Antimicrobial Activity of Origanum vulgare L. And Salvia rosmarinus Spenn (syn Rosmarinus officinalis L.) Essential Oil Combinations Against Escherichia coli and Salmonella typhimurium Isolated from Poultry
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil Extraction and Characterization
2.2. Microorganism Isolation from Poultry and Identification
2.3. Minimum Inhibitory and Bactericidal Concentrations
2.4. EO Interaction Assay
2.5. Statistical Analysis
3. Results
3.1. Essential Oil Extraction and Characterization
3.2. Minimum Inhibitory and Bactericidal Concentrations
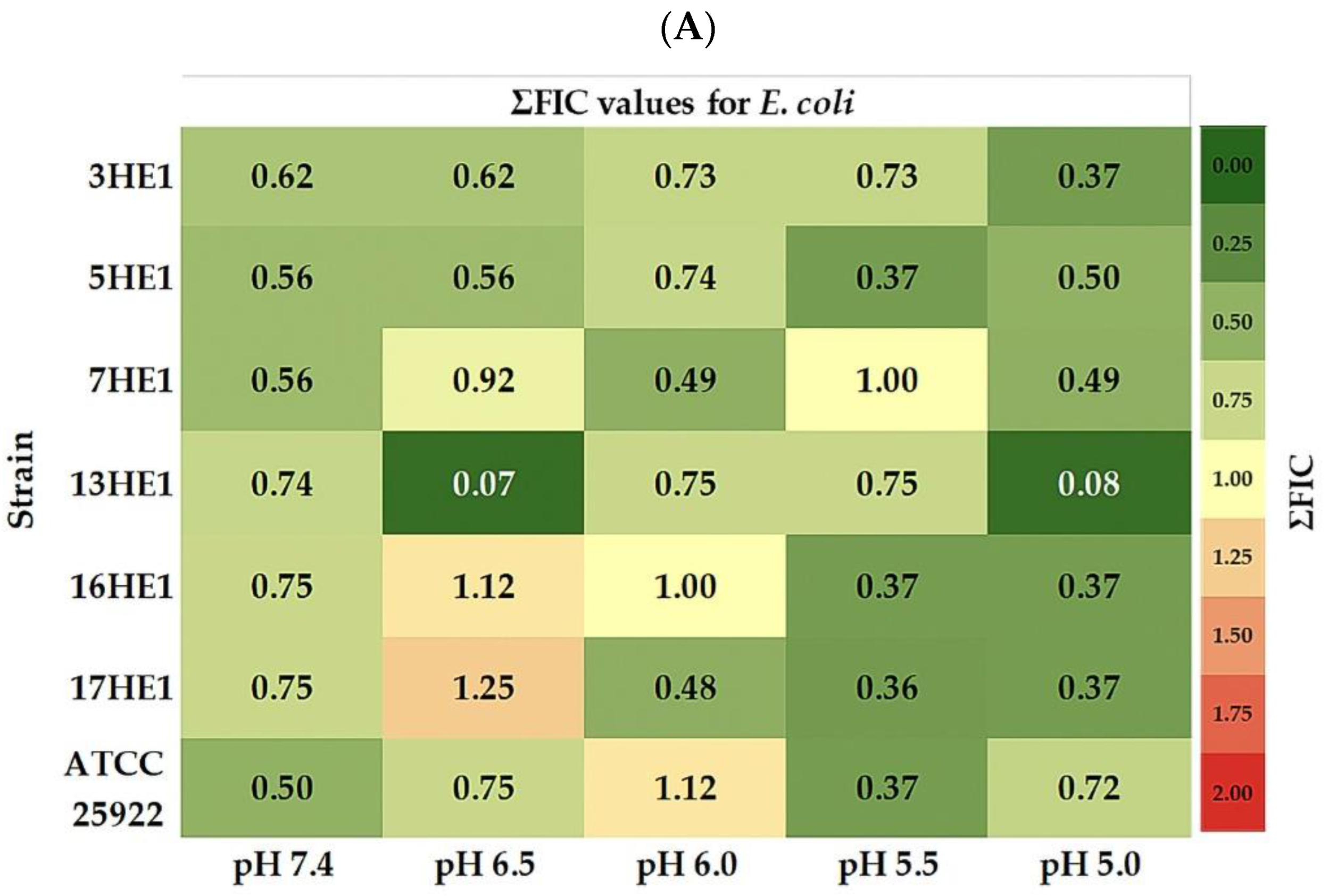
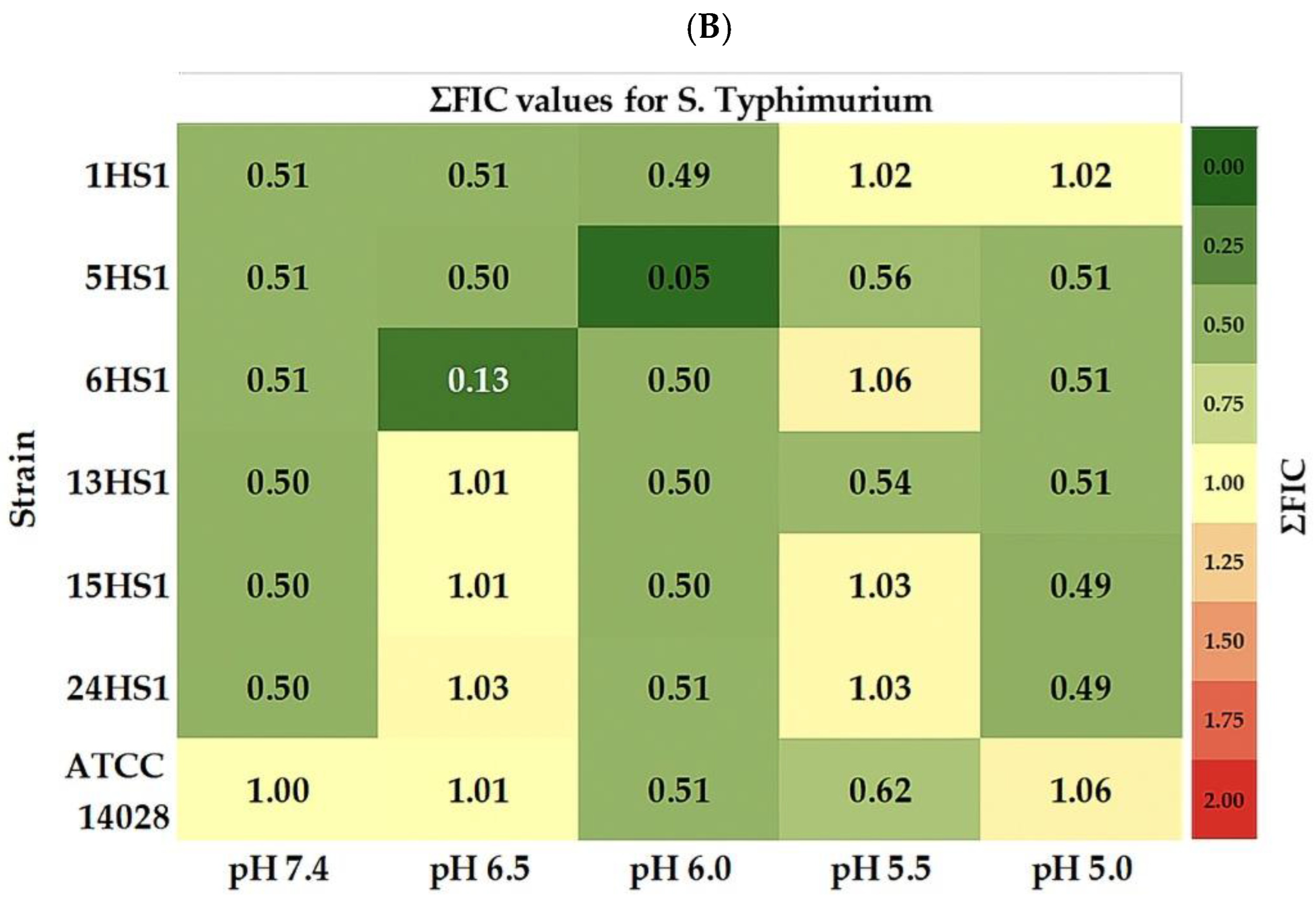
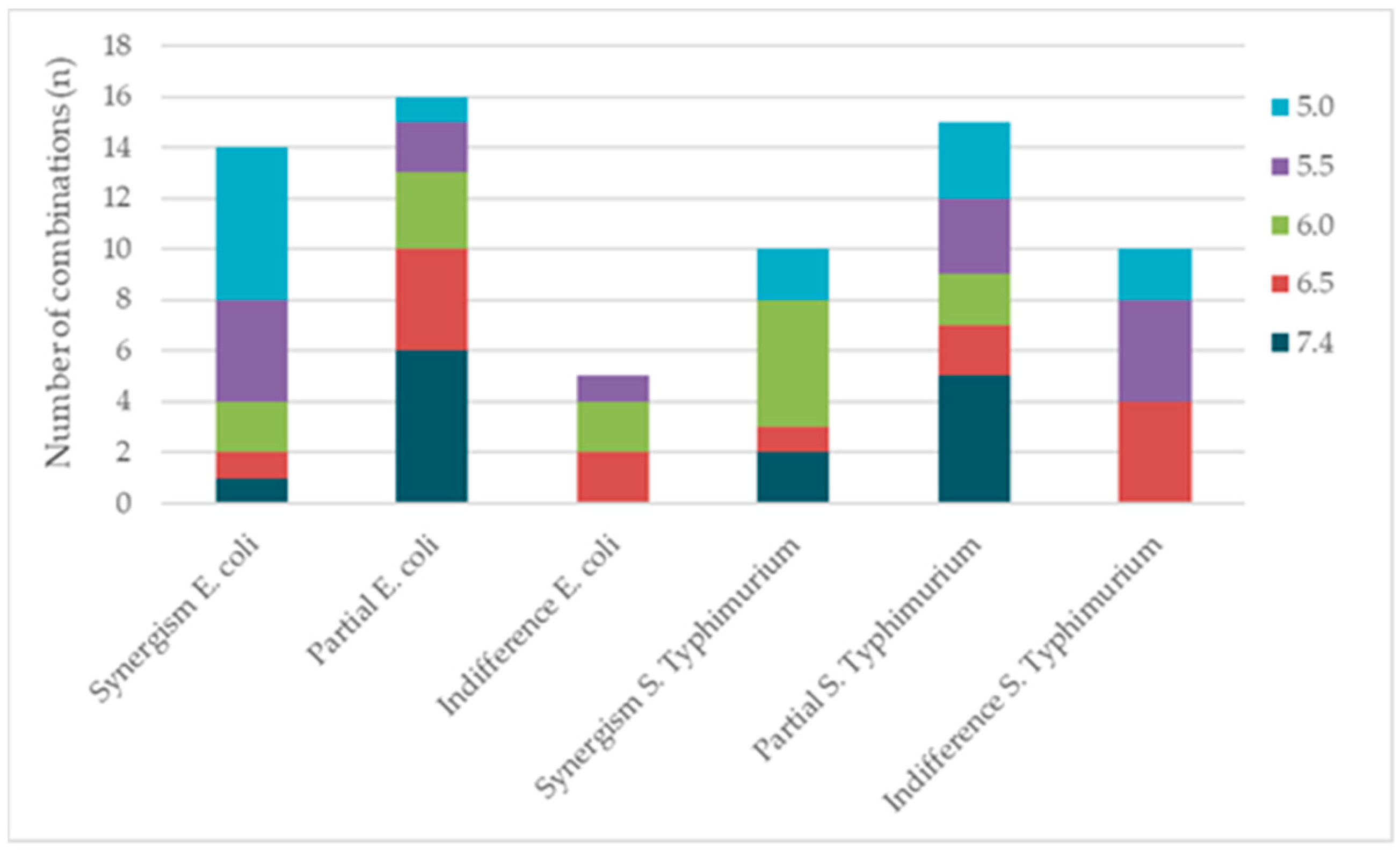
3.3. Antimicrobial Interaction Between OVEO and ROEO
4. Discussion
4.1. Essential Oil Composition
4.2. Antimicrobial Activity and pH-Dependency
4.3. Antimicrobial Interaction Between OVEO and ROEO
4.4. Implications for Poultry Production and AGP Alternatives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Wallace, R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004, 63, 621–629. [Google Scholar] [CrossRef]
- Castanon, J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Foreign Agricultural Service. Livestock and Poultry: World Markets and Trade. 2018. Available online: https://apps.fas.usda.gov/psdonline/circulars/livestock_poultry.pdf (accessed on 10 August 2025).
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef]
- Martinez Martinez, R.; Ortega Cerrilla, M.E.; Herrera Haro, J.G.; Kawas Garza, J.R.; Zárate Ramos, J.J.; Robles Soriano, R. Uso de aceites esenciales en animales de granja. Interciencia 2015, 40, 744–750. [Google Scholar]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef]
- Lupia, C.; Castagna, F.; Bava, R.; Naturale, M.D.; Zicarelli, L.; Marrelli, M.; Statti, G.; Tilocca, B.; Roncada, P.; Britti, D.; et al. Use of essential oils to counteract the phenomena of antimicrobial resistance in livestock species. Antibiotics 2024, 13, 163. [Google Scholar] [CrossRef]
