Hydroxyl Group-Dependent Effects of Alkanolamine Additives on Rheology, Hydration, and Performance of Early-Strength Cement Slurries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cement Slurry
2.3. Measurement of Cement Slurry Thickening Performance
2.4. Measurement of Cement Slurry Rheological Properties
- (1)
- The rheological properties were measured using a ZNN-D6S six-speed rotational viscometer (Qingdao Haitongda Special Instrument Factory, Qingdao, China). For viscometer testing: Freshly mixed cement slurry was placed in the atmospheric consistometer and allowed to stand for 20 ± 0.5 min at ambient conditions. It was then poured into the viscometer cup. The torque values were measured sequentially at rotational speeds of 600 r/min, 300 r/min, 200 r/min, 100 r/min, 6 r/min, and 3 r/min. The measurements were first taken in increasing order of rotational speed, then repeated in a decreasing order. The arithmetic mean of the readings at each corresponding speed (ascending/descending) was calculated. The rheological parameters were determined by substituting these values into Equations (1) and (2).: Flow index;: Consistency coefficient.
- (2)
- Truncated cone fluidity test: The glass base plate was wiped with a damp, lint-free cloth to ensure a moist, water-free surface. The truncated cone mold was positioned on the glass plate. The mold was rapidly filled with cement slurry and the top surface was leveled in a single pass using a spatula. The cone mold was lifted vertically and a stopwatch was started simultaneously. After 30 s, the maximum spread diameter of the cement slurry in two perpendicular directions was measured using a digital vernier caliper. The average of these two values was recorded as the fluidity of the cement slurry.
2.5. Measurement of Cement Slurry Density
2.6. Measurement of Cement Slurry Shrinkage
2.7. Determination of Cement Hydration Degree by Chemically Bound Water Method
- (1)
- Determination of Loss on Ignition (LOI): Approximately 1~2 g of cement paste powder (weighed to an accuracy of 0.0001 g) was placed into a pre-ignited and constant-weight porcelain crucible. The lid was placed askew on the crucible, which was then transferred into a muffle furnace. The temperature was gradually increased to and maintained at 950 ± 25 °C for 15 min~20 min. The crucible was removed, placed in a desiccator to cool to room temperature, and weighed. This process was repeated until a constant weight was achieved. The LOI of cement was calculated using Equation (4).
- (2)
- Removal of Non-Combined Water: The hardened cement samples were cured to the required test age. The samples were broken into small pieces, immersed in anhydrous ethanol to stop hydration, ground into powder, and dried in an oven at 60 °C until a constant weight was achieved. This process removed the non-chemically bound water from the sample.
- (3)
- Determination of Bound Water: A total of 1~2 g of the treated cement powder (weighed to an accuracy of 0.0001 g) was placed into a pre-ignited, constant-weight porcelain crucible. It was ignited following the LOI determination method until a constant weight was reached. The chemically bound water content (×1) of the tested cement sample was calculated using Equation (5).
- (4)
- Calculation of Hydration Degree: Based on the chemically bound water content measured at various ages and the chemically bound water content at complete hydration determined using the above methods, the hydration degree (α) at each age was calculated using Equation (6).
