Transcriptomic Response of Azospirillum brasilense Co-Cultured with Green Microalgae Chlorella sp. and Scenedesmus sp. During CO2 Biogas Fixation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
2.2. Experimental Culture Conditions
2.3. Microarray and Data Analyses
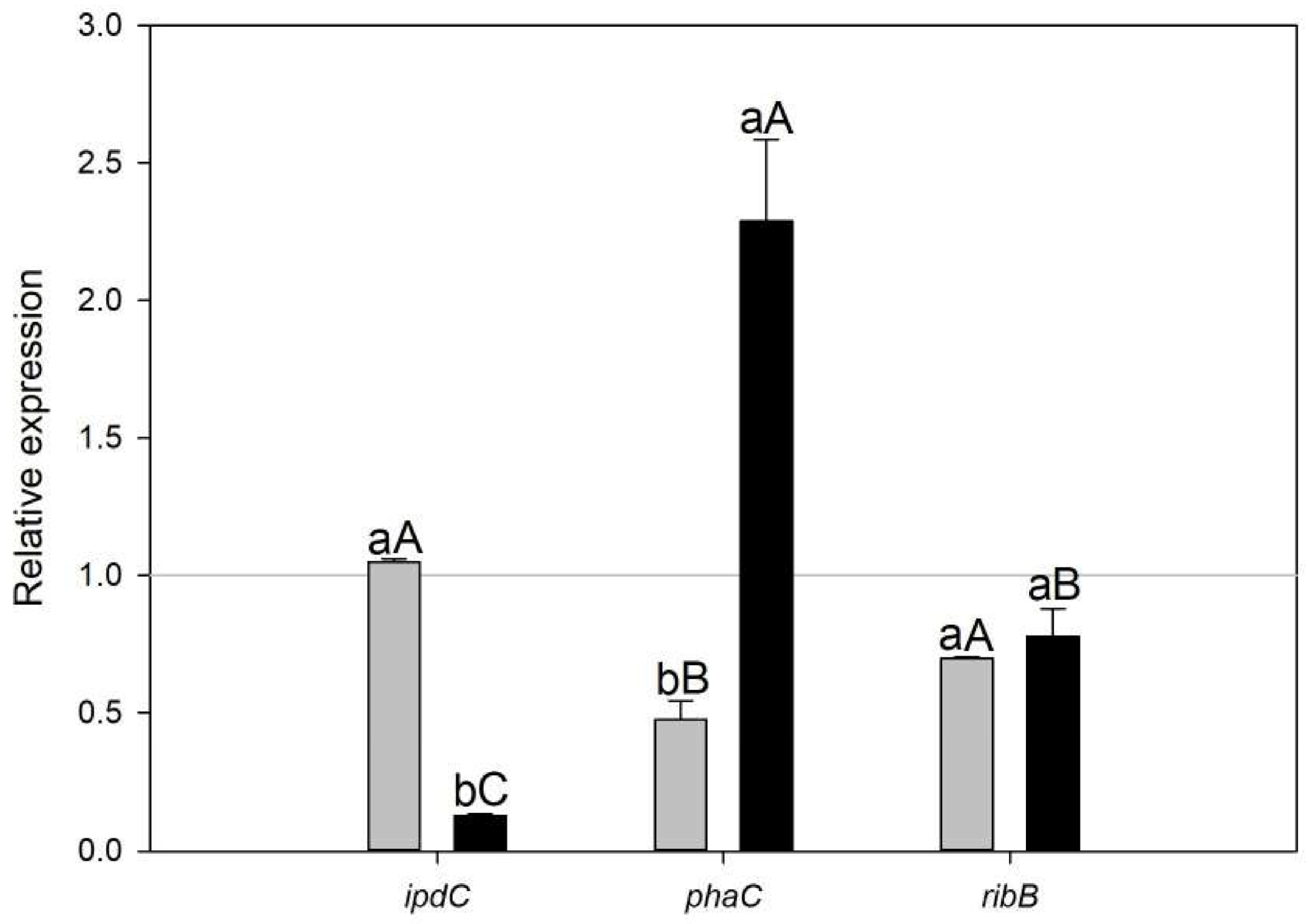
Pathway Enrichment Analyses and qRT-PCR Validation
2.4. Bacterium A. brasilense Effect on Microalgae Cell Density
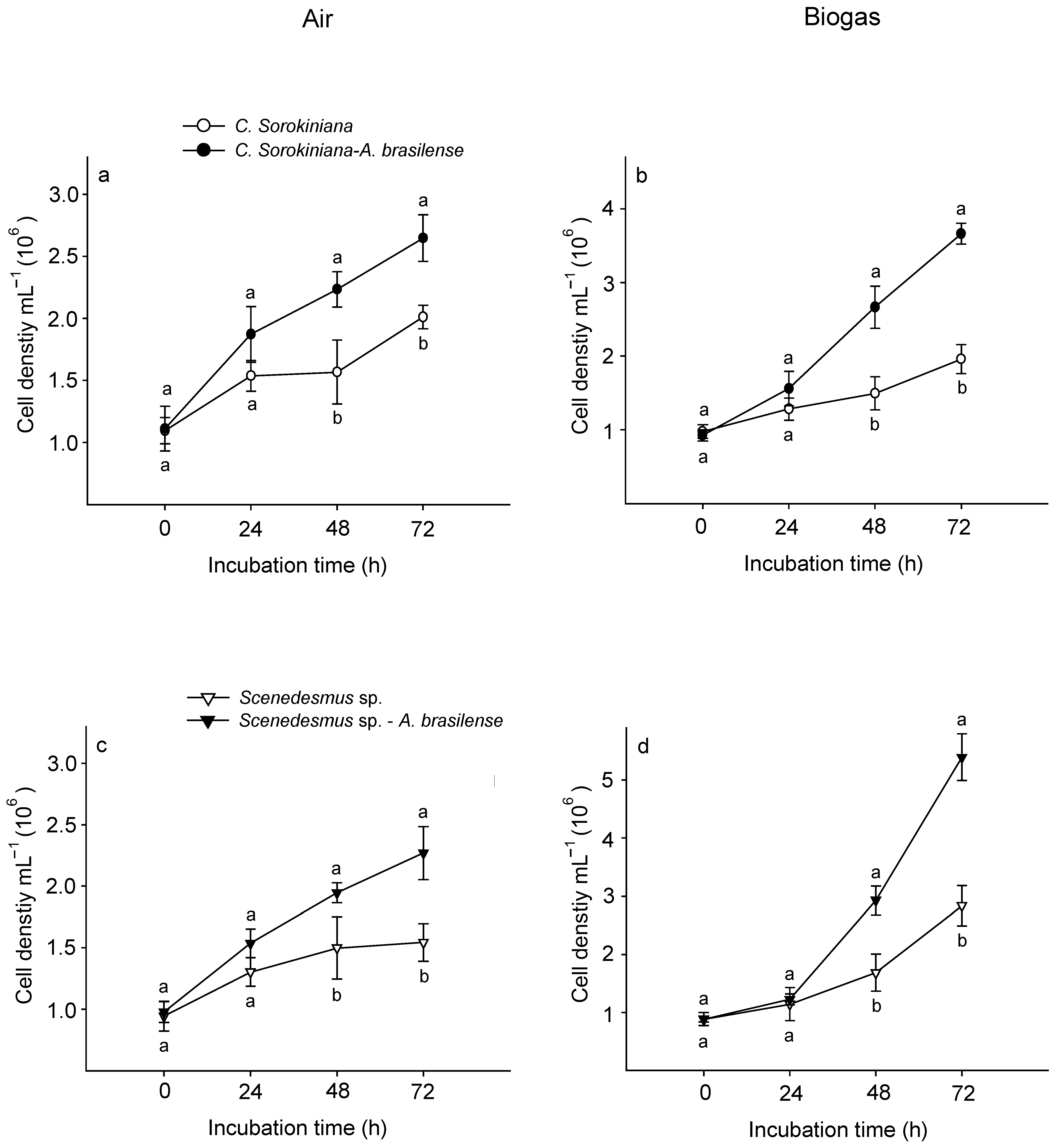
2.4.1. Counting Microorganisms
2.4.2. Determination of CO2 Fixation Rate
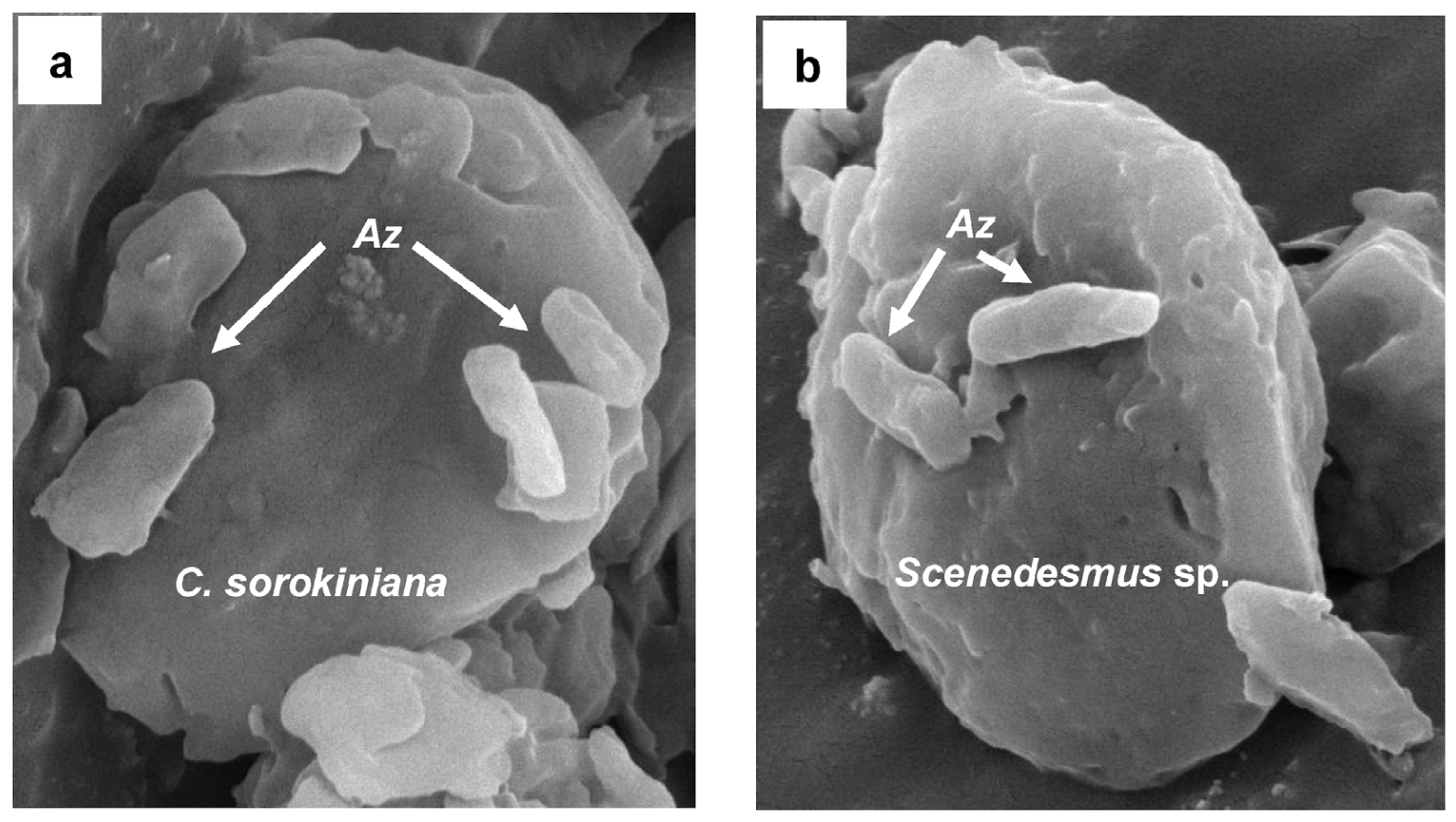
2.4.3. Visualization by Scanning Electron Microscopy (SEM)
2.4.4. Experimental Designs and Statistical Analyses
3. Results
3.1. Genes Differentially Expressed in Azospirillum brasilense Interacting with Microalgae
3.2. Functional Annotation and Enrichment Analyses
3.3. qRT-PCR Validation of Gene Expression Patterns
3.4. Cell Density of C. sorokiniana and Scenedesmus sp. associated with A. brasilense
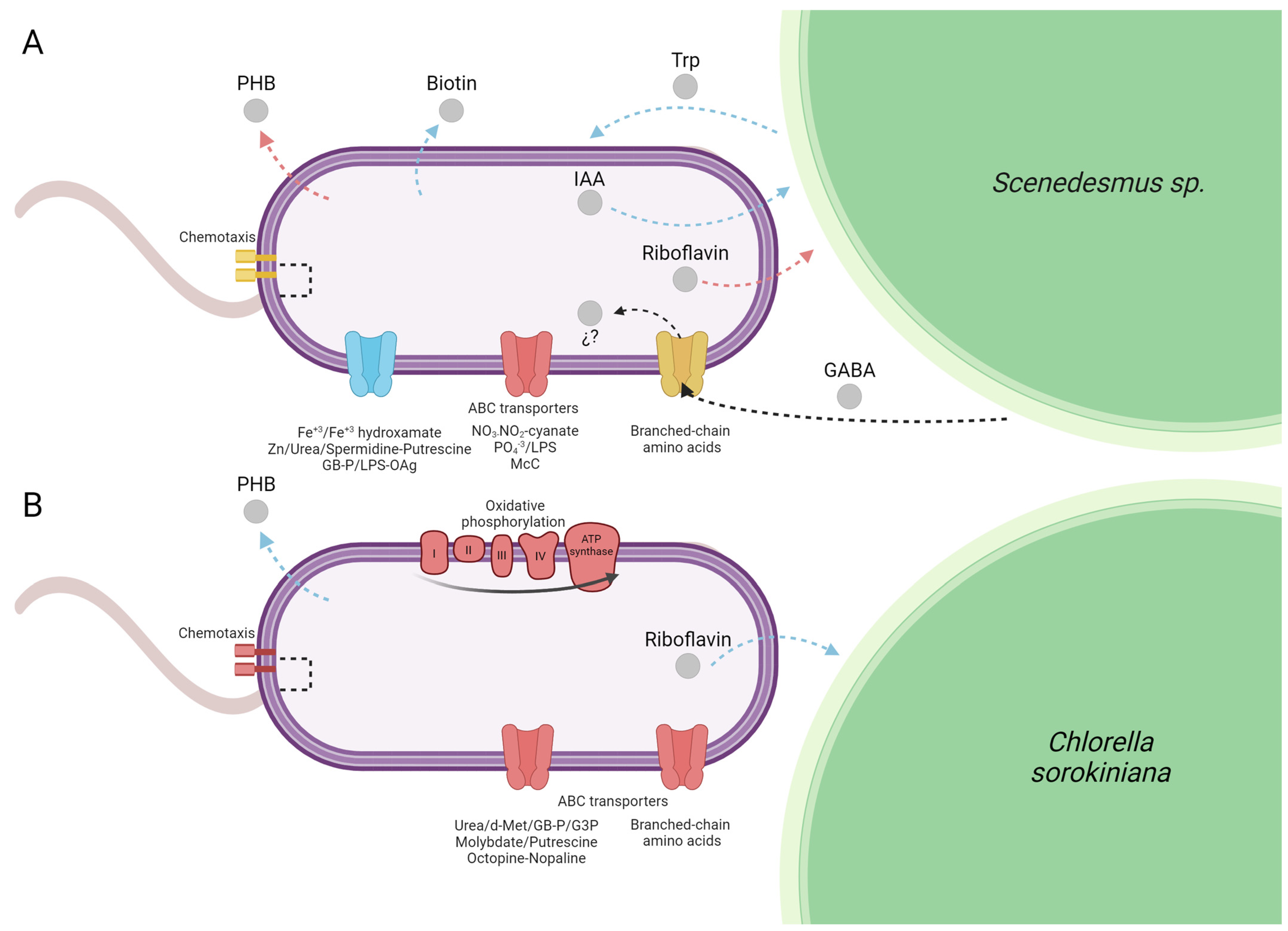
4. Discussion
5. Conclusions
6. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassan, F.; Coniglio, A.; Lopez, G.; Molina, R.; Nievas, S.; de Carlan, C.L.N.; Torres, D.; Rosas, S.; Pedrosa, F.O.; de Souza, E.; et al. Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Pereg, L.; de-Bashan, L.E.; Bashan, Y. Assessment of affinity and specificity of Azospirillum for plants. Plant Soil. 2016, 399, 389–414. [Google Scholar] [CrossRef]
- Palacios, O.A.; López, B.R.; de-Bashan, L.E. Microalga Growth-Promoting Bacteria (MGPB): A formal term proposed for beneficial bacteria involved in microalgal–bacterial interactions. Algal Res. 2022, 61, 102585. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Mayali, X.; Bebout, B.M.; Weber, P.K.; Detweiler, A.M.; Hernandez, J.P.; Prufert-Bebout, L.; Bashan, Y. Establishment of stable synthetic mutualism without co-evolution between microalgae and bacteria demonstrated by mutual transfer of metabolites (NanoSIMS isotopic imaging) and persistent physical association (Fluorescent in situ hybridization). Algal Res. 2016, 15, 179–186. [Google Scholar] [CrossRef]
- Contreras, C.A.; Palacios, O.A.; de-Bashan, L.E.; Choix, F.J. Microalga Growth-Promoting Bacteria as Strategy to Improve CO2 Removal from Biogas. BioEnergy Res. 2024, 17, 2082–2099. [Google Scholar] [CrossRef]
