Artificial Neural Network Modeling Enhancing Photocatalytic Performance of Ferroelectric Materials for CO2 Reduction: Innovations, Applications, and Neural Network Analysis
Abstract
1. Introduction
2. Photocatalytic CO2 Conversion Optimization
2.1. Photocatalysis for Sustainable CO2 Conversion
2.1.1. Light Absorption
2.1.2. Load Separation
2.1.3. Surface Reactivity
2.2. Materials and Methods
3. Enhancing Ferroelectric Catalytic Reactivity
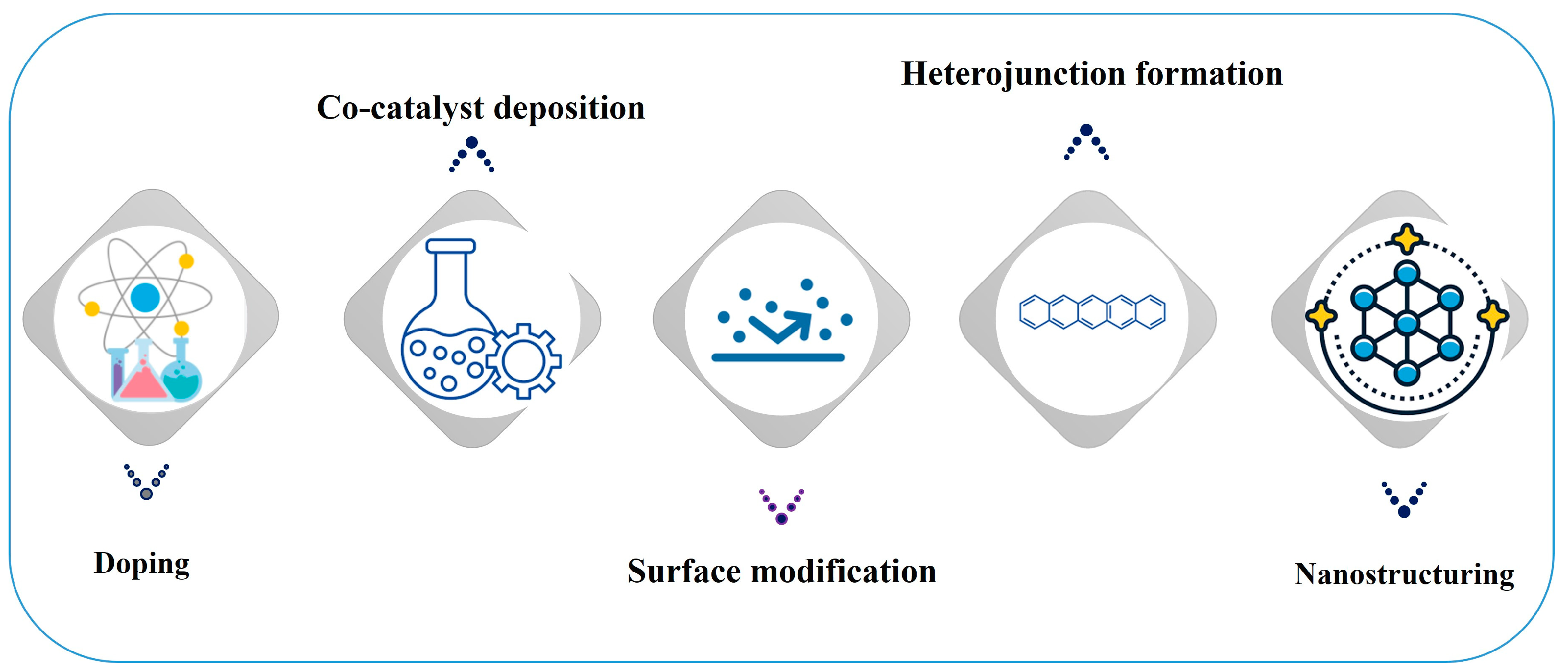
3.1. Surface Engineering
3.2. Defect Engineering
3.3. Surface Optimization Strategies
3.4. Adjusting the Electronic Structure
4. Advantage of Ferroelectric Materials as Photocatalysts
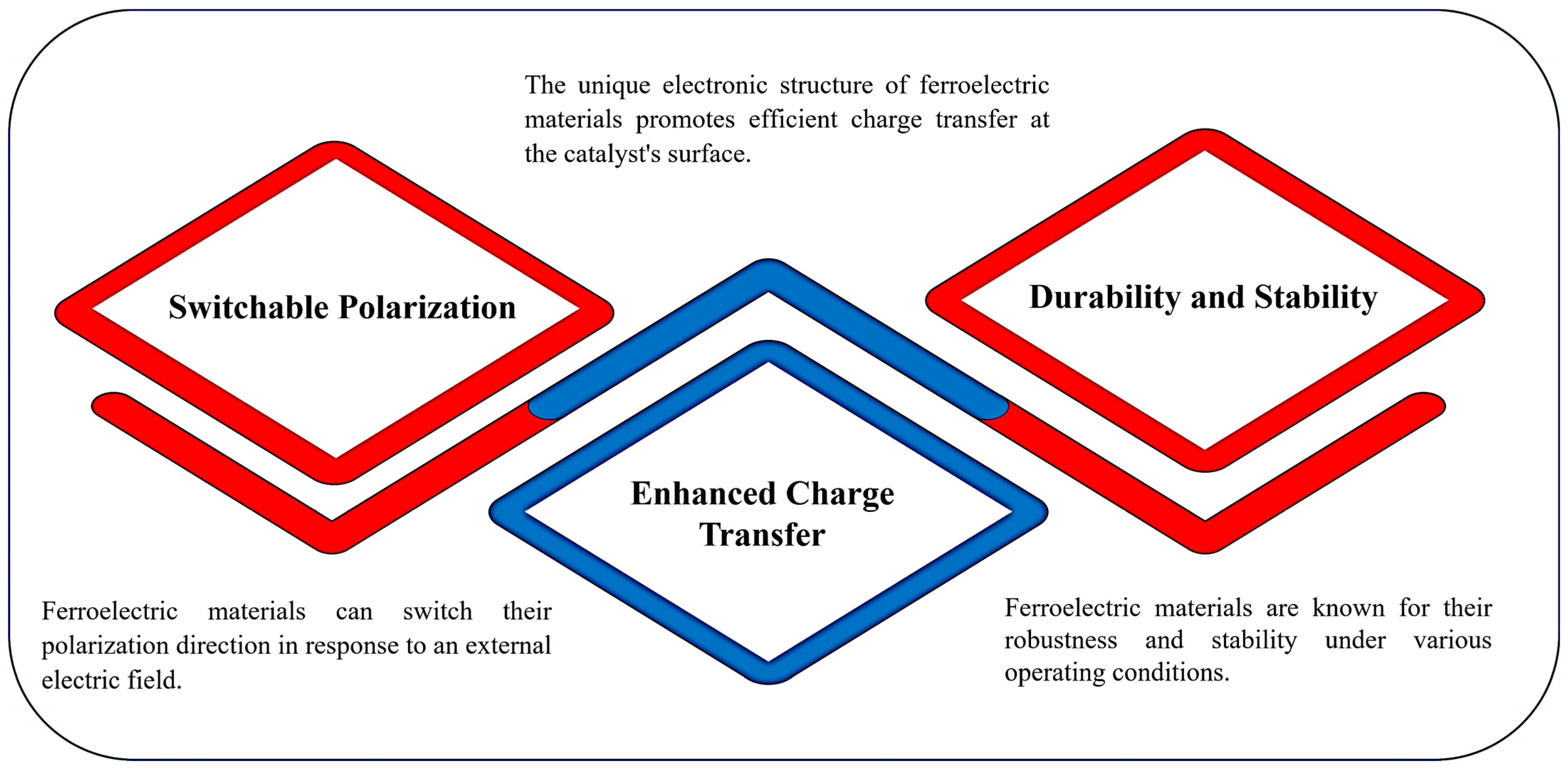
4.1. Electrical Properties
4.2. Optical Properties
4.3. Sustainability
5. Ferroelectric Material as a Photocatalyst to Reduce Carbon Dioxide
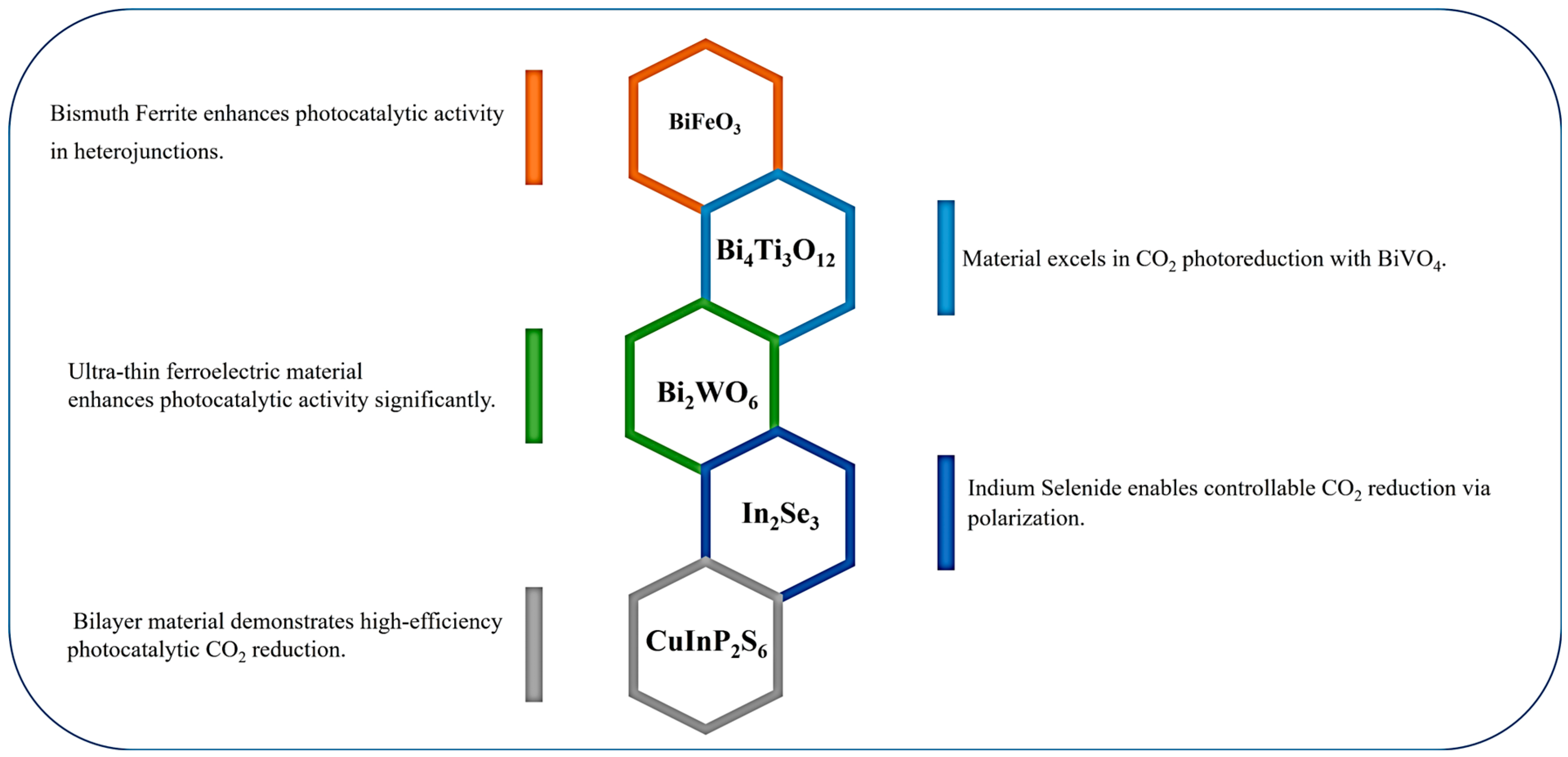
5.1. Bismuth Ferrite (BiFeO3)
5.2. Barium Titanate
5.3. Lead Zirconate Titanate (Pb(Zr,Ti)O3 or PZT)
5.4. Strontium Titanate (SrTiO3)
5.5. Barium Strontium Titanate (Ba,Sr)TiO3
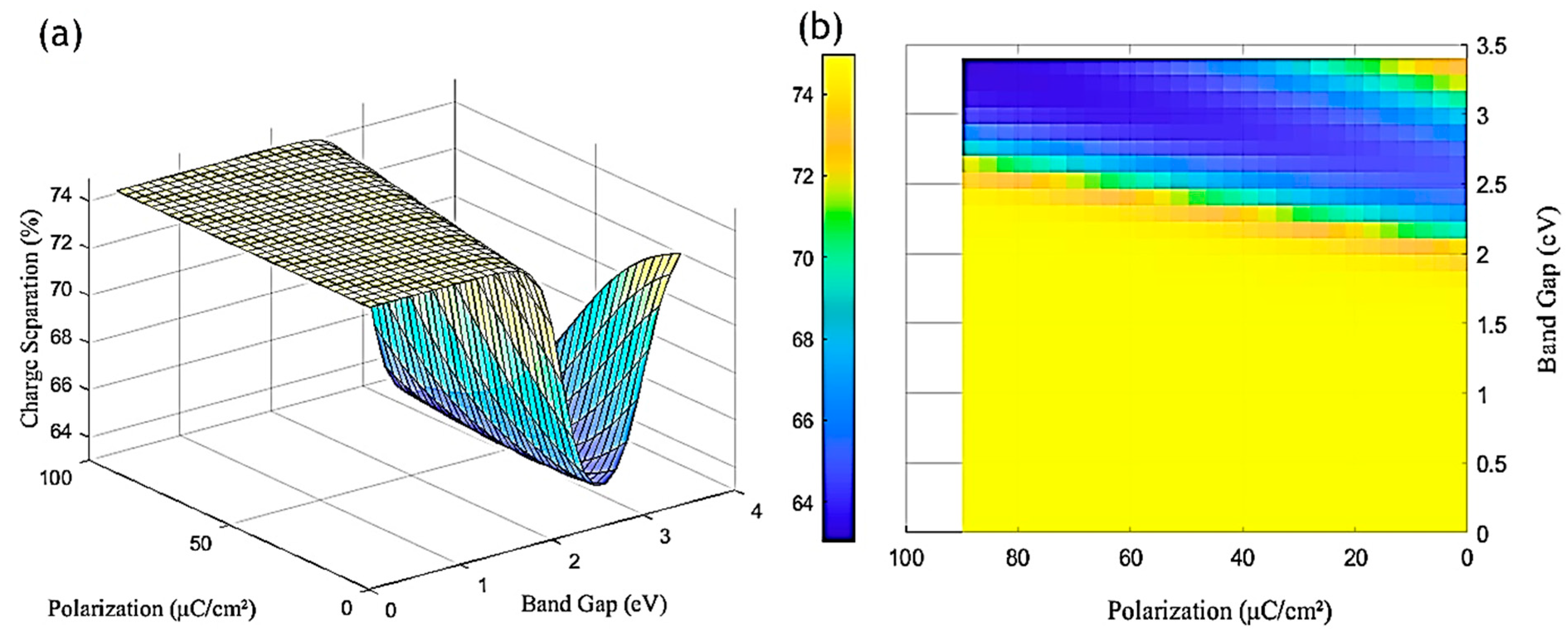
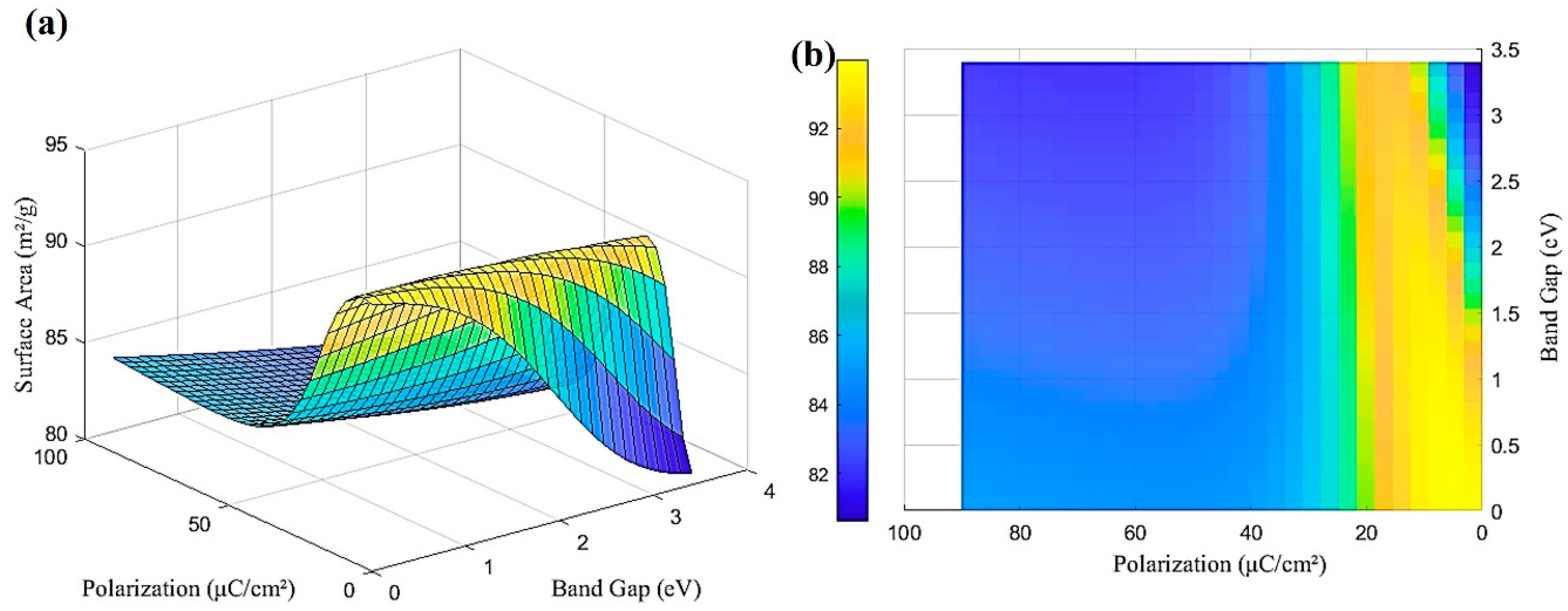
6. Results and Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef] [PubMed]
- Arif, A. The Role of Greenhouse Gases, Aerosols, and Deforestation in Climate Change: A Multidisciplinary Assessment of the Interaction Mechanisms between Human Activity and the Environment. Int. J. Med. Net. 2024, 2, 1–14. [Google Scholar]
- Talberth, J.; Carlson, E. Forest carbon tax and reward: Regulating greenhouse gas emissions from industrial logging and deforestation in the US. Environ. Dev. Sustain. 2024, 27, 1–22. [Google Scholar] [CrossRef]
- Tudor, M.; Borlan, R.; Maniu, D.; Astilean, S.; de la Chapelle, M.L.; Focsan, M. Plasmon-enhanced photocatalysis: New horizons in carbon dioxide reduction technologies. Sci. Total Environ. 2024, 932, 172792. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, F.; Liu, E.; Ouyang, H.; Lu, W.; Gu, H.; Ren, J.; Peng, W.; Hou, H.; He, Y. Carbon dioxide capture and green conversion to clean energy against global warming. Adv. Compos. Hybrid Mater. 2024, 7, 147. [Google Scholar] [CrossRef]
- Murugesan, P.; Narayanan, S.; Manickam, M. Photocatalytic Conversion of Carbon Dioxide into Hydrocarbons. Conversion of Carbon Dioxide into Hydrocarbons Vol. 1 Catalysis; Springer: Cham, Switzerland, 2020; pp. 133–163. [Google Scholar]
- Ochedi, F.O.; Liu, D.; Yu, J.; Hussain, A.; Liu, Y. Photocatalytic, electrocatalytic and photoelectrocatalytic conversion of carbon dioxide: A review. Environ. Chem. Lett. 2021, 19, 941–967. [Google Scholar] [CrossRef]
- Yuan, H.; Mei, J.H.; Gong, Y.N.; Zhong, D.C.; Lu, T.B. Cobalt-based heterogeneous catalysts for photocatalytic carbon dioxide reduction. Tungsten 2024, 6, 410–421. [Google Scholar] [CrossRef]
- Tang, S.; Yang, S.; Chen, Y.; Yang, Y.; Li, Z.; Zi, L.; Liu, Y.; Wang, Y.; Li, Z.; Fu, Z.; et al. Ionothermally synthesized S-scheme isotype heterojunction of carbon nitride with significantly enhanced photocatalytic performance for hydrogen evolution and carbon dioxide reduction. Carbon 2023, 201, 815–828. [Google Scholar] [CrossRef]
- Gulati, S.; Vijayan, S.; Kumar, S.; Harikumar, B.; Trivedi, M.; Varma, R.S. Recent advances in the application of metal-organic frameworks (MOFs)-based nanocatalysts for direct conversion of carbon dioxide (CO2) to value-added chemicals. Coord. Chem. Rev. 2023, 474, 214853. [Google Scholar] [CrossRef]
- Yao, Z.; Zhu, S.; Xu, J.; Xu, Q.; Shi, P.; Min, Y. Engineering of Ti/V bimetallene for selectivity boosting the photocatalytic carbon dioxide hydrogenation reaction. Chem. Eng. J. 2023, 461, 141981. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, J.; Lei, Y.; Liu, H.; Tang, X.; Yi, H.; Huang, X.; Zhao, S.; Zhou, Y.; Gao, F. Photocatalytic CO2 reduction reaction: Influencing factors, reaction pathways and dominant catalysts. Sep. Purif. Technol. 2024, 328, 125056. [Google Scholar] [CrossRef]
- Yu, M.; Wang, J.; Li, G.; Zhang, S.; Zhong, Q. Construction of 3D/2D indium vanadate/graphite carbon nitride with nitrogen defects Z-scheme heterojunction for improving photocatalytic carbon dioxide reduction. J. Mater. Sci. Technol. 2023, 154, 129–139. [Google Scholar] [CrossRef]
- Guo, W.; Guo, T.; Zhang, Y.; Yin, L.; Dai, Y. Progress on simultaneous photocatalytic degradation of pollutants and production of clean energy: A review. Chemosphere 2023, 339, 139486. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Lu, C.S.; Liu, F.Y.; Huang, H.H.; Lin, J.H.; Chen, C.C. Visible-light-driven photocatalysis of carbon dioxide by BiSeX and BiSeX/g-C3N4 (X = Cl, Br, I). Mater. Today Sustain. 2023, 23, 100473. [Google Scholar] [CrossRef]
