Abstract
Agresto is the unfermented juice traditionally obtained from boiled unripe grapes, typically using fruit that would otherwise be discarded, and enriched with spices, herbs, and fruit. In this study, the phenolic profile, antioxidant and antibacterial activity, and volatile organic compounds (VOCs) of Agresto produced from two grape varieties (Sangiovese, and Vermentino) harvested in Mount Amiata (Tuscany) were evaluated. Agresto from Vermentino showed a higher total phenolic content (TPC), 1.31 mg GAE/mL, as well as a greater total flavonoid and flavonol content and FRAP activity compared to Agresto from Sangiovese. The highest ORAC value was observed in Agresto from Vermentino, 41.01 mg TE/mL, compared to that from Sangiovese. TPC, flavonols, apocarotenes, sulfur derivatives, and non-terpene derivatives were positively correlated with antimicrobial activity against E. coli, FRAP, and ORAC. Overall, our results showed that grape variety significantly influences the chemical composition of Agresto, particularly in terms of both VOCs and phenolic compounds. The observed variations in phenolic composition also affected the antioxidant and antimicrobial activity of Agresto. These experimental findings clearly suggest the utmost importance of identifying the optimal chemical profile of “unripe grapes” used as raw material for Agresto production, considering both variety and the specific ripening degree achievable through vine green harvesting.
1. Introduction
In the last few decades, waste management has been regarded as an essential tool for driving the implementation of a circular economy [1,2].
Its goal is to close resource loops and retain valuable materials within the economic system. Notably, agro-industrial residues represent one of the most significant waste streams, both in terms of quantity and quality [3] and can be effectively utilized as a source of bioactive compounds to meet the growing demand for “healthy functional foods”. Among these, food waste from berries and grapes offers significant health benefits due to its high content of vitamins, minerals, lipids, and fiber, as well as phenolic compounds, which are the predominant phytochemicals in these fruits [4,5].
In this context, ‘vine green harvesting’ can also be considered a source of waste, as bunch thinning is carried out by removing unripe grape bunches to improve production quality. Some authors have reported the potential use of unripe grapes in winemaking to reduce the alcohol concentration and pH of wines [6,7].
Additionally, unripe grapes can serve as an emerging source of various nutraceutical compounds, including sugars, organic acids, phenolic compounds, vitamins, fibers, mineral salts, lipids, and other minor compounds [8].
In the Mediterranean area, where the vine is one of the main crops [2], unripe grapes have traditionally been used to produce various products, such as sauces, acidic juices, digestive drinks, and syrups, within the frame of circular economy and sustainability; they are also added to typical products as a dried or fresh ingredient [9,10,11,12,13,14]. The oldest use of unripe grapes is to turn them into an acidic juice called verjuice, which has different names depending on the country where it is produced: Vertjus or Verjus in France, Agraz in Spain and Germany, Agresto in Italy, Koruk in Turkey, and Abe-ghureh in Persia [14].
Agresto is a traditional seasoning from the Italian region of Tuscany made from the unfermented juices of unripe grapes, concentrated by heating. The main ingredient is unripe grape juice, which is then reduced by heating, flavored with spices and aromas, and finally mixed with a variable amount of vinegar and honey [15,16].
The biosynthesis of all phenolic compounds occurs through the phenylpropanoid pathway from the amino acid phenylalanine, leading to the production of different classes of compounds: phenolic acids (e.g., hydroxycinnamic and hydroxybenzoic acids), flavonoids (e.g., anthocyanins, flavonols and flavan-3-ols), and stilbenes [17,18].
The final content and composition of phenolic compounds are influenced by multiple factors, such as grape variety, climate, soil, and growing conditions [17]; these compounds play important roles in plant growth, reproduction, and defense reactions. Furthermore, these compounds have considerable influences on grape quality and sensory characteristics—particularly astringency, bitterness, and color intensity and stability [19,20].
Phenolic compounds in grape juice, grape seed, and wine have been investigated by many researchers to show their potent antioxidant, anti-inflammatory, anticancerogenic, antimutagenic, antibacterial, antiviral, antifungal, and antiulcer activities [21].
Moreover, volatile organic compounds (VOCs) are the primary contributors to fruit aroma, and the sensory quality of fruits is largely determined by their qualitative and quantitative composition [22]. Grape aroma is a complex of hundreds of VOCs belonging to different chemical classes such as alcohols, esters, acids, terpenes, aldehydes, furanones, pyrazines, isoprenoids and many others. VOCs play an important role as they determine the flavor of grapes and their derivatives with different nuances, such as specific fruits, flowers, honey, grass, vinegar, and others. They can be found in their free or bound forms in different proportions, depending on whether the cultivar is aromatic or non-aromatic [23].
To date, despite the long-standing history of this product and its recent resurgence in popularity across various countries—both in the form of Verjus and Agresto—no studies have been conducted on the development, characterization, and assessment of the bioactivities of Agresto produced in Tuscany from unripe grapes and subsequently infused with herbs. Further, few data are available [8,16] about the influence of grape variety on the chemical composition of Agresto.
Therefore, this study aimed to identify the differences in terms of antioxidant activity, antimicrobial activity, and the profile of phenolic compounds and volatile organic compounds (VOCs) between Agresto obtained from two different grape varieties (Vermentino and Sangiovese) selected among the most cultivated in Tuscany.
To focus our attention on the role of grape variety, the composition of the herb blend and the experimental conditions were kept constant across all experimental runs.
2. Materials and Methods
2.1. Chemicals and Reagents
All standards and reagents were of analytical grade. Hexane, methanol, Folin–Ciocalteu reagent, gallic acid, catechin, sodium carbonate, tripyridyltriazine, Trolox, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH), phosphate-buffered saline (PBS), and LC-MS/MS standards including gallic acid (GA), rosmarinic acid (RA), caffeic acid (CA), vanillic acid (VA), 1,3-Dicaffeoylquinic acid (1,3DQA), quercetin 3-O-glucoside (Q3G), kaempferol 7-O-glucoside (K7G), kaempferol 3-O-rutinoside (K3R), apigenin (AP), resveratrol 3-O-glucoside (R3G), hydroxytyrosol (HYT), verbascoside (VER), oleuropein (OLE), ligstroside (LIG), phloretin (PHL), luteolin (LUT), and naringenin (NAR) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). The other chromatographic standards, i.e., 3-O-caffeoylquinic acid (3CQA), p-coumaric acid (pCA), trans-ferulic acid (tFA), quercetin (Q), quercetin 3-O-rutinoside (Q3R), quercetin 3,4-O-diglucoside (QDG), kaempferol 3-O-glucoside (K3G), (+)-catechin (C), (−)-epicatechin (EC), resveratrol (RES), and phloridzin (PHZ), were acquired from Extrasynthese (Genay, France). The bacterial media Mueller Hinton Broth (MHB) and Mueller Hinton Agar (MHA) were purchased from VWR (Radnor, PA, USA).
2.2. Raw Material
2.2.1. Grapes
The selection criteria for the grape varieties were based on two main principles: the preliminary criterion involved choosing varieties that are generally suitable for green harvesting; the secondary criterion focused on selecting among the most widely cultivated varieties in Tuscany. Based on these criteria, Vermentino (white) and Sangiovese (red) were chosen to compare Agresto produced from white grapes with that obtained from red ones. Grapes were provided by two wine farms of the Amiata region: Salustri winery, Poggi del Sasso (Grosseto), Italy, for Vermentino and Sangiovese; Podere Giardino, Sant’Angelo in Colle, Montalcino (Siena), Italy, for Sangiovese.
