Abstract
With the popularization of nuclear energy in the field of energy application, the effective removal of radioactive iodine isotopes is crucial for the long-term development of nuclear energy. In this paper, bimetallic MOFs with different Cu/Bi ratios were synthesized by a simple solvothermal method, and a bimetallic nano-adsorbent Cux/Bi10−x-NC was prepared by one-step calcination. Adsorption experiments show that Cux/Bi10−x-NC exhibits excellent adsorption performance for iodide ions, gaseous iodine, and I2 in cyclohexane solution, with the maximum adsorption capacities reaching up to 484.08 and 233.11 mg g−1, respectively. Through the characterization of the material system before and after adsorption, this excellent adsorption performance is attributed to the synergistic effect between Cu and Bi, as well as the highly dispersed adsorption active sites derived from the MOF template. Therefore, the prepared Cux/Bi10−x-NC has great potential in the efficient and stable capture of various forms of iodine.
1. Introduction
With the increasingly serious problem of energy shortage, more and more researchers are dedicated to developing high-quality energy sources that can replace traditional fossil fuels [1]. Nuclear energy, due to its advantages of being clean, efficient, and free of greenhouse gas emissions, has received extensive attention and rapid development in recent years. However, the use of nuclear energy is accompanied by the release of large amounts of different forms of radioactive iodine isotopes (131I and 129I), which can cause extreme long-term radioactive toxicity to animals, plants, and humans [2,3]. Moreover, these radioactive iodines can accumulate in human thyroids through the atmosphere, water cycle, and food chain, thereby causing thyroid cell damage or even thyroid cancer, posing a serious threat to human health [4,5]. Therefore, the effective removal of radioactive iodine isotopes is crucial for the long-term development of nuclear energy.
Currently, various methods have been developed for capturing radioactive iodine, such as adsorption, ion exchange, catalytic oxidation, and wet scrubbing [6,7,8,9]. Among them, adsorption is considered the most promising method for capturing radioactive iodine, as it has the advantages of high removal rate, a simple process, and low cost [10,11,12]. In recent years, a variety of materials, including activated carbon [13,14], aerogels [15,16], metal-based adsorbents [17,18], and MOFs [19,20], have been used as adsorbents for capturing radioactive iodine. Activated carbon, with its large specific surface area and low cost, has been widely used for capturing radioactive iodine. However, since activated carbon mainly captures radioactive iodine in gas or liquid through physical adsorption, radioactive iodine is prone to desorption during the post-treatment of the adsorbent, leading to secondary pollution [21]. Metal-based composite materials, including silver-based, copper-based, and bismuth-based adsorbents, mainly capture radioactive iodine through chemical adsorption, which can form stable compounds with iodine, thereby avoiding secondary pollution in subsequent processing [22,23,24]. Among them, compared with expensive silver-based adsorbents, bismuth-based and copper-based adsorbents with lower costs have shown great potential in capturing radioactive iodine. For example, Zhou et al. found that the prepared Cu/Cu2O@NC-400 nanocomposite adsorbent can rapidly adsorb iodide ions in water and reach the equilibrium time within 40 min, attributed to the highly dispersed Cu2O nanoparticles on the material surface and the doping of a certain amount of Cu0 [25]. Han et al. prepared a nanocomposite material composed of Bi and graphene oxide (Bi-GO), with a maximum adsorption capacity of 230 mg g−1 [26]. More importantly, this nanocomposite material has a removal efficiency of ≥95% for iodide and iodate, which is superior to the existing commercial Ag-exchanged zeolite for iodine capture. However, although numerous studies have confirmed the excellent iodine capture potential of copper-based and bismuth-based adsorbents, traditional methods for preparing copper-based and bismuth-based adsorbents still face problems such as easy particle agglomeration, slow adsorption rate, and a high proportion of physical adsorption.
Metal–organic frameworks (MOFs) are a class of crystalline porous materials formed by the self-assembly of metal ions and organic ligands, which have the advantages of large specific surface area, high porosity, and high modifiability [27,28]. By using MOFs as self-sacrificing templates and precursors, various MOF-derived materials (such as porous carbon, metal oxides, and metal–carbon nanohybrids) can be prepared through simple pyrolysis. Metal ions in MOFs can form metal single substances/metal oxide particles, while common organic linkers can also be converted into porous carbon materials at a high yield. Existing studies have also shown that metal-based composite materials obtained from MOF derivatives usually exhibit significantly enhanced iodine capture capacity [29]. For example, Chen et al. used Cu-Zn ZIFs as precursors and prepared nwCu0@ZnO nanocomposite materials through two-step calcination for the removal of iodide ions in water [17]. The saturated adsorption capacity of nwCu0@ZnO for iodide ions is as high as 220.6 mg g−1, which is much higher than that of copper-based adsorbents prepared by traditional methods. Chen et al. prepared AC-Bi2S3-x@C nanocomposites by one-step sulfidation using CAU-17 as the precursor. Thanks to the synergy of amorphous structure and sulfur vacancy defects, AC-Bi2S3−x@C shows an adsorption capacity of up to 1763.9 mg g−1 for gaseous iodine and a high chemical adsorption ratio of 93.4%, reaching adsorption equilibrium within 15 min [30].
In this study, Cux/Bi10−x-NC nanocomposites were prepared by one-step calcination using Cu-Bi bimetallic MOFs as precursors, and the microstructure and chemical composition of the materials were comprehensively characterized. On this basis, the effects of Cu/Bi ratio, calcination temperature, adsorption temperature, initial pH, and coexisting ions on the adsorption of iodide ions and the capture of gaseous iodine by the nanocomposites were investigated. In addition, by characterizing the adsorbents before and after adsorbing radioactive substances, the potential mechanism of Cux/Bi10−x-NC nanocomposites for capturing iodide ions in solution and gaseous iodine was proposed.
2. Experiment
2.1. Chemicals & Reagents
The chemicals used in this study are described in Text S1.
2.2. Preparation of the Cux/Bi10−x-MOF and Cux/Bi10−x-NC
Cux/Bi10−x-MOFs were prepared with slight modifications to the existing method for the single-metal Bi-MOFs [31]. In a typical synthesis process, 485.07 mg of Bi(NO3)3·5H2O and 144.57 mg of Cu(NO3)2·3H2O were dissolved in 30 mL of ethylene glycol under ultrasonication, and 498.39 mg of H2BDC was dissolved in 30 mL of DMF. The two solutions were then mixed and stirred continuously for 2 h. Subsequently, the well-mixed solution was then transferred to the 100 mL Teflon autoclave and heated at 150 °C for 16 h. After the autoclave was naturally cooled to room temperature, the solid product obtained was collected by centrifugation and then washed with DMF and ethanol, repeated several times. After being dried under vacuum at 60 °C for 12 h, the final products were obtained and named as Cux/Bi10−x-MOFs. The total dosage of Cu(NO3)2·3H2O and Bi(NO3)3·6H2O used in the synthesis of Cux/Bi10−x-MOFs was 2 mmol, where x refers to the molar ratios of Cu in the two metals (the molar ratios were set to Cu:Bi = 5:5; 4:6; 3:7; 2:8; 1:9, respectively).
Subsequently, the prepared Cux/Bi10−x-MOFs were pyrolyzed at 600 °C for 2 h with a heating rate of 5 °C/min under a nitrogen atmosphere, and then Cux/Bi10−x-NC was obtained. Meanwhile, the Cu-NC and Bi-NC were also obtained by a similar method from single-metal Cu-MOF and Bi-MOF and compared with the iodine capture properties of Cux/Bi10−x-NC. The detailed synthesis methods for Cu-MOF and Bi-MOF are shown in the Supporting Materials.
2.3. Experiment of the Iodine Capture
2.3.1. Capture of Iodide Ions in Water
Due to the chemical toxicity and radioactivity of the radioisotope 131I, the non-radioactive isotope 127I (NaI), which has the same chemical properties as 131I, was used as a simulated adsorption object in this paper. The adsorption performance of Cux/Bi10−x-NC on iodide ions was verified by isothermal adsorption test, kinetic adsorption test, and the study of solution pH and interfering ions.
The adsorption performance was measured by the adsorption capacity, and the absorbance of the sample at a wavelength of 227 nm was determined using a UV spectrophotometer, which can be used to find the equilibrium mass concentration of iodide ions in the solution according to the standard curve, and then the equilibrium adsorption capacity of the adsorbent can be derived from Equation (1) [17].
where qe represents the equilibrium adsorption capacity (mg/g), and C0 and Ce denote the initial and equilibrium mass concentration of iodide ions (mg/L), respectively. Furthermore, V is the volume of iodide ion solution (mL), and m is the mass of Cux/Bi10−x-NC (mg).
2.3.2. Capture of Iodine Vapor
Iodine vapor uptake experiments based on gravimetric analysis were performed in the following steps. An amount of 100 mg of five samples was placed in open heat-resistant glass caps and an excess of iodine solids was placed in a gas-tight container and heated at 350 K and ambient pressure. After trapping the iodine vapor for some time, the samples were cooled to room temperature and weighed. The amount of iodine monomer vapor adsorbed by 100 mg of material at a given time was calculated by using the difference between the two weights through Equation (2) [32].
where Q represents the iodine capture capacities of Cux/Bi10−x-NC (wt%). The m1 is the mass of the Cux/Bi10−x-NC (g), and mt is the mass of the Cux/Bi10−x-NC at time t (g).
2.3.3. Capture of Iodine in Cyclohexane
An iodine-containing cyclohexane solution was used to evaluate the adsorption properties of the prepared materials for iodine in solution. The iodine adsorption experiments were carried out as follows: 10 mg of the sample was dispersed in 10 mL of iodine-containing cyclohexane solution and the mixture was stirred in a thermostatic shaker at room temperature (25 °C) with a constant speed of 150 rpm.
The residual iodine concentration in cyclohexane at different time points was then monitored by measuring the absorbance of the supernatant at 523 nm using a UV–Vis spectrophotometer. The equilibrium adsorption capacity of the sample for iodine (Qe, mg/g) was calculated using Equation (1).
