Ethyl Cellulose Co-Encapsulation of Steel Slag–Persulfate Long-Term Petroleum Hydrocarbon Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of (SS + SPS)/EC Composite Granules
2.3. Degradation Performance Assessment
2.4. Morphological and Elemental Characterization
2.5. Surface Area and Porosity Analysis
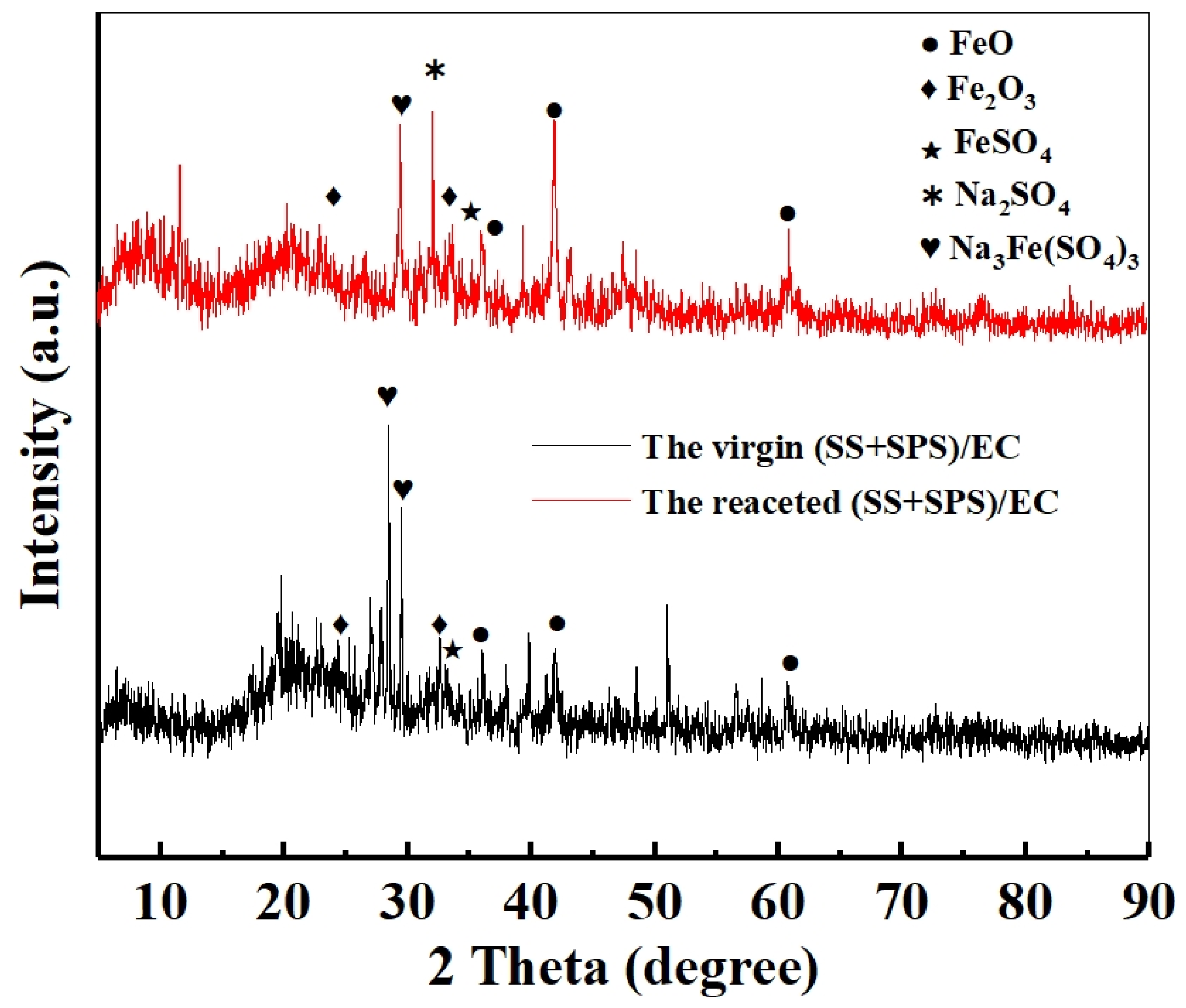
2.6. Crystalline Phase Evolution Analysis
2.7. Surface Chemical State Analysis
2.8. Batch Degradation Experiments
- (1)
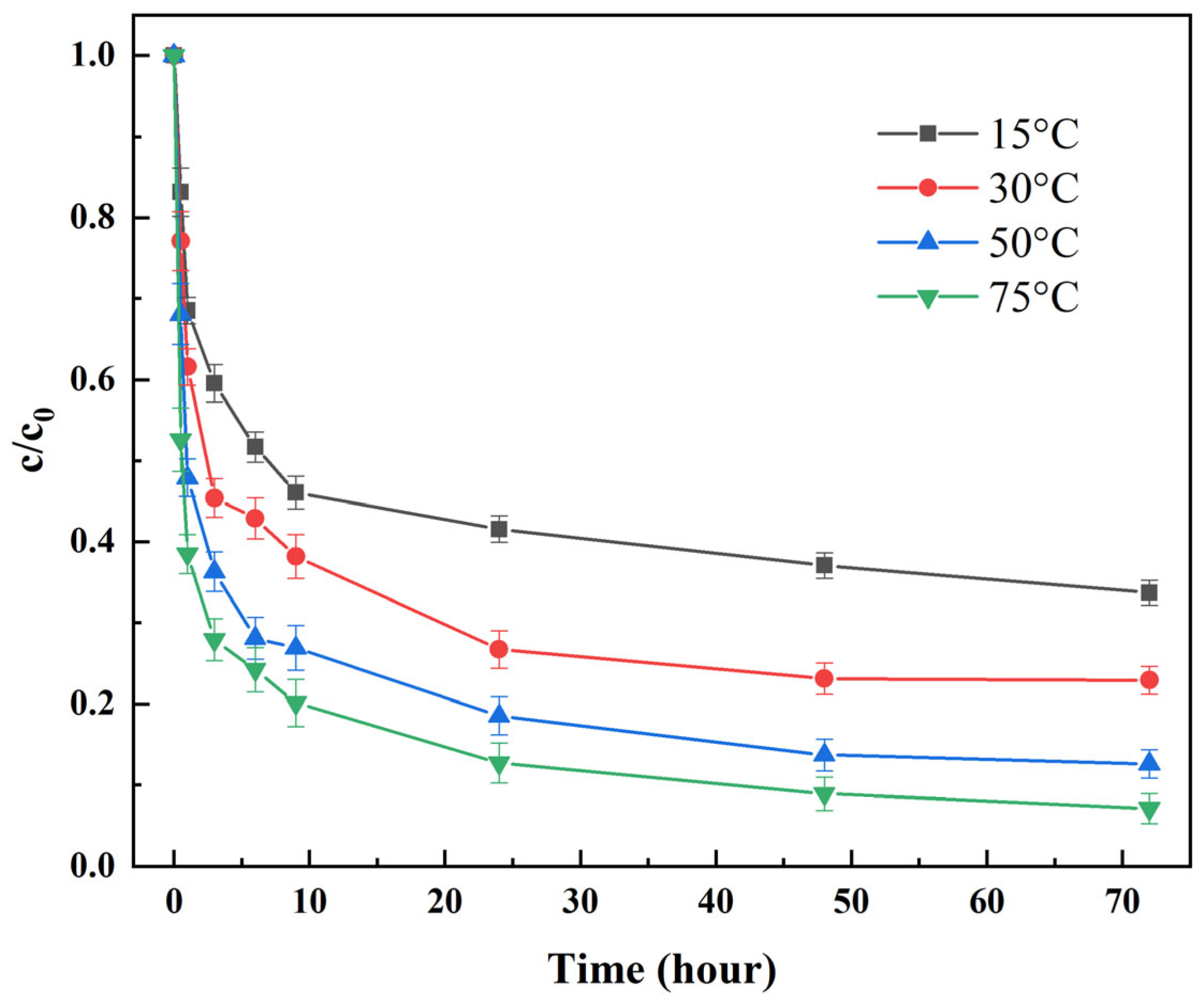
- Temperature effect: 10 g of composite granules were tested across temperatures (15, 30, 50, 75 °C) maintained by a water bath (±0.5 °C control).