Unripe grape juices were prepared at the winemaking wineries and then transported quickly in steel containers to the laboratory of Lombardi e Visconti SAS, Abbadia San Salvatore (Siena), Italy, where the Agresto was produced as described below.
2.2.2. Herbs
A preliminary consensus panel was conducted to identify the optimal blend of dried herbs among those traditionally cultivated and/or used for cooking in Tuscany. The final blend of dried herbs consisted of 43% juniper berries (Juniperus communis L.), 22% wild garlic (Allium ursinum L.), 21% chives Allium schoenoprasum L.), 8% onion (Allium cepa L.), 6% Carline root (Carlina acaulis L.). It was added to the concentrated must during the flavoring phase at a ratio of 4% w/vol. The herbs were supplied by Azienda Agricola Fonte Magria di Pacini Antonio, Abbadia San Salvatore (Siena), Italy and A. Minardi & Figli s.r.l. Bagnacavallo (Ravenna), Italy.
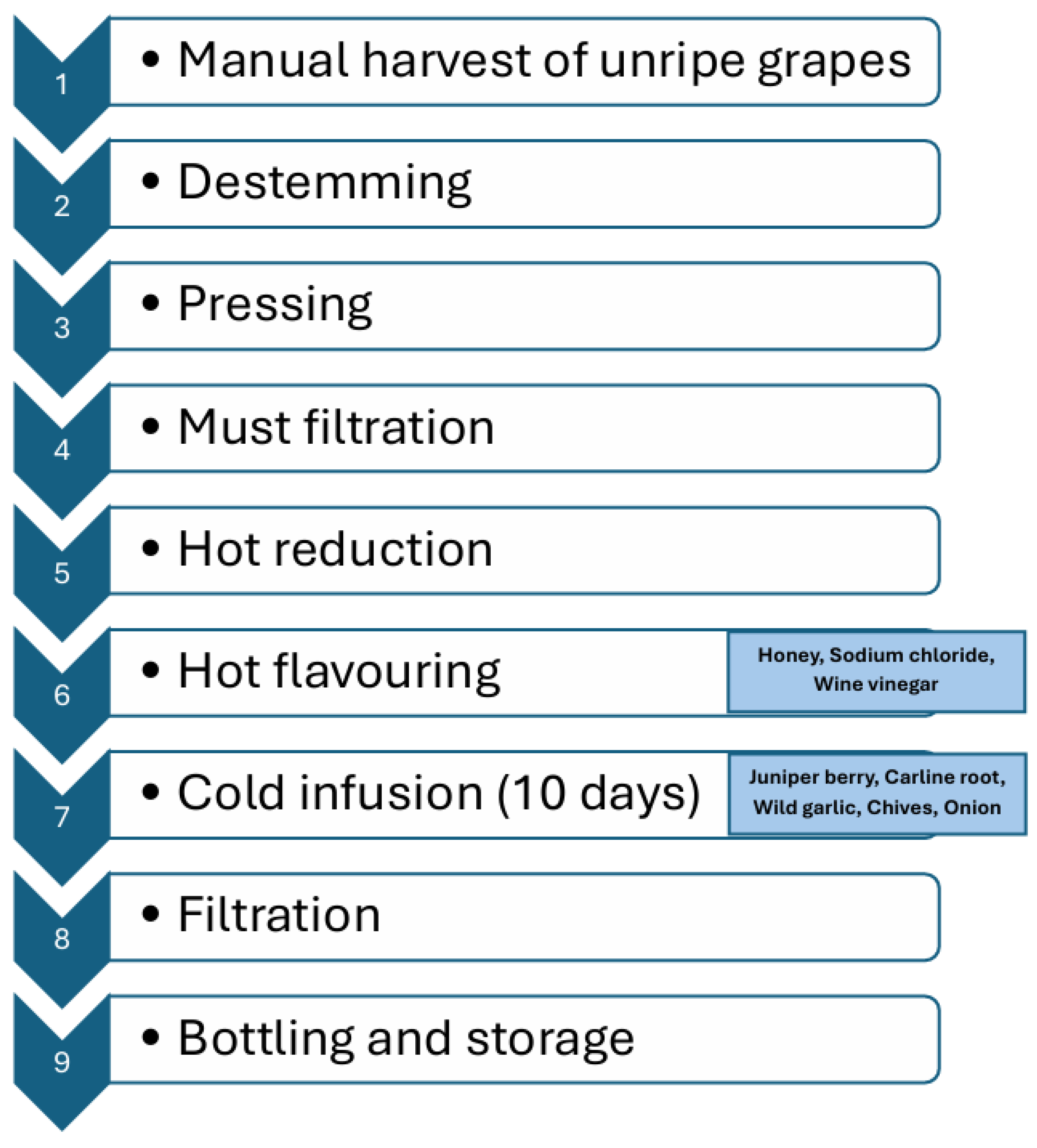
2.3. Production of Unripe Grape Juice
Two different grape varieties (Vermentino, and Sangiovese) were used. To produce Agresto, unripe grapes are manually harvested and the material is placed in crates. This harvest takes place between the pre-veraison stage and the beginning of veraison. Depending on the grape variety and the specific vintage, the harvest typically occurs between late July and early August. During this period, grape clusters generally have a sugar content of 80–140 g/L and a sugar-to-total acidity ratio (technological maturity) in the range of 11–12. Unripe grape clusters were destemmed and pressed using a small-scale stainless-steel basket press to obtain juice (Figure 1). The juice was filtered through a Hippocratic filter, then maintained at a constant boil over direct heat for nearly 12–15 h until its volume was reduced to one-third of the original. At this stage, honey (3%), sodium chloride (0.7%), and wine vinegar (15%) were added to the concentrated must (hot addition) before filtration. After cooling, juniper berry (Juniperus communis L.), Carline root (Carlina acaulis L.), wild garlic (Allium ursinum L.), chives (Allium schoenoprasum L.), and onion (Allium cepa L.) from Mount Amiata (Tuscany) were added by cold addition. The mixture was left to infuse at room temperature for 10 days and then filtered again using a Hippocratic filter. The resulting Agresto was bottled in 700 mL dark bottles and stored at 5 °C until analyses.
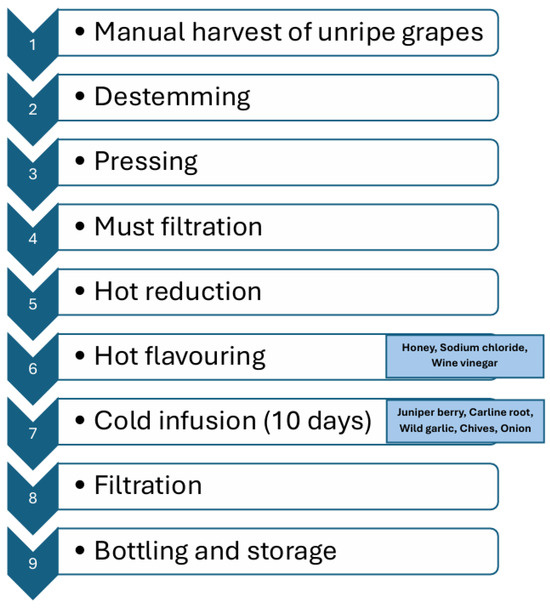
Figure 1.
Lab-scale processing of Agresto.
2.4. Grapes and Herbs Extraction
One gram of grapes, herbs and herbal mixture was exposed to double extraction in 10 mL of 80% methanol following the method of Quasim and colleagues [24] in order to obtain a final concentration of 50 mg/mL and stored at −20 °C until use.
