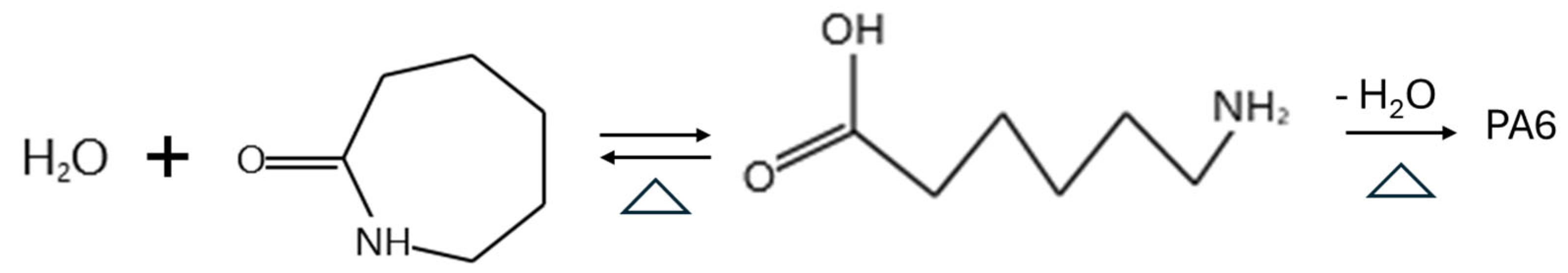
Figure 1.
Pathways for CPL polymerization into PA6 via polycondensation and ring-opening polymerization.
Figure 1.
Pathways for CPL polymerization into PA6 via polycondensation and ring-opening polymerization.
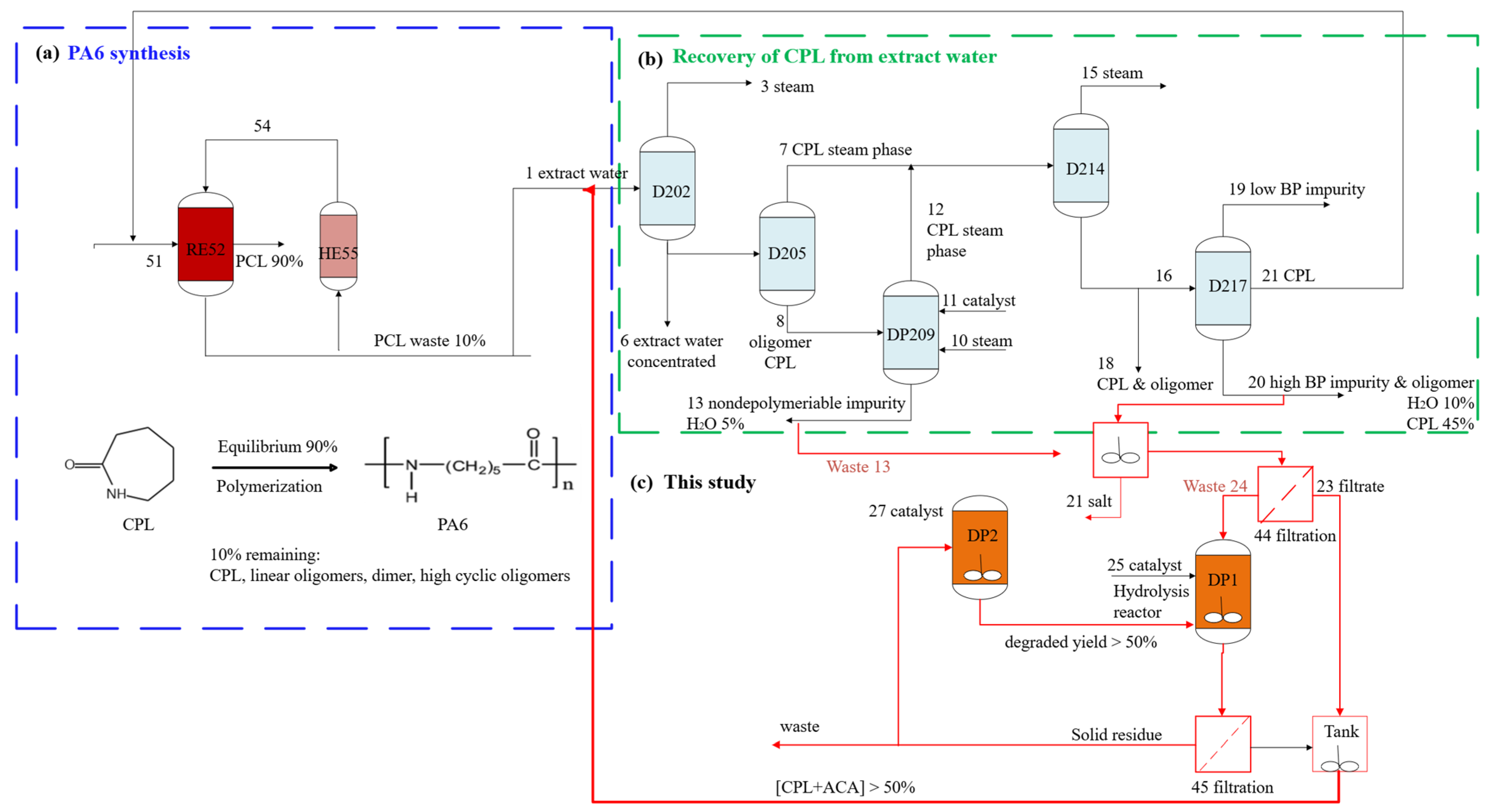
Figure 2.
