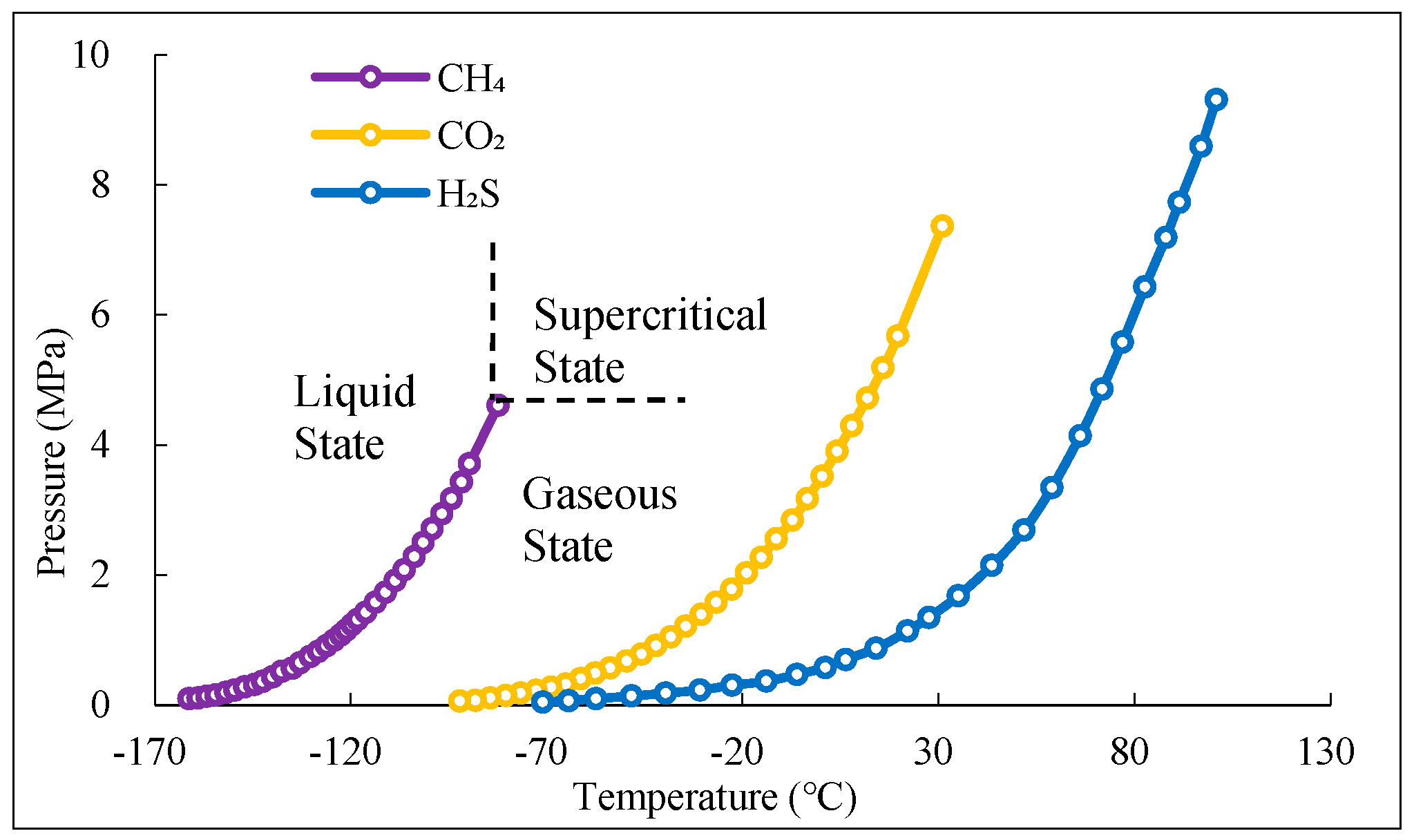
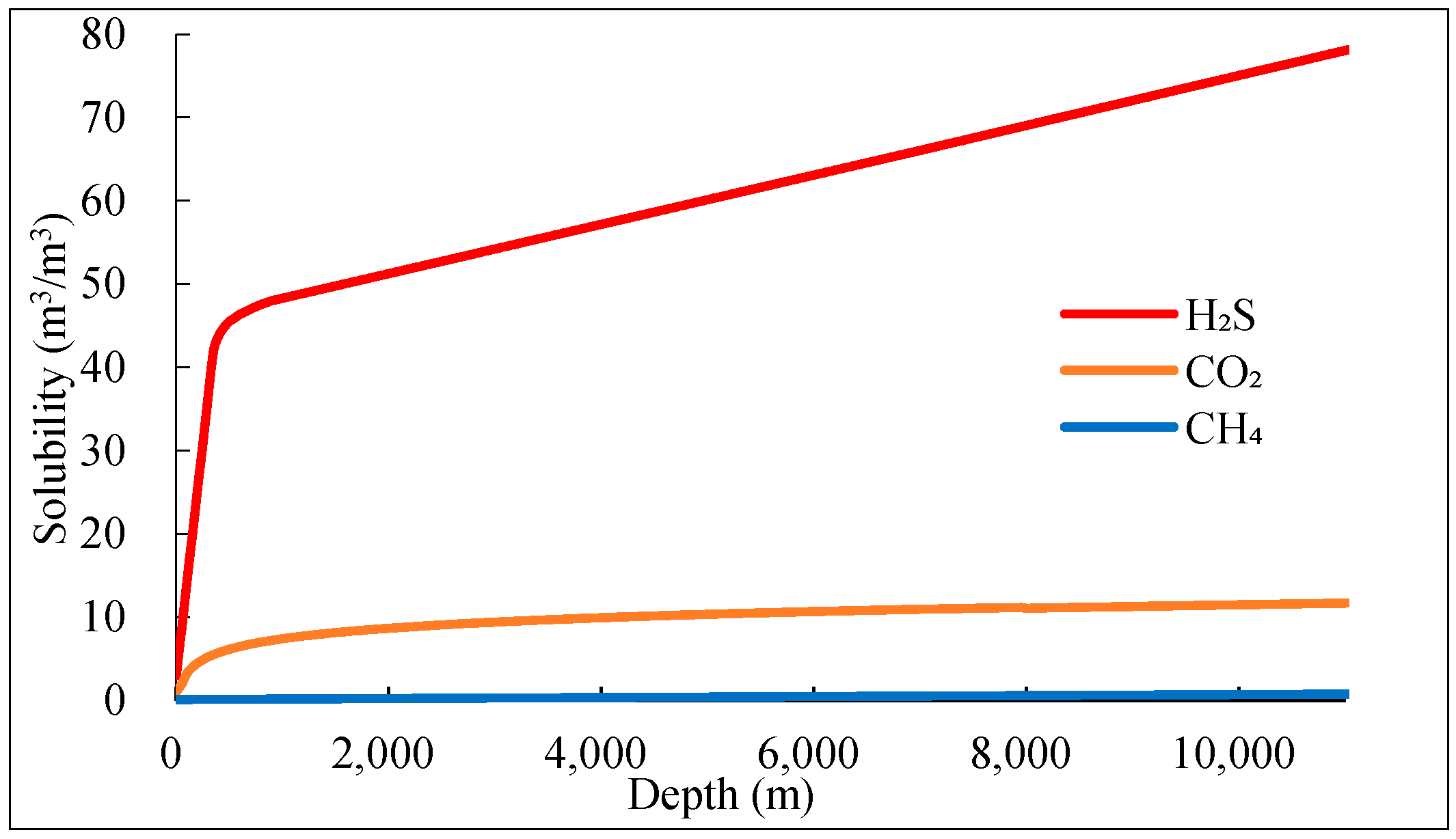
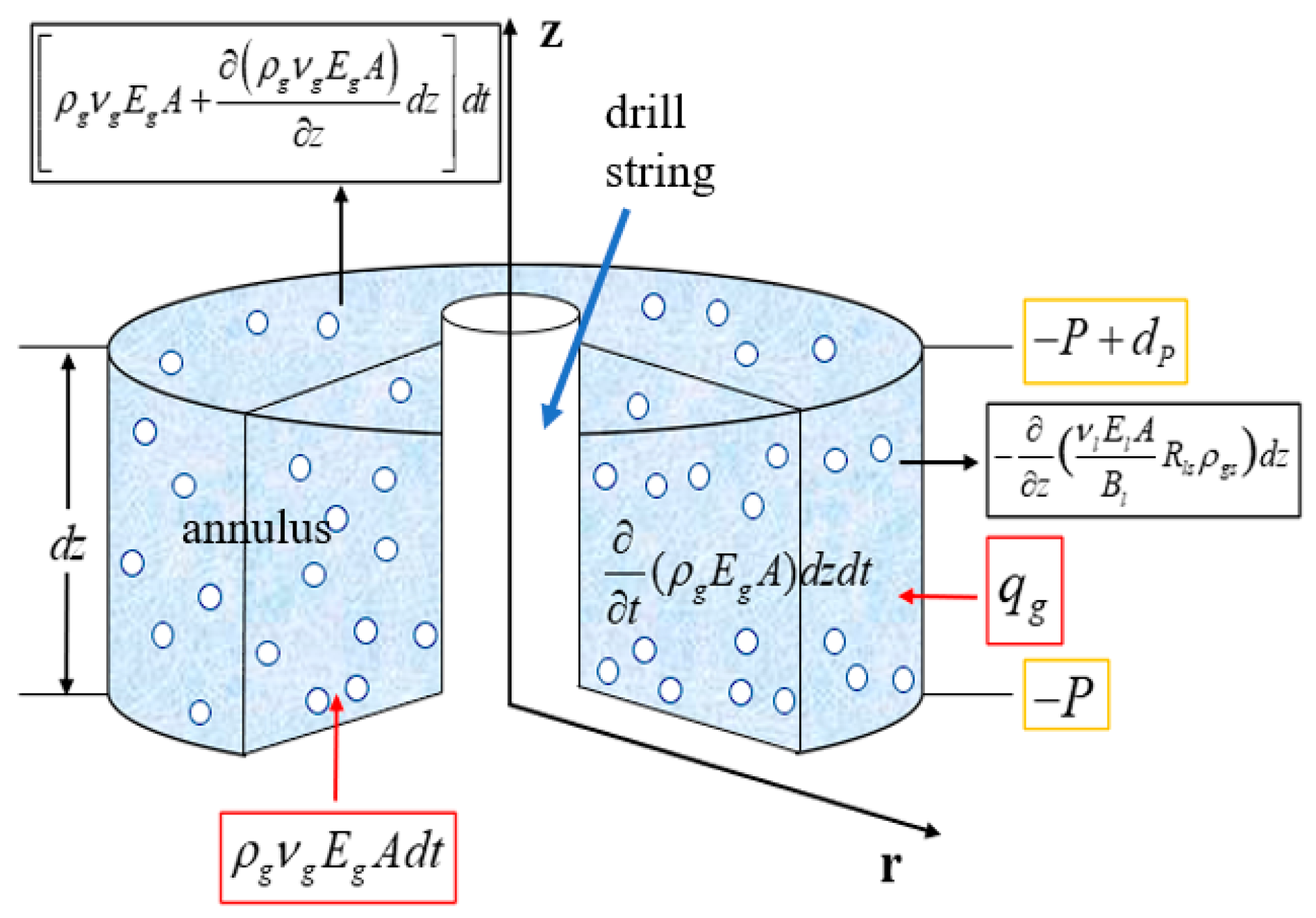
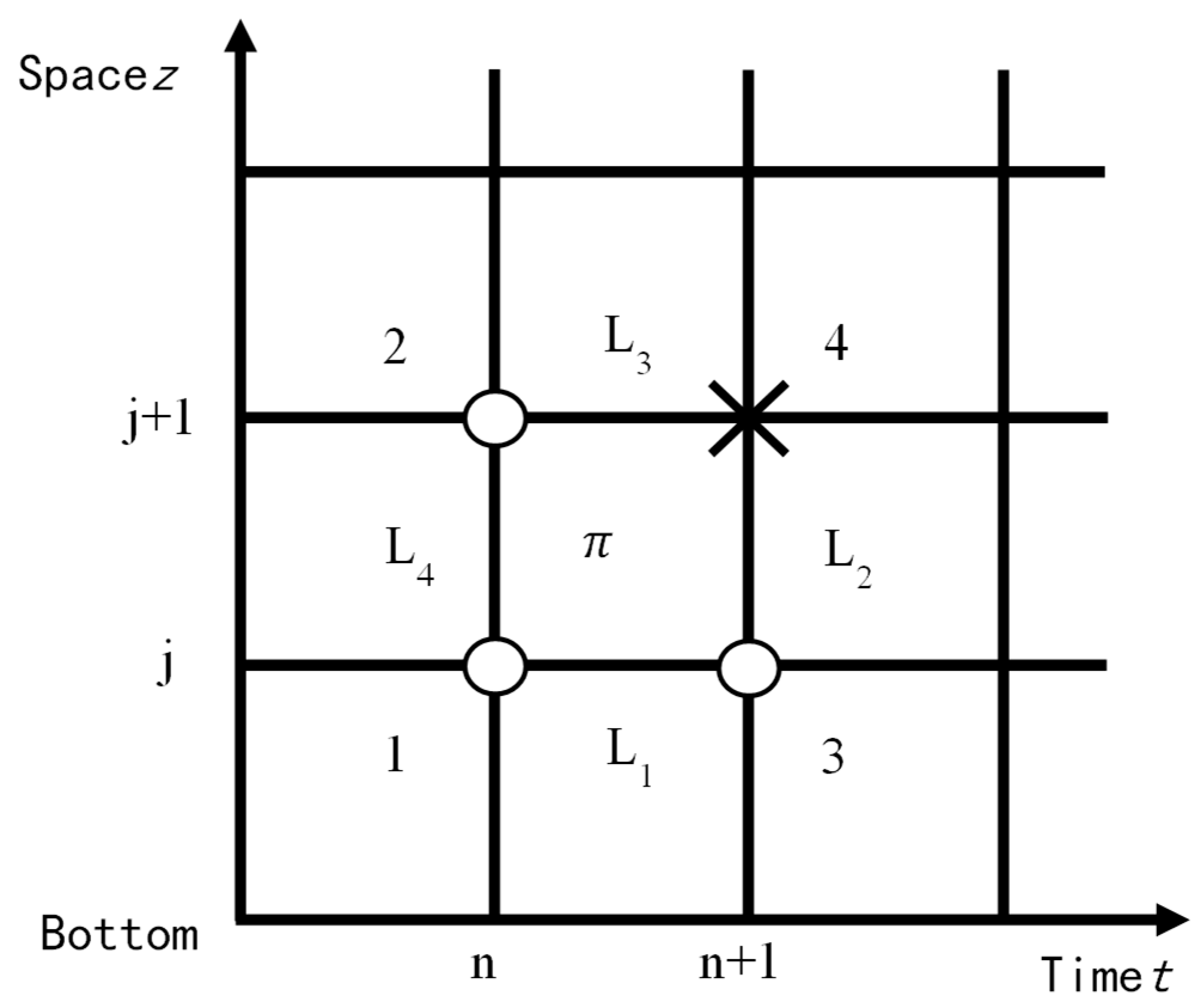
1. Introduction
The deep to ultra-deep conventional gas resources in major basins of PetroChina amount to 20 trillion cubic meters, indicating enormous exploration potential [
1,
2]. However, with increasing well depths, high-temperature, high-pressure, and high-sulfur conditions pose significant challenges to well control safety [
3,
4].
Table 1 presents statistical data on blowouts caused by acidic gas influx [
5], revealing that once gas invasion occurs in natural gas containing acidic components, there is a high probability of severe accidents [
6,
7,
8]. Therefore, it is imperative to investigate the migration mechanisms of acidic gas under high-temperature and high-pressure conditions in ultra-deep wells [
9], ensuring the safe and efficient development of acidic gas reservoirs [
10,
11].
Today, researchers both domestically and internationally have conducted extensive studies on wellbore gas kicks and gas pollution [
12,
13,
14].
In 2012, ref. [
15] considered the unique physical properties of high-sulfur gases and potential phase changes occurring in the wellbore, developing a mathematical model for wellbore flow and heat transfer during high-sulfur gas overflow. They coupled temperature, pressure, and physical property parameters of high-sulfur gases, and proposed a solution method. The study analyzed the wellbore pressure and gas properties during the ascent of gases with different H
