Tomato Seed Inoculation with Bacillus subtilis Biofilm Mitigates Toxic Effects of Excessive Copper in the Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Bacterial Activation and Biofilm Preparation
2.3. Laboratory Tests
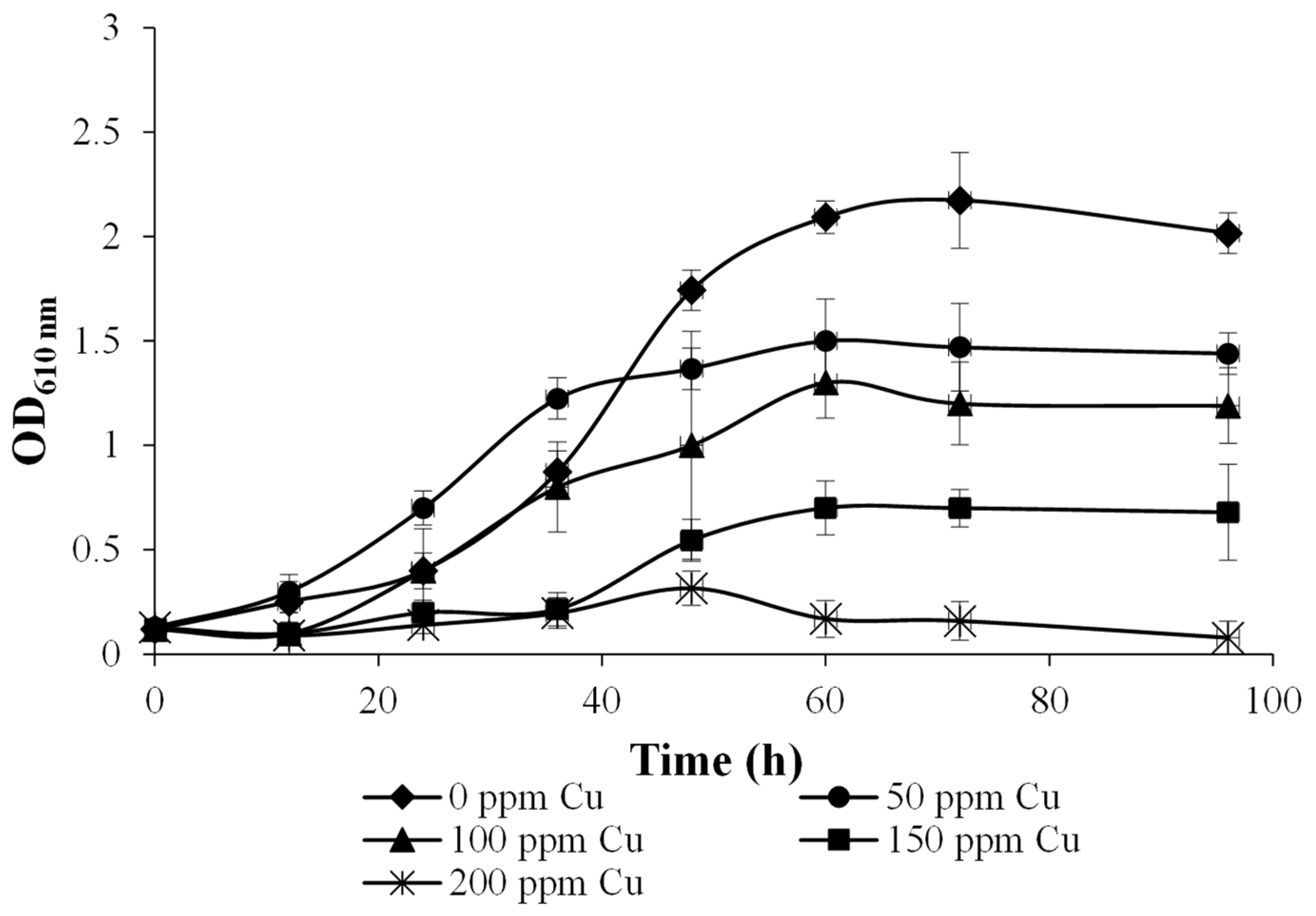
2.3.1. Effect of Cu Concentration on Bacterial Growth
2.3.2. In Vitro Seed Germination
2.4. Greenhouse Cultivation
2.4.1. Provision of a Cu-Rich Crop Substrate
2.4.2. Inoculation, Sowing, and Growth Conditions
2.4.3. Plant and Fruit Analysis at Harvest
2.5. Analysis of Cu on Tomato Fruit
2.6. Statistical Analysis
3. Results
3.1. Bacterial Tolerance to Increased Cu Concentrations in the Culture Medium