- Londero, A. Alimentos Funcionales: Obtención de un Producto Probiótico para Aves a partir de Suero de Quesería Fermentado con Microorganismos de Kéfir. Ph.D. Thesis, Facultad de Ciencias Exactas, Universidad Nacional de La Plata, La Plata, Argentina, 2012. [Google Scholar]
- Kmeť, V.; Piatnicová, E. Antibiotic resistance in commensal intestinal microflora. Folia Microbiol. 2010, 55, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Apajalahti, J.; Kettunen, A.; Graham, H. Characteristics of the gastrointestinal microbial communities with special reference to the chicken. World’s Poult. Sci. J. 2004, 60, 223–232. [Google Scholar] [CrossRef]
- Butaye, P.; Devriese, L.; Haesebrouck, F. Antimicrobial growth promoters used in animal feed: Effects of less well-known antibiotics on Gram-positive bacteria. Clin. Microbiol. Rev. 2003, 16, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Morales, R. Las Paredes Celulares de Levadura de Saccharomyces cerevisiae: Un Aditivo Natural Capaz de Mejorar la Productividad y Salud de Pollo de Engorde. Ph.D. Thesis, Universidad Autónoma de Barcelona, Barcelona, Spain, 2007. [Google Scholar]
- Lee, K.; Lillehoj, H.S.; Siragusa, G.R. Direct-fed microbials and their impact on the intestinal microflora and immune system of chickens. J. Poult. Sci. 2010, 47, 106–114. [Google Scholar] [CrossRef]
- Kizerwetter-Świda, M.; Binek, M. Bacterial microflora of the chicken embryos and newly hatched chicken. J. Anim. Feed Sci. 2008, 17, 224–232. [Google Scholar] [CrossRef][Green Version]
- Chambers, J.; Gong, J. The intestinal microbiota and its modulation for Salmonella control in chickens. Food Res. Int. 2011, 44, 3149–3159. [Google Scholar] [CrossRef]
- El-Sawah, A.A.; Dahshan, A.H.; El-Nahass, E.-S.; El-Mawgoud, A.A. Pathogenicity of Escherichia coli O157 in commercial broiler chickens. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 553–558. [Google Scholar] [CrossRef]
- FAO/WHO. The Need to Strengthen National Monitoring Programs; Joint FAO/WHO Expert Consultation: Rome, Italy, 2005. [Google Scholar]
