1. Introduction
The hydroxyl radical (
•OH) is widely acknowledged as the most powerful oxidizing species in natural aquatic environments, with second-order rate constants ranging from 10
8 to 10
10 M
−1s
−1 for its reactions with diverse organic compounds—making it central to advanced oxidation processes (AOPs) [
1,
2]. In aqueous systems, one of the principal pathways for
•OH generation involves the oxidation of photochemically produced Fe(II) by hydrogen peroxide, a sequence known as the photo-Fenton process [
3]. This mechanism combines the photolysis of iron(III) complexes with subsequent Fenton-like reactions, and it is collectively referred to as the photo-Fenton system. Due to the widespread presence of iron in atmospheric waters such as fog, rain, and aerosol particles [
4,
5], the photo-Fenton pathway is considered a major source of in situ hydroxyl radical production in these environmental compartments [
6,
7].
Many publications [
8,
9,
10,
11] have documented the chemistry and photochemistry of Fe(II) and Fe(III) complexes in the presence of oxalic acid. In an aqueous solution, oxalic acid is speciated via two ionization processes (
pKa1 = 1.25,
pKa2 = 4.27), where the species H
2C
2O
4, HC
2O
4−, and C
2O
42− coexist in equilibrium. These species distribute as H
2C
2O
4 for pH < 1, C
2O
42− for pH > 4.5, and HC
2O
4− for 1.5 ≤ pH ≤ 4. In the presence Fe
3+ ions, oxalate can form three kinds of complexes with Fe(III), i.e., Fe(C
2O
4)
+, Fe(C
2O
4)
2−, and Fe(C
2O
4)
3−3 (Equations (4)–(6) of
Table 1), in different proportions depending on the pH and the Fe(III)-to-oxalate ratio (see
Figures S1 and S2 of the
Supplementary Materials) [
9,
12]. When the iron-to-oxalate ratio is one, the dominating species is Fe
III(C
2O
4)
+, but, at greater concentrations, oxalate generates species such as Fe
III(C
2O
4)
2− and Fe
III(C
2O
4)
33−, which are more photoactive than Fe
III(C
2O
4)
+ and Fe
III(OH)
2+ [
12]. According to Lee et al. [
13], the most stable iron oxalate complex is Fe
III(C
2O
4)
33−, and all Fe
3+ oxalate complexes are more stable than Fe
2+ oxalate complexes. Vincze and Papp [
14] measured quantum yields at 254 nm of 0, 1.18, and 1.60 for Fe
III(C
2O
4)
+, Fe
III(C
2O
4)
2−, and Fe
III(C
2O
4)
33−, respectively. With regard to their markedly different photoactivity, a diverse photoredox chemistry of the photo-Ferrioxalate system (PFS) is expected, with changes in Fe speciation caused by the rapid consumption of oxalate.
In the near UV (300–400 nm) and a small portion of visible spectra, the Fe
3+ oxalate and Fe
2+ oxalate complexes show enhanced molar absorption coefficients (
Figure S3) [
20]. These substances are an attractive alternative for enhancing the performance of standard Fenton processes, since they can operate in circumstances close to neutrality and have a greater quantum yield [
11]. The photolysis process and subsequent reactions in the photo-Ferrioxalate system are summarized in Equations (7)–(31) of
Table 1. The mechanism of the primary photolysis of Ferrioxalate is either excitation followed by photodissolution without electron transfer between iron and an oxalate ligand, or by an intramolecular ligand-to-metal charge transfer process (Equations (7) and (8) of
Table 1) [
21,
22], where both mechanisms will lead to the formation of a Fe(II) and C
2O
4•− radical anion, which decomposes ultrafastly to yield a CO
2•− radical anion according to Equation (10) of
Table 1 (
k10 = 2×10
6 s
−1). CO
2•− is a powerful reducing agent (
E0 = 1.8 V vs. NHE) that may react with another Ferrioxalate molecule or, alternatively, reduce O
2 to a superoxide anion (O
2•−) at a near-diffusion-controlled rate (Equation (12) in
Table 1,
k12 = 2.4×10
9 M
−1 s
−1) [
12]. The O
2•− may initialize a chain of reaction (Equations (13)–(21) of
Table 1), which end after the formation of a hydroxyl radical through the Fenton process (Equations (22) and (23) of
Table 1). In addition of the pollutant destruction (Equation (24) of
Table 1), the yielded
•OH may recombine and react with other existing species, depending on the reactant’s loadings and other operational circumstances (Equations (25)–(31) of
Table 1).
Despite the recognized efficiency of the photo-Ferrioxalate system under ultraviolet and visible light irradiation, most studies have primarily employed a batch system with UV-based light sources, which often involve higher energy consumption and operational costs with limited scalability. Light-emitting diodes (LEDs), by contrast, offer a cost-effective, energy-efficient, and environmentally friendly alternative, yet comparative assessments of their performance against traditional light sources (e.g., fluorescent lamps) remain scarce. Moreover, few investigations have focused on dual-irradiation configurations—combining internal and external light paths—or on how reactor configurations affect radical production and pollutant degradation. The roles of various influencing parameters, such as light intensity, reactant concentrations, flow rates, and matrix constituents (e.g., salts), also require deeper investigations to optimize and understand the PFS-based processes.
The present study aims to fill this knowledge gap by systematically evaluating the performance of a dual-reactor photo-Ferrioxalate system for the degradation of Toluidine Blue (TB), a model dye pollutant, under visible blue light. The system incorporates two photochemical reactors: one illuminated internally using a submerged fluorescent lamp (tube), and the other externally using LED panels. By decoupling and combining these two irradiation modes, this study investigates the effect of light source type and intensity on TB degradation efficiency. Key operational parameters—such as Fe(III) and oxalate concentrations, dye loading, solution pH, flow rate, external addition of H2O2, ligand type, and the presence of background electrolytes—are optimized. Furthermore, scavenger experiments were conducted to elucidate the nature of the reactive species involved, confirming the predominant role of hydroxyl radicals in the degradation process. Total Organic Carbon (TOC) analysis was also performed to gain insight into the extent of mineralization.
2. Material and Methods
2.1. Reagents
All solutions used in this study were prepared using distilled water, and all chemical reagents were of analytical grade and used without further purification. Toluidine Blue (TB; Class: phenothiazine dye, CAS No. 92-31-9), with a molecular weight of 373.97 g mol−1, was obtained from Sigma-Aldrich. Ferric chloride hexahydrate (FeCl3·6H2O), with a molecular weight of 270.32 g mol−1, was also supplied by Sigma-Aldrich, and was used as the Fe(III) source. Sodium oxalate (Na2C2O4), with a molecular weight of 133.99 g mol−1, served as the main complexing agent in the Ferrioxalate system. All other reagents—ethylenediaminetetraacetic acid (EDTA), sodium citrate, sodium acetate, sodium hydroxide (NaOH), sulfuric acid (H2SO4), various inorganic salts (NaCl, Na2SO4, NaNO3, NaNO2, KBr, Na2SO3), 2-propanol, and sodium azide (NaN3)—were of analytical grade and also purchased from Sigma-Aldrich.
