1. Introduction
The average daily generation of livestock manure in the Republic of Korea was approximately 137.1 megagrams (Mg) in 2023, with swine and cattle manure accounting for 55.63 Mg (41%) and 45.697 Mg (33%), respectively, comprising approximately 74% of total livestock manure [
1]. Swine manure is mainly used for biogas production, as the biogas generated through anaerobic digestion typically contains a high proportion of methane (50–75%), which supports renewable energy generation and mitigates livestock odor [
2]. Accordingly, the use of anaerobic digestion has been promoted as a key policy strategy. In contrast, cattle and poultry manures are still treated solely through composting [
3]. However, the continuous decline in arable land area, along with nitrogen surplus—which reached 227.8 kg N/ha in 2019, among the highest levels in OECD countries—has exacerbated nutrient overload problems [
4]. The composting process, which emits significant amounts of methane (CH
4) and nitrous oxide (N
2O), also presents limitations in terms of greenhouse gas reduction [
5].
To overcome such limitations, many countries have begun to recognize livestock manure as a valuable energy and organic resource and have adopted a range of resource recovery technologies. In Germany, >40% of all biogas plants process livestock manure through co-digestion, with some facilities using only cattle manure [
6]. Denmark actively promotes biogas production by expanding governmental financial incentives and using the produced biogas as vehicle fuel for district heating [
7]. Similar policies and technologies are being implemented in places such as Italy and New York in the United States to expand livestock manure-based bioenergy production [
8,
9].
In China, approximately 2 × 10
9 Mg of livestock manure, including cattle manure, is generated annually, with the potential to produce 5.73 × 10
13 to 6.74 × 10
13 MJ of biogas per year [
10]. Furthermore, Shen et al. [
11]. analyzed the compositional characteristics and energy content of various types of animal manure in China, identifying cattle manure as a particularly promising resource due to its relatively high calorific value and fuel properties
However, despite the global adoption of manure-to-energy technologies, the Republic of Korea continues to rely entirely on composting for cattle and poultry manure treatment. This underscores the urgent need for alternative treatment strategies to achieve nutrient recovery and greenhouse gas reduction. A promising approach is the solid fuel conversion of cattle manure to produce a biomass-based energy source that is considered carbon neutral when combusted, thereby offering a viable and climate-resilient solution for sustainable manure management through thermochemical conversion technologies such as dry reforming and syngas synthesis [
12].
Domestic studies have reported that cattle manure pellets have an average lower heating value (LHV) of approximately 14.6 megajoules per kilogram (MJ/kg), which meets legal standards. However, depending on factors such as prior anaerobic degradation or the presence of foreign materials, the values can vary widely from 6.7 to 18.0 MJ/kg [
13].
In addition, recent studies have explored how thermal processing methods affect the fuel characteristics of cattle manure-derived materials, confirming that such treatments can significantly alter energy content and elemental composition, thus impacting combustion performance and suitability for resource recovery [
14].
The bottom ash produced after combustion contains nutrients such as P and K, indicating its potential for reuse as a fertilizer or soil conditioner. Nevertheless, the concentrations of certain heavy metals, particularly Cu and Zn, have been found to exceed permissible limits, suggesting the need for post-treatment technologies [
15,
16]. Furthermore, despite these findings, integrated studies evaluating the performance and resource recovery potential of cattle manure-based fuels in the Republic of Korea remain limited.
In this context, this study aimed to analyze the characteristics of solid fuel derived from cattle manure and assess the chemical composition of its combustion residues. Through this analysis, the suitability of cattle manure as a bioenergy source and its potential for resource recovery from bottom ash were quantitatively evaluated. The overarching goal was to determine whether solid fuel conversion can serve as a viable alternative within the livestock manure treatment framework in the Republic of Korea.
2. Literature Review
According to Annex 4-2 of the Enforcement Rule of the Act on the Management and Use of Livestock Manure [
17], the quality standards for solid fuel derived from livestock manure are as follows: ≤0.04 m length, ≥12.6 MJ/kg LHV, ≤20% moisture content, ≤30% ash content, and ≤2% sulfur content. In terms of heavy metals, the permissible limits are 1.2 mg/kg Hg, 9.0 mg/kg Cd, 200.0 mg/kg Pb, and 70.0 mg/kg Cr. The above quality standards are summarized in
Table 1.
A series of previous studies [
11,
13,
14] analyzed the characteristics of solid fuel derived from fully dried cattle manure. As the lower heating value (LHV) is a key indicator of fuel quality, particular attention has been given to its variation, which ranges approximately from 12.93 to 14.90 MJ/kg across the literature. The average values of volatile matter, ash, fixed carbon, LHV, and elemental compositions (C, H, O, N, S), based on proximate and ultimate analyses, are summarized in
Table 2.
Sahu et al. [
18] reported that the LHV of cattle manure-based solid fuel ranged from 11.57 to 18.41 MJ/kg, with an average of approximately 14.59 MJ/kg. The cellulose, hemicellulose, and lignin included in manure are known to reduce its combustion efficiency. These fibrous components tend to retain moisture, and the relatively low LHV observed in some samples suggests that this moisture retention ultimately reduces the energy density of the fuel. In addition, highly volatile fibrous organics may be lost during the thermal drying process, further contributing to the decrease in fuel quality [
19].
