The HDS Process: Origin, Process Evolution, Reaction Mechanisms, Process Units, Catalysts, and Health Risks
Abstract
1. Introduction
1.1. Current Status of Development and Relationships with the Hydrodesulfurization Process
1.2. Structure of the Brief Review
2. Origins
2.1. Petroleum
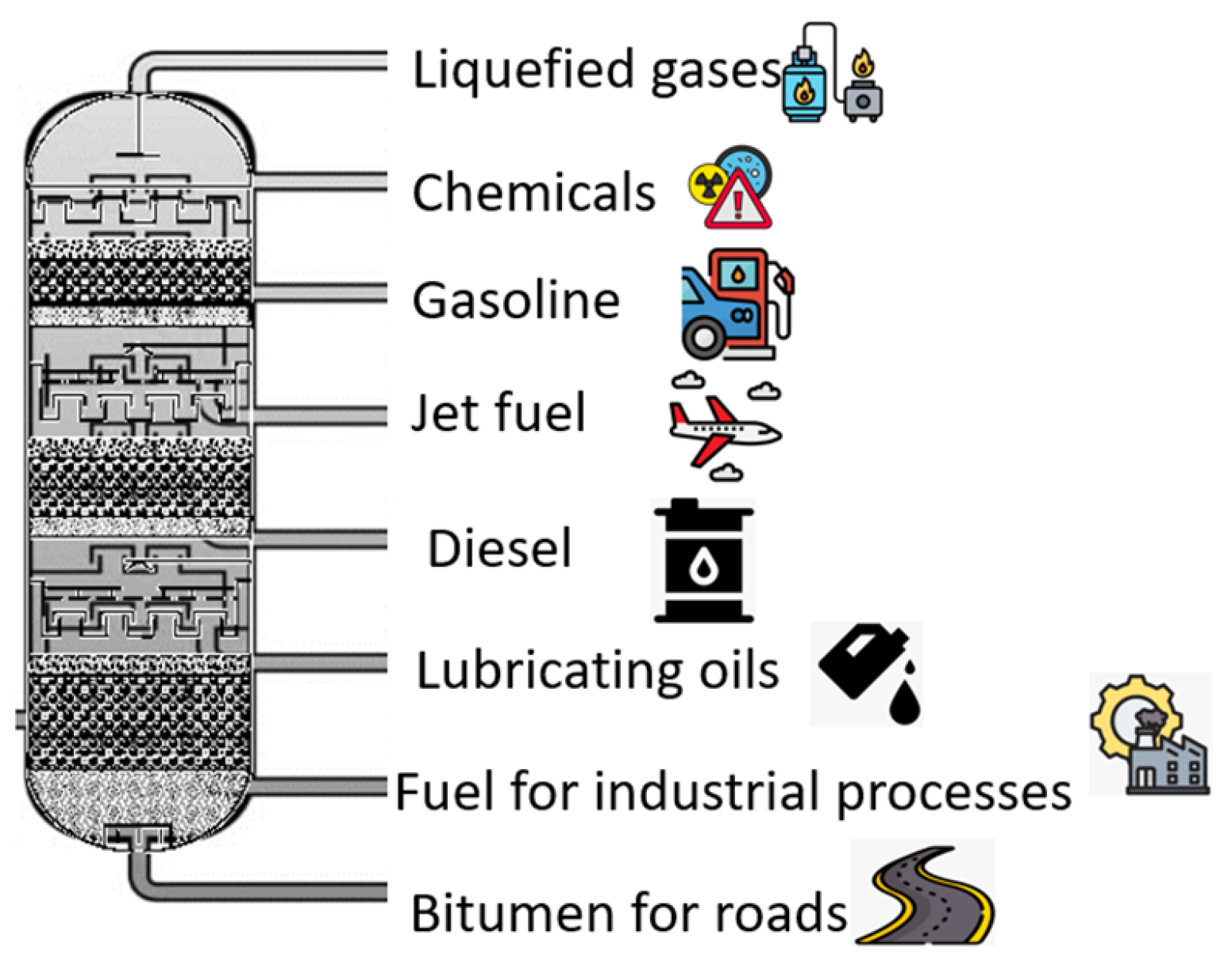
2.2. Oil Distillation
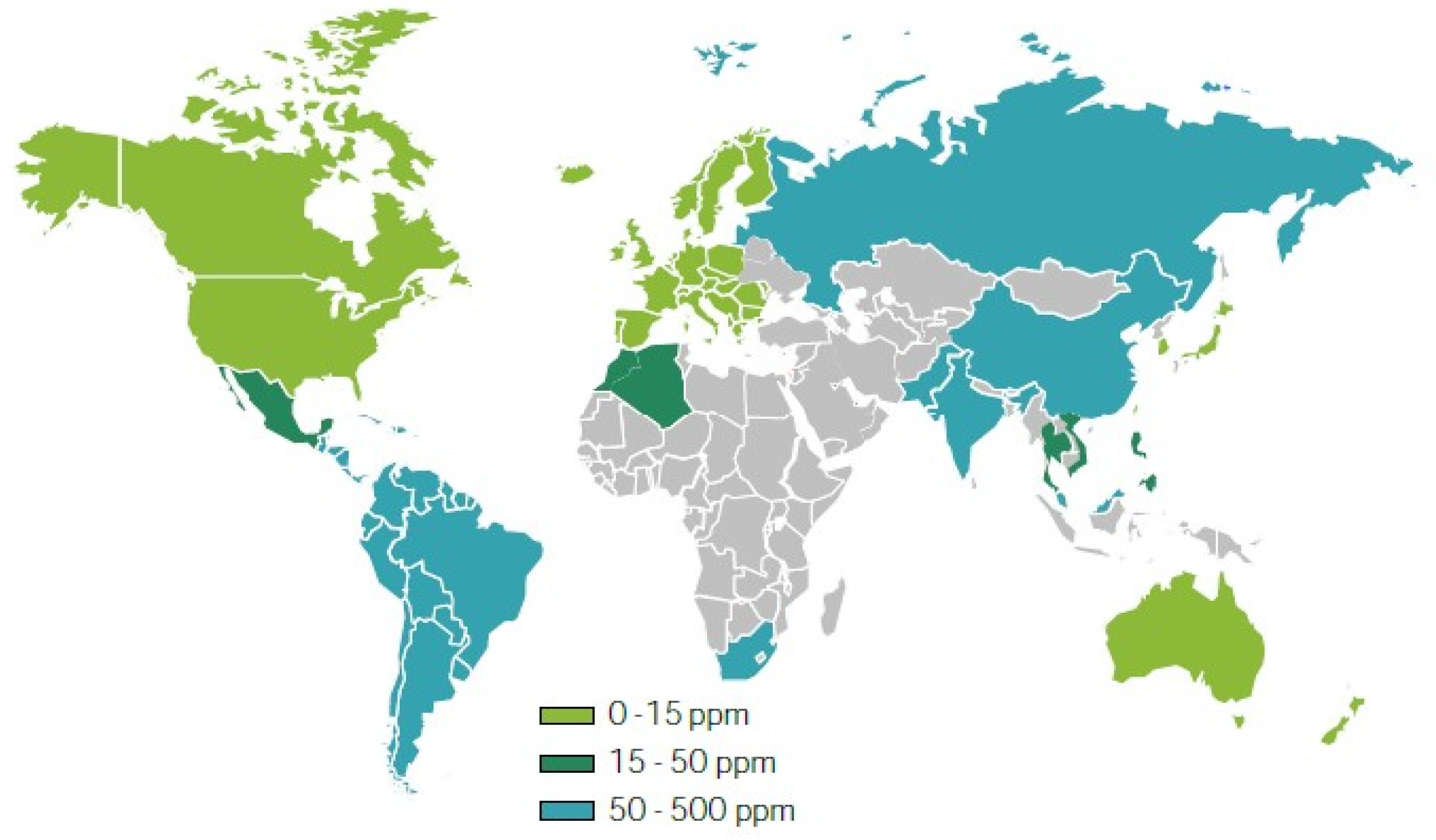
2.3. Heteroatoms in Petroleum
3. The Hydrodesulfurization Process
3.1. Hydrotreating Processes
- Hydrodesulfurization (HDS): This leads to the removal of sulfur from petroleum compounds by converting them to H2S and products in the form of hydrocarbons with lower molecular weight and boiling points.