2.2. Experimental Setup
The experimental configuration employed in this study is depicted in
Figure 1, and consists of a recirculating batch photochemical system equipped with two distinct photoreactors connected in series, each irradiated by visible blue light sources. The setup was specifically designed to evaluate the performance of Ferrioxalate photolysis for the degradation of TB, a representative persistent textile dye.
The operating solution (total volume: 500 mL) was stored in a 1 L glass reservoir and continuously circulated through the system using a peristaltic pump (Master Flex Console Drive 7520-47). The flowrate was precisely controlled and optimized to ensure uniform exposure and mixing throughout the system. The two photoreactors employed are described as follows:
Reactor 1 (R1): This reactor consists of a single-tube glass reactor with a diameter of 3 cm and a length of 45 cm. A fluorescent blue-light tube (12 W, Ø = 1 cm, length = 40 cm) was directly immersed into the center of the reactor, providing internal illumination. This lamp emits visible light primarily in the blue region (approx. 400–500 nm).
Reactor 2 (R2): LED-Based Double-Tube Reactor: R2 is a double-walled tubular reactor, composed of an inner tube (Ø = 1 cm) and an outer tube (Ø = 4 cm), both made of transparent glass. The reactive solution circulated through the annular space between the inner and outer tubes, while compressed air passed through the central inner tube to prevent overheating. The solution temperature increase did not exceed +3 °C above the ambient operating temperature (22–23 °C), a variation too small to influence the degradation results. External illumination is provided by two blue LED lamps (each 9 W; length = 30 cm; emissions: approx. 400–500 nm), which are tightly affixed to the outer surface of the reactor. This configuration ensures uniform external irradiation of the solution across the entire length of the reactor.
The circulation loop allows for the solution to be alternately exposed to internal and external light sources as it passes through both reactors. An air compressor, connected via a flowmeter, continuously supplies air to the system to enhance dissolved oxygen content during treatment. To ensure maximum light utilization within the system, the glass reservoir, the two photoreactors, and the connecting tubing were all covered with aluminum foils.
2.3. Procedures
Stock solutions were first prepared for the different components involved in the experiments. A TB stock solution (100 mg/L, natural pH), a Fe(III) solution (10 mM, pH 3), and an oxalate solution (20 mM, natural pH) were individually prepared by dissolving accurately weighed amounts of each compound in deionized water under magnetic stirring until complete dissolution was achieved. The pH of each solution was adjusted as required using either 0.1 M NaOH or 0.1 H2SO4. From the stock solutions, working solutions with the desired concentrations of TB, Fe(III), and oxalate were freshly prepared for each experiment by appropriate dilution. The total reaction volume was fixed at 500 mL, and the mixture was introduced into the system’s glass reservoir.
Before initiating the photoreaction, the solution containing TB, Fe(III), and oxalate was circulated through the two photoreactors for 10 min in the dark. This pre-circulation period allowed for sufficient time for the formation of the Fe(III) oxalate complexes, which are photoactive under visible light. During this phase, no irradiation was applied. Following this dark pre-equilibration, the light sources in both reactors (fluorescent in R1 and LEDs in R2) were switched on simultaneously, marking time zero (t = 0) for the kinetic study of dye degradation. To maintain oxygenated conditions throughout the experiment, the solution was continuously aerated using an air compressor connected to a flowmeter, allowing for air to be bubbled into the solution throughout the entire treatment. The air bubbling also served to mix and homogenize the stock solution.
