Coproduction of Biodiesel and Bioethanol from Ricinus communis Seed Through an Integrated Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Sample Collection
2.2. Stage 1: Biodiesel Production
2.2.1. R. communis Seed Oil Extraction
2.2.2. Physicochemical Characterization of the R. communis Oil
2.2.3. Gas Chromatography FAMEs Characterization
2.2.4. Ultrasound-Assisted Transesterification
2.3. Stage 2: Bioethanol Production
2.3.1. Starch Content Determination
2.3.2. Pretreatment of Castor Cake
2.3.3. Acid Hydrolysis of Starch Assisted by Ultrasound
2.3.4. Enzymatic Hydrolysis of Starch Assisted by Ultrasound
2.3.5. Fermentation of Castor Cake Hydrolysates
3. Results and Discussion
3.1. Biodiesel Production
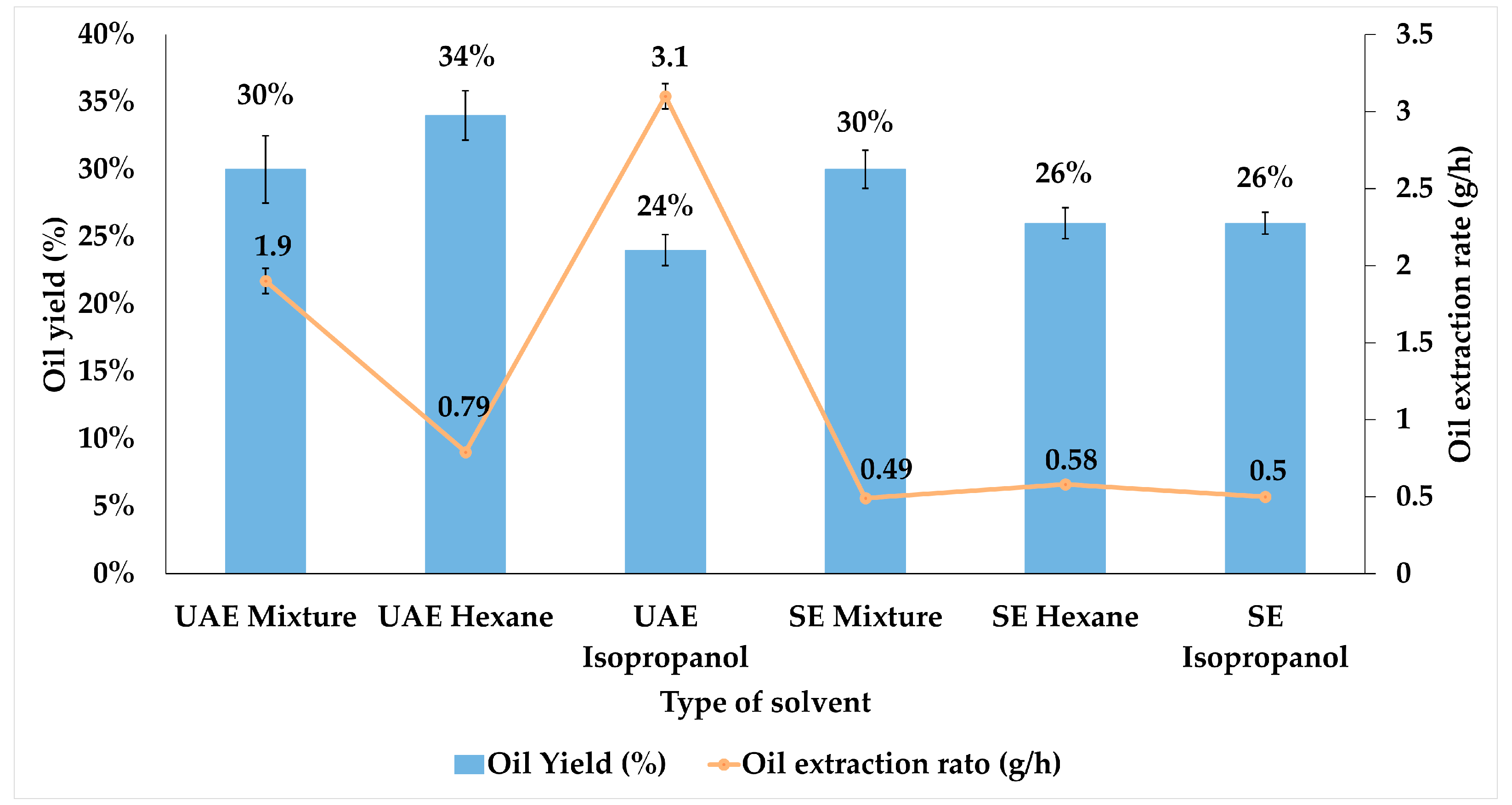
3.1.1. Castor Oil Extraction Assisted by Ultrasound
3.1.2. Physicochemical Properties of R. communis Oil
3.1.3. Gas Chromatography Characterization
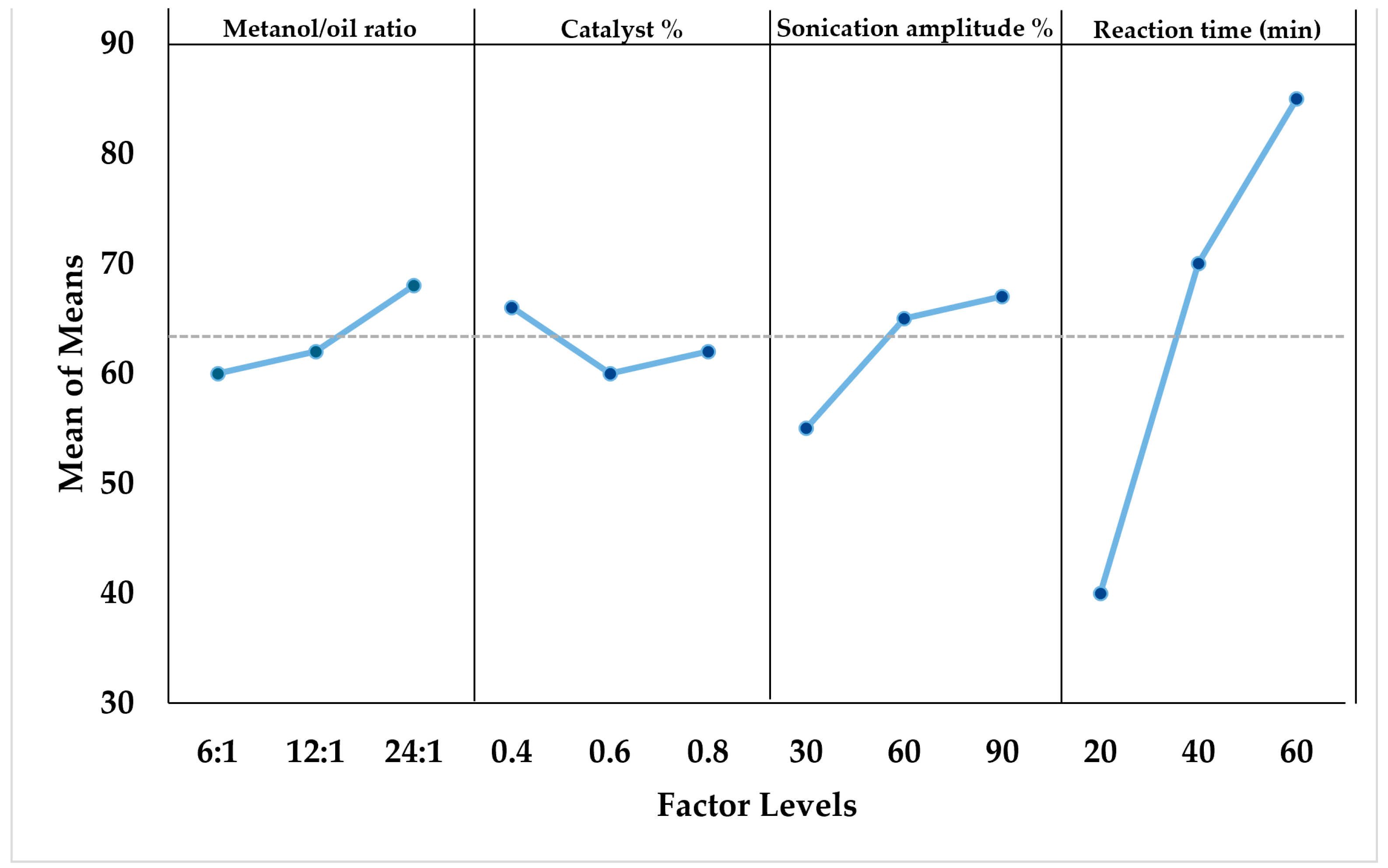
3.1.4. Optimization of Ultrasound-Assisted Transesterification
3.2. Bioethanol Production
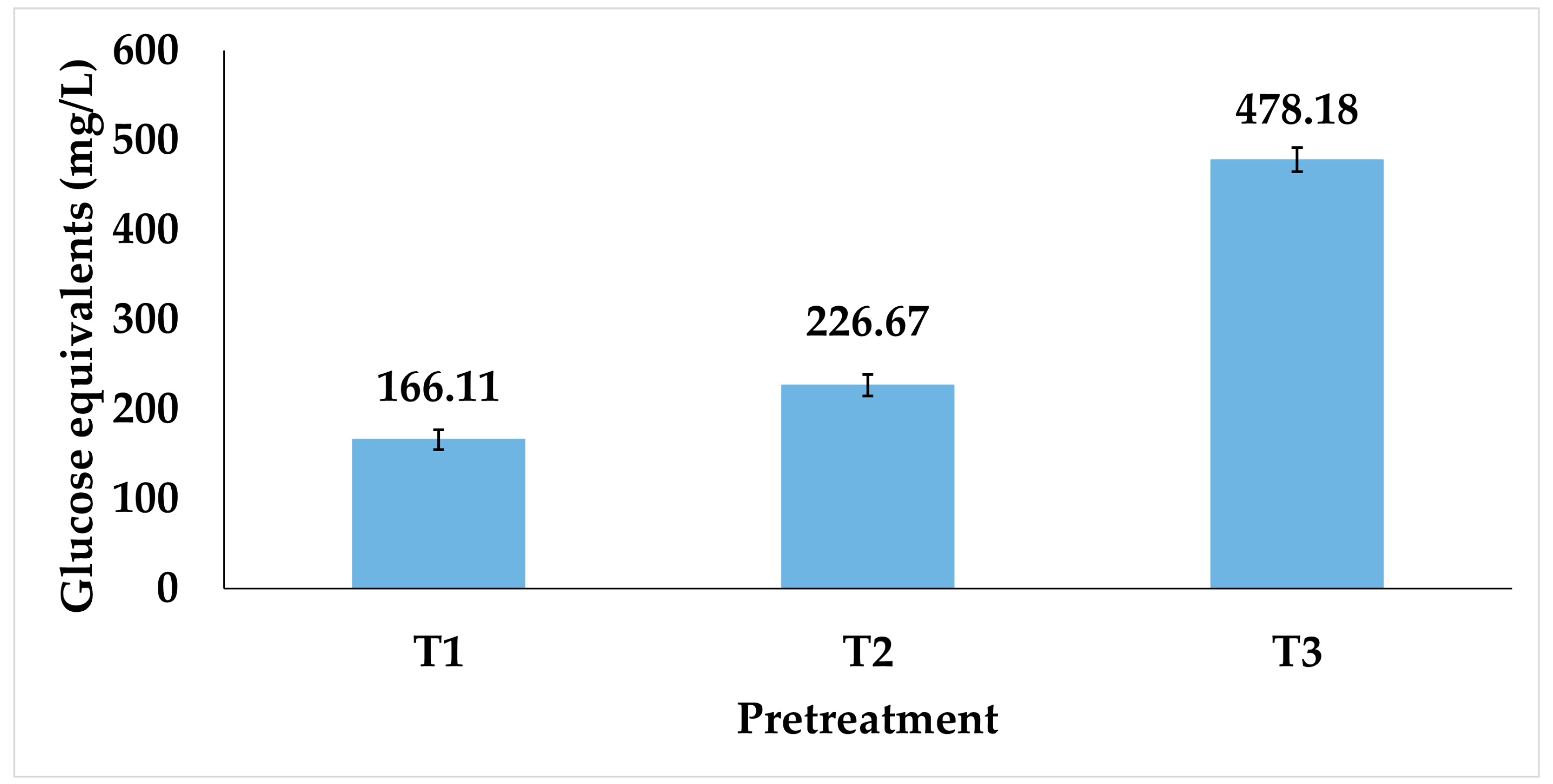
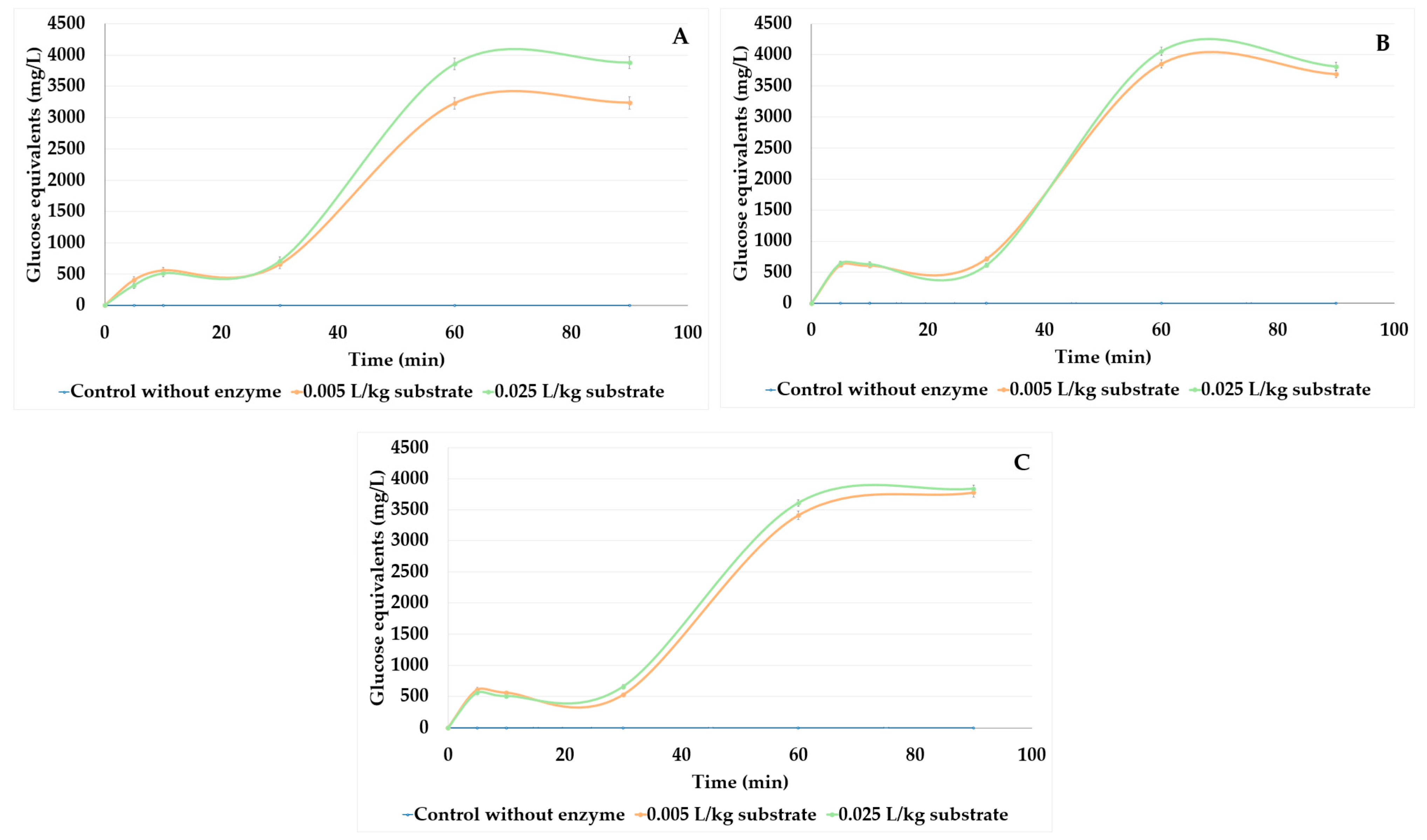
3.2.1. Effect of Pretreatment on Reducing Sugars Yield from Castor Cake
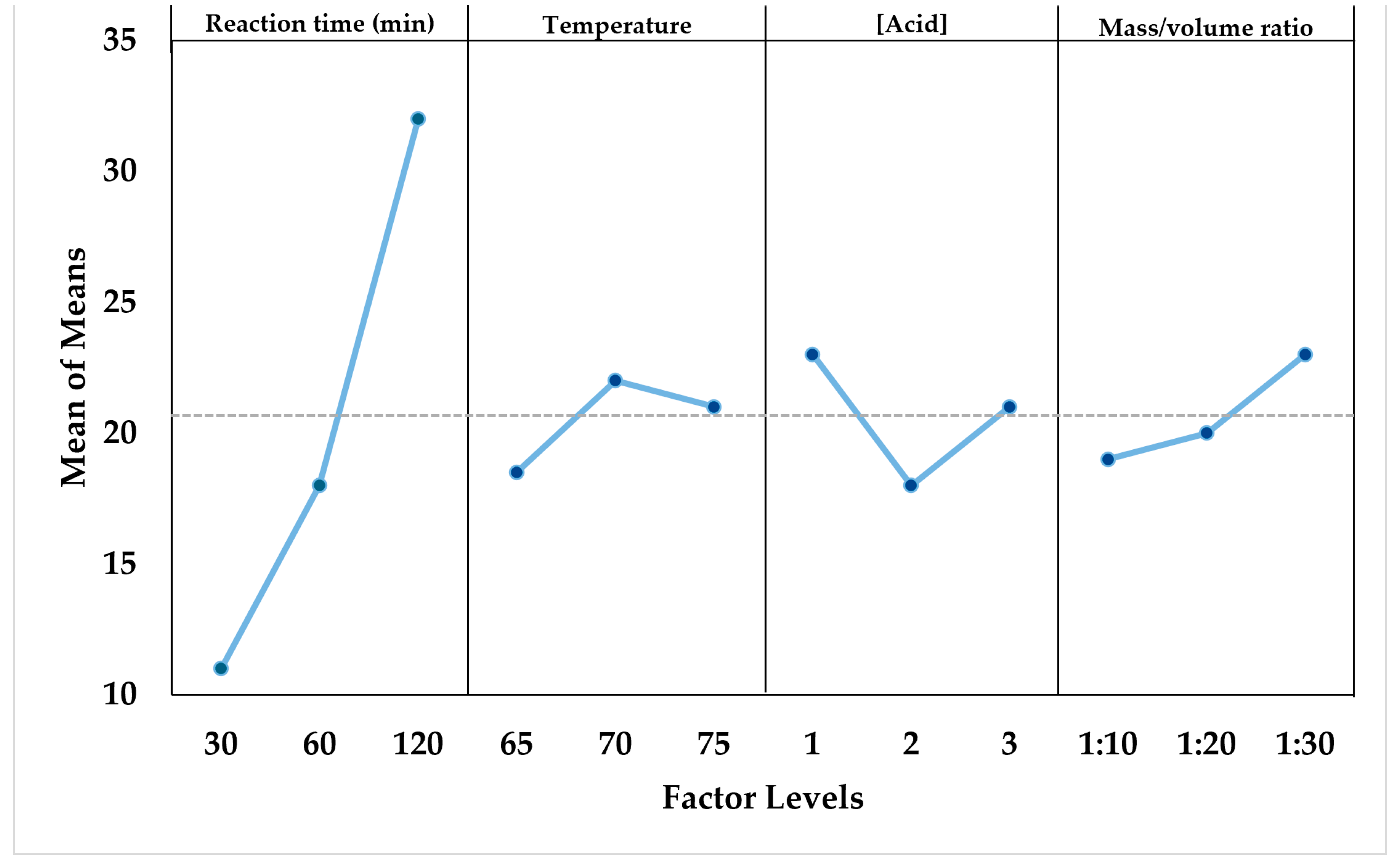
3.2.2. Effect of Ultrasound-Assisted Acid Hydrolysis (USAH) on Castor Cake
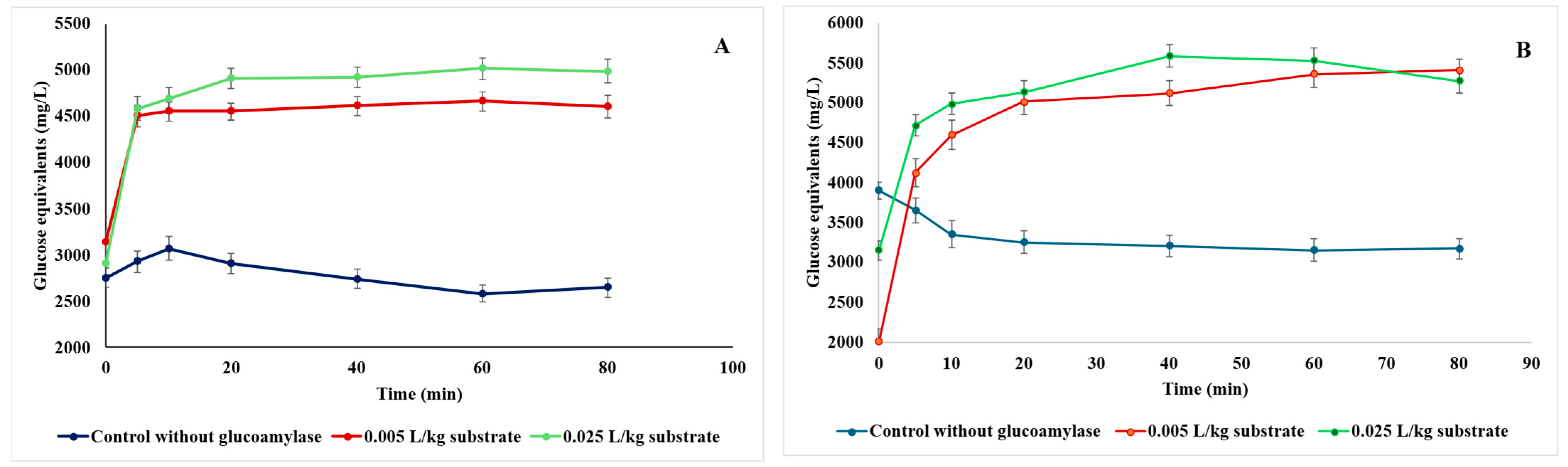
3.2.3. Effect of Ultrasound-Assisted Enzymatic Hydrolysis
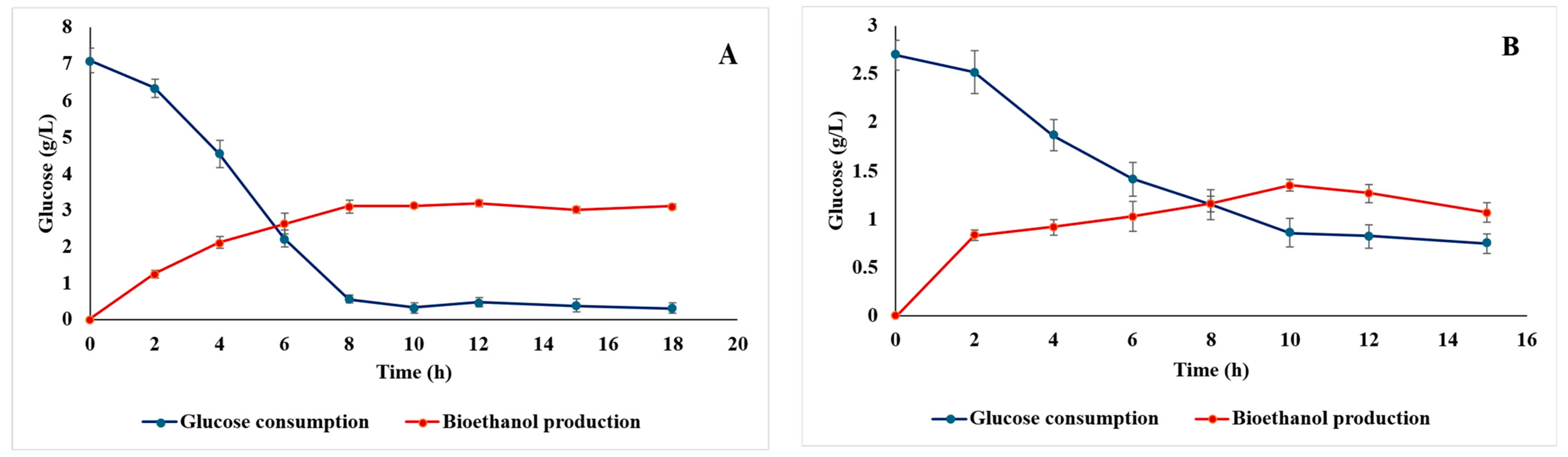