3. Results and Discussion
3.1. Characterization of the Cux/Bi10−x-NC
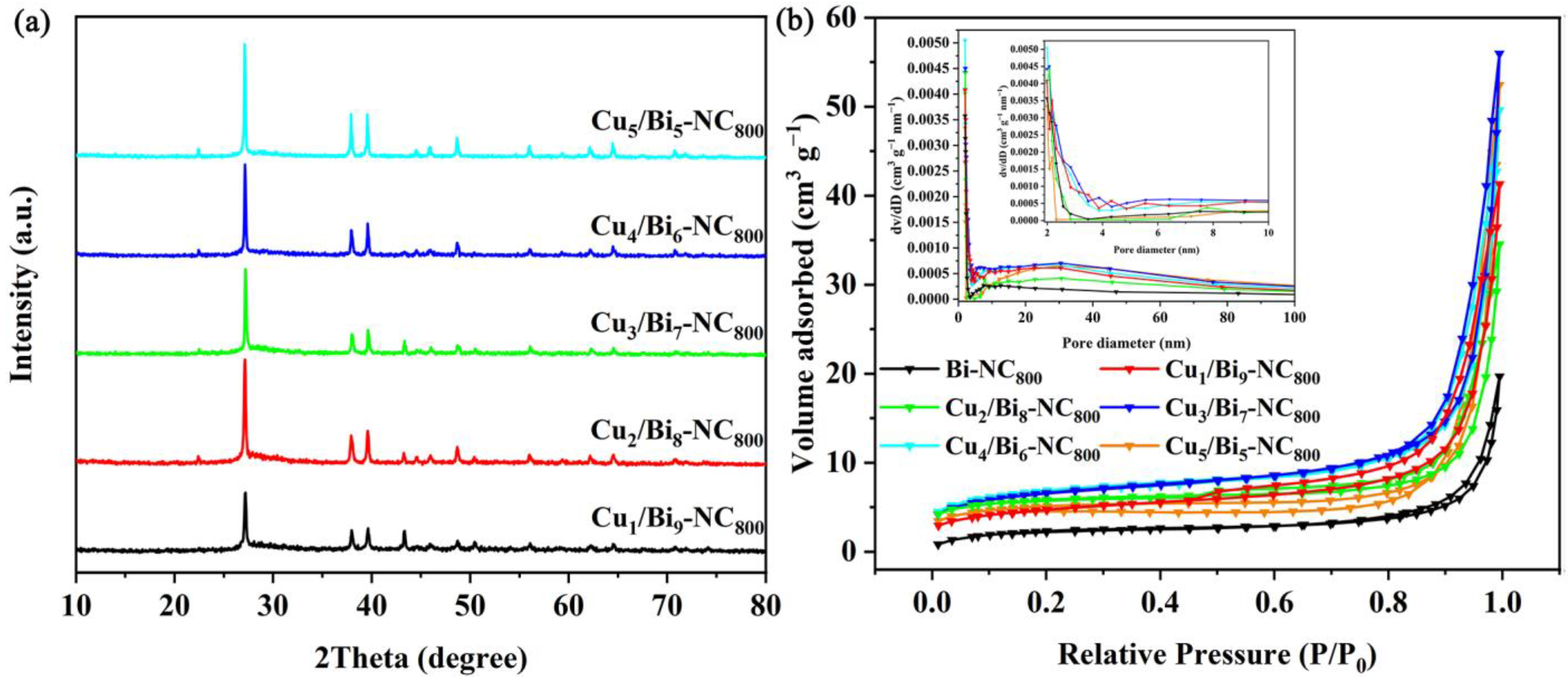
Firstly, the nanocomposites with different Cu/Bi ratios were characterized by XRD. As shown in Figure 1, the XRD patterns of Cux/Bi10−x-NC with different ratios of copper nitrate and bismuth nitrate did not show significant differences, indicating that the variation of the Cu/Bi ratio during the synthesis process did not affect the main crystalline components of the synthesized materials. As shown in Figure 1a, characteristic peaks of Bi0 were detected at 22.5°, 27.2°, 38.1°, 39.7°, 48.8°, 62.2°, and 64.5°, corresponding to the (003), (012), (104), (110), (202), (116), and (122) crystal planes, respectively. Diffraction peaks attributed to Cu species were detected at 43.4°, 50.5°, and 39.68°, corresponding to the (111) and (200) crystal planes of Cu0 and the (110) crystal plane of CuO, respectively. Meanwhile, it could be observed that with the increase in the Cu/Bi molar ratio, although the main crystalline phase remains the same, the relative intensity of the diffraction peaks changes significantly. This indicates that the main components of the synthesized Cux/Bi10−x-NC are Bi0, Cu0, and CuO. In addition, the XRD patterns of Cux/Bi10−x-NC calcined at 600 °C and 1000 °C were also analyzed. As shown in Figure S1, unlike Cux/Bi10−x-NC, Cux/Bi10−x-NC600 exhibited characteristic peaks at 27.9°, 31.7°, 32.7°, 46.2°, and 55.5°, which were attributed to Bi2O3 and corresponded to the (201), (002), (220), (222), and (421) crystal planes of Bi2O3, respectively. The characteristic peaks at 35.5° and 48.6° belonged to CuO, indicating that Cux/Bi10−x-NC600 was composed of Bi2O3 and CuO. When the pyrolysis temperature was 1000 °C, the XRD pattern of Cux/Bi10−x-NC1000 was similar to that of Cux/Bi10−x-NC800, but the characteristic peaks attributed to CuO were absent, suggesting that Cux/Bi10−x-NC1000 was composed of Bi0 and Cu0.
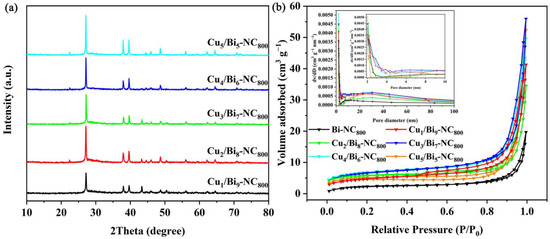
Figure 1.
(a) XRD spectra of Cux/Bi10−x-NC800 and (b) N2 adsorption–desorption isotherms of the Cux/Bi10−x-NC800 composites.
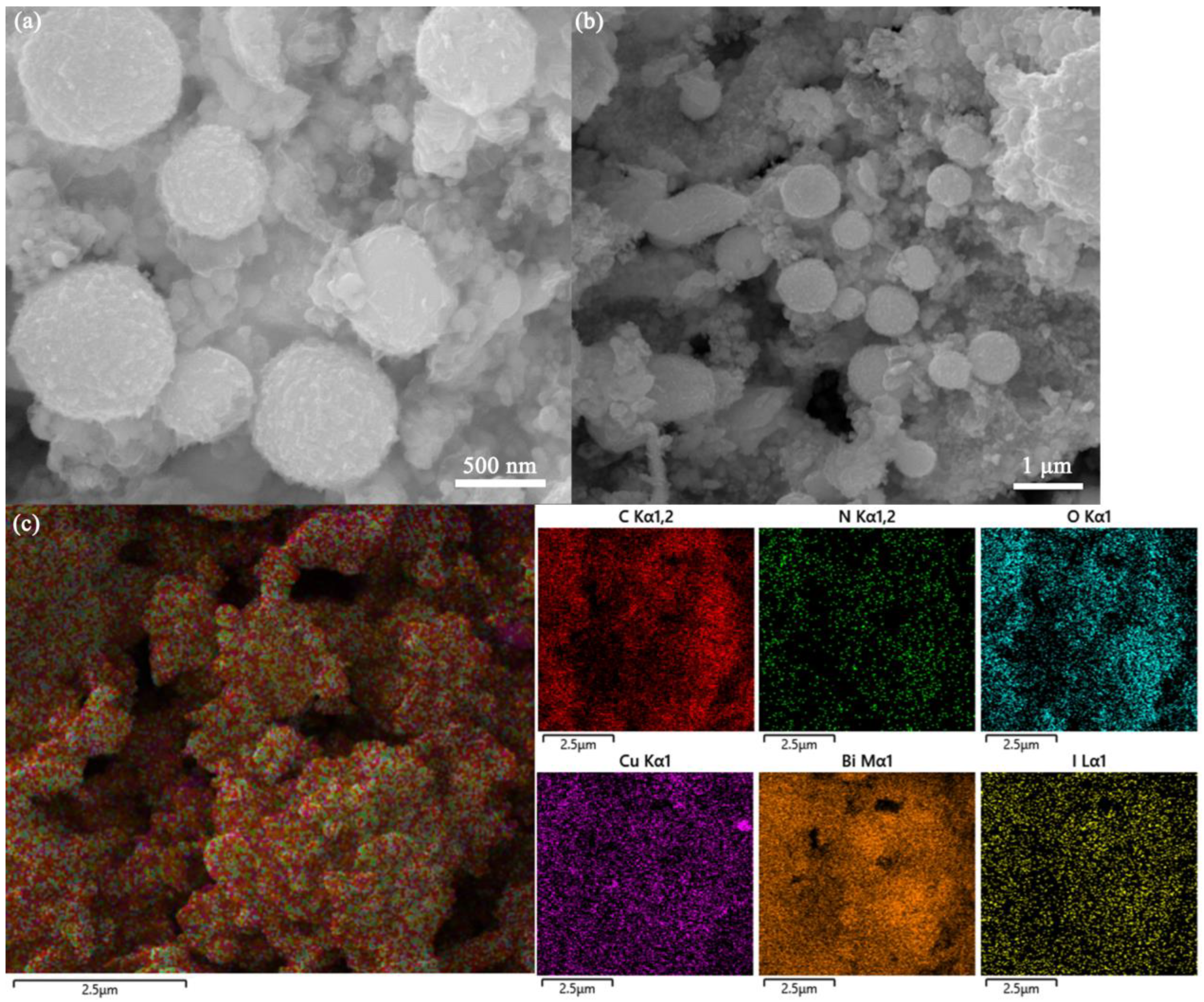
A larger specific surface area can provide more active adsorption sites for the capture of I2. Therefore, the effect of Cu introduction on the Cux/Bi10−x-NC800 composites was investigated by N2 adsorption–desorption isotherms. Figure 1b shows the specific surface area and pore size distribution of different proportions of Cux/Bi10−x-NC800. It can be observed that all Cux/Bi10−x-NC800 with different Cu/Bi ratios exhibit typical type IV N2 adsorption–desorption isotherms with an H3 hysteresis loop. However, a sharp increase in adsorption capacity around p/p0 ≈ 1.0 indicates that it is mainly external surface area or macropores, rather than true internal micropores or mesopores. The BET specific surface areas of Bi-NC800, Cu1/Bi9-NC800, Cu2/Bi8-NC800, Cu3/Bi7-NC800, Cu4/Bi6-NC800, and Cu5/Bi5-NC800 were calculated to be 12.7, 23.6, 27.6, 32.4, 41.4, and 24.5 m2 g−1, respectively. This indicates that the specific surface area of Cux/Bi10−x-NC800 gradually increases with the introduction of Cu, and reaches the maximum specific surface area when the Cu/Bi ratio is 4:6. Additionally, the pore size distribution curve obtained by the BJH method (assuming cylindrical pores) is shown in Figure 1b, indicating that the pores in the composite material are primarily distributed within the range of 2 to 4 nm. And with the increase in Cu content, the number of pores within the range of 3 to 4 nm gradually increases. However, this result contradicts the previously obtained conclusions. The adsorption–desorption isotherms and the relatively small BET specific surface area suggest that the material is likely to be macroporous or nonporous. As shown in Figure 2a, the SEM image reveals the microstructure of Cu4Bi6-NC800, which is an irregular spherical shape with a rough surface and an overall diameter of 500 to 800 nm. Additionally, the elemental mapping characterization also confirms the coexistence of Cu and Bi elements in the material.
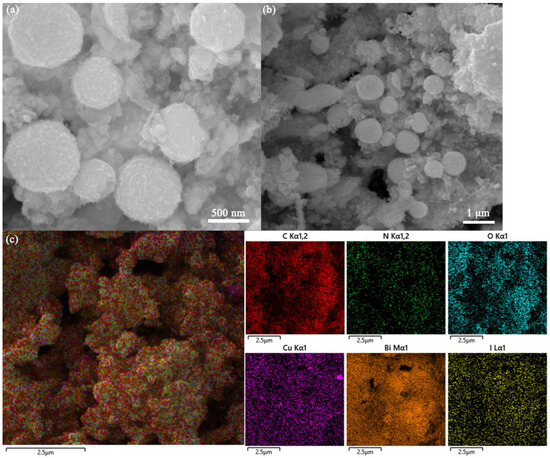
Figure 2.
(a) SEM image of Cu4/Bi6-NC800, (b) Cu4/Bi6-NC800 after the adsorption, and (c) the distribution of elements in Cu4/Bi6-NC800 after the adsorption.
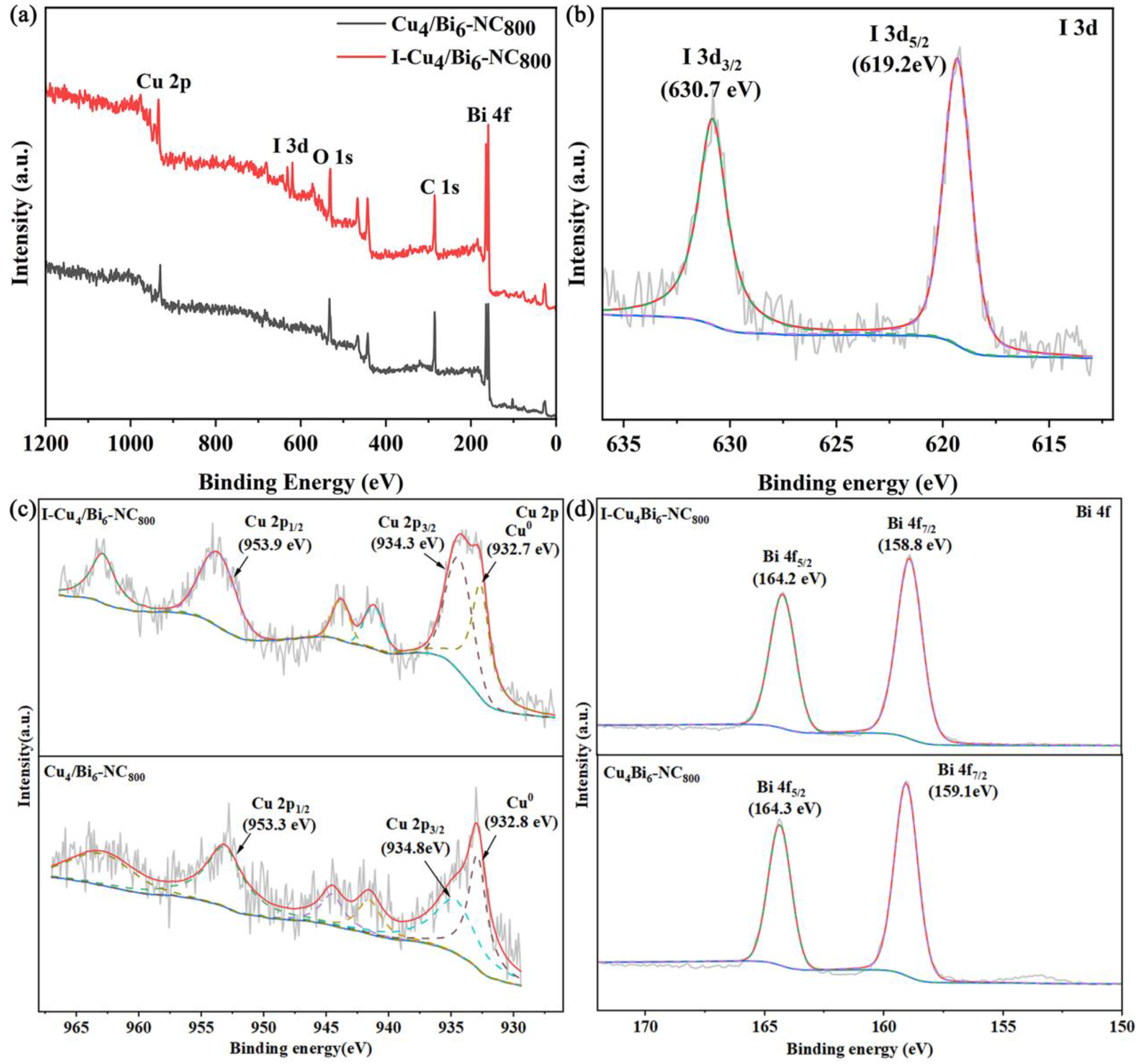
To determine the main components and the existence forms of elements in the Cu/Bi-NC composite materials, XPS characterization was conducted on Cu4/Bi6-NC800 and I-Cu4/Bi6-NC800, and the results are shown in Figure 3. The result indicates that the Cu4/Bi6-NC800 material contains characteristic peaks of elements such as Cu, Bi, O, and C. For the high-resolution XPS spectrum of Cu 2p (Figure 3c), a characteristic peak attributed to the Cu 2p1/2 orbital was detected at a binding energy of 953 eV, and characteristic peaks attributed to the Cu 2p3/2 orbital were detected at 934.3 eV and 932.7 eV. Combined with XRD, the characteristic peak at a binding energy of 932.7 eV indicates the presence of Cu0 in the material. The obvious Cu 2p satellite peaks also suggest that the material contains Cu2+, indicating that Cu in the Cu4/Bi6-NC800 material mainly exists in the forms of Cu2+ and Cu0. The two distinct characteristic peaks in the high-resolution XPS spectrum of Bi4f indicate that Bi in the material exists in the form of Bi3+.
