Research Progress on the Pyrolysis Characteristics of Oil Shale in Laboratory Experiments
Abstract
1. Introduction
Methodology of Literature Selection
2. Laboratory Research Progress on Oil Shale Pyrolysis Mechanism
2.1. Revealing Pyrolysis Stages and Kinetics via Thermogravimetric and Calorimetric Analyses
2.2. Online Product Analysis and Combined Spectroscopic Techniques

2.3. Pyrolysis Experiments Under Different Laboratory Reactors and Conditions
3. Effects of Pyrolysis Conditions on Oil Shale Pyrolysis Behavior
3.1. Research Progress on the Influence of Pyrolysis Temperature
3.2. Research Progress on the Influence of Heating Rate

3.3. Research Progress on the Influence of Pyrolysis Methods on Oil Shale Pyrolysis Behavior
3.3.1. Pyrolysis Characteristics of Oil Shale Under Conventional Heating
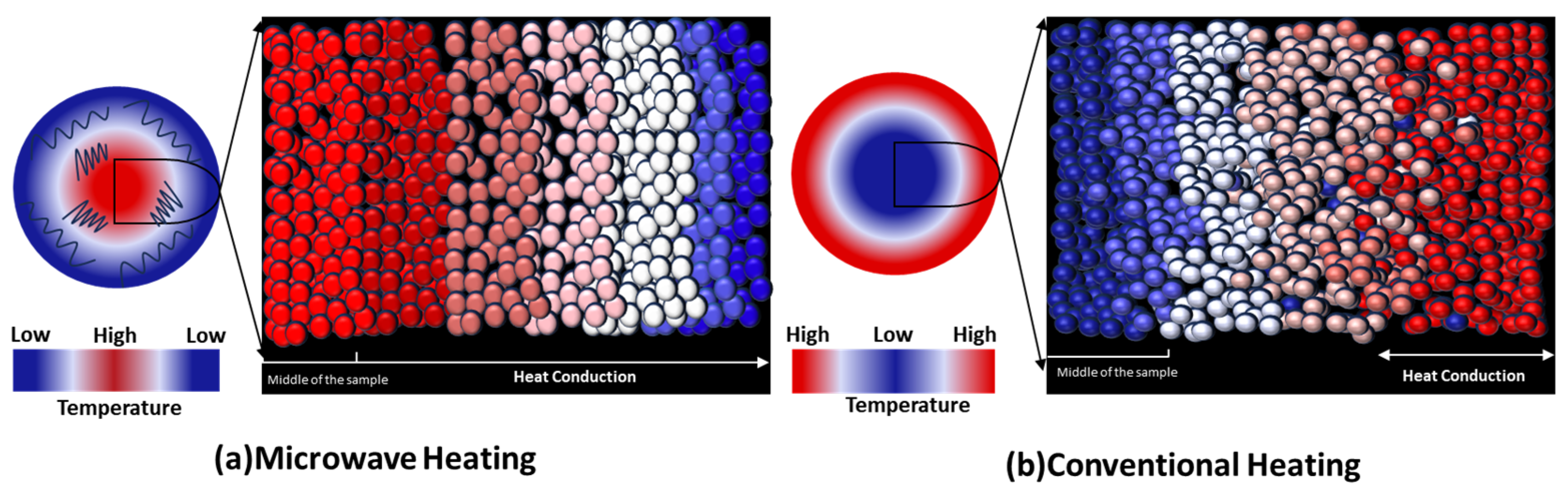
3.3.2. Pyrolysis Behavior of Oil Shale Under Microwave Heating
3.3.3. Pyrolysis Behavior of Oil Shale Under Auto-Thermal Heating
3.4. Research Progress on the Effects of Minerals and Catalysts on Oil Shale Pyrolysis Behavior
4. Mechanism Understanding: Current Insights and Challenges
5. Future Research Directions and Outlook
6. Conclusions
- (1)
- The thermal decomposition process includes three stages—evaporation of moisture and light volatiles (150–200 °C), kerogen pyrolysis (300–520 °C), and mineral decomposition (>600 °C). TGA–DSC provides effective characterization of mass and heat changes across these stages, with DSC quantifying the endothermic demand of each stage (e.g., kerogen decomposition consumes ~36% of total pyrolysis heat) and TGA-DTG enabling deconvolution of overlapping reaction peaks to identify stage-specific kinetic contributions [17,21].
- (2)
- Reaction temperature, heating rate, and particle size critically influence product distribution. Optimal oil yields are achieved at 450–550 °C under moderate heating rates (1–7 °C/min), with particle size affecting heat and mass transfer efficiency. Smaller particles increase gas yield by enhancing secondary cracking, while larger particles (1–3 mm) maintain higher oil yield by reducing vapor residence time [58,60]. Notably, heating rate also modulates kinetic parameters: fast heating (>50 °C/min) causes thermal lag, increasing apparent Ea by up to 30 kJ/mol and leading to incomplete pyrolysis, while moderate rates balance primary reaction efficiency and secondary reaction inhibition [53,55].
