Mechanochemical Loading of Doxorubicin on the Surface of Magnesium and Zinc-Based Layered Double Hydroxides
Abstract
1. Introduction
2. Materials and Methods
2.1. Magnesium (MgAl-NO3) and Zinc (ZnAl-CO3) Layered Double Hydroxides
2.2. Loading Attempt by Anion Exchange
2.3. Loading Attempt by Co-Precipitation
2.4. Loading Attempt by Mechanochemical Reaction
2.5. Solid-State Characterization
3. Results and Discussion
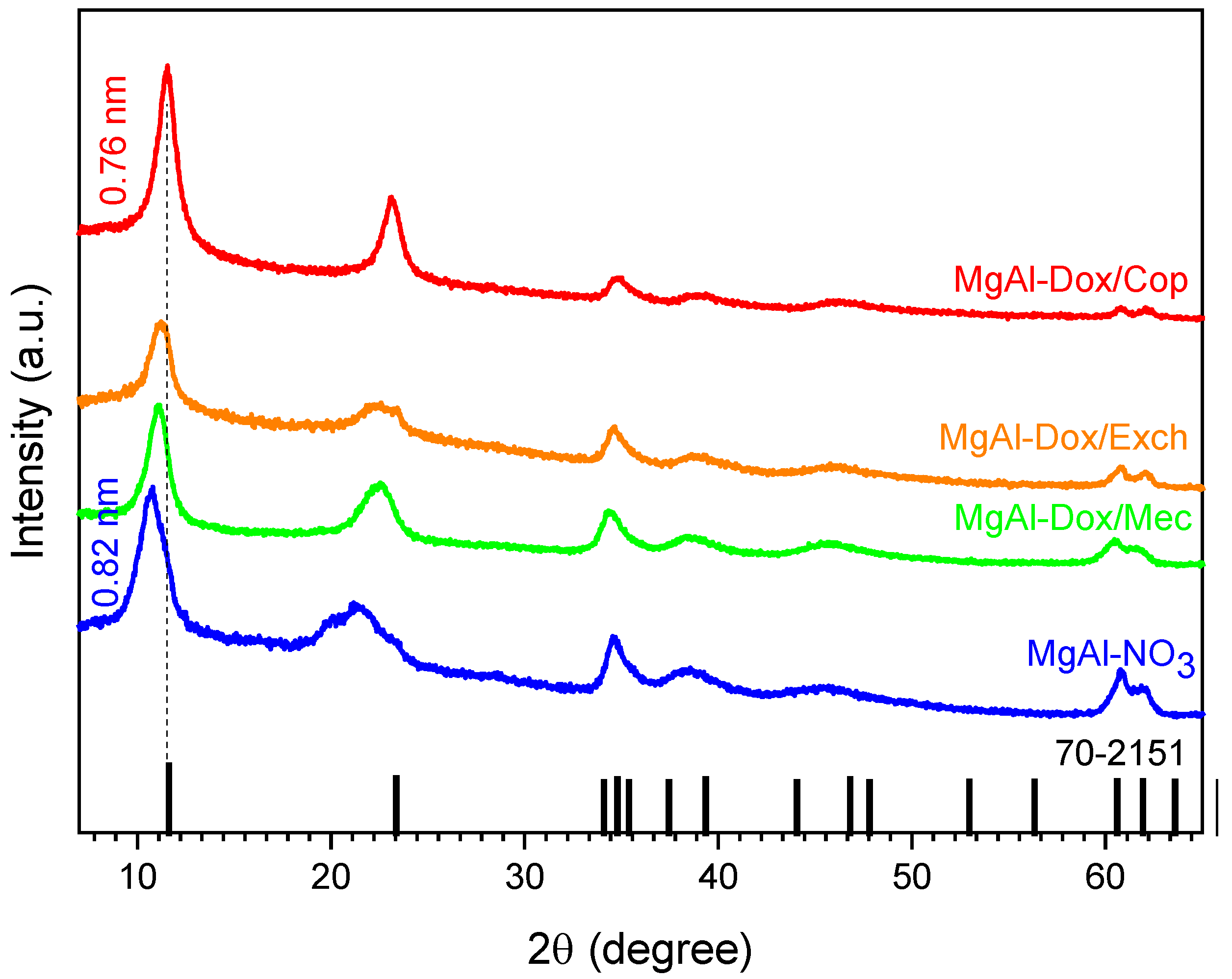
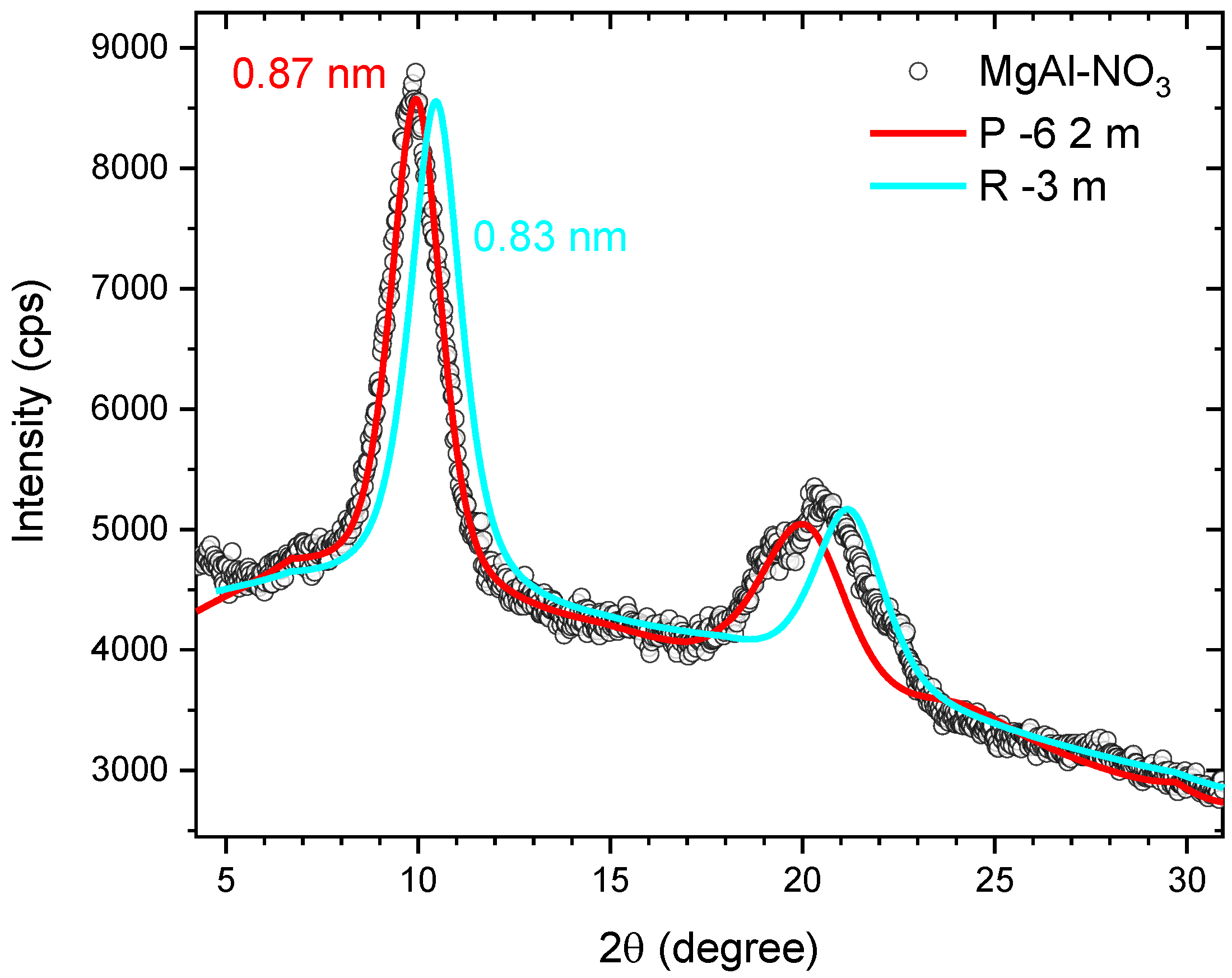
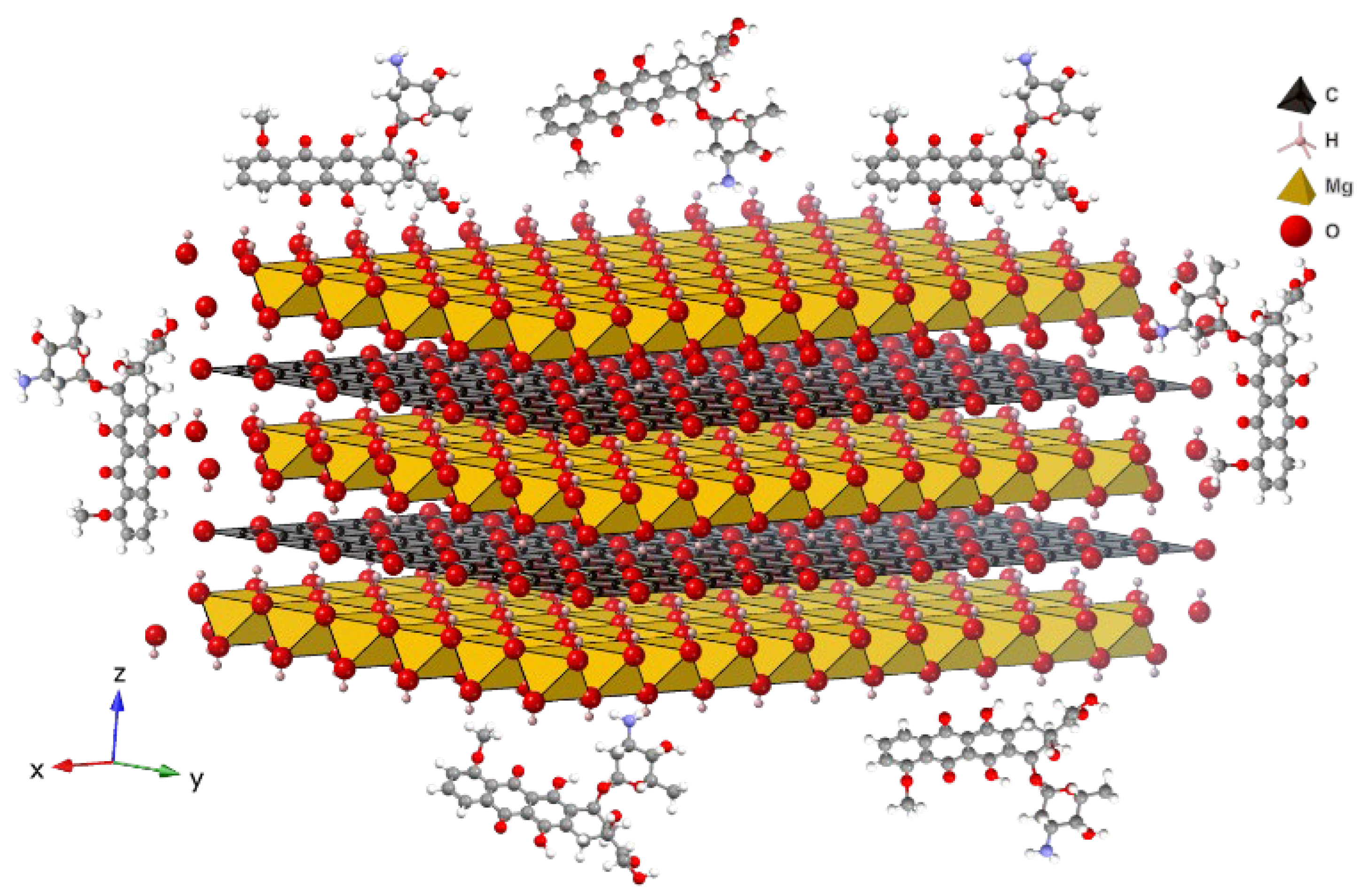
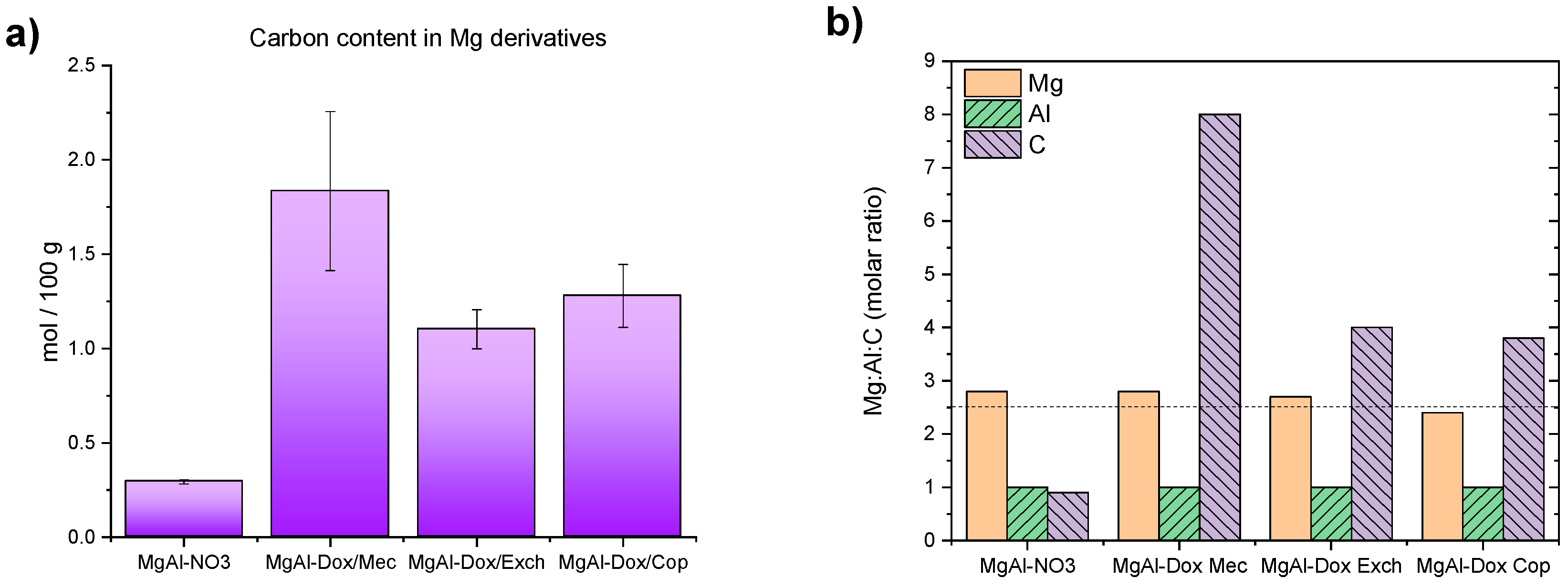
3.1. Magnesium-Based LDH Loaded with DOX
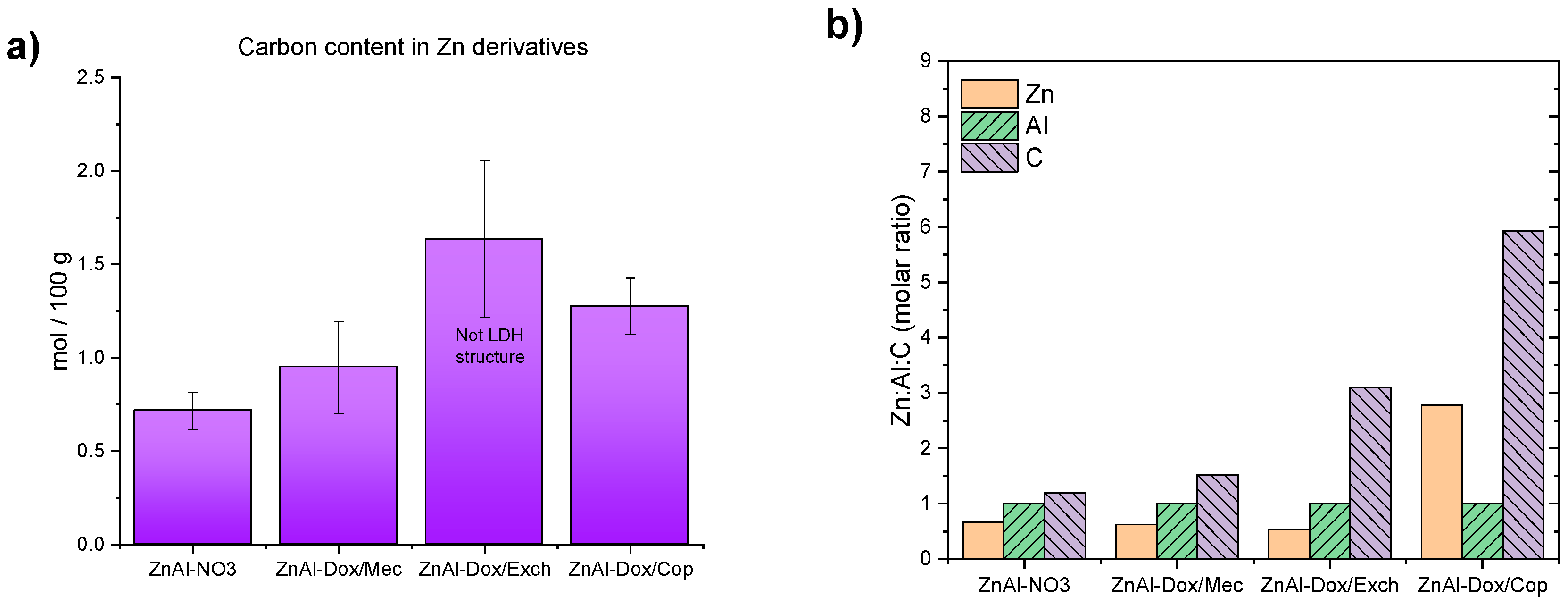
3.2. Zinc-Based LDH Loaded with DOX
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Liu, D.; Ai, H.; Chang, Q.; Liu, D.; Xia, Y.; Liu, S.; Peng, N.; Xi, Z.; Yang, X. Biological evaluation of layered double hydroxides as efficient drug vehicles. Nanotechnology 2010, 21, 105101. [Google Scholar] [CrossRef]
- Choi, G.; Piao, H.; Aalothman, Z.; Vinu, A.; Yun, C.O.; Choy, J.H. Anionic clay as the drug delivery vehicle: Tumor targeting function of layered double hydroxide- methotrexate nanohybrid in C33A orthotopic cervical cancer model. Int. J. Nanomed. 2016, 11, 337–348. [Google Scholar] [CrossRef]