- Puri, N.; Gupta, A. Water remediation using titanium and zinc oxide nanomaterials through disinfection and photo catalysis process: A review. Environ. Res. 2023, 227, 115786. [Google Scholar] [CrossRef]
- Etafo, N.O.; Bamidele, M.O.; Bamisaye, A.; Alli, Y.A. Revolutionizing photocatalysis: Unveiling efficient alternatives to titanium (IV) oxide and zinc oxide for comprehensive environmental remediation. J. Water Process Eng. 2024, 62, 105369. [Google Scholar] [CrossRef]
- Chowdhury, P.R.; Medhi, H.; Bhattacharyya, K.G.; Hussain, C.M. Photocatalysis: TiO2, ZnO, and species of iron oxides. In Nanoremediation; Elsevier: Amsterdam, The Netherlands, 2023; pp. 101–126. [Google Scholar]
- Ichipi, E.O.; Mugumo, R.; Tichapondwa, S.M.; Chirwa, E.M. A comparative study of metal-sulphide/ZnO for visible-light-induced photocatalysis and the six-flux photon absorption model of an external lamp photoreactor. J. Environ. Chem. Eng. 2024, 12, 113355. [Google Scholar] [CrossRef]
- Tong, F.; Liang, X.; Bao, X.; Zheng, Z. Photocatalysis on hybrid plasmonic nanomaterials: From catalytic mechanism study at single-particle level to materials design. ACS Catal. 2024, 14, 11425–11446. [Google Scholar] [CrossRef]
- Madanu, T.L.; Mouchet, S.R.; Deparis, O.; Liu, J.; Li, Y.; Su, B.L. Tuning and transferring slow photons from TiO2 photonic crystals to BiVO4 nanoparticles for unprecedented visible light photocatalysis. J. Colloid Interface Sci. 2023, 634, 290–299. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, T.; Huang, H.; Yu, M.H.; Fan, X.; Chang, Z.; Bu, X.H. Metal–organic-framework-based photocatalysts optimized by spatially separated cocatalysts for overall water splitting. Adv. Mater. 2020, 32, 2004747. [Google Scholar] [CrossRef]
- Sui, S.; Quan, H.; Hu, Y.; Hou, M.; Guo, S. A strategy of heterogeneous polyurethane-based sponge for water purification: Combination of superhydrophobicity and photocatalysis to conduct oil/water separation and dyes degradation. J. Colloid Interface Sci. 2021, 589, 275–285. [Google Scholar] [CrossRef]
- Teng, Z.; Cai, W.; Sim, W.; Zhang, Q.; Wang, C.; Su, C.; Ohno, T. Photoexcited single metal atom catalysts for heterogeneous photocatalytic H2O2 production: Pragmatic guidelines for predicting charge separation. Appl. Catal. B Environ. 2021, 282, 119589. [Google Scholar] [CrossRef]
- Long, C.; Long, X.; Cai, Y.; Wang, X.; Li, C.; Qing, Y.; Zhao, Y. Long-lived nanoparticle-embedded superhydrophobic membranes with rapid photocatalytic properties and continuous oil–water separation. Chem. Eng. J. 2024, 482, 148743. [Google Scholar] [CrossRef]
- Duan, C.; Liu, X.; Tian, G.; Zhang, D.; Wen, Y.; Che, Y.; Xie, Z.; Ni, Y. A one-stone-two-birds strategy for cellulose dissolution, regeneration, and functionalization as a photocatalytic composite membrane for wastewater purification. Int. J. Biol. Macromol. 2024, 274, 133317. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Pant, K.K.; Kumar, S.; Joshi, D.; Biswas, P. Mechanistic exploration in controlling the product selectivity via metals in TiO2 for photocatalytic carbon dioxide reduction. Appl. Catal. B Environ. Energy 2024, 352, 124054. [Google Scholar] [CrossRef]
- Zhang, L.; Hussain, S.; Li, Q.; Yang, J. PdCu alloy anchored defective titania for photocatalytic conversion of carbon dioxide into methane with 100% selectivity. J. Energy Chem. 2024, 91, 254–265. [Google Scholar] [CrossRef]
- Xu, J.; Roghabadi, F.A.; Luo, Y.; Ahmadi, V.; Wang, Q.; Wang, Z.; He, H. Recent advances in heterogeneous catalysis of solar-driven carbon dioxide conversion. J. Environ. Sci. 2024, 140, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Wang, Y.; Sui, X.; Wu, S.; Dai, W.; Zhang, Z.; Ding, Z.; Long, J. Insight into the Selectivity-Determining Step of Various Photocatalytic CO2 Reduction Products by Inorganic Semiconductors. ACS Catal. 2024, 14, 10760–10788. [Google Scholar] [CrossRef]
- Cheng, Y.; Jabeen, S.; Lei, S.; Liu, N.; Liu, Y.; Liu, Y.; Li, Y.; Wu, X.; Tong, Z.; Yu, J.; et al. N-doped carbon dots-modulated interfacial charge transfer and surface structure in FeNbO4 photocatalysts for enhanced CO2 conversion selectivity to CH4. Chem. Eng. J. 2024, 498, 155576. [Google Scholar] [CrossRef]
- She, H.; Hua, R.; Zhao, J.; Xia, Y.; Wang, L.; Huang, J.; Wang, Q. Synergetic regulation of interfacial electronic structure of Cu, N co-doped carbon modified TiO2 for efficient photocatalytic CO2 reduction. Chem. Eng. J. 2024, 496, 153799. [Google Scholar] [CrossRef]
