Enhancement of Phenolic and Polyacetylene Production in Chinese Lobelia (Lobelia chinensis Lour.) Plant Suspension Culture by Employing Silver, Iron Oxide Nanoparticles and Multiwalled Carbon Nanotubes as Elicitors
Abstract
1. Introduction
2. Results
2.1. Effects of AgNPs on Biomass Accumulation, Production of Bioactive Compounds, and Antioxidant Activity
2.1.1. Effect on Biomass Accumulation
2.1.2. Effect on Bioactive Compound Production
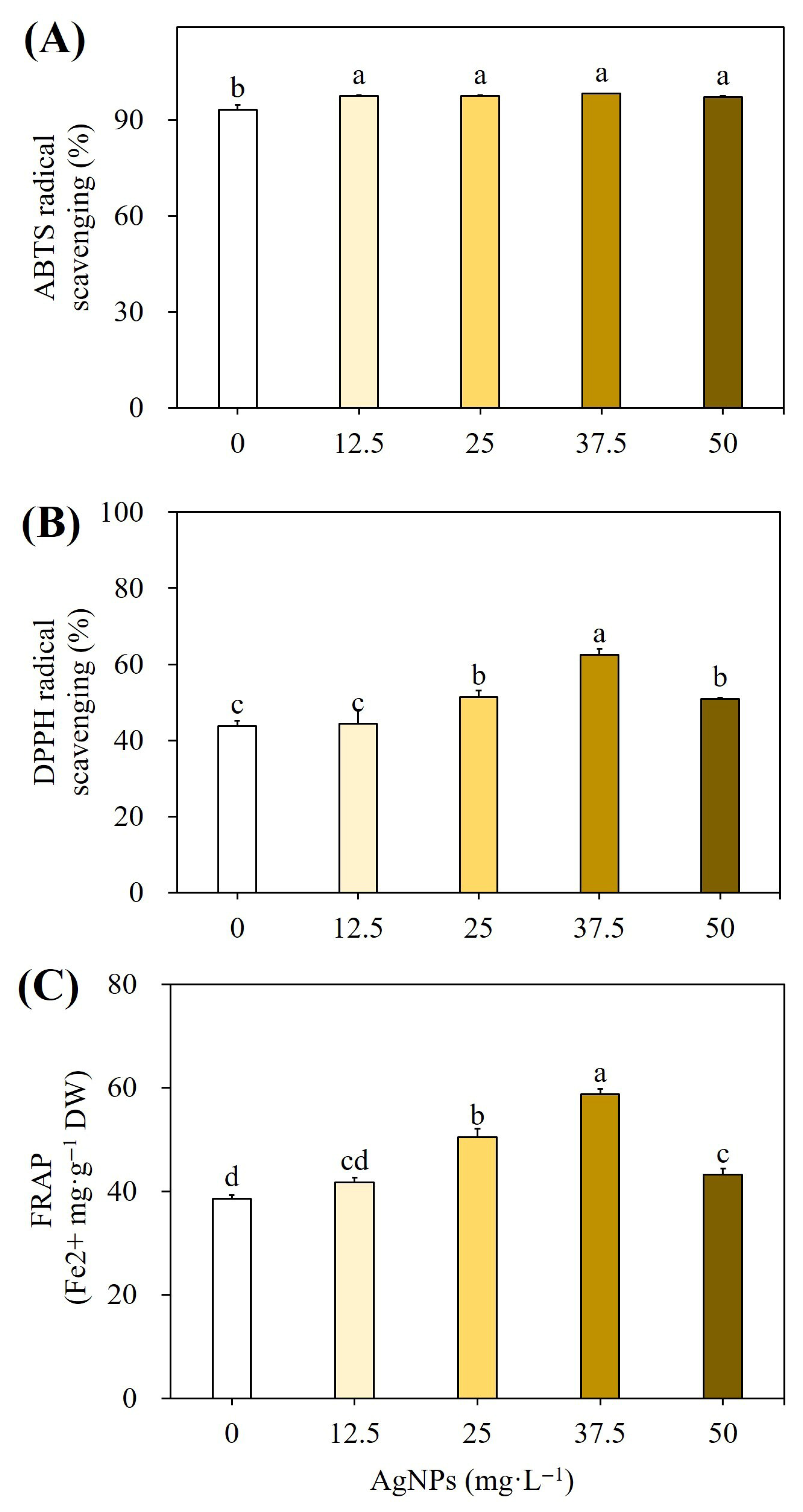
2.1.3. Effect on Antioxidant Activities
2.2. Effects of Fe2O4NPs on Biomass Accumulation, Production of Bioactive Compounds, and Antioxidant Activity
2.2.1. Effect on Biomass Accumulation
2.2.2. Effect on Bioactive Compound Production
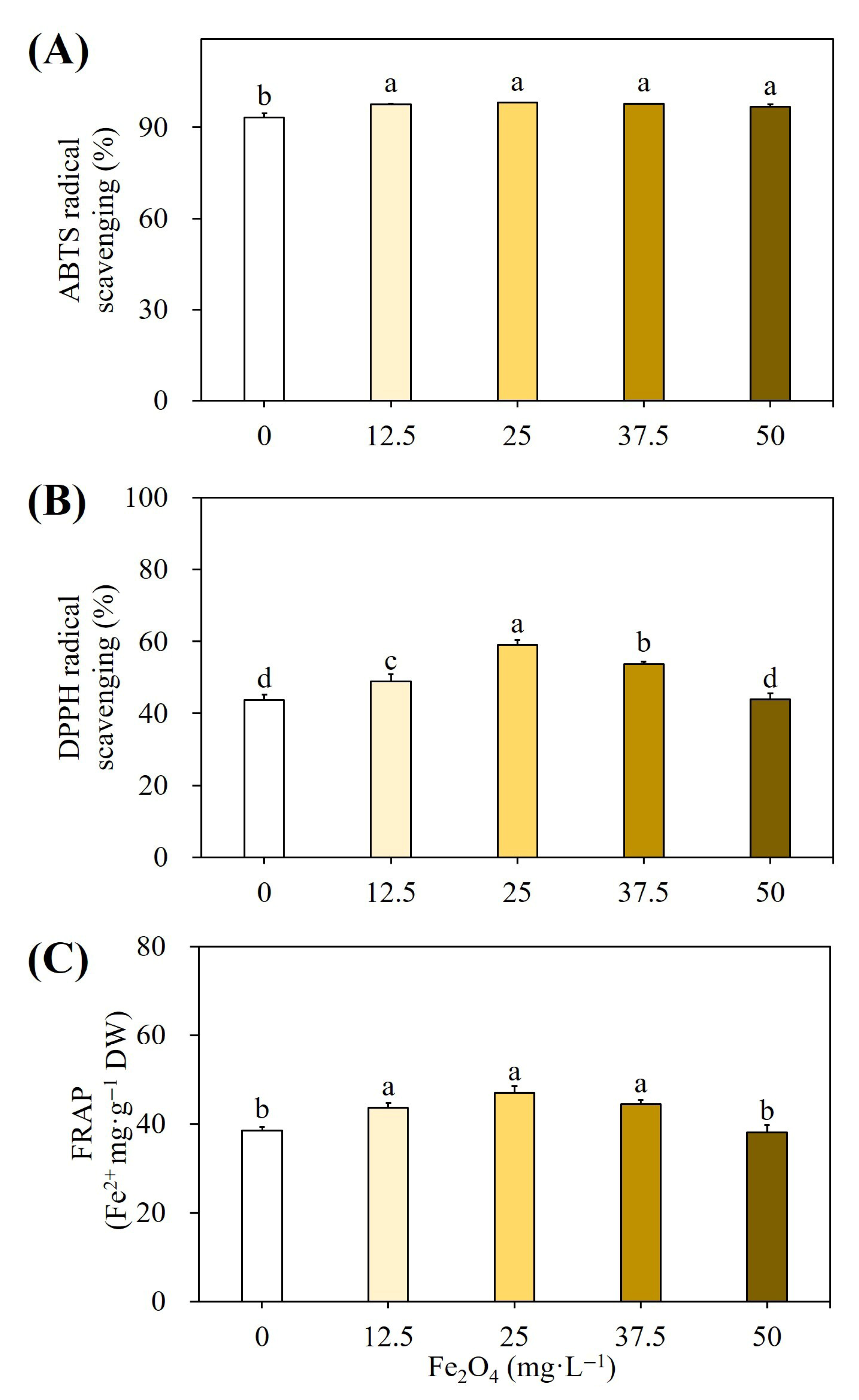
2.2.3. Effect on Antioxidant Activities
2.3. Effects of MWCNTs on Biomass Accumulation, Production of Bioactive Compounds, and Antioxidant Activity
2.3.1. Effect on Biomass Accumulation
2.3.2. Effect on Bioactive Compound Production
2.3.3. Effect on Antioxidant Activities
3. Discussion
3.1. Effect of Nanomaterials on the Growth Performance of Chinese Lobelia Plants In Vitro
3.2. Effect of Nanomaterials on the Accumulation of Bioactive Compounds and Antioxidant Activities in Chinese Lobelia Plant Cultures
4. Materials and Methods
4.1. Establishment of In Vitro Culture
4.2. Elicitation
4.3. Biomass Estimation
4.4. Plant Extract Preparation
4.5. Quantification of Phenolic Content
4.6. Quantification of Flavonoid Content
4.7. Analysis of Phenolics Using HPLC
4.8. Analysis of Polyacetylenes Using HPLC
4.9. Antioxidant Activity Assays
4.9.1. DPPH Assay
4.9.2. ABTS Assay
4.9.3. FRAP Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethybenzothiazoline-6-sulphonic acid |
| AgNPs | Silver nanoparticles |
| AAE | Ascorbic acid equivalent |
| CE | Catechin equivalent |
| DPPH | 2,2 Diphenyl 1 picrylhydrazyl |
| DW | Dry weight |
| FRAP | Ferric reducing antioxidant power |
| Fe2O4NPs | Iron oxide nanoparticles |
| FW | Fresh weight |
| HPLC | High-performance liquid chromatography |
| LEDs | Light-emitting diodes |
| NP | Nanoparticle |
| NM | Nanomaterial |
| MWCNTs | Multiwalled carbon nanotubes |
| MS | Murashige and Skoog medium |
| UV | Ultraviolet light |
References
- Li, S. The Ben Cao Gang Mu, 1st ed.; University of California Press: Oakland, CA, USA, 2016; p. 625. (In Chinese) [Google Scholar]
- Kuo, P.C.; Hwang, T.L.; Lin, Y.T.; Kuo, Y.C.; Leu, Y.L. Chemical constituents from Lobelia chinensis and their anti-virus and anti-inflammatory bioactivates. Arch. Pharmacal Res. 2011, 34, 715–722. [Google Scholar] [CrossRef]