- Hydrodenitrogenation (HDN): Nitrogen removal is performed to minimize catalyst poisoning in subsequent processes as they are a source of coke formation during catalytic cracking and inhibit reactions by adsorption on acid sites.
- Hydrodeoxygenation (HDO): Oxygenated compounds are present at low concentrations in petroleum, increasing with the boiling point. A process to remove the oxygen that is present is also carried out.
- Hydrodemetallization (HDM): Traces of nickel and vanadium (330 ppm Ni + V in Maya crude) are present in petroleum, generally in the form of porphyrins or chelating compounds. These compounds can be deposited on catalysts during the conversion process in the form of transition metal sulfides (Ni3S2, V3S4, and V2S3). This deposition poisons the catalytic material, reducing the number of active sites (the area where the substrate binds in catalysis) and impeding the transportation of reactants due to potential blockage of the pores [12,13].
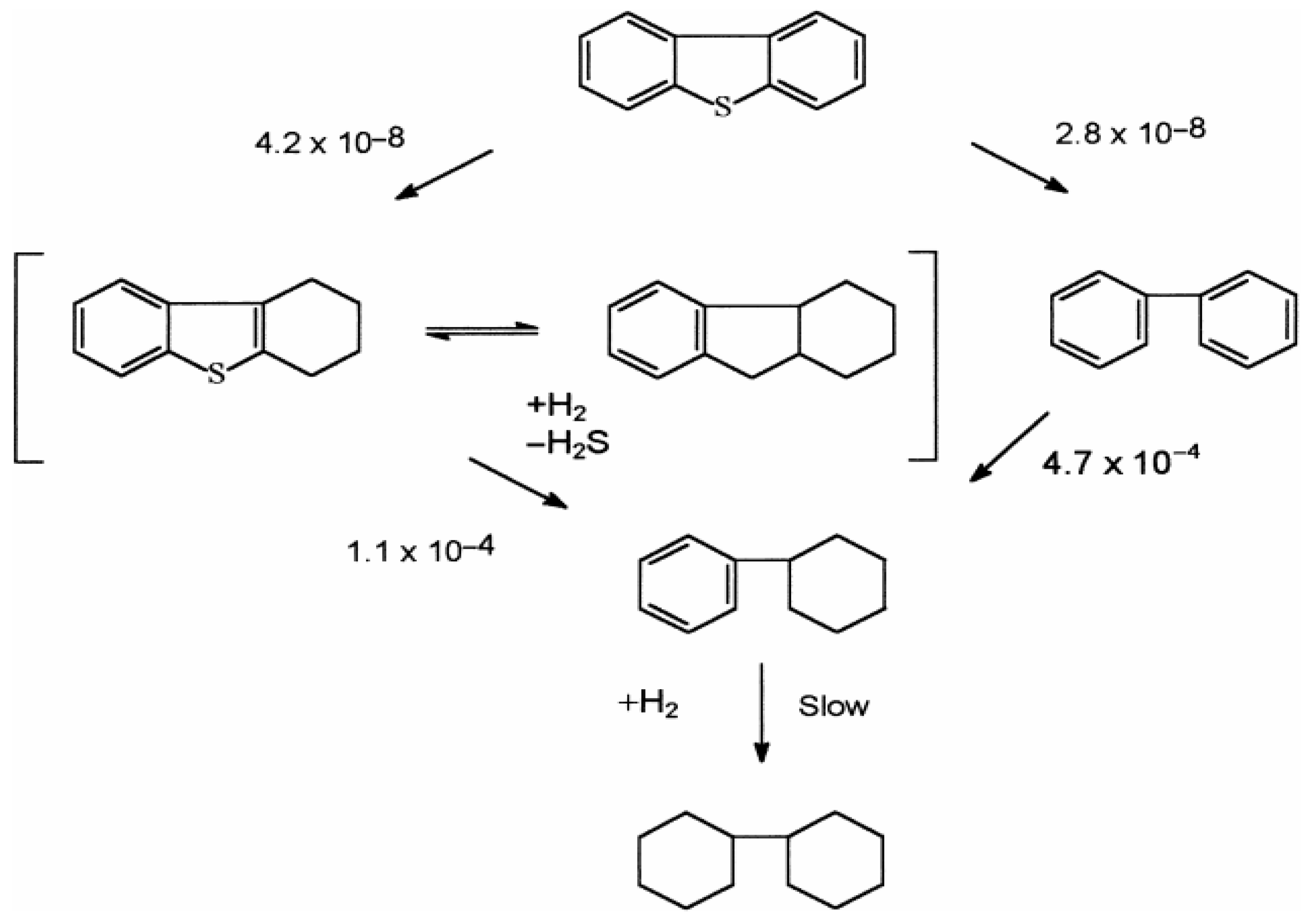
3.2. Mechanisms of Hydrodesulfuration Reactions
- Non-heterocyclic: thiols (mercaptans, SSR), sulfides (RSR), and disulfides (RSSR).
- Heterocyclic: compounds containing several thiophenes (one or more rings), sometimes with alkyl or aryl substituents.
| Sulfur Compounds | Structure | |||
|---|---|---|---|---|
| Thiols (mercaptans) | R-SH | R-S-R | R-S-S-R’ | |
| Thiophenes | 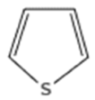 | 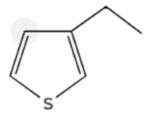 | ||
| Sulfides | 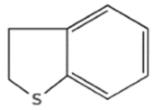 | 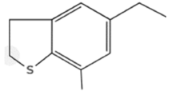 | ||
| Dibenzothiophenes |  | |||
3.3. Hydrodesulfurization Units
- Reactor section or reaction section.
- Recycle gas section.
- Product recovery section.

3.4. Three-Phase Reactors
- HDT (Hydrotreating of naphtha and heavy gas oils).
- Hydrocracking.
- “Hydrorefining” of lubricating oils.
3.5. Reactor Operating Variables
- Liquid hourly space velocity (LHSV)
- Temperature
- Pressure
- Hydrogen-to-hydrocarbon ratio
3.6. Nature of the Feed
4. Catalysts
- Active agent: This is the primary constituent responsible for catalytic function and includes metals, semiconductors, and insulators.
- Support: Materials frequently used as catalytic supports are porous solids with a high specific surface area, which are classified as follows:
- o Inert supports, such as silica (SiO2).
- o Supports with catalytic activity, such as alumina (Al2O3), aluminosilicates, and zeolites.
- o Supports that influence the catalytic activity of the active phase, such as titania (TiO2).