- (2)
- Dosage effect: At 15 °C, composite dosage levels corresponding to 1%, 2%, 4%, 6%, and 8% (w/v, relative to solution mass) were evaluated.
- (3)
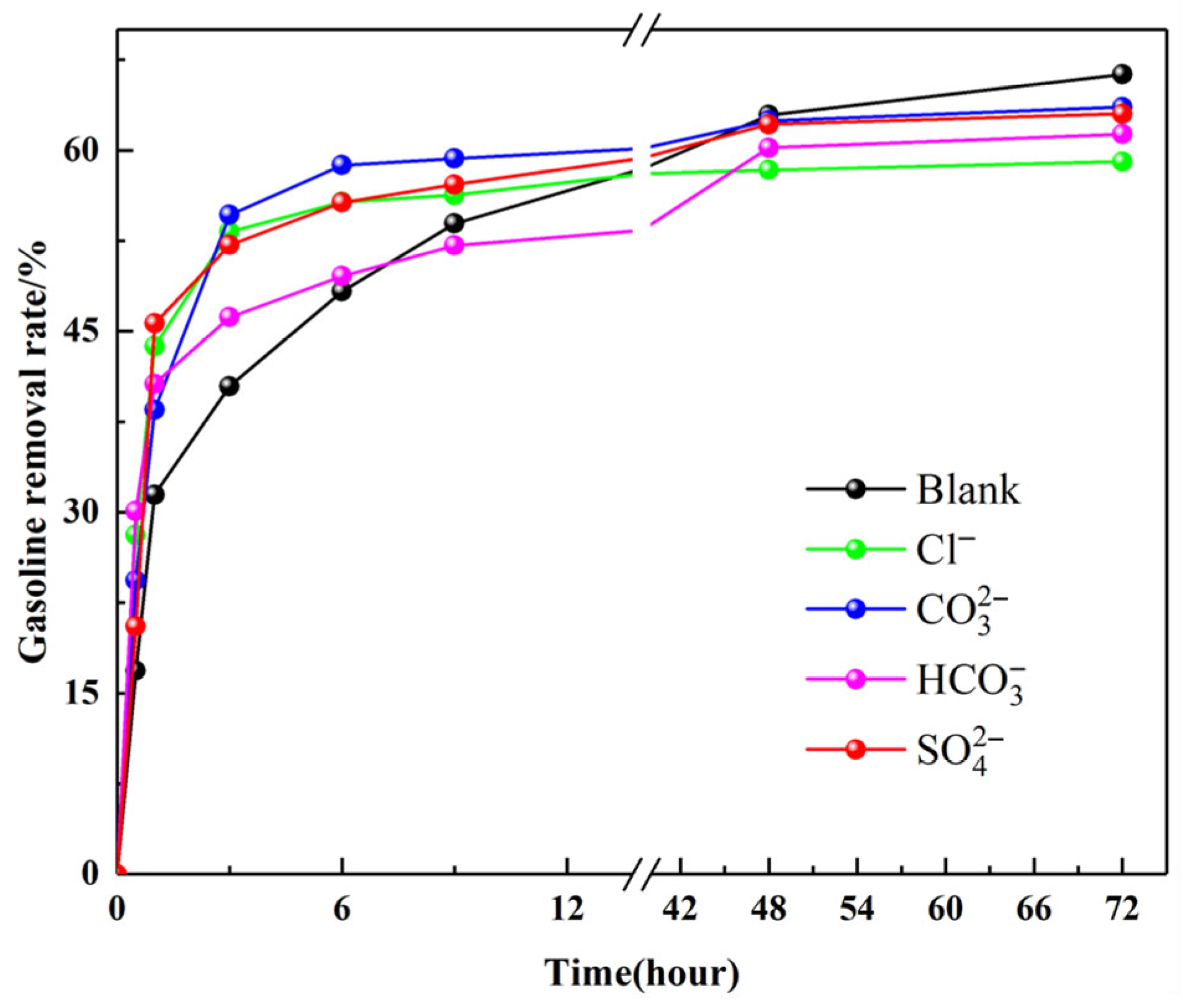
- Anion interference: Solutions containing 50 mg·L−1 of common groundwater anions (Cl−, , , ) were tested with 10 g composites at 15 °C, with ionic species introduced as sodium salts prior to PHs spiking.
- (4)
- Contaminant loading effect: PHs concentrations (1, 5, 20 mg·L−1) tested with 16 g composites in 400 mL solution for stoichiometric assessment.