- Chen, M.S.; Chen, W.R.; Zhang, J.M.; Long, X.Y.; Wang, Y.T. Lobelia chinesnis: Chemical constituents and anticancer activity perspective. Chin. J. Nat. Med. 2014, 12, 103–107. [Google Scholar] [CrossRef]
- Yang, S.; Shen, T.; Zhao, L.J.; Li, C.; Zhang, Y.; Lou, H.X.; Ren, D.M. Chemical constituents of Lobelia chinensis. Fitoterapia 2014, 93, 168–174. [Google Scholar] [CrossRef]
- Li, K.C.; Ho, Y.L.; Huang, G.J.; Chang, Y.S. Anti-oxidative and anti-inflammatory effects of Lobelia chinensis in vitro and in vivo. Am. J. Chin. Med. 2015, 43, 269–287. [Google Scholar] [CrossRef]
- Zhang, L.; Reddy, N.; Khoo, C.; Koyyalamudi, S.R.; Jones, C.E. Antioxidant and immunomodulatory activities and structural characterization of polysaccharides isolated from Lobelia chinensis Lour. Pharmacologia 2018, 9, 157–168. [Google Scholar]
- Kuo, Y.C.; Lee, Y.C.; Leu, Y.L.; Tsai, W.J.; Chang, S.C. Efficacy of orally administered Lobelia chinensis extracts on herpes simplex virus type 1 infection in BALB/c mice. Antivir. Res. 2008, 80, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, P.; Zhang, X.; Li, X. Chemical structure elucidation of an inulin-type fructan isolated from Lobelia chinensis Lour. with anti-obesity on diet-induced mice. Carbohydr. Polym. 2020, 240, 116357. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Lee, I.A. The anti-tubercular activity of Melia azedarach L. and Lobelia chinensis Lour. and their potential as effective anti-Mycobacterium tuberculosis candidate agents. Asian Pac. J. Trop. Biomed. 2016, 6, 830–835. [Google Scholar] [CrossRef]
- Shao, J.H.; Zhang, H. Influence of Lobelia chinses Lour. Decoction on expression of C-erbB-2 and P53 on H22 tumor-bearing mice. Chin. J. Clin. Pharm. 2010, 19, 372–375. [Google Scholar]
- Santosa, M.H.; Herzog, R.; Voelter, W. Antitumor activity of the hot water extract of Lobelia chinensis. Planta Med. 1986, 6, 555. [Google Scholar] [CrossRef]
- Bai, X.; Lee, H.S.; Murthy, H.N.; Kwon, H.J.; Yeon, S.H.; Ju, J.Y.; Park, S.Y. Micropropagation of Lobelia chinensis Lour.: Influence of medium parameters on plant regeneration, antioxidant activity and secondary metabolite accumulation. Korean J. Plant Res. 2024, 37, 225–234. [Google Scholar] [CrossRef]
- Bai, X.; Lee, H.S.; Han, E.J.; Murthy, H.N.; Paek, K.Y.; Park, S.Y. Stimulating synthesis of phenolics and polyacetylenes in Lobelia chinensis Lour. Plantlets using various bioreactor systems: A comparison of parameter effects. Plant Cell Tissue Organ Cult. 2024, 16, 36. [Google Scholar] [CrossRef]
