Preparation, Performance Evaluation and Mechanisms of a Diatomite-Modified Starch-Based Fluid Loss Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
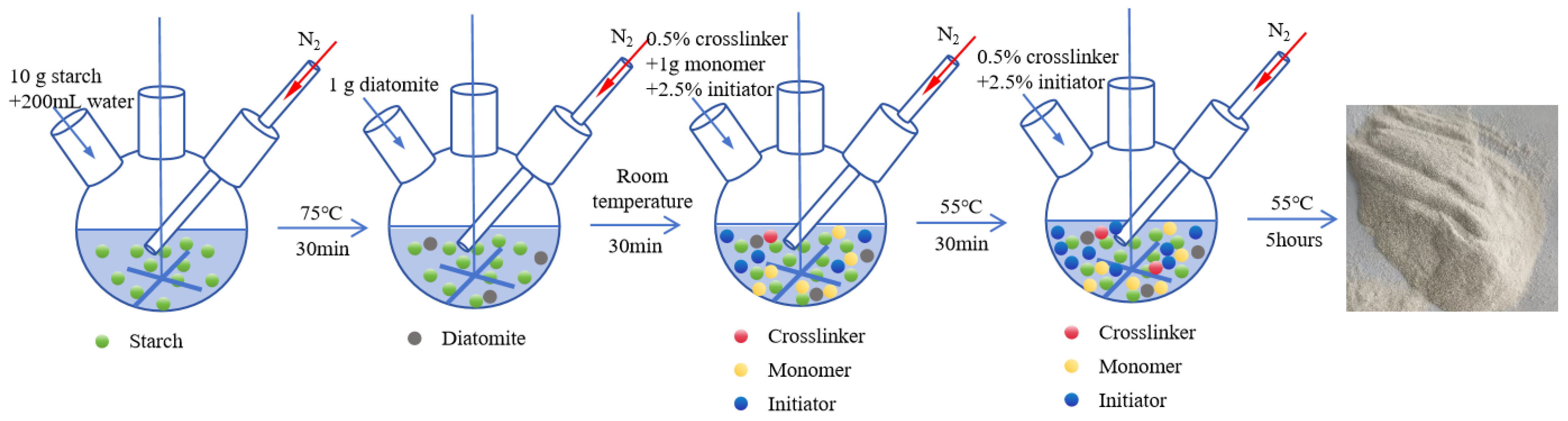
2.2.1. Preparation of Fluid Loss Agent
2.2.2. Fourier-Transform Infrared Spectroscopy
2.2.3. Rheological Properties of Fluid Loss Agent Suspensions
