Abstract
Water pollution is a global problem that severely impacts human and environmental health, water recycling, and the economy. In Mexico, due to water scarcity, potable water contains significant amounts of heavy metals (i.e., arsenic (As)); thus, there is a need for efficient and sustainable water treatment strategies. Bismuth oxyhalides, BiOX (X = Cl, Br, I), exhibit three-dimensional (3D) porous structures suitable for efficient adsorption activity. In addition, bismuth is an abundant and biocompatible element appropriate for fabricating sustainable environmental remediation technologies, such as adsorptive BiOX nanomaterials (NMs). In this study, we examine the adsorption capacity of BiOX (X = Cl, I), BiOX-GO (GO: graphene oxide) and GO NMs to remove methylene blue (MB), methyl orange (MO) and arsenite (AsO33−) from aqueous solution. BiOCl-GO 10%, BiOI, BiOI-GO 1%, BiOI-GO 10% and GO have an enhanced adsorption capacity, removing MB (20 ppm) within one hour using a low dose of NMs (1 mg/mL). In addition, BiOX-GO NMs can be easily separated from the solution and regenerated upon visible light activation due to the photocatalytic activity of the materials. The efficiency of the NMs under study for MO removal decreases, with the GO material having the highest efficiency (96%), followed by BiOX-GO 10% (78%). BiOCl-GO 1% removes arsenic from aqueous solution at low doses and short treatment times; 5 mg As/g adsorbent takes five hours; however, at longer adsorption times (24 h), BiOI-GO 1% excels in its arsenic removal capacity. Perlite-supported BiOCl NMs exhibit a weak capacity for water treatment due to the poor mechanical strength of perlite and the amount of surface-exposed BiOCl material. For the photocatalytic removal of arsenic (oxidation–adsorption), BiOI-GO 1% excels in arsenic removal with efficiencies > 70%.
Keywords:
bismuth oxyhalides; BiOCl-GO; BiOI-GO; adsorption; arsenic; groundwater; methylene blue; methyl orange; photocatalysis 1. Introduction
Water scarcity exacerbated by water pollution is a risk to human and environmental health and survival. Although water is a renewable resource, due to population growth and social development, its demand is high or misused, breaking its natural cycle and positioning numerous populations as having severe water shortage problems. Mexico has a low water availability per person, resulting in groundwater pumping in deep regions, causing minerals to be incorporated into potable water, among which are toxic elements such as arsenic or fluoride [1].
The chronic and acute toxicity of arsenic and the incorporation of this element in drinking water from natural or anthropogenic sources is well known. For example, recent studies report that chronic exposure to arsenic in drinking water produces metabolic alterations in humans, increasing the risk for obesity, hypertension and diabetes [2]. The effects of arsenic exposure are diverse and dependent on several variables (age, gender, nutrition, early exposure, genetic susceptibility, arsenic speciation); however, recent studies remark an increase in the rate of morbidity and mortality related to arsenic exposition [3].
Nowadays, substantial evidence supports the numerous side effects on human health due to the presence of arsenic in drinking water. Thus, the World Health Organization (WHO) adjusted the maximum allowable concentration of arsenic in water (which has changed over time) to 10 μg/L. This arsenic limit applies in almost every country except Mexico, Peru, Bolivia and Argentina (25 μg/L) [4]. In addition, the International Agency for Research in Cancer (IARC) recommends lowering the actual maximum allowable concentration of arsenic in water [5]. However, the accurate quantification of arsenic in drinking water is difficult since there is a lack of portable, efficient, economical and reliable technology for measurement. Finally, water scarcity worsens the problems associated with arsenic in the environment. Multidisciplinary efforts are needed to alleviate this challenge.
Latin America, India, China and some other countries face contamination and human exposure to arsenic at toxic levels, calling urgently for sustainable technologies for arsenic removal. Diverse techniques have been investigated for the sustainable removal of arsenic from water. Juarez-Aparicio et al. examined the adsorption capacity of natural limestones to remove arsenic and fluoride from water. This study demonstrates that the limestones with the highest content of calcium carbonate and the smallest particle sizes exhibit the highest removal efficiency for arsenic and fluoride [6].
Although the impact of arsenic on the environment and public health is increasing, there is a lack of reliable technology to remove this pollutant in real scenarios [7,8]. Nanotechnology has numerous applications for environmental remediation, mainly because its versatility allows for the design of a system with unique properties to solve the problems (remediate) of a specific polluted site [9]. Recent studies support this statement, highlighting the need for location-specific arsenic attenuation strategies. Furthermore, the WHO recommends groundwater treatment upon abstraction. The need for location-specific arsenic attenuation strategies is justified in several ways; for example, As(III) is more mobile and about 25–60 times more toxic than its oxidized state, As(V) [10]. The ratio of the As(III)/As(V) concentration depends on the redox properties present in the geological environment [11].
Nanomaterials (NMs) arose as highly engineered compounds with enhanced properties for several applications. NMs are a special class of materials with astounding properties related to their reduced size (at least one of their dimensions is below 100 nm). Because of their small size, NMs have high surface areas, an essential property for catalysis or environmental remediation (adsorption) applications [12]. NMs based on iron oxide or zero-valent iron (nZVI) are currently being investigated at the laboratory scale to remove arsenic from soil. Despite their wide bioavailability and compatibility, iron-based NMs easily oxidize, modifying their properties and activity. There is a significant affinity for As(V) to chemisorb to iron-based adsorbents [13]. However, iron oxides are not chemically stable at low pH values, resulting in their dissolution and leakage into treated water. In groundwater, arsenic is found primarily as As(III); this form of arsenic is more toxic and difficult to remove because it exists as a protonated species in a large pH interval [14]. Despite the numerous advantages of the use of nanotechnology for diverse applications, it is still necessary to investigate their environmental and health impacts (hazard identification, hazard characterization, exposure assessment and risk characterization) in a case-specific context, before their large-scale application [15,16,17].
Recently, bismuth-containing NMs have attracted enormous interest due to their easily tunable properties, abundance and biocompatibility. A current study positions bismuth-based NMs as one of the most solid solutions for water monitoring (sensing) and purification, since these NMs are suitable for the fabrication of cost-effective electrochemical sensors for water pollutant detection in real-time scenarios, highly efficient adsorbents or photocatalytic materials [18]. Regarding the photocatalytic activity of the BiOX NMs, BiOI is active under visible light; however, its activity is not optimal due to the high recombination of the photogenerated active species (e−/h+). Some strategies exist to improve the activity of BiOI NMs: (a) doping with other halogens, (b) physically joining to a different semiconducting material and (c) modifying with carbonaceous materials (the photocatalytic activity improves; still, a reduction in the surface area of the material can also occur) [19].
Other investigations describe the capacity of BiOI/γ-Fe2O3 to adsorb and oxidize As(III) to the less toxic As(V) species; the composite material can be easily separated from solution due to the magnetic properties of the iron oxide [10]. BiOX NMs are suitable for environmental remediation, with remarkable activity for the photocatalytic oxidation of toxic pollutants. Dyes represent a serious environmental problem due to their extensive use in the textile industry. Adsorption and photocatalysis have demonstrated suitability for the remediation of these pollutants from the environment [20].
A previous study from our research group demonstrates the outstanding capacity of BiOCl NMs for indoor air disinfection [21]. Aiming to expand the environmental applications of BiOX NMs, this study investigates the simultaneous photocatalytic oxidation and adsorption of water pollutants (methylene blue, methyl orange and arsenic) by BiOX and BiOX-GO. Dyes are toxic pollutants predominantly found in wastewater. In this study, we use MB (a cationic dye), methyl orange (an anionic dye) and arsenite (anionic inorganic pollutant) to demonstrate the capacity of the BiOX to adsorb positively and negatively charged pollutants. Researching the exact mechanism for the adsorption of these pollutants to the surface of the NMs is out of the scope of this study. Methylene blue and methyl orange efficiently adsorb to the surface of BiOX and BiOX-GO and oxidize upon the activation of the catalyst with visible light. Similarly, the NMs under study adequately remove the As(III) species, with BiOI-GO 1% being the NM with the highest performance for arsenic remediation. The main aim of this study was to select the BiOX or BiOX-GO NMs with the highest performance for arsenic removal under laboratory conditions. Future studies will investigate the activity, mechanism and suitability of BiOI-GO NMs for the development of sustainable technologies for groundwater purification.
2. Materials and Methods
2.1. Chemicals and Materials
All the reagents were analytical grade and were used with no further purification, including bismuth nitrate (III), KCl, KI, methylene blue (Baker, Phillipsburg, NJ, USA), sodium (meta), arsenite (Aldrich, Saint Louis, MO, USA, Product of India), ethylene glycol (EG, Golden Bell, Torreón, Coahuila, Mexico) and deionized water. The glassware was properly cleaned (first with nitric acid, then distilled water) before use.
Synthesis and Characterization of BiOX and BiOX-GO NMs
Details of the synthesis and characterization of BiOX and BiOX-GO NMs were previously published [21] by our research group. In brief, BiOX and BiOX-GO NMs were prepared using a microwave-activated solvothermal route. Bi(NO3)3∙5H2O and KX (X = Cl, I) were dissolved separately in an aqueous solution of EG (5:1) and stirred at 20 °C for one hour. Then the KX solution was added dropwise to Bi(NO3)3∙5H2O and stirred for one hour, followed by thermal treatment in a CEM microwave reactor (120 °C × 20 min). The materials were separated by centrifugation followed by purification (reflux in absolute ethanol for one hour).
GO was prepared using an improved Hummer method. In brief, a mixture of powdered graphite (1.0 g, Sigma Aldrich, Saint Louis, MO, USA) and sodium nitrate (0.5 g, NaNO3, Golden Bell, Torreón, Coahuila, Mexico) was put into and dissolved in concentrated H2SO4 (23 mL, J.T.Baker, Phillipsburg, NJ, USA) and stirred at 0 °C in an ice bath. After, 3 g of KMnO4 (J.T.Baker, Phillipsburg, NJ, USA) was added to the suspension and heated to 35 °C for 12 h. Then, 3 g of KMnO4 was added and allowed to stand for three hours, followed by the addition of deionized water (46 mL). Then, the solution was diluted with distilled water (140 mL) and treated with hydrogen peroxide (30% w/w) to reduce residual manganese oxides. After, the suspension was filtered, obtaining a yellow-brown precipitate, following purification with warm water (three times). The GO residue was suspended in 100 mL of HCl (10% w/w, Golden Bell). Finally, GO was separated by centrifugation followed by purification (water washing and drying in the oven at 60 °C for 12 h).
The synthesis of BiOX-GO composites proceeded by combining the methods previously described. After mixing bismuth and halide precursors in aqueous ethylene glycol (5:1), 10 mg of GO was added with continuous stirring and stirring continued for one hour. Then, the mixture was put in a CEM microwave reactor for the reaction. Finally, the product was separated by centrifugation and purified by washing with distilled water and absolute ethanol.
The materials were characterized using several techniques. The size, shape and composition of the NMs were investigated by Scanning Electron Microscopy (SEM-EDX; JSM-7800F de Jeol, Pleasanton, CA, USA)) and High-Resolution Transmission Electron Microscopy (HRTEM, JEOL ARM 200F (Akishima, Tokyo, Japan) operating at 200 kV). The Brunauer–Emmett–Teller (BET) surface area test was acquired using an autosorb iQ automated gas sorption analyzer from Quantachrome. For a more detailed characterization of the NMs, see [21].
2.2. Methylene Blue (MB) and Methyl Orange (MO) Adsorption Assay
2.2.1. Screening Test for MB Adsorption
A preliminary assay evaluated the adsorptive capacity of BiOCl to remove MB from aqueous solution (20 mg/L in deionized water). First, different doses of BiOCl NMs (1, 2 or 3 mg/mL) were investigated to optimize the amount of adsorbent material used for MB (20 mL, 20 ppm) removal. The suspensions were magnetically stirred (four hours). Then, the MB removal efficiency was monitored using UV-Vis spectroscopy (664 nm), withdrawing samples every hour (0.5 mL) to quantify dye removal.
2.2.2. Evaluation of the MB and MO Removal Efficiency
The adsorption capacity of BiOCl, BiOCl-GO, BiOI, BiOI-GO and GO was evaluated using methylene blue (cationic) and methyl orange (anionic) as model compounds (50 mL, 20 ppm) and 1 mg/mL of the catalyst. The suspensions were magnetically stirred (four hours). The removal of MB or MO was measured by UV-Vis spectroscopy (664 nm for MB and 450 nm for MO). Samples were withdrawn every hour (0.5 mL) to quantify dye removal.
2.3. Photocatalytic Removal of MB or MO
The photocatalytic activity of BiOX, BiOX-GO and GO NMs for the degradation (or mineralization) of model pollutants (MB or MO) was investigated using visible light for catalyst activation. First, the NMs (1 mg/mL) were added to the dye solutions (20 mg/L) and stirred in the dark for one hour. Then, the suspensions were magnetically stirred and radiated with visible light. The photocatalytic efficiency for MB or MO degradation was monitored using UV-Vis spectroscopy (664 nm for MB and 450 nm for MO), withdrawing samples every hour (0.5 mL).
2.4. Arsenic Adsorption Assay
The capacity of BiOX (X = Cl, I), BiOX-GO and GO NMs to remove arsenic from an aqueous solution was investigated. The arsenic standard solutions (20 mg/L) were prepared by dissolving sodium (meta) arsenite in deionized water (arsenic concentration refers to elemental arsenic, not arsenite). To 100 mL of arsenite solution, 1 mg/mL of adsorbent was added. The suspension was magnetically stirred for 5 h at 20 °C (same methodology was used to evaluate arsenic removal efficiency at 24 h of adsorption). Experiments were conducted in triplicate. After, the NMs were removed by decantation and centrifugation (to avoid the interference of any adsorbent residue during arsenic quantification). To quantify residual arsenic, an aliquot (45 mL) of the treated solution was placed in a flask, and concentrated nitric acid was added (5 mL). The solutions were stirred and digested for 24 h. The concentration of total arsenic was quantified in the acid-digested samples by hydride generation, utilizing a Perkin Elmer Atomic Absorption Spectrometer, PinAAcle 900H (Perkin Elmer, Waltham, MA, USA).