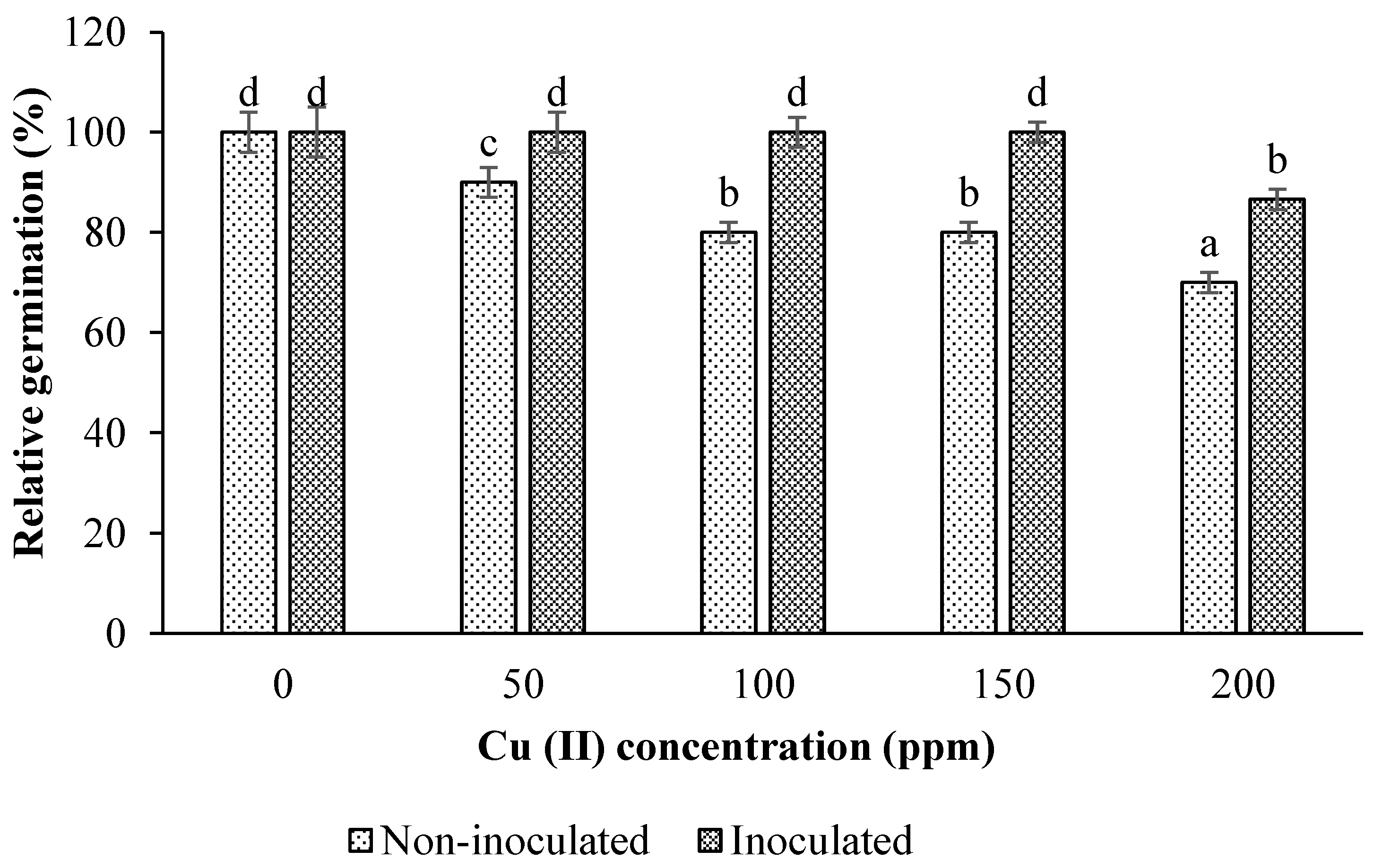
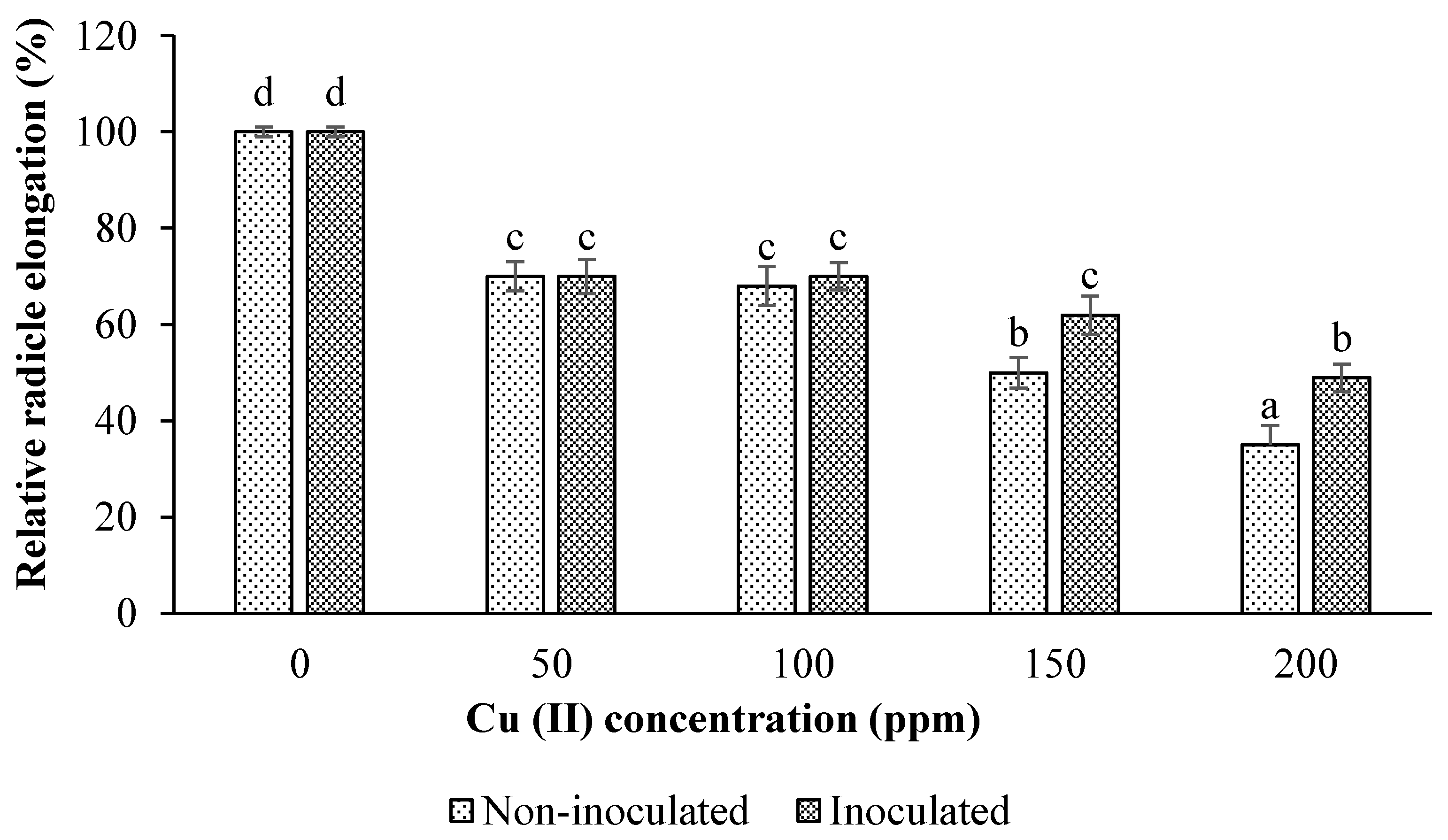
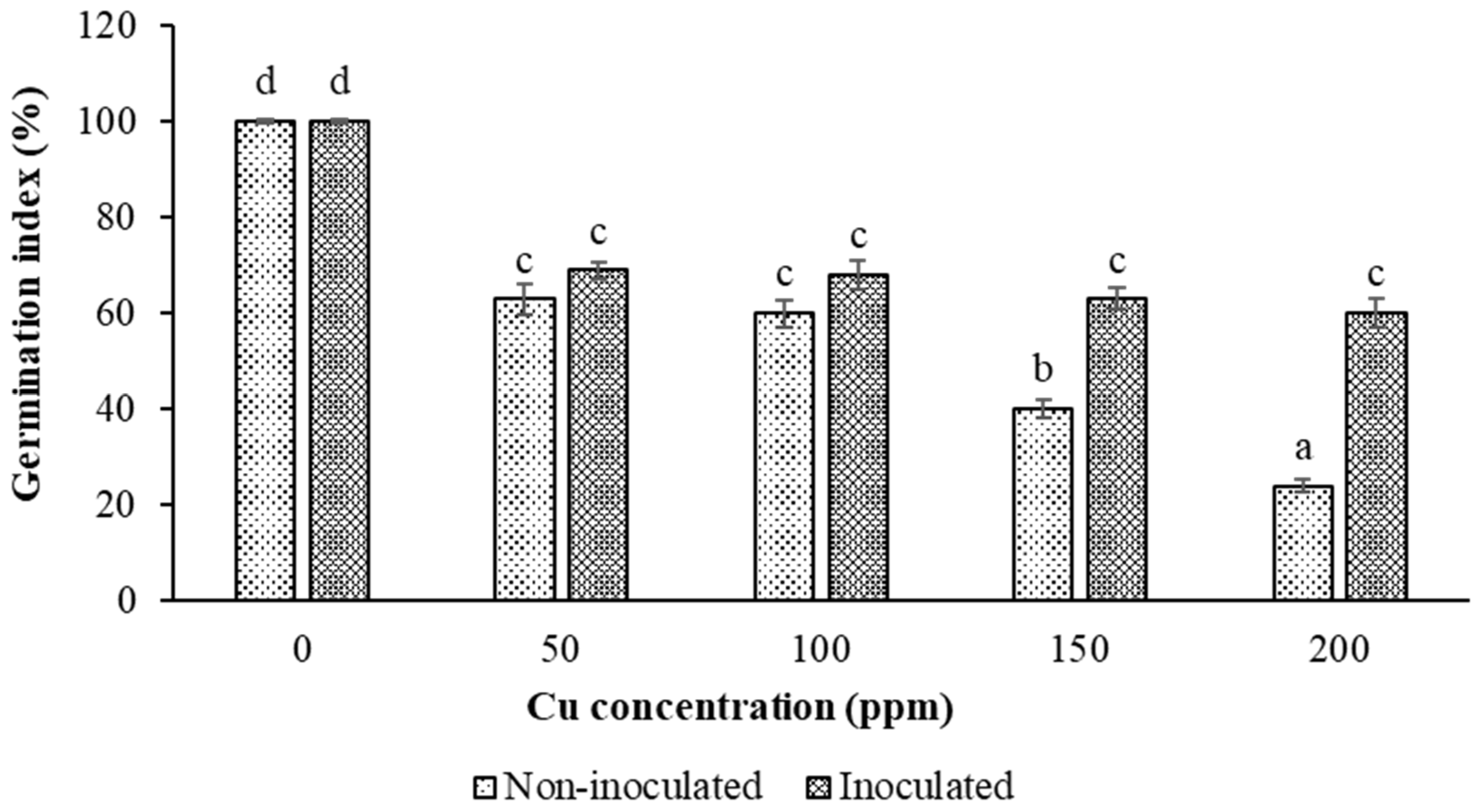
3.2. Impacts of Inoculation and Cu Levels on Seed Germination
3.2.1. Relative Germination Percentage
3.2.2. Relative Root Elongation
3.2.3. Germination Index
3.3. Inoculation and Excess Substrate Cu Impacts on Plants and Fruits
3.3.1. Plant Variables
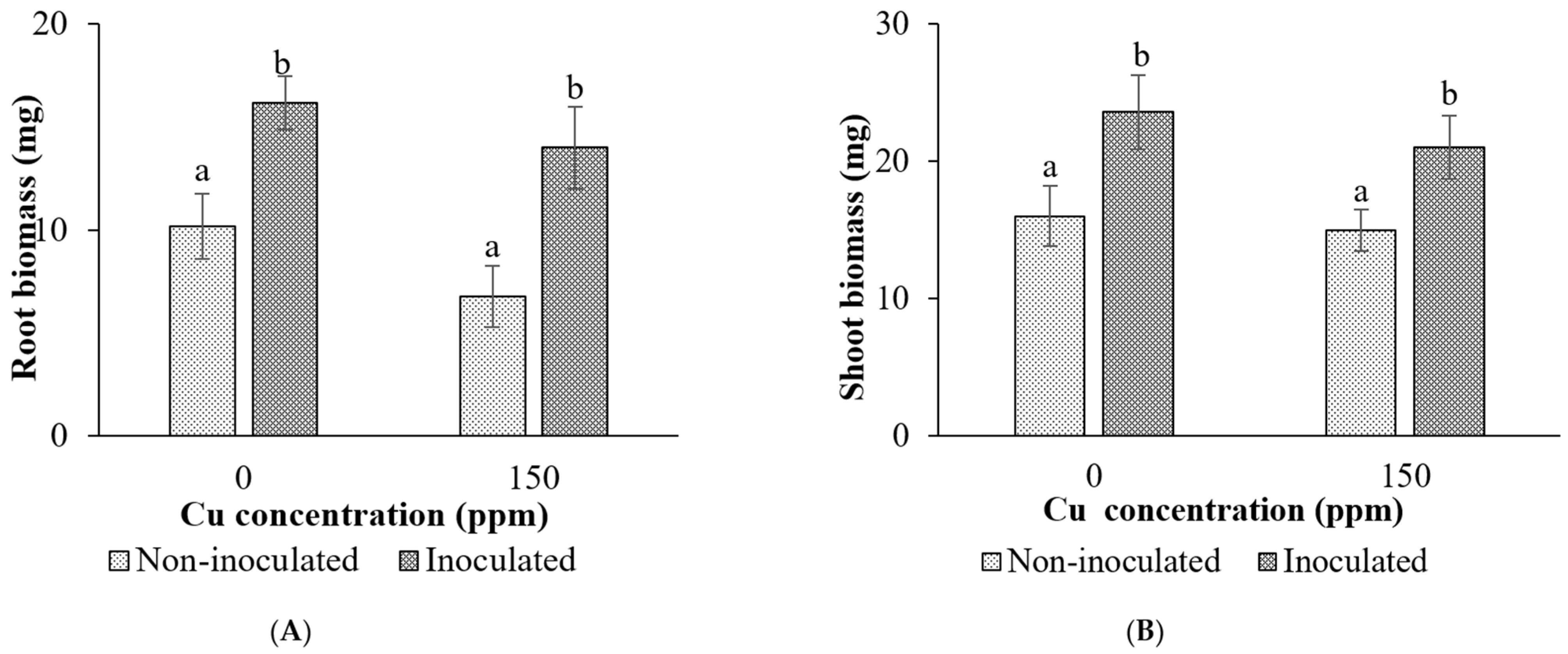
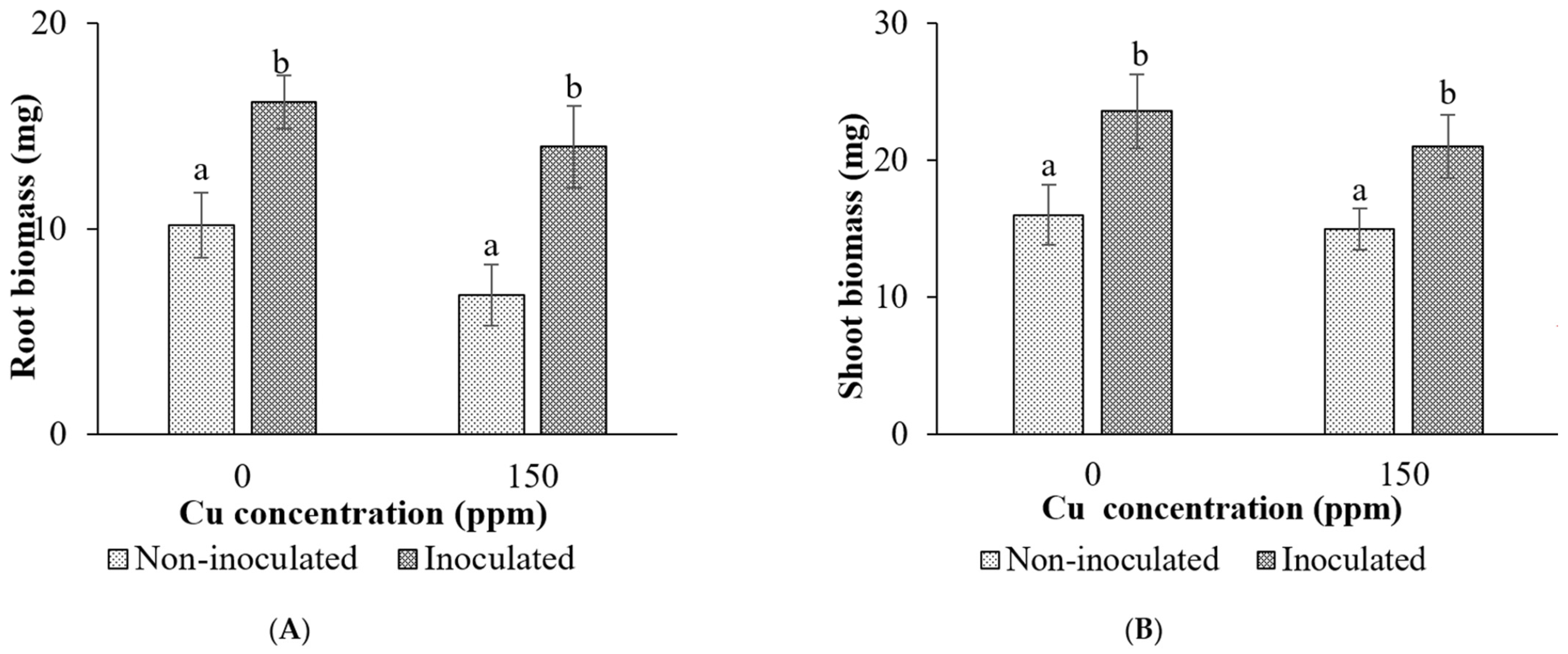
Root and Shoot Biomass
Height and Leaf Area
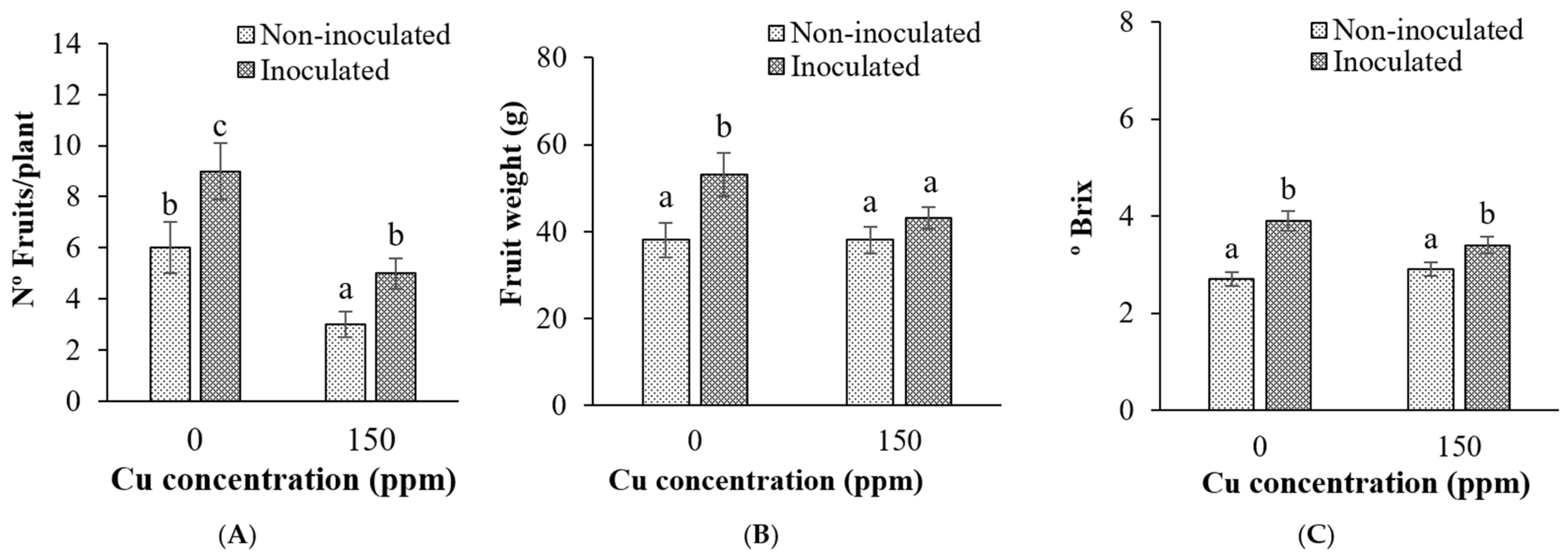
3.3.2. Fruit Yield and Quality
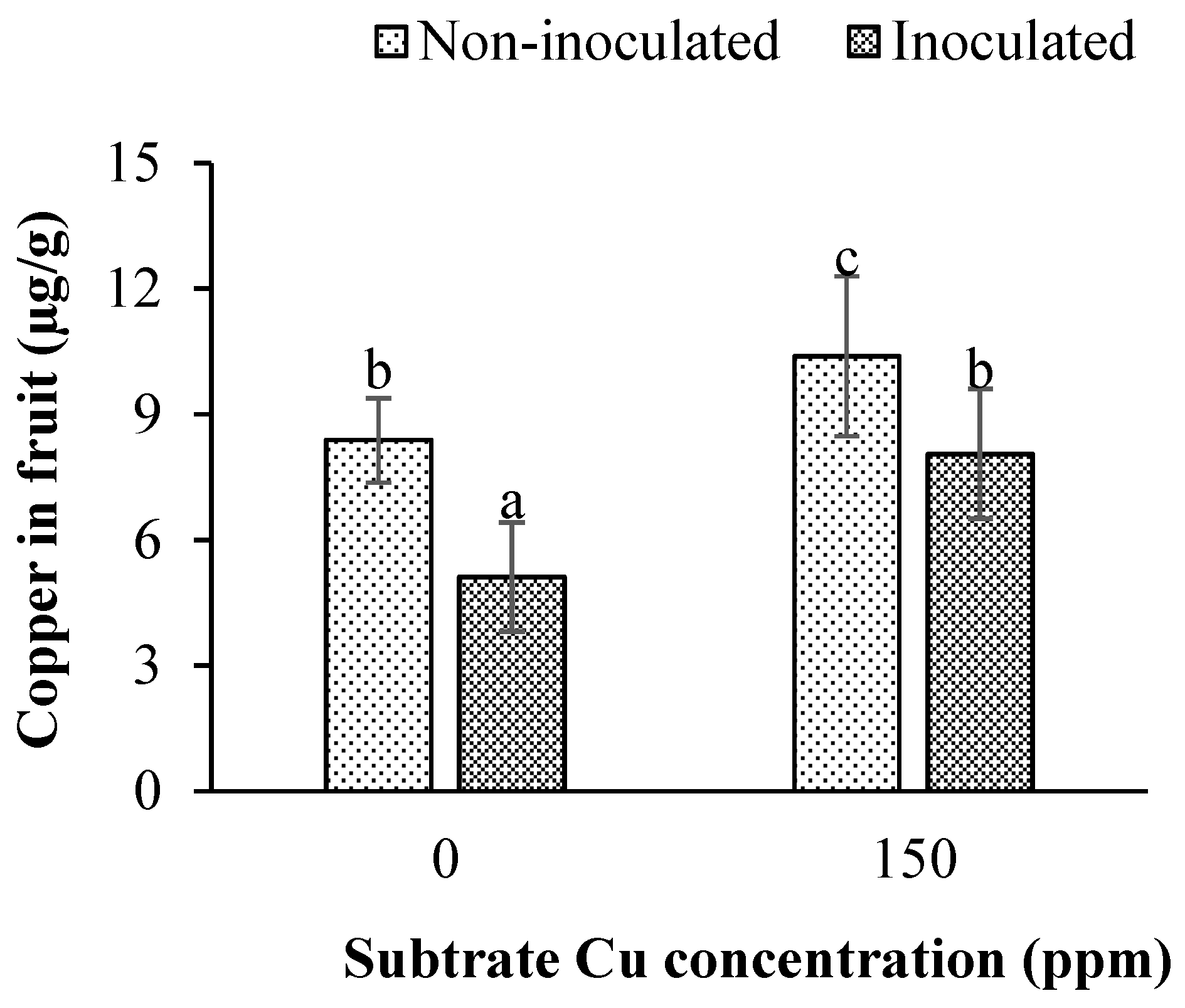