- Ruiz-Güereca, S.S.; Sánchez-Saavedra, M.P. Growth and phosphorus removal by Synechococcus elongatus coimmobilized in alginate beads with Azospirillum brasilense. J. Appl. Phycol. 2016, 28, 1501–1507. [Google Scholar] [CrossRef]
- Barbosa-Nuñez, J.A.; Palacios, O.A.; Mondragón-Cortez, P.; Ocampo-Alvarez, H.; Becerril-Espinosa, A.; Nevárez-Moorillón, G.V.; Choix, F.J. Chemical and physical affinity of microalga—Azospirillum consortium co-cultured in suspension during CO2 fixation from biogas. Bioenergy Res. 2022, 16, 579–592. [Google Scholar] [CrossRef]
- Lopez, B.R.; Bashan, Y.; Trejo, A.; de-Bashan, L.E. Amendment of degraded desert soil with wastewater debris containing immobilized Chlorella sorokiniana and Azospirillum brasilense significantly modifies soil bacterial community structure, diversity, and richness. Biol. Fert. Soils 2013, 49, 1053–1063. [Google Scholar] [CrossRef]
- Mulu, E.; M’Arimi, M.M.; Ramkat, R.C. A review of recent developments in application of low cost natural materials in purification and upgrade of biogas. Renew. Sust. Energ. Rev. 2021, 145, 111081. [Google Scholar] [CrossRef]