2.8. XRD Test
3. Results and Commentary
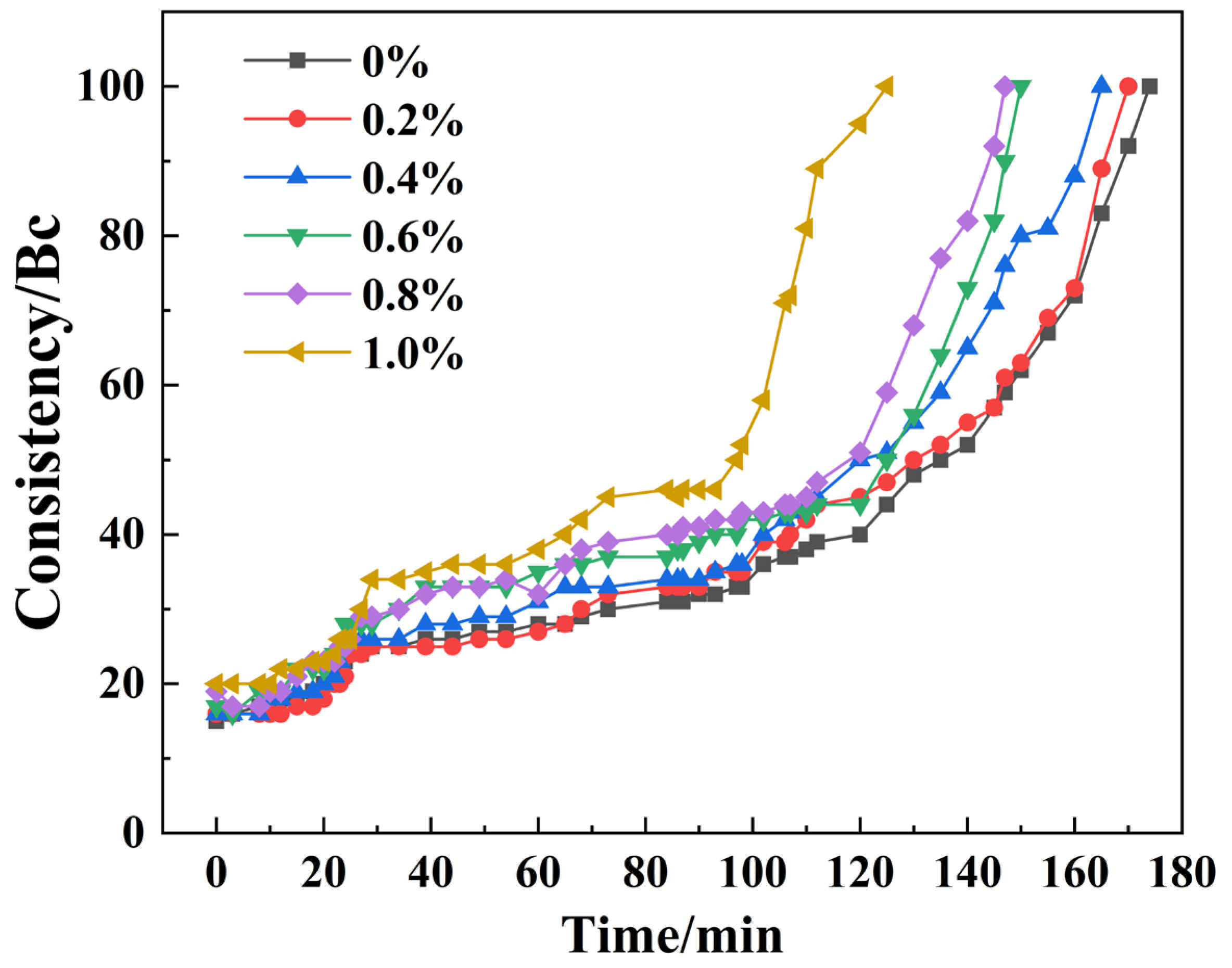
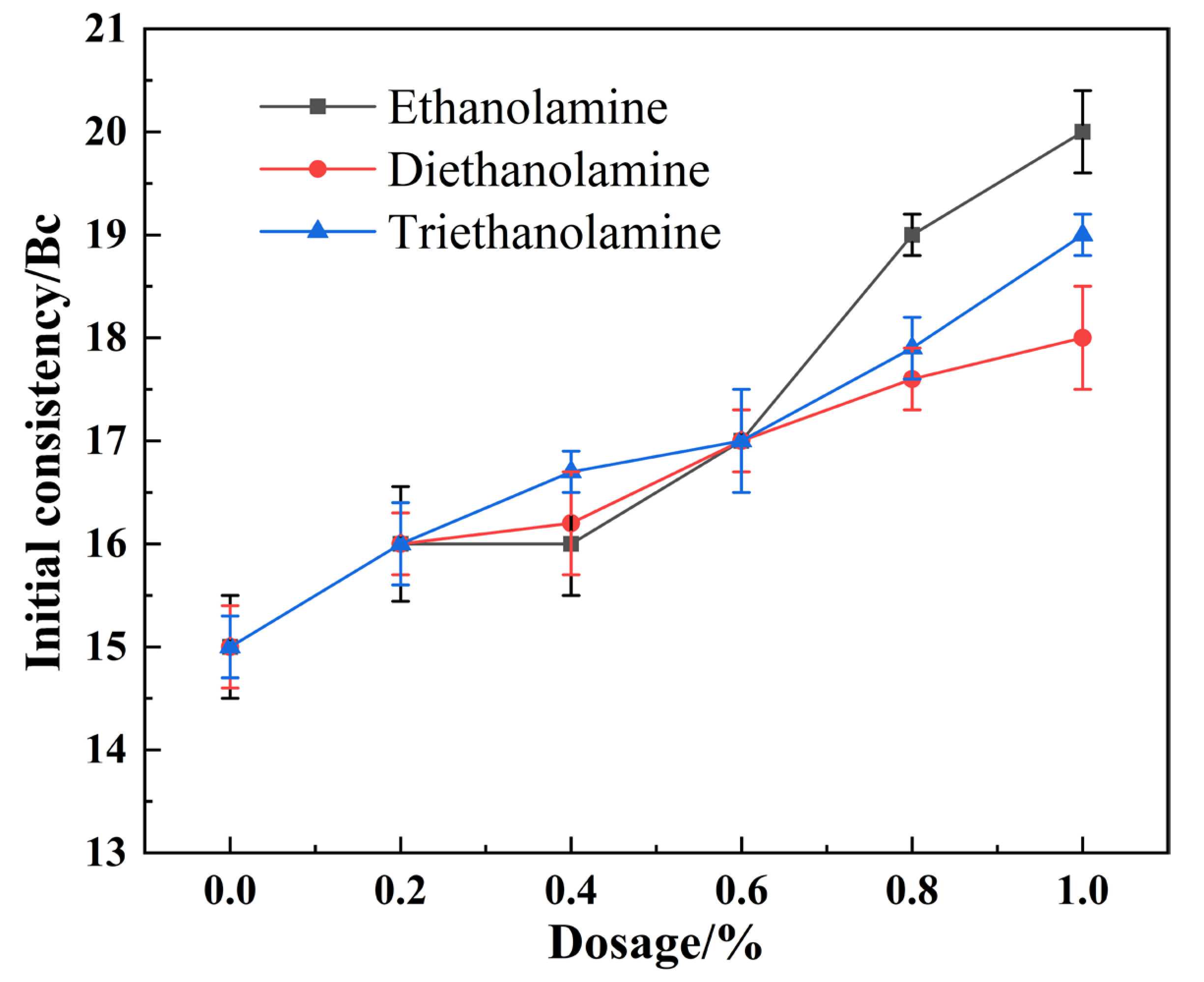
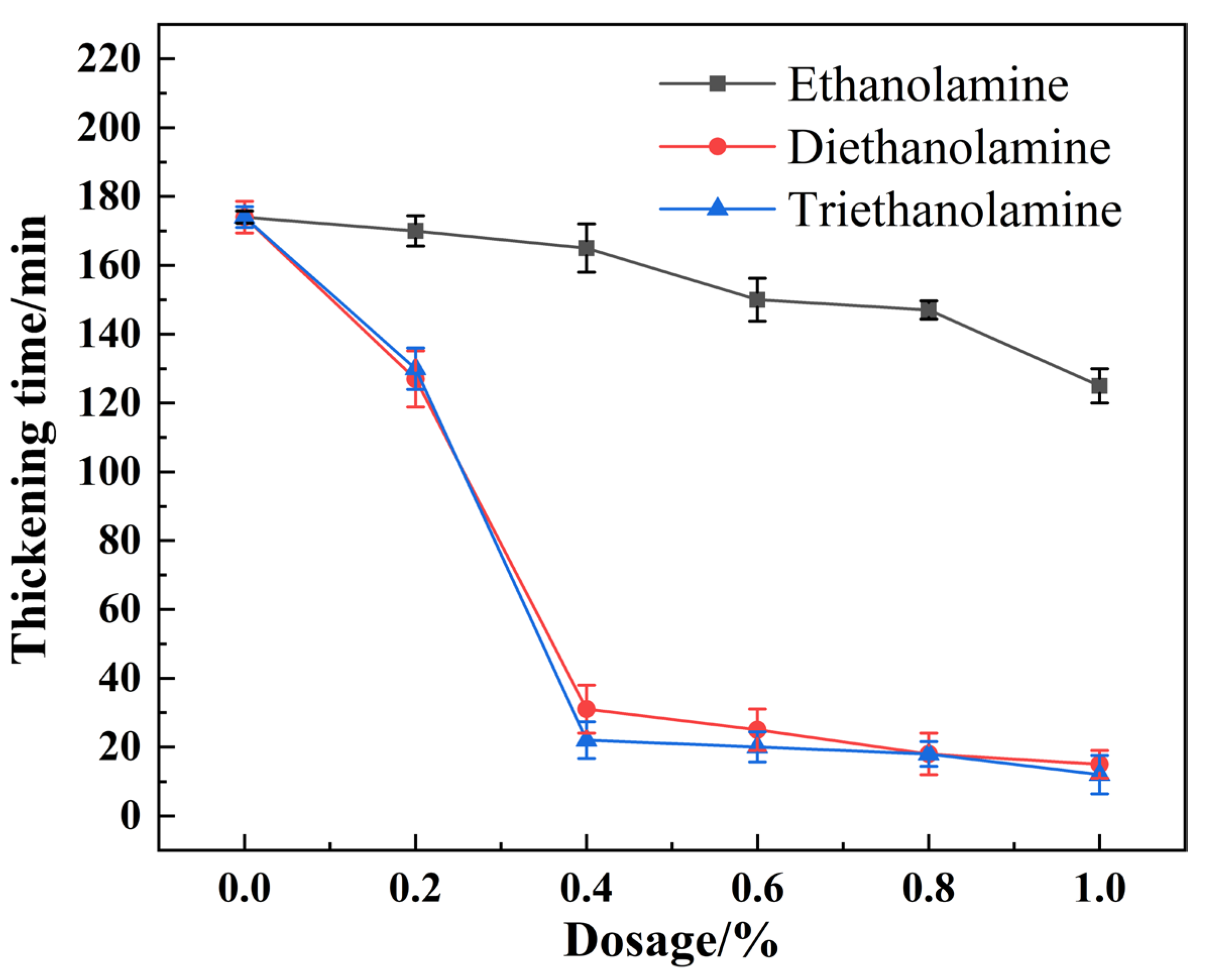
3.1. Effect of Alkanolamine Additives on Cement Slurry Thickening Behavior
3.2. Effect of Alkanolamine Additives on Rheological Properties of Cement Slurry
3.3. Effect of Alkanolamine Additives on Cement Slurry Density
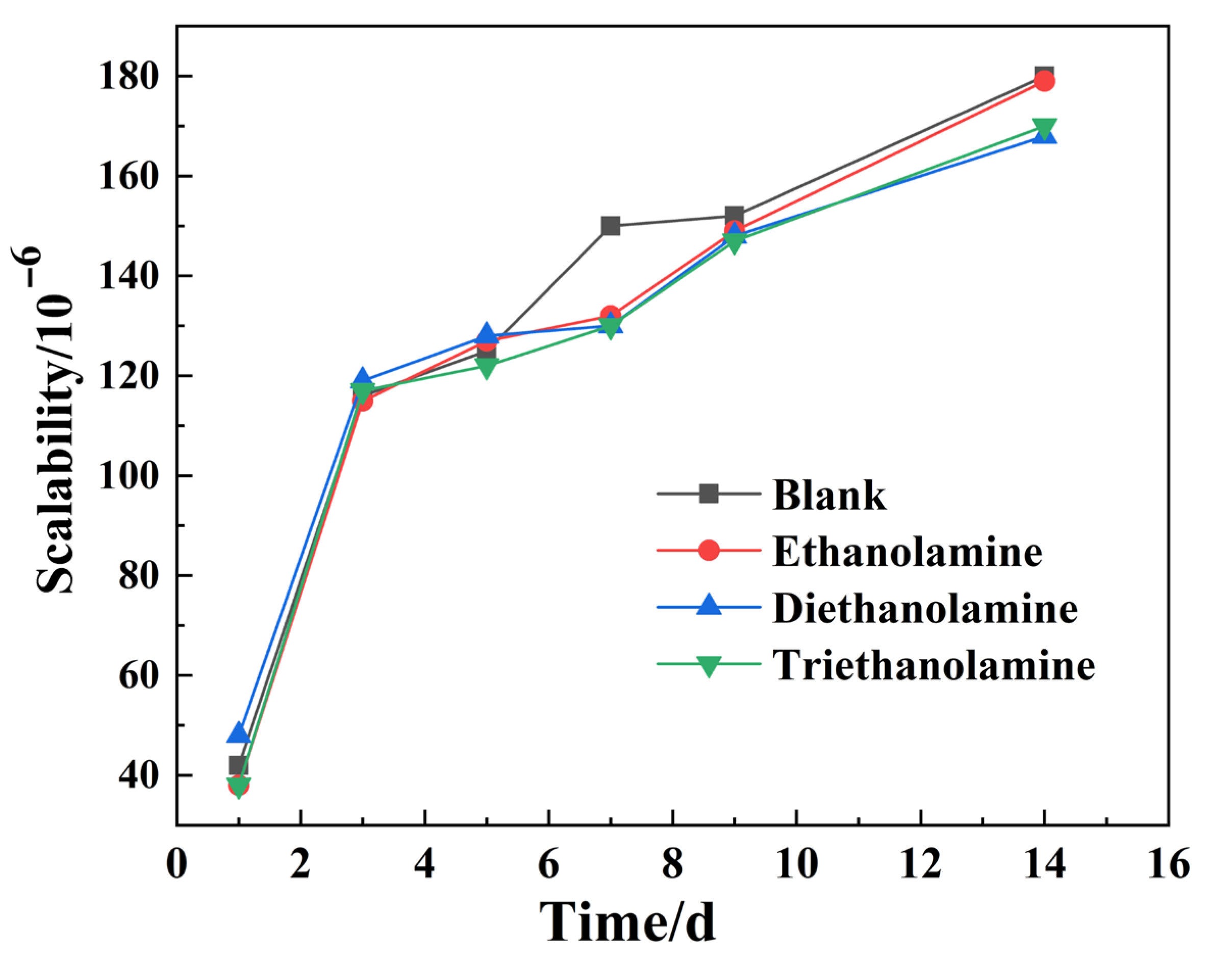
3.4. Effect of Alkanolamine Additives on Shrinkage and Hydration Degree
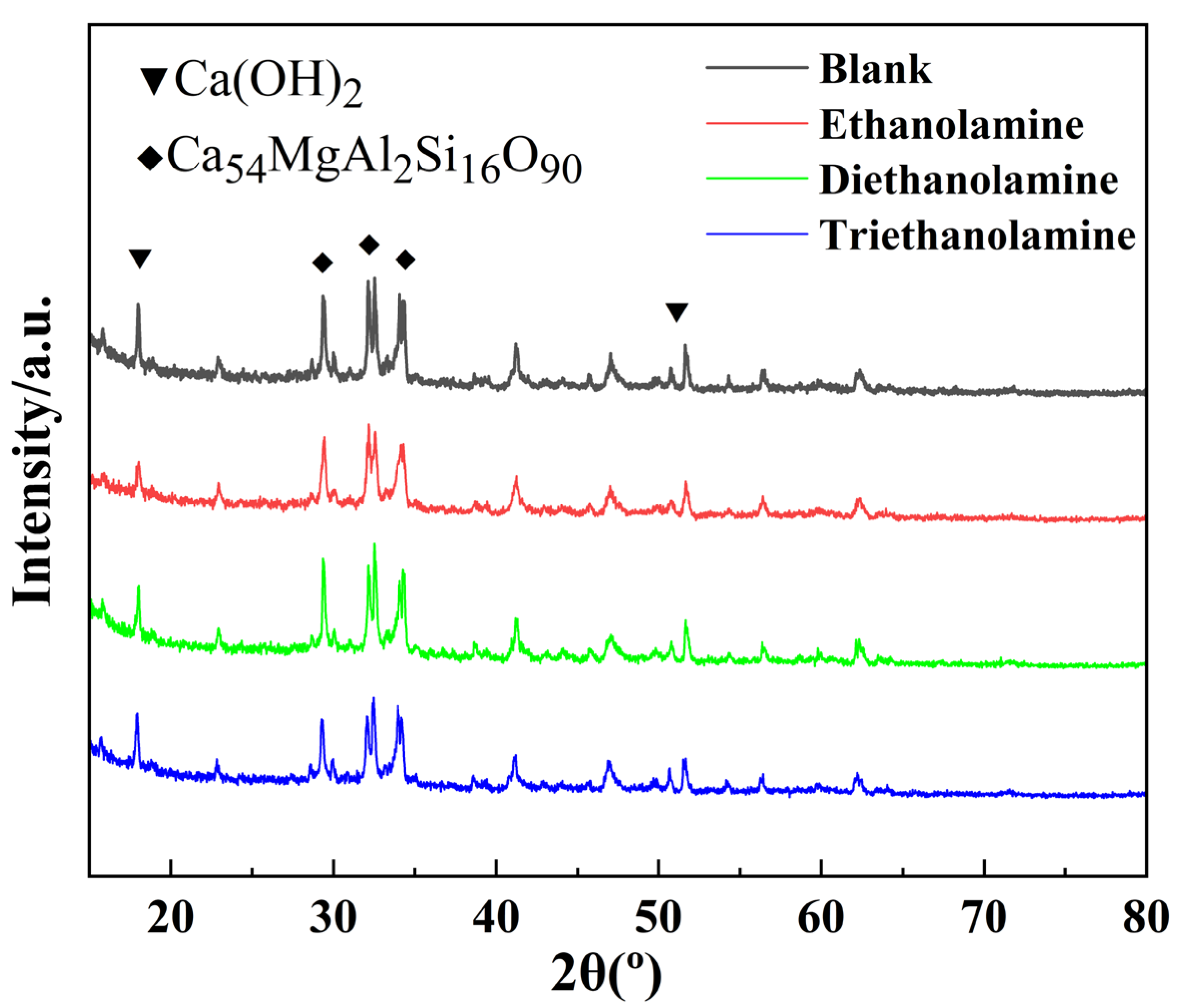
3.5. XRD Analysis
3.6. Mechanism of Alkanolamine Additives on Cement Slurry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aiad, I.; Abd El-Aleem, S.; El-Didamony, H. Effect of Delaying Addition of Some Concrete Admixtures on the Rheological Properties of Cement Pastes. Cem. Concr. Res. 2002, 32, 1839–1843. [Google Scholar] [CrossRef]
- Uliasz–Bocheńczyk, A.; Wiśniowski, R. Impact of Hydrogen on Cement Slurry: A Review. Renew. Sustain. Energy Rev. 2025, 214, 115541. [Google Scholar] [CrossRef]
- Cestari, A.R.; Vieira, E.F.S.; Silva, E.C.S.; Alves, F.J.; Andrade, M.A.S. Synthesis, Characterization and Hydration Analysis of a Novel Epoxy/Superplasticizer Oilwell Cement Slurry—Some Mechanistic Features by Solution Microcalorimetry. J. Colloid Interface Sci. 2013, 392, 359–368. [Google Scholar] [CrossRef]
