Effect of Acid Treatment on the Structure of Natural Zeolite from the Shankhanai Deposit
Abstract
1. Introduction
2. Materials and Methods
2.1. Acid Activation of Natural Zeolite
2.2. Thermal Treatment of Activated Zeolite
2.3. Characterization Techniques
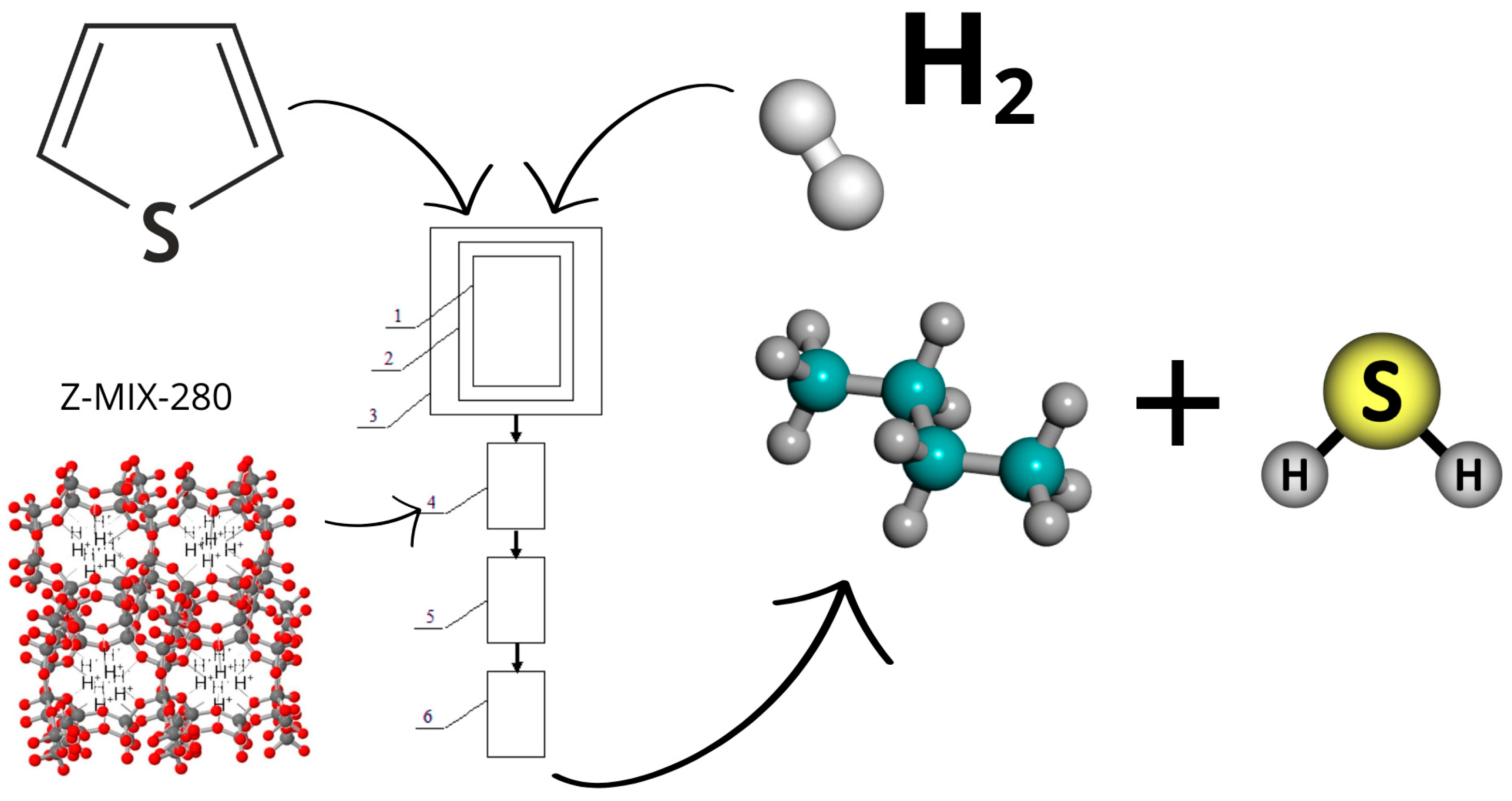
2.4. Catalytic Activity
3. Results
3.1. Selection of Acid Concentrations and Thermal Treatment Conditions
3.1.1. X-Ray Diffraction (XRD)
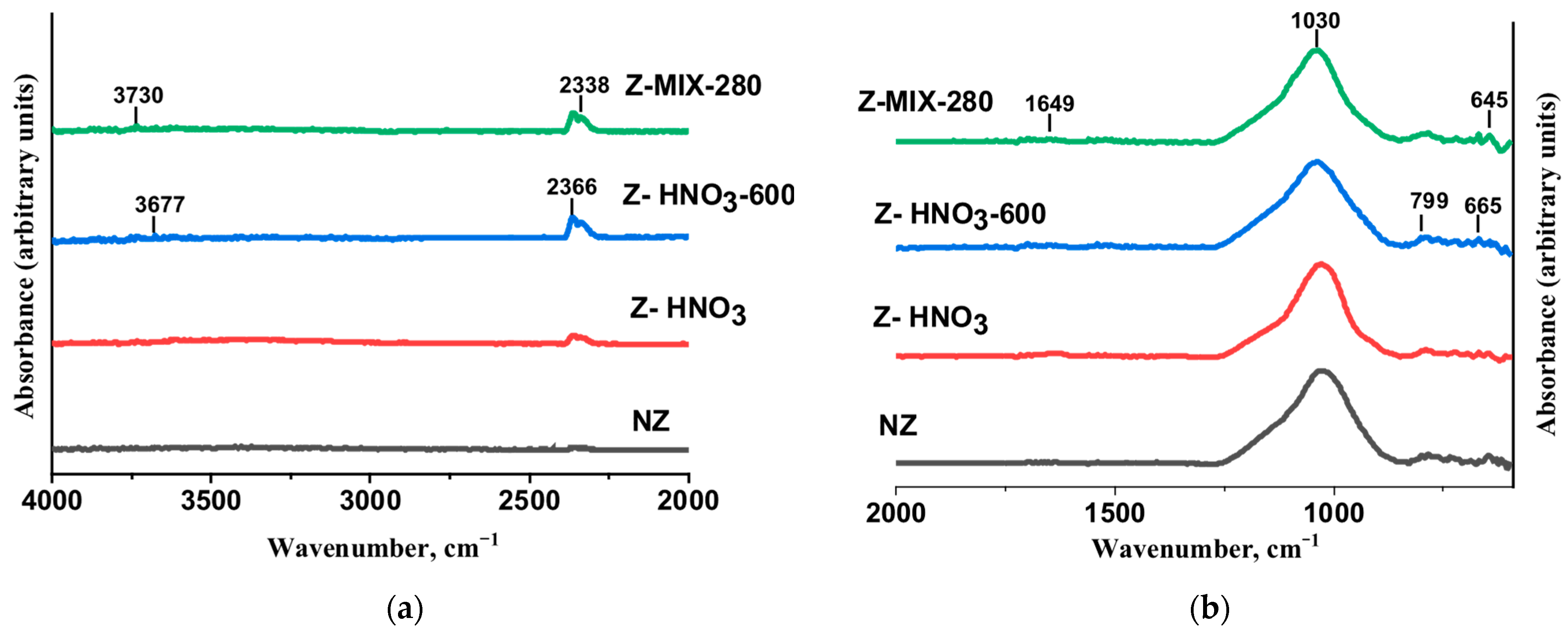
3.1.2. FTIR Analysis
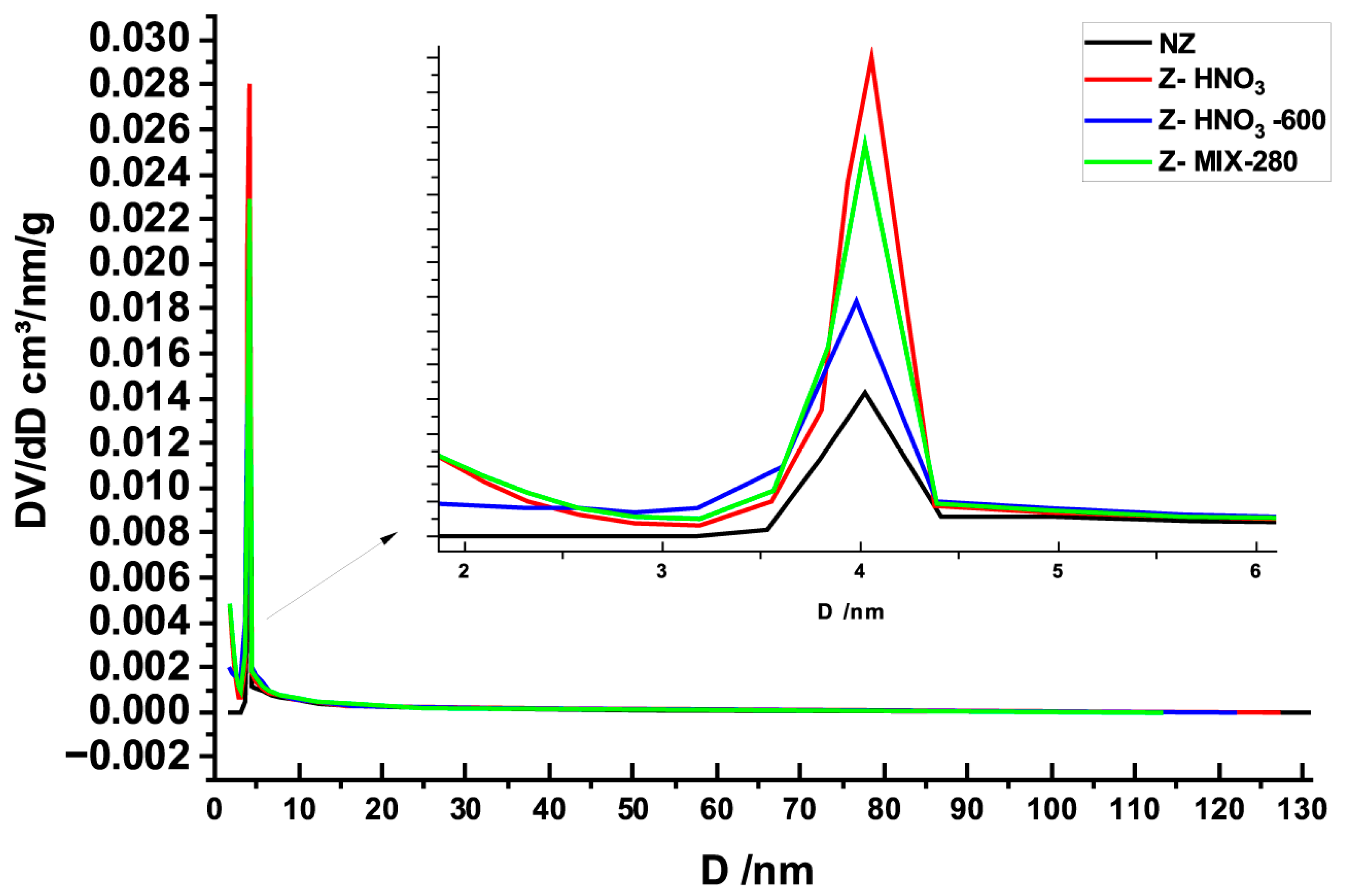
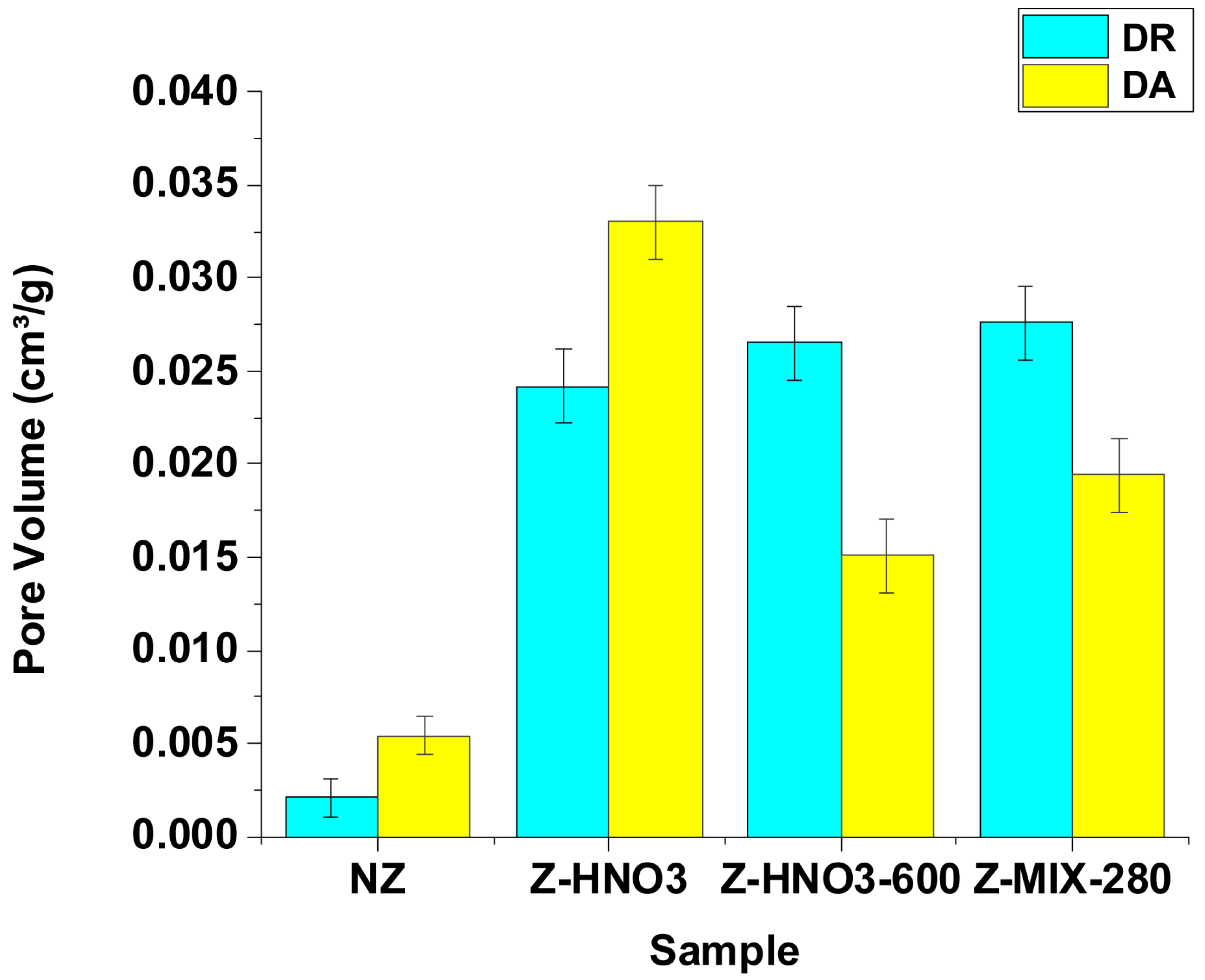
3.1.3. N2 Adsorption–Desorption Isotherms (BET)
3.1.4. Thermogravimetric Analysis (TGA)
3.1.5. SEM and EDX Analysis
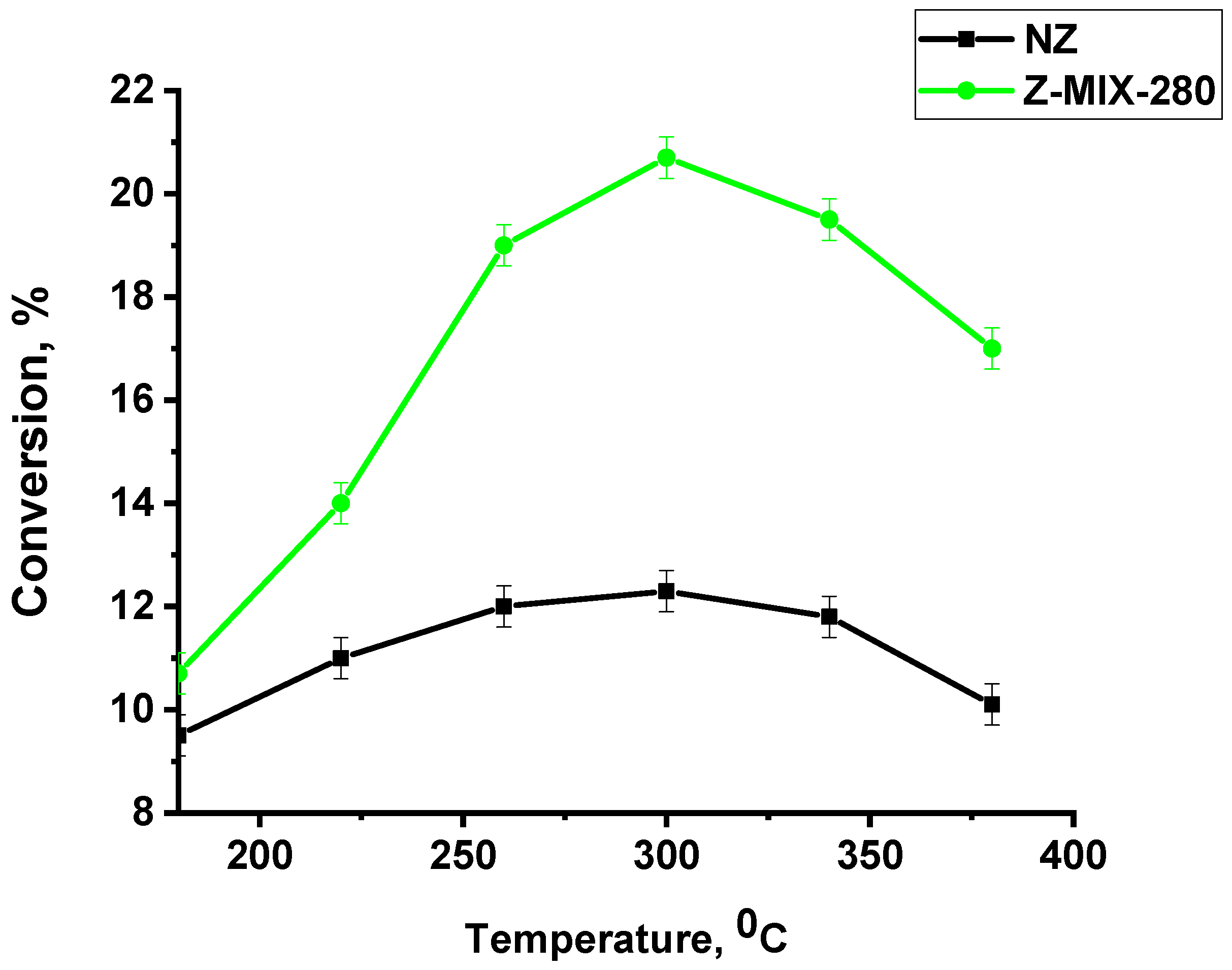
3.2. Catalytic Activity of Zeolite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FTIR | Fourier Transform Infrared Spectroscopy |
| XRD | X-ray Diffraction |
| EDAX | Energy Dispersive X-ray Analysis |
| EDTA | Ethylenediaminetetraacetic Acid |
| SEM | Scanning Electron Microscopy |
| DA | Dubinin–Astakhov method |
| DR | Dubinin–Radushkevich method |
| BJH | Barrett–Joyner–Halenda method |
| TGA | Thermogravimetric Analysis |
| DSC | Differential Scanning Calorimetry |
References
- European Parliament and Council. Directive 2009/30/EC amending Directive 98/70/EC as Regards the Specification of Petrol, Diesel and Gas-Oil and Introducing a Mechanism to Monitor and Reduce Greenhouse Gas Emissions; Official Journal of the European Union: Brussels, Belgium, 2009. [Google Scholar]
- International Maritime Organization (IMO). IMO 2020—Reducing Sulphur Oxide Emissions. London, UK. Available online: https://www.imo.org/en/mediacentre/hottopics/pages/sulphur-2020.aspx (accessed on 7 September 2025).