2.5. Determination of Phenolic Profile by UHPLC-ESI-MS/MS
Liquid chromatography (LC) separation and tandem mass spectrometry (MS/MS) detection of phenolic compounds of herbs extract, blend of herbs extract, grapes juice extract and diluted samples of Agresto produced from different grape varieties was performed employing a 5500+ QTrap mass spectrometer with a Turbo V ion-spray source (AB Sciex, Framingham, MA, USA) connected with an Exion LC AC system consisting of two ExionLC AC pumps, autosampler, controller, degasser, and tray (Shimadzu, Kyoto, Japan) and following the analytical conditions reported by Raffaelli and colleagues [25].
2.6. Determination of the Total Phenolic Content (TPC) and Flavonoid and Flavonol Content
Total phenolic content (TPC) was measured following the method of Quasim and colleagues [26]. Flavonoids were determined using the following the method of Kim and colleagues [27]. Flavonols were quantified as previously described by Souid et al. [28].
2.7. In Vitro Antioxidant Activity Assays
Antioxidant activity was evaluated using the FRAP method [29], the DPPH assay [30], and the ORAC assay [31].
2.8. Determination of the Antimicrobial Activity
The antimicrobial activity of Agresto produced from different grape varieties was analyzed against three Gram-negative bacteria and two Gram-positive bacteria. The effect of samples on final growth (O.D.) of bacteria was determined according to method detailed in a previous paper [32]. Equation (1) was applied for the calculation of the inhibition rate:
where:
Inhibition rate (%) = (100 − O.D.sample/O.D.control) × 100
O.D.sample = the optical density of the measurement with extract;
O.D.control = the optical density of control without extract.
2.9. Analysis of Volatile Organic Compounds (VOCs) by GC-MS
The headspace of samples was analyzed with the SPME (solid-phase micro-extraction). Samples of Agresto produced from the two different grape varieties were sealed with aluminum foil and left to equilibrate for 30 min at room temperature. To sample the headspace, Supelco fibers coated with PDMS (PolyDiMethylSiloxane, 100 μm thickness) were used, after the preconditioning recommended by the manufacturer. Sampling was performed by inserting the fiber into the headspace for 60 min. At the end of sampling, the fiber was retracted inside the needle and transferred into the injector of a Gas Chromatograph coupled to a Mass Spectrometry (GC-MS) apparatus, as described by Sanmartin et al. [33].
2.10. Statistical Analysis
Data were reported as mean ± standard deviation of triplicates. The statistically significant (p < 0.05) differences between extracts were evaluated with an analysis of variance (ANOVA) and Tukey’s test for the phenolic profile of herbs extract and with an independent Student’s t-test for all the other parameters. Pearson’s correlation analysis was conducted in order to evaluate the correlation between variables (TPC, antioxidant activity, antimicrobial activity, and VOCs). Pearson’s correlation and principal component analysis (PCA), for the analysis of multivariate data, were applied to characterize and separate Agresto from grapes from different vines (Vermentino and Sangiovese) in relation to variables (TPC, flavonoids, flavonols, antioxidant activity, antimicrobial activity, and contents of individual phenolics and of VOCs). Analyses were performed using XLSTAT software (version 2019).
3. Results and Discussion
As reviewed by Fia et al. [8], unripe grapes—considered a by-product of grapevine cultivation—have emerged as a promising source of valuable compounds, including organic acids, phenolics, vitamins, and minerals, with notable applications in the pharmaceutical, cosmetic, and food industries. Their bioactive components have demonstrated anti-inflammatory, cardioprotective, anticancer, and antidiabetic properties in various in vitro and in vivo studies. While low in simple sugars, unripe grapes are particularly rich in phenolic flavonoids, non-flavonoids, organic acids, stilbenes, condensed tannins, and glutathione [18], and their chemical composition is influenced by several factors, such as grape variety, vintage, and ripening stage [16,33].
Due to their high nutraceutical value, traditional products derived from unripe grapes have been increasingly investigated for innovative applications in food and beverage formulations [34,35], in response to the growing consumer demand for natural and health-enhancing products [7,36,37,38,39]. In this context, the following sections present an overview of the composition of the raw materials used in the experimental runs together with the two different samples of Agresto produced.
3.1. Phenolic Compound Profile of Herbs and Grapes Juice Extracts
The phenolic profiles of herb extracts (Table S1) and grape juices (Table S2) were analyzed using Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS). A total of 28 phenolic compounds were identified in each herb extract and the blend, while 27 compounds were found in each grape juice sample.
Among the herb extracts, the juniper berry extract was particularly rich in rutin (44.89 µg/100 mg), (+)-catechin (38.61 µg/100 mg), kaempferol 3-O-glucoside (17.73 µg/100 mg), kaempferol 7-O-glucoside (6.20 µg/100 mg), (–)-epicatechin (4.70 µg/100 mg), kaempferol 3-O-rutinoside (3.14 µg/100 mg), vanillic acid (1.67 µg/100 mg), and phloretin (1.54 µg/100 mg). In carline root extract, the predominant phenolics were chlorogenic acid (3.18 µg/100 mg), verbascoside (1.50 µg/100 mg), and vanillic acid (1.46 µg/100 mg). Wild garlic extract showed high levels of kaempferol 3-O-glucoside (27.02 µg/100 mg), p-coumaric acid (12.49 µg/100 mg), and kaempferol 7-O-glucoside (8.39 µg/100 mg).
Chives extract had the highest concentration of kaempferol 3-O-glucoside (60.92 µg/100 mg), followed by trans-ferulic acid (19.59 µg/100 mg), kaempferol 3-O-rutinoside (5.73 µg/100 mg), p-coumaric acid (4.88 µg/100 mg), quercetin 3,4′-O-diglucoside (2.07 µg/100 mg), and kaempferol 7-O-glucoside (1.47 µg/100 mg). Onion extract, in contrast, exhibited only trace amounts of phenolics, ranging from 0.002 to 0.541 µg/100 mg.
In the blended herb extract, the major phenolic compound was kaempferol 3-O-glucoside (30.50 µg/100 mg), contributed by juniper berry, wild garlic, and chives. Other abundant compounds included rutin (13.94 µg/100 mg; from juniper berry), chlorogenic acid (10.62 µg/100 mg; from carline root), trans-ferulic acid (6.30 µg/100 mg; from chives), (+)-catechin (5.69 µg/100 mg; from juniper berry), p-coumaric acid (4.05 µg/100 mg; from wild garlic and chives), kaempferol 3-O-rutinoside (3.21 µg/100 mg; from juniper berry and chives), kaempferol 7-O-glucoside (2.70 µg/100 mg; from juniper berry, wild garlic, and chives), and verbascoside (1.55 µg/100 mg; from carline root).
Regarding grape juices, the Vermentino sample contained significantly (p < 0.05) higher concentrations of several phenolic compounds compared to Sangiovese. These included chlorogenic acid (44.0 µg/100 mg), rosmarinic acid (13.8 µg/100 mg), quercetin (155.3 µg/100 mg), rutin (1336.1 µg/100 mg), quercetin 3,4-O-diglucoside (175.3 µg/100 mg), kaempferol 7-O-glucoside (244.1 µg/100 mg), kaempferol 3-O-glucoside (704.1 µg/100 mg), kaempferol 3-O-rutinoside (426.5 µg/100 mg), (+)-catechin (15,606.0 µg/100 mg), piceid (581.7 µg/100 mg), verbascoside (4313.9 µg/100 mg), phlorizin (762.2 µg/100 mg), phloretin (1254.5 µg/100 mg), apigenin (3.0 µg/100 mg), and luteolin (31.9 µg/100 mg).