- Raziq, F.; Rahman, M.Z.; Ali, S.; Ali, R.; Ali, S.; Zada, A.; Wu, X.; Gascon, J.; Wang, Q.; Qiao, L. Enhancing Z-scheme photocatalytic CO2 methanation at extended visible light (>600 nm): Insight into charge transport and surface catalytic reaction mechanisms. Chem. Eng. J. 2024, 479, 147712. [Google Scholar] [CrossRef]
- Kulandaivalu, T.; Mohamed, A.R.; Ali, K.A.; Mohammadi, M. Photocatalytic carbon dioxide reforming of methane as an alternative approach for solar fuel production-a review. Renew. Sustain. Energy Rev. 2020, 134, 110363. [Google Scholar] [CrossRef]
- Chen, J.; Abazari, R.; Adegoke, K.A.; Maxakato, N.W.; Bello, O.S.; Tahir, M.; Tasleem, S.; Sanati, S.; Kirillov, A.M.; Zhou, Y. Metal–organic frameworks and derived materials as photocatalysts for water splitting and carbon dioxide reduction. Coord. Chem. Rev. 2022, 469, 214664. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, J.; Yang, L.; An, Q.; Xu, Q.; Yang, Y.; Guo, H. Enhanced piezoelectric polarization by subtle structure distortion to trigger efficient photocatalytic CO2RR. Appl. Catal. B Environ. 2024, 340, 123177. [Google Scholar] [CrossRef]
- Guan, C.; Liao, Y.; Xiang, Q. Dual-facet engineering of surface carboxyl functionalization and interlayer potassium ions regulation in carbon nitride for enhanced CO2 photoreduction. Sci. China Mater. 2024, 67, 473–483. [Google Scholar] [CrossRef]
- Feng, G.; Mao, J.; Li, G.; Wu, G.; Chen, A.; Wang, J.; Liu, X.; Wei, Y.; Li, S.; Dong, X.; et al. Synergistic effect of oxygen vacancies and functionalized ligands selectively enhanced the photocatalytic oxidation of methane to carbon monoxide. Chem. Eng. J. 2024, 498, 155303. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Hosseinzadeh, A.; Zhou, J.L.; Huang, Z. Progress in the application of surface engineering methods in immobilizing TiO2 and ZnO coatings for environmental photocatalysis. Catal. Rev. 2023, 65, 822–873. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Huang, L.; Zaman, S.; Lei, K.; Yue, T.; Li, Z.A.; You, B.; Xia, B.Y. Engineering 2D photocatalysts toward carbon dioxide reduction. Adv. Energy Mater. 2021, 11, 2003159. [Google Scholar] [CrossRef]
- Prabhu, P.; Jose, V.; Lee, J.M. Heterostructured catalysts for electrocatalytic and photocatalytic carbon dioxide reduction. Adv. Funct. Mater. 2020, 30, 1910768. [Google Scholar] [CrossRef]
- Ji, G.; Zhao, Y.; Liu, Z. Design of porous organic polymer catalysts for transformation of carbon dioxide. Green Chem. Eng. 2022, 3, 96–110. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Wang, W.; Wang, H.; Chen, Q.; Chen, J.; Chen, D. Constructing functionalized carbon quantum dots on amino-rich graphitic carbon nitride to enhance CO2 photocatalytic reduction: Critical role of functional group modulation. Sep. Purif. Technol. 2024, 355, 129780. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.; Huang, Y.; Du, X.; He, Z.; Wang, D.; Song, S. Hydroxylated SiO2-modified {0 0 1}-TiO2 nanosheets as a surface multifunctional photocatalyst for enhanced degradation of gaseous toluene. Chem. Eng. J. 2024, 499, 156055. [Google Scholar] [CrossRef]
- Wang, H.; Xu, S.; Ni, B.; Xu, J.; Solan, G.A.; Gong, S.; Min, Y. Interface interaction mediated surface plasmon resonance enhancement promoted visible-light-driven CO2 reduction with water. Appl. Catal. B Environ. Energy 2024, 355, 124141. [Google Scholar] [CrossRef]
- Zhang, H.; Bian, H.; Xu, B.; Zhu, L.; Zhang, S.; Xia, D. Enhanced selectivity of photoreduction CO2 over Ag/C3N5 Schottky heterostructure by the SPR effect. J. Environ. Chem. Eng. 2024, 12, 112270. [Google Scholar] [CrossRef]
- Shi, J.; Wang, A.; An, Y.; Chen, S.; Bi, C.; Qu, L.; Shi, C.; Kang, F.; Sun, C.; Huang, Z.; et al. Core@ shell-structured catalysts based on Mg-O-Cu bond for highly selective photoreduction of carbon dioxide to methane. Adv. Compos. Hybrid Mater. 2024, 7, 2. [Google Scholar] [CrossRef]
- Zhao, S.; Wan, Y.; Han, L.; Tian, B.; Duan, Z.; Su, R.; Li, X. Advances in ferroelectric and piezoelectric photocatalysts with oxygen vacancy. Carbon Lett. 2024, 35, 1–22. [Google Scholar] [CrossRef]
- He, J.; Wang, X.; Lan, S.; Tao, H.; Luo, X.; Zhou, Y.; Zhu, M. Breaking the intrinsic activity barriers of perovskite oxides photocatalysts for catalytic CO2 reduction via piezoelectric polarization. Appl. Catal. B Environ. 2022, 317, 121747. [Google Scholar] [CrossRef]
- Li, S.; Chen, F.; Chu, S.; Zhang, Z.; Huang, J.; Wang, S.; Feng, Y.; Wang, C.; Huang, H. Synergy-Compensation Effect of Ferroelectric Polarization and Cationic Vacancy Collaboratively Promoting CO2 Photoreduction. Small 2023, 19, 2203559. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Xiao, H.; Jia, Y.; Chen, L.; Geng, H.; Bakhtiar, S.U.H.; Fu, Q.; Guo, Y. Engineering the defects and microstructures in ferroelectrics for enhanced/novel properties: An emerging way to cope with energy crisis and environmental pollution. Adv. Sci. 2022, 9, 2105368. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, H. Ferroelectrics in photocatalysis. Chem. A Eur. J. 2022, 28, e202103975. [Google Scholar] [CrossRef]