- Shafiq, I.; Shafique, S.; Akhter, P.; Yang, W.; Murid, H. Recent Developments in Alumina-Supported Hydrodesulfurization Catalysts. Catal. Rev. 2020, 62, 96–164. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Liu, X.; Guo, R.; Meng, B.; Li, G.; Ren, S.; Guo, Q.; Shen, B. Tuning effect of the zeolite Brønsted acidity on the FeZn bimetallic hydrodesulfurization catalyst. Energy Fuels 2021, 36, 527–538. [Google Scholar] [CrossRef]
- Mustapha, U.; Ismail, A.; Abdulkadir, T.; Khalid, A. Modification of ZSM-5 mesoporosity and application as catalyst support in hydrodesulfurization of dibenzothiophene: Experimental and DFT studies. J. Environ. Chem. Eng. 2021, 9, 106738. [Google Scholar] [CrossRef]
- Jiang, K.; Li, Z.; Zheng, Z.; Li, J.; Qi, X.; Zhou, J.; Wei, H.; He, Y.; Xue, M.; Chu, H. Enhanced adsorption performance for aromatic sulfur compounds over a hierarchical structured AgX zeolite. Environ. Sci. Atmos. 2021, 1, 569–576. [Google Scholar] [CrossRef]
- Song, Z.; Bi, M.; Li, J.; Guo, Y.; Xu, Q.; He, Y.; Zhao, N.; Chen, L.; Ren, D. Synthesis of TiO2-Modified Y Zeolite and Its Adsorption-Catalytic Oxidative Desulfurization Performance. Silicon 2024, 16, 4159–4172. [Google Scholar] [CrossRef]
- Molina-Pérez, R.I.; Cruces-Lira, J.; Klimova, T.E. Upgrading titania and alumina supports for NiMo hydrodesulfurization catalysts by the incorporation of 2D graphene oxide. MRS Adv. 2025, 10, 654–659. [Google Scholar] [CrossRef]
- Chen, L.-H.; Sun, M.-H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B.-L. Hierarchically Structured Zeolites: From Design to Application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef] [PubMed]
- Antosik, A.K.; Musik, M.; Miądlicki, P.; Weisbrodt, M.; Wilpiszewska, K. Influence of Acid-Modified Clinoptilolite on the Self-Adhesive Properties of Silicone Pressure-Sensitive Adhesives. Polymers 2023, 15, 707. [Google Scholar] [CrossRef]