3.2.4. Ethanol Production from Castor Cake Hydrolysates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joven, J.M.O.; Gadian, J.T.; Perez, M.A.; Caingles, J.G.; Mansalaynon, A.P.; Ido, A.L.; Arazo, R.O. Optimized ultrasonic-assisted oil extraction and biodiesel production from the seeds of Maesopsis eminii. Ind. Crops Prod. 2020, 155, 112772. [Google Scholar] [CrossRef]
- Karmakar, B.; Dhawane, S.H.; Halder, G. Optimization of biodiesel production from castor oil by Taguchi design. J. Environ. Chem. Eng. 2018, 6, 2684–2695. [Google Scholar] [CrossRef]
- Mahmud, S.; Redwan Haider, A.S.M.; Shahriar, S.T.; Salehin, S.; Monjurul Hasan, A.S.M.; Johansson, M.T. Bioethanol and biodiesel blended fuels—Feasibility analysis of biofuel feedstocks in Bangladesh. Energy Rep. 2022, 8, 1741–1756. [Google Scholar] [CrossRef]
- Palconite, C.L.; Edrolin, A.C.; Lustre, S.N.B.; Manto, A.A.; Caballero, J.R.L.; Tizo, M.S.; Ido, A.L.; Arazo, R.O. Optimization and characterization of bio-oil produced from Ricinus communis seeds via ultrasonic-assisted solvent extraction through response surface methodology. Sustain. Environ. Res. 2018, 28, 444–453. [Google Scholar] [CrossRef]
- Roy, D.K.; Abedin, M.Z. Potentiality of biodiesel and bioethanol production from feedstock in Bangladesh: A review. Heliyon 2022, 8, e11213. [Google Scholar] [CrossRef]
- Janaun, J.; Ellis, N. Perspectives on biodiesel as a sustainable fuel. Renew. Sustain. Energy Rev. 2010, 14, 1312–1320. [Google Scholar] [CrossRef]