- Mathlouthi, N.; Bouzaienne, T.; Oueslati, I.; Recoquillay, F.; Hamdi, M. Use of rosemary, oregano, and a commercial blend of essential oils in broiler chickens: In vitro antimicrobial activities and effects on growth performance. J. Anim. Sci. 2012, 90, 813–823. [Google Scholar] [CrossRef]
- Kachkoul, R.; Benjelloun Touimi, G.; Bennani, B.; El Mouhri, G.; El Habbani, R.; Zouhri, A.; El-Mernissi, Y.; Lahrichi, A. Optimisation of three essential oils against Salmonella spp. and Escherichia coli by mixture design. Chem. Biodivers. 2023, 20, e202301221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Xin, H.; Chen, S.; Yang, C.; Duan, Y.; Yang, X. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim. Sci. J. 2017, 88, 1414–1424. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mandal, G.; Patra, A.; Kumar, P.; Samanta, I.; Pradhan, S.; Samanta, A. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim. Feed Sci. Technol. 2018, 236, 39–47. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Christaki, E.; Fletouris, D.J.; Florou-Paneri, P.; Spais, A.B. The effect of dietary oregano essential oil on lipid oxidation in raw and cooked chicken during refrigerated storage. Meat Sci. 2002, 62, 259–265. [Google Scholar] [CrossRef]
- Koiyama, T.G.; Rosa, A.P.; Padilha, M.T.S.; Boemo, L.S.; Scher, A.; da Silva Melo, A.M.; de Oliveira Fernandes, M. Desempenho e rendimento de carcaça de frangos de corte alimentados com mistura de aditivos fitogênicos na dieta. Pesq. Agropec. Bras. 2014, 49, 225–231. [Google Scholar] [CrossRef]
- Buldain, D.; Gortari Castillo, L.; Marchetti, M.L.; Julca Lozano, K.; Bandoni, A.; Mestorino, N. Modeling the growth and death of Staphylococcus aureus against Melaleuca armillaris essential oil at different pH conditions. Antibiotics 2021, 10, 222. [Google Scholar] [CrossRef]
- Holmgren, P.K.; Holmgren, N.H.; Barnett, L.C. Index Herbariorum, Part I: The Herbaria of the World, 8th ed.; The New York Botanic Garden Press: New York, NY, USA, 1990. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Wiley/NIST. The Wiley/NBS Registry of Mass Spectral Data, 8th ed.; J. Wiley & Sons, Inc.: New York, NY, USA, 2008. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 7th ed.; CLSI Supplement VET01S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Eliopoulos, G.M.; Moellering, R.C. Antimicrobial combinations. In Antibiotics in Laboratory Medicine; Lorian, V., Ed.; Williams & Wilkins: Baltimore, MD, USA, 1996; pp. 330–396. [Google Scholar]
- Saviuc, C.; Gheorghe, I.; Coban, S.; Drumea, V.; Chifiriuc, M.; Banu, O.; Bezirtzoglou, E.; Laz, V. Rosmarinus officinalis essential oil and eucalyptol act as efflux pumps inhibitors and increase ciprofloxacin efficiency against Pseudomonas aeruginosa and Acinetobacter baumannii MDR strains. Rom. Biotechnol. Lett. 2016, 21, 11782–11790. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Sana, A.M.; van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Chowdhury, K.; Sharmin, T.; Islam, M.N. Comparison of chemical constituents of essential oils from Origanum vulgare and Rosmarinus officinalis grown in Bangladesh. Bangladesh J. Bot. 2018, 47, 1093–1100. [Google Scholar]
- Al-Maharik, N.; Jaradat, N.; Hawash, M.; Al-Lahham, S.; Qadi, M.; Shoman, I.; Jaber, S.; Rahem, R.A.; Hussein, F.; Issa, L. Chemical Composition, Antioxidant, Antimicrobial and Anti-Proliferative Activities of Essential Oils of Rosmarinus officinalis from five Different Sites in Palestine. Separations 2022, 9, 339. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Lira-Moreno, C.Y.; Guerrero-Legarreta, I.; Wild-Padua, G.; Di Pierro, P.; García-Almendárez, B.E.; Regalado-González, C. Effect of nanoemulsified and microencapsulated Mexican oregano (Lippia graveolens Kunth) essential oil coatings on quality of fresh pork meat. J. Food Sci. 2017, 82, 1423–1432. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.W.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [CrossRef]
- Bassolé, I.H.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
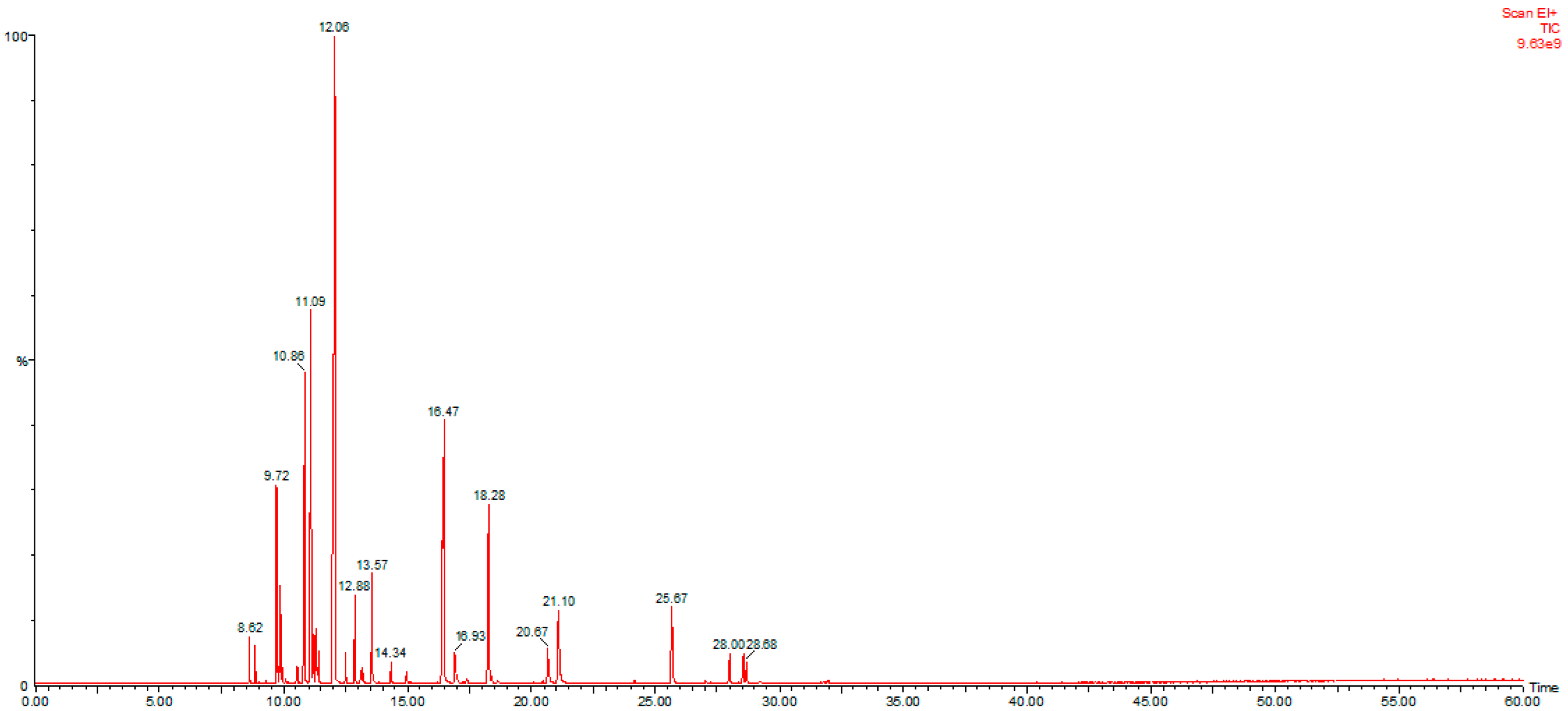
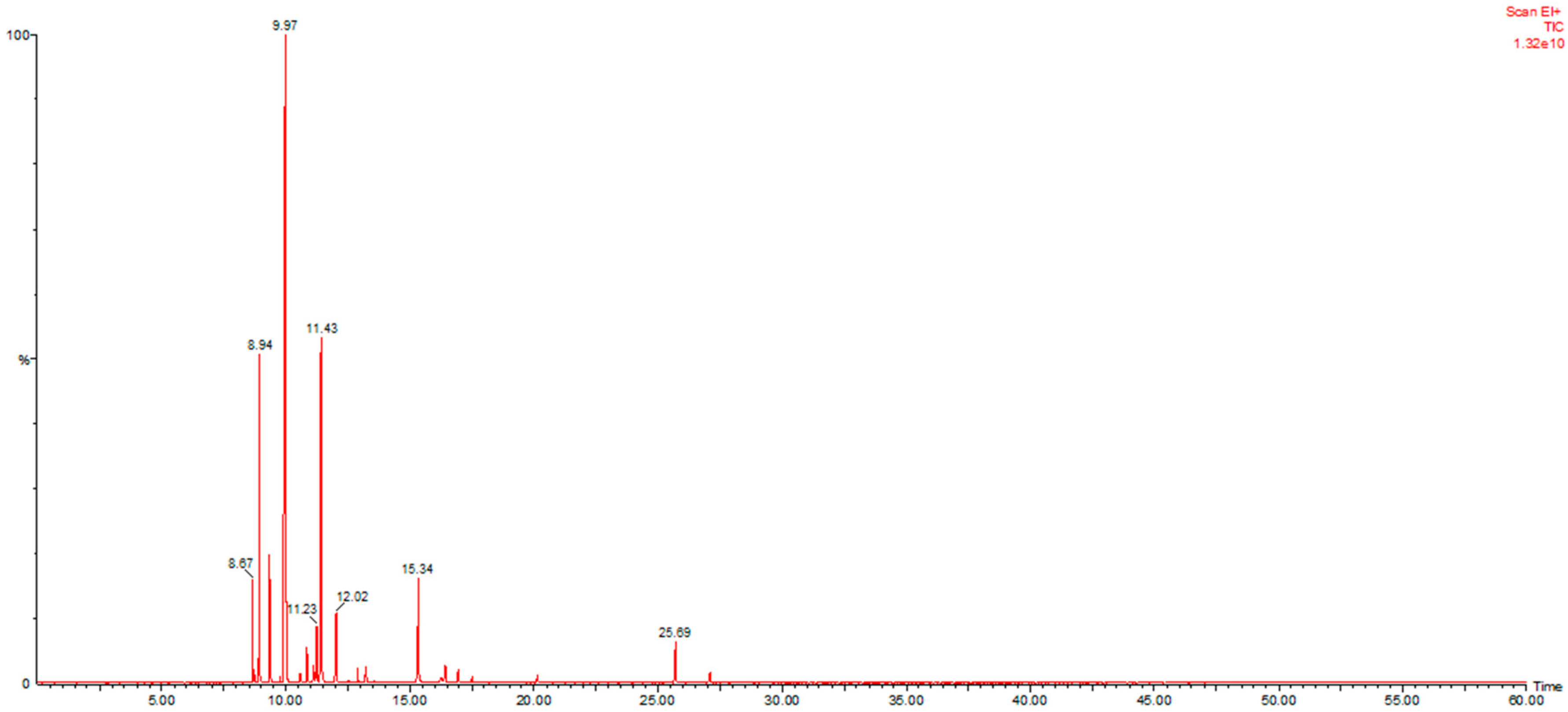
| Tr | LRIN | COMPOUND | % | Tr | LRIN | COMPOUND | % |
|---|---|---|---|---|---|---|---|