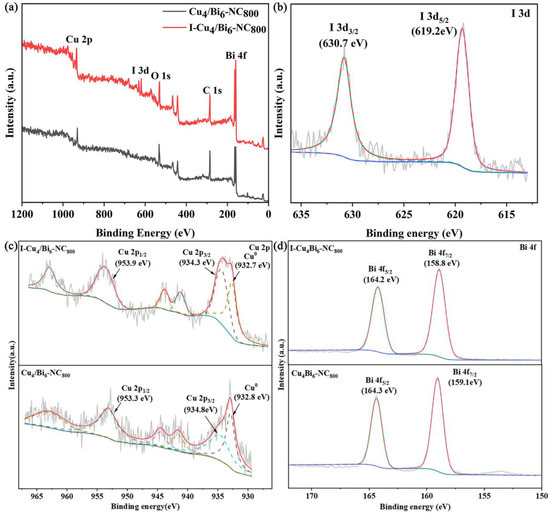
Figure 3.
XPS spectra of Cu4/Bi6-NC800 and Cu4/Bi6-NC800 after adsorption: (a) survey spectra; (b) I 3d spectra; (c) Cu 2p spectra; (d) Bi 4f spectra.
3.2. Capture Properties Evaluation of the Iodine Vapor
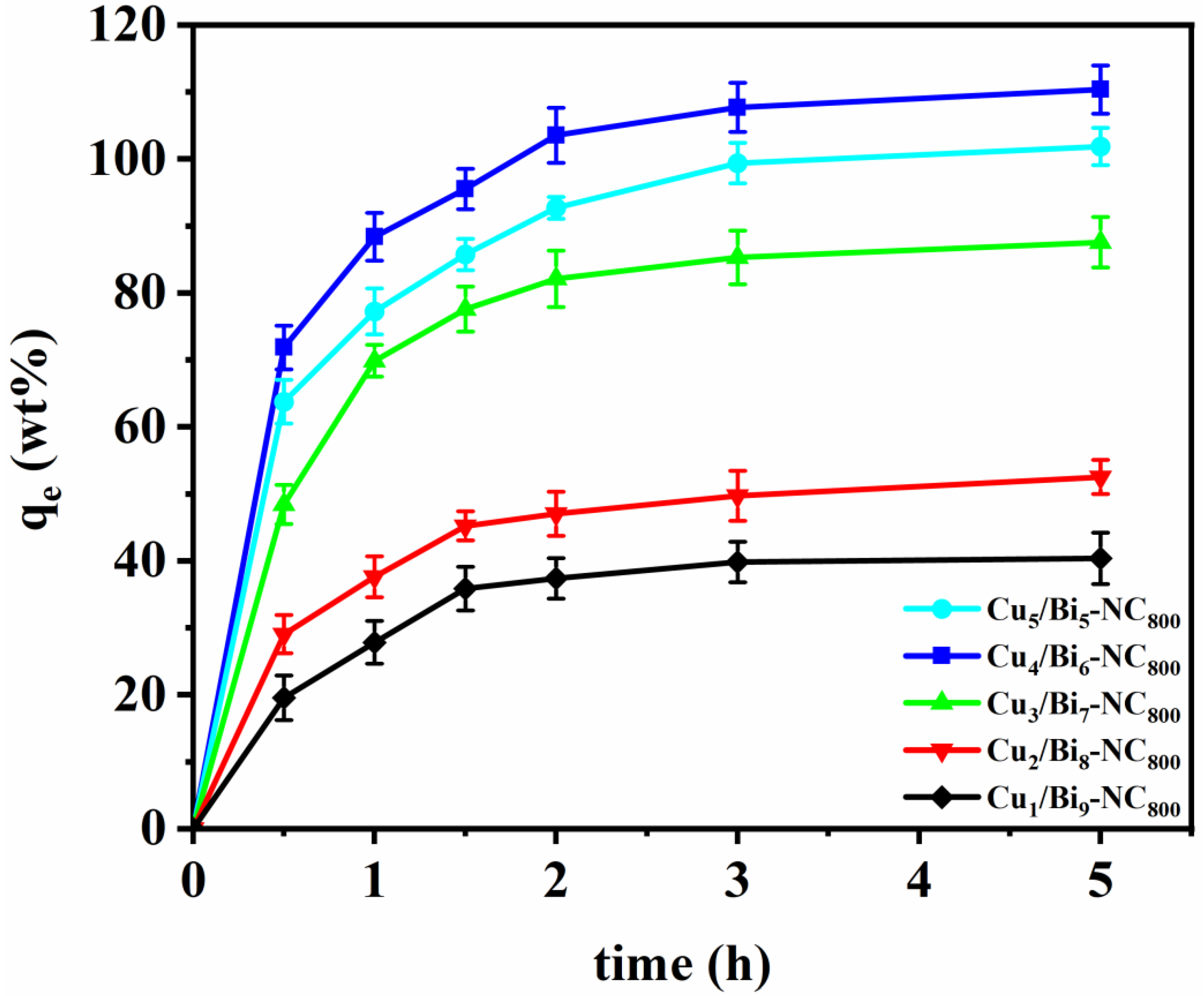
The iodine absorption capacity of Cux/Bi10−x-NC800 was evaluated by placing the samples in a sealed container filled with iodine vapor at 350 K and ambient pressure, conditions typical for nuclear fuel reprocessing. As illustrated in Figure 4, all samples exhibited a sharp increase in weight within the first 30 min, indicating rapid iodine adsorption. After 2 h, no significant weight change was observed, suggesting that adsorption equilibrium had been reached. The equilibrium adsorption capacities varied among Cux/Bi10−x-NC800 samples with different Cu doping ratios: 100.84 wt% (Cu5/Bi5-NC800), 110.36 wt% (Cu4/Bi6-NC800), 87.55 wt% (Cu3/Bi7-NC800), 51.49 wt% (Cu2/Bi8-NC800), and 40.35 wt% (Cu1/Bi9-NC800). It is evident that the adsorption capacity initially increased with higher Cu content, peaking at a Cu/Bi ratio of 4:6. However, further increases in Cu content led to a slight decrease in adsorption capacity. Notably, Cu4/Bi6-NC800, derived from Cu/Bi MOFs, demonstrated the highest I2 adsorption capacity of 110.36 wt%, surpassing other similar solid adsorbents. This suggests that Cux/Bi10−x-NC800 holds significant potential for capturing gaseous iodine. The enhanced performance may be attributed to the larger surface area and pore volume of the MOF-derived nanocomposites, which provide more binding sites and space for iodine adsorption. Furthermore, post-experiment color changes from black to dark brown in the Cu/Bi MOF-derived materials serve as visual confirmation of successful iodine capture.
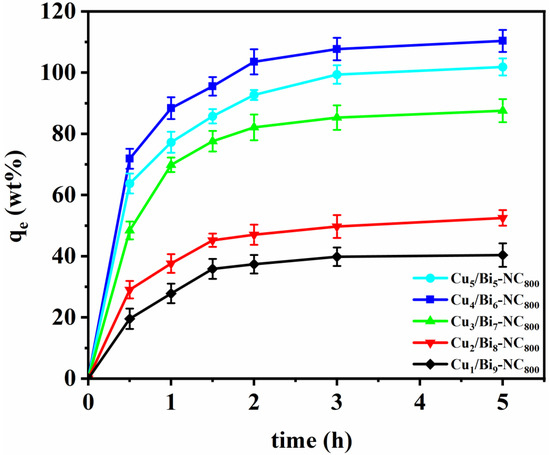
Figure 4.
Adsorption properties of Cux/Bi10−x-NC800 for gaseous iodine.
3.3. Capture Properties Evaluation of the Iodine in Cyclohexane
3.3.1. Adsorption Isotherms
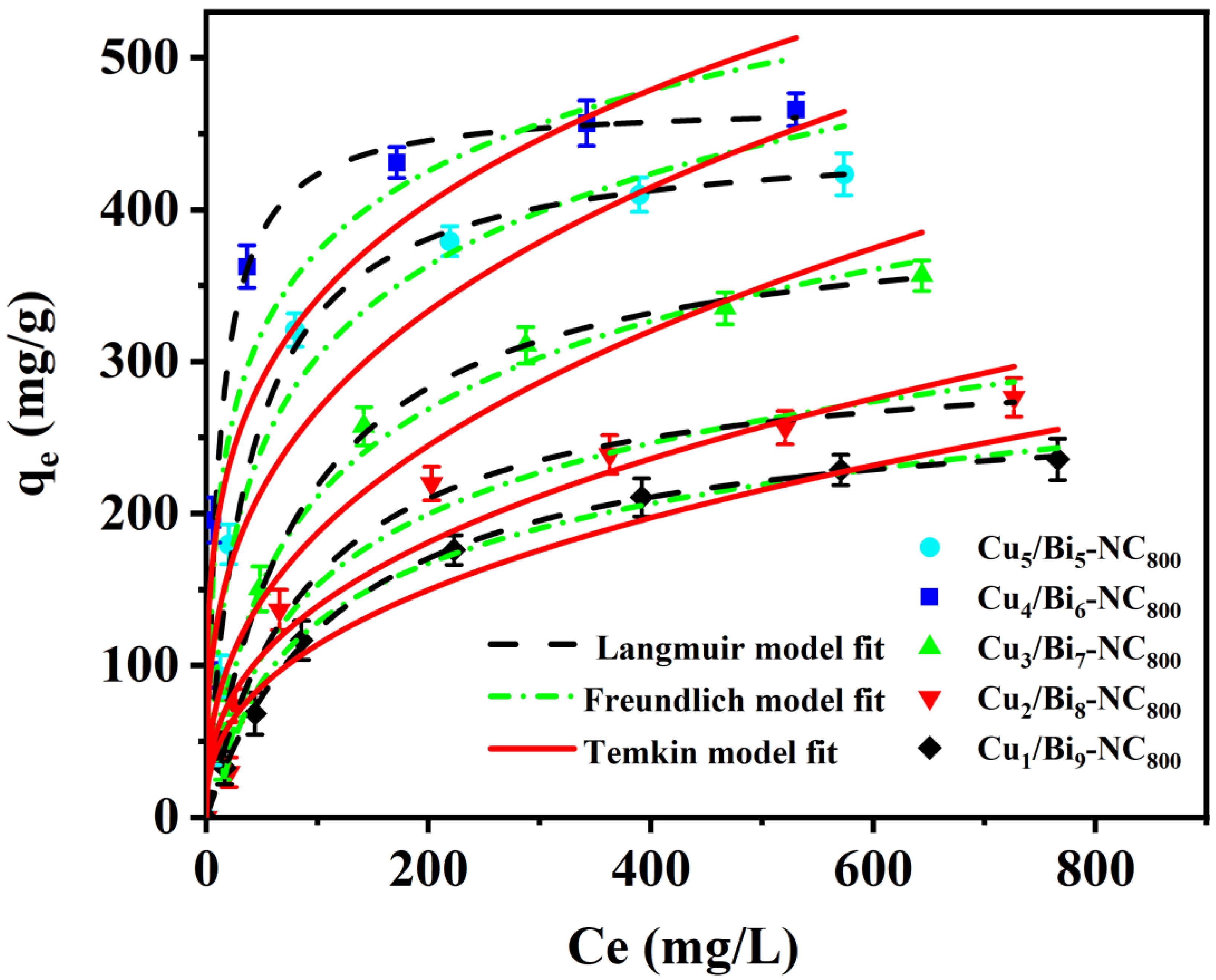
In addition, considering the significance of nuclear waste management, the adsorption performance of the prepared adsorbents for iodine in cyclohexane solution was further investigated. Firstly, the adsorption isotherms of Cux/Bi10−x-NC800 in cyclohexane solutions with different initial concentrations of I2 were measured at 25 °C. Figure 5 reveals that the I2 adsorption capacity of Cux/Bi10−x-NC800 increases with the increase in the initial concentration of I2 in the cyclohexane solution. Moreover, with the increase in the Cu doping ratio, the equilibrium adsorption capacity shows a trend of first increasing and then decreasing, which is consistent with the change trend of the adsorption performance of Cux/Bi10−x-NC800 for gaseous iodine. Through calculation, it could be concluded that the maximum adsorption capacities of Cu5/Bi5-NC800, Cu4/Bi6-NC800, Cu3/Bi7-NC800, Cu2/Bi8-NC800, and Cu1/Bi9-NC800 for I2 in cyclohexane solution are 461.93, 484.08, 412.98, 321.93, and 279.29 mg/g, respectively, which was similar to the data measured by the experiment (450.06, 470.34, 401.55, 308.69, and 276.24 mg/g).