- Li, W.; Du, W.; Liu, D. Optimization of whole cell-catalyzed methanolysis of soybean oil for biodiesel production using response surface methodology. J. Mol. Catal. B Enzym. 2007, 45, 122–127. [Google Scholar] [CrossRef]
- Patil, P.D.; Deng, S. Optimization of biodiesel production from edible and non-edible vegetable oils. Fuel 2009, 88, 1302–1306. [Google Scholar] [CrossRef]
- Avramović, J.M.; Stamenković, O.S.; Todorović, Z.B.; Lazić, M.L.; Veljković, V.B. The optimization of the ultrasound-assisted base-catalyzed sunflower oil methanolysis by a full factorial design. Fuel Process. Technol. 2010, 91, 1551–1557. [Google Scholar] [CrossRef]
- Jeyakumar, N.; Hoang, A.T.; Nižetić, S.; Balasubramanian, D.; Kamaraj, S.; Lakshmana Pandian, P.; Sirohi, R.; Nguyen, P.Q.P.; Nguyen, X.P. Experimental investigation on simultaneous production of bioethanol and biodiesel from macro-algae. Fuel 2022, 329, 125362. [Google Scholar] [CrossRef]
- Demirbaş, A. Progress and Recent Trends in Biodiesel Fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Bateni, H.; Karimi, K. Biodiesel production from castor plant integrating ethanol production via a biorefinery approach. Chem. Eng. Res. Des. 2016, 107, 4–12. [Google Scholar] [CrossRef]
- Acevedo-García, B.; Santibañez-Aguilar, J.E.; Alvarez, A.J. Integrated multiproduct biorefinery from Ricinus communis in Mexico: Conceptual design, evaluation, and optimization, based on environmental and economic aspects. Bioresour. Technol. Rep. 2022, 19, 101201. [Google Scholar] [CrossRef]