Process overview of (a) PA6 production, (b) CPL recovery from extract water, and (c) hydrolytic degradation of oligomeric wastes in this study.
Figure 2.
Process overview of (a) PA6 production, (b) CPL recovery from extract water, and (c) hydrolytic degradation of oligomeric wastes in this study.
Figure 3.
Representative PA6 waste feedstocks: WS-24, WS-13, C-fiber, and C-resin.
Figure 3.
Representative PA6 waste feedstocks: WS-24, WS-13, C-fiber, and C-resin.
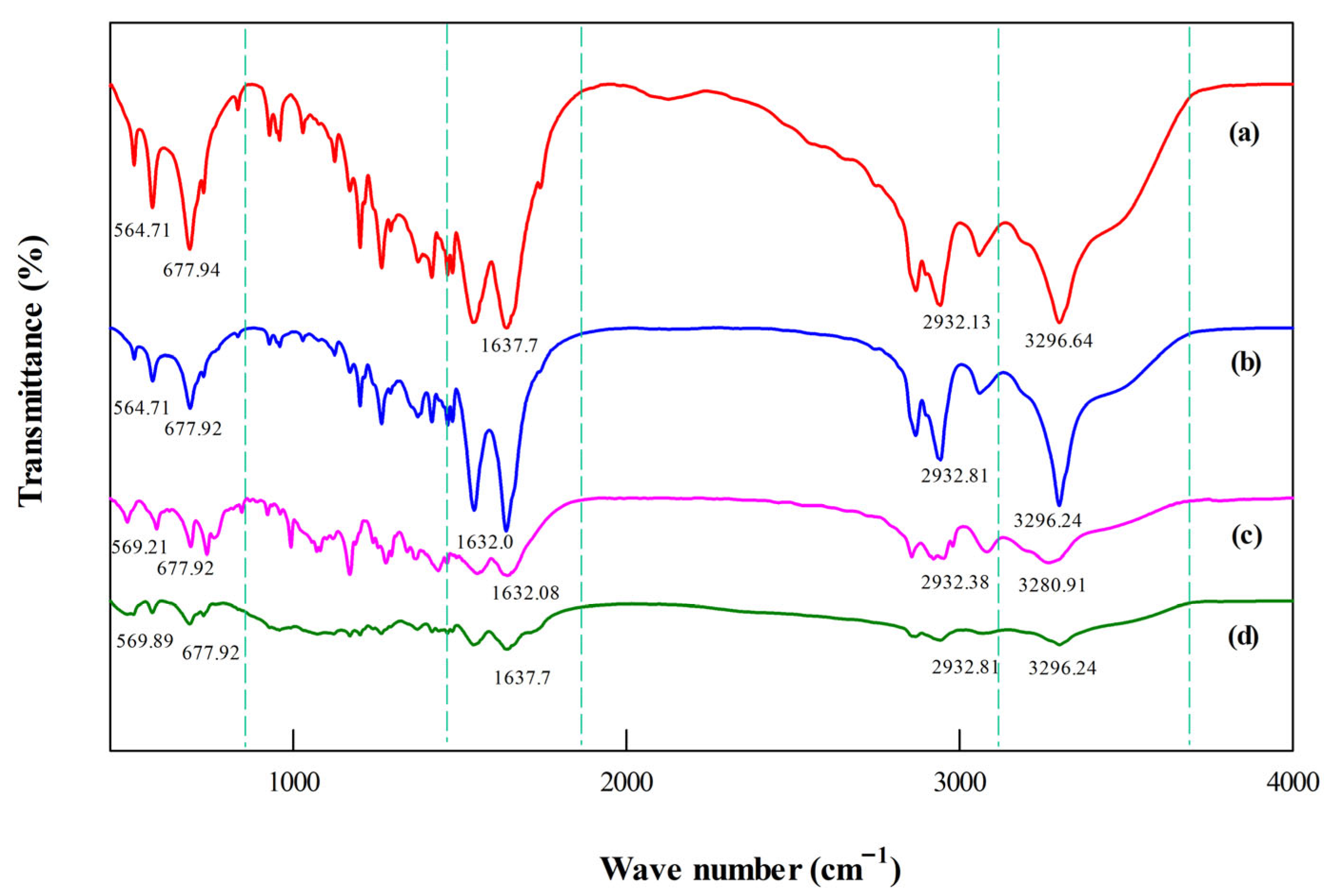
Figure 4.
FTIR spectra of PA6 feedstocks showing differences in functional groups. (a) C-fiber, (b) C-resin, (c) WS-13, (d) WS-24.
Figure 4.
FTIR spectra of PA6 feedstocks showing differences in functional groups. (a) C-fiber, (b) C-resin, (c) WS-13, (d) WS-24.
Figure 5.
