Electron-Shuttling and Bioenergy-Stimulating Properties of Mulberry Anthocyanins: A Mechanistic Study Linking Redox Activity to MFC Performance and Receptor Affinity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Extraction
2.2. Determination of Total Polyphenol Content
2.3. Determination of Total Flavonoid Content
2.4. Determination of Total Condensed Tannin Content
2.5. Determination of Total Anthocyanin Content
2.6. Determination of DPPH Free Radical Scavenging Activity
2.7. Ferric Reduction Antioxidant Power (FRAP) Assay
2.8. Electron Shuttling Activity Analysis
2.8.1. Double-Chambered Microbial Fuel Cells
2.8.2. Cyclic Voltammetry
2.9. Fermentation Reactor Configuration and Compartmental Modeling
- (1)
- The time at which the maximum concentration of B occurs in cultures:
- (2)
- Rate constant for the conversion from the reactants to the intermediates:
- (3)
- Prediction of the concentration of the intermediate over a time-course:
- (i)
- The given , equation for tmax could be used to determine α;
- (ii)
- Have some trial α value to satisfy RSS (i.e., ) for the most appropriate k1 value;
- (iii)
- Use obtained k1 value to determine k2 and plot for time courses.
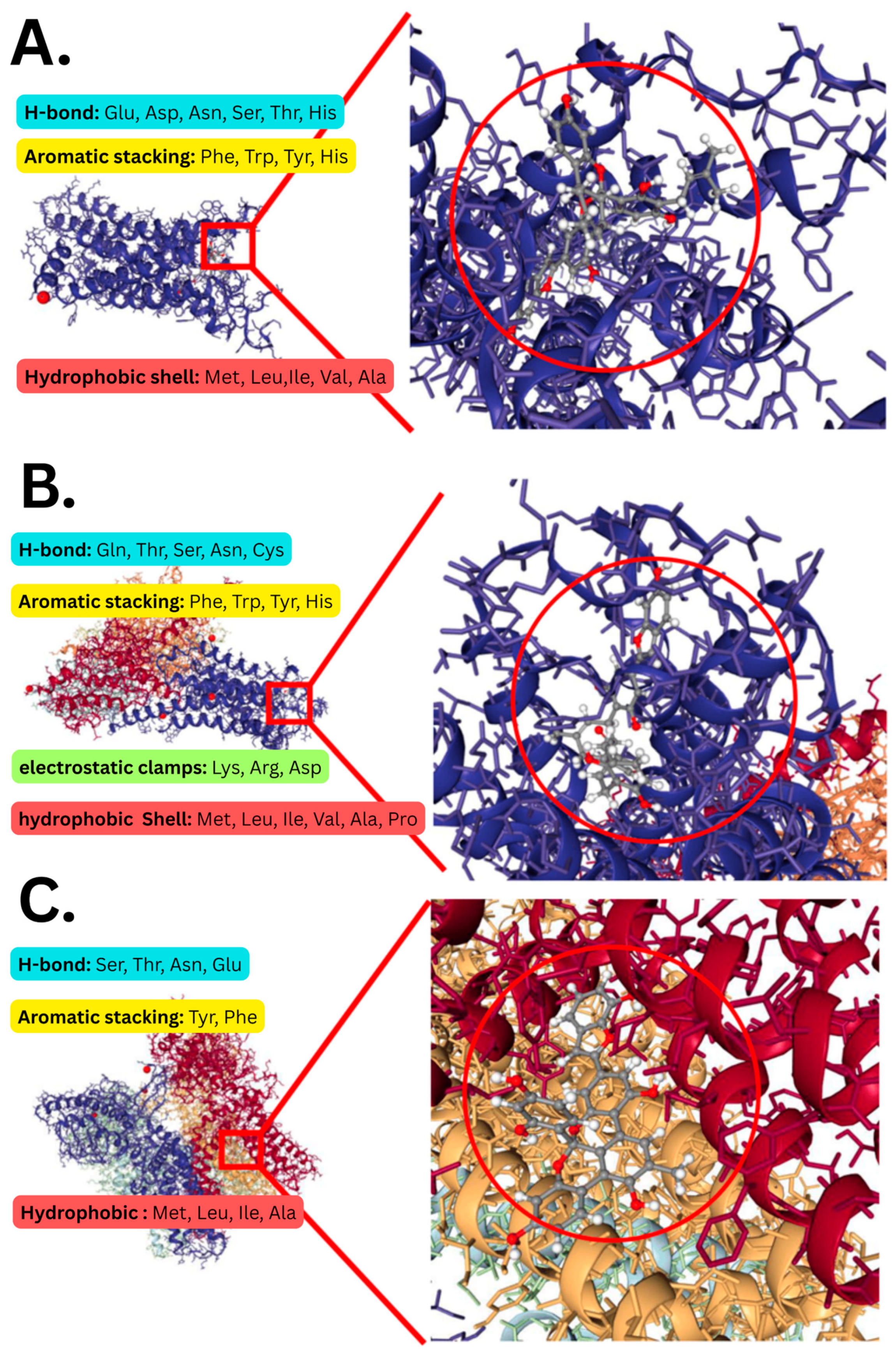
2.10. Multi-Receptor Virtual Screening
2.11. Cell Cultures
2.12. Anti-Cancer Assay
2.13. Nitric Oxide (NO) Anti-Inflammatory Inhibition Assay
3. Results
3.1. Phytochemical Analysis
3.2. Electron-Shuttling Capability of Mulberry Fruit
3.3. Anthocyanin Shows Persistence Against Degradation in Fermentation
3.4. Delphinidin, Peonidin, and Cyanidin Have the Highest Receptor Affinity
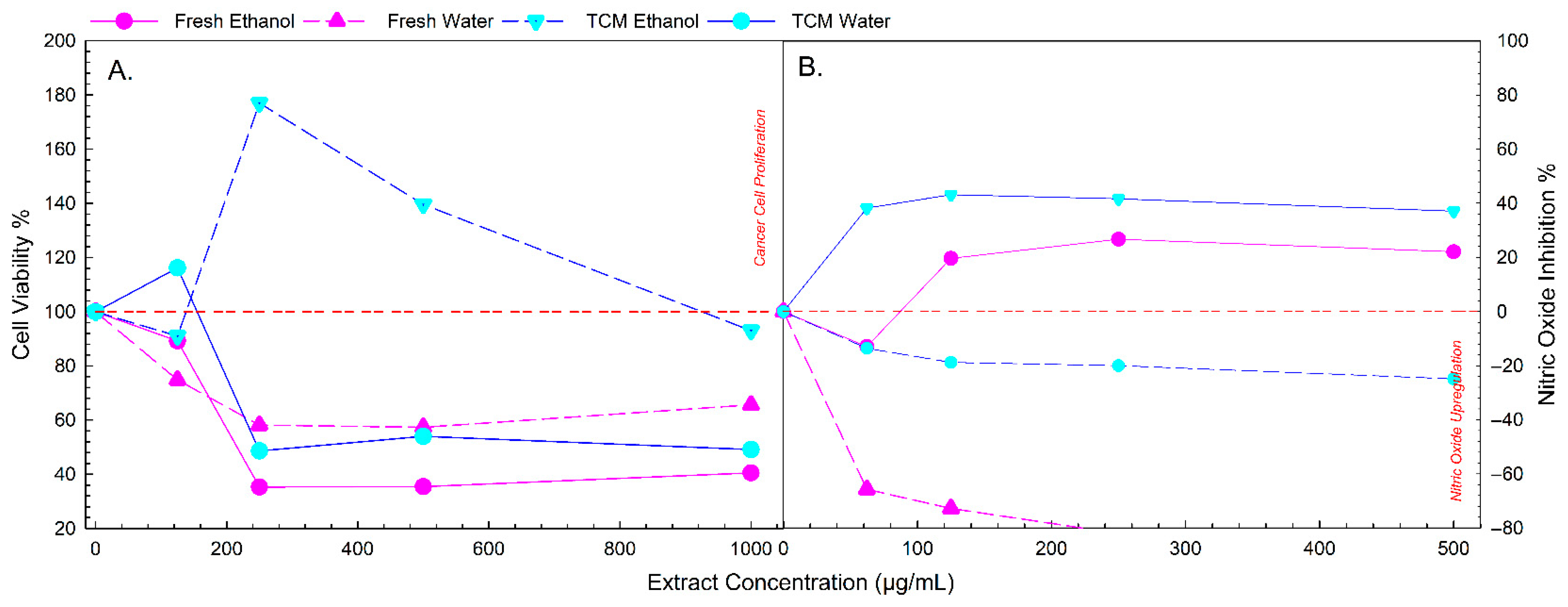
3.5. Fresh Mulberry Extract Shows Highest NO Inhibition and Anti-Cancer Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The Trends Projection Analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Chen, H.; Simoska, O.; Lim, K.; Grattieri, M.; Yuan, M.; Dong, F.; Lee, Y.S.; Beaver, K.; Weliwatte, S.; Gaffney, E.M.; et al. Fundamentals, Applications, and Future Directions of Bioelectrocatalysis. Chem. Rev. 2020, 120, 12903–12993. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, C.C.; Wu, C.C.; Chen, B.Y. Polyphenolic Compounds as Electron Shuttles for Sustainable Energy Utilization. Biotechnol. Biofuels 2019, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, K.; Zou, J.; Li, X.; Wang, Z.; Hu, C. Electron shuttles enhanced the removal of antibiotics and antibiotic resistance genes in anaerobic systems: A review. Front. Microbiol. 2022, 13, 1004589. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Di Meo, S. The Role of Reactive Oxygen Species in the Life Cycle of the Mitochondrion. Int. J. Mol. Sci. 2020, 21, 2173. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Manefield, M.; Lee, M.; Kouzuma, A. Electron Shuttles in Biotechnology. Curr. Opin. Biotechnol. 2009, 20, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. An in Vitro Batch Fermentation Protocol for Studying the Contribution of Food to Gut Microbiota Composition and Functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-W.; Hsieh, C.-Y.; Ting, J.U.; Rogio, K.G.G.; Lee, C.-J.; De Castro-Cruz, K.A.; Ciou, Y.-R.; Lien, T.-K.; Yang, L.-L.; Hsueh, C.-C.; et al. Unraveling the Bioenergy Production and Electron Transport Characteristics of Processed Rheum palmatum L. for Antiviral Drug Development. Ind. Crops Prod. 2023, 195, 116488. [Google Scholar] [CrossRef]
- Tsai, P.-W.; Tayo, L.L.; Ting, J.U.; Hsieh, C.-Y.; Lee, C.-J.; Chen, C.-L.; Yang, H.-C.; Tsai, H.-Y.; Hsueh, C.-C.; Chen, B.-Y. Interactive Deciphering Electron-Shuttling Characteristics of Coffea Arabica Leaves and Potential Bioenergy-Steered Anti-SARS-CoV-2 RdRp Inhibitor via Microbial Fuel Cells. Ind. Crops Prod. 2023, 191, 115944. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-W.; Mailem, R.C.; Tayo, L.L.; Hsueh, C.-C.; Tseng, C.-C.; Chen, B.-Y. Interactive Network Pharmacology and Electrochemical Analysis Reveals Electron Transport-Mediating Characteristics of Chinese Medicine Formula Jing Guan Fang. J. Taiwan. Inst. Chem. Eng. 2023, 147, 104898. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative Stress and Cancer: An Overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Alimirah, F.; Chen, J.; Basrawala, Z.; Xin, H.; Choubey, D. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: Implications for the androgen receptor functions and regulation. FEBS Lett. 2006, 580, 2294–2300. [Google Scholar] [CrossRef] [PubMed]