These findings suggest that dried cattle manure satisfies the legal standards for solid fuel, including an LHV of at least 12.6 MJ/kg and an ash content below 30%, indicating strong potential as a renewable energy resource. However, the LHV of cattle manure-based solid fuel may decrease owing to the inclusion of inorganic materials such as soil from barn floors, loss of organic matter during prolonged storage within livestock facilities, or addition of vermiculite and other inorganics during composting. Therefore, the use of organic bedding materials such as sawdust or rice husks is recommended to maintain fuel quality, and the incorporation of non-combustible substances should be minimized through appropriate management practices [
19].
A combustion experiment conducted by Szymajda et al. [
20] reported an average CO emission of 118.6 ppm, which meets the European standard of ≤200 ppm. Additionally, the average NO
x concentration was 63.2 ppm, which was lower than that of typical wood pellets (approximately 70–100 ppm) despite the N content in cattle manure pellets. Particulate matter emissions were also recorded at an average of ≤20 mg/Nm
3, demonstrating superior performance compared with the general emission range for biomass boilers (30–50 mg/Nm
3).
Various combustion systems have been investigated for burning livestock manure-based solid fuel, including small-scale domestic boilers, fluidized bed combustors, and traditional open fires. Rzeźnik et al. [
21] evaluated a 15 kW domestic boiler and reported that the combustion of 100% manure pellets led to high emissions of CO (1548 ± 555 mg/m
3), NO
x (554 ± 88 mg/m
3), and dust (482 ± 63 mg/m
3), exceeding the EU Ecodesign limits. However, co-combustion with wood pellets significantly improved combustion stability and reduced emissions. Guo et al. [
22] highlighted that thermochemical systems such as pyrolysis and gasification offer promising alternatives for stable energy recovery from manure, with LHV values ranging between 13 and 19 MJ/kg. Maj [
23] further emphasized that fuel properties and emissions are influenced by moisture content, ash, and inorganic impurities from bedding. Overall, proper feedstock management and system selection are essential for achieving efficient and environmentally compliant combustion performance.
3. Materials and Methods
3.1. Experimental Overview and Design
In this study, cattle manure generated from a cattle farm in Gyeongsangbuk-do, Republic of Korea, was converted into solid fuel, and the fuel characteristics and combustion residue composition were analyzed. Samples were collected from the cattle farm and categorized into three types: raw manure (pre-dried), dried manure, and pelletized solid fuel. Each type was sampled three times, and the average values were used for the analysis. After combustion, the residues were classified and collected as bottom ash from the boiler base and fly ash from the dust collector and bag filter. All samples were pretreated using a 0.002 m sieve, homogenized according to the relevant government guidelines, and stored in sealed containers in a cool, dark environment to prevent moisture absorption.
3.2. Solid Fuel Production and Combustion Test
Cattle manure was processed into solid fuel using a dedicated pelletizing system at a facility in Gyeongsangbuk-do, Republic of Korea.
The manure moisture content, which was approximately 60%, was mechanically dried to below 30%. A pelletizing machine with a production capacity of 1000 kg/h was then used to pelletize the manure, followed by cooling and screening to produce the final solid fuel.
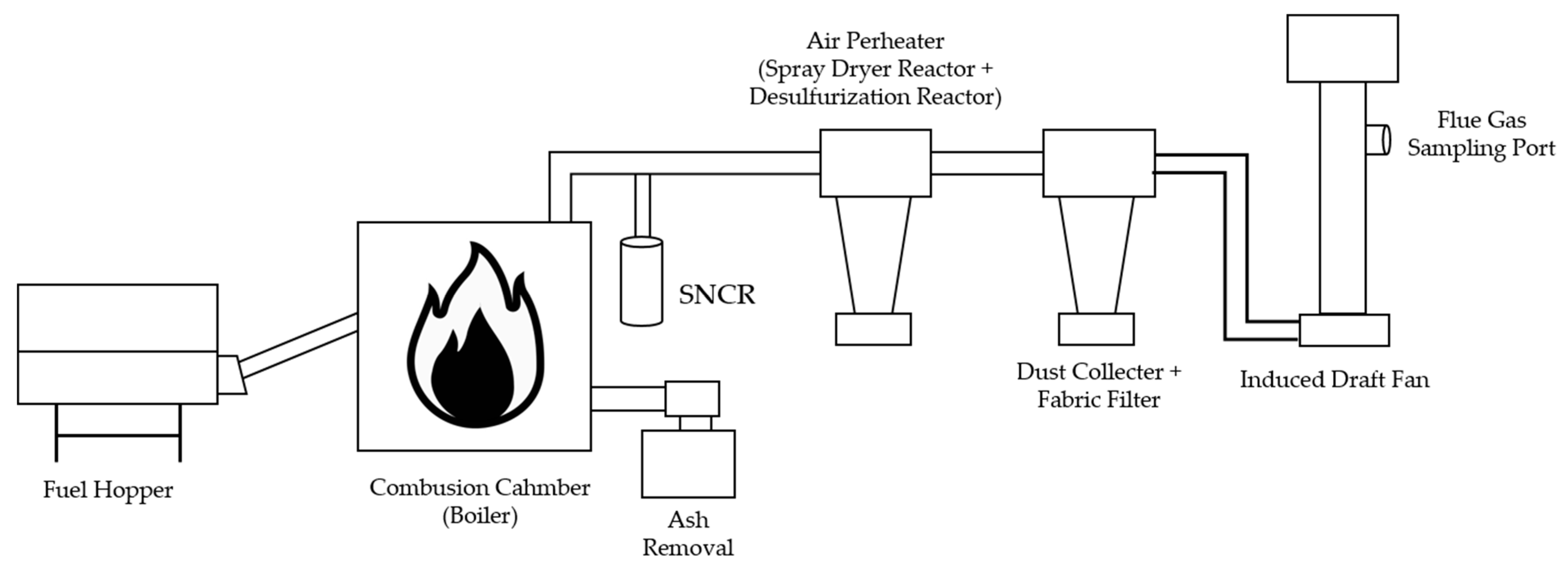
Combustion tests were conducted to assess the emission characteristics of the air pollutants generated during the combustion of the cattle manure-based solid fuel and evaluate their compliance with the relevant legal standards. A dedicated solid fuel boiler with a thermal capacity of 2.51 × 106 kJ/h and maximum processing capacity of 150 kg/h, located at the same facility, was used. This was equipped with a centrifugal dust collector, fabric filter, and selective noncatalytic reduction (SNCR) system, allowing for the effective reduction of major air pollutants. The tests were performed by a certified emissions measurement agency in accordance with the standards outlined in Annex 8 of the Enforcement Rules of the Clean Air Conservation Act. The emission limits for biomass and wood pellet boilers were applied. The cattle manure-based solid fuel was continuously fed into the boiler at a rate of 150 kg/h for 8 h. After combustion, the residues were collected separately and used for further analysis.
