Comparative Study of Variable-Flow Gas Injection Patterns on CH4 Diffusion Dynamics: Experimental Insights into Enhanced Coalbed Methane Recovery
Abstract
1. Introduction
2. Experimental Samples, Apparatus, and Procedures
2.1. Preparation of Coal Sample
2.2. Experimental Methods
2.2.1. Experimental Apparatus
2.2.2. Experimental Procedures
3. Experimental Results
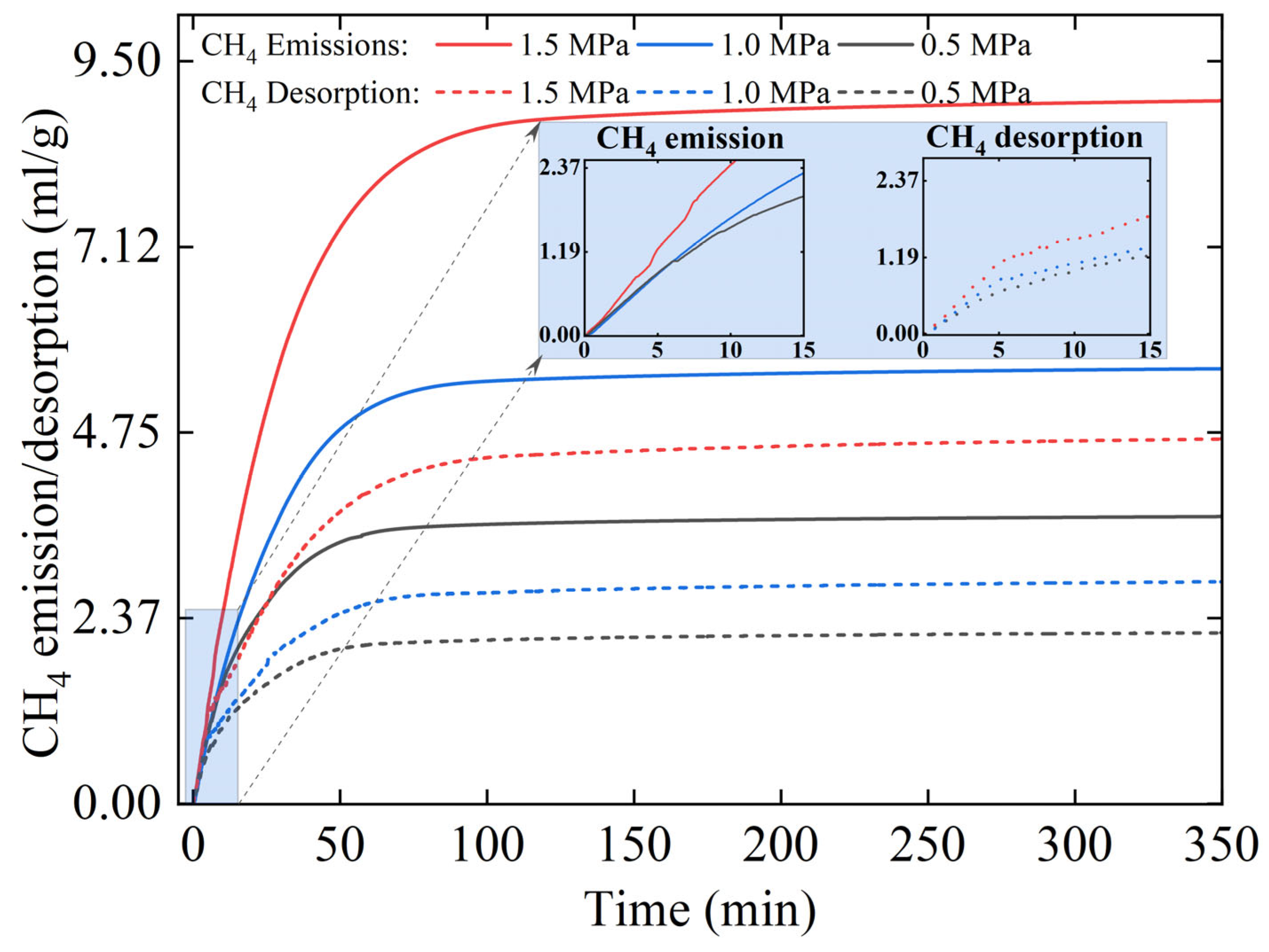
3.1. Unidirectional Diffusion Experiments with N2, CO2, CH4
3.2. Bidirectional Diffusion Experiments with Constant Flow Gas Injection
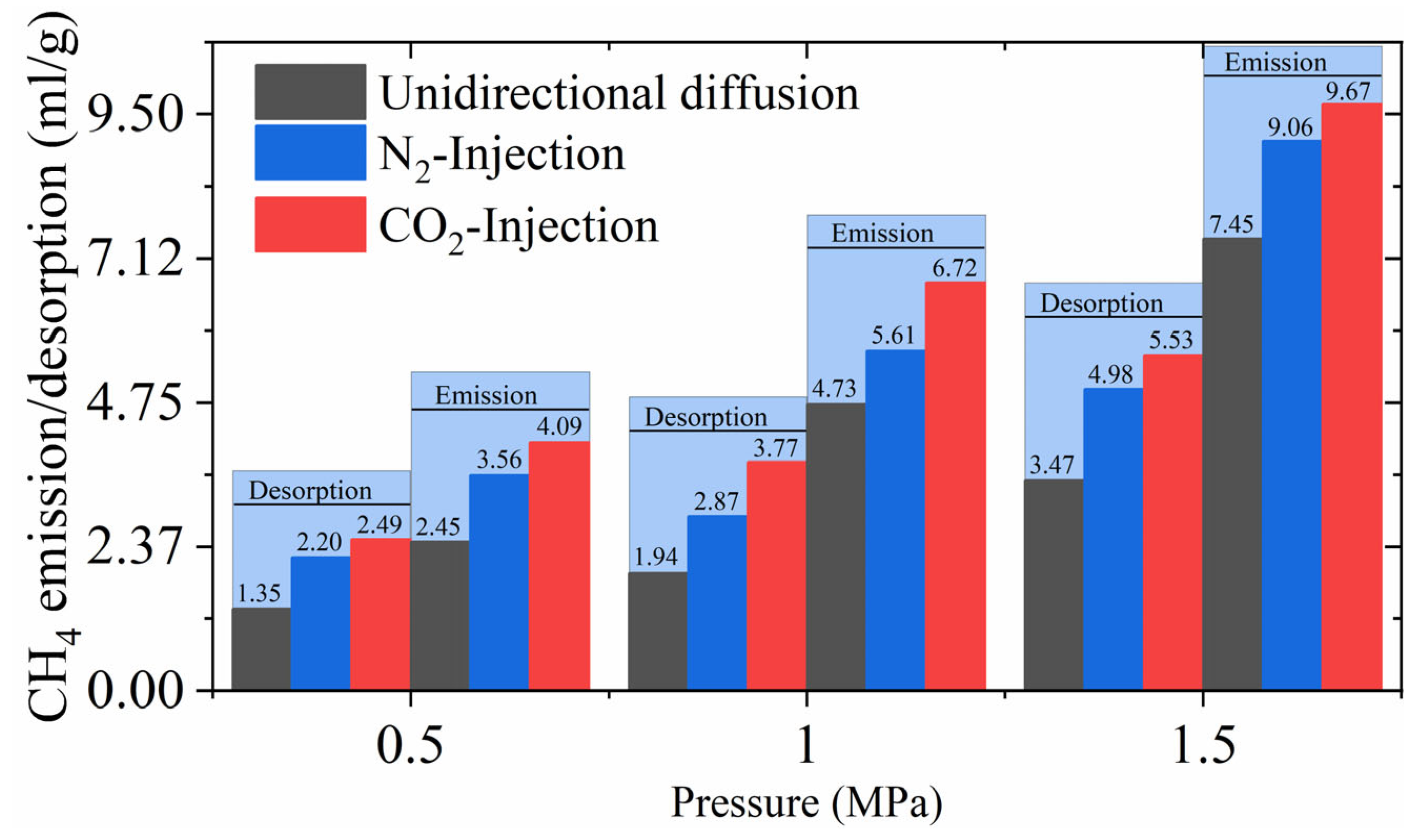
3.2.1. Effect of Pre-Adsorbed Gas Equilibrium Pressure on CH4 Diffusion During Constant Flow Injection
3.2.2. Effect of Different Injection Gas Types on the Diffusion of CH4
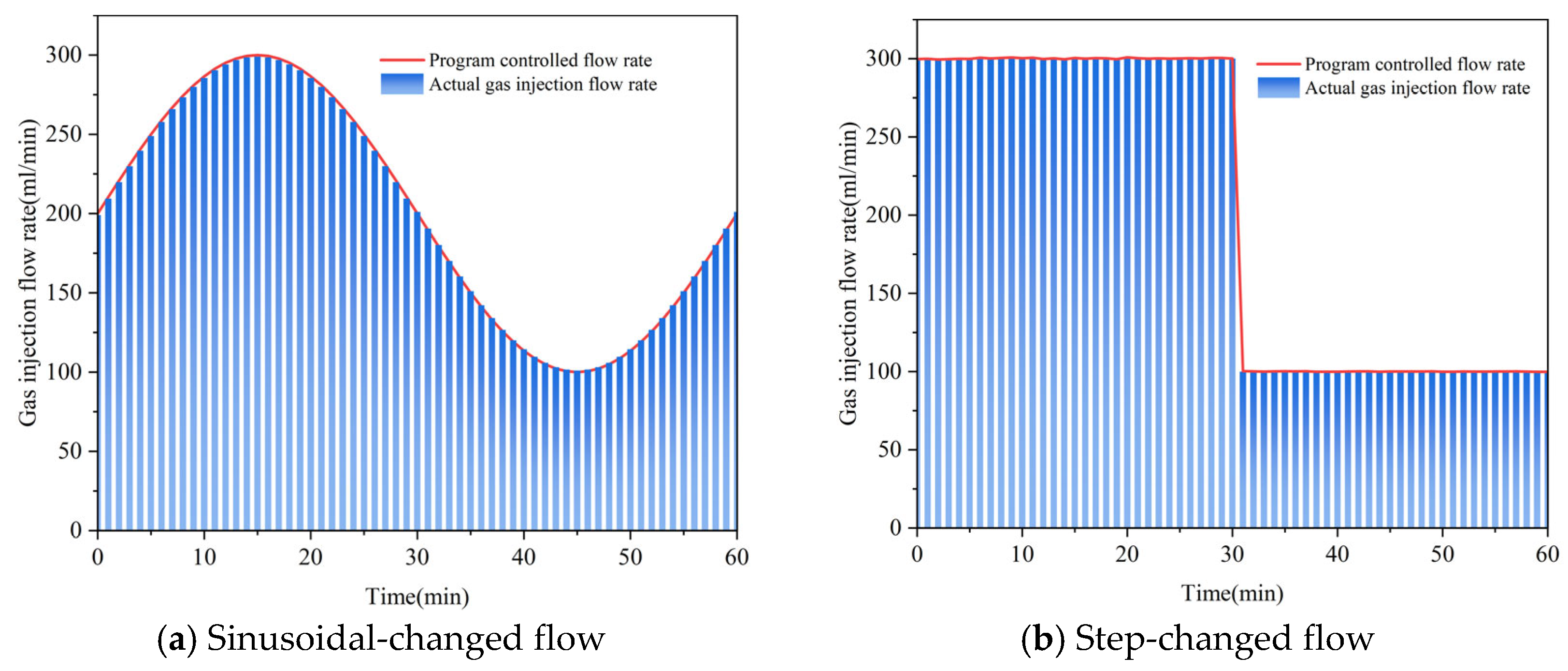
3.3. CH4 Diffusion Experiments for Variable-Flow Gas Injection
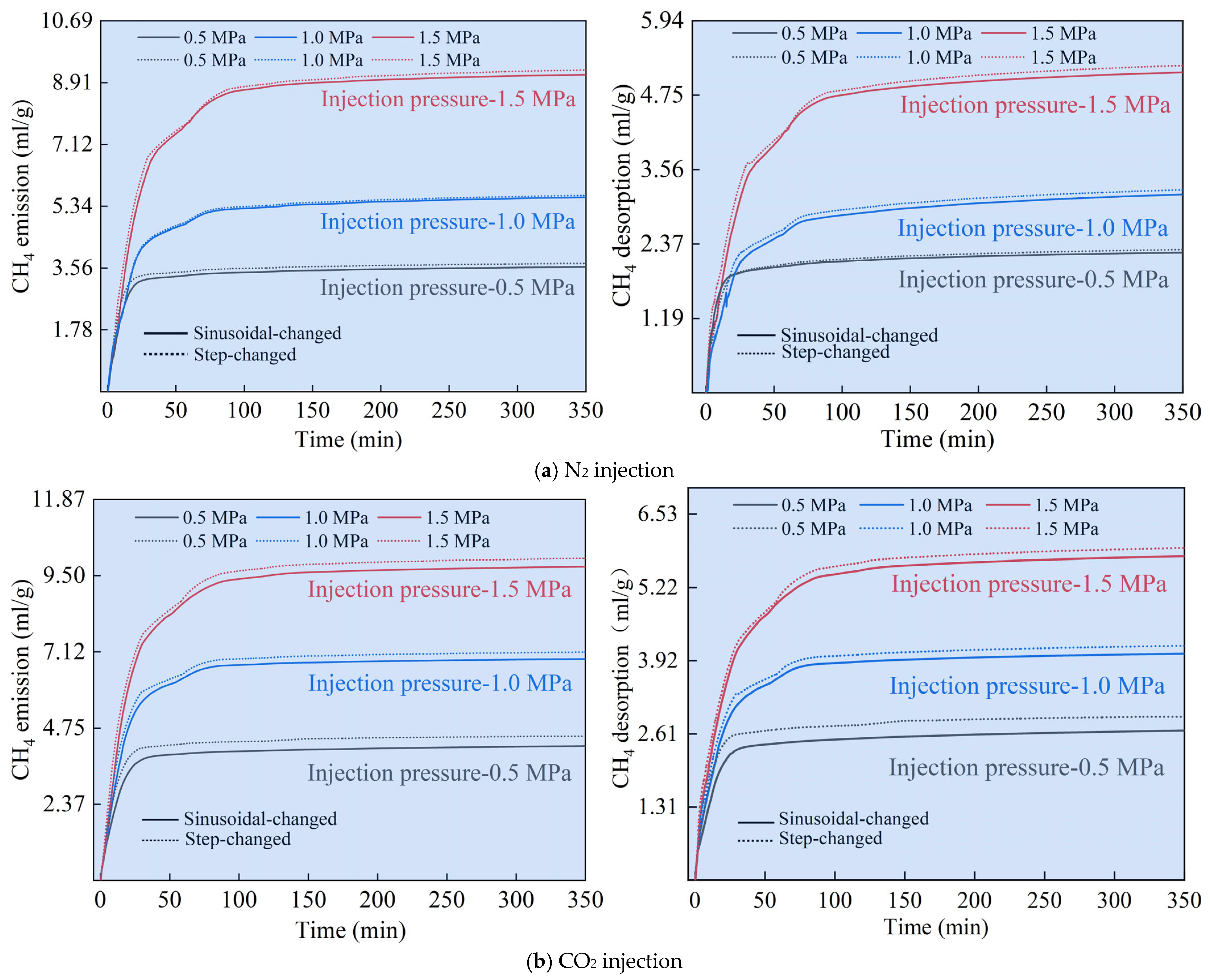
3.3.1. Effect of Gas Injection Types and Injection Modes on CH4 Diffusion
3.3.2. Analysis of the Effect of CH4 Replacement by the Principle of Equal-Time and Equal-Quantity Gas Injection
4. Analysis of the Applicability of the Gas Diffusion Model in Coal
4.1. Model of Gas Diffusion in Coal Particles
4.1.1. Unipore Diffusion Model
4.1.2. Bidisperse Diffusion Model
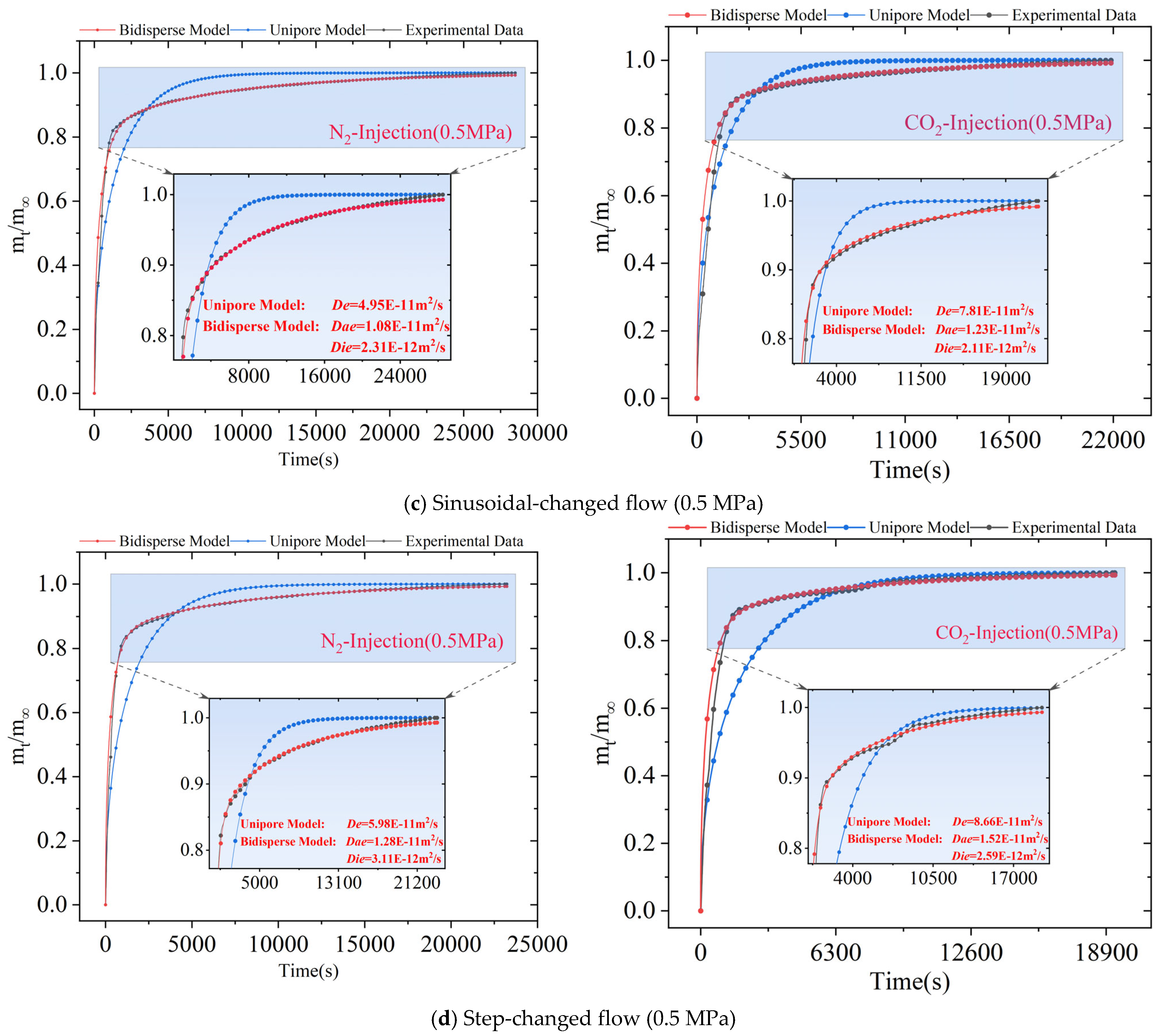
4.2. Diffusion Model Applicability Analysis
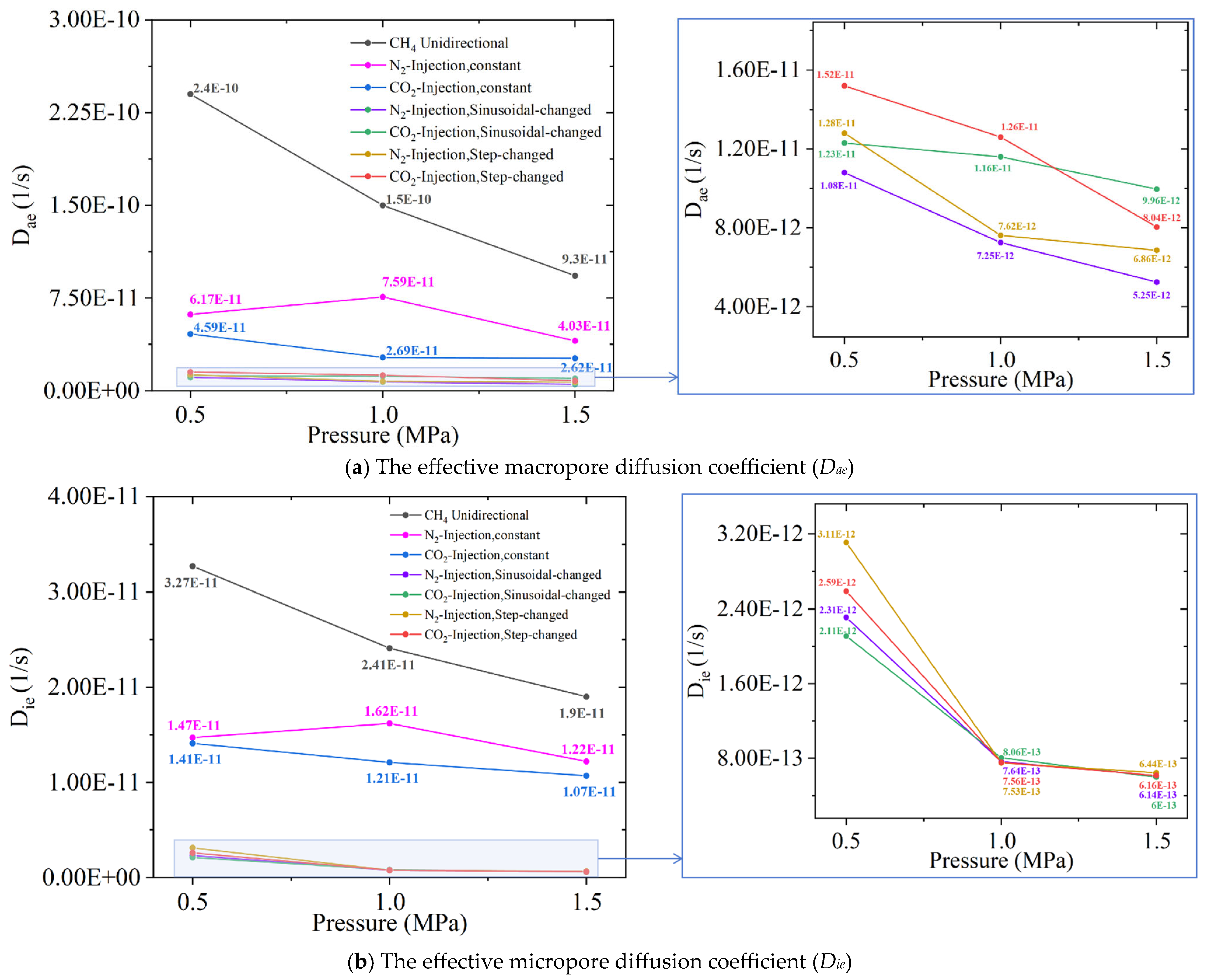
4.3. Effect of Different Gas Injection Methods on Diffusion Coefficients
4.4. Limitations of Unipore and Bidisperse Diffusion Models
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reeves, S.; Taillefert, A.; Pekot, L.; Clarkson, C. The Allison Unit CO2-ECBM Pilot: A Reservoir Modeling Study; Topical Report DE-FC26-0NT4092433; U.S. Department of Energy: Washington, DC, USA, 2003. [CrossRef]
- Fujioka, M.; Yamaguchi, S.; Nako, M. CO2-ECBM field tests in the Ishikari coal basin of Japan. Int. J. Coal Geol. 2010, 82, 287–298. [Google Scholar] [CrossRef]
- Wong, S.; Law, D.; Deng, X.; Robinson, J.; Kadatz, B.; Gunter, W.D.; Ye, J.; Feng, S.; Fan, Z. Enhanced coalbed methane and CO2 storage in anthracitic coals—Micro-pilot test at South Qinshui, Shanxi, China. Int. J. Greenh. Gas Control 2007, 1, 215–222. [Google Scholar] [CrossRef]