- Keneni, Y.G.; Marchetti, J.M. Oil extraction from plant seeds for biodiesel production. AIMS Energy 2017, 5, 316–340. [Google Scholar] [CrossRef]
- Bueno, A.V.; Pereira, M.P.B.; de Oliveira Pontes, J.V.; de Luna, F.M.T.; Cavalcante, C.L. Performance and emissions characteristics of castor oil biodiesel fuel blends. Appl. Therm. Eng. 2017, 125, 559–566. [Google Scholar] [CrossRef]
- Qenawy, M.; Khalaf, M.; Wang, J.; Tian, J.; Zuo, L.; Mustafa, H.M.M.; Esmail, M.F.C. Performance and emission of extracted biodiesel from mixed Jatropha-Castor seeds. Fuel 2024, 357, 130060. [Google Scholar] [CrossRef]
- Thakkar, K.; Kachhwaha, S.S.; Kodgire, P. A novel approach for improved in-situ biodiesel production process from gamma-irradiated castor seeds using synergistic ultrasound and microwave irradiation: Process optimization and kinetic study. Ind. Crops Prod. 2022, 181, 114750. [Google Scholar] [CrossRef]
- Abada, E.; Al-Fifi, Z.; Osman, M. Bioethanol production with carboxymethylcellulase of Pseudomonas poae using castor bean (Ricinus communis L.) cake. Saudi J. Biol. Sci. 2019, 26, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Baskar, G.; Aberna Ebenezer Selvakumari, I.; Aiswarya, R. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef]