2S contents, concluding that high-sulfur gases can easily lead to well control issues. In 2016, ref. [
16] developed a temperature field model for CO
2 in the wellbore and fractures to calculate the phase change of fluid and variations in thermophysical parameters during fracturing. The study analyzed the location of CO
2 phase transitions from liquid to supercritical state, finding that the transition point shifted from inside the wellbore toward the fractures. In 2017, ref. [
17] established a multiphase flow analysis model for wellbore considering factors such as wellbore temperature, fluid compressibility, annulus pressure loss, throttling pipeline friction loss, and gas slippage in order to determine the optimal casing depth and select the appropriate casing grade. Neglecting the effects of wellbore temperature and fluid compressibility and designing casing based on a single bubble model is unreliable, and in offshore oil wells, the frictional loss of throttling pipelines is an important factor that should be considered in casing design. In 2019, ref. [
18] considered the effect of solubility on gas migration and established a semi analytical model of annular air invasion based on a drift model. For two scenarios of single bubble migration and constant gas invasion rate, gas invasion behavior was simulated in water-based drilling fluid and oil-based drilling fluid, respectively. Mud pit increment and wellhead pressure were determined to be two key indicators for gas invasion detection. Ref. [
19] performed extensive experiments using detailed data on gas and brine compositions in the Sarawak Basin, Malaysia. He estimated the initial CO
2 solubility in brine reservoirs at 423.15 K and 36.0 MPa in the Sarawak Basin. In 2019, ref. [
20] conducted a quantitative analysis of the dissolution process of H
2S in NaCl solution, using normalized Raman peak intensities (peak area ratios and peak height ratios) to quantify the solubility of H
2S in the solution.
It is evident that experimental investigations on supercritical acidic gases have primarily focused on CH4, CO2, and their mixtures. However, due to the high toxicity of H2S, experimental studies on supercritical H2S and CO2–H2S mixtures are relatively limited. Given the challenges of conducting experiments with H2S, simulation and numerical modeling methods are commonly used. Moreover, most existing work focuses on how acidic gases and their mixtures dissolve in water-based systems. In contrast, there is limited understanding of how these gases—especially hydrogen sulfide (H2S)—behave in organic solvents.
In the study of annular multiphase flow, extensive efforts have been made to explore its characteristics and underlying mechanisms [
21,
22].