- van Bergen, F.; Krzystolik, P.; van Wageningen, N.; Pagnier, H.; Jura, B.; Skiba, J.; Winthaegen, P.; Kobiela, Z. Production of gas from coal seams in the Upper Silesian Coal Basin in Poland in the post-injection period of an ECBM pilot site. Int. J. Coal Geol. 2008, 77, 175–187. [Google Scholar] [CrossRef]
- Gong, H.; Wang, K.; Wang, G.; Yang, X.; Du, F. Underground coal seam gas displacement by injecting nitrogen: Field test and effect prediction. Fuel 2021, 306, 121646. [Google Scholar] [CrossRef]
- Yang, X.; Wang, G.; Du, F.; Jin, L.; Gong, H. N2 injection to enhance coal seam gas drainage (N2-ECGD): Insights from underground field trial investigation. Energy 2022, 239, 122247. [Google Scholar] [CrossRef]
- Lin, J.; Ren, T.; Cheng, Y.; Nemcik, J.; Wang, G. Cyclic N2 injection for enhanced coal seam gas recovery: A laboratory study. Energy 2019, 188, 116115. [Google Scholar] [CrossRef]
- Packham, R.; Connell, L.; Cinar, Y.; Moreby, R. Observations from an enhanced gas recovery field trial for coal mine gas management. Int. J. Coal Geol. 2012, 100, 82–92. [Google Scholar] [CrossRef]
- Reeves, S.R. The Coal-Seq project: Key results from field, laboratory, and modeling studies. In Proceedings of the 7th International Conference on Greenhouse Gas Control Technologies, Vancouver, BC, Canada, 5–9 September 2004. [Google Scholar] [CrossRef]
- Bustin, A.M.M.; Bustin, R.; Chikatamarla, L.; Downey, R.; Mansoori, J. Learnings from a failed nitrogen enhanced coalbed methane pilot: Piceance Basin, Colorado. Int. J. Coal Geol. 2016, 165, 64–75. [Google Scholar] [CrossRef]
- Mazzotti, M.; Pini, R.; Storti, G. Enhanced coalbed methane recovery. J. Supercrit. Fluids 2008, 47, 619–627. [Google Scholar] [CrossRef]
- Pan, Z.; Connell, L.D. Comparison of adsorption models in reservoir simulation of enhanced coalbed methane recovery and CO2 sequestration in coal. Int. J. Greenh. Gas Control 2008, 3, 77–89. [Google Scholar] [CrossRef]
- Shi, J.-Q.; Durucan, S.; Fujioka, M. A reservoir simulation study of CO2 injection and N2 flooding at the Ishikari coalfield CO2 storage pilot project, Japan. Int. J. Greenh. Gas Control 2008, 2, 47–57. [Google Scholar] [CrossRef]
