3. CP Methodology
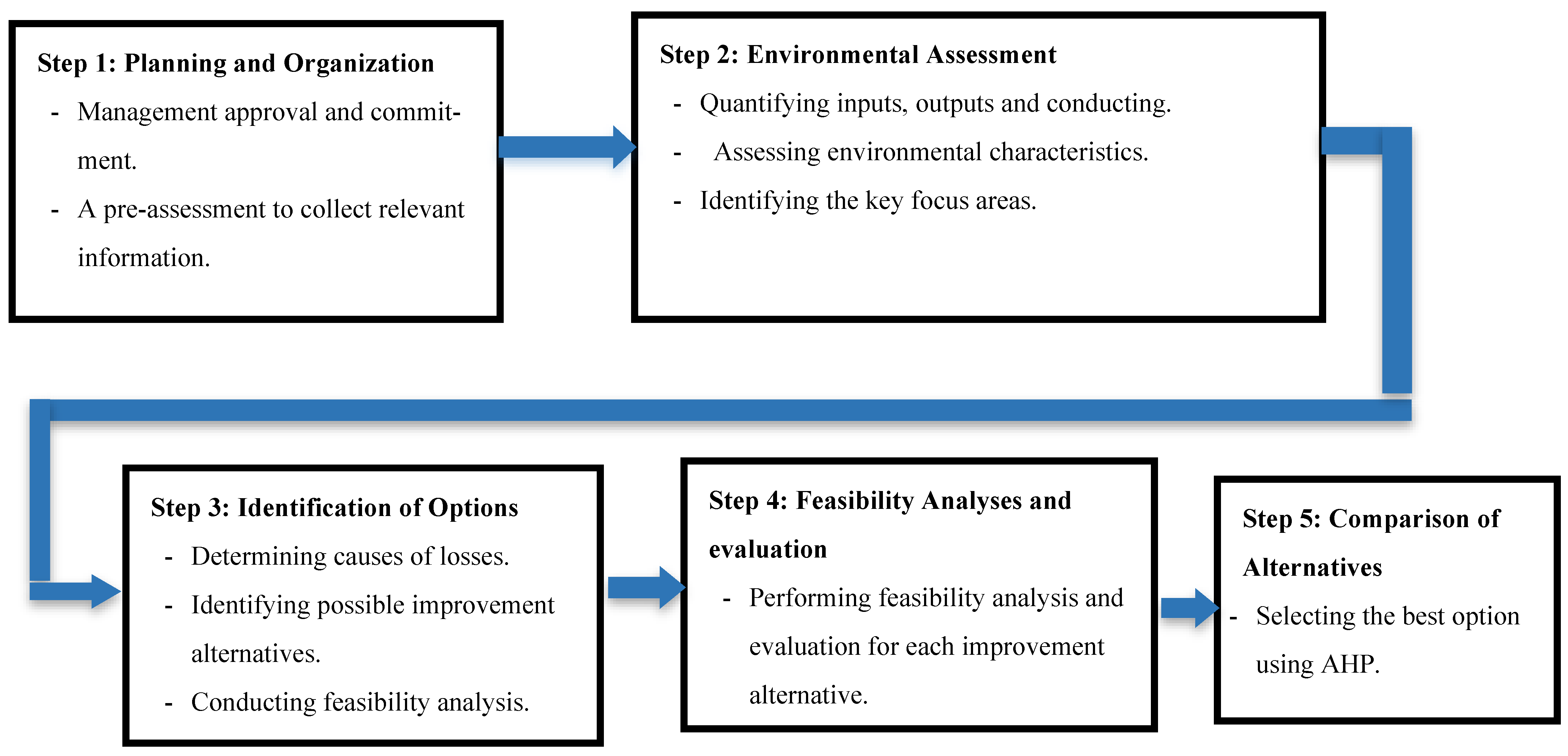
The CP approach is conducted as illustrated in
Figure 1.
The four steps and their tasks are presented as follows:
Step 1: Planning and Organization
The purpose of this step is to obtain commitment from top management to planning and organizing the environmental assessment. A pre-assessment is conducted to identify the biggest areas for savings. This information can be obtained through existing documentation and computer systems, a walkthrough of the plant, and simple monitoring, including production capacity, production data for the past year, preferably for each month, energy and other resource consumption data, and costs, preferably for each month and for each production step or department.
Step 2: Environmental Assessment
The environmental assessment was conducted and is presented as follows:
- (1)
Quantifying inputs and outputs for each process
The most important input resources to the technological process were determined, including electricity, fuels, water, raw materials, and chemicals. Also, the relevant outputs of the technological process were identified and measured, including solid waste, heat, emissions, noise, and wastewater.
- (2)
Assessing environmental characteristics
Five profiles were examined to assess the environmental characteristics for the technological processes, including raw material, energy, waste, product, and packaging profiles as follows [
25]:
- i.
Raw material intensity indicator
This indicator calculates the raw material intensity as a percentage of the annual NOP quantity. Let PR (tons/year) denote the annual NOP production. Let
Qm be the quantity (tons/year) of raw material
m used in the NOP production;
m = 1, …,
M. Then, the partial raw material intensity,
Rm, of material
m is estimated as follows:
Then, the total raw material intensity, RI, is calculated using Equation (2).
- ii.
Product indicator
This indicator assesses the quantity,
Np, of noxious material
p in the NOP production;
p = 1, …,
P. The partial product indicator,
Pp, is estimated as follows:
Considering
P types of noxious materials, the total product indicator,
PI, is calculated as follows:
- iii.
Energy unit indicator
In fertilizer production, various energy types are used to produce
PR of NOP, including electricity and heavy fuel oil (HFO). Let
De and
Ae denote the expected and actual consumptions of source
e in NOP production;
e = 1,…, E. Let
Ve represent the recovered quantity of source
e. Then, the partial energy index,
Ee, for energy source
e can be calculated as follows:
Then, the total energy indicator,
EI, from
E sources of energy is estimated as stated in Equation (6).
- iv.