In 2017, Na [
23] coupled wellbore temperature, pressure, and hydrate dissociation effects to establish a dynamic model for wellbore temperature, pressure, multiphase flow, and hydrate mass transfer during offshore natural gas hydrate drilling. In 2017, ref. [
24] introduced a new method to measure pressure drop and liquid hold-up to improve the closure relationships of the MAST multiphase flow simulator, which had been validated using a series of laboratory and field data collected by TEA Sistemi. In 2018, ref. [
25] found that gas diffusion in the annulus is the main parameter affecting the peak pressure of the casing. Considering the two extreme cases of single bubbles and dispersed bubbles rising along the annulus, ignoring the influence of annulus friction, casing pressure calculation models were established separately. The study found that under the same conditions, there is a significant difference in the casing pressure calculated by the two models, especially for situations where the bottomhole pressure is low. Karami and Akbari used commercial OLGA software to simulate the development law of gas invasion, and found that gas diffusion was most significant at low cycle displacements; When the circulation displacement is high, the time for gas invasion to reach the wellhead is the fastest. Ref. [
26] proposed a multiphase flow model for real-time calculations used in managed pressure drilling (MPD) control, considering gas solubility in the drilling fluid. The model accounts for the real-time adjustment of wellhead pressure and its impact on gas migration and phase changes. In 2020, ref. [
27] noted that existing two-phase flow models for the annulus rarely consider the effects of liquid viscosity, leading to poor predictions. The study examined the impact of liquid viscosity on two-phase flow regimes in vertical pipes and proposed two flow regime transitions: bubble flow (BL) and dispersed bubble flow (DB) for different bubble sizes. In 2019, ref. [
28] considered the compressibility and rheology of drilling fluids and established a transient compressible isothermal mathematical model to predict the mud pit increment and wellbore pressure transfer during gas invasion. Research has shown that the greater the compressibility of the fluid, the greater the difference between the increment of the mud pit and the volume of gas invasion. The rheological properties of the drilling fluid greatly affect gas invasion detection and pressure transmission. Ref. [
29] used CFD software to simulate gravity-driven gas–liquid two-phase flow and analyzed the variation in gravity displacement rates within fractures under two boundary conditions. Additionally, they developed a simplified model for gravity displacement in dual-boundary fractures, based on gas–liquid two-phase flow theory.
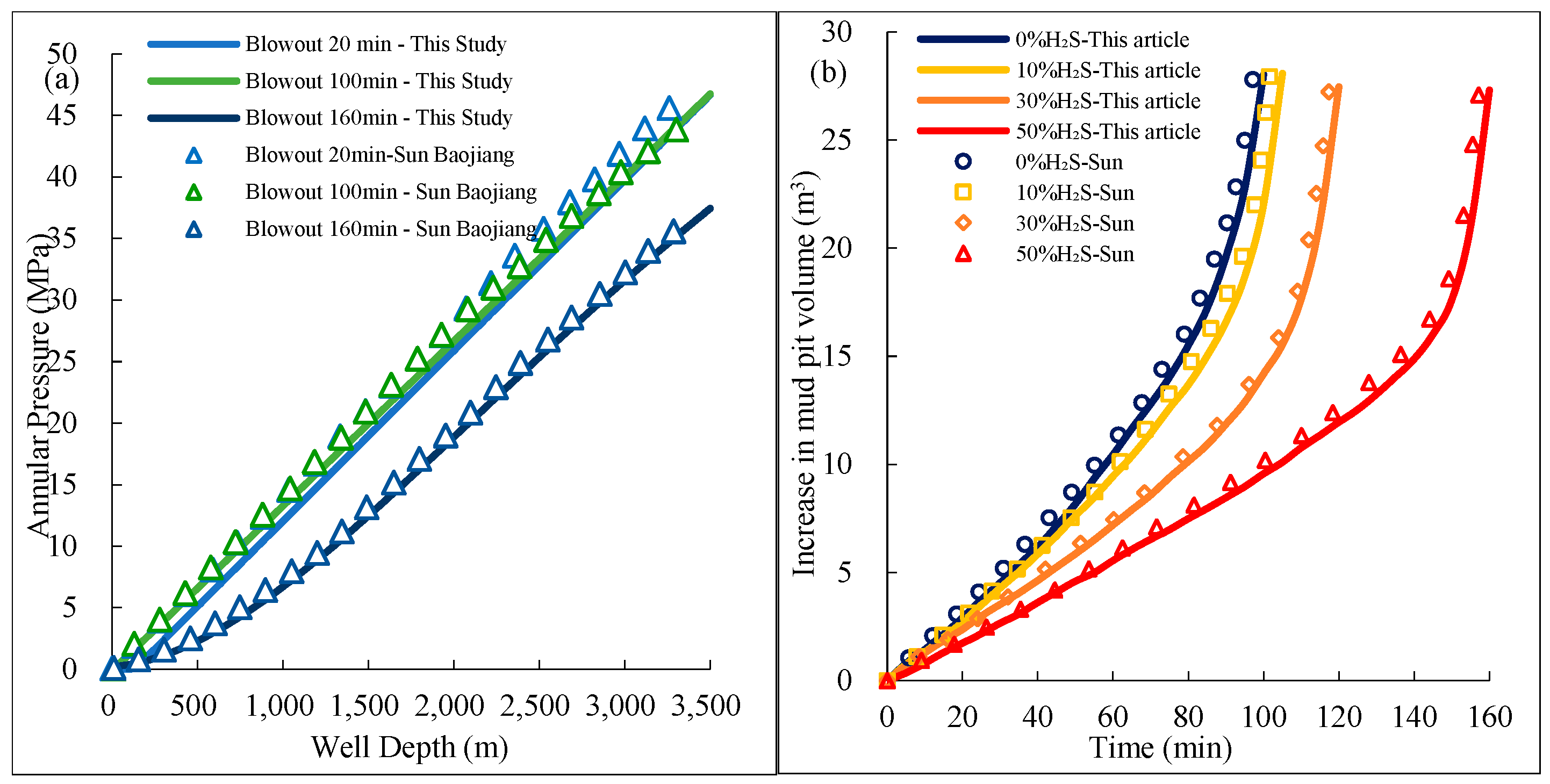
It is evident that studies on annular multiphase flow have primarily relied on simulation and numerical modeling, with a strong emphasis on single-component systems. However, most of these studies do not consider the effects of H2S invasion and the solubility of acidic gases in drilling fluids on transient multiphase flow in the annulus. A few studies analyze the impact of acidic gases by considering their phase behavior, but the fundamental causes are not discussed. This paper establishes a flow model based on the solubility of acidic gases and critical parameters, solving it using a four-point difference scheme. The model reveals the expansion and flow velocity variation in acidic gases at the wellhead and quantifies the significant impact of H2S on well control, providing theoretical support for the safety of well control in ultra-deep wells.