- Kacar, S.; Kar, F.; Hacioglu, C.; Kanbak, G.; Sahinturk, V. The effects of L-NAME on DU145 human prostate cancer cell line: A cytotoxicity-based study. Hum. Exp. Toxicol. 2020, 39, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-W.; Anderson, D. Reactive Oxygen Species-Induced DNA Damage and Its Modification: A Chemical Investigation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1997, 379, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Rosas-Murrieta, N.H.; Millán-Perez-Peña, L.; Maycotte, P. Reactive Oxygen Species: Role in Carcinogénesis, Cancer Cell Signaling and Tumor Progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kitamura, M. Bidirectional Regulation of NF-ΚB by Reactive Oxygen Species: A Role of Unfolded Protein Response. Free Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef] [PubMed]
- Policastro, L.L.; Ibañez, I.L.; Notcovich, C.; Duran, H.A.; Podhajcer, O.L. The Tumor Microenvironment: Characterization, Redox Considerations, and Novel Approaches for Reactive Oxygen Species-Targeted Gene Therapy. Antioxid. Redox Signal 2013, 19, 854–895. [Google Scholar] [CrossRef] [PubMed]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Fu, Y.; Liu, Y.; Liu, C.; Xu, B.; Zhang, Q.; Jiang, P. The Role of TRPV1 in RA Pathogenesis: Worthy of Attention. Front. Immunol. 2023, 14, 1232013. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-H.; Jang, J.; Oh, S.; Yoon, J.-H.; Jo, D.-G.; Yun, U.J.; Park, K.W. Nrf2 Induces Ucp1 Expression in Adipocytes in Response to Β3-AR Stimulation and Enhances Oxygen Consumption in High-Fat Diet-Fed Obese Mice. BMB Rep. 2021, 54, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic Nerve Development Contributes to Prostate Cancer Progression. Science (1979) 2013, 341, 1236361. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. AMP N1-Oxide, a Unique Compound of Royal Jelly, Induces Neurite Outgrowth from PC12 Vells via Signaling by Protein Kinase A Independent of That by Mitogen-Activated Protein Kinase. Evid.-Based Complement. Altern. Med. 2010, 7, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhu, C.; Xie, Q.; Wang, Y. Adenosine A2A Receptor Antagonists for Cancer Immunotherapy: Miniperspective. J. Med. Chem. 2020, 63, 12196–12212. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, C.; Chiang, C.; Xiao, T.; Chen, Y.; Zhao, Y.; Zheng, D. The Impact of TRPV1 on Cancer Pathogenesis and Therapy: A Systematic Review. Int. J. Biol. Sci. 2021, 17, 2034–2049. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Notarnicola, M.; Caruso, M.G.; Tutino, V.; Scilimati, A. Upregulation of Β3-Adrenergic Receptor MRNA in Human Colon Cancer: A Preliminary Study. Oncology 2008, 75, 224–229. [Google Scholar] [CrossRef] [PubMed]
- DeOliveira, C.C.; Gotardo, E.M.F.; Ribeiro, M.L.; Gambero, A. Role of A1 and A2A Adenosine Receptor Agonists in Adipose Tissue Inflammation Induced by Obesity in Mice. Eur. J. Pharmacol. 2017, 799, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Lan, J.C.; Sun, Q.; Hsueh, C.; Chen, B.-Y. Deciphering Optimal Biostimulation Strategy of Supplementing Anthocyanin-Abundant Plant Extracts for Bioelectricity Extraction in Microbial Fuel Cells. Biotechnol. Biofuels 2019, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Atnip, A.A.; Sigurdson, G.T.; Bomser, J.; Giusti, M.M. Time, Concentration, and PH-Dependent Transport and Uptake of Anthocyanins in a Human Gastric Epithelial (NCI-N87) Cell Line. Int. J. Mol. Sci. 2017, 18, 446. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-Y.; Hsueh, C.-C.; Tsai, P.-W.; Lin, Y.-H.; Tsai, P.-S.; Lien, T.-K.; Yang, C.-W.; Jiang, L.-D. Deciphering Biotransformation of Anthraquinone Electron Shuttles in Rheum palmatum L. for Value-Added Production. J. Taiwan. Inst. Chem. Eng. 2022, 139, 104508. [Google Scholar] [CrossRef]
- Sobremisana, G.; Ferrer, R.; Carpio, A.R.; Tayo, L.L.; Tsai, P.-W.; Hsueh, C.-C.; Chen, B.-Y. Exploring Characteristics of Value-Added Production of Anthraquinones in Rhubarb via Fermentation: Compartmental Modelling and Molecular Docking Analysis. J. Taiwan. Inst. Chem. Eng. 2023, 150, 105076. [Google Scholar] [CrossRef]