- Bai, X.L.; Murthy, H.N.; Park, S.Y. In vitro propagation of Lobelia chinensis Lour. under different LED lights. Rom. Biotechnol. Lett. 2023, 28, 3942–3949. [Google Scholar] [CrossRef]
- Ramirez-Estrda, K.; Limon, H.V.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cudio, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Production of specialized metabolites in plant cell and organo-cultures: The role of gamma radiation in eliciting secondary metabolism. Int. J. Rad. Biol. 2024, 100, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Light as an elicitor for enhanced production of secondary metabolites in plant cell, tissue, and organ cultures. Plant Growth Regul. 2024, 104, 31–49. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites in cell and organ cultures: Current status and future outlooks. Plant Growth Regul. 2024, 104, 5–30. [Google Scholar] [CrossRef]
- Sharafi, E.; Khayam Nekoei, S.M.; Fotokian, M.H.; Davoodi, D.; Hadavand Mirzaei, H.; Hsanloo, T. Improvement of hypericin and hyperforin production using zinc and iron nano-oxides as elicitors in cell suspension culture of St. John’s wort (Hypericum perforatum L.). J. Med. Plants By-Prod. 2013, 2, 177–184. [Google Scholar]
- Poboilova, Z.; Opatrilova, R.; Babula, P. Toxicity of aluminum oxide nanoparticles demonstrated using a BY-2 plant cell suspension culture model. Environ. Exp. Bot. 2013, 91, 1–11. [Google Scholar] [CrossRef]
- Chung, I.M.; Rajakumar, G.; Thiruvengadam, M. Effect of silver nanoparticles on phenolic compounds production and biological activities in hairy root cultures of Cucumis anguria. Acta Biol. Hung. 2018, 69, 97–109. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, Y.; Yoon, H.; Hwang, I.; Chang, Y.S. Iron nanoparticle-induced activation of plasma membrane H+-ATPase promotes stomatal opening in Arabidopsis thaliana. Environ. Sci. Technol. 2015, 49, 1113–1119. [Google Scholar] [CrossRef]
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2012, 27, 42–49. [Google Scholar] [CrossRef]
- Tariverdizadeh, N.; Mohebodini, M.; Chamani, E.; Ebadi, A. Iron and zinc oxide nanoparticles: An efficient elicitor to enhance trigonelline alkaloid production in hairy roots of fenugreek. Ind. Crops Prod. 2021, 162, 113240. [Google Scholar] [CrossRef]