- Vasilina, G.K.; Moisa, R.M.; Abildin, T.S.; Yesemaliyeva, A.S.; Kuanyshova, S.D. Influence of the structure of natural zeolites on their acidic characteristics. Izv. NAN RK. Ser. Khimii i Tekhnologii 2017, 6, 81–85. (In Russian) [Google Scholar]
- Mambetova, M.; Yergaziyeva, G.Y.; Zhoketayeva, A.B. Physicochemical characteristics and carbon dioxide sorption properties of natural zeolites. Gorenie i Plazmokhimiya 2022, 21, 81–87. (In Russian) [Google Scholar]
- Fatemeh, B.; Ahad, G. Modification and functionalization of zeolites to improve the efficiency of CO2 adsorption: A review. Case Stud. Chem. Environ. Eng. 2024, 9, 100564. [Google Scholar] [CrossRef]
- Khamzina, Z.B.; Belopukhov, S.L. Efficiency of using the Kazakhstan zeolites for the purification of water from iron ions. IOP Conf. Ser. Earth Environ. Sci. 2022, 965, 012010. [Google Scholar] [CrossRef]
- Akhalbedashvili, L.G. Catalytic and Ion-Exchange Properties of Modified Zeolites and Superconducting Cuprates. Ph.D. Thesis, Ivane Javakhishvili Tbilisi State University, Tbilisi, Georgia, 2006. (In Russian). [Google Scholar]
- Zhu, D.; Wang, L.; Cui, W.; Tan, J.; Tian, P.; Liu, Z. High-Silica Zeolite Y: Seed-Assisted Synthesis, Characterization and Catalytic Properties. Inorg. Chem. Front. 2022, 9, 2213–2220. [Google Scholar] [CrossRef]
- Çakıcıoğlu-Özkan, F.; Becer, M. Effect of the acid type on the natural zeolite structure. JOTCSB 2019, 2, 69–74. [Google Scholar]
- Fani, K.; Lycourghiotis, S.; Bourikas, K.; Kordouli, E. Influence of Natural Mordenite Activation Mode on Its Efficiency as Support of Nickel Catalysts for Biodiesel Upgrading to Renewable Diesel. Nanomaterials 2023, 13, 1603. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wei, C.; Zhao, L.; Gao, J.; Xu, C. Modification of the Acidic and Textural Properties of HY Zeolite by AHFS Treatment and Its Coke Formation Performance in the Catalytic Cracking Reaction of N-Butene. Catalysts 2022, 12, 640. [Google Scholar] [CrossRef]
