Effect of Storage Conditions on the Composition and Bioactivity of Freeze-Dried Lemongrass Oil Nanoemulsions Stabilized by Salt-Sensitive Cellulose Nanocrystals and Tween 80
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoemulsion Preparation and Freeze-Drying
2.3. Physicochemical Characterization of Redispersed Nanoemulsion
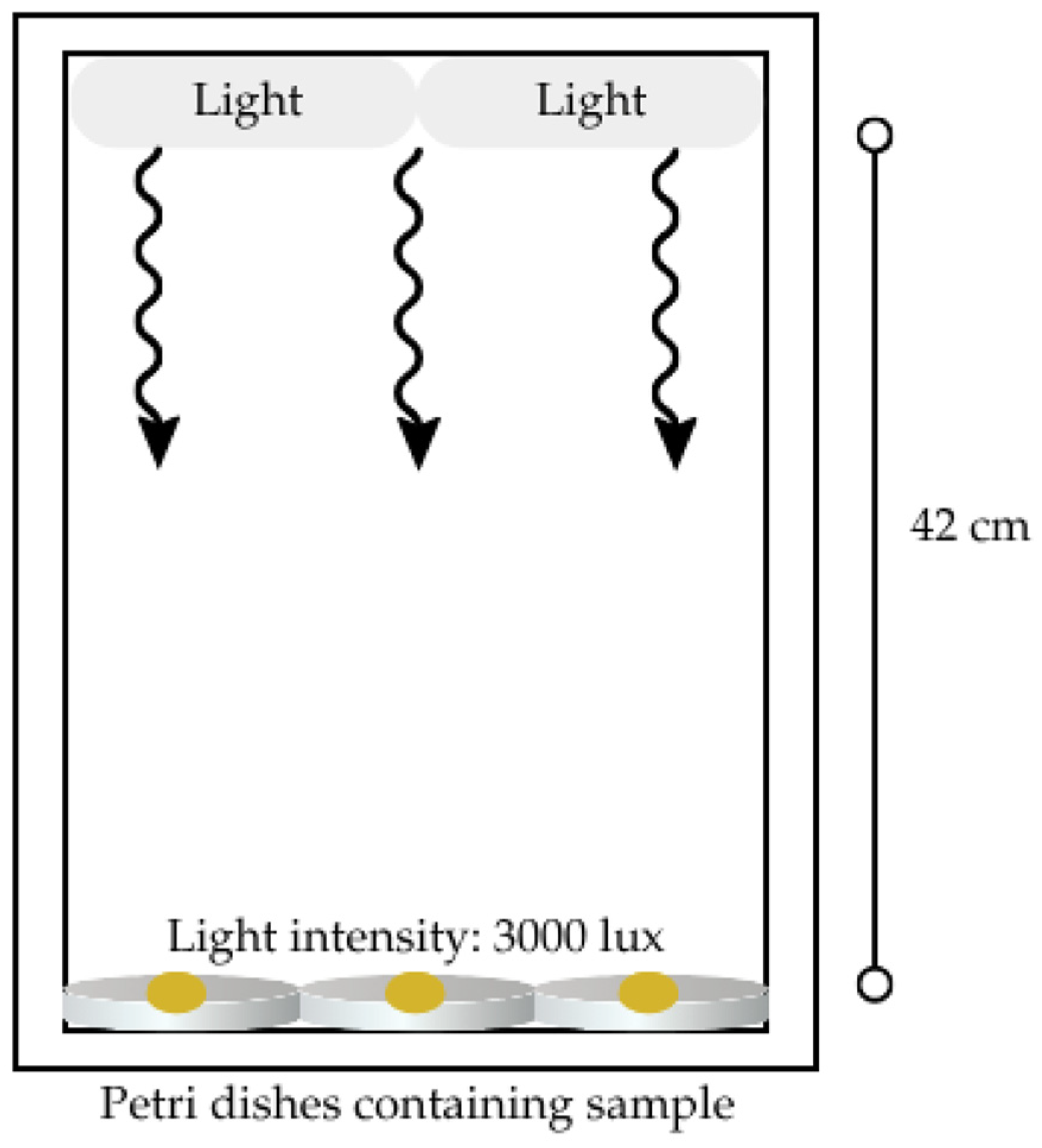
2.4. Effect of Light Exposure and Room Temperature Storage on Essential Oil Composition
2.5. Antioxidant Activity of Redispersed Nanoemulsion
2.6. Fungal Cultures
2.7. Antifungal Activity of Redispersed Nanoemulsion
2.8. GC/Q-TOF Characterization and Quantification of Redispersed Nanoemulsion
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Characterization of Redispersed Nanoemulsion
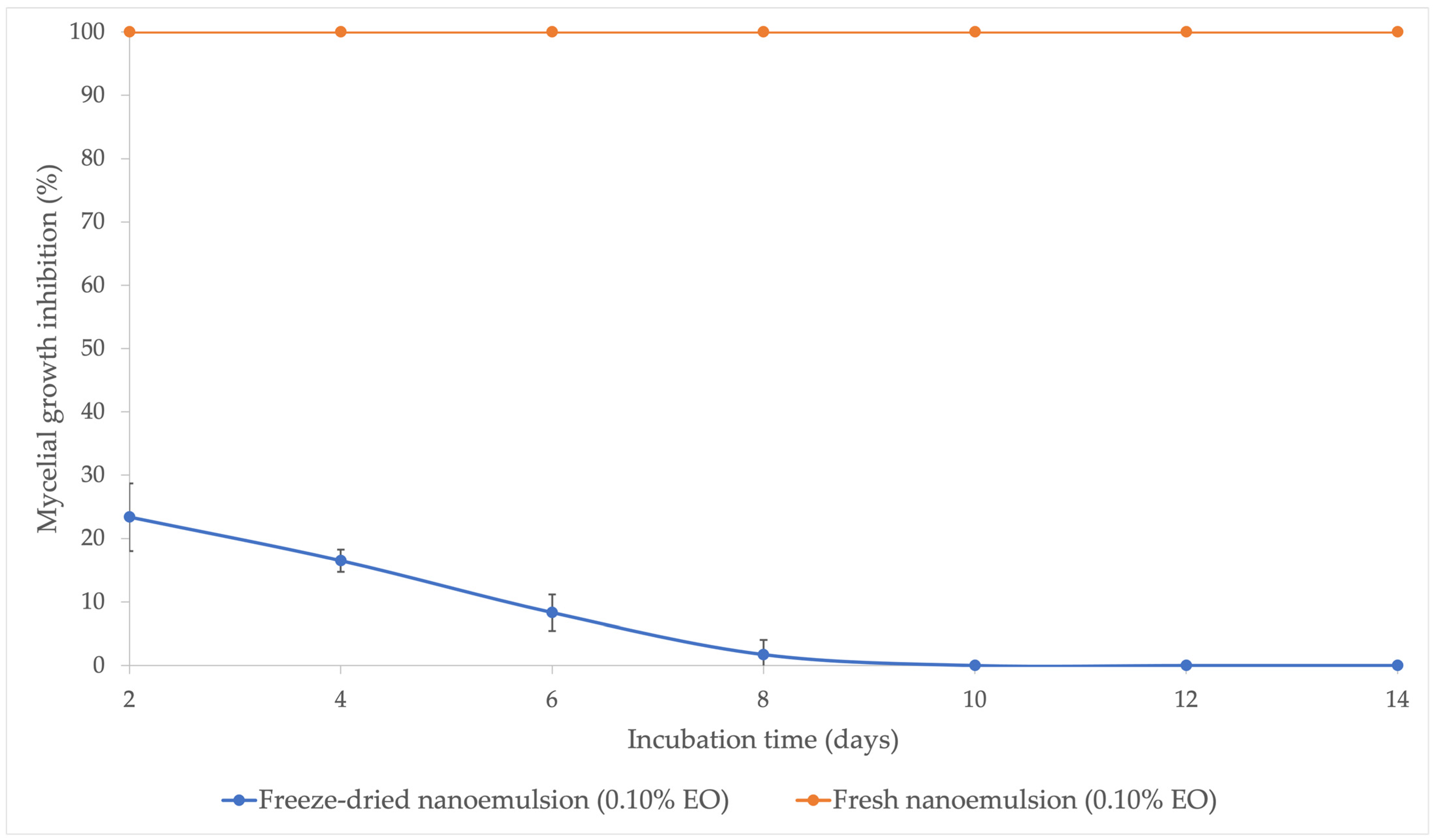
3.2. Effect of Freeze-Drying on the Antifungal Activity of Nanoemulsion Containing LGEO Against Aspergillus Flavus
3.3. Antioxidant Activity of LGEO, Nanoemulsion, and Redispersed Nanoemulsion over 30-Day Period
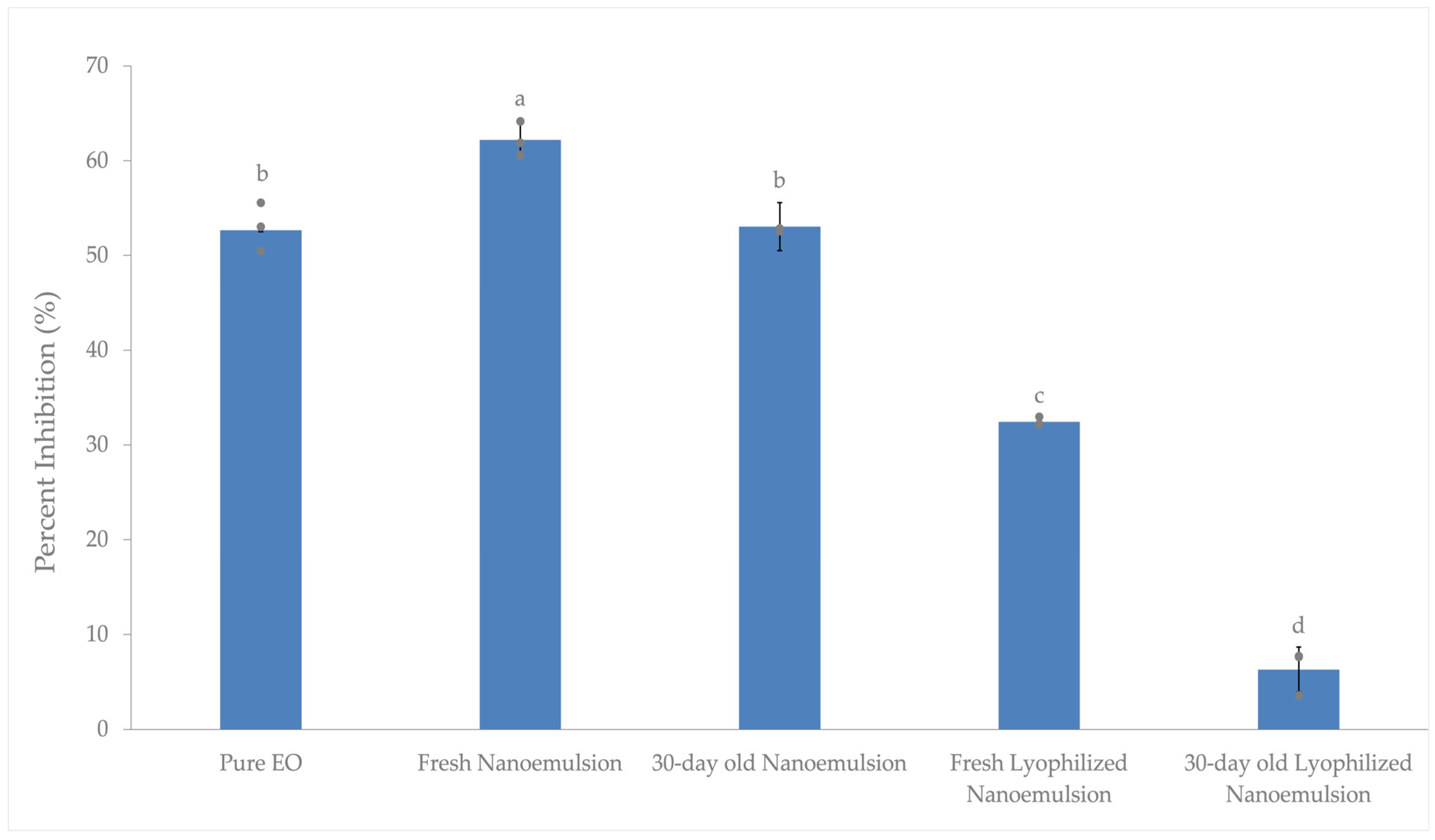
3.3.1. Antioxidant Activity of Pure Essential Oil, Fresh Nanoemulsion, and Freshly Redispersed Freeze-Dried Nanoemulsion
3.3.2. Antioxidant Activity of Pure Essential Oil, Fresh Nanoemulsion, and Freeze-Dried Nanoemulsion After 30 Days of Room Temperature Storage
3.4. GC/QTOF Analysis of the Chemical Composition and Concentration of Volatile Components in Lemongrass Essential Oil and Its Nanoemulsion
3.4.1. GC/Q-TOF Analysis of Lemongrass Essential Oil
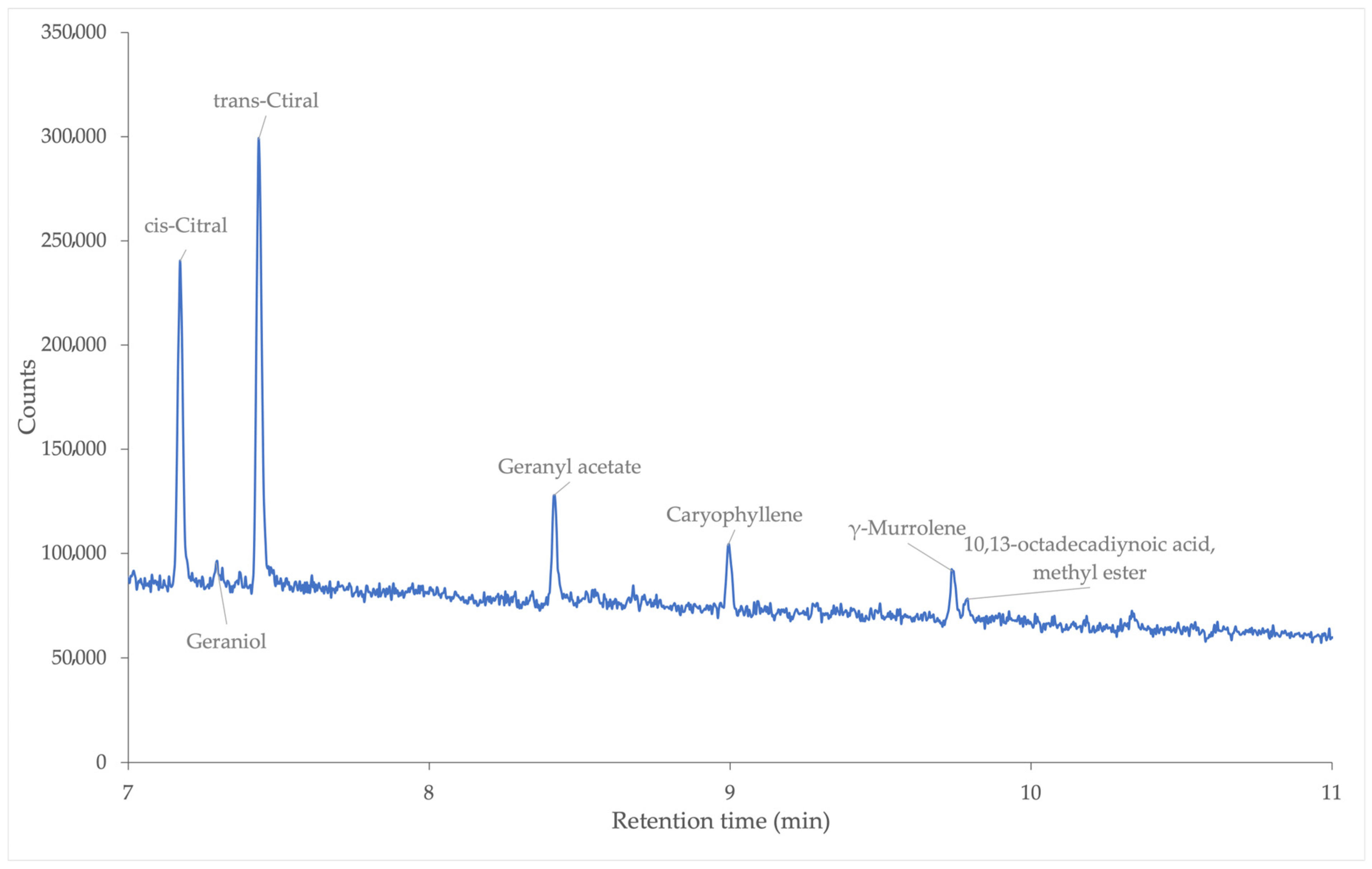
3.4.2. GC/Q-TOF Analysis of Fresh Nanoemulsion Containing Lemongrass Essential Oil
3.5. Effect of Light Exposure and Room Temperature Storage on Freeze-Dried Nanoemulsion Containing LGEO
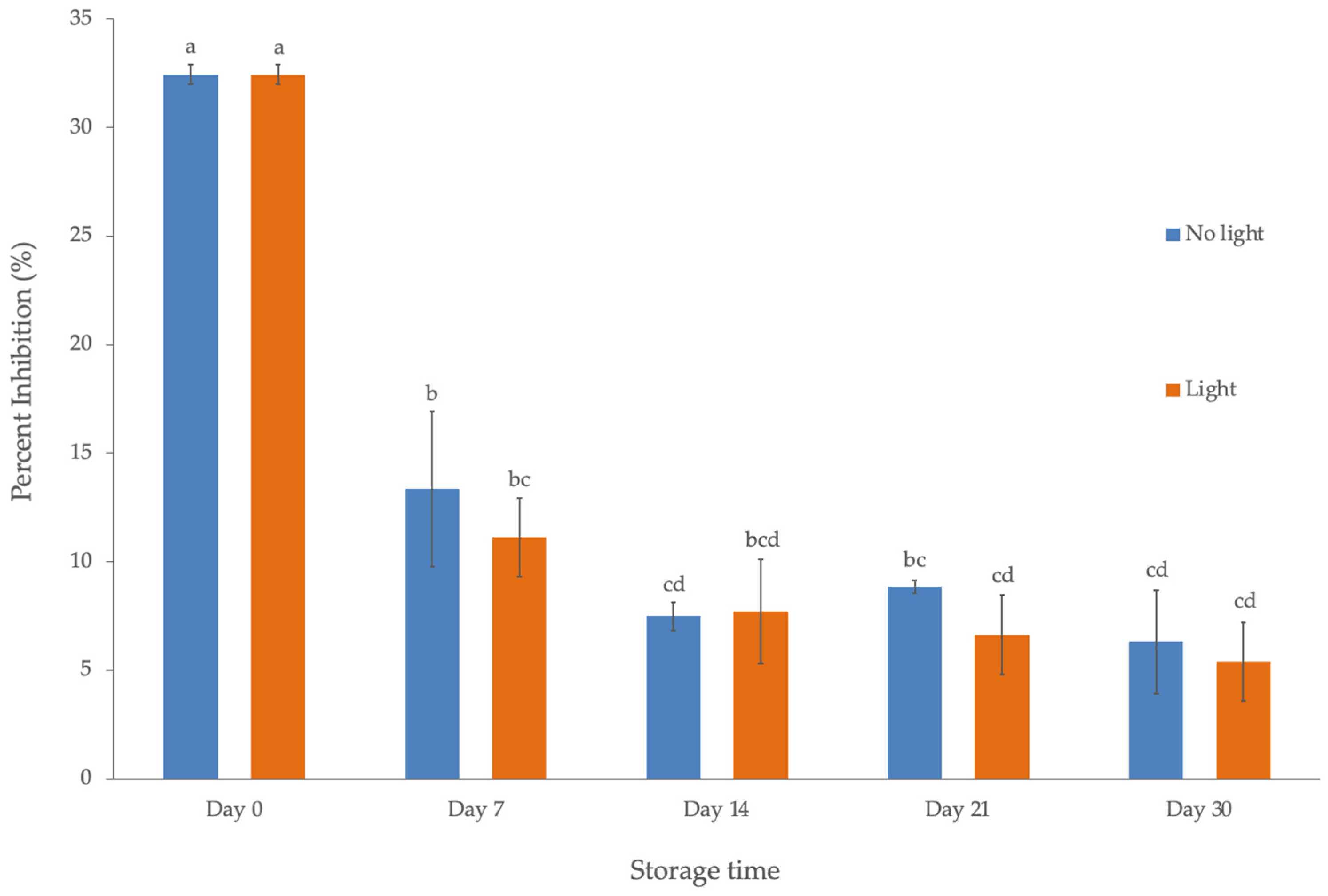
3.5.1. Effect of Light Exposure and Room Temperature Storage on the Antioxidant Activity of Freeze-Dried Nanoemulsion
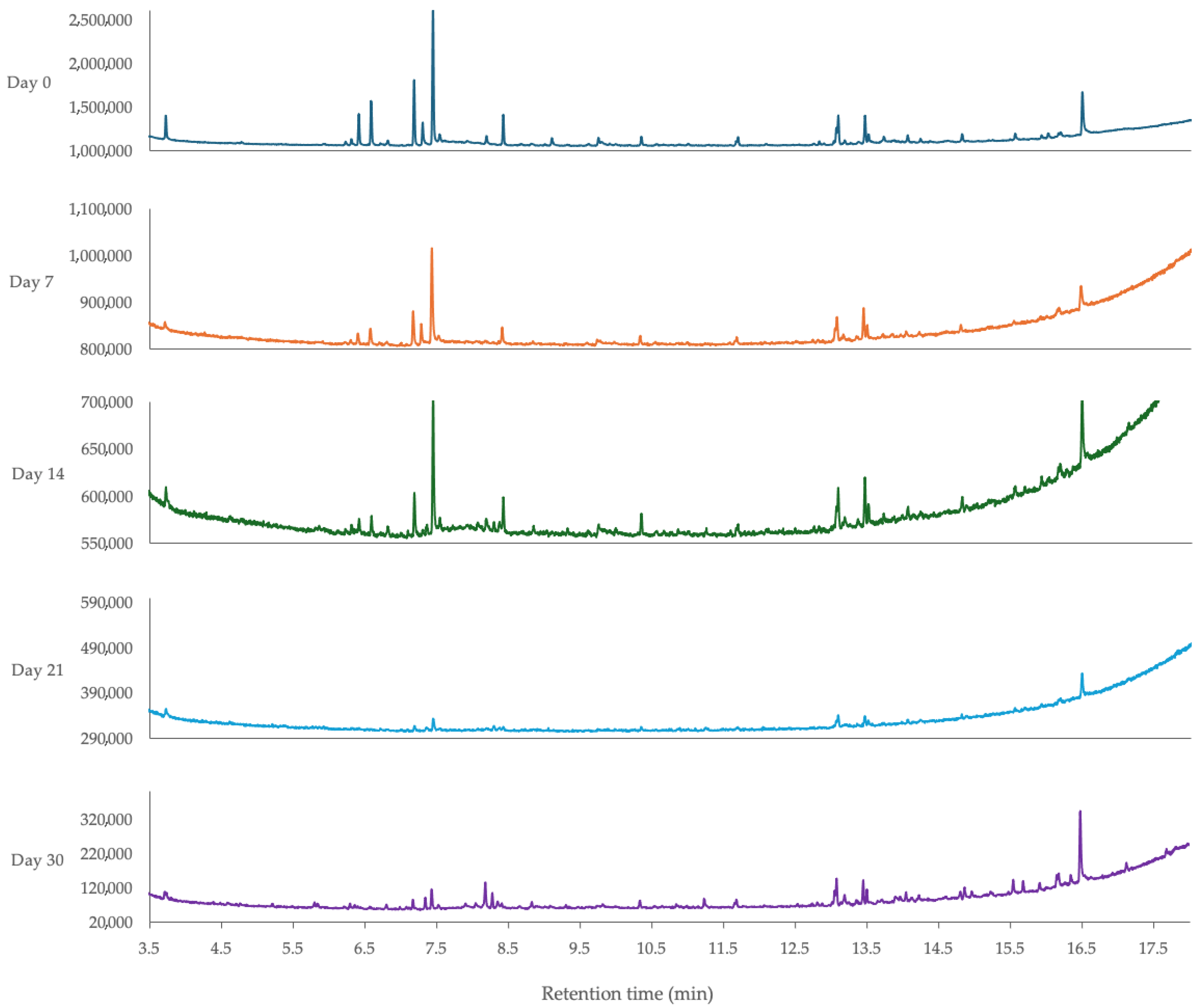
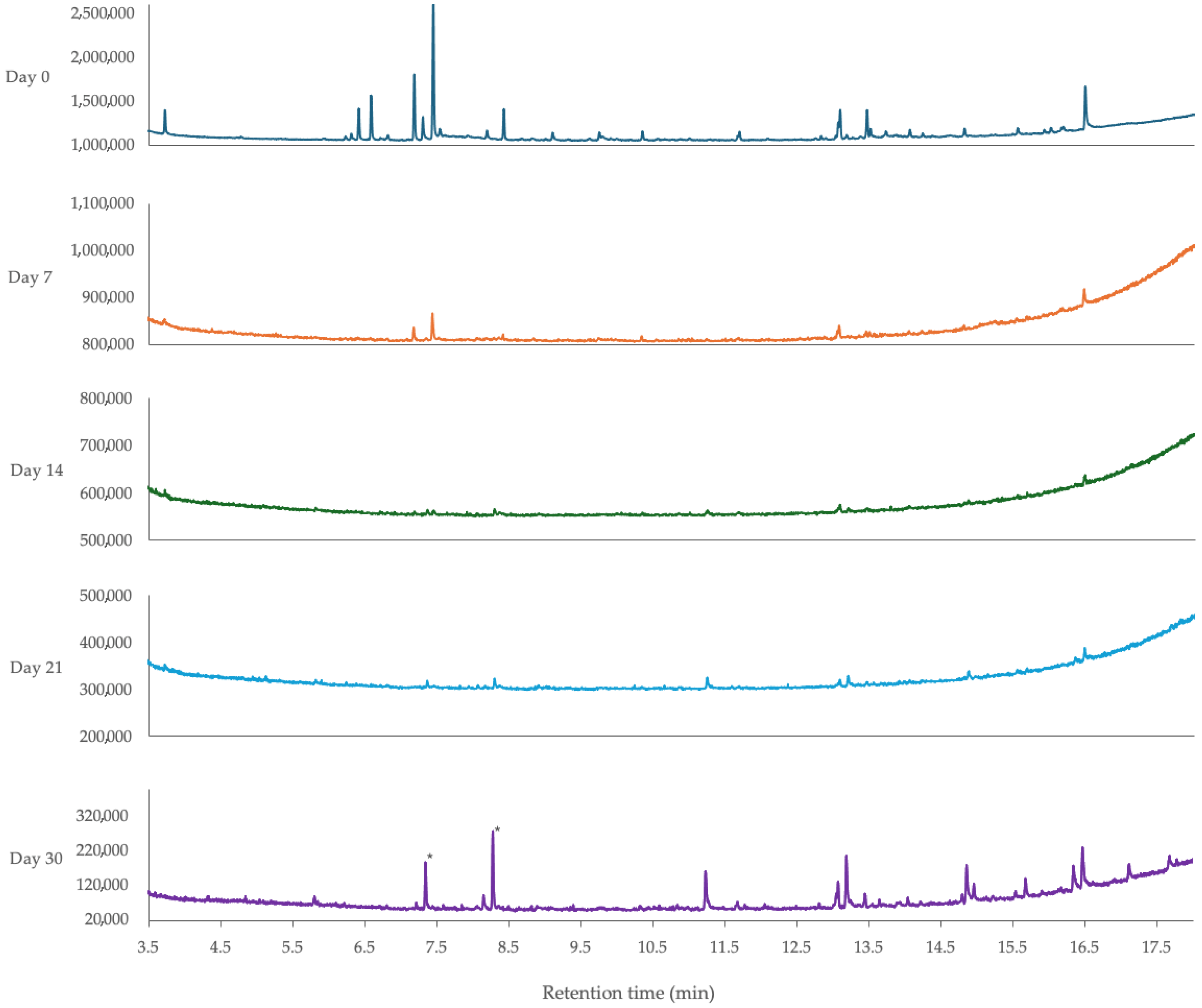
3.5.2. Effect of Light Exposure and Room Temperature Storage Time on the Composition of Lemongrass Essential Oil-Loaded Nanoemulsion
3.5.3. Effect of Light Exposure and Room Temperature Storage on the Concentration of Four Volatile Components in Lemongrass Essential Oil-Loaded Nanoemulsion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SBS-CNC | Soybean stover-derived cellulose nanocrystals |
| O/W | Oil-in-water |
| LGEO | Lemongrass essential oil |
| EO | Essential oil |
References
- Hüsnü Can Baser, K.; Demirci, F. Chemistry of essential oils. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; pp. 43–86. [Google Scholar]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; St. Rose, T.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef]
- Hu, F.; Tu, X.; Thakur, K.; Hu, F.; Li, X.; Zhang, Y.; Zhang, J.; Wei, Z. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef]
- Andoğan, B.C.; Baydar, H.; Kaya, S.; Demirci, M.; Özbaşar, D.; Mumcu, E. Antimicrobial activity and chemical composition of some essential oils. Arch. Pharmacal Res. 2002, 25, 860–864. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants 2022, 11, 20. [Google Scholar] [CrossRef]
- Martinazzo, A.P.; Oliveira, F.S.; Teodoro, C.E.S. Antifungal activity of Cymbopogon citratus essential oil against Aspergillus flavus. Cienca E Nat. 2022, 41, e20. [Google Scholar] [CrossRef]