- Promoter: Substances added to enhance the physical and chemical functions of the catalyst are known as promoters. Their purpose is to improve catalytic properties, increasing their activity, selectivity, and resistance to deactivation. Although promoters are added in relatively small quantities, their selection is often decisive regarding the catalyst’s properties. Promoters can be incorporated into the catalyst at some stage in the chemical processing of the catalyst components. In some cases, promoters are added during the reaction. There are two types of promoters:
- o Textural promoters, which give greater stability to the active phase.
- o Electronic promoters, which increase activity.
4.1. Transition Metal Sulfides (TMS)
- Third row: RuS2 > Rh2S3 > PdS > MoS2 > NbS2 > ZrS2.
- Second row: OsSx > IrSx > ReS2 > PtS > WS2 > TaS2.
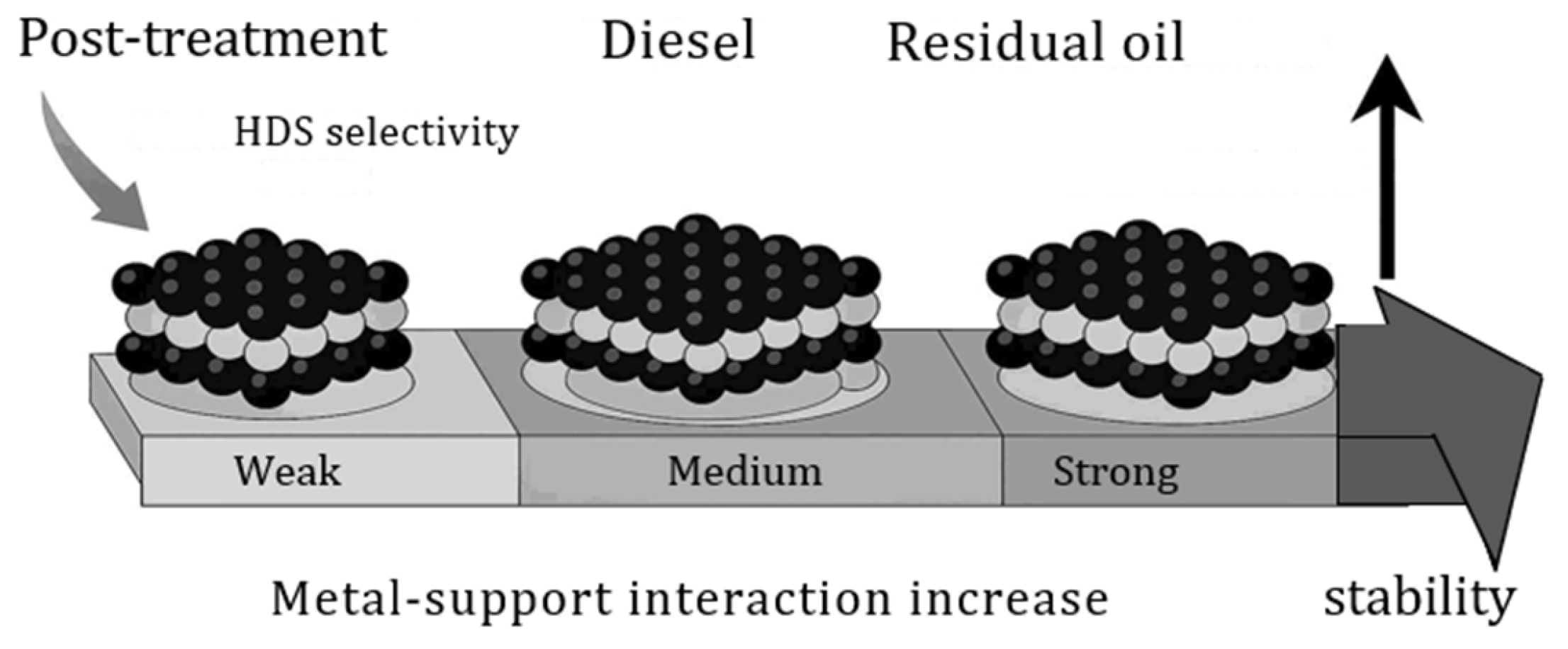
4.2. Supported Catalysts for HDS: The Role of the Support in Active Sulfide Phases
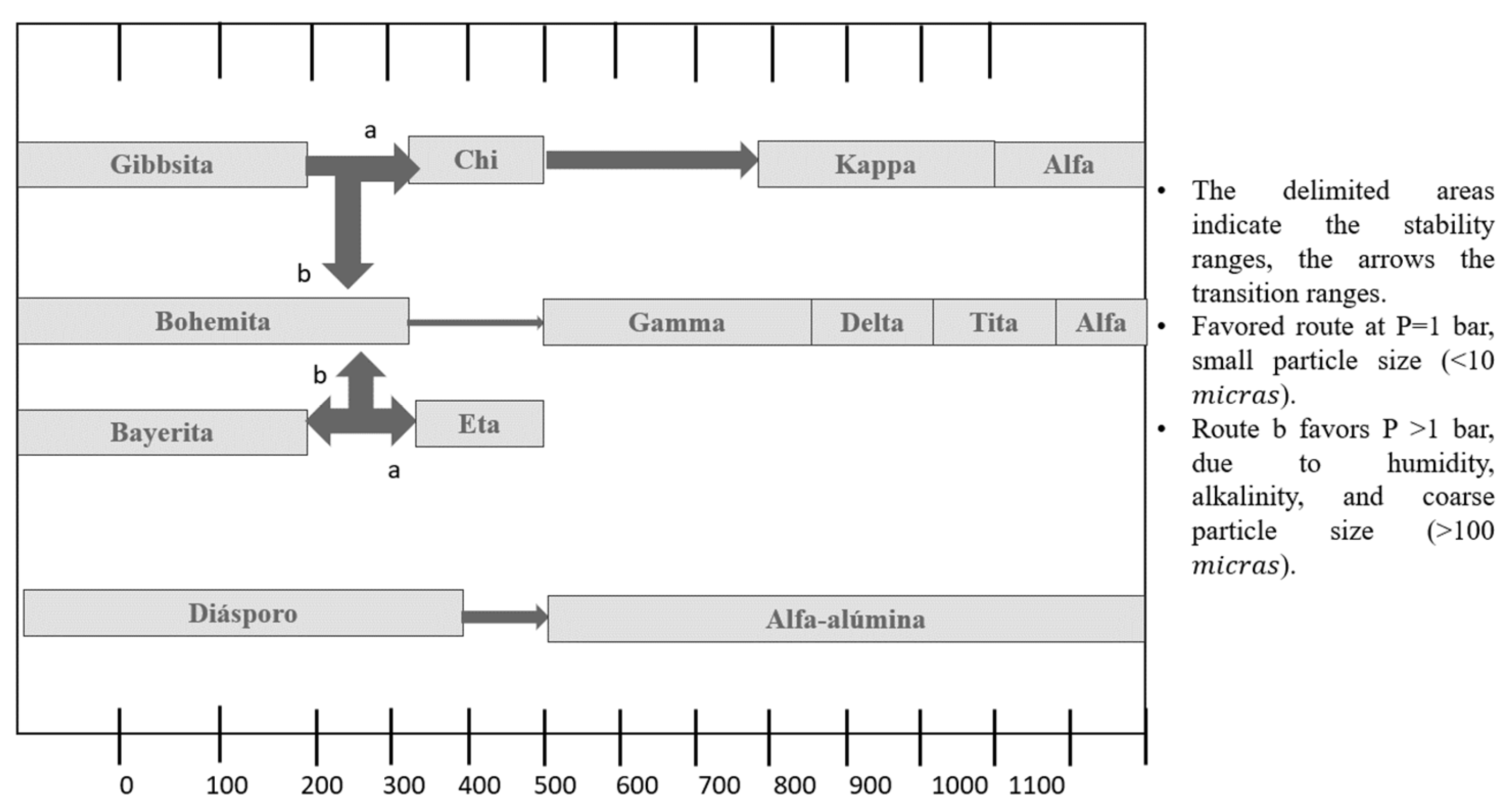
4.3. Al2O3 Supports
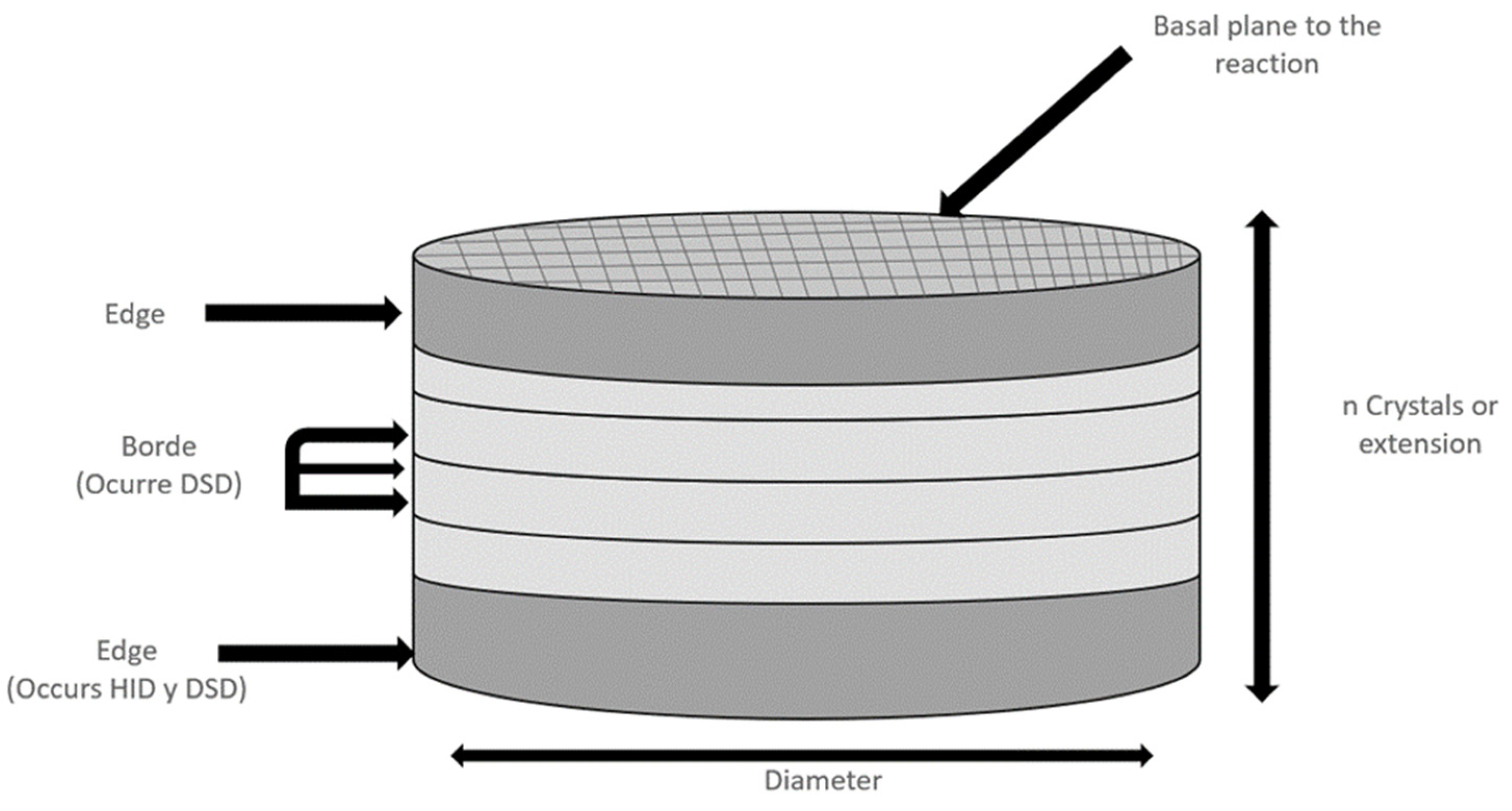
4.4. Structure of γ-Al2O3
4.5. Incorporation of Metal Ions into γ-Al2O3
- 1.
- Solid substitution solution
- A.
- Atomic size: The relative difference between the atomic diameters of the two species must be less than 15%; otherwise, the solubility is very limited.
- B.
- Crystal structure: The solvent and solute atoms must crystallize in the same structure, e.g., face-centered cubic, hexagonal, etc.
- C.
- Valence: The solute and solvent atoms must have the same valency.
- D.
- Reactivity: The two species must be chemically similar and must be close in the electrochemical series. Chemical reactions between species tend to favor the formation of stable compounds before forming solid solutions.
- 2.
- Interstitial solid solution
- A.
- Atomic size: The diameters of the solute atom must be small compared to the solvent atom (diameter ratio of less than 0.59).
- B.
- Crystal structure: The structure of the solvent and solute atoms does not matter.
- C.
- Solubility in metals: Solute atoms dissolve much more easily in transition metals than in other metals due to their electron configuration (d and f orbitals, free).