- He, X.; Li, W.; Su, Y.; Zheng, Z.; Fu, J.; Zeng, J.; Tan, H.; Wu, Y.; Yang, J. Recycling of Plastic Waste Concrete to Prepare an Effective Additive for Early Strength and Late Permeability Improvement of Cement Paste. Constr. Build. Mater. 2022, 347, 128581. [Google Scholar] [CrossRef]
- Cheung, J.; Jeknavorian, A.; Roberts, L.; Silva, D. Impact of Admixtures on the Hydration Kinetics of Portland Cement. Cem. Concr. Res. 2011, 41, 1289–1309. [Google Scholar] [CrossRef]
- Sakai, E.; Ishida, A.; Ohta, A. New Trends in the Development of Chemical Admixtures in Japan. J. Adv. Concr. Technol. 2006, 4, 211–223. [Google Scholar] [CrossRef]
- Huang, T.; Wang, D.; Liu, Z. The Influence of Alcohol Amine Admixtures on the Macroscopic Properties of Portland Cement Paste. Appl. Mech. Mater. 2014, 525, 573–579. [Google Scholar] [CrossRef]
- Heren, Z.; Ölmez, H. The Influence of Ethanolamines on the Hydration and Mechanical Properties of Portland Cement. Cem. Concr. Res. 1996, 26, 701–705. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Hydration of Cement—Role of Triethanolamine. Cem. Concr. Res. 1976, 6, 623–631. [Google Scholar] [CrossRef]
- Gartner, E.; Myers, D. Influence of Tertiary Alkanolamines on Portland Cement Hydration. J. Am. Ceram. Soc. 1993, 76, 1521–1530. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Dong, B.; Chen, Q.; Gu, L.; Yang, X.; Wang, W. Differences between Their Influences of TEA and TEA·HCl on the Properties of Cement Paste. Constr. Build. Mater. 2020, 239, 117797. [Google Scholar] [CrossRef]