Waste generation unit indicator
Three types of waste are examined, including liquid, gaseous, and solid waste. Let W denote the quantity of liquid waste generated from NOP production. Let
TL denote the relative toxicity indicator of liquid waste, which is derived from a scale based on environmental hazards, effects on aquatic life, and global warming potential. Then, the partial unit indicator,
LI, is calculated as follows:
Further, the indicator,
GIge, of gaseous waste
g,
g = 1, …, G, due to the use of energy source
e is calculated as follows. Let
TGge denote the relative toxicity indicator of gaseous waste g due to using energy source
e, fuel or electricity, in specific stages of NOP production. Then,
GIge is calculated as follows:
Then, the total gaseous waste indicator,
GI, of G gaseous waste from E energy sources is estimated using Equation (9).
Finally, the solid waste indicator,
SIs, is calculated as follows. Let the quantity of solid waste
s from NOP processes be denoted as
Ss;
s = 1,…, S. Let
TSs indicate the relative toxicity indicator of solid waste s. Then,
SIs is calculated as follows:
Then, the total solid waste indicator,
SI, from S solid wastes is estimated using Equation (11).
The calculated
LI,
GI, and
SI are then used to calculate the overall waste index,
WI, as follows:
- v.
Packaging Unit Indicator
Let
k be the indicator of the material type of bag used for packaging NOP products, where
k = 1, …, K. Let c
k and
wk denote the capacity (bags/ton NOP) and weight (kg) of packaging type
k. Finally, let
yk and
lk denote the percentage of type
k of bags used for packaging NOP products and the relative environmental loading of
kth bag material type based on the qualitative environmental assessment of packaging materials utilizing subjective experts’ evaluations of each material type regarding environmental degradation, consumption of natural resources and energy, emissions, waste, and effects on human health and safety, respectively. Then, the total quantity (kg),
Qk, required for packaging
yk of NOP products is calculated as follows:
The packaging unit indicator,
Kk, for packaging type
k is estimated using Equation (14).
The total packaging indicator,
KI, from K types of packaging materials will be
The same approach can be used to calculate the partial packaging indices for wooden hooding films.
- (3)
Identifying the focus areas
Generally, the focus areas could include the entire plant, waste generation, raw material, products, and packaging materials that contribute to significant environmental impacts. To identify the key focus areas, the degree of the environmental impacts from all profiles related to the technological processes of NOP is assessed by estimating the total integrated environmental assessment indicator,