The flue gas emissions were measured at the outlet of the induced draft fan. The combustion system used for the tests is illustrated in
Figure 1.
3.3. Fuel and Residue Characterization and Analysis
3.3.1. Fuel Analysis
The calorific value, moisture, ash, volatile matter, and fixed carbon contents, and elemental composition (C, H, N, S) of the cattle manure and pelletized solid fuel were analyzed in accordance with Annex 1 of the Quality Test and Analysis Method for Solid Recovered Fuel Products, as specified by the Ministry of Environment [
24].
3.3.2. Air Pollutant Analysis
The air pollutants emitted during combustion were measured by an accredited analytical agency in accordance with the Air Pollution Test Methods published by the National Institute of Environmental Research [
25]. The applied methods were as follows: semi-automatic method (ES 01301.1e) for particulate matter, automatic method (ES 01307.1b) for sulfur oxide (SO
x), automatic method (ES 01308.1b) for nitrogen oxide (NO
x), atomic absorption spectrometry (ES 01400.1d) for heavy metals, and inductively coupled plasma atomic emission spectrometry (ES 01400.2d).
3.3.3. Combustion Residue Analysis
To evaluate the resource recovery potential of solid fuel derived from cattle manure, the combustion residues were analyzed by categorizing them into bottom and fly ash. The samples were obtained from the combustion of the same cattle manure pellets used in the tests. This analysis assesses whether these combustion byproducts could be reused as raw materials for fertilizers. The results were compared to the heavy metal limits and nutrient content standards defined in the Fertilizer Specification Guidelines.
The bottom and fly ash components generated after combustion, including total N, P
2O
5, and potassium (K
2O), salinity, and organic matter, as well as heavy metal (Cd, Pb, Cu, Zn, Ni, As, and Hg) content, were analyzed. The analysis followed the physicochemical test methods outlined in Annex 1 of the Fertilizer Specification and Test Methods issued by the Rural Development Administration [
23]. The parameters were reported as either percentages (%) or milligrams per kilogram (mg/kg).
4. Results
4.1. Physicochemical Properties of Livestock Manure and Derived Solid Fuel
To ensure the stability of the thermal properties of cattle manure-based solid fuel, this study analyzed the physicochemical characteristics of raw manure samples according to the time elapsed since excretion (
Table 3). During composting or prolonged storage, organic matter in manure tends to degrade, resulting in a decrease in heating value. A significant difference in the LHV was observed between samples collected within one month of excretion and those stored for four to six months.
To assess the effect of storage duration while minimizing farm-specific variability, certain samples were collected from the same farms at different excretion times. Notably, both the solid fuel and Manure A originated from the same farm; however, the solid fuel was produced from manure stored for six months, while Manure A was derived from manure collected within three months of excretion. Similarly, Manures C-1 and C-2, as well as Manures D-1 and D-2, were obtained from the same farms but differed in terms of storage period. These paired samples enabled a controlled evaluation of the impact of storage duration on fuel quality.
Manure A, which had been dried to a moisture content of 3.5%, exhibited a relatively high LHV of 13.77 MJ/kg. In contrast, the solid fuel, produced with a moisture content of 15%, showed a substantially lower LHV of 10.18 MJ/kg. This highlights the combined effects of extended storage and elevated moisture content on fuel quality.
Although the moisture content was adjusted to below 15% during solid fuel production, the resulting LHV remained below the legal minimum standard of 12.6 MJ/kg, as specified in the Enforcement Rules of the Act on the Management and Use of Livestock Manure. These findings underscore the importance of raw material quality and age management in the production of cattle manure-based solid fuels.
The solid fuel characteristics of raw and dried cattle manure, along with the solid fuel derived from them, are presented in
Table 4. All samples originated from the same farm (Farm A), with Manure 1 and Manure 2 representing two separate collection events. The raw manure samples showed high moisture contents (over 70%) and relatively low ash contents (<7%), which indicates the need for drying prior to use as a solid fuel feedstock.