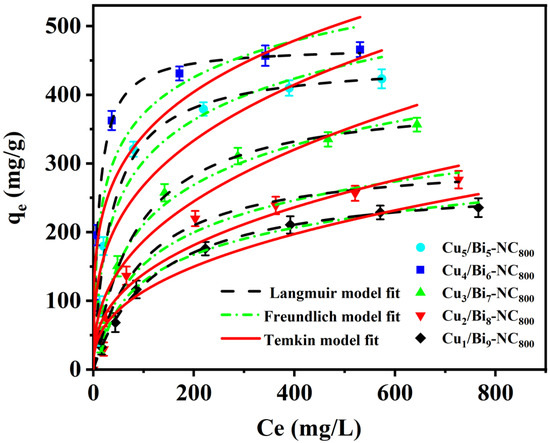
Figure 5.
Adsorption isotherms of I2 on Cux/Bi10−x-NC800 composites.
To better understand the potential mechanism of I2 adsorption by Cux/Bi10−x-NC800 in cyclohexane, the adsorption isotherm data were analyzed using the Langmuir, Freundlich, and Temkin isotherm models.
The adsorption process of iodine in cyclohexane solution by Cu/Bi MOF-derived materials was fitted by the Langmuir and Freundlich models, and the fitting results are shown in Figure 5. It can be observed that the shape of the experimental adsorption isotherm is more in line with the plateau saturation characteristic of Langmuir type I isotherms than the continuous slow-rise type that is more common with Freundlich. In addition, the correlation coefficients R2 of the Langmuir model for Cu5/Bi5-NC800, Cu4/Bi6-NC800, Cu3/Bi7-NC800, Cu2/Bi8-NC800, and Cu1/Bi9-NC800 are 0.9937, 0.9892, 0.9953, 0.9886, and 0.9994, respectively, while the correlation coefficients of the Freundlich model are 0.9297, 0.9075, 0.9526, 0.9453, and 0.9696, respectively. Meanwhile, the correlation coefficients R2 of the Temkin model are 0.9759, 0.9564, 0.9923, 0.9813, and 0.9936, which are also lower than those of the Langmuir model. This suggests that the adsorption process of iodine in cyclohexane solution by this material may have better correlation with the Langmuir model. Therefore, under the current conditions, it may be more appropriate to assume the Langmuir model to describe the isothermal adsorption process of iodine in cyclohexane solution by this material.
The adsorption favorability of the Langmuir model could be evaluated by the dimensionless coefficient RL, where C0 (mg/L) represents the initial concentration of the iodine-containing cyclohexane solution [33,34]. Based on the RL parameter value, the adsorption process could be considered irreversible (RL = 0), favorable (0 < RL < 1), or linear (RL = 1). Here, the RL values are within the range of 0 to 1, indicating that the adsorption process is favorable and iodine could be easily adsorbed by Cu/Bi MOF-derived materials.
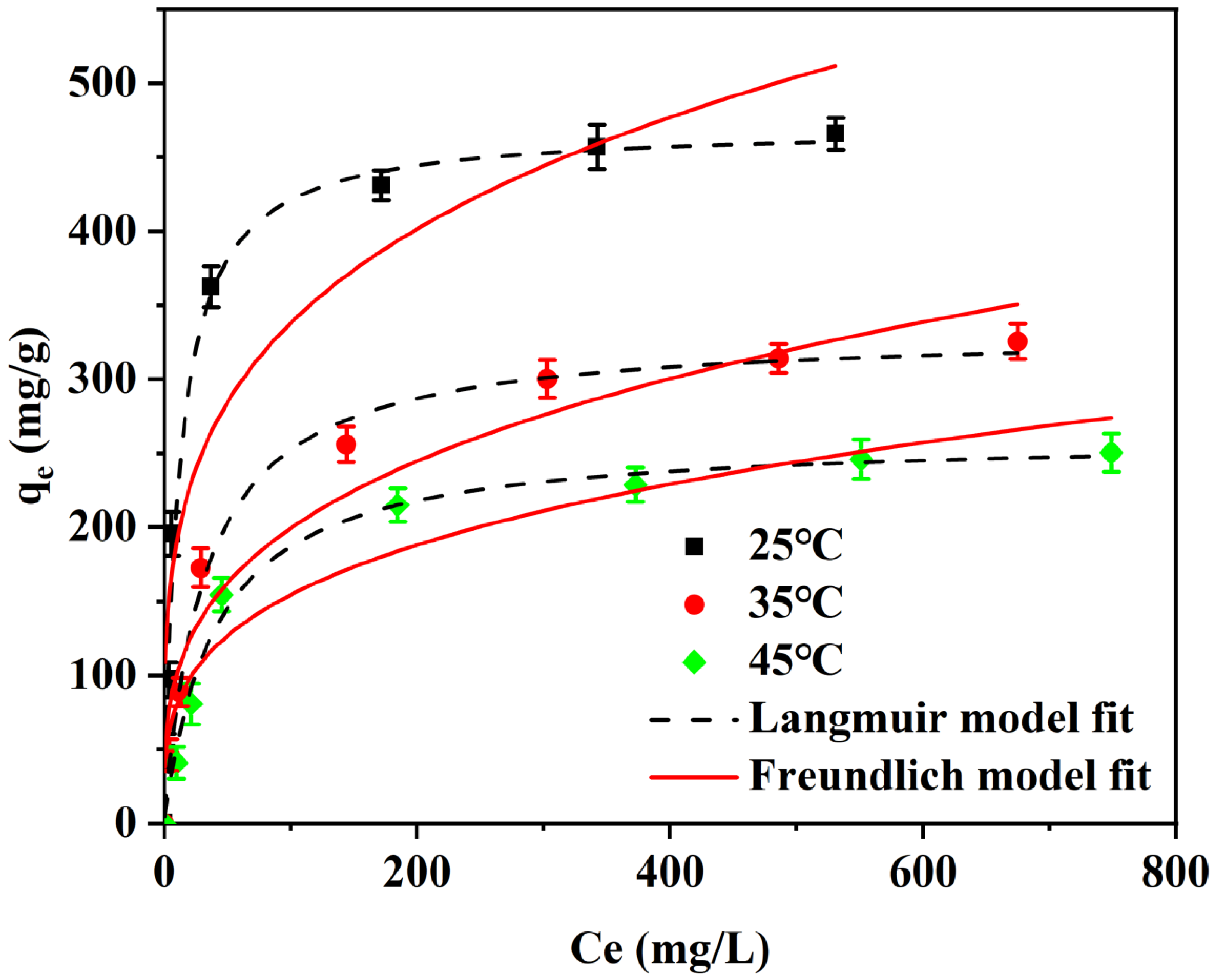
To further explore the adsorption process of I2 in cyclohexane by the adsorption material, thermodynamic adsorption experiments were also conducted. As shown in Figure 6, the adsorption performance of Cu4/Bi6-NC800 for I2 in cyclohexane at different temperatures (25, 35, 45 °C) was investigated. It can be seen that the adsorption capacity of Cu4/Bi6-NC800 for I2 gradually decreased from 484.03 (25 °C) to 340.2 (35 °C) and 268.8 mg/g (45 °C) as the temperature increased from 25 °C to 45 °C. This indicates that the adsorption of I2 in cyclohexane solution by Cu4/Bi6-NC800 is an exothermic process. The adsorption process of Cu4/Bi6-NC800 for I2 in cyclohexane solution at different temperatures was fitted by the Langmuir and Freundlich models. The correlation coefficients R2 of the Langmuir model at 25 °C, 35 °C, and 45 °C were 0.985, 0.992, and 0.988, respectively, while those of the Freundlich model were 0.878, 0.946, and 0.889, respectively. This indicates that the adsorption process of the material for iodine-containing cyclohexane solution has a better correlation with the Langmuir isothermal adsorption model. Therefore, the Langmuir model is more suitable for describing the adsorption process of iodine in cyclohexane solution by this material. This result is consistent with the analysis of the adsorption isotherm, further confirming that the adsorption of I2 in cyclohexane solution by Cu4/Bi6-NC800 is monolayer adsorption and that the process is uniformly distributed on the surface of Cu4/Bi6-NC800.
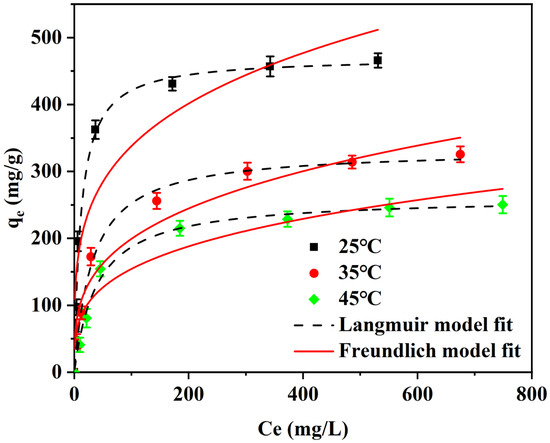
Figure 6.
Adsorption thermodynamic curves of Cu4/Bi6-NC800 composites.