TI, given by Equation (16).
Based on
TI and the ranking of indices for the five profiles, the profiles with significant environmental impacts are identified as the focus areas for which improvement opportunities are needed [
26,
27].
Table 1 displays the environmental nuisance classification scheme for
TI.
Step 3: Determining improvement alternatives
After identifying the critical focus areas, it is necessary to investigate the causes behind significant negative environmental impacts. Improvement actions can include modifications to production processes, adopting new technologies, input materials substitution, implementing alternative energy technologies and solutions, on-site reuse/recovery, and production of useful by-products.
Step 4: Feasibility analysis and evaluation of alternatives
Feasibility analysis and evaluation should be conducted for each improvement alternative, including technical, financial, and environmental aspects, to assess its capability to improve the efficiency and sustainability of the NOP production line. Technical feasibility assesses the need for new equipment, space availability, and impact on product quality. The economic feasibility in this research considers the payback period method. Finally, the environmental feasibility evaluates the impact on energy consumption, greenhouse gas emissions, solid waste, and wastewater.
Step 5: Comparing alternatives. AHP is a multi-criteria decision-making approach that can be used to solve complex decision problems [
28,
29]. It uses a multi-level hierarchical structure of objectives, criteria, sub-criteria, and alternatives. The pertinent data are derived by using a set of pairwise comparisons. These comparisons are used to obtain the weights of importance of the decision criteria and the relative performance measures of the alternatives in terms of each decision criterion. AHP was applied for decision-making, planning, priority setting, and economic analysis in various industrial applications, such as water security assessment and environmental protection measures, and the quality evaluation indicator system of the ecological environment [
30].
Table 2 shows the recommended importance and ratings for AHP.
In this research, AHP is utilized for the evaluation of the suggested improvement alternatives and solutions based on multi-criteria including cost, environmental impact, warranty, robustness and sensitivity, maintenance, payback period, and performance. This helps determine the best improvement
4. Application of CP to NOP Production
The CP methodology is applied as follows:
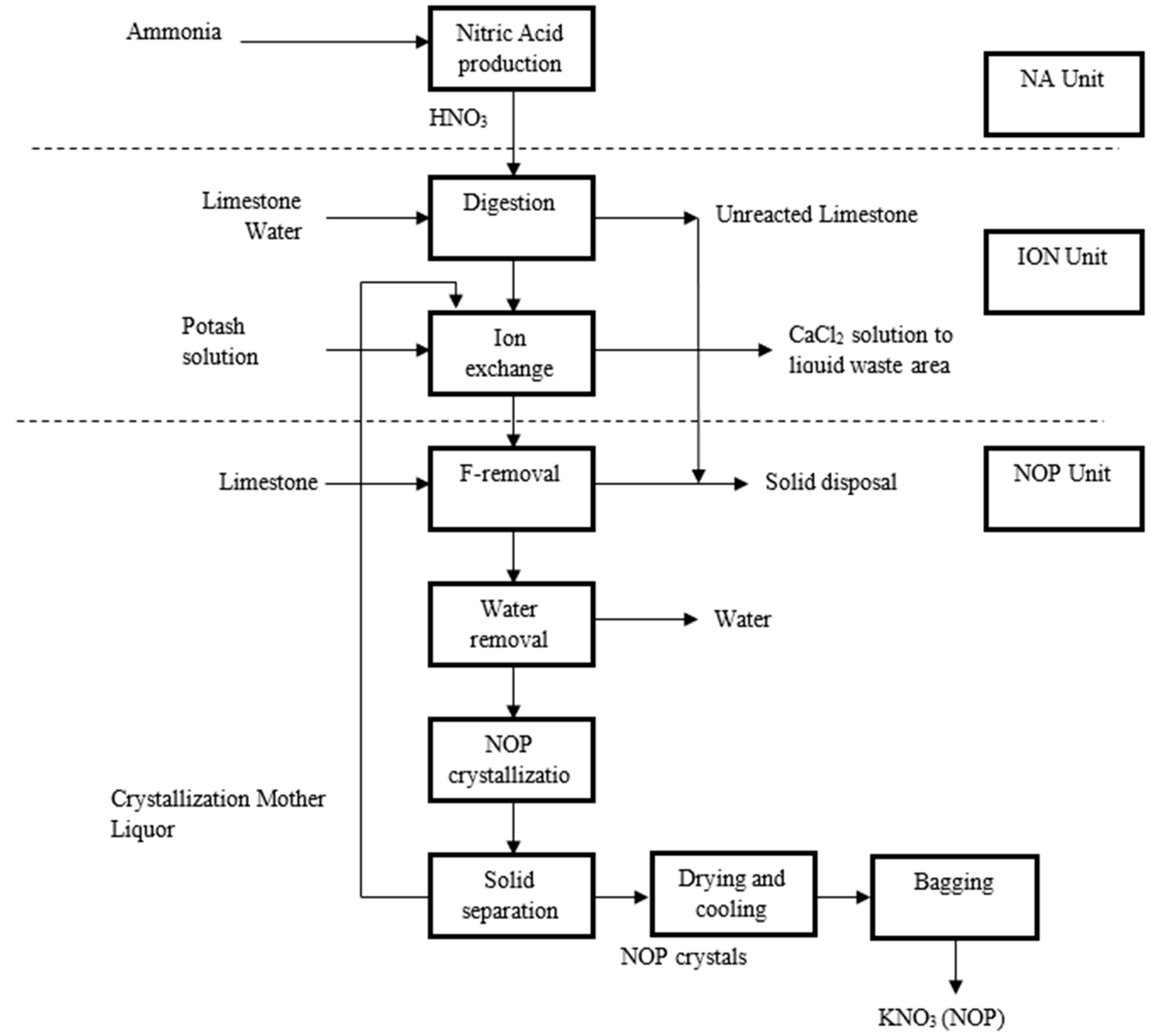
Step 1: The NOP production line is shown in
Figure 2. Information about production capacity, production data for energy and resources consumption, and costs was collected and is described as follows:
The capacity of the Nitric Acid plant is 350 mt/day of HNO3 (expressed at 100% concentration). The product is delivered as 60% by weight. Liquid ammonia is evaporated, superheated, mixed with air, and oxidized in the burner. The resulting nitrous gases are absorbed by water, forming 60% HNO3 (Nitric Acid).
- ii.
Ion Exchange Production
In the digestion unit, phosphate reacts with Nitric Acid. The reaction slurry is fed to a thickener. The slurry from digestion is then pumped through a selected number of columns in the Ion Exchange unit, where the calcium and acid hydrogen are exchanged for K-ion. Resin regeneration is done by a concentrated potash solution, which replaces calcium on the resin with potassium. The effluent from regeneration is a calcium chloride solution, sent to an evaporation pond after neutralization with limestone. Urea is finally added to the digester to reduce NOx.
- iii.
Potassium Nitrate (NOP) unit
In the NOP unit, the product leaving the ion exchange unit and the underflow from the lamella separator are treated by limestone slurry to precipitate and separate fluorine compounds. The Potassium Nitrate is crystallized from the rich mother liquor. This is done by up-concentrating the liquor in a three-step vacuum evaporation system (falling film evaporators) with subsequent crystallization of the potassium nitrate in the draft tube crystallizer. The crystals from the produced slurry are dewatered in a pre-thickener, followed by a centrifuge, and are dried in a shaking and static dryer. The pure dry NOP product is sent to the bagging unit. The NOP unit consists of a sophisticated energy and condensate recovery system. The most significant waste stream is the concentrated mother liquid (CML) leaving the pre-thickener and the centrifuge. CML is returned to the Ion Exchange unit for recovery/re-processing of the nutrients. The capacity is 150,000 tons of potassium nitrate per year, with actual production of 78,960 tons. The average monthly NOP production is 9017.2 tons.
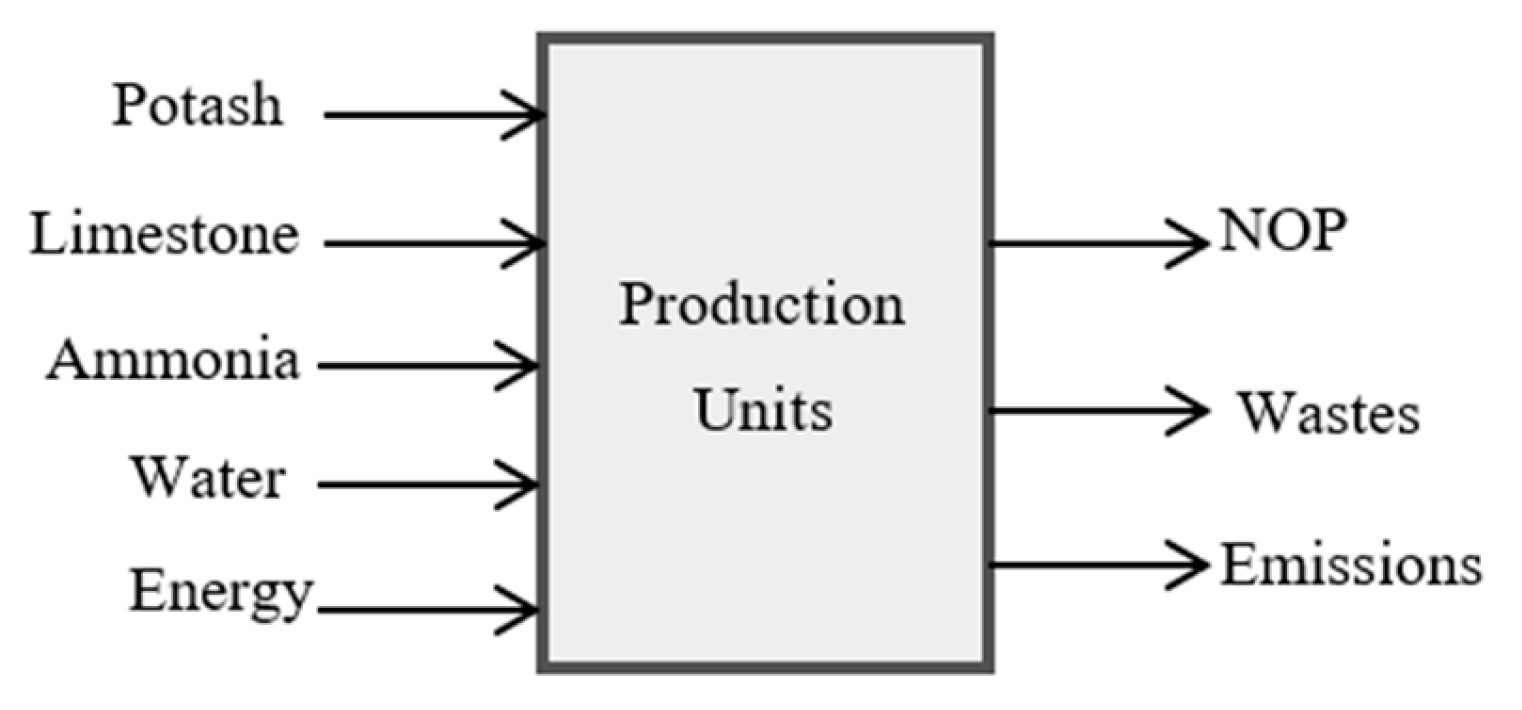
Step 2: Conducting an environmental assessment
Information was collected regarding the inputs comprising the raw materials, resources, and energy required to produce NOP, and the outputs covering the main products, liquid waste, solid wastes, and emissions, as shown in
Figure 3.
An environmental assessment was then conducted to assess the environmental unit indices for five key profiles involving raw materials, energy sources, waste, product, and packaging materials as follows:
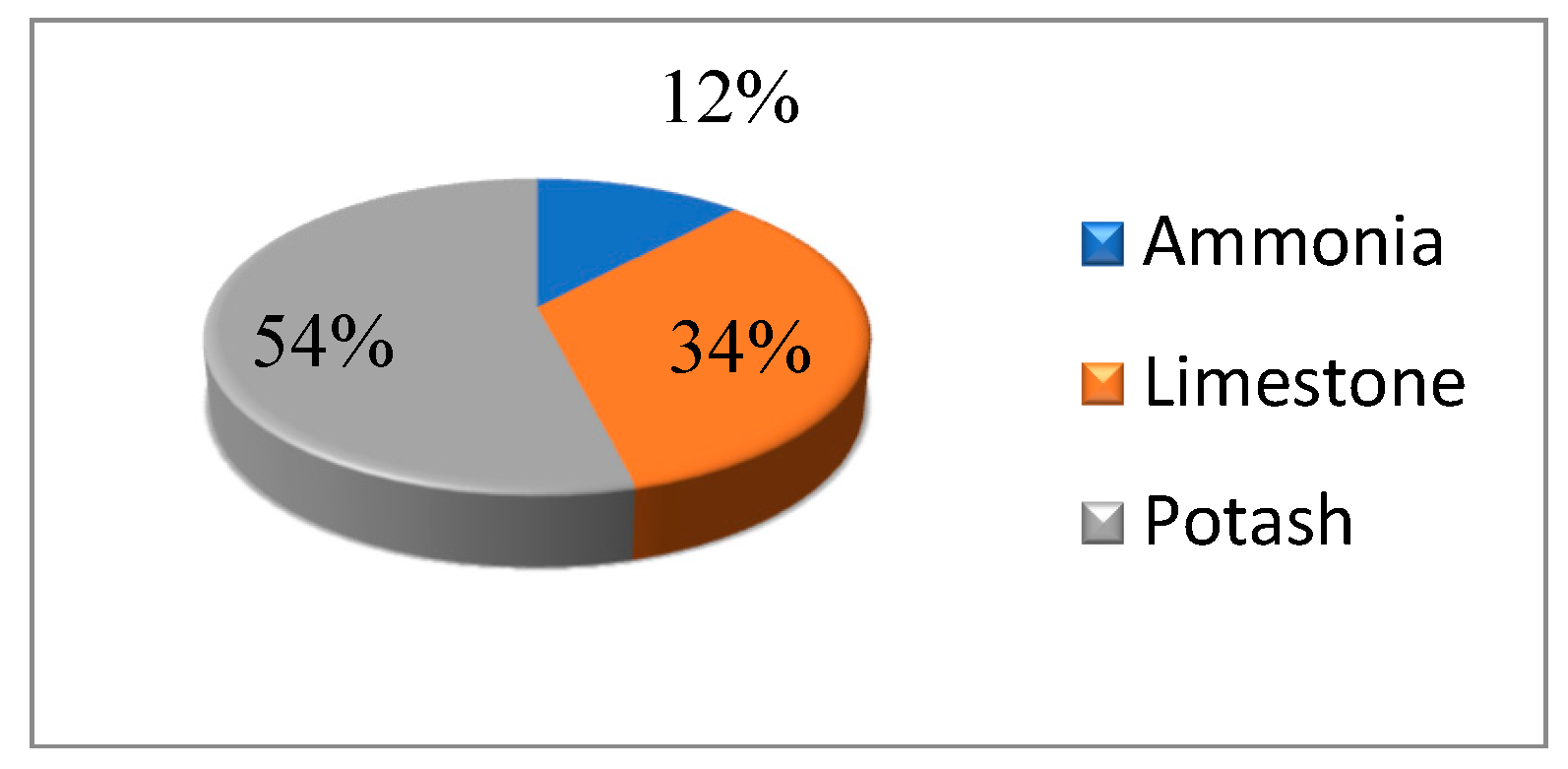
The annual NOP production is 120,000 tons. Three raw materials (M = 3) of NOP are used, including ammonia, limestone, and potash. The raw materials quantities are shown in
Table 3.