- Mejias, N.; Vega-Galvez, A.; Pasten, A.; Uribe, E.; Andrés, A.; Muñoz-Pina, S.; Khvostenko, K.; García-Segovia, P. Evaluating the Microstructure and Bioaccessibility of Bioactive Compounds and Antioxidant Activity After the Dehydration of Red Cabbage. Foods 2025, 14, 1932. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Crystallogr. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chao, H.; Chen, L.; Craig, P.A.; Crichlow, G.V.; Dalenberg, K.; Duarte, J.M.; et al. RCSB Protein Data Bank (RCSB.org): Delivery of Experimentally-Determined PDB Structures alongside One Million Computed Structure Models of Proteins from Artificial Intelligence/Machine Learning. Nucleic Acids Res. 2023, 51, D488–D508. [Google Scholar] [CrossRef] [PubMed]
- Doré, A.S.; Robertson, N.; Errey, J.C.; Ng, I.; Hollenstein, K.; Tehan, B.; Hurrell, E.; Bennett, K.; Congreve, M.; Magnani, F.; et al. Structure of the Adenosine A2A Receptor in Complex with ZM241385 and the Xanthines XAC and Caffeine. Structure 2011, 19, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Nagiri, C.; Kobayashi, K.; Tomita, A.; Kato, M.; Kobayashi, K.; Yamashita, K.; Nishizawa, T.; Inoue, A.; Shihoya, W.; Nureki, O. Cryo-EM Structure of the Β3-Adrenergic Receptor Reveals the Molecular Basis of Subtype Selectivity. Mol. Cell 2021, 81, 3205–3215.e5. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, A.; Oda, M.; Nikolaev, Y.A.; Nadezhdin, K.D.; Gracheva, E.O.; Bagriantsev, S.N.; Sobolevsky, A.I. Human TRPV1 Structure and Inhibition by the Analgesic SB-366791. Nat. Commun. 2023, 14, 2451. [Google Scholar] [CrossRef] [PubMed]
- Gajos, M.; Jasnovidova, O.; van Bömmel, A.; Freier, S.; Vingron, M.; Mayer, A. Conserved DNA Sequence Features Underlie Pervasive RNA Polymerase Pausing. Nucleic Acids Res. 2021, 49, 4402–4420. [Google Scholar] [CrossRef] [PubMed]
- Krivák, R.; Hoksza, D. P2Rank: Machine Learning Based Tool for Rapid and Accurate Prediction of Ligand Binding Sites from Protein Structure. J. Cheminform. 2018, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Lee, J. Variations in Anthocyanin Profiles and Antioxidant Activity of 12 Genotypes of Mulberry (Morus spp.) Fruits and Their Changes during Processing. Antioxidants 2020, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, Z.-F.; Wu, N.; Gou, G.-Z.; Chen, X.-L.; Yang, G.-M.; Liu, W. One-Pot Synthesis of N-Doped Reduced Graphene Oxide Decorated with Au-Pd@ Au Alloy Nanodendrites for Electrochemical Detection of Chrysophanol. Int. J. Electrochem. Sci. 2020, 15, 3660–3671. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Liao, J.-H.; Hsueh, C.-C.; Qu, Z.; Hsu, A.-W.; Chang, C.-T.; Zhang, S. Deciphering Biostimulation Strategy of Using Medicinal Herbs and Tea Extracts for Bioelectricity Generation in Microbial Fuel Cells. Energy 2018, 161, 1042–1054. [Google Scholar] [CrossRef]
- Sobremisana, G.; Tayo, L.L.; Tsai, P.-W.; Hsueh, C.-C.; Chen, B.-Y. Machine Learning-Assisted Optimized Production of Quorum Quenching Anthraquinones in Rhubarb. J. Taiwan. Inst. Chem. Eng. 2024, 160, 105358. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Biotransformation of Green Tea (Camellia sinensis) by Wine Yeast Saccharomyces Cerevisiae. J. Food Sci. 2020, 85, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-L.; Qin, L.-J.; Xu, B.; Wang, X.-Z.; Hsueh, C.-C.; Chen, B.-Y. Deciphering Electron-Shuttling Characteristics of Epinephrine and Dopamine for Bioenergy Extraction Using Microbial Fuel Cells. Biochem. Eng. J. 2019, 148, 57–64. [Google Scholar] [CrossRef]
- Huang, D.-Q.; Chen, C.; Wu, Y.-M.; Zhang, H.; Sheng, L.-Q.; Xu, H.-J.; Liu, Z.-D. The Determination of Dopamine Using Glassy Carbon Electrode Pretreated by a Simple Electrochemical Method. Int. J. Electrochem. Sci. 2012, 7, 5510–5520. [Google Scholar] [CrossRef]
- Ali, S.; Shultz, J.L. High Performance Microbiological Transformation of L-Tyrosine to L-Dopa by Yarrowia Lipolytica NRRL-143. BMC Biotechnol. 2007, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-Y.; Wu, Y.-C.; Lin, Y.-H.; Tayo, L.L.; Tacas, A.C.; Hsueh, C.-C.; Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Deciphering Electron-Shuttling Characteristics of Parkinson’s Disease Medicines via Bioenergy Extraction in Microbial Fuel Cells. Ind. Eng. Chem. Res. 2020, 59, 17124–17136. [Google Scholar] [CrossRef]