- (3)
- Novel pyrolysis techniques—including steam-assisted, microwave, and autothermic methods—offer improved efficiency and selectivity. Each approach presents specific benefits and technical challenges, especially in energy management and control precision: microwave heating reduces Ea by 13–39% and shortens heating time (750 °C in 9 min vs. 55 min for conventional heating) but risks local overheating [73,74]; autothermic heating achieves an energy efficiency of 3.46 (6.78 times that of nitrogen injection) but requires precise oxygen control to avoid over-oxidation [84]; steam-assisted methods provide hydrogen radicals to lower secondary reaction Ea, increasing light oil yield by 15–20% [70,96].
- (4)
- Carbonate minerals promote hydrocarbon generation (e.g., increasing oil yield by 5–8% via catalytic kerogen cleavage), while silicates inhibit pyrolysis and lead to aromatization (e.g., quartz raises apparent Ea by 20–25 kJ/mol) [87,88]. Catalysts such as Fe2O3 and ZSM-5 regulate reaction pathways and lower activation temperatures: Fe2O3 reduces kerogen pyrolysis Ea by 13–39% to enhance oil yield, while ZSM-5 promotes cracking of long-chain hydrocarbons into light fractions (C5–C13) but may cause excessive secondary cracking, reducing oil yield by 5–10% [90,91].
- (5)
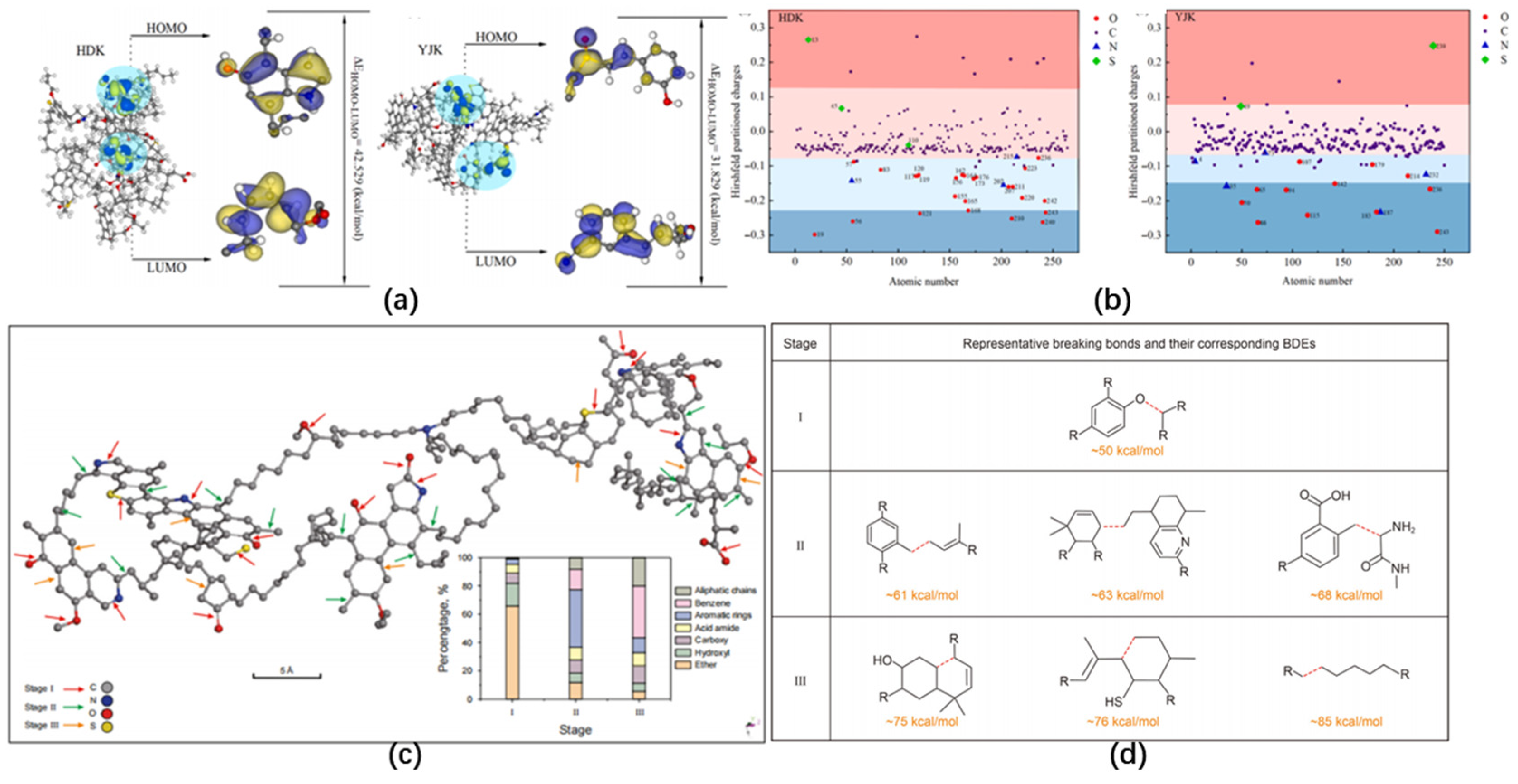
- The structural heterogeneity of kerogen limits unified kinetic modeling [107] —Type I kerogen (Green River) has Ea of 205–280 kJ/mol, Type II (Fushun) of 225–295 kJ/mol, and Type III (Yaojie) of 280–350 kJ/mol, while process controllability remains a challenge. Existing kinetic models often simplify multi-step reactions (primary + secondary) or rely on single mechanisms (e.g., Coats-Redfern), leading to Ea deviations of up to 40 kJ/mol for Type III kerogen compared to DAEM [18,38,87]. Advanced characterization tools (e.g., in situ TG-FTIR-MS) and molecular simulations (ReaxFF MD) are essential for mechanism elucidation, and real-time monitoring and intelligent systems (e.g., coupling kinetic models with reactor temperature control) are needed to enhance process regulation and efficiency.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kılıç, M.; Pütün, A.E.; Uzun, B.B.; Pütün, E. Converting of oil shale and biomass into liquid hydrocarbons via pyrolysis. Energy Convers. Manag. 2014, 78, 461–467. [Google Scholar] [CrossRef]
- World Oil Group. EIA estimates global recoverable shale oil resources at 345 billion bbl. World Oil 2013, 234, 13. [Google Scholar]
- Energy Information Administration. Technically Recoverable Shale Oil and Shale Gas Resources: An Assessment of 137 Shale Formations in 41 Countries Outside the United States; US Department of Energy: Washington, DC, USA, 2013.
