Simulation of the Adsorption Bed Process of Activated Carbon with Zinc Chloride from Spent Coffee Grounds for the Removal of Parabens in Treatment Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Information
2.2. Physicochemical Properties of Parabens
2.3. Simulations in Aspen Adsorption®
2.3.1. Mass/Momentum Balance
2.3.2. Kinetic Model
2.3.3. Adsorption Isotherm
2.3.4. Column Performance Study
3. Results and Discussion
3.1. Physical and Chemical Properties of Parabens
3.2. Adsorption Column Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowak, K.; Ratajczak-Wrona, W.; Górska, M.; Jabłońska, E. Parabens and Their Effects on the Endocrine System. Mol. Cell. Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef]
- Tsubouchi, L.M.S.; de Almeida, E.A.; Santo, D.E.; Bona, E.; Pereira, G.L.D.; Jegatheesan, V.; Cardozo-Filho, L.; Peron, A.P.; Junior, O.V. Production and Characterization of Graphene Oxide for Adsorption Analysis of the Emerging Pollutant Butylparaben. Water 2024, 16, 3703. [Google Scholar] [CrossRef]
- Correa-Navarro, Y.M.; Rivera-Giraldo, J.D.; Cardona-Castaño, J.A. Modified Cellulose for Adsorption of Methylparaben and Butylparaben from an Aqueous Solution. ACS Omega 2024, 9, 30224–30233. [Google Scholar] [CrossRef]
- Giulivo, M.; Lopez de Alda, M.; Capri, E.; Barceló, D. Human Exposure to Endocrine Disrupting Compounds: Their Role in Reproductive Systems, Metabolic Syndrome and Breast Cancer. A Review. Environ. Res. 2016, 151, 251–264. [Google Scholar] [CrossRef]
- Boberg, J.; Taxvig, C.; Christiansen, S.; Hass, U. Possible Endocrine Disrupting Effects of Parabens and Their Metabolites. Reprod. Toxicol. 2010, 30, 301–312. [Google Scholar] [CrossRef]
- Chen, M.H.; Yu, B.; Zhang, Z.F.; Ma, W.L. Occurrence of Parabens in Outdoor Environments: Implications for Human Exposure Assessment. Environ. Pollut. 2021, 282, 117058. [Google Scholar] [CrossRef] [PubMed]
- Derisso, C.R.; Pompei, C.M.E.; Spadoto, M.; da Silva Pinto, T.; Vieira, E.M. Occurrence of Parabens in Surface Water, Wastewater Treatment Plant in Southeast of Brazil and Assessment of Their Environmental Risk. Water Air Soil Pollut. 2020, 231, 231–468. [Google Scholar] [CrossRef]