2.9. Continuous-Flow Column Experiment
3. Results and Discussion
3.1. Optimization of SS:SPS Mass Ratio in (SS + SPS)/EC Composites
3.2. pH Evolution Dynamics and Buffering Mechanisms
3.3. Morphological Evolution and Sodium Distribution Dynamics
3.4. Pore Structure Evolution and Reactivity Implications
3.5. Batch Experiments of Simulated Gasoline Pollution Solution Degradation by (SS + SPS)/EC
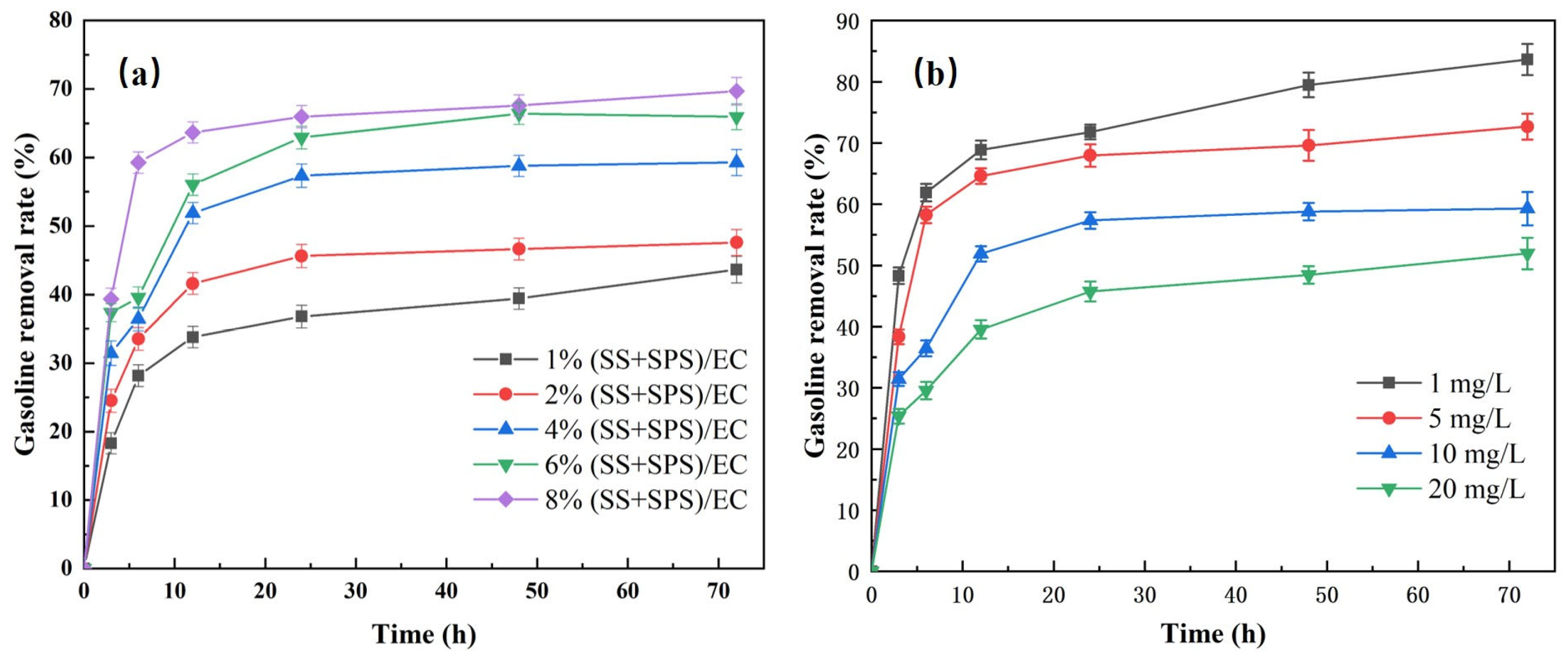
3.5.1. The Effect of the (SS + SPS)/EC Dosage
3.5.2. The Influence of Reaction Temperature
3.5.3. The Influence of Coexisting Anions
3.6. Analysis of the Critical Iron Redox Behavior of the (SS + SPS)/EC
3.7. Sustained Remediation in Continuous-Flow Column of the (SS + SPS)/EC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PH | Petroleum Hydrocarbon |
| SS | Steel Slag |
| SPS | Sodium Persulfate |
| EC | Ethyl Cellulose |
References
- Yuan, L.; Wang, K.; Zhao, Q.; Yang, L.; Wang, G.; Jiang, M.; Li, L. An overview of in situ remediation for groundwater co-contaminated with heavy metals and petroleum hydrocarbons. J. Environ. Manag. 2024, 349, 119342. [Google Scholar] [CrossRef]
- Sanchez-Huerta, C.; Zhang, S.; Alahmari, M.; Humam, A.A.; Hong, P.-Y. Remediation of petroleum hydrocarbons in contaminated groundwater with the use of surfactants and biosurfactants. Chemosphere 2025, 376, 144290. [Google Scholar] [CrossRef]
- Ahmadnezhad, Z.; Vaezihir, A.; Schueth, C.; Zarrini, G. Combination of zeolite barrier and bio sparging techniques to enhance efficiency of organic hydrocarbon remediation in a model of shallow groundwater. Chemosphere 2021, 273, 128555. [Google Scholar] [CrossRef]
- Saini, A.; Bekele, D.N.; Chadalavada, S.; Fang, C.; Naidu, R. A review of electrokinetically enhanced bioremediation technologies for PHs. J. Environ. Sci. 2020, 88, 31–45. [Google Scholar] [CrossRef]
- Lee, T.H.; Tsang, D.C.W.; Chen, W.H.; Verpoort, F.; Sheu, Y.T.; Kao, C.M. Application of an emulsified polycolloid substrate biobarrier to remediate petroleum-hydrocarbon contaminated groundwater. Chemosphere 2019, 219, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Daghio, M.; Franzetti, A.; Papini, M.P.; Aulenta, F. The bioelectric well: A novel approach for insitu treatment of hydrocarbon-contaminated groundwater. Microb. Biotechnol. 2018, 11, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.-H.; Ma, J.; Xi, B.-D.; Yu, M.-D.; Cui, J.; Chen, B.-L.; Li, Y.; Gu, Q.-B.; He, X.-S. Recent progress on in-situ chemical oxidation for the remediation of petroleum contaminated soil and groundwater. J. Hazard. Mater. 2022, 432, 128738. [Google Scholar] [CrossRef]