4.6. Acidity of the Alumina-Based Support
4.7. Catalysts Supported on SBA-15
4.8. Deactivation of HDT Catalysts
4.9. Catalytic Reactivation
4.10. Ex-Situ Reactivation
4.11. In-Situ Reactivation
4.12. Industrial Regeneration
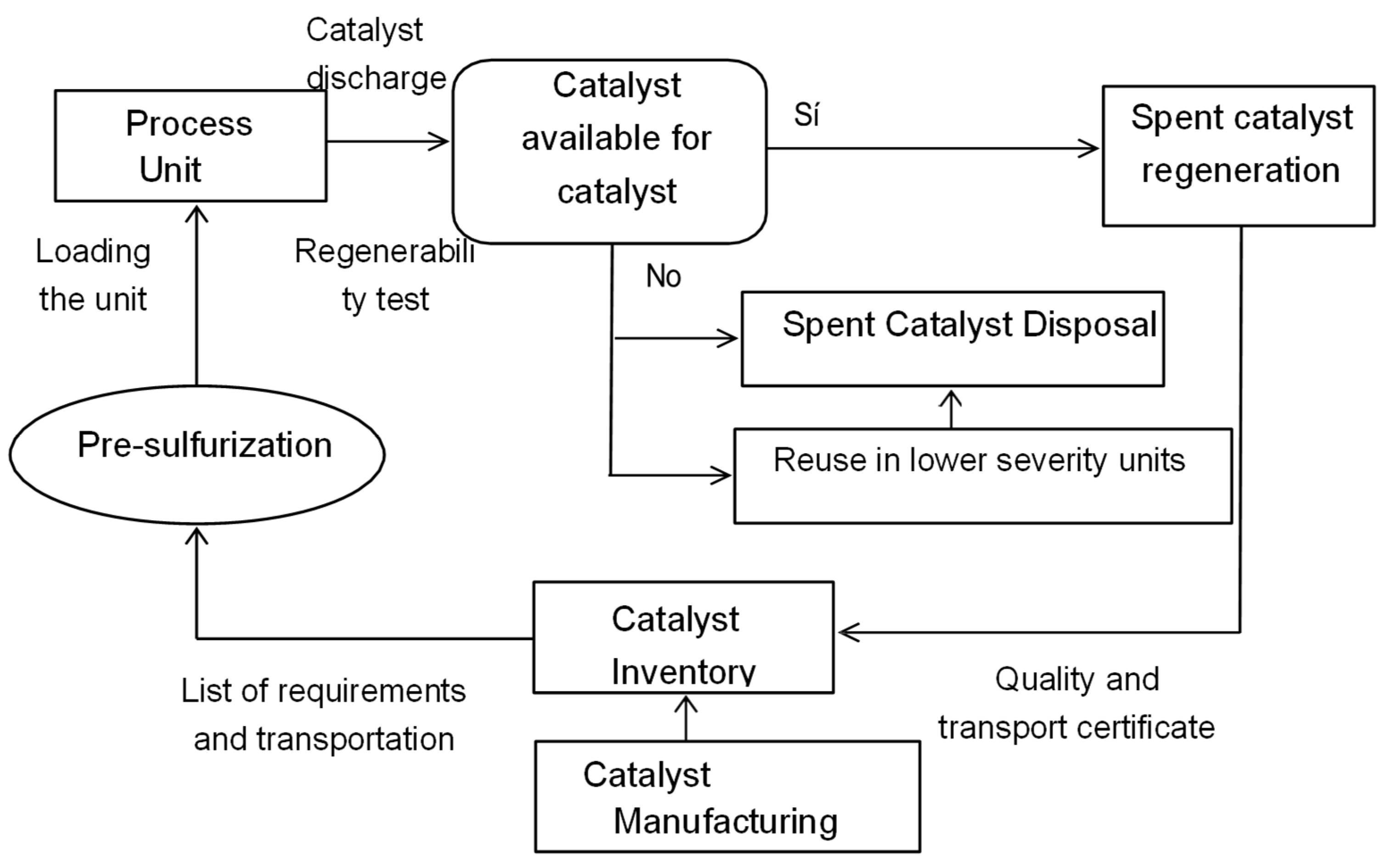
4.13. Spent Catalysts and Management Alternatives
5. Risks to Human Health, Environmental Issues, and Mexican Standards
5.1. Risks to Human Health
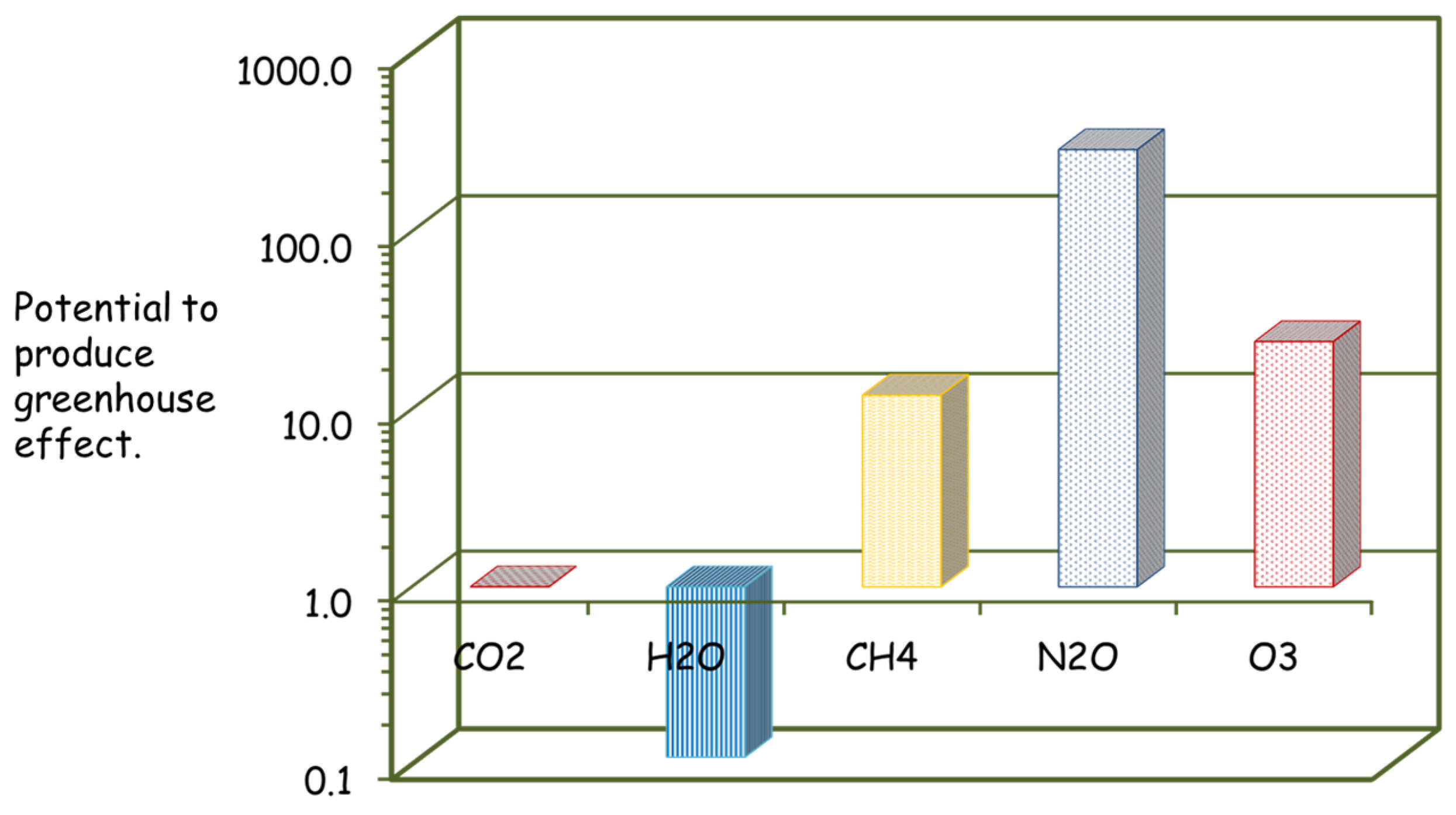
5.2. Environmental Damage
5.3. Mexican Regulations
6. Effects of Desulfurized Fuels on Motor Engines
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Al3+ | Aluminum (III) ions |
| Al2O3 | Alumina |
| BCH | Bicyclohexyl |
| BF | Biphenyl |
| CHB | Cyclohexylbenzene |
| Co | Cobalt |
| CO | Carbon monoxide |
| CoMoS | Cobalt and molybdenum sulfur |
| COP | Conference of the Parties |
| CS2 | Carbon disulfide |
| DBT | Dibenzothiophene |
| DSD | Direct desulfurization reactions |
| HC | Hydrocarbons |
| HHDBT | Hexahydrodibenzothiophene |
| HDM | Hydrodemetallization reactions |
| HDN | Hydrodenitrogenation reactions |
| HDO | Hydrodeoxygenation reactions |
| HDS | Hydrodesulfurization reactions |
| HDT | Hydrotreatment processes |
| HYD | Hydrogenation reactions |
| IUPAC | International Union of Pure and Applied Chemistry |
| LHSV | Liquid hourly space velocity, h−1 |
| MoDTCs | Molybdenum di-thio-carbamates, (Mo2O2S2(OSNR2)2)-type compounds |
| MoS2 | Molybdenum disulfide |
| Ni | Nickel |
| N2O | Nitrous oxide |
| NOX | Nitrogen oxides, (X = {either 2 or 3 or both}) |
| O2− | Oxygen ions |
| -OH | Hydroxyl substituent |
| OH−1 | Hydroxyl ion |
| R or -R | Alquil substituent group |
| SOX | Sulfur oxides, (X = {either 2 or 3 or both}) |
| THDBT | Tetrahydrodibenzothiophene |
| UNFCCC | United Nations Framework Convention on Climate Change |
| VGO | Vacuum gas oil |
| WS2 | Tungsten disulfide |
| ZDDPs | Zinc dialkyl-dithio-phosphates, (Zn((RS)2PO2))-type compounds. |
References
- COP29 UN Climate Conference Agrees to Triple Finance to Developing Countries, Protecting Lives and Livelihoods. 2025. Available online: https://unfccc.int/news/cop29-un-climate-conference-agrees-to-triple-finance-to-developing-countries-protecting-lives-and (accessed on 17 June 2025).
