Abstract
The genus Anisosciadium, belonging to the Apiaceae family, has been traditionally recognized for its anti-inflammatory, antioxidant, and antimicrobial properties. However, scientific research on this genus is still limited, highlighting the need for a comprehensive review of its chemical composition and pharmacological characteristics. A comprehensive compilation of data was conducted using major databases such as Google Scholar, Research Gate, Web of Science, Scopus, and ScienceDirect. In this review, we collected and organized the available information of identified compounds from different species of the genus Anisosciadium, covering the literature from 2003 to 2024. In total, 64 phytoconstituents were detected. The findings suggest that the traditional therapeutic properties of Anisosciadium are well supported by the reported pharmacological activities from previous studies. Notably, these studies highlight its antioxidant, antibacterial, and cytotoxic effects, emphasizing the potential of this genus in the development of new therapeutic agents. Nonetheless, the lack of comparative studies among Anisosciadium species and the scarcity of in vivo studies and clinical trials limit the full realization of its therapeutic potential. Specifically, comparative studies could be crucial in identifying species with unique chemical profiles and understanding how variations in secondary metabolite compositions may influence their pharmacological activities.
1. Introduction
Herbal medicine has gained significant popularity over the past few decades due to its ease of use, availability, affordability, and minimal or absence of side effects [1]. Natural compounds found in medicinal plants serve as an invaluable source of bioactive molecules, contributing to the discovery of effective new drugs for treating various ailments due to their ability to interact selectively with specific proteins. In contrast to synthetic compounds, natural substances tend to exhibit greater structural complexity and molecular rigidity, which enhance their potent and specific biological interactions. Additionally, the high content of oxygen and carbon atoms in natural products, coupled with lower levels of halogens and nitrogen, suggests a favorable pharmacokinetic profile. This combination leads to improved bioavailability and fewer adverse effects. It is estimated that over 50% of the medicines approved in the 21st century are directly or indirectly derived from natural products, underscoring the importance of exploring the world’s flora for new therapeutics [2]. Ethnopharmacology, the discipline that studies the traditional use of medicinal plants, has become an essential tool in the quest for new drugs. The correlation between traditional uses and modern pharmacological activities is noteworthy; one study found that 80% of plant-derived compounds used therapeutically align with their ethnopharmacological applications. This finding emphasizes the validity of traditional knowledge as a foundation for pharmacological research and highlights the importance of systematically documenting and analyzing ancestral medicinal practices [2]. With the growing importance of ethnopharmacological studies in the discovery of new medications, the potential of Anisosciadium species is often underestimated. The Anisosciadium genus is part of the Apiaceae family, one of the largest and oldest families of aromatic plants. This genus is well-known for its distinctive flavor, which arises from secretory cavities containing schizogenous oil ducts filled with oil, resin, or mucilage. These cavities can be found in the stems, leaves, roots, and fruits of the plants [3].
This paper aims to provide a comprehensive review of the ethnopharmacological statements, chemical compositions, and biological activities of extracts and compounds isolated from various species within this genus. Therefore, this review seeks to enhance our understanding of these medicinal plants, laying a solid foundation for future research that could lead to the discovery of new therapeutic agents derived from Anisosciadium.
2. Methodology
2.1. Study Design
This review was structured according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to ensure transparency, reproducibility, and methodological rigor. It aims to systematically gather and synthesize published data regarding the taxonomy, traditional uses, phytochemistry, and pharmacological activities of species belonging to the genus Anisosciadium.
2.2. Data Sources and Search Strategy
A systematic literature search was performed in the PubMed, Scopus, Science Direct, Web of Science, and Google Scholar databases to retrieve articles published between January 2003 and April 2024. The search included the following terms and Boolean operators: “Anisosciadium” OR “Anisosciadium lanatum” OR “Anisosciadium orientale” OR “Anisosciadium isosciadium” AND (“ethnobotany” OR “traditional use” OR “phytochemistry” OR “essential oil” OR “antioxidant” OR “antimicrobial” OR “cytotoxic”).
Manual searches of the reference lists of relevant articles were also conducted to identify additional studies.
2.3. Eligibility Criteria
The following inclusion criteria were applied to select relevant papers:
- -
- Studies discussing the taxonomic classification of the Apiaceae family, the Anisosci adium genus, and the species Anisosciadium lanatum, Anisosciadium orientale, and Anisosciadium isosciadium, including their synonyms. Credible global databases such as Plants of the World Online (POWO) and the World Flora Online Plant List (WFO) were consulted to obtain accurate information on the different species of the Anisosciadium genus.
- -
- Studies examining the traditional uses of Anisosciadium species in various practices, including cooking, health, and cosmetic applications.
- -
- Studies providing detailed information on the phytochemical compositions, properties, and bioactive compounds isolated from Anisosciadium species.
- -
- Articles investigating the biological and pharmacological activities of Anisosciadium species, including both in vitro and in vivo studies.
- -
- Taxonomic considerations regarding the genus Anisosciadium, which is classified in the tribe Echinophoreae, subfamily Apioideae, family Apiaceae, order Apiales, clade Asterids, and clade Eudicots [4,5,6].
The following exclusion criteria were applied:
- -
- Non-English publications;
- -
- Reviews or meta-analyses not presenting new data;
- -
- Studies not directly related to Anisosciadium species;
- -
- Abstracts, theses, patents, or book chapters without peer review.
2.4. Selection Process
All records were independently screened by two reviewers based on the titles and abstracts. Full-text articles were then assessed for eligibility. Any discrepancies were resolved through discussion or by consulting a third reviewer. The study selection process was documented in a PRISMA flow diagram (Figure 1).
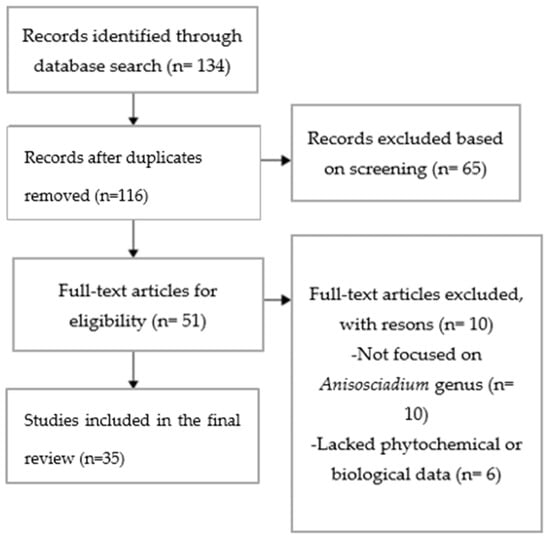
Figure 1.
PRISMA flow diagram.
2.5. Data Extraction and Analysis
For each included article, the following relevant data were extracted into a standardized table:
- -
- Authors, year of publication;
- -
- Country of study;
- -
- Studied species;
- -
- Part of the plant used;
- -
- Extraction methods;
- -
- Identified phytochemicals;
- -
- Documented pharmacological activities;
- -
- Reported traditional uses.
Due to the heterogeneity of experimental designs, a qualitative narrative synthesis was conducted. When applicable, comparative tables were constructed to visualize differences in chemical compositions and biological effects among species.
3. Taxonomic Considerations for the Genus Anisosciadium
3.1. Apiaceae Family
The Apiaceae family, formerly known as Umbelliferae, is a large and taxonomically complex family that includes 434 genera and approximately 3780 species [7]. It was highlighted by various researchers that the classification of the Apiaceae has primarily focused on fruit morphology [8,9,10,11,12,13,14]. One prominent classification system was proposed by Drude in 1898 [13], which divides the family into three subfamilies: Apioideae, Saniculoideae, and Hydrocotyloideae. This system is further delineated into 12 tribes and 15 subtribes.
The Apiaceae family is mainly found in temperate regions and includes several well-known vegetables and herbs, such as Apium graveolens L. (celery), Foeniculum vulgare (fennel), and Centella asiatica L. (Indian pennywort) [15,16]. Many members of this family exhibit various biological activities, such as antibacterial, hepatoprotective, cyclooxygenase inhibitory, anti-inflammatory, and anti-tumor properties. Most species within the Apiaceae family are generally considered safe for consumption, including Anethum graveolens (dill), Anthriscus cerefolium (chervil), and Carum carvi (caraway) [5]. Additionally, numerous species are recognized as significant sources of essential oils (EOs), such as Peucedanum ruthenicum M. Bieb. and Grammosciadium platycarpum Boiss [17].