- Ma, S.; Li, W.; Shen, X. Study on the Physical and Chemical Properties of Portland Cement with THEED. Constr. Build. Mater. 2019, 213, 617–626. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X. Properties of Coal Gangue-Portland Cement Mixture with Carbonation. Fuel 2019, 245, 1–12. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Tan, H.; Gu, B.; Zhang, T.; Chen, P.; Li, H.; Mei, J. Effect of Aluminum Sulfate on the Hydration of Portland Cement, Tricalcium Silicate and Tricalcium Aluminate. Constr. Build. Mater. 2020, 232, 117179. [Google Scholar] [CrossRef]
- Hu, M.; Guo, J.; Xu, Y.; Fan, J.; Cao, L.; Wang, M.; Feng, Y. Influence of Triethanolamine on Reactivity of Hydrated Matrix in Sodium Silicate Self-Healing System and the Mechanism. Constr. Build. Mater. 2018, 185, 445–452. [Google Scholar] [CrossRef]
- Liu, H.; Lin, H.; Liu, X.; Wang, J.; Pang, X.; Kong, X. Effects of Triethanolamine on Autogenous Shrinkage and Drying Shrinkage of Cement Mortar. Constr. Build. Mater. 2021, 304, 124620. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.; Lu, Z.; Lu, Z.; Zhang, Q.; Dong, B.; Xing, F. Influence of Triethanolamine on the Hydration Product of Portlandite in Cement Paste and the Mechanism. Cem. Concr. Res. 2016, 87, 64–76. [Google Scholar] [CrossRef]