- Transformación Energética. Available online: https://www.pemex.com/nuestro-negocio/dte/Paginas/default.aspx (accessed on 18 June 2025).
- López-Salinas, E.; Espinosa, J.; Hernández-Cortez, J.; Sánchez-Valente, J.; Nagira, J. Long-Term Evaluation of Nimo/Alumina–carbon Black Composite Catalysts in Hydroconversion of Mexican 538 °C+ Vacuum Residue. Catal. Today 2005, 109, 69–75. [Google Scholar] [CrossRef]
- Climent, M. Principales Aplicaciones Industriales; Editorial Limusa: Mexico City, Mexico, 2021. [Google Scholar]
- Speight, J.G. Handbook of Petrochemical Processes; Taylor & Francis: London, UK, 2019; ISBN 9780429155611. [Google Scholar]
- Kabe, T.; Ishihara, A.; Qian, W. Hydrodesulfurization and Hydrodenitrogenation: Chemistry and Engineering; Wiley: Weinheim, Germany, 1999. Available online: https://www.osti.gov/etdeweb/biblio/20101494 (accessed on 1 August 2025).
- Ancheyta, J.; Angeles, M.J.; Macías, M.J.; Marroquín, G.; Morales, R. Changes in Apparent Reaction Order and Activation Energy in the Hydrodesulfurization of Real Feedstocks. Energy Fuels 2001, 16, 189–193. [Google Scholar] [CrossRef]
- Bara, C.; Lamic-Humblot, A.-F.; Fonda, E.; Gay, A.-S.; Taleb, A.-L.; Devers, E.; Digne, M.; Pirngruber, G.D.; Carrier, X. Surface-Dependent Sulfidation and Orientation of MoS2 Slabs on Alumina-Supported Model Hydrodesulfurization Catalysts. J. Catal. 2016, 344, 591–605. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Domínguez-García, S.; Maya-Yescas, R.; Béjar-Gómez, L. Reduction of lubricant life in lubrication systems for internal combustion engines due to high lubricant supply rates. Mater. Lett. 2022, 313, 131785. [Google Scholar] [CrossRef]
- Alonso, G.; Chianelli, R.R.; Fuentes, S.; Torres, B. Molybdenum Sulfide/Carbide Catalysts. U.S. Patent US7223713B2, 29 May 2007. [Google Scholar]
- Gosselink, J.W. A critical review of the sulfided catalysts and their use for the hydroprocessing. Sulfide catalysts in refineries. CatTech 1998, 2, 127–144. [Google Scholar]
- Mouli, K.C.; Mohanty, S.; Hu, Y.; Dalai, A.; Adjaye, J. Effect of Hetero Atom on Dispersion of NiMo Phase on M-SBA-15 (M=Zr, Ti, Ti-Zr). Catal. Today 2013, 207, 133–144. [Google Scholar] [CrossRef]
- NOM-086-SEMARNAT-SENER-SCFI-2011. NORMA Oficial Mexicana, Especificaciones de los Combustibles Fósiles para la Protección Ambiental. Secretaría de Medio Ambiente y Recursos Naturales. Mexico. Available online: https://www.dof.gob.mx/normasOficiales/892/semarna/semarna.htm (accessed on 1 June 2025).
- Flores, O.; Fabela, M.; Blake, C.; Vazquez, D.; Hernandez, R. Regulación De Emisiones Contaminantes De Los Motores De Combustión Interna; Instituto Mexicano Del Petroleo: Mexico City, Mexico, 2020; Available online: https://imt.mx/resumen-boletines.html?IdArticulo=396&IdBoletin=149. (accessed on 22 September 2020).
- Alonso Nunez, G.; Zepeda Partida, T.A.; Fuentes Moyados, S.; Smolentseva, E.; Diaz de Leon Hernandez, J.N. Catalyst for Hydrotreatment of Gasoline and Diesel and Method of Preparation. U.S. Patent US9675968B2, 13 June 2017. [Google Scholar]
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- Yang, Y. Determination of nitrogen compounds in catalytic diesel oil using gas chromatography. Chin. J. Chromatogr. 2008, 26, 478–483. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A.; Furimsky, E. Handbook of Spent Hydroprocessing Catalyst; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Levenspiel, O. Ingeniería De Las Reacciones Químicas, 3rd ed.; Limusa Wiley: Hoboken, NJ, USA, 2007; p. 677. [Google Scholar]
- Houalla, M.; Nag, N.K.; Sapre, A.V.; Broderick, D.H.; Gates, B.C. Hydrodesulfurization of Dibenzothiophene Catalyzed by Sulfided CoO-MoO3/γ-Al2O3: The Reaction Network. AIChE J. 1978, 24, 1015–1021. [Google Scholar] [CrossRef]
- Ledoux, M.J.; Micraux, O.; Agostini, G.; Pannisod, P. The Influence of Sulfide Structures on the Hydrodesulfuration Activity of Carbon-Supported Catalysts. J. Catal. 1986, 102, 275–288. [Google Scholar] [CrossRef]
- Klimova, T.; Gutiérrez, O.; Lizama, L.; Amezcua, J. Advantages of ZrO2- and TiO2–SBA-15 Mesostructured Supports for Hydrodesulfurization Catalysts Over Pure TiO2, ZrO2 and SBA-15. Microporous Mesoporous Mater. 2010, 133, 91–99. [Google Scholar] [CrossRef]
- Toledo-Chávez, G.; Paniagua-Rodríguez, J.-C.; Zárate-Medina, J.; Maya-Yescas, R. Reactions analysis during the synthesis of pseudo-boehmite as precursor of gamma-alumina. Catal. Today 2016, 271, 207–212. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Rivera-Muñoz, E.M.; Pawelec, B.; Ostrooumov, M.; Maya-Yescas, R.; Rico, J.L. The Use of a Natural Mexican Zeolite as Support of NiMoW Sulphide Hydrotreating Catalysts. Catal. Today 2014, 220–222, 301–309. [Google Scholar] [CrossRef]
