Extraction and Spray Drying-Based Encapsulation of Anthocyanin Pigments from Jabuticaba Sabará Peel (Myrciaria jaboticaba (Vell.) O. Berg)
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Preparation
2.2. ANC Extraction
2.3. ANC Encapsulation
Spray Drying
2.4. ANCs Quantification
2.5. Water Activity (aw), Color Parameters (L*, a*, b*), and Moisture Content Determination
2.6. Statistical Analysis
3. Results and Discussion
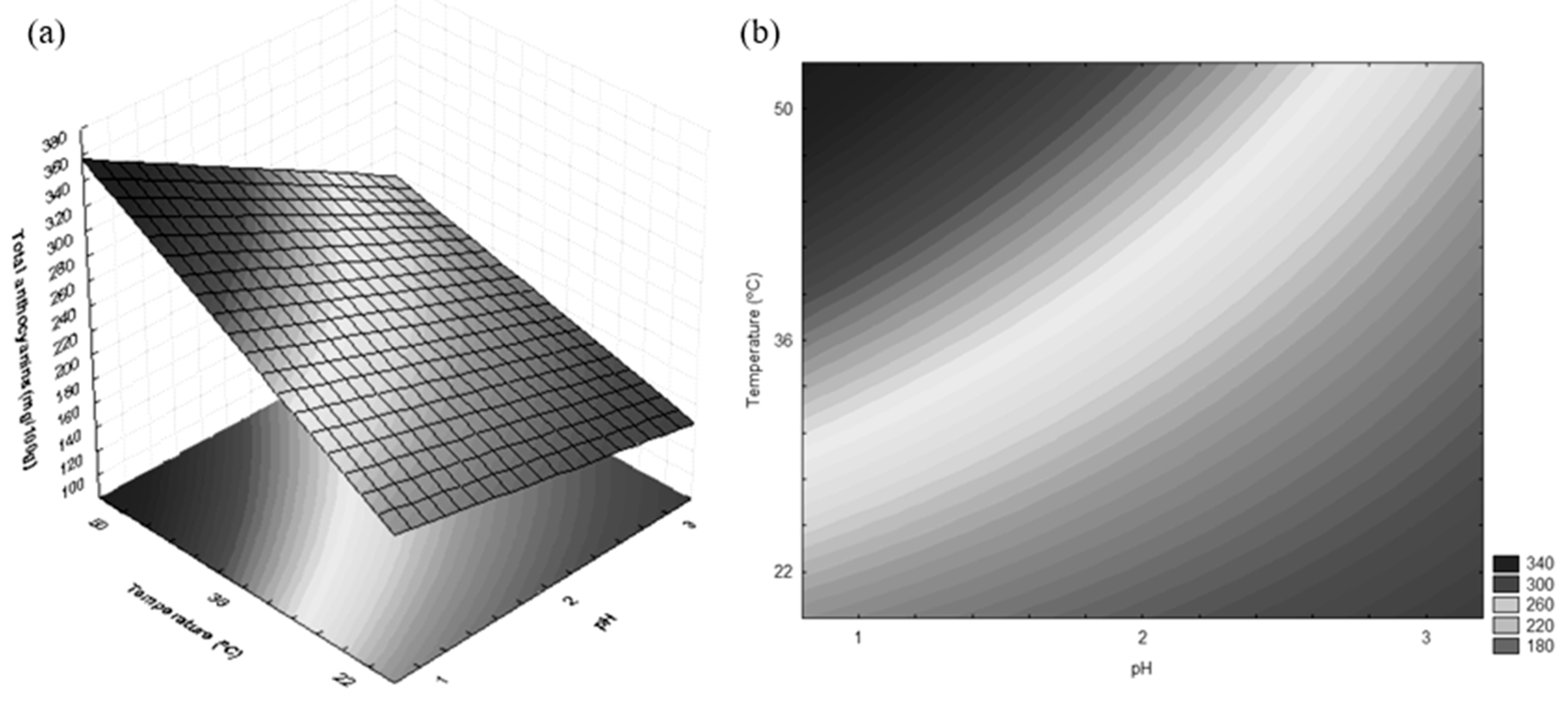
3.1. ANC Extraction
3.2. Characterization of Encapsulated Material
4. Applications of Encapsulated Anthocyanins in Functional Foods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borges, L.L.R.; Freitas, V.V.; Nascimento, A.L.A.A.; Fernandes, J.G.; de Barros Kobi, H.; Eller, M.R.; de Barros, F.A.R.; de Souza, L.Â.; Castro, G.A.D.; de Carvalho, A.F.; et al. Enhancement of Phenolic Compounds Bioaccessibility in Jabuticaba Wine through Fermentation by Saccharomyces cerevisiae. Food Bioprod. Process. 2024, 148, 198–207. [Google Scholar] [CrossRef]
- Lima, N.M.; Santos, G.F.; de Jesus, A.S.A.T.; Dias, L.S.; Silva, P.A.; Castro, S.B.R.; Carli, A.P.; Alves, C.C.S.; Lima, G.S.; Vaz, B.G. Metabolic Signatures by LC-HRMS/MS of Jabuticaba (Plinia cauliflora) Juice, Liqueur, and Wines Reveal the Wealthiest Sources of Bioactive Metabolites. Talanta 2025, 287, 127602. [Google Scholar] [CrossRef]
- do Nascimento, R.d.P.; Rizzato, J.S.; Polezi, G.; Moya, A.M.T.M.; Silva, M.F.; da Fonseca Machado, A.P.; Franchi Junior, G.C.; Borguini, R.G.; de Araújo Santiago, M.C.P.; Paiotti, A.P.R.; et al. Freeze-Dried Jaboticaba (Myrciaria jaboticaba) Peel Powder, a Rich Source of Anthocyanins and Phenolic Acids, Mitigates Inflammation-Driven Colorectal Cancer in Mice. Food Biosci. 2023, 53, 102578. [Google Scholar] [CrossRef]
- Geraldi, M.V.; de Souza, Á.C.; Norde, M.M.; Berni, P.R.; Reguengo, L.M.; Geloneze, B.; Marostica, M.R. Jaboticaba Peel Improves Postprandial Glucose and Inflammation: A Randomized Controlled Trial in Adults with Metabolic Syndrome. Nutr. Res. 2024, 125, 36–49. [Google Scholar] [CrossRef]