- Kang, Z.; Zhao, Y.; Yang, D. Review of oil shale in-situ conversion technology. Appl. Energy 2020, 269, 115121. [Google Scholar] [CrossRef]
- Pantilov, P.V.; Gorbunova, M.V.; Krivtsov, E.B. Influence of Mineral Components on the Composition of Oil Shale Organic Matter Cracking Products. Solid Fuel Chem. 2024, 58, 124–128. [Google Scholar] [CrossRef]
- Bai, F.; Liu, Y.; Lai, C.; Sun, Y.; Wang, J.; Sun, P.; Xue, L.; Zhao, J.; Guo, M. Thermal degradations and processes of four kerogens via thermogravimetric–fourier-transform infrared: Pyrolysis performances, products, and kinetics. Energy Fuels 2020, 34, 2969–2979. [Google Scholar] [CrossRef]
- Moratori, C.C.; Luz Lisbôa, A.C. Mass and heat transfer to and from oil shale exposed to a gas stream at constant temperature. Oil Shale 2023, 40, 151–165. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Zhang, D.; Chen, X.; Luo, H.; Wang, J.; Ma, Z.; Wang, Q.; Liu, P.; Chen, Z.; et al. Hydrocarbon generation-expulsion-retention mechanisms in marine organic-richmarl: Insights from semi-closed hydrous pyrolysis simulation experiments. J. Anal. Appl. Pyrolysis 2025, 107224. [Google Scholar] [CrossRef]
- Ma, Y.; He, L.; Li, S.; Teng, J. Heat transfer of oil shale in a small-scale fixed bed. J. Therm. Anal. Calorim. 2016, 124, 461–469. [Google Scholar] [CrossRef]
- Tiwari, P. Oil Shale Pyrolysis: Benchscale Experimental Studies and Modeling; Department of Chemical Engineering, University of Utah: Salt Lake City, UT, USA, 2012. [Google Scholar]
- Skala, D.; Kopsch, H.; Sokić, M.; Neumann, H.J.; Jovanović, J.A. Kinetics and modelling of oil shale pyrolysis. Fuel 1990, 69, 490–496. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, C.; Han, X.; Jiang, X. A TGA-MS investigation of the effect of heating rate and mineral matrix on the pyrolysis of kerogen in oil shale. Oil Shale 2016, 33, 125–141. [Google Scholar] [CrossRef]
- Berkovich, A.J.; Levy, J.H.; Schmidt, S.J.; Young, B.R. Heat capacities and enthalpies for some Australian oil shales from non-isothermal modulated DSC. Thermochim. Acta 2000, 357, 41–45. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, Y.; Fan, X.; Xu, M.; Pang, Y.; Wang, J.; Yang, S.; Shi, L. Covalent bonding structures of eight oil shales and the characteristics of bond cleavage during the pyrolysis process. Fuel 2024, 370, 131821. [Google Scholar] [CrossRef]
- Levy, M.; Riri, K. Comparative TGA and DSC studies of oil shales. Thermochim. Acta 1988, 134, 327–331. [Google Scholar] [CrossRef]
- Abduhani, H.; Tursun, Y.; Abulizi, A.; Talifu, D.; Huang, X. Characteristics and kinetics of the gas releasing during oil shale pyrolysis in a micro fluidized bed reactor. J. Anal. Appl. Pyrolysis 2021, 157, 105187. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, Y.; Lü, X.; Li, Q. Energy consumption and product release characteristics evaluation of oil shale non-isothermal pyrolysis based on TG-DSC. J. Pet. Sci. Eng. 2020, 187, 106812. [Google Scholar] [CrossRef]
- Bai, F.; Guo, W.; Lü, X.; Liu, Y.; Guo, M.; Li, Q.; Sun, Y. Kinetic study on the pyrolysis behavior of Huadian oil shale via non-isothermal thermogravimetric data. Fuel 2015, 146, 111–118. [Google Scholar] [CrossRef]
- Warne, S.S.J.; Dubrawski, J.V. Applications of DTA and DSC to coal and oil shale evaluation. J. Therm. Anal. 1989, 35, 219–242. [Google Scholar] [CrossRef]
- Patterson, J.H. A review of the effects of minerals in processing of Australian oil shales. Fuel 1994, 73, 321–327. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Han, X.X.; Li, Q.Y.; Huang, Y.R.; Jiang, X.M. TG–DSC analysis of pyrolysis process of two Chinese oil shales. J. Therm. Anal. Calorim. 2014, 116, 511–517. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Q.; Lu, H.; Wang, G.; Song, Z.; Jin, H.; Guo, L. Insight into kerogen II pyrolysis mechanism by master plot method based on TG-FTIR analysis. Fuel 2025, 395, 135237. [Google Scholar] [CrossRef]