- Oxenham, S.K.; Svoboda, K.P.; Walters, D.R. Antifungal activity of the essential oil of basil (Ocimum basilicum). J. Phytopathol. 2005, 153, 174–180. [Google Scholar] [CrossRef]
- Daouk, R.K.; Dagher, S.M.; Sattout, E.J. Antifungal activity of the essential oil of Origanum syriacum L. J. Food Prot. 1995, 58, 1147–1149. [Google Scholar] [CrossRef]
- Barradas, T.N.; Gyselle de Holanda e Silva, K. Nanoemulsions of essential oils to improve solubility, stability and permeability: A review. Environ. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]
- Weisheimer, V.; Miron, D.; Silva, C.B.; Guterres, S.S.; Schapoval, E.E.S. Microparticles containing lemongrass volatile oil: Preparation, characterization and thermal stability. Die Pharm. Int. J. Pharm. Sci. 2010, 65, 885–890. [Google Scholar]
- Rowshan, V.; Bahmanzadegan, A.; Saharkhiz, M.J. Influence of storage conditions on the essential oil composition of Thymus daenensis Celak. Ind. Crops Prod. 2013, 49, 97–101. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Ghasemi Pirbalouti, A.; Moghaddam, M. Storage stability of essential oil of cumin (Cuminum cyminum L.) as a function of temperature. Int. J. Food Prop. 2017, 20 (Suppl. S2), 1742–1750. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Impact of different storage conditions on the quality of selected essential oils. Food Res. Int. 2012, 46, 341–353. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Chu, Y.; Xu, T.; Gao, C.; Liu, X.; Zhang, N.I.; Feng, X.; Liu, X.; Shen, X.; Tang, X. Evaluations of physicochemical and biological properties of pullulan-based films incorporated with cinnamon essential oil and Tween 80. Int. J. Biol. Macromol. 2019, 122, 388–394. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Dubey, N.K. Fabrication, characterization, and bioactivity assessment of chitosan nanoemulsion containing allspice essential oil to mitigate Aspergillus flavus contamination and aflatoxin B1 production in maize. Food Chem. 2022, 372, 131221. [Google Scholar] [CrossRef]
- Harleen, K.; Pranav, P.; Ramneek, K.; Shriya, A.; Manisha, S. Synthesis and characterization of Citrus limonum essential oil based nanoemulsion and its enhanced antioxidant activity with stability for transdermal application. J. Biomater. Nanobiotechnology 2020, 11, 215–236. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Y.; Chen, L. Enhanced emulsifying properties of wood-based cellulose nanocrystals as Pickering emulsion stabilizer. Carbohydr. Polym. 2017, 169, 295–303. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- do Vale Morais, A.R.; do Nascimento Alencar, É.; Júnior, F.H.X.; De Oliveira, C.M.; Marcelino, H.R.; Barratt, G.; Fessi, H.; Do Egito, E.S.T.; Elaissari, A. Freeze-drying of emulsified systems: A review. Int. J. Pharm. 2016, 503, 102–114. [Google Scholar] [CrossRef] [PubMed]
- El-Messery, T.M.; Altuntas, U.; Altin, G.; Özçelik, B. The effect of spray-drying and freeze-drying on encapsulation efficiency, in vitro bioaccessibility and oxidative stability of krill oil nanoemulsion system. Food Hydrocoll. 2020, 106, 105890. [Google Scholar] [CrossRef]
- Alarcón-Moyano, J.; Acuña, D.; Matiacevich, S.; Caballero, L.; Melo, F.; Quero, F.; Díaz-Calderón, P. Physico-chemical and structural characterization of cellulose nanocrystals obtained by two drying methods: Freeze-drying and spray-drying. Food Hydrocoll. 2023, 140, 108571. [Google Scholar] [CrossRef]
