Abstract
Microalgal–bacterial consortia are the environmentally sustainable biotechnological strategy to enhance the potential of microalgae. Understanding the regulatory mechanisms that enable bacteria to adapt to culture conditions of each bioprocess is crucial to ensure a successful synergic interaction. Thus, the present study evaluated the transcriptomic response of microalgal growth-promoting bacteria (MGPB) A. brasilense separately co-cultured with both green microalgae Scenedesmus sp. and Chlorella sorokiniana during CO2 fixation from biogas through a microarray-based approach. The transcriptome profiling revealed a total of 416 differentially expressed genes (DEGs) in A. brasilense: 228 (140 upregulated and 88 downregulated) interacting with Scenedesmus sp. and 188 (40 upregulated and 148 downregulated) associated with C. sorokiniana. These results support the modulation of signal molecules: indole-3-acetic acid (IAA), riboflavin, and biotin, during co-cultivation with both microalgae. The findings suggest that the metabolic A. brasilense adaptation was mainly favored during the mutualistic interaction with Scenedesmus sp. Finally, a valuable contribution is provided to the biotechnological potential of the microalga–Azospirillum consortium as an environmentally sustainable strategy to improve the bio-refinery capacity of these microalgae and biogas upgrading by valorizing CO2 of these gaseous effluent.
1. Introduction
Azospirillum spp. are gram-negative, microaerophilic, non-fermentative, and nitrogen-fixing bacteria [1], which are the most studied plant-growth-promoting bacteria (PGPB) due to their capacity to positively influence physiology, enhancing growth, development, and productivity of multiple agricultural plants [2] and microalgae [3]. Thus, Azospirillum spp. are also considered as microalgal growth-promoting bacteria (MGPB). Specifically, this genus improves the metabolism of microalgae through multiple growth-promoting mechanisms, such as: (1) CO2 released from their carbon catabolism; (2) vitamin production; (3) phytohormone production; (4) nitrogen fixation or catabolism of complex nitrogen compounds; (5) siderophore production; (6) organic matter release; and (7) avoidance of negative bacterial presence [1,3,4]. To date, many studies demonstrate that Azospirillum spp. stimulates higher photosynthetic efficiency. Thus, CO2 fixation, cell density, and high-value compound production are enhanced in diverse microalgae genera, such as Chlorella. Scenedesmus, Chlamydomonas, Spirulina, and Synechococuus under different culture conditions, even stressful environments [3,5]. For this reason, the consortium microalga–Azospirillum has been used in different environmental processes, such as wastewater bioremediation [6], CO2 fixation [7], and eroded soil restoration [8]. Nonetheless, environmental factors and specific culture conditions of each bioprocess can negatively affect their mechanisms, hence the positive influence of Azospirillum on microalgal physiology. Therefore, comprehending the effect of the particular conditions of each bioprocess on Azospirillum growth-promoting mechanisms is vital to warrant the successful biotechnological application of this microalga–bacteria consortium.
On the other hand, biogas is a low-cost fuel mainly composed of methane (CH4; 40–80%, v/v) and carbon dioxide (CO2; 20–40%, v/v) generated during organic material anaerobic digestion process [9]. At present, biogas is supplied as a carbon source for diverse microalgal genera, since CO2 fixed through its photosynthetic process is converted into biomass with high-value metabolites [5]. This activity upgrades CH4 content from biogas to take advantage of it as an alternative energy source, since CO2 content reduces CH4 caloric value, thus preventing its use for this purpose [9,10]. To date, the green microalgae of Scenedesmus and Chlorella genus are widely studied for CO2 capture in biogas [11]. Whilst Barbosa-Nuñez et al. [7] demonstrated that Azospirillum brasilense can establish a synergic association with Scenedesmus and Chlorella under the stressful biogas composition, increasing CO2 fixation of both microalgae. In this regard, Garciglia-Mercado et al. [12] revelated the physiological adaptations of A. brasilense through a microarray-based transcriptome approach to maintain active its metabolism cultured alone under biogas (75% CH4–25% CO2). Nonetheless, the transcriptomic regulation of multiple growth-promoting mechanisms of Azospirillum to maintain its synergic association with microalgae have not been evaluated during CO2 fixation of biogas. Therefore, understanding gene regulatory mechanisms enabling A. brasilense metabolic adaptations and sustaining its mutualistic interaction with the green microalgae Scenedesmus sp. and Chlorella sorokiniana is imperative for implementing this consortium as an environmentally sustainable biotechnological strategy to biogas upgrading and enhancing the bio-refinery capacity of microalgae.
Considering the above, the hypothesis of this study is that A. brasilense would modulate its genes involved in growth-promoting mechanisms, enabling it to maintain its mutualistic interaction with Scenedesmus sp. and Chlorella sorokiniana microalgae under a biogas atmosphere. Therefore, the aim of the present study is to determine A. brasilense transcriptomic response through a microarray-based approach separately co-cultured in suspension with the microalgae Scenedesmus sp. and Chlorella sorokiniana under synthetic biogas.
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
The bacterium A. brasilense Cd (DMSZ 1843, Leibniz-Institute DMSZ, Braunschweig, Germany) and green microalgae Scenedesmus sp. and Chlorella sorokiniana were isolated from Lago de Chapala (Jalisco, Mexico; 20°15′27″ N, 103°02′33″ W) and used in the present study. The bacterium was maintained in modified Organo–Acrylate Binding (OAB) medium [13] at 36 ± 0.5 °C and stirred at 80 revolutions/min (RPM) for 24 h. Both microalgae were maintained in medium C30 + M [7] at 27 ± 2 °C, 200 μmol photons m−2 s−1, and stirred at 100 RPM for seven days.
2.2. Experimental Culture Conditions
The synthetic biogas atmosphere (75% CH4–25% CO2, Praxair-Mexico) effect was assessed on A. brasilense at the stationary growth phase (72 h) co-cultured in suspension with the microalgae Scenedesmus sp. and Chlorella sorokiniana. Hydrocarbon-free air (Praxair-Mexico) was used for control. Then, 40 mL of A. brasilense Cd (109 colony forming units (CFU)·mL−1) and 40 mL of Scenedesmus sp. or C. sorokiniana (106 cell·mL−1) were inoculated into 320 mL of C30 + M medium in a flask sealed with glass stoppers. A total of eight modified Kitasato flasks were used, four for air atmosphere (control) of which two were for A. brasilense + Scenedesmus sp. (A.b + S) and two were for A. brasilense + C. sorokiniana (A.b + C), and the other four for biogas atmosphere (treatment): two flasks for A.b + S, and two for A.b + C. Both gases (air or biogas) were supplied daily with a flow rate of 3 mL min−1 by a side flask feed to maintain biogas or air in the headspace, incubated at 25 ± 0.5 °C, 200 μmol photons m−2 s−1, and 200 RPM in a shaking incubator for 72 h. Two biological replicates were made for each treatment and control, and four sub-replicates of each replicate were taken for further analyses.
It is important to highlight that 72 h is the time at which A. brasilense metabolism is adapted to biogas [12]; similarly, the microalga–Azospirillum interaction is stronger at this time [14]. Therefore, the gene expression changes in A. brasilense associated with each microalga were evaluated through a microarray-based transcriptome at 72 h.
2.3. Microarray and Data Analyses
The transcriptome analysis was performed with a custom A. brasilense gene expression microarray [12] that targets the main pathways involved in A. brasilense metabolism under non-oxygen conditions (e.g., energy processes, nitrogen fixation, transport, and motility). Ribonucleic acid (RNA) extraction, sample preparation, microarray hybridization, and data analysis were performed as previously reported [12]. The present study considered upregulated (positive z-score) and downregulated (negative z-score) genes when a z-score ≥ 1.5 or ≤−1.5 was observed. Overlapping differentially expressed genes (DEGs) were identified at the different treatments using a Venn diagram tool (https://bioinformatics.psb.ugent.be/webtools/Venn/).