- Ariffin, M.M.; Azmi, A.H.M.; Saleh, N.M.; Mohamad, S.; Rozi, S.K.M. Surfactant Functionalisation of Magnetic Nanoparticles: A Greener Method for Parabens Determination in Water Samples by Using Magnetic Solid Phase Extraction. Microchem. J. 2019, 147, 930–940. [Google Scholar] [CrossRef]
- Han, J.H.; Cui, Y.Y.; Yang, C.X. Tailored Amino/Hydroxyl Bifunctional Microporous Organic Network for Efficient Stir Bar Sorptive Extraction of Parabens and Flavors from Cosmetic and Food Samples. J. Chromatogr. A 2021, 1655, 462521. [Google Scholar] [CrossRef]
- Bolujoko, N.B.; Unuabonah, E.I.; Alfred, M.O.; Ogunlaja, A.; Ogunlaja, O.O.; Omorogie, M.O.; Olukanni, O.D. Toxicity and Removal of Parabens from Water: A Critical Review. Sci. Total Environ. 2021, 792, 148092. [Google Scholar] [CrossRef]
- Rocha, B.C.d.S.; de Moraes, L.E.Z.; Santo, D.E.; Peron, A.P.; Souza, D.C.d.; Bona, E.; Valarini, O. Removal of Bentazone Using Activated Carbon from Spent Coffee Grounds. J. Chem. Technol. Biotechnol. 2024, 99, 1342–1355. [Google Scholar] [CrossRef]
- do Nascimento, R.F.; de Lima, A.C.A.; Vidal, C.B.; Melo, D.d.Q.; Raulino, G.S.C. Adsorção: Aspectos Teóricos e Aplicações Ambientais; Fortaleza Imprensa Universitária: Fortaleza, Brazil, 2020; p. 308. [Google Scholar]
- Ribas, F.B.T.; da Silva, W.L. Biosorption: A Review of Promising Alternative Methods in Wastewater Treatment. Rev. Mater. 2022, 27, e13212. [Google Scholar] [CrossRef]
- dos Santos Gonçalves Nascimento, G.C.; da Cunha Barros, D.G.; Ratuchinski, L.S.; Okon, C.; Bressiani, P.A.; Santo, D.E.; Duarte, C.C.S.; Ferreira, P.M.P.; Junior, O.V.; Pokrywiecki, J.C.; et al. Adverse Effects of Octocrylene on Cultivated and Spontaneous Plants and in Soil Animal. Water Air Soil Pollut. 2023, 234, 109688. [Google Scholar] [CrossRef]
- Siregar, C.A.; Siregar, A.M.; Lubis, R.W.; Marpaung, D. Rancang Bangun Mesin Giling Kopi Untuk Menunjang Dan Membuka Unit Usaha Baru Mitra Deli Coffe. ABDI SABHA J. Pengabdi. Kpd. Masy. 2022, 3, 174–180. [Google Scholar] [CrossRef]
- Saberian, M.; Li, J.; Donnoli, A.; Bonderenko, E.; Oliva, P.; Gill, B.; Lockrey, S.; Siddique, R. Recycling of Spent Coffee Grounds in Construction Materials: A Review. J. Clean. Prod. 2021, 289, 125837. [Google Scholar] [CrossRef]