3.2. Apioideae Subfamily
The Apioideae subfamily consists of 400 genera and 2900 species [18]. The first classification of Apioideae, based on molecular phylogenetic evidence, was presented by Downie et al. [19]. They divided the subfamily into ten monophyletic tribes: Aciphylleae (M.F. Watson & S.R. Downie), Bupleureae (Spreng.), Careae (Baill.), Echinophoreae (Benth. & Hook. f.), Heteromorpheae (M.F. Watson & S.R. Downie), Oenantheae (Dumort.), Pleurospermeae (M.F. Watson & S.R. Downie), Pyramidoptereae (Boiss.), Smyrnieae (Spreng.), and Scandiceae (Spreng.). A key morphological characteristic of the subfamily is the arrangement of flowers, which are typically grouped in terminal simple or, more commonly, compound umbels. Members of this subfamily can be annual or perennial, ranging from dwarf forms to those reaching up to 3 m in height. Most are herbaceous and may be monocarpic or polycarpic. The fruits are composed of two fused carpels that split at maturity into two mericarps, each containing a single seed [20]. Notable and economically significant members include carrot (Daucus carota L. subsp. sativus), celery (Apium graveolens L.), coriander (Coriandrum sativum L.), cumin (Cuminum cyminum L.), and parsley (Petroselinum crispum (Mill.) Fuss) [21].
3.3. Echinophoreae Tribe
The Echinophoreae tribe is characterized by unisexual and hermaphroditic sessile flowers arranged in central ultimate umbels [19,22,23]. A crucial diagnostic feature of this tribe is the fusion of all bracts, flowers, and mericarps in the ultimate umbels [24,25].
3.4. Anisosciadium Genus
The genus Anisosciadium comprises three species. The first species is Anisosciadium isosciadium Bornm., commonly known as besbes. Its synonyms include Dicyclophora caucalioides Velen., Anisosciadium isosciadium var. idumaeum zohary, Chamaesciadium caucalioides (Velen.) M. Hiroe, and Echinophora isolophme (Bornm. ex Dinsm.) M. Hiroe. The second species is named Anisosciadium orientale DC; the vernacular name is Zeret besbes [26]. The local name for the plant in Iran is “Khaliloo” [27]. Its synonyms include Dicyclophora morphologica Velen., as listed in Mem. Soc. Sci. Boheme 1921–22, No. 6, 5 (1923) [28]; Chamaesciadium morphologicum (Velen.) M. Hiroe, Umbelliferae World 996 (1979) [28]; and Echinophora orientale (DC.) M. Hiroe, Umbelliferae World 649 (1979) [28]. The third species is Anisosciadium lanatum Boiss, locally known as “besbes om thariss.” Its synonym is Echinosciadium arabicum Zohary [29].
4. Geographical Distribution of Anisosciadium Genus
4.1. Geographical Distribution of Anisosciadium lanatum Boiss
A. lanatum Boiss. is an accepted species that was first described in “Flora Orientalis, Supplement” on page 261 in 1888. Its native range extends from Iraq to the Arabian Peninsula, including countries such as Kuwait and Saudi Arabia. This species is annual and predominantly grows in desert or dry shrubland biomes (Figure 2) [30,31,32,33,34,35,36].
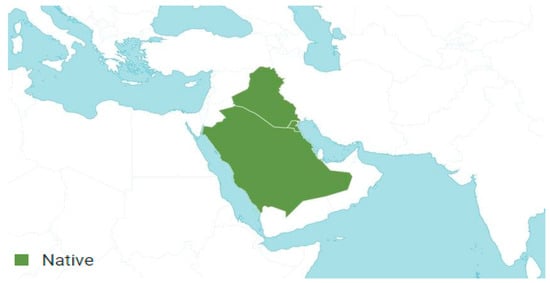
Figure 2.
Geographical distribution of Anisosciadium lanatum Boiss. according to Plants of the World Online [37].
4.2. Geographical Distribution of Anisosciadium orientale DC
A. orientale is an accepted species that was first published in Prodr. 4: 234 in 1830 [28]. Its native range includes Syria, Lebanon, Afghanistan, Kuwait, Iraq, and Iran. This species is an annual plant and primarily grows in subtropical biomes (Figure 3) [24,32,33].
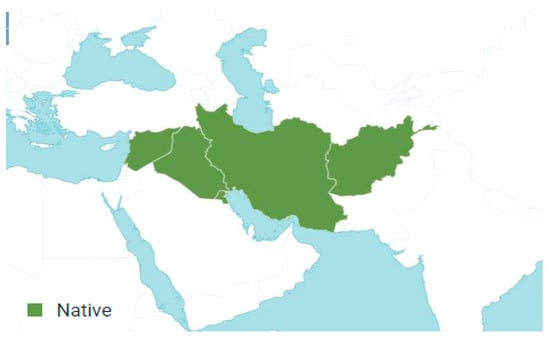
Figure 3.
Geographical distribution of Anisosciadium orientale DC. according to Plants of the World Online [38].
4.3. Geographical Distribution of Anisosciadium isosciadium Bornm
A. isosciadium was accepted and first published in Repert. Spec. Nov. Regni Veg. 10: 468 (1912) [32]. This species is native to Syria, Lebanon, Iraq, Saudi Arabia, and Palestine. It is an annual plant that primarily grows in subtropical regions (Figure 4) [32,33].
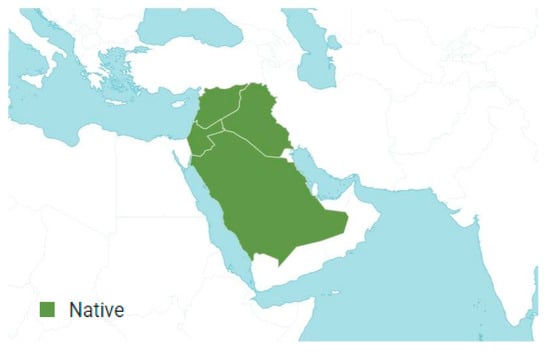
Figure 4.
Geographical distribution of Anisosciadium isosciadium Bornm. according to Plants of the World Online [39].
5. Descriptions of the Species of the Genus Anisosciadium
5.1. Anisosciadium lanatum Boiss
A. lanatum (Figure 5) is an annual herb and chamaephyte [40]. It typically flowers from March to April and thrives in sandy desert plains at an altitude of 75 m [29]. This species grows following the winter rainfall [41]. The morphology of the leaves is either bi- or tri-pinnatisect, decreasing in size towards the top. They are triangular-ovate in outline, with lanceolate segments that are densely covered in short whitish hairs. The inflorescences have umbel branches that are flattened and become somewhat broader and turbinate below the partial umbel when the fruit ripens. The umbels are opposite the leaves, usually with about seven radiating rays. The rays and bracts of the involucre, especially when young, are characterized by long white hairs [42]. The flowers are small with pinkish-white petals. The bracts feature spiny heads. The fruits of each secondary navel initially aggregate before maturity but later separate into spiny units [35,36,43,44]. Pollen from this species is perprolate, with a polar axis measuring 29.68 μm and an equatorial axis measuring 13.45 μm. The outline of the pollen is oblong with rounded poles, featuring striate-rugulose ornamentation. The apertures are di-ectocolpate [45].

Figure 5.
Anisosciadium lanatum Boiss as depicted in (A) [36] and (B) [46].
5.2. Anisosciadium orientale DC
A. orientale (Figure 6) is a therophyte [47]. This annual herbaceous plant has a grooved stem and rigid, sulcate, glabrous, or downy branches that branch dichotomously from about halfway. The leaves are dill-shaped, arranged alternately, and are often sheathed [48]. The leaf surface is glabrous or nearly so [49]. The morphology of the inflorescence is characterized by umbel branches that become broad and flattened when the fruit is ripe. The turbinate base is located below the partial umbel. The umbels are positioned opposite the leaves, typically numbering between three to five, and they radiate outward. There are three to six involucral bracts that are lanceolate in shape, and those belonging to the involucel grow larger during fruit development, becoming elongated, oblong, and foliose [42]. The flowers are white and typically bisexual. The calyx segments are accrescent and very unequal in size, with the larger ones being oblong-lanceolate and consisting of five contiguous sepals. The fruit is a schizocarp, and the pollen has a perprolate shape with polar axes measuring 30.02 μm and equatorial axes measuring 32.2 μm. The outline of the pollen is oblong with rounded poles, featuring striate-granulate ornamentation. The apertures are di-ectocolpate [45,48,50].