- Bose, A.; Lin, R.; Rajendran, K.; O’Shea, R.; Xia, A.; Murphy, J.D. How to optimize photosynthetic biogas upgrading: A perspective on system design and microalgae selection. Biotechnol. Adv. 2019, 37, 107444. [Google Scholar] [CrossRef]
- Solovchenko, A.; Khozin-Goldberg, I. High-CO2 tolerance in microalgae: Possible mechanisms and implications for biotechnology and bioremediation. Biotechnol. Lett. 2013, 35, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Garciglia-Mercado, C.; Contreras, C.A.; Choix, F.J.; de-Bashan, L.E.; Gomez-Anduro, G.A.; Palacios, O.A. Metabolic and physiological adaptations of microalgal growth-promoting bacterium Azospirillum brasilense growing under biogas atmosphere: A microarray-based transcriptome analysis. Arch. Microbiol. 2024, 206, 173. [Google Scholar] [CrossRef]
- Bashan, Y.; Hernandez, J.P.; Leyva, L.A.; Bacilio, M. Alginate microbeads as inoculant carrier for plant growth-promoting bacteria. Biol. Fertil. Soils 2002, 35, 359–368. [Google Scholar] [CrossRef]
- Lopez, B.R.; Palacios, O.A.; Bashan, Y.; Hernández-Sandoval, F.E.; de-Bashan, L.E. Riboflavin and lumichrome exuded by the bacterium Azospirillum brasilense promote growth and changes in metabolites in Chlorella sorokiniana under autotrophic conditions. Algal Res. 2019, 44, 101696. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
- Mcmillan, M.; Pereg, L. Evaluation of reference genes for gene expression analysis using quantitative RT-PCR in Azospirillum brasilense. PLoS ONE 2014, 9, e98162. [Google Scholar] [CrossRef]
- Palacios, O.A.; Gomez-Anduro, G.; Bashan, Y.; de-Bashan, L.E. Tryptophan, thiamine and indole-3-acetic acid exchange between Chlorella sorokiniana and the plant growth-promoting bacterium Azospirillum brasilense. FEMS Microbiol. Ecol. 2016, 92, fiw077. [Google Scholar] [CrossRef]
- Gonzales-Infante, E.J. Metabolismo de polihidroxibutirato en Azospirillum brasilense Cd durante la interacción Azospirillum brasilense con Chlorella sorokiniana. Master’s Thesis, Universidad Autónoma de Baja California Sur, La Paz, Mexico, 2020. Available online: https://biblio.uabcs.mx/tesis/tesis/te4530.pdf (accessed on 25 March 2024).
