1. Introduction
The modern development of industry and energy worldwide is accompanied by a steady increase in anthropogenic waste, which poses a serious threat to ecosystems. This problem is particularly acute in regions with well-developed fuel-energy and metallurgical sectors, where large volumes of waste such as oil sludges, industrial dusts, metallurgical slags, plastics, and rubber accumulate and contribute to long-term environmental degradation [
1]. The challenge is both global and local, necessitating solutions that are scalable and adaptable to diverse industrial contexts.
Traditional waste management practices, including landfilling and incineration, are often insufficient and environmentally damaging, leading to land degradation and contamination of soil and water resources [
2]. Moreover, complex and mixed industrial waste—containing hydrocarbon residues, heavy metals, and mineral components—requires advanced treatment technologies that are not widely available [
3]. In this context, the development of innovative, low-impact technologies for the efficient processing and utilization of such waste is an urgent task for the global scientific and engineering community.
Simultaneously, the construction industry is facing increasing demand for sustainable, cost-effective, and environmentally friendly materials. With global efforts aimed at reducing the carbon footprint and improving the energy efficiency of buildings, the conversion of industrial waste into valuable construction materials has gained significant attention. Utilizing waste from fuel-energy and metallurgical industries for the production of ceramics and composites can reduce environmental burden and preserve natural resources, in line with the principles of sustainable development and the circular economy.
However, international studies have shown that ceramics produced from industrial waste may demonstrate heterogeneous physical properties and variable long-term durability due to inconsistent waste composition and the presence of harmful impurities [
4,
5,
6]. Additionally, some field evaluations conducted over extended service periods have revealed susceptibility to environmental degradation, including leaching and structural weakening under harsh climatic conditions [
7,
8,
9]. These findings underscore the importance of careful material selection, pretreatment, and performance monitoring when using such ceramics in construction. To address these challenges, researchers in Europe, East Asia, and North America have proposed multi-stage stabilization techniques, hybrid formulations, and standardization protocols aimed at improving reproducibility and environmental performance [
10,
11,
12].
The production of ceramic construction materials from ash and slag waste can alter the strength, thermal conductivity, water absorption, and other properties of the ceramic body. This opens up opportunities for manufacturing ceramics with tailored properties, adapted to specific requirements.
The aim of the study is to investigate the physicochemical properties and elemental composition of ash and slag waste from long-flame coal for its application in ceramic mixtures used in construction.
This research contributes to these global efforts by focusing on the development and optimization of ceramic materials synthesized from industrial waste. While the Republic of Kazakhstan is used as a representative case study—given its extensive metallurgical and energy sectors—the methods and technologies proposed are designed to be broadly applicable. The work falls under applied chemistry and targets practical innovations for industrial and solid household waste (SHIW) utilization.
The program’s main objective is to develop scientifically grounded formulations and processing regimes for ceramic materials derived from waste, supported by the construction of a pilot-scale production unit. The expected outcomes include the following:
A substantial reduction in environmental impact by diverting waste from landfills;
Economically viable and scalable technologies for producing construction materials from secondary raw materials;
Potential integration into industrial enterprises not only in Kazakhstan but also in other countries facing similar waste challenges.
The scientific novelty lies in the development of a universal high-efficiency pyrolysis technology capable of processing complex waste streams without prior sorting. This approach differs from traditional methods by eliminating the need for elaborate gas purification systems while achieving high detoxification levels [
13]. The technology is suitable for various waste types—including oil sludge, plastics, rubber, dust, and metallurgical slag—and can be integrated into existing industrial infrastructures to reduce capital and operational expenditures [
14].
By transforming industrial waste into construction ceramics and composites with high strength, chemical resistance, and thermal insulation properties, this research opens pathways for sustainable materials innovation [
15,
16,
17,
18]. The use of waste-based materials not only reduces production costs but also lessens dependency on natural raw materials, aligning with the strategic goals of global sustainable construction.
Overall, the program supports international priorities in circular economy, pollution reduction, and sustainable industrial development. Its relevance extends beyond national boundaries and aligns with a number of global initiatives focused on waste valorization, environmental protection, and resource-efficient technologies [
19,
20,
21,
22].
2. Materials and Methods
Sampling and sample preparation were carried out in accordance with the standard method specified in GOST 57789-2017 [
23].
For the purpose of studying ash, slag, and ash–slag mixtures from their storage sites, no fewer than ten spot samples were taken to form a composite sample.
Spot samples weighing at least 4–5 kg each were collected from a depth of 0.2 m to 3 m below the surface. The spot samples of ash, slag, and ash–slag mixtures intended for testing were hermetically sealed in waterproof bags.
The overall methodology of the study, including the preparation and characterization steps, is shown in
Scheme 1. Illustrations of the experimental workflow.
In the study of lignite (brown coal), a comprehensive set of methodologies was used to determine its physical and chemical properties. The core methodology included proximate analysis; theoretical analysis; as well as techniques for elemental analysis, particle size distribution, and X-ray phase analysis.
The main types of waste generated from the use of long-flame coal from Kazakhstan’s deposits as fuel are slag and ash [
24,
25,
26].
We conducted a study of ash and slag waste resulting from the combustion of lignite. The waste produced during the extraction and processing of lignite includes ash and slag, which are the mineral residues left after coal combustion.
To utilize by-products in the form of ash and slag, a closed hydraulic ash removal system is used at the deposits. It consists of units for washing, transporting, and accumulating ash and slag mass. Ash and slag are removed together and transported as a pulp through pipelines using dredgers to ash dump No. 1, which is a settling pond with an area of 60.4 hectares. It is located in the floodplain of nearby rivers, approximately 800–1000 m west of the main industrial site.
As a result of determining the elemental composition, the ash and slag formed during the combustion of long-flame coal consist mainly of aluminosilicate compounds, among which quartz, hematite, mullite, magnetite, and particles of unburned coal predominate. The material is a fine-grained mass of gray shades—from light to dark—depending on the concentration of unburned carbon components. Physically settled ash and slag contain microspheres—rounded glassy formations formed as a result of melting quartz, as well as angular particles of various shapes, which make up the bulk of the material. The size of ash particles ranges from 1.1 to 2.8 mm, while the proportion of particles smaller than 0.13 mm ranges from 13% to 93% depending on the distance from the ash dump crest where the sample is taken. In general, the granulometric composition of the material mainly consists of fractions smaller than 0.27 mm with a high content of dust particles—about 36–41% (less than 0.07 mm).