Pathway Enrichment Analyses and qRT-PCR Validation
Functional assignment and pathway enrichment analysis of the DEGs were performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthology-Based Annotation System (KOBAS v.3.0; A. brasilense sp7 GenBank assembly accession: GCA_001315015.1 Complete Genome, BioProject: 293508) bioinformatics tool [15] as described previously [12]. Significance was calculated by Fisher’s exact test with a false discovery rate (FDR)-corrected p-value < 0.05. Data and visualization analyses were performed in a bioinformatics mapping platform (http://www.bioinformatics.com.cn/). The DEGs were validated by performing a quantitative real-time PCR analysis (qRT-PCR) of the selected genes (primer sequences are listed in Table 1) as described by Garciglia-Mercado et al. [12]. The statistical analyses were made by a two-way analysis of variance (ANOVA) and Fisher’s least significant differences (LSD) post hoc test (p < 0.05).

Table 1.
List of primers used for quantitative reverse transcription polymerase chain reaction (qRT-PCR) validation of differentially expressed mRNAs in the current study.
2.4. Bacterium A. brasilense Effect on Microalgae Cell Density
To evaluate the effect of A. brasilense on cell density and CO2 biogas fixation of the microalgae C. sorokiniana and Scenedesmus sp., the setup of the experiments was a separate culture of C. sorokiniana and Scenedesmus sp. growing alone (control) and associated with the bacterium A. brasilense (C. sorokiniana–A. brasilense and Scenedesmus sp. –A. brasilense; treatments) supplied with air or synthetic biogas.
2.4.1. Counting Microorganisms
Microalga cell density was determined each 24 h by cell count with a Neubauer hemocytometer under a light microscopy (Olympus BX40, Tokyo, Japan). Samples of one mL of culture were directly taken. Growth rate (µ) was calculated by the following formula:
where N is the number of cells at initial (ti) and final (tf) sampling time. Biomass production (X; g L−1) was measured by dry weight at the beginning and end of experimental time (72 h); fifty mL of suspension culture were centrifuged at 6800× g for 10 min. Then, the pellet was washed thrice with 20 mL of distilled water and dried at 80 °C in a HERA thermal oven, Model OGS100 (Waltman, MA, USA) for 24 h. Biomass productivity (P; g L−1 d−1) was calculated according to Equation (2):
where X is biomass concentration at the beginning (ti) and end (tf) of experimental time. The bacterium A. brasilense was counted after serial dilution by the plate count method on Congo red solid medium [19].
2.4.2. Determination of CO2 Fixation Rate
The CO2 fixation rate (RCO2 in g L−1 d−1) was determined by Equation (3), according to Barbosa-Nuñez et al. [7] considering the molecular formula of microalgal biomass, CO0.48H1.83N0.11P0.01 [20].
where P is biomass productivity and Cc is the carbon content of the microalgal cell; Mc is carbon molecular weight, and MCO2 the molecular weight of CO2; pH in the culture medium was determined with a pH meter (Thermo-Orion Model 720A, MA, USA).
2.4.3. Visualization by Scanning Electron Microscopy (SEM)
At the end of experimentation time (72 h), 20 mL of each culture in suspension was centrifuged at 6800× g for 5 min; the pellet was washed twice with distilled water and then lyophilized. The samples were visualized in a high resolution (1 nm for high vacuum) scanning electron microscope: TESCAN-MIRA 3 LMU (Czech Republic). Each sample was exposed to gold for 30 s with a power of 10 kV and then analyzed with a working plan of 15 mm and magnifications of 5.000X–20.000X.
2.4.4. Experimental Designs and Statistical Analyses
Each experiment was performed in triplicate and repeated thrice. Data from each treatment from the three replicates were combined for analyses, first by one-way analysis of variance (ANOVA) and then by least significant difference (LSD) post hoc analysis with significance set at p < 0.05 using Statistica 6.0 software (StatSoft, Tulsa, OK, USA).
3. Results
3.1. Genes Differentially Expressed in Azospirillum brasilense Interacting with Microalgae
Utilizing a microarray-based gene expression analysis, DEGs in A. brasilense co-cultured in suspension with Scenedesmus sp. (A.b + S) Chlorella sorokiniana (A.b + C) under a biogas atmosphere were identified with air as control.
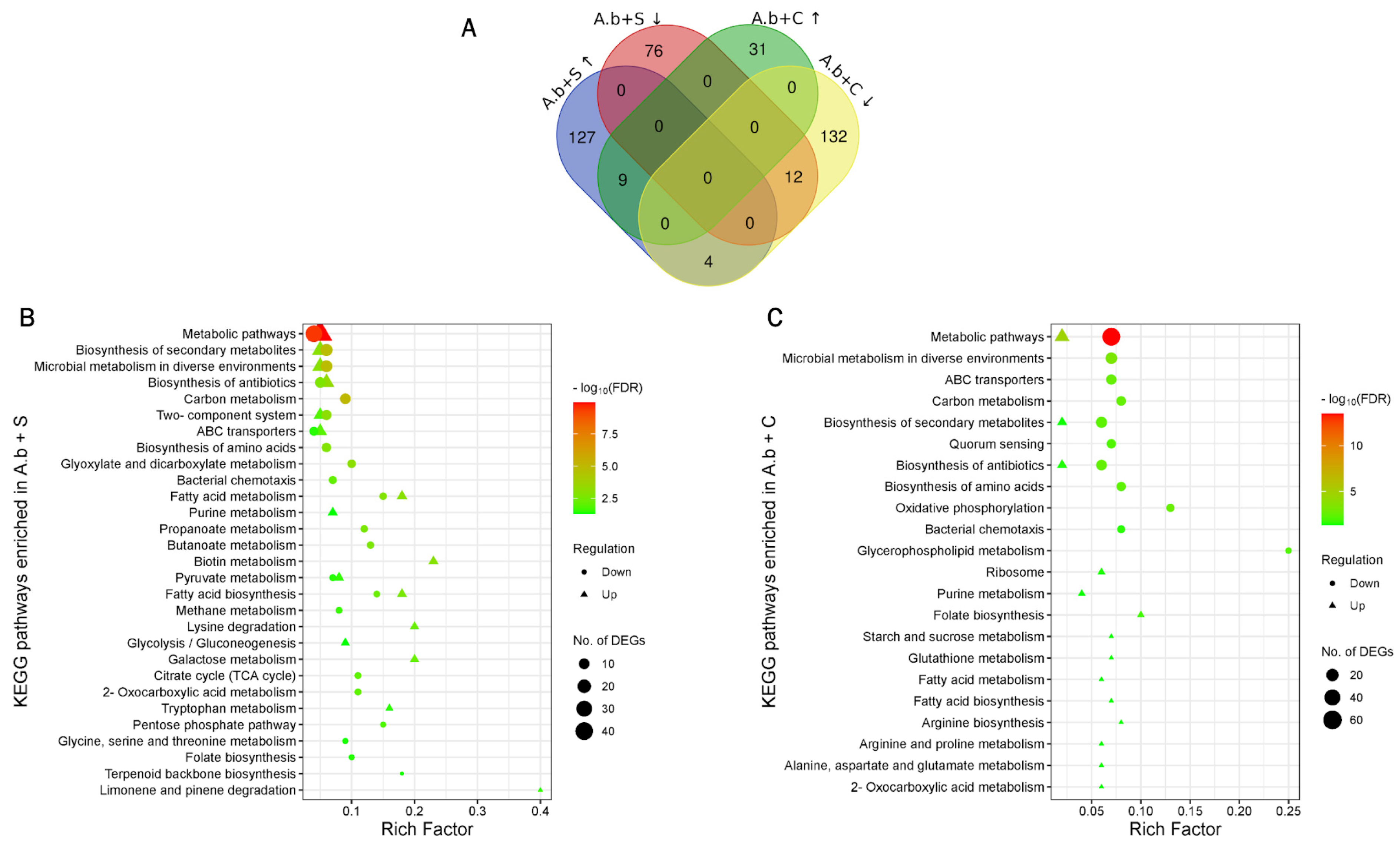
Overall, 416 DEGs were found in both treatments: 140 upregulated and 88 downregulated after A.b + S treatment, and 40 upregulated and 148 downregulated after A.b + C treatment (Supplementary Table S1). A.b + S treatment led to a higher number of DEGs than A.b + C. Moreover, more genes were upregulated in A.b + S and downregulated in A.b + C. Interestingly, only 25 DEGs were shared among treatments (Figure 1A).