- Senapati, S.; Thakur, R.; Verma, S.P.; Duggal, S.; Mishra, D.P.; Das, P.; Shripathi, T.; Kumar, M.; Rana, D.; Maiti, P. Layered double hydroxides as effective carrier for anticancer drugs and tailoring of release rate through interlayer anions. J. Control. Release 2016, 224, 186–198. [Google Scholar] [CrossRef]
- Li, B.; He, J.; Evans, D.G.; Duan, X. Enteric-coated layered double hydroxides as a controlled release drug delivery system. Int. J. Pharm. 2004, 287, 89–95. [Google Scholar] [CrossRef]
- Kovalenko, V.; Kotok, V.; Murashevych, B. Layered double hydroxides as the unique product of target ionic construction for energy, chemical, foods, cosmetics, medicine and ecology applications. Chem. Rec. 2023, 24, e202300260. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Sekhar, V.C. Fabrication of functionalized layered double hydroxide/chitosan nanocomposite with dual responsive drug release for the targeted therapy of breast cancer. Eur. Polym. J. 2020, 139, 109993. [Google Scholar] [CrossRef]
- Huynh, K.K.; Tieu, A.K.; Lu, C.; Smillie, L.; Nguyen, C.; Pham, S.T. In-situ engineering catalytically active surfaces for tribocatalysis with layered double hydroxide nanoparticles. Carbon 2024, 228, 119324. [Google Scholar] [CrossRef]
- Pillai, S.K.; Kleyi, P.; de Beer, M.; Mudaly, P. Layered double hydroxides: An advanced encapsulation and delivery system for cosmetic ingredients—An overview. Appl. Clay Sci. 2020, 199, 105868. [Google Scholar] [CrossRef]