- Gunter, W.D.; Mavor, M.J.; Robinson, J.R. CO2 storage and enhanced methane production: Field testing at the Fenn-Big Valley, Alberta, Canada, with application. In Proceedings of the 7th International Conferenceon Greenhouse Gas Control Technologies, Vancouver, BC, Canada, 5–9 September 2004. [Google Scholar]
- Durucan, S.; Shi, J.-Q. Improving the CO2 well injectivity and enhanced coalbed methane production performance in coal seams. Int. J. Coal Geol. 2008, 77, 214–221. [Google Scholar] [CrossRef]
- Battistutta, E.; Van Hemert, P.; Lutynski, M.; Bruining, H.; Wolf, K.H. Swelling and sorption experiments on methane, nitrogen and carbon dioxide on dry Selar Cornish coal. Int. J. Coal Geol. 2010, 84, 39–48. [Google Scholar] [CrossRef]
- Fitzgerald, J.E.; Pan, Z.; Sudibandriyo, M.; Robinson, R.L., Jr.; Gasem, K.A.M.; Reeves, S. Adsorption of methane, nitrogen, carbon dioxide and their mixtures on wet Tiffany coal. Fuel 2005, 84, 2351–2363. [Google Scholar] [CrossRef]
- Cui, X.; Bustin, R.M.; Dipple, G. Selective transport of CO2, CH4, and N2 in coals: Insights from modeling of experimental gas adsorption data. Fuel 2004, 83, 293–303. [Google Scholar] [CrossRef]
- Tang, X. Surface thermodynamics of hydrocarbon vapors and carbon dioxide adsorption on shales. Fuel 2019, 238, 402–411. [Google Scholar] [CrossRef]
- Lin, J.; Ren, T.; Wang, G.; Booth, P.; Nemcik, J. Experimental investigation of N2 injection to enhance gas drainage in CO2-rich low permeable seam. Fuel 2018, 215, 665–674. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Xue, J.; Zhang, C.; Fang, X. Experimental studies on the changing characteristics of the gas flow capacity on bituminous coal in CO2-ECBM and N2-ECBM. Fuel 2021, 291, 120115. [Google Scholar] [CrossRef]
- Akbarzadeh, H.; Abbaspour, M.; Salemi, S.; Akbari, M. Injection of mixture of shale gases in a nanoscale pore of graphite and their displacement by CO2/N2 gases using molecular dynamics study. J. Mol. Liq. 2017, 248, 439–446. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Li, K.; Chen, H. Comparison of enhanced coalbed methane recovery by pure N2 and CO2 injection: Experimental observations and numerical simulation. J. Nat. Gas Sci. Eng. 2015, 23, 363–372. [Google Scholar] [CrossRef]
- Ji, P.; Lin, H.; Kong, X.; Li, S.; Cai, Y.; Wang, R.; Tian, Y.; Zhao, T.-S. Experimental study on enhanced coal seam gas extraction by uniform pressure/pulse pressure N2 injection. Fuel 2023, 351, 128988. [Google Scholar] [CrossRef]