The raw material percentages are depicted in
Figure 4, where the largest (=54%) and lowest (=12%) contributions belong to Potash and Ammonia, respectively.
- 2.
Product indicator
For assessing the product indicator, Ammonia (p = 1) is the only significantly noxious material in NOP production. The partial product unit indicator, P1, and the environmental nuisance indicator, PI, for ammonia (23,400 tons) are calculated as 0.195 (=23,400/120,000), indicating an insignificant environmental nuisance of NOP.
- 3.
Energy unit indicator
The monthly NOP-produced quantities are displayed in
Table 4.
Two main energy sources (E = 2) are used in NOP processes, including electricity and heavy fuel oil. The energy flow analysis considers the following [
29].
- (a)
Electricity
The annual electricity consumption for the facility is estimated to be approximately 20.945 GWh of electricity (equivalent to JOD 1.88 million) for the 12 months. The average cost of the electricity tariff is calculated to be 3.79 JOD/kW for the maximum demand, 82 Fils/kWh for the day tariff, and 70 Fils/kWh for the night tariff. The electrical energy consumption for the factory facilities has an irregular pattern. The main electricity consumers in the factory are divided between the nitric acid plant, the ion exchange plant, and the NOP plant. A significant portion of the overall electricity consumption goes to plant auxiliary systems that are required for the processes. The auxiliary systems are lighting, a compressed air system, pumps, and motors. Based on the annual NOP production, the estimated electricity consumption is 20.95 GWh.
- (b)
Heavy Fuel Oil (HFO)
HFO is the main fuel utilized by steam boilers in production line processes. The annual estimate of HFO consumption is about 6113.74 metric tons. The estimated HFO consumption per ton of NOP is about 56.5 kg/ton of NOP (=6113.74 GWh/108,206.55 tons). Then, the annual HFO demand to produce 120,000 tons of NOP is 6780.1 tons. The average price of HFO is 462 JOD/metric ton. For technological processes of NOP, inputs of a mass of 51 kg of HFO and 174.54 kWh of electricity are required to produce one ton of NOP. For 120,000 tons of NOP, the average annual demands of HFO and electricity are 6120 (=51 × 120,000) tons and 20,945,000 (= 174.54 × 120,000) kWh, respectively. About 7 kg of high-pressure steam (HPS) at 40 bars and 390 °C is recovered per ton of NOP. The annual amount of recovered steam is 840 tons HPS. The actual consumption of energy sources exceeds the required consumption due to variations in production rates, heat losses, lighting, and the compressed air system. The energy unit indices E
1 and E
2 for HFO and electricity, respectively, were calculated, and the results are displayed in
Table 5.
For illustration, the actual consumption and the recovered energy of HFO are 6780.1 and 67.2 tons, respectively. Then, the partial unit indicator,
E1, is 1.097 (= (6780.1 − 67.2)/6120). From
Table 5,
E1 and
E2 are 1.097 and 1.11 for HFO and electricity, respectively. Then, the resultant energy indicator,
EI, is calculated as 2.207.
- 4.
Waste generation unit indicator
Three types of waste are generated in the process: solid, liquid, and gaseous. Solid waste is 20 kg of unreacted limestone generated per ton of NOP, liquid waste is 3 m
3 of calcium chloride byproduct per ton of NOP with a density of 1390 kg/m
3, and two types of gaseous waste are emitted in the process in a significant amount: CO
2 and N
2O. For HFO, the average of the emission factor is 3.114 g of CO
2 per 1 g HFO (Lower calorific value = 40.5 MJ/kg HFO) and 0.00018 g N
2O per g HFO [
31,
32,
33]. A relatively low toxicity indicator is given to Limestone, as limestone has no recognized unusual toxicity to plants or animals. Calcium chloride does not biodegrade or bio-accumulate and remains in a dissolved state. However, it is still toxic to aquatic life in high concentrations, hence the relatively medium toxicity indicator assigned to it. For the gaseous emissions, toxicity was mostly based on the global warming potential (GWP), where the GWP for N
2O and CO
2 are 273 and 1, respectively [
34].
The toxicity indicator for each waste type is shown in
Table 6. The partial waste generation unit indicator is then calculated for each type of waste as shown in
Table 7.