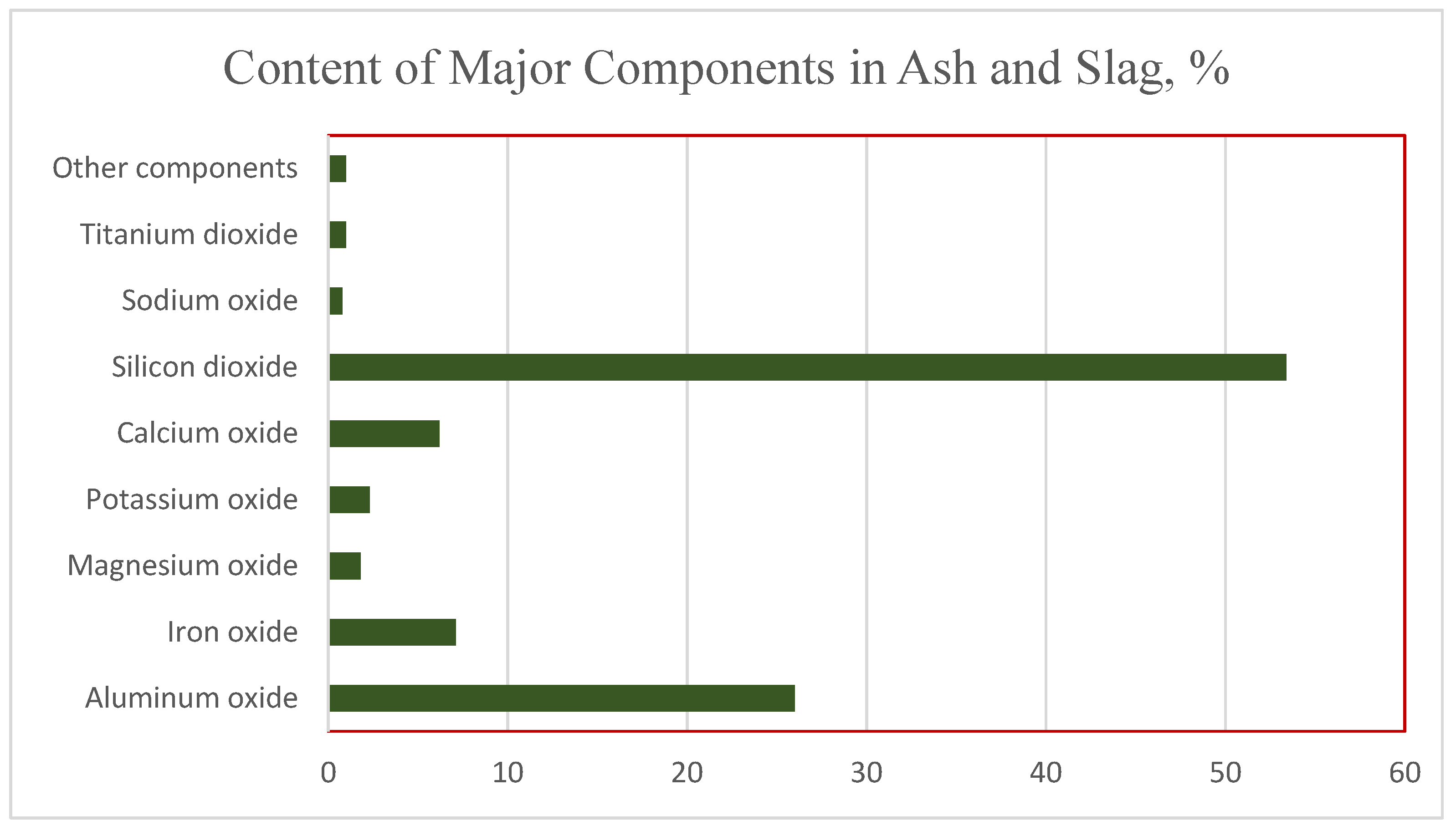
The chemical composition of ash and slag is shown in
Figure 1 and
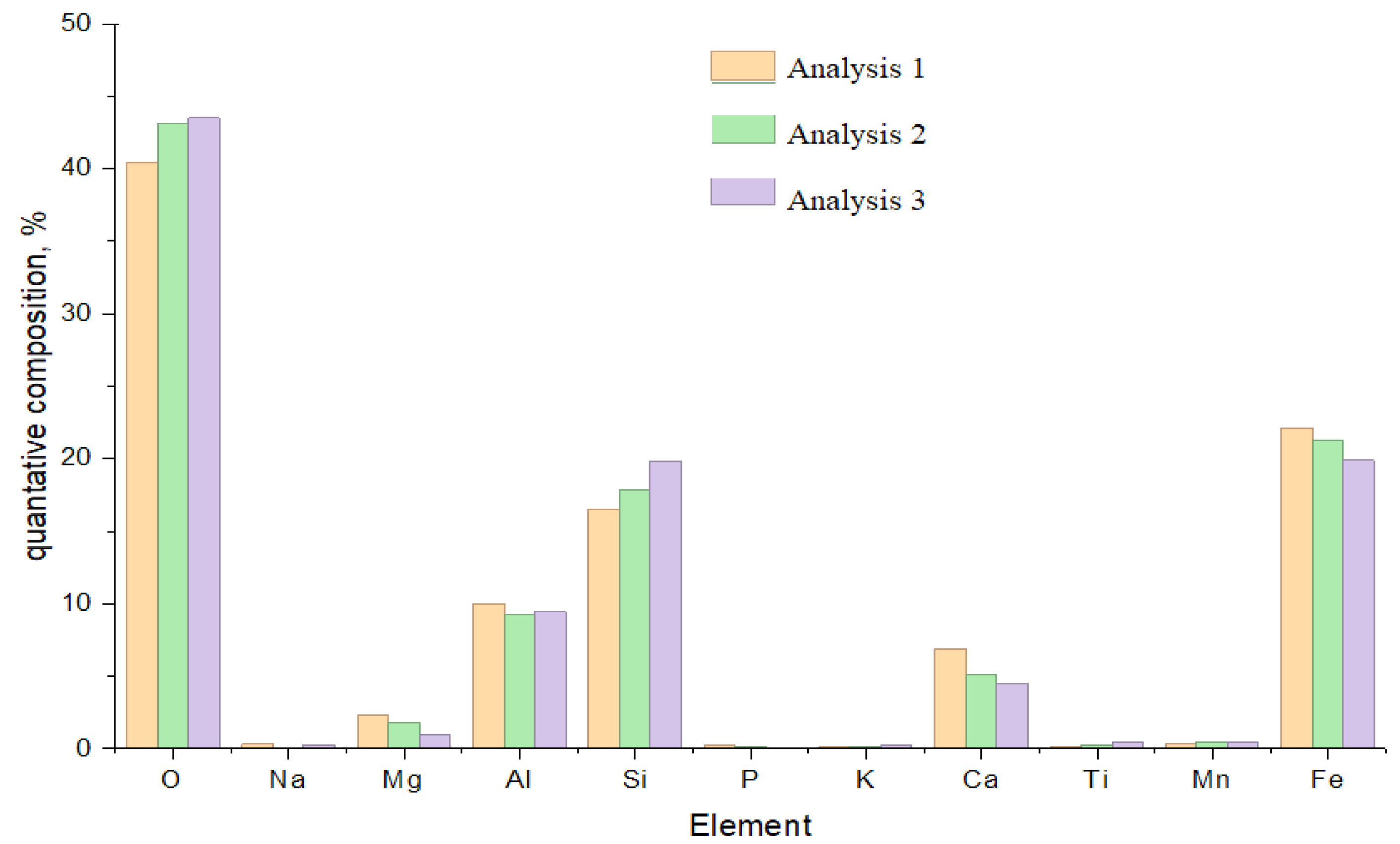
Figure 2. The dominant components are silicon, aluminum, iron, and calcium oxides, the total content of which is up to 93% of the sample mass. An analysis shows that silicon dioxide makes up about 53.4% of the material mass, and about 33.1% is accounted for by aluminum and iron oxides. The proportion of alkaline and alkaline earth components in the ash residue does not exceed 8%.