- da Silva-Maia, J.K.; Nagalingam, A.; Cazarin, C.B.B.; Marostica Junior, M.R.; Sharma, D. Jaboticaba (Myrciaria jaboticaba) Peel Extracts Induce Reticulum Stress and Apoptosis in Breast Cancer Cells. Food Chem. Mol. Sci. 2023, 6, 100167. [Google Scholar] [CrossRef]
- Lima, A.d.J.B.; Corrêa, A.D.; Saczk, A.A.; Martins, M.P.; Castilho, R.O. Anthocyanins, Pigment Stability and Antioxidant Activity in Jabuticaba [Myrciaria cauliflora (Mart.) O. Berg]. Rev. Bras. Frutic. 2011, 33, 877–887. [Google Scholar] [CrossRef]
- Plaza, M.; Batista, Â.G.; Cazarin, C.B.B.; Sandahl, M.; Turner, C.; Östman, E.; Maróstica Júnior, M.R. Characterization of Antioxidant Polyphenols from Myrciaria jaboticaba Peel and Their Effects on Glucose Metabolism and Antioxidant Status: A Pilot Clinical Study. Food Chem. 2016, 211, 185–197. [Google Scholar] [CrossRef]
- Fernandes, I.d.A.A.; Maciel, G.M.; Maroldi, W.V.; Bortolini, D.G.; Pedro, A.C.; Haminiuk, C.W.I. Bioactive Compounds, Health-Promotion Properties and Technological Applications of Jabuticaba: A Literature Overview. Meas. Food 2022, 8, 100057. [Google Scholar] [CrossRef]
- Chaves, V.C.; Soares, M.S.P.; Spohr, L.; Teixeira, F.; Vieira, A.; Constantino, L.S.; Pizzol, F.D.; Lencina, C.L.; Spanevello, R.M.; Freitas, M.P.; et al. Blackberry Extract Improves Behavioral and Neurochemical Dysfunctions in a Ketamine-Induced Rat Model of Mania. Neurosci. Lett. 2020, 714, 134566. [Google Scholar] [CrossRef]
- Godlewska, K.; Pacyga, P.; Najda, A.; Michalak, I. Investigation of Chemical Constituents and Antioxidant Activity of Biologically Active Plant-Derived Natural Products. Molecules 2023, 28, 5572. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Machado, G.H.A.; Marques, T.R.; de Carvalho, T.C.L.; Duarte, A.C.; de Oliveira, F.C.; Gonçalves, M.C.; Piccoli, R.H.; Corrêa, A.D. Antibacterial Activity and In Vivo Wound Healing Potential of Phenolic Extracts from Jaboticaba Skin. Chem. Biol. Drug Des. 2018, 92, 1333–1343. [Google Scholar] [CrossRef]