- Benaliouche, F.; Boucheffa, Y.; Ayrault, P.; Mignard, S.; Magnoux, P. NH3-TPD and FTIR Spectroscopy of Pyridine Adsorption Studies for Characterization of Ag- and Cu-exchanged X Zeolites. Microporous Mesoporous Mater. 2008, 111, 80–88. [Google Scholar] [CrossRef]
- Chizallet, C.; Bouchy, C.; Larmier, K.; Pirngruber, G. Molecular views on mechanisms of Brønsted acid catalyzed reactions in zeolites. Chem. Rev. 2023, 123, 6107–6196. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, H.; Wu, Q.; Xi, X.; Li, Z. Preparation of Al–Si composite from high-alumina coal fly ash by mechanical–chemical synergistic activation. Ceram. Int. 2017, 43, 6532–6541. [Google Scholar] [CrossRef]
- Huntley, G.M.; Luck, R.L.; Mullins, M.E.; Newberry, N.K. Hydrochloric Acid Modification and Lead Removal Studies on Naturally Occurring Zeolites from Nevada, New Mexico, and Arizona. Processes 2021, 9, 1238. [Google Scholar] [CrossRef]
- Wang, T.; Yu, H.; Bian, B.; Liu, Y.; Liu, S.; Yu, S.; Wang, Z. One-Pot Synthesis of Anthraquinone Catalyzed by Microwave Acetic Acid Modified Hβ Zeolite. Catal. Lett. 2020, 150, 3007–3016. [Google Scholar] [CrossRef]
- Yi, F.; Xing, M.; Cao, J.-P.; Guo, S.; Yang, Y. Comparison of Brønsted Acidic Silanol Nests and Lewis Acidic Metal Sites in Ti-Beta Zeolites for Conversion of Butenes. Catalysts 2024, 14, 749. [Google Scholar] [CrossRef]
- Qi, K.; Gao, L.; Li, X.; He, F. Research Progress in Gas Separation and Purification Based on Zeolitic Materials. Catalysts 2023, 13, 855. [Google Scholar] [CrossRef]
- Tanirbergenova, S.; Tagayeva, A.; Rossi, C.O.; Porto, M.; Caputo, P.; Kanzharkan, E.; Tugelbayeva, D.; Zhylybayeva, N.; Tazhu, K.; Tileuberdi, Y. Studying the Characteristics of Tank Oil Sludge. Processes 2024, 12, 2007. [Google Scholar] [CrossRef]