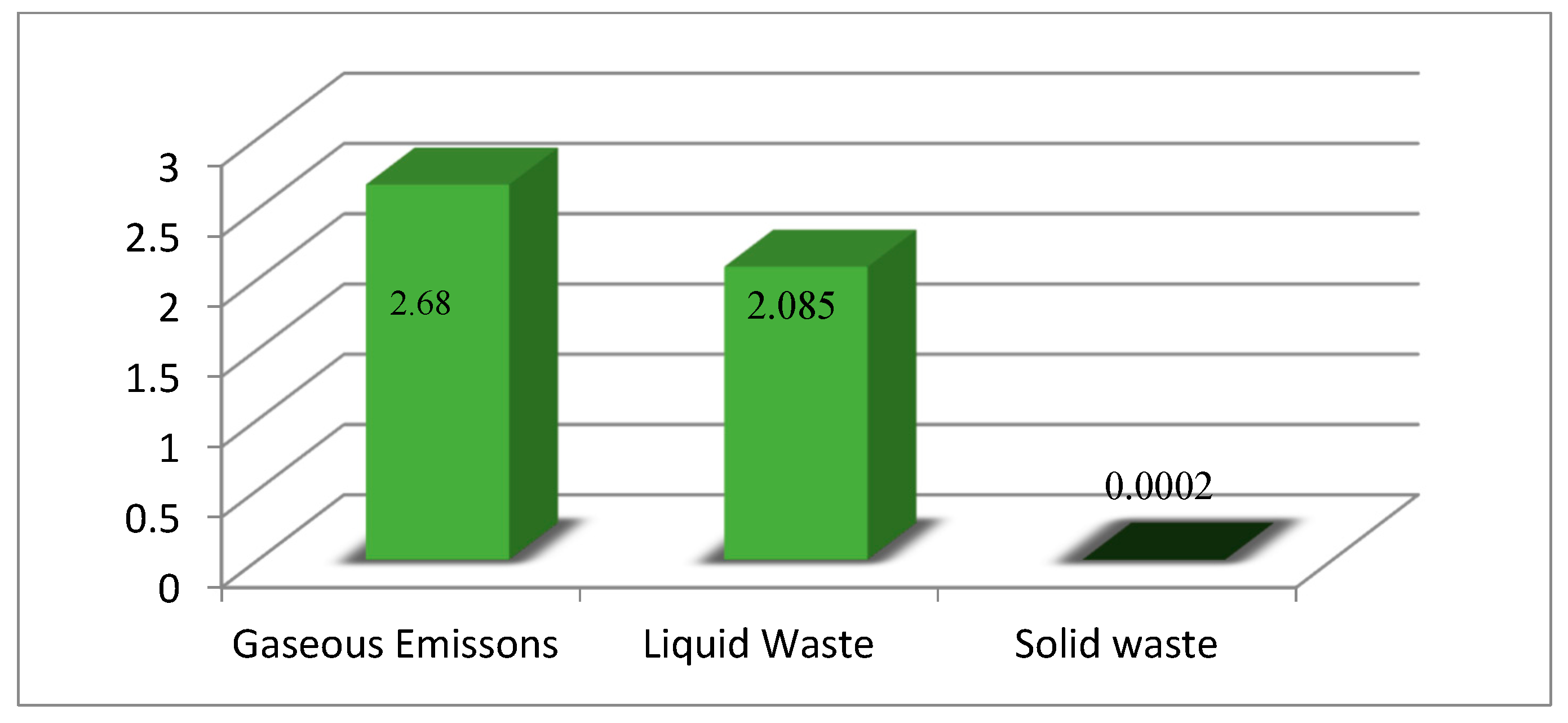
Figure 5 shows a histogram for the partial waste unit indices, where the highest partial unit indicator (=2.68) and the minimum (=0.0002) correspond to the gaseous and solid wastes, respectively. The total gaseous waste indicator,
WI, is found to be 4.684.
- 5.
Packaging Unit Indicator
A percentage of 40% of NOP is packaged in two types of Jumbo bags; the first type has a capacity of 1 ton of NOP and weighs 2.3 kg per bag, and the second type has a capacity of 1.2 tons of the product and weighs 2.6 kg per bag. About 10% and 50% of NOP are packaged into small 25 kg PP and PE bags, respectively. The 25 kg PP and PE bags weigh 0.14 and 0.1 Kg, respectively. Each ton of the small 25 kg bags is grouped on a wooden pallet that weighs 18 kg and is wrapped with a polystyrene hooding film, which weighs 0.62 kg. The mass of each of the packaging materials is calculated as follows:
Jumbo bags (1 ton) = 120,000 t NOP × 20% × 1 bag/t NOP × 2.3 kg/bag = 55,200 kg
Jumbo bags (1.2 ton) = 120,000 t NOP × 20% × 1 bag/t NOP × 2.6 kg/bag = 62,400 kg
Small 25 kg PP bags= 120,000 t NOP × 10% × 40 bag/t NOP × 0.14 kg/bag = 67,200 kg
Small 25 kg PE bags= 120,000 t NOP × 50% × 40 bag/t NOP × 0.10 kg/bag = 240,000 kg
Wooden pallets = 120,000 t NOP × 60% × 1 pallet/t NOP × 18 kg/pallet = 1,296,000 kg
Hooding film = 120,000 t NOP × 60% × 0.62 kg/t NOP = 44,640 kg
The partial packaging unit indicator and relative environmental loading indicator of packaging, pallets, and hooding film are shown in
Table 8.
The results for environmental assessment revealed that the highest indicator values correspond to energy and waste profiles. High energy consumption, waste, effluents, and gaseous emissions are common outcomes for plants with large production lines. The integrated environmental assessment indicator is calculated as the square root of the sum of the squares for the five profiles and found to be 5.18, indicating, as shown in
Table 1, a moderate environmental nuisance. The reduction in high consumption of HFO and/or electricity as energy sources can be seen as an area for improvement of interest to enhance the environmental performance.
Step 3. Improvement alternatives
Two improvement alternatives were suggested—based on production and expert opinions—to reduce energy consumptions that result from high production demands of HFO and electricity, including the following: A1, installation of a solar thermal system to support the steam boilers; and A2, adoption of photovoltaic solar cells to support the electrical generator. Nevertheless, the high investment costs necessitate conducting a feasibility analysis and multi-criteria evaluation for each option.
Step 4: Feasibility analysis of alternatives
Alternative 1: Solar thermal system installation
The solar thermal system collects heat by absorbing sunlight using a collector that captures sunlight radiation. Lighter and easier collectors with high reliability, efficiency, and the capability to heat water to higher temperatures. Installing 250 evacuated tube collectors requires 1000 square meters. In the production process, two boilers generate steam: a waste heat boiler and an auxiliary boiler. The auxiliary boiler operates at 44 bar and 400 °C. The capacity of the auxiliary boiler is 33 metric tons of steam per hour. The waste heat boiler is mainly an economizer installed to recover the generated heat from burning nitrate. Significant amounts of HFO are consumed to generate steam and hot water. The system delivers 100 m
3/day (100,000 Lit/day) hot water, at an average temperature of 55 C°, as depicted in
Table 9.