- Jafari, S.M.; Mahdavi-Khazaei, K.; Hemmati-Kakhki, A. Microencapsulation of Saffron Petal Anthocyanins with Cress Seed Gum Compared with Arabic Gum through Freeze Drying. Carbohydr. Polym. 2016, 140, 20–25. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A Novel Nano-Encapsulation Approach for Bioactive Compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Weber, F.; Boch, K.; Schieber, A. Influence of Copigmentation on the Stability of Spray Dried Anthocyanins from Blackberry. LWT-Food Sci. Technol. 2017, 75, 72–77. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Retention of Polyphenols in Encapsulated Sour Cherry Juice in Dependence of Drying Temperature and Wall Material. LWT-Food Sci. Technol. 2017, 83, 110–117. [Google Scholar] [CrossRef]
- Atay, E.; Fabra, M.J.; Martínez-Sanz, M.; Gomez-Mascaraque, L.G.; Altan, A.; Lopez-Rubio, A. Development and Characterization of Chitosan/Gelatin Electrosprayed Microparticles as Food Grade Delivery Vehicles for Anthocyanin Extracts. Food Hydrocoll. 2018, 77, 699–710. [Google Scholar] [CrossRef]
- Oro, C.E.D.; Paroul, N.; Mignoni, M.L.; Zabot, G.L.; Backes, G.T.; Dallago, R.M.; Tres, M.V. Microencapsulation of Brazilian Cherokee Blackberry Extract by Freeze-Drying Using Maltodextrin, Gum Arabic, and Pectin as Carrier Materials. Food Sci. Technol. Int. 2023, 29, 255–265. [Google Scholar] [CrossRef]
- Capello, C.; Leandro, G.C.; Maduro Campos, C.E.; Hotza, D.; Mattar Carciofi, B.A.; Valencia, G.A. Adsorption and Desorption of Eggplant Peel Anthocyanins on a Synthetic Layered Silicate. J. Food Eng. 2019, 262, 162–169. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, J.; Wang, Y.; Shi, Z.; Xie, K.; Liao, X.; Tan, J. Factors Affecting the Stability of Anthocyanins and Strategies for Improving Their Stability: A Review. Food Chem. X 2024, 24, 101883. [Google Scholar] [CrossRef] [PubMed]
- Šturm, L.; Osojnik Črnivec, I.G.; Istenič, K.; Ota, A.; Megušar, P.; Slukan, A.; Humar, M.; Levic, S.; Nedović, V.; Kopinč, R.; et al. Encapsulation of Non-Dewaxed Propolis by Freeze-Drying and Spray-Drying Using Gum Arabic, Maltodextrin and Inulin as Coating Materials. Food Bioprod. Process. 2019, 116, 196–211. [Google Scholar] [CrossRef]