- Atheba, P.; Allou, N.B.; Drogui, P.; Trokourey, A. Adsorption Kinetics and Thermodynamics Study of Butylparaben on Activated Carbon Coconut Based. J. Encapsulation Adsorpt. Sci. 2018, 8, 39–57. [Google Scholar] [CrossRef]
- Moraes, L.E.Z.d.; Marcoti, F.A.O.; Lucio, M.A.N.; Rocha, B.C.d.S.; Rocha, L.B.; Romero, A.L.; Bona, E.; Peron, A.P.; Junior, O.V. Analysis and Simulation of Adsorption Efficiency of Herbicides Diuron and Linuron on Activated Carbon from Spent Coffee Beans. Processes 2024, 12, 1952. [Google Scholar] [CrossRef]
- Yousef, R.; Qiblawey, H.; El-Naas, M.H. Evaluation of Activated Carbon Fiber Packed-Bed for the Treatment of Gas-to-Liquid Wastewater: Experimental, Modeling and ASPEN Adsorption Simulation. Emergent Mater. 2024, 8, 1591–1603. [Google Scholar] [CrossRef]
- Rocha, S.A.F.d.; Rocha, B.C.d.S.; Moraes, L.E.Z.d.; Villaça, J.M.P.; Scapin, D.; Santo, D.E.; Gonzalez, R.d.S.; Junior, O.V.; Peron, A.P. Evaluation and Simulation of the Adsorption Capacity of Octocrylene Sunscreen on Commercial Carbon and Biochar from Spent Coffee Beans. Processes 2024, 12, 1249. [Google Scholar] [CrossRef]
- Juela, D.M. Comparison of the Adsorption Capacity of Acetaminophen on Sugarcane Bagasse and Corn Cob by Dynamic Simulation. Sustain. Environ. Res. 2020, 30, 23. [Google Scholar] [CrossRef]
- Bouillot, B.; Teychené, S.; Biscans, B. An Evaluation of Thermodynamic Models for the Prediction of Drug and Drug-like Molecule Solubility in Organic Solvents. Fluid Phase Equilib. 2011, 309, 36–52. [Google Scholar] [CrossRef]
- Tavan, Y.; Hosseini, S.H.; Ahmadi, G.; Olazar, M. Mathematical Model and Energy Analysis of Ethane Dehydration in Two-Layer Packed-Bed Adsorption. Particuology 2019, 47, 33–40. [Google Scholar] [CrossRef]
- Bueno, M.d.l.Á.B.-R.d.H.; Boluda-Botella, N.; Prats Rico, D. Removal of Emerging Pollutants in Water Treatment Plants: Adsorption of Methyl and Propylparaben onto Powdered Activated Carbon. Adsorption 2019, 25, 983–999. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Bono, A.; Krishnaiah, D.; Tan, Y.Z. A Study on Dynamic Simulation of Phenol Adsorption in Activated Carbon Packed Bed Column. J. King Saud Univ.-Eng. Sci. 2016, 28, 47–55. [Google Scholar] [CrossRef]