Thermal decomposition profiles of PA6 feedstocks: (a) TG curves, (b) DTG curves.
Figure 5.
Thermal decomposition profiles of PA6 feedstocks: (a) TG curves, (b) DTG curves.
Figure 6.
DSC thermograms of PA6 feedstocks showing melting and crystallization transitions. (a) WS-24, (b) WS-13, and (c) C-resin (d) C-fiber.
Figure 6.
DSC thermograms of PA6 feedstocks showing melting and crystallization transitions. (a) WS-24, (b) WS-13, and (c) C-resin (d) C-fiber.
Figure 7.
KSA plot of polycaprolactam for different values of conversion.
Figure 7.
KSA plot of polycaprolactam for different values of conversion.
Figure 8.
The apparent activation energy at different conversion rates (a) WS-24, (b) WS-13, (c) C-fiber, (d) C-resin.
Figure 8.
The apparent activation energy at different conversion rates (a) WS-24, (b) WS-13, (c) C-fiber, (d) C-resin.
Figure 9.
Comparison of acid types on PA6 (WS-13) depolymerization efficiency. WS-13 = 10 g; T= 200 °C; t = 1 h; H3PO4 = 10 g; volume of water 10 mL.
Figure 9.
Comparison of acid types on PA6 (WS-13) depolymerization efficiency. WS-13 = 10 g; T= 200 °C; t = 1 h; H3PO4 = 10 g; volume of water 10 mL.
Figure 10.
Influence of reaction time on the degradation efficiency of different PA6 feedstocks using phosphoric acid. PA6 mass = 10 g; T = 200 °C; t = 1 h; H3PO4 = 5 g; volume of water 5 mL.
Figure 10.
Influence of reaction time on the degradation efficiency of different PA6 feedstocks using phosphoric acid. PA6 mass = 10 g; T = 200 °C; t = 1 h; H3PO4 = 5 g; volume of water 5 mL.
Figure 11.
Effect of phosphoric acid dosage on the residual fraction of WS-13 after hydrolysis.
Figure 11.
Effect of phosphoric acid dosage on the residual fraction of WS-13 after hydrolysis.
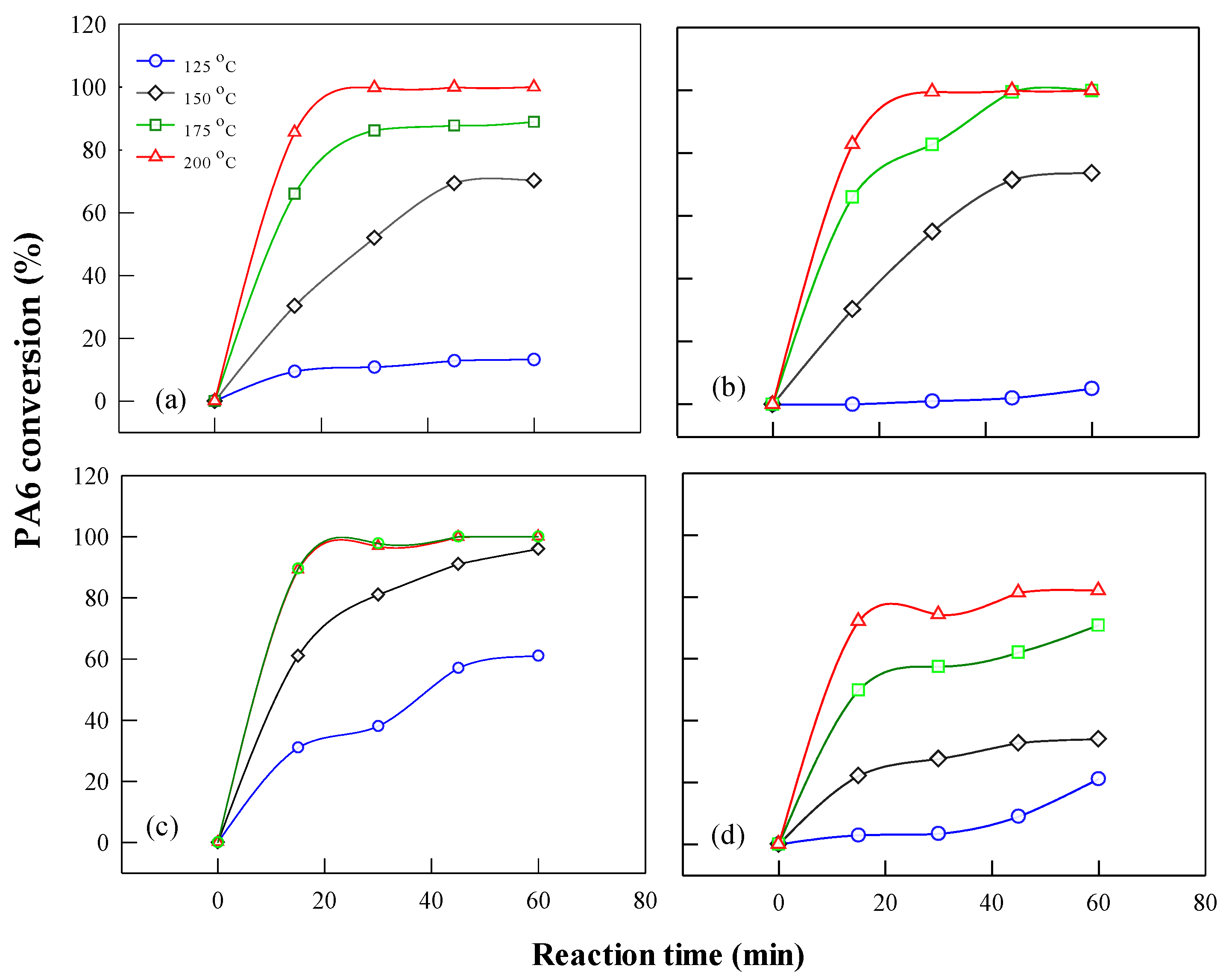
Figure 12.