3.4. Impact of Inoculation and Cu Substrate Excess on Cu Fruit Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, D.A.E.A.; Slima, D.F.; Al-Yasi, H.M.; Hassan, L.M.; Galal, T.M. Risk assessment of trace metals in Solanum lycopersicum L. (tomato) grown under wastewater irrigation conditions. Environ. Sci. Pollut. Res. 2023, 30, 42255–42266. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.G.; Scott, A.J. Multifunctional Green Belts: A planning policy assessment of Green Belts wider functions in England. Land Use Policy 2023, 132, 106799. [Google Scholar] [CrossRef]
- Zhou, L.; Gong, Y.; López-Carr, D.; Huang, C. A critical role of the capital green belt in constraining urban sprawl and its fragmentation measurement. Land Use Policy 2024, 141, 107148. [Google Scholar] [CrossRef]
- Paladino, I.; Clara, S.A.; Barbara, P.M.C.; Wolski, J.E.; Navas, R.H.M. Soil Quality Problems Associated with Horticulture in the Southern Urban and Peri-Urban Area of Buenos Aires, Argentina. In Urban Horticulture-Necessity of the Future; IntechOpen: London, UK, 2020; p. 180. [Google Scholar] [CrossRef]
- Giuffré, L.; Ratto, S.; Marbán, L.; Schonwald, J.; Romaniuk, R. Heavy metals risk in urban agriculture. Cienc. Suelo 2005, 23, 101–106. [Google Scholar]
- Rieumont, S.O.; Céspedes, D.G.; Cazorla, L.L.; Sánchez, I.S.; Casals, A.L.; Pérez, P.Á. Niveles de Cadmio, Plomo, Cobre y Zinc en Hortalizas cultivadas en una zona altamente urbanizada de la ciudad de la Habana, Cuba. Rev. Int. Contam. Ambie. 2013, 29, 285–294. [Google Scholar]
- von Hoffen, L.P.; Säumel, I. Orchards for edible cities: Cadmium and lead content in nuts, berries, pome and stone fruits harvested within the inner city neighbourhoods in Berlin, Germany. Ecotoxicol. Environ. Saf. 2014, 101, 233–239. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. (Eds.) Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; Available online: http://www.crcpress.com (accessed on 8 May 2019).