From
Table 9, installing a Solar Thermal system reduces HFO consumption, thereby reducing gaseous emissions. Knowing that the average temperature difference (∆T) in the summer season is 35.4 °C, and the specific heat capacity of water is 4.179 KJ/kg °C, the amount of heat gained by water is then calculated as follows:
Finally, the amount of saved HFO is calculated as
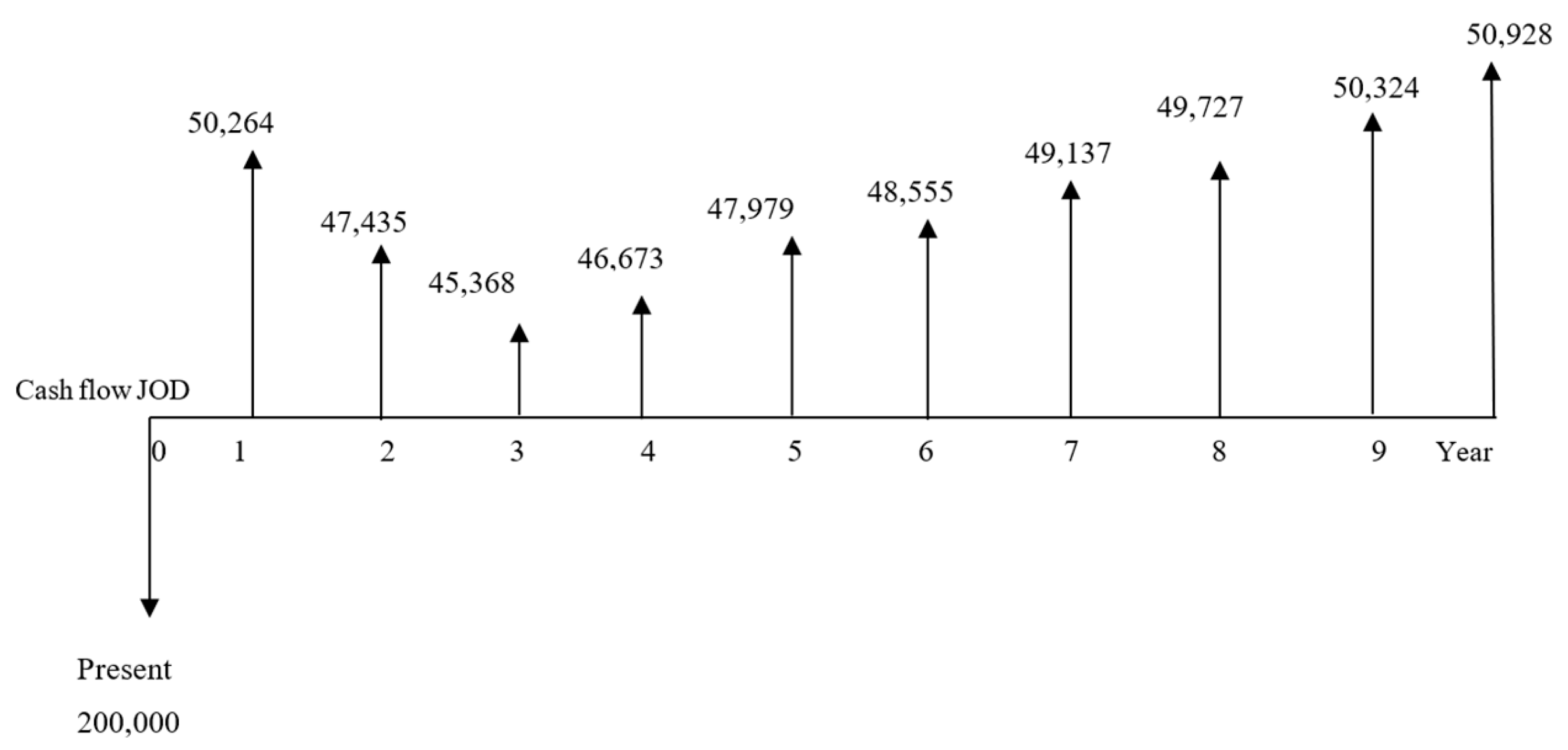
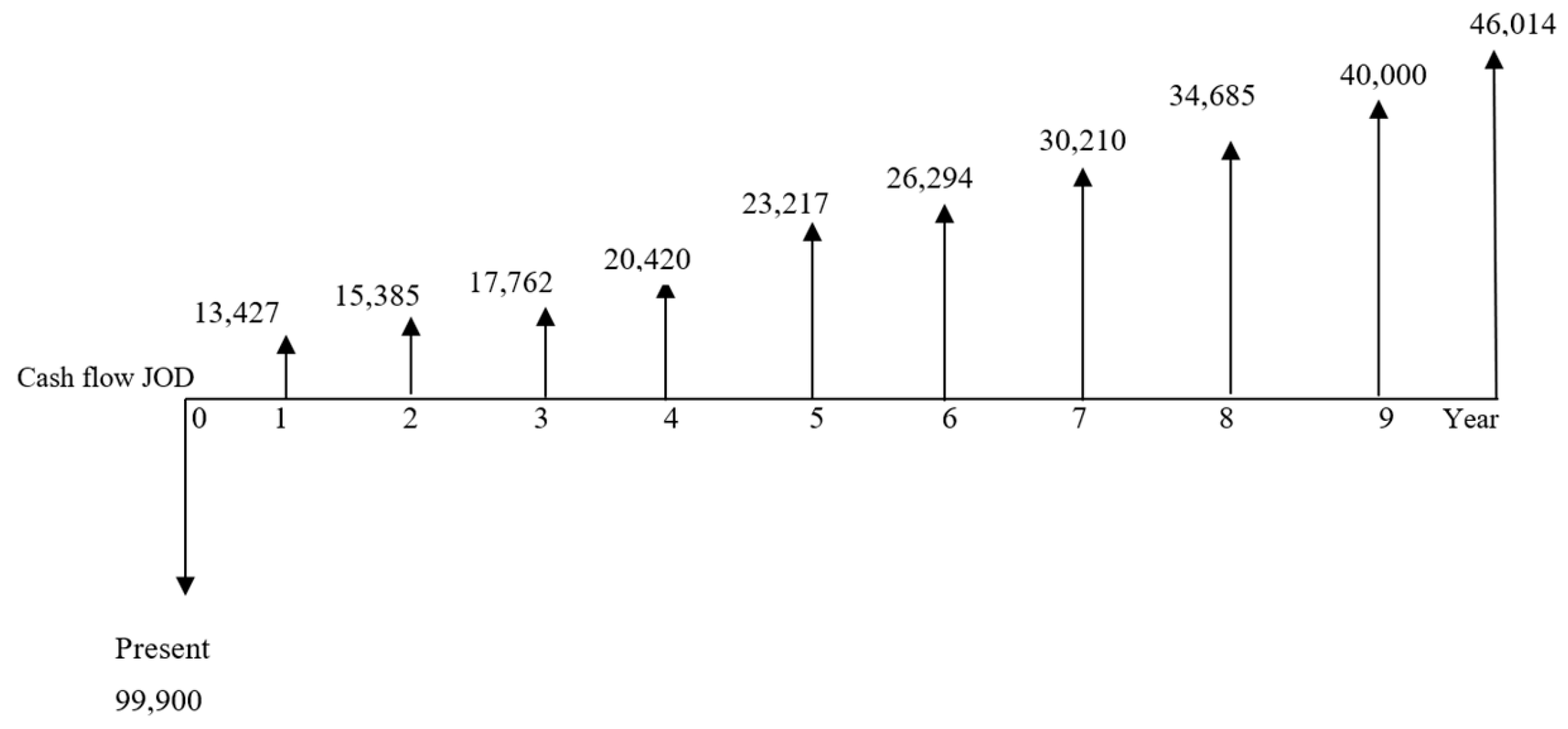
The economic benefits due to installing a solar thermal system are determined using three criteria: net present value (NPV), internal rate of return (IRR), and payback period. The initial investment cost, including collectors, tanks, and installation, is about JOD 200,000, and the actual annual consumption for HFO is about 6114 tons/year. Knowing that the amount saved of HFO after installing the system would be 121 tons/year, the new annual consumption of HFO would be 5993 tons/year. The depreciation in each year of the first 10 years would be JOD 20,000. Considering the 14% tax rate, the NPV shown in
Figure 6 is calculated at an inflation rate of 11% using Equation (19).
where
dk: depreciation in year k;
B: cost Basis;
SVN: salvage value;
N: depreciable life.
Figure 6.
Cash flows over the years for the A1 system.
Figure 6.
Cash flows over the years for the A1 system.
The reason for the pattern is that the tariff for HFO will decrease by 6.3% in year 1 and 5.7% in year 2 according to the information in Jordan’s market, and then the tariff starts increasing again in the years after.
Alternative 2: Photovoltaic Cells Installation (PV)
The average electrical consumption is 1,745,416.67 kWh per month. Considering that the electricity tariff for industrial institutions will increase by 15%, PV cells generate a portion of this consumption independently. There are several types of PV cells: Polycrystalline Silicon (multi-Si) cells made of high-purity and multi-crystalline silicon used as raw material [
35]. The power output to these cells is proportional to the sun’s intensity, and unlike solar thermal systems, they are extremely sensitive to shading. The available area of 1000 m
2 enables the installation of 333 panels (1200 JOD, 3 m
2 per panel, with an average annual energy output of 250 kWh/m
2) [
36,
37,
38,
39,
40]. The total cost is JOD 99,900. Consequently, the transition to using PV solar systems can substitute about 250 MWh annually. Electricity itself does not cause pollution or gaseous emissions; however, the production of electricity does. Indirect GHG emissions from the consumption of purchased electricity, heat, or steam are as noxious to the environment, and their effect must be accounted for. When producing 1 kWh, an average of 424 g of CO
2 is emitted, or 59.39 kg CO
2/kWh [
39,
40,
41,
42]. Consequently, the monthly emissions resulting are 740 tons, or 8880 tons annually. The proposed PV system has an annual capacity of 250 MWh, resulting in a CO