Upon drying, both manure samples showed substantial improvements in fuel quality. Dried Manure 1 and Dried Manure 2 had moisture contents of 3.2% and 2.6%, respectively, with LHV values of 13.57 MJ/kg and 15.28 MJ/kg. These results demonstrate that, with appropriate moisture reduction, the raw material can meet legal solid fuel standards even prior to pelletization.
The solid fuel used in the combustion tests, which was produced from the dried manure samples, showed an LHV of 13.27 MJ/kg at a moisture content of 13.2%, satisfying the legal threshold. When the solid fuel was further dried to 1.6%, its LHV significantly increased to 15.11 MJ/kg. This was accompanied by increases in fixed carbon content (from 7.9% to 12.7%) and elemental carbon content (from 42.4% to 43.0%), indicating improved combustion efficiency. These findings confirm that both raw material quality and precise moisture control during production are critical to the performance of cattle manure-based solid fuels.
C is the primary energy-contributing element in fuels because it plays a key role in heat generation during combustion. The calorific value of solid biomass fuels is largely proportional to their C content, with higher C concentrations generally indicating better thermal performance. In this study, the cattle manure-based solid fuel exhibited a C content of 42.4%, which is comparable to—or in some cases exceeds—that of major domestic and international biomass fuels (typically 40–45%) [
26,
27], demonstrating favorable fuel characteristics.
In contrast, O exists primarily in the form of oxides and generally does not contribute to heat generation; instead, it can reduce fuel efficiency during combustion. Higher O content tends to be associated with LHVs and is often linked to an increased proportion of combustion residues. In this study, the measured O content was 31.83%, which falls within the typical range of 30–35% for solid fuels derived from livestock manure and serves as a critical indicator of fuel quality. The C and O contents observed in this study suggest that solid fuel not only meets the legal criteria for biomass fuels but also demonstrates potential as a substitute for coal.
4.2. Emission Characteristics and Analysis of Solid Fuel Combustion Air Pollutants
4.2.1. Combustion Conditions
Two combustion tests were conducted, with the first in September 2024. Due to a malfunction in the hot water circulation system, the hot water tank temperature exceeded the setpoint (90 °C), preventing the combustion chamber from reaching a stable operating temperature. This led to unstable combustion conditions, and the measured carbon monoxide (CO) concentration reached 1766.0 ppm, significantly exceeding the legal limit of 200 ppm. In the second test conducted in October 2024, the same fuel and equipment were used; however, the hot water circulation system was improved to ensure stable temperature control. Consequently, the CO concentration decreased to 129.9 ppm, and all measured parameters were within the permitted emission limits. Both SO
x and NO
x were recorded at very low levels in both tests, and particulate matter and heavy metals were either below the detection limit or present only in trace amounts. All emission values were corrected to a standard oxygen concentration of 12% O
2. All measurements were performed while the SNCR system was in operation
Table 5.
4.2.2. Combustion Temperature Conditions
To quantitatively compare air pollutant emissions under different combustion conditions, variations in the combustion chamber temperature, exhaust gas temperature, and return water temperature were analyzed over time during the combustion tests.
Figure 2a shows the temperature profile of the first test, in which the hot water tank temperature increased rapidly, making it difficult to control the combustion chamber temperature. This likely led to incomplete combustion and a corresponding spike in CO emissions.
Figure 2b illustrates the second test, during which the hot water circulation system operated stably and maintained a consistent internal combustion temperature. This stable condition contributed to significant reductions in key air pollutants such as CO and NOx
4.3. Combustion Ash Characteristics and Mass Balance Analysis
4.3.1. Bottom and Fly Ash Composition
The analysis results in
Table 6 indicate that the bottom ash meets the regulatory limits for all tested heavy metals (As, Cd, Hg, Pb, Cr, Cu, Ni, and Zn), in accordance with the Fertilizer Specification Guidelines issued by the Rural Development Administration [
28]. The contents of organic matter, nitrogen (N), phosphorus pentoxide (P
2O
5), and potassium (K) were at levels considered suitable for agricultural use. In particular, the P
2O
5 content ranged from 9.4% to 11.7%, and the K content ranged from 8.1% to 11.8%, indicating strong potential for use as a source of phosphorus and potassium in fertilizer production. According to the Fertilizer Specification Guidelines, three-component compound fertilizers must contain a combined total of N, P
2O
5, and K of at least 12%. The bottom ash contains sufficiently high levels of P
2O
5 and K to serve as an alternative to other P- and K-rich ingredients in compound fertilizer formulations. Although its nitrogen content (0.19–0.25%) and organic matter content (4.7–5.5%) are relatively low, these components can be supplemented with other raw materials to meet the standard requirements. Meanwhile, the fly ash showed elevated concentrations of zinc (4320–6200 mg/kg), significantly exceeding the limit of 900 mg/kg. This is likely due to the volatilization of metallic elements during combustion, followed by their capture and concentration in the bag filter system. Accordingly, caution is needed when considering the reuse of fly ash as a fertilizer material.
According to thermodynamic modeling and combustion residue analysis, trace elements such as Zn, Cu, Cr, Cd, and As are known to exist in ashes in the form of stable oxides (e.g., ZnO and CuO), spinel-type compounds (e.g., ZnFe
2O
4), or as complex salts and phosphates, depending on combustion temperature and ash composition. Volatile elements, like Cd and As, may also partially volatilize and concentrate in the fly ash through condensation on fine particulates during flue gas cooling [
29].