- Fadhil, R.; Hayati, R.; Agustina, R. Quality Characteristics of Sauerkraut from Cabbage (Brassica oleracea) during Fermentation and Variation of Salt Concentration. Parameters 2019, 1, K3. [Google Scholar]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yang, J.; Ma, L.; Cai, J.; Li, J. Comparison of Polyphenol Profile and Inhibitory Activities against Oxidation and A-glucosidase in Mulberry (Genus Morus) Cultivars from China. J. Food Sci. 2015, 80, C2440–C2451. [Google Scholar] [CrossRef] [PubMed]
- Hazafa, A.; Rehman, K.-U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Thangapazham, R.L.; Passi, N.; Maheshwari, R.K. Green Tea Polyphenol and Epigallocatechin Gallate Induce Apoptosis and Inhibit Invasion in Human Breast Cancer Cells. Cancer Biol. Ther. 2007, 6, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Gong, C.; Song, H.; Cui, Y. Effects of Anthocyanins on the Prevention and Treatment of Cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- ben Sghaier, M.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin Inhibits Proliferation, Attenuates Superoxide Production and Decreases Adhesion and Migration of Human Cancerous Cells. Biomed. Pharmacother. 2016, 84, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and Cancer: New Insights into Its Therapeutic Effects on Ovarian Cancer Cells. Cell Biosci. 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Bawadi, H.A.; Bansode, R.R.; Trappey, A., II; Truax, R.E.; Losso, J.N. Inhibition of Caco-2 Colon, MCF-7 and Hs578T Breast, and DU 145 Prostatic Cancer Cell Proliferation by Water-Soluble Black Bean Condensed Tannins. Cancer Lett. 2005, 218, 153–162. [Google Scholar] [CrossRef] [PubMed]
- AlMalki, F.A.; Hassan, A.M.; Klaab, Z.M.; Abdulla, S.; Pizzi, A. Tannin Nanoparticles (NP99) Enhances the Anticancer Effect of Tamoxifen on ER+ Breast Cancer Cells. J. Renew. Mater. 2021, 9, 2077–2092. [Google Scholar] [CrossRef]
- Du, Q.-Z.; Zheng, J.; Xu, Y. Composition of Anthocyanins in Mulberry and Their Antioxidant Activity. J. Food Compos. Anal. 2008, 21, 390–395. [Google Scholar] [CrossRef]
- Katsube, T.; Yamasaki, M.; Shiwaku, K.; Ishijima, T.; Matsumoto, I.; Abe, K.; Yamasaki, Y. Effect of Flavonol Glycoside in Mulberry (Morus alba L.) Leaf on Glucose Metabolism and Oxidative Stress in Liver in Diet-induced Obese Mice. J. Sci. Food Agric. 2010, 90, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-Y.; Ma, C.-M.; Han, K.; Yueh, P.-L.; Qin, L.-J.; Hsueh, C.-C. Influence of Textile Dye and Decolorized Metabolites on Microbial Fuel Cell-Assisted Bioremediation. Bioresour. Technol. 2016, 200, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Saensouk, S.; Senavongse, R.; Papayrata, C.; Chumroenphat, T. Evaluation of Color, Phytochemical Compounds and Antioxidant Activities of Mulberry Fruit (Morus alba L.) during Ripening. Horticulturae 2022, 8, 1146. [Google Scholar] [CrossRef]
- Sangteerakij, D.; Rodyu, S.; Junden, S. Nutrition Composition and Anthocyanin Content of Mulberry Fruits (Morus alba L.) by Different Ripening Stage Grown in the South of Thailand, Applicants in Sorbet and Sherbet Ice-Cream and Analysis of Physical, Chemical and Sensory Evaluation. Emir. J. Food Agric. 2022, 35, 130–138. [Google Scholar] [CrossRef]
- Petrovic, S.C. Correlation of Perceived Wine Astringency to Cyclic Voltammetric Response. Am. J. Enol. Vitic. 2009, 60, 373–378. [Google Scholar] [CrossRef]
- Qin, L.; Guo, L.; Xu, B.; Hsueh, C.-C.; Jiang, M.; Chen, B.-Y. Exploring Community Evolutionary Characteristics of Microbial Populations with Supplementation of Camellia Green Tea Extracts in Microbial Fuel Cells. J. Taiwan. Inst. Chem. Eng. 2020, 113, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, D.R. Evidence for Involvement of an Electron Shuttle in Electricity Generation by Geothrix fermentans. Appl. Environ. Microbiol. 2005, 71, 2186–2189. [Google Scholar] [CrossRef] [PubMed]
- Sund, C.J.; McMasters, S.; Crittenden, S.R.; Harrell, L.E.; Sumner, J.J. Effect of electron mediators on current generation and fermentation in a microbial fuel cell. Appl. Microbiol. Biotechnol. 2007, 76, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yu, H.; Wang, S.; Wang, W.; Liang, Z. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Choudhary, Y.S.; Jothi, L.; Nageswaran, G. Electrochemical Characterization. In Spectroscopic Methods for Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 19–54. [Google Scholar]