2.5. Photocatalytic Arsenic Removal Assay
Arsenic standard solutions (30 mg/L) were prepared by dissolving sodium (meta) arsenite in deionized water. The pH of the solution was adjusted to 12 using NaOH, and the ionic strength was 0.01 M, NaNO3 (Golden Bell, Torreón, Coahuila, Mexico). Aliquots (50 mL) of the standard arsenic solution were placed in Erlenmeyer flasks. The assays were conducted in triplicate considering the following groups: (A) control group (without NMs), (B) BiOCl, (C) BiOCl-GO 1%, (D) BiOCl-GO 10%, (E) BiOI, (F) BiOI-GO 1%, (G) BiOI-GO 10% and (H) GO. Arsenic solutions were treated using 1 mg/mL of the different NMs. To evaluate NM ion (Bi5+, Cl−, I−) lixiviation, NMs were suspended in deionized water and treated under the same conditions as all other samples. The different solutions (suspensions) were placed in an orbital shaker and irradiated with visible light for 5 h. Then, the NMs were removed by decantation and centrifugation (to avoid the interference of any adsorbent residue during arsenic quantification). To quantify the amount of residual arsenic, an aliquot (45 mL) of the treated solution was placed in a flask and concentrated nitric acid was added (5 mL). The solutions were stirred and digested for 24 h. The concentration of total arsenic was quantified in the acid-digested samples by hydride generation, utilizing a Perkin Elmer Atomic Absorption Spectrometer, PinAAcle 900H (Waltham, MA, USA).
3. Results and Discussion
3.1. Synthesis and Characterization of BiOX (X = Cl, I) and BiOX-GO NMs
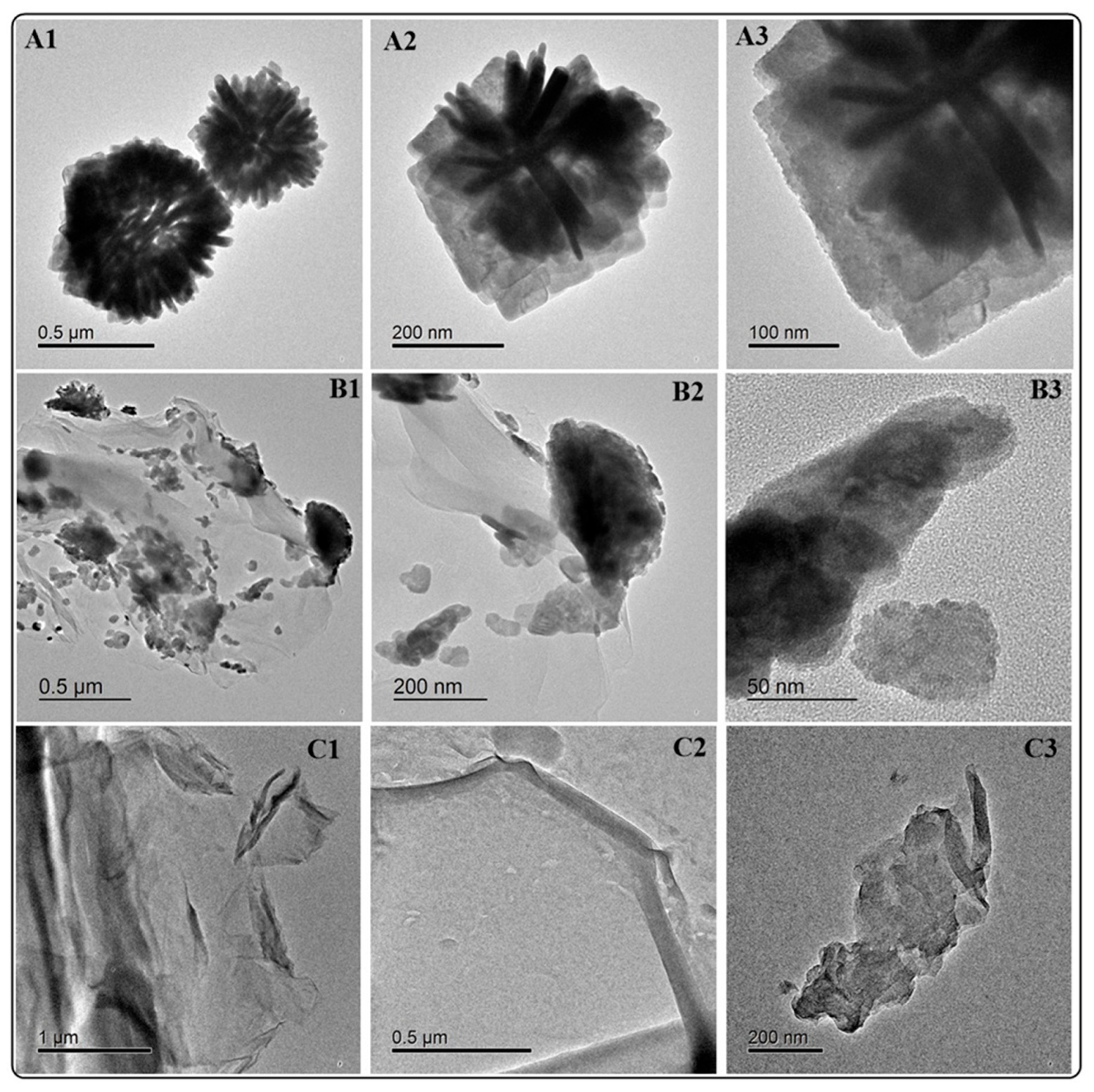
In a previous study, the detailed characterization of BiOCl and BiOCl-GO was reported. In summary, the materials exhibit hierarchical 3D structures (Figure 1) composed of ordered thin platelets of BiOCl (Figure 1(A1–A3)). BiOCl-GO composites consist of BiOCl deposited on the surface of thin GO layers (Figure 1(B1–B3)). Graphene oxide (GO) shows a sheet-like morphology; its transparency denotes the formation of a few layers of GO material. More details on the properties of GO are included in Appendix B (Figure A1).
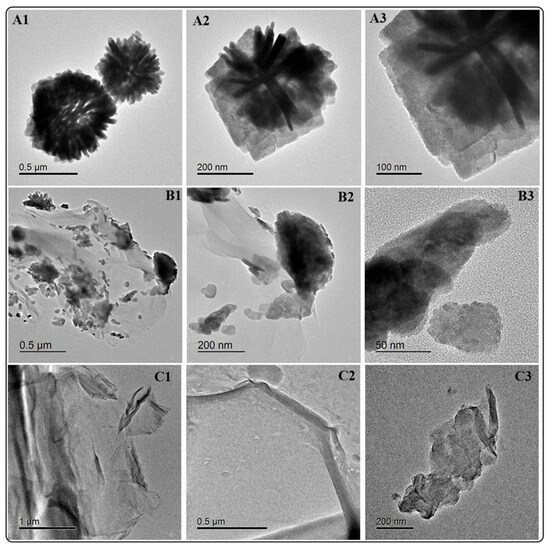
Figure 1.
HRTEM analysis of BiOCl, BiOCl-GO and GO NMs. (A1–A3) BiOCl 3D ordered structures at different magnifications. The 3D structures are composed of BiOCl thin platelets. (B1–B3) BiOCl deposited on the surface of GO at different magnifications. The layered GO structures supporting 3D BiOCl nanostructures. (C1–C3) GO NMs at different magnifications.
Numerous previous reports describe the formation of BiOX NMs with hierarchical 3D-layered structures. The properties (size, thickness, composition, 3D arrangement) of these NMs can be easily varied, favoring their tuning for enhanced activity [22]. For example, the solvent and surfactant determine the thickness of the platelets; this property greatly influences adsorption and photocatalytic activity. This was demonstrated by [23] by varying the synthesis conditions of BiOCl using the following: (a) ethylene glycol, water, and Polyvinylpyrrolidone; (b) ethylene glycol and (c) water. The conditions in (a) render the thinnest and most catalytically active material. Numerous reports rely on using a solvothermal route that demands several hours for reaction completion; in this study, using a microwave-activated synthesis approach allows for the evaluation of different synthesis conditions in a short time frame and produces BiOX NMs with varied properties.
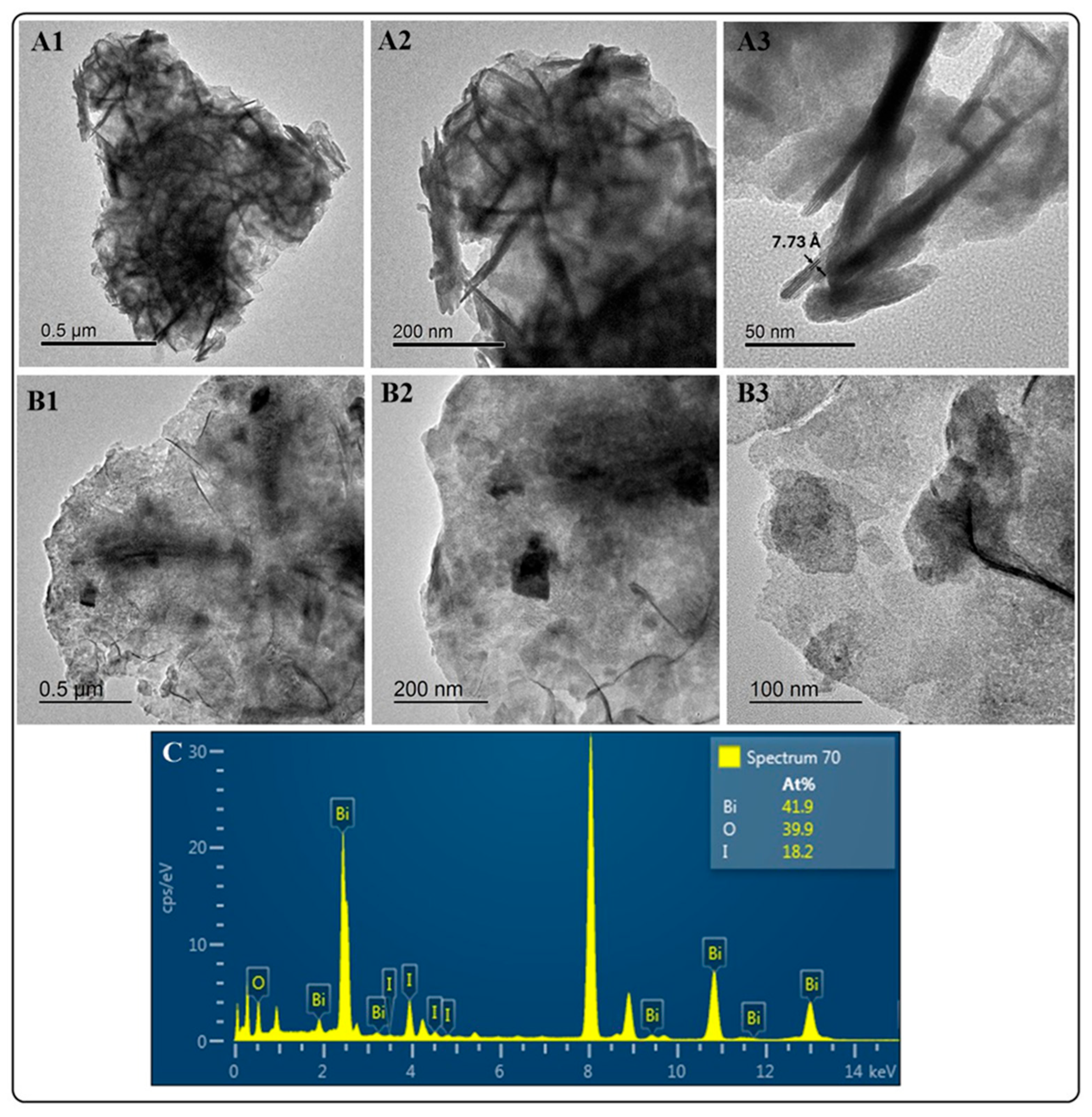
Figure 2 depicts the images from the HRTEM analysis of BiOI and BiOI-GO NMs. The top row (Figure 2(A1–A3)) shows the BiOI platelets at different magnifications. In Figure 2A3, the lattice spacing of 7.73 Å matches the layer thickness of BiOI. The middle row (Figure 2(B1–B3)) shows the BiOI-GO NMs at different magnifications, demonstrating the presence of the BiOI NMs on the surface of GO. At the highest magnifications, it is possible to distinguish the shape of the BiOI platelets. In addition, Figure 2C depicts the elemental composition of BiOI NMs; according to this analysis, the NMs under study are BiOI iodine-deficient materials (BiOI0.5).
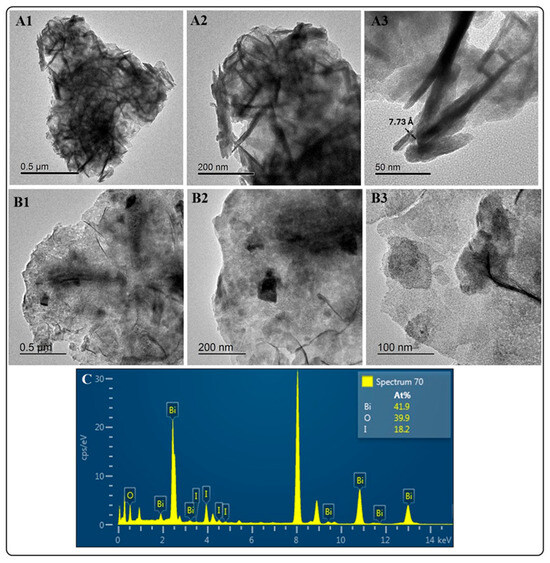
Figure 2.