- Poi, G.; Shahsavari, E.; Aburto-Medina, A.; Mok, P.C.; Ball, A.S. Large scale treatment of total petroleum-hydrocarbon contaminated groundwater using bioaugmentation. J. Environ. Manag. 2018, 214, 157–163. [Google Scholar] [CrossRef]
- Liu, J.-W.; Wei, K.-H.; Xu, S.-W.; Cui, J.; Ma, J.; Xiao, X.-L.; Xi, B.-D.; He, X.-S. Surfactant-enhanced remediation of oil-contaminated soil and groundwater: A review. Sci. Total Environ. 2021, 756, 144142. [Google Scholar] [CrossRef]
- Zhang, S.; Su, X.; Lin, X.; Zhang, Y.; Zhang, Y. Experimental study on the multi-media PRB reactor for the remediation of petroleum-contaminated groundwater. Environ. Earth Sci. 2015, 73, 5611–5618. [Google Scholar] [CrossRef]
- Zhao, Q.; Liao, C.; Jiang, E.; Yan, X.; Su, H.; Tian, L.; Li, N.; Lobo, F.L.; Wang, X. Dual-purpose elemental sulfur for capturing and accelerating biodegradation of petroleum hydrocarbons in anaerobic environment. Water Res. X 2025, 26, 100290. [Google Scholar] [CrossRef]
- Freidman, B.L.; Terry, D.; Wilkins, D.; Spedding, T.; Gras, S.L.; Snape, I.; Stevens, G.W.; Mumford, K.A. Permeable bio-reactive barriers to address petroleum hydrocarbon contamination at subantarctic Macquarie Island. Chemosphere 2017, 174, 408–420. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Wu, W.; Yan, M.; Xie, Y. Research and application of arsenic-contaminated groundwater remediation by manganese ore permeable reactive barrier. Environ. Technol. 2021, 42, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Ren, Y.; Zhou, Y.; Sun, S.; Cui, M.; Khim, J. Machine learning vs. statistical model for prediction modeling and experimental validation: Application in groundwater permeable reactive barrier width design. J. Hazard. Mater. 2024, 469, 133825. [Google Scholar] [CrossRef] [PubMed]
- Yee, J.-J.; Justo Arida, C.V.; Futalan, C.M.; Daniel Garrido de Luna, M.; Wan, M.-W. Treatment of Contaminated Groundwater via Arsenate Removal Using Chitosan-Coated Bentonite. Molecules 2019, 24, 2464. [Google Scholar] [CrossRef]
- Gibert, O.; Assal, A.; Devlin, H.; Elliot, T.; Kalinc, R.M. Performance of a field-scale biological permeable reactive barrier for in-situ remediation of nitrate-contaminated groundwater. Sci. Total Environ. 2019, 659, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-H.; Chen, K.-F.; Kao, C.-M.; Liang, S.-H.; Chen, T.-Y. Application of persulfate to remediate petroleum hydrocarbon-contaminated soil: Feasibility and comparison with common oxidants. J. Hazard. Mater. 2011, 186, 2097–2102. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Z.; Cai, Y.; Li, X.; Huang, Y.; Hou, J.; Liu, W. Response of petroleum-contaminated soil to chemical oxidation combined with biostimulation. Ecotoxicol. Environ. Saf. 2024, 282, 116694. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Zhong, S.; Zhang, L. AOPs-based remediation of petroleum hydrocarbons-contaminated soils: Efficiency, influencing factors and environmental impacts. Chemosphere 2020, 246, 125726. [Google Scholar] [CrossRef]