Figure 6.
Anisosciadium orientale DC as depicted in (A) [48] and (B) [49].
5.3. Anisosciadium isosciadium Bornm
A. isosciadium (Figure 7) is an annual plant classified as a therophyte, with a flowering period that occurs between March and April [51]. This species thrives in sandy desert plains at altitudes of around 200 m. The flowers are small, white, and have four petals with yellow stamens. The seeds are small, dark brown, and ovoid in shape, while the seedlings are small, dark green, and feature a single pair of leaves. Propagation of this species can be achieved through seeds or cuttings. For seed propagation, seeds should be sown in a well-draining soil mix at a depth of 1/4 inch and kept moist. Cuttings should be taken during the spring or summer and rooted in a well-draining soil mix. The plants should be placed in a warm, bright location and watered regularly. Notably, the outer row of flowers does not conspicuously radiate, and the accrescent calyx segments are of nearly equal size. The surface of the leaves is covered with abundant short, stiff white hairs [49]. Pollen characteristics show a perprolate shape, with polar axes measuring 30.79 μm and equatorial axes measuring 11.87 μm. The outline of the pollen is oblong with rounded poles, and the ornamentation is described as striate-granulate. The apertures are di-ectocolpate [45].

Figure 7.
Anisosciadium isosciadium Bornm as depicted in (A) [39] and (B) [52].
6. Traditional Medicine
The three species of Anisosciadium have been widely used in traditional medicine across several countries to treat a broad range of ailments, in addition to their applications in food and ornamentation (Table 1).

Table 1.
Ethnotraditional uses of Anisosciadium species.
7. Phytochemical Studies
The phytochemical studies of Anisosciadium species are presented below. All compounds identified in both A. lanatum (A.l) and A. orientale (A.o) are illustrated in Figure 8. In total, 64 different compounds were detected within the Anisosciadium genus; 45 compounds were found in A. lanatum and 34 compounds were found in A. orientale.
7.1. Anisosciadium lanatum
The first study on A. lanatum was published in 2003 by Al-Mazroa [36], who collected this plant from the Riyadh region of Saudi Arabia. The essential oil was extracted from the aerial parts (flowers, leaves, and stems) using steam distillation. The resulting essential oil was light yellow in color and had a pleasant fragrance, with a yield of 1.23% of steam-volatile oil. This oil was subsequently analyzed using a Shimadzu GC-MS-QP 5050 A (Kyoto, Japan) with a DB-1 glass column (30 m, 0.25 mm). Eleven components were identified in the oil: α-pinene (1) (2.47%), ρ-myrcene (2) (1.83%), α-phellandrene (3) (2.20%), ρ-cymene (4) (2.20%), limonene (5) (5.39%), 2-carene (6) (7%), ρ-cymen-8-ol (7) (2.88%), trans-caryophyllene (8) (3.20%), α-farnesene (9) (3.62%), α-humulene (10) (6.41%), and guaiol (11) (2.27%) (Figure 8). A second study on the essential oil of A. lanatum was published in 2022 by Khalil et al. [59]. In this study, the authors collected aerial parts of the plant from Riyadh, Saudi Arabia, in 2014 and extracted the essential oil using a Clevenger-type apparatus. The yield from this extraction was 0.46% by volume per unit of dried plant weight. A total of 38 components were detected and quantified, which accounted for 94.68% of the total composition: α-pinene (1) (10.14%), camphene (12) (4.4%), β-pinene (13) (6.2%), β-myrcene (14) (8.45%), α-phellandrene (3) (0.2%), car-4-ene (15) (5.8%), α-terpinene (16) (3.62%), limonene (5) (6.7%), ρ-cymene (4) (6.58%), terpinolene (17) (2.07%), eucalyptol (18) (6.35%), linalool (19) (4.34%), camphor (20) (3.1%), isoborneol (21) (0.1%), terpinen-4-ol (22) (2.4%), α-terpineol (23) (1.5%), fenchyl acetate (24) (0.1%), nerol (25) (4.3%), bornyl acetate (26) (0.6%), methyl geranate (27) (0.2%), neryl acetate (28) (0.1%), α-copaene (29) (0.9%), β-cubebene (30) (1.3%), trans-caryophyllene (8) (7.25%), trans-α-bergamotene (31) (0.1%), α-guaiene (32) (2.02%), α-humulene (10) (6.45%), β-farnesene (33) (8.25%), germacrene D (34) (2.31%), β-selinene (35) (0.6%), β-bisabolene (36) (0.2%), γ-cadinene (37) (0.2%), caryophyllene oxide (38) (7.25%), α-eudesmol (39) (0.8%), chavicol (40) (0.07%), eugenol (41) (1.5%), methyl eugenol (42) (0.2%), and ethyl isovalerate (43) (0.2%) (Figure 8).
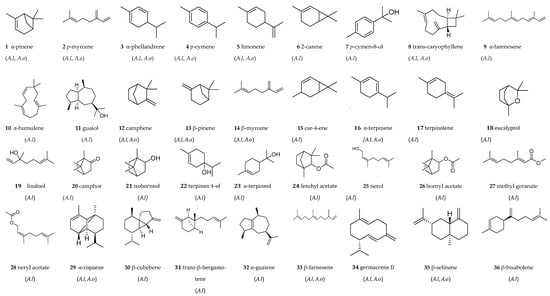
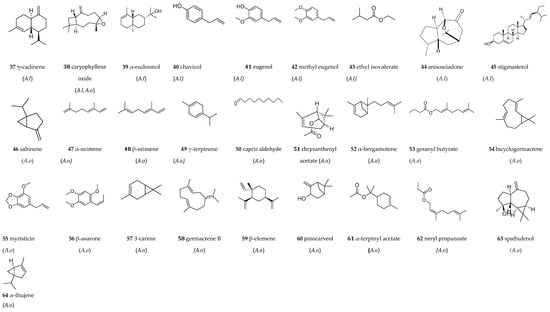
Figure 8.
Chemical compounds identified in the Anisosciadium genus (1–64). A.l: Anisosciadium lanatum, A.o: Anisosciadium orientale.
El-Sayed et al. [6] analyzed the contents of flavonoids, lycopene, chlorophyll a and b, and β-carotene in four different extracts from the aerial parts of A. lanatum collected from the Al-Nayrrian Ajman Valley in Saudi Arabia. The extract with a ratio of CH2Cl2:MeOH:petroleum ether (1:1:1) exhibited a moderate flavonoid content, equivalent to 25 mg of quercetin per gram. In contrast, the H2O:MeOH (1:1) extract, the 100% water extract, and the essential oil showed low flavonoid contents, while the n-Hexane extract was nearly devoid of flavonoids and contained a very low amount of lycopene. Conversely, all other extracts and the essential oil demonstrated moderate levels of lycopene. The CH2Cl2:MeOH:petroleum ether (1:1:1) extract had the highest lycopene content, approximately 41 mg/g, followed by the H2O:MeOH (1:1) extract, which contained 30 mg/g of lycopene. The essential oil also had a significant β-carotene content of 31 mg/g, and this was mirrored in the polar extracts (H2O:MeOH (1:1) with 31 mg/g and H2O 100% with 27 mg/g). These latter two extracts exhibited the highest levels of chlorophyll a and b. Additionally, the researchers isolated a new guaiane sesquiterpene containing rare epoxide structural elements, 10β,11β−epoxy−1α,4β,5β,7αH-guaiane-9-one, anisosciadone (44), and stigmasterol (45), from the methylene chloride and methanol extract of A. lanatum (Figure 8). The isolated compounds were identified using various spectroscopic techniques, including IR, GC-MS, HRFABMS, and 400 MHz 1D and 2D NMR analyses (1H, 13C NMR, DEPT, 1H-1H COSY, HMQC, HMBC, and NOESY) [60]. In another study, the preliminary phytochemical screening was conducted on petroleum ether, dichloromethane, ethyl acetate, butanol, and aqueous fractions derived from the leaves, stems, and flowers of A. lanatum collected in Riyadh, Saudi Arabia [61]. Using an alkaline solution test, the researchers identified the presence of flavonoids in the ethyl acetate, butanol, and aqueous fractions of the stems, leaves, and flowers. A foam test demonstrated the presence of saponins in the ethyl acetate, butanol, and aqueous fractions of the leaves and stems, as well as in the dichloromethane extract.
