Effect of Adding Citrus Fruits on the Behavior of Reducing Sugars During the Fermentation of Criollo Cocoa Beans
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Materials and Reagents
2.2. Fermentation of Criollo Cocoa Beans and Sampling
2.3. Sample Treatment
2.4. Sugar Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Dynamics of Sucrose, Glucose, and Fructose During the Fermentation of Criollo Cocoa with Citrus Fruit Additions
3.2. Quantification of Total Sugars in SF, FF5, and CF10 Criollo Cocoa Beans
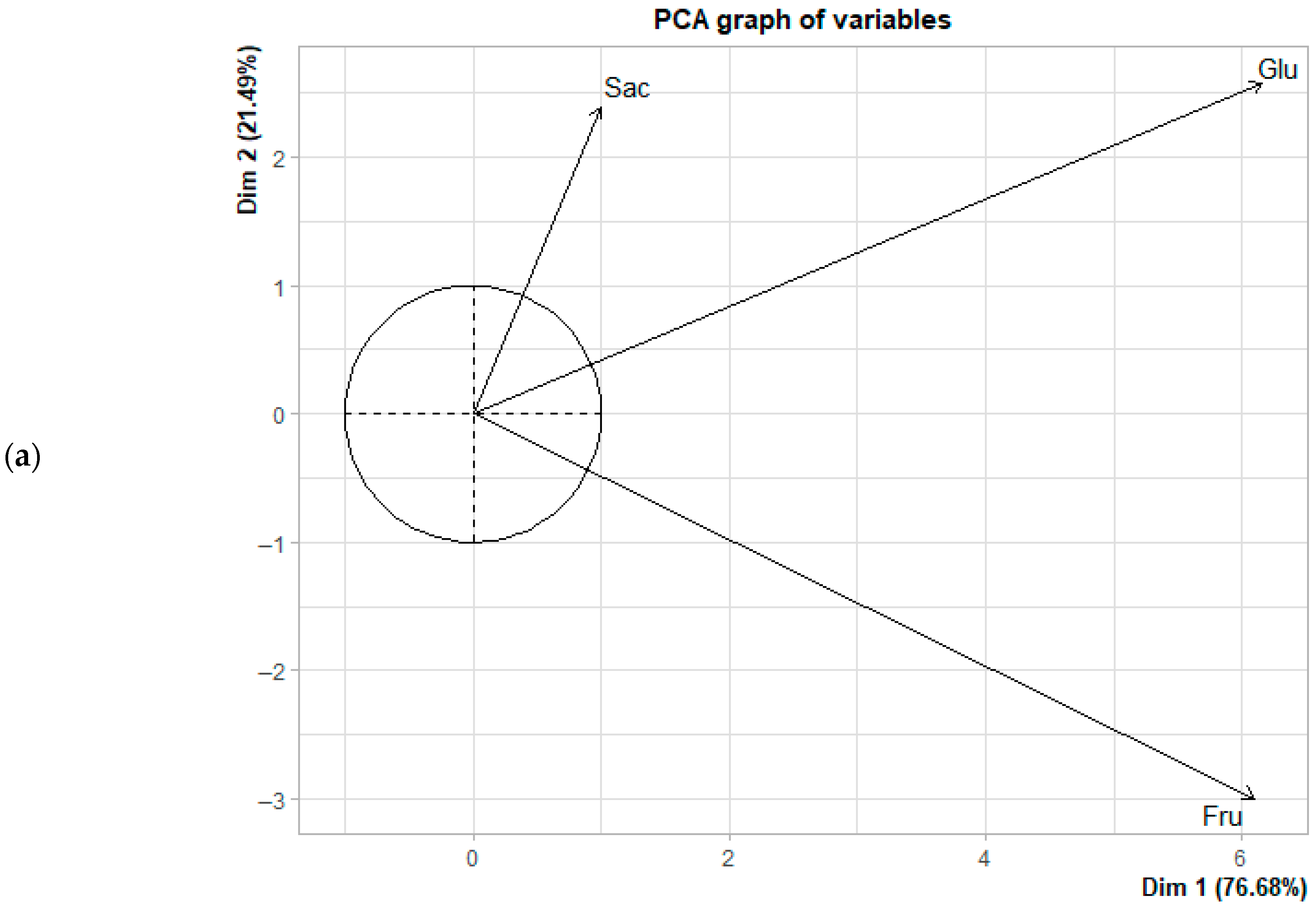
3.3. Principal Component Analysis (PCA) in the Formation of Reducing Sugars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becerra, L.D.; Zuluaga, M.; Mayorga, E.Y.; Moreno, F.L.; Ruíz, R.Y.; Escobar, S. Cocoa Seed Transformation under Controlled Process Conditions: Modelling of the Mass Transfer of Organic Acids and Reducing Sugar Formation Analysis. Food Bioprod. Process. 2022, 136, 211–225. [Google Scholar] [CrossRef]
- Castro, E.M.; Idrogo, G.; Siche, R.; Cardenas, F.P. Formation of Aromatic Compounds Precursors during Fermentation of Criollo and Forastero Cocoa. Heliyon 2019, 5, e01157. [Google Scholar] [CrossRef]
- Huamán, A.F.; Torres, M.; Aime, M.C.; Leiva, S.T.; Oliva, S.M.; Díaz, J.R. First Report of Thread Blight Caused by Marasmius tenuissimus on Cacao (Theobroma cacao) in Peru. Plant Dis. 2023, 107, 219. [Google Scholar] [CrossRef]
- Sánchez, V.; Zambrano, J.; Iglesias, C. La Cadena de Valor Del Cacao En América Latina y El Caribe; INIAP: Quito, Ecuador, 2019; ISBN 978-9942-36-465-4. Available online: https://repositorio.iniap.gob.ec/handle/41000/5382 (accessed on 27 August 2025).