- Rambacher, K.M.; Moniri, N.H. Cysteine redox state regulates human β2-adrenergic receptor binding and function. Sci. Rep. 2020, 10, 2934. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kang, J.; Kwon, H.; Frueh, D.; Seung, H.Y.; Wagner, G.; Park, S. Effects of redox potential and Ca2+ on the inositol 1,4,5-trisphosphate receptor L3-1 loop region: Implications for receptor regulation. J. Biol. Chem. 2008, 283, 25567–25575. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Smith, J.W. Mechanism of integrin activation by disulfide bond reduction. Biochemistry 2001, 40, 8861–8867. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Martin, E.; Sharina, I.; Esposito, I.; Szabo, C.; Bucci, M.; Cirino, G.; Papapetropoulos, A. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. Pharmacol. Res. 2016, 111, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Saura, P.; Frey, D.M.; Gamiz-Hernandez, A.P.; Kaila, V.R.I. Electric field modulated redox-driven protonation and hydration energetics in energy converting enzymes. Chem. Commun. 2019, 55, 6078–6081. [Google Scholar] [CrossRef] [PubMed]
- Olsbu, I.K.; Zoppellaro, G.; Andersson, K.K.; Boucher, J.; Hersleth, H. Importance of Val567 on Heme Environment and Substrate Recognition of Neuronal Nitric Oxide Synthase. FEBS Open Bio 2018, 8, 1553–1566. [Google Scholar] [CrossRef] [PubMed]
- Oue, S.; Okamoto, A.; Nakai, Y.; Nakahira, M.; Shibatan, T.; Hayashi, H.; Kagamiyama, H. Paracoccus Denitrificans Aromatic Amino Acid Aminotransferase: A Model Enzyme for the Study of Dual Substrate Recognition Mechanism. J. Biochem. 1997, 121, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.; Comini, M.; Salinas, G.; Trujillo, M. Redox Chemistry and Biology of Thiols; Academic Press: Cambridge, MA, USA, 2022; ISBN 0323915663. [Google Scholar]
- Chan, A.W.E.; Laskowski, R.A.; Selwood, D.L. Chemical Fragments That Hydrogen Bond to Asp, Glu, Arg, and His Side Chains in Protein Binding Sites. J. Med. Chem. 2010, 53, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Oezguen, N.; Urvil, P.; Ferguson, C.; Dann, S.M.; Savidge, T.C. Regulation of Protein-Ligand Binding Affinity by Hydrogen Bond Pairing. Sci. Adv. 2016, 2, e1501240. [Google Scholar] [CrossRef] [PubMed]
- Uyaver, S. Tyrosine, Phenylalanine, and Tryptophan Undergo Self-Aggregation in Similar and Different Manners. Atmosphere 2022, 13, 1448. [Google Scholar] [CrossRef]
- Patil, R.; Das, S.; Stanley, A.; Yadav, L.; Sudhakar, A.; Varma, A.K. Optimized Hydrophobic Interactions and Hydrogen Bonding at the Target-Ligand Interface Leads the Pathways of Drug-Designing. PLoS ONE 2010, 5, e12029. [Google Scholar] [CrossRef] [PubMed]
- Bruunsgaard, H. Physical Activity and Modulation of Systemic Low-Level Inflammation. J. Leukoc. Biol. 2005, 78, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.; Vyas, P.; Attur, M.; Leszczynska-Piziak, J.; Patel, I.R.; Weissmann, G.; Abramson, S.B. The Mode of Action of Aspirin-like Drugs: Effect on Inducible Nitric Oxide Synthase. Proc. Natl. Acad. Sci. USA 1995, 92, 7926–7930. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.H.; Lee, S.O.; Kim, S.Y.; Yang, S.J.; Lim, Y. Anti-Inflammatory and Antiobesity Effects of Mulberry Leaf and Fruit Extract on High Fat Diet-Induced Obesity. Exp. Biol. Med. 2013, 238, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Mirmalek, S.A.; Faraji, S.; Ranjbaran, S.; Aryan, H.; Arani, H.Z.; Jangholi, E.; Marzouni, H.Z.; Salimi-Tabatabaee, S.A. Cyanidin 3-glycoside induced apoptosis in MCF-7 breast cancer cell line. Arch. Med. Sci. AMS 2020, 19, 1092. [Google Scholar] [CrossRef] [PubMed]
- Sargara, P.; Nataraj, M.; Subramanian, R.B.; Thakkar, A.B. Hydromethanolic extracts of Morus alba L. (Mulberry) young and ripe fruit induces apoptosis in lung carcinoma cells (A549). Braz. J. Pharm. Sci. 2025, 61, e24112. [Google Scholar] [CrossRef]