- Sadenova, M.A.; Abdulina, S.A.; Tungatarova, S.A. The use of natural Kazakhstan zeolites for the development of gas purification catalysts. Clean Technol. Environ. Policy 2016, 18, 449–459. [Google Scholar] [CrossRef]
- Aitugan, A.N.; Tanirbergenova, S.K.; Tileuberdi, Y.; Yucel, O.; Tugelbayeva, D.; Mansurov, Z.; Ongarbayev, Y.K. A carbonised cobalt catalyst supported by acid-activated clay for the selective hydrogenation of acetylene. React. Kinet. Mech. Catal. 2021, 133, 277–292. [Google Scholar] [CrossRef]
- Król, M.; Dechnik, J.; Szymczak, P.; Handke, B.; Szumera, M.; Stoch, P. Thermal Behavior of Clinoptilolite. Crystals 2024, 14, 646. [Google Scholar] [CrossRef]
- Cadar, O.; Senila, M.; Hoaghia, M.-A.; Scurtu, D.; Miu, I.; Levei, E.A. Effects of Thermal Treatment on Natural Clinoptilolite-Rich Zeolite Behavior in Simulated Biological Fluids. Molecules 2020, 25, 2570. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, M.; Yan, W.; Yu, J. Regulation of the Si/Al ratios and Al distributions of zeolites and their impact on properties. Chem. Sci. 2023, 14, 1935–1959. [Google Scholar] [CrossRef]
- Alhassan, A.M.; Tanimu, A.; Yusuf, B.O.; Siddiqui, M.A.B.; Aitani, A.; Alhooshani, K.R.; Ganiyu, S.A. Insights into Si/Al ratios for enhanced performance of β-zeolites in thermocatalytic cracking of crude oil to light olefins. J. Energy Inst. 2024, 117, 101792. [Google Scholar] [CrossRef]
- Zahid, M.; Doszhanov, Y.; Saurykova, K.; Ahmadi, N.; Bolatova, D.; Kurmanbayeva, M.; Aydarbek, A.; Ihsas, R.; Seitzhanova, M.; Akhmetzhanova, D.; et al. Modification and Application of Natural Clinoptilolite and Mordenite from Almaty Region for Drinking Water Purification. Molecules 2025, 30, 2021. [Google Scholar] [CrossRef]
- Kuldeyev, E.I.; Orynbekov, Y.S.; Mansurov, Z.A.; Nurlybayev, R.E.; Zhumadilova, Z.O.; Murzagulova, A.A. Applicability of Zeolite from the Daubabinsk and Chankanai Deposits as a Sorbent for Natural Waters. Water 2023, 15, 2231. [Google Scholar] [CrossRef]