- Yaphary, Y.L.; Yu, Z.; Lam, R.H.W.; Lau, D. Effect of Triethanolamine on Cement Hydration toward Initial Setting Time. Constr. Build. Mater. 2017, 141, 94–103. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Influence of Triethanolamine on the Hydration Characteristics of Tricalcium Silicate. J. Appl. Chem. Biotechnol. 1972, 22, 1125–1138. [Google Scholar] [CrossRef]
- Huang, H.; Shen, X.; Zheng, J. Modeling, Analysis of Interaction Effects of Several Chemical Additives on the Strength Development of Silicate Cement. Constr. Build. Mater. 2010, 24, 1937–1943. [Google Scholar] [CrossRef]
- Han, J.; Wang, K.; Shi, J.; Wang, Y. Mechanism of Triethanolamine on Portland Cement Hydration Process and Microstructure Characteristics. Constr. Build. Mater. 2015, 93, 457–462. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Li, H.; Xu, L.; Ren, Q.; Li, L. Effect of Triethanolamine Hydrochloride on the Performance of Cement Paste. Constr. Build. Mater. 2019, 200, 218–225. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Action of Triethanolamine on the Hydration of Tricalcium Aluminate. Cem. Concr. Res. 1973, 3, 41–54. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, J.; Yang, Q.; Li, Q.; Lu, X.; Ye, Z. Mechanisms of Mechanical Performance Variations in Cement with Diethanol-Isopropanolamine Addition: Insights from a Hydration Perspective. J. Build. Eng. 2025, 107, 112747. [Google Scholar] [CrossRef]