- Talwan, P.; Gautam, D.; Kumar, R.; Sharma, S.; Dhiman, S.; Gill, R.; Thakur, A.; Sharma, D.; Sharma, S.; Kumar, A. A review of the chemical composition, modification, and biomedical application of Ricinus communis. Indian J. Nat. Prod. Resour. 2024, 15, 21–42. [Google Scholar] [CrossRef]
- Ngew, E.; Phue, W.H.; Liu, Z.; George, S. Composite of layered double hydroxide with casein and carboxymethylcellulose as a white pigment for food application. Foods 2022, 11, 1120. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, X.; Zhu, Y.; Wang, Z.; He, X.; Wu, Z.; Xue, L.; Fan, W.; Huang, R.; Xu, Z.; et al. Immunomodulatory layered double hydroxide nanoparticles enable neurogenesis by targeting transforming growth factor-β receptor 2. ACS Nano 2021, 15, 2812–2830. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, E.E.; Ha, T.; Hamilton, A.R. Ibuprofen intercalation and release from different layered double hydroxides. Ther. Deliv. 2018, 9, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Perioli, L.; Blasi, F.; Bastianini, M.; Chiesi, C.; Cossignani, L. Optimisation of phenol extraction from wine using layered double hydroxides and technological evaluation of the bioactive-rich powder. Int. J. Food Sci. Technol. 2017, 52, 2582–2588. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Likholobov, V.A. Mechanochemical synthesis of layered double hydroxides as a promising method for the preparation of adsorbents and catalysts. Kinet. Catal. 2022, 63, 615–641. [Google Scholar] [CrossRef]