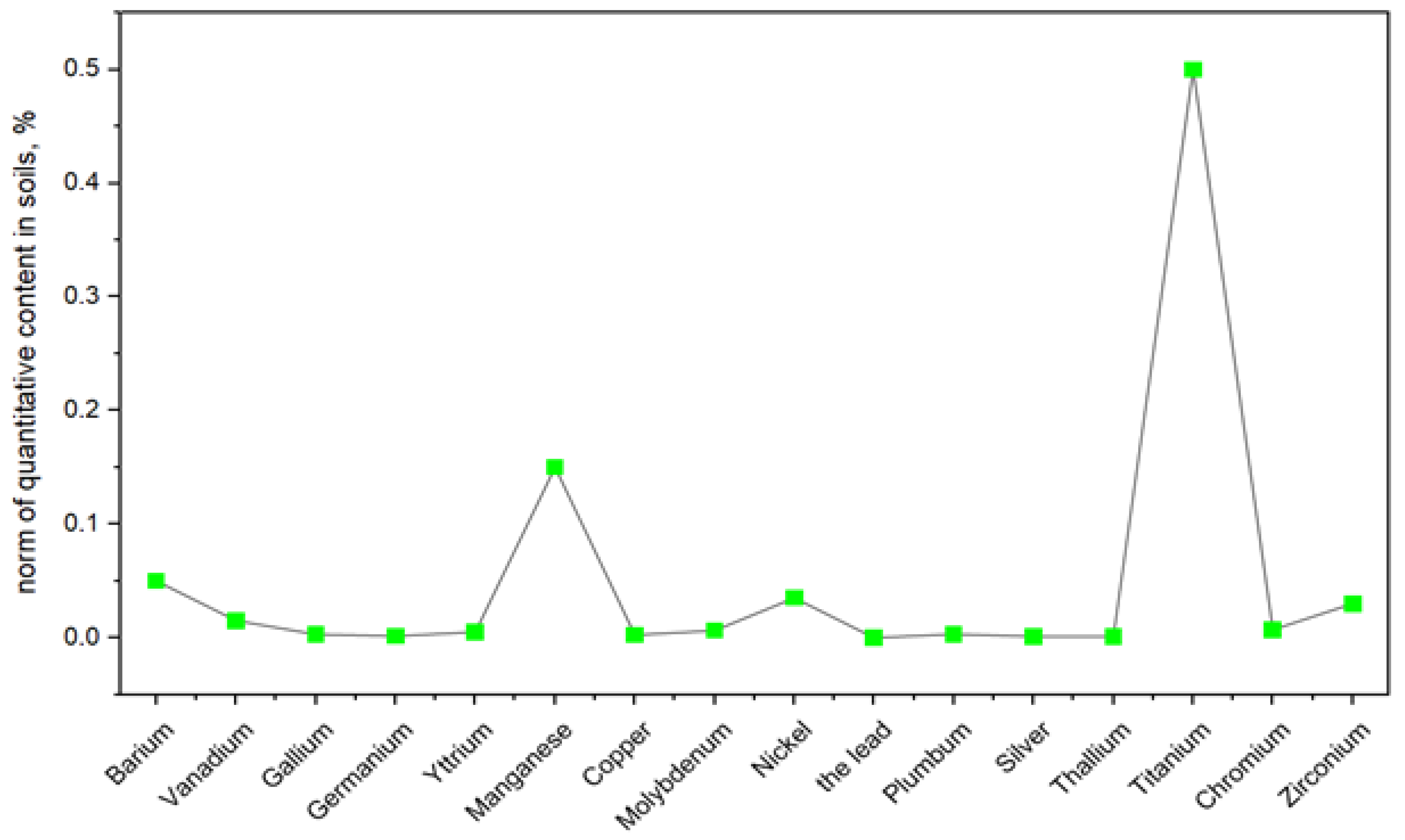
The concentrations of V, Cu, As, Ni, and Pb correspond to the maximum permissible concentrations (MPC) in soils, while the remaining elements are compared to their average content in soils. Based on the data presented in peak 2 and their comparison with soil (according to average concentrations), three groups of elements can be identified:
Group 1—elements (Ag, Bi, Cu, Sr, Ti, and W) present in coal in very low amounts (virtually absent);
Group 2—elements (Co, Ba, V, Mn, Ni, Cd, Cr, Li, Pb, Sb, Ta, and Y), whose concentrations in coal are comparable to their average concentrations in soils;
Group 3—elements (Al, As, B, Mo, and Sc) whose concentrations are one to two orders of magnitude higher than in soils, which allows them to be classified as associative and environmentally hazardous elements, requiring constant monitoring.
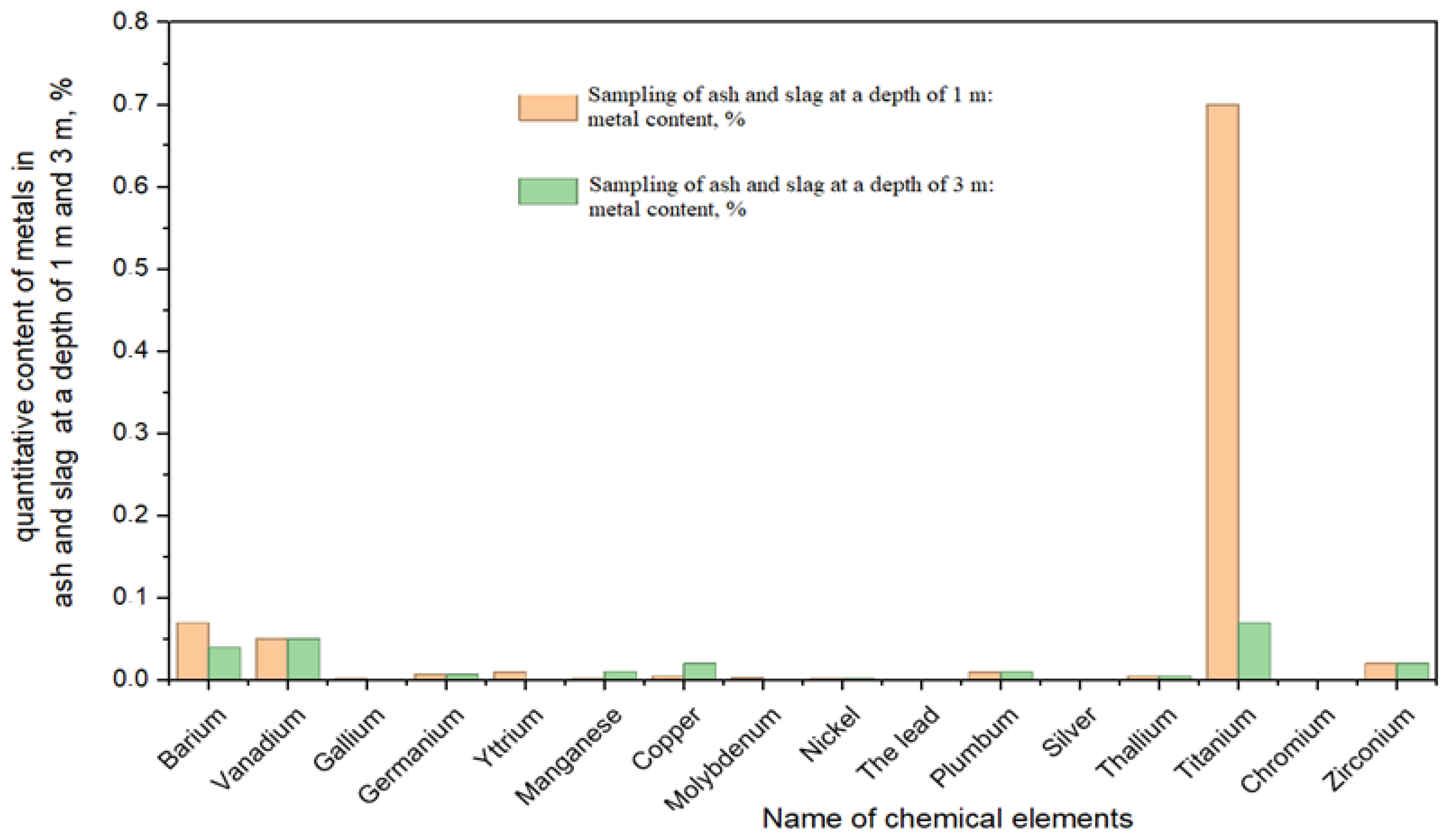
To study the trace element composition of ash and slag, a sampling profile was established in the basin of ash dump No. 1, with soil samples collected according to lithological horizons. In total, three samples of aged ash and slag were collected at depths of 1, 2, and 3 m. The samples were prepared and analyzed at the State Scientific Production Association of Ecology “Kazmekhanobr”. The results of the chemical composition analysis of aged ash and slag are presented in
Figure 3 and
Figure 4.
