY, USA, 1978; pp. 33–54. ISBN 0895731029. [Google Scholar]
- Singh, J.; Kumar, S.; Garg, M.O. Kinetic modelling of thermal cracking of petroleum residues: A critique. Fuel Process. Technol. 2012, 94, 131–144. [Google Scholar] [CrossRef]
- Kapadia, P.R.; Kallos, M.S.; Gates, I.D. A new kinetic model for pyrolysis of Athabasca bitumen. Can. J. Chem. Eng. 2013, 91, 889–901. [Google Scholar] [CrossRef]
- Cabrales-Navarro, F.A.; Pereira-Almao, P. Reactivity and comprehensive kinetic modeling of deasphalted vacuum residue thermal cracking. Energy Fuels 2017, 31, 4318–4332. [Google Scholar] [CrossRef]
- Handle, F.; Füssl, J.; Neudl, S.; Grossegger, D.; Eberhardsteiner, L.; Hofko, B.; Hospodka, M.; Blab, R.; Grothe, H. The bitumen microstructure: A fluorescent approach. Mater. Struct. 2016, 49, 167–180. [Google Scholar] [CrossRef]
- Yokota, T.; Striven, F.; Montgomery, D.S.; Strausz, O.P. Absorption and emission spectra of Athabasca asphaltene in the visible and near ultraviolet regions. Fuel 1985, 65, 1142–1149. [Google Scholar] [CrossRef]
- Aguilar, R.A.; Ancheyta, J. Modeling coil and soaker reactors for visbreaking. Ind. Eng. Chem. Res. 2016, 55, 912–924. [Google Scholar] [CrossRef]
- Guerra, A.; Symonds, R.; Bryson, S.; Kirney, C.; Di Bacco, B.; Macchi, A.; Hughes, R. Five-lump mild thermal cracking reaction model of crude oils and bitumen with VLE calculations. Ind. Eng. Chem. Res. 2019, 58, 16417–16430. [Google Scholar] [CrossRef]
- Alemán-Vázquez, L.O.; Torres-Mancera, P.; Ancheyta, J.; Ramírez-Salgado, J. Use of hydrogen donors for partial upgrading of heavy petroleum. Energy Fuels 2016, 30, 9050–9060. [Google Scholar] [CrossRef]
- Zachariah, A.; Wang, L.; Yang, S.; Prasad, V.; De Klerk, A. Suppression of coke formation during bitumen pyrolysis. Energy Fuels 2013, 27, 3061–3070. [Google Scholar] [CrossRef]
- Yokono, T.; Obara, T.; Sanada, Y.; Shimomura, S.; Imamura, T. Characterization of carbonization reaction of petroleum residues by means of high-temperature ESR and transferable hydrogen. Carbon 1986, 24, 29–32. [Google Scholar] [CrossRef]
- De Klerk, A. Thermal conversion modeling of visbreaking at temperatures below 400 °C. Energy Fuels 2020, 34, 15285–15298. [Google Scholar] [CrossRef]
- Poutsma, M.L. Atom-transfer and substitution reactions. In Free Radicals. Vol. II; Kochi, J.K., Ed.; Wiley-Interscience: New York, NY, USA, 1973; pp. 113–158. ISBN 9780471497028. [Google Scholar]
- Zhao, Y.; Gray, M.R.; Chung, K.H. Molar kinetics and selectivity in cracking of Athabasca asphaltenes. Energy Fuels 2001, 15, 751–755. [Google Scholar] [CrossRef]
- Yan, T.Y. Characterization of visbreaker feeds. Fuel 1990, 69, 1062–1064. [Google Scholar] [CrossRef]
| Property | Oilsands Bitumen Characterization | ||
|---|---|---|---|