- Tsitsishvili, V.; Dolaberidze, N.; Mirdzveli, N.; Nijaradze, M.; Dzhakipbekova, N.; Harutyunyan, L.; Amiridze, Z.; Khutsishvili, B. Acid treatment of Georgian, Kazakhstani and Armenian natural heulandite-clinoptilolites II. Adsorption and porous structure. In Proceedings of the 6th ISPC «International Scientific Discussion: Problems, Tasks and Prospects», Brighton, UK, 19–20 March 2023. [Google Scholar] [CrossRef]
- Cui, T.-Y.; Rajendran, A.; Fan, H.X.; Feng, J.; Li, W.Y. Review on Hydrodesulfurization over Zeolite-Based Catalysts. Ind. Eng. Chem. Res. 2021, 60, 3295–3323. [Google Scholar] [CrossRef]
- Khare, R.; Weindl, R.; Kim, S.; Kovarik, L.; Jentys, A.; Reuter, K.; Lercher, J.A. Hydrogen Activation on Zeolite Stabilized Ni–Mo Sulfide Clusters. JACS Au 2025, 5, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Guo, X.; Bao, W.; Chang, L.; Liao, J. Metal Modified NaY Zeolite as Sorbent for the Ultra-Deep Removal of Thiophene in Simulated Coke Oven Gas. Energies 2022, 15, 2620. [Google Scholar] [CrossRef]
- Boukoberine, Y.; Boudjema, H. Thiophene hydrodesulfurization over CoMo/Al2O3-CuY catalysts: Temperature effect study. Arab. J. Chem. 2011, 9, S522–S527. [Google Scholar] [CrossRef]

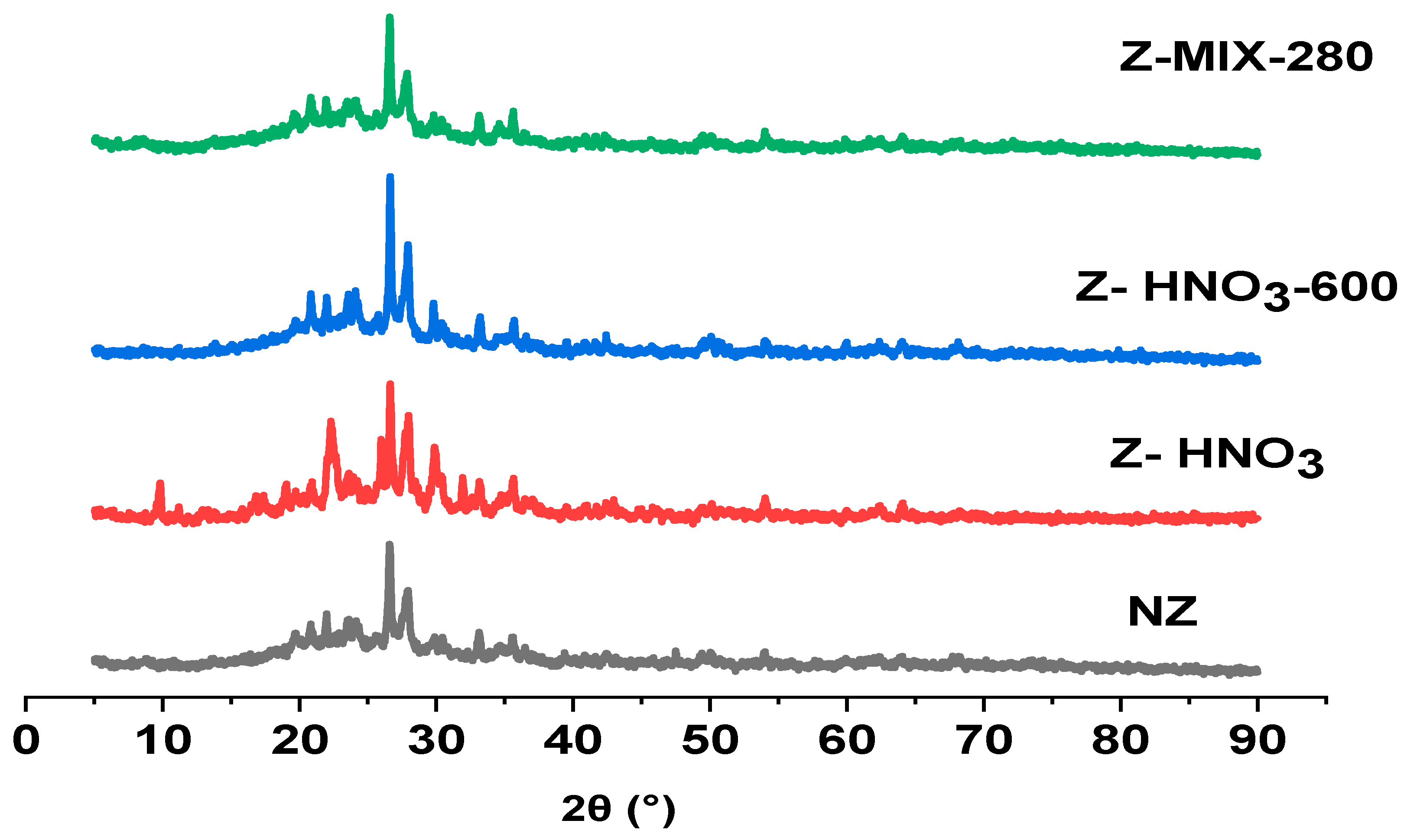


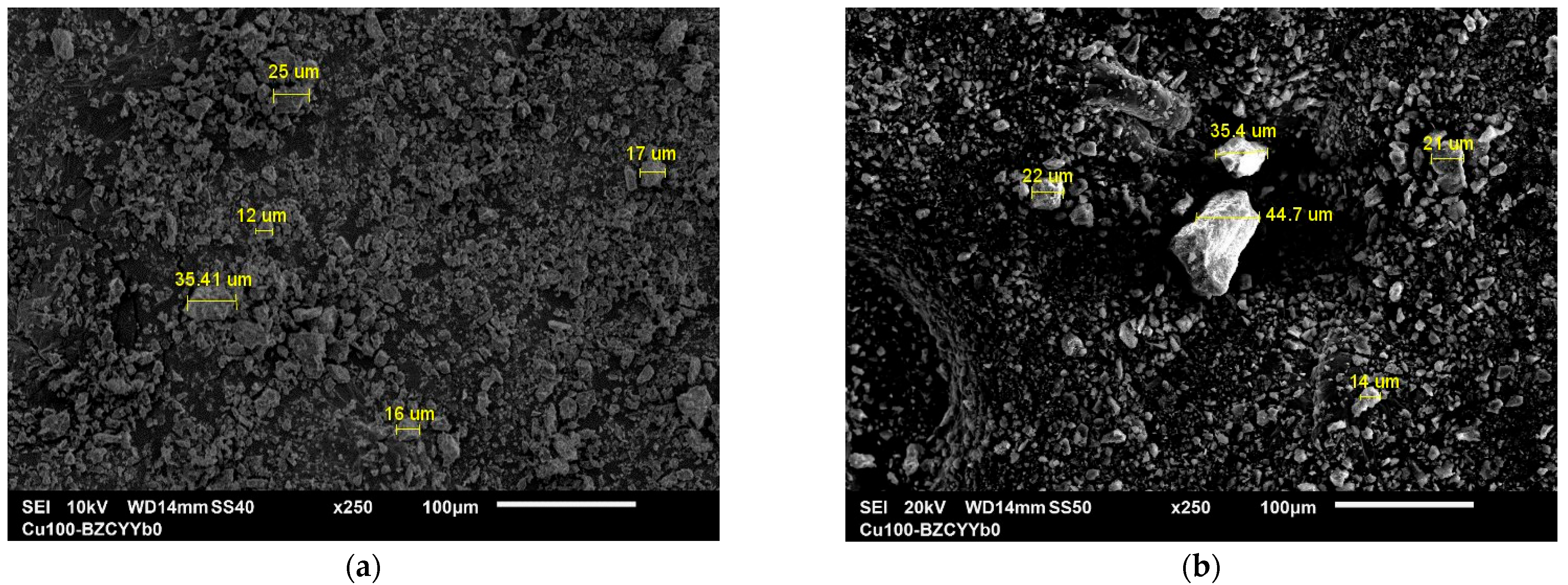
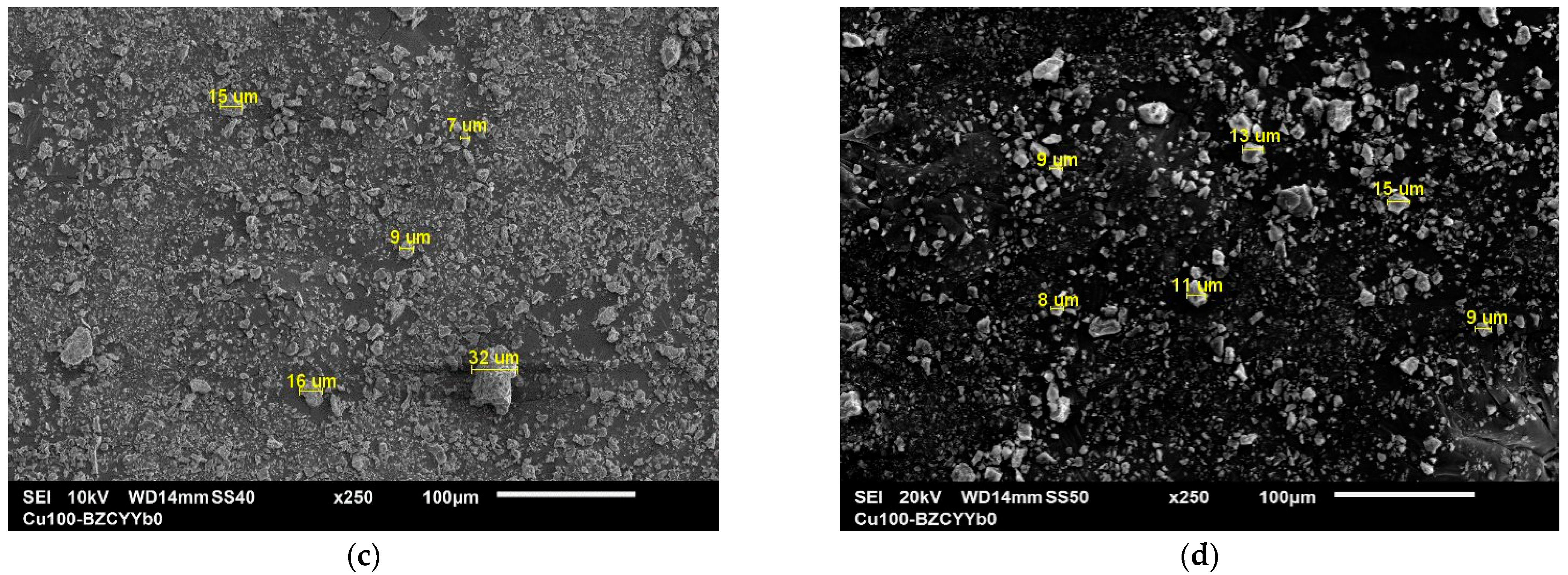
| Sample | Phases Identified | Semi-Quantitative Composition (wt.%) |
|---|---|---|
| NZ (Natural zeolite) | NaAlSi3O8, SiO2, KAlSi3O8, Na0.3Fe2Si4O10(OH)2·4H2O, Fe2O3 | 35.8, 23.9, 19.1, 15.6, 5.6 |
| Z–HNO3 | (Ca,Na)2–3Al3(Al,Si)2Si13O36·12H2O (heulandite), SiO2, NaAlSi3O8, B-containing phase, Fe2O3 | 43, 20, 14, 16, 6 |
| Z–HNO3-600 | (Na,Ca)Al(Si,Al)3O8, SiO2, KAlSi3O8, NaAlSi3O8, Fe2O3 | 48, 22, 24, 18, 6 |