- Liu, J.; Chen, M.; Zhang, Y.; Zheng, B. Analyses of the oil content, fatty acid composition, and antioxidant activity in seeds of Thlaspi arvense L. from different provenances and correlations with environmental factors. Chem. Biol. Technol. Agric. 2022, 9, 11. [Google Scholar] [CrossRef]
- Lugo-Méndez, H.; Sánchez-Domínguez, M.; Sales-Cruz, M.; Olivares-Hernández, R.; Lugo-Leyte, R.; Torres-Aldaco, A. Synthesis of biodiesel from coconut oil and characterization of its blends. Fuel 2021, 295, 120595. [Google Scholar] [CrossRef]
- Sakthivel, R.; Ramesh, K.; Purnachandran, R.; Mohamed Shameer, P. A review on the properties, performance and emission aspects of the third generation biodiesels. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]
- ASTM D445-00; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2000.
- Wypych, G.; Pionteck, J. Chapter 3: Typical Methods of Quality Control of Antistatics. In Handbook of Antistatics, 2nd ed.; ChemTec Publishing: Toronto, ON, Canada, 2016; pp. 39–63. [Google Scholar] [CrossRef]
- ASTM D5558-24; Standard Test Method for Determination of the Saponification Value of Fats and Oils. ASTM International: West Conshohocken, PA, USA, 2024.
- Bockisch, M. Chapter 9: Analytical Methods. In Fats and Oils Handbook; AOCS Press: Champaign, IL, USA, 1998; pp. 803–808. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. BF3–Methanol Protocol. 2013. Available online: https://tools.thermofisher.com/content/sfs/brochures/AN-GC-FID-FAMEs-Oils-AN20733_E.pdf (accessed on 8 September 2025).