- Yin, G.; Deng, B.; Li, M.; Zhang, D.; Wang, W.; Li, W.; Li, W.; Shang, D. Impact of injection pressure on CO2–enhanced coalbed methane recovery considering mass transfer between coal fracture and matrix. Fuel 2017, 196, 288–297. [Google Scholar] [CrossRef]
- Kumar, H.; Elsworth, D.; Mathews, J.P.; Liu, J.; Pone, D. Effect of CO2 injection on heterogeneously permeable coalbed reservoirs. Fuel 2014, 135, 509–521. [Google Scholar] [CrossRef]
- Fan, Z.; Fan, G.; Zhang, D.; Zhang, L.; Zhang, S.; Liang, S.; Yu, W. Optimal injection timing and gas mixture proportion for enhancing coalbed methane recovery. Energy 2021, 222, 119880. [Google Scholar] [CrossRef]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G.; Wei, C.H. An experimental investigation of applicability of CO2 enhanced coal bed methane recovery to low rank coal. Fuel 2017, 189, 391–399. [Google Scholar] [CrossRef]
- Vishal, V.; Singh, T.N.; Ranjith, P.G. Influence of sorption time in CO2-ECBM process in Indian coals using coupled numerical simulation. Fuel 2015, 139, 51–58. [Google Scholar] [CrossRef]
- Sander, R.; Connell, L.D.; Pan, Z.; Camilleri, M.; Heryanto, D.; Lupton, N. Core flooding experiments of CO2 enhanced coalbed methane recovery. Int. J. Coal Geol. 2014, 131, 113–125. [Google Scholar] [CrossRef]
- Connell, L.D.; Sander, R.; Pan, Z.; Camilleri, M.; Heryanto, D. History matching of enhanced coal bed methane laboratory core flood tests. Int. J. Coal Geol. 2011, 87, 128–138. [Google Scholar] [CrossRef]
- Deisman, N.; Khajeh, M.; Chalaturnyk, R.J. Using geological strength index (GSI) to model uncertainty in rock mass properties of coal for CBM/ECBM reservoir geomechanics. Int. J. Coal Geol. 2013, 112, 76–86. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, Y.; Wang, Y.; Wang, L.; Li, W. Experimental investigation of CO2 injection into coal seam reservoir at in-situ stress conditions for enhanced coalbed methane recovery. Fuel 2019, 236, 709–716. [Google Scholar] [CrossRef]
- Sun, J.; Chen, C.; Zhang, Y.; Li, W.; Song, Y. Competitive adsorption characteristics based on partial pressure and adsorption mechanism of CO2/CH4 mixture in shale pores. Chem. Eng. J. 2022, 430, 133172. [Google Scholar] [CrossRef]