The same research group, Matar et al. [61], utilized the Liebermann–Burchard test to detect steroids and triterpenoids, which were found in the petroleum ether, dichloromethane, ethyl acetate, and butanol extracts of the flowers and leaves. However, these compounds were only present in the ethyl acetate and butanol extracts of the stems. The presence of carbohydrates was assessed using Molisch’s test, which revealed their presence in the ethyl acetate, butanol, and aqueous fractions of the leaves, stems, and flowers. Tannins were found only in the ethyl acetate and butanol extracts. Notably, cardiac glycosides, alkaloids, and anthraquinones were absent in all tested extracts. The same authors (Matar et al. [61]) also conducted a qualitative study on the total phenolic and flavonoid contents, which were expressed as milligrams of standard gallic acid per gram of dried plant extract (mg GAE/g) and milligrams of rutin per gram of dried plant extract (mg RE/g), respectively. The ethyl acetate and butanol extracts of leaves exhibited the highest total phenolic contents, measuring 44 mg GAE/g and 42 mg GAE/g of dry extract, respectively, followed by the aqueous fraction at 15 mg GAE/g of dry extract. Similarly, the ethyl acetate and butanol extracts were the richest in flavonoid content, with the aqueous extracts also showing notable amounts [61]. Figure 8 includes the chemical structures of the 45 compounds identified in the essential oils and extracts of A. lanatum.
7.2. Anisosciadium orientale
The first study on A. orientale was conducted by Rowshan et al. [62]. The species was collected from wild plants in Fars Province (southern Iran) during May and June of 2011. The aerial parts of the plants and the fruits, harvested at the flowering stage, were air-dried and subjected to hydrodistillation using a Clevenger-type apparatus. The essential oils were analyzed using gas chromatography (GC) and gas chromatography–mass spectrometry (GC-MS). A total of twenty-one compounds were identified in the fruit oil, accounting for 98.9% of the total oil composition, while fifteen compounds, representing 96.5% of the oil, were identified in the aerial parts. The essential oils derived from the fruit and aerial parts of A. orientale contain a variety of volatile compounds, including α-pinene (1) (4.3–2.9%), sabinene (46) (0.2–0.2%), β-pinene (13) (0.2–0.1%), β-myrcene (14) (5–5.9%), α-phellandrene (3) (6.3–4.4%), p-cymene (4) (0.2–0.3%), limonene (5) (19.5–19.7%), α-ocimene (47) (0.2–0.3%), β-ocimene (48) (1–1.6%), γ-terpinene (49) (0.9–1%), α-terpinolene (17) (22–25.8%), capric aldehyde (50) (0.5–0.3%), trans-caryophyllene (8) (0.5–0.2%), bicyclogemacrene (54) (1.4–0.1%), and myristicin (55) (33.5–33.7%). Germacrene-D (34) (1.9%), chrysanthenyl acetate (51) (0.1%), α-bergamotene (52) (0.1%), geranyl butyrate (53) (0.8%), and β-asarone (56) (0.2%) were only detected in the fruit oil. Another research group from Iran investigated the essential oil of A. orientale using the same method. They identified sixteen compounds that accounted for 92.86% of the total components in the oil. The major constituents included myristicin (55) (30.07%), α-terpinolene (17) (24.54%), and limonene (5) (20.99%) [56]. Additionally, Izadi et al. [63] analyzed the essential oil of A. lanatum collected from Iran using GC-MS. Their results revealed the presence of fourteen compounds, including α-pinene (1) (6.58%), β-pinene (13) (0.24%), 3-carene (57) (21.02%), α-phellandrene (3) (4.26%), limonene (5) (40.93%), β-ocimene (48) (0.67%), γ-terpinene (49) (1%), β-elemene (59) (0.19%), trans-caryophyllene (8) (0.41%), E-β-farnesene (33) (0.2%), germacrene D (34) (2.19%), β-selinene (35) (0.16%), germacrene B (58) (1.03%), and caryophyllene oxide (38) (1.52%). Masroorbabanari et al. [27] conducted a study on the essential oil of A. orientale, which was also collected from Iran.
The oil was analyzed using gas chromatography–flame ionization detection (GC-FID) and gas chromatography–mass spectrometry (GC-MS). A total of fifteen compounds were identified in the oil through GC retention indices and mass spectral analysis. Notably, this essential oil was rich in myristicin (55) (30.6%), limonene (5) (21%), α-terpinolene (17) (19.7%), germacrene D (34) (6.1%), p-myrcene (2) (5.6%), α-pinene (1) (3.2%, β-ocimene (48) (1.3%), and γ-terpinene (49) (1.2%). The minor compounds found in this oil were α-phellandrene (3) (0.7%), β-pinene (13) (0.6%), bicyclogermacrene (54) (0.6%), pinocarveol (60) (0.5%), trans-caryophyllene (8) (0.5%), sabinene (46) (0.1%), and β-ocimene (48) (0.04%).
In this study, the total phenol contents of the methanol and 80% methanol extracts, as well as those of the essential oil, were determined using the Folin–Ciocalteu method. The results are expressed as milligrams of gallic acid equivalents (EG) per gram of essential oil (EO) or plant material (PM). The findings revealed that the total phenol content of the essential oil was 8.5 ± 0.2 mg EG/g EO, while the methanol extract contained 10.3 ± 0.5 mg EG/g PM, and the 80% methanol extract had 15.9 ± 0.2 mg EG/g PM.
A study conducted in 2024 by Ghani and Mohtashami [57] analyzed the essential oil composition of various parts of A. orientale (flower, leaf, fruit, and stem) in Iran, identifying limonene as the predominant compound (5) (56.29%), and also identifying the presence of p-myrcene (2) (14.80%), α-pinene (1) (11.10%), p-cymene (4) (5.79%), terpinolene (17) (3.78%), myristicin (55) (2.56%), p-cymen-8-ol (7) (1.02%), α-thujene (64) (0.21%), camphene (12) (0.29%), sabinene (47) (0.42%), β-pinene (13) (0.55%), α-phellandrene (3) (0.40%), β-ocimene (48) (0.55%), γ-terpinene (49) (0.11%), chrysanthenyl acetate (51) (<0.05%), α-terpinyl acetae (61) (<0.05%), α-copaene (29) (0.21%), trans-caryophyllene (8) (0.30%), α-bergamotene (52) (<0.05%), neryl propanoate (62) (0.44%), germacrene D (34) (0.44%), β-selinene (35) (0.14%), bicyclogermacrene (54) (0.19%), spathulenol (63) (0.21%), and caryophyllene oxide (38) (0.14%). When comparing different parts of this plant, the flowers exhibited the highest concentration of essential oil at 1.01%. The greatest amounts of flavones and flavonols were found in the flowers, followed by the leaves and stems, with a measurement of 0.49 mg of quercetin equivalent per gram of dry sample. The fruit sample had the lowest concentration of these compounds at just 0.02 mg. In terms of total flavonoids, the fruit sample again stood out with a content of 99.82 mg of quercetin equivalent per gram of dry sample. The fruit also contained 24.72 mg of phenolic compounds and 420.23 mg of carbohydrates per gram of dry sample. Among the various organs of the plant, the leaves had the highest tannin content at 2.09 mg of catechin equivalent per gram of dry sample, followed by the flowers at 1.45 mg and the stems at 1.25 mg. The lowest tannin concentration was found in the fruit at 0.6 mg of catechin equivalent per gram of dry sample. Additionally, the fruit sample demonstrated the highest carbohydrate content, with 420.23 mg of glucose equivalent per gram of dry sample, while the leaves contained 280.67 mg of glucose equivalent per gram of dry sample [57]. Figure 8 includes the chemical structures of the 34 compounds identified in the essential oils of A. orientale.
7.3. Anisosciadium isosciadium
Currently, no research has been published regarding the chemical composition of A. isosciadium.
8. Biological and Pharmacological Properties
The Anisosciadium genus has garnered significant scientific interest due to its broad spectrum of pharmacological activities, including antibacterial, anti-inflammatory, antioxidant, anticancer, antimutagenic, and insecticidal potential. Figure 9 and Table 2 provide a visual overview of the principal biological properties attributed to Anisosciadium extracts, as reported in recent studies.