- Nomicisio, C.; Taviot-Guého, C.; Ruggeri, M.; Forano, C.; Vigani, B.; Viseras, C.; Rossi, S.; Sandri, G. Layered double hydroxides for biomedical purposes: Sustainable and green synthesis. Appl. Clay Sci. 2024, 258, 107480. [Google Scholar] [CrossRef]
- Li, L.; Soyhan, I.; Warszawik, E.; van Rijn, P. Layered double hydroxides: Recent progress and promising perspectives toward biomedical applications. Adv. Sci. 2024, 11, e2306035. [Google Scholar] [CrossRef]
- Bellotto, M.; Rebours, B.; Clause, O.; Lynch, J.; Bazin, D.; Elkaïm, E. A reexamination of hydrotalcite crystal chemistry. J. Phys. Chem. 1996, 100, 8527–8534. [Google Scholar] [CrossRef]
- Arakcheeva, A.V.; Pushcharovskii, D.Y.; Rastsvetaeva, R.K.; Atencio, D.; Lubman, G.U. Crystal structure and comparative crystal chemistry of Al2Mg4(OH)12(CO3)·3H2O, a new mineral from the hydrotalcite-manasseite group. Crystallogr. Rep. 1996, 41, 1024–1034. (In Russian) [Google Scholar]
- Soulé, S.; Durand, P.; El-Kirat-Chatel, S.; Quilès, F.; Carteret, C. Structural features and dynamic behaviour of the interlayer space of layered double hydroxide coatings. Mater. Today Chem. 2024, 35, 101897. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Q.; Hong, X.; Chen, J.; Liu, D. Synthesis of layered double hydroxides with nitrate and its adsorption properties of phosphate. Water Sci. Technol. 2021, 83, 100–110. [Google Scholar] [CrossRef]