At predetermined time intervals, 3 mL aliquots of the reaction solution were withdrawn from the reservoir using a syringe. The residual concentration of TB was determined by UV–Visible spectrophotometry. Absorbance measurements were performed using a JASCO V-730 UV-Vis spectrophotometer. A scan of the TB solution confirmed its maximum absorbance wavelength (λmax) at 626 nm in the visible region (Abs = 0.63 over pH 2–5). To quantify TB concentration from absorbance data, a calibration curve was established in accordance with Beer–Lambert’s law (A = εLC), where A is the absorbance, L is the optical path length (1 cm), C is the TB concentration in mg/L, and ε is the molar absorptivity. The slope of the calibration curve (εL) was determined to be 0.126 L·mg−1, which enabled direct calculation of TB concentrations during kinetic analyses for pH 2–5.
3. Results and Discussion
3.1. Preliminary Results
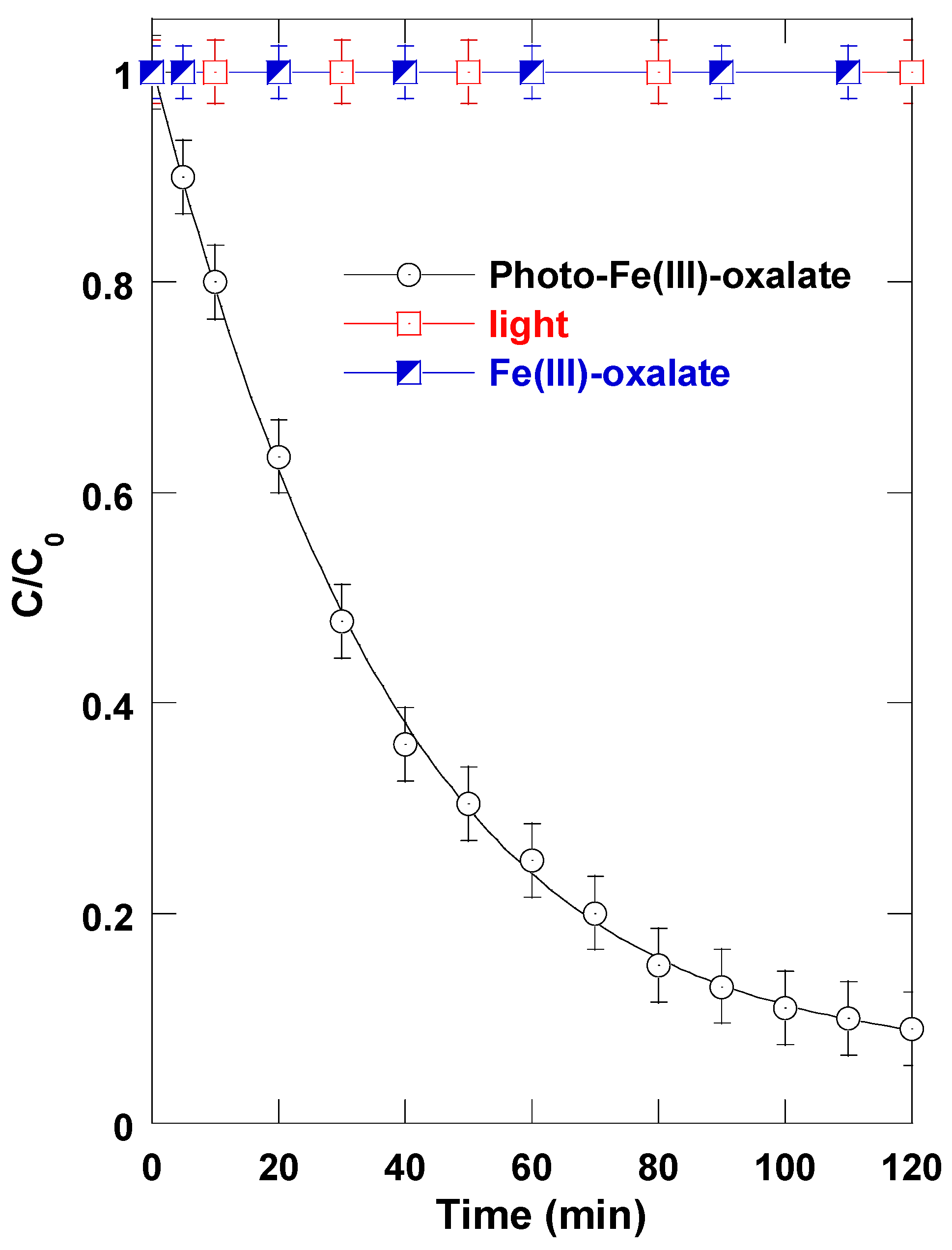
Initial experiments were conducted to evaluate the effectiveness of the visible-light-activated Ferrioxalate system in degrading TB. Aqueous TB solutions (C
0 = 5 mg L
−1) were treated under the experimental configuration shown in
Figure 1, using 0.1 mM Fe(III) and 1 mM oxalate at a circulating flowrate of 25 mL s
−1. As illustrated in
Figure 2, the TB concentration decreased exponentially with time, indicating that the degradation followed apparent pseudo-first-order kinetics. Specifically, 50% of the dye was removed within 28 min, and over 85% degradation was achieved after 120 min of treatment. Control experiments confirmed that neither visible-light irradiation alone nor Fe(III) oxalate complexes in the dark induced any measurable changes in TB concentration (
Figure 2). This highlights the necessity of simultaneous exposure to both light and the Fe(III) oxalate complexes to activate the degradation pathway. The observed degradation is therefore attributed to the photoactivation of Ferrioxalate complexes, which is known to generate highly reactive oxidizing species upon visible light absorption [
23]. As discussed in the introduction (paragraph 3), the proposed mechanism (outlined in
Table 1) involves the formation of
•OH as the principal reactive species.
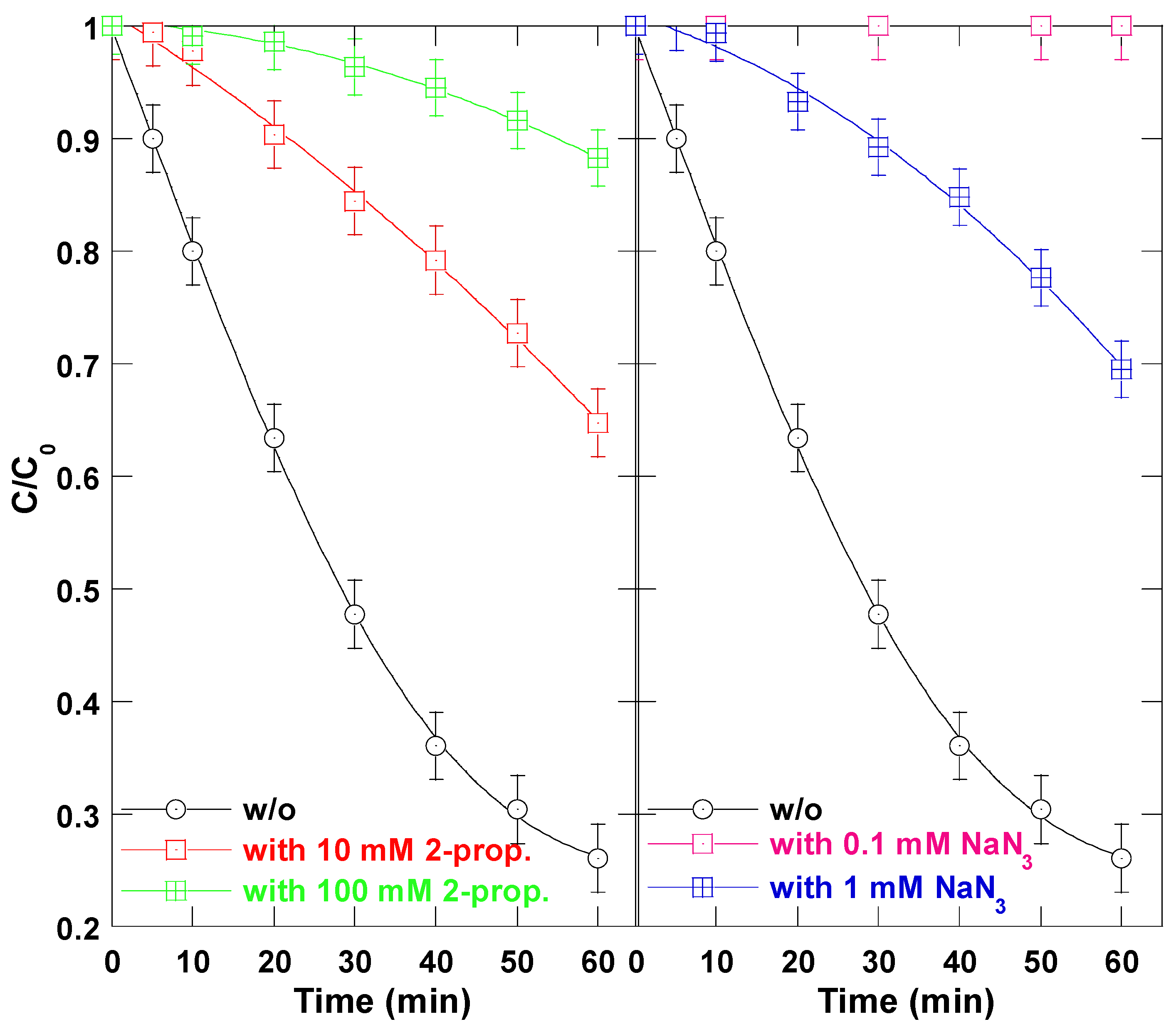
To confirm the involvement of
●OH radicals in the degradation process, 2-propanol and azide ions (NaN
3) were introduced into the reaction medium as selective hydroxyl radical scavengers. These scavengers exhibit high second-order rate constants with
•OH:
k2-prop-•OH = 1.9 × 10
9 M
1−s
−1 and
kN3−-•OH = 1.4 × 10
10 M
1−s
−1 [
17]. As shown in
Figure 3, the addition of these scavengers significantly suppressed the degradation efficiency. In the presence of 2-propanol, the TB removal after 60 min decreased from 74% (without scavenger) to 35% with 10 mM and 11% with 100 mM, respectively. Even more strikingly, the addition of only 1 mM NaN
3 resulted in the complete inhibition of the dye degradation process. These findings demonstrate that hydroxyl radicals were the dominant oxidative species responsible for the photodegradation of TB in the visible-light-driven Ferrioxalate system. The strong inhibitory effects of the radical scavengers confirm the critical role of
•OH radicals in the reaction mechanism.