Notably, during the first test, insufficient circulation in the hot water system led to inadequate combustion temperatures, resulting in slightly higher heavy metal concentrations in the bottom ash. In the second test, stable temperature control facilitated greater volatilization and transfer of metals into the fly ash, leading to higher concentrations in the collected particulates. These results indicate that the combustion temperature significantly influenced the distribution of elements in the ash residues. Thus, optimizing the combustion parameters is critical for ensuring the quality and safety of recycling ash as a fertilizer.
According to [
30], the ash from waste biomass combustion exhibits high alkalinity (pH ≈ 13) and contains substantial amounts of Ca and P. P in ash primarily exists as hydroxyapatite and mixed phosphate (P
2O
5) salts, which can be efficiently recovered (up to 90%) via acid leaching under optimized conditions. According to [
31], the chemical and physical characteristics of biomass ash derived from various agricultural residues indicate that such materials, due to their high alkalinity and abundant nutrients (K, Ca, Mg, and P), can be effectively utilized as soil amendments when properly managed. By extension, the ash generated from the combustion of cattle manure solid fuel may also exhibit similar potential for soil application, provided that its composition is thoroughly evaluated and adequate treatment or management strategies are in place.
4.3.2. Application of Bottom Ash in Three-Component Compound Fertilizer Production
The nutrient composition of compound fertilizers produced by blending bottom ash at different ratios is shown in
Table 7. Among the samples, the fertilizer containing 20% bottom ash (Compound Fertilizer 1) showed the highest combined content of N, P
2O
5, and K at 28.78%, along with the highest organic matter content at 22.15%, indicating superior nutrient quality. However, even with a 50% bottom ash blend (Compound Fertilizer 3), the fertilizer still met the regulatory requirement of at least 12% total N + P
2O
5 + K and 10% organic matter. These results demonstrate the potential to use higher ratios of bottom ash in fertilizer formulations while still meeting the standards.
Utilizing bottom ash derived from cattle manure in compound fertilizer formulations presents notable cost-saving opportunities. As a byproduct of biomass combustion, bottom ash is readily available and often regarded as waste. Its integration into fertilizer production can reduce dependence on costlier mineral sources of phosphorus and potassium, thereby lowering raw material expenses [
32]. Moreover, this approach aligns with circular economy principles by transforming waste into value-added agricultural inputs.
4.3.3. Material Balance
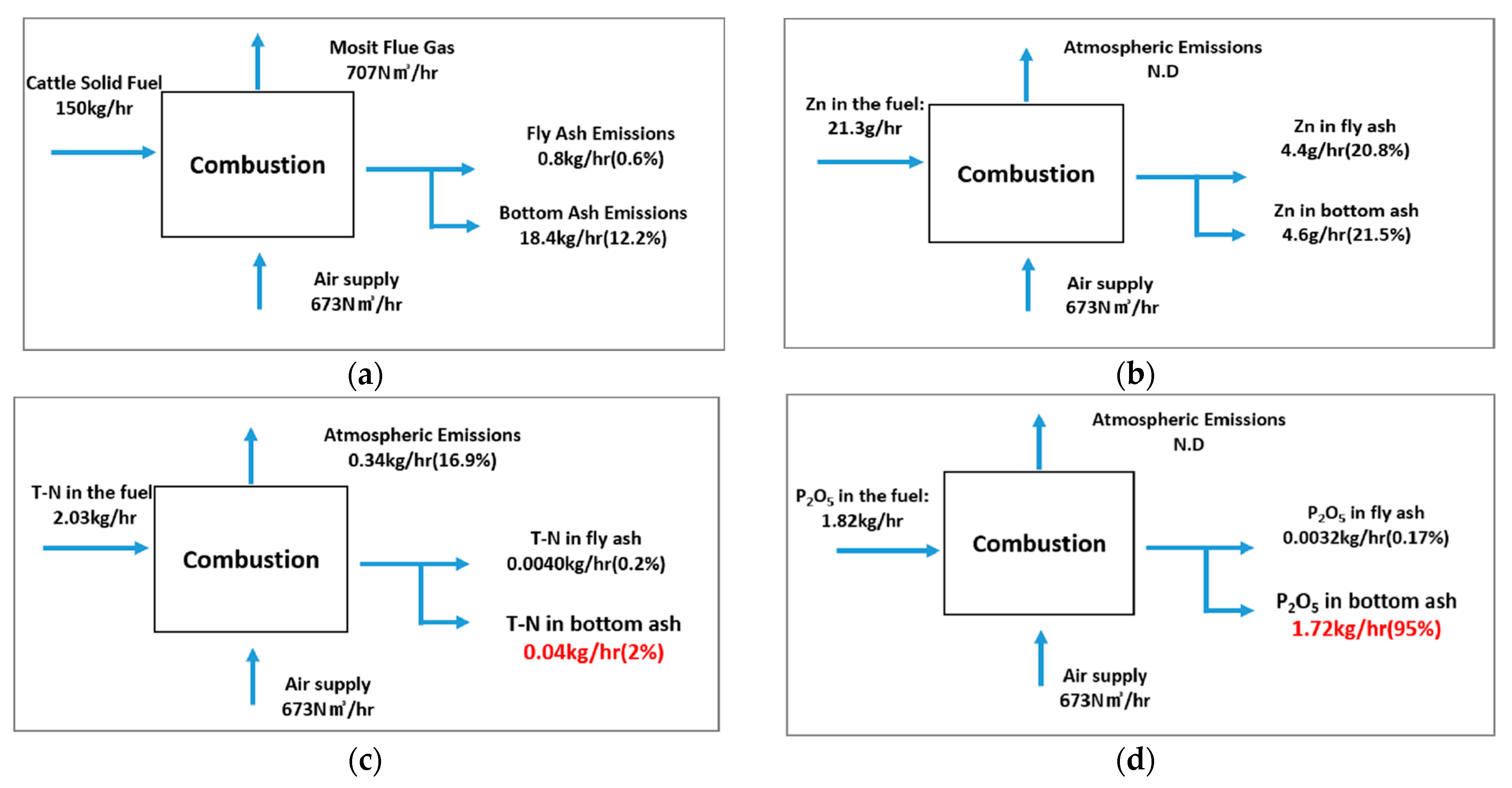
A material balance analysis was conducted to quantitatively trace the flow of the nutrients and hazardous elements during the combustion of the solid fuel from cattle manure. The analysis was based on a fuel input rate of 150 kg/h, and the distribution of major elemental components into atmospheric emissions, residual ash, and process losses was estimated (
Figure 3a).