- Suhane, A.; Sarviya, R.M.; Siddiqui, A.R.; Khaira, H.K. Optimization of Wear Performance of Castor Oil based Lubricant using Taguchi Technique. Mater. Today Proc. 2017, 4, 2095–2104. [Google Scholar] [CrossRef]
- Shinde, K.; Kaliaguine, S. A Comparative Study of Ultrasound Biodiesel Production Using Different Homogeneous Catalysts. ChemEngineering 2019, 3, 18. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Masjuki, H.H.; Kalam, M.A.; Ong, H.C.; Gul, M.; Farooq, M.; Soudagar, M.E.M.; Ahmed, W.; Harith, M.H.; Yusoff, M.N.A.M. Ultrasound-assisted process optimization and tribological characteristics of biodiesel from palm-sesame oil via response surface methodology and extreme learning machine—Cuckoo search. Renew. Energy 2020, 158, 202–214. [Google Scholar] [CrossRef]
- Pan, P.; Tang, Y.; Sun, D.; Jiang, J.; Song, X. Effect of Ultrasonic-assisted Pretreatment on Hydrolysis and Fermentation of Acorn Starch. BioResources 2014, 9, 2705–2716. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Perea-Flores, M.J.; Chanona-Pérez, J.J.; Garibay-Febles, V.; Calderón-Dominguez, G.; Terrés-Rojas, E.; Mendoza-Pérez, J.A.; Herrera-Bucio, R. Microscopy techniques and image analysis for evaluation of some chemical and physical properties and morphological features for seeds of the castor oil plant (Ricinus communis). Ind. Crops Prod. 2011, 34, 1057–1065. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Tang, S.; Yu, Z.; Zhang, Y.; Zheng, B. Ultrasound-assisted production of biodiesel from field pennycress (Thlaspi arvense L.) seeds: Process optimization and quality evaluation. Ind. Crops Prod. 2023, 203, 117224. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Zaini, M.A.A. Characterization and process optimization of castor oil (Ricinus communis L.) extracted by the soxhlet method using polar and non-polar solvents. J. Taiwan Inst. Chem. Eng. 2015, 47, 99–104. [Google Scholar] [CrossRef]
- Abayeh, O.J.; Aina, A.E.; Okuonghae, C.O. Oil content and oil quality characteristics of some Nigerian oil seeds. J. Pure Appl. Sci. 1998, 1, 17–23. [Google Scholar]
- Sangeetha, B.; Mohana Priya, S.; Pravin, R.; Tamilarasan, K.; Baskar, G. Process optimization and technoeconomic assessment of biodiesel production by one-pot transesterification of Ricinus communis seed oil. Bioresour. Technol. 2023, 376, 128880. [Google Scholar] [CrossRef]
- Dasari, S.R.; Goud, V.V. Effect of pre-treatment on solvents extraction and physico-chemical properties of castor seed oil. J. Renew. Sustain. Energy 2014, 6, 063108. [Google Scholar] [CrossRef]
- Nangbes, J.G.; Nvau, J.B.; Buba, W.M.; Zukdimma, A.N. Extraction and characterization of castor (Ricinus communis) seed oil. Int. J. Eng. Sci. 2013, 2, 105–109. [Google Scholar]
- Codex Alimentarius Commission. Codex Standard for Named Vegetable Oils (CODEX STAN 210-1999, Amended 2015); FAO/WHO: Rome, Italy, 1999; Available online: https://www.fao.org/input/download/standards/336/CXS_210e_2015.pdf (accessed on 20 September 2024).
- Woldetensay, H.Z.; Zeleke, D.S.; Tibba, G.S. Data set of production of castor oil and characterization of cotton and castor mixed seed oil additives with diesel fuel. Data Brief 2024, 53, 110210. [Google Scholar] [CrossRef]
- Phewphong, S.; Roschat, W.; Ratchatan, T.; Suriyafai, W.; Khotsuno, N.; Janlakorn, C.; Leelatam, T.; Namwongsa, K.; Moonsin, P.; Yoosuk, B.; et al. Physicochemical exploration of castor seed oil for high-quality biodiesel production and its sustainable application in agricultural diesel engines. Chem. Eng. Res. Des. 2024, 205, 207–220. [Google Scholar] [CrossRef]
- Sbihi, H.M.; Nehdi, I.A.; Mokbli, S.; Romdhani-Younes, M.; Al-Resayes, S.I. Hexane and ethanol extracted seed oils and leaf essential compositions from two castor plant (Ricinus communis L.) varieties. Ind. Crops Prod. 2018, 122, 174–181. [Google Scholar] [CrossRef]
- Gopinath, A.; Puhan, S.; Govindan, N. Effect of unsaturated fatty acid esters of biodiesel fuels on combustion, performance and emission characteristics of a DI diesel engine. Int. J. Energy Environ. 2010, 1, 411–430. [Google Scholar]
- Bokhari, A.; Chuah, L.F.; Yusup, S.; Klemeš, J.J.; Kamil, R.N.M. Optimisation on pretreatment of rubber seed (Hevea brasiliensis) oil via esterification reaction in a hydrodynamic cavitation reactor. Bioresour. Technol. 2016, 199, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Encinar, J.M.; González, J.F.; Rodríguez-Reinares, A. Biodiesel from Used Frying Oil. Variables Affecting the Yields and Characteristics of the Biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Colucci, J.A.; Borrero, E.E.; Alape, F. Biodiesel from an alkaline transesterification reaction of soybean oil using ultrasonic mixing. J. Am. Oil Chem. Soc. 2005, 82, 525–530. [Google Scholar] [CrossRef]
- Chai, M.; Tu, Q.; Lu, M.; Yang, Y.J. Esterification pretreatment of free fatty acid in biodiesel production, from laboratory to industry. Fuel Process. Technol. 2014, 125, 106–113. [Google Scholar] [CrossRef]