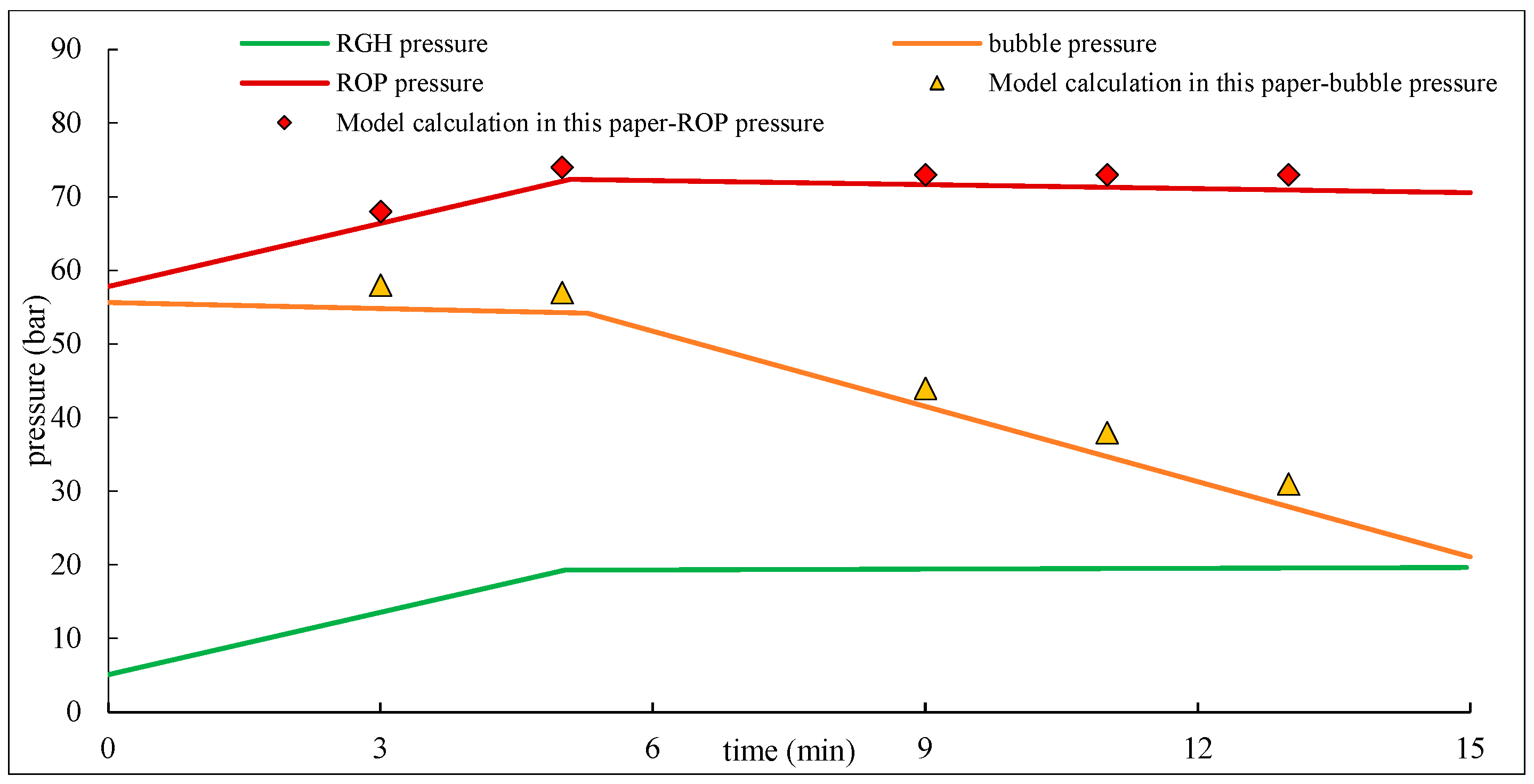
6. Discussion
The migration laws of acidic gas overflow obtained in this study provide important practical guidance for well control safety in HPHT gas wells. The results can be applied in three main aspects: well control design, emergency planning, and well-killing operations.
In terms of well control design optimization, the simulations show that acidic gas exsolution and rapid expansion near the wellhead lead to nonlinear pressure drops and accelerated flow velocities. In engineering design, this requires incorporating larger design safety margins in wellhead equipment and casing strength to accommodate sudden annular pressure fluctuations. And selecting drilling fluids with improved H2S and CO2 solubility to delay gas exsolution and reduce the intensity of overflow expansion.
Compared to CO2, the invasion of H2S is more dangerous because of its higher solubility and delayed but stronger dissolution. This means that we should strengthening on-site safety equipment, including emergency ventilation, breathing apparatus, and protective shelters, to address the higher probability of H2S release during overflow.
The findings indicate that H2S intrusion produces greater wellhead expansion and mud pit displacement, which requires us to adjust the well killing strategy, such as: Kill mud density calculations should be corrected for acidic gas solubility and pseudo-critical behavior rather than methane-only assumptions. Foam or polymer-enhanced drilling fluids can be considered to suppress bubble growth and improve stability during acid gas kicks.
Field cases and model results suggest that when H2S concentration exceeds “20–30% in the invading gas phase”, migration behavior changes significantly: solubility increases sharply, exsolution is delayed to shallower depths, and volume expansion near the wellhead intensifies. This threshold marks the point at which overflow transitions from manageable to high-risk. Engineering practice should therefore treat influxes with H2S content above 20% as high-risk scenarios requiring enhanced monitoring, higher mud density margins, and stricter surface safety protocols. For H2S concentrations above “40%”, the model predicts critical nonlinear effects: compressibility factor reductions >0.5 and wellhead volume expansion exceeding 20% compared to methane. Such conditions should be considered “severe hazard,” and pre-planned contingency measures must be activated immediately.