| Z–MIX-280 | NaAlSi3O8, KAlSi3O8, Na0.3Fe2Si4O10(OH)2·4H2O, SiO2, Fe2O3 | 53, 23, 11, 9, 4 |
| 2θ (°) JCPDS | hkl | Rel. Int. JCPDS (%) | 2θ (°) NZ | Rel. Int. NZ (%) | 2θ (°) Z–HNO3 | Rel. Int. Z–HNO3 (%) | 2θ (°) Z–HNO3-600 | Rel. Int. (%) Z–HNO3-600 | 2θ (°) Z–MIX-280 | Rel. Int. (%) Z–MIX-280 |
|---|---|---|---|---|---|---|---|---|---|---|
| 9.8 | (020) | 100 | 9.79 | 44 | ||||||
| 11.2 | (110) | 20 | 11.18 | 28 | ||||||
| 19.7 | (111) | 35 | 19.7 | 41 | 19.73 | 38 | 19.72 | 33 | 19.77 | 41 |
| 22.4 | (400) | 60 | 21.96 | 52 | 22.32 | 79 | 21.99 | 42 | 21.97 | 50 |
| 26.6 | (402) | 40 | 26.59 | 100 | 26.63 | 100 | 26.62 | 100 | 26.57 | 100 |
| 27.9 | (511) | 35 | 27.9 | 69 | 27.96 | 83 | 27.91 | 70 | 27.85 | 65 |
| 29.9 | (222) | 30 | 29.82 | 38 | 29.89 | 65 | 29.81 | 40 | 29.81 | 41 |
| 33.1 | (440) | 25 | 33.13 | 42 | 33.15 | 44 | 33.17 | 35 | 33.13 | 41 |
| 36.5 | (600) | 15 | 36.5 | 32 | 36.48 | 28 | 36.53 | 25 | 36.45 | 30 |
| Wavenumber (cm−1) | Assignment | Samples Observed |
|---|---|---|
| 3800–3300 | O–H stretching (water, hydroxyl groups) | All (more intense in modified samples) |
| ~1640 | H–O–H bending (adsorbed water) | Stronger in Z–HNO3, reduced after heating |
| 1030–1032 | Asymmetric Si–O–Si/Si–O–Al stretching | All samples |
| 799–787 | Symmetric stretching of tetrahedral units | All samples |
| 645–616 | Framework bending vibrations | All samples |
| 2400–2300 | CO2 background interference (not structural) | All spectra (artifact) |
| Sample | BET SSA (m2/g) | DR Micropore SSA (m2/g) | DR Micropore Volume (cm3/g) | DA Micropore Volume (cm3/g) | DR Avg. Pore Diameter (nm) | Average Pore Diameter (4V/A, nm) |
|---|---|---|---|---|---|---|
| NZ | 4.95 ± 0.25 | 5.91 ± 0.30 | 0.002 ± 0.001 | 0.005 ± 0.001 | 2.09 ± 0.10 | 15.99 ± 0.80 |
| Z–HNO3 | 59.86 ± 2.99 | 67.98 ± 3.40 | 0.024 ± 0.001 | 0.033 ± 0.001 | 1.36 ± 0.07 | 3.26 ± 0.16 |