Conversely, Sangiovese juice exhibited significantly (p < 0.05) higher levels of vanillic acid (698.1 µg/100 mg), p-coumaric acid (134.7 µg/100 mg), quercetin 3-O-glucoside (426.7 µg/100 mg), (–)-epicatechin (1043.4 µg/100 mg), resveratrol (11.6 µg/100 mg), and naringenin (8.5 µg/100 mg), distinguishing its phenolic composition from Vermentino.
3.2. Phenolic Compound Profile of Agresto Samples
The phenolic composition of Agresto samples analyzed by LC-MS/MS (Table 1) revealed a dominance of phenolic acids in Vermentino. This sample showed significantly (p < 0.05) higher levels of gallic acid (809.82 µg/mL), chlorogenic acid (779.27 µg/mL), caffeic acid (809.96 µg/mL), p-coumaric acid (1922.67 µg/mL), and trans-ferulic acid (1297.36 µg/mL) compared to Sangiovese. Vermentino also had greater amounts of quercetin (7.77 µg/mL), quercetin 3,4-O-diglucoside (31.18 µg/mL), kaempferol 3-O-rutinoside (65.82 µg/mL), piceid (17.99 µg/mL), resveratrol (1.13 µg/mL), hydroxytyrosol (323.46 µg/mL), and phloridzin (45.26 µg/mL). In contrast, Sangiovese Agresto contained higher levels of rosmarinic acid (0.59 µg/mL), cynarin (0.46 µg/mL), quercetin 3-O-glucoside (76.40 µg/mL), rutin (196.61 µg/mL), kaempferol 7-O-glucoside (536.84 µg/mL), oleuropein (85.24 µg/mL), apigenin (0.85 µg/mL), luteolin (0.55 µg/mL), and naringenin (9.16 µg/mL).

Table 1.
Content of individual phenolic compounds of Agresto from grapes from different vines (µg/100 mL).
3.3. Total Phenolic Content (TPC) and Flavonoid and Flavonol Content
The assay with Folin–Ciocalteu reagent was performed to determine the total phenolic content (TPC) of samples of Agresto obtained from grapes from different vines (Vermentino and Sangiovese). The results expressed based on mg of gallic acid equivalent (GAE) per mL are reported in Table 2. The TPC ranged from 0.76 to 1.31 mg GAE/mL. Agresto from Sangiovese showed the lowest TPC, while Agresto from Vermentino showed the highest. The total flavonoid content values are expressed as mg of catechin equivalents (CE) per mL and varied from 0.06 to 0.10 mg CE/mL (Table 2), and no significant differences were found between the two samples. The total flavonol content values are expressed as mg of quercetin equivalent (QE) per mL and ranged between 0.10 mg QE/mL in Agresto from Sangiovese and 0.21 mg QE/mL in Agresto from Vermentino, which showed the significantly highest content (Table 2).

Table 2.
Total phenolic content (TPC) and flavonoid and flavonol content of Agresto from different grape varieties.
3.4. Antioxidant Activity
Antioxidant activity of Agresto samples was evaluated using FRAP, DPPH, and ORAC assays (Table 3). FRAP values ranged from 2.78 to 4.11 mg TE/mL, with Agresto from Vermentino showing significantly higher activity than Sangiovese. DPPH scavenging activity showed no significant differences between the two samples. ORAC values ranged from 26.76 to 41.01 mg TE/mL, with Vermentino again exhibiting significantly higher antioxidant capacity (p < 0.0001). FRAP values were positively and significantly (p < 0.05) correlated with total phenolic content (TPC), flavonols, apocarotenes, sulfur compounds, and non-terpene derivatives. ORAC values also showed significant positive correlations (p < 0.05) with TPC, flavonols, sulfur derivatives, and non-terpene compounds. In contrast, DPPH results did not correlate with any of the measured components (Table S3).

Table 3.
Ferric-reducing antioxidant power (FRAP), DPPH scavenging activity, and oxygen radical absorbance capacity (ORAC) of Agresto from grapes from different vines.
3.5. Antimicrobial Activity
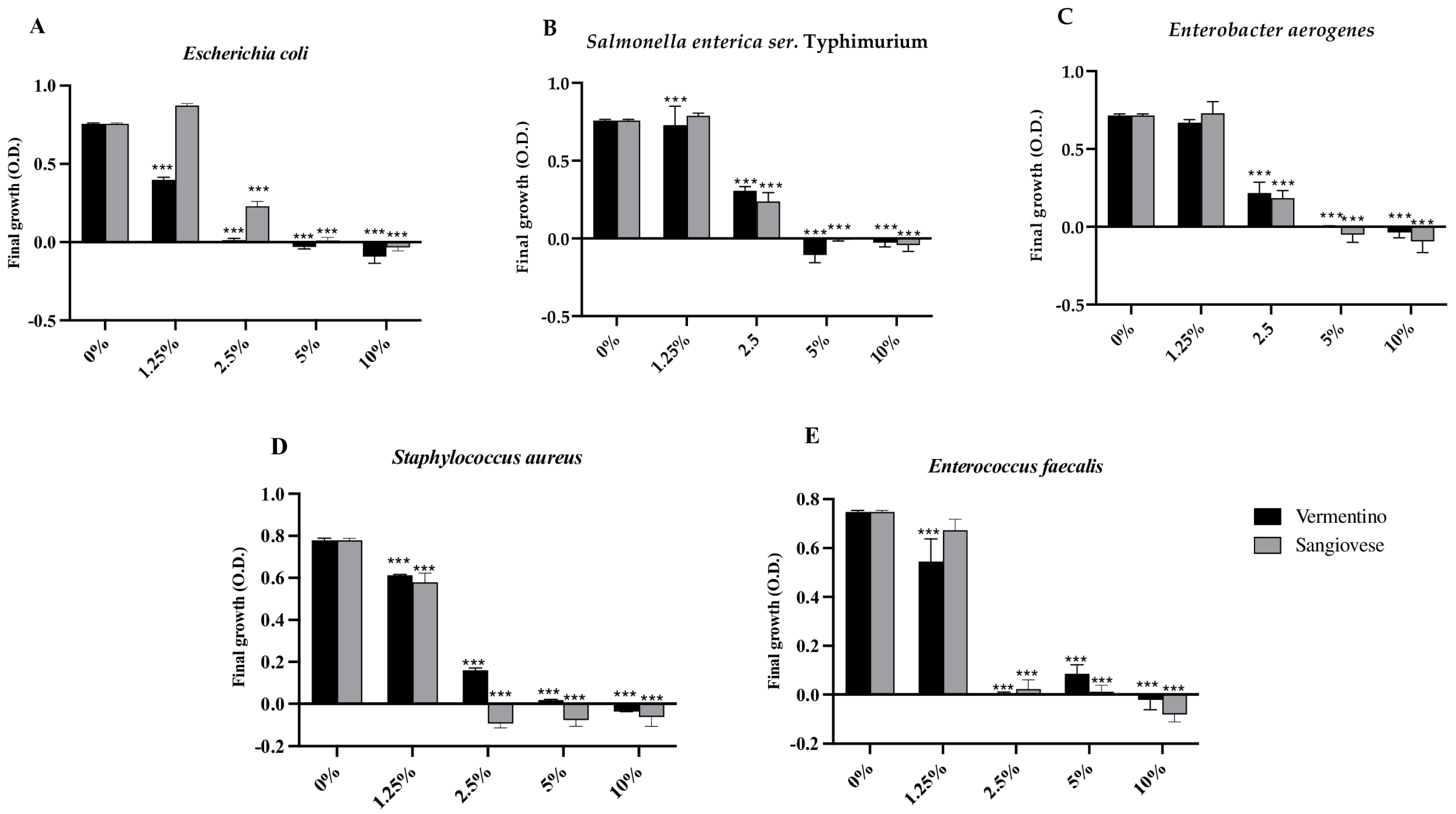
The antimicrobial properties of Agresto samples from Vermentino and Sangiovese were assessed against selected Gram-negative (Escherichia coli, Salmonella enterica ser. Typhimurium, Enterobacter aerogenes) and Gram-positive (Staphylococcus aureus, Enterococcus faecalis) bacteria by evaluating growth inhibition (Figure 2).