- Oliva-Cruz, M.; Goñas, M.; Bobadilla, L.G.; Rubio, K.B.; Escobedo-Ocampo, P.; García Rosero, L.M.; Rojas Briceño, N.B.; Maicelo-Quintana, J.L. Genetic Groups of Fine-Aroma Native Cacao Based on Morphological and Sensory Descriptors in Northeast Peru. Front. Plant Sci. 2022, 13, 896332. [Google Scholar] [CrossRef]
- Ruiz-Santiago, F.L.; Márquez-Rocha, F.J.; García-Alamilla, P.; Carrera-Lanestosa, A.; Ramírez-López, C.; Ocaranza-Sánchez, E.; Jiménez-Rodríguez, D.J. Physicochemical and Biochemical Changes in Cocoa during the Fermentation Step. Fermentation 2024, 10, 405. [Google Scholar] [CrossRef]
- Jagtap, U.B.; Bapat, V.A. Wines from Fruits Other than Grapes: Current Status and Future Prospectus. Food Biosci. 2015, 9, 80–96. [Google Scholar] [CrossRef]
- Subroto, E.; Djali, M.; Indiarto, R.; Lembong, E.; Baiti, N. Microbiological Activity Affects Post-Harvest Quality of Cocoa (Theobroma cacao L.) Beans. Horticulturae 2023, 9, 805. [Google Scholar] [CrossRef]
- Calvo, A.M.; Botina, B.L.; García, M.C.; Cardona, W.A.; Montenegro, A.C.; Criollo, J. Dynamics of Cocoa Fermentation and Its Effect on Quality. Sci. Rep. 2021, 11, 16746. [Google Scholar] [CrossRef]
- Grassia, M.; Salvatori, G.; Roberti, M.; Planeta, D.; Cinquanta, L. Polyphenols, Methylxanthines, Fatty Acids and Minerals in Cocoa Beans and Cocoa Products. Food Meas. 2019, 13, 1721–1728. [Google Scholar] [CrossRef]
- Besançon, L.; Lorn, D.; Kouamé, C.; Grabulos, J.; Lebrun, M.; Fontana, A.; Schorr-Galindo, S.; Boulanger, R.; Strub, C.; Colas de la Noue, A. Influence of Yeast Interactions on the Fermentation Process and Aroma Production in Synthetic Cocoa Pulp vs. Real Mucilage Media. Fermentation 2024, 10, 662. [Google Scholar] [CrossRef]
- Campos, S.d.M.; Martínez-Burgos, W.J.; dos Reis, G.A.; Ocán-Torres, D.Y.; dos Santos Costa, G.; Rosas Vega, F.; Alvarez Badel, B.; Sotelo Coronado, L.; Lima Serra, J.; Soccol, C.R. The Role of Microbial Dynamics, Sensorial Compounds, and Producing Regions in Cocoa Fermentation. Microbiol. Res. 2025, 16, 75. [Google Scholar] [CrossRef]
- Viesser, J.A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Rogez, H.; Góes-Neto, A.; Azevedo, V.; Brenig, B.; Aburjaile, F.; Soccol, C.R. Co-Culturing Fructophilic Lactic Acid Bacteria and Yeast Enhanced Sugar Metabolism and Aroma Formation During Cocoa Beans Fermentation. Int. J. Food Microbiol. 2021, 339, 109015. [Google Scholar] [CrossRef]
- Gutiérrez-Ríos, H.G.; Suárez-Quiroz, M.L.; Hernández-Estrada, Z.J.; Castellanos-Onorio, O.P.; Alonso-Villegas, R.; Rayas-Duarte, P.; Cano-Sarmiento, C.; Figueroa-Hernández, C.Y.; González-Rios, O. Yeasts as Producers of Flavor Precursors During Cocoa Bean Fermentation and Their Relevance as Starter Cultures: A Review. Fermentation 2022, 8, 331. [Google Scholar] [CrossRef]
- Balcázar-Zumaeta, C.R.; Fernández-Romero, E.; Lopes, A.S.; Ferreira, N.R.; Chagas-Júnior, G.C.A.; Yoplac, I.; López-Trigoso, H.A.; Tuesta-Occ, M.L.; Maldonado-Ramirez, I.; Maicelo-Quintana, J.L.; et al. Amino Acid Profile Behavior during the Fermentation of Criollo Cocoa Beans. Food Chem. X 2024, 22, 101486. [Google Scholar] [CrossRef]
- Mayra, O.C.; Manosalvas-Quiroz, L.-A.; Mosquera, N.P.; Samaniego, I. Effect of Fermentation Parameters on the Antioxidant Activity of Ecuadorian Cocoa (Theobroma cacao L.). AIMS Agric. Food 2024, 9, 872–886. [Google Scholar] [CrossRef]
- Guillen-Guerrero, K.M.; de la Rosa-Millan, J. Effects of Fermentation Temperature on the Physicochemical Properties, Bioactive Compounds, and In Vitro Digestive Profile of Cacao (Theobroma cacao) Seeds. Fermentation 2025, 11, 167. [Google Scholar] [CrossRef]
- Rohan, T.A.; Stewart, T. The Precursors of Chocolate Aroma: Production of Reducing Sugars during Fermentation of Cocoa Beans. J. Food Sci. 1967, 32, 399–402. [Google Scholar] [CrossRef]
- Rojas-Rojas, K.; Hernández-Aguirre, C.; Mencía-Guevara, A.; Rojas-Rojas, K.; Hernández-Aguirre, C.; Mencía-Guevara, A. Transformaciones bioquímicas del cacao (Theobroma cacao L.) durante un proceso de fermentación controlada. Agron. Costarric. 2021, 45, 53–65. [Google Scholar] [CrossRef]
- Silva, L.C.F.; Pereira, P.V.R.; Cruz, M.A.D.d.; Costa, G.X.R.; Rocha, R.A.R.; Bertarini, P.L.L.; Amaral, L.R.d.; Gomes, M.S.; Santos, L.D. Enhancing Sensory Quality of Coffee: The Impact of Fermentation Techniques on Coffea arabica cv. Catiguá MG2. Foods 2024, 13, 653. [Google Scholar] [CrossRef]
- Guzmán-Armenteros, T.M.; Ruales, J.; Villacís-Chiriboga, J.; Guerra, L.S. Experimental Prototype of Electromagnetic Emissions for Biotechnological Research: Monitoring Cocoa Bean Fermentation Parameters. Foods 2023, 12, 2539. [Google Scholar] [CrossRef] [PubMed]
- Balcázar-Zumaeta, C.R.; Maicelo-Quintana, J.L.; Salón-Llanos, G.; Barrena, M.; Muñoz-Astecker, L.D.; Cayo-Colca, I.S.; Torrejón-Valqui, L.; Castro-Alayo, E.M. A Novel Technique Using Confocal Raman Spectroscopy Coupled with PLS-DA to Identify the Types of Sugar in Three Tropical Fruits. Appl. Sci. 2024, 14, 8476. [Google Scholar] [CrossRef]
- Hu, L.; Yang, C.; Zhang, L.; Feng, J.; Xi, W. Effect of Light-Emitting Diodes and Ultraviolet Irradiation on the Soluble Sugar, Organic Acid, and Carotenoid Content of Postharvest Sweet Oranges (Citrus sinensis (L.) Osbeck). Molecules 2019, 24, 3440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, X.; Ali, M.M.; Rizwan, H.M.; Li, B.; Li, H.; Jia, K.; Yang, X.; Ma, S.; Li, S.; et al. Changes in the Content of Organic Acids and Expression Analysis of Citric Acid Accumulation-Related Genes during Fruit Development of Yellow (Passiflora edulis f. Flavicarpa) and Purple (Passiflora edulis f. Edulis) Passion Fruits. Int. J. Mol. Sci. 2021, 22, 5765. [Google Scholar] [CrossRef] [PubMed]