HRTEM analysis of BIOI and BiOI-GO NMs. (A1–A3) Micrographs of BiOI at different magnifications. BiOI exhibits a highly crystalline (A3) thin-sheet morphology (A1,A2). (B1–B3) BiOI-GO NMs at different magnifications. Figure B3 shows the presence of thin BiOI nanosheets on the surface of GO. (C) EDX analysis of BiOI NMs. The EDX spectrum denotes the formation of iodine-deficient BiOI (BiOI0.5) NMs.
The HRTEM images of BiOI show their thin-sheet-like morphology and crystallinity. In contrast to BiOCl, BiOI NMs do not exhibit a 3D-ordered structure. The 3D-ordered structure is more temperature-dependent. For example, Hu et al. reported that the 3D assembly of the BiOI nanosheets depends on the solvent viscosity and reaction temperature [22]. In addition, a further study investigates the effect of synthesis conditions on the physicochemical and photocatalytic properties of BiOI NMs, indicating that high temperatures render BiOI NMs with enhanced activity due to iodine vacancies [24]. In this study, the solvent (EG–water), reaction temperature and activation (microwave-mediated) of the reactants contribute to forming crystalline, ultrathin and iodine-deficient BiOI NMs.
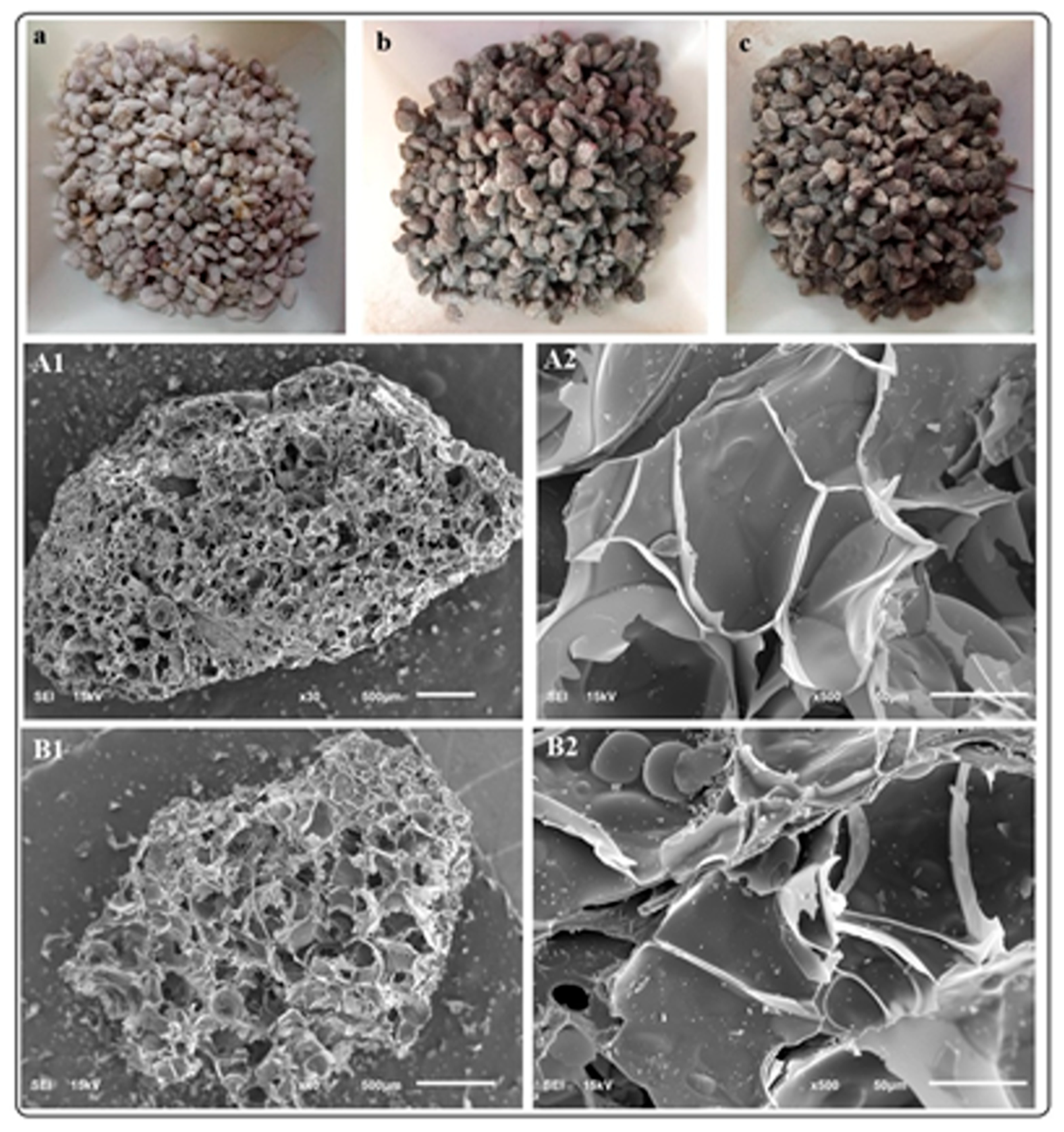
SEM analysis of the perlite-supported BiOCl-GO materials is depicted in Figure 3. The top row includes pictures of bare perlite (a) and BiOCl (b) or BiOCl-GO supported on perlite (c). The middle row shows the low-magnification images of BiOCl perlite-supported materials. Image A2 shows the presence of BiOCl NMs on the surface of the support. EDS analysis confirms the presence of Bi-containing materials on the support. There are no significant differences between BiOCl or BiOCl-GO supported materials. EDX analysis (Appendix A, Table A1) indicates a low percentage of the NMs within the support.
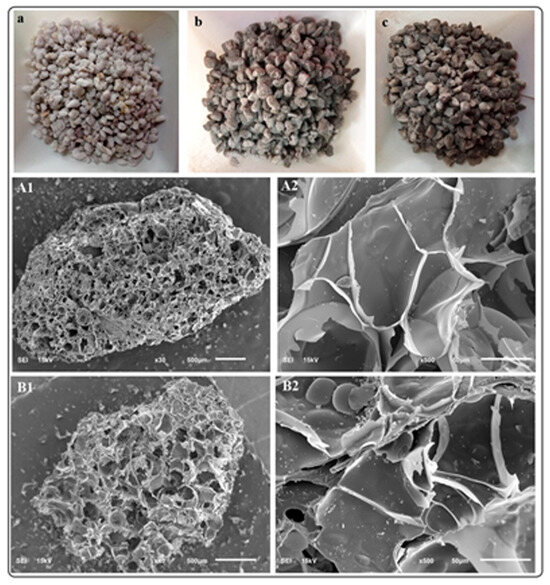
Figure 3.
Photographs and SEM analysis of perlite-supported BiOCl and BiOCl-GO NMs. (a–c) Pictures of cleaned bare perlite, perlite-supported BiOCl and BiOCl-GO perlite-supported NMs. (A1,A2) SEM analysis of BiOCl perlite-supported NMs at different magnifications. (500 and 50 μm, respectively). (B1,B2) SEM analysis of BiOCl-GO perlite-supported NMs at different magnifications.
Figure 3(A1,B1) exhibit the porous structure of perlite (scale, 500 μm). The magnification of these images (Figure 3(A2,B2); scale 50 μm) allows for the visualization of bismuth-containing NMs on the surface and pores of perlite. Previous studies from our research group demonstrated the excellent activity of perlite-supported NMs for air disinfection [21]. The support allows for the free entrance of air and its circulation through the photocatalytic column and contributes to pollutant adsorption. The catalyst is very stable and can be reused for several cycles without a detriment to their efficiency. However, perlite-supported BiOX catalysts are not suitable for water treatment (the following sections show the low capacity of perlite-supported NMs for the removal of arsenite in aqueous solution) due to the low mechanical resistance of this support.
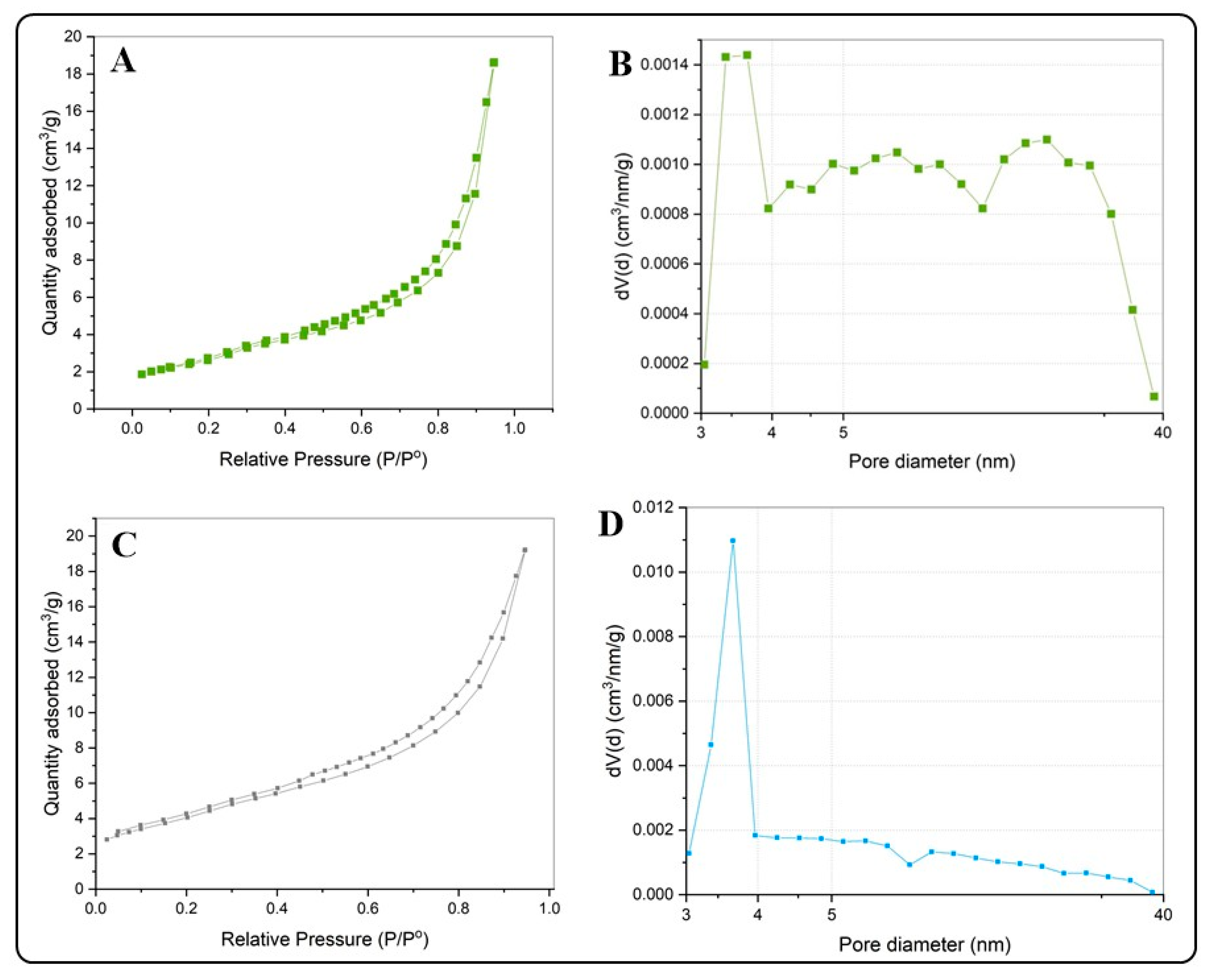
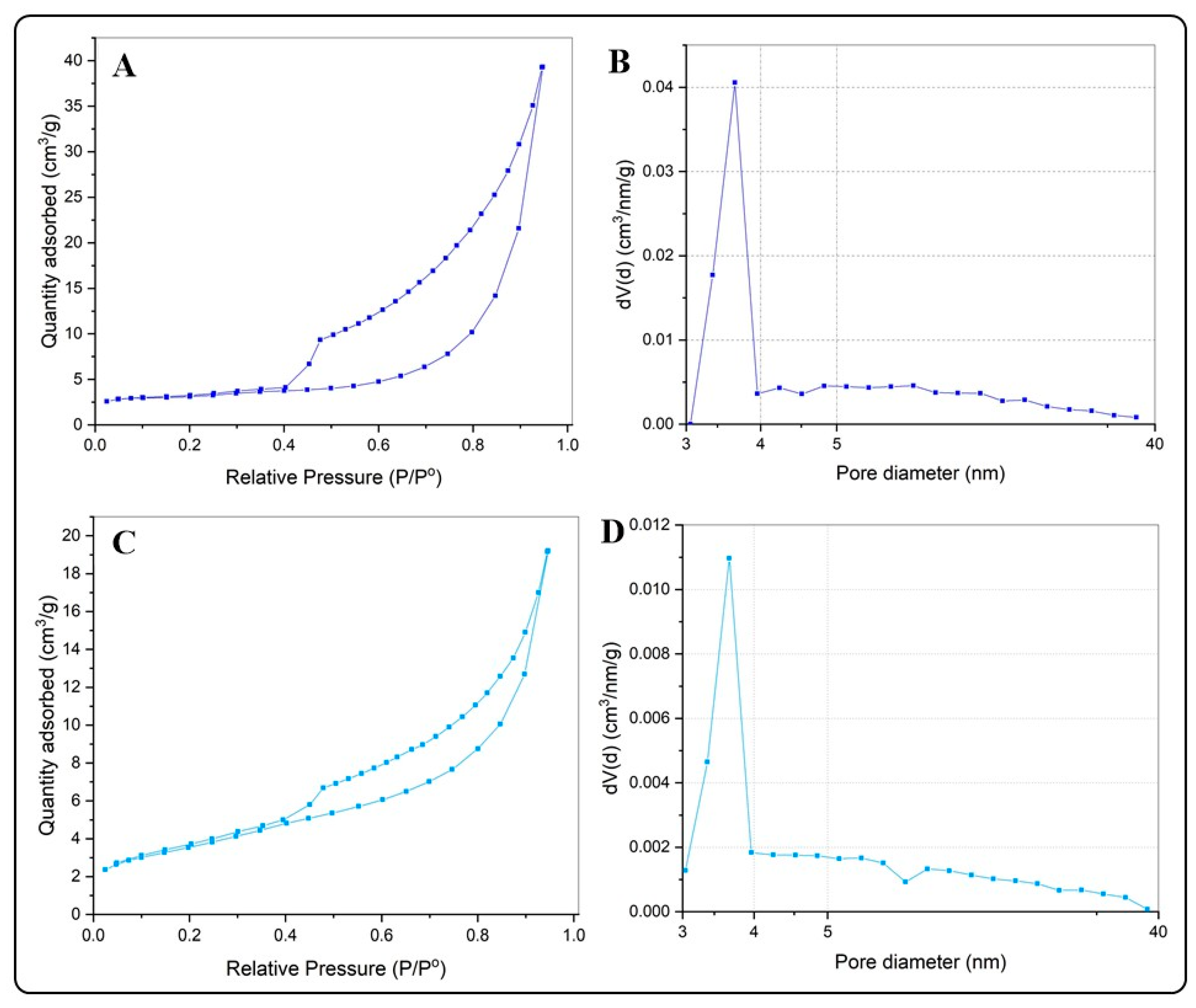
Table 1 summarizes the results of the N2 adsorption–desorption isotherms and the pore diameter distributions for the BiOX and BiOX-GO NMs. Figure 4 and Figure 5 and Figure A2 show the N2 adsorption–desorption isotherms; all the NMs possess a type IV isotherm with a hysteresis loop of (according to IUPAC classification) mesoporous materials. The specific surface areas of BiOI NMs are higher than those of BiOCl NMs. In addition, pure BiOI possesses a higher surface area than BiOI-GO; the opposite happens for BiOCl and BiOCl-GO NMs. This behavior is due to the ordered 3D structures of BiOCl responsible for diminishing the exposed area of the materials. An earlier study describes the synthesis of BiOI/BiOCl composite microflowers: the ordered arrangement of the nanoplates decreases the surface area (SBET, m2/g) of the materials (BiOI: 5.71; BiOI0.75Cl0.25: 13.67; BiOI0.5Cl0.5: 16.41; BiOI0.25Cl0.75: 17.40; BiOCl: 9.02) [25].