- Palayangoda, S.S.; Nguyen, Q.P. Thermal behavior of raw oil shale and its components. Oil Shale 2015, 32, 160–171. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.; Han, X.; Liu, J. A TG–FTIR investigation to the catalytic effect of mineral matrix in oil shale on the pyrolysis and combustion of kerogen. Fuel 2013, 104, 307–317. [Google Scholar] [CrossRef]
- Hao, J.; Feng, W.; Qiao, Y.; Tian, Y.; Zhang, J.; Che, Y. Thermal cracking behaviors and products distribution of oil sand bitumen by TG-FTIR and Py-GC/TOF-MS. Energy Convers. Manag. 2017, 151, 227–239. [Google Scholar] [CrossRef]
- Raja, M.A.; Zhao, Y.; Zhang, X.; Li, C.; Zhang, S. Practices for modeling oil shale pyrolysis and kinetics. Rev. Chem. Eng. 2017, 34, 21–42. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Fu, B.; Hu, G. Chemical structure and gas products of different rank coals during pyrolysis: Based on in-situ FTIR and TG/MS analysis techniques. J. Therm. Anal. Calorim. 2019, 136, 2017–2031. [Google Scholar] [CrossRef]
- Han, F.; Meng, A.; Li, Q.; Zhang, Y. Thermal decomposition and evolved gas analysis (TG-MS) of lignite coals from Southwest China. J. Energy Inst. 2016, 89, 94–100. [Google Scholar] [CrossRef]
- Pan, L.; Dai, F.; Li, G.; Liu, S. A TGA/DTA-MS investigation to the influence of process conditions on the pyrolysis of Jimsar oil shale. Energy 2015, 86, 749–757. [Google Scholar] [CrossRef]
- Tian, B.; Qiao, Y.; Tian, Y.; Liu, Q. Investigation on the effect of particle size and heating rate on pyrolysis characteristics of a bituminous coal by TG–FTIR. J. Anal. Appl. Pyrolysis 2016, 121, 376–386. [Google Scholar] [CrossRef]
- Chen, B.; Han, X.; Jiang, X. In situ FTIR analysis of the evolution of functional groups of oil shale during pyrolysis. Energy Fuels 2016, 30, 5611–5616. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, H.; Guo, J.; Liu, G.; Wang, Z.; Guo, Y. Pyrolysis Characteristics and Effect on Pore Structure of Jimsar Oil Shale Based on TG-FTIR-MS Analysis. Geofluids 2022, 2022, 7857239. [Google Scholar] [CrossRef]
- Kok, M.V.; Varfolomeev, M.A.; Nurgaliev, D.K.; Kandasamy, J. Application of TGA-MS technique for oil shale characterization and kinetics. J. Therm. Anal. Calorim. 2022, 147, 10767–10774. [Google Scholar] [CrossRef]
- Khraisha, Y.H. Flash pyrolysis of oil shales in a fluidized bed reactor. Energy Convers. Manag. 2000, 41, 1729–1739. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, S.; Li, J.; Li, Y.; Chen, K.; Zhang, Y.; Fu, D. Pyrolysis characteristics analysis of Chang-7 oil shale using thermal analysis and pyrolysis-gas chromatograph-mass spectrometry. Energy Explor. Exploit. 2018, 36, 1006–1021. [Google Scholar] [CrossRef]
- Williams, P.T.; Ahmad, N. Investigation of oil-shale pyrolysis processing conditions using thermogravimetric analysis. Appl. Energy 2000, 66, 113–133. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Al-Ayed, O.; Robinson, J.; Kingman, S.; Al-Harahsheh, A.; Tarawneh, K.; Saeid, A.; Barranco, R. Effect of demineralization and heating rate on the pyrolysis kinetics of Jordanian oil shales. Fuel Process. Technol. 2011, 92, 1805–1811. [Google Scholar] [CrossRef]
- Pan, N.; Li, D.; Lü, W.; Dai, F. Kinetic study on the pyrolysis behavior of Jimsar oil shale. Oil Shale 2019, 36, 462–482. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Linghu, R.; Wang, C.; Zhang, S. Comparative kinetics of coal and oil shale pyrolysis in a micro fluidized bed reaction analyzer. Carbon Resour. Convers. 2019, 2, 217–224. [Google Scholar] [CrossRef]
- Williams, P.T.; Ahmad, N. Influence of process conditions on the pyrolysis of Pakistani oil shales. Fuel 1999, 78, 653–662. [Google Scholar] [CrossRef]
- Nazzal, J.M. Influence of heating rate on the pyrolysis of Jordan oil shale. J. Anal. Appl. Pyrolysis 2002, 62, 225–238. [Google Scholar] [CrossRef]
- Lin, L.; Lai, D.; Guo, E.; Zhang, C.; Xu, G. Oil shale pyrolysis in indirectly heated fixed bed with metallic plates of heating enhancement. Fuel 2016, 163, 48–55. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, J. Developments in the understanding of gas–solid contact efficiency in the circulating fluidized bed riser reactor: A review. Chin. J. Chem. Eng. 2016, 24, 53–62. [Google Scholar] [CrossRef]