- Lei, Y.-J.; Zhang, J.; Tian, Y.; Yao, J.; Duan, Q.-S.; Zuo, W. Enhanced degradation of total petroleum hydrocarbons in real soil by dual-frequency ultrasound-activated persulfate. Sci. Total Environ. 2020, 748, 141414. [Google Scholar] [CrossRef]
- Chen, X.; Mu, S.; Luo, Y. Removal of total petroleum hydrocarbons from oil-based drilling cuttings by a heat activation persulfate-based process. Environ. Technol. 2024, 45, 835–844. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Xi, P.; Qiao, X.; Chen, J.; Cai, X. Interrelated effects of soils and compounds on persulfate oxidation of petroleum hydrocarbons in soils. J. Hazard. Mater. 2021, 408, 124845. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Xiao, X.; He, X.; Ji, C.; Li, L.; Zhou, M.; Yin, X.; Shan, Y.; Wang, M.; Zhao, Y. Activation of persulfate by g-C3N4/nZVI@SBC for degradation of total petroleum hydrocarbon in groundwater. J. Environ. Manag. 2024, 356, 120612. [Google Scholar] [CrossRef] [PubMed]
- Yasin, H.; Al-Taani, B.; Salem, M.S. Preparation and characterization of ethylcellulose microspheres for sustained-release of pregabalin. Res. Pharm. Sci. 2021, 16, 1–15. [Google Scholar] [CrossRef]
- Zhu, X.; Ji, H.; Hua, G.; Zhou, L. Dynamic Release Characteristics and Kinetics of a Persulfate Sustained-Release Material. Toxics 2023, 11, 829. [Google Scholar] [CrossRef]
- Fernando Castilla-Acevedo, S.; Andres Betancourt-Buitrago, L.; Dionysiou, D.D.; Machuca-Martinez, F. Ultraviolet light-mediated activation of persulfate for the degradation of cobalt cyanocomplexes. J. Hazard. Mater. 2020, 392, 122389. [Google Scholar] [CrossRef]
- Lalas, K.; Arvaniti, O.S.; Panagopoulou, E.I.; Thomaidis, N.S.; Mantzavinos, D.; Frontistis, Z. Acesulfame degradation by thermally activated persulfate: Kinetics, transformation products and estimated toxicity. Chemosphere 2024, 352, 141260. [Google Scholar] [CrossRef]
- Liang, C.; Weng, C.Y. Evaluation of alkaline activated sodium persulfate sustained release rod for the removal of dissolved trichloroethylene. J. Hazard. Mater. 2022, 439, 129657. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Gu, X.; Danish, M.; Shan, A.; Ali, M.; Qiu, Z.; Sui, Q.; Lyu, S. Mechanism of surfactant in trichloroethene degradation in aqueous solution by sodium persulfate activated with chelated-Fe(II). J. Hazard. Mater. 2021, 407, 124814. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, J.; Ji, Q.; Cheng, H.; Komarneni, S. Visible-light-driven activation of sodium persulfate for accelerating orange II degradation using ZnMn2O4 photocatalyst. Chemosphere 2021, 278, 130404. [Google Scholar] [CrossRef]