- Danaci, D.; Webley, P.A.; Petit, C. Guidelines for Techno-Economic Analysis of Adsorption Processes. Front. Chem. Eng. 2020, 2, 602430. [Google Scholar] [CrossRef]
- USACE. Design, Construction, and Operation Small Wastewater Systems; EM 1110-2-501; USACE Publications-Engineer Manuals: Washington, DC, USA, 1999. [Google Scholar]
- Rietveld, L.; Van Der Helm, A.; Van Schagen, K.; Van Der Aa, R.; Van Dijk, H. Integrated Simulation of Drinking Water Treatment. J. Water Supply Res. Technol.-AQUA 2008, 57, 133–141. [Google Scholar] [CrossRef][Green Version]
- Lakshmipathy, R.; Sarada, N.C. A Fixed Bed Column Study for the Removal of Pb2+ Ions by Watermelon Rind. Environ. Sci. Water Res. Technol. 2015, 1, 244–250. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Balsamo, M.; Montagnaro, F. Liquid-Solid Mass Transfer in Adsorption Systems-An Overlooked Resistance? Ind. Eng. Chem. Res. 2020, 59, 22007–22016. [Google Scholar] [CrossRef]
- Biswas, S.; Sharma, S.; Mukherjee, S.; Meikap, B.C.; Sen, T.K. Process Modelling and Optimization of a Novel Semifluidized Bed Adsorption Column Operation for Aqueous Phase Divalent Heavy Metal Ions Removal. J. Water Process Eng. 2020, 37, 101406. [Google Scholar] [CrossRef]
- Zhang, Y.; Sivakumar, M.; Yang, S.; Enever, K.; Ramezanianpour, M. Application of Solar Energy in Water Treatment Processes: A Review. Desalination 2018, 428, 116–145. [Google Scholar] [CrossRef]
- Luukkonen, T.; Pehkonen, S.O. Peracids in Water Treatment: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1–39. [Google Scholar] [CrossRef]
- Teodosiu, C.; Gilca, A.F.; Barjoveanu, G.; Fiore, S. Emerging Pollutants Removal through Advanced Drinking Water Treatment: A Review on Processes and Environmental Performances Assessment. J. Clean. Prod. 2018, 197, 1210–1221. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- El-Sayed Abdel-Raouf, M.; Maysour, N.E.; Kamal Farag, R.; Mahmoud Abdul-Raheim, A.-R. Wastewater Treatment Methodologies, Review Article. Int. J. Environ. Agric. Sci. 2019, 3, 018. [Google Scholar]
- Dhote, J.; Ingole, S.; Chavhan, A. Review on Wastewater Treatment Technologies. Int. J. Eng. Res. Technol. 2012, 1, 111–126. [Google Scholar]