- Maaten, B.; Siirde, A.; Vahur, S.; Kirsimäe, K. Influence of the end-temperature on the oil shale fast pyrolysis process and its products. J. Therm. Anal. Calorim. 2023, 148, 1647–1655. [Google Scholar] [CrossRef]
- Luo, W. Experimental Study on Pyrolysis Process and Product Precipitation Characteristics of Oil Shale. Ph.D. Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2016. [Google Scholar]
- Le Doan, T.V.; Bostrom, N.W.; Burnham, A.K.; Kleinberg, R.L.; Pomerantz, A.E.; Allix, P. Green River oil shale pyrolysis: Semi-open conditions. Energy Fuels 2013, 27, 6447–6459. [Google Scholar] [CrossRef]
- Li, J.; Shan, X.; Song, X.; He, W. Evaluation of the organic matter product of Huadian oil shale during pyrolysis using multiple approaches: Guidance for the in situ conversion of oil shale. J. Anal. Appl. Pyrolysis 2022, 167, 105656. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, J.; Qiao, P.; Li, J.; Zhang, W. Effects of secondary reaction of primary volatiles on oil/gas yield and quality in oil shale pyrolysis. J. Fuel Chem. Technol. 2021, 49, 924–932. [Google Scholar] [CrossRef]
- Ding, H.; Ma, Y.; Li, S.; Wang, Q.; Hong, W.; Jiang, H.; Li, H.; Jiang, M. Pyrolytic characteristics of Fushun oil shale and its by-products. J. Therm. Anal. Calorim. 2022, 147, 5255–5267. [Google Scholar] [CrossRef]
- Syed, S.; Qudaih, R.; Talab, I.; Janajreh, I. Kinetics of pyrolysis and combustion of oil shale sample from thermogravimetric data. Fuel 2011, 90, 1631–1637. [Google Scholar] [CrossRef]
- Yang, Q.; Qian, Y.; Kraslawski, A.; Zhou, H.; Yang, S. Framework for advanced exergoeconomic performance analysis and optimization of an oil shale retorting process. Energy 2016, 109, 62–76. [Google Scholar] [CrossRef]
- Foltin, J.P.; Lisboa, A.C.L.; de Klerk, A. Oil shale pyrolysis: Conversion dependence of kinetic parameters. Energy Fuels 2017, 31, 6766–6776. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Zheng, J.; Pan, Y.; Ji, C. Comprehensive review: Study on heating rate characteristics and coupling simulation of oil shale pyrolysis. J. Anal. Appl. Pyrolysis 2024, 177, 106289. [Google Scholar] [CrossRef]
- Shi, W. Investigation on Influencing Factors of Low-Temperature Retorting of Sunite Oil Shale. Coal 2013, 22, 20–21. [Google Scholar]
- Al-Ayed, O.S.; Al-Harahsheh, A.; Khaleel, A.M.; Al-Harahsheh, M. Oil shale pyrolysis in fixed-bed retort with different heating rates. Oil Shale 2009, 26, 139–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Z.; Wu, H.; Lai, D.; Glarborg, P.; Xu, G. Interactive matching between the temperature profile and secondary reactions of oil shale pyrolysis. Energy Fuels 2016, 30, 2865–2873. [Google Scholar] [CrossRef]
- Lisbôa, A.C.L. Investigations on Oil Shale Particle Reactions. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1997. [Google Scholar]
- Miura, K. Mild conversion of coal for producing valuable chemicals. Fuel Process. Technol. 2000, 62, 119–135. [Google Scholar] [CrossRef]
- Chang, Z.; Chu, M. The chemical composition and pyrolysis characteristics of thermal bitumen derived from pyrolyzing Huadian oil shale, China. Oil Shale 2019, 36, 62–75. [Google Scholar] [CrossRef]
- Ahmad, N.; Williams, P.T. Influence of particle grain size on the yield and composition of products from the pyrolysis of oil shales. J. Anal. Appl. Pyrolysis 1998, 46, 31–49. [Google Scholar] [CrossRef]
- Khalil, A.M. Oil shale pyrolysis and effect of particle size on the composition of shale oil. Oil Shale 2013, 30, 136–146. [Google Scholar] [CrossRef]
- Pan, L.; Dai, F.; Pei, S.; Huang, S.; Liu, S. Influence of particle size and temperature on the yield and composition of products from the pyrolysis of Jimsar (China) oil shale. J. Anal. Appl. Pyrolysis 2021, 157, 105211. [Google Scholar] [CrossRef]
- Burnham, A.K.; McConaghy, J.R. Comparison of the Acceptability of Various Oil Shale Processes; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Sun, T.; Liu, H.; Zhang, Y.; Li, Y. Numerical simulation and optimization study of In-Situ Heating for three-dimensional oil shale exploitation with different well patterns. Case Stud. Therm. Eng. 2024, 55, 104089. [Google Scholar] [CrossRef]