Zn was present in the fuel at a concentration of 21.3 g/h. After combustion, approximately 4.4 g/h (20.8%) fly ash and 4.6 g/h (21.5%) bottom ash were produced. The remaining 9 g/h was not accounted for in the material balance, possibly due to inefficiencies in the filtration system or particle size-related issues. Although the Zn concentration in fly ash exceeded the permissible level for fertilizer use, the fly ash only accounted for 0.6% of the total fuel input, suggesting that disposal as general waste would not pose a significant environmental burden (
Figure 3b).
The N content of the fuel was approximately 2.03 kg/h. During combustion, a small portion of N (0.04 kg/h, 2%) was detected in the bottom ash, likely influenced by the injection of urea solution (ammonia water) through the SNCR system. The majority of the nitrogen was oxidized to NO
x during the combustion process, with approximately 0.34 kg/h (16.9%) emitted. This value corresponds to emissions measured after the selective non-catalytic reduction (SNCR) process. The N detected in the ash may be attributed to ammonia slip during urea injection (
Figure 3c).
The P
2O
5 content in the fuel was 1.82 kg/h, and approximately 95% (1.72 kg/h) remained in the bottom ash after combustion. This indicates that P tends to remain in a solid ash form rather than volatilizing or being emitted into the atmosphere, resulting in minimal loss during fuel processing and demonstrating a high potential for resource recovery. Given that P is a critical component of phosphate rocks, the importance of cattle manure combustion residues as raw materials for P-based fertilizers is highlighted (
Figure 3d).
5. Discussion
The experimental results obtained in this study demonstrate that the solid fuel derived from cattle manure has significant potential for both energy production and nutrient recovery. The physicochemical properties of the tested fuel met the quality standards stipulated in the Enforcement Rules of the Act on the Management and Use of Livestock Manure, with a LHV of 13.27 MJ/kg, a moisture content of 13.2%, and an ash content of 12.8%. These values are consistent with those of previous studies [
16,
17], thereby supporting the replicability of cattle manure as a viable biomass fuel under domestic and international conditions.
The results of the combustion tests demonstrated that cattle manure-based solid fuels can meet all regulatory emission limits for air pollutants, provided that proper combustion conditions and environmental control systems are in place. The air pollutant emissions varied markedly depending on the combustion conditions. In particular, the CO emissions, which are highly sensitive to the combustion efficiency, varied significantly depending on the combustion temperature and operational parameters.
In the first combustion test, malfunctioning of the hot water circulation system resulted in incomplete combustion and a high CO concentration exceeding the permissible limit. However, in the second test, the stabilization of the temperature control significantly reduced the emission levels of CO (129.9 ppm) and NOx (41.5 ppm). These results highlight the critical role of the combustion temperature and oxygen supply in reducing atmospheric pollutants. Notably, the dramatic reduction of CO and NOx in the second test suggests the necessity of engineering control for the solid fuel combustion systems. With stable combustion temperature and airflow regulation, pollutant formation can be minimized, suggesting that the deployment of this technology at scale should incorporate automated combustion management.
The low emissions of SOx and NOx can be attributed to the low S content of the cattle manure and stable combustion reaction under a sufficient O supply. Additionally, the results for heavy metals and particulate matter confirmed that the use of the solid fuel did not pose a significant environmental risk. Therefore, the results of this study serve as a foundational reference for future environmental assessments, expanding the use of solid fuels derived from livestock manure.
The analysis of the combustion residues also provided meaningful insights. The bottom ash contained high levels of available nutrients, such as P2O5 and K, and met the heavy metal limit for use as a fertilizer or soil conditioner. In contrast, fly ash exceeded the Zn threshold; however, its generation accounted for only 0.6% of the total ash, indicating minimal environmental impact and a manageable environmental risk. Potential treatment solutions include stabilization via immobilization agents or integration into cementitious materials, where Zn leaching is minimized.
Mass flow analysis further showed that the total N was primarily released into the atmosphere as NOx, with some retained in the ash owing to the injection of urea solution via the SNCR system. Despite the N component being less efficiently retained, this limitation can be addressed through combustion optimization and supplementary nitrogen recovery systems such as ammonia stripping or catalytic reduction. Furthermore, the trace retention of N in ash underscores the potential for co-blending it with other organic nutrient sources to formulate complete fertilizers.