Time–temperature dependence of PA6 feedstocks conversion during phosphoric acid-catalyzed depolymerization (a) C-resin (b) C-fiber (c) WS-13 and (d) WS-24 PA6: 10 g; acid = 10 g; power = 400 W; volume of H2O = 10 mL).
Figure 12.
Time–temperature dependence of PA6 feedstocks conversion during phosphoric acid-catalyzed depolymerization (a) C-resin (b) C-fiber (c) WS-13 and (d) WS-24 PA6: 10 g; acid = 10 g; power = 400 W; volume of H2O = 10 mL).
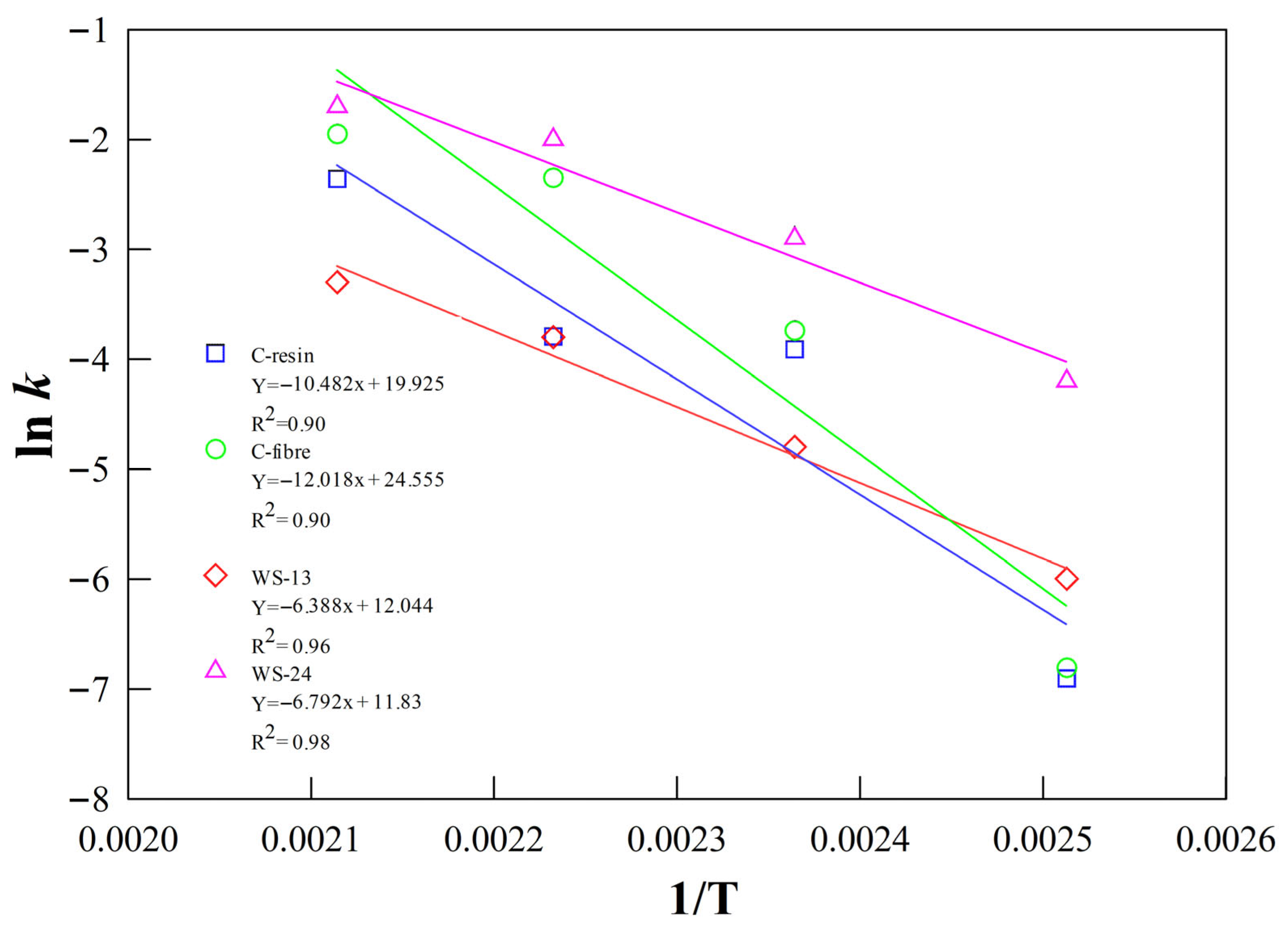
Figure 13.