The average content in soils is provided for comparison. The concentrations of elements were measured at different sampling depths of ash and slag (1.0 m, 2.0 m, and 3.0 m). The average content of elements V, Mn, Cu, and Ni corresponds to the maximum permissible concentrations in soils. The chemical composition analysis of the coals burned at [CHP plant], three groups of elements can be distinguished:
First group—Cr and Mn;
Second group—Ba, Ge, Ni, V, Ti, Zr, and Ag;
Third group—Ta, Cu, Pb, Ga, Mo, and Y.
An analysis shows that a number of elements present in coal within normal limits become enriched in ash and slag products after combustion, thus entering the list of environmentally potentially hazardous elements (such as copper, lead, gallium, and yttrium).
The data indicate that ash and slag undergo physicochemical weathering during their storage in the dump. The surface horizons of aged ash and slag contain higher amounts of barium, chromium, copper, manganese, and titanium. However, many potentially hazardous minerals containing associative elements (thallium, lead, gallium, molybdenum, and yttrium) are quite resistant to weathering.
Ash and slag wastes, produced by the combustion of coal in thermal power plants, are a physical mixture of ash and slag. On average, the ash to slag ratio is approximately 4:1 [
15]. Ash and slag wastes include microscopic spherical particles formed by the melting of high-temperature minerals (primarily quartz), as well as irregularly shaped particles composed of other mineral components.
Ash is usually a finely dispersed mineral powder, colored in various shades of gray—from light to dark. Particle sizes vary from 0.1–0.2 mm to a maximum of 1–2.5 mm. Slag inclusions, on the contrary, are characterized by a larger dispersion, from 0.1 to 20 mm, with individual particles reaching 40–60 mm, and the minimum size is about 0.03–0.04 mm [
16]. The most significant physical parameters of ash and slag are bulk density, true density, granulometric composition, and specific surface. Although fly ash has a high specific surface, its active components are often either enclosed in a glassy phase or covered with a glassy layer, which hinders effective interaction with water. Therefore, when using ash as a component in the production of binders, preliminary grinding is required to destroy the glassy shell and release the active phases. The granulometric composition of ash and slag is influenced by several factors: coal type, coal preparation method, combustion temperature and duration, as well as the ash capture system. Globally, the degree of ash fineness is evaluated by specific surface area, which should be no less than 2600–4000 cm
3/g.
The chemical–mineralogical and fractional composition of ash directly affects its bulk density, which varies for different ashes in the range of 500–1300 kg/m
3 [
27,
28]. True density, depending on the coal feedstock, ranges from 1.7 to 3.5 g/cm
3, averaging 2.1–2.4 g/cm
3 [
17].
Below are the physical and mechanical properties of slag waste:
Bulk density: 700–800 kg/m3;
Specific surface area: 4000–6700 cm3/g;
Content of unburned carbonaceous inclusions: 16–25%;
Moisture content: 2–15%;
Porosity coefficient: 1.03–1.44 (total porosity 50.1–58.9%);
Maximum grain size: 1.0–2.5 mm;
Dust fractions (particles less than 0.05 mm) range from 15% to 95%, depending on the distance of the sampling point from the crest of the ash dump dam;
Specific surface area and granulometric composition strongly depend on the sampling location.
By nature, fuel ash and slag are products of thermal and phase transformations of the mineral part of the fuel and also include minerals originally present in the source rocks.
During the combustion of solid fuel at thermal power plants (TPPs), the resulting ash and slag materials include both organic and inorganic phases. The inorganic part consists of crystalline and amorphous components. The amorphous phase may contain glassy formations and amorphous clay compounds. The crystalline fraction includes altered minerals originally present in the fuel (such as quartz, feldspars, and other refractory minerals), as well as new crystalline phases formed during combustion—such as mullite, hematite, and calcium aluminates [
18,
19,
20,
21,
22].
According to studies [
24,
25,
26], the main component of ash and especially slag is the glassy phase. The glass in ashes can range from silicate to aluminosilicate and sometimes iron silicate types. Its color depends on the chemical composition and may be transparent, brown, yellow, or even black.
The amorphous phase significantly influences the chemical activity of ash and slag wastes and determines the shape and surface texture of ash particles (metakaolinite, partially sintered amorphous clay substances, and glassy structures).
If the combustion temperature is insufficiently high or the ash components of the fuel have high refractory properties, ashes predominantly containing amorphized clay material form. This material consists of porous, irregularly shaped particles with a high moisture absorption capacity.
Unlike ashes, slags contain significantly fewer organic residues and amorphous clay compounds, while the proportion of the glassy phase in slags can reach up to 95%. This is explained by the longer residence time of slags in the high-temperature combustion zone [
5,
8].
One of the key properties of TPP ash and slag wastes is their chemical activity, which determines their potential use as independent binding materials or as part of complex binder mixtures.
According to chemical composition, ashes and slags are divided into acidic, basic, and neutral. Basic ashes and slags contain hydraulically active substances and can serve as independent binders. Acidic ashes have typical pozzolanic properties and are used as active mineral additives.
Fuel ash and slag generally do not react directly with water, except those containing free calcium and magnesium oxides. The amorphous fractions of ash and slag exhibit pozzolanic activity—the ability to react at ambient temperatures with calcium hydroxide to form insoluble compounds. The accumulation of such compounds can lead to hydraulic hardening of binder compositions based on lime or Portland cement with ash and slag additives [
27].