- Wu, Q.; Olafsen, A.; Vistad, Ø.B.; Roots, J.; Norby, P. Delamination and restacking of a layered double hydroxide with nitrate as counter anion. J. Mater. Chem. 2005, 15, 4695. [Google Scholar] [CrossRef]
- Ram Reddy, M.K.; Xu, Z.P.; Lu, G.Q.; Diniz da Costa, J.C. Layered double hydroxides for CO2 capture: Structure evolution and regeneration. Ind. Eng. Chem. Res. 2006, 45, 7504–7509. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, J.; Zhang, C.; Duan, T.; Li, Y.; Zou, L.; Qian, G. Synthesis of layered double hydroxides with fermentation liquid of organic waste to extract short-chain fatty acids as a biodenitrification carbon source. ACS Sustain. Chem. Eng. 2017, 5, 9095–9101. [Google Scholar] [CrossRef]
- Khitous, M.; Salem, Z.; Halliche, D. Removal of phosphate from industrial wastewater using uncalcined MgAl-NO3 layered double hydroxide: Batch study and modeling. Desalination Water Treat. 2016, 57, 15920–15931. [Google Scholar] [CrossRef]
- Bansal, R.; Singh, R.; Kaur, K. Quantitative analysis of doxorubicin hydrochloride and arterolane maleate by mid IR spectroscopy using transmission and reflectance modes. BMC Chem. 2021, 15, 27. [Google Scholar] [CrossRef]