- Xue, J.; Liu, Z. Numerical Simulation of Temperature Field Distribution in In-Situ Retorting of Oil Shale by Electric Heating Method. Chin. J. Undergr. Space Eng. 2015, 11, 669–672. [Google Scholar]
- Bai, Y.; Shu, Y.; Dang, H.; Yun, Y.; Tu, X.; Zhang, L.; Gao, T.; Zhang, M.; Zhao, X.; Yang, S. A review on the application of numerical simulation of oil shale electrical heating technology. Chem. Technol. Fuels Oils 2024, 60, 69–79. [Google Scholar] [CrossRef]
- Lei, G.; Li, Z.; Yao, C.; Zheng, Y.; Wang, N.; Wang, Z. Numerical simulation on in-situ upgrading of oil shale via steam injection. J. Univ. Pet. China 2017, 41, 100–107. [Google Scholar]
- Liu, S.; Gai, H.; Cheng, P. Technical scheme and application prospects of oil shale in situ conversion: A review of current status. Energies 2023, 16, 4386. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.; Wang, G.; Zhao, J.; Yang, D.; Kang, Z.; Zhao, Y. Effect of long reaction distance on gas composition from organic-rich shale pyrolysis under high-temperature steam environment. Int. J. Coal Sci. Technol. 2024, 11, 34. [Google Scholar] [CrossRef]
- Inaba, H. New challenge in advanced thermal energy transportation using functionally thermal fluids. Int. J. Therm. Sci. 2000, 39, 991–1003. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Gao, L.; Guo, S.; He, F. Microwave treatment of minerals and ores: Heating behaviors, applications, and future directions. Minerals 2024, 14, 219. [Google Scholar] [CrossRef]
- Lan, X.; Luo, W.; Song, Y.; Zhou, J.; Zhang, Q. Effect of the temperature on the characteristics of retorting products obtained by Yaojie oil shale pyrolysis. Energy Fuels 2015, 29, 7800–7806. [Google Scholar] [CrossRef]
- He, L.; Ma, Y.; Yue, C.; Li, S.; Tang, X. The heating performance and kinetic behaviour of oil shale during microwave pyrolysis. Energy 2022, 244, 123021. [Google Scholar] [CrossRef]
- Zhu, J.; Li, F.; Wang, H.; Yang, Z.; Chen, H.; Zhu, H. Numerical analysis of microwave-enhanced oil shale pyrolysis by rotation turntable based on the Arbitrary Lagrangian-Eulerian method. Fuel 2024, 371, 131925. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, Z.; Li, X.; Qi, S.; Fang, Q.; Ding, Y. The experimental study of microwave heating on the microstructure of oil shale samples. Energy Sci. Eng. 2019, 7, 809–820. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. The influence of electromagnetic waves on the gas condensate characterisation: Experimental evaluation. J. Pet. Sci. Eng. 2018, 166, 568–576. [Google Scholar] [CrossRef]
- Miura, M.; Kaga, H.; Sakurai, A.; Kakuchi, T.; Takahashi, K. Rapid pyrolysis of wood block by microwave heating. J. Anal. Appl. Pyrolysis 2004, 71, 187–199. [Google Scholar] [CrossRef]
- Chen, P.; Xie, Q.; Addy, M.; Zhou, W.; Liu, Y.; Wang, Y.; Cheng, Y.; Li, K.; Ruan, R. Utilization of municipal solid and liquid wastes for bioenergy and bioproducts production. Bioresour. Technol. 2016, 215, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Mutyala, S.; Fairbridge, C.; Paré, J.R.J.; Bélanger, J.M.R.; Ng, S.H.; Hawkins, R. Microwave applications to oil sands and petroleum: A review. Fuel Process. Technol. 2010, 91, 127–135. [Google Scholar] [CrossRef]
- Xu, S.; Sun, Y.; Yang, Q.; Wang, H.; Kang, S.; Guo, W.; Shan, X.; He, W. Product migration and regional reaction characteristics in the autothermic pyrolysis in-situ conversion process of low-permeability Huadian oil shale core. Energy 2023, 283, 128525. [Google Scholar] [CrossRef]
- Yang, Q. Theoretical and Laboratory Experimental Study on In-Situ Pyrolysis of Oil Shale by Autogenous Heating Method. Ph.D. Thesis, Jilin University, Changchun, China, 2022. [Google Scholar]
- Guo, H.; Yang, Y.; Wang, K.; Pei, Y.; Wu, Q.; Liu, Y. Strengthening the applicability of self-heating retorting process to oil shale via co-retorting. Fuel 2015, 143, 1–8. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, W.; Xu, S.; Zhu, C. The autothermic pyrolysis in-situ conversion process for oil shale recovery: Effect of gas injection parameters. Energy 2023, 283, 129134. [Google Scholar] [CrossRef]