- Veljković, V.B.; Avramović, J.M.; Stamenković, O.S. Biodiesel production by ultrasound-assisted transesterification: State of the art and the perspectives. Renew. Sustain. Energy Rev. 2012, 16, 1193–1209. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Optimization of the Synthesis of Biodiesel via Ultrasound-Enhanced Base-Catalyzed Transesterification of Soybean Oil Using a Multifrequency Ultrasonic Reactor. Energy Fuels 2009, 23, 2757–2766. [Google Scholar] [CrossRef]
- Rodriguez-de la Garza, J.A.; Castillo-Quiroz, D.; Rios-González, L.J.; Morales-Martínez, T.K.; González-Fuentes, J.A.; Valdez-Aguilar, L.A.; Medina-Morales, M.A. Autohydrolysis Pretreatment of Castor Plant Pruning Residues to Enhance Enzymatic Digestibility and Bioethanol Production. Bioresources 2020, 15, 6206–6216. [Google Scholar] [CrossRef]
- Melo, W.; dos Santos, A.S.; Santa Anna, L.M.M.; Pereira, N., Jr. Acid and Enzymatic Hydrolysis of the Residue from Castor Bean (Ricinus communis L.) Oil Extraction for Ethanol Production: Detoxification and Biodiesel Process Integration. J. Braz. Chem. Soc. 2008, 19, 418–425. [Google Scholar] [CrossRef]
- de Castro, A.M.; Castilho, L.d.R.; Freire, D.M.G. Characterization of babassu, canola, castor seed and sunflower residual cakes for use as raw materials for fermentation processes. Ind. Crops Prod. 2016, 83, 140–148. [Google Scholar] [CrossRef]
- Bateni, H.; Karimi, K.; Zamani, A.; Benakashani, F. Castor plant for biodiesel, biogas, and ethanol production with a biorefinery processing perspective. Appl. Energy 2014, 136, 14–22. [Google Scholar] [CrossRef]
- Deshmukh, M.; Pande, A.; Marathe, A. Different particle size study of castor deoiled cake for biofuel production with an environmental sustainability perspective. Heliyon 2022, 8, e09710. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.M.; Gogate, P.R. Intensification of dilute acid hydrolysis of spent tea powder using ultrasound for enhanced production of reducing sugars. Ultrason. Sonochem. 2020, 61, 104843. [Google Scholar] [CrossRef] [PubMed]
- Shangdiar, S.; Cheng, P.-C.; Chen, S.-C.; Amesho, K.T.T.; Ponnusamy, V.K.; Lin, Y.-C. Enhancing sugar yield for bioconversion of rice straw: Optimization of Microwave-assisted Pretreatment using dilute acid hydrolysis. Environ. Technol. Innov. 2023, 32, 103313. [Google Scholar] [CrossRef]
- Werle, L.B.; Garcia, J.C.; Kuhn, R.C.; Schwaab, M.; Foletto, E.L.; Cancelier, A.; Jahn, S.L.; Mazutti, M.A. Ultrasound-assisted acid hydrolysis of palm leaves (Roystonea oleracea) for production of fermentable sugars. Ind. Crops Prod. 2013, 45, 128–132. [Google Scholar] [CrossRef]
- Pereira Ramos, L. The Chemistry Involved in the Steam Treatment of Lignocellulosic Materials. Química Nova 2003, 26, 863–871. [Google Scholar] [CrossRef]
- Ramon, A.P.; Taschetto, L.; Lunelli, F.; Mezadri, E.T.; Souza, M.; Foletto, E.L.; Jahn, S.L.; Kuhn, R.C.; Mazutti, M.A. Ultrasound-assisted acid and enzymatic hydrolysis of yam (Dioscorea sp.) for the production of fermentable sugars. Biocatal. Agric. Biotechnol. 2015, 4, 98–102. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Babu, N.R.; Gogate, P.R. Intensification of enzymatic hydrolysis of waste newspaper using ultrasound for fermentable sugar production. Ultrason. Sonochem. 2014, 22, 326–332. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, X.; Zhang, Z.; Tian, M.; Zhang, F. Investigation of the potential mechanisms of α-amylase and glucoamylase through ultrasound intensification. LWT 2024, 198, 115979. [Google Scholar] [CrossRef]
- Lan, W.; Chen, S. Chemical kinetics, thermodynamics and inactivation kinetics of dextransucrase activity by ultrasound treatment. React. Kinet. Mech. Catal. 2020, 129, 843–864. [Google Scholar] [CrossRef]
- Li, F.; Tang, Y. The activation mechanism of peroxidase by ultrasound. Ultrason. Sonochem. 2021, 71, 105362. [Google Scholar] [CrossRef]
- Ali, I.; Sultan, S.; Mahmood, R.T.; Tariq, M.; Shamim, Z.; Mushtaq, A.; Asiri, M. Production and Characterization of α-Amylase from Indigenously Isolated Streptomyces sp. BioResources 2022, 18, 6–18. BioResources 2022, 18, 6–18. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Jia, L.; Gui, S.; Fu, Y.; Zheng, D.; Guo, W.; Lu, F. Improvement of the acid stability of Bacillus licheniformis alpha amylase by site-directed mutagenesis. Process Biochem. 2017, 58, 174–180. [Google Scholar] [CrossRef]
- Ma, X.; Wang, D.; Yin, M.; Lucente, J.; Wang, W.; Ding, T.; Ye, X.; Liu, D. Characteristics of pectinase treated with ultrasound both during and after the immobilization process. Ultrason. Sonochem. 2017, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Triplett, O.A.; Nguyen, K.T.; Melchior, W.B., Jr.; Taylor, K.; Jackson, L.S.; Tolleson, W.H. Thermal inactivation reaction rates for ricin are influenced by pH and carbohydrates. Food Chem. Toxicol. 2013, 58, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Kuligowski, K.; Świerczek, L.; Cenian, A. Review of Lignocellulosic Biomass Pretreatment Using Physical, Thermal and Chemical Methods for Higher Yields in Bioethanol Production. Sustainability 2025, 17, 287. [Google Scholar] [CrossRef]