- Purbaningrum, K.; Hidayat, C.; Witasari, L.D.; Utami, T. Flavor Precursors and Volatile Compounds Improvement of Unfermented Cocoa Beans by Hydrolysis Using Bromelain. Foods 2023, 12, 820. [Google Scholar] [CrossRef]
- He, Z.; Cao, L.; Zhao, L.; Zhou, Y.; Gong, J.; Zhu, C.; Tan, C. Effects of the Mixed Fermentation of Apples and Yeast on the Sensory Evaluation, Physicochemical Composition and Flavor of Coffee Beans. Food Biosci. 2025, 68, 106337. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and Grape Polyphenols—A Chemical Perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Kim, I.J.; Park, S.; Kyoung, H.; Song, M.; Kim, S.R. Microbial Valorization of Fruit Processing Waste: Opportunities, Challenges, and Strategies. Curr. Opin. Food Sci. 2024, 56, 101147. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Q.; Wu, C.; Li, T.; Tu, K. Characterization of Soluble Sugars, Glycosidically Bound and Free Volatiles in Fresh-Cut Pineapple Stored at Different Temperature. Food Biosci. 2021, 43, 101329. [Google Scholar] [CrossRef]
- Castro-Alayo, E.M.; Torrejón-Valqui, L.; Medina-Mendoza, M.; Cayo-Colca, I.S.; Cárdenas-Toro, F.P. Kinetics Crystallization and Polymorphism of Cocoa Butter throughout the Spontaneous Fermentation Process. Foods 2022, 11, 1769. [Google Scholar] [CrossRef]
- Alghamdi, B.A.; Alshumrani, E.S.; Saeed, M.S.B.; Rawas, G.M.; Alharthi, N.T.; Baeshen, M.N.; Helmi, N.M.; Alam, M.Z.; Suhail, M. Analysis of Sugar Composition and Pesticides Using HPLC and GC–MS Techniques in Honey Samples Collected from Saudi Arabian Markets. Saudi J. Biol. Sci. 2020, 27, 3720–3726. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Reyes, D.; Rodrí guez-Campos, J.; Avendaño-Arrazate, C.; Gschaedler, A.; Alcázar-Valle, M.; Lugo-Cervantes, E. Forastero and Criollo Cocoa Beans, Differences on the Profile of Volatile and Non-Volatile Compounds in the Process from Fermentation to Liquor. Heliyon 2023, 9, e15129. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Gloria, M.B.A.; Martins, L.H.D.S.; Lopes, A.S. Chemical Implications and Time Reduction of On-Farm Cocoa Fermentation by Saccharomyces cerevisiae and Pichia kudriavzevii. Food Chem. 2021, 338, 127834. [Google Scholar] [CrossRef]
- Afoakwa, E.O. Chocolate Science and Technology, 1st ed.; Wiley: Hoboken, NJ, USA, 2016; ISBN 978-1-118-91378-9. [Google Scholar] [CrossRef]
- Cartas, J.; Alvarenga, N.; Partidário, A.; Lageiro, M.; Roseiro, C.; Gonçalves, H.; Leitão, A.E.; Ribeiro, C.M.; Dias, J. Influence of Geographical Origin in the Physical and Bioactive Parameters of Single Origin Dark Chocolate. Eur. Food Res. Technol. 2024, 250, 2569–2580. [Google Scholar] [CrossRef]
- Peña González, M.A.; Ortiz Urgiles, J.P.; Santander Pérez, F.A.; Lazo Vélez, M.A.; Caroca Cáceres, R.S. Physicochemical Changes during Controlled Laboratory Fermentation of Cocoa (CCN-51) with the Inclusion of Fruits and on-Farm Inoculation. Braz. J. Food Technol. 2023, 26, e2023013. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Wang, S.; Zhu, F. Chemical and Biological Properties of Cocoa Beans Affected by Processing: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8403–8434. [Google Scholar] [CrossRef] [PubMed]
- Megías-Pérez, R.; Grimbs, S.; D’Souza, R.N.; Bernaert, H.; Kuhnert, N. Profiling, Quantification and Classification of Cocoa Beans Based on Chemometric Analysis of Carbohydrates Using Hydrophilic Interaction Liquid Chromatography Coupled to Mass Spectrometry. Food Chem. 2018, 258, 284–294. [Google Scholar] [CrossRef]
- Vizcaino-Almeida, C.R.; Guajardo-Flores, D.; Caroca-Cáceres, R.; Serna-Saldívar, S.O.; Briones-García, M.; Lazo-Vélez, M.A. Non-Conventional Fermentation at Laboratory Scale of Cocoa Beans: Using Probiotic Microorganisms and Substitution of Mucilage by Fruit Pulps. Int. J. Food Sci. Technol. 2022, 57, 4307–4315. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Fleet, G.H.; Zhao, J. Unravelling the Contribution of Lactic Acid Bacteria and Acetic Acid Bacteria to Cocoa Fermentation Using Inoculated Organisms. Int. J. Food Microbiol. 2018, 279, 43–56. [Google Scholar] [CrossRef]
- Moreira, I.M.D.V.; Miguel, M.G.D.C.P.; Duarte, W.F.; Dias, D.R.; Schwan, R.F. Microbial Succession and the Dynamics of Metabolites and Sugars during the Fermentation of Three Different Cocoa (Theobroma cacao L.) Hybrids. Food Res. Int. 2013, 54, 9–17. [Google Scholar] [CrossRef]
- Kouamé, C.; Loiseau, G.; Grabulos, J.; Boulanger, R.; Mestres, C. Development of a Model for the Alcoholic Fermentation of Cocoa Beans by a Saccharomyces Cerevisiae Strain. Int. J. Food Microbiol. 2021, 337, 108917. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Zambrano, M.; Grimbs, S.; Ullrich, M.S.; Hütt, M.-T. A Mathematical Model of Cocoa Bean Fermentation. R. Soc. Open Sci. 2018, 5, 180964. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, L.G.; Mendoza, L.M.; Van-Nieuwenhove, C.P.; Pescuma, M.; Mozzi, F.B. Fermentación de Jugos y Bebidas a Base de Frutas; Instituto Danone: Barcelona, Spain, 2020; ISBN 978-987-25312-2-5. Available online: https://ri.conicet.gov.ar/handle/11336/120385 (accessed on 27 August 2025).