- Sun, Y.H.; Bai, F.T.; Lü, X.S.; Li, Q.; Liu, Y.M.; Guo, M.Y.; Guo, W.; Liu, B.C. A novel energy-efficient pyrolysis process: Self-pyrolysis of oil shale triggered by topochemical heat in a horizontal fixed bed. Sci. Rep. 2015, 5, 8290. [Google Scholar] [CrossRef]
- Xu, S.T.; Lü, X.S.; Wang, H.; Sun, Y.H.; Kang, S.J.; Wang, Z.D.; Guo, W.; Deng, S.H. Characterization of oxygen initiation process in the autothermic pyrolysis in-situ conversion of Huadian oil shale. Pet. Sci. 2024, 21, 4481–4496. [Google Scholar] [CrossRef]
- Yu, D.; Fu, H.; Deng, S.; Xu, S.; Tang, W.; Sun, Y.; Guo, W. Effects of maturity on the oxidative pyrolysis characteristics and heat balance in autothermic in-situ conversion of low-medium maturity organic-rich shales. Energy 2025, 314, 134334. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhou, S.; Meng, B.; Wu, T. A review on thermogravimetric analysis-based analyses of the pyrolysis kinetics of oil shale and coal. Energy Sci. Eng. 2024, 12, 329–355. [Google Scholar] [CrossRef]
- Chang, Z.; Chu, M.; Zhang, C.; Bai, S.; Lin, H.; Ma, L. Influence of inherent mineral matrix on the product yield and characterization from Huadian oil shale pyrolysis. J. Anal. Appl. Pyrolysis 2018, 130, 269–276. [Google Scholar] [CrossRef]
- Hetényi, M. Simulated thermal maturation of type I and III kerogens in the presence, and absence, of calcite and montmorillonite. Org. Geochem. 1995, 23, 121–127. [Google Scholar] [CrossRef]
- Yu, H.; Li, S.; Jin, G. Catalytic hydrotreating of the diesel distillate from Fushun shale oil for the production of clean fuel. Energy Fuels 2010, 24, 4419–4424. [Google Scholar] [CrossRef]
- Williams, P.T.; Chishti, H.M. Two stage pyrolysis of oil shale using a zeolite catalyst. J. Anal. Appl. Pyrolysis 2000, 55, 217–234. [Google Scholar] [CrossRef]
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Behar, F.; Vandenbroucke, M.; Teermann, S.C.; Hatcher, P.G.; Leblond, C.; Lerat, O. Experimental simulation of gas generation from coals and a marine kerogen. Chem. Geol. 1995, 126, 247–260. [Google Scholar] [CrossRef]
- Zhong, M.; Huang, H.; Xu, P.; Hu, J. Catalysis of minerals in pyrolysis experiments. Minerals 2023, 13, 515. [Google Scholar] [CrossRef]
- Lai, D.; Shi, Y.; Geng, S.; Chen, Z.; Gao, S.; Zhan, J.H.; Xu, G. Secondary reactions in oil shale pyrolysis by solid heat carrier in a moving bed with internals. Fuel 2016, 173, 138–145. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, C.; Meng, X.; Ma, Y.; Xu, L.; Du, X. Pyrolysis mechanism and reservoir simulation study of organic-rich shale during the in situ conversion via supercritical water heating. Energy Fuels 2024, 38, 14246–14261. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Pan, J.; Zhu, C.; Deng, S. In-situ pyrolysis of oil shale in pressured semi-closed system: Insights into products characteristics and pyrolysis mechanism. Energy 2024, 286, 129608. [Google Scholar] [CrossRef]
- Shi, Y.; Weng, D.; Cai, B.; Zhang, Y.; Zhang, Y.; Wang, B.; Wang, H. Flow and Heat Transfer of Shale Oil Reservoir during CO2 Enhanced Pyrolysis: A Pore-Scale Modeling. Processes 2024, 12, 1694. [Google Scholar] [CrossRef]
- Lü, X.; Sun, Y.; Lu, T.; Bai, F.; Viljanen, M. An efficient and general analytical approach to modelling pyrolysis kinetics of oil shale. Fuel 2014, 135, 182–187. [Google Scholar] [CrossRef]
- Baruah, B.; Tiwari, P. Effect of high pressure on nonisothermal pyrolysis kinetics of oil shale and product yield. Energy Fuels 2020, 34, 15855–15869. [Google Scholar] [CrossRef]
- Pan, S.; Zhou, H.; Wang, Q.; Bai, J.; Cui, D.; Wang, X. Experimental and molecular simulation studies of Huadian oil shale kerogen. ACS Omega 2022, 7, 17253–17269. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Zhang, Y.; Bai, J.; Wang, Z.; Cui, D.; Wang, Q. Pyrolysis mechanism of kerogen: Model construction and multi-scale molecular simulations. J. Anal. Appl. Pyrolysis 2024, 183, 106837. [Google Scholar] [CrossRef]