- Zhou, G.; Duan, X.; Chang, J.; Bo, Y.; Huang, Y. Investigation of CH4/CO2 competitive adsorption-desorption mechanisms for enhanced shale gas production and carbon sequestration using nuclear magnetic resonance. Energy 2023, 278, 127964. [Google Scholar] [CrossRef]
- Wang, F.; Yao, Y.; Wen, Z.; Sun, Q.; Yuan, X. Effect of water occurrences on methane adsorption capacity of coal: A comparison between bituminous coal and anthracite coal. Fuel 2020, 266, 117102. [Google Scholar] [CrossRef]
- Jia, J.; Wang, D.; Li, B.; Wu, Y.; Zhao, D. Molecular simulation study on the effect of coal metamorphism on the competitive adsorption of CO2/CH4 in binary system. Fuel 2023, 335, 127046. [Google Scholar] [CrossRef]
- Liu, W.; Chu, X.; Xu, H.; Yang, T.; Qin, Y.; Zhao, W. Migration behavior of two-component gases among CO2, N2 and O2 in coal particles during adsorption. Fuel 2022, 313, 123003. [Google Scholar] [CrossRef]
- Wu, S.; Deng, C.; Wang, X. Molecular simulation of flue gas and CH4 competitive adsorption in dry and wet coal. J. Nat. Gas Sci. Eng. 2019, 71, 102980. [Google Scholar] [CrossRef]
- Li, Z.; Sun, X.; Zhao, K.; Lei, C.; Wen, H.; Ma, L.; Shu, C.-M. Deformation mechanism and displacement ability during CO2 displacing CH4 in coal seam under different temperatures. J. Nat. Gas Sci. Eng. 2022, 108, 104838. [Google Scholar] [CrossRef]
- Rani, S.; Prusty, B.K.; Pal, S.K. Adsorption kinetics and diffusion modeling of CH4 and CO2 in Indian shales. Fuel 2018, 216, 61–70. [Google Scholar] [CrossRef]
- Charrière, D.; Pokryszka, Z.; Behra, P. Effect of pressure and temperature on diffusion of CO2 and CH4 into coal from the Lorraine basin (France). Int. J. Coal Geol. 2010, 81, 373–380. [Google Scholar] [CrossRef]
- Wang, G.; Ren, T.; Qi, Q.; Lin, J.; Liu, Q.; Zhang, J. Determining the diffusion coefficient of gas diffusion in coal: Development of numerical solution. Fuel 2017, 196, 47–58. [Google Scholar] [CrossRef]
- Shi, J.Q.; Durucan, S. A bidisperse pore diffusion model for methane displacement desorption in coal by CO2 injection. Fuel 2003, 82, 1219–1229. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Zhao, W.; Huang, W.; Ling, H.; Wang, L.; Cheng, Y. A novel bidisperse diffusion model for coal particles considering micropores as the major sites of CH4 adsorption. Fuel 2023, 333, 126505. [Google Scholar] [CrossRef]