Table 1.
Physicochemical properties of BiOX and BiOX-GO NMs.
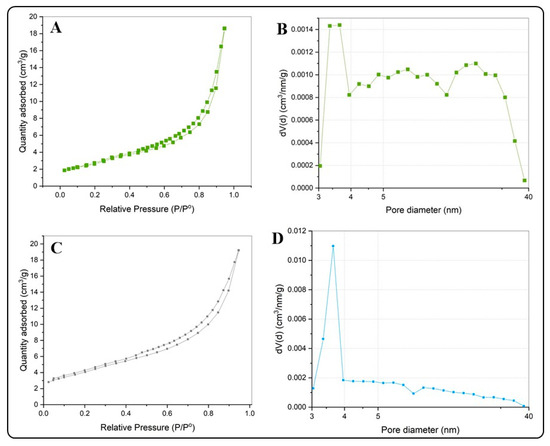
Figure 4.
(A,B) N2 adsorption isotherms for BiOCl (A) and BiOCl-GO (C). (B,D) Pore size distribution curves for BiOCl (B) and BiOCl-GO (D).
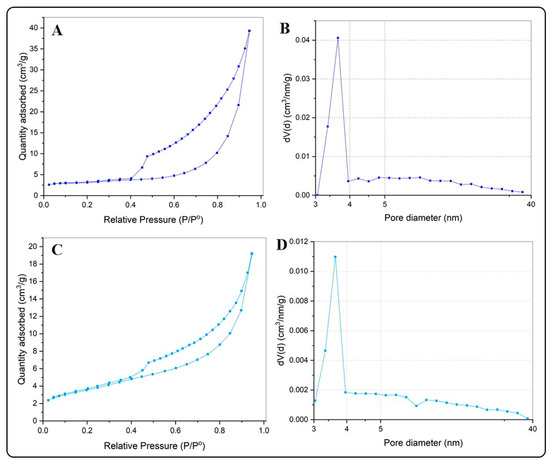
Figure 5.
(A,B) N2 adsorption isotherms for BiOI (A) and BiOI-GO (C). (B,D) Pore size distribution curves for BiOI (B) and BiOI-GO (D).
GO presents the highest surface area of the NMs under study (Table 1 and Figure A2), a characteristic that suits it as a proper sorbent material; thus, our results evince the high capacity of GO to remove MB and MO from aqueous solution; however, its capacity to remove arsenic is lower than that observed for BiOI NMs. Thus, although the surface area of the adsorbents is a relevant property to act efficiently for the removal of organic or inorganic pollutants, the removal efficiency also depends on other mechanisms that derive from the presence of different chemical species in solution and the sorption mechanism of the pollutant with the sorbent under study. For example, a recent study investigates the effect of the oxidation degree (OD) of GO on the adsorption (efficiency and mechanism) of MB [26]. The study remarks that a higher OD favors the removal capacity of the material; in addition, at a high OD, the mechanism for MB uptake changes from a Freundlich- to a Langmuir-type adsorption scheme.
Earlier studies discuss the physicochemical properties of BiOX NMs and their influence on the catalytic and adsorptive capacity of the materials. By increasing the surface area of the materials, their adsorption capacity improves. BiOI NMs have the highest surface area of the NMs under study. In addition, this material has improved properties compared to those previously reported by different researchers. For example, Wang et al. report the synthesis of pure BiOI and BiOI supported on mesoporous carbon microspheres [19]. The pure BiOI material exhibits a surface area of 8.28 m2g−1. Furthermore, a different study reports the fabrication of thin platelets of BiOCl NMs with large surface areas (39 m2g−1) [23].
3.2. Methylene Blue and Methyl Orange Adsorption Assay
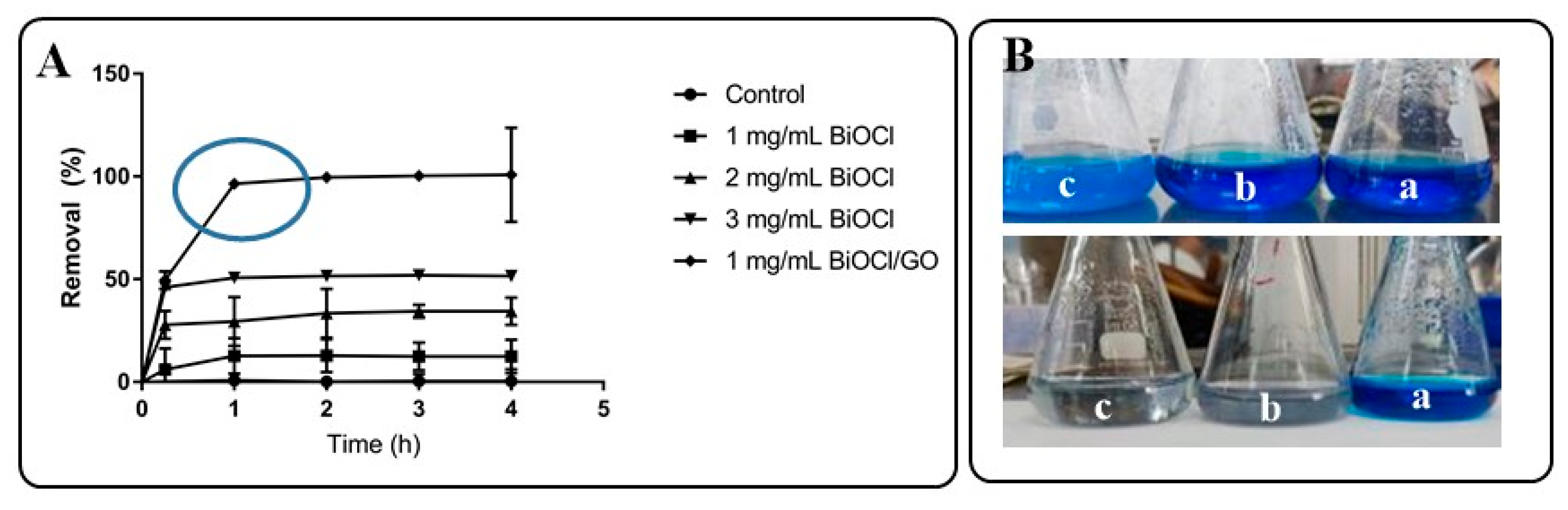
Methylene blue was used as model pollutant to evaluate the adsorption capacity of the materials (BiOCl or BiOCl-GO) at different doses. Dyes can be used as model compounds because of the simplicity of evaluating their removal. There are significant differences in the adsorption capacity of BiOCl or BiOCl-GO NMs. As illustrated in Figure 6, BiOCl-GO 1% removes the dye completely in a short period (60 min) at low doses (1 mg/mL).
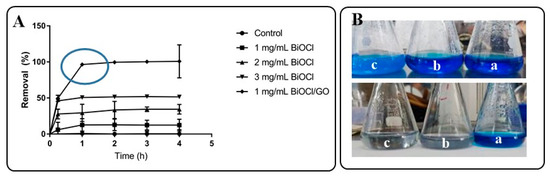
Figure 6.
Evaluation of the adsorption capacity of BiOCl and BiOCl-GO NMs for the removal of MB from aqueous solution. (A) Adsorption capacity of BiOCl (at different doses) and BiOCl-GO at different times. (B) Schematic representation of the adsorption capacity of BiOCl (top) and BiOCl-GO (bottom) NMs to remove MB from aqueous solution. a: t = 0 h; b: t = 1 h; c: t = 4 h.
On the other hand, BiOCl only removes the dye partially (Figure 6A), independent of the dose of the NMs (1, 2 and 3 mg/mL were evaluated). Figure 6B illustrates the efficiency of MB removal of the different BiOCl NMs. The top image (from right to left) shows the appearance of the MB solution at the beginning and the end of the adsorption process. As portrayed in the figure, the solution stays blue since the removal efficiency of BiOCl oscillates around 50%; on the contrary, Figure 6B (bottom row) illustrates the efficiency of BiOCl-GO NMs in removing MB from aqueous solution. The transparency of the final solution demonstrates the removal of the dye and the catalyst after the adsorption process.
Recent investigations incentivize the research on BiOCl with hierarchical 3D structures; however, the adsorption capacity of the materials decreases due to the ordered array of the thin platelets forming the 3D structure. TEM images of the BiOCl and BiOCl-GO under study illustrate the dispersion of ordered 3D BiOCl NMs on the surface of the thin GO; in addition, there is an increase in the surface area of the composites, enhancing their adsorptive capacity. By dispersing the BiOCl materials, their adsorption capacity increases, observing better performances of MB removal for the composite with the highest amount of GO in its composition. An earlier report discusses the modulation of the physicochemical properties and photocatalytic activity of BiOCl NMs by varying the nature or the amount of the surfactant directing agent. Their results demonstrate the better photocatalytic performance of the NMs with reduced thickness, increased exposed facets and the smallest crystallite size [27].
It is important to note that scarce research exists regarding the effect of heterostructures (i.e., BiOCl-GO) on the adsorption performance of BiOX NMs. BiOX NMs belong to the layered Aurivillius-related oxides family, consisting of a [Bi2O2]2+ layer sandwiched by chloride ions. The crystal structure of these compounds can be tuned by doping (or mixing) different halides at different proportions. Further studies from our research group will investigate the adsorption capacity of BiOCl1−xIx.
In addition, following adsorption, BiOCl-GO NMs exhibit photocatalytic activity upon visible light activation, causing the mineralization of the adsorbed organic pollutant. Our research group is optimizing the properties, biocompatibility and feasible large-scale fabrication of BiOX NMs for diverse applications (environmental remediation, energy production). Although BiOCl has the highest Eg value, it is also the cheapest, most abundant and biocompatible bismuth oxyhalide compound, justifying research efforts to optimize BiOCl activity for practical applications.