2.2.4. Performance Evaluation of Fluid Loss Agent
Evaluation of Thermal Stability of Fluid Loss Agent
Evaluation of Salt Pollution Resistance
Evaluation of Calcium Pollution Resistance
3. Results and Discussion
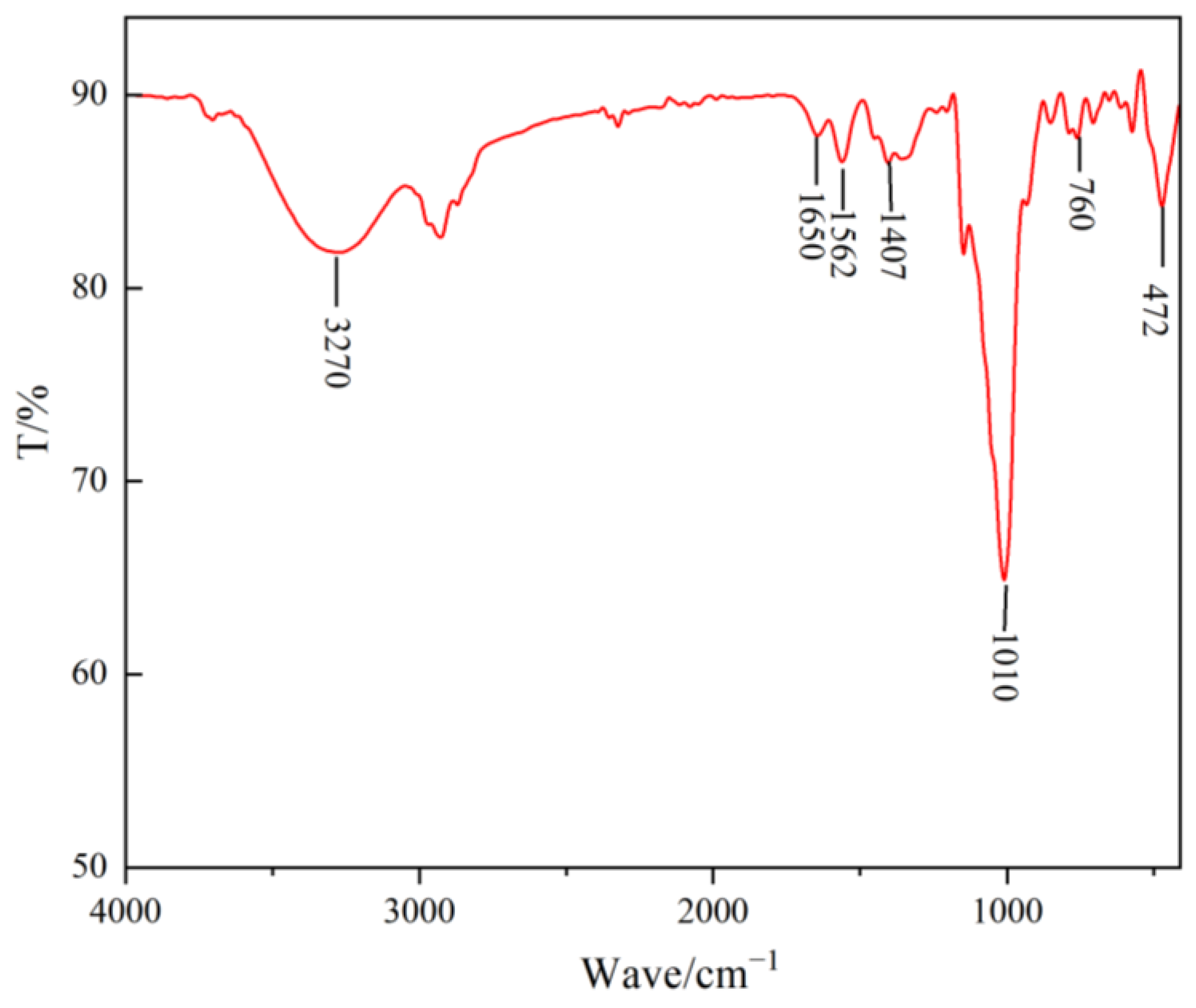
3.1. Infrared Spectral Analysis
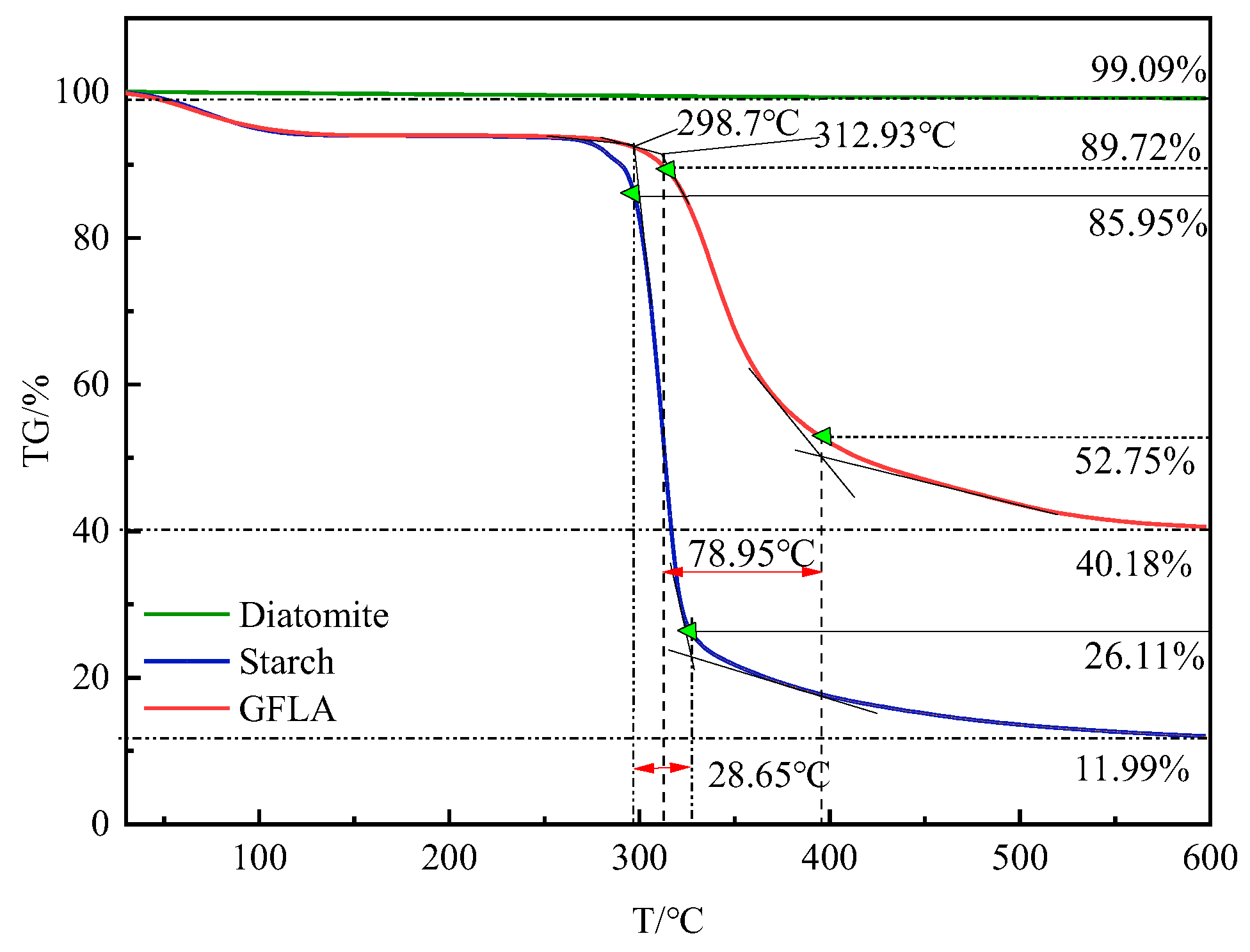
3.2. Thermogravimetric Analysis
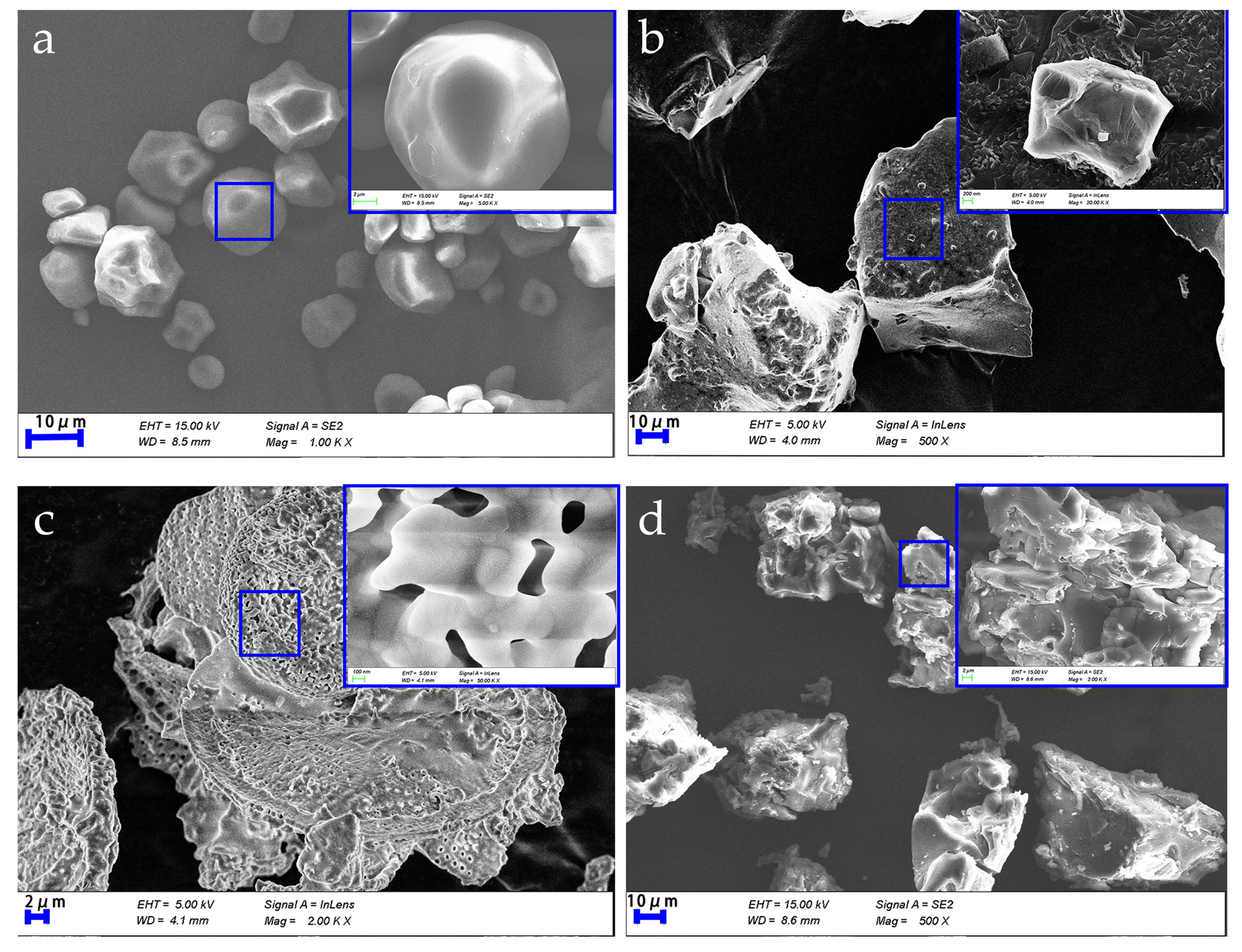