- Ren, Y.; Cui, M.; Zhou, Y.; Lee, Y.; Ma, J.; Han, Z.; Khim, J. Zero-valent iron based materials selection for permeable reactive barrier using machine learning. J. Hazard. Mater. 2023, 453, 131349. [Google Scholar] [CrossRef] [PubMed]
- Ganbat, N.; Hamdi, F.M.; Ibrar, I.; Altaee, A.; Alsaka, L.; Samal, A.K.; Zhou, J.; Hawari, A.H. Iron slag permeable reactive barrier for PFOA removal by the electrokinetic process. J. Hazard. Mater. 2023, 460, 132360. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, X.; Huang, W.; Cheng, X.; Wang, F.; Fang, S.; Cao, J.; Liu, J.; Cheng, S. Novel calcium oxide activated peroxymonosulfate system for methylene blue removal: Identification of key influencing factors, transformation pathway and toxicity assessment. Chemosphere 2024, 349, 140955. [Google Scholar] [CrossRef]
- Zheng, M.-W.; Lin, C.-W.; Chou, P.-H.; Chiang, C.-L.; Lin, Y.-G.; Liu, S.-H. Highly effective degradation of ibuprofen by alkaline metal-doped copper oxides via peroxymonosulfate activation: Mechanisms, degradation pathway and toxicity assessments. J. Hazard. Mater. 2024, 462, 132751. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Cui, X.; Liu, W.; Zou, B.; Liao, W. Cobalt/calcium bimetallic oxides based on bio-waste eggshells for the efficient degradation of norfloxacin by peroxymonosulfate activation. J. Colloid Interface Sci. 2022, 621, 1–11. [Google Scholar] [CrossRef]
- Xu, Q.; Shi, F.; You, H.; Wang, S. Integrated remediation for organic-contaminated site by forcing running-water to modify alkali-heat/persulfate via oxidation process transfer. Chemosphere 2021, 262, 128352. [Google Scholar] [CrossRef]
- Li, Y.-T.; Li, D.; Lai, L.-J.; Li, Y.-H. Remediation of petroleum hydrocarbon contaminated soil by using activated persulfate with ultrasound and ultrasound/Fe. Chemosphere 2020, 238, 124657. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, C.; Ai, D.; Fan, Z. Activation of persulfate by green nano-zero-valent iron-loaded biochar for the removal of p-nitrophenol: Performance, mechanism and variables effects. J. Hazard. Mater. 2021, 417, 126106. [Google Scholar] [CrossRef]
- Ike, I.A.; Linden, K.G.; Orbell, J.D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Rong, X.; Xie, M.; Kong, L.; Natarajan, V.; Ma, L.; Zhan, J. The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation. Chem. Eng. J. 2019, 372, 294–303. [Google Scholar] [CrossRef]