- Hallet, V.; Hertel, T.; Eykens, L.; Belie, N.; Pontikes, Y. Alkanolamines as Grinding Aids and Hydration Enhancers in Blended Cements with Iron-Rich Non-Ferrous Metallurgy Slag. Constr. Build. Mater. 2025, 470, 140575. [Google Scholar] [CrossRef]
- Zeng, B.; Han, W.; Jia, S.; Mo, L. Effects of a Novel Amide-Alcohol Bearing Admixture on the Hydration, Microstructure and Strength of Portland Cement. Constr. Build. Mater. 2024, 435, 136910. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standardization Administration of the People’s Republic of China: Beijing, China, 2021.





| Dosage, % | θ600 | θ300 | θ200 | θ100 | θ6 | θ3 | n | K |
|---|---|---|---|---|---|---|---|---|
| 0 | 150 | 120 | 96 | 79 | 36 | 30 | 0.3805 | 5.3540 |
| 0.2 | 150 | 124 | 99 | 80 | 37 | 30 | 0.3989 | 4.9328 |
| 0.4 | 152 | 125 | 98 | 81 | 36 | 31 | 0.3949 | 5.0980 |
| 0.6 | 156 | 124 | 99 | 81 | 36 | 31 | 0.3876 | 5.2932 |
| 0.8 | 155 | 128 | 100 | 84 | 37 | 31 | 0.3834 | 5.6091 |
| 1.0 | 158 | 130 | 102 | 85 | 38 | 30 | 0.3868 | 5.5793 |
| Dosage/% | θ600 | θ300 | θ200 | θ100 | θ6 | θ3 | n | K |
|---|---|---|---|---|---|---|---|---|
| 0 | 150 | 120 | 96 | 79 | 36 | 30 | 0.381 | 5.3542 |
| 0.2 | 159 | 122 | 99 | 81 | 37 | 30 | 0.373 | 5.7111 |
| 0.4 | 163 | 125 | 100 | 83 | 38 | 29 | 0.373 | 5.8550 |
| 0.6 | 168 | 128 | 102 | 85 | 38 | 29 | 0.373 | 5.9991 |