| 8.6 | 933 | α-Thujene | 1.1 | 13.6 | 1099 | trans-Sabinene hydrate | 4.0 |
| 8.9 | 945 | α-Pinene | 0.9 | 14.3 | 1105 | Linalool | 0.4 |
| 9.3 | 961 | Camphene | 0.1 | 14.9 | 1110 | 3-Octen-1-yl acetate | 0.3 |
| 9.7 | 971 | 3-Octen-1-ol | 1.0 | 16.4 | 1197 | Terpinen-4-ol | 10.6 |
| 9.8 | 985 | Sabinene | 5.4 | 17.0 | 1211 | α-Terpineol | 1.0 |
| 9.9 | 992 | β-Myrcene | 2.0 | 18.3 | 1243 | Carvacrol methyl ether | 5.9 |
| 10.0 | 994 | ß-Pinene | 0.4 | 20.7 | 1295 | Thymol | 2.0 |
| 10.6 | 1017 | α-Phellandrene | 0.4 | 21.1 | 1298 | Carvacrol | 4.5 |
| 10.9 | 1025 | α-Terpinene | 7.5 | 25.7 | 1437 | ß-Caryophyllene | 5.0 |
| 11.1 | 1034 | p-Cymene | 7.5 | 27.8 | 1458 | allo-Aromadendrene | 0.2 |
| 11.2 | 1038 | Limonene | 0.9 | 28.0 | 1496 | Germacrene D | 1.0 |
| 11.3 | 1043 | ß-Phellandrene | 1.2 | 28.6 | 1508 | Bicyclogermacrene | 1.2 |
| 11.4 | 1048 | trans-ß-Ocimene | 0.3 | 28.7 | 1510 | ß-Bisabolene | 0.8 |
| 12.1 | 1060 | γ -Terpinene | 24.0 | 32.1 | 1596 | Spathulenol | 0.1 |
| 12.5 | 1067 | cis-Sabinene hydrate | 0.9 | 32.2 | 1601 | Caryophyllene oxide | 0.1 |
| 12.9 | 1095 | Terpinolene | 2.8 | ||||
| TOTAL | 93.5 | ||||||
| Tr | LRIN | COMPOUND | % | Tr | LRIN | COMPOUND | % |
|---|---|---|---|---|---|---|---|
| 8.6 | 933 | α-Thujene | 3.3 | 12.0 | 1060 | γ-Terpinene | 3.3 |
| 8.7 | 937 | Tricyclene | 0.1 | 12.9 | 1095 | Terpinolene | 0.8 |
| 8.9 | 945 | α-Pinene | 13.3 | 13.2 | 1105 | Linalool | 0.7 |
| 9.4 | 961 | Camphene | 5.4 | 15.4 | 1141 | Camphor | 7.6 |
| 9.8 | 985 | Sabinene | 0.3 | 16.2 | 1163 | Borneol | 1.0 |
| 9.9 | 992 | β-Myrcene | 29.8 | 16.4 | 1197 | Terpinen-4-ol | 0.9 |
| 10.0 | 994 | ß-Pinene | 4.1 | 17.5 | 1204 | Verbenone | 0.7 |
| 10.6 | 1017 | α-Phellandrene | 0.4 | 16.9 | 1211 | α-Terpineol | 0.8 |
| 10.9 | 1025 | α-Terpinene | 1.3 | 20.1 | 1285 | Bornyl acetate | 0.3 |
| 11.1 | 1034 | p-Cymene | 0.5 | 25.7 | 1437 | ß-Caryophyllene | 1.0 |
| 11.2 | 1038 | Limonene | 2.7 | 27.0 | 1455 | α-Humulene | 0.9 |
| 11.4 | 1040 | Eucalyptol (1,8-Cineole) | 15.2 | 32.2 | 1601 | Caryophyllene oxide | 0.1 |
| TOTAL | 94.5 | ||||||