Pozzolanic activity in ash and slag is attributed to the firing products of clays: amor-phized clay material, metakaolinite, amorphous silica, and alumina oxides, as well as aluminosilicate glass. The reactivity of these components toward calcium hydroxide varies depending on the temperature at which kaolinite clays were transformed during fuel combustion. Metakaolinite Al
2O
3•2SiO
2 with a high specific surface area actively reacts with Ca(OH)
2 under normal conditions to form calcium hydrosilicate and hydrogelinite [
28,
29] according to the following reaction:
Amorphous SiO
2 and Al
2O
3 oxides formed at higher temperatures show lower activity due to a sharp decrease in specific surface area caused by sintering and crystallization processes of minerals such as mullite and cristobalite [
7].
High-temperature melting and sintering of clay minerals lead to a significant reduction in their specific surface area, which in turn reduces chemical activity. As a result, the glassy phase of ashes and slags becomes poorly reactive under normal conditions. Exceeding the permissible fuel combustion temperature leads to a decrease in the activity of fuel ash and slag [
10].
The analysis of the thermal characteristics of TPP ashes and slags enables assessment of their applicability in manufacturing of artificial porous ceramic materials, mineral wool, refractory concrete, and other products involving high-temperature processes.
During heating from 100 to 300 °C, powdery ashes lose adsorbed moisture. The higher the content of amorphous clay substances and organic inclusions in the ash, the more moisture it can retain. Between 400 and 700 °C, organic fuel residues combust. Subsequently, in the range of 700–850 °C, carbonate decomposition occurs in the ash, and at about 920–980 °C, crystallization of amorphous clay and glass phases takes place [
29,
30].
High-temperature processes include softening of ash and slag particles, their sintering upon contact, drying, and crystallization during cooling. Ash fusibility is characterized by three stages: deformation onset, softening onset, and transition to a liquid phase. Depending on composition, ashes soften between 1050 and 1470 °C and melt at temperatures from 1250 to 1710 °C [
13]. Ash and slag are classified by melting temperature into five groups: low-melting (<1200–1230 °C), medium-melting (1200–1350 °C), refractory (1350–1500 °C), very refractory (1500–1165 °C), and fire-resistant (>1650 °C) [
31,
32].
There are several main types of ash and slag wastes, each with its own characteristics and processing requirements. Metallurgical slag formed during metal smelting contain significant metallic impurities and can be used as secondary raw material for building materials production [
16,
17]. Electric arc furnace slag, produced during metal scrap processing, has high heavy metal content and requires special disposal conditions. Thermal slag is formed during coal or other fuel combustion for power generation (TPP ash and slag).
To assess the suitability of raw materials for ceramic production, their composition is studied. These properties depend on chemical, mineralogical, and granulometric composition. In
Figure 5 and
Figure 6 are shown a photo of slag and a photo of the clay raw material, respectively.
The following methods were used to study the physico-chemical characteristics of the selected raw material components:
Determination of granulometric composition;
Chemical and phase composition analysis;
Differential thermal analysis (DTA);
X-ray fluorescence analysis;
X-ray phase analysis;
Thermodynamic analysis.
Granulometric composition is one of the key characteristics determining the rheological properties of the raw components used for preparing (the “dough” of) ceramic materials, as well as structure formation during firing. One of the main rheological properties of clays and slags is their plasticity. The higher the content of microdispersed particles, the greater the plasticity of the material.
Consequently, the raw material will possess high cohesiveness, which positively affects the strength properties of the finished products. Granulometric composition also plays a key role in determining the sorption capacities of the material [
25,
29].
When analyzing granulometric composition, particle size and the percentage share of particles within each size fraction are determined. Methods for granulometric analysis include microscopic examination; sieve analysis; particle separation by settling velocity; counting methods; centrifugal separation; and simpler methods such as decantation and screening.
Sieve analysis is based on the mechanical separation of particles by size using sieves with openings of various sizes corresponding to size classes. A set of standard sieves is used for sieve analysis, e.g., GOST 66-13-86, 3826-82 Laboratory Sieves for Grain Bulk Materials, commonly applied in construction laboratories [
32].
Sampling of raw materials and their preparation for analysis were carried out according to the generally accepted methodology in accordance with GOST 25818-2017 for slag and for clay raw materials, according to GOST 32026 [
33,
34].
Figure 7 presents the granulometric composition results of the raw materials.
3. Results and Discussion
Study of the Granulometric and Material Composition of the Investigated Technogenic Raw Material
For this study, a sample was taken from the near-furnace zone, where coarse-grained slag material is present. The granulometric composition of the sample, as well as the distribution of gold (determined by atomic absorption analysis) and iron across particle size fractions, are presented in
Figure 8.
The mineralogical and chemical composition of the sample was studied comprehensively: in addition to microscopy, an X-ray phase analysis was applied, and chemical analysis data were also taken into account. For the first time, all fractions and raw materials were analyzed in detail, with the results presented in
Figure 8,
Figure 9,
Figure 10 and
Figure 11.
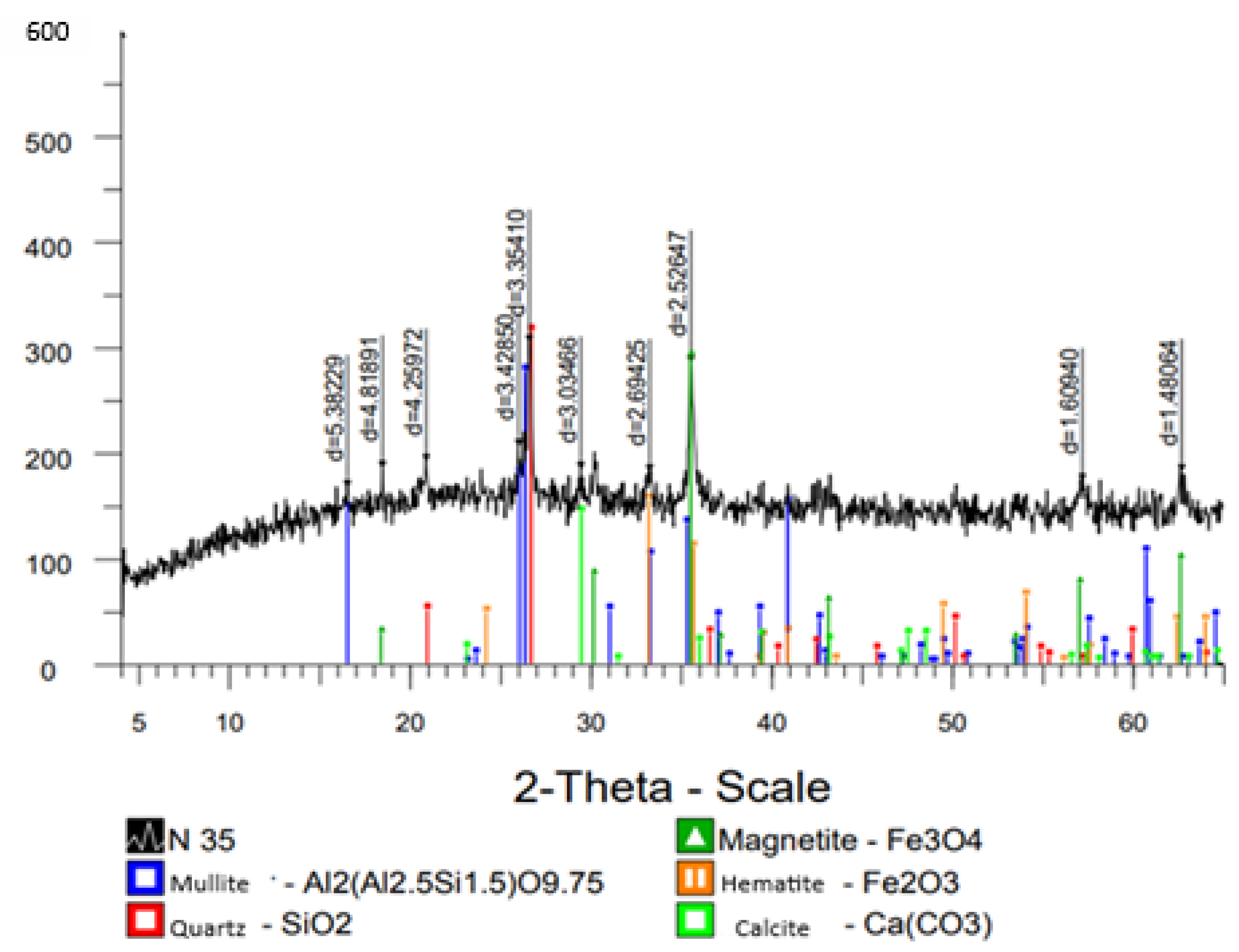
Composition of Minerals in Ash–Slag Waste.