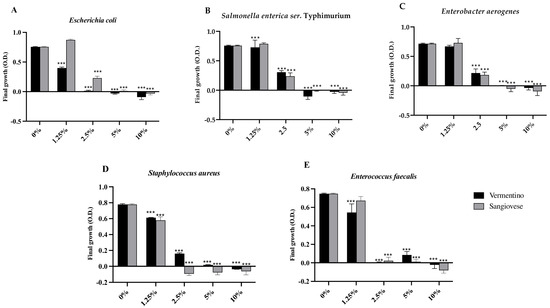
Figure 2.
Final growth expressed as optical density (O.D.) of Escherichia coli (A), Salmonella enterica ser. Typhimurium (B), Enterobacter aerogenes (C), Staphylococcus aureus (D), and Enterococcus faecalis (E) in the presence of Agresto from Vermentino and Sangiovese at different percentage (%). Results are reported as mean and standard deviation (n = 3). ***, p < 0.0001.
For E. coli, Agresto from Vermentino significantly (p < 0.0001) inhibited growth at 1.25%, with a minimum inhibitory concentration (MIC) of 2.5%, while Agresto from Sangiovese was effective at 2.5% (p < 0.0001) with an MIC of 5%. S. Typhimurium growth was reduced at 1.25% by Vermentino and at 2.5% by Sangiovese (both p < 0.0001), with an MIC of 5% for both. E. aerogenes showed significant inhibition at 2.5% for both samples, with an MIC of 5%.
Among Gram-positive strains, S. aureus was significantly inhibited at 1.25% by both extracts (p < 0.0001), with a MIC of 2.5% for Sangiovese and 5% for Vermentino. E. faecalis growth was significantly reduced by Vermentino at 1.25% (MIC 2.5%) and by Sangiovese at 2.5% (MIC 5%).
Positive correlations (p < 0.05) were observed between antimicrobial activity against E. coli and Agresto’s TPC, flavonols, apocarotenes, sulfur compounds, and non-terpene derivatives (Table S3).
3.6. Analysis of VOCs
Overall, 47 different volatiles, belonging to seven main chemical classes, were identified in the headspace of the three samples (Table 4), accounting for 97.4–99.3% of total emission. The most representative classes were those of sulfur derivatives, monoterpene hydrocarbons, and oxygenated monoterpenes. However, they were largely unevenly distributed depending on the sample type. It is interesting to note that sulfur derivatives, clearly the predominant chemical class in Vermentino (83.10%), were considerably less represented in the Sangiovese sample (20.75%). On the contrary, Sangiovese was rich in monoterpenes, both in their hydrocarbon (54.65%) and oxygenated (19.20%) forms, which in the other samples reached only 1.39–8.89%.

Table 4.
Content of VOCs in the headspace of Agresto from grapes from different vines (%).
Regarding sulfur derivatives, the most represented in Vermentino were methyl (Z)-1-propenyl disulfide (14.04%, methyl propyl disulfide 13.67%) and methyl allyl disulfide (19.4%). In Sangiovese, only three sulfur derivatives exceeded 3%, i.e., methyl (Z)-1-propenyl disulfide (4.35%), methyl allyl disulfide (3.75%), and methyl (E)-1-propenyl disulfide (3.25%).
Sangiovese was particularly rich in monoterpene hydrocarbons, with limonene (30.60%) as the main representative, followed by p-cymene (9.20%) and γ-terpinene (7.95%). Among oxygenated monoterpenes, 4-terpineol (8.50%), 1,4-cineole (4.97%), and 1,8-cineole (4.65%) should be mentioned.
Terpenes were decidedly less represented in Vermentino. Among these, only limonene (3.10%), 4-terpineol (0.49%), and 1.4-cineole (0.61%) reached appreciable percentages.
3.7. Overall Rate of Results with Pearson’s Correlation and Principal Component Analysis
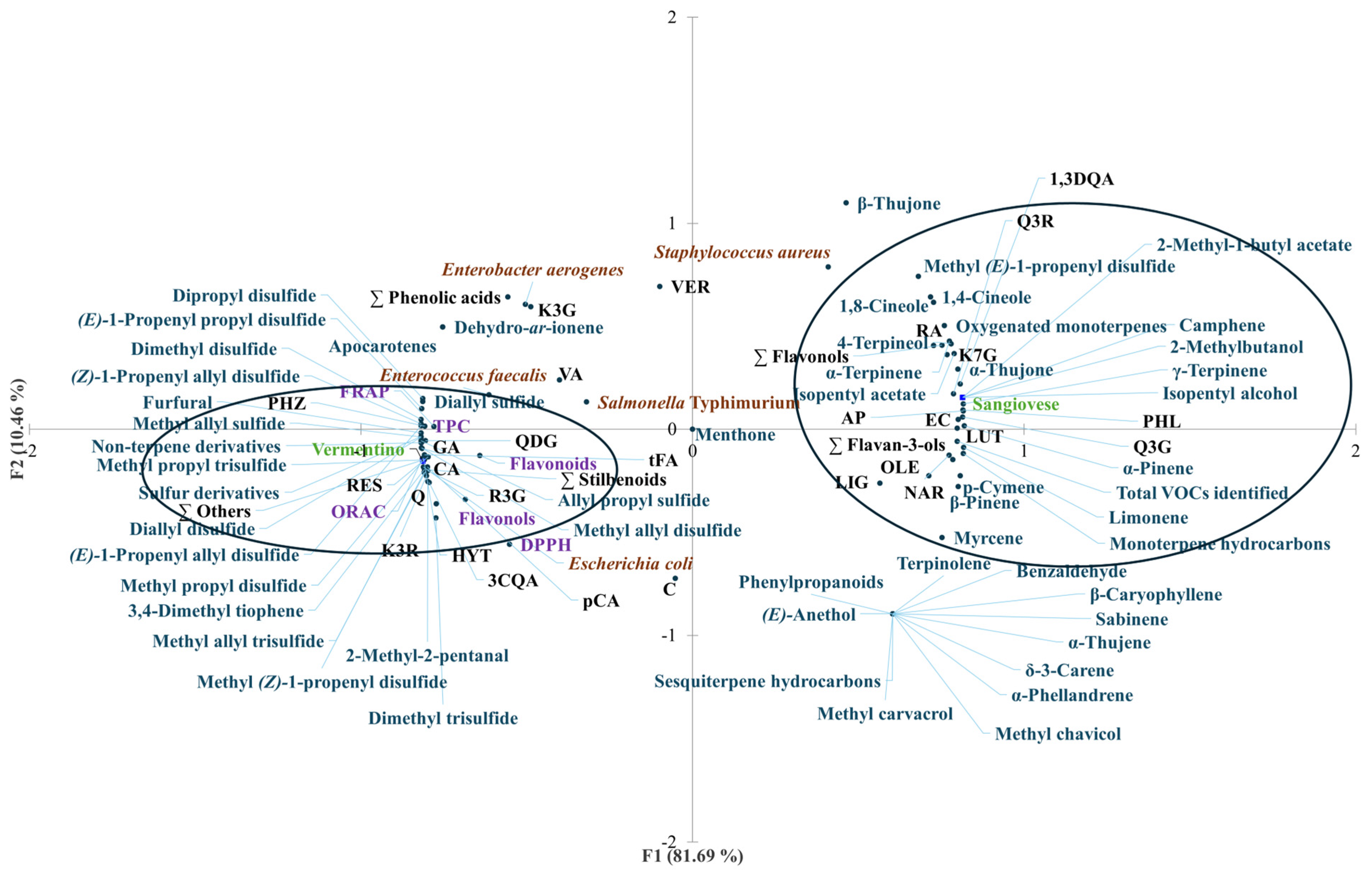
Principal component analysis (PCA) was conducted to explore the relationships between Agresto samples from two grape varieties, Vermentino and Sangiovese, and a range of variables including total phenolic content (TPC), phenolic profiles, volatile organic compounds (VOCs), antioxidant activity, and antimicrobial effectiveness. The PCA results are depicted in Figure 3, illustrating the distribution of samples and variables.