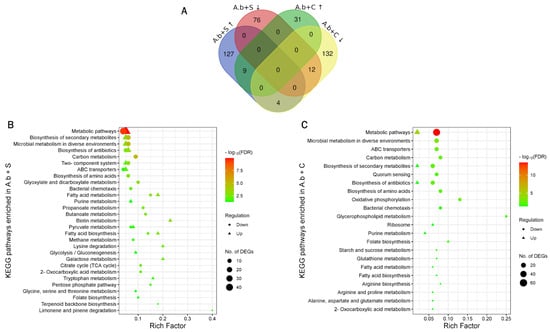
Figure 1.
Identification of differentially expressed genes (DEGs) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis in Azospirillum brasilense growing under biogas co-cultured in suspension with the microalgae Scenedesmus sp. (A.b + S) and Chlorella sorokiniana (A.b + C) at 72 h compared to an air atmosphere. (A) Venn diagram highlighting the overlap between DEGs in A.b + S and A.b + C. Upward arrows denote upregulated DEGs, whereas downward arrows denote downregulated DEGs. Scatter plot of the enriched KEGG pathways in (B) A.b + S and (C) A.b + C. Rich factor is the ratio of the number of DEGs to the total gene number in a certain pathway. Dots and triangles represent the downregulated and upregulated genes, respectively. The color and size of dots and triangles represent the range of −log10(FDR) corrected p-value < 0.05, and the number of DEGs mapped to the indicated pathways, respectively.
3.2. Functional Annotation and Enrichment Analyses
Functional annotation and identification of significantly enriched pathways among these DEGs allowed understanding their biological role in A. brasilense under both treatments. In total, 38 and 25 pathways were identified as significantly enriched for A.b + S and A.b + C treatments, respectively (Supplementary Table S2). In A.b + S, 16 pathways were enriched in positive regulation of amino acid metabolism (e.g., lysine degradation and tryptophan metabolism), carbohydrate metabolism (e.g., glycolysis/gluconeogenesis, galactose metabolism, and pyruvate metabolism), signal transduction (e.g., two-component system), membrane transport (e.g., ABC transporters), and lipid metabolism (e.g., fatty acid biosynthesis and metabolism). Biotin metabolism and limonene and pinene degradation were the most highly enriched in this treatment (Rich Factor > 0.20 and corrected p-value < 0.05). These pathways included DEGs coding for several hydro-lyases, acyltransferases, oxidoreductases, and dehydrogenases. In negative regulation, 22 pathways were enriched, including carbohydrate metabolism (e.g., pyruvate metabolism, butanoate metabolism, propanoate metabolism, etc.), energy-generating processes (e.g., methane metabolism), metabolism of terpenoids and polyketides (e.g., terpenoid backbone biosynthesis), metabolism of cofactors and vitamins (e.g., folate biosynthesis), signal transduction (e.g., two-component system), membrane transport (e.g., ABC transporters), and cell motility (e.g., bacterial chemotaxis) (Figure 1B). Under the A.b + C treatment, 14 pathways were enriched in positive regulation of amino acid metabolism (arginine, proline, alanine, aspartate and glutamate metabolism, and arginine biosynthesis), carbohydrate metabolism (starch and sucrose metabolism), metabolism of cofactors and vitamins (folate biosynthesis), and lipid metabolism (fatty acid biosynthesis and metabolism). In comparison, 11 pathways were enriched in negative regulation of cellular community and motility (quorum sensing and bacterial chemotaxis) and membrane transport (ABC transporters). The highest enriched pathways in this treatment were oxidative phosphorylation and glycerophospholipid metabolism (Rich Factor > 0.10 and corrected p-value < 0.05) with DEGs coding for type 1 NAD(P)H-quinone oxidoreductase subunits, cytochrome c oxidases, succinate dehydrogenases, the ATPase complex, and transferases, lysophospholipases, acyltransferases, and dehydrogenases, respectively (Figure 1C). Finally, among the 25 DEGs shared in both treatments, the tyrosine metabolism pathway was the most enriched (Rich Factor = 0.22 and corrected p-value < 0.05) (Figure 1A).
3.3. qRT-PCR Validation of Gene Expression Patterns
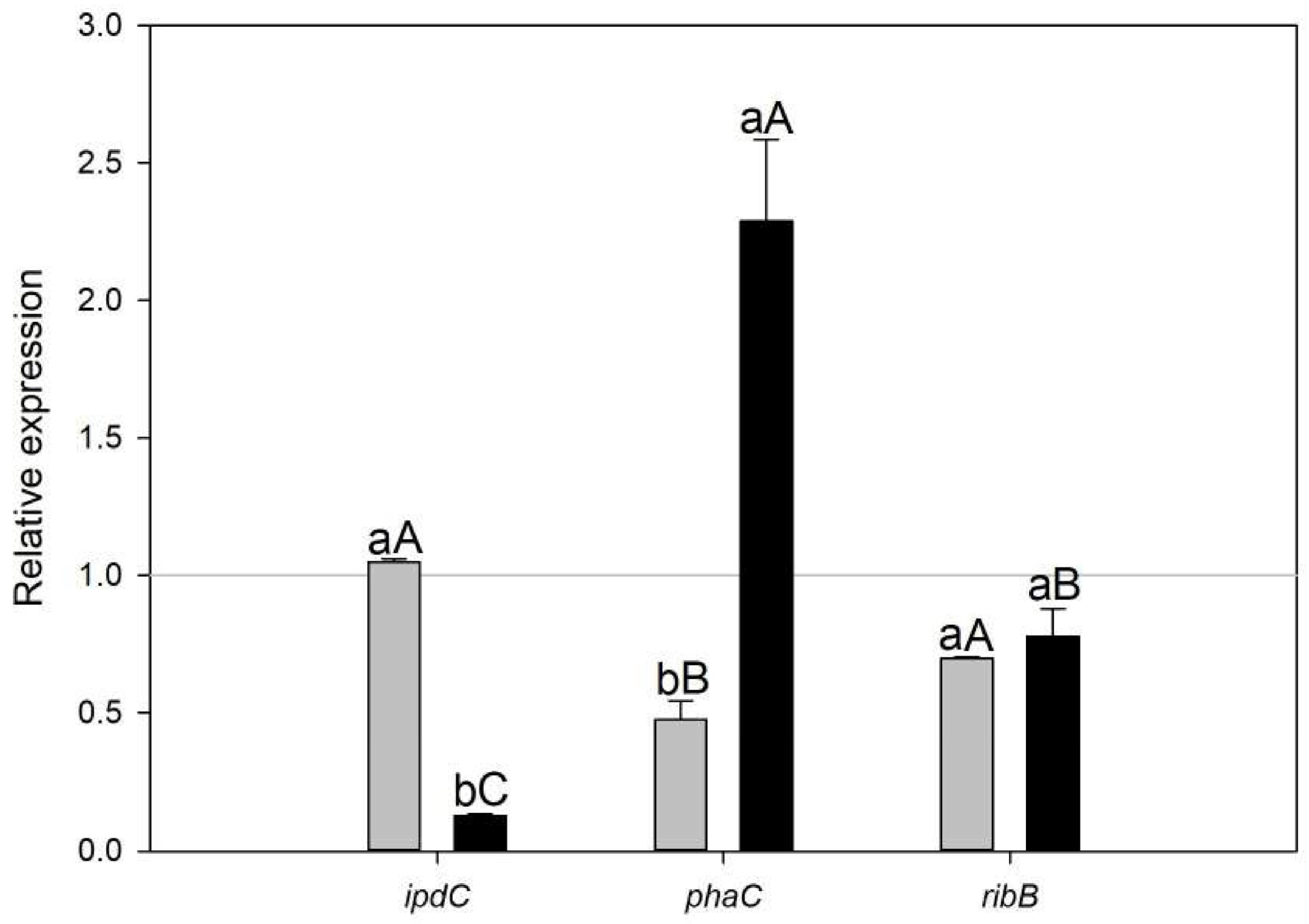
Quantitative real-time PCR (qRT-PCR) was performed for three selected genes to validate the expression patterns observed in the microarray-based transcriptome analysis. The qRT-PCR results showed a consistent trend for the assessed genes, confirming the reliability of the microarray data. The expression levels were significantly different (p < 0.05) between treatments for ipdC and phaC (Figure 2, lower letters) and between genes in A.b + C treatment (Figure 2, capital letters).