- Khoshkava, V.; Kamal, M.R. Effect of drying conditions on cellulose nanocrystal (CNC) agglomerate porosity and dispersibility in polymer nanocomposites. Powder Technol. 2024, 261, 288–298. [Google Scholar] [CrossRef]
- Abdallah, W.; Kamal, M.R. Influence of process variables on physical characteristics of spray freeze dried cellulose nanocrystals. Cellulose 2018, 25, 5711–5730. [Google Scholar] [CrossRef]
- Liu, L.; Abiol, K.A.; Friest, M.A.; Fisher, K.D. Synergistic stabilization of nanoemulsion using nonionic surfactants and salt-sensitive cellulose nanocrystals. Polymers 2023, 15, 4682. [Google Scholar] [CrossRef]
- Liu, L.; Fisher, K.D.; Bussey, W.D. Comparison of emulsion stabilizers: Application for the enhancement of the bioactivity of lemongrass essential oil. Polymers 2024, 16, 415. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Sánchez-Moreno, C.; Saura-Calixto, F. Evaluation of free radical scavenging of dietary carotenoids by the stable radical 2, 2-diphenyl-1-picrylhydrazyl. J. Sci. Food Agric. 2000, 80, 1686–1690. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, A.; Savic, S.; Vuleta, G.; Mueller, R.H.; Keck, C.M. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): Effects on size, physical stability and particle matrix structure. Int. J. Pharm. 2011, 406, 163–172. [Google Scholar] [CrossRef]
- Farouil, L.; Dias, R.P.; Popotte-Julisson, G.; Bibian, G.; Adou, A.I.; de la Mata, A.P.; Muriel, S.; James, J.H.; Cebrián-Torrejón, G. The Metabolomic Profile of the Essential Oil from Zanthoxylum caribaeum (syn. chiloperone) Growing in Guadeloupe FWI using GC× GC-TOFMS. Metabolites 2022, 12, 1293. [Google Scholar] [CrossRef]
- Bashlouei, S.G.; Karimi, E.; Zareian, M.; Oskoueian, E.; Shakeri, M. Heracleum persicum essential oil nanoemulsion: A nanocarrier system for the delivery of promising anticancer and antioxidant bioactive agents. Antioxidants 2022, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- de Godoi, S.N.; Quatrin, P.M.; Sagrillo, M.R.; Nascimento, K.; Wagner, R.; Klein, B.; Ourique, A.F. Evaluation of stability and in vitro security of nanoemulsions containing Eucalyptus globulus oil. BioMed Res. Int. 2017, 1, 2723418. [Google Scholar] [CrossRef]
- Pinto, L.D.A.; Machado, F.P.; Esteves, R.; Farias, V.M.; Köptcke, F.B.N.; Ricci-Junior, E.; Rocha, L.; Keller, L.A.M. Characterization and inhibitory effects of essential oil and nanoemulsion from Ocotea indecora (Shott) Mez in Aspergillus species. Molecules 2023, 28, 3437. [Google Scholar] [CrossRef]
- Soliman, W.S.; Salaheldin, S.; Amer, H.M. Chemical Composition Evaluation of Egyptian Lemongrass, Cymbopogon citratus, Essential Oil. Int. J. Sci. Eng. Res. 2017, 8, 630–634. [Google Scholar]
- Hanaa, A.M.; Sallam, Y.I.; El-Leithy, A.S.; Aly, S.E. Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods. Ann. Agric. Sci. 2012, 57, 113–116. [Google Scholar] [CrossRef]
- Silva, C.D.B.D.; Guterres, S.S.; Weisheimer, V.; Schapoval, E.E. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz. J. Infect. Dis. 2008, 12, 63–66. [Google Scholar]
- Tang, X.; Shao, Y.L.; Tang, Y.J.; Zhou, W.W. Antifungal activity of essential oil compounds (geraniol and citral) and inhibitory mechanisms on grain pathogens (Aspergillus flavus and Aspergillus ochraceus). Molecules 2018, 23, 2108. [Google Scholar] [CrossRef] [PubMed]
