Mechanoluminescent-Boosted NiS@g-C3N4/Sr2MgSi2O7:Eu,Dy Heterostructure: An All-Weather Photocatalyst for Water Purification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of g-C3N4
2.3. Preparation of NiS@g-C3N4
2.4. Preparation of Sr2MgSi2O7:Eu,Dy
2.5. Preparation of the NiS@g-C3N4/Sr2MgSi2O7:Eu,Dy Composite
2.6. Characterizations
2.7. Photocatalytic Activity Test
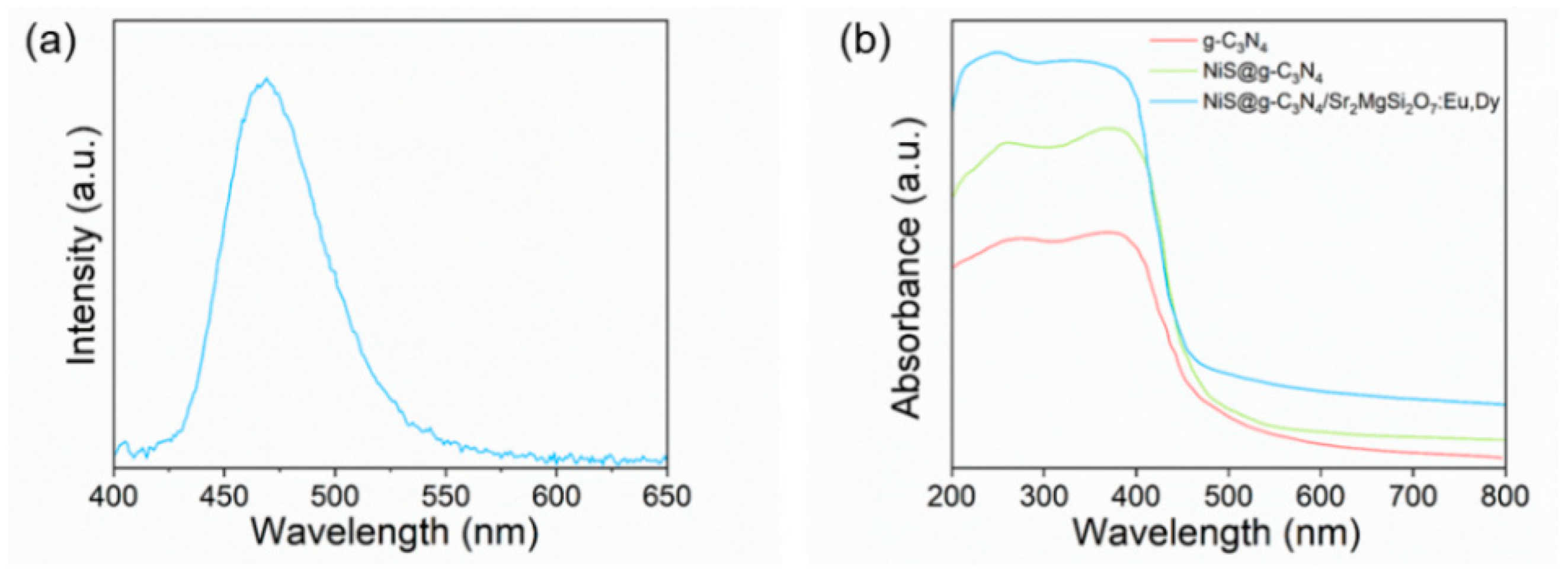
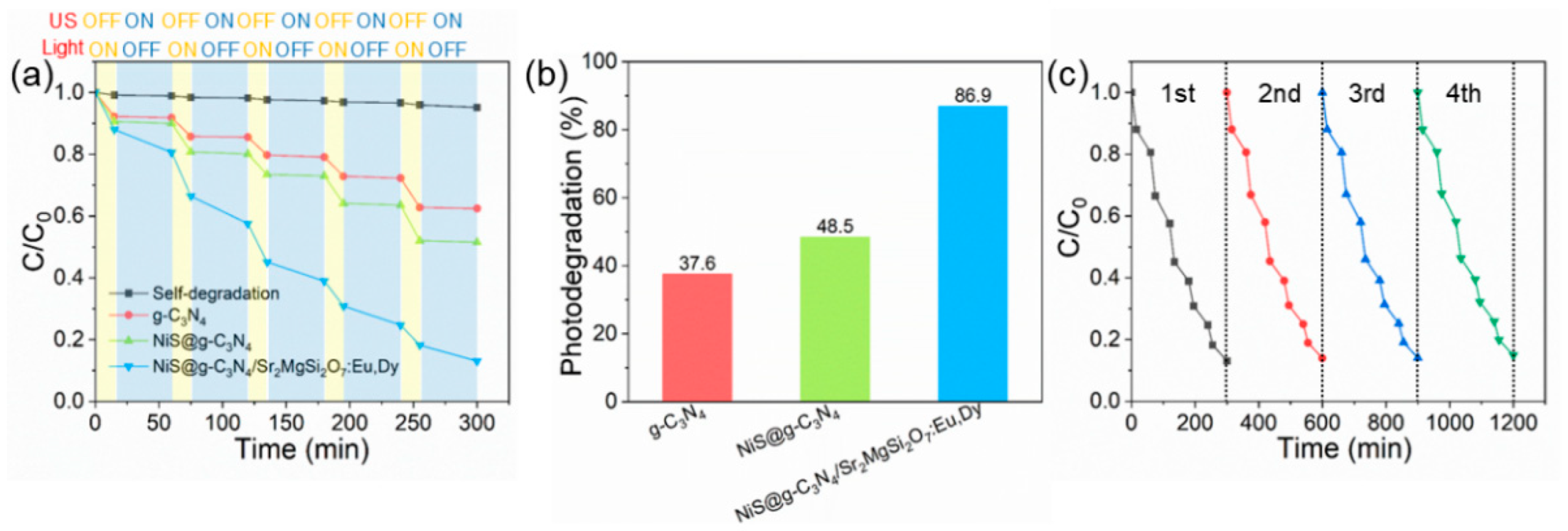
3. Results
Results of the Characterizations and Photocatalytic Activity Tests
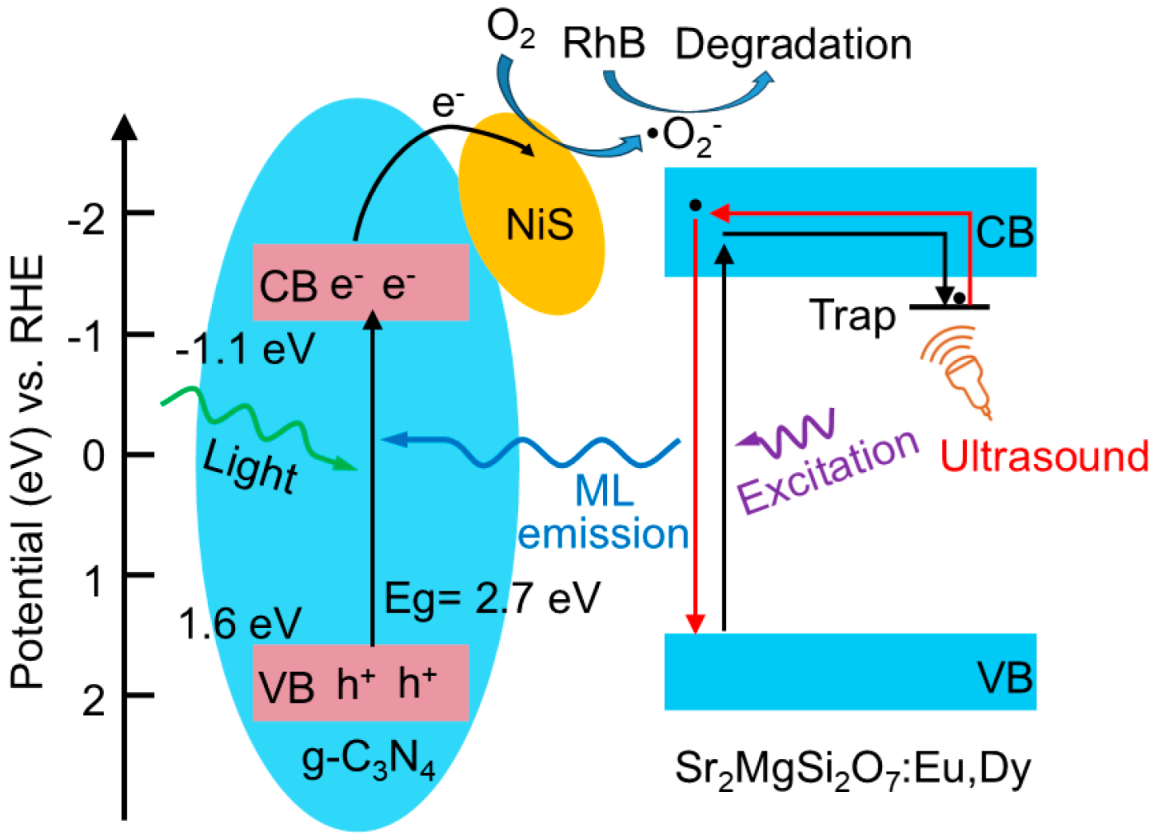
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotox. Environ. Safe 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Wang, Z. Construction of WO3/g-C3N4 heterojunction via cold plasma for enhanced visible-light-driven photocatalysis. Mater. Lett. 2025, 389, 138413. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Hu, Z.; Liu, F.; Zuo, Z.; Gao, Z.; Zhang, H.; Zhu, Y.; Liu, R.; Yin, Y.; et al. Boosted Charge Transfer for Highly Efficient Photosynthesis of H2O2 over Z-Scheme I−/K+ Co-Doped g-C3N4/Metal–Organic-Frameworks in Pure Water under Visible Light. Adv. Energy Mater. 2024, 14, 2401873. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, S.; Deng, J.; Gangadharan, D.T.; Yang, F.; Xu, Z.; Giorgi, G.; Palummo, M.; Chaker, M.; Ma, D. Ice-Assisted Synthesis of Black Phosphorus Nanosheets as a Metal-Free Photocatalyst: 2D/2D Heterostructure for Broadband H2 Evolution. Adv. Funct. Mater. 2019, 29, 1902486. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, Y.; Cai, C.; Lai, S.; Cheng, L.; Zhang, J.; Zhu, W.; Guo, Y.; Hou, M.; Ma, L.; et al. Flash joule heating synthesis of nitrogen-rich defective g-C3N4 for highly efficient photocatalytic hydrogen evolution. Small 2025, e2503335. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, K.; Li, Z.; Chen, Z. W18O49/ZnIn2S4 S-Scheme photocatalyst with full-spectrum response for efficient H2O2 production. J. Mater. Sci. Technol. 2026, 245, 309–321. [Google Scholar] [CrossRef]
- Cao, K.; Ge, X.; Li, S.; Tian, Z.; Cui, S.; Guo, G.; Yang, L.; Li, X.; Wang, Y.; Bai, S.; et al. Facile preparation of a 3D rGO/g-C3N4 nanocomposite loaded with Ag NPs for photocatalytic degradation. RSC Adv. 2025, 15, 17089–17101. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Q.; Sun, N.; Su, H.; Chen, P.; Wu, J.; Fu, Y.; Ma, J. Giant improvement in piezo-photocatalytic capability of colloidal g-C3N4 quantum dots. Prog. Nat. Sci. 2025, in press. [Google Scholar] [CrossRef]
- Samaniego-Benitez, J.E.; Jimenez-Rangel, K.; Lartundo-Rojas, L.; García-García, A.; Mantilla, A. Enhanced photocatalytic H2 production over g-C3N4/NiS hybrid photocatalyst. Mater. Lett. 2021, 290, 129476. [Google Scholar] [CrossRef]
- Shan, X.; Yang, X.; Liu, F.; Zhang, X.; Chen, J.; Liu, D.; Zhang, Q.; Liu, R.; Yin, Y.; Cai, Y. Photoreduction and extraction of U(VI) from mining wastewater using magnetically recoverable ZnIn2S4-based Z-scheme photocatalysts. Chem. Eng. J. 2025, 517, 164483. [Google Scholar] [CrossRef]
- Shen, R.; Zhang, L.; Chen, X.; Jaroniec, M.; Li, N.; Li, X. Integrating 2D/2D CdS/α-Fe2O3 ultrathin bilayer Z-scheme heterojunction with metallic β-NiS nanosheet-based ohmic-junction for efficient photocatalytic H2 evolution. Appl. Catal. B Environ. Energy 2020, 266, 118619. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, Z.; Deng, P.; Zhang, L.; Hou, Y. NiSe2/Cd0.5Zn0.5S as a type-II heterojunction photocatalyst for enhanced photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 15389–15397. [Google Scholar] [CrossRef]