3.3.2. Adsorption Kinetics
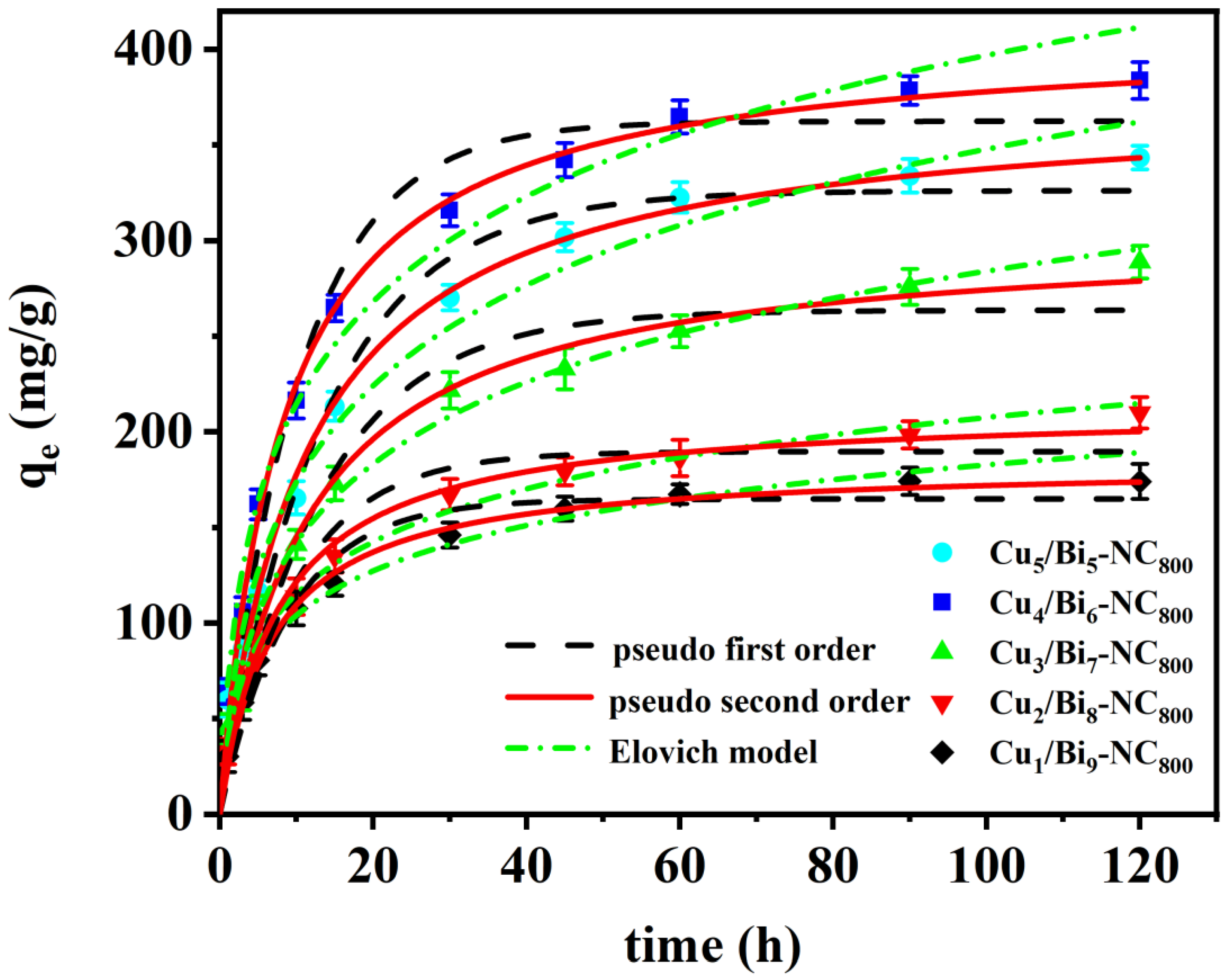
To determine the adsorption equilibrium time of the adsorbent material, adsorption kinetics experiments were conducted in cyclohexane solution with an I2 concentration of 1000 mg/L. As shown in Figure 7, with the increase in contact time, the iodine adsorption capacity rose sharply within the initial reaction time of 1 min, because the adsorbent has a large number of accessible active sites and a considerable amount of iodine could easily bind to the active binding sites. However, as the contact time increased, the concentration of active binding sites and iodine-containing cyclohexane solution decreased, and the adsorption rate dropped sharply, reaching adsorption equilibrium within 3 min. The saturated adsorption capacities of Cu5/Bi5-NC800, Cu4/Bi6-NC800, Cu3/Bi7-NC800, Cu2/Bi8-NC800, and Cu1/Bi9-NC800 were 335.91, 372.14, 272.78, 196.51, and 169.71 mg/g, respectively. The adsorption process was fitted by the following classical kinetic models.
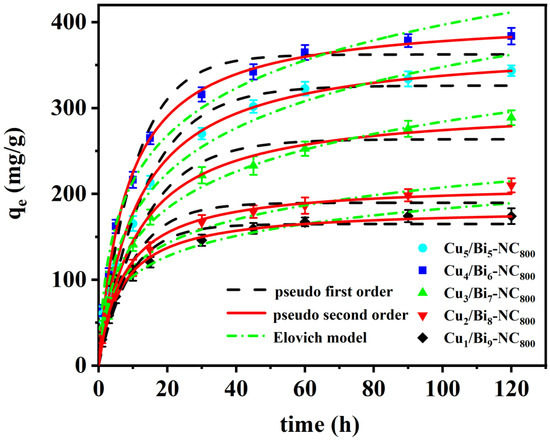
Figure 7.
Adsorption kinetics curve of the Cux/Bi10−x-NC800 composites.
The pseudo-first-order kinetic model (6) and pseudo-second-order kinetic model (7) are classic kinetic models mainly used to determine the rate-controlling steps of mass transfer and physicochemical reactions in adsorption.
where Qe and Qt (mg/g) are the adsorption capacities at equilibrium and at other times, respectively. k1 (min−1) and k2 (mg/g−1 min−1) are the pseudo-first-order and pseudo-second-order rate constants, respectively. The curves and corresponding parameters were fitted based on the experimental results and are shown in Figure 7 and the tables.
The adsorption process of Cu/Bi MOF-derived materials on iodine-containing cyclohexane solution was fitted by the pseudo-first-order and pseudo-second-order kinetic equations. The correlation coefficients R2 of the pseudo-first-order kinetic model for Cu5/Bi5-NC800, Cu4/Bi6-NC800, Cu3/Bi7-NC800, Cu2/Bi8-NC800, and Cu1/Bi9-NC800 are 0.9943, 0.9981, 0.9738, 0.9948, and 0.9975, respectively. The correlation coefficients R2 of the pseudo-second-order kinetic model are 0.9957, 0.9985, 0.9848, 0.9955, and 0.9958, respectively. The results indicate that the adsorption process of Cu/Bi MOF-derived materials on iodinated cyclohexane solutions is slightly better correlated with the pseudo-second-order kinetic model than with the pseudo-first-order kinetic model. In addition, the calculated equilibrium adsorption capacity is in good agreement with the experimentally measured values. Considering that the R2 value of the pseudo-second-order kinetic model is only slightly higher than that of the pseudo-second-order kinetic model, the experimental data were also fitted through the Elovich model. As shown in Figure 7, the R2 values of the Elovich model are 0.9844, 0.9831, 0.9819, 0.9943, and 0.9845, respectively, which are still slightly lower than those of the pseudo-second-order kinetic model. Therefore, due to the universal applicability of the pseudo-second-order kinetic model in describing chemical adsorption or site-controlled processes, as well as its conceptual compatibility with the Langmuir model, it is assumed that the pseudo-second-order kinetic model may be more appropriate for describing the adsorption kinetics of this system under the current conditions. This indicates that the adsorption rate of this material on iodine-containing cyclohexane solution is less affected by diffusion and more influenced by chemical adsorption.
3.4. Capture Mechanisms
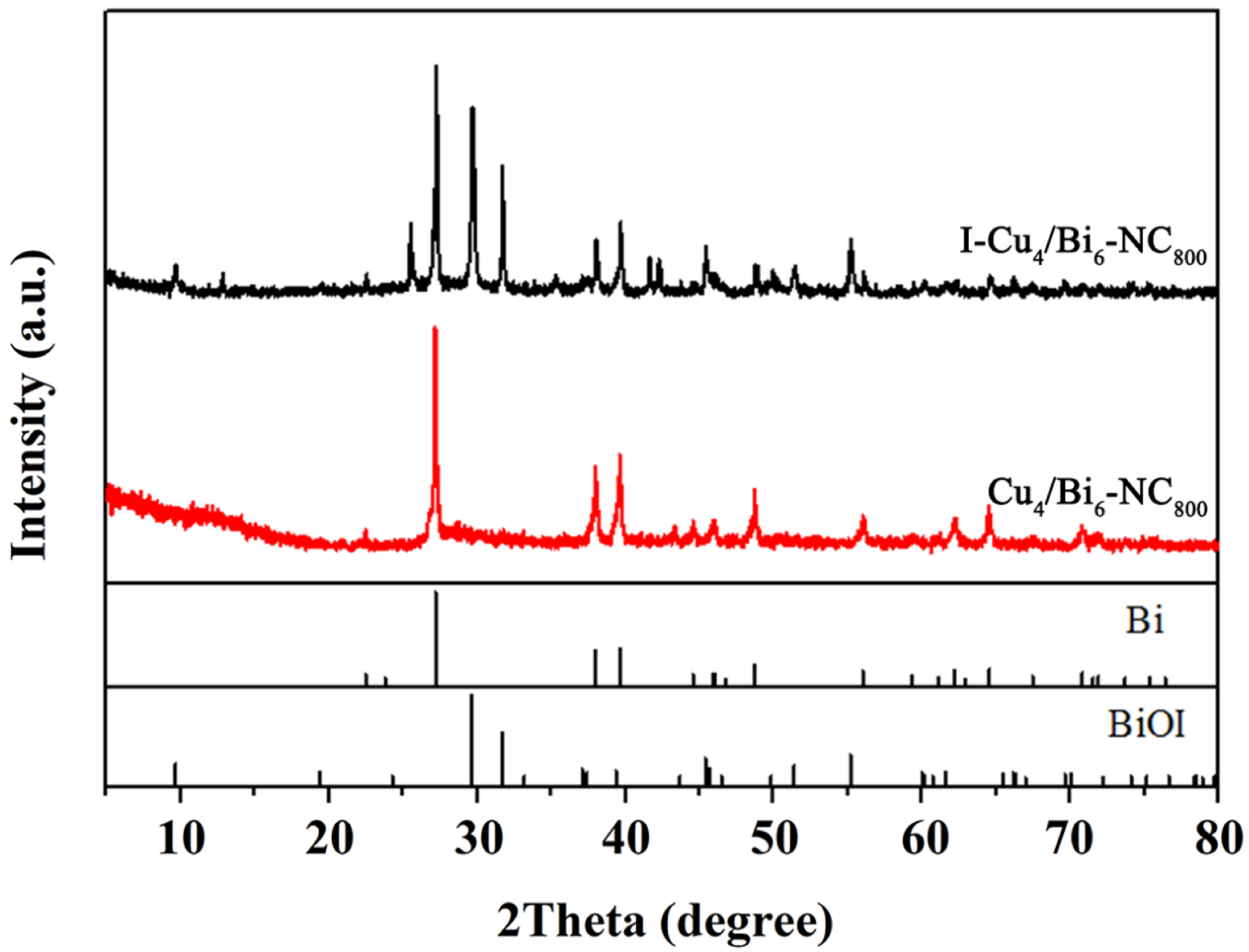
A series of I2 adsorption experiments indicated that the adsorption process of I2 by the adsorption materials involved both chemical and physical adsorption, but chemical adsorption played a major role in the removal of I2, with most of the iodine being chemically captured by the adsorption materials. To accurately reveal the potential removal mechanism of I2 by the adsorption materials, systematic characterizations were conducted on the materials before and after adsorption. Figure 2 shows the SEM images of the adsorption materials before and after adsorbing I2. By comparison, it could be observed that more flaky structures appeared on the surface of the adsorption materials after adsorption, indicating that chemical adsorption occurred between the adsorption materials and I2. Additionally, EDS spectra revealed that a large amount of iodine elements were distributed on the surface of the adsorption materials after adsorption, further confirming that a significant amount of I2 was captured by the adsorption materials. Considering that Cu4/Bi6-NC800 exhibited the strongest adsorption performance for I2, XRD and XPS characterizations were conducted on Cu4/Bi6-NC800 before and after adsorption.
As shown in Figure 8, the main components of the product before adsorption were elemental bismuth, elemental copper, and copper oxide in crystalline phases. However, new characteristic peak signals appeared at 2θ = 14.3°, 27.1°, and 41.7° in the XRD pattern of the product after adsorption, which were attributed to the (101), (200), and (300) crystal planes of BiI3 (JCPDS No. 48-1795). Additionally, characteristic peaks at 2θ = 29.7°, 31.8°, and 55.6° were found, which were attributed to the (012), (110), and (122) crystal planes of BiOI (JCPDS No. 73-2062), while the characteristic peaks originally belonging to Bi0 slightly decreased. This indicates that elemental bismuth in the adsorbent played an important role in capturing I2, and it also suggests that the adsorption process of iodine by the adsorbent is actually a chemical reaction process.
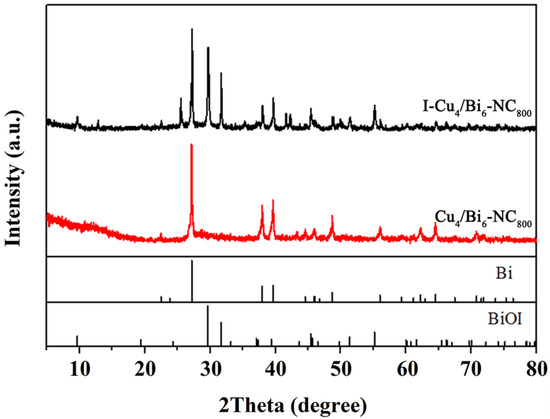
Figure 8.