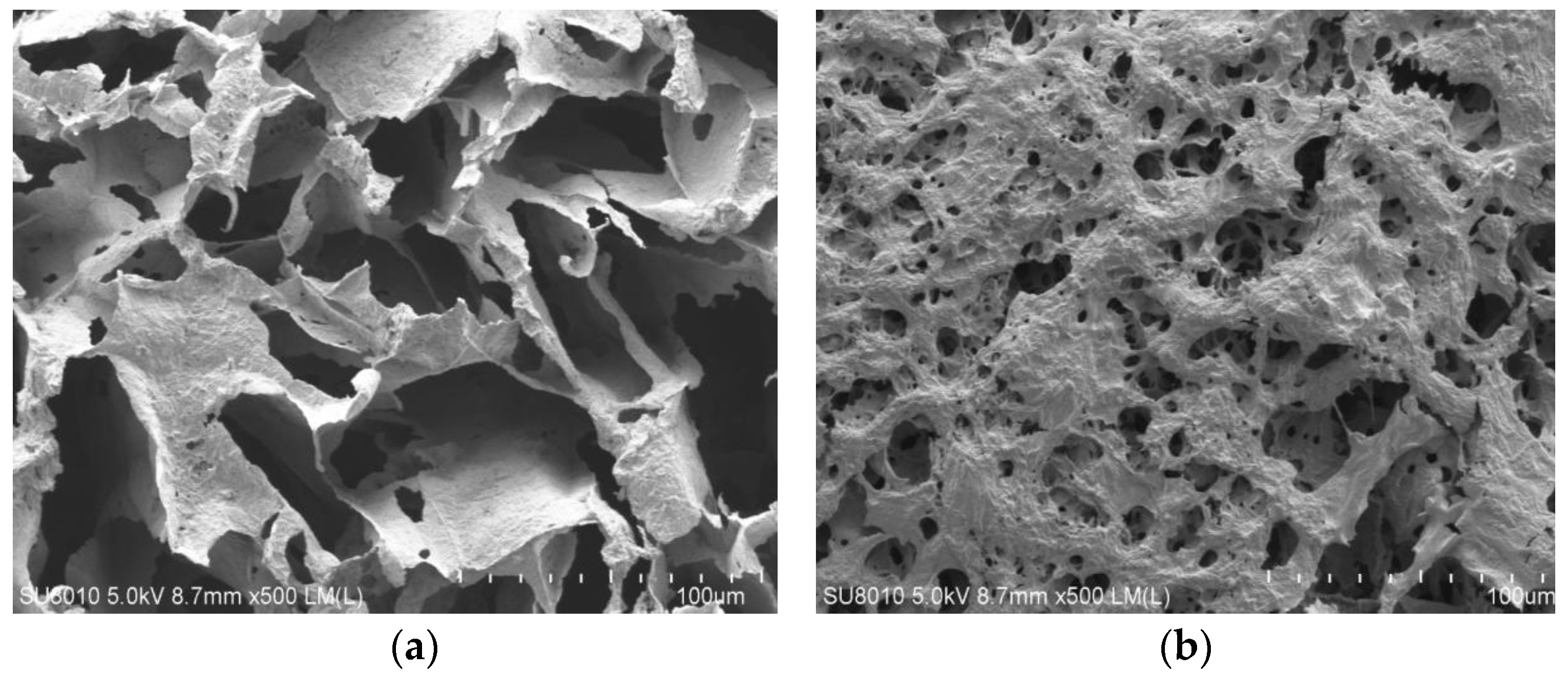
3.3. Scanning Electron Microscope Analysis
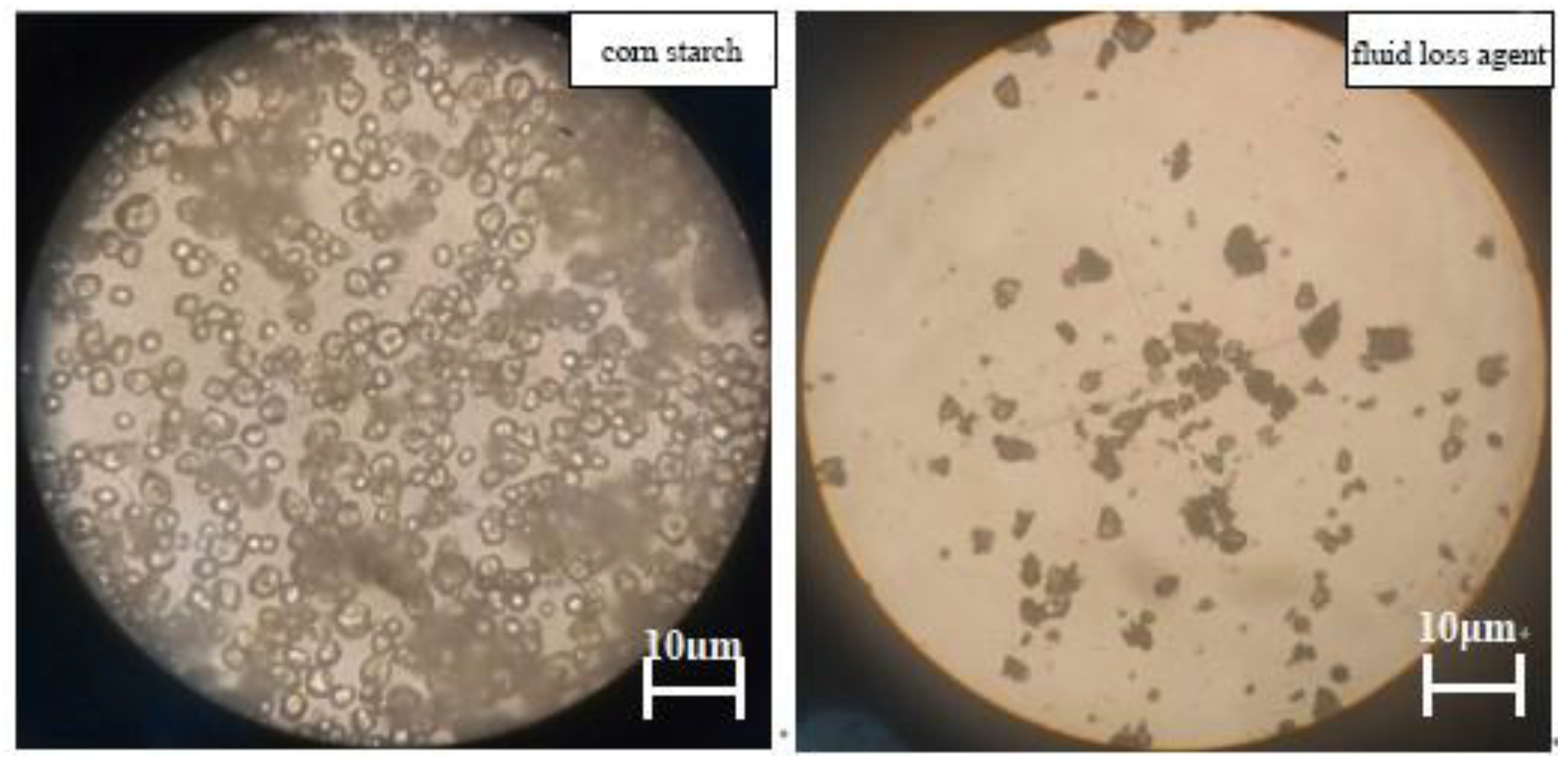
3.4. Polarizing Microscope Analysis
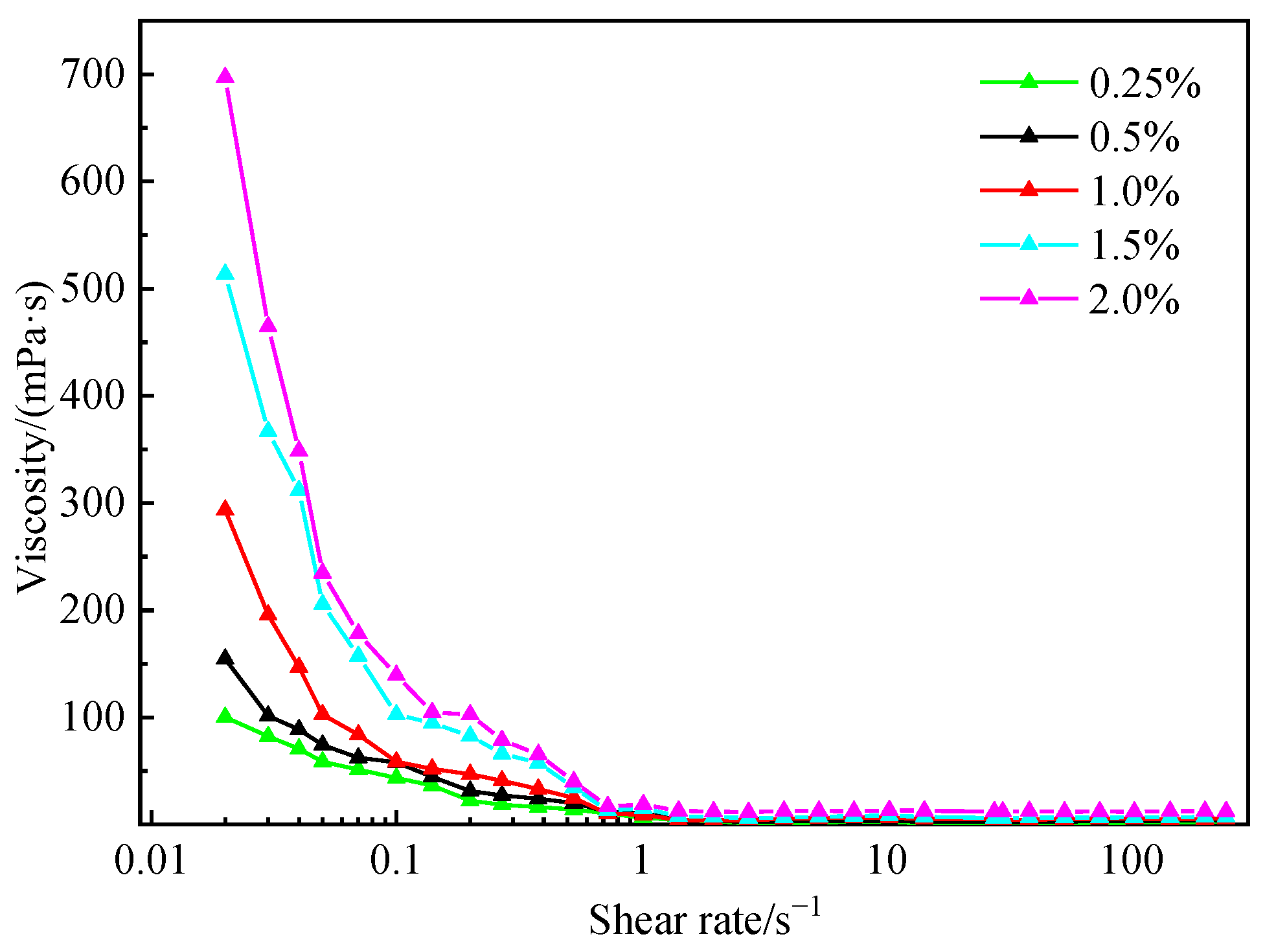
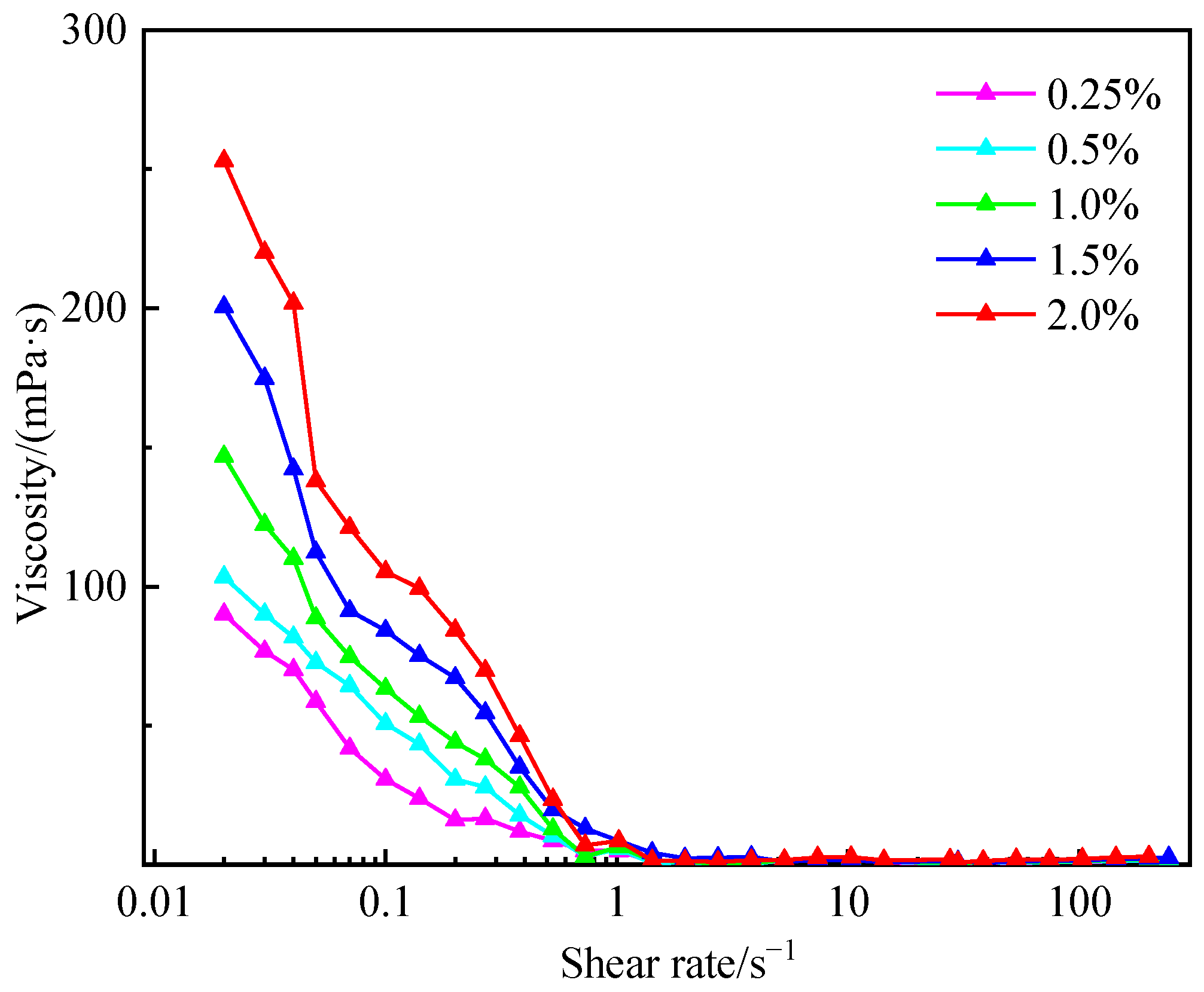
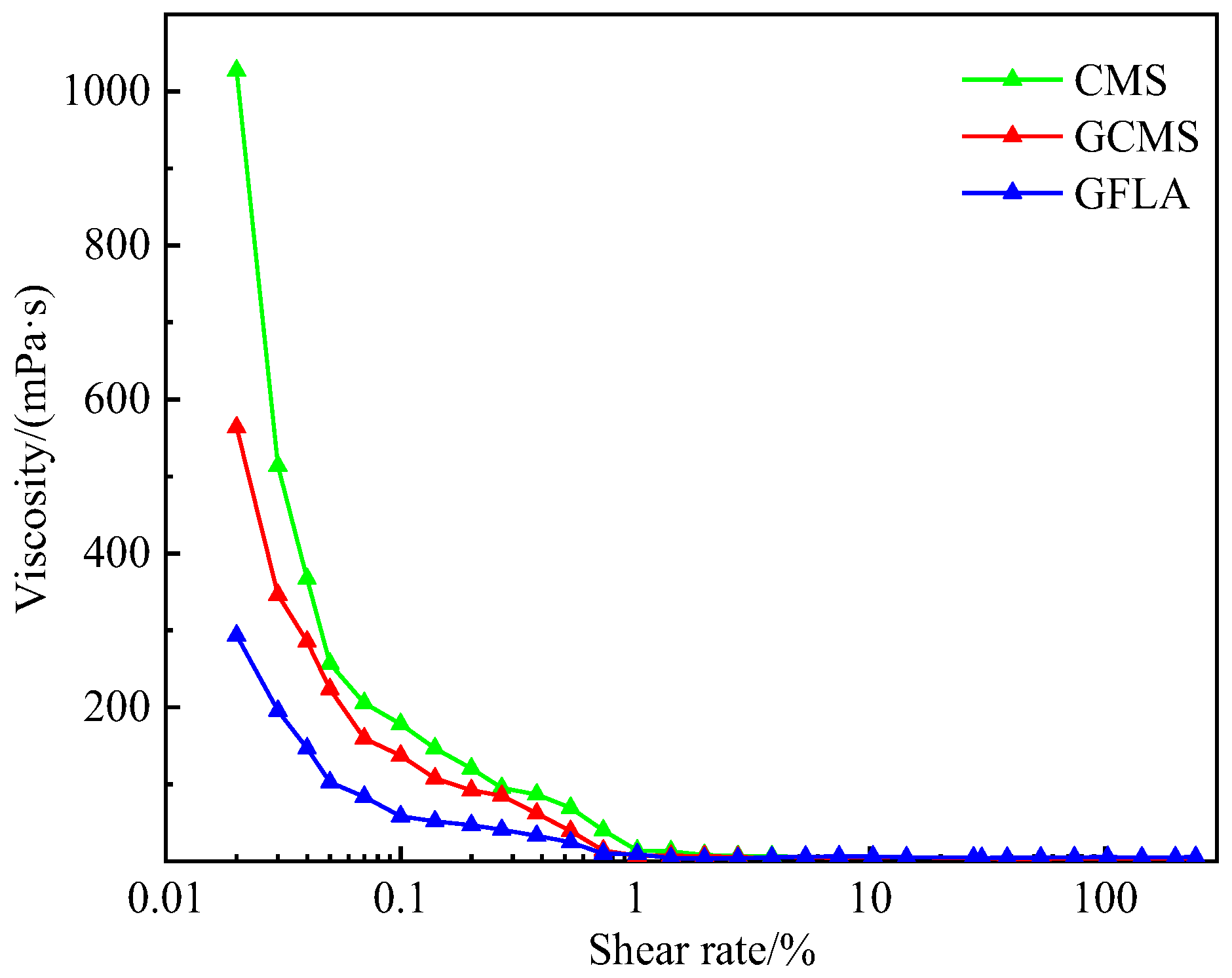
3.5. Performance Evaluation of Fluid Loss Agent Water Suspension