- Liu, W.; Du, M.; Wang, Y.; Liu, Y.; Kang, S. Pyroelectric Photocatalysis: Polarization mechanism insight, in situ characterizations and challenges. Chem. Eng. J. 2024, 485, 149627. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, L.; Lei, N.; Khetab, U.; Wei, X.; Zhang, J.; Niu, Y. Piezoelectric field and defect engineering synergistically improved photocatalytic performance of K0.5Na0.5BixNb1−xO3 for degradation of organic pollutants. J. Catal. 2024, 429, 115282. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.Y.; Komarneni, S. The piezoelectric and piezo-photoelectric effects: Emerging strategies for catalytic performance enhancement of low-dimensional nanostructures. Appl. Catal. A Gen. 2023, 670, 119550. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, K.; Li, D.; Li, N.; Xu, J.; Bahnemann, D.W.; Wang, C. Polarization-enhanced photocatalytic activity in non-centrosymmetric materials based photocatalysis: A review. Chem. Eng. J. 2021, 426, 131681. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, D.; Wang, D.; Liu, X.; Wu, Z.; Liu, X.; Tang, W.; Lan, Y.; Yuan, Y.; Liu, F. A novel perovskite ferroelectric KNbO3-Bi (Ni1/2Ti1/2) O3 nanofibers for photocatalytic hydrogen production. Appl. Surf. Sci. 2022, 572, 151359. [Google Scholar] [CrossRef]
- Pal, D.; Maity, D.; Sarkar, A.; Khan, G.G. Photocatalyst Perovskite Ferroelectric Nanostructures. In Solar Fuels; Scrivener Publishing LLC.: Beverly, MA, USA, 2023; pp. 285–339. [Google Scholar]
- Gohar, O.; Khan, M.Z.; Bibi, I.; Bashir, N.; Tariq, U.; Bakhtiar, M.; Karim, M.R.A.; Ali, F.; Hanif, M.B.; Motola, M. Nanomaterials for advanced energy applications: Recent advancements and future trends. Mater. Des. 2024, 241, 112930. [Google Scholar] [CrossRef]
- Zeng, S.; Kar, P.; Thakur, U.K.; Shankar, K. A review on photocatalytic CO2 reduction using perovskite oxide nanomaterials. Nanotechnology 2018, 29, 052001. [Google Scholar] [CrossRef]
- Hu, C.; Tu, S.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Photocatalysis enhanced by external fields. Angew. Chem. Int. Ed. 2021, 60, 16309–16328. [Google Scholar] [CrossRef]
- Liu, L.; Hu, J.; Ma, Z.; Zhu, Z.; He, B.; Chen, F.; Lu, Y.; Xu, R.; Zhang, Y.; Ma, T.; et al. One-dimensional single atom arrays on ferroelectric nanosheets for enhanced CO2 photoreduction. Nat. Commun. 2024, 15, 305. [Google Scholar] [CrossRef]
- Rao, Z.; Huang, R.; Cai, W.; Wang, F.; Yan, Y.; Yang, X.; Long, F.; Wang, Z.; Ma, Y.; Gao, R.; et al. Bi6Fe2Ti3O18 ferroelectrics with various morphologies under mild conditions by crystal growth control for promoted adsorption-piezo-photocatalytic degradation performances. Sep. Purif. Technol. 2025, 354, 129293. [Google Scholar] [CrossRef]
- Alsubaie, M.N.; Alabbad, S.S.; Alrashidi, W.; Alomair, N.A.; Kochkar, H.; Berhault, G.; Jomni, F.; Ercan, I. Ferroelectric-assisted enhancement of the photocatalytic activity of g-C3N4/TiO2 nanotubes heterojunctions through the addition of strontium. J. Photochem. Photobiol. A Chem. 2024, 454, 115699. [Google Scholar]
- Jia, P.; Li, J.; Huang, H. Piezocatalysts and Piezo-Photocatalysts: From Material Design to Diverse Applications. Adv. Funct. Mater. 2024, 34, 2407309. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, S.; Li, L.; Liu, C.; Li, Z.; Luo, D. Piezoelectric effect-assisted Z-scheme heterojunction ZnIn2S4/BaTiO3 for improved photocatalytic reduction of CO2 to CO. Chem. Eng. J. 2024, 483, 149058. [Google Scholar] [CrossRef]
- Yu, L.Q.; Guo, R.T.; Guo, S.H.; Yan, J.S.; Liu, H.; Pan, W.G. Research progress on photocatalytic reduction of CO2 based on ferroelectric materials. Nanoscale 2024, 16, 1058–1079. [Google Scholar] [CrossRef]
- Batool, M.; Nazar, M.F.; Zafar, M.N.; Tahir, A.A. Applications of metal ferrites as photocatalyst for solar fuel production, water splitting and carbon dioxide reduction. In Photoelectrochemical Engineering for Solar Harvesting; Elsevier: Amsterdam, The Netherlands, 2024; pp. 109–139. [Google Scholar]
- Govedarica, M.; Milosevic, I.; Jankovic, V.; Mitrovic, R.; Kundacina, I.; Nastasijevic, I.; Radonic, V. A Cost-Effective and Rapid Manufacturing Approach for Electrochemical Transducers with Magnetic Beads for Biosensing. Micromachines 2025, 16, 343. [Google Scholar] [CrossRef]
- Du, M.; Liu, W.; Liu, N.; Ling, Y.; Kang, S. Mechanisms of noble metal-enhanced ferroelectric spontaneous polarized photocatalysis. Nano Energy 2024, 124, 109495. [Google Scholar] [CrossRef]