- Gbadegesin, A.; Olabode, M.A. Soil properties in the metropolitan region of Ibadan, Nigeria: Implications for the management of the urban environment of developing countries. Environmentalist 2000, 20, 205–214. [Google Scholar] [CrossRef]
- Afzal, H.; Ali, M.; Sajjad, A.; Nawaz, F.; Saeed, S. Heavy metal concentrations of copper and nickel in peri urban vegetable agro-ecosystem of Multan. Pak. J. Anim. Plant Sci. 2023, 33, 296–302. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.J.; He, L.Y.; Sheng, X.F. Increased biomass and quality and reduced heavy metal accumulation of edible tissues of vegetables in the presence of Cd-tolerant and immobilizing Bacillus megaterium H3. Ecotoxicol. Environ. Saf. 2018, 148, 269–274. [Google Scholar] [CrossRef]
- Stamenković, S.; Beškoski, V.; Karabegović, I.; Lazić, M.; Nikolić, N. Microbial fertilizers: A comprehensive review of current findings and future perspectives. Span. J. Agric. Res. 2018, 16, e09R01. [Google Scholar] [CrossRef]
- Azam, M.S.; Shafiquzzaman, M.; Haider, H. Arsenic release dynamics of paddy field soil during groundwater irrigation and natural flooding. J. Environ. Manag. 2023, 343, 118204. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Bashir, S.; Saleem, M.H.; Chen, C.; Peng, D.; Siddique, K.H. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol. Biochem. 2019, 138, 121–129. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Fincheira, P.; Parada, J.; de Oliveira, H.C.; Benavides-Mendoza, A.; Leiva, S.; Fernandez-Baldo, M.; Seabra, A.B. Copper nanoparticles as a potential emerging pollutant: Divergent effects in the agriculture, risk-benefit balance and integrated strategies for its use. Emerg. Contam. 2024, 10, 100352. [Google Scholar] [CrossRef]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef]
- Morales, L.; Jiménez, G.S. El daño por oxidación causado por cobre y la respuesta antioxidante de las plantas. Interciencia 2012, 37, 805–811. [Google Scholar]
- Li, L.; Hou, M.; Cao, L.; Xia, Y.; Shen, Z.; Hu, Z. Glutathione S-transferases modulate Cu tolerance in Oryza sativa. Environ. Exp. Bot. 2018, 155, 313–320. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, X.; Lin, Y.; Yang, C.; Tang, W.; Wu, S.; Li, D.; Lou, W. Effects of copper ions on removal of nutrients from swine wastewater and on release of dissolved organic matter in duckweed systems. Water Res. 2019, 158, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Steinman, A.D.; Wan, X.; Xie, L. Combined toxicity of microcystin-LR and copper on lettuce (Lactuca sativa L.). Chemosphere 2018, 206, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Villegas, M.; Tejeda, C.; Umaña, R.; Iranzo, E.C.; Salgado, M. Are Reactive Oxygen Species (ROS) the Main Mechanism by Which Copper Ion Treatment Degrades the DNA of Mycobacterium avium subsp. paratuberculosis Suspended in Milk. Microorganisms 2022, 10, 10112272. [Google Scholar] [CrossRef]
- Wairich, A.; De Conti, L.; Lamb, T.I.; Keil, R.; Neves, L.O.; Brunetto, G.; Sperotto, R.A.; Ricachenevsky, F.K. Throwing Copper Around: How Plants Control Uptake, Distribution, and Accumulation of Copper. Agronomy 2022, 12, 994. [Google Scholar] [CrossRef]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. BioMetals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Han, H.; Sheng, X.; Hu, J.; He, L.; Wang, Q. Metal-immobilizing Serratia liquefaciens CL-1 and Bacillus thuringiensis X30 increase biomass and reduce heavy metal accumulation of radish under field conditions. Ecotoxicol. Environ. Saf. 2018, 161, 526–533. [Google Scholar] [CrossRef]
- Mallick, I.; Bhattacharyya, C.; Mukherji, S.; Dey, D.; Sarkar, S.C.; Mukhopadhyay, U.K.; Ghosh, A. Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci. Total Environ. 2018, 610–611, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Fang, L.; Cai, P.; Huang, Q.; Chen, H.; Liang, W.; Rong, X. Influence of extracellular polymeric substances (EPS) on Cd adsorption by bacteria. Environ. Pollut. 2011, 159, 1369–1374. [Google Scholar] [CrossRef]
- Kalpana, R.; Angelaalincy, M.J.; Kamatchirajan, B.V.; Vasantha, V.S.; Ashokkumar, B.; Ganesh, V.; Varalakshmi, P. Exopolysaccharide from Bacillus cereus VK1: Enhancement, characterization and its potential application in heavy metal removal. Colloids Surf. B. Biointerfaces 2018, 171, 327–334. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ma, Y.; Freitas, H. Improvement of Ni phytostabilization by inoculation of Ni resistant Bacillus megaterium SR28C. J. Environ. Manag. 2013, 128, 973–980. [Google Scholar] [CrossRef]
- Labra-Cardón, D.; Guerrero-Zúñiga, A.; Rodríguez-Tovar, A.V.; Montes-Villafán, S.; Pérez-jiménez, S.; Rodríguez-Dorantes, A. Respuesta de crecimiento y tolerancia a metales pesados de Cyperus elegans y Echinochloa polystachya inoculadas con una rizobacteria aislada de un suelo contaminado con hidrocarburos derivados del petróleo. Rev. Int. Contam. Ambient. 2012, 28, 7–16. [Google Scholar]
- Ali, H.H.; Bibi, S.; Zaheer, M.S.; Iqbal, R.; Khan, W.U.D.; Mustafa, A.E.Z.M.A.; Elshikh, M.S. Control of copper-induced physiological damage in okra (Abelmoschus esculentus L.) via Bacillus subtilis and farmyard manure: A step towards sustainable agriculture. Plant Stress 2024, 11, 100309. [Google Scholar] [CrossRef]
- Kumar, G.; Lal, S.; Maurya, S.K.; Kumar, D.; Shukla, S.K. Exploration of PGPR microorganisms and their application to enhance the crop production. Krishi Sci. 2021, 2, 5–7. [Google Scholar]