- Aw, Y.Z.; Lim, H.P.; Low, L.E.; Singh, C.K.S.; Chan, E.S.; Tey, B.T. Cellulose nanocrystal (CNC)-stabilized Pickering emulsion for improved curcumin storage stability. Lwt 2022, 159, 113249. [Google Scholar] [CrossRef]
- Anggraeni, N.I.; Hidayat, I.W.; Rachman, S.D.; Ersanda. Bioactivity of essential oil from lemongrass (Cymbopogon citratus Stapf) as antioxidant agent. AIP Conf. Proc. 2018, 1927, 030007. [Google Scholar] [CrossRef]
- Mirghani, M.E.S.; Liyana, Y.; Parveen, J. Bioactivity analysis of lemongrass (Cymbopogan citratus) essential oil. Int. Food Res. J. 2012, 19, 569–575. [Google Scholar]
- Pérez-Rosés, R.; Risco, E.; Vila, R.; Peñalver, P.; Cañigueral, S. Antioxidant activity of Tween-20 and Tween-80 evaluated through different in-vitro tests. J. Pharm. Pharmacol. 2015, 67, 666–672. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, L.; Wang, W.; Zeng, W.; Mustapha, A.; Lin, M. Soy protein-based films incorporated with cellulose nanocrystals and pine needle extract for active packaging. Ind. Crops Prod. 2018, 112, 412–419. [Google Scholar] [CrossRef]
- Physical Properties of Pure Methanol. Available online: https://www.methanol.org/wp-content/uploads/2016/06/Physical-Properties-of-Pure-Methanol.pdf (accessed on 27 August 2025).
- Ali, M.M.; Yusuf, M.A.; Abdalaziz, M.N. GC-MS Analysis and Antimicrobial Screening of Essential Oil from Lemongrass (Cymbopogon citratus). Int. J. Pharm. Chem. 2017, 3, 72–76. [Google Scholar] [CrossRef]
- Praveen, T.; Krishnamoorthy, A.S.; Nakkeeran, S.; Sivakumar, U.; Amirtham, D. Antifungal volatiles from medicinal herbs suppress Fusarium oxysporum f. sp. lycopersici. J. Entomol. Zool. Stud. 2021, 9, 1083–1093. [Google Scholar]
- Altameme, H.J.M. GC-MS and FTIR analysis Phytocomponents on different parts of Capparis spinosa L. (Capparidaceae) in Iraq. J. Chem. Pharm. Sci. 2016, 4, 3269–3282. [Google Scholar]
- Pal, A.; Ray, R.; Acharya, K.; Paul, S. Assessment of the anti-leukemic and antioxidant potential of the methanol extract of a wild, edible, and novel mushroom, Astraeus hygrometricus, and unraveling its metabolomic profile. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 388–404. [Google Scholar] [CrossRef]
- Lu, W.C.; Huang, D.W.; Wang, C.C.; Yeh, C.H.; Tsai, J.C.; Huang, Y.T.; Li, P.H. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J. Food Drug Anal. 2018, 26, 82–89. [Google Scholar] [CrossRef]
- Bazana, M.T.; da Silva, S.S.; Codevilla, C.F.; de Deus, C.; Lucas, B.N.; Ugalde, G.A.; Mazutti, M.A.; Flores, E.M.M.; Barin, J.S.; da Silva, C.D.B.; et al. Development of nanoemulsions containing Physalis peruviana calyx extract: A study on stability and antioxidant capacity. Food Res. Int. 2019, 125, 108645. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Choi, S.J.; Alamed, J.; Henson, L.; Popplewell, M.; McClements, D.J.; Decker, E.A. Citral stability in oil-in-water emulsions with solid or liquid octadecane. J. Agric. Food Chem. 2010, 58, 533–536. [Google Scholar] [CrossRef]
- Irmak, S.; Solakyildirim, K.; Hesenov, A.; Erbatur, O. Study on the stability of supercritical fluid extracted rosemary (Rosmarinus offcinalis L.) essential oil. J. Anal. Chem. 2010, 65, 899–906. [Google Scholar] [CrossRef]