- Qi, J.C.; Peng, H.; Xu, Z.K.; Wang, Z.X.; Tang, Y.Y.; Liao, W.Q.; Zou, G.; You, Y.M.; Xiong, R.G. Discovery of molecular ferroelectric catalytic annulation for quinolines. Nat. Commun. 2024, 15, 6738. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, K.; Wang, Y.; Wang, B.; Zhang, L.; Gao, F.; Zhao, Y.; Feng, W.; Zhang, S.; Liu, P. Enhanced charge carrier separation to improve hydrogen production efficiency by ferroelectric spontaneous polarization electric field. Appl. Catal. B Environ. 2018, 227, 322–329. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Li, L.; Yan, W.; Wang, H.; Mao, W.; Cui, Y.; Li, Y.; Zhu, X. Synergizing the internal electric field and ferroelectric polarization of the BiFeO3/ZnIn2S4 Z-scheme heterojunction for photocatalytic overall water splitting. J. Mater. Chem. A 2023, 11, 434–446. [Google Scholar] [CrossRef]
- Huang, R.; Cai, W.; Wang, F.; Zhao, Y.; Rao, Z.; Wang, Z.; Gao, R.; Chen, G.; Deng, X.; Lei, X.; et al. Achieving remarkable piezo-photocatalytic performances in Bi6Fe2Ti3O18/BiOCl S-scheme heterojunction through ferroelectric polarization effect. Appl. Mater. Today 2024, 39, 102308. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, P.; Yang, F.; Zhu, Y.; Pan, Y.; Xue, H.; Mao, W.; Chu, L. Ferroelectric polarization in Bi0.9Dy0.1FeO3/g-C3N4 Z-scheme heterojunction boosts photocatalytic hydrogen evolution. Sci. China Mater. 2024, 67, 3160–3167. [Google Scholar] [CrossRef]
- Liu, S.; Ruan, M.; Guo, Z.; Wang, C.; Nie, W.; Liu, X.; Zhang, L.; Pan, J.; Jia, K. A dual optimization approach for photo-electric catalysis: Heterojunction of BiOBr/Ba0.7Sr0.3TiO3 system construction and ferroelectric polarization. J. Electroanal. Chem. 2024, 952, 117999. [Google Scholar] [CrossRef]
- Yin, X.; Li, Y.; Yang, D.; Xi, R.; Liu, M.; Han, W.; Zhang, M.; Li, X.; Sun, S. Revealing the role of ferroelectric size effect on photocatalyst activity. Appl. Surf. Sci. 2024, 645, 158839. [Google Scholar] [CrossRef]
- Assavachin, S.; Osterloh, F.E. Ferroelectric polarization in BaTiO3 nanocrystals controls photoelectrochemical water oxidation and photocatalytic hydrogen evolution. J. Am. Chem. Soc. 2023, 145, 18825–18833. [Google Scholar] [CrossRef]
- Fei, H.; Zhao, T.; Guo, W.; Wang, X.; Zhang, J.; Fei, Z.; Feng, Z.; Liu, G. Strategies for enhancing activities of typical piezo-photocatalytic material and its applications in environmental remediation: A review. J. Environ. Chem. Eng. 2023, 12, 111650. [Google Scholar] [CrossRef]
- Naz, A.; Bibi, I.; Majid, F.; Dahshan, A.; Jilani, K.; Taj, B.; Ghafoor, A.; Nazeer, Z.; Alzahrani, F.M.; Iqbal, M. Cu and Fe doped NiCo2O4/g-C3N4 nanocomposite ferroelectric, magnetic, dielectric and optical properties: Visible light-driven photocatalytic degradation of RhB and CR dyes. Diam. Relat. Mater. 2024, 141, 110592. [Google Scholar] [CrossRef]
- Mustafa, G.; Bibi, I.; Majid, F.; Sultan, M.; Taj, B.; Nazeer, Z.; Umair, H.M.; Elqahtani, Z.M.; Alwadai, N.; Khan, M.I.; et al. Zn and Ni doping effect on barium hexaferrite ferroelectric, optical properties and photocatalytic activity under visible light irradiation. Phys. B Condens. Matter 2023, 663, 415006. [Google Scholar] [CrossRef]
- Nazeer, Z.; Bibi, I.; Majid, F.; Kamal, S.; Ghafoor, A.; Ali, A.; Kausar, A.; Elqahtani, Z.M.; Alwadai, N.; Iqbal, M. Microemulsion synthesis of Ga and Sr doped BiFeO3 nanoparticles and evaluation of their ferroelectric, optical, dielectric and photocatalytic properties. Phys. B Condens. Matter 2023, 657, 414788. [Google Scholar] [CrossRef]
- Fei, D.; Peng, Y.; Tang, F.; Liu, Z.; Wang, R.; Chen, L.; Liu, X.; Chen, X.; Song, M.; Hao, H. Simultaneous enhancement of the optical absorption, ferroelectric polarization, and photocatalytic activity by designing the Ti/Fe ratio for Bi5Ti3FeO15. Ceram. Int. 2024, 50, 12017–12027. [Google Scholar] [CrossRef]
- Jia, P.; Li, Y.; Zheng, Z.; Liu, Y.; Wang, Y.; Liu, T. A dual optimization approach for photoreduction of CO2 to alcohol in g-C3N4/BaTiO3 system: Heterojunction construction and ferroelectric polarization. Appl. Surf. Sci. 2022, 602, 154310. [Google Scholar] [CrossRef]
- Sakar, M.; Prakash, R.M.; Hussain, C.M.; Shankar, M.V. Ferroelectric semiconductors for photocatalytic energy and environmental applications. In Handbook of Smart Photocatalytic Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–19. [Google Scholar]