- Absi–Halabi, M.; Stanislaus, A.Y.; Trimm, D.L. Coke formation on catalysts during the hydroprocessing of heavy oils. Appl. Catal. 1991, 72, 193–215. [Google Scholar] [CrossRef]
- Furimsky, E.; Massoth, F.E. Deactivation of Hydroprocessing Catalysts. Catal. Today 1999, 52, 381–495. [Google Scholar] [CrossRef]
- Topsøe, H.; Clausen, B.S.; Massoth, F.E. Catalysis: Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 11. [Google Scholar]
- Berkner, L.V.; Marshall, L.C. The history of growth of oxygen in the Earth’s atmosphere. In The Origin and Evolution of Atmospheres and Oceans, Proceedings of a Conference, Held at the Goddard Institute for Space Studies, NASA, New York, NY, USA, 8–9 April 1963; Brancazio, P.J., Cameron, A.G.W., Eds.; Wiley: New York, NY, USA, 1964; pp. 102–126. [Google Scholar]
- Johnson, F.S. The balance of atmospheric oxygen and carbon dioxide. Biol. Conserv. 1970, 2, 83–89. [Google Scholar] [CrossRef]
- Seinfield, J.H. Atmospheric Chemistry and Physics of Air Pollution; John Wiley and Sons: New York, NY, USA, 1986; Chapter 4. [Google Scholar]
- Gitzen, W. Aluminas as Ceramic Material; American Ceramic Society: Columbus, OH, USA, 1970. [Google Scholar]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.; Chmelka, B.; Stucky, G. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Escobar, J.; Barrera, M.C.; de los Reyes, J.A.; Toledo, J.A.; Santes, V.; Colín, J.A. Effect of Chelating Ligands on Ni–Mo Impregnation over Wide-Pore ZrO2–TiO2. J. Mol. Catal. A Chem. 2008, 287, 33–40. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Valencia, D.; Fuentes, G.A.; Klimova, T. Mo and NiMo Catalysts Supported on SBA-15 Modified by Grafted ZrO2 Species: Synthesis, Characterization and Evaluation in 4,6-Dimethyldibenzothiophene Hydrodesulfurization. J. Catal. 2007, 249, 140–153. [Google Scholar] [CrossRef]
- Klimova, T.; Reyes, J.; Gutiérrez, O.; Lizama, L. Novel Bifunctional NiMo/Al-SBA-15 Catalysts for Deep Hydrodesulfurization: Effect of Support Si/Al Ratio. Appl. Catal. A Gen. 2008, 335, 159–171. [Google Scholar] [CrossRef]
- Peña, L.; Valencia, D.; Klimova, T. CoMo/SBA-15 Catalysts Prepared with EDTA and Citric Acid and Their Performance in Hydrodesulfurization of Dibenzothiophene. Appl. Catal. B Environ. 2014, 147, 879–887. [Google Scholar] [CrossRef]
- Silva Neto, A.V.; Leite, E.R.; da Silva, V.T.; Zotin, J.L.; Urquieta-González, E.A. NiMoS HDS Catalysts—The Effect of the Ti and Zr Incorporation into the Silica Support and of the Catalyst Preparation Methodology on the Orientation and Activity of the Formed MoS2 Slabs. Appl. Catal. A Gen. 2016, 528, 74–85. [Google Scholar] [CrossRef]
- Tung, S.C.; McMillan, M.I. Automotive tribology overview of current advances and challenges for the future. Tribol. Int. 2004, 37, 517–536. [Google Scholar] [CrossRef]
- Olomolehin, Y.; Kapadia, R.; Spikes, H. Antagonistic actions of antiwear additives and carbon black. Tribol. Lett. 2010, 37, 49–58. [Google Scholar] [CrossRef]
- Domínguez-García, S.; Aguilar-Ramírez, C.E.; Béjar-Gómez, L.; Maya-Yescas, R. Mass Balance of the Tribofilm in Lubricated Systems. Tribol. Int. 2021, 155, 106757. [Google Scholar] [CrossRef]
- Morina, A.; Neville, A. Tribofilms: Aspects of formation, stability and removal. J. Phys. D Appl. Phys. 2007, 40, 5476–5487. [Google Scholar] [CrossRef]
- Morina, A.; Neville, A.; Priest, M.; Green, J.H. ZDDP and MoDTC interactions in boundary lubrication—The effect of temperature and ZDDP/MoDTC ratio. Tribol. Int. 2006, 39, 1545–1557. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Zhang, Y.; Jiang, Z.; Li, C. Ultra-Deep Hydrodesulfurization of Diesel Fuels on Trimetallic NiMoW Sulfide Catalysts. Chem. Eur. J. 2009, 15, 12571–12575. [Google Scholar] [CrossRef]
- Domínguez-García, S.; Béjar-Gómez, L.; López-Velázquez, A.; Maya-Yescas, R.; Nápoles-Rivera, F. Maximizing Lubricant Life for Internal Combustion Engines. Processes 2022, 10, 2070. [Google Scholar] [CrossRef]
- Domínguez-García, S.; Béjar-Gómez, L.; Maya-Yescas, R.; Lara-Romero, J.; Castro-Cedeño, B.; Espinosa-Medina, M.A. Friction Coefficient Dynamics of Tribological Coatings from Engine Lubricants: Analysis and Interpretation. Coatings 2023, 13, 1753. [Google Scholar] [CrossRef]
- INECC, Instituto Nacional de Ecología y Cambio Climático. Available online: https://www.gob.mx/inecc/articulos/informe-nacional-de-calidad-del-aire-2017. (accessed on 1 June 2025).

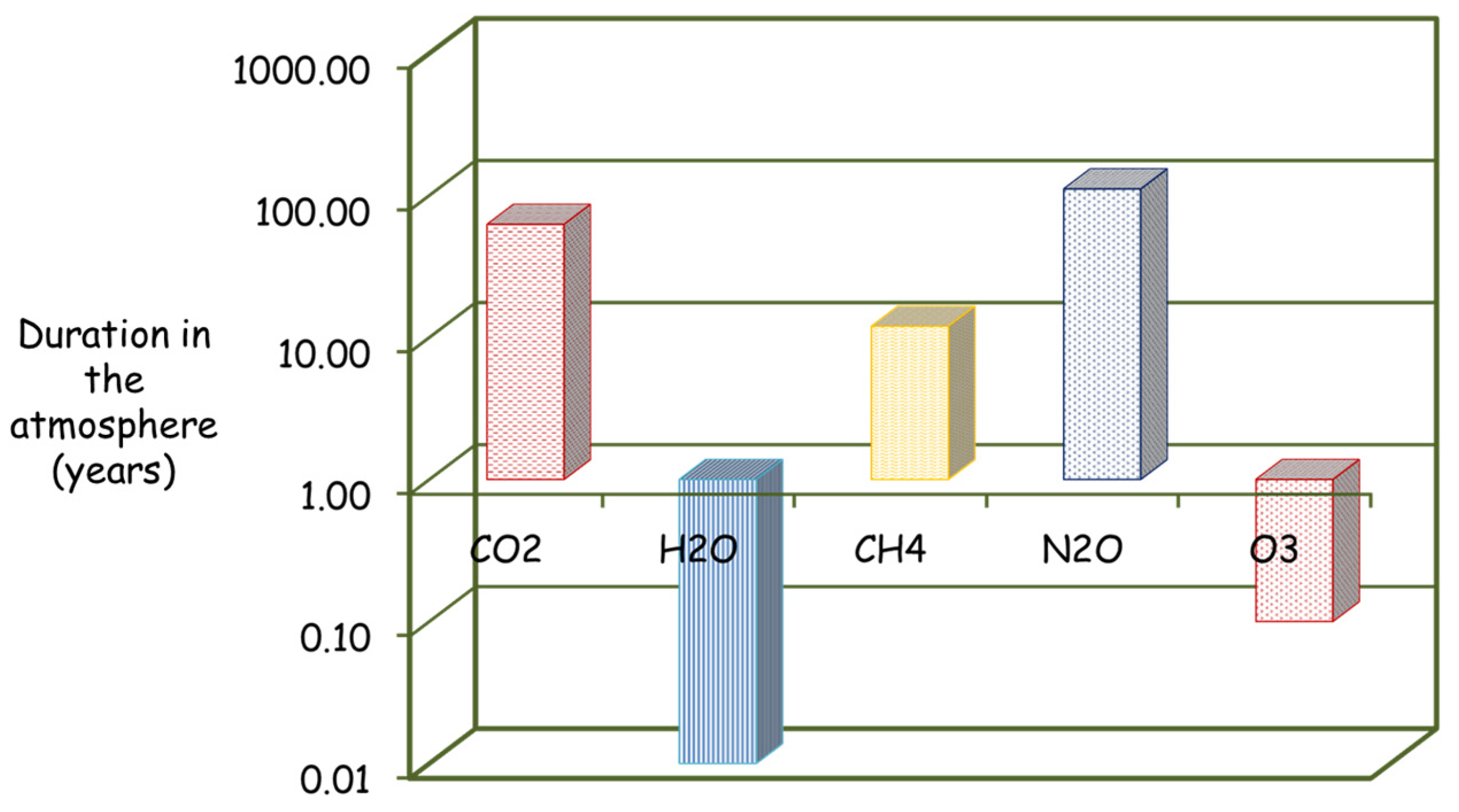
| Characteristics | Mayan | Istmo | Olmeca | Altamira |