- Sun, B.; Liu, X.P.; Liu, J.; Liu, T.; Hua, Z.X.; Peng, W.D. Evolution and generation mechanism of retained oil in lacustrine shales: A combined ReaxFF-MD and pyrolysis simulation perspective. Pet. Sci. 2025, 22, 29–41. [Google Scholar] [CrossRef]
- He, H.; Gong, Y.; Peng, M.; Zheng, H.; Feng, T.; Zhang, M.; Li, Q.; Liu, P. Study on mechanism of in-situ hydrogen generation based on molecular dynamics simulation from pyrolysis of heavy oil. Fuel 2025, 398, 135534. [Google Scholar] [CrossRef]
- Meuwly, M.; Becker, O.M.; Stote, R.; Karplus, M. NO rebinding to myoglobin: A reactive molecular dynamics study. Biophys. Chem. 2002, 98, 183–207. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, J.H.; Lai, D.; Liu, X.; Zhang, Z.; Xu, G. Initial pyrolysis mechanism of oil shale kerogen with reactive molecular dynamics simulation. Energy Fuels 2015, 29, 2987–2997. [Google Scholar] [CrossRef]
- Vandenbroucke, M. Kerogen: From types to models of chemical structure. Oil Gas Sci. Technol. 2003, 58, 243–269. [Google Scholar] [CrossRef]


| Origin of Oil Shale | Temperature Range (°C) | Main Products | Optimal Temperature for Maximum Oil Yield (°C) |
|---|---|---|---|
| Yaojie, Gansu [45] | 300–1000 | Liquid: alkanes, cycloalkanes, aromatics, and other compounds; Gases: CO2, CH4, C2–C6, C3H6, C3H8, sulfurous gases. | 550 |
| Green River, USA [46] | 150–600 | Early: low-molecular hydrocarbons, bitumen; Middle: CH4, C2H6, C3H8, NGLs, bitumen, coke; Late: CO2, CH4, coke. | 400–500 |
| Huadian, Jilin [47,48] | 200–650 | Liquid: alkanes and derivatives, phenols, aromatics, oxides, PAHs, cycloalkanes, olefins; Gases: H2, CO2, CO, CH4, C2H6, C2H4, sulfurous gases. | 475 |
| Fushun, Liaoning [49] | 200–700 | Early: mainly H2O; Middle: aromatics, saturated and unsaturated hydrocarbons, SO2, NO2, NH3; Late: H2O, aromatics, CO2, saturated hydrocarbons, trace SO2 and NO2. | 496 |
| Lujjin, Jordan [50] | 20–830 | 20–280 °C: H2O; 280–540 °C: hydrocarbons; 540–830 °C: CO2, CO. | 420–550 |
| Heating Method | Energy Efficiency | Limitations | Key Mechanism |
|---|---|---|---|
| Conventional Heating [63,64,65,66,67,68,69,70] | High heat loss in electric heating; improved but still limited in thermal fluid heating. | -Electric heating: severe heat loss, prolonged heating time (9 years to reach 500 °C), uneven temperature distribution. -Thermal fluid heating: large heat injection volume, significant heat loss along the path. | Relies on heat conduction and convection; steam provides hydrogen radicals to promote kerogen cracking. |
| Microwave Heating [71,72,73,74,75,76,77,78,79] | 750 °C reached in 9 min vs. 55 min in conventional heating; 13–39% lower activation energy. | Local overheating at high power may cause secondary cracking of oil/gas, reducing oil yield. | Volumetric heating via electromagnetic waves interacting with polar molecules/minerals. |
| Auto-Thermal Heating [80,81,82,83,84,85,86] | Energy efficiency of 3.46, 6.78 times that of high-temperature nitrogen injection. | Requires precise oxygen control to avoid under-oxidation or over-oxidation of organic matter. | Heat from partial oxidation of organic matter/minerals drives pyrolysis; residual carbon oxidation sustains reaction. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yi, R.; Zhao, D.; Luo, W.; Huang, L.; Su, J.; Zhu, J. Research Progress on the Pyrolysis Characteristics of Oil Shale in Laboratory Experiments. Processes 2025, 13, 2787. https://doi.org/10.3390/pr13092787
Liu X, Yi R, Zhao D, Luo W, Huang L, Su J, Zhu J. Research Progress on the Pyrolysis Characteristics of Oil Shale in Laboratory Experiments. Processes. 2025; 13(9):2787. https://doi.org/10.3390/pr13092787
Chicago/Turabian StyleLiu, Xiaolei, Ruiyang Yi, Dandi Zhao, Wanyu Luo, Ling Huang, Jianzheng Su, and Jingyi Zhu. 2025. "Research Progress on the Pyrolysis Characteristics of Oil Shale in Laboratory Experiments" Processes 13, no. 9: 2787. https://doi.org/10.3390/pr13092787
APA StyleLiu, X., Yi, R., Zhao, D., Luo, W., Huang, L., Su, J., & Zhu, J. (2025). Research Progress on the Pyrolysis Characteristics of Oil Shale in Laboratory Experiments. Processes, 13(9), 2787. https://doi.org/10.3390/pr13092787