- Long, H.L.; Zhang, F.F.; Wang, H.L.; Yang, W.S.; Hou, H.T.; Yu, J.K.; Liu, B. Mulberry anthocyanins improves thyroid cancer progression mainly by inducing apoptosis and autophagy cell death. Kaohsiung J. Med. Sci. 2018, 34, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Dalkiliç, S.; Dalkiliç, L.K.; İnci, Ş.; Korkmaz, İ.; Kırbağ, S. Investigation of Cytotoxic Effect of Black Mulberry (Morus nigra L.) Fruit. Indian J. Tradit. Knowl. (IJTK) 2021, 20, 54–58. [Google Scholar] [CrossRef]
- Xiang, Y.; Lai, F.; He, G.; Li, Y.; Yang, L.; Shen, W.; Huo, H.; Zhu, J.; Dai, H.; Zhang, Y. Alleviation of Rosup-induced oxidative stress in porcine granulosa cells by anthocyanins from red-fleshed apples. PLoS ONE 2017, 12, e0184033. [Google Scholar] [CrossRef] [PubMed]
- Pahlke, G.; Ahlberg, K.; Oertel, A.; Janson-Schaffer, T.; Grabher, S.; Mock, H.P.; Matros, A.; Marko, D. Antioxidant Effects of Elderberry Anthocyanins in Human Colon Carcinoma Cells: A Study on Structure–Activity Relationships. Mol. Nutr. Food Res. 2021, 65, 2100229. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Samani, M.; Rafieian-Kopaei, M.; Lorigooini, Z.; Shirzad, H. A Screening of Growth Inhibitory Activity of Iranian Medicinal Plants on Prostate Cancer Cell Lines. Biomedicine 2018, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-N.; Chu, S.-C.; Chiou, H.-L.; Chiang, C.-L.; Yang, S.-F.; Hsieh, Y.-S. Cyanidin 3-Glucoside and Peonidin 3-Glucoside Inhibit Tumor Cell Growth and Induce Apoptosis in Vitro and Suppress Tumor Growth in Vivo. Nutr. Cancer 2005, 53, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Katsube, N.; Iwashita, K.; Tsushida, T.; Yamaki, K.; Kobori, M. Induction of Apoptosis in Cancer Cells by Bilberry (Vaccinium myrtillus) and the Anthocyanins. J. Agric. Food Chem. 2003, 51, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-Rich Extract from Aronia meloncarpa E. Induces a Cell Cycle Block in Colon Cancer but Not Normal Colonic Cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Sotero, M.Y.; Cruz-Hernández, C.D.; Oliart-Ros, R.M.; Chávez-Servia, J.L.; Guzmán-Gerónimo, R.I.; González-Covarrubias, V.; Cruz-Burgos, M.; Rodríguez-Dorantes, M. Anthocyanins of Blue Corn and Tortilla Arrest Cell Cycle and Induce Apoptosis on Breast and Prostate Cancer Cells. Nutr. Cancer 2020, 72, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Liu, Y.; Liu, J.; Wang, E. Stability of blueberry anthocyanin, anthocyanidin and pyranoanthocyanidin pigments and their inhibitory effects and mechanisms in human cervical cancer HeLa cells. RSC Adv. 2019, 9, 10842–10853. [Google Scholar] [CrossRef] [PubMed]
- Jing, N.; Song, J.; Liu, Z.; Wang, L.; Jiang, G. Glycosylation of anthocyanins enhances the apoptosis of colon cancer cells by handicapping energy metabolism. BMC Complement. Med. Ther. 2020, 20, 312. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Chung, E.Y.; Jang, H.-Y.; Hong, O.-Y.; Chae, H.S.; Jeong, Y.-J.; Kim, S.-Y.; Kim, B.-S.; Yoo, D.J.; Kim, J.-S.; et al. Anti-Cancer Effect of Cyanidin-3-Glucoside from Mulberry via Caspase-3 Cleavage and DNA Fragmentation in Vitro and in Vivo. Anti-Cancer Agents Med. Chem. 2017, 17, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Zhao, Y.; Shi, Z.; Xing, L.; Li, X.; Jia, L.; Lin, K. Plant-derived paclitaxel-loaded ultra-small Fe3O4 nanoparticles for MR imaging-mediated antitumor therapy. Ind. Crops Prod. 2025, 228, 120902. [Google Scholar] [CrossRef]
- Matsumura, E.; Matsuda, M.; Sato, F.; Minami, H.; Ramawat, K.G.; Mérillon, J.-M. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Ali, H.M.; Almagribi, W.; Al-Rashidi, M.N. Antiradical and Reductant Activities of Anthocyanidins and Anthocyanins, Structure–Activity Relationship and Synthesis. Food Chem. 2016, 194, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Wahyuningsih, S.; Wulandari, L.; Wartono, M.W.; Munawaroh, H.; Ramelan, A.H. The Effect of PH and Color Stability of Anthocyanin on Food Colorant. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 193, p. 12047. [Google Scholar]
- Kostka, T.; Ostberg-Potthoff, J.J.; Briviba, K.; Matsugo, S.; Winterhalter, P.; Esatbeyoglu, T. Pomegranate (Punica granatum L.) Extract and Its Anthocyanin and Copigment Fractions—Free Radical Scavenging Activity and Influence on Cellular Oxidative Stress. Foods 2020, 9, 1617. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Blesso, C.N. Antioxidant Properties of Anthocyanins and Their Mechanism of Action in Atherosclerosis. Free Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef] [PubMed]