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and Infrared Spectroscopy of Carbohydrates: A Review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Díaz-Muñoz, C.; De Vuyst, L. Functional Yeast Starter Cultures for Cocoa Fermentation. J. Appl. Microbiol. 2022, 133, 39–66. [Google Scholar] [CrossRef]
- Sari, A.B.T.; Fahrurrozi; Marwati, T.; Djaafar, T.F.; Hatmi, R.U.; Purwaningsih; Wanita, Y.P.; Lisdiyanti, P.; Perwitasari, U.; Juanssilfero, A.B.; et al. Chemical Composition and Sensory Profiles of Fermented Cocoa Beans Obtained from Various Regions of Indonesia. Int. J. Food Sci. 2023, 2023, 5639081. [Google Scholar] [CrossRef]
- Megias-Perez, R.; Moreno-Zambrano, M.; Behrends, B.; Corno, M.; Kuhnert, N. Monitoring the Changes in Low Molecular Weight Carbohydrates in Cocoa Beans During Spontaneous Fermentation: A Chemometric and Kinetic Approach. Food Res. Int. 2020, 128, 108865. [Google Scholar] [CrossRef]
- Ghisolfi, R.; Bandini, F.; Vaccari, F.; Bellotti, G.; Bortolini, C.; Patrone, V.; Puglisi, E.; Morelli, L. Bacterial and Fungal Communities Are Specifically Modulated by the Cocoa Bean Fermentation Method. Foods 2023, 12, 2024. [Google Scholar] [CrossRef]
- Tigrero-Vaca, J.; Maridueña-Zavala, M.G.; Liao, H.-L.; Prado-Lince, M.; Zambrano-Vera, C.S.; Monserrate-Maggi, B.; Cevallos-Cevallos, J.M. Microbial Diversity and Contribution to the Formation of Volatile Compounds during Fine-Flavor Cacao Bean Fermentation. Foods 2022, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Chen, K.-F.; Changchien, L.-L.; Chen, K.-C.; Peng, R.Y. Volatile Variation of Theobroma cacao Malvaceae L. Beans Cultivated in Taiwan Affected by Processing via Fermentation and Roasting. Molecules 2022, 27, 3058. [Google Scholar] [CrossRef]