The XRD spectra of Cu4/Bi6-NC800 and I-Cu4/Bi6-NC800.
To further understand the changes in the surface chemical composition of the adsorption materials during the I2 adsorption process, XPS characterizations were conducted on the adsorbents before and after adsorption. As shown in Figure 9, in the total spectrum, the I 3d orbital was not present in the material before adsorption, but was observed in the total spectrum after adsorption, indicating that iodine elements were successfully adsorbed in our adsorption process. We performed peak fitting on the iodine 3d orbitals and found that the iodine 3d orbitals were mainly composed of 3d5/2 and 3d3/2, and the two orbitals corresponded to the −1 valence state of iodide ions.
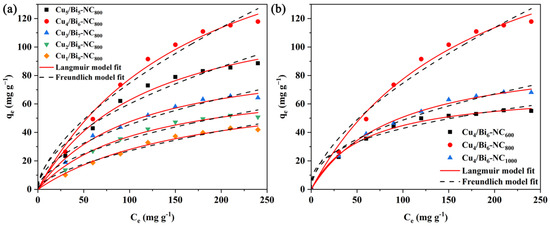
Figure 9.
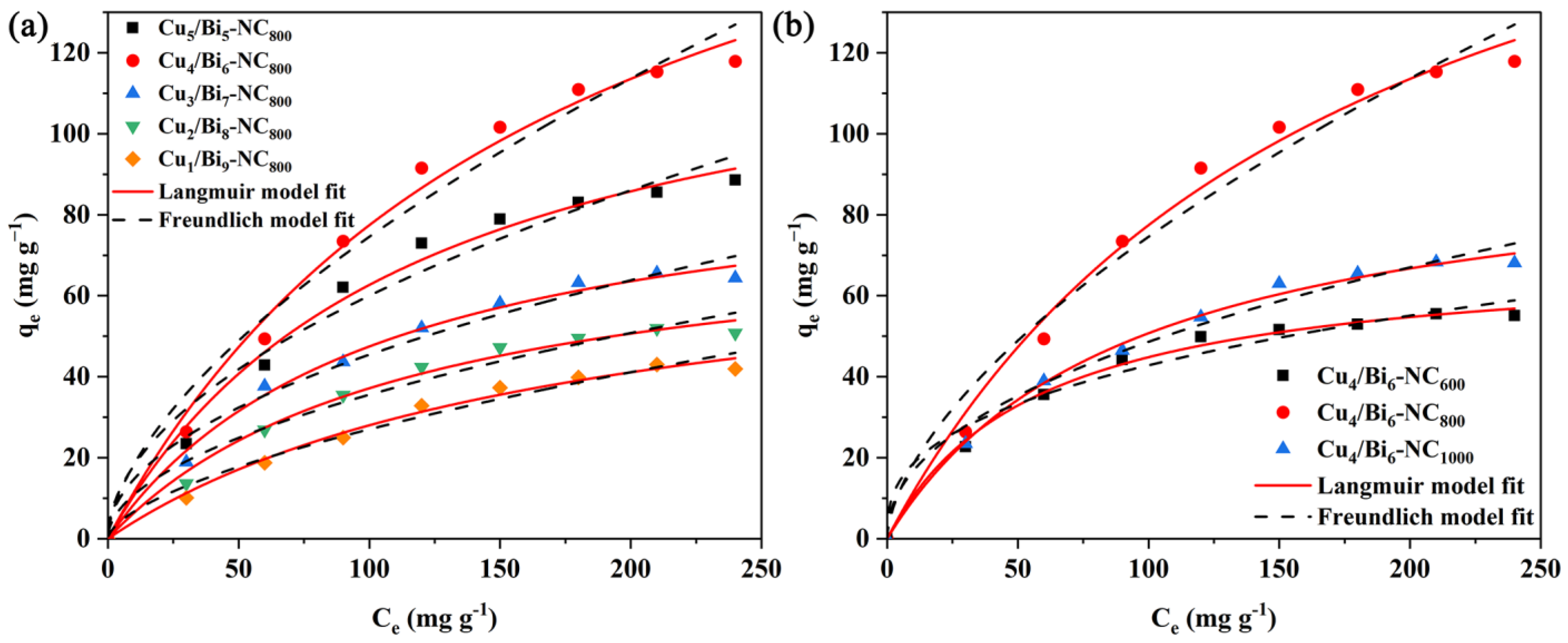
(a) Adsorption isotherms of Cux/Bi1−x-NC800 for iodide anion elimination; (b) the effect of pyrolysis temperature on iodide adsorption by Cu4/Bi6-NC.
Figure 3d shows the 4f orbital of Bi in the material. It could be seen that after adsorbing iodine, the 4f orbital of Bi in the material shifts towards higher binding energy. This indicates that during the adsorption process, Bi undergoes electron loss, which is consistent with the formation of BiOI as indicated by XRD. In BiOI, Bi is in the +3 oxidation state, and the transition from elemental Bi to trivalent Bi is an electron loss process. Therefore, the shift of the Bi 4f orbital towards higher binding energy in XPS is observed. The trend of the Cu 2p orbital shift before and after adsorption is basically the same as that of the Bi 4f orbital, with a slight shift towards higher binding energy. Previously, the copper element in the material mainly existed in the form of elemental copper and monovalent copper ions. After adsorbing iodine, the material mainly exists in the form of monovalent and divalent copper ions, indicating that copper also undergoes electron loss during the adsorption process. In summary, throughout the adsorption reaction, both copper and bismuth elements in the adsorption process provide electrons in an electron loss state to form BiOI and promote the reaction.
3.5. Capture Properties Evaluation of the Iodine Ions
3.5.1. Adsorption Isotherms of the Iodine Ions
To compare the effects of different Cu/Bi molar ratios and different pyrolysis temperatures on the adsorption performance of the materials, isothermal adsorption tests were conducted. The results are shown in Figure 9, which illustrates the influence of ion concentration and pyrolysis temperature on the adsorption of iodide ions by Cux/Bi10−x-NC. As can be seen from Figure 9a, in the initial stage, the adsorption capacity of the adsorbent increases rapidly with the increase in the equilibrium mass concentration of iodide ions. When the equilibrium mass concentration increases to a certain extent, the change in equilibrium adsorption capacity gradually stabilizes. This is because at the beginning, there are sufficient active adsorption sites on the adsorbent, resulting in a fast adsorption rate; after a period of adsorption, the active adsorption sites are gradually occupied, leading to a decrease in the adsorption rate. Additionally, as the Cu/Bi molar ratio increases, the adsorption capacity of the material for iodide ions gradually increases. When the Cu/Bi molar ratio increases from 1:9 to 5:5, the saturated adsorption capacity of iodide ions increases from 84.75 mg/g to 85.48 mg/g, 101.81 mg/g, 233.11 mg/g, and 149.92 mg/g, respectively. This indicates that Cu is the main capture site for iodide ions. As shown in Figure 9b, it can be seen that among the three temperatures of 600, 800, and 1000 °C, the best adsorption performance is at 800 °C, followed by 1000 °C, and then 600 °C. The saturated equilibrium adsorption capacities obtained from the isothermal adsorption experiments of the materials with a Cu/Bi molar ratio of 4:6 at the three different temperatures are 23.3 mg/g, 117.8 mg/g, and 74.3 mg/g, respectively.
To further investigate the adsorption characteristics of Cux/Bi10−x-NC towards iodide ions, the data were fitted using the Langmuir and Freundlich isothermal adsorption models. The fitting results are shown in Figure 9b and Table 1. According to Table 1, the correlation coefficient obtained by fitting with the Langmuir isothermal adsorption model is higher than that of the Freundlich isothermal adsorption model. Meanwhile, as shown in Figure 9b, the Langmuir isothermal adsorption model has a better fit with the iodide ion adsorption data of Cux/Bi10−x-NC. Based on the Langmuir isothermal adsorption curve, the maximum adsorption capacities of Cu4/Bi6-NC800, Cu4/Bi6-NC600, and Cu4/Bi6-NC1000 for iodide ions were calculated to be 233.11, 72.45, and 102.33 mg g−1, respectively, which were close to the adsorption capacities obtained from the isothermal adsorption experiments.

Table 1.
Fitting parameters of Langmuir and Freundlich isotherms.
3.5.2. Adsorption Kinetics of the Iodine Ions
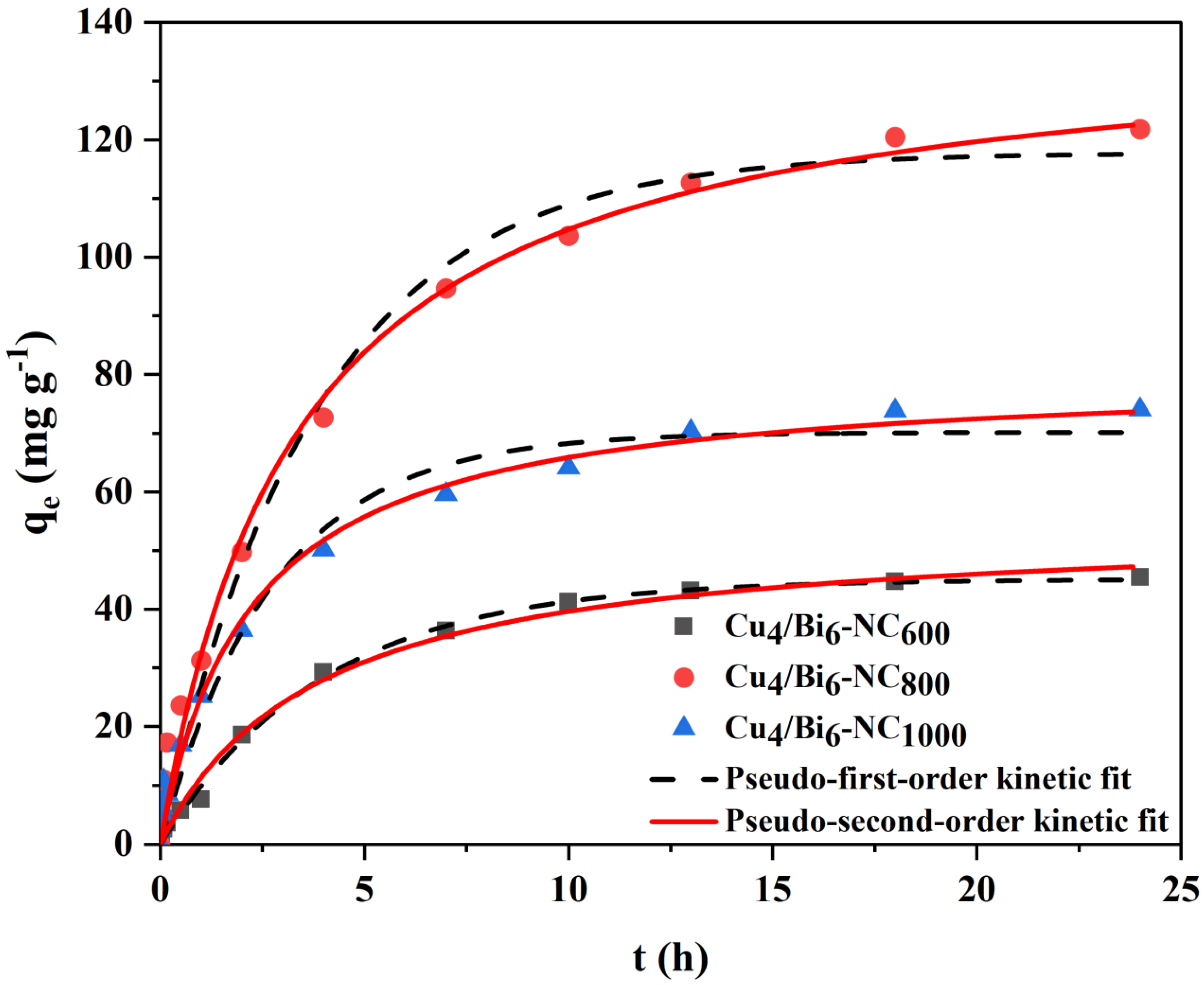
To understand the adsorption kinetics characteristics of Cu/Bi-NC composite materials, kinetic adsorption tests were conducted. Figure 10 shows the adsorption kinetics curve of Cux/Bi10−x-NC material for iodide ions. It can be seen from Figure 10 that at the initial stage of adsorption, the adsorption capacity of Cux/Bi10−x-NC for iodide ions increases rapidly; as the adsorption time increases, the adsorption process slows down. Eventually, Cux/Bi10−x-NC600, Cux/Bi10−x-NC800, and Cux/Bi10−x-NC1000 reach adsorption equilibrium at 24 h, 13 h, and 18 h, respectively, with saturated adsorption capacities of 121.8, 45.5, and 70.8 mg g−1. The reason for the rapid adsorption of iodide ions by Cux/Bi10−x-NC at the initial stage is mainly due to the sufficient effective active sites on the material surface, which makes it easy for iodide ions to be captured by the active sites; however, as the adsorption time extends, the active sites decrease, and the resistance to the adsorption of free iodide ions increases, thus entering a slow adsorption stage. Additionally, the increase in the Cu/Bi molar ratio could expose more Cu active components on the matrix surface, which not only helps to increase the adsorption capacity for iodide ions but also shortens the time required to reach adsorption equilibrium.