- Puente, M.L.; Maroniche, G.A.; Panepucci, M.; Sabio y Garcia, J.; Garcia, J.E.; Criado, M.V.; Molina, R.; Cassan, F. Localization and survival of Azospirillum brasilense Az39 in soybean leaves. Lett. Appl. Microbiol. 2021, 72, 626–633. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Contreras-Angulo, J.R.; Mata, T.M.; Cuellar-Bermudez, S.P.; Caetano, N.S.; Chandra, R.; Garcia-Perez, J.S.; Muylaert, K.; Parra-Saldivar, R. Symbiotic Co-Culture of Scenedesmus sp. and Azospirillum brasilense on N-Deficient media with biomass production for biofuels. Sustainability 2019, 11, 707. [Google Scholar] [CrossRef]
- Peng, H.; de-Bashan, L.E.; Higgins, B.T. Azospirillum brasilense reduces oxidative stress in the green microalgae Chlorella sorokiniana under different stressors. J. Biotechnol. 2021, 325, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.F. Vitamin formation from fatty acid precursors. In Biogenesis of Fatty Acids, Lipids and Membranes, Handbook of Hydrocarbon and Lipid Microbiology; Geiger, O., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 259–271. [Google Scholar] [CrossRef]
- Dahm, H.; Rózycki, H.; Strzelczyk, E.; Li, C.Y. Production of B-group vitamins by Azospirillum spp. grown in media of different pH at different temperatures. Zentralblatt Mikrobiol. 1993, 148, 195–203. [Google Scholar] [CrossRef]
- Tandon, P.; Jin, Q.; Huang, L. A promising approach to enhance microalgae productivity by exogenous supply of vitamins. Microb. Cell Fact. 2017, 16, 219. [Google Scholar] [CrossRef] [PubMed]
- Cassán, F.; Okon, Y.; Creus, C.M. Handbook for Azospirillum: Technical Issues and Protocols; Springer International Publishing: Cham, Switzerland, 2015; Volume 10. [Google Scholar] [CrossRef]
- Müller-Santos, M.; Koskimäki, J.J.; Silveira-Alves, L.P.; de Souza, E.M.; Jendrossek, D.; Pirttilä, A.M. The protective role of PHB and its degradation products against stress situations in bacteria. FEMS Microbiol. Rev. 2021, 45, fuaa058. [Google Scholar] [CrossRef]
- Orlova, M.V.; Tarlachkov, S.V.; Dubinina, G.A.; Belousova, E.V.; Tutukina, M.N.; Grabovich, M.Y. Genomic insights into metabolic versatility of a lithotrophic sulfur-oxidizing diazotrophic Alphaproteobacterium Azospirillum thiophilum. FEMS Microbiol. Ecol. 2016, 92, fiw199. [Google Scholar] [CrossRef][Green Version]
- Muñoz-Velasco, I.; Garcia-Ferris, C.; Hernandez-Morales, R.; Lazcano, A.; Pereto, J.; Becerra, A. Methanogenesis on early stages of life: Ancient but not primordial. Orig. Life Evol. Biosph. 2018, 48, 407–420. [Google Scholar] [CrossRef]
- Malinich, E.A.; Bauer, C.E. Transcriptome analysis of Azospirillum brasilense vegetative and cyst states reveals large-scale alterations in metabolic and replicative gene expression. Microb. Genom. 2018, 4, e000200. [Google Scholar] [CrossRef]