3.2. Evaluation of Internal and External Irradiation Modes
To compare the performance of the two light sources integrated into the system and assess the effect of light intensity, a series of TB degradation experiments were conducted by selectively activating the photoreactors. Reactor 1 was equipped with a 12 W fluorescent tube lamp, delivering internal irradiation, while Reactor 2 featured two 9 W LED lamps (total power 18 W) providing external irradiation. Each reactor was operated individually and then in combination under identical experimental conditions: an initial TB concentration of 5 mg L
−1, 0.1 mM Fe(III), 1 mM oxalate, and a circulating flowrate of 25 mL s
−1. The results of these trials are presented in
Figure 4.
When operating the reactors individually, both configurations exhibited similar removal efficiencies, with approximately 45% TB degradation after 1 h of treatment. However, when both reactors were operated simultaneously, the removal efficiency significantly increased to approximately 74%, demonstrating a clear enhancement due to increased overall light intensity and dual irradiation modes. This enhanced performance is attributed to the faster photodecomposition of Fe(III) oxalate complexes under higher light exposure, which leads to a more rapid generation of reactive species—particularly •OH—in the aqueous phase. Both internal irradiation (fluorescent lamp) and external irradiation (LEDs) were effective in photoactivating the Ferrioxalate complexes, confirming the flexibility of the system in utilizing various light sources to drive the reaction.
Nevertheless, it should be noted that the combined operation of both reactors did not result in a synergistic or even additive effect. Specifically, the overall degradation yield (~75%) was lower than the sum of the individual reactor efficiencies (~45% + 45% = 90%). This behavior is likely due to the formation of degradation intermediates and by-products in Reactor 1, which may strongly compete with the remaining parent dye molecules for reactive radicals in Reactor 2. This effect is particularly pronounced, given that the dye concentration entering Reactor 2 is already significantly reduced due to prior degradation. Moreover, it is important to emphasize that increasing light intensity does not always produce a proportional improvement in degradation performance. At excessively high irradiation levels, a degradation limit may be reached, where the excessive generation of reactive radicals promotes undesirable radical–radical recombination reactions (e.g.,
•OH +
•OH → H
2O
2,
k31 = 5.5 × 10
9 M
−1 s
−1), i.e., a phenomenon largely reported in UV-based AOPs at higher radicals generation yield [
24,
25,
26]. These side reactions lower the effective concentration of radicals available to attack the pollutant, thereby reducing the overall efficiency of the process, despite the higher photon input.
3.3. Effect of Solution pH
The effect of initial solution pH on the photodegradation efficiency of TB was examined across the pH range of 2–5 under constant operating conditions: C
0 = 5 mg L
−1, [Fe(III)]
0 = 0.1 mM, [oxalate]
0 = 1 mM, and a recirculation flow rate of 25 mL s
−1. As shown in
Figure 5a, high and comparable degradation efficiencies were achieved at acidic pH values between 2 and 4, with approximately 75% of TB removed within 1 h of irradiation. The corresponding initial degradation rate was around 0.1 mg L
−1 min
−1 (
Figure 5b). However, a significant decline in dye degradation was observed at pH 5, where the removal efficiency dropped sharply to 22%, and the initial degradation rate decreased to 0.027 mg L
−1 min
−1, representing a ~70% reduction compared to the rate at lower pH. This trend clearly demonstrates the strong pH dependence of the photo-Ferrioxalate system, which can be attributed to both speciation and photoreactivity changes in Fe(III) oxalate complexes.
As detailed in the introduction, Fe(C
2O
4)
2− and Fe(C
2O
4)
33− are the most stable and photoactive complexes in the system [
27]. These species predominate between pH 2 and 4 over a broad range of Fe(III) and oxalate concentrations (
Figure S2a,b). Their photolysis (Reactions 7 and 8,
Table 1) proceeds via ligand-to-metal charge transfer (LMCT), generating the oxalate radical anion (C
2O
4•−), which further decomposes rapidly to form CO
2•− radicals. These reactive intermediates are key precursors for H
2O
2 generation via a cascade of reactions (Reactions 10 and 12–15,
Table 1), ultimately enabling
•OH formation through Fenton-type reactions (Reaction 22 and 23,
Table 1).
At pH values above 4, the concentration of Fe(C
2O
4)
2− declines sharply (
Figure S2b), leading to diminished photoreactivity and reduced radical production, thereby explaining the observed inhibition of TB degradation at pH 5 (
Figure 5). Similar pH-sensitive behavior has been reported by Kocar and Inskeep [
15] during photo-Ferrioxalate-mediated oxidation of As(III) using 18 µM Fe(III) and 1 mM oxalate under visible light (250 W halogen lamp, 300–500 nm). They observed a notable decrease in the oxidation rate from 255 µM h
−1 at pH 3 to 118 µM h
−1 at pH 5, which coincided with a drop in H
2O
2 production (861 µM h
−1 at pH 3 vs. 259 µM h
−1 at pH 5). Additional tests using excess 2-propanol (a hydroxyl radical scavenger) confirmed that
•OH was the primary oxidant responsible for As(III) oxidation under these conditions. Palmer and Sulzberger [
9] also investigated the pH-dependent degradation of atrazine in a photo-Ferrioxalate system (6 µM Fe, 0–180 µM oxalate) under simulated sunlight. They reported optimal atrazine degradation around pH 4, with hardly any inhibition at pH 7.5. Their mechanistic explanation involved two key pH effects: (i) The concentration of the highly photolyzable species Fe(C
2O
4)
2− and Fe(C
2O
4)
33− peaks around pH 4. (ii) The formation of Fe(II) oxalate complexes (Fe(II)(C
2O
4)), which react with H
2O
2 at a much higher rate (
k23 = 3.1 × 10
4 M
−1 s
−1, Reaction 23—
Table 1) than free Fe
2+ (
k22 = 53 M
−1 s
−1, Reaction 22—
Table 1), also increases with rising pH from 2 to 4, before declining thereafter. Therefore, maintaining the pH around 4 is perfect for maximizing the efficiency of the photo-Ferrioxalate system, as it ensures favorable iron speciation, optimal photoreactivity, and robust radical-mediated degradation pathways at relatively lowered H
+ concentrations.
3.4. Effect of Oxalate Dosage
The influence of oxalate initial concentration on the degradation of TB via the photo-Ferrioxalate process was assessed over the range of 0.2–20 mM at fixed conditions: [Fe(III)]
0 = 0.1 mM, pH 4, and a recirculating flow rate of 25 mL s
−1.