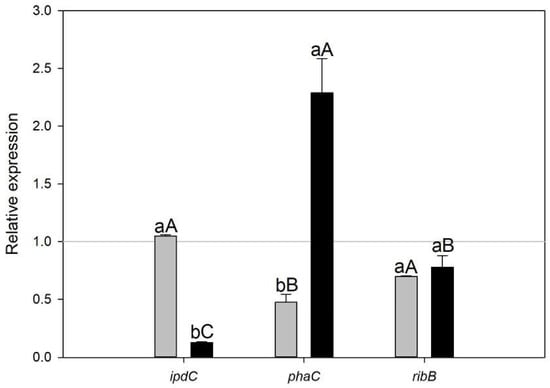
Figure 2.
Relative expression of ipdC, phaC, and ribB transcripts in Azospillum brasilense growing under biogas co-cultured with the microalgae Scenedesmus sp. (A.b + S, gray) and Chlorella sorokiniana (A.b + C, black) was assessed at 72 h and compared to an air atmosphere (=1). Data points represent the mean, and whisker lines represent standard error (SE), n = 3. Different lower letters between treatments in the same gene and different capital letters between genes in the same treatment indicate significant differences in relative expression after analysis of variance (ANOVA) and Fisher’s Least Significant Difference (LSD) post hoc test (p < 0.05).
3.4. Cell Density of C. sorokiniana and Scenedesmus sp. associated with A. brasilense
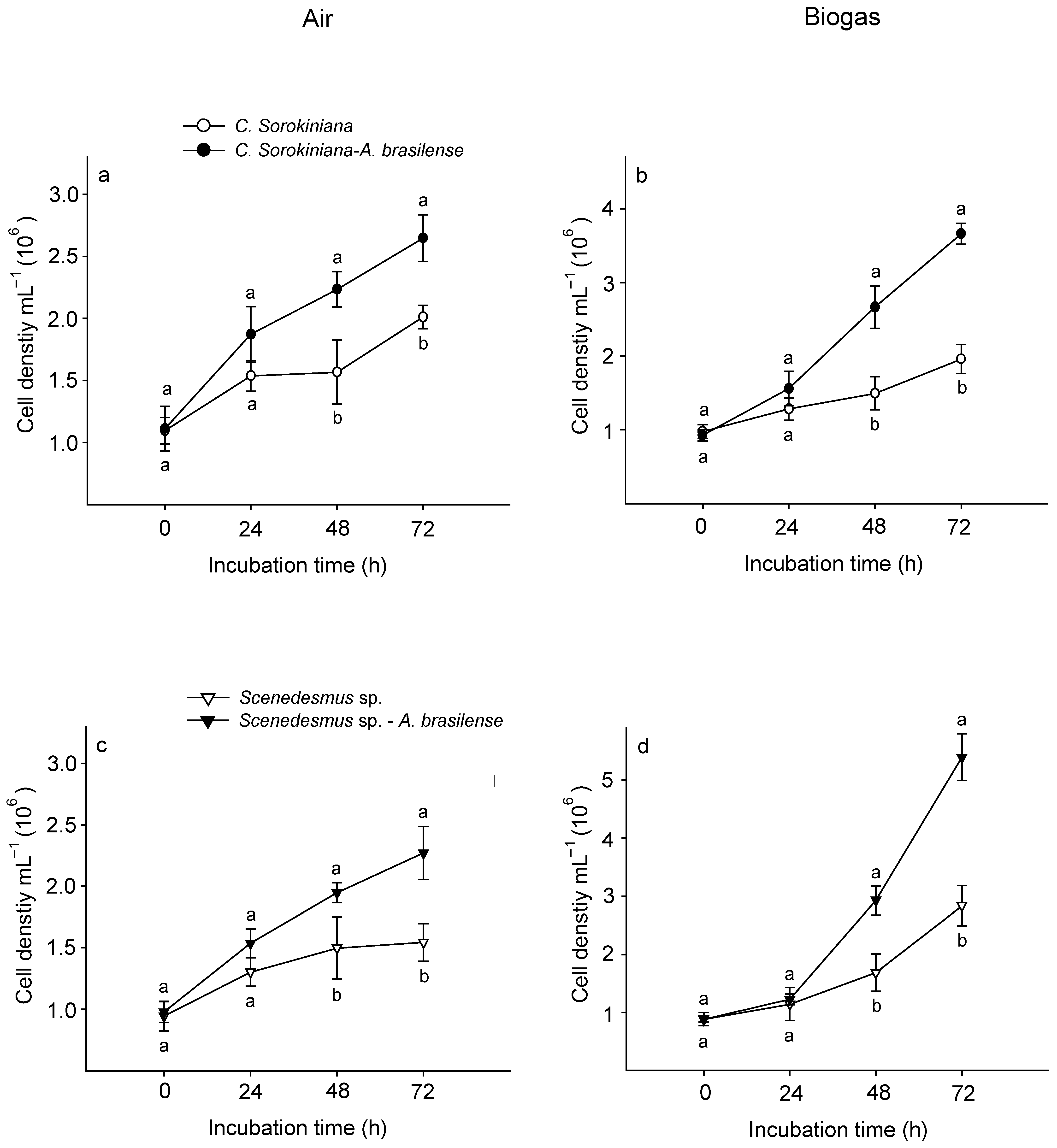
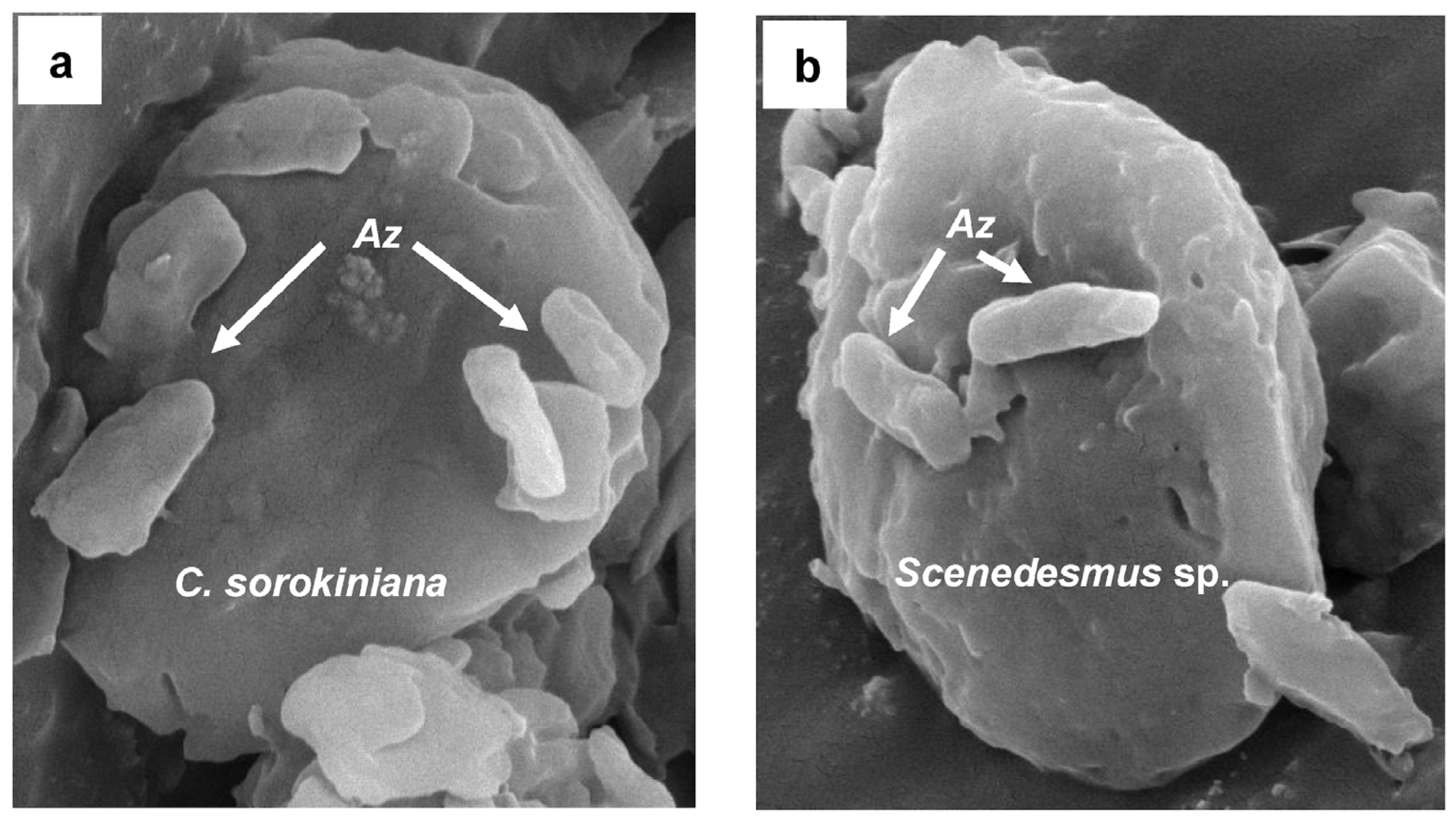
In both treatments (air and biogas), the microalga C. sorokiniana showed a significantly higher growth co-cultured with A. brasilense than growing alone (Figure 3a,b). Supplied with air, this microalga recorded a cell density of 2.6 × 106 ± 0.2 cel mL−1 at the end of experimental time (72 h) (Figure 3a). Whilst when supplied with biogas it attained 3.7 × 106 ± 0.3 cel mL−1 (Figure 3b). Similarly, at 72 h Scenedesmus sp. co-cultured with this bacterium reached 2.2 × 106 ± 0.2 and 5.3 × 106 ± 0.4 cel mL−1 supplied with air and biogas, respectively (Figure 3c,d). At this time, A. brasilense maintained a physical affinity with each microalga supplied with biogas (Figure 4). Similarly, CO2 fixation of biogas and growth rates were also significantly higher when both microalgae were interacting with A. brasilense than growing alone (Table 2).
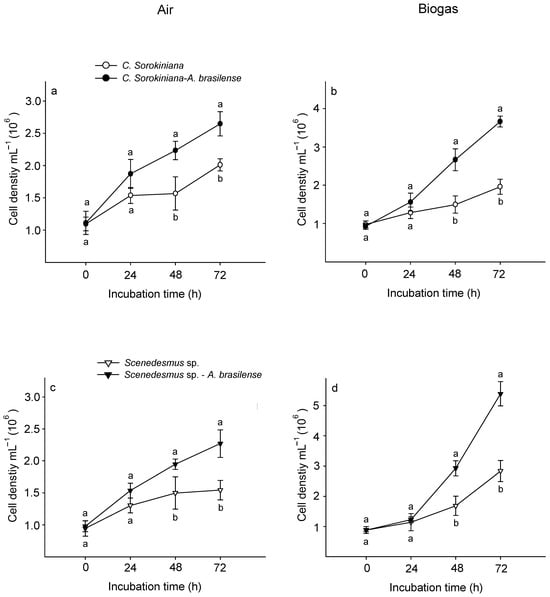
Figure 3.
Cell density of Chlorella sorokiniana (a,b) and Scenedesmus sp. (c,d) growing alone and associated with Azospirillum brasilense supplied with air or biogas. Different lowercase letters in each time interval indicate significant differences when each microalga was growing alone or associated with the bacterium least significant difference (LSD post hoc analysis at p < 0.05). Bars represent standard error.
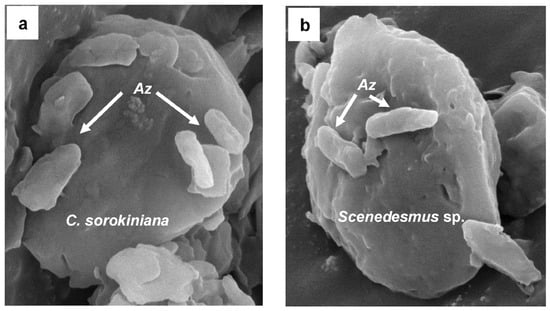
Figure 4.