- Fayek, N.M.; Xiao, J.; Farag, M.A. A Multifunctional Study of Naturally Occurring Pyrazines in Biological Systems; Formation Mechanisms, Metabolism, Food Applications and Functional Properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 5322–5338. [Google Scholar] [CrossRef] [PubMed]
- Laemont, J.; Barringer, S. Effect of pH, Reducing Sugars, and Protein on Roasted Sunflower Seed Aroma Volatiles. Foods 2023, 12, 4155. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Fuentes, L.F.; García-Jerez, A.; Rodríguez-Negrette, A.C.; Hoyos-Merlano, N.T.; Alvis-Bermúdez, A. Impact of Spontaneous Fermentation on the Physicochemical and Sensory Qualities of Cacao. Fermentation 2025, 11, 377. [Google Scholar] [CrossRef]
- Tonin, I.P.; Baqueta, M.R.; Hantao, L.W.; Marini, F.; de Souza Silveira, P.T.; Martins, M.O.P.; Efraim, P. Volatiles from Cocoa Bean to Nibs, Liquor, and Chocolate: An Integrated Chemometric Analysis of Fermentation Treatments with Pulp Reduction, Malt Residues, and Banana Peel. Food Chem. 2025, 487, 144774. [Google Scholar] [CrossRef]
- Barišić, V.; Kopjar, M.; Jozinović, A.; Flanjak, I.; Ačkar, Đ.; Miličević, B.; Šubarić, D.; Jokić, S.; Babić, J. The Chemistry behind Chocolate Production. Molecules 2019, 24, 3163. [Google Scholar] [CrossRef] [PubMed]
- Rottiers, H.; Tzompa Sosa, D.A.; De Winne, A.; Ruales, J.; De Clippeleer, J.; De Leersnyder, I.; De Wever, J.; Everaert, H.; Messens, K.; Dewettinck, K. Dynamics of Volatile Compounds and Flavor Precursors During Spontaneous Fermentation of Fine Flavor Trinitario Cocoa Beans. Eur. Food Res. Technol. 2019, 245, 1917–1937. [Google Scholar] [CrossRef]