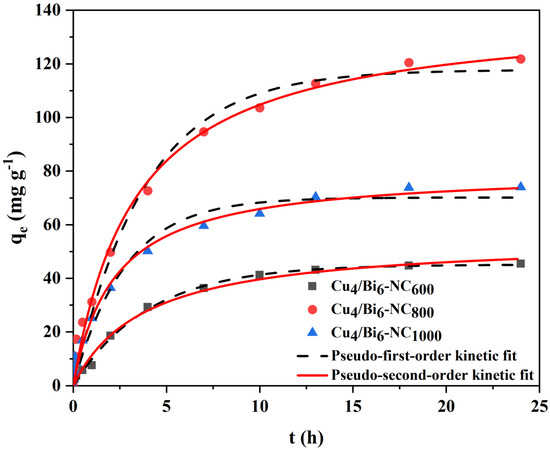
Figure 10.
Kinetic adsorption of iodide by Cu4Bi6-NC under air atmosphere.
To further study the adsorption characteristics of Cux/Bi10−x-NC1000 for iodide ions, the data were fitted using pseudo-first-order and pseudo-second-order kinetic equations. It could be seen from Figure 10 and Table 2 that the adsorption of iodide ions by Cu/Bi composites conforms to both the pseudo-first-order kinetic model and the pseudo-second-order kinetic model. Therefore, it could be considered that the adsorption process of iodide ions by Cu/Bi composites includes both physical adsorption and chemical adsorption.

Table 2.
The fitting parameters of the quasi-first-order kinetic equation and quasi-second-order kinetic equation.
3.5.3. Influencing Factors of the Iodine Ion Adsorption
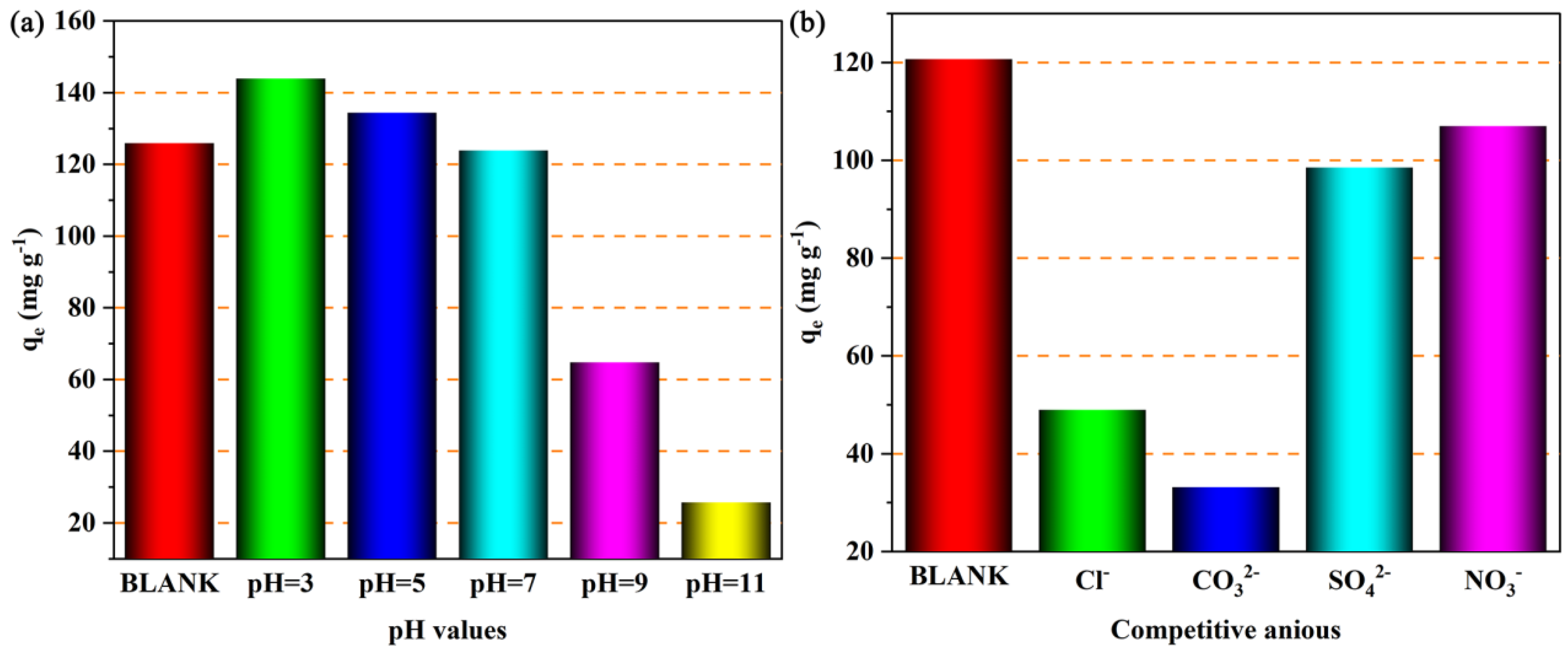
The pH of the solution could affect the number of active sites on the adsorbent surface and the form of the adsorbate in the solution, so the influence of pH on the adsorption performance of the adsorbent needs to be considered. Figure 11a shows the adsorption capacity of Cu4/Bi6-NC800 for iodide ions when the initial pH of the solution is in the range of 3 to 11. It is obvious that the adsorption capacity of Cu4/Bi6-NC800 for iodide ions gradually decreases as the initial pH of the solution increases. This is similar to the copper-based adsorbents reported in the existing literature. This is mainly because in acidic conditions, the excess H+ in the solution will react with Cu0, promoting the rapid release of Cu+ and reacting with I− in the solution to form CuI precipitate [35]. On the other hand, Cu0 will react with molecular oxygen in the environment to in situ generate Cu+ and H2O2 [36,37]. The generated H2O2 could oxidize I− in the solution to I3−, which is then captured by Cu sites and Bi sites [38,39]. This means that the generation of H2O2 will promote the adsorption of I−, and this process may be enhanced under acidic conditions [36,40]. In summary, acidic conditions are favorable for the adsorption of iodide ions by Cu4/Bi6-NC800.
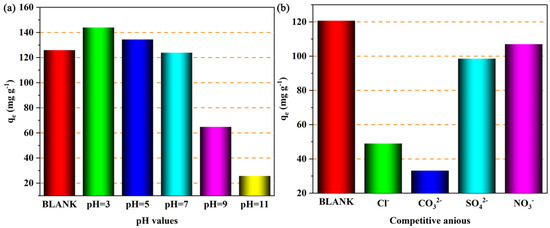
Figure 11.
(a) The effect of solution pH on the adsorption of iodine ions by Cu4/Bi6-NC800. (b) The effect of interference ions on the adsorption of iodine ions by Cu4/Bi6-NC800.
Figure 11b shows the adsorption capacity of Cu4/Bi6-NC800 for iodide ions in the presence of Cl−, CO32−, SO42−, and NO3−. It can be seen from Figure 11 that when there are no competitive ions in the solution, the adsorption capacity of Cu4/Bi6-NC800 for iodide ions is 120.7 mg g−1. However, when Cl−, CO32−, SO42−, and NO3− coexist with I−, the adsorption capacity of Cu4/Bi6-NC800 for iodide ions all show different degrees of reduction, with the adsorption capacities being 49.0, 33.1, 98.5, and 106.9 mg g−1, respectively. Clearly, when SO42− and NO3− are present, the adsorption capacity of Cu5/Bi5-NC800 for iodide ions is only slightly inhibited. This may be due to the strong acidity of SO42− and NO3−, which enhances the acidity of the reaction solution, thereby enhancing the adsorption performance of the adsorption material for iodide ions [41,42]. However, when Cl− and CO32− are present, the adsorption performance of Cu5/Bi5-NC800 for iodide ions is severely inhibited. This inhibitory effect is mainly because CO32− is a weak acid anion, and its presence could increase the pH of the solution. Based on the influence of different pH values on the adsorption performance of Cu5/Bi5-NC800 for iodide ions, it could be inferred that the increase in pH caused by CO32− inhibits the adsorption performance of Cu5/Bi5-NC800 for iodide ions [43,44]. In addition, Cl− in the solution has similar chemical properties to I−, and competes for active adsorption sites during the adsorption process of I−, and reacts with Cu+ on the surface of Cu5/Bi5-NC800 to form insoluble CuCl electrolyte (Ksp = 1.7 × 10−7), thereby inhibiting the adsorption performance of Cu5/Bi5-NC800 [41,44].
4. Conclusions
In summary, bimetallic MOFs with different Cu/Bi ratios were synthesized through a simple solvothermal method and a bimetallic nano-adsorbent Cux/Bi10−x-NC was prepared by one-step calcination. The adsorption performance of the material for iodide ions and elemental iodine was investigated. When the Cu/Bi ratio was 4:6 and the calcination temperature was 800 °C, the prepared material exhibited the highest adsorption performance, with an adsorption capacity of 117.81 mg/g for iodide ions. Additionally, the maximum adsorption capacity for gaseous iodine was 1103.64 mg/g, and it also demonstrated excellent capture ability for I2 dissolved in cyclohexane solution, with a maximum adsorption capacity of 484.08 mg/g. We believe that the synergistic effect between Cu and Bi is the main reason for the high adsorption performance of Cux/Bi10−x-NC for various forms of iodine. In addition, the advantages brought by the MOF-derived material, such as high specific surface area and low aggregation degree of adsorption active sites, also improved the adsorption performance of Cux/Bi10−x-NC. Based on the results of this study, it could be considered that Cux/Bi10−x-NC has the potential to provide new ideas for the capture of different forms of iodine.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13092678/s1, Text S1: Chemicals; Text S2: Characterization; Figure S1: XRD spectra of Cux/Bi10−x-NC600; Figure S2: XRD spectra of Cux/Bi10−x-NC1000; Figure S3: FTIR spectra of Cux/Bi10−x-NC800; Figure S4: UV-Vis absorption spectra of I2/ethanol solutions during desorption (10-fold dilution); Figure S5: The effect of TBA on iodide elimination.