Arrhenius plots and activation energy determination for PA6 degradation with phosphoric acid for different kinds of PA6.
Figure 13.
Arrhenius plots and activation energy determination for PA6 degradation with phosphoric acid for different kinds of PA6.
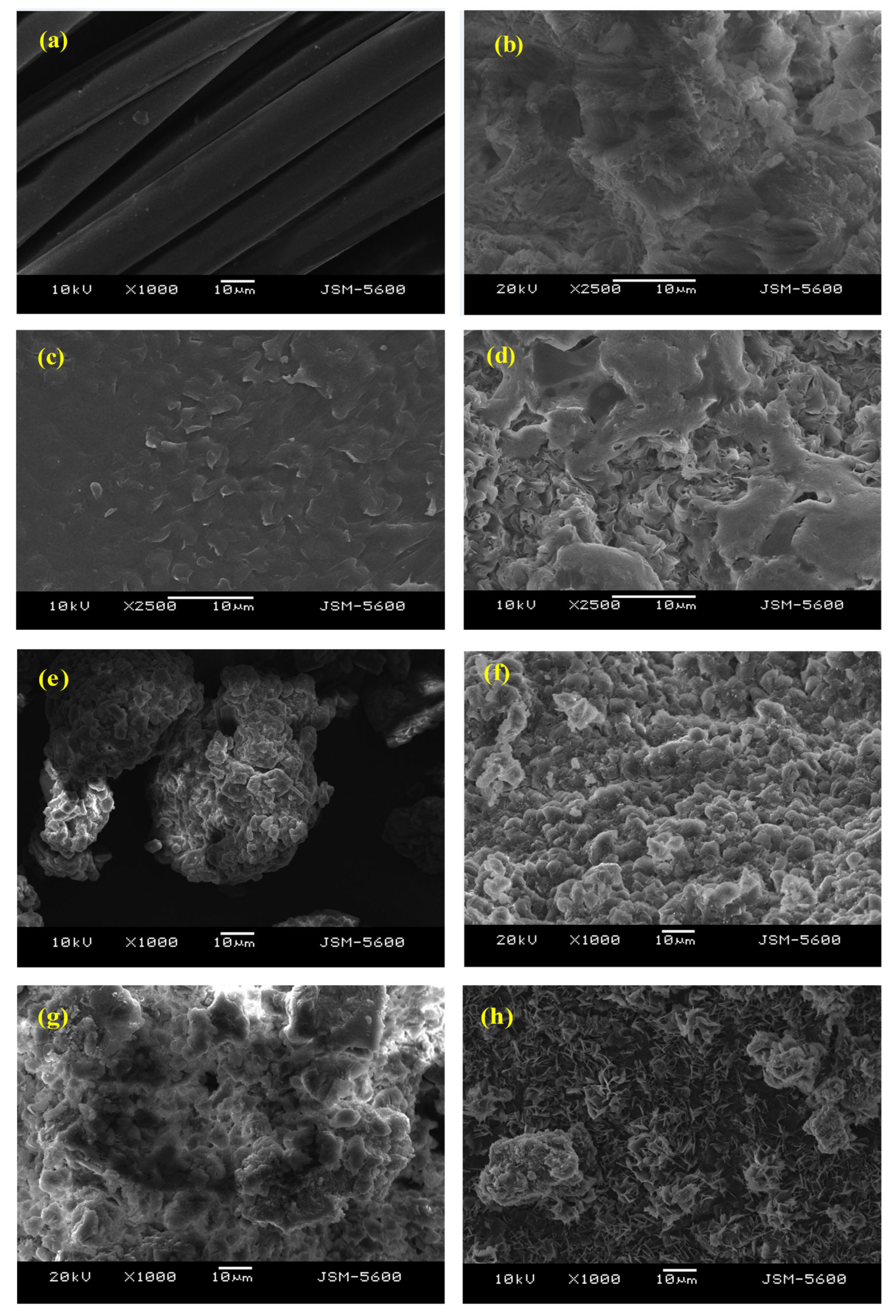
Figure 14.
Surface Morphology of Raw PA6 Feedstocks Observed via SEM Imaging (a) C-fiber, (c) C-resin, (e) WS-13, and (g) WS-24, and solid residue after phosphoric acid hydrolysis at 150 °C for 15 min (b) C-fiber, (d) C-resin, (f) WS-13, and (h) WS-24.
Figure 14.
Surface Morphology of Raw PA6 Feedstocks Observed via SEM Imaging (a) C-fiber, (c) C-resin, (e) WS-13, and (g) WS-24, and solid residue after phosphoric acid hydrolysis at 150 °C for 15 min (b) C-fiber, (d) C-resin, (f) WS-13, and (h) WS-24.