| Ref. [26] | Ref. [27] | Ref. [28] | |
| region of origin | Cold Lake | Athabasca | Athabasca |
| elemental analysis (wt%) | |||
| carbon | 82.6 ± 0.1 | 83.0 ± 0.1 | 83.4 ± 0.1 |
| hydrogen | 10.3 ± 0.1 | 10.3 ± <0.1 | 10.4 ± 0.1 |
| nitrogen | 0.6 ± 0.1 | 0.5 ± <0.1 | 0.5 ± <0.1 |
| sulfur | 4.7 ± 0.1 | 5.3 ± 0.3 | 4.9 ± 0.1 |
| asphaltene content (wt%) | 16.5 a | 11.5 b,c | 15.4 a,d |
| density (kg/m3) | |||
| 20 °C | 1024.0 ± 1.2 | 1014.2 ± 2.1 | 1008.3 ± 1.8 |
| 40 °C | 1011.3 ± 1.4 | 1001.6 ± 2.2 | 995.8 ± 1.7 |
| dρ/dT (kg/m3.K) | −0.629 | −0.643 | −0.626 |
| refractive index at 598 nm | |||
| 20 °C | 1.5844 ± 0.0008 | 1.5791 ± 0.0001 | 1.5748 ± 0.0002 |
| 40 °C | 1.5768 ± 0.0007 | 1.5716 ± 0.0002 | 1.5673 ± 0.0001 |
| dn/dT (1/K) | −3.80 × 10−4 | −3.74 × 10−4 | −3.76 × 10−4 |
| viscosity (Pa.s) | |||
| 20 °C | 1655 ± 2 | 2461 ± 26 | - |
| 40 °C | 88 ± <0.1 | - | 36.3 ± 0.2 |
| Pressure (MPa) | Reaction Time (min) | Fluorescence Intensity Per µg/g Sample Concentration | ||
|---|---|---|---|---|
| 389 nm | 407 nm | 435 nm | ||
| Athabasca bitumen feed | 882 | 767 | 618 | |
| 0.5 | 77 | 4075 | 4054 | 3600 |
| 0.5 | 83 | 7439 | 7289 | 6185 |
| 1.0 | 74 | 12,385 | 11,388 | 9297 |
| 1.0 | 45 | 10,620 | 9505 | 7874 |
| 1.0 | 85 | 14,624 | 13,091 | 10,593 |
| 1.5 | 74 | 3111 | 2921 | 2443 |
| 1.5 | 70 | 3598 | 3343 | 2741 |
| 1.5 | 68 | 8242 | 7737 | 6695 |
| 2.0 | 62 | 3221 | 2934 | 2425 |
| 2.0 | 61 | 3948 | 3674 | 3101 |
| 2.0 | 80 | 3607 | 3235 | 2637 |
| 2.0 | 90 | 3047 | 2698 | 2199 |
| 2.5 | 75 | 5424 | 4952 | 4063 |
| 2.5 | 72 | 1773 | 1631 | 1333 |
| 2.5 | 70 | 3866 | 3611 | 3051 |
| 3.0 | 72 | 2885 | 2602 | 2087 |
| 3.0 | 75 | 4213 | 3810 | 3089 |
| 3.0 | 59 | 3643 | 3311 | 2674 |
| 4.0 | 83 | 5638 | 4950 | 4152 |
| 4.0 | 83 | 4070 | 3583 | 2924 |
| 4.0 | 82 | 3590 | 3209 | 2558 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Divyajeetsinh, R.; Yañez Jaramillo, L.M.; Nascimento, P.T.H.; de Klerk, A. Fluorescence Spectroscopy Applied to Thermal Conversion of Bitumen. Processes 2025, 13, 2901. https://doi.org/10.3390/pr13092901
Divyajeetsinh R, Yañez Jaramillo LM, Nascimento PTH, de Klerk A. Fluorescence Spectroscopy Applied to Thermal Conversion of Bitumen. Processes. 2025; 13(9):2901. https://doi.org/10.3390/pr13092901
Chicago/Turabian StyleDivyajeetsinh, Raj, Lina M. Yañez Jaramillo, Priscila T. H. Nascimento, and Arno de Klerk. 2025. "Fluorescence Spectroscopy Applied to Thermal Conversion of Bitumen" Processes 13, no. 9: 2901. https://doi.org/10.3390/pr13092901
APA StyleDivyajeetsinh, R., Yañez Jaramillo, L. M., Nascimento, P. T. H., & de Klerk, A. (2025). Fluorescence Spectroscopy Applied to Thermal Conversion of Bitumen. Processes, 13(9), 2901. https://doi.org/10.3390/pr13092901