3.6. Properties of GFLA and Other Similar Products
3.7. Evaluation of Thermal Stability of GFLA
3.8. Evaluation of Salt Pollution Resistance of GFLA
3.9. Evaluation of Calcium Pollution Resistance of Fluid Loss Agent
3.10. Mechanism Analysis of GFLA
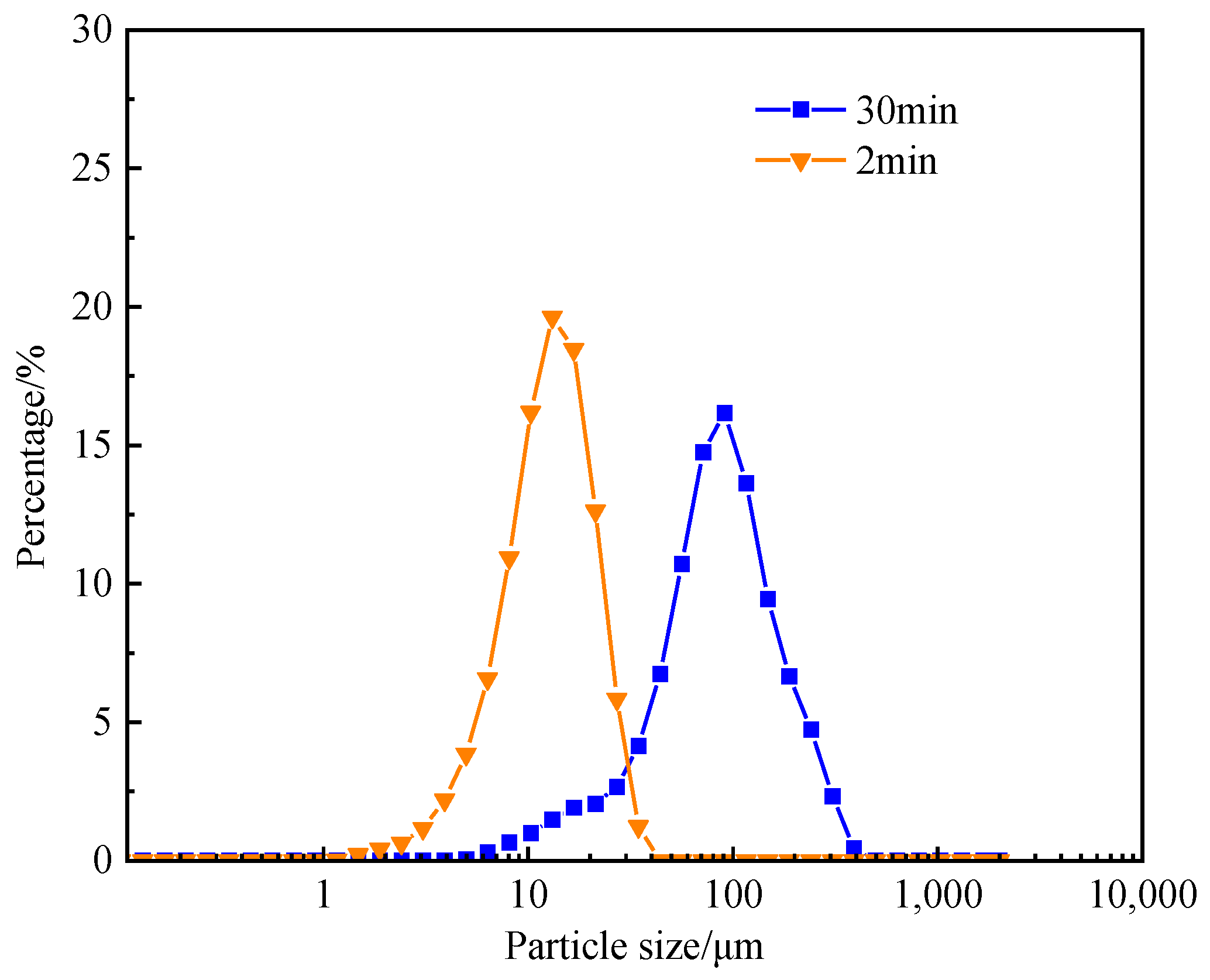
3.10.1. Water Swelling
3.10.2. The Formation of the Grid Structure