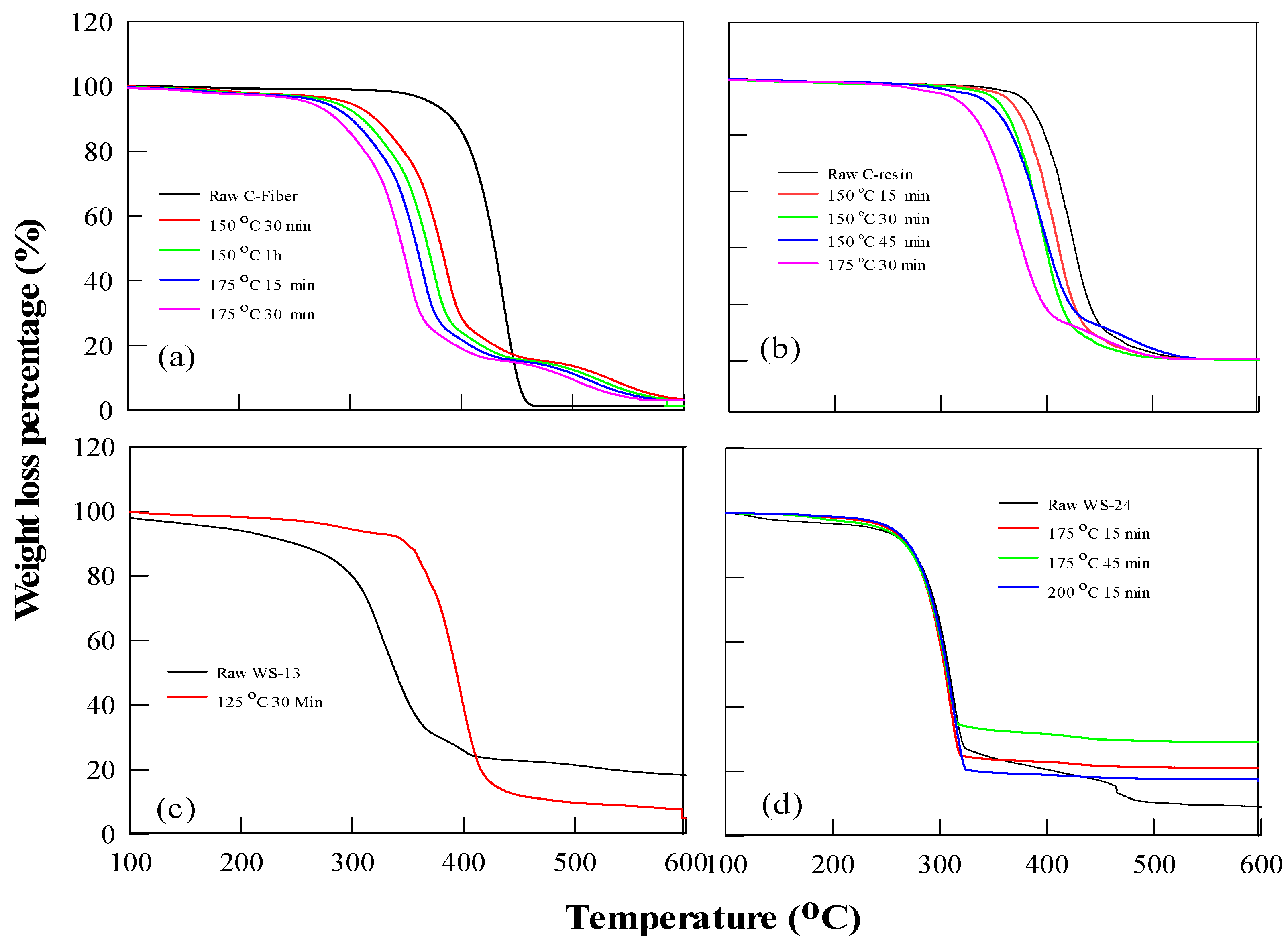
Figure 15.
Thermal stability analysis of PA6 before and after hydrolysis, using TGA (a) C-fiber (b) C-resin (c) WS-13 (d) WS-24.
Figure 15.
Thermal stability analysis of PA6 before and after hydrolysis, using TGA (a) C-fiber (b) C-resin (c) WS-13 (d) WS-24.
Table 1.
Physicochemical and thermal properties of PA6 feedstocks.
Table 1.
Physicochemical and thermal properties of PA6 feedstocks.
| Type | Mw | RV | Moisture Content (%) | TGA | DSC |
|---|
| Ti (°C) | Tp (°C) | Residue (%) | Tm (°C) | Tc (°C) |
|---|
| WS-24 | 1363 | 1.47 | 21.8 | 257 | 302 | 8.90 | 127 | 138 |
| WS-13 | 7268 | 1.66 | 5.60 | 275 | 323 | 4.38 | 192 | 163 |
| C-fiber | 12,583 | 2.15 | 2.56 | 367 | 422 | 0.03 | 217 | 166 |
| C-resin | 16,055 | 2.47 | 1.82 | 372 | 436 | 0.52 | 235 | 168 |
Table 2.
Thermal transition parameters (Tm, Tc, ΔHm, ΔHc) of PA6 feedstocks determined by DSC.
Table 2.
Thermal transition parameters (Tm, Tc, ΔHm, ΔHc) of PA6 feedstocks determined by DSC.
| Type | Tm (°C) | Tc (°C) | ΔHm (J/g) | ΔHc (J/g) |
|---|
| WS-24 | 127 | −138 | 4.17 | −5.20 |
| WS-13 | 192 | −163 | 27.3 | −42.0 |
| C-fiber | 235 | −168 | 25.7 | −52.9 |
| C-resin | 218 | −166 | 51.9 | −52.8 |
Table 3.