- da Fonseca Machado, A.P.; Rezende, C.A.; Rodrigues, R.A.; Barbero, G.F.; e Rosa, P.d.T.V.; Martínez, J. Encapsulation of Anthocyanin-Rich Extract from Blackberry Residues by Spray-Drying, Freeze-Drying and Supercritical Antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Handb. Food Anal. Chem. 2001, 2, 19–31. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, Y.; Xu, G.; Li, M.; Du, L.; An, L.; Xiu, Z. Extraction and Purification of Anthocyanins from the Fruit Residues of Vaccinium uliginosum Linn. J. Chromatogr. Sep. Tech. 2013, 4, 1000167. [Google Scholar] [CrossRef]
- Guan, Y.; Zhong, Q. The Improved Thermal Stability of Anthocyanins at PH 5.0 by Gum Arabic. LWT-Food Sci. Technol. 2015, 64, 706–712. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin Extraction from Plant Tissues: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Stafussa, A.P.; Maciel, G.M.; Da Silva Anthero, A.G.; Da Silva, M.V.; Zielinski, A.A.F.; Haminiuk, C.W.I. Biosorption of Anthocyanins from Grape Pomace Extracts by Waste Yeast: Kinetic and Isotherm Studies. J. Food Eng. 2016, 169, 53–60. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The Physicochemical Properties of Spray-Dried Watermelon Powders. Chem. Eng. Process. Process. Intensif. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Valduga, E.; Lima, L.; do Prado, R.; Padilha, F.F.; Treichel, H. Extração, Secagem Por Atomização e Microencapsulamento de Antocianinas Do Bagaço Da Uva Isabel (Vitis labrusca). Cienc. Agrotecnol. 2008, 32, 1568–1574. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation Efficiency and Oxidative Stability of Flaxseed Oil Microencapsulated by Spray Drying Using Different Combinations of Wall Materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Santos, D.T.; Albarelli, J.Q.; Beppu, M.M.; Meireles, M.A.A. Stabilization of Anthocyanin Extract from Jabuticaba Skins by Encapsulation Using Supercritical CO2 as Solvent. Food Res. Int. 2013, 50, 617–624. [Google Scholar] [CrossRef]