3.11. Biological Toxicity and Degradability
4. Conclusions
- (1)
- The graft-modified GFLA exhibits an irregular morphology with an increased specific surface area. This unique structural configuration facilitates enhanced adsorption potential, effectively reducing free water content in the drilling fluid and consequently achieving a significant filtrate reduction.
- (2)
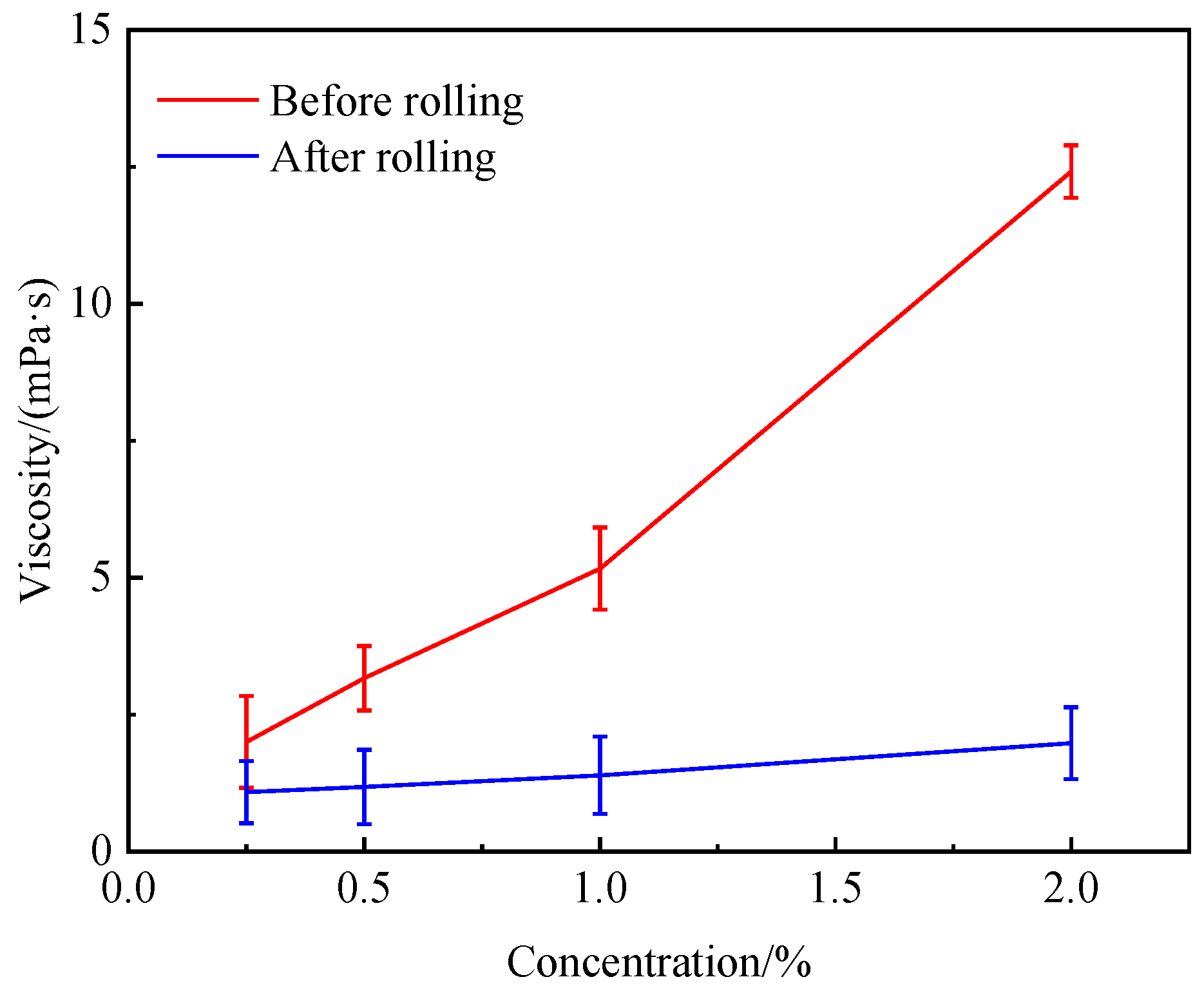
- Compared to conventional starch-based fluid loss agents, the developed GFLA demonstrates a minimized viscosity effect after 160 °C hot rolling while maintaining temperature resistance up to 180 °C. Notably, even prior to rolling, it exhibits a negligible impact on the rheological properties of drilling fluids (Table 1).