Figure 6a,b presents the initial degradation rate (r
0) and the time-dependent TB removal profiles under different oxalate dosages. An increase in oxalate concentration from 0.2 mM to 5 mM led to a progressive enhancement in the dye degradation performance. The initial degradation rate doubled from 0.061 mg L
−1 min
−1 to 0.125 mg L
−1 min
−1, while the dye removal efficiency after 60 min rose from 32% to 75%. This enhancement is attributed to the increased formation of photoactive Fe(III) oxalate complexes, mainly Fe(C
2O
4)
2− and Fe(C
2O
4)
33−, which absorb visible light and undergo ligand-to-metal charge transfer to generate oxalate radicals. These radicals subsequently led to H
2O
2 formation and Fenton-type reactions (as described in Reactions 7–15,
Table 1), contributing to hydroxyl radical generation.
The speciation diagram (
Figure S1a), constructed for 0.1 mM Fe(III) and up to 1 mM oxalate, confirms that Fe(C
2O
4)
2− and Fe(C
2O
4)
33− dominate under such conditions. However, the continuous increase in degradation efficiency up to 5 mM oxalate suggests that higher oxalate levels beyond 1 mM further shift the complexation equilibrium toward LMCT-active species, sustaining radical production. In contrast, when oxalate concentrations exceeded 5 mM, the degradation rate began to decline: r
0 dropped from 0.125 to 0.091 and 0.053 mg L
−1 min
−1 for 10 and 20 mM oxalate, respectively. This inhibition is likely due to the radical-scavenging effects caused by the excess oxalate and Fe(III) oxalate complexes. Specifically, Fe(C
2O
4)
2− and Fe(C
2O
4)
33− are known to quench key radical intermediates such as superoxide (O
2•−) and hydroperoxyl (HO
2•) radicals with second-order rate constants of 1.0 × 10
5 and 1.2 × 10
4 M
−1 s
−1, respectively (Equations (18) and (19),
Table 1). Additionally, Fe(C
2O
4)
33− can quench CO
2•− radicals (Reaction 11,
Table 1), while the oxalate itself could scavenge
•OH at a higher-rate constant (Equation (25) of
Table 1,
k25 = 7.7 × 10
6 M
−1 s
−1) Given that O
2•−, HO
2•, and CO
2•− act as precursors for H
2O
2 generation (Equations (12)–(15),
Table 1), their quenching leads to diminished H
2O
2 availability, thereby reducing hydroxyl radical formation via the photo-Fenton pathway. This mechanistic inhibition is consistent with the observed decline in TB degradation efficiency at oxalate dosages above 5 mM (
Figure 6).
3.5. Effect of Fe(III) Dosage
The impact of Fe(III)’s initial concentration on the degradation efficiency of TB was evaluated in the photo-Ferrioxalate system using 1 mM oxalate at pH 4. As illustrated in
Figure 7a,b, dye removal improved significantly with increasing Fe(III) concentration from 0.05 mM to 0.1 mM. Specifically, the degradation efficiency rose from ~42% to ~75% after 60 min of treatment, and the corresponding initial degradation rate (r
0) increased from 0.058 mg L
−1 min
−1 to 0.1 mg L
−1 min
−1. Beyond 0.1 mM Fe(III), however, further increases in iron concentration had a marginal effect. Although r
0 slightly increased to 0.11 mg L
−1 min
−1 at 0.3 mM, this change was not substantial compared to the value at 0.1 mM, suggesting diminishing returns. Therefore, 0.1 mM Fe(III) can be considered the optimal concentration for this system, offering effective dye degradation while minimizing reagent consumption.
The initial increase in degradation performance with Fe(III) concentration (0.05–0.1 mM) is attributed to the enhanced formation of photoreactive Fe(III) oxalate complexes—primarily Fe(C
2O
4)
2− and Fe(C
2O
4)
33−. These complexes absorb visible light and undergo ligand-to-metal charge transfer, leading to oxalate radical formation, H
2O
2 production, and subsequent
•OH generation. This is consistent with the oxalate effect discussed earlier (
Section 3.4).
At Fe(III) concentrations exceeding 0.1 mM, however, the degradation efficiency plateaued or slightly decreased. This may result from radical scavenging phenomena due to excess Fe(III) and its complexes. The speciation diagrams in
Figure S1 confirms that increasing Fe(III) from 0.1 to 0.4 mM (with 1 mM oxalate) raises the fraction of Fe(C
2O
4)
2− from 0.7 to 0.9, while Fe(C
2O
4)
33− drops from 0.4 to 0.15. This suggests that Fe(C
2O
4)
2− becomes the dominant species at elevated Fe(III), yet both Fe(C
2O
4)
2− and Fe(C
2O
4)
33− may act as radical scavengers when in excess. Indeed, these species are known to quench reactive oxygen species (ROS), particularly O
2•−, HO
2•, and CO
2•−, as shown in Reactions 11 and 18–19 (
Table 1). In addition, free Fe(III) can also directly quench O
2•− and HO
2•, yielding molecular oxygen (Reactions 16–17 in
Table 1) with even higher rate constants of
k16 = 1.5 × 10
8 M
−1 s
−1 and
k17 = 1.0 × 10
6 M
−1 s
−1. The collective scavenging of these radical species limits H
2O
2 accumulation and hydroxyl radical generation, thereby impairing the efficiency of the photo-Fenton pathway. This explains the observed plateau or slight decline in TB degradation at Fe(III) concentrations above 0.1 mM. Therefore, the optimal Fe(III) dosage of 0.1 mM offers a balance between complex formation and radical availability, maximizing the photo-Ferrioxalate system’s effectiveness while minimizing potential side reactions due to excess iron.