- Yoom, H.; Shin, J.; Ra, J.; Son, H.; Ryu, D.; Kim, C.; Lee, Y. Transformation of Methylparaben during Water Chlorination: Effects of Bromide and Dissolved Organic Matter on Reaction Kinetics and Transformation Pathways. Sci. Total Environ. 2018, 634, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Ji, F.; Wang, W.; Wang, Q.; Hu, Z.; Yuan, S. Chlorination of Parabens: Reaction Kinetics and Transformation Product Identification. Environ. Sci. Pollut. Res. 2016, 23, 23081–23091. [Google Scholar] [CrossRef] [PubMed]
- López-Timoner, R.; Duarte-Alvarado, V.; Castillo, M.Á.; Santos-Juanes, L.; Arques, A.; Amat, A.M. Parabens and Methylisotiazolinone (MIT): Preservatives with Different Behaviors When Subjected to Ozone and Ultraviolet Light Treatments. Water 2023, 15, 3837. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. The Removal of Heavy Metals in a Packed Bed Column Using Immobilized Cassava Peel Waste Biomass. J. Ind. Eng. Chem. 2015, 21, 635–643. [Google Scholar] [CrossRef]
- Soriano, A.N.; Orfiana, O.N.; Pangon, M.B.J.; Nieva, A.D.; Adornado, A.P. Simulated Biosorption of Cd(II) and Cu(II) in Single and Binary Metal Systems by Water Hyacinth (Eichhornia crassipes) Using Aspen Adsorption®. ASEAN J. Chem. Eng. 2016, 16, 21–43. [Google Scholar] [CrossRef]

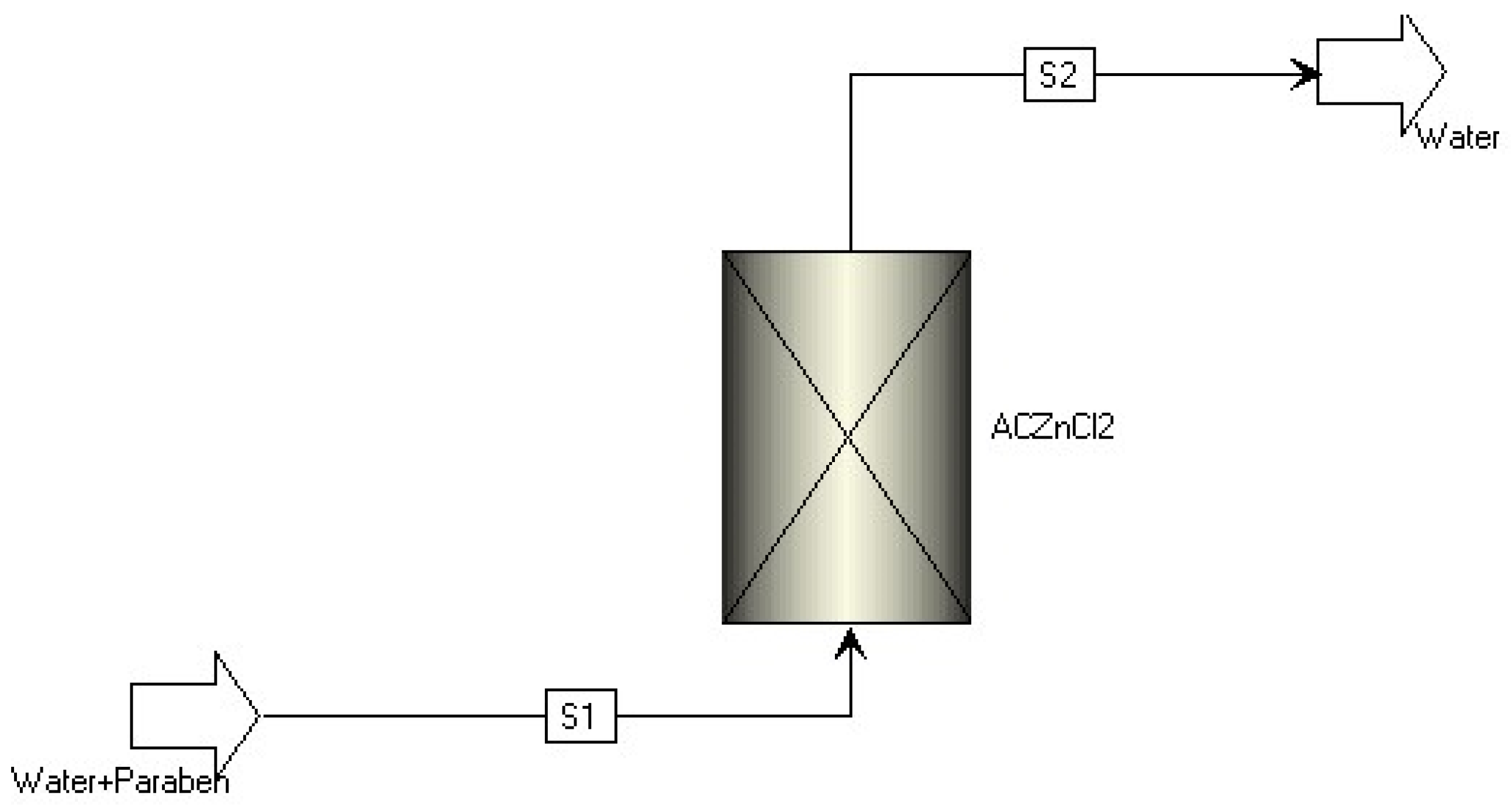
| Property | Value | References |
|---|---|---|
| Concentration paraben—mg/L | 50 and 100 | Variable |
| Concentration water—kmol/m3 | 55.55 | Fixed |
| Height of adsorbent layer—(Hb) m | 3 and 4 | Variable |
| Particle radius—(RP) m | 8.47 × 10−8 | Fixed |
| Pore radius—(rp) m | 1.11 × 10−9 | Fixed |
| Diameter of adsorbent layer—(dp) m | 1.69 × 10−7 | Fixed |
| Inter-particle voidage— m3∙void/m3∙bed | 0.50 | Fixed |
| Particle porosity— | 0.42 | Fixed |
| Bulk density kg/m3 | 740 | Fixed |
| Parameters-Unit | MeP | EtP | PrP | BuP |
|---|---|---|---|---|
| Sc | 0.0119 | 0.0112 | 0.0106 | 0.0101 |
| Dm-m2/s | 8.41 × 10−5 | 8.94 × 10−5 | 9.47 × 10−5 | 9.97 × 10−5 |
| Dk-m2/s | 4.37 × 10−7 | |||
| Dp-m2/s | 3.37 × 10−7 | 3.37 × 10−7 | 3.37 × 10−7 | 3.38 × 10−7 |
| Ki-s−1 | 1.48 × 108 | |||
| IP1-mmol/g | 1.10 × 10−3 | 1.00 × 10−3 | 8.38 × 10−4 | 3.87 × 10−5 |
| IP2-L/mol | 8.22 × 105 | 8.97 × 105 | 4.02 × 106 | 1.81 × 104 |
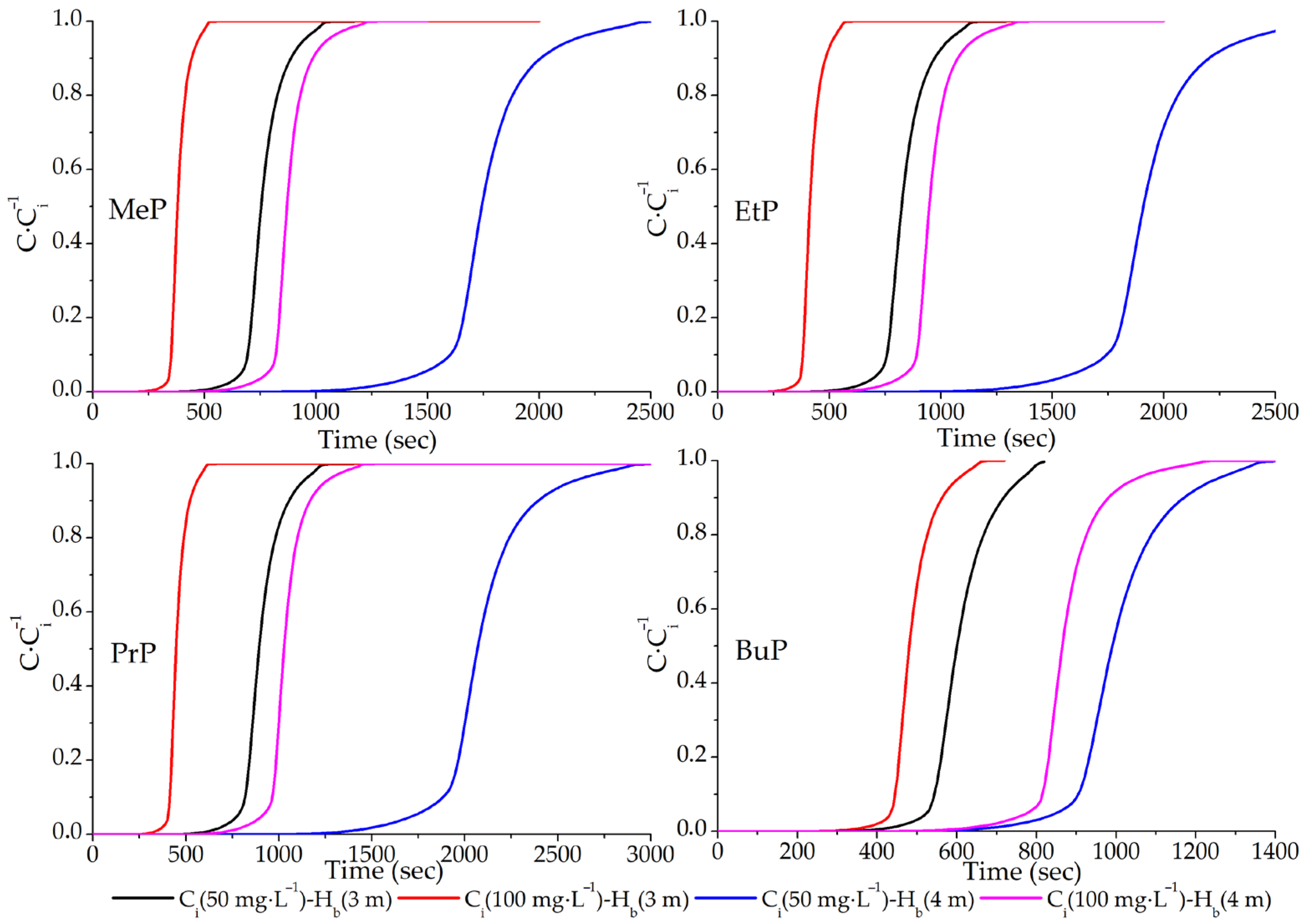
| Parabens | Ci mg/L | Hb m | tbreak min | tsat min | Veff m3 | qmax mg | qe mg/g |