- Aguirre-Becerra, H.; Pineda-Nieto, S.A.; García-Trejo, J.F.; Guevara-González, R.G.; Feregrino-Pérez, A.A.; Álvarez-Mayorga, B.L.; Pastrana, D.M.R. Jacaranda flower (Jacaranda mimosifolia) as an alternative for antioxidant and antimicrobial use. Heliyon 2002, 6, e05802. [Google Scholar] [CrossRef]
- Ahmed, R.; Abdel-Rahman, A. Effect of Papaya Wastes on Quality Characteristics of Meat Burger. New Val. J. Agric. Sci. 2022, 2, 483–511. [Google Scholar] [CrossRef]
- Ahuchaogu, A.A.; Igara, C.E. Antibacterial, GC-MS and FTIR Chemical Profiling of Methanol Leaf Extract of Cymbopogon citratus Linn. ARC J. Pharm. Sci. 2011, 7, 1–9. [Google Scholar]
- Almalki, G.; Rabah, S.; Al-Faifi, Z.; Alharbi, A.; Sharma, M. Phytochemistry Screening, Antioxidant and Antimicrobial Activities of Euphorbia inarticulata Schweinf Plant Extract. Pharmacophore 2022, 13, 91–99. [Google Scholar] [CrossRef]
- Antwi-Agyakwa, A.K.; Yusuf, A.A.; Pirk, C.W.; Mohamed, S.A.; Ekesi, S.; Torto, B. Exploring non-host plant-based management strategy with lemongrass, garlic and guava volatiles for the African citrus triozid. J. Appl. Entomol. 2021, 145, 757–766. [Google Scholar] [CrossRef]
- Ayalew, A.A. Chromatographic and spectroscopic determination of solvent-extracted Lantana camara leaf oil. J. Int. Med. Res. 2020, 48, 300060520962344. [Google Scholar] [CrossRef]
- Begum, N.; Akhtar, K.; Ahanger, M.A.; Iqbal, M.; Wang, P.; Mustafa, N.S.; Zhang, L. Arbuscular mycorrhizal fungi improve growth, essential oil, secondary metabolism, and yield of tobacco (Nicotiana tabacum L.) under drought stress conditions. Environ. Sci. Pollut. Res. 2021, 28, 45276–45295. [Google Scholar] [CrossRef]
- Bennett, V.; Godwin, J. Proximate, Selected Metals and Metabolites Profile of Citrullus lanatus (Watermelon) Leaves. Int. J. Adv. Res. Chem. Sci. 2023, 10, 14–21. [Google Scholar] [CrossRef]
- Chairgulprasert, V.; Prasertsongskun, S.; Junpra-ob, S.; Sangjun, M. Chemical constituents of the essential oil, antioxidant and antibacterial activities from Elettariopsis curtisii Baker. Songklanakarin J. Sci. Technol. 2008, 30, 591–596. [Google Scholar]
- Chanthai, S.; Prachakoll, S.; Ruangviriyachai, C.; Luthria, D.L. Influence of extraction methodologies on the analysis of five major volatile aromatic compounds of citronella grass (Cymbopogon nardus) and lemongrass (Cymbopogon citratus) grown in Thailand. J. AOAC Int. 2012, 95, 763–772. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Xiao, J.; Liu, J.; Tang, N.; Zhou, A. Variations of volatile flavour compounds in finger citron (Citrus medica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2020, 138, 109717. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Fung, P.K.; Kim, J.S. Aroma impact components in commercial plain sufu. J. Agric. Food Chem. 2005, 53, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.A.; Singh, T.B. Composition of Essential Oil of Indigenous and Exotic Species of Lemongrass Growing in Manipur. Ann. Multidiscip. Res. Innov. Technol. 2023, 2, 58–64. [Google Scholar]
- Elsharkawy, E.R.; Abdallah, E.M.; Shiboob, M.H.; Alghanem, S. Phytochemical, antioxidant and antibacterial potential of Ducrosia anethifolia in northern border region of Saudi Arabia. J. Pharm. Res. Int. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Ghosh, S.; Derle, A.; Ahire, M.; More, P.; Jagtap, S.; Phadatare, S.D.; Patil, A.B.; Jabgunde, A.M.; Sharma, G.K.; Shinde, V.S.; et al. Phytochemical Analysis and Free Radical Scavenging Activity of Medicinal Plants Gnidia glauca and Dioscorea bulbifera. PLoS ONE 2012, 8, e82529. [Google Scholar] [CrossRef]