Photograph by scanning electron microscope (SEM) of the bacterium Azospirillum brasilense attached with the microalga Chlorella sorokiniana (a) and Scenedesmus sp. (b) growing under biogas.

Table 2.
CO2 fixation rate, growth rate, and biomass productivity of Chlorella sorokiniana and Scenedesmus sp. interacting with Azospirillum brasilenese.

Table 3.
Colony forming unit of Azospirillum brasilense growing alone or co-cultured with Chlorella sorokiniana or Scenedesmus sp.
4. Discussion
The viability of the multiple growth-promoting mechanisms of Azospirillum is essential to boost the physiological performance of microalgae during each biotechnological process. The present study evaluated the genetic modulation of the growth-promoting mechanisms of this bacterium to maintain its synergic association with microalgae supplied with biogas. Thus, the aim was to determine A. brasilense transcriptomic response through a microarray-based approach separately co-cultured in suspension with Scenedesmus sp. and Chlorella sorokiniana microalgae during CO2 fixation of biogas.
The interaction between microalgae and A. brasilense has been studied with various species of microalgae, revealing different effects of this bacterium depending on the microalgae species. Under nitrogen limitation, Scenedesmus sp. exhibits increased growth and fatty acid production when interacting with A. brasilense [21]. Nonetheless, when this bacterium interacted with Chlorella species under nitrogen limitation, it showed growth promotion of the microalgae; however, its effect on lipid production by the microalgae was not evident [22]. Moreover, their impact is more noticeable on starch accumulation. The results demonstrated that A. brasilense had the ability to modulate several metabolic pathways to establish a synergic interaction with both microalgae. The interaction between A. brasilense and Scenedesmus sp. or C. sorokiniana induces the production of different vitamins, such as biotin and riboflavin, respectively. Biotin is a well-recognized cofactor of carboxylase enzymes, and riboflavin is a precursor of flavin cofactors, which are essential for basic energy metabolism for electron transport. Both vitamins can induce higher growth of microalgae, but they affect energy metabolism at different levels.