| Methanol-to-Oil Ratio | Catalyst Concentration % (w/w) | Sonication Amplitude (%) | Reaction Time (min) | |
|---|---|---|---|---|
| Level 1 | 6:1 | 0.4 | 30 | 20 |
| Level 2 | 12:1 | 0.6 | 60 | 40 |
| Level 3 | 24:1 | 0.8 | 90 | 60 |
| Reaction Time (min) | Temperature (°C) | Sulfuric Acid Concentration % (w/w) | Mass/Volume Ratio | |
|---|---|---|---|---|
| Level 1 | 30 | 65 | 1 | 1:10 |
| Level 2 | 60 | 70 | 2 | 1:20 |
| Level 3 | 120 | 75 | 3 | 1:30 |
| Property | ||||||
|---|---|---|---|---|---|---|
| Type of Seed | Density (g/mL) | Viscosity (cSt) | Moisture Content (%) | Saponification Index (mgKOH/g Oil) | Acid Index (mgKOH/g Oil) | Ref. |
| R. communis | 0.963 | 266.81 | 0.17 | 180.5 | 1.7 | This study |
| R. communis | 0.960 | 257.82 | 0.07 | ---- | 1.67 | [37] |
| R. communis | 0.961 | 270 | ---- | ---- | 9.12 | [37] |
| R. communis | 0.976 | 230 | ---- | 176.38 | 2.71 | [38] |
| R. communis | 0.948 | ---- | 0.3 | 180.77 | ---- | [39] |
| T. arvense | 0.889 | ---- | 0.52 | 233.7 | 0.26 | [34] |
| Cotton | ---- | 29.22 | 0.02 | 187.94 | 0.24 | [8] |
| Fatty Acid Name | Chemical Structure | Fatty Acid Relative Content (%) |
|---|---|---|
| Saturated fatty acids (SFA) | ||
| Palmitic acid (C 16:0) | C16H32O2 | 1 |
| Stearic acid (C 18:0) | C18H36O2 | 1 |
| Oleic acid (C 18:1) | C18H34O3 | 3 |
| Saturated fatty acids (UFA) | ||
| Linoleic acid (C 18:2) | C18H32O2 | 5 |
| Linolenic acid (C 18:3) | C18H30O2 | 1 |
| Ricinoleic acid (C 18:1) | C18H34O3 | 89 |
| Run | Methanol to Oil Ratio | Catalyst Concentration % (w/w) | Sonication Amplitude % | Reaction Time (min) | Biodiesel Yield (%) |
|---|---|---|---|---|---|
| 1 | 6:1 | 0.4 | 30 | 20 | 24.6 ± 1.9 |
| 2 | 6:1 | 0.6 | 60 | 40 | 67.7 ± 4.8 |
| 3 | 6:1 | 0.8 | 90 | 60 | 87.5 ± 6.0 |
| 4 | 12:1 | 0.4 | 60 | 60 | 90.8 ± 6.4 |
| 5 | 12:1 | 0.6 | 90 | 20 | 31.2 ± 2.3 |
| 6 | 12:1 | 0.8 | 30 | 40 | 61.7 ± 4.3 |
| 7 | 24:1 | 0.4 | 90 | 40 | 81.6 ± 5.7 |
| 8 | 24:1 | 0.6 | 30 | 60 | 81.2 ± 5.3 |
| 9 | 24:1 | 0.8 | 60 | 20 | 36.8 ± 2.7 |
| Source | DF | SS | MS | F-Value | Impact Factor % |
|---|---|---|---|---|---|
| Methanol/oil ratio | 2 | 143.14 | 71.57 | 5.96 | 0.836 |
| Catalyst concentration | 2 | 130.04 | 65.02 | 5.42 | 0.744 |
| Sonication amplitude | 2 | 670.77 | 335.39 | 27.96 | 4.54 |
| Reaction time | 2 | 13,090.35 | 6545.17 | 545.36 | 91.69 |
| Experimental error | 18 | 216.03 | 12.0 | 2.19 | |
| Total | 26 | 14,250.33 | 100 |
| Run | Reaction Time (min) | Temperature (°C) | Acid Concentration % (w/w) | Mass/Volume Ratio | Conversion (%) |
|---|---|---|---|---|---|
| 1 | 30 | 65 | 1 | 1:10 | 9.30 |
| 2 | 30 | 70 | 2 | 1:20 | 9.70 |
| 3 | 30 | 75 | 3 | 1:30 | 14.20 |
| 4 | 60 | 65 | 2 | 1:30 | 17.10 |
| 5 | 60 | 70 | 3 | 1:10 | 18.48 |
| 6 | 60 | 75 | 1 | 1:20 | 19.91 |
| 7 | 120 | 65 | 3 | 1:20 | 30.10 |
| 8 | 120 | 70 | 1 | 1:30 | 37.10 |
| 9 | 120 | 75 | 2 | 1:10 | 27.40 |
| Source | DF | SS | MS | F-Value | Impact Factor % |
|---|---|---|---|---|---|
| Reaction time | 2 | 1923.74 | 961.87 | 559.16 | 88.89 |
| Temperature | 2 | 38.4 | 19.2 | 11.2 | 1.62 |
| Acid concentration | 2 | 76.7 | 38.33 | 22.29 | 3.4 |
| Mass/volume ratio | 2 | 90.45 | 45.22 | 26.29 | 4.03 |
| Experimental error | 18 | 30.96 | 1.72 | 2.07 | |
| Total | 26 | 2160.24 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva-Rodríguez, A.G.; Salinas De León, F.; Morales-Martínez, T.K.; Rodríguez-De la Garza, J.A.; Medina-Morales, M.A.; Cruz-Requena, M.; Neyra-Escobedo, G.A.; Ríos-González, L.J. Coproduction of Biodiesel and Bioethanol from Ricinus communis Seed Through an Integrated Process. Processes 2025, 13, 2877. https://doi.org/10.3390/pr13092877
Oliva-Rodríguez AG, Salinas De León F, Morales-Martínez TK, Rodríguez-De la Garza JA, Medina-Morales MA, Cruz-Requena M, Neyra-Escobedo GA, Ríos-González LJ. Coproduction of Biodiesel and Bioethanol from Ricinus communis Seed Through an Integrated Process. Processes. 2025; 13(9):2877. https://doi.org/10.3390/pr13092877
Chicago/Turabian StyleOliva-Rodríguez, Alejandra G., Fernando Salinas De León, Thelma K. Morales-Martínez, José Antonio Rodríguez-De la Garza, Miguel A. Medina-Morales, Marisol Cruz-Requena, Gustavo A. Neyra-Escobedo, and Leopoldo J. Ríos-González. 2025. "Coproduction of Biodiesel and Bioethanol from Ricinus communis Seed Through an Integrated Process" Processes 13, no. 9: 2877. https://doi.org/10.3390/pr13092877
APA StyleOliva-Rodríguez, A. G., Salinas De León, F., Morales-Martínez, T. K., Rodríguez-De la Garza, J. A., Medina-Morales, M. A., Cruz-Requena, M., Neyra-Escobedo, G. A., & Ríos-González, L. J. (2025). Coproduction of Biodiesel and Bioethanol from Ricinus communis Seed Through an Integrated Process. Processes, 13(9), 2877. https://doi.org/10.3390/pr13092877