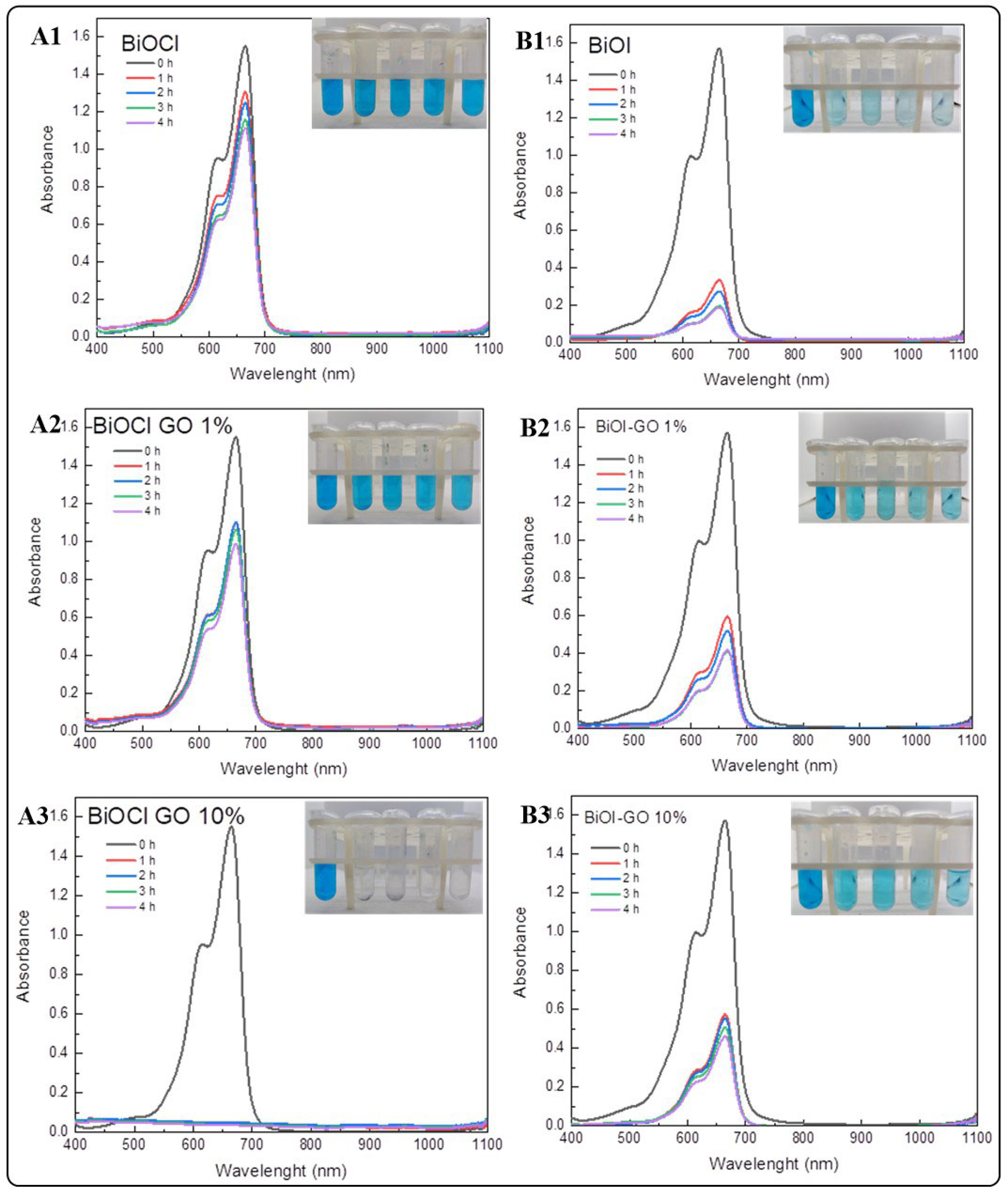
Figure 7 illustrates the efficiency of BiOX and BiOX-GO NMs for removing MB from aqueous solution. Table 2 summarizes the percentage efficiencies for the materials under study (at different times). As previously discussed, BiOCl-GO exerts enhanced activity because the surface area of the materials increases upon composite formation. BiOI NMs exhibit the opposite behavior, since, as illustrated by HRTEM analysis, BiOI NMs do not exhibit compact 3D ordered structures like BiOCl NMs (in addition, BiOI NMs possess the highest surface area of the NMs under study). In addition, BiOI platelets are thinner, increasing their surface area and adsorption capacity. The adsorption efficiencies of the materials after four hours of treatment vary as follows: BiOI-GO 10% and GO (99%) ˃ BiOCl-GO 10% and BiOI (98%) ˃ BiOI-GO 1% (97%) ˃ BiOCl-GO 1% (37%) ˃ BiOCl (28%).
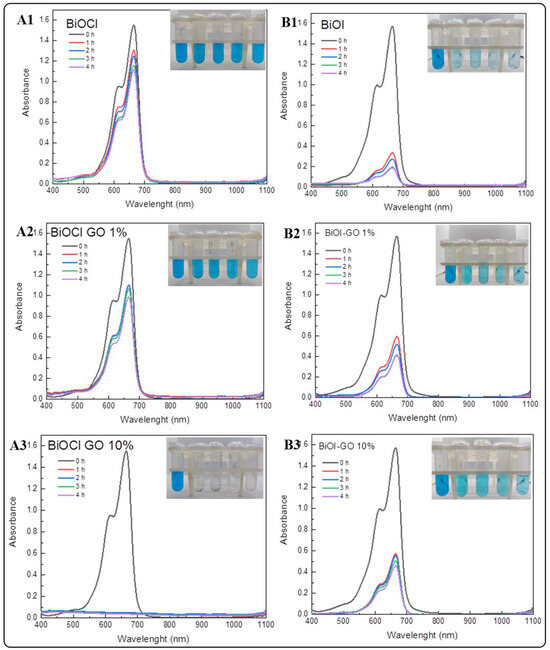
Figure 7.
Removal of methylene blue using BiOX and BiOX-GO NMs (1 mg/mL) at different time intervals (0, 1, 2, 3 and 4 h). (A1–A3) BiOCl, BiOCl-GO 1% and BiOCl-GO 10%. (B1–B3) BiOI, BiOI-GO 1% and BiOI-GO 10%.

Table 2.
MB removal efficiencies of the NMs under study at different time intervals.
In addition, after the removal of MB from the solution, the photocatalyst is activated with visible light, causing the oxidation of the dye. Once the adsorption process finishes, the BiOCl-GO powder shows a blue color due to the presence of the dye on its surface. Resuspending the catalyst in water and irradiating it with visible light results in dye degradation, regenerating the powder catalyst for reuse. BiOI exhibits a better MB removal capacity than BiOI-GO NMs. After four hours of treatment, the efficiencies are higher than 90%. Table A2 and Table A3 summarize the photocatalytic degradation efficiencies of the NMs under study.
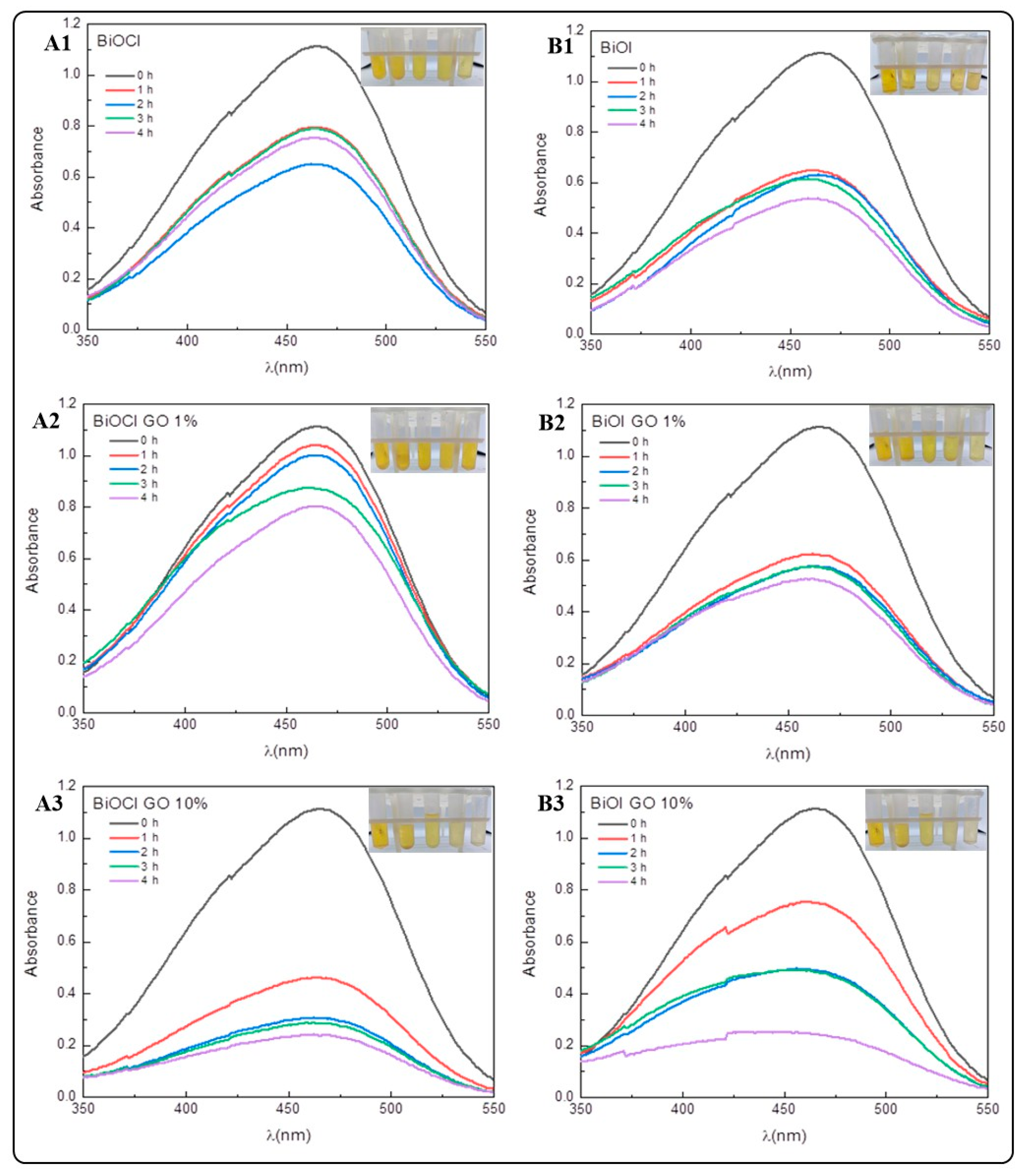
The removal efficiencies for MO (Figure 8 and Table 3) are lower compared to the MB removal efficiencies. This result might indicate a higher affinity of the NMs under study for positively charged pollutants. However, the removal efficiencies for MO are higher in comparison to the earlier literature reports [20]. Table 4 and Figure A3 summarize the adsorption efficiencies of GO for the removal of the dyes under study. Our results indicate that GO is the NM with the highest capacity for MO removal. The efficiencies vary as follows: GO (96%) > BiOCl-GO 10% and BiOI-GO 10% (78%) > BiOI-GO 1% (53%) > BiOI (51%) > BiOCl (41%) > BiOCl-GO 1% (27%).
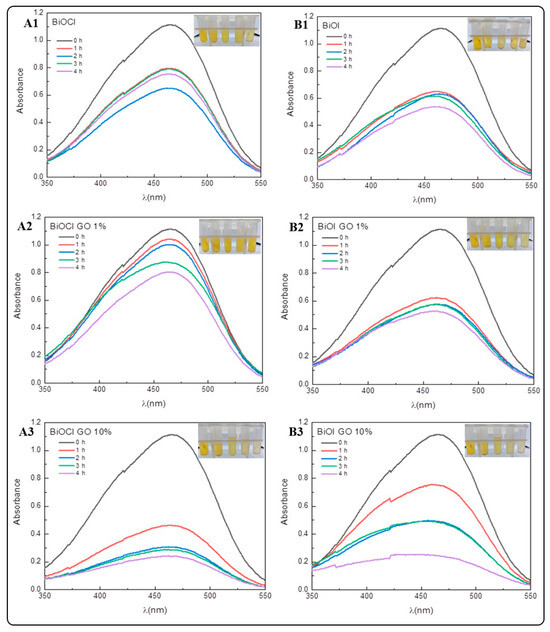
Figure 8.
Removal of methyl orange (MO) using BiOX and BiOX-GO NMs (1 mg/mL) at different time intervals (0, 1, 2, 3 and 4 h). (A1–A3) BiOCl, BiOCl-GO 1% and BiOCl-GO 10%. (B1–B3) BiOI, BiOI-GO 1% and BiOI-GO 10%.

Table 3.
MO removal efficiencies of the NMs under study at different time intervals.

Table 4.
MB and MO adsorption removal and photocatalytic efficiencies of GO at different time intervals.
Previous studies investigated the adsorption capacity of Bi2MoO6@BiOCl heterostructures to remove dyes and chromium from aqueous solution. The materials show good activity in the adsorption of rhodamine blue and poor performance in removing methyl orange and methylene blue. In addition, the chromium adsorption capacity of the materials is moderate, achieving a removal efficiency of 33% [28]. The photocatalytic degradation of MO for the NMs under study indicates efficiencies of 94% for BiOI-GO 10% (Figure A4 and Figure A5, Table A2 and Table A3).
Arsenic Adsorption Assay
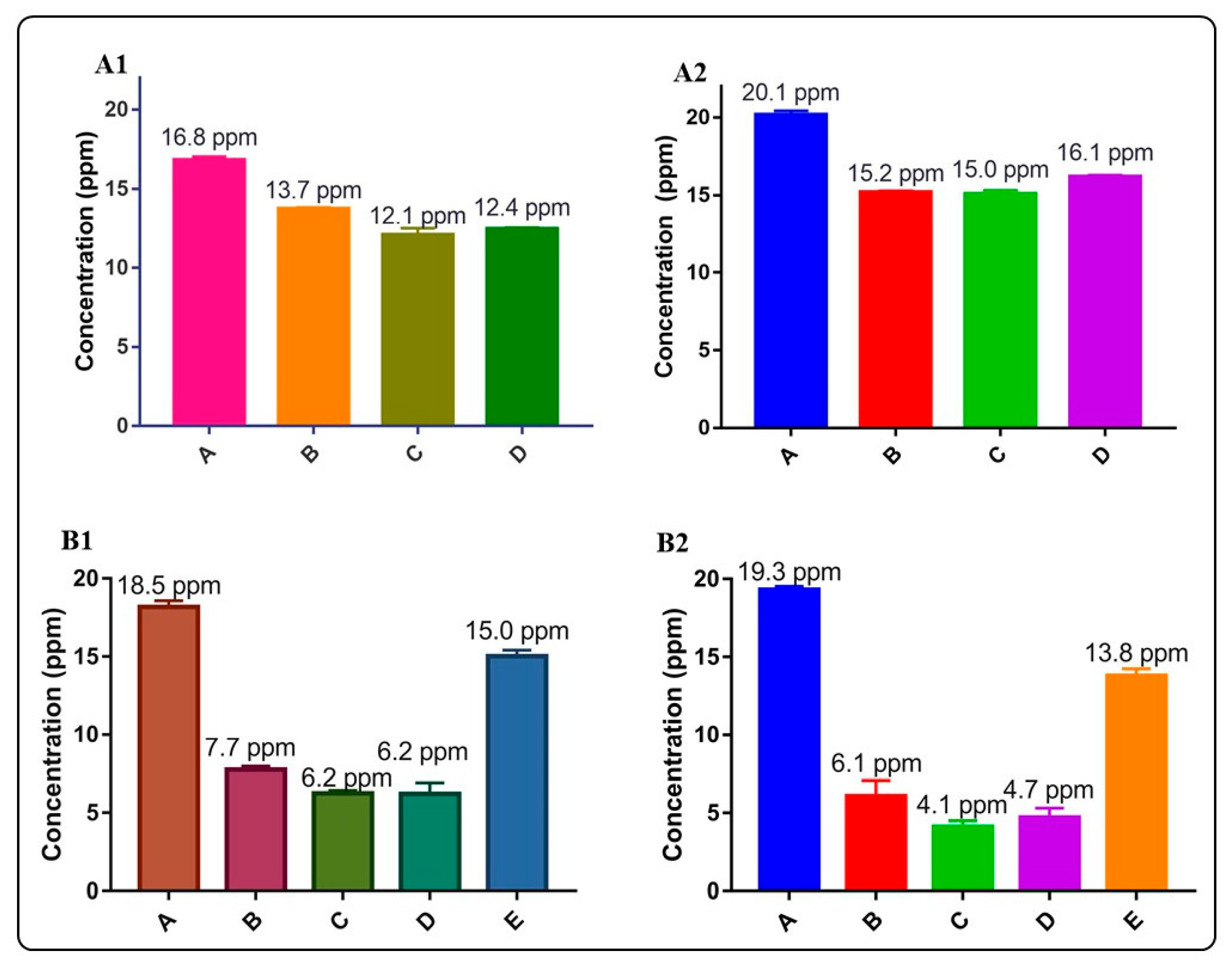
Figure 9 and Figure 10 and Table 5 summarize the arsenic adsorptive capacity of the different BiOX and BiOX-GO materials. As illustrated in Figure 9 and Table 5, BiOI-GO (1%) excels as an arsenic adsorbent under the evaluated conditions (24 h, 20 ppm). However, as illustrated in Figure 9B and Table 5, the BiOCl-GO composites decrease the adsorption capacity at long exposure times (24 h). On the other hand, pure BiOCl improves arsenic removal at longer exposure times. Although BiOCl-GO NMs have a greater surface area, other variables affect their performance. The removal efficiencies (~50%) of BiOI-GO NMs are high, considering that arsenic is present as arsenite and is removed at a neutral pH and without the adjustment of the ionic strength of the medium. The arsenic removal efficiencies for BiOI NMs are higher than the performance of GO NMs (24%), contrary to the observed data for the dye removal assays.