- Bai, X.; Lee, H.S.; Han, J.E.; Murthy, H.N.; Park, S.Y. Enhancement of phenolic and polyacetylene accumulation in Lobelia chinensis (Chinese lobelia) plantlet cultures through yeast extract and salicylic acid elicitation. Horticulturae 2025, 11, 612. [Google Scholar] [CrossRef]
- Wilson, S.A.; Roberts, S.C. Recent advances towards development and commercialization of plant cell culture processes for synthesis of biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; De-silva, K.; Nedosekin, D.A.; Dervishi, A.; Biris, A.S.; Shashkov, E.V.; Galnzha, E.V.; Galanzha, E.J.; Zharov, V.P. Complex genetic, photochemical, and photacoustic analysis of nanoparticle-plant interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Inokuchi, T.; Fujioka, S.; Kimura, Y. Phenolic compounds and flavonoids as plant growth regulators from fruit and leaf of Vitex rotundifolia. Z. Naturforschung C 2004, 59, 509–514. [Google Scholar] [CrossRef]
- Kaveh, R.; Li, Y.S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Aken, B.V. Changes in Arabidopsis thaliana gene expression in response silver nanoparticles and silver ions. Environ. Sci. Technol. 2013, 47, 10637–10644. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Gurunathn, S.; Chung, I.M. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma 2015, 252, 1031–1046. [Google Scholar] [CrossRef]
- Khan, M.A.; Ali, A.; Mohammed, S.; Ali, H.; Khan, T.; Mashwani, Z.R.; Jan, A.; Ahmad, P. Iron nono modulated growth and biosynthesis of steviol glycosides in Stevia rebaudiana. Plant Cell Tissue Organ Cult. 2020, 143, 121–130. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadin, J. Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 2015, 94, 749–759. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, Y.; Lou, W.; Su, J.; Wei, S.; Yang, X.; Wang, R.; Guan, R.; Pu, H.; Shen, W. Nitrate reductase-dependent nitric oxide is crucial for multi-walled carbon nanotube-induced plant tolerance against salinity. Nanoscale 2019, 11, 10511–10523. [Google Scholar] [CrossRef]
- Sareea Al-Rekaby, L. Influence of multiwalled carbon nanotubes and biostimulators on growth and content of bioactive constituents of karkade (Hibiscus sabadariffa L.). J. Bot. 2018, 2018, ID9097363. [Google Scholar] [CrossRef]
- Ghasempour, M.; Iranbakhsh, A.; Ebadi, M.; Oraghi Aardebili, Z. Multi-walled carbon nanotubes improved growth, anatomy, physiology, secondary metabolism, and callus performance in Catharanthus roseus: An in vitro study. Biotech 2019, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Dilshad, E.; Ismail, H.; Khan, M.A.; Cusido, R.M.; Mirza, B. Metabolite profiling of Artemisia carvifolia Buch. transgenic plants and estimation of their anticancer and antidiabetic potential. Bioctal. Agric. Biotechnol. 2020, 25, 101539. [Google Scholar] [CrossRef]