| * OVEO E. coli | * ROEO E. coli | MIC ROEO/OVEO (µL/mL/µL/mL) | ΣFIC | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | pH | MIC (µL/mL) | MBC (µL/mL) | R MBC/MIC | MIC (µL/mL) | MBC (µL/mL) | R MBC/MIC | |||
| 3HE1 | 7.4 | 6 | 6 | 1 | 50 | 100 | 2 | 6/3 | 0.62 | PS |
| 6.5 | 3 | 3 | 1 | 12.5 | 50 | 4 | 1.5/1.5 | 0.62 | PS | |
| 6.0 | 3 | 3 | 1 | 6 | 25 | 4 | 3/0.7 | 0.73 | PS | |
| 5.5 | 3 | 3 | 1 | 6 | 12.5 | 2 | 3/0.7 | 0.73 | PS | |
| 5.0 | 6 | 12.5 | 2 | 12.5 | 50 | 4 | 1.5/1.5 | 0.37 | S | |
| 5HE1 | 7.4 | 6 | 6 | 1 | 25 | 100 | 4 | 1.5/3 | 0.56 | PS |
| 6.5 | 6 | 6 | 1 | 25 | 100 | 4 | 1.5/3 | 0.56 | PS | |
| 6.0 | 3 | 3 | 1 | 12.5 | 50 | 4 | 3/1.5 | 0.74 | PS | |
| 5.5 | 6 | 6 | 1 | 12.5 | 50 | 4 | 1.5/1.5 | 0.37 | S | |
| 5.0 | 3 | 3 | 1 | 12.5 | 12.5 | 1 | 1.5/1.5 | 0.50 | S | |
| 7HE1 | 7.4 | 6 | 6 | 1 | 25 | 100 | 4 | 1.5/3 | 0.56 | PS |
| 6.5 | 3 | 3 | 1 | 25 | 100 | 4 | 12.5/1.25 | 0.92 | PS | |
| 6.0 | 6 | 6 | 1 | 12.5 | 50 | 4 | 3/1.5 | 0.49 | S | |
| 5.5 | 3 | 3 | 1 | 3 | 25 | 8 | 1.5/1.5 | 1.00 | I | |
| 5.0 | 6 | 6 | 1 | 12.5 | 50 | 4 | 3/1.5 | 0.49 | S | |
| 13HE1 | 7.4 | 3 | 3 | 1 | 3 | 100 | 8 | 1.5/3 | 0.74 | PS |
| 6.5 | 3 | 3 | 1 | 12.5 | 100 | 8 | 0.15/0.3 | 0.07 | S | |
| 6.0 | 3 | 3 | 1 | 6 | 25 | 4 | 1.5/1.5 | 0.75 | PS | |
| 5.5 | 3 | 3 | 1 | 6 | 12.5 | 2 | 1.5/1.5 | 0.75 | PS | |
| 5.0 | 6 | 6 | 1 | 3 | 6 | 2 | 1.5/1.5 | 0.08 | S | |
| 16HE1 | 7.4 | 6 | 6 | 1 | 6 | 50 | 8 | 1.5/3 | 0.75 | PS |
| 6.5 | 12.5 | 12.5 | 1 | 12.5 | 100 | 8 | 1.5/12.5 | 1.12 | I | |
| 6.0 | 3 | 6 | 2 | 6 | 25 | 4 | 3/1.5 | 1.00 | I | |
| 5.5 | 6 | 6 | 1 | 12.5 | 25 | 2 | 1.5/1.5 | 0.37 | S | |
| 5.0 | 6 | 6 | 1 | 12.5 | 12.5 | 1 | 1.5/1.5 | 0.37 | S | |
| 17HE1 | 7.4 | 6 | 6 | 1 | 6 | 25 | 4 | 1.5/3 | 0.75 | PS |
| 6.5 | 25 | 25 | 1 | 12.5 | 25 | 2 | 1.5/6 | 1.25 | I | |
| 6.0 | 12.5 | 12.5 | 1 | 12.5 | 25 | 2 | 3/6 | 0.48 | S | |
| 5.5 | 25 | 25 | 1 | 12.5 | 50 | 4 | 1.5/6 | 0.36 | S | |
| 5.0 | 6 | 6 | 1 | 12.5 | 12.5 | 1 | 1.5/1.5 | 0.37 | S | |
| ATCC 25922 | 7.4 | 3 | 6 | 2 | 6 | 25 | 4 | 1.5/1.5 | 0.50 | S |
| 6.5 | 3 | 3 | 1 | 6 | 100 | 8 | 1.5/3 | 0.75 | PS | |