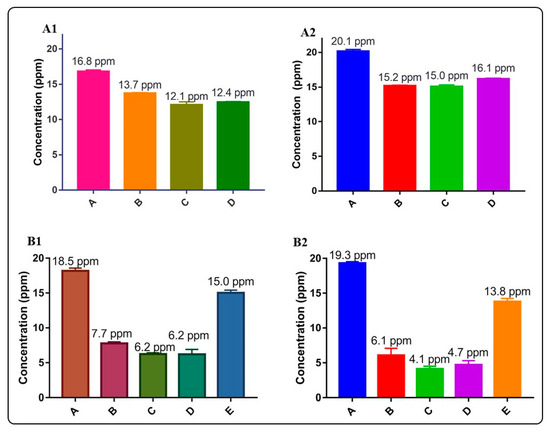
Figure 9.
Evaluation of arsenic removal using BiOX, BiOX-GO and GO NMs at different adsorption times (5 and 24 h). (A1) Arsenic removal efficiency of BiOCl and BiOCl-GO NMs. Control (A), BiOCl (B), BiOCl-GO 1% (C), BiOCl-GO 10% (D). Adsorption time: 5 h. The concentrations on top of the bars are the final concentrations of arsenic in the different groups. (A2) Same as A1, changing the adsorption time to 24 h. (B1) Arsenic removal efficiency of BiOI, BiOI-GO and GO NMs. Control (A), BiOI (B), BiOI-GO 1% (C), BiOI-GO 10% (D), GO (E). Adsorption time: 5 h. (B2) Same as (B1), changing the adsorption time to 24 h.
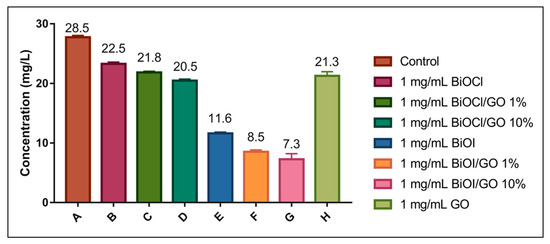
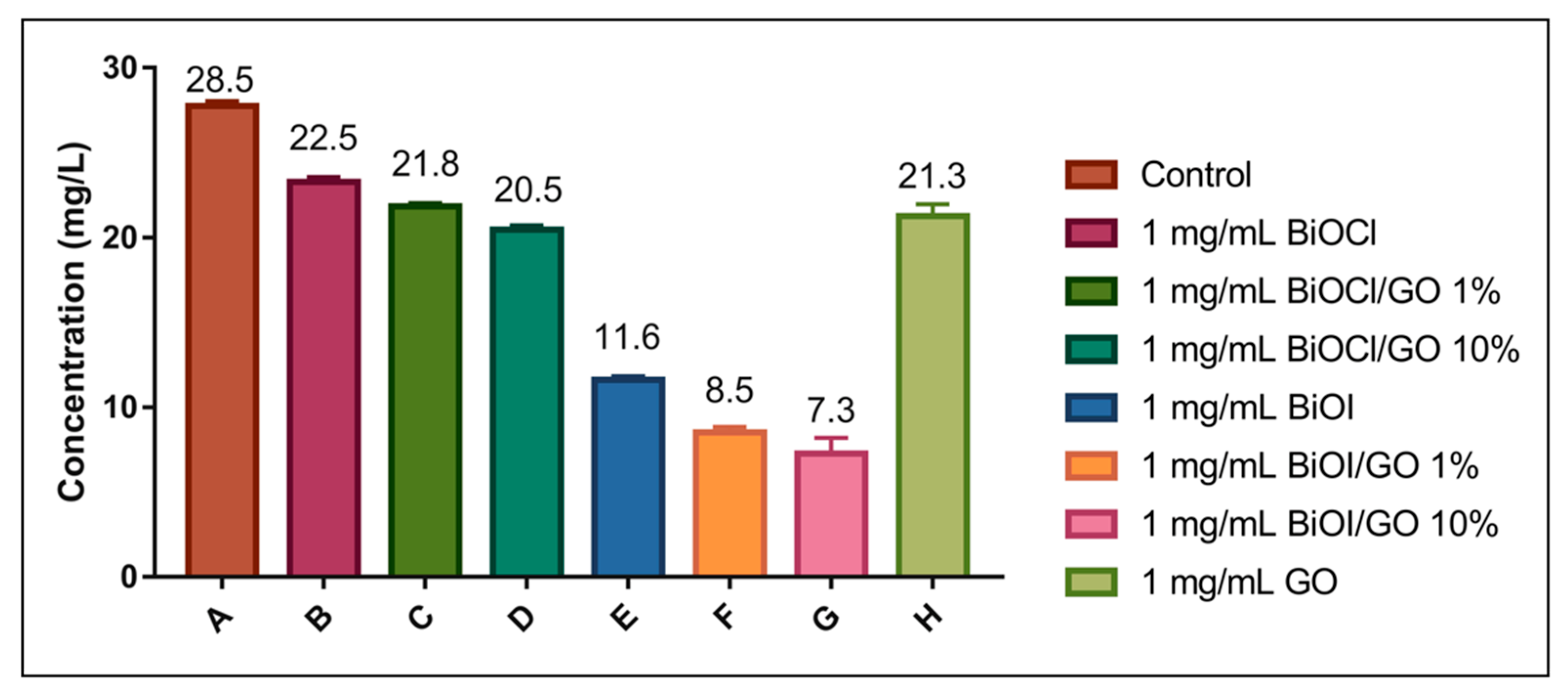
Figure 10.
Photocatalytic removal efficiencies of BiOX, BiOX-GO and GO NMs for arsenite. The assays were conducted at pH 12 and radiated with visible light (fluorescent bulb, day light CE, 120 V/60 Hz, 20 W).

Table 5.
Arsenic removal efficiencies under different conditions.
The adsorption capacity of the NMs under study to remove arsenite from an aqueous solution might seem low; however, the NMs are exposed to high As(III) concentrations at neutral pH values. The interest in investigating the As(III) removal capacity of these materials under these conditions is to mimic the purification of potable water under real scenarios (pH value). On the other hand, even though the As(III) concentration is high (20 ppm), BiOI-GO removes 38% of As at low doses (1 mg/mL) of adsorbent and short exposure times (5 h).
International standards for arsenic in drinking water have changed with time due to the evidence of chronic arsenic toxicity. Nowadays, several countries have adjusted to the World Health Organization (WHO) regulation that sets a maximum contaminant level of 10 ppb (for arsenic in drinking water). However, Mexico and some other countries in South America (Argentina, Bolivia and Peru) still adopt an MCL of 25 ppb for arsenic in drinking water [4]. This study demonstrates the arsenic removal capacity of BiOX and BiOX-GO NMs. We use concentrations above the MCL due to the detection limit of the analytical technique available in our research group. However, nowadays, the bottleneck of analytical chemistry is the need for real-time measurements. In this regard, aware of the need for highly sensitive techniques for arsenic in situ determination, further research will aim to develop colorimetric or electrochemical sensors with high sensitivity and reproducibility for arsenic monitoring.
In addition, the adsorption capacity of supported (perlite) BiOCl materials was investigated. As illustrated in Figure A6, due to the low amount of BiOCl NMs on the support, the performance of the materials resembles the activity of the supporting material (perlite). Despite the numerous advances in the fabrication of supported photocatalytic materials, finding appropriate supports is complex. Perlite offers many advantages as a support for photocatalytic materials for air treatment (purification or disinfection); however, it performs poorly as a support for water treatment due to its fragility to mechanical stirring.
A recent study investigates the photocatalytic oxidation–adsorption of As(III) by ZrO2-coated BiOCl0.5I0.5 under visible light. The heterostructure completely oxidizes 5 mg/mL of As(III) within 90 min upon activation with visible light. The oxidized As(V) is then efficiently removed by adsorption [14]. In this study, we evaluated the removal efficiencies of the BiOX and BiOX-GO NMs at high pH values (to favor arsenite deprotonation) under visible light (fluorescent bulb, 120 V/60 Hz, 20 W). Under these conditions, BiOI and BiOI-GO NMs excel in their capacity for arsenic removal. BiOI-GO shows efficiencies greater than 70%. These results might indicate the photocatalytic capacity of BiOI and BiOI-GO to oxidize arsenite followed by adsorption. BiOI is a visible-light active semiconducting material; however, its narrow band gap results in the fast recombination of active (e/h) species. Furthermore, GO incorporation inhibits the (e−/h+) recombination, creating NMs with enhanced photocatalytic activity.
Earlier studies demonstrated the enhanced photocatalytic activity of BiOI–carbonaceous material (graphene oxide, reduced graphene oxide, graphene aerogel, gC3N4) composites due to reduced e−/h+ recombination [19,29,30,31]. For example, the composite of BiOI encapsulated in graphene aerogel exerts excellent photocatalytic activity toward methyl orange degradation [31]. A different study reports the improved photocatalytic activity of g-C3N4/BiOI/Bi2O2CO3 for the degradation of rhodamine B [29]. In addition, the composite of iodine-deficient BiOI and carbon nanotubes efficiently degrades dyes and phenol [30]. Although numerous studies exist regarding the photocatalytic activity of BiOX NMs for the mineralization of organic pollutants, scarce information exists about their performance on the removal and oxidation of toxic oxyanions (i.e., arsenic). In this sense, an earlier study discusses the photocatalytic oxidation and adsorption activity of BiOX/r-Fe2O3 NMs for arsenite removal, observing higher efficiencies for BiOI in arsenic removal compared to BiOCl or BiOBr [10].
The advantage of the above-mentioned study over the present investigation is the magnetic nature of the catalyst; however, for the photocatalytic activation, the author used a 500 W Xenon lamp and quartz tube containers. The present study investigates arsenite removal using indoor fluorescent bulbs for catalyst activation and glass containers (Figure A7).
4. Conclusions
This study investigated the physicochemical and adsorptive properties of BiOX (X: Cl, I) and BiOX-GO NMs. BiOI NMs exhibit an enhanced capacity for arsenic removal due to their large surface area and photocatalytic oxidative capacity. BiOX NMs also show an excellent capacity to degrade aqueous organic pollutants. The NMs can remove arsenic from laboratory-prepared samples (water under natural conditions: neutral pH, indoor light), offering a sustainable and affordable process suitable for large-scale applications. Further studies will investigate the capacity and the mechanism of BiOI, BiOI-GO and supported BiOI NMs, aiming to provide an efficient and specific-site mitigation technology for arsenic remediation in groundwater.
Author Contributions
Conceptualization, I.E.M.-R.; methodology, J.H.M.-M., M.L.J.-G., A.G.-P., M.M., F.J.A.-G. and I.E.M.-R.; validation, J.H.M.-M., F.J.A.-G., M.M. and I.E.M.-R.; formal analysis, J.H.M.-M., I.E.M.-R. and J.E.M.-D.; investigation, M.M., F.J.A.-G., J.H.M.-M., I.E.M.-R. and J.E.M.-D.; resources, F.J.A.-G. and I.E.M.-R.; writing—original draft preparation, I.E.M.-R.; writing—review and editing, all authors; supervision, I.E.M.-R. and F.J.A.-G.; project administration, I.E.M.-R.; funding acquisition, I.E.M.-R. and J.E.M.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Elemental composition of perlite supported BiOX and BiOX-GO NMs.
Table A1.
Elemental composition of perlite supported BiOX and BiOX-GO NMs.
| Element (Wt. %) | BiOI | BiOI-GO 10% | BiOCl | BiOCl-GO 10% |
| Carbon | 12.57 | 15.28 | 10.84 | 18.63 |
| Oxygen | 19.21 | 20.62 | 27.34 | 23.16 |
| Sodium | 1.82 | 1.16 | 2.54 | 1.8 |
| Aluminum | 7.36 | 7.19 | 7.62 | 7.41 |
| Silicon | 39.72 | 36.59 | 38.35 | 36.63 |
| Potassium | 4.69 | 4.3 | 4.57 | 4.04 |
| Bismuth | 14.63 | 14.85 | 8.75 | 8.34 |

Table A2.