The primary mechanism that sustains the mutualistic interaction between microalgae and Azospirillum is the metabolite exchange that acts as signal molecules. In particular, A. brasilense produces and releases phytohormone indole-3-acetic acid (IAA) by utilizing the tryptophan (Trp) and thiamine released by the microalga. Additionally, A. brasilense also produces riboflavin and its degradation compound, lumichrome, stimulating the microalgal metabolism and the induction of stress tolerance [3,13].
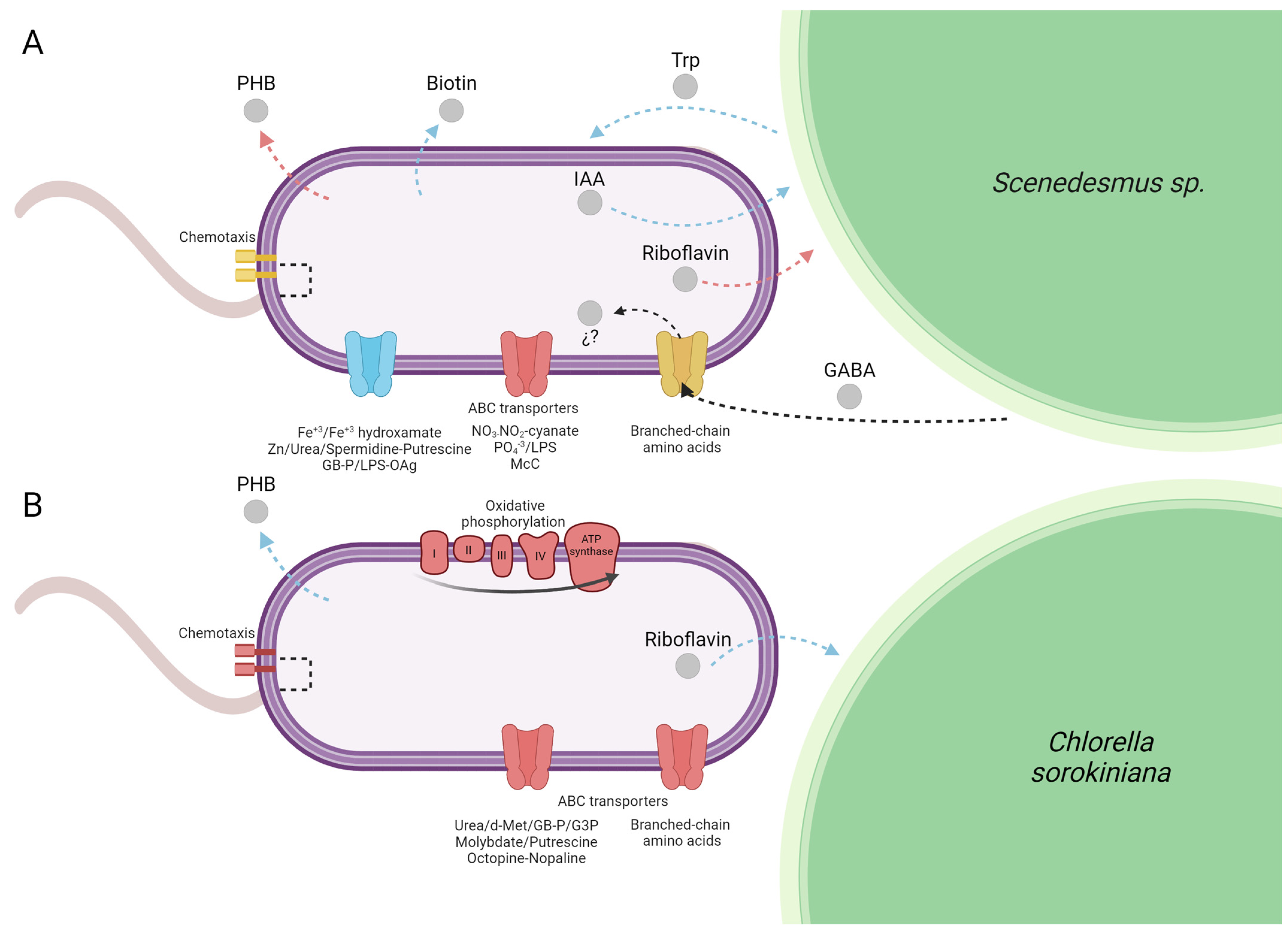
In the present study, the principal enzyme activity of the Trp-dependent indole-3-pyruvic acid (IPA) pathway and the main route for IAA biosynthesis, indole-3-pyruvate decarboxylase (encoded by the gene ipdC, AMK58_11540) was more elevated in A.b + S (z-score = 0.758) than in A.b + C (z-score = 0.113). This expression pattern was also confirmed by RT-qPCR (Figure 2). Moreover, enrichment in positive regulation of the tryptophan metabolism pathway in A.b + S (Rich Factor = 0.16 and corrected p-value < 0.05) likely contributed to the observed differences between treatments since Trp is a precursor of IAA biosynthesis by A. brasilense (Supplementary Table S2). This finding supports the metabolic exchange of IAA and Trp between A. brasilense and Scenedesmus sp., respectively (Figure 5A).
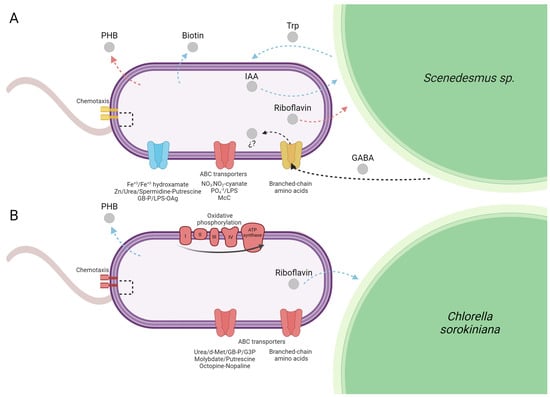
Figure 5.
Overview of the major Azospirilum brasilense pathways and functions differentially expressed growing under biogas co-cultured in suspension with microalgae at 72 h compared to an air atmosphere. (A) Scenedesmus sp. (A.b + S) and (B) Chlorella sorokiniana (A.b + C). The color of the figures and arrows represent a general expression of the pathway or function: blue for upregulation, red for downregulation, and yellow for differentially expressed genes (DEGs), both upregulated and downregulated. Gray circles represent metabolites and signal molecules. The question mark indicates that the role of GABA in A. brasilense fitness remains unclear. Created with BioRender.com.
On the other hand, the gene coding for the riboflavin biosynthetic enzyme, 3,4-dihydroxy-2-butanone-4-phosphate synthase (AMK58_08300, ribB), was transcriptionally elevated in A.b + C (z-score = 0.187) and repressed in A.b + S (z-score = −0.716). The above can be attributed to biogas atmosphere since it can impose a stressful condition, while the riboflavin production is essential as a cofactor in the antioxidation and peroxidation metabolism helping to mitigate the osmotic and environmental stress [3]. Consequently, A. brasilense may upregulate ribB co-cultured with C. sorokiniana to cope with oxidative stress induced by biogas atmosphere (Figure 5B). Similarly, the metabolism of biotin production was one of the most highly enriched pathways in A.b + S (Rich Factor > 0.20 and corrected p-value < 0.05) with upregulation of three enzymes (AMK58_07400, FabF; AMK58_26095, FabG; AMK58_06305, FabZ) involved in the elongation of fatty acids, which are precursors for biotin novo synthesis [23]. Nowadays, several studies demonstrate that the exogenous supply of vitamins, such as biotin, thiamine, and cobalamin, enhance the physiological performance of microalgae [24,25]. These results indicate A. brasilense transcriptomic capacity to genetically modulate its growth-promoting mechanisms by stimulating a synergic association establishment with microalgae supplied with biogas.