- Su, A.; Yu, Q.; Luo, Y.; Yang, J.; Wang, E.; Yuan, H. Metabolic engineering of microorganisms for the production of multifunctional non-protein amino acids: γ-aminobutyric acid and δ-aminolevulinic acid. Microb. Biotechnol. 2021, 14, 2279–2290. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, S.; Li, B.; Li, J.; Wang, X.; Zhang, J.; Guan, C.; Ji, J. The combined use of a plant growth promoting Bacillus sp. strain and GABA promotes the growth of rice under salt stress by regulating antioxidant enzyme system, enhancing photosynthesis and improving soil enzyme activities. Microbiol. Res. 2023, 266, 127225. [Google Scholar] [CrossRef]
- Teng, C.S.; Xue, C.; Lin, J.Y.; Ng, I.S. Towards high-level protein, beta-carotene, and lutein production from Chlorella sorokiniana using aminobutyric acid and pseudo seawater. Biochem. Eng. J. 2022, 184, 108473. [Google Scholar] [CrossRef]
- Teng, C.S.; Ng, I.S. Optimization of 4-aminobutyric acid feeding strategy and clustered regularly interspaced short palindromic repeats activation for enhanced value-added chemicals in halophilic Chlorella sorokiniana. Bioresour. Technol. 2023, 387, 129599. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, X.; Zhong, D.B.; Yu, L.; Yu, X. γ-Aminobutyric acid (GABA) regulates lipid production and cadmium uptake by Monoraphidium sp. QLY-1 under cadmium stress. Bioresour. Technol. 2020, 297, 122500. [Google Scholar] [CrossRef]
- Planamente, S.; Mondy, S.; Hommais, F.; Vigouroux, A.; Moréra, S.; Faure, D. Structural basis for selective GABA binding in bacterial pathogens. Mol. Microbiol. 2012, 86, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, S.; Bell, T.A.S.; Dahlin, L.R.; Kunde, Y.; LaButti, K.; Louie, K.B.; Kuftin, A.; Treen, D.; Dilworth, D.; Mihaltcheva, S.; et al. A multi-omic characterization of temperature stress in a halotolerant Scenedesmus strain for algal biotechnology. Commun. Biol. 2021, 4, 333. [Google Scholar] [CrossRef]
- Planamente, S.; Moréra, S.; Faure, D. In planta fitness-cost of the Atu4232-regulon encoding for a selective GABA-binding sensor in agrobacterium. Commun. Integr. Biol. 2013, 6, e23692. [Google Scholar] [CrossRef] [PubMed]
- Silva-Dias, B.H.; Jung, S.H.; Castro-Oliveira, J.V.D.; Ryu, C.M. C4 bacterial volatiles improve plant health. Pathogens 2021, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Baz, L.; Abulfaraj, A.A.; Tashkandi, M.A.; Baeissa, H.M.; Refai, M.Y.; Barqawi, A.A.; Shami, A.; Abuauf, H.W.; Ashy, R.A.; Jalal, R.S. Predicted functional shifts due to type of soil microbiome and watering of two wild plants in western region of Saudi Arabia. Phyton 2022, 91, 2249. [Google Scholar] [CrossRef]
- Ganusova, E.E.; Vo, L.T.; Abraham, P.E.; O’Neal-Yoder, L.; Hettich, R.L.; Alexandre, G. The Azospirillum brasilense core chemotaxis proteins CheA1 and CheA4 link chemotaxis signaling with nitrogen metabolism. mSystems 2021, 6, e01354-20. [Google Scholar] [CrossRef]
- Ganusova, E.E.; Vo, L.T.; Mukherjee, T.; Alexandre, G. Multiple CheY proteins control surface associated lifestyles of Azospirillum brasilense. Front. Microbiol. 2021, 12, 664826. [Google Scholar] [CrossRef]
- Ganusova, E.E.; Rost, M.; Aksenova, A.; Abdulhussein, M.; Holden, A.; Alexandre, G. Azospirillum brasilense AerC and Tlp4b cytoplasmic chemoreceptors are promiscuous and interact with the two membrane-bound chemotaxis signaling clusters mediating chemotaxis responses. J. Bacteriol. 2023, 205, e00484-22. [Google Scholar] [CrossRef]