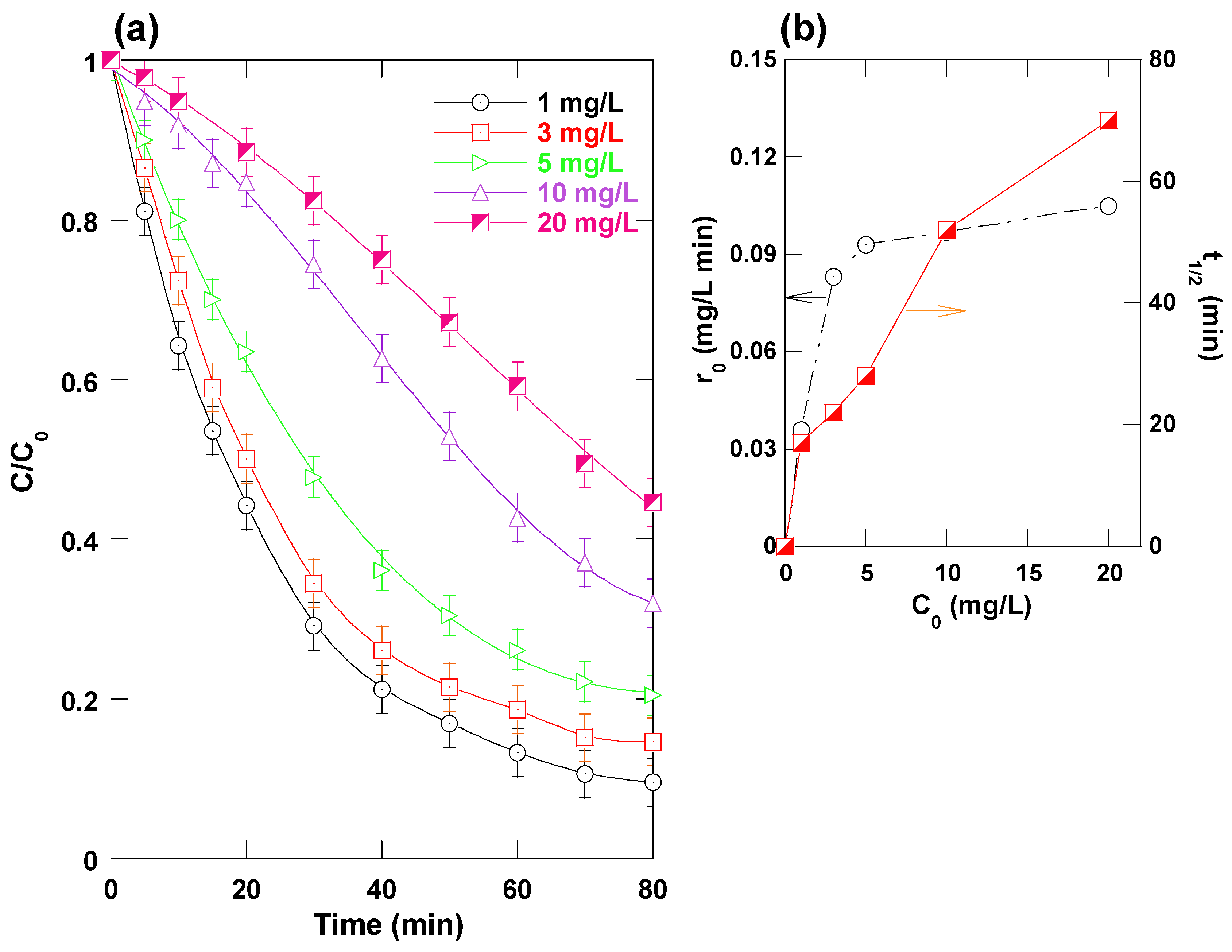
3.6. Effect of Dye Concentration
The influence of initial dye concentration (1–20 mg L
−1) on TB degradation efficiency and initial degradation rate (r
0) in the photo-Ferrioxalate system was investigated under fixed conditions: 0.1 mM Fe(III), 1 mM oxalate, pH 4, and a recirculating flow rate of 25 mL s
−1. The results are illustrated in
Figure 8a,b.
As shown in
Figure 8a, the overall removal efficiency decreased with increasing initial dye concentration. After 80 min of irradiation, the removal efficiencies were approximately 90%, 80%, 70%, and 55% for initial dye concentrations of 1, 5, 10, and 20 mg L
−1, respectively. In parallel, the half-reaction time (t
1/
2) markedly increased from 17 min at 1 mg L
−1 to 28, 52, and 70 min for 5, 10, and 20 mg L
−1, respectively. This decline in degradation efficiency at higher dye concentrations can be attributed to several interrelated factors. First, increased dye load leads to the accumulation of intermediate degradation products and by-products in the solution, which can act as additional scavengers of reactive oxygen species—particularly
•OH. This competitive consumption of
•OH reduces its availability for direct attack on the parent dye molecules, thereby slowing down the overall degradation process [
18,
24,
28,
29,
30].
On the other hand,
Figure 8b reveals that the initial degradation rate significantly increased with dye concentration, rising from 0.035 mg L
−1 min
−1 at 1 mg L
−1 to 0.092 and 0.10 mg L
−1 min
−1 at 5 and 20 mg L
−1, respectively. This trend is commonly observed in advanced oxidation processes (AOPs) [
18,
24,
28,
29,
30,
31] and can be explained by the constant generation rate of hydroxyl radicals under fixed operational conditions. At the early stages of reaction—when intermediate products are negligible—the higher pollutant concentration increases the likelihood of encounters between dye molecules and
•OH radicals, thereby enhancing the rate of degradation. Thus, while higher dye concentrations initially promote faster degradation due to increased reaction probability, the accumulation of by-products and competition for reactive species during the reaction adversely affects the overall removal efficiency and kinetics in the later stages.
3.7. Effect of Recirculating Flow Rate
The influence of the recirculating flow rate on the degradation of TB in the photo-Ferrioxalate process [0.1 mM Fe(III), 1 mM oxalate, pH 4] was examined over the range of 8.3–33.3 mL s
−1, with the results presented in
Figure 9a,b.
As shown, increasing the flow rate from 8.3 to 25 mL s−1 significantly enhanced the degradation performance. The initial degradation rate increased from 0.060 mg L−1 min−1 (corresponding to 62% removal at 60 min) at 8.3 mL s−1 to 0.100 mg L−1 min−1 (75% removal) at 25 mL s−1. However, further increasing the flow rate to 33.3 mL s−1 led to a decline in performance, with r0 dropping to 0.057 mg L−1 min−1 and only 60% dye removal after 60 min. This clearly identifies 25 mL s−1 as the optimal flow rate for this recirculating batch-loop photo-Ferrioxalate reactor system. The observed trend can be explained by the hydrodynamic influence of flow rate on photon utilization and residence time in the photoreactor.
At low flow rates (e.g., 8.3 mL s−1), the residence time of the dye solution within the illuminated zone is longer, but insufficient mixing and limited mass transport may restrict the exposure of Fe(III) oxalate complexes to photons, leading to lower rates of photolysis and radical generation. As the flow rate increases to 25 mL s−1, the solution experiences improved circulation and mixing. This enhances the uniform exposure of reactants to light, promotes more effective photodissociation of the Fe(III) oxalate complexes, and facilitates faster generation and distribution of reactive species (e.g., C2O4•− and •OH), resulting in improved degradation efficiency. However, at excessively high flow rates (e.g., 33.3 mL s−1), the residence time of the solution within the irradiated zone becomes too short, limiting the time available for light-driven activation of the complexes. Despite better mixing, this reduced photon contact time per volume element hampers photochemical efficiency, thus decreasing the overall degradation rate.
3.8. Effect of Dissolved Gas Type
The role of dissolved gases, specifically O
2 and N
2, was investigated to assess their influence on the photo-Ferrioxalate process efficiency for TB degradation. In this experiment, either O
2 or N
2 was continuously bubbled for 10 min prior to irradiation and maintained throughout the reaction. The results were compared to a control run under ambient air (control), using 0.1 mM Fe(III), 1 mM oxalate, pH 4, and a recirculating flow rate of 25 mL s
−1. The degradation performances under different gas conditions are presented in
Figure 10.