Author Contributions
Formal analysis, J.R., A.G. and P.W.; investigation, J.R., A.G. and Y.Y.; data curation, C.G. and K.C. (Kaiwei Chen); writing—original draft, A.G.; writing—review and editing, P.M.; visualization, Y.J.; project administration, K.C. (Kai Chen); funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 52470120 and No. 12475310); the Fundamental Research Funds for the Central Universities (No. 30921013110); Chinese Postdoctoral Science Foundation (No. 2022M711631); the Provincial Ecological Environment Research Project of Jiangsu (No. 2022017); the Opening Foundation of Key Laboratory for Palygorskite Science and Applied Technology of Jiangsu Province (No. HPK202001); the Open Fund by Jiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control (No. KHK2210); Lianyungang Key Research and Development Plan Projects (No. CG2330); and Lianyungang Basic Research Program (No. JCYJ2329).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Veliscek-Carolan, J. Separation of actinides from spent nuclear fuel: A review. J. Hazard. Mater. 2016, 318, 266–281. [Google Scholar] [CrossRef]
- Robshaw, T.J.; Griffiths, S.M.; Canner, A.; Bezzina, J.P.; Waller, A.G.L.; Hammond, D.B.; van Meurs, S.; Ogden, M.D. Insights into the interaction of iodide and iodine with Cu(II)-loaded bispicolylamine chelating resin and applications for nuclear waste treatment. Chem. Eng. J. 2020, 390, 124647. [Google Scholar] [CrossRef]
- Chapman, K.W.; Chupas, P.J.; Nenoff, T.M. Radioactive Iodine Capture in Silver-Containing Mordenites through Nanoscale Silver Iodide Formation. J. Am. Chem. Soc. 2010, 132, 8897–8899. [Google Scholar] [CrossRef]
- Luo, J.; Du, X.; Gao, F.; Ma, P.; Hao, X.; Guan, G.; Scialdone, O.; Li, J. Electrochemically triggered iodide-vacancy BiOI film for selective extraction of iodide ion from aqueous solutions. Sep. Purif. Technol. 2021, 259, 118120. [Google Scholar] [CrossRef]
- Lee, S.-H.; Takahashi, Y. Selective immobilization of iodide onto a novel bismuth-impregnated layered mixed metal oxide: Batch and EXAFS studies. J. Hazard. Mater. 2020, 384, 121223. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, N.; Zhang, Y.; Li, Y.; Han, Z.; Na, P. Efficient removal of radioactive iodide ions from water by three-dimensional Ag2O–Ag/TiO2 composites under visible light irradiation. J. Hazard. Mater. 2015, 284, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, Q.; Zhang, X.; Liu, Y.; Wang, P.; Jiao, Y.; Yang, Y. Nanometer mixed-valence silver oxide enhancing adsorption of ZIF-8 for removal of iodide in solution. Sci. Total Environ. 2019, 646, 634–644. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, P.; Zhou, S.; Li, X.; Zhang, G.; Dong, L. Enhanced removal of iodide ions by nano Cu2O/Cu modified activated carbon from simulated wastewater with improved countercurrent two-stage adsorption. Sci. Total Environ. 2018, 626, 612–620. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, P.; Yang, Y.; Jia, L.; Zhang, M.; Zhang, G. Removal of radioactive iodide from simulated liquid waste in an integrated precipitation reactor and membrane separator (PR-MS) system. Sep. Purif. Technol. 2016, 171, 221–228. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, B.; Du, X.; Zhang, J.; Fu, H.; Gao, H.; Liao, Y. Construction of N-loaded conjugated polymer for highly effective removal of iodine in organic solution. J. Environ. Chem. Eng. 2023, 11, 109125. [Google Scholar] [CrossRef]
- Lin, G.; Zhu, L.; Duan, T.; Zhang, L.; Liu, B.; Lei, J. Efficient capture of iodine by a polysulfide-inserted inorganic NiTi-layered double hydroxides. Chem. Eng. J. 2019, 378, 122181. [Google Scholar] [CrossRef]
- Huang, M.; Yang, L.; Li, X.; Chang, G. An indole-derived porous organic polymer for the efficient visual colorimetric capture of iodine in aqueous media via the synergistic effects of cation–π and electrostatic forces. Chem. Commun. 2020, 56, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, P.; Gong, C.; Sun, Y.; Zhu, B.; Yang, Y.; Liu, F. Bimetal ZIFs-derived Cu0 embedded in nitrogen-doped carbon framework activation of molecular oxygen for efficient iodide elimination. J. Environ. Chem. Eng. 2024, 12, 112235. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, L.; Cheng, X.; Ding, P.; Zhu, W.; Duan, T.; He, G.; Wei, Y.; Sun, D.; Zhou, Y.; et al. In-site interface growth of bismuth-based hydrothermal carbon using collagen fiber for selective removal of iodide ion from wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131177. [Google Scholar] [CrossRef]
- Riley, B.J.; Chong, S.; Asmussen, R.M.; Bourchy, A.; Engelhard, M.H. Polyacrylonitrile Composites of Ag–Al–Si–O Aerogels and Xerogels as Iodine and Iodide Sorbents. ACS Appl. Polym. Mater. 2021, 3, 3344–3353. [Google Scholar] [CrossRef]
- Zhou, X.; Mao, P.; Jin, H.; Huang, W.; Gu, A.; Chen, K.; Yun, S.; Chen, J.; Yang, Y. Cu/Al2O3 aerogels for high-efficiency and rapid iodide elimination from water. J. Hazard. Mater. 2023, 443, 130349. [Google Scholar] [CrossRef]
- Chen, J.; Gu, A.; Miensah, E.D.; Liu, Y.; Wang, P.; Mao, P.; Gong, C.; Jiao, Y.; Chen, K.; Yang, Y. Cu-Zn bimetal ZIFs derived nanowhisker zero-valent copper decorated ZnO nanocomposites induced oxygen activation for high-efficiency iodide elimination. J. Hazard. Mater. 2021, 416, 126097. [Google Scholar] [CrossRef]
- Reda, A.T.; Zhang, D.; Xu, X.; Xu, S. Highly stable iodine capture by pillared montmorillonite functionalized Bi2O3@g-C3N4 nanosheets. Sep. Purif. Technol. 2022, 292, 120994. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, P.; Wang, D.; Li, T.-T.; Wu, L.-H.; Zheng, S.-R. A mixed-ligand Co(ii) MOF synthesized from a single organic ligand to capture iodine and methyl iodide vapour. Dalton Trans. 2023, 52, 7709–7717. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Ren, W.; Li, S.; Jin, C. Rapid and selective removal of radioactive iodide ions from wastewater via bismuth-based metal-organic frameworks. J. Environ. Chem. Eng. 2024, 12, 111906. [Google Scholar] [CrossRef]
- Chen, K.-W.; Gu, A.-T.; Zhou, X.-Y.; Wang, P.; Gong, C.-H.; Mao, P.; Jiao, Y.; Chen, K.; Yang, Y. Core-shell structured Bi2S3-ZnS@C derived from ZIF-8 for efficient capture and reliable storage of volatile radioactive iodine. Sep. Purif. Technol. 2023, 322, 124380. [Google Scholar] [CrossRef]
- Dai, X.-J.; Chen, K.-W.; He, M.-L.; Chen, K.; Zhou, X.-Y.; Chen, Y.-T.; Gong, C.-H.; Wang, P.; Mao, P.; Yang, Y. Removal of radioactive iodine by Cu2O prepared with PVP as an active agent: Role of crystal facets and oxygen vacancy in adsorption mechanisms. Chem. Eng. J. 2024, 493, 152515. [Google Scholar] [CrossRef]
- Liao, L.; Song, S.; Liang, G.; Wang, X.; Pan, N.; Lei, H.; Zhang, Y.; Zou, H.; Cheng, J.; Wen, J.; et al. Resource recovery from iodine-containing silver-loaded silica gel: Recycling of silver and immobilization of iodine. J. Hazard. Mater. 2025, 493, 138306. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.-J.; Chen, K.-W.; He, M.-L.; Zhou, X.-Y.; Chen, Y.-T.; Li, S.-X.; Gong, C.-H.; Wang, P.; Mao, P.; Lu, J.-G.; et al. Efficient removal of radioactive iodine anions using δ-Bi2O3/MOF-808 through photo-oxidation and adsorption: Performance evaluation and investigation of synergistic mechanisms. Sep. Purif. Technol. 2025, 360, 130973. [Google Scholar] [CrossRef]
- Zhou, L.-W.; Chen, K.-W.; Dai, X.-J.; He, M.-L.; Wang, P.; Gong, C.-H.; Gu, A.-T.; Lu, J.-G.; Yang, Y. Tunable Cu(0)/Cu(I) hybrids derived from Cu-MOF for enhanced removal of iodide anions. Sep. Purif. Technol. 2025, 357, 130046. [Google Scholar] [CrossRef]
- Han, S.; Um, W.; Kim, W.-S. Development of bismuth-functionalized graphene oxide to remove radioactive iodine. Dalton Trans. 2019, 48, 478–485. [Google Scholar] [CrossRef]
- Kamal, S.; Khalid, M.; Khan, M.S.; Shahid, M.; Ahmad, M. A bifunctionalised Pb-based MOF for iodine capture and dye removal. Dalton Trans. 2023, 52, 4501–4516. [Google Scholar] [CrossRef]
- Agarwal, R.A. Selective and Reversible Capture of Volatile I2 Modifying As-Synthesized 2D Cd-MOF to 3D. Cryst. Growth Des. 2021, 21, 2046–2055. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Tang, J.; Salunkhe, R.R.; Jiang, X.; Yu, A.; Wu, K.C.W.; Yamauchi, Y. Nanoarchitectured Design of Porous Materials and Nanocomposites from Metal-Organic Frameworks. Adv. Mater. 2017, 29, 1604898. [Google Scholar] [CrossRef]
- Chen, K.-W.; Zhou, X.-Y.; Chen, Y.-T.; Dai, X.-J.; Li, S.-X.; Gu, A.-T.; Gong, C.-H.; Wang, P.; Mao, P.; Lu, J.-G.; et al. Tunable sulfur vacancies in amorphous-crystalline AC-Bi2S3−x@C derived from CAU-17 for efficient capture of radioiodine: Comparison of sulfur vacancies in amorphous and crystalline structures. Chem. Eng. J. 2024, 499, 156002. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Pham, A.L.H.; Nguyen, V.-H.; Lee, T.; Nguyen, T.D. Facile synthesis of bismuth(III) based metal-organic framework with difference ligands using microwave irradiation method. Chem. Eng. Res. Des. 2022, 177, 321–330. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, X.; Cheng, Z.; Liao, Y.; Pu, Q.; Duan, M. Millimeter-sized Bi2S3@polyacrylonitrile hybrid beads for highly efficient iodine capture. New J. Chem. 2020, 44, 16759–16768. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, R.; Neogi, S. Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water. J. Hazard. Mater. 2020, 392, 122441. [Google Scholar] [CrossRef] [PubMed]
- Nekouei, F.; Nekouei, S.; Tyagi, I.; Gupta, V.K. Kinetic, thermodynamic and isotherm studies for acid blue 129 removal from liquids using copper oxide nanoparticle-modified activated carbon as a novel adsorbent. J. Mol. Liq. 2015, 201, 124–133. [Google Scholar] [CrossRef]
- Choung, S.; Um, W.; Kim, M.; Kim, M.G. Uptake mechanism for iodine species to black carbon. Environ. Sci. Technol. 2013, 47, 10349–10355. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Wang, J. Fenton-like degradation of sulfamethoxazole in Cu-0/Zn-0-air system over a broad pH range: Performance, kinetics and mechanism. Chem. Eng. J. 2021, 403, 126320. [Google Scholar] [CrossRef]
- Feng, H.; Tang, L.; Tang, J.; Zeng, G.; Dong, H.; Deng, Y.; Wang, L.; Liu, Y.; Ren, X.; Zhou, Y. Cu-Doped Fe@Fe2O3 core-shell nanoparticle shifted oxygen reduction pathway for high-efficiency arsenic removal in smelting wastewater. Environ. Sci. Nano 2018, 5, 1595–1607. [Google Scholar] [CrossRef]
- Li, H.-P.; Yeager, C.M.; Brinkmeyer, R.; Zhang, S.; Ho, Y.-F.; Xu, C.; Jones, W.L.; Schwehr, K.A.; Otosaka, S.; Roberts, K.A.; et al. Bacterial production of organic acids enhances H2O2-dependent iodide oxidation. Environ. Sci. Technol. 2012, 46, 4837–4844. [Google Scholar] [CrossRef]
- Changani, Z.; Razmjou, A.; Taheri-Kafrani, A.; Warkiani, M.E.; Asadnia, M. Surface modification of polypropylene membrane for the removal of iodine using polydopamine chemistry. Chemosphere 2020, 249, 126079. [Google Scholar] [CrossRef]
- Li, W.; Chen, C.; Zhu, J.; Zhou, L.; Lan, Y. Efficient removal of aniline by micro-scale zinc-copper (mZn/Cu) bimetallic particles in acidic solution: An oxidation degradation mechanism via radicals. J. Hazard. Mater. 2019, 366, 482–491. [Google Scholar] [CrossRef]
- Mao, P.; Qi, L.; Liu, X.; Liu, Y.; Jiao, Y.; Chen, S.; Yang, Y. Synthesis of Cu/Cu2O hydrides for enhanced removal of iodide from water. J. Hazard. Mater. 2017, 328, 21–28. [Google Scholar] [CrossRef]
- Jiao, H.; Li, Y.; Gao, K.; Zhao, J.; Wang, C.; Li, M.; Na, P. Efficient removal of radioactive iodide by three-dimensional Cu@Cu2O: An adsorption and electrocatalytic oxidation coupling process. Colloids Surf. A-Physicochem. Eng. Asp. 2020, 602, 124964. [Google Scholar] [CrossRef]
- Chen, J.; Gu, A.; Miensah, E.D.; Liu, Y.; Wang, P.; Mao, P.; Gong, C.; Jiao, Y.; Chen, K.; Zhang, Z.; et al. Core-shell ZnO@Cu2O encapsulated Ag NPs nanocomposites for photooxidation-adsorption of iodide anions under visible light. Sep. Purif. Technol. 2021, 262, 118328. [Google Scholar] [CrossRef]
- Seon, J.; Hwang, Y. Cu/Cu2O-immobilized cellulosic filter for enhanced iodide removal from water. J. Hazard. Mater. 2021, 409, 124415. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).