Figure 9.
Schematic of the biological activities of the Anisosciadium genus.

Table 2.
Summary of the biological and pharmacological activities of the Anisosciadium genus.
8.1. Anti-Proliferative Activity
El-Sayed et al. [6] demonstrated the anti-proliferative effects of various extracts from A. lanatum. The extracts tested included hexane (100%); a mixture of methylene chloride, methanol, and petroleum ether; a combination of water and methanol; and water alone against several cell lines: rat hepatic H4IIE1, human colon HT-29, human breast MCF-7, human lung, and human prostate cells. The combination of methylene chloride, petroleum ether, and methanol, along with the methanol and water mixture, exhibited the strongest anticancer effects, achieving a growth inhibition 50 (GI50) at concentrations as low as 80–90 ng against lung, liver, prostate, and breast cell lines. The 100% hexane extract was less effective, with a GI50 ranging from 120 to 860 ng. Notably, the water extract inhibited the growth of colon cells at a very low dose of 60 ng. The hexane extract, as well as the mixtures of methylene chloride with methanol and petroleum ether and water with methanol, showed total growth inhibition of breast, liver, and lung cell lines at concentrations between 420 and 810 ng. These two mixtures also affected prostate cells similarly, with total growth inhibition values recorded at 560 ng and 480 ng, respectively. The aqueous extract demonstrated a total growth inhibition of 380 ng. In a study by Mahmoud and El-Sayed [60], the anti-proliferative effects of isolated compounds from A. lanatum were tested against various human cell lines, including HepG2, MCF7, HT29, and PC3. Among these compounds, anisosciadone showed significant anti-proliferative activity specifically against liver, colon, and lung cells. Stigmasterol, on the other hand, exhibited substantial activity against liver, colon, and breast cells. Importantly, neither of these constituents caused cytotoxicity in normal fibroblasts. The compound anisosciadone increased the expression and activity of Caspase 3 and p53 in HepG2 cells, while having no effect on Caspase 9. Additionally, it resulted in approximately 50% downregulation of cdk1 expression. In a 2022 study by Khalil et al. [59], the cell viability of A. lanatum essential oil was tested in the NIH-3T3 fibroblast cell line, resulting in an IC50 of 52.1 µg/mL. Use of this essential oil led to increased cell proliferation and viability, with insignificant inhibition up to a concentration of 25 µg/mL. Furthermore, the same study demonstrated the anti-proliferative activity of this volatile oil in hepatoma HepG2 liver cancer cells, resulting in an IC50 of 11.3 µg/mL, which was linked to the regulation of the apoptotic markers BCL-2 and CASPASE-3.
Regarding A. orientale, the cytotoxic activities of essential oil, methanol, and 80% methanol extracts were assessed against three human cancer cell lines: K562 (human chronic myelogenous leukemia), LS180 (human colon adenocarcinoma), and MCF-7 (human breast adenocarcinoma). The IC50 values for the essential oil were 396.8 ± 64 µg/mL, 183.2 ± 42.8 µg/mL, and 159.5 ± 29.0 µg/mL, respectively. In contrast, the plant extracts showed no significant activity [27].
8.2. Antioxidant Activity
El-Sayed et al. [6] conducted tests to evaluate the metal chelating activities and free radical scavenging activities of various extracts from A. lanatum. They utilized the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method to assess these activities alongside total antioxidant activity, superoxide scavenging activity, and peroxide scavenging activity. The extracts tested included hexane, a mixture of methylene chloride + methanol + petroleum ether, a mixture of water + methanol, water, and essential oil samples. The hexane extract demonstrated the highest metal chelating activity, comparable to that of the reference product tocopherol. This was followed by the water/methanol and the methylene chloride + methanol + petroleum ether extracts. In terms of free radical scavenging activity, the methylene chloride + methanol + petroleum ether extract, along with the water/methanol extract, exhibited the highest levels, with the aqueous extract and essential oil following. Notably, the hexane extract showed the lowest activity. The water/methanol extract displayed a significant total antioxidant activity, equivalent to approximately 101 μg Trolox/g of extract. It also demonstrated noteworthy peroxide and superoxide scavenging activities. All A. lanatum samples tested in the study showed peroxide scavenging activities greater than that of Trolox, with the essential oil revealing the highest activity at about 99%, compared to Trolox’s 32%. Furthermore, the water/methanol and aqueous extracts exhibited the highest superoxide scavenging activities, measuring 91.7 ± 9.4% and 99.7 ± 9.4%, respectively. Matar et al. [61] investigated the petroleum ether, dichloromethane, ethyl acetate, n-butanol, and aqueous fractions of the leaves, flowers, and stems of A. lanatum. The results revealed moderate ABTS scavenging activities across all samples, with IC50 values ranging from 13.83 ± 1.9 μg/mL to 39.03 ± 1.0 μg/mL. These values were compared to the positive standard, ascorbic acid, which had an IC50 of 5.97 ± 0.7 μg/mL. The ethyl acetate, n-butanol, and aqueous fractions from the leaves showed the best antioxidant results, followed by the extracts from the stems and flowers. In terms of the second species, A. orientale, only one study on its antioxidant activity was found. Jassbi et al. [56] assessed the antioxidant activity using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay on oil, methanol, 80% methanol, and dichloromethane extracts of A. orientale. The 80% methanol extract exhibited the highest antioxidant activity. Furthermore, Masroorbabanari et al. [27] also evaluated the antioxidant properties of the essential oil, methanol extract, and 80% methanol extract using the DPPH method and reported that the IC50 values were as follows: essential oil = 633.2 ± 0.2 µg EO/mL, methanol extract = 400 ± 0.2 µg plant material/mL DPPH, and 80% methanol extract = 220 ± 0.0 µg plant material/mL DPPH. Lastly, Ghani et al. [57] examined the antioxidant activity of various parts of A. orientale using 70% methanol extracts of leaves, flowers, fruits, and stems with the DPPH method. They found that the flower extract had the best antioxidant activity (90%), followed by the leaves (80%), fruits (72%), and stems extracts (31.70%).
8.3. Anti-Inflammatory Activity
The methanol extracts of the leaves, stems, and flowers of A. lanatum demonstrated significant lipoxygenase inhibition activity, with IC50 values of 4.88, 5.40, and 6.05 μg/mL, respectively [61].
8.4. Antimutagenic Activity
El-Sayed et al. [6] evaluated the extracts using the Ames test in Salmonella typhimurium against two mutagens: sodium azide (NaN3) and benzo(a)pyrene (B[a]P). The hexane, dichloromethane/methanol/petroleum ether, and water/methanol extracts exhibited significant antimutagenic activity against NaN3 in a dose-dependent manner that was independent of the exposure conditions. The water sample also showed strong antimutagenic activity (≥40%) but only under pre-exposure conditions at high concentrations, where the extract was incubated with the bacteria before adding NaN3. All extracts tested in this study demonstrated important antimutagenic activity against B[a]P under both exposure regimens investigated in a concentration-independent manner.
8.5. Antibacterial Activity
The antibacterial activities of the volatile oil and the methanol, 80% methanol, and dichloromethane extracts of A. orientale have been evaluated using the micro-dilution method against a Gram-positive bacterium, Bacillus subtilis, and a Gram-negative bacterium, Escherichia coli. Among these, the methanolic extract exhibited the highest antibacterial activity, while the volatile oil showed no detectable antibacterial effect [56]. In a separate study by El-Sayed et al. [6], the viability of Salmonella typhimurium was tested in the presence of hexane, a solvent mixture of dichloromethane, methanol, and petroleum ether, as well as water and 100% water extracts of A. lanatum using nutrient agar plates. Not all extracts affected bacterial viability at concentrations of 5 or 10 mg/mL; however, at a concentration of 20 mg/mL, significant reductions in bacterial viability were observed. The essential oil and the mixture of dichloromethane, methanol, and petroleum ether extract were the most effective, reducing bacterial viability by 94% and 93%, respectively.