|---|---|---|---|---|---|---|---|
| MeP | 50 | 3 | 8.6 | 16.6 | 0.0387 | 3.869 | 0.77 |
| 100 | 3 | 5.2 | 8.8 | 0.0206 | 2.057 | 0.41 | |
| 50 | 4 | 18.2 | 41.5 | 0.0968 | 9.683 | 1.94 | |
| 100 | 4 | 10.5 | 20.5 | 0.0478 | 4.783 | 0.96 | |
| EtP | 50 | 3 | 9.5 | 25.5 | 0.0595 | 5.950 | 1.19 |
| 100 | 3 | 5.3 | 12.9 | 0.0301 | 3.006 | 0.60 | |
| 50 | 4 | 10.2 | 40.2 | 0.0937 | 9.372 | 1.87 | |
| 100 | 4 | 10.8 | 22.5 | 0.0525 | 5.250 | 1.05 | |
| PrP | 50 | 3 | 8.3 | 20.8 | 0.0486 | 4.861 | 0.97 |
| 100 | 3 | 4.7 | 10.4 | 0.0242 | 2.419 | 0.48 | |
| 50 | 4 | 19.2 | 47.7 | 0.1112 | 11.122 | 2.22 | |
| 100 | 4 | 10.1 | 24.2 | 0.0564 | 5.639 | 1.13 | |
| BuP | 50 | 3 | 7.1 | 13.6 | 0.0318 | 3.181 | 0.64 |
| 100 | 3 | 5.9 | 10.8 | 0.0253 | 2.528 | 0.51 | |
| 50 | 4 | 10.9 | 22.8 | 0.0533 | 5.328 | 1.07 | |
| 100 | 4 | 10.2 | 20.7 | 0.0482 | 4.822 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, W.V.; Santos, A.R.D.; Tractz, G.T.; Bonfim-Rocha, L.; Peron, A.P.; Junior, O.V. Simulation of the Adsorption Bed Process of Activated Carbon with Zinc Chloride from Spent Coffee Grounds for the Removal of Parabens in Treatment Plants. Processes 2025, 13, 2481. https://doi.org/10.3390/pr13082481
Martins WV, Santos ARD, Tractz GT, Bonfim-Rocha L, Peron AP, Junior OV. Simulation of the Adsorption Bed Process of Activated Carbon with Zinc Chloride from Spent Coffee Grounds for the Removal of Parabens in Treatment Plants. Processes. 2025; 13(8):2481. https://doi.org/10.3390/pr13082481
Chicago/Turabian StyleMartins, Wagner Vedovatti, Adriele Rodrigues Dos Santos, Gideã Taques Tractz, Lucas Bonfim-Rocha, Ana Paula Peron, and Osvaldo Valarini Junior. 2025. "Simulation of the Adsorption Bed Process of Activated Carbon with Zinc Chloride from Spent Coffee Grounds for the Removal of Parabens in Treatment Plants" Processes 13, no. 8: 2481. https://doi.org/10.3390/pr13082481
APA StyleMartins, W. V., Santos, A. R. D., Tractz, G. T., Bonfim-Rocha, L., Peron, A. P., & Junior, O. V. (2025). Simulation of the Adsorption Bed Process of Activated Carbon with Zinc Chloride from Spent Coffee Grounds for the Removal of Parabens in Treatment Plants. Processes, 13(8), 2481. https://doi.org/10.3390/pr13082481