First-order rate constants of PA6 feedstocks at different temperatures during acid hydrolysis from Arrhenius plots.
Table 3.
First-order rate constants of PA6 feedstocks at different temperatures during acid hydrolysis from Arrhenius plots.
| Type | k (1/min) | ln k | T (K) | 1/T (1/K) | R2 |
|---|
| WS-24 | 0.0011 | −4.200 | 398 | 0.0025 | 0.986 |
| 0.0028 | −2.900 | 423 | 0.0024 | 0.917 |
| 0.0079 | −2.000 | 448 | 0.0022 | 0.912 |
| 0.0151 | −1.700 | 473 | 0.0021 | 0.992 |
| WS-13 | 0.0068 | −6.000 | 398 | 0.0025 | 0.962 |
| 0.0229 | −4.800 | 423 | 0.0024 | 0.998 |
| 0.0609 | −3.800 | 448 | 0.0022 | 0.998 |
| 0.0808 | −3.300 | 473 | 0.0021 | 0.971 |
| C-fiber | 0.0010 | −6.810 | 398 | 0.0025 | 0.667 |
| 0.0240 | −3.740 | 423 | 0.0024 | 0.967 |
| 0.0950 | −2.350 | 448 | 0.0022 | 0.899 |
| 0.1410 | −1.950 | 473 | 0.0021 | 0.591 |
| C-resin | 0.0010 | −6.908 | 398 | 0.0025 | 0.781 |
| 0.0200 | −3.912 | 423 | 0.0024 | 0.932 |
| 0.0230 | −3.794 | 448 | 0.0022 | 0.766 |
| 0.094 | −2.361 | 473 | 0.0021 | 0.931 |
Table 4.
Activation energies of various PA6 types calculated from Arrhenius plots under H3PO4.
Table 4.
Activation energies of various PA6 types calculated from Arrhenius plots under H3PO4.
| PA6 | Ea (kJ/mol) | R2 | ±SD (kJ mol−1) |
|---|
| WS-24 | 56.5 | 0.98 | ±2.1 |
| WS-13 | 53.1 | 0.91 | ±1.8 |
| C-fiber | 99.9 | 0.91 | ±3.5 |
| C-resin | 87.1 | 0.90 | ±2.9 |
Table 5.
Hydrolysis yields of WS-24 under various temperature and time conditions.
Table 5.
Hydrolysis yields of WS-24 under various temperature and time conditions.
| T (°C) | Time (min) | Conversion Degradation (%) | Yield (ACA + CPL) | Yield ACA (%) | Yield CPL (%) |
|---|
| 125 | 30 | 10.8 | 8.69 | 8.35 | 0.35 |
| | 45 | 12.8 | 8.84 | 8.09 | 0.75 |
| | 60 | 13.2 | 8.87 | 7.61 | 1.26 |
| 150 | 15 | 30.2 | 22.1 | 20.8 | 1.3 |
| | 30 | 51.9 | 34.1 | 32.7 | 1.4 |
| | 45 | 69.4 | 57.3 | 56.5 | 0.8 |
| | 60 | 70.2 | 55.4 | 54.7 | 0.7 |
| 175 | 15 | 66 | 34.5 | 33.15 | 1.35 |
| | 30 | 86.1 | 40.3 | 38.84 | 1.46 |
| | 45 | 87.7 | 56.2 | 55.36 | 0.84 |
| | 60 | 88.9 | 57 | 56.19 | 0.81 |
| 200 | 15 | 86.5 | 42.1 | 39.65 | 2.45 |
| | 30 | 99.7 | 45.4 | 42.89 | 2.51 |
| | 45 | 99.9 | 58.2 | 56.5 | 1.7 |
| | 60 | 99.9 | 62 | 61.14 | 1.26 |
Table 6.
Hydrolysis product yields of WS-13 at different reaction conditions using Phosphoric Acid.
Table 6.
Hydrolysis product yields of WS-13 at different reaction conditions using Phosphoric Acid.
| T (°C) | Time (min) | Conversion Degradation (%) | Yield (ACA + CPL) | Yield ACA (%) | Yield CPL (%) |
|---|
| 125 | 30 | 3.4 | 1.6 | 1.6 | 0 |
| | 45 | 3.9 | 4.3 | 4.3 | 0 |
| | 60 | 21.1 | 4.6 | 4.6 | 0 |
| 150 | 15 | 22.2 | 7 | 5.7 | 1.3 |
| | 30 | 22.7 | 7.21 | 6.1 | 1.1 |
| | 45 | 32.7 | 5.2 | 4.9 | 0.3 |
| | 60 | 34.1 | 5.04 | 4.9 | 0.14 |
| 175 | 15 | 49.8 | 4.9 | 7.2 | 0 |
| | 30 | 57.5 | 59 | 59 | 0 |
| | 45 | 62.1 | 59 | 41 | 0 |
| | 60 | 70.8 | 41 | 28 | 0.2 |
| 200 | 15 | 72.1 | 28.2 | 27.3 | 0.9 |
| | 30 | 74.3 | 62 | 43 | 0.01 |
| | 45 | 81.3 | 62.4 | 46 | 16.4 |
| | 60 | 82.3 | 58.1 | 56 | 2.03 |
Table 7.