8.6. Ovicidal Activity
The acaricidal, persistent, and ovicidal activities of methanolic extracts from the leaves of A. orientale were evaluated against Tetranychus urticae Koch (the two-spotted spider mite) in a laboratory bioassay. The results indicated that this extract caused a mortality rate of 23.16%. In persistence tests, mortality rates significantly varied at 1, 24, 48, and 72 h after treatment, with the highest mortality occurring one hour post-treatment. Additionally, the ovicidal effect was substantially greater than that of the control group, with an egg mortality rate of 41.40% [64].
9. Discussion
In recent years, natural treatments derived from herbs, plants, and other biological materials have gained significant attention due to their structural diversity and broad pharmacological properties. One notable example is the Anisosciadium genus, which includes species such as A. lanatum, A. orientale, and A. isosciadium. Both A. lanatum and A. orientale are recognized as promising sources of bioactive compounds, emphasizing their potential for various therapeutic applications, particularly due to their richness in essential oils.
In fact, 64 compounds were identified in the Anisosciadium genus. Two studies have examined the chemical composition of the essential oil from A. lanatum collected in Saudi Arabia and identified 43 intriguing compounds. A third study mainly focused on isolating two pure compounds from this species. Additionally, five studies analyzed the essential oil composition of A. orientale sourced from different regions in Iran, detecting 34 compounds. Limonene is a major common compound found in all studies of Anisosciadium essential oils. This volatile and aromatic monoterpene is known for its diverse biological activities. Limonene exhibits remarkable anticancer, antidiabetic, and gastroprotective properties. Its anti-inflammatory and antioxidant effects help combat oxidative stress, while its antinociceptive and antihyperalgesic properties provide pain relief. Moreover, limonene demonstrates antiviral capabilities, underscoring its potential in the prevention and treatment of infectious diseases [65]. Importantly, limonene also aids in cancer prevention and does not pose risks of cancer, kidney damage, and mutation in humans [66]. α-Pinene, a significant monoterpene identified in various studies on Anisosciadium, has demonstrated a range of beneficial effects, including cytoprotective, gastroprotective, neuroprotective, anxiolytic, and anticonvulsant properties [67]. Other common compounds found in Anisosciadium species include myrcene, α-phellandrene, trans-caryophyllene, β-pinene, β-myrcene, β-farnesene, and caryophyllene. Myristicin, which is exclusively found in A. orientale, is the major compound in the essential oil of A. orientale, as analyzed in studies by Rowshan et al. [62], Jassbi et al. [56], and Masroorbabanari et al. [27]. This phenylpropanoid boasts a remarkable array of biological benefits, including anti-inflammatory, antimicrobial, anticarcinogenic, analgesic, antioxidant, antidiabetic, insecticidal, and hepatoprotective properties [68,69]. Traditionally valued for its healing potential, myristicin has been used to address various gastrointestinal and respiratory issues, showcasing its versatility in promoting health and well-being.
This review highlights the significance of the genus Anisosciadium in ethnopharmacology, emphasizing its diverse array of bioactive compounds with considerable therapeutic potential for future medicinal applications. A comparative study of the chemical compositions and pharmacological activities of different species reveals significant differences that underscore their unique therapeutic potentials. By examining these differences, we can develop deeper insight into how they may influence efficacy in medical applications and guide targeted treatment strategies. For instance, the extract from A. lanatum leaves is notable for its high levels of phenolic compounds and flavonoids, contributing to its impressive antioxidant activity [61]. Additionally, the essential oil from A. orientale flowers exhibited the strongest antioxidant activity and presented the highest flavonoid content [64]. In the case of A. orientale, there is a significant connection between its traditional ethnopharmacological applications, the documented biological activities, and the chemical composition found in various studies. Traditionally, this plant has been used for addressing gastrointestinal issues and respiratory ailments, as well as for alleviating toothaches and epilepsy. The antibacterial properties observed in the essential oil and extracts of A. orientale may support their use in managing digestive disorders. Furthermore, its antioxidant properties align with its traditional role in alleviating respiratory issues, which are often linked to oxidative stress. Several studies suggest that the high concentration of myristicin in this species may contribute to its ovicidal properties. In contrast, A. isosciadium species have demonstrated ethnobotanical uses but lack phytochemical and biological studies. Cancer is a leading cause of death worldwide, prompting significant efforts to develop both synthetic and natural therapies. Natural treatments derived from plants and herbs are gaining attention due to their diverse chemical structures and pharmacological properties. Several extracts, pure compounds, and essential oils derived from Anisosciadium species have been tested against various cell lines, including rat hepatic H4IIE1, human colon HT-29, human breast MCF-7, human lung cancer cells, human prostate PC3, human hepatoblastoma Hep G2, human chronic myelogenous leukemia, and human colon adenocarcinoma.
The cytotoxic mechanism of 10β, 11β−epoxy−1α, 4β, 5β, 7αH-guaiane-9-one, anisosciadone isolated from A. lanatum has been studied and shown to elevate the expression and activity of Caspase 3, as well as p53 expression, without affecting Caspase 9 in HepG2 cells. In another study, the essential oil from the same species demonstrated anti-proliferative activity in HepG2 liver cancer cells through the regulation of apoptotic markers BCL-2 and CASPASE-3. Additionally, the essential oil of A. orientale exhibited significant cytotoxic activity against chronic myelogenous leukemia, colon adenocarcinoma, and MCF-7 cancer cells.
10. Conclusions and Perspectives
In conclusion, the Anisosciadium genus shows considerable potential as a source of various secondary metabolites. This review highlights gaps in the current literature. To fully unlock the therapeutic potential of Anisosciadium species, future research should focus on less-explored domains and use advanced methodologies. Investigating its secondary metabolites, such as alkaloids and phenolic compounds, utilizing modern chromatographic techniques like GC-MS, HPLC, and LC-MS could reveal novel bioactive compounds that contribute to its anticancer, antimicrobial, anti-inflammatory, and antioxidant properties. All current research on Anisosciadium mainly depends on in vitro studies.
Although these studies are informative, they provide limited understanding of the complex interactions that occur within living organisms. Future research should integrate thorough in vivo experiments to confirm these findings, focusing on the safety, efficacy, and pharmacodynamic and pharmacokinetic profiles of phytoconstituents derived from Anisosciadium.