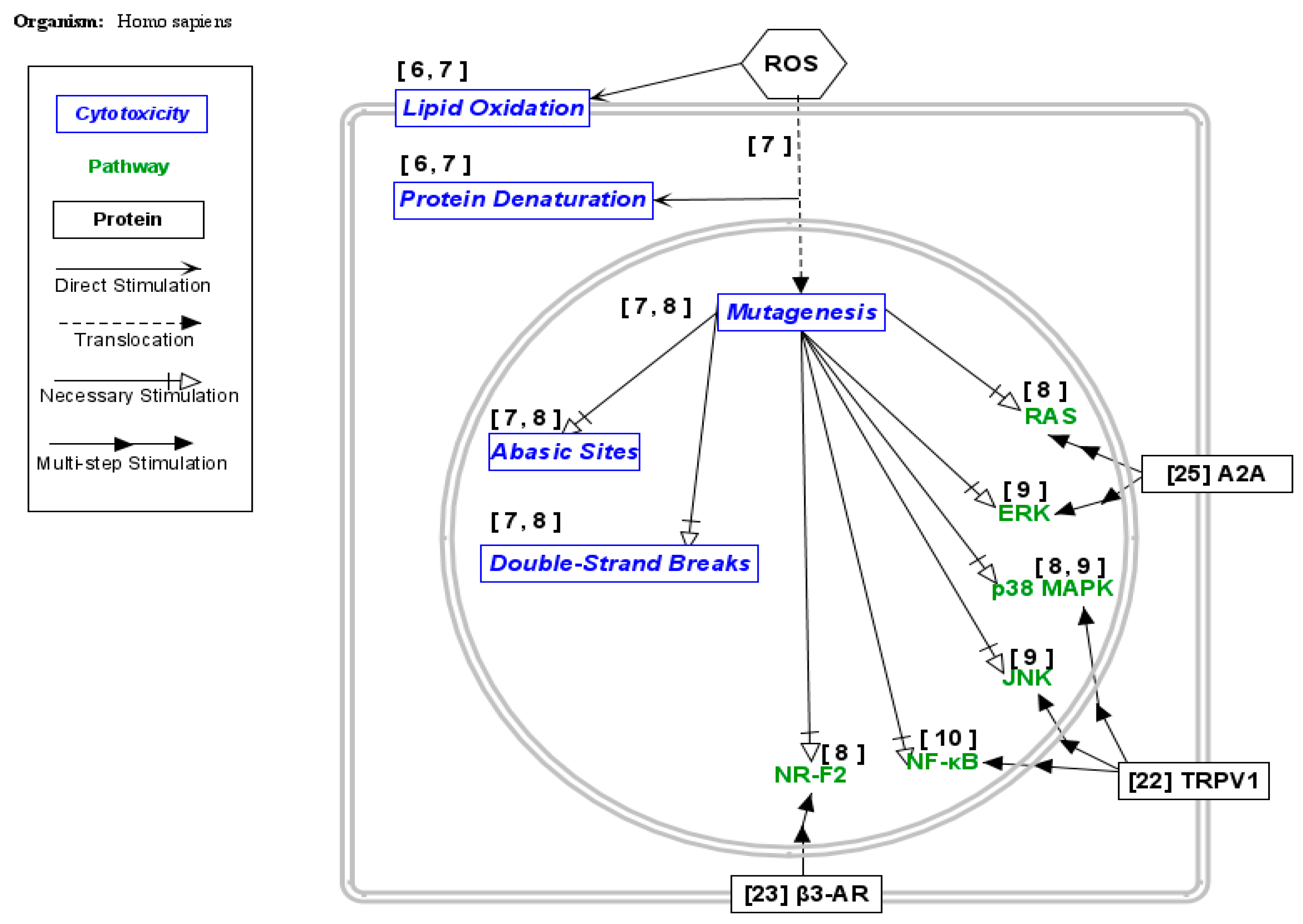

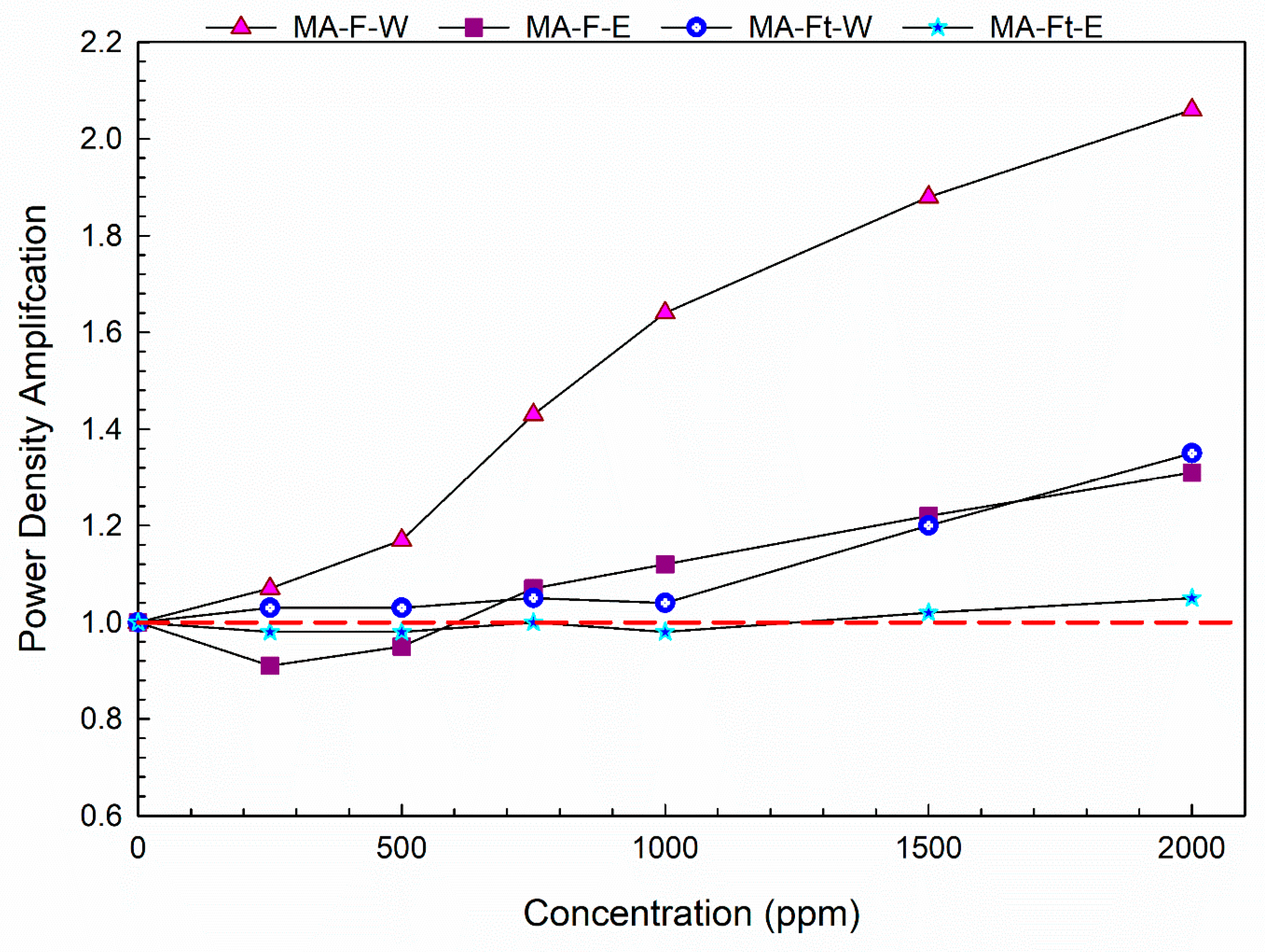

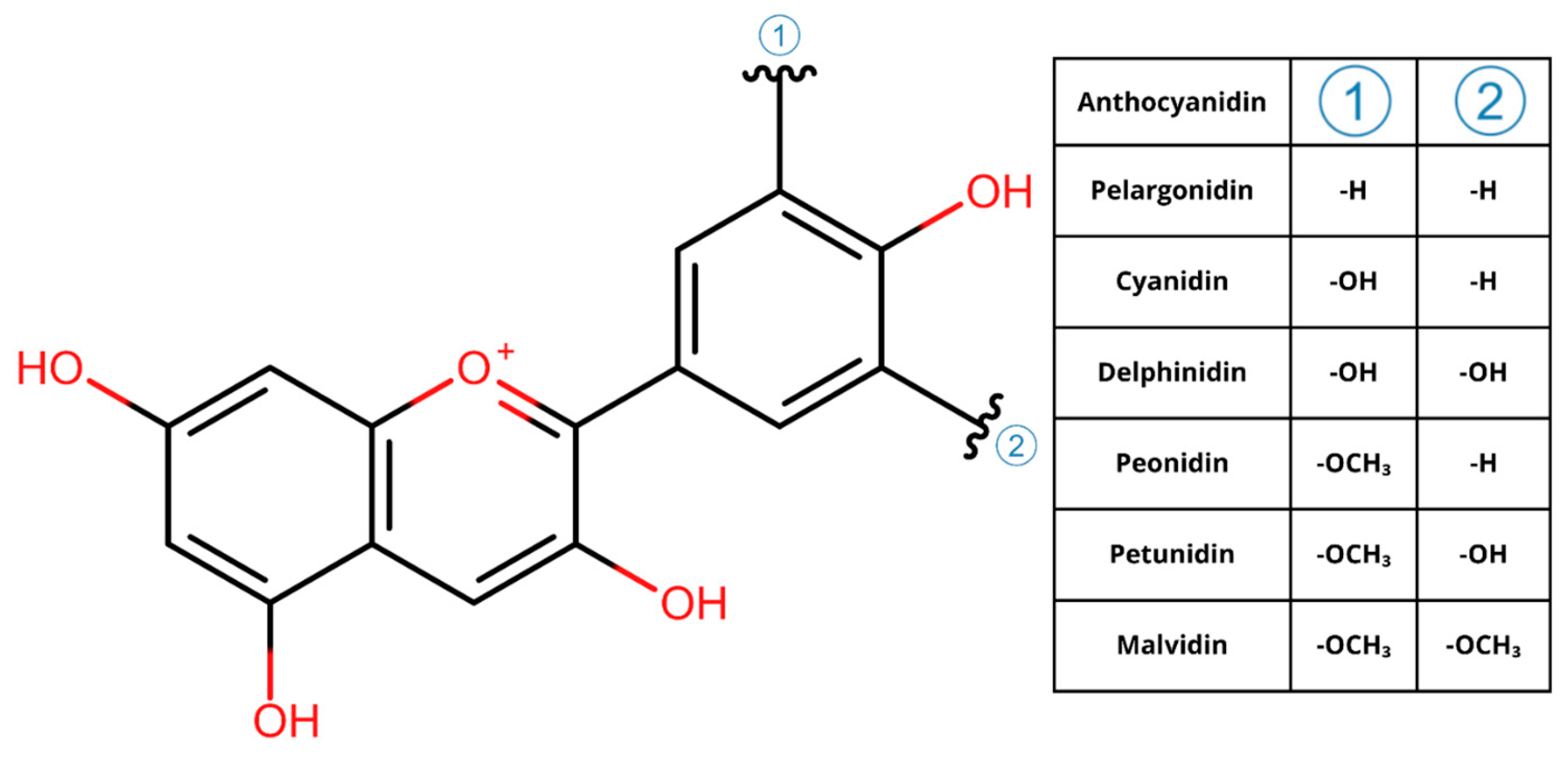
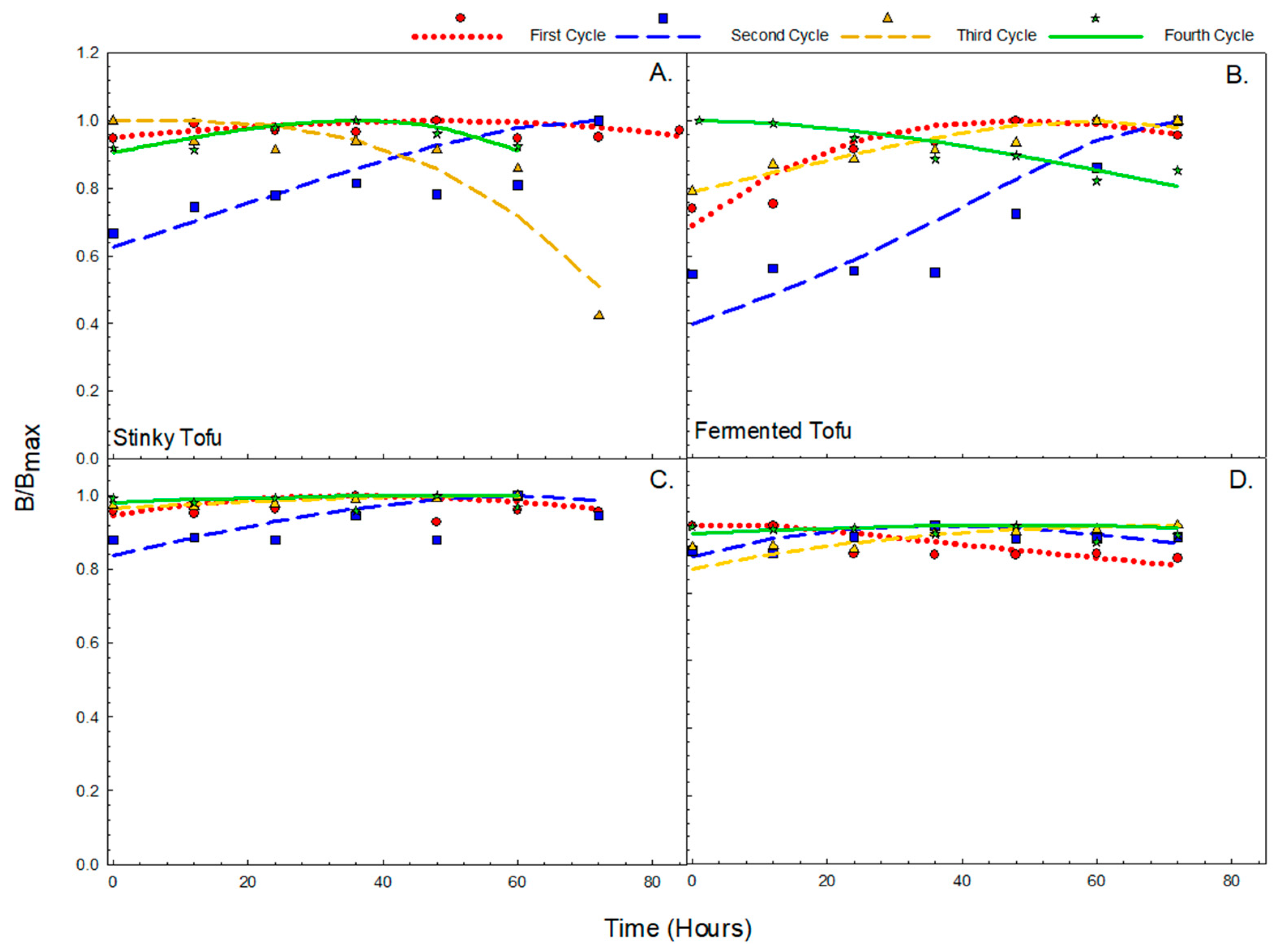

| Legend | Percent Yield | Description |
|---|---|---|
| MA-F-W | 33.30 ± 2.069 | Commercially obtained ripe Morus alba fruit water extract |
| MA-F-E | 15.99 ± 0.421 | Ripe Morus alba fruit ethanol extract |
| MA-Ft-W | 13.18 ± 0.251 | Dried unripe Morus alba traditional Chinese medicine water extract |
| MA-Ft-E | 5.087 ± 0.314 | Dried unripe Morus alba traditional Chinese medicine ethanol extract |
| Protein Receptor | Coordinates | ||
|---|---|---|---|
| X | Y | Z | |
| TRPV1 | 103.8511 | 81.0341 | 85.8088 |
| β3-adrenergic (β3-AR) | 77.6744 | 73.631 | 120.2751 |
| A2A | 9.7198 | −34.0464 | −32.6645 |
| Compound | Identification Number |
|---|---|
| Cyanidin hexose deoxyhexose hexoside | Pubchem: 56671053 |
| Cyanidin dihexoside | Pubchem: 44256718 |
| Cyanidin 3-O-glucoside | Pubchem: 197081 |
| Cyanidin 3-O-rutinoside | Pubchem: 441674 |
| Pelargonidin 3-O-glucoside | Pubchem: 443648 |
| Malvidin 3-O-beta-D-glucoside | Pubchem: 443652 |
| pelargonidin 3-O-rutinoside | CHEBI: 31968 |
| Peonidin 3-glucoside | Pubchem: 443654 |
| Cyanidin pentoside | Pubchem: 12137509 |
| cyanidin 3-O-(6-O-malonyl-β-D-glucoside) | CHEBI: 31442 |
| Peonidin-3,5-O-di-β-glucopyranoside | Chemspider: 4589999 |
| Cyanidin deoxyhexoside | Pubchem: 14138147 |
| Delphinidin 3-glucoside chloride | Pubchem: 165558 |
| Sample | Total Polyphenol Content Analysis (Gallic Acid mg/g) | Total Flavonoids Content Analysis (Rutin mg/g) | Total Condensed Tannin Content Analysis (Catechin mg/g) | Total Anthocyanin Content Analysis (C3G mg/g) |
|---|---|---|---|---|
| MA-F-E | 16.335 ± 0.397 | 5.434 ± 0.265 | 8.152 ± 0.652 | 2.325 ± 0.342 |
| MA-F-W | 46.730 ± 1.703 | 6.895 ± 0.171 | 12.216 ± 0.741 | 8.906 ± 1.206 |
| MA-Ft-E | 17.217 ± 0.065 | 21.248 ± 0.257 | 1.891 ± 0.036 | N.D. |
| MA-Ft-W | 13.110 ± 0.065 | 2.500 ± 0.029 | 19.557 ± 0.571 | N.D. |
| Sample | DPPH Assay IC50 (mg/mL) | FRAP Assay (mg Trolox/g Extract) |
|---|---|---|
| MA-F-W | 1.475 ± 0.020 | 45.031 ± 0.199 |
| MA-F-E | 5.171 ± 0.013 | 21.518 ± 0.229 |
| MA-Ft-W | 4.546 ± 0.235 | 23.476 ± 0.057 |
| MA-Ft-E | 4.206 ± 0.015 | 28.911 ± 0.118 |
| Test | One-Way ANOVA | Two-Way ANOVA (Maturity:Solvent) | Multivariate ANOVA (Phytochemical Profile) | |||
|---|---|---|---|---|---|---|
| p-Value | F Value | p-Value | F Value | p-Value | F Value | |
| TPC | 7.99 × 10−11 | 1114 | 3.34 × 10−10 | 1346.1 | 2.20 × 10−16 | 6 |
| TFC | 6.52 × 10−15 | 11,753 | 1.40 × 10−14 | 16,796 | ||
| TCT | 1.84 × 10−9 | 506.8 | 3.15 × 10−8 | 427.1 | ||
| TAC | 1.07 × 10−6 | 100.9 | 6.69 × 10−5 | 56.81 | ||
| DPPH | 1.45 × 10−12 | 3043 | 2.10 × 10−12 | 4801 | ||
| FRAP | 7.36 × 10−14 | 6410 | 2.54 × 10−12 | 4577 | ||
| Concentration (µg/mL) | MA-F-W | MA-F-E | MA-Ft-W | MA-Ft-E |
|---|---|---|---|---|
| 250 | 0.0814 | 0.105 | 0.476 | 0.419 |
| 500 | 0.0215 | 0.296 | 0.996 | 0.348 |
| 750 | 0.00199 | 0.385 | 0.495 | 0.681 |
| 1000 | 0.00184 | 0.216 | 0.748 | 0.52 |
| 1500 | 0.00209 | 0.157 | 0.174 | 0.946 |
| 2000 | 0.00132 | 0.0794 | 0.066 | 0.587 |
| Sample | Area (uW) | Decay Ratio | Redox Ratio | ||
|---|---|---|---|---|---|
| 10th Cycle | 20th Cycle | 50th Cycle | |||
| MA-F-W | 15.22 | 10.93 | 5.71 | 62.48% | 1.65 |
| MA-F-E | 10.77 | 3.34 | 2.17 | 79.85% | 1.27 |
| MA-Ft-W | 21.17 | 20.22 | 18.42 | 12.99% | No peaks |
| MA-Ft-E | 17.37 | 15.22 | 6.32 | 63.61% | No peaks |
| Fermented Tofu | TFC | Cycle | α | RSS | K1 | K2 | RMSD | RMSE |
| 1 | 0.978 | 0.011528 | 0.013354 | 0.01306 | 0.044481 | 0.117687 | ||
| 2 | 3.829 | 0.021213 | −0.01684 | −0.0645 | 0.099317 | 0.262769 | ||
| 3 | 3.961 | 0.003175 | −0.00771 | −0.03054 | 0.027256 | 0.072112 | ||
| 4 | 2.47 | 0.004284 | 0.007407 | 0.018295 | 0.030015 | 0.079413 | ||
| Anthocyanin | Cycle | α | RSS | K1 | K2 | RMSD | RMSE | |
| 1 | 0.00479 | 0.005869 | 0.450736 | 0.002159 | 0.030851 | 0.081623 | ||
| 2 | 0.0491 | 0.005954 | −0.0359 | −0.00176 | 0.038532 | 0.10194 | ||
| 3 | 0.471 | 0.000163 | 0.008743 | 0.004118 | 0.012894 | 0.034115 | ||
| 4 | 0.71 | 0.005954 | −0.00591 | −0.00419 | 0.021622 | 0.057205 |