- Yu, H.; Chen, F.; Li, X.; Huang, H.; Zhang, Q.; Su, S.; Wang, K.; Mao, E.; Mei, B.; Mul, G.; et al. Synergy of ferroelectric polarization and oxygen vacancy to promote CO2 photoreduction. Nat. Commun. 2021, 12, 4594. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Garg, S.; Bhandari, H.; Sharma, V. A review on recent progressions of Bismuth ferrite modified morphologies as an effective photocatalyst to curb water and air pollution. Inorg. Chem. Commun. 2022, 144, 109834. [Google Scholar] [CrossRef]
- Kharbanda, S.; Dhanda, N.; Sun, A.C.A.; Thakur, A.; Thakur, P. Multiferroic perovskite bismuth ferrite nanostructures: A review on synthesis and applications. J. Magn. Magn. Mater. 2023, 572, 170569. [Google Scholar] [CrossRef]
- Bhat, S.A.; Rashid, A.; Majeed, M.; Tantray, A.M.; Rani, A.; Yousuf, S.; Ikram, M. Enhanced magnetic, ferroelectric, and photocatalytic properties of Nd and Co doped bismuth ferrite (Bi1−xNdxFe1−yCoyO3) ceramics. Inorg. Chem. Commun. 2024, 170, 113148. [Google Scholar] [CrossRef]
- Chen, X.; Sun, D.; He, Z.; Kang, S.; Miao, Y.; Li, Y. Ferrite bismuth-based nanomaterials: From ferroelectric and piezoelectric properties to nanomedicine applications. Colloids Surf. B Biointerfaces 2024, 233, 113642. [Google Scholar] [CrossRef]
- Gulati, S.; Goyal, K.; Arora, A.; Kumar, S.; Trivedi, M.; Jain, S. Bismuth ferrite (BiFeO3) perovskite-based advanced nanomaterials with state-of-the-art photocatalytic performance in water clean-up. Environ. Sci. Water Res. Technol. 2022, 8, 1590–1618. [Google Scholar] [CrossRef]
- Dewan, M.; Majumder, S.B. Investigations on the multifunctionality of bismuth iron oxide. Trans. Indian Inst. Met. 2019, 72, 2061–2072. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, H.; Yamamoto, A.; Yoshida, H. Barium titanate photocatalysts with silver-manganese dual cocatalyst for carbon dioxide reduction with water. Dalton Trans. 2024, 53, 10712–10719. [Google Scholar] [CrossRef]
- Cai, W.; Ma, X.; Chen, J.; Shi, R.; Wang, Y.; Yang, Y.; Jing, D.; Yuan, H.; Du, J.; Que, M. Synergy of oxygen vacancy and piezoelectricity effect promotes the CO2 photoreduction by BaTiO3. Appl. Surf. Sci. 2023, 619, 156773. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Jang, H.J.; Kim, Y.J.; Maeng, J.Y.; Rhee, C.K.; Sohn, Y. Eu (III)–BaTiO3 nanoparticles and BaTiO3/TiO2/Ti sheets; photocatalytic and electrocatalytic CO2 reduction. Mater. Sci. Semicond. Process. 2023, 153, 107134. [Google Scholar] [CrossRef]
- Masekela, D.; Hintsho-Mbita, N.C.; Sam, S.; Yusuf, T.L.; Mabuba, N. Application of BaTiO3-based catalysts for piezocatalytic, photocatalytic and piezo-photocatalytic degradation of organic pollutants and bacterial disinfection in wastewater: A comprehensive review. Arab. J. Chem. 2023, 16, 104473. [Google Scholar] [CrossRef]
- Singh, K.; Verma, R.; Chauhan, A.; Jasrotia, R.; Saini, S.; Thakur, P.; Kumar, V.; Thakur, P.; Thakur, A. Waste Water Treatment Using Piezoelectric Materials: A Review on Piezo-photocatalysis. Top. Catal. 2024, 67, 1–28. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Liu, J.; Yan, K.; Zhu, K. Recent advancements in the use of novel piezoelectric materials for piezocatalytic and piezo-photocatalytic applications. Appl. Catal. B Environ. 2024, 341, 123335. [Google Scholar] [CrossRef]
- Li, T.; Deng, S.; Liu, H.; Chen, J. Insights into strain engineering: From ferroelectrics to related functional materials and beyond. Chem. Rev. 2024, 124, 7045–7105. [Google Scholar] [CrossRef]
- Cheng, H.; Bai, Z.; Cong, R.; Zhou, Z.; Zhang, Z. SrTiO3: Al/Cd0.6Zn0.4S Type-II Heterojunction Photocatalysts for Efficient Solar Water Splitting. J. Phys. Chem. C 2024, 128, 12427–12436. [Google Scholar] [CrossRef]
- Aa, S.; HYb, H.; CEc, N.; ABd, S.; Je, M.; AAf, M. Recent Advances and Perspectives of Perovskites-Based Materials for Efficient Solar Fuel (Hydrogen) Generation via Photocatalytic Water-Splitting. Glob. J. Mater. Sci. Eng. 2024, 6, 1–13. [Google Scholar]
- Ye, X.; Zhong, H.; Zhang, Y.; Wu, Y.; Cai, S.; Chen, S.; Ma, L.; Wang, Q. Fabrication of Ti3C2/TiO2/SrTiO3 ternary heterojunction photocatalyst with efficient charge separation for enhanced hydrogen evolution. Int. J. Hydrogen Energy 2024, 51, 1109–1121. [Google Scholar] [CrossRef]
- Kassim, H.; Aljaafreh, M.J.; Prasad, S.; AlSalhi, M.S.; Asemi, N.N.; Manikandan, E. Investigating the effect of gamma irradiation on the structural, optical, and electrical properties of bismuth-modified strontium titanate ceramics. J. Mater. Sci. Mater. Electron. 2023, 34, 617. [Google Scholar] [CrossRef]
- Arun, K.; Therese, P.S. Novel Green Synthesis of Strontium Titanate (SrTiO3) Nanoparticle-a Perovskite Material. J. Jilin Univ. 2023, 42, 53–62. [Google Scholar]
- Kuspanov, Z.; Umirzakov, A.; Serik, A.; Baimenov, A.; Yeleuov, M.; Daulbayev, C. Multifunctional strontium titanate perovskite-based composite photocatalysts for energy conversion and other applications. Int. J. Hydrogen Energy 2023, 48, 38634–38654. [Google Scholar] [CrossRef]