In contrast, P2O5 remained largely in the bottom ash (approximately 95%), demonstrating a low loss of P and high potential for resource recovery. The consistent retention of P following combustion suggests a highly efficient pathway for nutrient recovery. In a country such as the Republic of Korea, where P is largely imported for fertilizer use, the reuse of ash as a P2O5-rich soil amendment could reduce dependency on non-renewable phosphate rock. This aligns with international trends that emphasize circular nutrient economies and contribute to domestic resource self-sufficiency.
These comprehensive results highlight not only the technical feasibility of converting cattle manure into solid biofuels but also its practical implications for environmental management and agricultural sustainability. The insights support the development of integrated manure management frameworks that combine energy recovery with nutrient recycling and pollution control, thereby promoting climate-smart and resource-efficient agriculture.
Despite these findings, some limitations remain. The study was limited to samples collected from a single cattle farm in one region and thus did not account for seasonal variations or differences in manure characteristics due to farming practices or storage duration. Future studies should include assessments of the fuel stability under various environmental and management conditions, economic feasibility of large-scale systems, and validation of heavy metal treatment and fertilizer application in agricultural settings.
6. Conclusions
This study evaluated the potential of cattle manure as a biomass energy and nutrient recovery resource by converting it into solid fuel and analyzing the composition of the combustion residues. The cattle manure-based solid fuel satisfied the national quality standards, with an LHV of 13.27 MJ/kg, a moisture content of 13.2%, and an ash content of 12.8%, confirming its viability as a renewable energy source. Elemental analysis also revealed appropriate C content, indicating its reliability as a biomass fuel. Based on the emission, combustion residue, and mass flow analyses, the importance of precise system management for enhancing both fuel efficiency and environmental safety was underscored, and recommendations to minimize environmental impact and nutrient loss and enhance the recyclability of combustion residues were provided.
In conclusion, the solid fuel conversion of cattle manure offers a sustainable waste-to-energy solution, achieving the dual goals of renewable energy production and nutrient recycling. Further research should focus on fuel quality stability under diverse production conditions, applicability to medium- and large-scale systems, and field validation of combustion residue use. These efforts will support the establishment of solid fuel technology as a practical and sustainable model for livestock manure valorization.
Author Contributions
Conceptualization, E.L. and J.H.; methodology, E.L. and S.O.; validation, J.H., and S.O.; formal analysis, E.L.; investigation, E.L.; data curation, E.L.; writing—original draft preparation, E.L.; writing—review and editing, J.H. and S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry and Energy (MOTIE) of the Republic of Korea (Project Number: 20227410100080), under the “Public Energy Leading Investment and New Industry Creation Support Project (R&D)”.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank all contributors and collaborators involved in the project for their valuable support and assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Korea Energy Economics Institute. Research on Expanding Biogas Production and Promoting Utilization (KEEI Research Report 23-08). 2023. Available online: https://www.keei.re.kr (accessed on 5 June 2025). (In Korean).
- Poeschl, M.; Ward, S.; Owende, P. Prospects for expanded utilization of biogas in Germany. Renew. Sustain. Energy Rev. 2010, 14, 1782–1797. [Google Scholar] [CrossRef]
- National Institute of Environmental Research. National Pollution Source Survey Data. 2023. Available online: https://www.data.go.kr/data/3045217/fileData.do (accessed on 5 June 2025).
- OECD. Agri-Environmental Indicators: Nutrient Balances. 2023. Available online: https://stats.oecd.org (accessed on 15 June 2025).
- Rural Development Administration. Study on Methane and Nitrous Oxide Emissions During Composting (Research Report No. TRKO201200000010). 2010. Available online: https://scienceon.kisti.re.kr/srch/selectPORSrchReport.do?cn=TRKO201200000010 (accessed on 6 June 2025).