- (3)
- At a 2% dosage concentration, GFLA shows excellent contamination resistance with a 30% NaCl and a 0.6% CaCl2 tolerance. This indicates that GFLA is suitable for complex salt systems containing ≥ 20% monovalent salt ions, which enables GFLA to be effectively applied in high-salinity drilling environments.
- (4)
- Water absorption analysis reveals that GFLA’s 30 min uptake capacity significantly surpasses its 2 min absorption (Figure 10). Filter cake characterization (Figure 11) indicates improved compactness in additive-containing systems. These findings confirm dual working mechanisms: (i) hydration-induced expansion and (ii) three-dimensional network formation, synergistically contributing to effective fluid loss control.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fanta, G.F.; Shogren, R.L. Modification of starch–poly(methyl acrylate) graft copolymers by steam jet cooking. J. Appl. Polym. Sci. 1997, 65, 1021–1029. [Google Scholar] [CrossRef]
- Khalil, M.I.; Mo, K.M.; Hebeish, A. Synthesis of Poly(Methacrylic Acid-)Starch Graft Copolymers Using Mn-IV-Acid System. Starch 1990, 42, 107–111. [Google Scholar] [CrossRef]
- Biswas, A.; Shogren, R.L.; Stevenson, D.G.; Willett, J.L.; Bhowmik, P.K. Liquids as solvents for biopolymers: Acylation of starch and zein protein. Carbohydr. Polym. 2006, 66, 546–550. [Google Scholar] [CrossRef]
- Wang, Z.H. Progress in research and application of starch graft copolymer used for oilfield. Fault-Block Oil Gas Field 2010, 17, 239–245. [Google Scholar]
- Wang, Z.H. Preparation and Application of Modified Starch Additives for Drilling Fluid. Spec. Petrochem. 2009, 10, 12–16. [Google Scholar]
- Wang, Z.H. AMPS/AM/Starch graft copolymer as fluid loss controller for drilling muds. Oilfield Chem. 1997, 21, 77–78. [Google Scholar]
- Wang, Z.H. Research and application of modified tannin drilling fluid additives. Spec. Petrochem. 2008, 9, 10–13. [Google Scholar]
- Guo, D.R.; Gao, J.P.; Lv, K.H.; Xia, J.Y. Biodegradable filtrate loss controller SPS: Synthesis and properties. Oilfield Chem. 1995, 53, 165–166. [Google Scholar]
- Sifferman, T.R.; Muijs, H.M.; Fanta, G.F.; Felker, F.C.; Erhan, S.M. Starch-lubricant compositions for improved lubricity and fluid loss in water-based drilling muds. SPE Int. Conf. Oilfield Chem. 2003, SPE-80213. [Google Scholar] [CrossRef]
- Kainuma, K.; French, D. Naegeli amylodextrin and its relationship to starch granule structure. II. Role of water in crystallization of B-starch. Biopolymers 1972, 11, 2241–2250. [Google Scholar]
- Yang, Y.L.; Li, Z.J.; Wang, Z.F.; Pu, C.S. Synthesis of modified corn starch filtrate reducer for water-based drilling fluid. Oilfield Chem. 2006, 34, 198–200. [Google Scholar]
- Chen, Y.F.; Wei, X.G. Research advance and application on modified starch with high-temperature tolerance used for drilling fluid. Petrochem. Ind. Appl. 2020, 39, 16–20. [Google Scholar]
- API RP 13B-1; Field Testing Water-based Drilling Fluids. API: New York, NY, USA, 2020.
- Sinhmar, A.; Sharma, S.; Pathera, A.K.; Nehra, M.; Thory, R.; Nain, V.; Godara, S.K. Comprehensive Characterization of Starch from Diverse Sources: Physicochemical, and Functional Properties. Starch 2024, 76, 2300280. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR–ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef]
- Man, J.M.; Cai, C.H.; Yan, Q.X.; Hu, M.Z.; Liu, Q.Q.; Wei, C.X. Applications of Infrared Spectroscopy in the Analysis of Ordered Structure of Starch Grain. Acta Agron. Sin. 2012, 38, 505–513. [Google Scholar] [CrossRef]
- Perez, E.; Alviso, D.; Manrique, E.; Artana, G. Estimation of the rheological curve of HPAM solutions from measurements using the Brookfield viscometer. J. Pet. Sci. Eng. 2022, 216, 110793. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, F.J.; Han, C.Z.; Li, B.; Shao, Z.Q. Comparative Study on the Viscosity of CMC Solution in Different Viscometers. J. Cellul. Sci. Technol. 2010, 18, 38–42. [Google Scholar]
- Mischenko, S.V.; Mordasov, M.M.; Savenkov, A.P.; Sychev, V.A. Analysis of the Influence of Sizes of a Vessel with a Liquid on the Readings of Brookfield Viscometer. Meas. Tech. 2020, 63, 288–294. [Google Scholar] [CrossRef]
- Kong, C.L.; Sun, Y.X.; Li, C.L.; Zhao, J.Y.; Zhu, X.Y. Numerical simulation study on impact factors to dynamic filtration loss. J. Pet. Explor. Prod. Technol. 2024, 14, 593–607. [Google Scholar] [CrossRef]
- Ma, S.K.; Zhang, G.L.; Shi, C.H.; Dong, Q.Y.; Ji, T. Achieving Practical Venue Recycle of Waste Oil-Based Drilling Fluids with Vacuum Distillation Technology. ACS Omega 2023, 8, 16306. [Google Scholar] [CrossRef]
- Eutamene, M.; Benbakhti, A.; Khodja, M.; Jada, A. Preparation and Aqueous Properties of Starch-grafted Polyacrylamide Copolymers. Starch 2009, 61, 81–89. [Google Scholar] [CrossRef]
- Youcefi, M.R.; Hadjadj, A.; Bentriou, A.; Boukredera, F.S. Real-Time Prediction of Plastic Viscosity and Apparent Viscosity for Oil-Based Drilling Fluids Using a Committee Machine with Intelligent Systems. Arab. J. Sci. Eng. 2021, 47, 11145–11158. [Google Scholar] [CrossRef]
- Blaz, S.; Zima, G.; Jasinski, B. Laboratory studies on the use of trimanganese tetroxide as a weighting material for drilling fluids. Naft.-Gaz 2022, 3, 208–218. [Google Scholar]
- Jain, R.; Mahto, V.; Sharma, V.P. Evaluation of polyacrylamide-grafted-polyethylene glycol/silica nanocomposite as potential additive in water based drilling mud for reactive shale formation. J. Nat. Gas Scinece Eng. 2016, 26, 526–537. [Google Scholar] [CrossRef]
- Saboori, R.; Azin, R.; Osfouri, S.; Sabbaghi, S.; Bahramian, A. Wettability alteration of carbonate rocks from strongly liquid-wetting to strongly gas-wetting by fluorine-doped silica coated by fluorosilane. J. Dispers. Sci. Technol. 2018, 39, 767–776. [Google Scholar] [CrossRef]
- Sadeghalvaad, M.; Sabbaghi, S. Application of TiO2/Polyacrylamide Core–Shell Nanocomposite as an Additive for Controlling Rheological and Filtration Properties of Water-Based Drilling Fluid. J. Nanofluid 2017, 6, 205–212. [Google Scholar] [CrossRef]
- He, Z.; Yang, Y.; Qi, J.; Lin, X.Y.; Wang, N.; Wang, L.; Dai, H.M.; Lu, H.S. Hyperbranched Polymer Nanocomposite as a Potential Shale Stabilizer in Water-Based Drilling Fluids for Improving Wellbore Stability. J. Mol. Liq. 2024, 395, 123903. [Google Scholar] [CrossRef]
- SY/T 6787-2010; Technical Requirements for Environmental Protection of Water-Soluble Oilfield Chemicals. National Energy Administration of the People’s Republic of China: Beijing, China, 2010.