- Sarti, G.C.; Galelli, M.E.; Arreghini, S.; Cristóbal-Miguez, J.A.E.; Curá, J.A.; Paz-González, A. Inoculation with Biofilm of Bacillus subtilis Promotes the Growth of Lactuca sativa. Sustainability 2023, 15, 15406. [Google Scholar] [CrossRef]
- Sarti, G.C.; Galelli, M.E.; Cristóbal-Miguez, J.A.E.; Cárdenas-Aguiar, E.; Chudil, H.D.; García, A.R.; Paz-González, A. Inoculation with Biofilm of Bacillus subtilis Is a Safe and Sustainable Alternative to Promote Tomato (Solanum lycopersicum) Growth. Environments 2024, 11, 54. [Google Scholar] [CrossRef]
- Hobley, L.; Harkins, C.; MacPhee, C.E.; Stanley-Wall, N.R. Giving structure to the biofilm matrix: An overview of individual strategies and emerging common themes. FEMS Microbiol. Rev. 2015, 39, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Penha, R.O.; Vandenberghe, L.P.S.; Faulds, C.; Soccol, V.T.; Soccol, C.R. Bacillus lipopeptides as powerful pest control agents for a more sustainable and healthy agriculture: Recent studies and innovations. Planta 2020, 251, 70. [Google Scholar] [CrossRef]
- Marbán, L.; De López Camelo, L.G.; Ratto, S.; Agostini, A. Contaminación con metales pesados en un suelo de la cuenca del río Reconquista. Ecol. Austral 1999, 9, 15–19. [Google Scholar]
- De Cabo, L.I.; Faggi, A.; Miguel, S.; Basílico, G. Rehabilitación de las riberas de un sitio de la cuenca baja del río Matanza-Riachuelo. Biol. Acuát. 2019, 33, 005. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2022; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Ministerio de Economía Argentina. Producción de Tomate en Argentina. Evolución del Cultivo Hasta la Temporada 2021/22; Ministerio de Economía Argentina: Buenos Aires, Argentina, 2023.
- Rosa-Martínez, E.; García-Martínez, M.D.; Adalid-Martínez, A.M.; Pereira-Dias, L.; Casanova, C.; Soler, E.; Figàs, M.R.; Raigón, M.D.; Plazas, M.; Soler, S.; et al. Fruit composition profile of pepper, tomato and eggplant varieties grown under uniform conditions. Food Res. Int. 2021, 147, 110531. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-haq, I.; Ur-rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Martí, L.A. Efecto de la Salinidad y de la Temperatura en la Germinación de Semillas de Limonium mansanetianum. Bachelor´s Thesis, Universidad Politécnica de Valencia, Valencia, Spain, 2010. Available online: https://riunet.upv.es/server/api/core/bitstreams/f61596d8-cbee-40bf-aa29-cfcd46ed1cf0/content (accessed on 16 May 2025).
- Emino, E.R.; Warman, P.R. Biological assay for compost quality. Compost. Sci. Util. 2004, 12, 342–348. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeny, D.R. (Eds.) Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties Second Edition; The American Society of Agronomy and the Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- US EPA. Method 3051a Microwave Assisted acid Digestion of Sediments, Sluges, Solis and Oils; US EPA: Washington, DC, USA, 2007.
- Santiago, J.; Mendoza, M.; Borrego, F. Evaluación de tomate (Lycopersicum esculentum, MILL) en invernadero: Criterios fenológicos y fisiológicos. Agron. Mesoam. 1998, 9, 59–65. [Google Scholar] [CrossRef]
- Galelli, M.E.; Sarti, G.C.; Miyazaki, S.S. Lactuca sativa biofertilization using biofilm from Bacillus with PGPR activity. J. App. Hortic. 2015, 17, 186–191. [Google Scholar] [CrossRef]
- Sarti, G.C.; Miyazaki, S.S. Actividad antifúngica de extractos crudos de Bacillus subtilis contra fitopatógenos de soja (Glycine max) y efecto de su coinoculación con Bradyrhizobium japonicum. Agrociencia 2013, 47, 373–383. [Google Scholar]
- Wang, R.-X.; Wang, Z.-H.; Sun, Y.-D.; Wang, L.-L.; Li, M.; Liu, Y.-T.; Zhang, H.-M.; Jing, P.-W.; Shi, Q.-F.; Yu, Y.-H. Molecular mechanism of plant response to copper stress: A review. Environ. Exp. Bot. 2024, 218, 105590. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Shan, X.; Zhu, Y.G. Toxicity of arsenate and arsenite on germination, seedling growth and amylolytic activity of wheat. Chemosphere 2005, 61, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Baruah, N.; Mondal, S.C.; Farooq, M.; Gogoi, N. Influence of Heavy Metals on Seed Germination and Seedling Growth of Wheat, Pea, and Tomato. Water Air Soil Pollut. 2019, 230, 273. [Google Scholar] [CrossRef]
- El-Tayeb, M.A.; El-Enany, A.E.; Ahmed, N.L. Salicylic acid-induced adaptive response to copper stress in sunflower (Helianthus annuus L.). Plant Growth Regul. 2006, 50, 191–199. [Google Scholar] [CrossRef]
- Pandey, S.; Ghosh, P.K.; Ghosh, S.; De, T.K.; Maiti, T.K. Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 2013, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Poveda, J.; González-Andrés, F. Bacillus as a source of phytohormones for use in agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 8629–8645. [Google Scholar] [CrossRef]
- Seneviratne, G.; Weerasekara, M.L.M.A.W.; Seneviratne, K.A.C.N.; Zavahir, J.S.; Kecskés, M.L.; Kennedy, I.R. Importance of Biofilm Formation in Plant Growth Promoting Rhizobacterial Action. In Plant Growth and Health Promoting Bacteria; Maheshwari, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 81–95. [Google Scholar] [CrossRef]
- Ferreira, C.M.H.; Vilas-Boas, Â.; Sousa, C.A.; Soares, H.M.V.M.; Soares, E.V. Comparison of five bacterial strains producing siderophores with ability to chelate iron under alkaline conditions. AMB Express 2019, 9, 78. [Google Scholar] [CrossRef]