The results clearly show that oxygen plays a pivotal role in sustaining high degradation efficiency. After 60 min, TB removal reached ~75% under both oxygen-saturated and air-equilibrated conditions. In stark contrast, nitrogen saturation (i.e., oxygen-deprived conditions) drastically reduced the degradation efficiency to only ~20%. This drastic decline under nitrogen highlights the central role of O2 in the photo-Ferrioxalate system. Although ambient air already contains sufficient oxygen for the reaction to proceed effectively, complete depletion of O2—via N2 bubbling—disrupts key oxidative pathways.
Mechanistically, oxygen is essential for ROS formation and propagation. Upon photolysis of Fe(III) oxalate complexes, the CO
2•− radical is formed, which rapidly reduces O
2 to generate the O
2•− (Reaction 12,
Table 1,
k12 = 2.4 × 10
9 M
−1 s
−1). This step is critical because O
2•− is a precursor to H
2O
2, which in turn undergoes a reaction with Fe(II)/Fe
II(C
2O
4) to produce hydroxyl radicals (Equations (22) and (23),
Table 1). Therefore, in the absence of O
2, this cascade is interrupted, greatly limiting
•OH production and overall degradation performance. In conclusion, the experiment confirms that, while air is sufficient to sustain efficient photo-Ferrioxalate activity, the complete absence of O
2 significantly suppresses degradation, underlining the necessity of oxygen for ROS-mediated pollutant removal in this system.
3.9. Effect of Complexing Agent Type
To evaluate the specificity of oxalate as a ligand in the photo-Ferrioxalate system, alternative Fe(III) complexing agents—including citrate, acetate, and ethylenediaminetetraacetic acid (EDTA)—were tested under the same experimental conditions: 0.1 mM Fe(III), 1 mM ligand, pH 4, air bubbling, and a recirculating flow rate of 25 mL s
−1. The results, presented in
Figure 11, show substantial differences in degradation performance depending on the ligand used.
The removal efficiencies of TB after 60 min follow the order Oxalate (75%) > Citrate (25%) >> Acetate ≈ EDTA (0%). These results highlight the unique suitability of oxalate as a ligand for promoting the efficient photoactivation of Fe(III) complexes in the studied system. The superior performance of oxalate is attributed to its favorable photochemical properties, particularly its ability to undergo intramolecular ligand-to-metal charge upon visible irradiation [
22]. This photoreaction efficiently reduces Fe(III) to Fe(II) while generating CO
2•− radicals, which initiate the cascade of ROS formation leading to pollutant degradation. In contrast, EDTA and acetate complexes failed to induce any photoreactivity under the same conditions. This is likely due to their photochemical inertness or unfavorable LMCT dynamics, which prevent effective Fe(III) photoreduction and ROS generation. EDTA, despite forming very stable complexes with Fe(III), is known to inhibit photoredox cycling and acts more as a radical quencher than a ROS generator. Citrate, a weak Fe(III) chelator, showed only modest activity (~25% TB removal). While citrate-Fe(III) complexes may absorb some photons and undergo limited photoreduction, the process appears to be inefficient compared to oxalate. Furthermore, the complex structure and possible radical scavenging behavior of citrate may further suppress efficient ROS production.
Consequently, oxalate’s ability to stabilize Fe(III) in photoactive forms and undergo efficient LMCT makes it uniquely effective for driving the photo-Ferrioxalate process. The poor performance of other ligands underlines the need for careful ligand selection in designing photo-assisted AOP, especially in visible-light photo-recirculation systems.
3.10. Effect of Water Matrix Components
The influence of various inorganic salts—NaCl, Na
2SO
4, NaNO
3, NaNO
2, KBr, and Na
2SO
3—at concentrations ranging from 0.1 to 50 mM was assessed under standard photo-Ferrioxalate conditions (0.1 mM Fe(III), 1 mM ligand, pH 4, air bubbling, 25 mL s
−1 recirculation rate). As illustrated in
Figure 12, the degradation efficiency of TB varied significantly depending on the anion type, indicating both chemical reactivity (i.e., radical scavenging capacity) and impact on complexation equilibria [
11].
Table 2 provides some scavenging reaction between the water matrix anions and hydroxy radicals.
Chloride showed a minimal impact on degradation up to 20 mM; however, at higher concentrations, a decline in TB removal efficiency was observed (from 75% at 0 mM to 60% at 50 mM). This can be attributed to the scavenging of
•OH by Cl
− forming reactive chlorine species (e.g., Cl
•, Cl
2•−), which are less oxidative than hydroxyl radicals and prone to recombination and termination reactions (see Reactions 32–43,
Table 2). These secondary chlorine radicals may partially maintain oxidation, explaining the moderate decline.
The addition of sulfate ions (Na
2SO
4) displayed a minor influence at low concentrations, but significantly inhibited dye degradation at higher concentrations (50% removal at 50 mM). While sulfate itself is not a strong
•OH scavenger, its ionic strength effect can destabilize the Fe(III) oxalate complexes and alter photoreactive species formation, particularly Fe(C
2O
4)
33−, thereby impacting radical generation efficiency. However, Nitrate induced only a slight reduction in degradation efficiency. Although NO
3− can react with
•OH to form NO
3• (Reaction 44,
Table 2), the low reactivity and low-rate constant of this pathway result in minimal interference in the overall process.
A dramatic inhibitory effect was observed with NO
2−, even at low concentrations: TB removal dropped to 10% at 1 mM, and was completely suppressed at 50 mM. NO
2− is a potent scavenger of
●OH (
k45 = 1 × 10
10 M
−1 s
−1, Reaction 45—
Table 2), forming NO
2•, a weak oxidant. This reaction directly competes with dye oxidation and depletes reactive radicals, explaining the near-total inhibition. Br
− also significantly inhibited the process, but to a lesser extent than NO
2−. At 1 mM Br
−, the removal was 30%, decreasing to 10% at 50 mM. Br
− reacts with
•OH (k
46 = 1.1 × 10
10 M
−1 s
−1, Reaction 46—
Table 2), forming Br
• and Br
2•− (Reactions 47–53—
Table 2). These bromine radicals are relatively less reactive and more selective compared to
•OH, leading to reduced degradation capacity. Similarly, the presence of sulfite (Na
2SO
3) resulted in the immediate and complete suppression of TB degradation even at 0.1 mM. Sulfite is a very strong radical scavenger, reacting rapidly with •OH and superoxide, and also acts as a reducing agent, likely leading to Fe(III) reduction and decomposition of the photoactive Fe oxalate complexes.