2 emissions reduction of 106 tons. The monthly power needed after installing the system would be calculated using Equation (20).
Knowing that the factory is subject to a 14% tax and the depreciation for the system, which has a depreciable life of 30 years, the depreciation rate is calculated as JOD 9990 over the ten years. The cash flow timeline for the cost reduction is shown in
Figure 7 and then discounted back to the present to have an NPV value of JOD 29,236.62.
The cash flow shown in
Figure 7 is a gradient, as the tariff for electricity keeps increasing each year by 15%.
Step 5: Comparing alternatives using AHP
A comparison between the two proposed alternatives is displayed in
Table 10, where the solar thermal system (STS) results in a reduction in the annual consumption of HFO by 120.681 tons/year, thereby reducing emissions of CO
2 and N
2O by 375.6 tons and 21.685 kg, respectively.
AHP optimizes decision-making based on multiple criteria, including cost, environmental impact, sensitivity to surrounding conditions, maintenance, warranty, payback period, and performance. Utilizing the ratings displayed in
Table 2, the pairwise comparisons between multiple criteria were conducted, and the results are presented in
Table 11.
The weights in
Table 11 are then standardized by dividing the rate in each cell by the sum of weights in the corresponding column.
Table 12 displays the standardized weights. For illustration, for the cost criteria, the weight of 0.0444 for the cell (C-C) is calculated by dividing the corresponding weight (=1) in
Table 11 by the sum of the weights of the cost column (=22.5). The other standardized weights are calculated similarly. Next, the sum of the weights in each row is calculated and then divided by the number of criteria (=7) to obtain the weight of each criterion. The consistency check was then performed and found acceptable (consistency ratio = 0).
Based on expert opinions and management preferences, the rating of the two alternative systems, STS and PV, was conducted concerning the selected multiple criteria, as shown in
Table 13. The weights are then standardized as shown in
Table 14. Finally, the weight of each alternative for each criterion is calculated by multiplying the weighted alternative rating by the corresponding criterion weight. The overall alternative weight is the sum of alternative weights for all criteria, as shown in
Table 15. It is found that the overall weight for solar thermal (=0.96) is larger than the weight (=0.71) of the PV system. The ratios of the STS and PV are 57.6% (=0.96/1.67) and 42.4%, respectively.
The AHP results in
Table 14 reveal that the solar thermal system (A2) is the best alternative to improve organizational goals for the NOP products and production processes based on multiple environmental and technical criteria.