7. Conclusions
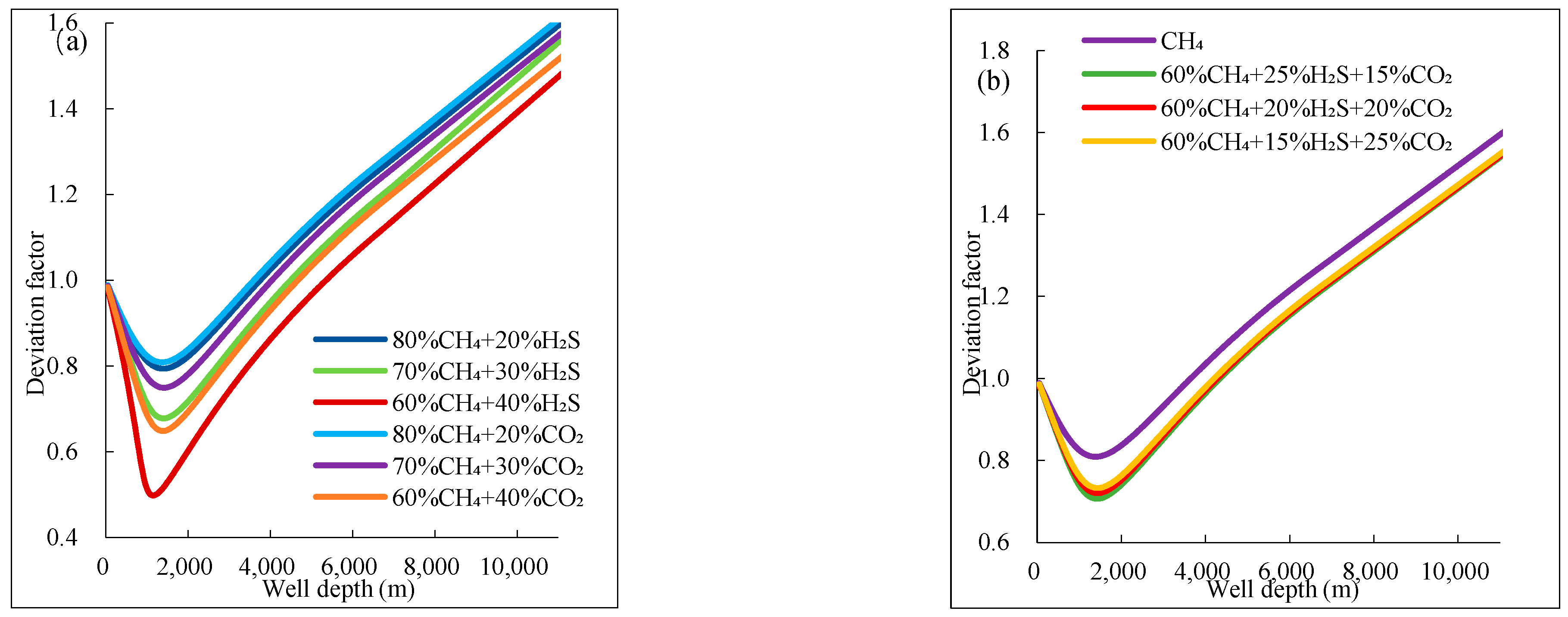
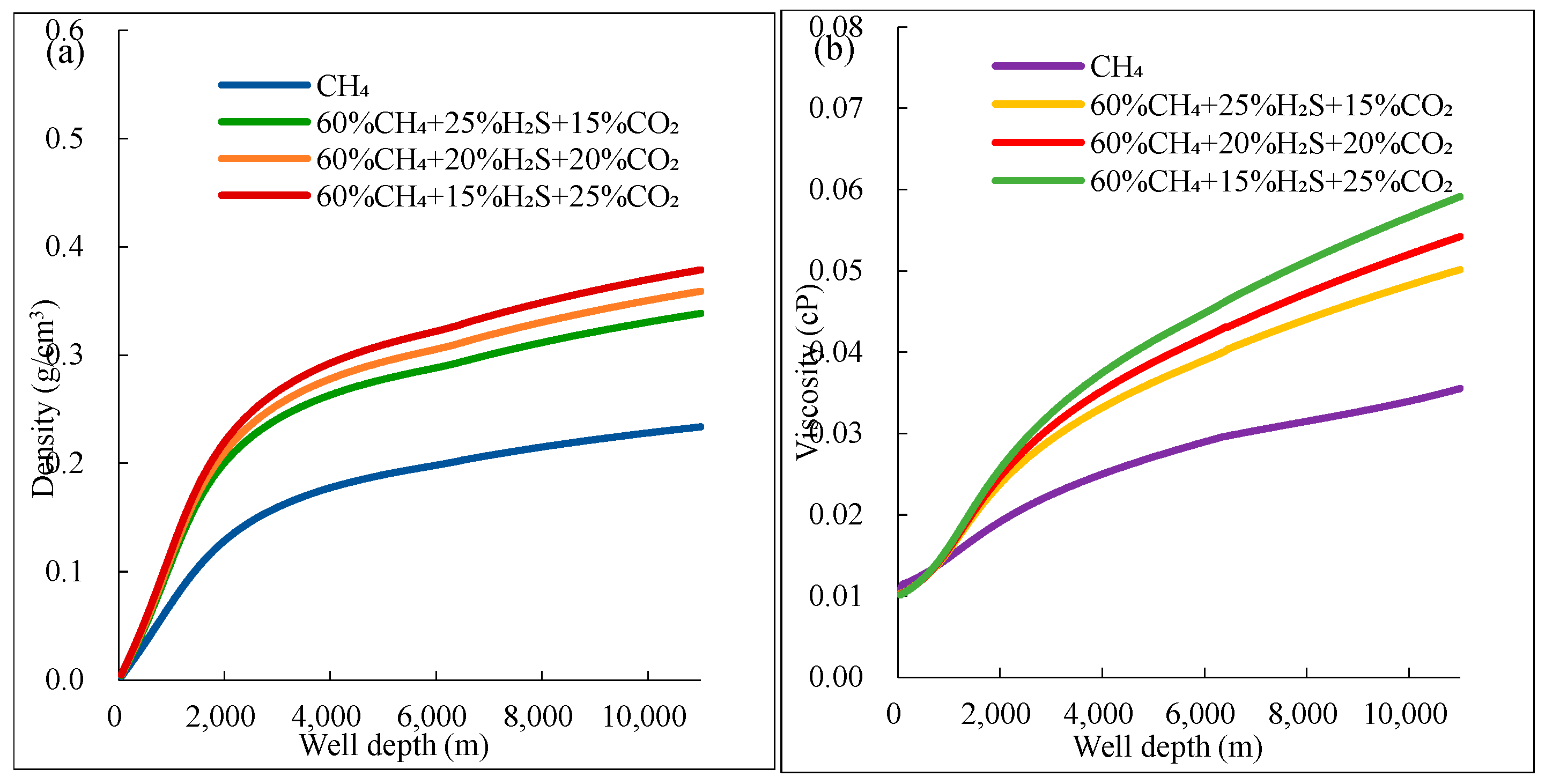
(1) For gas mixtures with >30% H2S, the compressibility factor decreases by 40–50% near the pseudo-critical point, while density increases by 35–45% and viscosity rises by 20–30% compared to pure CH4. These changes are most severe at depths of 1000–1500 m, where temperature/pressure approach pseudo-critical conditions.