The above can be corroborated through A. brasiliense secondary metabolites since it accumulates polyhydroxybutyrate (PHB) as a carbon and energy storage metabolite in response to various stress factors [26,27]. During the mutualistic microalgal–bacterial consortium, A. brasilense can also uptake the extracellular organic carbon released from the microalgae photosynthesis or metabolism of starch reserves. However, according to Palacios et al. [3], the type and quantity of these exuded carbon compounds differ even at strain level. For instance, two strains of C. sorokiniana (UTEX 2714 and UTEX 2805) release myo-inositol and lactate, but only one (UTEX 2714) exuded glycerol when grown with A. brasilense in an oligotrophic medium. Such variations in exudate profiles can define the affinity and establishment of the microalgal–bacterial associations [2]. In the present study, A. brasilense showed greater transcriptional modulation of growth-promoting mechanisms when associated with Scenedesmus sp., a trend that aligned with higher CO2 fixation and cell density observed in this microalga compared to C. sorokiniana. Although the specific composition of exudates from C. sorokiniana and Scenedesmus sp. was not analyzed in the present study, future studies will address this aspect in greater detail.
Furthermore, the co-culture conditions induced by biogas characterized by elevated carbon availability, nitrogen limitation (high C/N ratio), and low oxygen levels favored the synthesis and intracellular accumulation of PHB in A. brasilense when interacting with both microalgal species [28,29,30]. These findings highlight the importance of characterizing each microbial consortium under specific bioprocess conditions.
Under the experimental conditions of the present study, the transcriptomic and RT-qPCR results showed the upregulation of the gene involved in (R)-3-Hydroxybutanoyl-CoA conversion to PHB (AMK58_06740, phaC) in A.b + C (Figure 2 and Figure 5B). These results suggest that besides the carbon oversupply from continuous CO2 transfer from biogas to culture media, the constant exogenous carbon source provided by C. sorokiniana allows A. brasilense to sustain the synthesis and accumulation of PHB, even in the stationary phase, to store excess carbon for future periods of starvation, particularly under fluctuating environmental conditions. In contrast, in A.b + S, the butanoate metabolism pathway was enriched in negative regulation (Rich Factor = 0.13 and corrected p-value < 0.05). Since PHB is a metabolite in the butanoate metabolism, the transcriptomic analysis also showed the downregulation of the phaC gene, which was further confirmed by RT-qPCR (Figure 2 and Figure 5A). Recently, Garciglia-Mercado et al. [12] demonstrated an increased expression of the phaC gene at 24 h and a decrease at 72 h in A. brasilense growing alone under biogas, implying PHB accumulation at the exponential phase followed by PHB mobilization at the stationary phase. Therefore, this shift from PHB accumulation to its utilization on A.b + S could indicate that the external carbon sources provided by Scenedesmus sp. might be intermittent or insufficient, prompting A. brasilense to degrade stored PHB to meet its metabolic needs. However, this hypothesis deserves further investigation.
Likewise, nutrient exchange in A. brasilense energy-generating processes, such as micro and macronutrients, CO2, vitamins, and phytohormones, are essential for the mutualistic interaction between microalgae and bacteria. In the present study, two enzymes involved in formaldehyde conversion to CO2 via tetrahydromethanopterin (H4MTP) pathway were downregulated in A.b + S; a methenyl-H4MPT cyclohydrolase (AMK58_22330, Mch) that hydrolyses 5,10-Methylenyl-H4MPT to N5-formyl-H4MPT and a formate dehydrogenase (FDH) that catalyze oxidation to CO2 (AMK58_26610, FdhA) [31,32,33]. Since CO2 production at the stationary phase (72 h) stabilizes as growth rates plateau due to nutrient limitations or accumulation of waste products, enzyme downregulation was expected to be involved in CO2 production by intracellular oxidation.
Moreover, several enzymes involved in oxidative phosphorylation in A.b + C were downregulated. This finding aligns with a recent study that observed a reduction in the expression of various enzymes involved in the phosphorylation process in A. brasilense under a biogas atmosphere [12]. This reduction is likely due to the different affinities of various oxidases to oxygen [31]. Nevertheless, the reduced phosphorylation activity is balanced by alternative energy production pathways like nitrate/nitrite reduction and nitrogen fixation [33]. Similarly, γ-aminobutyric acid (GABA) is a non-protein amino acid broadly distributed in prokaryotic and eukaryotic organisms as a metabolic product with multi-physiological functions [34,35]. In microalgae, GABA has been shown to act as a pH regulator and improve lipids, astaxanthin, and biomass production by regulating ROS-scavenging system and carbon/nitrogen metabolism [36,37]. Particularly in the green microalgae Monoraphidium sp., Chlorella sp., and Scenedesmus sp., in which GABA accumulation is involved in the physiological adaptation mechanisms by which microalgae responds to and tolerates different stressors, such as cadmium [38,39], salinity [36], and cold [40]. In microbe–host interactions, GABA is used for nutrients or as interspecies signals [41,42]. The uptake of GABA into bacterial cells involves its transport through periplasmic binding proteins (PBPs) that bring GABA to the appropriate transporters and later to the cytoplasm [43].
In plant-growth-promoting rhizobacteria Rhizobium leguminosarum and soil pathogen Agrobacterium tumefaciens, these GABA transporters belong to ATP-binding cassette (ABC) transporters [40]. Although GABA function in Rhizobium is yet unclear, in A. tumefaciens, GABA modulates the severity of the symptoms provoked on the plant host and limits the progression of a quorum-sensing signal infection, reducing this pathogen virulence [43,44]. Recently, livF and livK genes of liv operon (livKHMGF) encoding for an ABC transporter have been reported as involved in the branched-chain amino acid selective stimulated transport in rhizosphere soil microbiomes, as a response to GABA release by plants to regulate decomposition to succinic semialdehyde (SSA), later oxidized to succinate to feed into the TCA cycle [44]. In this study, several genes from the liv operon were modulated in A.b + S, which participates in both quorum-sensing and ABC transporter pathways. Alternatively, this operon was downregulated in A.b + C (Supplementary Table S2). These findings suggest the possible GABA release by Scenedesmus sp. and A. brasilense uptake and transport as a nitrogen feedstock and quorum-sensing signal molecule, adjusting gene expression accordingly (Figure 5A). However, the role of GABA in A. brasilense fitness is still unclear and remains to be experimentally demonstrated.
In the present study, A. brasilense also showed physical attachment to the phycosphere of each microalga, since the nutrients and organic carbon present in this microenvironment act as signal molecules stimulating bacterial chemotaxis [7], the latter of which allowed higher CO2 fixation rate and biomass productivity attained by the two microalgae co-cultured with A. brasilense. As well-established, chemotaxis signal transduction controls the polar flagellum rotation, thus, the swimming pattern in A. brasilense is principally through two chemotaxis systems (Che1 and Che4) [41,42,43]. Additionally, A. brasilense genome encodes two chemosensory-like (Che2 and Che3) systems that control flagella biosynthesis in Rhodospirillum centenum and flocculation in A. brasilense, respectively [44]. Similarly, as in quorum sensing, the genesis modulation observed in A.b + S is involved in the chemotaxis systems Che2 and Che4 (upregulation of AMK58_02410, cheY4; AMK58_13775, mcp; AMK58_19980, mcp; AMK58_23635, cheW2, and downregulation of AMK58_23615, cheY2; AMK58_14210, mcp; AMK58_04635, mcp; AMK58_04405, mcp; AMK58_25380, motB, and AMK58_13745, cheY7) while several genes were downregulated in A.b + C (Supplementary Table S2). Besides the principal Che4 system and consistent with previous findings, Che2 appears to be required to maintain cell motility processes in A. brasilense under a biogas atmosphere [12] and mainly favored by chemoattractants or signal molecules exuded by Scenedesmus sp., inducing its physical association with each microalga.