- de Melo Ramos, F.; Silveira Júnior, V.; Prata, A.S. Assessing the Vacuum Spray Drying Effects on the Properties of Orange Essential Oil Microparticles. Food Bioprocess Technol. 2019, 12, 1917–1927. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of Extracts of Bioactive Compounds Obtained from Acerola (Malpighia emarginata DC) Pulp and Residue by Spray and Freeze Drying: Chemical, Morphological and Chemometric Characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Guizani, N.; Al-Ruzeiki, M.H. D- and Z-Values of Microflora in Tuna Mince during Moist- and Dry-Heating. LWT-Food Sci. Technol. 2004, 37, 93–98. [Google Scholar] [CrossRef]
- Abadio, F.D.B.; Domingues, A.M.; Borges, S.V.; Oliveira, V.M. Physical Properties of Powdered Pineapple (Ananas comosus) Juice—Effect of Malt Dextrin Concentration and Atomization Speed. J. Food Eng. 2004, 64, 285–287. [Google Scholar] [CrossRef]
- Borges, J.M.; Figueiredo, A.P. Estudo Da Estabilidade de Antocianinas Em Diferentes Alcoóis Alifáticos Para Uso Como Indicador de PH. RECEN-Rev. Ciênc. Exatas Nat. 2014, 16, 129–142. [Google Scholar] [CrossRef][Green Version]
- Da Silva, R.D.C.S.; Camponogara, J.A.; Farias, C.A.A.; Dos Reis, A.R.; dos Santos, B.A.; Pinton, M.B.; Corrêa, L.P.; Campagnol, P.C.B.; Dantas, G.A.; Santos, R.C.V.; et al. Synergistic Effects Evaluation of Jabuticaba and Strawberry Extracts on Oxidative Stability of Pork Burgers. Meat Sci. 2025, 219, 109685. [Google Scholar] [CrossRef] [PubMed]
- Fracari, P.R.; Tomasevic, I.; Massia, A.G.; Laroque, D.A.; Balzan, M.M.; dos Santos, B.A.; Cichoski, A.J.; Wagner, R.; Carciofi, B.A.M.; Campagnol, P.C.B. Pulsed Light and Jabuticaba Peel Extract for Nitrite Reduction and Quality Enhancement in Sliced Mortadella. Meat Sci. 2025, 224, 109777. [Google Scholar] [CrossRef]
- Coelho, V.S.; Aguiar, L.L.; Grancieri, M.; Lourenço, J.M.P.; Braga, D.P.; Saraiva, S.H.; Costa, A.G.V.; Silva, P.I. Incorporation of Microencapsulated Polyphenols from Jabuticaba Peel (Plinia spp.) into a Dairy Drink: Stability, In Vitro Bioaccessibility, and Glycemic Response. Food Res. Int. 2024, 189, 114567. [Google Scholar] [CrossRef]