Figure 11.
Diffractogram of minerals comprising the ash and slag waste.
Figure 11.
Diffractogram of minerals comprising the ash and slag waste.
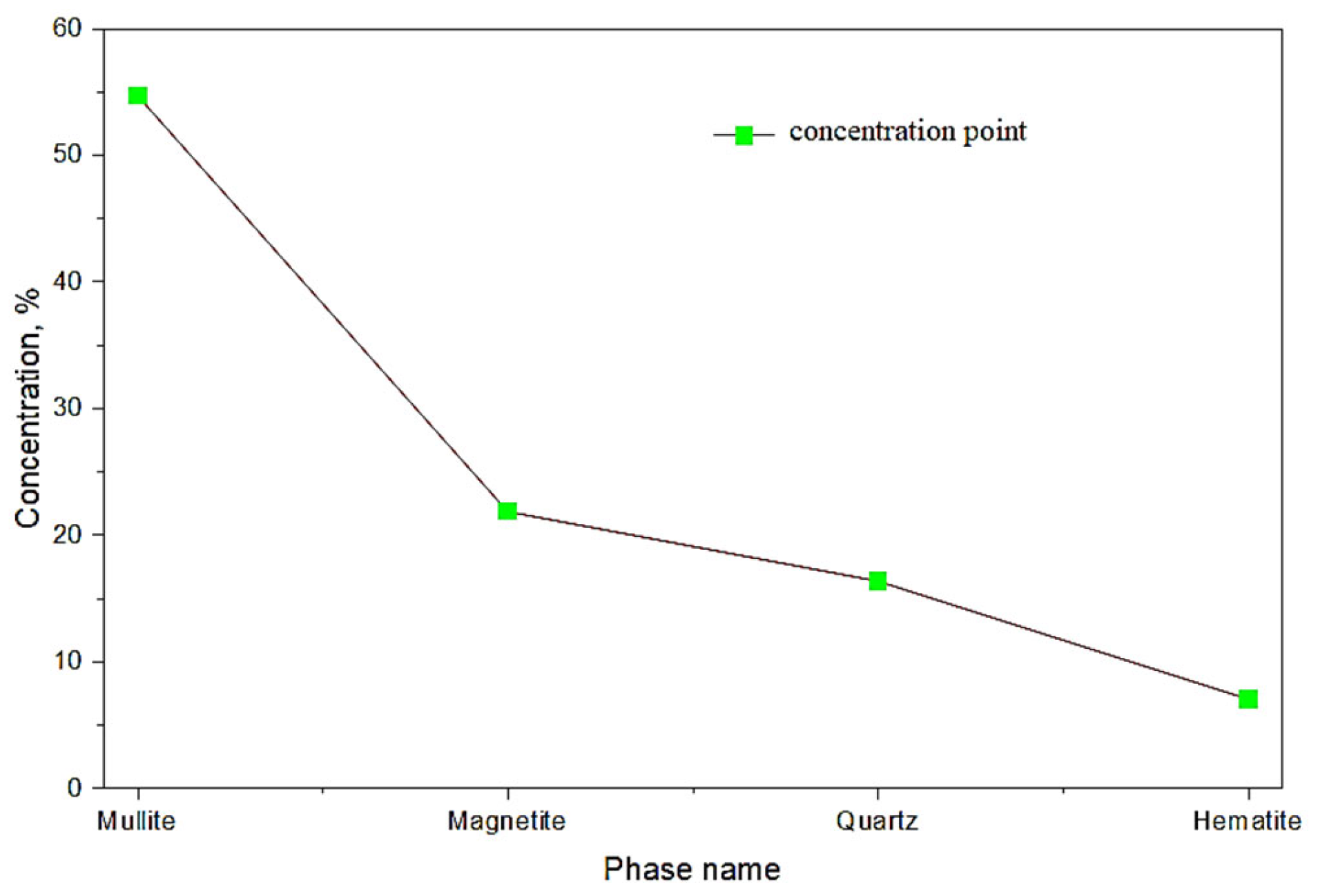
The main minerals in the coarse-grained fraction of the ash–slag waste include mullite, magnetite, and quartz, with the presence of hematite, cristobalite, calcite, and gypsum. The chemical composition is predominantly represented by aluminum, silicon, iron, and calcium. Additionally, stable and significant impurities were identified: titanium, manganese, and occasionally chromium.
The mineral and chemical composition of the ash and slag waste from the thermal power plant is presented in
Figure 12.
The chemical composition of ash and slag waste makes it possible to consider them as fluxing and structure-forming components of building materials.
A high content of fluxing components (K2O, CaO, and MgO) ensures a reduction in the initial sintering temperature, while Al2O3 and SiO2 provide structural stability under thermal exposure. The presence of Fe2O3 contributes to the formation of an intense coloration. Al2O3 enhances thermal resistance and shape stability during firing. An increased content of Fe2O3 results in a characteristic dark red coloration of the products.
This combination of properties indicates the feasibility of mixing ash and slag waste with construction components to optimize the composition of ceramic bodies.
The mineralogical and chemical composition of the coarse fraction (+2 mm) of ash and slag waste from the thermal power plant is presented in
Figure 13,
Figure 14,
Figure 15 and
Figure 16, respectively.
Elemental and X-ray phase analyses made it possible to determine the proportion of major oxides in the studied samples: ash and slag. SiO
2 and Al
2O
3 dominate in the composition of ash and slag, indicating their aluminosilicate nature (
Figure 12 and
Figure 13). A significant content of Fe
2O
3, as well as fluxing oxides such as K
2O, CaO, and MgO—with a combined content exceeding 10%—makes these materials effective components for lowering the sintering temperature.