Photocatalytic degradation of MB.
Table A2.
Photocatalytic degradation of MB.
| Time (Hours) | BiOCl | BiOCl-GO 1% | BiOCl-GO 10% | BiOI | BiOI-GO 1% | BiOI-GO 10% |
| Degradation Percentage | ||||||
| 0 | 2 ± 0.5 | 6 ± 0.5 | 7 ± 0.5 | 5 ± 0.1 | 5 ± 0.1 | 7 ± 0.5 |
| 1 | 19 ± 0.1 | 22 ± 0.9 | 94 ± 0.3 | 72 ± 0.2 | 83 ± 0.5 | 83 ± 0.2 |
| 2 | 23 ± 0.5 | 28 ± 0.1 | 97 ± 0.1 | 83 ± 0.9 | 84 ± 0.3 | 93 ± 0.6 |
| 3 | 25 ± 0.6 | 32 ± 0.4 | 99 ± 0.9 | 88 ± 0.3 | 85 ± 0.2 | 97 ± 0.9 |
| 4 | 27 ± 0.4 | 43 ± 0.3 | 99 ± 0.2 | 93 ± 0.7 | 86 ± 0.2 | 97 ± 0.5 |
| 5 | 33 ± 0.6 | 45 ± 0.5 | 99 ± 0.3 | 95 ± 0.5 | 88 ± 0.4 | 98 ± 0.3 |

Table A3.
Photocatalytic degradation of MO.
Table A3.
Photocatalytic degradation of MO.
| Time (Hours) | BiOCl | BiOCl-GO 1% | BiOCl-GO 10% | BiOI | BiOI-GO 1% | BiOI-GO 10% |
| Degradation Percentage | ||||||
| 0 | 2 ± 0.3 | 6 ± 0.5 | 7 ± 0.5 | 3 ± 0.1 | 6 ± 0.1 | 7 ± 0.5 |
| 1 | 23 ± 0.2 | 25 ± 0.6 | 67 ± 0.9 | 55 ± 0.4 | 53 ± 0.5 | 64 ± 0.6 |
| 2 | 37 ± 0.1 | 34 ± 0.5 | 75 ± 0.7 | 63 ± 0.6 | 60 ± 0.9 | 67 ± 0.3 |
| 3 | 42 ± 0.3 | 33 ± 0.4 | 79 ± 0.7 | 67 ± 0.1 | 70 ± 0.6 | 81 ± 0.7 |
| 4 | 45 ± 0.6 | 37 ± 0.8 | 78 ± 0.8 | 73 ± 0.8 | 79 ± 0.8 | 87 ± 0.9 |
| 5 | 49 ± 0.6 | 42 ± 0.6 | 80 ± 0.4 | 80 ± 0.9 | 85 ± 0.7 | 94 ± 0.7 |
Appendix B
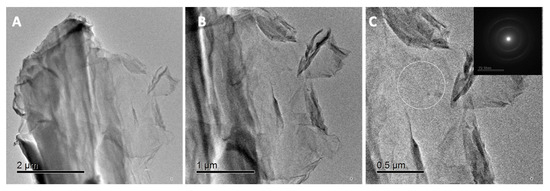
Figure A1.
TEM images of GO NMs at different magnifications. (A,B) TEM image of GO showing its sheet like morphology (C) TEM and SAED analysis (insert) denoting the poor crystallinity of GO NMs.
Figure A1.
TEM images of GO NMs at different magnifications. (A,B) TEM image of GO showing its sheet like morphology (C) TEM and SAED analysis (insert) denoting the poor crystallinity of GO NMs.
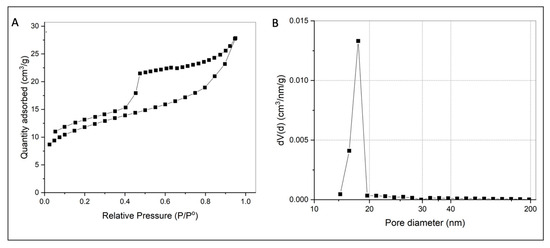
Figure A2.
(A) N2 adsorption isotherms for GO. (B) Pore size distribution curves for GO.
Figure A2.
(A) N2 adsorption isotherms for GO. (B) Pore size distribution curves for GO.
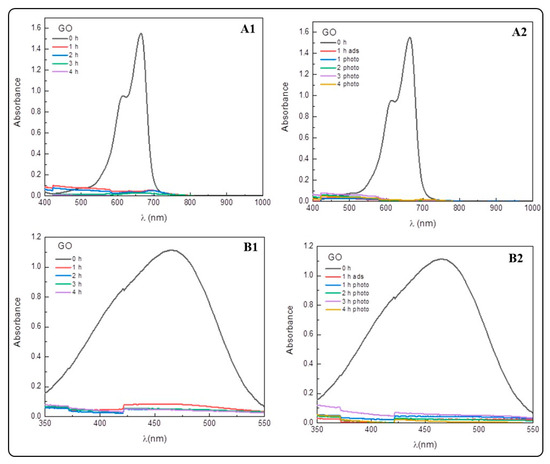
Figure A3.
Evaluation of the removal efficiencies of GO for MB and MO. (A1,B1) Adsorptive capacity of GO to remove MB (A1) and Mo (B1) at different interval times. (A2,B2) Photocatalytic degradation of MB (A2) and MO (B2) using GO and visible light.
Figure A3.
Evaluation of the removal efficiencies of GO for MB and MO. (A1,B1) Adsorptive capacity of GO to remove MB (A1) and Mo (B1) at different interval times. (A2,B2) Photocatalytic degradation of MB (A2) and MO (B2) using GO and visible light.
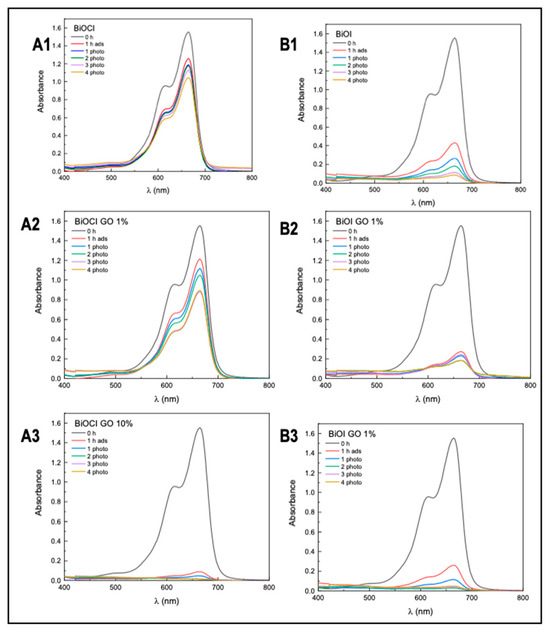
Figure A4.
Photocatalytic degradation of methylene blue. Photocatalytic degradation of MB at different times, using BiOCl (A1), BiOCl-GO 1% (A2) and BiOCl-GO 10% (A3). Photocatalytic degradation of MB at different times, using BiOI (B1), BiOI-GO 1% (B2) and BiOI-GO 10% (B3).
Figure A4.
Photocatalytic degradation of methylene blue. Photocatalytic degradation of MB at different times, using BiOCl (A1), BiOCl-GO 1% (A2) and BiOCl-GO 10% (A3). Photocatalytic degradation of MB at different times, using BiOI (B1), BiOI-GO 1% (B2) and BiOI-GO 10% (B3).
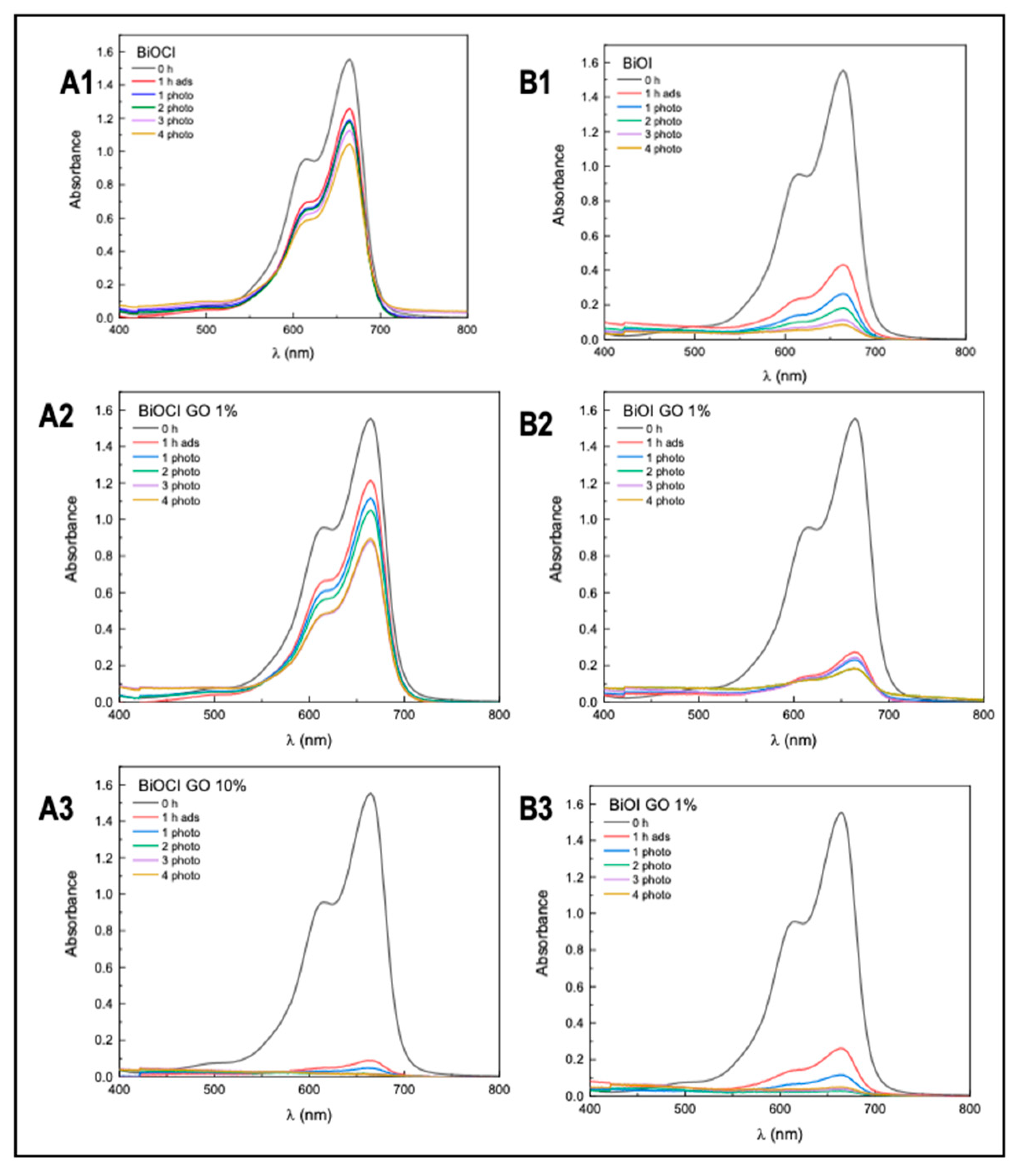
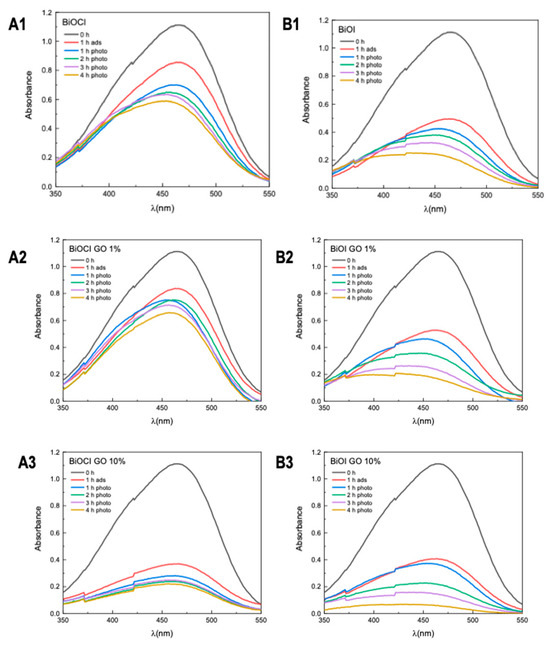
Figure A5.
Photocatalytic degradation of MO. Photocatalytic degradation of MO at different times, using BiOCl (A1), BiOCl-GO 1% (A2) and BiOCl-GO 10% (A3). Photocatalytic degradation of MO at different times, using BiOI (B1), BiOI-GO 1% (B2) and BiOI-GO 10% (B3).
Figure A5.
Photocatalytic degradation of MO. Photocatalytic degradation of MO at different times, using BiOCl (A1), BiOCl-GO 1% (A2) and BiOCl-GO 10% (A3). Photocatalytic degradation of MO at different times, using BiOI (B1), BiOI-GO 1% (B2) and BiOI-GO 10% (B3).
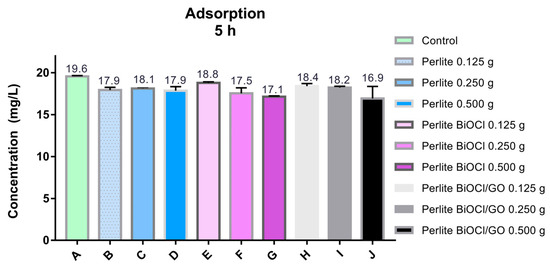
Figure A6.
Evaluation of the arsenic removal efficiency of BiOCl and BiOCl-GO supported materials. Perlite was used as the support. The activity of perlite for arsenic removal was also evaluated under the same conditions as the supported catalysts.