| API Gravity | 21.3 | 33.1 | 38.7 | 16.5 |
| Elemental analysis (wt.%) | ||||
| Carbon | 83.96 | 85.4 | 85.91 | 84.96 |
| Hydrogen | 1.8 | 12.68 | 12.8 | 1.7 |
| Oxygen | 0.35 | 0.33 | 0.23 | 0.36 |
| Nitrogen | 0.32 | 0.14 | 0.07 | 0.34 |
| Sulfur | 3.57 | 1.45 | 0.99 | 6.0 |
| H/C Ratio | 1.687 | 1.782 | 1.788 | 1.69 |
| Metals (ppm) | ||||
| Nickel | 53.4 | 10.2 | 1.6 | 53.9 |
| Vanadium | 298.1 | 52.7 | 8 | 299 |
| Asphaltenes (wt.%) | ||||
| n-C5 | 14.1 | 3.63 | 1.05 | 15 |
| n-C7 | 11.32 | 3.34 | 0.75 | 12 |
| Fraction | Number of Carbon Atoms per Molecule |
|---|---|
| Non-condensable gas | C1–C2 |
| Liquefied gas (LPG) | C3–C4 |
| Gasoline | C5–C9 |
| Kerosene | C10–C14 |
| Diesel | C15–C23 |
| Lubricants and paraffins | C20–C35 |
| Heavy fuel oil | C25–C35 |
| Asphalts | >C39 |
| Process | Function |
|---|---|
| Hydrocracking | Converts diesel fuel to gasoline and eliminate heterocompounds |
| Hydrodesulfurization of gasoline | Eliminates undesirable products such as sulfur and nitrogen from gasoline |
| Catalytic naphtha hydrodesulfurization | Reduces the sulfur content to below 15 ppm in gasoline |
| Hydrodesulfurization of cooking oil and vacuum gas oil | Reduces the sulfur content in diesel and gas oil products |
| Nitrogen Compounds | Structure |
|---|---|
| Pyrroles |  |
| Indoles |  |
| Pyridine | 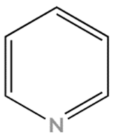 |
| Acridines | 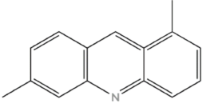 |
| Quinolines | 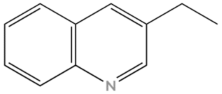 |
| Carbazols | 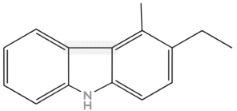 |
| Catalytic Process | Catalyst | Cause of Deactivation | ||
|---|---|---|---|---|
| Coke Deposits | Sintering of the Active Phase | Poisoning | ||
| Diesel hydrodesulfurization | CoMo-NiMo/Al2O3 | +++ | ++ | + a |
| Hydrotreatment of waste | NiMo-CoMo/Al2O3 | +++ | + | +++ b |
| Product | Sulfur Content (ppm S Weight) |
|---|---|
| Premium gasoline | October 2011: 80 |
| MAGNA gasoline | January 2011: 500 October 2011: 80 January 2009 30 |
| PEMEX diesel | January 2011: 500 January 2011: 15 January 2011: 10 |
| Agricultural and marine diesel | 5000 |
| Industrial diesel | 500 |
| Jet fuel | 3000 |
| LP gas | 140 |
| Domestic diesel | 500 |
| Refinery | Construction |
|---|---|
| Cadereyta | A 42,500-barrel-per-day catalytic gasoline desulfurization plant, with an amine regeneration unit, elevated burner, pumping equipment for hydrocarbons and sour water, complementary facilities, and integrations |
| Madero | Two catalytic gasoline desulfurization plants with a capacity of 20,000 barrels per day, two amine regeneration units, an elevated burner, pumping equipment for hydrocarbons and bitter waters, complementary facilities, and integrations |
| Minatitlán | A 25,000-barrel-per-day catalytic gasoline desulfurization plant, an amine regeneration plant, complementary auxiliary service systems, and their integration into the refinery |
| Salina Cruz | Two catalytic gasoline desulfurization plants with a capacity of 25,000 barrels per day, two amine regeneration plants, complementary auxiliary service systems, and their integration into the refinery |
| Tula | A 30,000-barrel-per-day catalytic gasoline desulfurization plant, an amine regeneration plant, complementary auxiliary service systems, and their integration into the refinery |
| Salamanca | A 25,000-barrel-per-day catalytic gasoline desulfurization plant, an amine regeneration plant, complementary auxiliary service systems, and their integration into the refinery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arevalo-Basañez, E.; Jiménez-García, G.; Villalón-López, U.A.; Maya-Yescas, R. The HDS Process: Origin, Process Evolution, Reaction Mechanisms, Process Units, Catalysts, and Health Risks. Processes 2025, 13, 2817. https://doi.org/10.3390/pr13092817
Arevalo-Basañez E, Jiménez-García G, Villalón-López UA, Maya-Yescas R. The HDS Process: Origin, Process Evolution, Reaction Mechanisms, Process Units, Catalysts, and Health Risks. Processes. 2025; 13(9):2817. https://doi.org/10.3390/pr13092817
Chicago/Turabian StyleArevalo-Basañez, Edgar, Gladys Jiménez-García, Ulises Alejandro Villalón-López, and Rafael Maya-Yescas. 2025. "The HDS Process: Origin, Process Evolution, Reaction Mechanisms, Process Units, Catalysts, and Health Risks" Processes 13, no. 9: 2817. https://doi.org/10.3390/pr13092817
APA StyleArevalo-Basañez, E., Jiménez-García, G., Villalón-López, U. A., & Maya-Yescas, R. (2025). The HDS Process: Origin, Process Evolution, Reaction Mechanisms, Process Units, Catalysts, and Health Risks. Processes, 13(9), 2817. https://doi.org/10.3390/pr13092817