| Z–HNO3-600 | 19.39 ± 0.97 | 21.09 ± 1.05 | 0.026 ± 0.001 | 0.015 ± 0.001 | 1.75 ± 0.09 | 6.21 ± 0.31 |
| Z–MIX-280 | 48.07 ± 2.40 | 54.50 ± 2.73 | 0.027 ± 0.001 | 0.019 ± 0.001 | 1.52 ± 0.08 | 3.84 ± 0.19 |
| Sample | Phase Composition, wt.% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | SiO2 | Fe2O3 | MgO | Na2O | K2O | CaO | TiO2 | H2O | Total | |
| NZ | 15.1 | 62.2 | 5.8 | 3.6 | 5.8 | 2.1 | 4.1 | 0.7 | 0.6 | 100.0 |
| Z–HNO3 | 16.7 | 71.4 | 2.9 | 2.28 | 2.6 | 1.5 | 2.1 | 0.02 | 0.5 | 100.0 |
| Z–HNO3-600 | 18.2 | 73.5 | 2.1 | 1.9 | 2.2 | 1.1 | 1.9 | 0.01 | 0.09 | 100.0 |
| Z–MIX-280 | 20.2 | 76.04 | 0.6 | 0.8 | 0.7 | 0.7 | 0.9 | 0.01 | 0.05 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanirbergenova, S.; Tugelbayeva, D.; Zhylybayeva, N.; Aitugan, A.; Tazhu, K.; Moldazhanova, G.; Mansurov, Z. Effect of Acid Treatment on the Structure of Natural Zeolite from the Shankhanai Deposit. Processes 2025, 13, 2896. https://doi.org/10.3390/pr13092896
Tanirbergenova S, Tugelbayeva D, Zhylybayeva N, Aitugan A, Tazhu K, Moldazhanova G, Mansurov Z. Effect of Acid Treatment on the Structure of Natural Zeolite from the Shankhanai Deposit. Processes. 2025; 13(9):2896. https://doi.org/10.3390/pr13092896
Chicago/Turabian StyleTanirbergenova, Sandugash, Dildara Tugelbayeva, Nurzhamal Zhylybayeva, Aizat Aitugan, Kairat Tazhu, Gulya Moldazhanova, and Zulkhair Mansurov. 2025. "Effect of Acid Treatment on the Structure of Natural Zeolite from the Shankhanai Deposit" Processes 13, no. 9: 2896. https://doi.org/10.3390/pr13092896
APA StyleTanirbergenova, S., Tugelbayeva, D., Zhylybayeva, N., Aitugan, A., Tazhu, K., Moldazhanova, G., & Mansurov, Z. (2025). Effect of Acid Treatment on the Structure of Natural Zeolite from the Shankhanai Deposit. Processes, 13(9), 2896. https://doi.org/10.3390/pr13092896