Author Contributions
Conceptualization, M.B., A.H. and H.C.; Methodology, M.B., A.H., W.B.A.-S. and H.C.; Software, M.B., A.A.A., A.H. and D.A., Validation, H.C., A.A.A. and H.B.J., Resources, W.B.A.-S. and D.A., Writing—original draft preparation, M.B., A.H. and H.C.; Writing-review and editing, M.B., A.H. and H.B.J.; Supervision, H.B.J.; Project administration, M.B. and H.B.J.; Funding acquisition. M.B., H.C., W.B.A.-S., D.A., A.A.A. and H.B.J. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that financial support was received for the research and publication of this review. This research was supported by the University of Ha’il, grant number RG- 24 081.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research has been funded by the Scientific Research Deanship at the University of Ha’il—Saudi Arabia through project number <<RG-24 081>>.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| mg/mL | milligram per milliliter |
| GAE/g | Galic Acid Equivalent per gram |
| mg RE/g | milligram Rutin Equivalent per gram |
| GC-MS | Gas Chromatography-Mass Spectroscopy |
| GC-FID | Gas Chromatography-Flame Ionization Detection |
| PM | Plant Material |
| H4IIE1 | Rat hepatic cancer cells |
| HT-29 | Human colon cancer cells |
| MCF-7 | Human breast cancer cells |
| A549 | Human lung cancer cells |
| PC3 | Human prostate cancer cells |
| GI50 | Growth Inhibition 50 |
| ng | Nanogram |
| HepG2 | Hepatoblastoma cell line |
| NIH-3T3 | Fibroblast cell line |
| IC50 | Half maximal inhibitory concentration |
| K562 | Lymphoblast cells |
| LS180 | Human colon adenocarcinoma |
| h | hour |
| DPPH | 2-Dipheny1-1-picryhydrazy1 |
| µg/mL | microgram per mL |
References
- McKenna, D.J.; Jones, K.; Hughes, K.; Tyler, V.M. Botanical Medicines: The Desk Reference for Major Herbal Supplements, 2nd ed.; Routledge: New York, NY, USA, 2012. [Google Scholar]
- Fabricantd, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar]
- Sayed-Ahmad, B.; Thierry, T.; Zeinab, S.; Akram, H.; Othmane, M. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Ghahraman, A. Flora de L’Iran; Research Institute of Forest and Rangeland: Tehran, Iran, 1997; Volume 16, No 1970. [Google Scholar]
- Amiri, M.S.; Joharchi, M.R. Ethnobotanical knowledge of Apiaceae family in Iran: A review. Avicenna J. Phytomedicine 2016, 6, 6–62. [Google Scholar]
- El-Sayed, W.M.; Hussin, W.A.; Mahmoud, A.A.; AlFredan, M.A. Antimutagenic activities of Anisosciadium lanatum extracts could predict the anticancer potential in different cell lines. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 197–206. [Google Scholar] [CrossRef][Green Version]
- Calvino, C.I.; Teruel, F.E.; Downie, S.R. The role of the southern hemisphere in the evolutionary history of Classification of Apioideae in Iran 107 Apiaceae, a mostly north temperate plant family. J. Biogeogr. 2016, 43, 398–409. [Google Scholar] [CrossRef]
- Bentham, G. Umbelliferae. In Genera Plantarum; Bentham, G., Hooker, J.D., Eds.; Reeve: London, UK, 1867; Volume I, pp. 859–931. [Google Scholar]
- Boissier, P.E. Echinophoreae Umbelliferae. In Flora Orientalis; Boissier, P.E., Ed.; H. Georg (Genevae et Basiliae): Geneve, Switzerland, 1872; Volume 2, pp. 819–1090. [Google Scholar]
- Calestani, V. Conspectus specierum europaearum generis Peucedani. Bull. Soc. Bot. Ital. Ann. 1905, 1905, 193–201. [Google Scholar]
- De Candolle, A.P. Umbelliferae. In Prodromus Systematis Naturalis Regni Vegetabilis; de Candolle, A.P., Ed.; Sumptibus Sociorum Treuttel et Würtz: Paris, France, 1830; Volume 4, pp. 55–250. [Google Scholar]
- Cerceau-Larrival, M.T. Plantules et pollens d’ombelliferes. Leur intérêt systematique et phylogenique. Mem. Mus. Nat. Hist. Nat. Ser. B Bot. 1962, 14, 1–166. [Google Scholar]
- Drude, C.G.O. Umbelliferae. In Die Natürlichen Pflanzenfamilien; Engler, A., Prantl, K., Eds.; Verlag von Wilhelm Engelmann: Leipzig, Germany, 1898; Volume 3, pp. 63–250. [Google Scholar]
- Koso-Poljansky, B.M. Sciado phytorum systematis lineamenta: Mantissa prior. Bull. Soc. Nat. Mosc 1916, 30, 277–290. [Google Scholar]
- Maulidiani, M.; Abas, F.; Khatib, A.; Shaari, K.; Lajis, N. Chemical characterization and antioxidant activity of three medicinal Apiaceae species. Ind. Crops Prod. 2014, 55, 238–247. [Google Scholar] [CrossRef]
- Maggi, F.; Barboni, L.; Papa, F.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Vittori, S. A forgotten vegetable (Smyrnium olusatrum L., Apiaceae) as a rich source of isofuranodiene. Food Chem. 2012, 135, 2852–2862. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of Innovation in Health and Disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef]
- Pimenov, M.G.; Leonov, M.V.E. The Genera of the Umbelliferae: A Nomenclator, 2nd ed.; Royal Botanic Gardens: London, UK, 1993; pp. 8–21. [Google Scholar]
- Downie, S.R.; Plunkett, G.M.; Watson, M.F.; Spalik, K.; Katz-Downie, D.S.; Valiejo-Roman, C.M.; Terentieva, E.I.; Troitsky, A.V.; Lee, B.Y.; Lahham, J.; et al. Tribes and clades within Apiaceae subfamily Apioideae: The contribution of molecular data. Edinb. J. Bot. 2001, 58, 301–330. [Google Scholar] [CrossRef]
- Simpson, M.G. Plant Systematics, 2nd ed.; Elsevier Science Publishing Co. Inc.: Amsterdam, The Netherlands, 2011; p. 360. [Google Scholar]
- Mousavi, S.; Mozaffarian, V.; Mummenhoff, K.; Downie, S.R.; Zarre, S. An updated lineage-based tribal classification of Apiaceae subfamily Apioideae with special focus on Iranian genera. Syst. Biodivers. 2020, 19, 89–109. [Google Scholar] [CrossRef]
- Downie, S.R.; Katz-Downie, D.S.; Watson, M.F. A Phylogeny of the Flowering Plant Family Apiaceae Based on Chloroplast DNA rpl16 and rpoC1 Intron Sequences: Towards a Suprageneric Classification of Subfamily Apioideae. Am. J. Bot. 2000, 87, 273–292. [Google Scholar] [CrossRef]
- Valiejo-Roman, C.M.; Terentieva, E.I.; Samigullin, T.H.; Pimenov, M.G.; Ghahremani- Nejad, F.; Mozaffarian, V. Molecular data (nrITS- sequencing) reveal relationships among Iranian endemic taxa of the Umbelliferae. Feddes Repert. 2006, 117, 367–388. [Google Scholar] [CrossRef]
- Hedge, I.C.; Lamond, J.M.; Rechinger, K.H. Flora Iranica; Akademische Druck-u. Verlagsanstalt: Graz, Austria, 1987; Volume 162, pp. 1–555. [Google Scholar]
- Mozaffarian, V. Flora of Iran; Umbelliferae; Research Institute of Forests and Rangelands: Tehran, Iran, 2007; Volume 54. [Google Scholar]
- Boukezoula, F.; Chenikher, H.; Smaali, S.; Boughanbouz, I.; Soualmia, D. Ethnopharmacological survey of medicinal plants used in the traditional treatment of gastrointestinal disorders in a region of eastern algeria (tebessa). Phytothérapie 2022, 20, 72–79. [Google Scholar] [CrossRef]
- Masroorbabanari, M.; Miri, R.; Firuzi, O.; Jassbi, A.R. Antioxidant and Cytotoxic Activities of the Essential Oil and Extracts of Anisosciadium orientale. J. Chem. Soc. Pak. 2014, 36, 457–461. [Google Scholar]
- Khajehpiri, M.; Saeidi, H.; La Farge, C.; Ghahremaninejad, F. Typification notes on Iranian Apiaceae (tribe Echinophoreae). Rostaniha 2022, 23, 238–251. [Google Scholar] [CrossRef]
- Abdullah, M.T.; Al-Dosari, M.E. Vegetation of the State of Kuwait; IUCN and Kuwait, State of Kuwait, Environment Public Authority: Safat, Kuwait, 2022. [Google Scholar]
- Collenette, S. Wildflowers of Saudi Arabia; Camacho-Corona, M.R., Ed.; National Commission for Wildlife Conservation and Development (NCWCD), Kingdom of Saudi Arabia: Riyadh, Saudi Arabia, 1999; pp. 1–799. [Google Scholar]
- Daoud, H.S.; Al-Rawi, A. Flora of Kuwait 1: Volume 1 Dicotyledoneae; Kegan Paul: London, UK, 1985; pp. 1–236. [Google Scholar] [CrossRef]
- Daoud, H.S.; Al-Rawi, A. Flora of Iraq; Edmondson, J.R., Ed.; Ministry of Agriculture & Agrarian Reform: Baghdad, Iraq, 2013; Volume 5, pp. 1–349. [Google Scholar]
- Govaerts, R. World Checklist of Seed Plants; MIM: Deurne, Belgium, 1995; Volume 1, pp. 1–483. [Google Scholar]
- Llewellyn, O.A.; Hall, M.; Miller, A.G.; Al-Abbasi, T.M.; Al-Wetaid, A.H.; Al-Harbi, R.J.; Al-Shammari, K.F. Important plant areas in the Arabian peninsula: 4. Jabal Aja. Edinb. J. Bot. 2011, 68, 199–224. [Google Scholar] [CrossRef]
- Sher, H.; Aldosari, A. Ethnobotanical survey on plants of veterinary importance around Al-Riyadh (Saudi Arabia). Afr. J. Pharm. Pharmacol. 2013, 7, 1404–1410. [Google Scholar] [CrossRef]
- Al-Mazroa, S. Chemical constituents of A. Lanatum, H. Tuberculatum, A. Garcini, S. Spinosa, H. Bacciferum, and A. Ludwigh grown in Saudi Arabia. J. Saudi Chem. 2003, 7, 255–258. [Google Scholar]
- Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:837841-1 (accessed on 30 March 2025).