- Staib, G.; Sakurovs, R.; Gray, E.M.A. Dispersive diffusion of gases in coals. Part I: Model development. Fuel 2015, 143, 612–619. [Google Scholar] [CrossRef]
- Staib, G.; Sakurovs, R.; Gray, E.M.A. Dispersive diffusion of gases in coals. Part II: An assessment of previously proposed physical mechanisms of diffusion in coal. Fuel 2015, 143, 620–629. [Google Scholar] [CrossRef]
- Ajoma, E.; Saira; Sungkachart, T.; Ge, J.; Le-Hussain, F. Water-saturated CO2 injection to improve oil recovery and CO2 storage. Appl. Energy 2020, 266, 114853. [Google Scholar] [CrossRef]
- Hao, Y.; Li, Z.; Su, Y.; Kong, C.; Chen, H.; Meng, Y. Experimental investigation of CO2 storage and oil production of different CO2 injection methods at pore-scale and core-scale. Energy 2022, 254, 124349. [Google Scholar] [CrossRef]
- Zanganeh, P.; Dashti, H.; Ayatollahi, S. Comparing the effects of CH4, CO2, and N2 injection on asphaltene precipitation and deposition at reservoir condition: A visual and modeling study. Fuel 2018, 217, 633–641. [Google Scholar] [CrossRef]
- Mohammadi, K.; Ameli, F. Toward mechanistic understanding of Fast SAGD process in naturally fractured heavy oil reservoirs: Application of response surface methodology and genetic algorithm. Fuel 2019, 253, 840–856. [Google Scholar] [CrossRef]
- Li, D.; Saraji, S.; Jiao, Z.; Zhang, Y. CO2 injection strategies for enhanced oil recovery and geological sequestration in a tight reservoir: An experimental study. Fuel 2021, 284, 119013. [Google Scholar] [CrossRef]
- Badrouchi, N.; Pu, H.; Smith, S.; Badrouchi, F. Evaluation of CO2 enhanced oil recovery in unconventional reservoirs: Experimental parametric study in the Bakken. Fuel 2022, 312, 122941. [Google Scholar] [CrossRef]
- Xue, H.; Gui, X.; Wang, G.; Yang, X.; Gong, H.; Du, F. Prediction of gas drainage changes from nitrogen replacement: A study of a TCN deep learning model with integrated attention mechanism. Fuel 2024, 357, 129797. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Jian, X.; Guan, P.; Zhang, W. Carbon dioxide sorption and diffusion in coals: Experimental investigation and modeling. Sci. China Earth Sci. 2012, 55, 633–643. [Google Scholar] [CrossRef]