- Harlina, P.W.; Ma, M.; Shahzad, R.; Gouda, M.M.; Qiu, N. Effect of clove extract on lipid oxidation, antioxidant activity, volatile compounds and fatty acid composition of salted duck eggs. J. Food Sci. Technol. 2018, 55, 4719–4734. [Google Scholar] [CrossRef]
- Jaafar, F.M.; Osman, C.P.; Ismail, N.H.; Awang, K. Analysis of essential oils of leaves, stems, flowers and rhizomes of Etlingera elatior (Jack) RM Smith. Malays. J. Anal. Sci. 2007, 11, 269–273. [Google Scholar]
- Javaid, A.; Qudsia, H.; Khan, I.H.; Anwar, A.; Ferdosi, M.F. Antifungal activity of Senna occidentalis root extract against Macrophomina phaseolina and its GC-MS analysis. Pak. J. Weed Sci. Res. 2022, 28, 115–122. [Google Scholar] [CrossRef]
- Jisim, K.G.D.D.S.; Terbang, M. Analysis of essential oils of Etlingera sphaerocephala var. grandiflora by two-dimensional gas chromatography with time-of-flight mass spectrometry. Malays. J. Anal. Sci. 2010, 14, 32–40. [Google Scholar]
- Kamel, D.G.; Mansour, A.I.; El-Diin, M.A.N.; Hammam, A.R.; Mehta, D.; Abdel-Rahman, A.M. Using rosemary essential oil as a potential natural preservative during stirred-like yogurt making. Foods 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Kothiyal, G.; Singh, K.; Kishor, K.; Kamal, R.; Kunar, N. GC-MS Analysis For Determination of Bioactive Compounds of Phellinus Pectinatus: A Species of Wild Mushroom from Uttarakhand Himalaya, India. J. Chem. Health Risks 2024, 14, 2005–2013. [Google Scholar]
- Mahavirsing Dinore, J.; Shivaji Patil, H.; Farooqui, S.; Pradhan, V.; Farooqui, M. GC/MS and LC/MS phytochemical analysis of Vigna unguiculata L. Walp pod. Chem. Biodivers. 2023, 20, e202200048. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Rahmah, A.N.; Abdel-Megeed, A. Evaluation of some plant extracts for their antifungal and antiaflatoxigenic activities. J. Med. Plants Res. 2011, 5, 231–4238. [Google Scholar]
- Mathkoor, M.M.; Alkhfaji, E.N.; Oda, N.A. Using GC-MS Technology to Identify the Compounds Resulting from Mixing of Alcoholic Extracts of Some Medicinal Plants. J. Med. Chem. Sci. 2022, 6, 486–499. [Google Scholar]
- Mestour, S.; Meniai, A.H.; Melloul, S.; Louaer, M. Sustainability by extraction of bioactive compounds from onion seeds. Comptes Rendus Chimie 2024, 27 (Suppl. S3), 55–67. [Google Scholar] [CrossRef]
- Moni, J.N.R.; Adnan, M.; Tareq, A.M.; Kabir, M.I.; Reza, A.A.; Nasrin, M.S.; Capasso, R. Therapeutic potentials of syzygium fruticosum fruit (seed) reflected into an array of pharmacological assays and prospective receptors-mediated pathways. Life 2021, 11, 155. [Google Scholar] [CrossRef]
- Mwithiga, G.; Maina, S.; Muturi, P.; Gitari, J. Lemongrass (Cymbopogon flexuosus) agronomic traits, oil yield and oil quality under different agro-ecological zones. J. Agric. Food Res. 2022, 10, 100422. [Google Scholar] [CrossRef]
- Mwithiga, G.; Maina, S.; Muturi, P.; Gitari, J. Lemongrass (Cymbopogon flexuosus) growth rate, essential oil yield and composition as influenced by different soil conditioners under two watering regimes. Heliyon 2024, 10, e25540. [Google Scholar] [CrossRef] [PubMed]
- Ojewumi, M.E.; Adedokun, S.O.; Omodara, O.J.; Oyeniyi, E.A.; Taiwo, O.S.; Ojewumi, E.O. Phytochemical and antimicrobial activities of the leaf oil extract of Mentha spicata and its efficacy in repelling mosquito. Int. J. Pharm. Res. Allied Sci. 2017, 6, 17–27. [Google Scholar]