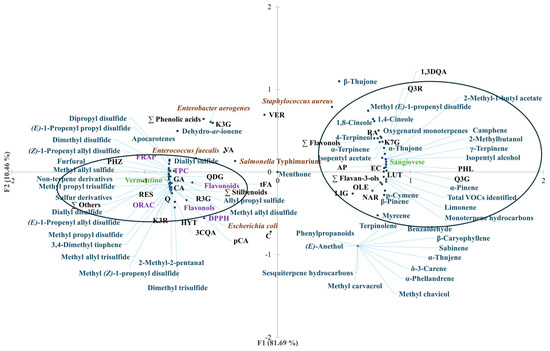
Figure 3.
Principal component analysis (PCA) plot with distribution of the variables including total phenolic content (TPC), flavonols, flavonoids, antioxidant activity (DPPH assay, FRAP, and ORAC), antimicrobial activity against Escherichia coli, Salmonella enterica ser. Typhimurium, Enterobacter aerogenes, Staphylococcus aureus, and Enterococcus faecalis, and contents of individual phenolic compounds and VOCs of Agresto from grapes from different vines (Vermentino, Sangiovese). The acronyms at the black dots correspond to the names of the compounds given in Table 1. FRAP, ferric-reducing antioxidant power; ORAC, oxygen radical absorbance capacity; DPPH assay, assay with 2,2-diphenyl-1-picrylhydrazyl radical; PC1, first principal component; PC2, second principal component.
The first two principal components (PC1 and PC2) accounted for 92.15% of the total variance, demonstrating a clear separation between the two Agresto types. PC1 alone explained 81.69% of this variance and distinctly separated Vermentino and Sangiovese samples into two clusters.
Agresto from Vermentino grouped closely with higher TPC, flavonols, and antioxidant activities measured by FRAP and ORAC assays. It was also associated with stronger antimicrobial activity against Escherichia coli. Several individual phenolic compounds were enriched in Vermentino samples, including gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, trans-ferulic acid, total phenolic acids, quercetin, quercetin 3,4-O-diglucoside, kaempferol 3-O-rutinoside, piceid, resveratrol, total stilbenoids, hydroxytyrosol, phloridzin, and other minor phenolics.
Volatile compounds correlating with Vermentino included sulfur-containing compounds such as methyl allyl sulfide, dimethyl disulfide, methyl allyl disulfide, methyl propyl disulfide, dimethyl trisulfide, diallyl disulfide, as well as aldehydes like 2-methyl-2-pentanal and furfural. Other associated VOCs were apocarotenes, dehydro-ar-ionene, and various sulfur and non-terpene derivatives.
Conversely, Agresto from Sangiovese was associated with a different set of phenolics and volatiles. These included rosmarinic acid, cynarin, quercetin 3-O-glucoside, rutin, kaempferol 7-O-glucoside, total flavonols, (−)-epicatechin, total flavan-3-ols, oleuropein, phloretin, apigenin, luteolin, and naringenin. The Sangiovese samples also clustered with VOCs such as isopentyl alcohol, 2-methylbutanol, isopentyl acetate, 2-methyl-1-butyl acetate, methyl (E)-1-propenyl disulfide, and a series of monoterpenes including α-pinene, camphene, β-pinene, myrcene, 1,4-cineole, α-terpinene, p-cymene, limonene, 1,8-cineole, γ-terpinene, α-thujone, and 4-terpineol, alongside oxygenated monoterpenes and the total VOC content.
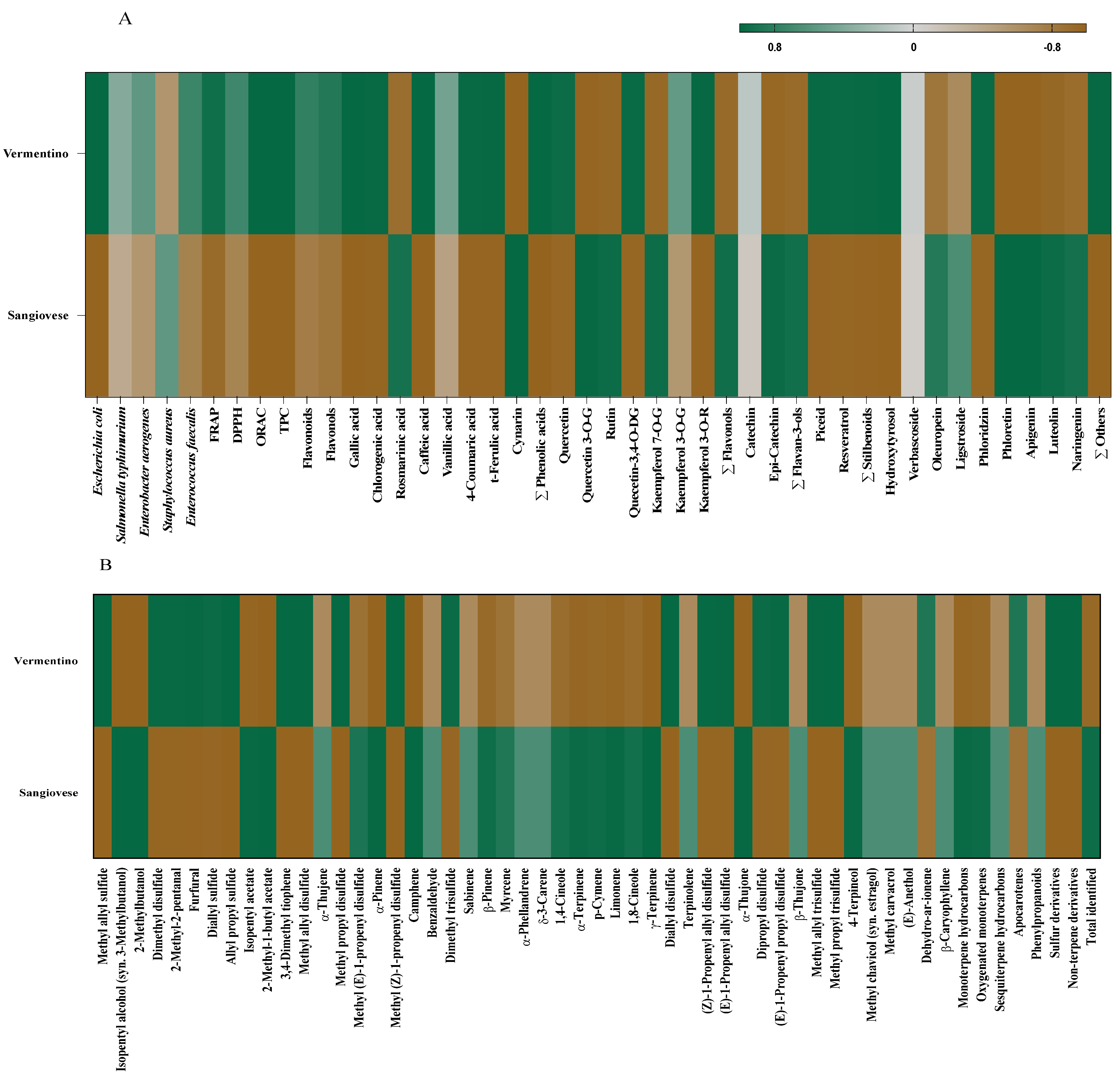
The PCA clustering reflected the results from Pearson’s correlation analysis (Figure 4A,B), which revealed significant positive correlations among TPC, flavonoids, flavonols, antioxidant activities (FRAP, DPPH, ORAC), antimicrobial efficacy, individual phenolic compounds, and volatile compounds.