- Yousaf, R.; Khan, M.A.; Ullah, N.; Khan, I.; Hayat, O.; Shehzad, M.A.; Khan, I.; Taj, F.; Ud Din, N.; Khan, A. Biosynthesis of anti-leishmanial natural products in callus cultures of Artemisia scoparia. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Peng, X.; Han, X.; Ren, J.; Sun, L.; Fu, Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 2013, 25, 1947–1956. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Villagarcia, H.; Dervishi, E.; de Silva, K.; Biris, A.S.; Khodakovskaya, M.V. Surface chemistry of carbon nanotubes impacts the growth and expression of water channel protein in tomato plants. Small 2012, 8, 2328–2334. [Google Scholar] [CrossRef]
- Keller, A.A.; Lazareva, A. Predicted releases of engineered nano-materials: From global to regional to local. Environ. Sci. Technol. Lett. 2014, 1, 65–70. [Google Scholar] [CrossRef]
- Pokhrel, L.R.; Dubey, B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci. Total Environ. 2013, 452, 321–332. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Armendariz, R.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of silver nanoparticles on radish sprouts: Root growth reduction and modifications in the nutritional value. Front. Plant Sci. 2016, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Riaz, M.S.; Ullah, N.; Alid, H.; Nadhmane, A. Plant cell nanomaterials interaction: Growth, physiology and secondary metabolism. Analysis, fate, and toxicity of engineered nanomaterials in plants. Compr. Anal. Chem. 2019, 84, 23–54. [Google Scholar] [CrossRef]
- Fazal, H.; Abbaisi, B.H.; Ahmad, N.; Ali, M. Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L. Appl. Biochem. Biotechnol. 2016, 180, 1076–1092. [Google Scholar] [CrossRef] [PubMed]
- Begaum, S.; Zahid, A.; Khan, T.; Khan, N.Z. Comparative analysis of the effects of chemically and biologically synthesized silver nanoparticles on biomass accumulation and secondary metabolism in callus cultures of Fagonia indica. Physiol. Mol. Biol. Plants 2020, 26, 1739–1750. [Google Scholar] [CrossRef]
- Chung, I.M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Elicitation of silver nanoparticles enhanced the secondary metabolites and pharmacological activities in cell suspension cultures of bitter gourd. 3 Biotech 2018, 8, 412. [Google Scholar] [CrossRef]