- Olayemi, R.F.; Jawonisi, I.O.; Samuel, J.A. Characterization and physico-chemical analysis of essential oil of Cymbopogon citratus leaves. Bayero J. Pure Appl. Sci. 2018, 11, 74–81. [Google Scholar] [CrossRef]
- Oso, B.J.; Boligon, A.A.; Oladiji, A.T. Metabolomic profiling of ethanolic extracts of the fruit of Xylopia aethiopica (Dunal) a. rich using gas chromatography and high-performance liquid chromatography techniques. J. Pharmacogn. Phytochem. 2018, 7, 2083–2090. [Google Scholar]
- Prakash, N.K.U.; Sripriya, N.S.; Raj, D.D.; Deepa, S.; Bhuvaneswari, S. Antioxidant potency and GC-MS composition of Origanum majorana Linn. Pak. J. Pharm. Sci. 2019, 32, 2117–2122. [Google Scholar] [PubMed]
- Selim, S.A.; Aziz, M.H.A.; Mashait, M.S.; Warrad, M.F. Antibacterial activities, chemical constitutes and acute toxicity of Egyptian Origanum majorana L., Peganum harmala L. and Salvia officinalis L. essential oils. Afr. J. Pharm. Pharmacol. 2013, 7, 725–735. [Google Scholar]
- Shruthi, C.N.; Kotresha, D. Chemical Profiling and in vitro Cytotoxic Properties of Themeda triandra Forssk. Against Human Bone Osteosarcoma Cell Line (MG-63). Asian J. Biol. Life Sci. 2024, 13, 83. [Google Scholar] [CrossRef]
- Singh, P.K.; Krishnan, N.; Raman, P. Quantification of hormones and profiling of metabolites from vegetative to reproductive growth phase shift in Lagenaria siceraria (Molina) Standl. Sci. Hortic. 2024, 323, 112464. [Google Scholar] [CrossRef]
- Steele, D.H.; Thornburg, M.J.; Stanley, J.S.; Miller, R.R.; Brooke, R.; Cushman, J.R.; Cruzan, G. Determination of styrene in selected foods. J. Agric. Food Chem. 1994, 42, 1661–1665. [Google Scholar] [CrossRef]
- Strelyaeva, A.V.; Kharitonova, A.G.; Vaskova, L.B.; Luferov, A.N.; Bokov, D.O.; Bondar, A.A.; Bobkova, N.V.; Jeremic, N.; Lazareva, Y.B.; Antsyshkina, A.M.; et al. Research on External Signs and Chemical Composition of Medicinal Plant Raw Material-Leaves of Ficus Elastica. Pharmacogn. J. 2022, 14, 958–972. [Google Scholar] [CrossRef]
- Tavanappanavar, A.N.; Mulla, S.I.; Seth, C.S.; Bagewadi, Z.K.; Rahamathulla, M.; Ahmed, M.M.; Farhana, S.A. Phytochemical analysis, GC–MS profile and determination of antibacterial, antifungal, anti-inflammatory, antioxidant activities of peel and seeds extracts (chloroform and ethyl acetate) of Tamarindus indica L. Saudi J. Biol. Sci. 2024, 31, 103878. [Google Scholar] [CrossRef]
- Ulqodry, T.Z.; Nugroho, R.Y.; Khotimah, N.N.; Putri, W.A.E.; Aryawati, R.; Mohamed, C.A.R. Insecticidal Activity and Phytochemical Profiles of Avicennia marina and Excoecaria agallocha Leaves Extracts. Indones. J. Mar. Sci. 2023, 28, 148–160. [Google Scholar]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Zárate-Reyes, J.A.; Cruz-Durán, R.; Lozoya-Gloria, E. Volatile composition and biological activities of the leaf essential oil from Zanthoxylum limoncello grown in Oaxaca, México. Chem. Biodivers. 2019, 16, e1800498. [Google Scholar] [CrossRef]
- Vintisen, R.; Krishnan, K.T.; Veloo, K.V.; Rajandas, H.; Parimannan, S.; Ravichandran, M. Gas chromatography-mass spectrometry (GC-MS) analysis of phytocompounds isolated from Hydnophytum formicarum Jack tuber extracts. J. Trop. Resour. Sustain. Sci. 2024, 12, 36–46. [Google Scholar] [CrossRef]