- Khan, I.; Azam, A.; Mahmood, A. The impact of enhanced atmospheric carbon dioxide on yield, proximate composition, elemental concentration, fatty acid and vitamin C contents of tomato (Lycopersicon esculentum). Environ. Monit. Assess. 2013, 185, 205–214. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Kapoulas, N.; Šunić, L.; Beković, D.; Mirecki, N. Heavy metals and nitrate content in tomato fruit grown in organic and conventional production systems. Pol. J. Environ. Stud. 2014, 23, 2027–2032. [Google Scholar] [CrossRef]
- Nektarios, P.A.; Ischyropoulos, D.; Kalozoumis, P.; Savvas, D.; Yfantopoulos, D.; Ntoulas, N.; Tsaniklidis, G.; Goumenaki, E. Impact of substrate depth and fertilizer type on growth, production, quality characteristics and heavy metal contamination of tomato and lettuce grown on urban green roofs. Sci. Hortic. 2022, 305, 111318. [Google Scholar] [CrossRef]
- Pimenta, T.M.; Souza, G.A.; Brito, F.A.L.; Teixeira, L.S.; Arruda, R.S.; Henschel, J.M.; Zsögön, A.; Ribeiro, D.M. The impact of elevated CO2 concentration on fruit size, quality, and mineral nutrient composition in tomato varies with temperature regimen during growing season. Plant Growth Regul. 2023, 100, 519–530. [Google Scholar] [CrossRef]
- Trebolazabala, J.; Maguregui, M.; Morillas, H.; García-Fernandez, Z.; de Diego, A.; Madariaga, J.M. Uptake of metals by tomato plants (Solanum lycopersicum) and distribution inside the plant: Field experiments in Biscay (Basque Country). J. Food Compos. Anal. 2017, 59, 161–169. [Google Scholar] [CrossRef]
- Thérien, M.; Kiesewalter, H.T.; Auria, E.; Charron-Lamoureux, V.; Wibowo, M.; Maróti, G.; Kovács, Á.T.; Beauregard, P.B. Surfactin production is not essential for pellicle and root-associated biofilm development of Bacillus subtilis. Biofilm 2020, 2, 100021. [Google Scholar] [CrossRef] [PubMed]
- Stoll, A.; Salvatierra-Martínez, R.; González, M.; Araya, M. The role of surfactin production by bacillus velezensis on colonization, biofilm formation on tomato root and leaf surfaces and subsequent protection (ISR) against Botrytis cinerea. Microorganisms 2021, 9, 2251. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Chao, Y.; Chen, Y.; Wang, S.; Qiu, R. The effect of interaction between Bacillus subtilis DBM and soil minerals on Cu(II) and Pb(II) adsorption. J. Environ. Sci. 2019, 78, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Pallavi; Barh, A.; Sharma, I.P. Plant Growth Promoting Bacteria: A Gateway to Sustainable Agriculture. In Microbial Biotechnology in Environmental Monitoring and Cleanup; IGI Global: Hershey, PA, USA, 2018; pp. 318–338. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Mathivanan, K.; Chandirika, J.U.; Vinothkanna, A.; Yin, H.; Liu, X.; Meng, D. Bacterial adaptive strategies to cope with metal toxicity in the contaminated environment—A review. Ecotoxicol. Environ. Saf. 2021, 226, 112863. [Google Scholar] [CrossRef]
- Khan, M.; Kamran, M.; Kadi, R.H.; Hassan, M.M.; Elhakem, A.; ALHaithloul, H.A.S.; Soliman, M.H.; Mumtaz, M.Z.; Ashraf, M.; Shamim, S. Harnessing the Potential of Bacillus altitudinis MT422188 for Copper Bioremediation. Front. Microbiol. 2022, 13, 878000. [Google Scholar] [CrossRef]
- Lu, H.; Xia, C.; Chinnathambi, A.; Nasif, O.; Narayanan, M.; Shanmugam, S.; Chi, N.T.L.; Pugazhendhi, A.; On-uma, R.; Jutamas, K.; et al. Optimistic influence of multi-metal tolerant Bacillus species on phytoremediation potential of Chrysopogon zizanioides on metal contaminated soil. Chemosphere 2023, 311, 136889. [Google Scholar] [CrossRef]
- STAN 193-1995; General Standard for Contaminants and Toxins in Food and Feed (Codex Stan 193-1995). FAO/WHO: Geneva, Switzerland, 1995. Available online: www.fao.org/input/download/standards/17/CXS_193e_2015.pdf (accessed on 16 May 2025).
- Murtic, S.; Zahirovic, C.; Civic, H.; Karic, L.; Jurokkovic, J. Uptake of Heavy Metals by Tomato Plants (Lycopersicum esculentum Mill.) and their distribution inside the Plant. Agric. For. 2018, 64, 251–261. [Google Scholar] [CrossRef]
| Germination Index (GI%) | Toxicity Level |
|---|---|
| <50% | High |
| 50–80% | Moderate |
| >80% | No toxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarti, G.C.; Paz-González, A.; Cristóbal-Míguez, J.A.E.; Arnedillo, G.; García, A.R.; Galelli, M.E. Tomato Seed Inoculation with Bacillus subtilis Biofilm Mitigates Toxic Effects of Excessive Copper in the Substrate. Processes 2025, 13, 2509. https://doi.org/10.3390/pr13082509
Sarti GC, Paz-González A, Cristóbal-Míguez JAE, Arnedillo G, García AR, Galelli ME. Tomato Seed Inoculation with Bacillus subtilis Biofilm Mitigates Toxic Effects of Excessive Copper in the Substrate. Processes. 2025; 13(8):2509. https://doi.org/10.3390/pr13082509
Chicago/Turabian StyleSarti, Gabriela Cristina, Antonio Paz-González, Josefina Ana Eva Cristóbal-Míguez, Gonzalo Arnedillo, Ana Rosa García, and Mirta Esther Galelli. 2025. "Tomato Seed Inoculation with Bacillus subtilis Biofilm Mitigates Toxic Effects of Excessive Copper in the Substrate" Processes 13, no. 8: 2509. https://doi.org/10.3390/pr13082509
APA StyleSarti, G. C., Paz-González, A., Cristóbal-Míguez, J. A. E., Arnedillo, G., García, A. R., & Galelli, M. E. (2025). Tomato Seed Inoculation with Bacillus subtilis Biofilm Mitigates Toxic Effects of Excessive Copper in the Substrate. Processes, 13(8), 2509. https://doi.org/10.3390/pr13082509