- Augurio, A. Ferroelectric-Photocatalyst Nanocomposite Thin Films for Enhanced Photocatalytic Activity. Ph.D. Thesis, Queen Mary University of London, London, UK, 2023. [Google Scholar]
- Avcıoglu, C.; Avcıoglu, S.; Bekheet, M.F.; Gurlo, A. Photocatalytic overall water splitting by SrTiO3: Progress report and design strategies. ACS Appl. Energy Mater. 2023, 6, 1134–1154. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Zhu, F.; Li, Y.; Tian, W.; Wang, W.; Guo, R.; Liu, L.; Shi, J. Surface oxygen vacancy engineering in weak Bi–O bonded ferroelectric bismuth sodium titanate for boosting the photocatalytic CO2 reduction reaction. J. Mater. Chem. A 2024, 12, 9661–9671. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, J.; Sheng, X.; Jing, Q.; Wang, X.; Duan, L.; Guo, H. Understanding the synergistic effect of piezoelectric polarization and the extra electrons contributed by oxygen vacancies on an efficient piezo-photocatalysis CO2 reduction. Inorg. Chem. Front. 2023, 10, 2939–2950. [Google Scholar] [CrossRef]
- Zhan, F.; Wen, G.; Li, R.; Feng, C.; Liu, Y.; Liu, Y.; Zhu, M.; Zheng, Y.; Zhao, Y.; La, P. A Comprehensive Review on Oxygen Vacancies Modified Photocatalysts: Synthesis, Characterization, and Applications. Phys. Chem. Chem. Phys. 2024, 26, 11182–11207. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Chen, X.Z.; Sanchis-Gual, R.; Veciana, A.; Franco, C.; Kim, D.; Surin, I.; Pérez-Ramírez, J.; Mattera, M.; et al. Tuning oxygen vacancies in Bi4Ti3O12 nanosheets to boost piezo-photocatalytic activity. Nano Energy 2023, 108, 108202. [Google Scholar] [CrossRef]
- Jiang, W.; Loh, H.; Low, B.Q.L.; Zhu, H.; Low, J.; Heng, J.Z.X.; Tang, K.Y.; Li, Z.; Loh, X.J.; Ye, E.; et al. Role of oxygen vacancy in metal oxides for photocatalytic CO2 reduction. Appl. Catal. B Environ. 2023, 321, 122079. [Google Scholar] [CrossRef]
- Ma, J.; Li, L.; Zhang, Y.; Qian, J.; Wang, X. Covalent Organic Frameworks: Synthesis, Structures, Characterizations and Progress of Photocatalytic Reduction of CO2. Chin. J. Struct. Chem. 2024, 43, 100466. [Google Scholar] [CrossRef]
- Cao, K.; Ge, X.; Li, S.; Tian, Z.; Cui, S.; Guo, G.; Yang, L.; Li, X.; Wang, Y.; Bai, S.; et al. Facile preparation of a 3D rGO/g-C3N4 nanocomposite loaded with Ag NPs for photocatalytic degradation. RSC Adv. 2025, 15, 17089–17101, Erratum in RSC Adv. 2025, 15, 20199. [Google Scholar] [CrossRef]
- Tian, G.; Li, Z.; Zhang, C.; Liu, X.; Fan, X.; Shen, K.; Meng, H.; Wang, N.; Xiong, H.; Zhao, M.; et al. Upgrading CO2 to sustainable aromatics via perovskite-mediated tandem catalysis. Nat. Commun. 2024, 15, 3037. [Google Scholar] [CrossRef]
- Fan, W.; Xin, Q.; Dai, Y.; Chen, Y.; Liu, S.; Zhang, X.; Yang, Y.; Gao, X. Competitive transport and adsorption of CO2/H2O in the graphene nano-slit pore: A molecular dynamics simulation study. Sep. Purif. Technol. 2025, 353, 128394. [Google Scholar] [CrossRef]



| Application | Description |
|---|---|
| Air Purification | Photocatalytic systems can decompose volatile organic compounds (VOCs), bacteria, and viruses using TiO2 under UV light. |
| Water Treatment | It can break down harmful contaminants into harmless byproducts, making it an essential technology for clean water initiatives. |
| Hydrogen Production | Water splitting using photocatalysts can generate hydrogen fuel from water under sunlight. |
| CO2 Reduction | Converting CO2 into hydrocarbons or alcohols provides a method for carbon recycling and energy storage. |
| Environmental Remediation | Photocatalytic systems can be used for the decomposition of pollutants in air and water. |
| Energy Conversion | Photocatalysts can be used in the production of renewable energy, such as hydrogen fuel. |
| Utilizing Polarization Effects | Switchable Polarization The inherent polarization in ferroelectric materials can influence surface chemistry. By switching the polarization direction, the adsorption strengths and reaction pathways for reactants can be altered, leading to enhanced catalytic activity. This phenomenon allows selective adsorption of reactants based on the polarization state, effectively overcoming limitations imposed by traditional catalytic principles like Sabatier’s principle. |
| Band Bending The polarization-induced band bending at the ferroelectric/semiconductor interface promotes charge separation and transfer, enhancing photocatalytic performance. This built-in electric field facilitates the separation of photogenerated electrons and holes, increasing their availability for redox reactions. | |