- Gumerov, V.M.; Ortega, D.R.; Adebali, O.; Ulrich, L.; Zhulin, I.B. MiST 3.0: An updated microbial signal transduction database with an emphasis on chemosensory systems. Nucleic Acids Res. 2020, 48, D459–D464. [Google Scholar] [CrossRef] [PubMed]

| Name | Forward | Reverse | Reference |
|---|---|---|---|
| recA * | GTCGAACTGCCTGGTGAT | GACGGAGGCGTAGAACTT | McMillan & Pereg, [16] |
| ipdC | AGTCGTCCAGGTCGTTGAAG | ACCCCATCGTGATCCTG | Palacios et al. [17] |
| phaC | AGCTTCTACCTGCGCAACAT | AAGAAGGACGGGGTCTTCAC | Gonzales Infante, [18] |
| ribB | ACGAAGGCGACGTGATCTAC | AGGATCAGGCAGACGATCC | Not published (Environmental Microbiology Group, The Northwestern Center for Biological Research CIBNOR) |
| Microalga | Growth Rate (µ; d−1) | Biomass Productivity (p; g L−1d−1) | CO2 Fixation Rate (RCO2; g L−1d−1) | |||
|---|---|---|---|---|---|---|
| Air | Biogas | Air | Biogas | Air | Biogas | |
| C. sorokiniana | 0.06 ± 0.02 b | 0.11 ± 0.01 b | 0.08 ± 0.02 b | 0.09 ± 0.03 b | 0.12 ± 0.03 a | 0.19 ± 0.02 a |
| C. sorokiniana—Az | 0.12 ± 0.1 a | 0.21 ± 0.04 a | 0.14 ± 0.02 a | 0.16 ± 0.02 a | 0.16 ± 0.03 a | 0.23 ± 0.03 a |
| Scenedesmus sp. | 0.07 ± 0.02 b | 0.13 ± 0.02 b | 0.09 ± 0.02 b | 0.12 ± 0.03 b | 0.10 ± 0.02 b | 0.22 ± 0.03 b |
| Scenedesmus sp.—Az. | 0.13 ± 0.03 a | 0.25 ± 0.02 a | 0.15 ± 0.03 a | 0.18 ± 0.01 a | 0.16 ± 0.02 a | 0.28 ± 0.02 a |
| Bacterium | Log CFU mL−1 | |
|---|---|---|
| Air | Biogas | |
| A. brasilense | 6.1 ± 0.8 a | 6.4 ± 0.6 a |
| A. brasilense—C. sorokiniana | 6.59 ± 1.0 a | 5.65 ± 0.4 a |
| A. brasilense—Scenedesmus sp. | 4.93 ± 1.0 a | 5.83 ± 0.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garciglia-Mercado, C.; Palacios, O.A.; Contreras-Godínez, C.A.; Torres-Velázquez, J.R.; Choix, F.J. Transcriptomic Response of Azospirillum brasilense Co-Cultured with Green Microalgae Chlorella sp. and Scenedesmus sp. During CO2 Biogas Fixation. Processes 2025, 13, 2177. https://doi.org/10.3390/pr13072177
Garciglia-Mercado C, Palacios OA, Contreras-Godínez CA, Torres-Velázquez JR, Choix FJ. Transcriptomic Response of Azospirillum brasilense Co-Cultured with Green Microalgae Chlorella sp. and Scenedesmus sp. During CO2 Biogas Fixation. Processes. 2025; 13(7):2177. https://doi.org/10.3390/pr13072177
Chicago/Turabian StyleGarciglia-Mercado, Carolina, Oskar A. Palacios, Claudia A. Contreras-Godínez, Jony Ramiro Torres-Velázquez, and Francisco J. Choix. 2025. "Transcriptomic Response of Azospirillum brasilense Co-Cultured with Green Microalgae Chlorella sp. and Scenedesmus sp. During CO2 Biogas Fixation" Processes 13, no. 7: 2177. https://doi.org/10.3390/pr13072177
APA StyleGarciglia-Mercado, C., Palacios, O. A., Contreras-Godínez, C. A., Torres-Velázquez, J. R., & Choix, F. J. (2025). Transcriptomic Response of Azospirillum brasilense Co-Cultured with Green Microalgae Chlorella sp. and Scenedesmus sp. During CO2 Biogas Fixation. Processes, 13(7), 2177. https://doi.org/10.3390/pr13072177