- Yang, F.; Kim, S.J.; Wu, X.; Cui, H.; Hahn, S.K.; Hong, G. Principles and applications of sono-optogenetics. Adv. Drug Deliver. Rev. 2023, 194, 114711. [Google Scholar] [CrossRef]
- Zhang, J.C.; Wang, X.; Marriott, G.; Xu, C.N. Trap-controlled mechanoluminescent materials. Prog. Mater. Sci. 2019, 103, 678–742. [Google Scholar] [CrossRef]
- Yang, F.; Wu, X.; Cui, H.; Jiang, S.; Ou, Z.; Cai, S.; Hong, G. Palette of rechargeable mechanoluminescent fluids produced by a biomineral-inspired suppressed dissolution approach. J. Am. Chem. Soc. 2022, 144, 18406–18418. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wu, J.; Li, H.; Liu, D.; Zhang, Q.; Li, K. Mechanoluminescent-Boosted NiS@g-C3N4/Sr2MgSi2O7:Eu,Dy Heterostructure: An All-Weather Photocatalyst for Water Purification. Processes 2025, 13, 2416. https://doi.org/10.3390/pr13082416
Huang Y, Wu J, Li H, Liu D, Zhang Q, Li K. Mechanoluminescent-Boosted NiS@g-C3N4/Sr2MgSi2O7:Eu,Dy Heterostructure: An All-Weather Photocatalyst for Water Purification. Processes. 2025; 13(8):2416. https://doi.org/10.3390/pr13082416
Chicago/Turabian StyleHuang, Yuchen, Jiamin Wu, Honglei Li, Dehao Liu, Qingzhe Zhang, and Kai Li. 2025. "Mechanoluminescent-Boosted NiS@g-C3N4/Sr2MgSi2O7:Eu,Dy Heterostructure: An All-Weather Photocatalyst for Water Purification" Processes 13, no. 8: 2416. https://doi.org/10.3390/pr13082416
APA StyleHuang, Y., Wu, J., Li, H., Liu, D., Zhang, Q., & Li, K. (2025). Mechanoluminescent-Boosted NiS@g-C3N4/Sr2MgSi2O7:Eu,Dy Heterostructure: An All-Weather Photocatalyst for Water Purification. Processes, 13(8), 2416. https://doi.org/10.3390/pr13082416