- POWO. Plants of The World Online. Available online: https://powo.science.kew.org/results?q=ANisosciadium%20orientale (accessed on 30 March 2025).
- POWO. Plants of The World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:837840-1 (accessed on 30 March 2025).
- ALSobeai, S.M. Floristic diversity, life form, and plant life chorology at al-fawzan reserve, kingdom of Saudi Arabia. Appl. Ecol. Environ. Res. 2024, 22, 3709–3726. [Google Scholar] [CrossRef]
- Arnulf, H.; Muhammad, A.; Ricardo, E.; Taymā, I. Archaeological Exploration, Palaeoenvironment, Cultural Contacts; Archaeopress Archaeology: Oxford, UK, 2018; pp. 1–278. [Google Scholar] [CrossRef]
- Ghazanfar, S.A. Flora of Iraq; Volume 5 Part 2: Lythraceae to Campanulaceae; Edmondson, J.R., Ed.; Ministry of Agriculture & Agrarian Reform: Baghdad, Iraq, 2014. [Google Scholar]
- Al-Yemeny, M.N.; Al-Yemeny, A. A check list of weeds in Al-kharj area of Saudi Arabia. Pak. J. Biol. Sci. 1999, 2, 7–13. [Google Scholar] [CrossRef]
- Joachim, W.; Kadereit, J.W.; Bittrich, V. The Families and Genera of Vascular Plants; Springer International Publishing AG: Steinhausen, Switzerland, 2018; p. 101. [Google Scholar]
- Al-Newani, H.R.H.; Aliway, S.A.; Aloubaidid, A.E.; Qasim, S.A. Scanning electron microscope of pollen grains of selected species of Iraqi Apiaceae. Innovaciencia Fac. Cienc. Exactas Nat. Agropecu. 2018, 6, 1–5. [Google Scholar] [CrossRef]
- Available online: https://fla7h.com/products/%D8%A8%D8%B0%D9%88%D8%B1-%D9%86%D8%A8%D8%A7%D8%AA-%D8%A7%D9%84%D8%A8%D8%B3%D8%A8%D8%A7%D8%B3-%D8%A7%D9%84%D8%A8%D8%B1%D9%8A-30-%D8%A8%D8%B0%D8%B1%D8%A9-anisosciadium-lanatum (accessed on 30 March 2025).
- Mirhashemi, H.; Moradi, F.; Pourbabaei, H.; Mezbani, A. Changes in flora, life forms and geographical distribution of herbaceous plant species along an altitudinal gradient in oak forests, Iran. Casp. J. Environ. Sci. 2021, 19, 619–627. [Google Scholar]
- Mozaffarian, V. Knowledge of Medicinal and Aromatic Plants of Iran; Farhang Moaser Publishers: Tehran, Iran, 2013; p. 1350. (In Persian) [Google Scholar]
- Townsend, C.C. Notes on Umbelliferae of Iraq: I. Kew Bull. 1964, 17, 427–439. [Google Scholar] [CrossRef]
- Mozaffarian, V. A Dictionary of Iranian Plant Names, 3rd ed.; Farhang Moaser Publishers: Tehran, Iran, 2003; p. 671. (In Persian) [Google Scholar]
- Al-Huqail, A.A.; Al-Harbi, H.F.; Lowaifeer, A.M.; El-Sheikh, M.A.; Assaeed, A.M.; Alsaleem, T.S.; Abd-ElGawad, A.M. Correlation between above ground vegetation composition and soil seed bank of Raudhat desert habitat: A case study of Raudhat Alkhafs, Saudi Arabia. BMC Plant Biol. 2025, 25, 136. [Google Scholar] [CrossRef]
- Available online: https://commons.wikimedia.org/wiki/File:Anisosciadium_isosciadium_kz02.jpg (accessed on 30 March 2025).
- Mandaville, J.P. Bedouin Ethnobotany: Plant Concepts and Uses in a Desert Pastoral World; University of Arizona Press: Tucson, AZ, USA, 2019; p. 355. [Google Scholar]
- Middleditch, B.S. Kuwaiti Plants: Distribution, Traditional Medicine, Pytochemistry, Pharmacology and Economic Value; Elsevier: New York, NY, USA, 1991; p. 10. [Google Scholar]
- Eddouks, M.; Ajebli, M.; Hebi, M. Ethnopharmacological survey of medicinal plants used in Daraa-Tafilalet region (Province of Errachidia), Morocco. J. Ethnopharmacol. 2017, 198, 516–530. [Google Scholar] [CrossRef]
- Jassbi, A.; Masroor, M.; Miri, R.; Firuzi, O.; Javanmardi, N. Composition and biological activities of the essential oil and extracts of Anisosciadium orientale. Res. Pharm. Sci. 2012, 7, 710. [Google Scholar]
- Ghani, A.; Mohtashami, S. Phytochemical Study of Anisosciadium orientale DC.: A Valuable Plant with Medicinal and Nutritional Potentials. Med. Plant Sci. 2024, in press. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Hannan, M.A.; Owayss, A.A.; Engel, M.S. The indigenous honey bees of Saudi Arabia (Hymenoptera, Apidae, Apis mellifera jemenitica Ruttner): Their natural history and role in beekeeping. ZooKeys 2011, 134, 83–98. [Google Scholar] [CrossRef]
- Khalil, H.E.; Ibrahim, H.-I.M.; Darrag, H.M.; Matsunami, K. Insight into Analysis of Essential Oil from Anisosciadium Lanatum Boiss.—Chemical Composition, Molecular Docking, and Mitigation of Hepg2 Cancer Cells through Apoptotic Markers. Plants 2022, 11, 66. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; El-Sayed, W.M. The anti-proliferative activity of anisosciadone: A new guaiane sesquiterpene from Anisosciadium lanatum. Anti-Cancer Agents Med. Chem. 2019, 19, 1114–1119. [Google Scholar] [CrossRef]
- Matar, M.W.; Alqahtani, S.M.N.; Alghafli, D.A.; Altaweel, A.A.; Alasoom, A.J.; Burshed, H.A.; Khalil, H.E. Phytochemical Approach Including Total Phenolic and flavonoid Contents and Evaluation of in vitro ABTS Antioxidant Capacity and Lipoxygenase Inhibition of Anisosciadium lanatum. Pharmacogn. J. 2022, 14, 928–932. [Google Scholar] [CrossRef]
- Rowshan, V.; Hatami, A.; Bahmanzadegan, A.; Yazdani, M. Chemical Constituents of the Essential Oil from Aerial Parts and Fruit of Anisosciadium Orientale. Nat. Prod. Commun. 2012, 7, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Izadi, S.; Farjam, M.H.; Bazregari, F.; Mohammadi, M. Phytochemical analysis of Anisosciadium orientale, Semin Iran. Semin. Org. Chem. Issue 2014, 21, 218. [Google Scholar]
- Ghaderi, S.; Minari, K.; Rowshan, V.; Ghamadyari, M. Toxicity and ovicidal activity of different plant extracts on two spotted spider mite, Tetranychus urticae Koch Acari: Tetranychidae. Arch. Phytopathol. Plant Prot. 2012, 46, 120–126. [Google Scholar] [CrossRef]
- Vieira, J.N.; Gonçalves, C.L.; Villarreal, J.P.V.; Gonçalves, V.M.; Lund, R.G.; Freitag, R.A.; Nascente, P.S. Chemical composition of essential oils from the Apiaceae family, cytotoxicity, and their antifungal activity in vitro against candida species from oral cavity. Braz. J. Biol. 2018, 79, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. D-Limonene Safety and Clinical Applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.L.D.; Jayaweera, S.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Chieng, T.C.; Assim, Z.B.; Fasihuddin, B.A. Toxicity and antitermite activities of the essential oils from Piper sarmentosum. Malays. J. Anal. Sci. 2008, 12, 234–239. [Google Scholar]
- Ramírez-Alarcón, K.; Martorell, M.; Gürer, E.S.; Laher Lam, H.L.; Mohieldin, E.A.M.; Muddathir, A.M.; Akram, M.; Iqbal, M.; Shafique, H.; Leyva-Gómez, G. Myristicin: From its biological effects in traditional medicine in plants to preclinical studies and use as ecological remedy in plant protection. eFood 2023, 4, 90. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).