Figure A6.
Evaluation of the arsenic removal efficiency of BiOCl and BiOCl-GO supported materials. Perlite was used as the support. The activity of perlite for arsenic removal was also evaluated under the same conditions as the supported catalysts.
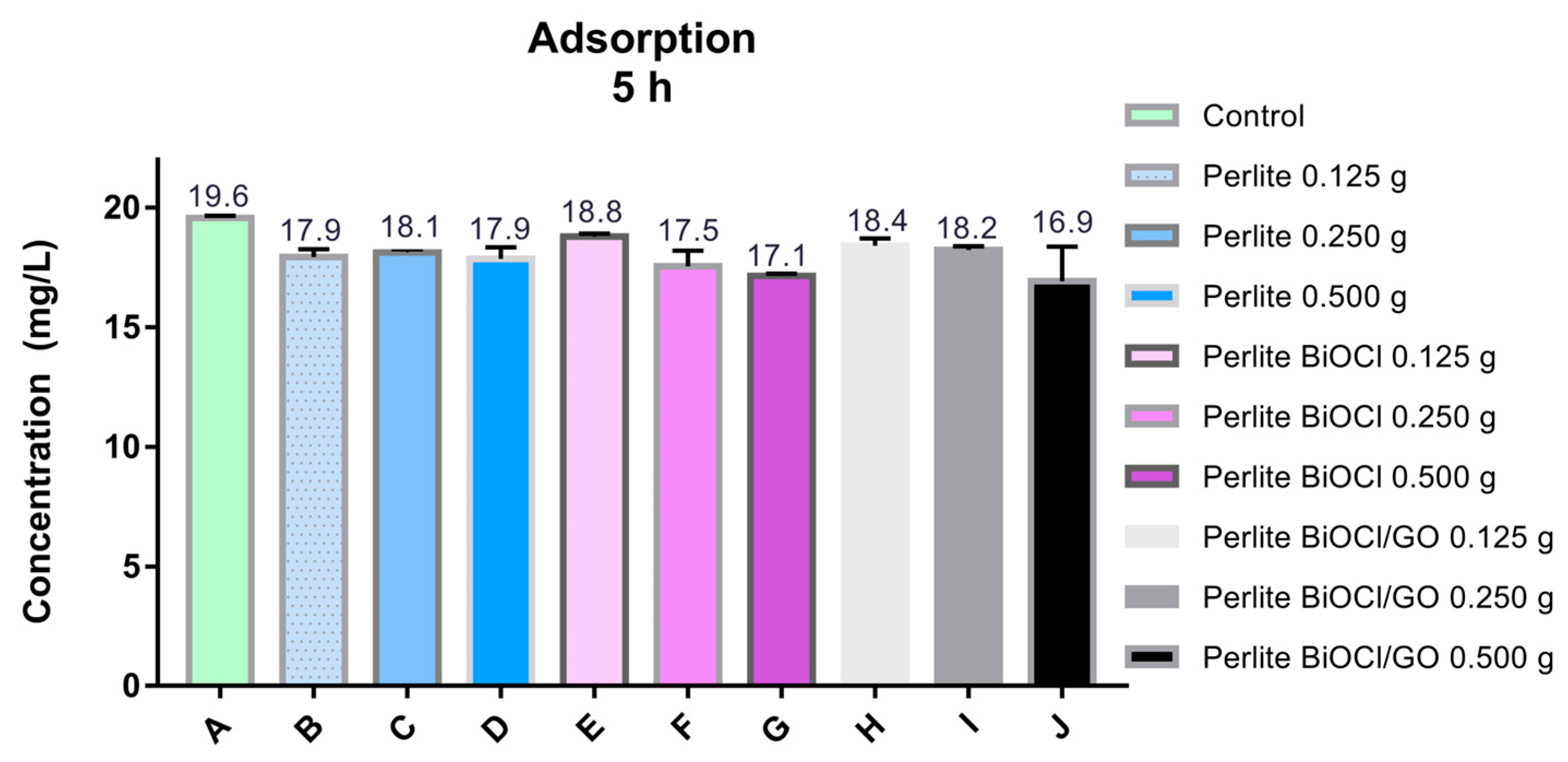

Figure A7.
Experimental setup for the photocatalytic degradation of arsenic, using indoor light for the catalyst activation.
Figure A7.
Experimental setup for the photocatalytic degradation of arsenic, using indoor light for the catalyst activation.

References
- Mahlknecht, J.; Aguilar-Barajas, I.; Farias, P.; Knappett, P.S.K.; Torres-Martínez, J.A.; Hoogesteger, J.; Lara, R.H.; Ramírez-Mendoza, R.A.; Mora, A. Hydrochemical Controls on Arsenic Contamination and Its Health Risks in the Comarca Lagunera Region (Mexico): Implications of the Scientific Evidence for Public Health Policy. Sci. Total Environ. 2023, 857, 159347. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, B.L.; Castillo-Maldonado, I.; Pedroza-Escobar, D.; Delgadillo-Guzmán, D.; Soto-Jiménez, M.F. Association of Obesity, Diabetes, and Hypertension with Arsenic in Drinking Water in the Comarca Lagunera Province (North-Central Mexico). Sci. Rep. 2023, 13, 9244. [Google Scholar] [CrossRef] [PubMed]
- García Salcedo, J.J.; Roh, T.; Nava Rivera, L.E.; Betancourt Martínez, N.D.; Carranza Rosales, P.; San Miguel Salazar, M.F.; Rivera Guillén, M.A.; Serrano Gallardo, L.B.; Niño Castañeda, M.S.; Guzmán Delgado, N.E.; et al. Comparative Biomonitoring of Arsenic Exposure in Mothers and Their Neonates in Comarca Lagunera, Mexico. Int. J. Environ. Res. Public Health 2022, 19, 16232. [Google Scholar] [CrossRef]
- Fisher, A.T.; López-Carrillo, L.; Gamboa-Loira, B.; Cebrián, M.E. Standards for Arsenic in Drinking Water: Implications for Policy in Mexico. J. Public Health Policy 2017, 38, 395–406. [Google Scholar] [CrossRef]
- Ahmad, A.; Bhattacharya, P. Arsenic in Drinking Water: Is 10 Μg/L a Safe Limit? Curr. Pollut. Rep. 2019, 5, 1–3. [Google Scholar] [CrossRef]
- Juárez-Aparicio, F.; Morales-Arredondo, J.I.; Armienta Hernández, M.A. Simultaneous Removal of Fluoride and Arsenic from Drinking Groundwater Using Limestones from Bajío Guanajuatense, Mexico. Arab. J. Geosci. 2024, 17, 109. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Ali, G.A.M.; Bashir, M.J.K.; Copty, N.K.; Amr, S.S.A.; Abushammala, M.F.M.; Al Maskari, T. Recent Advances of Nanoremediation Technologies for Soil and Groundwater Remediation: A Review. Water 2021, 13, 2186. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Naushad, M.; Chaudhry, S.A. Promising Prospects of Nanomaterials for Arsenic Water Remediation: A Comprehensive Review. Process Saf. Environ. Prot. 2019, 126, 60–97. [Google Scholar] [CrossRef]
- Maity, J.P.; Chen, C.Y.; Bhattacharya, P.; Sharma, R.K.; Ahmad, A.; Patnaik, S.; Bundschuh, J. Advanced Application of Nano-Technological and Biological Processes as Well as Mitigation Options for Arsenic Removal. J. Hazard. Mater. 2021, 405, 123885. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Chen, Q.; Zhang, T.C.; Ouyang, L.; Yuan, S. Simultaneous Photocatalytic Oxidation and Adsorption for Efficient As(III) Removal by Magnetic BiOI/γ-Fe2O3 Core–Shell Nanoparticles. Mater. Today Chem. 2022, 24, 100823. [Google Scholar] [CrossRef]
- Rahidul Hassan, H. A Review on Different Arsenic Removal Techniques Used for Decontamination of Drinking Water. Environ. Pollut. Bioavailab. 2023, 35, 2165964. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Dudek, S.; Kołodyńska, D. Arsenic(V) Removal on the Lanthanum-Modified Ion Exchanger with Quaternary Ammonium Groups Based on Iron Oxide. J. Mol. Liq. 2022, 347, 117985. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, M.; Guo, J.; Liu, W.; Tong, M. Facile Synthesis of ZrO2 Coated BiOCl0.5I0.5 for Photocatalytic Oxidation-Adsorption of As(III) under Visible Light Irradiation. Chemosphere 2018, 211, 934–942. [Google Scholar] [CrossRef]
- Jimenez-Chavez, A.; Pedroza-Herrera, G.; Betancourt-Reyes, I.; De Vizcaya Ruiz, A.; Masuoka-Ito, D.; Zapien, J.A.; Medina-Ramirez, I.E. Aluminum Enhances the Oxidative Damage of ZnO NMs in the Human Neuroblastoma SH-SY5Y Cell Line. Discov. Nano 2024, 19, 36. [Google Scholar] [CrossRef]
- Khosrovyan, A.; Vodovnik, M.; Mortimer, M. Omics Approaches in Environmental Effect Assessment of Engineered Nanomaterials and Nanoplastics. Environ. Sci. Nano 2025, 12, 2551–2579. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Y.; Li, C.; Liu, J.; Jia, J.; Zhou, H.; Yan, B. Nano-Cell and Nano-Pollutant Interactions Constitute Key Elements in Nanoparticle-Pollutant Combined Cytotoxicity. J. Hazard. Mater. 2021, 418, 126259. [Google Scholar] [CrossRef]
- Franceschini, F.; Jagdale, P.; Bartoli, M.; Tagliaferro, A. Perspectives on the Use of Bismuth-Based Materials for Sensing and Removal of Water Pollutants. Curr. Opin. Environ. Sci. Health 2022, 26, 100345. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Jiang, S.; Ma, X.; Shao, X.; Liu, Y.; Wang, D.; Li, X.; Li, B. Controllable Self-Assembly of BiOI/Oxidized Mesocarbon Microbeads Core-Shell Composites: A Novel Hierarchical Structure Facilitated Photocatalytic Activities. Chem. Eng. Sci. 2020, 221, 115653. [Google Scholar] [CrossRef]
- Akash, S.; Rameshwar, S.S.; Rajamohan, N.; Rajasimman, M.; Vo, D.V.N. Metal Oxide Nanobiochar Materials to Remediate Heavy Metal and Dye Pollution: A Review. Environ. Chem. Lett. 2024, 22, 2091–2112. [Google Scholar] [CrossRef]
- Martínez-Montelongo, J.H.; Pineda-Arellano, C.A.; Hernandez-Rangel, R.; Jiménez-González, M.; Betancourt, I.; Peralta-Hernández, J.M.; Medina-Ramírez, I.E. Bismuth-Based Nanocomposites as Potential Materials for Indoor Air Treatment. Chemosphere 2024, 367, 43539. [Google Scholar] [CrossRef]
- Hu, J.; Weng, S.; Zheng, Z.; Pei, Z.; Huang, M.; Liu, P. Solvents Mediated-Synthesis of BiOI Photocatalysts with Tunable Morphologies and Their Visible-Light Driven Photocatalytic Performances in Removing of Arsenic from Water. J. Hazard. Mater. 2014, 264, 293–302. [Google Scholar] [CrossRef]
- Li, B.; Shao, L.; Zhang, B.; Wang, R.; Zhu, M.; Gu, X. Understanding Size-Dependent Properties of BiOCl Nanosheets and Exploring More Catalysis. J. Colloid Interface Sci. 2017, 505, 653–663. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Zhou, C.; Zhang, R. Iodine-Vacancy BiOI1-x Ultrathin Sheets for Improved Visible-Light Photooxidation Activities. Appl. Surf. Sci. 2019, 493, 657–664. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Room Temperature Synthesis and Highly Enhanced Visible Light Photocatalytic Activity of Porous BiOI/BiOCl Composites Nanoplates Microflowers. J. Hazard. Mater. 2012, 219–220, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tao, X.; Yang, Z.; Li, K.; Yang, H.; Li, A.; Cheng, R. Effects of the Oxidation Degree of Graphene Oxide on the Adsorption of Methylene Blue. J. Hazard. Mater. 2014, 268, 191–198. [Google Scholar] [CrossRef]
- Guo, T.; Fan, X.; Jiang, X.; Qi, Y.; Du, J.; Zhang, A.; Wang, H. Engineering Shape of BiOCl Nanosheets with Improved Visible-Light Response for Superior Photocatalytic Degradation of Rhodamine B. J. Alloys Compd. 2023, 948, 169586. [Google Scholar] [CrossRef]
- Shah, A.H.; Gu, W.; Abideen, Z.U.; Teng, F. Removal of Chromium from Aqueous Solution by Porous Bi2MoO6@BiOCl Nanostructure. J. Solid State Chem. 2020, 292, 121719. [Google Scholar] [CrossRef]
- Zhong, S.; Zhou, H.; Shen, M.; Yao, Y.; Gao, Q. Rationally Designed a G-C3N4/BiOI/Bi2O2CO3 Composite with Promoted Photocatalytic Activity. J. Alloys Compd. 2021, 853, 157307. [Google Scholar] [CrossRef]
- Sharma, N.; Pap, Z.; Székely, I.; Focsan, M.; Karacs, G.; Nemeth, Z.; Garg, S.; Hernadi, K. Combination of Iodine-Deficient BiOI Phases in the Presence of CNT to Enhance Photocatalytic Activity towards Phenol Decomposition under Visible Light. Appl. Surf. Sci. 2021, 565, 150605. [Google Scholar] [CrossRef]
- Arumugam, M.; Seralathan, K.K.; Praserthdam, S.; Tahir, M.; Praserthdam, P. Synthesis of Novel Graphene Aerogel Encapsulated Bismuth Oxyiodide Composite towards Effective Removal of Methyl Orange Azo-Dye under Visible Light. Chemosphere 2022, 303, 135121. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).