| 6.0 | 3 | 3 | 1 | 12.5 | 100 | 8 | 1.5/3 | 1.12 | I | |
| 5.5 | 6 | 6 | 1 | 12.5 | 12.5 | 1 | 1.5/1.5 | 0.37 | S | |
| 5.0 | 3 | 3 | 1 | 12.5 | 12.5 | 1 | 1.5/1.5 | 0.72 | PS | |
| * OVEO S. typhimurium | * ROEO S. typhimurium | MIC ROEO/OVEO (µL/mL/µL/mL) | ΣFIC | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | pH | MIC (µL/mL) | MBC (µL/mL) | R MBC/MIC | MIC (µL/mL) | MBC (µL/mL) | R MBC/MIC | |||
| 1HS1 | 7.4 | 12.5 | 12.5 | 1 | 50 | >100 | >2 | 1.5/6 | 0.51 | PS |
| 6.5 | 12.5 | 12.5 | 1 | 50 | >100 | >2 | 1.5/6 | 0.51 | PS | |
| 6.0 | 12.5 | 12.5 | 1 | 100 | >100 | >1 | 1.5/6 | 0.49 | S | |
| 5.5 | 12.5 | 12.5 | 1 | 100 | >100 | >1 | 1.5/12.5 | 1.02 | I | |
| 5.0 | 6 | 6 | 1 | 100 | >100 | >1 | 1.5/6 | 1.02 | I | |
| 5HS1 | 7.4 | 12.5 | 12.5 | 1 | 50 | 100 | 2 | 1.5/6 | 0.51 | PS |
| 6.5 | 12.5 | 12.5 | 1 | 25 | 100 | 4 | 0.7/6 | 0.50 | PS | |
| 6.0 | 12.5 | 12.5 | 1 | 100 | >100 | >1 | 0.3/0.6 | 0.05 | S | |
| 5.5 | 6 | 6 | 1 | 50 | 100 | 2 | 3/6 | 0.56 | PS | |
| 5.0 | 12.5 | 12.5 | 1 | 50 | 50 | 1 | 1.5/6 | 0.51 | PS | |
| 6HS1 | 7.4 | 12.5 | 12.5 | 1 | 50 | 100 | 2 | 1.5/6 | 0.51 | PS |
| 6.5 | 12.5 | 12.5 | 1 | 50 | 100 | 2 | 0.7/1.5 | 0.13 | S | |
| 6.0 | 12.5 | 12.5 | 1 | 100 | >100 | >1 | 1.5/6 | 0.50 | S | |
| 5.5 | 6 | 6 | 1 | 50 | 100 | 2 | 3/6 | 1.06 | I | |
| 5.0 | 12.5 | 12.5 | 1 | 50 | 100 | 2 | 1.5/6 | 0.51 | PS | |
| 13HS1 | 7.4 | 125 | 12.5 | 1 | 100 | >100 | >1 | 1.5/6 | 0.50 | S |
| 6.5 | 6 | 12.5 | 2 | 50 | 100 | 2 | 0.7/6 | 1.01 | I | |
| 6.0 | 12.5 | 12.5 | 1 | 100 | >100 | >1 | 1.5/6 | 0.50 | S | |
| 5.5 | 6 | 6 | 1 | 50 | 100 | 2 | 1.5/6 | 0.54 | PS | |
| 5.0 | 125 | 12.5 | 1 | 100 | 100 | 1 | 3/6 | 0.51 | PS | |
| 15HS1 | 7.4 | 12.5 | 12.5 | 1 | 100 | 100 | 1 | 1.5/6 | 0.50 | PS |
| 6.5 | 6 | 12.5 | 1 | 50 | 100 | 2 | 0.7/6 | 1.01 | I | |
| 6.0 | 12.5 | 12.5 | 1 | 100 | >100 | >1 | 1.5/6 | 0.50 | S | |
| 5.5 | 6 | 6 | 1 | 50 | 100 | 2 | 1.5/6 | 1.03 | I | |
| 5.0 | 12.5 | 12,5 | 1 | 100 | 100 | 1 | 1.5/6 | 0.49 | S | |
| 24HS1 | 7.4 | 12.5 | 12.5 | 1 | 100 | >100 | >1 | 1.5/6 | 0.50 | S |