| Temperature/°C | Test Sample | AV /(mPa·s) | PV /(mPa·s) | YP /Pa | YP/PV | Gel10s/Gel10min /(Pa/Pa) | FLAPI /mL | Hk /mm | pH |
|---|---|---|---|---|---|---|---|---|---|
| Room temperature | Mud | 5 | 3 | 2 | 0.67 | 1.5/2.5 | 28 | 1 | 11 |
| Mud with GFLA | 6.25 | 5.5 | 0.75 | 0.14 | 0/0 | 6.4 | 0.5 | 10 | |
| 160 | Mud | 2.5 | 2 | 0.5 | 0.25 | 0/0 | 38 | 1.5 | 10 |
| Mud with GFLA | 2.5 | 2 | 0.5 | 0.25 | 0/0 | 7.6 | 0.5 | 9 | |
| 170 | Mud | 1.5 | 1 | 0.5 | 0.5 | 0/0 | 40 | 1.5 | 9 |
| Mud with GFLA | 2.75 | 2.5 | 0.25 | 0.10 | 0/0 | 7.2 | 0.5 | 9 | |
| 180 | Mud | 1.75 | 1.5 | 0.25 | 0.17 | 0/0 | 41 | 1.5 | 9 |
| Mud with GFLA | 2.25 | 1.5 | 0.75 | 0.5 | 0/0 | 10.0 | 0.5 | 9 |
| Concentrations of NaCl /% | Term | AV /(mPa·s) | PV /(mPa·s) | YP /Pa | YP/PV | FLAPI /mL | pH | HK /mm | FLHTHP /mL |
|---|---|---|---|---|---|---|---|---|---|
| 0 | Before rolling | 17.5 | 12 | 5.5 | 0.46 | 6.4 | 9 | 0.5 | 36 |
| After rolling | 9 | 8 | 1 | 0.13 | 7.0 | 7 | 0.5 | ||
| 0.5 | Before rolling | 16.5 | 11 | 5.5 | 0.50 | 6.8 | 10 | 0.5 | 32 |
| After rolling | 10 | 8 | 2 | 0.25 | 6.0 | 9 | 0.5 | ||
| 4.0 | Before rolling | 20 | 10 | 10 | 1.00 | 8.8 | 10 | 0.5 | 33 |
| After rolling | 15 | 9 | 6 | 0.67 | 5.0 | 8 | 0.5 | ||
| 10.0 | Before rolling | 20 | 7 | 13 | 1.86 | 14.4 | 10 | 1 | 34 |
| After rolling | 11 | 6 | 5 | 0.83 | 6 | 7 | 0.5 | ||
| 30.0 | Before rolling | 22 | 7 | 15 | 2.14 | 20.2 | 10 | 1 | 43 |
| After rolling | 8.5 | 5 | 3.5 | 0.70 | 16 | 7 | 0.5 |
| Concentrations of NaCl/% | 0 | 0.5 | 4.0 | 10.0 | 30.0 |
| Filtration loss before hot rolling/mL | 28 | 40 | 80 | 106 | 144 |
| Filtration loss after hot rolling/mL | 37 | 52 | 120 | 160 | 220 |
| Concentrations of CaCl2/% | Term | AV /(mPa·s) | PV /(mPa·s) | YP /Pa | YP/PV | FLAPI /mL | pH | HK /mm | FLHTHP /mL |
|---|---|---|---|---|---|---|---|---|---|
| 0 | Before rolling | 17.5 | 12 | 5.5 | 0.46 | 6.4 | 9 | 0.5 | 36 |
| After rolling | 9 | 8 | 1 | 0.13 | 7.0 | 7 | 0.5 | ||
| 0.1 | Before rolling | 21 | 17 | 4 | 0.24 | 6.8 | 9 | 0.5 | 38 |
| After rolling | 7.5 | 6 | 1.5 | 0.25 | 6 | 9 | 0.5 | ||
| 0.3 | Before rolling | 14 | 11 | 3 | 0.27 | 4.2 | 7 | 0.5 | 41 |
| After rolling | 6.5 | 5 | 1.5 | 0.30 | 4.0 | 7 | 0.5 | ||
| 0.6 | Before rolling | 15 | 12 | 3 | 0.25 | 10.0 | 7 | 0.5 | 46 |
| After rolling | 6 | 4.5 | 1.5 | 0.33 | 9.0 | 7 | 0.5 | ||
| 1.0 | Before rolling | 16 | 9 | 7 | 0.78 | 42 | 7 | 3 | 120 |
| After rolling | 11 | 7 | 4 | 0.57 | 39.2 | 7 | 3 |
| Material | BOD5 | CODCr | BOD5/CODCr | EC50 (×104 mg·L−1) |
|---|---|---|---|---|
| GFLA | 232.7 | 823.7 | 28.3% | 8.4 |
| Starch | 117.4 | 380.2 | 30.9% | 11.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; Zhang, X.; Yan, W.; Qiu, Z. Preparation, Performance Evaluation and Mechanisms of a Diatomite-Modified Starch-Based Fluid Loss Agent. Processes 2025, 13, 2427. https://doi.org/10.3390/pr13082427
Zhou G, Zhang X, Yan W, Qiu Z. Preparation, Performance Evaluation and Mechanisms of a Diatomite-Modified Starch-Based Fluid Loss Agent. Processes. 2025; 13(8):2427. https://doi.org/10.3390/pr13082427
Chicago/Turabian StyleZhou, Guowei, Xin Zhang, Weijun Yan, and Zhengsong Qiu. 2025. "Preparation, Performance Evaluation and Mechanisms of a Diatomite-Modified Starch-Based Fluid Loss Agent" Processes 13, no. 8: 2427. https://doi.org/10.3390/pr13082427
APA StyleZhou, G., Zhang, X., Yan, W., & Qiu, Z. (2025). Preparation, Performance Evaluation and Mechanisms of a Diatomite-Modified Starch-Based Fluid Loss Agent. Processes, 13(8), 2427. https://doi.org/10.3390/pr13082427