- Chacón, C.Y.; Mori, P.L.; Chavez, S.G. Antioxidantes y Polifenoles Totales de Chocolate Negro Con Incorporación de Cacao (Theobroma cacao L.) Crudo. Rev. Investig. Altoandin. 2021, 23, 266–273. [Google Scholar] [CrossRef]
- Nascimento, L.L.; Pereira, M.S.; de Almeida, L.S.; da Silveira Ferreira, L.; de Moura Pita, B.L.; de Souza, C.O.; Ribeiro, C.D.F.; Fricks, A.T. Innovation in Cocoa Fermentation: Evidence from Patent Documents and Scientific Articles. Fermentation 2024, 10, 251. [Google Scholar] [CrossRef]
- Santander Muñoz, M.; Rodríguez Cortina, J.; Vaillant, F.E.; Escobar Parra, S. An Overview of the Physical and Biochemical Transformation of Cocoa Seeds to Beans and to Chocolate: Flavor Formation. Crit. Rev. Food Sci. Nutr. 2020, 60, 1593–1613. [Google Scholar] [CrossRef]
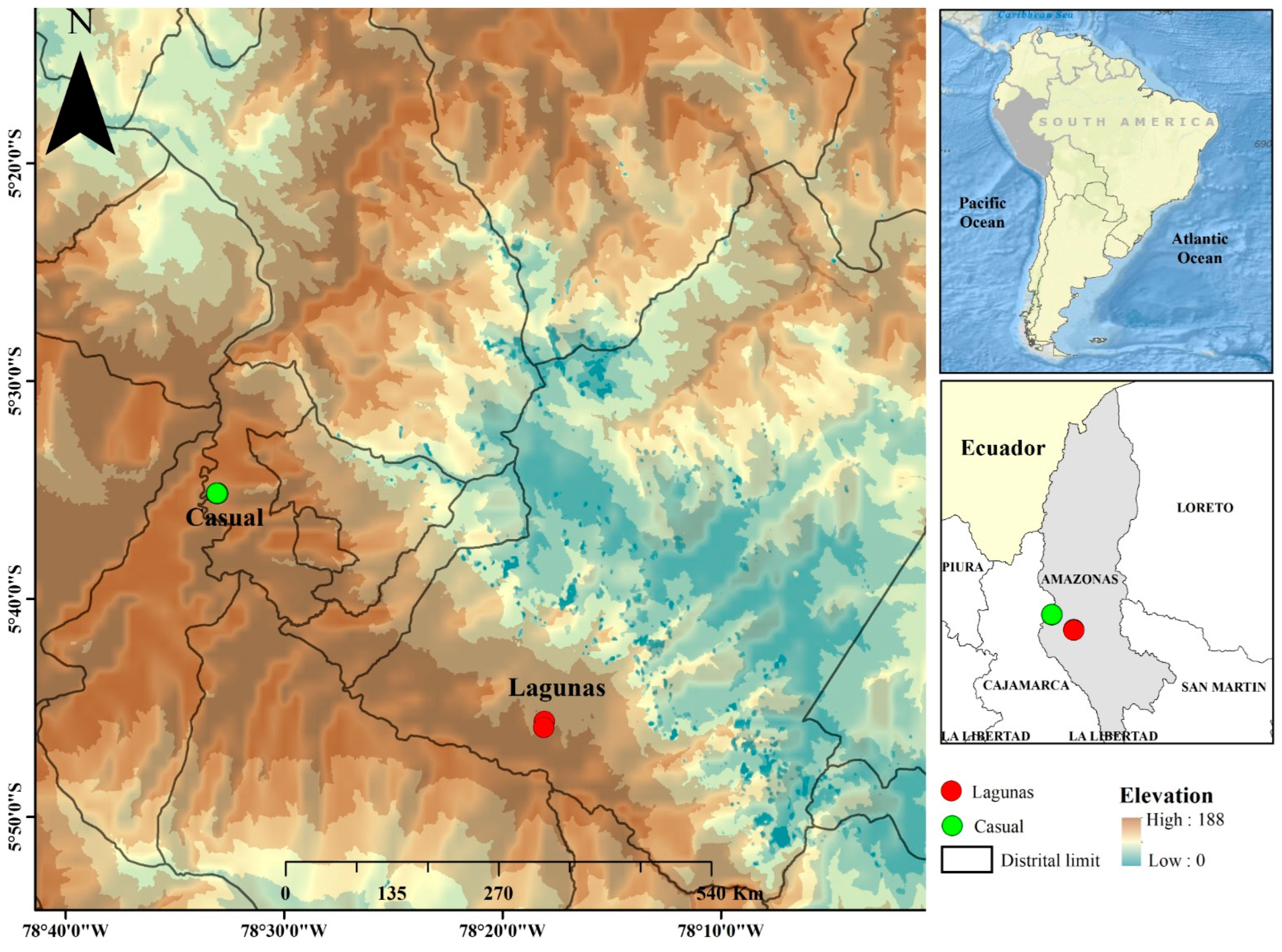

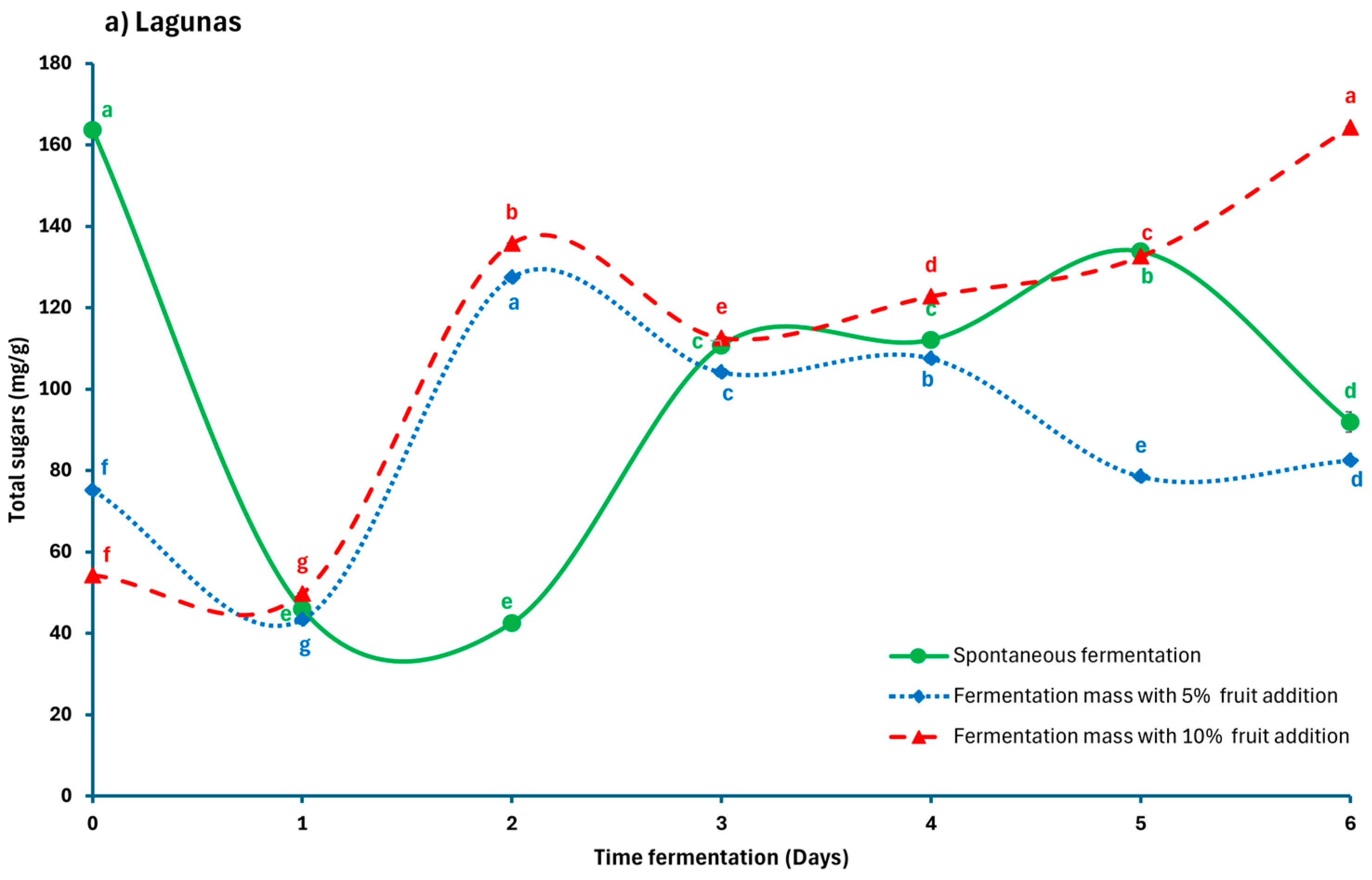
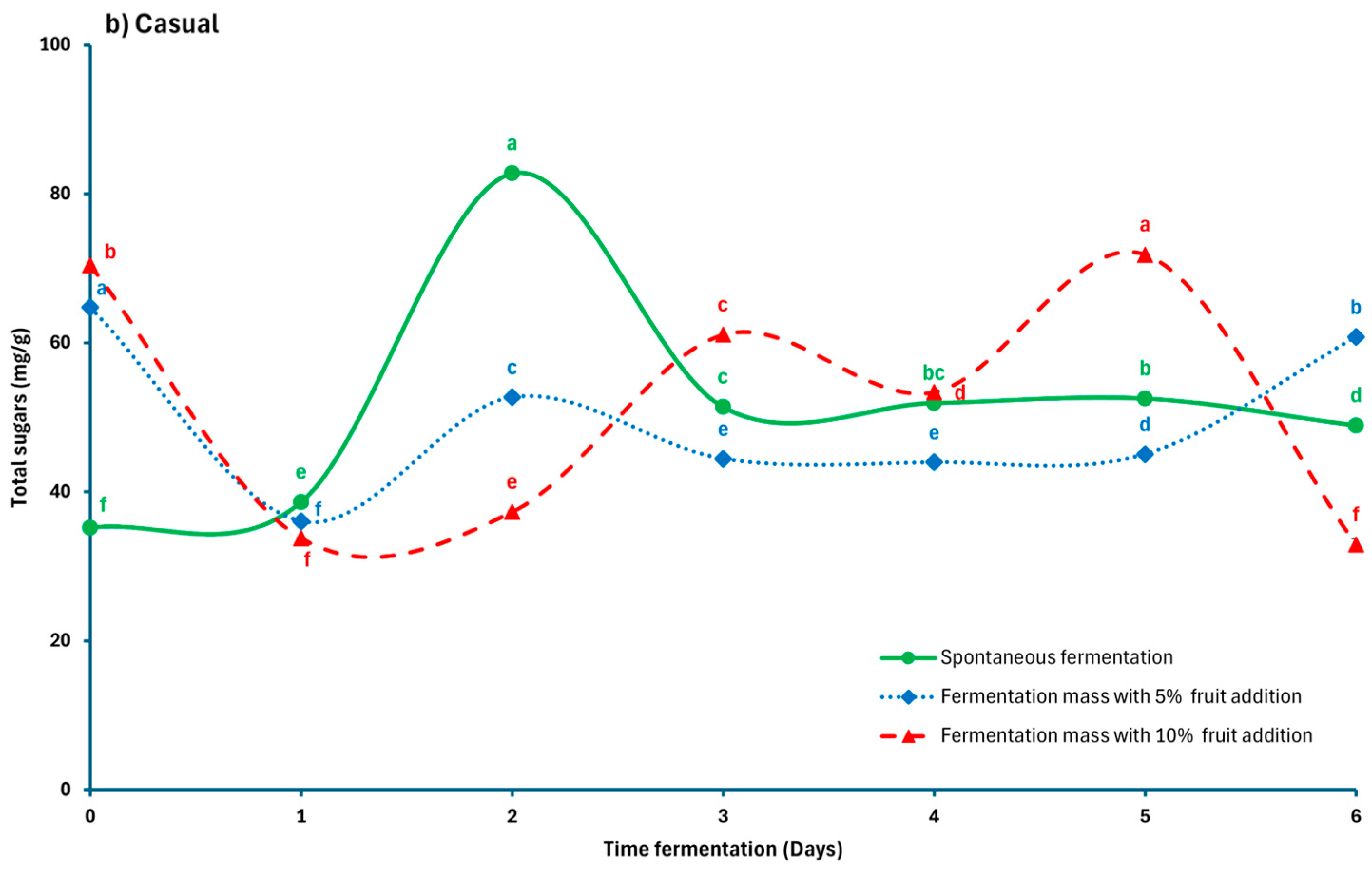
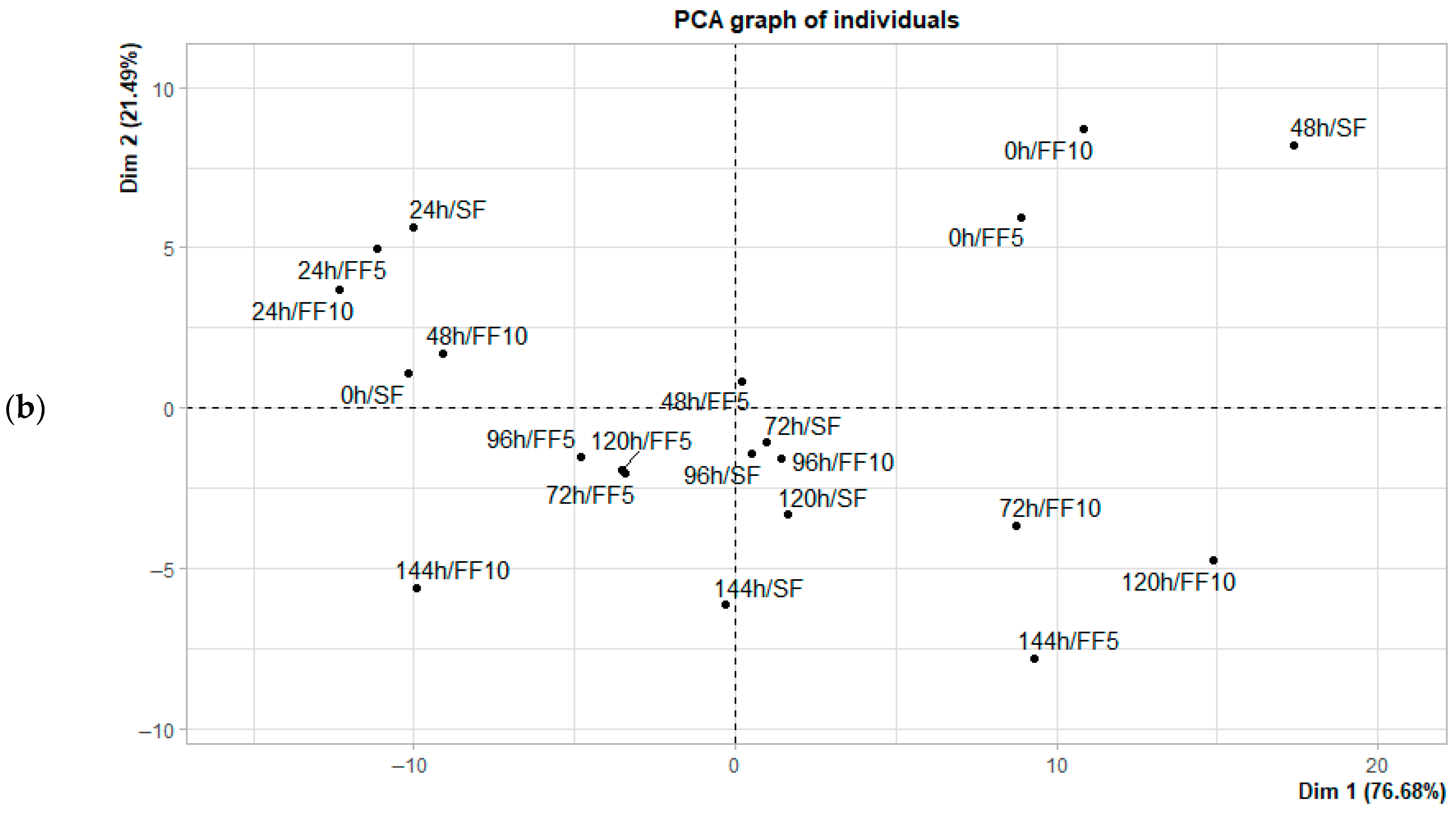
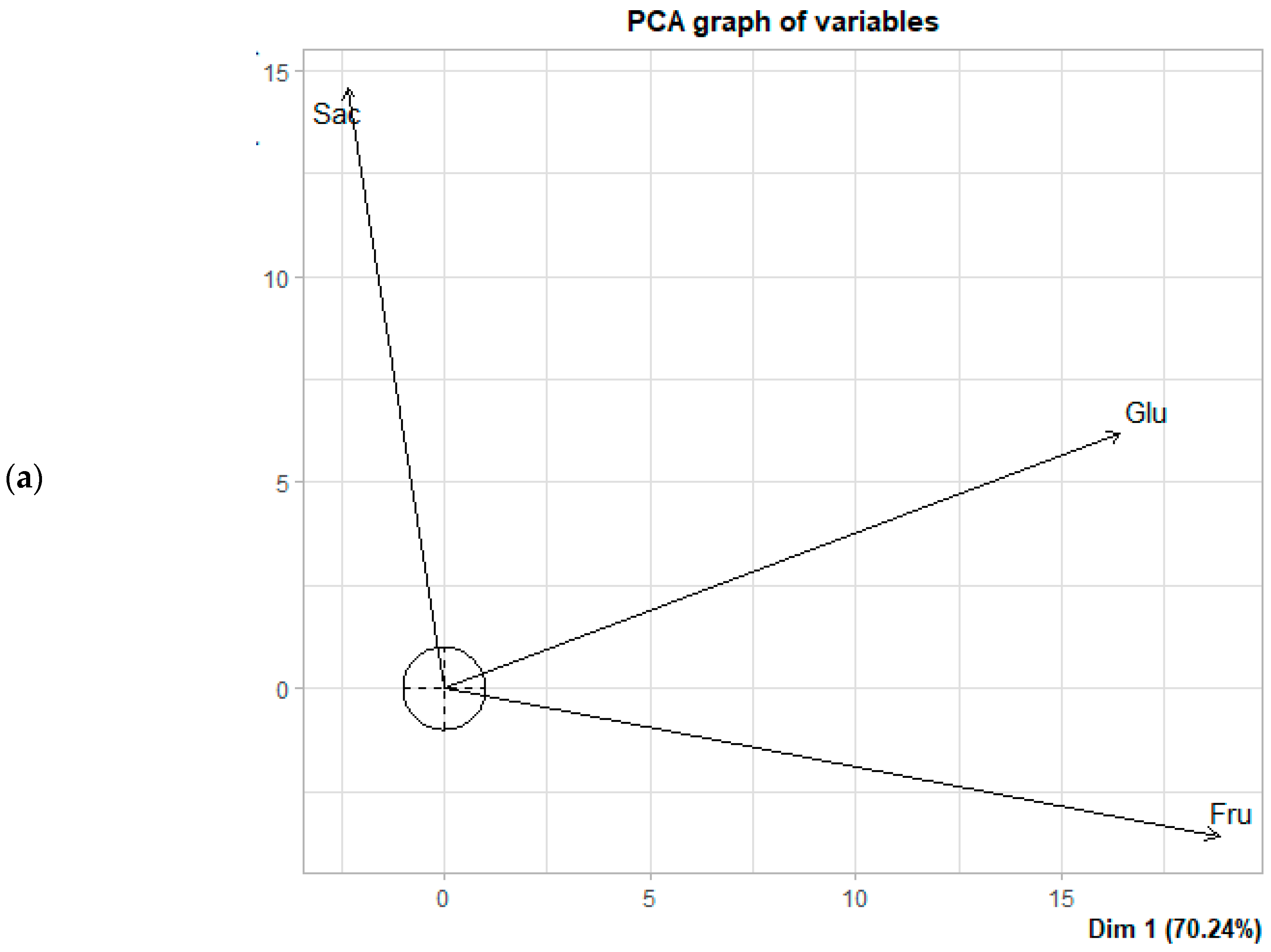

| Locations | Time Fermentation | Spontaneous Fermentation | Fermentation with Added Fruit (5%) | Fermentation with Added Fruit (10%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sac | Glu | Fru | Sac | Glu | Fru | Sac | Glu | Fru | ||
| Casual | 0 h | 6.3 ± 0.02 e | 17.54 ± 0.02 e | 11.34 ± 0.01 f | 9.25 ± 0.03 b | 34.61 ± 0.03 a | 20.91 ± 0.02 b | 12.68 ± 0.01 a | 36.6 ± 0.03 a | 21.13 ± 0.02 d |
| 24 h | 10.15 ± 0.13 b | 19.44 ± 0.12 d | 9.04 ± 0.04 g | 9.01 ± 0.05 c | 18.61 ± 0.1 f | 8.44 ± 0.02 g | 8.27 ± 0.07 b | 17.03 ± 0.05 f | 8.47 ± 0.02 g | |