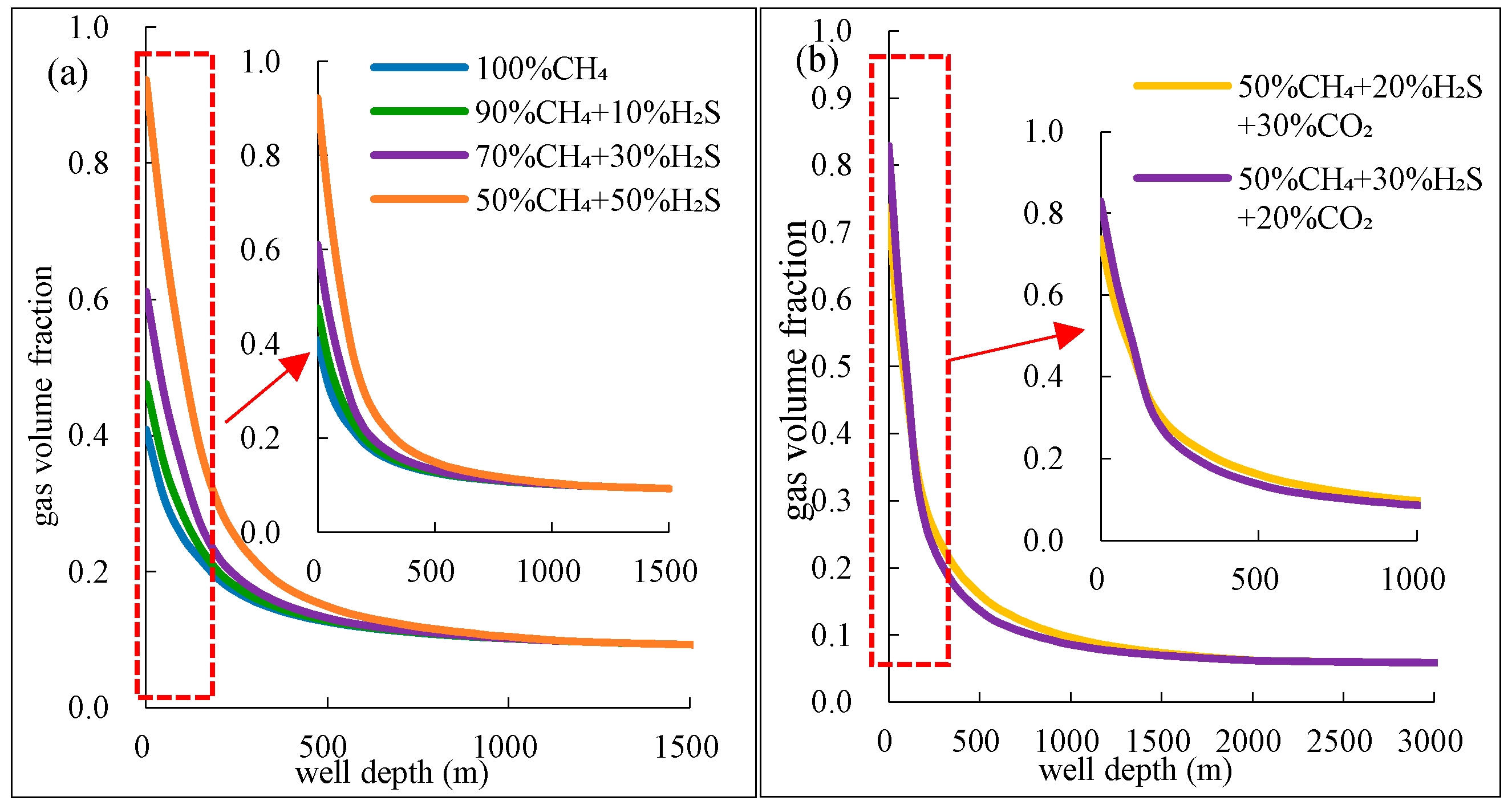
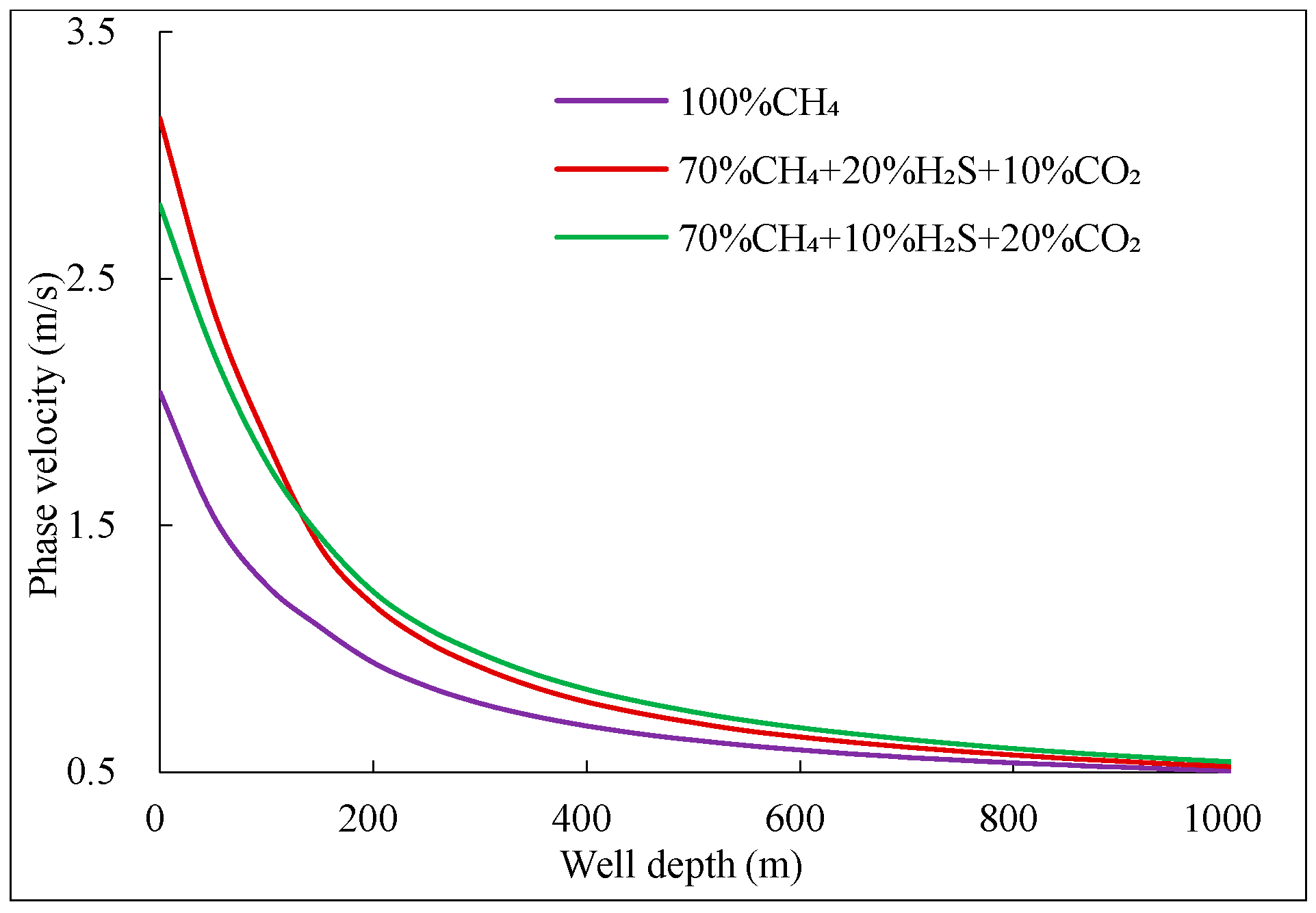
(2) For mixtures with ≥20% H2S, the gas phase volume fraction expands by 20–25% at the wellhead, and flow velocity surges by 10–15% due to abrupt exsolution below 1500 m. Ternary mixtures (e.g., 20% CO2 + 20% H2S) show 30% greater expansion than binary mixtures (e.g., 40% CO2).