Consequently, the observed impacts highlight the importance of water matrix composition when applying photo-Ferrioxalate systems in real-world wastewater settings. The nature and reactivity of the anion significantly influence the performance of the photo-Ferrioxalate process. Anions like NO2−, Br−, and SO32− act as strong radical scavengers, while Cl− and SO42− alter both radical chemistry and the Fe(III)-ligand complexation behavior. NO3−, by contrast, exerts negligible interference.
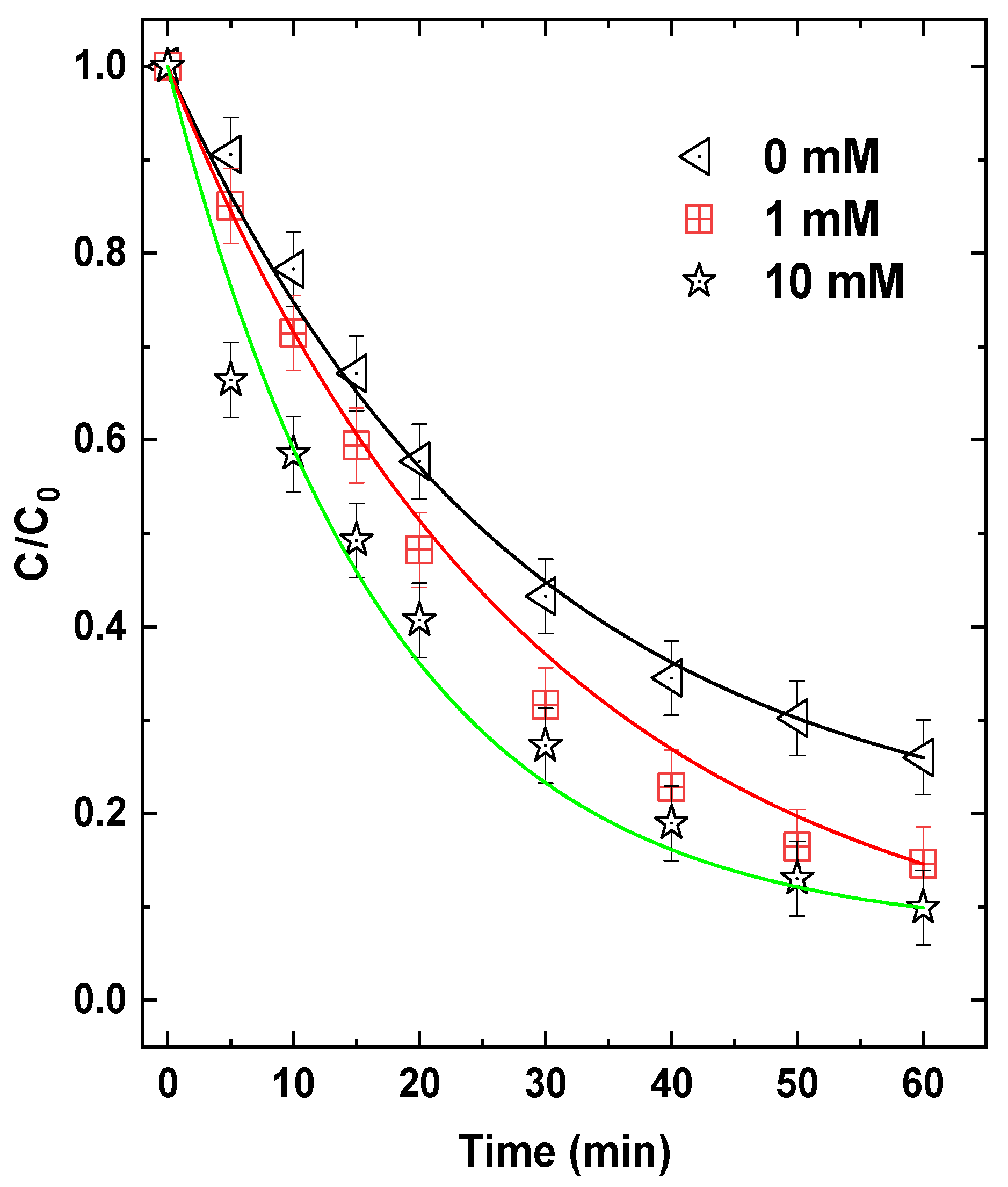
3.11. Effect of External Addition of H2O2
To evaluate the potential enhancement of the photo-Ferrioxalate process via the Fenton pathway, external H
2O
2 was added at concentrations of 1 and 10 mM under standard operating conditions (C
0 = 5 mg/L TB, [Fe(III)]
0 = 0.1 mM, [Oxalate]
0 = 1 mM, pH 4, 25 mL s
−1). The resulting TB degradation profiles are presented in
Figure 13.
The addition of H
2O
2 led to an improvement in dye removal: 95% and 90% degradation were achieved within 60 min for 10 and 1 mM H
2O
2, respectively, compared to 75% in the absence of H
2O
2. Correspondingly, t
1/
2 decreased from 25 min (control) to 20 min and 15 min for 1 and 10 mM H
2O
2, respectively, indicating improved kinetics. This enhanced performance can be attributed to the additional generation of
•OH via classical photo-Fenton reactions involving Fe(II)/Fe
II(C
2O
4) and H
2O
2 (Reactions 22–23—
Table 1).
Despite this improvement, the overall enhancement was less pronounced than what has been reported in other photo-Fenton studies (e.g., [
36,
37,
38]). This can be attributed to the limited availability of Fe(II) in our system. Since Fe(III) oxalate complexes dominate under the applied pH and ligand conditions, the photoreduction of Fe(III) to Fe(II) may not be sufficiently rapid to sustain a strong Fenton cycle, thereby limiting the generation of
•OH through H
2O
2 activation. In such a scenario, specifically, higher H
2O
2 concentrations may lead to scavenging of
•OH through reactions such as Reaction 28 in
Table 1 (
k28 = 2.7 × 10
7 M
−1 s
−1). This could partly explain the modest performance of H
2O
2 addition.
3.12. TOC Analysis of TB Degradation
The results of Total Organic Carbon (TOC) analysis for TB degradation under photo-Ferrioxalate conditions, with and without external H
2O
2 addition (10 mM), are presented in
Table 3. While a significant reduction in the dye concentration (C/C
0) was achieved within 120 min—with 90% decolorization being reached—the corresponding TOC removal remained relatively modest. At 120 min, only 30% of the initial TOC was removed without H
2O
2, and 36% with 10 mM H
2O
2.
This discrepancy between dye degradation and mineralization indicates that the photo-Ferrioxalate system primarily promotes the cleavage of the dye’s chromophoric structure, leading to the formation of non-colored but persistent organic intermediates. The addition of 10 mM H2O2 moderately improved TOC removal, likely due to enhanced hydroxyl radical production through the Fenton reaction. However, the limited mineralization observed suggests that the resulting intermediates are refractory and resistant to further oxidative breakdown under the current conditions. These findings underscore the importance of complementing dye degradation with a subsequent biological treatment step to achieve higher mineralization efficiency while maintaining a cost-effective overall process.