- dos Santos, B.A.; da Fontoura, A.M.; Correa, L.P.; Pinton, M.B.; Padilha, M.; Fracari, P.R.; Ribeiro, S.R.; Wagner, R.; Cichoski, A.J.; Barin, J.S.; et al. Jabuticaba Peel Extract and Nisin: A Promising Combination for Reducing Sodium Nitrite in Bologna-Type Sausages. Meat Sci. 2023, 204, 109273. [Google Scholar] [CrossRef]
- Gabriel da Rosa, R.; Sganzerla, W.G.; Barroso, T.L.C.T.; Buller, L.S.; Berni, M.D.; Forster-Carneiro, T. Sustainable Production of Bioactive Compounds from Jabuticaba (Myrciaria cauliflora): A Bibliometric Analysis of Scientific Research over the Last 21 Years. Sustain. Chem. Pharm. 2022, 27, 100656. [Google Scholar] [CrossRef]
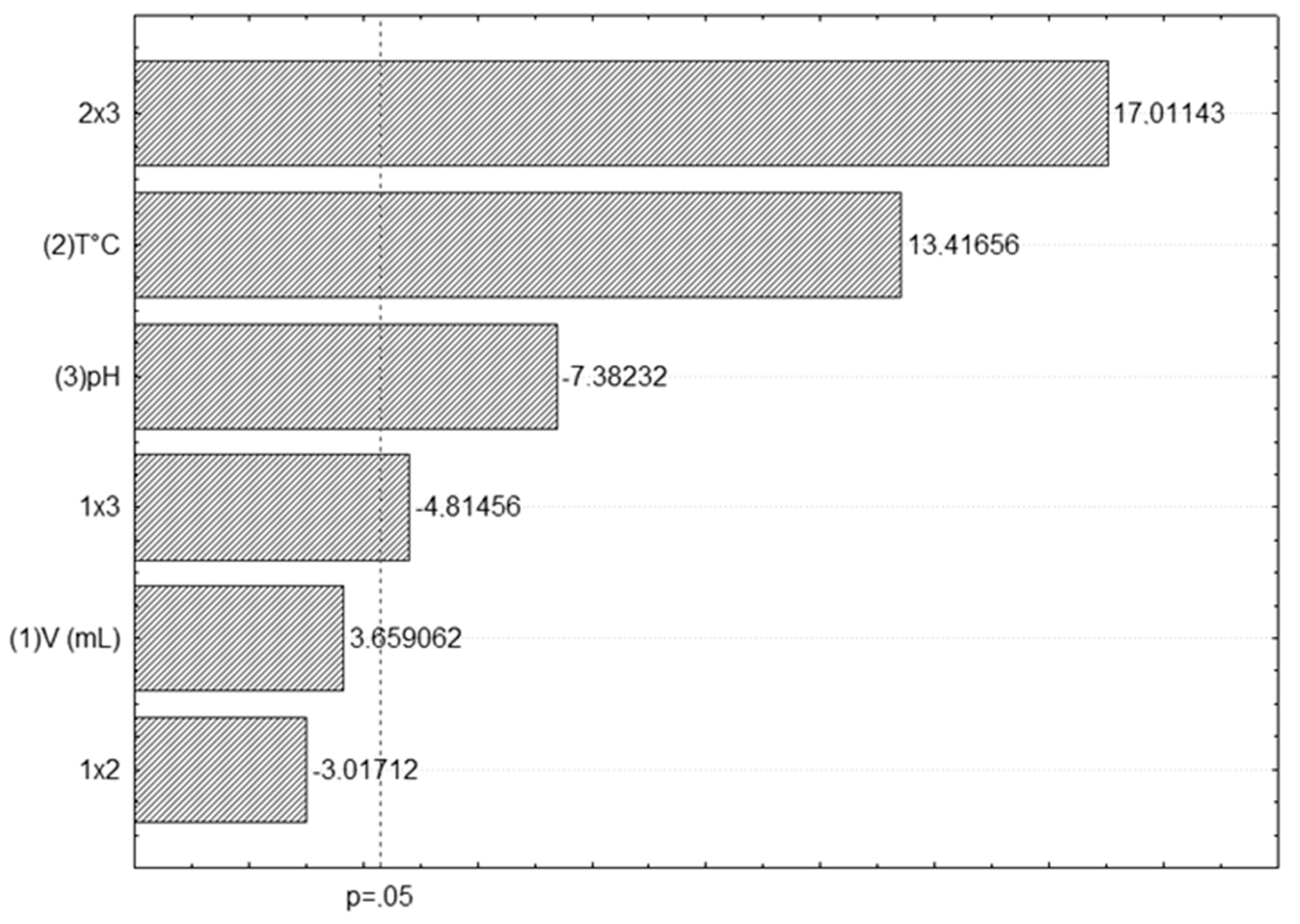
| Tests | Independent Variables | Total ANCs (mg/100 g) | ||
|---|---|---|---|---|
| Solvent (mL) | Temperature (°C) | pH | ||
| 1 | (−1) 100 | (−1) 14 | (−1) 1 | 115 |
| 2 | (+1) 250 | (−1) 14 | (−1) 1 | 161 |
| 3 | (−1) 100 | (+1) 40 | (−1) 1 | 114 |
| 4 | (+1) 250 | (+1) 40 | (−1) 1 | 134 |
| 5 | (−1) 100 | (−1) 14 | (+1) 3 | 40 |
| 6 | (+1) 250 | (−1) 14 | (+1) 3 | 46 |
| 7 | (−1) 100 | (+1) 40 | (+1) 3 | 169 |
| 8 | (+1) 250 | (+1) 40 | (+1) 3 | 154 |
| 9 | (0) 175 | (0) 27 | (0) 2 | 155 |
| 10 | (0) 175 | (0) 27 | (0) 2 | 150 |
| 11 | (0) 175 | (0) 27 | (0) 2 | 161 |
| Tests | Independent Variables | Total ANCs (mg/100 g) | |
|---|---|---|---|
| pH | Temperature (°C) | ||
| 1 | −1 (1) | −1 (22) | 203.52 |
| 2 | +1 (3) | −1 (22) | 151.33 |
| 3 | −1 (1) | +1 (50) | 328.13 |
| 4 | +1 (3) | +1 (50) | 218.96 |
| 5 | 0 (2) | 0 (36) | 233.78 |
| 6 | 0 (2) | 0 (36) | 220.84 |
| 7 | 0 (2) | 0 (36) | 254.24 |
| Tests | Gum Arabic/Maltodextrin (w/w) * | Total ANCs (mg/100 g) | aw | Moisture (%) |
|---|---|---|---|---|
| 1 | 1:1 | 155.79 d ± 7.52 | 0.25 d ± 0.003 | 7.65 b ± 0.05 |
| 2 | 1:2 | 315.37 a ± 10.55 | 0.27 cd ± 0.009 | 6.95 b ± 0.25 |
| 3 | 1:3 | 241.47 c ± 2.97 | 0.34 b ± 0.004 | 6.95 b ± 0.25 |
| 4 | 1:4 | 297.28 b ± 14.85 | 0.33 b ± 0.004 | 5.95 b ± 0.05 |
| 5 | 2:1 | 80.21 f ± 4.21 | 0.27 c ± 0.005 | 6.10 b ± 0.30 |
| 6 | 3:1 | 72.52 f ± 1.39 | 0.40 a ± 0.006 | 9.95 a ± 1.85 |