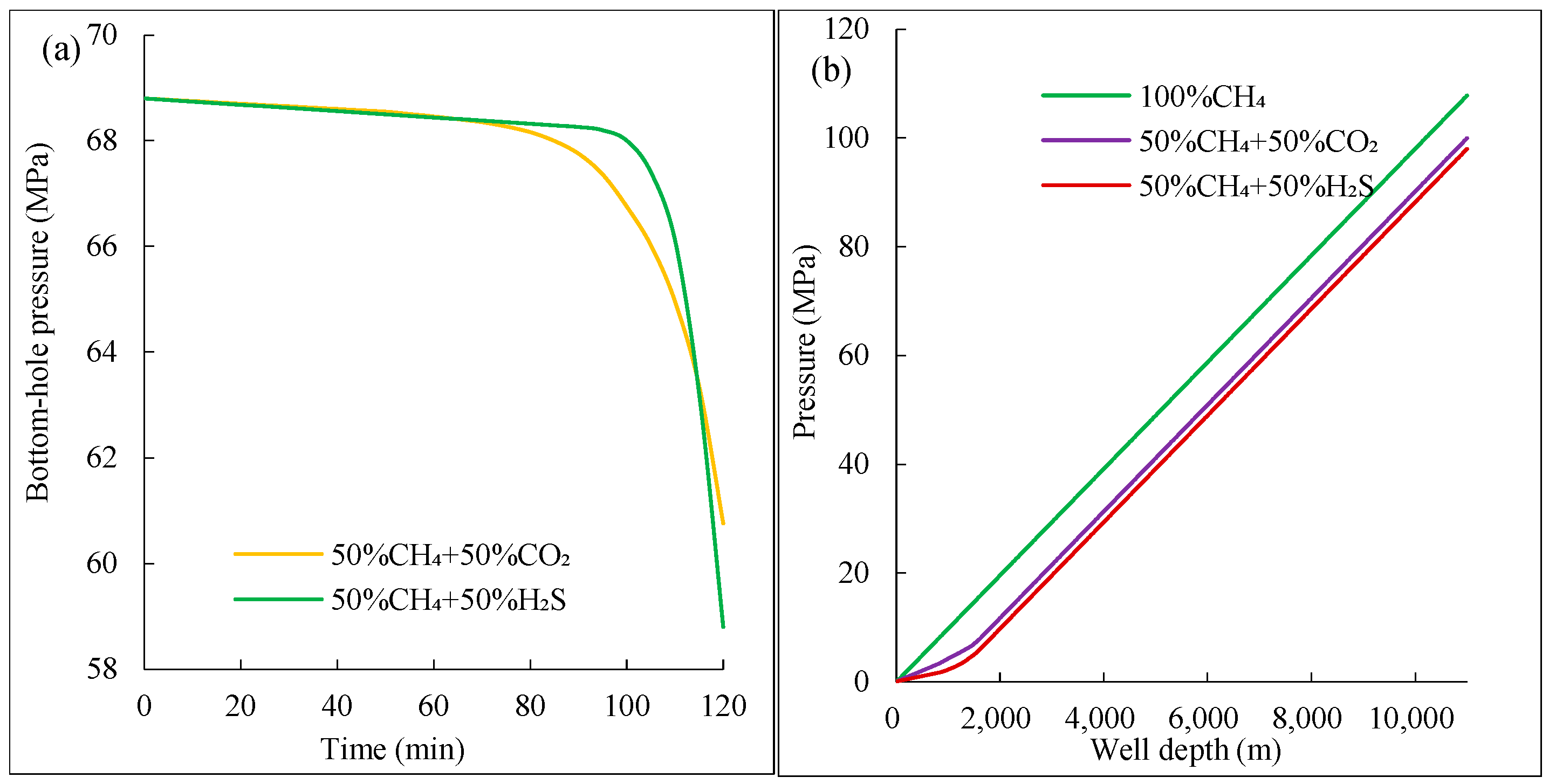
(3) Annular pressure drops nonlinearly by 15–20% near the wellhead for mixtures with >15% acidic gas, due to greater solubility in drilling fluid and increased expansion near the wellhead as temperature and pressure decrease, thereby exacerbating well control risks.
(4) The greater the density of acidic gases, the larger the increase in mud pit volume. Compared to CO2-rich gases, H2S intrusion causes more significant volume expansion due to its lower compressibility coefficient, resulting in greater displacement of drilling fluid near the wellhead. Therefore, H2S has the greatest impact on well control (difficulty in overflow monitoring, pressure control, etc.), followed by CO2, and CH4 has the least impact.