- Yan, L.; Xie, Y.; Wang, Y.; Zhu, J.; Li, M.; Liu, X.; Zhao, D. Variation in contents of active components and antibacterial activity in different parts of Thunb. Asian Biomed. 2020, 14, 19–26. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Cantrell, C.L.; Astatkie, T.; Cannon, J.B. Lemongrass productivity, oil content, and composition as a function of nitrogen, sulfur, and harvest time. Agron. J. 2011, 103, 805–812. [Google Scholar] [CrossRef]


| Parameter | Result |
|---|---|
| Zeta Potential (mV) | −30.6 ± 6.3 |
| Particle Size (nm) | 158.4 ± 34.9 |
| Polydispersity Index | 0.6 ± 0.2 |
| Essential Oil Recovery Percentage (%) | 68.7 ± 12.5 |
| Compound | Retention Time (min) | Relative Percentage (%) |
|---|---|---|
| cis-Citral | 7.172 | 30.00 |
| Geraniol | 7.295 | 2.14 |
| trans-Citral | 7.433 | 43.20 |
| Geranyl acetate | 8.417 | 11.65 |
| Caryophyllene | 8.996 | 6.13 |
| γ-Muurolene | 9.736 | 4.98 |
| 10,13-octadecadiynoic acid, methyl ester | 9.789 | 1.90 |
| Compound | Retention Time (min) | Relative Percentage (%) |
|---|---|---|
| Limonene oxide | 6.578 | 0.783 |
| cis-Citral | 7.174 | 18.799 |
| Geraniol | 7.295 | 0.680 |
| trans-Citral | 7.439 | 25.644 |
| Geranyl acetate | 8.416 | 7.702 |
| Caryophyllene | 8.998 | 3.170 |
| γ-Muurolene | 9.742 | 2.454 |
| Cadina-1(10),4-diene | 9.785 | 1.562 |
| Caryophyllene oxide | 10.341 | 1.631 |
| (1) | 11.683 | 1.832 |
| (2) | 12.818 | 0.884 |
| Methyl 5,8,11-heptadecatrienoate | 13.052 | 3.181 |
| 1,8,15,22-Tricosatetrayne | 13.079 | 6.815 |
| (3) | 13.45 | 3.460 |
| (4) | 13.712 | 0.883 |
| α-N-Normethadol | 14.046 | 1.485 |
| (5) | 14.806 | 1.812 |
| (6) | 15.536 | 1.534 |
| W-18 | 16.476 | 16.474 |
| Day | Condition | Cis-Citral Concentration (ppm) | Trans-Citral Concentration (ppm) | Geraniol Concentration (ppm) | Geranyl Acetate Concentration (ppm) |
|---|---|---|---|---|---|
| 0 | Day 0 | 1427.16 | 3804.20 | 898.81 | 107.41 |
| 7 | No light | 582.21 | 1674.69 | 557.66 | 111.76 |
| 14 | No light | 207.38 | 679.18 | 128.31 | 60.45 |
| 21 | No light | 82.98 | 169.50 | 0 | 0 |
| 30 | No light | 35.52 | 75.10 | 0 | 0 |
| 7 | Light | 51.35 | 62.43 | 0 | 17.96 |
| 14 | Light | 9.36 | 4.60 | 0 | 0 |
| 21 | Light | 0 | 0 | 0 | 0 |
| 30 | Light | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, K.D.; Liu, L. Effect of Storage Conditions on the Composition and Bioactivity of Freeze-Dried Lemongrass Oil Nanoemulsions Stabilized by Salt-Sensitive Cellulose Nanocrystals and Tween 80. Processes 2025, 13, 2752. https://doi.org/10.3390/pr13092752
Fisher KD, Liu L. Effect of Storage Conditions on the Composition and Bioactivity of Freeze-Dried Lemongrass Oil Nanoemulsions Stabilized by Salt-Sensitive Cellulose Nanocrystals and Tween 80. Processes. 2025; 13(9):2752. https://doi.org/10.3390/pr13092752
Chicago/Turabian StyleFisher, Kaleb D., and Lingling Liu. 2025. "Effect of Storage Conditions on the Composition and Bioactivity of Freeze-Dried Lemongrass Oil Nanoemulsions Stabilized by Salt-Sensitive Cellulose Nanocrystals and Tween 80" Processes 13, no. 9: 2752. https://doi.org/10.3390/pr13092752
APA StyleFisher, K. D., & Liu, L. (2025). Effect of Storage Conditions on the Composition and Bioactivity of Freeze-Dried Lemongrass Oil Nanoemulsions Stabilized by Salt-Sensitive Cellulose Nanocrystals and Tween 80. Processes, 13(9), 2752. https://doi.org/10.3390/pr13092752