- Jamshidi, M.; Ghanati, F.; Rezaei, A.; Bemani, E. Change of antioxidant enzymes activity of hazel (Corylus avellana L.) cells by AgNPs. Cytotechnology 2016, 68, 525–530. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, G.; Ramachandran, R.; Abirami, S.M.; Mohan, N.; Kalaichelvan, P.T. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri L. Wettst. plant growth metabolism. Process Biochem. 2012, 47, 651–658. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Nasibi, F.; Kalntari, K.M.; Ghanati, F. Evaluation of secondary metabolites and antioxidant activity in Dracocephalum polychaetum Bornm. cell suspension culture under magnetite nanoparticles and static magnetic field cultivation. Plant Cell Tissue Organ Cult. 2019, 136, 489–498. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Fraklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef]
- Lindermayr, C.; Durner, J. Interplay of reactive oxygen species homeostasis. Plant Physiol. 2015, 167, 1209–1210. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.J.; Wang, Y.J.; Zhou, Y.H.; Tao, Y.; Mao, W.H.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.Q. Reactive oxygen species are involved in barassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, A.; Zhang, Z.; Huang, Z.; Lu, P.; Zhang, D.; Liu, X.; Zhang, Z.F.; Huang, R. Ethylene response factor TREF1, regulated by ETHYLEND-RESPONSE3-like factors, functions in reactive oxygen species (ROS) scavenging in tobacco (Nicotiana tabacum L.). Sci. Rep. 2016, 6, 29948. [Google Scholar] [CrossRef]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Abdollahi Mandoulakani, B.A. Iron oxide nanoparticles: A novel elicitor to enhance anticancer flavonoid production and gene expression in Dracocephalum kotschyi hairy-root cultures. J. Sci. Food Agric. 2019, 99, 6418–6430. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Anbazhagn, V.; Dhankher, O.M.; Vara Prasad, P.V. Uptake, tranlocaiton, toxicity, and impact of nanoparticles on plant physiological processes. Plants 2024, 13, 3137. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 373–497. [Google Scholar] [CrossRef]
- Harborne, A.J. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Springer: Dordrecht, The Netherlands, 1994. [Google Scholar] [CrossRef]
- Burin, V.M.; Arari, S.G.; Costa, L.L.F.; Bordingon-Luiz, A.M.T. Determination of some phenolic compounds in red wind by RP-HPLC: Method development and validation. J. Chromatogr. Sci. 2011, 49, 647–651. [Google Scholar] [CrossRef]