- Xu, X.; Qin, J.; Wei, Y.; Ye, S.; Shen, J.; Yao, Y.; Ding, B.; Shu, Y.; He, G.; Chen, H. Heterogeneous activation of persulfate by NiFe2−xCoxO4-RGO for oxidative degradation of bisphenol A in water. Chem. Eng. J. 2019, 365, 259–269. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Tian, S.; Li, K.; Yan, F.; Liu, N.; Yang, M.; Chen, X. BOF steel slag as a low-cost sorbent for vanadium (V) removal from soil washing effluent. Sci. Rep. 2017, 7, 11177. [Google Scholar] [CrossRef]
- Hu, R.; Xie, J.; Wu, S.; Yang, C.; Yang, D. Study of Toxicity Assessment of Heavy Metals from Steel Slag and Its Asphalt Mixture. Materials 2020, 13, 2768. [Google Scholar] [CrossRef]
- Scattolin, M.; Peuble, S.; Pereira, F.; Paran, F.; Moutte, J.; Menad, N.; Faure, O. Aided-phytostabilization of steel slag dumps: The key-role of pH adjustment in decreasing chromium toxicity and improving manganese, phosphorus and zinc phytoavailability. J. Hazard. Mater. 2021, 405, 124225. [Google Scholar] [CrossRef]
- Zhao, Q.; Chu, X.; Mei, X.; Meng, Q.; Li, J.; Liu, C.; Saxen, H.; Zevenhoven, R. Co-treatment of Waste From Steelmaking Processes: Steel Slag-Based Carbon Capture and Storage by Mineralization. Front. Chem. 2020, 8, 571504. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Huang, B.; Zhang, T.; Liu, N.; Mao, Z. Preparation of Steel-Slag-Based Hydrotalcite and Its Adsorption Properties on Cl− and SO42−. Materials 2023, 16, 7402. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Niu, X.; Gao, J.; Waclawek, S.; Tang, L.; Dionysiou, D. Comparison of sulfate radical with other reactive species. Curr. Opin. Chem. Eng. 2022, 38, 100867. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, F.; Li, J.; Yang, H.; Wei, L.; Li, Q.; Jiang, J.; Zhang, G.; Zhao, Q. A Review Study on Sulfate-Radical-Based Advanced Oxidation Processes for Domestic/Industrial Wastewater Treatment: Degradation, Efficiency, and Mechanism. Front. Chem. 2020, 8, 592056. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Dong, J.; Xu, L.; Li, Y.; Wang, N.; Li, S.; Ma, R. Enhancement of Stability and Conductivity of α-Fe2O3 Anodes by Doping with Cs+ for Lithium-Ion Battery. ACS Appl. Energy Mater. 2024, 7, 9953–9961. [Google Scholar] [CrossRef]
- Tadic, M.; Trpkov, D.; Kopanja, L.; Vojnovic, S.; Panjan, M. Hydrothermal synthesis of hematite (α-Fe2O3) nanoparticle forms: Synthesis conditions, structure, particle shape analysis, cytotoxicity and magnetic properties. J. Alloys Compd. 2019, 792, 599–609. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, L.; Han, S.-J.; Liu, C.-H.; Li, Y.-B.; Fu, M.-L.; Yuan, B. Fe-MOF derived Fe3O4/C-based hydrogel for efficient solar-driven photothermal evaporation. Desalination 2024, 592, 118138. [Google Scholar] [CrossRef]