| 6.5 | 6 | 12.5 | 2 | 50 | 50 | 1 | 1.5/6 | 1.03 | I | |
| 6.0 | 12.5 | 12.5 | 1 | 100 | >100 | >1 | 3/6 | 0.51 | PS | |
| 5.5 | 6 | 12.5 | 2 | 100 | >100 | >1 | 3/6 | 1.03 | I | |
| 5.0 | 12.5 | 12.5 | 1 | 100 | >100 | >1 | 1.5/6 | 0.49 | S | |
| ATCC 14028 | 7.4 | 6 | 12.5 | 2 | 25 | 50 | 2 | 12.5/3 | 1.00 | PS |
| 6.5 | 6 | 6 | 1 | 25 | 25 | 2 | 0.7/6 | 1.01 | I | |
| 6.0 | 12.5 | 12.5 | 1 | 50 | >100 | >2 | 1.5/6 | 0.51 | PS | |
| 5.5 | 6 | 6 | 1 | 12.5 | 12.5 | 1 | 1.5/3 | 0.62 | PS | |
| 5.0 | 6 | 6 | 1 | 25 | 50 | 2 | 1.5/6 | 1.06 | I | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toso, F.; Buldain, D.; Retta, D.; Di Leo Lira, P.; Marchetti, M.L.; Mestorino, N. Antimicrobial Activity of Origanum vulgare L. And Salvia rosmarinus Spenn (syn Rosmarinus officinalis L.) Essential Oil Combinations Against Escherichia coli and Salmonella typhimurium Isolated from Poultry. Processes 2025, 13, 2856. https://doi.org/10.3390/pr13092856
Toso F, Buldain D, Retta D, Di Leo Lira P, Marchetti ML, Mestorino N. Antimicrobial Activity of Origanum vulgare L. And Salvia rosmarinus Spenn (syn Rosmarinus officinalis L.) Essential Oil Combinations Against Escherichia coli and Salmonella typhimurium Isolated from Poultry. Processes. 2025; 13(9):2856. https://doi.org/10.3390/pr13092856
Chicago/Turabian StyleToso, Federico, Daniel Buldain, Daiana Retta, Paola Di Leo Lira, María Laura Marchetti, and Nora Mestorino. 2025. "Antimicrobial Activity of Origanum vulgare L. And Salvia rosmarinus Spenn (syn Rosmarinus officinalis L.) Essential Oil Combinations Against Escherichia coli and Salmonella typhimurium Isolated from Poultry" Processes 13, no. 9: 2856. https://doi.org/10.3390/pr13092856
APA StyleToso, F., Buldain, D., Retta, D., Di Leo Lira, P., Marchetti, M. L., & Mestorino, N. (2025). Antimicrobial Activity of Origanum vulgare L. And Salvia rosmarinus Spenn (syn Rosmarinus officinalis L.) Essential Oil Combinations Against Escherichia coli and Salmonella typhimurium Isolated from Poultry. Processes, 13(9), 2856. https://doi.org/10.3390/pr13092856