- Qiao, C.F.; He, Z.D.; Han, Q.B.; Hu, H.X.; Jiang, R.W.; Li, S.L.; Zhang, Y.B.; But, P.P.H.; Shaw, P.C. The use of lobetyolin and HPLC-UV fingerprints for quality assessment of radix codonopsis. J. Food Drug. Anal. 2007, 15, 258–264. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]









|
AgNPs x (mg·L−1) |
Fresh Biomass (g·L−1) |
Dry Biomass (g·L−1) | Growth Ratio |
|---|---|---|---|
| 0 | 181.0 c | 17.6 b | 29.2 b |
| 12.5 | 191.0 b | 19.6 a | 30.8 b |
| 25 | 216.4 a | 21.6 a | 35.1 a |
| 37.5 | 213.4 a | 21.0 a | 34.6 a |
| 50 | 185.4 b | 17.0 b | 29.9 b |
|
Fe2O4 x (mg·L−1) |
Fresh Biomass (g·L−1) |
Dry Biomass (g·L−1) | Growth Ratio |
|---|---|---|---|
| 0 | 181.0 e | 17.6 c | 29.2 c |
| 12.5 | 220.0 b | 23.0 a | 35.7 b |
| 25 | 230.0 a | 23.4 a | 37.3 a |
| 37.5 | 205.6 c | 20.4 b | 33.3 b |
| 50 | 188.0 d | 17.4 c | 30.3 c |
|
MWCNTs (mg·L−1) x |
Fresh Biomass (g·L−1) |
Dry Biomass (g·L−1) | Growth Ratio |
|---|---|---|---|
| 0 | 181.0 d | 17.6 d | 29.2 c |
| 12.5 | 240.6 a | 22.6 a | 39.1 a |
| 25 | 217.4 b | 20.6 b | 35.2 b |
| 37.5 | 195.4 c | 19.4 c | 31.6 b,c |
| 50 | 185.6 c,d | 17.0 d | 29.9 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Lee, H.-S.; Han, J.-E.; Murthy, H.N.; Park, S.-Y. Enhancement of Phenolic and Polyacetylene Production in Chinese Lobelia (Lobelia chinensis Lour.) Plant Suspension Culture by Employing Silver, Iron Oxide Nanoparticles and Multiwalled Carbon Nanotubes as Elicitors. Processes 2025, 13, 2370. https://doi.org/10.3390/pr13082370
Bai X, Lee H-S, Han J-E, Murthy HN, Park S-Y. Enhancement of Phenolic and Polyacetylene Production in Chinese Lobelia (Lobelia chinensis Lour.) Plant Suspension Culture by Employing Silver, Iron Oxide Nanoparticles and Multiwalled Carbon Nanotubes as Elicitors. Processes. 2025; 13(8):2370. https://doi.org/10.3390/pr13082370
Chicago/Turabian StyleBai, Xinlei, Han-Sol Lee, Jong-Eun Han, Hosakatte Niranjana Murthy, and So-Young Park. 2025. "Enhancement of Phenolic and Polyacetylene Production in Chinese Lobelia (Lobelia chinensis Lour.) Plant Suspension Culture by Employing Silver, Iron Oxide Nanoparticles and Multiwalled Carbon Nanotubes as Elicitors" Processes 13, no. 8: 2370. https://doi.org/10.3390/pr13082370
APA StyleBai, X., Lee, H.-S., Han, J.-E., Murthy, H. N., & Park, S.-Y. (2025). Enhancement of Phenolic and Polyacetylene Production in Chinese Lobelia (Lobelia chinensis Lour.) Plant Suspension Culture by Employing Silver, Iron Oxide Nanoparticles and Multiwalled Carbon Nanotubes as Elicitors. Processes, 13(8), 2370. https://doi.org/10.3390/pr13082370