| 48 h | 16.24 ± 0.01 a | 39.37 ± 0.03 a | 27.13 ± 0.02 a | 9.55 ± 0.01 a | 23.6 ± 0.01 c | 19.55 ± 0.01 c | 7.03 ± 0.04 d | 18.47 ± 0.11 e | 11.8 ± 0.02 f | |
| 72 h | 6.65 ± 0.02 d | 24.09 ± 0.16 b | 20.64 ± 0.1 e | 5.82 ± 0.15 f | 20.36 ± 0.21 d | 18.26 ± 0.07 e | 5.03 ± 0.01 e | 28.71 ± 0.03 c | 27.31 ± 0.01 b | |
| 96 h | 8.08 ± 0.11 c | 22.71 ± 0.18 c | 21.13 ± 0.05 d | 7.67 ± 0.04 d | 18.78 ± 0.1 f | 17.52 ± 0.05 f | 8.27 ± 0.05 b | 23.2 ± 0.08 d | 21.9 ± 0.02 c | |
| 120 h | 6.88 ± 0.08 d | 22.62 ± 0.14 c | 23.03 ± 0.04 c | 6.75 ± 0.02 e | 19.91 ± 0.04 e | 18.4 ± 0.06 d | 7.4 ± 0.02 c | 31.29 ± 0.09 b | 33.13 ± 0.06 a | |
| 144 h | 5.86 ± 0.11 f | 19.32 ± 0.19 d | 23.71 ± 0.11 b | 3.75 ± 0.00 g | 26.36 ± 0.03 b | 30.68 ± 0.03 a | 3.09 ± 0.12 f | 13.85 ± 0.48 g | 15.98 ± 0.4 e | |
| Lagunas | 0 h | 69.22 ± 0.69 a | 69.39 ± 0.06 a | 24.94 ± 0.22 e | 33.08 ± 0.14 a | 29.65 ± 0.06 f | 12.48 ± 0.06 f | 23.38 ± 0.15 b | 21.64 ± 0.23 f | 9.22 ± 0.07 g |
| 24 h | 18.1 ± 0.05 b | 18.76 ± 0.08 e | 9.03 ± 0.02 g | 15.7 ± 0.05 c | 19.09 ± 0.12 g | 8.56 ± 0.02 g | 19.27 ± 0.01 c | 20.3 ± 0.13 g | 10.23 ± 0.01 f | |
| 48 h | 6.91 ± 0.07 f | 20.35 ± 0.13 e | 15.24 ± 0.04 f | 32.16 ± 0.04 b | 57.43 ± 0.19 a | 37.86 ± 0.08 d | 43.25 ± 0.04 a | 59.74 ± 0.11 c | 32.77 ± 0.01 e | |
| 72 h | 14.81 ± 0.04 c | 51.34 ± 0.23 c | 44.5 ± 0.24 c | 15.64 ± 0.2 c | 48.77 ± 0.49 c | 39.67 ± 0.44 b | 14.6 ± 0.02 d | 52.7 ± 0.2 e | 45.25 ± 0.27 d | |
| 96 h | 11.56 ± 0.15 e | 52.75 ± 0.17 c | 47.76 ± 0.3 b | 12.4 ± 0.13 d | 51.42 ± 0.21 b | 43.68 ± 0.12 a | 14.65 ± 0.05 d | 57.43 ± 0.07 d | 50.74 ± 0.14 c | |