- Kabekkodu, S.N.; Dosen, A.; Blanton, T.N. PDF-5+: A comprehensive Powder Diffraction FileTM for materials characterization. Powder Diffr. 2024, 39, 47–59. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Li, Z.; Liu, Q.; Yang, P.; Jing, X.; Zhang, M. Design of magnetic and fluorescent Mg–Al layered double hydroxides by introducing Fe3O4 nanoparticles and Eu3+ ions for intercalation of glycine. Mater. Res. Bull. 2010, 45, 640–645. [Google Scholar] [CrossRef]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Dubadi, R.; Huang, S.D.; Jaroniec, M. Mechanochemical synthesis of nanoparticles for potential antimicrobial applications. Materials 2023, 16, 1460. [Google Scholar] [CrossRef]
- Penczner, S.H.; Kumar, P.; Patel, M.; Bouchard, L.-S.; Iacopino, D.; Patel, R. Innovations in mechanochemical synthesis: Luminescent materials and their applications. Mater. Today Chem. 2024, 39, 102177. [Google Scholar] [CrossRef]
- Alshimaysawee, S.; Obaid, R.F.; Al-Gazally, M.E.; Ramírez-Coronel, A.A.; Bathaei, M.S. Recent advancements in metallic drug-eluting implants. Pharmaceutics 2023, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Functional PEO layers on magnesium alloys: Innovative polymer-free drug-eluting stents. Surf. Innov. 2018, 6, 237–243. [Google Scholar] [CrossRef]
- Al-Hawary, S.I.S.; Habash, R.T.; Abosaooda, M.; Hjazi, A.; Saleh, E.A.M.; Hassan, Z.F.; Bathaei, M.S. TiO2/PEG as smart anticorrosion and drug-eluting platforms in inflammatory conditions. Heliyon 2024, 10, e25605. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Yarmand, B.; Sharifi-Asl, S.; Mozafari, M. Improved corrosion performance of biodegradable magnesium in simulated inflammatory condition via drug-loaded plasma electrolytic oxidation coatings. Mater. Chem. Phys. 2020, 239, 122003. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura Muñoz, M.G.; Lara Cerón, J.A.; Gallegos Saucedo, M.d.J.; Carbajal Arizaga, G.G. Mechanochemical Loading of Doxorubicin on the Surface of Magnesium and Zinc-Based Layered Double Hydroxides. Processes 2025, 13, 931. https://doi.org/10.3390/pr13040931
Ventura Muñoz MG, Lara Cerón JA, Gallegos Saucedo MdJ, Carbajal Arizaga GG. Mechanochemical Loading of Doxorubicin on the Surface of Magnesium and Zinc-Based Layered Double Hydroxides. Processes. 2025; 13(4):931. https://doi.org/10.3390/pr13040931
Chicago/Turabian StyleVentura Muñoz, Minerva Guadalupe, Jesús Alfredo Lara Cerón, Manuel de Jesús Gallegos Saucedo, and Gregorio Guadalupe Carbajal Arizaga. 2025. "Mechanochemical Loading of Doxorubicin on the Surface of Magnesium and Zinc-Based Layered Double Hydroxides" Processes 13, no. 4: 931. https://doi.org/10.3390/pr13040931
APA StyleVentura Muñoz, M. G., Lara Cerón, J. A., Gallegos Saucedo, M. d. J., & Carbajal Arizaga, G. G. (2025). Mechanochemical Loading of Doxorubicin on the Surface of Magnesium and Zinc-Based Layered Double Hydroxides. Processes, 13(4), 931. https://doi.org/10.3390/pr13040931