| Surface Modification | Atomic Dispersion Modifying the surface with atoms that bind strongly can increase reactivity. These atoms can remain dispersed on the surface, enhancing catalytic properties without clustering, which often diminishes reactivity. |
| Functional Group Introduction The incorporation of various functional groups (e.g., -OH, -O, -F) on the surfaces of ferroelectric materials like MXenes can modify their electronic properties and enhance their chemical reactivity. This approach tailors the surface for specific reactions by adjusting the electronic environment. | |
| Nanostructuring and Thin Films | Thin Film Technologies Utilizing ultrathin ferroelectric films (e.g., less than 3 unit cells thick) allows for easier polarization switching and enhanced surface reactivity due to increased surface-to-volume ratios. This also facilitates better control over the material’s properties and enhances its catalytic performance. |
| 2D Ferroelectric Materials Emerging 2D ferroelectric materials exhibit high stability and larger reaction surfaces compared to traditional bulk materials. Their unique properties make them promising candidates for high-efficiency catalysis processes. | |
| Electrostatic Potential Engineering | Charge Imbalance Compensation The charge imbalance created by polarization can be compensated through electronic reconstruction or atomic rearrangement on the material’s surface. This process can enhance the catalytic efficiency by optimizing the active sites available for reactions. |
| Hybrid Systems with Catalytic Metals | Supported Catalytic Metals Integrating ferroelectric materials with metal catalysts can enhance overall reactivity. However, challenges arise from metal clustering; thus, maintaining a well-dispersed state is crucial for effective interaction between the metal and ferroelectric surfaces. |
| Material | Synthesis Method | Morphology | Band Gap (eV) | CO2 Conversion Rate (%) |
|---|---|---|---|---|
| BiFeO3 | Sol–gel | NPs | 2.1 | 20 |
| BaTiO3 | Hydrothermal | Nanosheets | 3.2 | 15 |
| PZT | Solvothermal | Nanofibers | 3.9 | 25 |
| SrTiO3 | Co-precipitation | NPs | 3.2 | 18 |
| KNbO3 | Solid-state | Nanosheets | 3.0 | 22 |
| PbTiO3 | Sol–gel | Nanofibers | 3.4 | 30 |
| Bi2WO6 | Hydrothermal | Nanosheets | 2.9 | 24 |
| LaFeO3 | Solvothermal | NPs | 2.5 | 21 |
| CaTiO3 | Co-precipitation | Nanosheets | 3.1 | 19 |
| ZnO | Solid-state | Nanofibers | 3.2 | 27 |
| Material | Synthesis Method | Morphology | Band Gap (eV) | Polarization (μC/cm2) | Charge Separation (%) | Light Absorption (%) | Surface Area (m2/g) | CO2 Conversion Rate (μmol/g·h) | Product Selectivity (%) | Stability (h) | Sensor Integration (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BiFeO3 | Sol–gel | Nanoparticles | 2.2 | 90 | 75 | 80 | 85 | 120 | 85 (CH4) | 100 | 92 |
| BaTiO3 | Hydrothermal | Nanofibers | 3.2 | 26 | 65 | 70 | 95 | 90 | 75 (HCOOH) | 80 | 88 |
| Pb(Zr,Ti)O3 | Solvothermal | Nanosheets | 3.4 | 35 | 72 | 75 | 90 | 110 | 80 (CH4) | 90 | 90 |
| SrTiO3 | Solid-state | Nanocubes | 3.2 | 18 | 68 | 65 | 80 | 85 | 72 (HCOOH) | 70 | 85 |
| (Ba,Sr)TiO3 | Co-precipitation | Nanoparticles | 3.0 | 30 | 70 | 75 | 92 | 100 | 82 (CH4) | 85 | 92 |
| Bi4Ti3O12 | Molten-salt | Nanosheets | 2.9 | 40 | 75 | 80 | 88 | 115 | 78 (HCOOH) | 95 | 88 |
| KNbO3 | Hydrothermal | Nanofibers | 3.1 | 22 | 62 | 68 | 90 | 80 | 70 (CH4) | 75 | 85 |
| Pb(Mg1/3Nb2/3)O3 | Sol–gel | Nanoparticles | 3.0 | 38 | 68 | 72 | 85 | 95 | 75 (HCOOH) | 80 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, M.; Li, X.; Zu, G.; Wang, L.; Wu, H. Artificial Neural Network Modeling Enhancing Photocatalytic Performance of Ferroelectric Materials for CO2 Reduction: Innovations, Applications, and Neural Network Analysis. Processes 2025, 13, 2670. https://doi.org/10.3390/pr13092670
Tong M, Li X, Zu G, Wang L, Wu H. Artificial Neural Network Modeling Enhancing Photocatalytic Performance of Ferroelectric Materials for CO2 Reduction: Innovations, Applications, and Neural Network Analysis. Processes. 2025; 13(9):2670. https://doi.org/10.3390/pr13092670
Chicago/Turabian StyleTong, Meijuan, Xixiao Li, Guannan Zu, Liangliang Wang, and Hong Wu. 2025. "Artificial Neural Network Modeling Enhancing Photocatalytic Performance of Ferroelectric Materials for CO2 Reduction: Innovations, Applications, and Neural Network Analysis" Processes 13, no. 9: 2670. https://doi.org/10.3390/pr13092670
APA StyleTong, M., Li, X., Zu, G., Wang, L., & Wu, H. (2025). Artificial Neural Network Modeling Enhancing Photocatalytic Performance of Ferroelectric Materials for CO2 Reduction: Innovations, Applications, and Neural Network Analysis. Processes, 13(9), 2670. https://doi.org/10.3390/pr13092670