| Stinky Tofu | TFC | Cycle | α | RSS | K1 | K2 | RMSD | RMSE |
| 1 | 3.291 | 0.008915 | −0.00414 | −0.0136 | 0.02551 | 0.072153 | ||
| 2 | 1.821 | 0.076641 | −0.01369 | −0.024 | 0.08842 | 0.25009 | ||
| 3 | 2.656 | 0.001033 | −0.00744 | −0.0197 | 0.07454 | 0.21083 | ||
| 4 | 3.016 | 0.001576 | −0.00868 | −0.0261 | 0.018227 | 0.051553 | ||
| Anthocyanin | Cycle | α | RSS | K1 | K2 | RMSD | RMSE | |
| 1 | 0.94 | 0.015361 | 0.008393 | 0.00789 | 0.030334 | 0.085796 | ||
| 2 | 0.946 | 0.033728 | −0.0123 | −0.0116 | 0.05193 | 0.146879 | ||
| 3 | 0.748 | 0.000224 | −0.00561 | −0.0041 | 0.006284 | 0.017773 | ||
| 4 | 0.811 | 0.004884 | −0.00477 | −0.0038 | 0.02131 | 0.060273 |
| Compound (Sample) | PDamplification | Ortho/Para Constituents | Rratio | Remarks | Ref. | |
|---|---|---|---|---|---|---|
| Chrysophanol (Rhubarb) | 2.39 ± 0.77 | Yes | 0.185 | 1.07 | Electron shuttle; Demonstrated quorum quenching ability. | [35,36,46] |
| Catechin (Green tea) | 2.67 | Yes | 0.0026 | 1.05 | Electron shuttle; Polyphenols were found to inhibit breast cancer cells growth. | [47] |
| Dopamine (pure) | 3.72 ± 0.33 | Yes | 0.4581 | 0.788 | Electron Shuttle; Demonstrated potential activity for Parkinson’s disease | [46,48,49,50,51,52,53] |
| Ascorbic Acid (pure) | 2.11± 0.27 | No | 1.3 | 1.7 | Not an Electron Shuttle; Widely used as a vitamin and antioxidant supplement. | [46,51] |
| Anthocyanin (Mulberry) | 2.06 ± 0.10 | Yes | 0.0269 | 1.65 | Electron shuttle; Shows Dose-dependent activity against cancer. | This study |
| A2A | Score | B3-Andregenic | Score | TRPV1 | Score |
|---|---|---|---|---|---|
| Delphinidin 3-glucoside chloride | −11.187 | Peonidin 3-glucoside | −10.639 | Cyanidin 3-O-rutinoside | −10.286 |
| Cyanidin 3-O-rutinoside | −10.961 | Malvidin hexoside | −10.414 | Malvidin hexoside | −10.024 |
| Cyanidin-3-glucoside | −10.64 | Cyanidin 3-O-rutinoside | −10.409 | Peonidin 3-glucoside | −9.916 |
| Pelargonidin 3-glucoside | −10.616 | Delphinidin 3-glucoside chloride | −10.323 | Cyanidin pentoside | −9.391 |
| Cyanidin pentoside | −10.52 | Pelargonidin 3-glucoside | −10.049 | Delphinidin 3-glucoside chloride | −9.131 |
| Caffeine * | −7.893 | Miragebron * | −9.915 | Capsaicin * | −9.332 |
| Comparison | One-Way ANOVA | One-Sample, One-Tailed t-Test |
|---|---|---|
| Between Anthocyanins | 0.959 | - |
| Between Receptors | 0.00154 | - |
| Anthocyanin vs. Caffeine | - | 1.034 × 10−5 |
| Anthocyanin vs. Miragebron | - | 4.49 × 10−3 |
| Anthocyanin vs. Capsaicin | - | 0.06033 |
| Sample | IC50 (µg/mL) | One-Tailed t-Test | ||
|---|---|---|---|---|
| NO Inhibition Assay | Anti-Cancer Assay | NO Inhibition Assay | Anti-Cancer Assay | |
| MA-F-W | - | 626.349 | 0.987 | 0.001 |
| MA-F-E | 0.486 | - | 0.022 | 0.015 |
| MA-Ft-W | - | - | 0.925 | 0.845 |
| MA-Ft-E | 4.614 | 843.532 | 0.005 | 0.069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobremisana, G.S.; Tsai, P.-W.; Rejano, C.J.F.; Tayo, L.L.; Hsueh, C.-C.; Hsieh, C.-Y.; Chen, B.-Y. Electron-Shuttling and Bioenergy-Stimulating Properties of Mulberry Anthocyanins: A Mechanistic Study Linking Redox Activity to MFC Performance and Receptor Affinity. Processes 2025, 13, 2290. https://doi.org/10.3390/pr13072290
Sobremisana GS, Tsai P-W, Rejano CJF, Tayo LL, Hsueh C-C, Hsieh C-Y, Chen B-Y. Electron-Shuttling and Bioenergy-Stimulating Properties of Mulberry Anthocyanins: A Mechanistic Study Linking Redox Activity to MFC Performance and Receptor Affinity. Processes. 2025; 13(7):2290. https://doi.org/10.3390/pr13072290
Chicago/Turabian StyleSobremisana, Gilbert S., Po-Wei Tsai, Christine Joyce F. Rejano, Lemmuel L. Tayo, Chung-Chuan Hsueh, Cheng-Yang Hsieh, and Bor-Yann Chen. 2025. "Electron-Shuttling and Bioenergy-Stimulating Properties of Mulberry Anthocyanins: A Mechanistic Study Linking Redox Activity to MFC Performance and Receptor Affinity" Processes 13, no. 7: 2290. https://doi.org/10.3390/pr13072290
APA StyleSobremisana, G. S., Tsai, P.-W., Rejano, C. J. F., Tayo, L. L., Hsueh, C.-C., Hsieh, C.-Y., & Chen, B.-Y. (2025). Electron-Shuttling and Bioenergy-Stimulating Properties of Mulberry Anthocyanins: A Mechanistic Study Linking Redox Activity to MFC Performance and Receptor Affinity. Processes, 13(7), 2290. https://doi.org/10.3390/pr13072290