- Lindstroem, N.; Talreja, T.; Linnow, K.; Stahlbuhk, A.; Steiger, M. Crystallization behavior of Na2SO4-MgSO4 salt mixtures in sandstone and comparison to single salt behavior. Appl. Geochem. 2016, 69, 50–70. [Google Scholar] [CrossRef]
- Salame, P.H. Synthesis and Electrical studies of Na3Fe(SO4)3 Cathode Material for Sodium Ion Batteries. In Proceedings of the 63rd DAE Solid State Physics Symposium (DAE-SSPS), Hisar, Haryana, India, 18–22 December 2018; Guru Jambheshwar University of Science and Technology: Hisar, India, 2019. [Google Scholar]
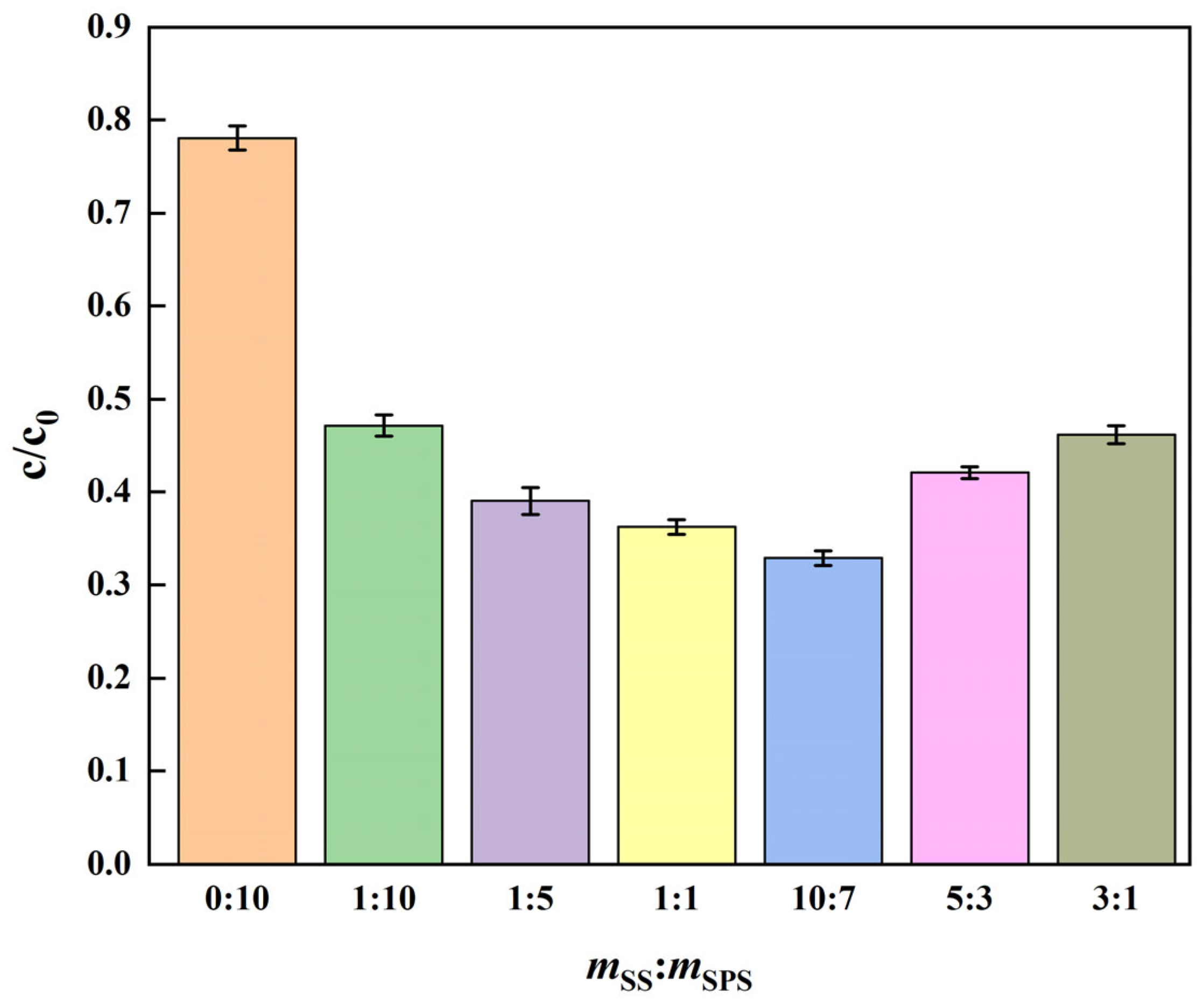
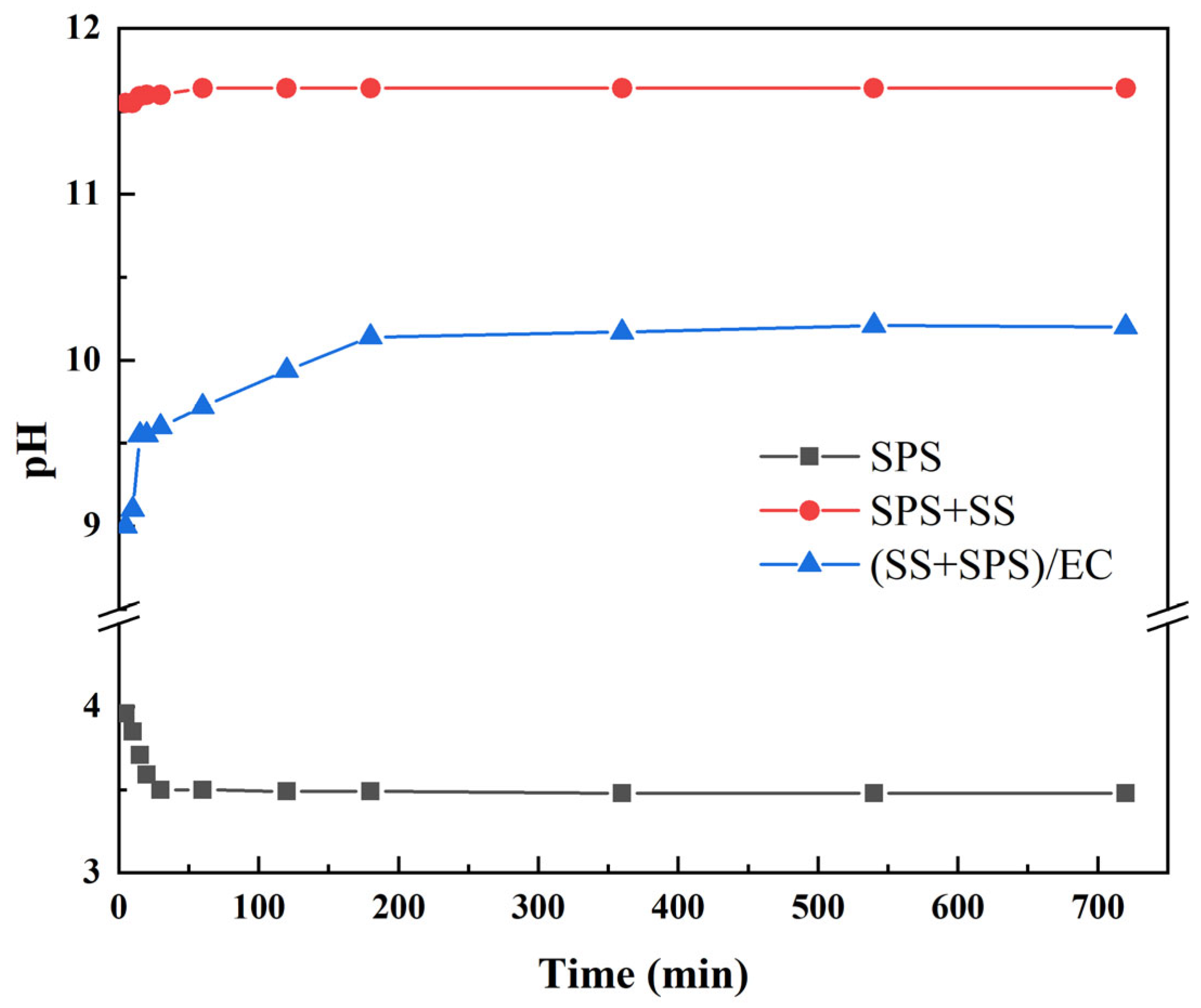
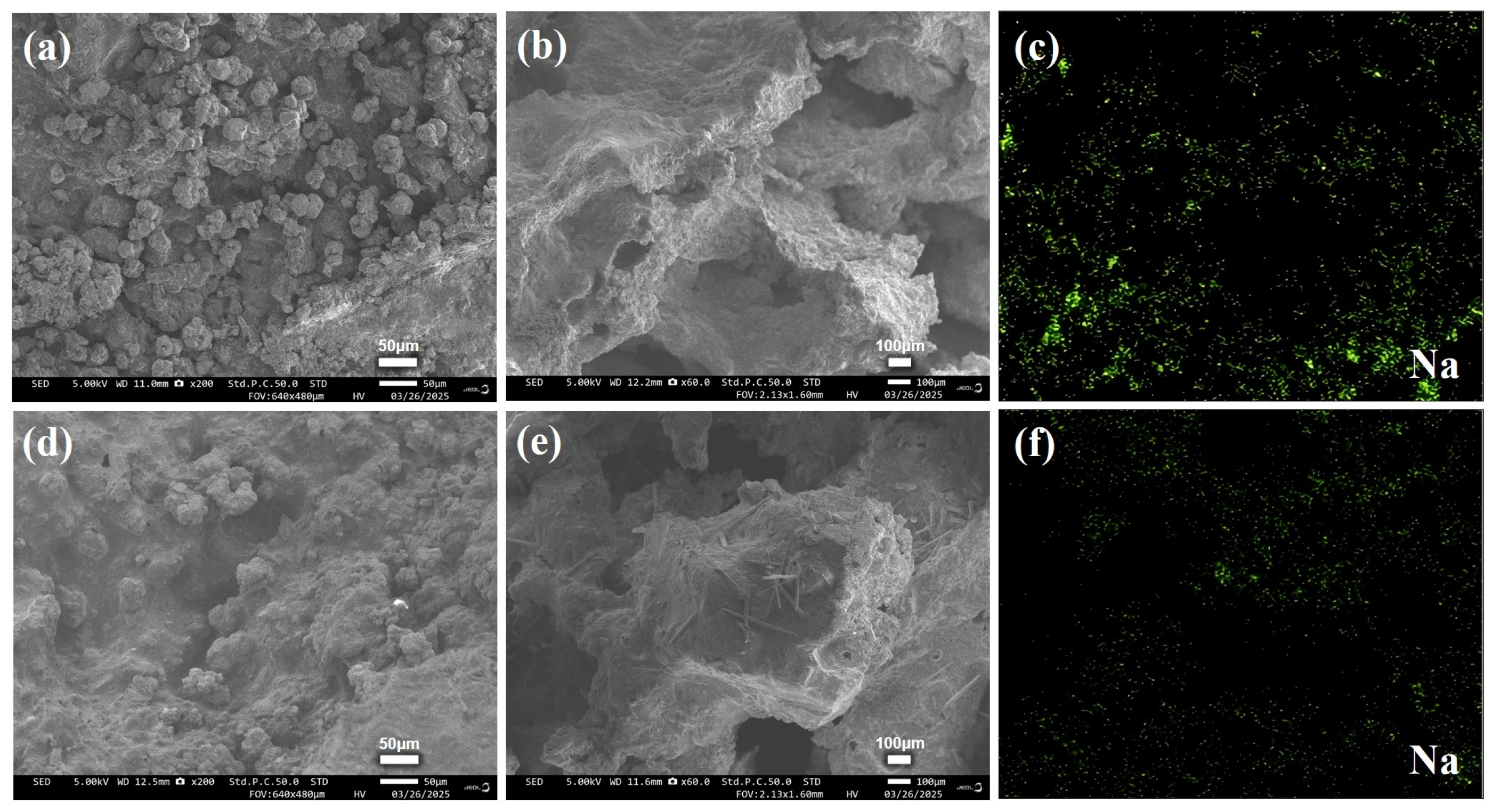
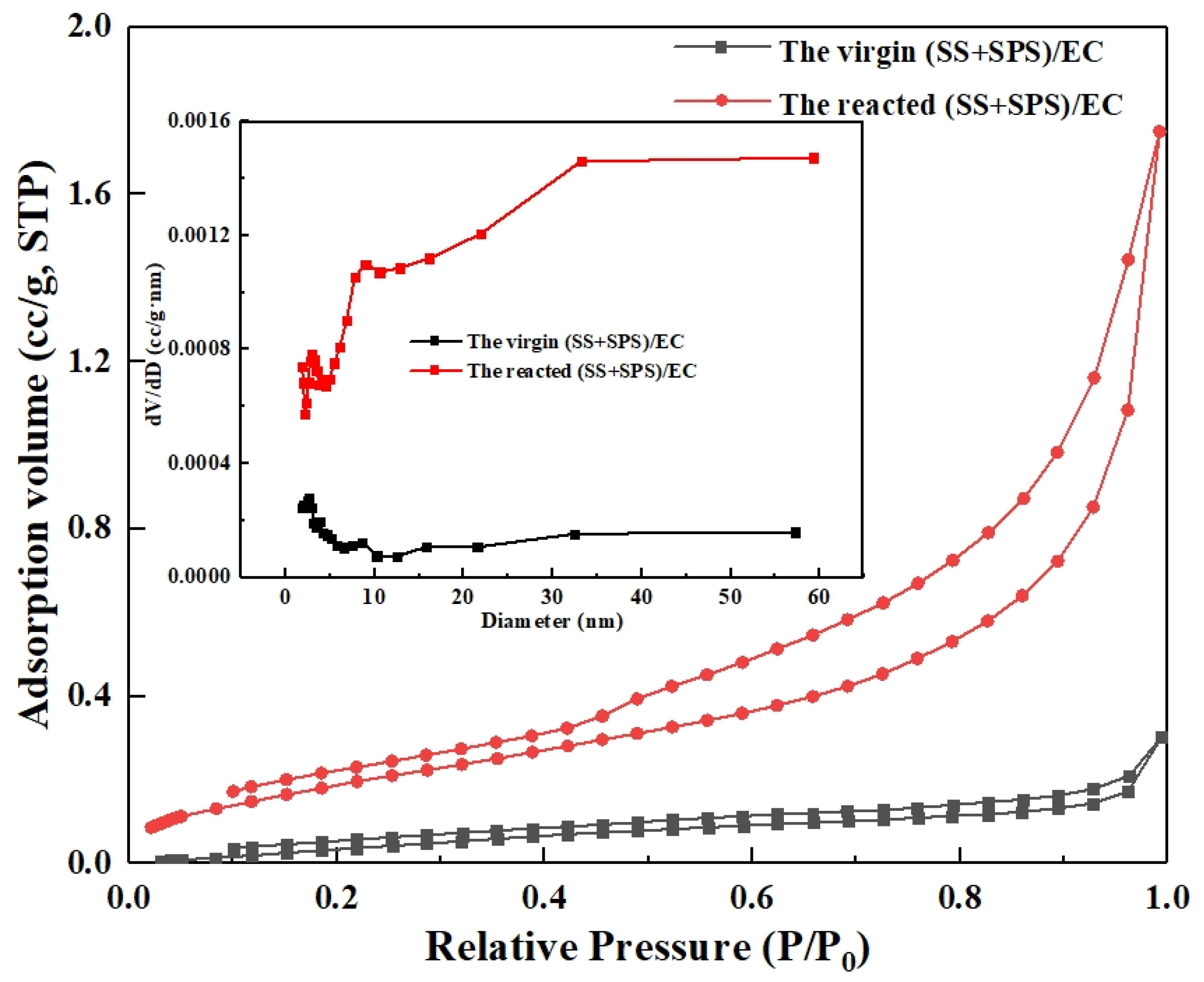
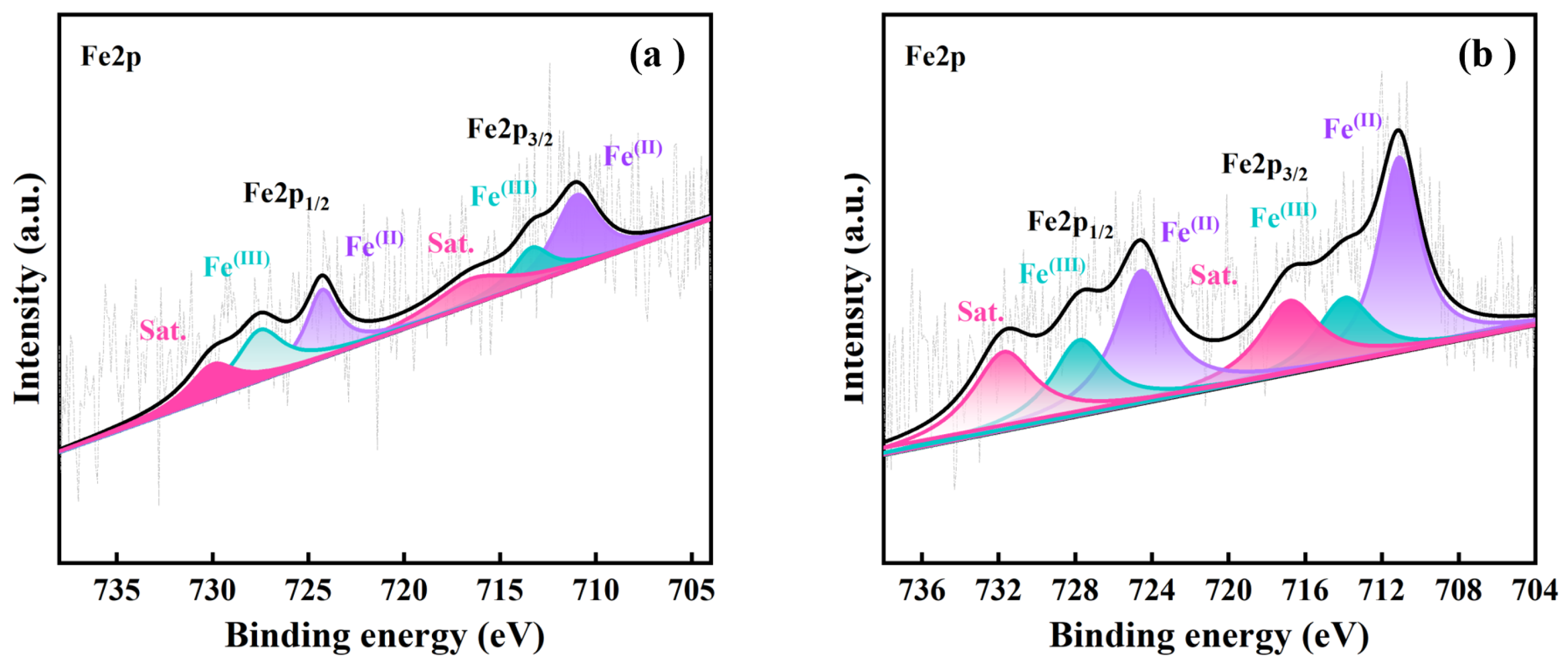
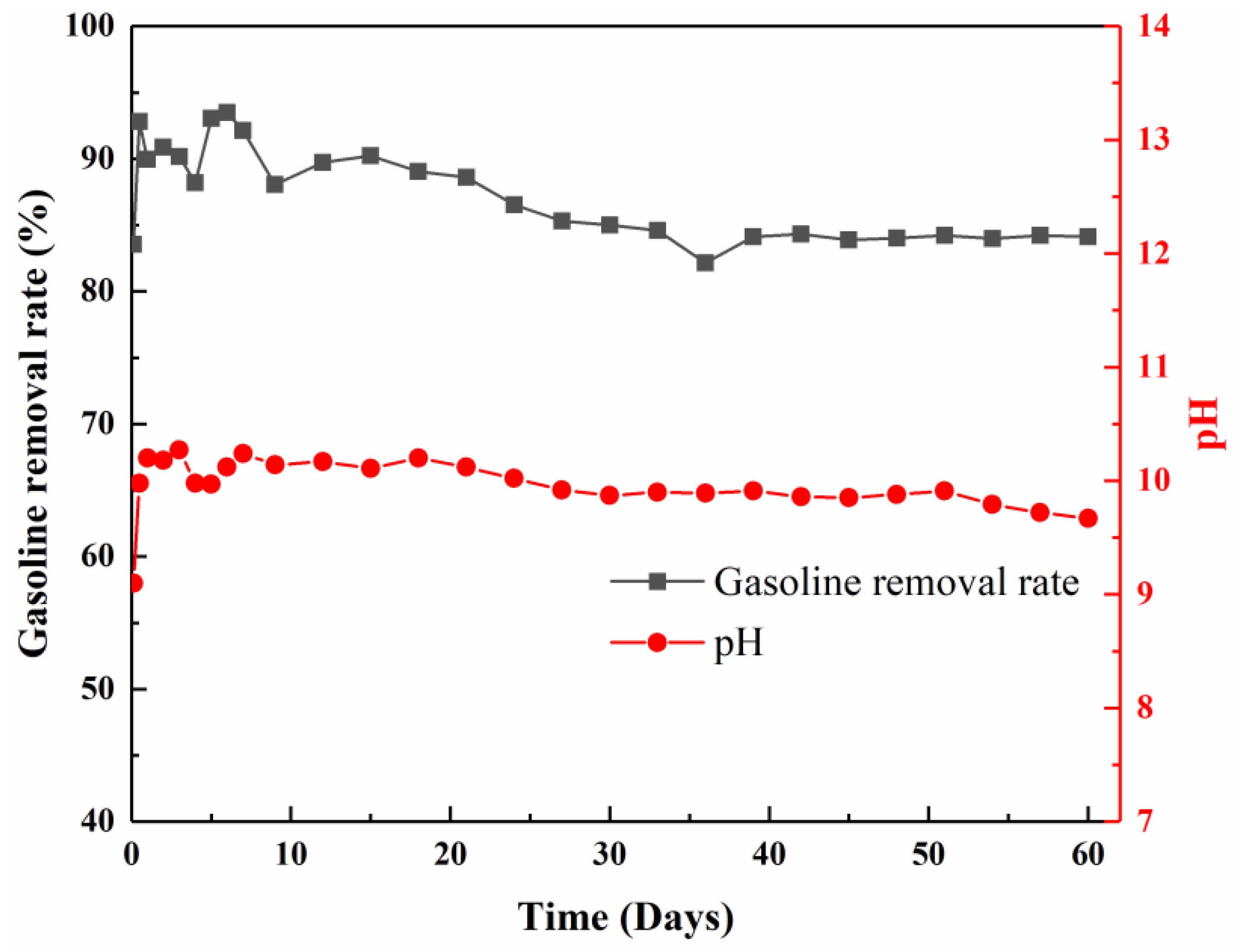
| Sample ID | SS:SPS Mass Ratio | SS Content (wt%) | EC Content (wt%) |
| SS0 | 0:10 | 0.0 | 50.0 |
| SS1 | 1:10 | 4.5 | 50.0 |
| SS2 | 1:5 | 8.3 | 50.0 |
| SS3 | 1:1 | 25.0 | 50.0 |
| SS4 | 10:7 | 29.4 | 50.0 |
| SS5 | 5:3 | 31.3 | 50.0 |
| SS6 | 3:1 | 37.5 | 50.0 |
| Variable | Tested Levels | Fixed Parameters |
| Temperature | 15, 30, 50, 75 °C | 10 g (SS + SPS)/EC, 400 mL PHs (10 mg·L−1) |
| Treatment Agent Dosage | 1%, 2%, 4%, 6%, 8% (w/v) | 15 °C, 400 mL PHs (10 mg·L−1) |
| Coexisting Anion | 50 mg·L−1 Cl−, , , | 15 °C, 400 mL PHs (10 mg·L−1), 10 g (SS + SPS)/EC |
| Parameter | Pre-Reaction | Post-Reaction | Δ% |
| Specific Surface Area | 0.3024 m2/g | 0.7753 m2/g | +156% |
| Total Pore Volume | 0.00047 cm3/g | 0.00271 cm3/g | +476% |
| Average Pore Diameter | 6.24 nm | 13.99 nm | +124% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Qu, C.; Xu, D. Ethyl Cellulose Co-Encapsulation of Steel Slag–Persulfate Long-Term Petroleum Hydrocarbon Remediation. Processes 2025, 13, 2501. https://doi.org/10.3390/pr13082501
Lin S, Qu C, Xu D. Ethyl Cellulose Co-Encapsulation of Steel Slag–Persulfate Long-Term Petroleum Hydrocarbon Remediation. Processes. 2025; 13(8):2501. https://doi.org/10.3390/pr13082501
Chicago/Turabian StyleLin, Shuang, Changsheng Qu, and Dongyao Xu. 2025. "Ethyl Cellulose Co-Encapsulation of Steel Slag–Persulfate Long-Term Petroleum Hydrocarbon Remediation" Processes 13, no. 8: 2501. https://doi.org/10.3390/pr13082501
APA StyleLin, S., Qu, C., & Xu, D. (2025). Ethyl Cellulose Co-Encapsulation of Steel Slag–Persulfate Long-Term Petroleum Hydrocarbon Remediation. Processes, 13(8), 2501. https://doi.org/10.3390/pr13082501