- Švábová, M.; Weishauptová, Z.; Přibyl, O. The effect of moisture on the sorption process of CO2 on coal. Fuel 2012, 92, 187–196. [Google Scholar] [CrossRef]
- Pone, J.D.N.; Halleck, P.M.; Mathews, J.P. Sorption capacity and sorption kinetic measurements of CO2 and CH4 in confined and unconfined bituminous coal. Energy Fuels 2009, 23, 4688–4695. [Google Scholar] [CrossRef]
- Mazumder, S.; Wolf, K.H. Differential swelling and permeability change of coal in response to CO2 injection for ECBM. Int. J. Coal Geol. 2008, 74, 123–138. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 1. Isotherms and pore volume distributions. Fuel 1999, 78, 1333–1344. [Google Scholar] [CrossRef]
- Thomas, N.L.; Windle, A.H. A theory of case II diffusion. Polymer 1982, 23, 529–542. [Google Scholar] [CrossRef]








| Coal Sample | Moisture (%) | Ash (%) | Volatile Matter (%) | Porosity (%) |
|---|---|---|---|---|
| Xiao Jiawa 13# | 2.09 | 31.39 | 29.87 | 4.82 |
| Number | Adsorbed Gas | Adsorption Equilibrium Pressure/MPa |
|---|---|---|
| 1 | CH4 | 0.5, 1.0, 1.5 |
| 2 | N2 | |
| 3 | CO2 |
| Number | Injected Gas | Adsorbed Gas | Gas Injection Method | Gas Injection Flow Rate/(mL/min) | Adsorption Equilibrium Pressure/MPa | Replacement Pressure/MPa |
|---|---|---|---|---|---|---|
| 1 | N2 | CH4 | Constant | 200 | 0.5, 1.0, 1.5 | 0.5, 1.0, 1.5 |
| Sinusoidal-changed | ||||||
| Step-changed | ||||||
| 2 | CO2 | CH4 | Constant | 200 | 0.5, 1.0, 1.5 | 0.5, 1.0, 1.5 |
| Sinusoidal-changed | ||||||
| Step-changed |
| Gas Type | Adsorption Equilibrium Pressure (MPa) | Gas Emission (mL/g) | Gas Desorption (mL/g) |
|---|---|---|---|
| CH4 | 0.5 | 2.45 | 1.35 |
| 1.0 | 4.73 | 1.94 | |
| 1.5 | 7.44 | 3.47 | |
| CO2 | 0.5 | 2.85 | / |
| 1.0 | 5.79 | ||
| 1.5 | 8.84 | ||
| N2 | 0.5 | 2.27 | / |
| 1.0 | 4.43 | ||
| 1.5 | 6.37 |
| Type of Gas Injection | Adsorption Equilibrium Pressure/(MPa) | Unipore Model | Bidisperse Model | |
|---|---|---|---|---|
| De/(m2/s) | /(m2/s) | |||
| CH4 unidirectional diffusion | 0.5 | 8.39 × 10−10 | 2.41 × 10−10 | 3.27 × 10−11 |
| 1.0 | 7.21 × 10−10 | 1.53 × 10−10 | 2.41 × 10−11 | |
| 1.5 | 8.07 × 10−10 | 9.32 × 10−11 | 1.94 × 10−11 | |
| Constant flow injection of N2 to replace CH4 | 0.5 | 3.78 × 10−10 | 6.17 × 10−11 | 1.47 × 10−11 |
| 1.0 | 8.21 × 10−10 | 7.59 × 10−11 | 1.62 × 10−11 | |