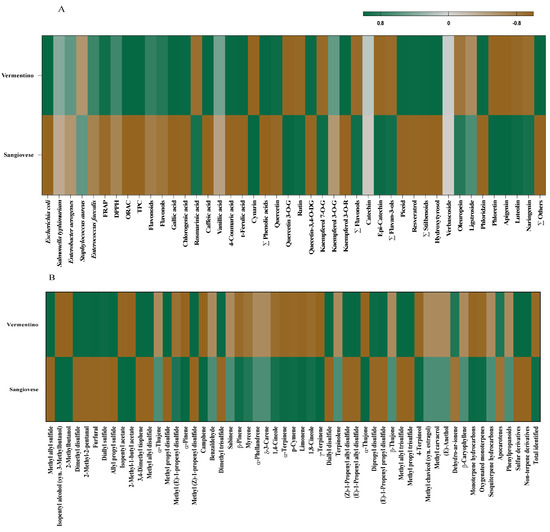
Figure 4.
Heatmap correlation matrix between Agresto from Vermentino and Sangiovese vines and antimicrobial activity (% inhibition), antioxidant capacity and phenolic profile (A) and between Agresto from Vermentino and Sangiovese vines and VOCs (B).
Specifically, Agresto from Vermentino showed positive correlations (p < 0.05) with antimicrobial activity against E. coli, FRAP, and ORAC antioxidant measures, TPC, flavonols, and phenolic acids including gallic, chlorogenic, caffeic, p-coumaric, and trans-ferulic acids. Additionally, it correlated with quercetin, quercetin 3,4-O-diglucoside, kaempferol 3-O-rutinoside, piceid, resveratrol, total stilbenoids, hydroxytyrosol, phloridzin, and other minor phenolics. Regarding VOCs, this sample showed significant associations with sulfur volatiles such as methyl allyl sulfide, dimethyl disulfide, methyl allyl disulfide, methyl propyl disulfide, dimethyl trisulfide, diallyl disulfide, (Z)- and (E)-1-propenyl allyl disulfides, dipropyl disulfide, methyl allyl trisulfide, methyl propyl trisulfide, along with aldehydes (2-methyl-2-pentanal, furfural), apocarotenes, and non-terpene derivatives.
In contrast, Agresto from Sangiovese correlated positively (p < 0.05) with phenolics like rosmarinic acid, cynarin, quercetin 3-O-glucoside, rutin, kaempferol 7-O-glucoside, total flavonols, (−)-epicatechin, total flavan-3-ols, oleuropein, phloretin, apigenin, luteolin, and naringenin. Its VOC profile showed positive associations with alcohols (isopentyl alcohol, 2-methylbutanol), esters (isopentyl acetate, 2-methyl-1-butyl acetate), methyl (E)-1-propenyl disulfide, and numerous monoterpenes including α-pinene, camphene, β-pinene, myrcene, 1,4-cineole, α-terpinene, p-cymene, limonene, 1,8-cineole, γ-terpinene, α-thujone, and 4-terpineol, along with oxygenated monoterpenes and the total VOC pool.
This comprehensive analysis highlights the distinct chemical and bioactive profiles of Agresto derived from Vermentino and Sangiovese grapes, underscoring the influence of grape variety on the phenolic, volatile, antioxidant, and antimicrobial characteristics of the final product.
4. Conclusions
According to tradition, Agresto is primarily made from an undefined form of “unripe grape juice,” which is then heated until reduced, infused with spices and aromas, and optionally blended with varying amounts of vinegar and honey. To the best of our knowledge, few data are currently available in the literature [17,20] regarding the influence of grape variety on the chemical composition and, consequently, the aromatic and nutritional profile of Agresto.
To address this gap, our research focused on the use of two different grape varieties (Sangiovese and Vermentino), widely cultivated in Tuscan vineyards, as raw materials for Agresto production. These varieties are characterized by distinct chemical compositions, phenological cycles, and harvest periods. More specifically, Vermentino is a white grape variety, while Sangiovese is red. Among these two varieties, the harvest period of Vermentino occurs significantly earlier than that of Sangiovese.
Overall, our results demonstrated that the grape variety significantly influences the chemical composition of Agresto, particularly in terms of VOCs and phenolic compounds. The observed variations in phenolic composition also affected the antioxidant and antimicrobial activity of Agresto.
These experimental findings clearly highlight the importance of identifying the optimal chemical profile of unripe grapes used as raw material for Agresto production, considering both variety and specific ripening stage of each variety available from ‘vine green harvesting’.
In the context of a circular economy, maximizing the value of unripe grapes for Agresto production requires identifying the ideal timing for green harvesting for each grape variety. Since unripe grapes serve as the raw material for Agresto, the optimal harvesting time should consider both the vineyard’s needs for primary wine production and the specific grape composition required for Agresto. To achieve this goal, further research will focus on exploring the impact of different ripening stages on the chemical composition and organoleptic profile of Agresto for each grape variety.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13072174/s1, Table S1: Content of individual phenolic compounds of herbs (µg/100 mg); Table S2: Content of individual phenolic compounds of grape juices from different vines (µg/100 mg); Table S3: Coefficients of Pearson’s correlations among antimicrobial and antioxidant activities and total phenolic content and VOCs of Agresto from grapes from different vines (Vermentino and Sangiovese).
Author Contributions
Conceptualization, L.P., S.F., F.V. and A.T.; methodology, A.R., T.G. and G.F.; software, L.P., A.R. and C.S.; investigation, L.P., A.R., T.G., S.F., V.L., F.V., C.S., G.F. (Giuseppe Ferroni), G.F. (Guido Flamini) and A.T.; data curation, L.P., A.R., F.V. and A.T.; writing—original draft preparation, L.P., F.V. and A.T.; writing—review and editing, A.R., T.G., V.L., C.S., G.F. (Giuseppe Ferroni) and G.F. (Guido Flamini).; supervision, F.V., C.S. and A.T.; funding acquisition, L.P., F.V. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
The current study was conducted within the AGRENO project by Tuscany Region PSR 2014–2020 (DBA.AD005.182, https://agreno.ciatoscana.eu/, accessed on 16 December 2024).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to express their appreciation to Aurelio Visconti (Visconti srl) for his assistance in Agresto preparation and precious collaboration.
Conflicts of Interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper. They also declare that their funding sources had no direct role in the study design, data collection, analysis and interpretation of the data, writing of the manuscript, or decision to publish the work.