When ash and slag are used as components in construction clay, which is characterized by a high content of Al2O3 and Fe2O3 and a relatively moderate proportion of SiO2, it is possible to obtain a dense ceramic body with an intense coloration tendency when fired at temperatures of 1100–1200 °C.
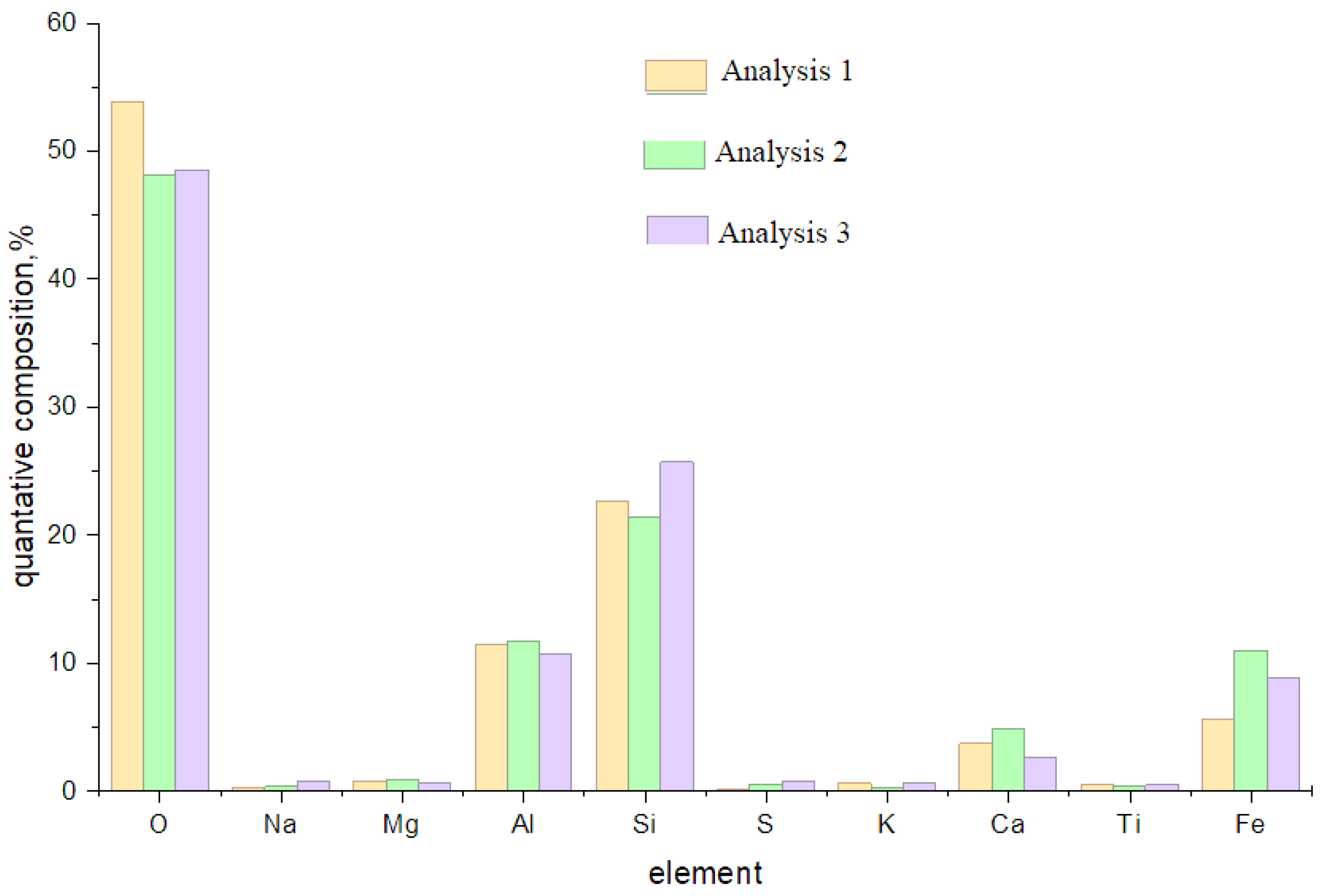
A comparative analysis of the elemental composition results for ash (
Figure 13) and the data on its rational composition shows good consistency, confirming the high reliability of the results.
The effectiveness of using ash and slag is determined by their compliance with physicochemical requirements such as particle dispersion, phase composition, oxide and impurity content.
Ash and slag from long-flame coal represent a fine-grained gray material consisting of microspheres and angular particles. According to the analysis, 88% of the particles range in size from 0.25 to 0.01 mm, which provides favorable conditions for the formation of a dense ceramic structure.
The composition of long-flame coal ash and slag is represented by aluminosilicates, including mullite, quartz, hematite, and magnetite (
Figure 14). The chemical composition is dominated by the oxides SiO
2, Al
2O
3, Fe
2O
3, CaO, MgO, and TiO
2 (
Figure 17 and
Figure 18).
The identified characteristics confirm the potential of using ash and slag as functional components in construction ceramics.
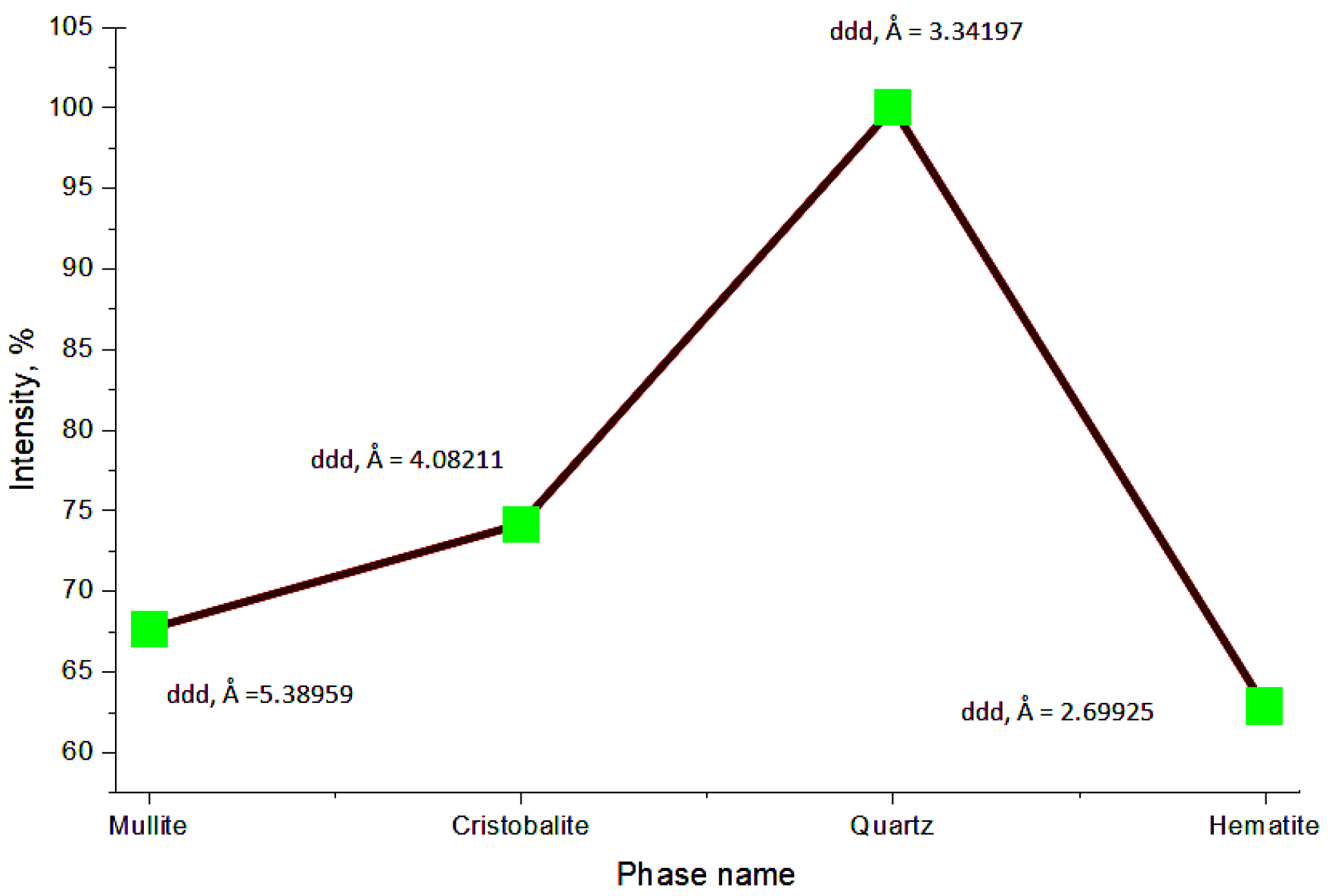
An X-ray phase analysis performed in the 2θ range from 5° to 70° made it possible to determine the mineral composition of the studied ash and slag samples, as shown in
Figure 18.