| 7 | 4:1 | 131.63 e ± 5.12 | 0.41 a ± 0.009 | 9.70 a ± 0.10 |
| Tests | Gum Arabic/Maltodextrin (w/w) | L* | a* | b* |
|---|---|---|---|---|
| 1 | 1:1 | 66.36 a ± 1.23 | 39.40 b ± 2.19 | 8.46 a ± 1.40 |
| 2 | 1:2 | 66.82 a ± 2.16 | 42.50 a ± 0.82 | 7.41 abc ± 0.89 |
| 3 | 1:3 | 67.67 a ± 1.09 | 42.19 ab ± 1.21 | 6.80 abc ± 0.53 |
| 4 | 1:4 | 68.25 a ± 1.92 | 39.89 b ± 0.28 | 5.69 c ± 0.89 |
| 5 | 2:1 | 71.15 a ± 0.98 | 37.21 bc ± 0.57 | 7.00 abc ± 0.27 |
| 6 | 3:1 | 68.18 a ± 2.27 | 34.47 c ± 0.83 | 6.22 b ± 0.16 |
| 7 | 4:1 | 63.50 a ± 3.41 | 33.42 c ± 0.74 | 6.10 b ± 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauletto, F.B.; Hentz, R.; Oro, C.E.D.; Borgmann, C.; Camargo, S.; Dallago, R.M.; Cansian, R.L.; Tres, M.V.; Valduga, E.; Paroul, N. Extraction and Spray Drying-Based Encapsulation of Anthocyanin Pigments from Jabuticaba Sabará Peel (Myrciaria jaboticaba (Vell.) O. Berg). Processes 2025, 13, 2490. https://doi.org/10.3390/pr13082490
Pauletto FB, Hentz R, Oro CED, Borgmann C, Camargo S, Dallago RM, Cansian RL, Tres MV, Valduga E, Paroul N. Extraction and Spray Drying-Based Encapsulation of Anthocyanin Pigments from Jabuticaba Sabará Peel (Myrciaria jaboticaba (Vell.) O. Berg). Processes. 2025; 13(8):2490. https://doi.org/10.3390/pr13082490
Chicago/Turabian StylePauletto, Fernanda B., Renata Hentz, Carolina E. Demaman Oro, Caroline Borgmann, Sabrina Camargo, Rogério M. Dallago, Rogério L. Cansian, Marcus V. Tres, Eunice Valduga, and Natalia Paroul. 2025. "Extraction and Spray Drying-Based Encapsulation of Anthocyanin Pigments from Jabuticaba Sabará Peel (Myrciaria jaboticaba (Vell.) O. Berg)" Processes 13, no. 8: 2490. https://doi.org/10.3390/pr13082490
APA StylePauletto, F. B., Hentz, R., Oro, C. E. D., Borgmann, C., Camargo, S., Dallago, R. M., Cansian, R. L., Tres, M. V., Valduga, E., & Paroul, N. (2025). Extraction and Spray Drying-Based Encapsulation of Anthocyanin Pigments from Jabuticaba Sabará Peel (Myrciaria jaboticaba (Vell.) O. Berg). Processes, 13(8), 2490. https://doi.org/10.3390/pr13082490