Degradation Efficiency of C-Resin under Varying Thermal Conditions with Phosphoric Acid.
Table 7.
Degradation Efficiency of C-Resin under Varying Thermal Conditions with Phosphoric Acid.
| T (°C) | Time (min) | Conversion Degradation (%) | Yield (ACA + CPL) | Yield ACA (%) | Yield CPL (%) |
|---|
| 125 | 30 | 10.8 | 4.3 | 4.2 | 0.1 |
| | 45 | 12.8 | 4.3 | 4.3 | 0 |
| | 60 | 13.2 | 4.4 | 4.3 | 0.1 |
| 150 | 15 | 30.2 | 5.8 | 5.6 | 0.2 |
| | 30 | 51.9 | 7.3 | 7.2 | 0.1 |
| | 45 | 69.4 | 7.7 | 7.3 | 0.5 |
| | 60 | 70.2 | 7.4 | 7.3 | 0.1 |
| 175 | 15 | 66 | 10.3 | 10.1 | 0.2 |
| | 30 | 86.1 | 39.9 | 39.6 | 0.3 |
| | 45 | 87.7 | 37.8 | 37.6 | 0.2 |
| | 60 | 88.9 | 42.8 | 42.6 | 0.2 |
| 200 | 15 | 85.5 | 15.3 | 14.1 | 1.2 |
| | 30 | 99.4 | 45.9 | 44.6 | 1.3 |
| | 45 | 99.7 | 48.1 | 46.7 | 1.4 |
| | 60 | 99.9 | 53.8 | 50.7 | 3.1 |
Table 8.
Product yields from phosphoric acid hydrolysis of C-fiber under different conditions.
Table 8.
Product yields from phosphoric acid hydrolysis of C-fiber under different conditions.
| T (°C) | Time (min) | Conversion Degradation (%) | Yield (ACA + CPL) | Yield ACA (%) | Yield CPL (%) |
|---|
| 125 | 30 | 1 | 0.2 | 0.2 | 0 |
| | 45 | 2 | 0.34 | 0.3 | 0 |
| | 60 | 5 | 4.53 | 4.5 | 0 |
| 150 | 15 | 30.3 | 13.24 | 13.2 | 0 |
| | 30 | 55 | 13.01 | 13 | 0.01 |
| | 45 | 71.5 | 33 | 33 | 0 |
| | 60 | 73.6 | 44.05 | 44 | 0.02 |
| 175 | 15 | 66 | 23.02 | 23 | 0 |
| | 30 | 82.8 | 48.05 | 48 | 0.05 |
| | 45 | 99.5 | 56.99 | 56.9 | 0.12 |
| | 60 | 99.9 | 61.01 | 60.9 | 0.12 |
| 200 | 15 | 80.2 | 60.29 | 60.1 | 0.19 |
| | 30 | 82.8 | 66.37 | 66.2 | 0.17 |
| | 45 | 99.5 | 69.05 | 69 | 0.05 |
| | 60 | 99.9 | 69.7 | 69.5 | 0.2 |
Table 9.
Summary of chemical recycling of PA6 waste.
Table 9.
Summary of chemical recycling of PA6 waste.
| Material | Product (%) | Method | Catalyst | T (°C) | Time | Solid: Solution | Ref. |
|---|
| PA6 | 77.9% CPL | hydrolysis | HPA | 330 | 85 min | 1:15 | [33] |
| PA6 | 55% Oligomer | hydrogenative | Ruthenium | 150 | 48 h | 1:2.5 | [34] |
| Teabag waste | 59.2% CPL | pyrolysis | - | 700 | - | - | [35] |
| PA6 plastic | 86% CPL | ionic liquids | PP13 TFSI | 300 | 6 h | - | [36] |
| PA6 | 85% ACA | ionic liquids | HCl, 30% | 109 | 24 h | - | [37] |
| PA6 fiber | 93% ACA | hydrolysis | HCl, 30% | 90 | 4 h | 1:25 | [17] |
| PA6 | 85% CPL | hydrothermal | - | 360 | 60 min | - | [11] |
| PA6 | 62.3% CPL | hydrolysis | 3 g Hβ-25 | 345 | 30 min | 1:10 | [38] |
| PA6 carpet waste | monomeric | hydrolysis | HCl 10% | 200 | 3 h | 1:20 | [10] |
| WS-24 | 62.0% (CPL + ACA) | hydrolysis | H3PO4 50% | 200 | 1 h | 1:1 | This study |
| WS-13 | 62.4% (CPL + ACA) | hydrolysis | H3PO4 50% | 200 | 1 h | 1:1 | This study |
| C-fiber | 69.7% (CPL + ACA) | hydrolysis | H3PO4 50% | 200 | 1 h | 1:1 | This study |
| C-resin | 53.8% (CPL + ACA) | hydrolysis | H3PO4 50% | 200 | 1 h | 1:1 | This study |