The ash and slag sample is characterized by an intense peak at approximately 26.6° and 36.2°, corresponding to the main reflection of quartz (SiO2). Additionally, reflections corresponding to mullite (Al6Si2O13) were identified.
The presence of a broad background in the range of 20–55° indicates the existence of an amorphous glassy phase. Secondary peaks may be attributed to hematite (Fe
2O
3) (
Figure 17), which supports the chemical analysis data indicating the presence of Fe and Ti.
The presence of hematite is confirmed by peaks in the ranges of 33–35° and around 54°. The quartz peak (~26.6°) is dominant, indicating its significant content. The amorphous component, expressed as a broad background, is typical of fine-grained phases and poorly ordered silicates.
The mineralogical and chemical composition of the fine-grained fraction (–0.1 + 0.044 mm) of ash and slag waste from the thermal power plant is presented in
Figure 19,
Figure 20,
Figure 21 and
Figure 22.
One of the key indicators of raw materials is their particle size distribution.
Figure 19 shows the analysis of the dust fraction (−0.044 mm) of ash and slag waste. The presence of this fine fraction indicates that the ash and slag waste contains a high proportion of fine particles. The higher the content of micro-dispersed particles, the greater the plasticity of the material.
Therefore, the raw material will exhibit high cohesion, which positively affects the strength characteristics of the final products. Moreover, particle size distribution is important for determining the material’s adsorption capacity.
Based on the above, it can be concluded that fine-dispersed ash and slag waste offers the potential to produce construction materials with enhanced plasticity.
The presence of fluxing oxides (K2O, CaO, and MgO) and amorphous glassy phases in ash and slag promotes low-temperature sintering, while aluminosilicates (SiO2 and Al2O3) provide structural integrity and thermal resistance of the ceramic body.
The results of this study show that ash and slag consist of both inorganic and organic phases. The inorganic phase includes amorphous and crystalline components. The amorphous component may appear as a glass or amorphized clay substance, indicating the potential for use in construction material production.
As shown in the figure, the crystalline component includes the following:
The glass in ash may be silicate or aluminosilicate in composition. The nature of the amorphous phase (e.g., poorly sintered amorphized clay substance, and sintered and partially vitrified glass) determines the chemical reactivity of the ash, as well as the shape and surface characteristics of the ash particles.
The reactivity of phases formed during high-temperature coal sintering, especially those rich in amorphous SiO2 and Al2O3, is significantly reduced due to a sharp decrease in specific surface area caused by sintering and crystallization of new phases (e.g., mullite and cristobalite). High-temperature sintering and melting drastically reduce the specific surface area and, accordingly, the activity of the material. Therefore, the glassy phase in ashes and slags may exhibit low activity under standard conditions.
Based on these findings, it is demonstrated that excessive fuel combustion temperatures beyond the permissible limit lead to a decline in the activity of most ashes and slags generated from coal combustion.
4. Conclusions
The conducted study confirmed the technological potential of using ash and slag waste generated from the combustion of coal as a secondary raw material for the production of construction ceramics. A comprehensive analysis of the material revealed a high content of silicon, aluminum, iron, and calcium oxides, which are essential for ceramic synthesis, along with a range of trace elements, some of which (e.g., As, Pb, and Mo) are ecotoxicologically significant and require careful monitoring and environmentally responsible handling.
Notably, this study established that prolonged storage of ash and slag in open settling ponds leads to physicochemical weathering and redistribution of elements, which alters the material’s original properties. These changes must be considered in the development of formulations and synthesis parameters for ceramic products.
To expand the raw material base and supply the construction industry with essential resources; develop resource-saving technologies in the production of building products; reduce their production costs; and address, to some extent, environmental issues, there is a pressing need for in-depth research into the technological properties of technogenic raw materials and the improvement of ceramic construction material production technologies.
The results of the conducted research show that ash and slag waste generated from coal combustion contain a high amount of silicon, quartz, and cristobalite (up to 70% combined) and can be used in the production of ceramic materials. In particular, ash and slag can serve as components in the manufacture of construction materials such as bricks, as well as in the creation of artificial porous aggregates used in lightweight concrete.
The X-ray phase analysis confirmed the presence of phases such as quartz, mullite, hematite, and magnetite. The use of ash and slag waste allows for the optimization of ceramic body formulations, improving both plasticity and strength.
The application of ash and slag waste in construction ceramics not only ensures technological efficiency but also addresses environmental challenges by reducing the volume of industrial waste, expanding the raw material base, increasing production profitability, and supporting the concept of sustainable development.
The results indicate a substantial transformation of the elemental and mineralogical composition during coal combustion, suggesting that combustion residues should be treated as new mineral systems rather than simple waste. These findings were used to formulate evidence-based recommendations for the design of ceramic compositions and the optimization of thermal treatment regimes.
To strengthen the scientific validity and international relevance of this study, the results were compared with data from international research on ash and slag reuse. The comparative analysis confirms the alignment of this work with current global efforts in waste valorization, sustainable construction, and circular economy development.
In summary, this research provides a technologically and environmentally justified framework for the utilization of ash–slag waste from the fuel-energy and metallurgical sectors of the Republic of Kazakhstan. It supports both waste reduction and the production of value-added ceramic materials, contributing to environmental sustainability and industrial innovation.