| 1.5 | 7.06 × 10−10 | 4.03 × 10−11 | 1.22 × 10−11 | |
| Constant flow injection of CO2 to replace CH4 | 0.5 | 1.49 × 10−10 | 4.59 × 10−11 | 1.41 × 10−11 |
| 1.0 | 1.66 × 10−10 | 2.69 × 10−11 | 1.21 × 10−11 | |
| 1.5 | 0.97 × 10−11 | 2.62 × 10−11 | 1.07 × 10−11 | |
| Sinusoidal-changed flow injection of N2 to replace CH4 | 0.5 | 4.95 × 10−11 | 1.08 × 10−11 | 2.31 × 10−12 |
| 1.0 | 2.23 × 10−11 | 7.25 × 10−12 | 7.64 × 10−13 | |
| 1.5 | 1.92 × 10−11 | 5.25 × 10−12 | 6.14 × 10−13 | |
| Sinusoidal-changed flow injection of CO2 to replace CH4 | 0.5 | 7.81 × 10−11 | 1.23 × 10−11 | 2.11 × 10−12 |
| 1.0 | 5.19 × 10−11 | 1.16 × 10−11 | 8.06 × 10−13 | |
| 1.5 | 3.55 × 10−11 | 9.96 × 10−12 | 6.00 × 10−13 | |
| Step-changed flow injection of N2 to replace CH4 | 0.5 | 5.98 × 10−11 | 1.28 × 10−11 | 3.11 × 10−12 |
| 1.0 | 2.79 × 10−11 | 7.62 × 10−12 | 7.53 × 10−13 | |
| 1.5 | 2.16 × 10−11 | 6.86 × 10−12 | 6.44 × 10−13 | |
| Step-changed flow injection of CO2 to replace CH4 | 0.5 | 8.66 × 10−11 | 1.52 × 10−11 | 2.59 × 10−12 |
| 1.0 | 5.54 × 10−11 | 1.26 × 10−11 | 7.56 × 10−13 | |
| 1.5 | 3.86 × 10−11 | 8.02 × 10−12 | 6.16 × 10−13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Gong, H.; Zhang, G.; Lou, Z.; Hu, J. Comparative Study of Variable-Flow Gas Injection Patterns on CH4 Diffusion Dynamics: Experimental Insights into Enhanced Coalbed Methane Recovery. Processes 2025, 13, 2642. https://doi.org/10.3390/pr13082642
Wu J, Gong H, Zhang G, Lou Z, Hu J. Comparative Study of Variable-Flow Gas Injection Patterns on CH4 Diffusion Dynamics: Experimental Insights into Enhanced Coalbed Methane Recovery. Processes. 2025; 13(8):2642. https://doi.org/10.3390/pr13082642
Chicago/Turabian StyleWu, Jingang, Haoran Gong, Guang Zhang, Zhen Lou, and Jiaying Hu. 2025. "Comparative Study of Variable-Flow Gas Injection Patterns on CH4 Diffusion Dynamics: Experimental Insights into Enhanced Coalbed Methane Recovery" Processes 13, no. 8: 2642. https://doi.org/10.3390/pr13082642
APA StyleWu, J., Gong, H., Zhang, G., Lou, Z., & Hu, J. (2025). Comparative Study of Variable-Flow Gas Injection Patterns on CH4 Diffusion Dynamics: Experimental Insights into Enhanced Coalbed Methane Recovery. Processes, 13(8), 2642. https://doi.org/10.3390/pr13082642