- Daniel-Gromke, J.; Rensberg, N.; Denysenko, V.; Stinner, W.; Schmalfuß, T.; Scheftelowitz, M.; Nelles, M.; Liebetrau, J. Current Developments in Production and Utilization of Biogas and Biomethane in Germany. Chem. Ing. Tech. 2018, 90, 17–35. [Google Scholar] [CrossRef]
- Thygesen, O.; Sommer, S.G.; Shin, S.G.; Triolo, J.M. Residual biochemical methane potential (BMP) of concentrated digestate from full-scale biogas plants. Fuel 2014, 132, 44–46. [Google Scholar] [CrossRef]
- Hou, Y.; Velthof, G.L.; Case, S.D.C.; Oelofse, M.; Grignani, C.; Balsari, P.; Zavattaro, L.; Gioelli, F.; Bernal, M.P.; Fangueiro, D.; et al. Stakeholder perceptions of manure treatment technologies in Denmark, Italy, the Netherlands and Spain. J. Clean. Prod. 2018, 172, 1620–1630. [Google Scholar] [CrossRef]
- Kassem, N.; Sills, D.; Posmanik, R.; Blair, C.; Tester, J.W. Combining anaerobic digestion and hydrothermal liquefaction in the conversion of dairy waste into energy: A techno-economic model for New York state. Waste Manag. 2020, 103, 228–239. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, J.; Lin, J.G.; Zhang, N. Biogas energy generated from livestock manure in China: Current situation and future trends. J. Environ. Manag. 2022, 319, 113324. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Huang, G.; Yang, Z.; Han, L. Compositional characteristics and energy potential of Chinese animal manure by type and as a whole. Appl. Energy 2015, 160, 108–119. [Google Scholar] [CrossRef]
- Rosha, P.; Rosha, A.K.; Ibrahim, H.; Kumar, S. Recent advances in biogas upgrading to value added products: A review. Int. J. Hydrogen Energy 2021, 46, 21318–21337. [Google Scholar] [CrossRef]
- Lee, S.; Yu, B.; Ju, S.; Kang, Y.; Jung, G. Characteristics of solid fuel from cattle manure. New Renew. Energy 2016, 12, 64–69. [Google Scholar] [CrossRef]
- Song, E.; Kim, H.; Kim, K.W.; Yoon, Y.-M. Characteristic evaluation of different carbonization processes for hydrochar, torrefied char, and biochar produced from cattle manure. Energies 2023, 16, 3265. [Google Scholar] [CrossRef]
- Olowoboko, T.B.; Azeez, J.O.; Olujimi, O.O.; Babalola, O.A. Availability and dynamics of organic carbon and nitrogen indices in some soils amended with animal manures and ashes. Int. J. Recycl. Org. Waste Agric. 2018, 7, 287–304. [Google Scholar] [CrossRef]
- Jeong, K.H.; Lee, D.J.; Lee, D.H.; Lee, S.H. Combustion characteristics of cow manure pellet as a solid fuel source. J. Korea Org. Resour. Recycl. Assoc. 2019, 27, 31–40. [Google Scholar] [CrossRef]
- Ministry of Environment. Act on the Management and Use of Livestock Excreta (Article 13-2, Amended on January 1, 2024). 2024. Available online: https://elaw.klri.re.kr/kor_service/lawView.do?hseq=56253&lang=ENG (accessed on 10 June 2025).
- Sahu, P.K.; Chakradhari, S.; Dewangan, S.; Patel, K.S. Combustion characteristics of animal manures. J. Environ. Prot. 2016, 7, 951–960. [Google Scholar] [CrossRef]
- Park, D.; Kim, C.W.; Yoo, D.H.; Lee, G.M.; Huh, J.S.; Lim, J.O. Feasibility study on improving lower heating value of cattle manure-based solid fuel by utilization of urban waste. New Renew. Energy 2019, 15, 61–68. [Google Scholar] [CrossRef]
- Szymajda, A.; Łaska, G.; Joka, M. Assessment of cow dung pellets as a renewable solid fuel in direct combustion technologies. Energies 2021, 14, 1192. [Google Scholar] [CrossRef]
- Rzeźnik, W.; Rzeźnik, I.; Mielcarek-Bocheńska, P.; Urbański, M. Air pollutants emission during co-combustion of animal manure and wood pellets in 15 kW boiler. Energies 2023, 16, 6691. [Google Scholar] [CrossRef]
- Guo, M.; Li, H.; Baldwin, B.; Morrison, J. Thermochemical processing of animal manure for bioenergy and biochar. In Animal Manure: Production, Characteristics, Environmental Concerns, and Management; Waldrip, H.M., Pagliari, P.H., He, Z., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 2020; pp. 255–274. [Google Scholar] [CrossRef]
- Maj, I. Significance and challenges of poultry litter and cattle manure as sustainable fuels: A review. Energies 2022, 15, 8981. [Google Scholar] [CrossRef]
- Ministry of Environment. Quality Test and Analysis Method for Solid Recovered Fuel Products (Notification No. 2024-10). 2024. Available online: https://www.law.go.kr/ (accessed on 10 June 2025). (In Korean).
- ES 01301.1e–ES 01400.2d; Air Pollution Test Methods. National Institute of Environmental Research: Incheon, Republic of Korea (Amended 2024). Ministry of Environment. 2024. Available online: https://www.law.go.kr/ (accessed on 10 June 2025). (In Korean).
- Kim, H.; Kim, Y.; Song, J. A experiment of combustion behavior of biomass fuels. Trans. Korean Hydrog. New Energy Soc. 2018, 29, 503–511. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Rural Development Administration. Fertilizer Specification and Test Methods (Notification No. 2024-15). 2024. Available online: https://www.law.go.kr/ (accessed on 10 June 2025). (In Korean)
- Jerzak, W. Experimental and thermodynamic analysis of trace element speciation during the combustion of ground cedar nut shells. Energy Fuels 2017, 31, 1969–1979. [Google Scholar] [CrossRef]
- Leng, L.; Bogush, A.A.; Roy, A.; Stegemann, J.A. Characterisation of ashes from waste biomass power plants and phosphorus recovery. Sci. Total Environ. 2019, 690, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Megel, A.; Parker, D.; Mitra, R.; Sweeten, J. Assessment of chemical and physical characteristics of bottom, cyclone, and baghouse ashes from the combustion of manure (Paper No. 062035). In Proceedings of the ASABE Annual International Meeting, Portland, OR, USA, 9–12 July 2006; American Society of Agricultural and Biological Engineers: Portland, OR, USA, 2006. [Google Scholar] [CrossRef][Green Version]
- Silva, F.C.; Cruz, N.C.; Tarelho, L.A.C.; Rodrigues, S.M. Use of biomass ash-based materials as soil fertilisers: Critical review of the existing regulatory framework. J. Clean. Prod. 2019, 214, 112–124. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).