References
- Morsetto, P. Targets for a circular economy. Resour. Conserv. Recycl. 2020, 153, 104553. [Google Scholar] [CrossRef]
- Perra, M.; Cuena-Lombraña, A.; Bacchetta, G.; Manca, M.L.; Manconi, M.; Maroun, R.G.; Muntoni, A.; Tuberoso, C.I.G.; Gil, K.A.; De Gioannis, G. Combining Different Approaches for Grape Pomace Valorization: Polyphenols Extraction and Composting of the Exhausted Biomass. Sustainability 2022, 14, 10690. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S. Valorization of agro-industrial wastes for biorefinery process and circular bioeconomy: A critical review. Bioresour. Technol. 2022, 343, 126126. [Google Scholar] [CrossRef]
- Gnanavinthan, A. Bioactives in Fruit: Health Benefits and Functional Foods; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 1–18. [Google Scholar] [CrossRef]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem. 2021, 12, 100149. [Google Scholar] [CrossRef]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canalis, J.M.; Zamora, F. Use of unripe grapes harvested during cluster thinning as a method for reducing alcohol content and pH of wine. Aust. J. Grape Wine Res. 2011, 17, 230–238. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Evaluation of antibrowning and antioxidant activities in unripe grapes recovered during bunch thinning. Aust. J. Grape Wine Res. 2017, 23, 33–41. [Google Scholar] [CrossRef]
- Fia, G.; Bucalossi, G.; Proserpio, C.; Vincenzi, S. Unripe grapes: An overview of the composition, traditional and innovative applications, and extraction methods of a promising waste of viticulture. Aust. J. Grape Wine Res. 2021, 28, 1322–7130. [Google Scholar] [CrossRef]
- Karapinar, M.; Sengun, I.Y. Antimicrobial effect of koruk (unripe grape—Vitis vinifera) juice against Salmonella typhimurium on salad vegetables. Food Control 2007, 18, 702–706. [Google Scholar] [CrossRef]
- Nikfardjam, M.S.P. General and polyphenolic composition of unripe grape juice (verjus/verjuice) from various producers. Mitt. Klosterneuburg. 2008, 58, 28–31. [Google Scholar]
- Hayoglu, I.; Basyigit, B.; Hayoglu, G.; Atasoy, A.F. Cream zahter: A functional food some chemical and sensory properties. Curr. Res. Nutr. Food Sci. 2016, 4, 32–36. [Google Scholar] [CrossRef]
- Hayoglu, I.; Kola, O.; Kaya, C.; Özer, S.; Turkoglu, H. Chemical and sensory properties of verjuice, a traditional Turkish non-fermented beverage from Kabarcik and Yediveren grapes. J. Food Process. Preserv. 2009, 33, 252–263. [Google Scholar] [CrossRef]
- Öncül, N.; Karabıyıklı, Ş. Survival of foodborne pathogens in unripe grape products. LWT 2016, 74, 168–175. [Google Scholar] [CrossRef]
- Öncül, N.; Karabıyıklı, Ş. Factors affecting the quality attributes of unripe grape functional foods products. J. Food Biochem. 2015, 39, 689–695. [Google Scholar] [CrossRef]
- Scalabrelli, G.; Toffanin, A.; Frassinetti, S.; Visconti, A. Agresto: A natural product from unripe grape. In Proceedings of the 4th International Conference and Exhibition on Food Processing & Technology, London, UK, 10–12 August 2015; Available online: https://hdl.handle.net/20.500.14243/304426 (accessed on 3 March 2025).
- Vasile Simone, G.; Montevecchi, G.; Masino, F.; Matrella, V.; Imazio, S.A.; Antonelli, A.; Bignami, C. Ampelographic and chemical characterization of Reggio Emilia and Modena (northern Italy) grapes for two traditional seasonings: ‘saba’ and ‘agresto’. J. Sci. Food Agric. 2013, 93, 3502–3511. [Google Scholar] [CrossRef]
- Flamini, R. Recent Applications of Mass Spectrometry in the Study of Grape and Wine Polyphenols. Int. Sch. Res. Notices 2013, 813563, 45. [Google Scholar] [CrossRef]
- Adams, D. Phenolics and ripening in grape berries. Am. J. Enol. Vitic. 2006, 57, 249–256. [Google Scholar] [CrossRef]
- Brossaud, F.; Cheynier, V.; Noble, A.C. Bitterness and astringency of grape and wine polyphenols. Aust. J. Grape Wine Res. 2001, 7, 33–39. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Fernández-Fernández, J.I. Phenolic Compounds and Color Stability of Red Wines: Effect of Skin Maceration Time. Am. J. Enol. Vitic. 2001, 52, 266–270. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Vilela, A. Phenolic Compounds and Antioxidant Activity in Grape Juices: A Chemical and Sensory View. Beverages 2018, 4, 22. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Aziz, I.; Rasheed, M.; Gul, B.; Khan, M.A. Effect of Extraction Solvents on Polyphenols and Antioxidant Activity of Medicinal Halophytes. Pak. J. Bot. 2016, 48, 621–627. [Google Scholar]
- Raffaelli, A.; Saba, A. Ion Scanning or Ion Trapping: Why Not Both? Mass Spectrom. Rev. 2021, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Oxidants and Antioxidants Part A; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Souid, A.; Gabriele, M.; Longo, V.; Pucci, L.; Bellani, L.; Smaoui, A.; Abdelly, C.; Hamed, K.B. Salt tolerance of the halophyte Limonium delicatulum is more associated with antioxidant enzyme activities than phenolic compounds. Funct. Plant Biol. 2016, 43, 607–619. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef]
- Pozzo, L.; Grande, T.; Raffaelli, A.; Longo, L.; Weidner, S.; Amarowicz, R.; Karamać, M. Characterization of Antioxidant and Antimicrobial Activity and Phenolic Compound Profile of Extracts from Defatted Seeds of Different Vitis Species. Molecules 2023, 28, 4924. [Google Scholar] [CrossRef]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed Cake as a Tool for the Improvement of Nutraceutical and Sensorial Features of Sourdough Bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Curioni, A.; Bakalinsky, A.T.; Marangon, M.; Pasini, G.; Vincenzi, S. Chemical and sensory analysis of verjuice: An acidic food ingredient obtained from unripe grape berries. Inn. Food Sci. Emerg. Technol. 2017, 44, 9–14. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Magli, M.; Marangon, M.; Curioni, A.; Pasini, G.; Vincenzi, S. Use of verjuice as an acidic salad seasoning ingredient: Evaluation by consumers’ liking and Check-All-That-Apply. Eur. Food Res. Technol. 2018, 244, 2117–2125. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Gomes Reis, M.; Maggs, R.; Hort, J. Understanding consumer acceptability of verjuice, its potential applications and sensory and chemical drivers of liking. Food Res. Int. 2024, 188, 114480. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Sengun, I.Y. Inactivation effect of marination liquids prepared with koruk juice and dried koruk pomace on Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes inoculated on meat. Int. J. Food Microbiol. 2019, 304, 32–38. [Google Scholar] [CrossRef]
- Tinello, F.; Mihaylova, D.; Lante, A. Effect of dipping pre-treatment with unripe grape juice on dried “Golden Delicious” apple slices. Food Bioproc. Technol. 2018, 11, 2275–2285. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Goztepe, E.; Ozturk, B. Efficiency of marination liquids prepared with koruk (Vitis vinifera L.) on safety and some quality attributes of poultry meat. LWT Food Sci. Technol. 2019, 113, 108317. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).