| 120 h | 12.58 ± 0.1 d | 62.24 ± 0.49 b | 58.95 ± 0.34 a | 6.3 ± 0.52 f | 38.14 ± 0.14 d | 34.02 ± 0.16 e | 7.56 ± 0.02 e | 65.11 ± 0.13 b | 59.94 ± 0.02 b | |
| 144 h | 6.26 ± 0.59 f | 45.1 ± 2.73 d | 40.5 ± 0.9 d | 7.09 ± 0.07 e | 36.63 ± 0.08 e | 38.7 ± 0.15 c | 7.05 ± 0.04 f | 76.24 ± 0.13 a | 81.06 ± 0.21 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuesta-Occ, M.L.; Calderón-Díaz, E.J.; Mori-Mestanza, D.; Pérez-Ramos, H.A.; Pajuelo-Muñoz, A.J.; Castro-Alayo, E.M.; Balcázar-Zumaeta, C.R. Effect of Adding Citrus Fruits on the Behavior of Reducing Sugars During the Fermentation of Criollo Cocoa Beans. Processes 2025, 13, 2834. https://doi.org/10.3390/pr13092834
Tuesta-Occ ML, Calderón-Díaz EJ, Mori-Mestanza D, Pérez-Ramos HA, Pajuelo-Muñoz AJ, Castro-Alayo EM, Balcázar-Zumaeta CR. Effect of Adding Citrus Fruits on the Behavior of Reducing Sugars During the Fermentation of Criollo Cocoa Beans. Processes. 2025; 13(9):2834. https://doi.org/10.3390/pr13092834
Chicago/Turabian StyleTuesta-Occ, Mery L., Edward J. Calderón-Díaz, Diner Mori-Mestanza, Harvey A. Pérez-Ramos, Alexa J. Pajuelo-Muñoz, Efraín M. Castro-Alayo, and César R. Balcázar-Zumaeta. 2025. "Effect of Adding Citrus Fruits on the Behavior of Reducing Sugars During the Fermentation of Criollo Cocoa Beans" Processes 13, no. 9: 2834. https://doi.org/10.3390/pr13092834
APA StyleTuesta-Occ, M. L., Calderón-Díaz, E. J., Mori-Mestanza, D., Pérez-Ramos, H. A., Pajuelo-Muñoz, A. J., Castro-Alayo, E. M., & Balcázar-Zumaeta, C. R. (2025). Effect of Adding Citrus Fruits on the Behavior of Reducing Sugars During the Fermentation of Criollo Cocoa Beans. Processes, 13(9), 2834. https://doi.org/10.3390/pr13092834