| 0.8 | 170 | 129 | 103 | 86 | 39 | 28 | 0.369 | 6.1822 |
| 1.0 | 172 | 131 | 105 | 88 | 40 | 28 | 0.362 | 6.5543 |
| Dosage, % | θ600 | θ300 | θ200 | θ100 | θ6 | θ3 | n | K |
|---|---|---|---|---|---|---|---|---|
| 0 | 150 | 120 | 96 | 79 | 36 | 30 | 0.381 | 5.3541 |
| 0.2 | 155 | 120 | 95 | 80 | 38 | 29 | 0.369 | 5.7502 |
| 0.4 | 159 | 122 | 93 | 82 | 39 | 30 | 0.362 | 6.1230 |
| 0.6 | 162 | 123 | 92 | 83 | 38 | 29 | 0.358 | 6.3146 |
| 0.8 | 168 | 123 | 90 | 84 | 39 | 28 | 0.347 | 6.7588 |
| 1.0 | 170 | 125 | 90 | 86 | 39 | 29 | 0.340 | 7.1634 |
| Dosage, % | Density (g/cm3) | Density Difference (g/cm3) | ||
|---|---|---|---|---|
| Upper | Center | Below | ||
| 0 | 1.80 | 1.82 | 1.83 | 0.3 |
| 0.2 | 1.80 | 1.81 | 1.83 | 0.3 |
| 0.4 | 1.81 | 1.82 | 1.84 | 0.3 |
| 0.6 | 1.82 | 1.82 | 1.84 | 0.2 |
| 0.8 | 1.82 | 1.82 | 1.84 | 0.2 |
| 1.0 | 1.83 | 1.83 | 1.85 | 0.2 |
| Dosage, % | Density (g/cm3) | Density Difference (g/cm3) | ||
|---|---|---|---|---|
| Upper | Center | Below | ||
| 0 | 1.80 | 1.82 | 1.83 | 0.3 |
| 0.2 | 1.80 | 1.80 | 1.82 | 0.2 |
| 0.4 | 1.81 | 1.82 | 1.83 | 0.2 |
| 0.6 | 1.81 | 1.81 | 1.83 | 0.2 |
| 0.8 | 1.82 | 1.82 | 1.83 | 0.1 |
| 1.0 | 1.82 | 1.82 | 1.83 | 0.1 |
| Dosage, % | Density (g/cm3) | Density Difference (g/cm3) | ||
|---|---|---|---|---|
| Upper | Center | Below | ||
| 0 | 1.80 | 1.82 | 1.83 | 0.3 |
| 0.2 | 1.80 | 1.81 | 1.82 | 0.2 |
| 0.4 | 1.81 | 1.81 | 1.83 | 0.2 |
| 0.6 | 1.81 | 1.81 | 1.82 | 0.1 |
| 0.8 | 1.82 | 1.82 | 1.83 | 0.1 |
| 1.0 | 1.83 | 1.83 | 1.84 | 0.1 |
| Alcoholic Amine Type | Thickening Time (min) | Fluidity (cm) |
|---|---|---|
 | 125 | 15.8 |
 | 25 | 14.6 |
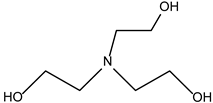 | 15 | 14.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Shi, Y.; Wang, L.; Zhuang, Y.; Li, Y.; Chen, G. Hydroxyl Group-Dependent Effects of Alkanolamine Additives on Rheology, Hydration, and Performance of Early-Strength Cement Slurries. Processes 2025, 13, 2681. https://doi.org/10.3390/pr13092681
Zhao Y, Shi Y, Wang L, Zhuang Y, Li Y, Chen G. Hydroxyl Group-Dependent Effects of Alkanolamine Additives on Rheology, Hydration, and Performance of Early-Strength Cement Slurries. Processes. 2025; 13(9):2681. https://doi.org/10.3390/pr13092681
Chicago/Turabian StyleZhao, Yifei, Ya Shi, Longjiang Wang, Yan Zhuang, Yongfei Li, and Gang Chen. 2025. "Hydroxyl Group-Dependent Effects of Alkanolamine Additives on Rheology, Hydration, and Performance of Early-Strength Cement Slurries" Processes 13, no. 9: 2681. https://doi.org/10.3390/pr13092681
APA StyleZhao, Y., Shi, Y., Wang, L., Zhuang, Y., Li, Y., & Chen, G. (2025). Hydroxyl Group-Dependent Effects of Alkanolamine Additives on Rheology, Hydration, and Performance of Early-Strength Cement Slurries. Processes, 13(9), 2681. https://doi.org/10.3390/pr13092681