5. Conclusions
Overall, the present study proves that A. brasilense growing under biogas co-cultured with Scenedesmus sp. and Chlorella sorokiniana successfully adapts and maintains its mutualistic interaction. Transcriptomic evidence demonstrated signal molecule modulation, such as IAA, riboflavin, and biotin, in A. brasilense during co-cultivation with both microalgae. During Scenedesmus sp.–Azospirillum mutualistic interaction, the activation of transporters for quorum-sensing molecule GABA and Che2 pathway were observed supporting cell motility. Conversely, the synthesis and PHB accumulation were sustained in C. sorokiniana–Azospirillum by the exogenous carbon source provided by this microalga. The present findings suggest that A. brasilense metabolic adaptation was mainly favored during the mutualistic interaction with Scenedesmus sp. Finally, the microalga–Azospirillum consortium biotechnological potential is demonstrated as an environmentally sustainable strategy improving the bio-refinery capacity of microalgae and valorizing CO2 from biogas.
6. Future Perspectives
Future studies could extend the experimental design to include a greater number of biological replicates, thereby enhancing the reliability of differential gene expression detection, particularly for genes showing subtle changes or borderline significance. Additionally, incorporating complementary RNA-Seq analyses would enable the exploration of novel transcripts and provide further validation of the present microarray-based findings. Expanding qRT-PCR validation to a broader range of differentially expressed genes across diverse functional categories should also strengthen the conclusions drawn. Moreover, while transcriptomic profiling offers valuable insights into potential metabolic interactions within the microalgal–Azospirillum consortium, direct metabolomic evidence of compound-level exchanges remains to be confirmed, since the rapid synthesis and immediate assimilation of these metabolites pose significant detection challenges. Addressing these aspects will deepen understanding of the molecular mechanisms underlying this mutualistic interaction and support its biotechnological application in CO2 biogas valorization.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pr13072177/s1, Supplementary Table S1. List of differentially expressed Azospirillum brasilense genes growing under biogas co-cultured in suspension with the microalgae Scenedesmus sp. (A.b + S) and Chlorella sorokiniana (A.b + C) at 72 h compared to an air atmosphere. Differential expression testing was performed using genArise software. Genes were considered significantly differentially expressed between biogas and air atmosphere conditions when they showed a z-score ≥ 1.5 or ≤−1.5. Supplementary Table S2. List of enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in Azospirillum brasilense growing under biogas co-cultured in suspension with microalgae Scenedesmus sp. (A.b + S) and Chlorella sorokiniana (A.b + C) at 72 h compared to an air atmosphere. Input refers to the number of differentially expressed genes (DEGs) mapped to the indicated pathway; background number is the total number of genes in a specific pathway; and the rich factor is the ratio of the number of DEGs to the total gene number in a specific pathway. Pathways were considered significantly enriched with false recovery rate (FDR)-corrected p-value < 0.05.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, experimental and microarray designs, and the first draft were performed by C.G.-M.; C.A.C.-G. contributed to data acquisition and analyses; J.R.T.-V. contributed to data analyses and discussion of the final draft; O.A.P. contributed to the experimental design, analysis of results, and discussion; F.J.C. contributed to financial support and the design of the experiment. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by CONAHCYT-Frontiers of Science 2019 Project 15769.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Francisco J. Choix acknowledges SECIHTI (Secretaría de Ciencia, Humanidades, Tecnología e Innovación, Mexico) for the support under the Program-Project 90 Catedras CONAHCYT, as well as CONAHCYT-Frontiers of Science 2019 Project 15769; CIBNOR (Centro de Investigaciones Biológicas del Noroeste, S.C.) technical support of Julio Antonio Hernandez; Lorena Chavez Gonzalez, Simon Guzman Leon, and Jorge Ramirez for technical assistance in the microarray determinations; Diana Fischer for English edition.
Conflicts of Interest
The authors have no relevant financial or non-financial interest to disclose.
References
- Cassan, F.; Coniglio, A.; Lopez, G.; Molina, R.; Nievas, S.; de Carlan, C.L.N.; Torres, D.; Rosas, S.; Pedrosa, F.O.; de Souza, E.; et al. Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Pereg, L.; de-Bashan, L.E.; Bashan, Y. Assessment of affinity and specificity of Azospirillum for plants. Plant Soil. 2016, 399, 389–414. [Google Scholar] [CrossRef]
- Palacios, O.A.; López, B.R.; de-Bashan, L.E. Microalga Growth-Promoting Bacteria (MGPB): A formal term proposed for beneficial bacteria involved in microalgal–bacterial interactions. Algal Res. 2022, 61, 102585. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Mayali, X.; Bebout, B.M.; Weber, P.K.; Detweiler, A.M.; Hernandez, J.P.; Prufert-Bebout, L.; Bashan, Y. Establishment of stable synthetic mutualism without co-evolution between microalgae and bacteria demonstrated by mutual transfer of metabolites (NanoSIMS isotopic imaging) and persistent physical association (Fluorescent in situ hybridization). Algal Res. 2016, 15, 179–186. [Google Scholar] [CrossRef]
- Contreras, C.A.; Palacios, O.A.; de-Bashan, L.E.; Choix, F.J. Microalga Growth-Promoting Bacteria as Strategy to Improve CO2 Removal from Biogas. BioEnergy Res. 2024, 17, 2082–2099. [Google Scholar] [CrossRef]
- Ruiz-Güereca, S.S.; Sánchez-Saavedra, M.P. Growth and phosphorus removal by Synechococcus elongatus coimmobilized in alginate beads with Azospirillum brasilense. J. Appl. Phycol. 2016, 28, 1501–1507. [Google Scholar] [CrossRef]
- Barbosa-Nuñez, J.A.; Palacios, O.A.; Mondragón-Cortez, P.; Ocampo-Alvarez, H.; Becerril-Espinosa, A.; Nevárez-Moorillón, G.V.; Choix, F.J. Chemical and physical affinity of microalga—Azospirillum consortium co-cultured in suspension during CO2 fixation from biogas. Bioenergy Res. 2022, 16, 579–592. [Google Scholar] [CrossRef]
- Lopez, B.R.; Bashan, Y.; Trejo, A.; de-Bashan, L.E. Amendment of degraded desert soil with wastewater debris containing immobilized Chlorella sorokiniana and Azospirillum brasilense significantly modifies soil bacterial community structure, diversity, and richness. Biol. Fert. Soils 2013, 49, 1053–1063. [Google Scholar] [CrossRef]
- Mulu, E.; M’Arimi, M.M.; Ramkat, R.C. A review of recent developments in application of low cost natural materials in purification and upgrade of biogas. Renew. Sust. Energ. Rev. 2021, 145, 111081. [Google Scholar] [CrossRef]
- Bose, A.; Lin, R.; Rajendran, K.; O’Shea, R.; Xia, A.; Murphy, J.D. How to optimize photosynthetic biogas upgrading: A perspective on system design and microalgae selection. Biotechnol. Adv. 2019, 37, 107444. [Google Scholar] [CrossRef]
- Solovchenko, A.; Khozin-Goldberg, I. High-CO2 tolerance in microalgae: Possible mechanisms and implications for biotechnology and bioremediation. Biotechnol. Lett. 2013, 35, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Garciglia-Mercado, C.; Contreras, C.A.; Choix, F.J.; de-Bashan, L.E.; Gomez-Anduro, G.A.; Palacios, O.A. Metabolic and physiological adaptations of microalgal growth-promoting bacterium Azospirillum brasilense growing under biogas atmosphere: A microarray-based transcriptome analysis. Arch. Microbiol. 2024, 206, 173. [Google Scholar] [CrossRef]
- Bashan, Y.; Hernandez, J.P.; Leyva, L.A.; Bacilio, M. Alginate microbeads as inoculant carrier for plant growth-promoting bacteria. Biol. Fertil. Soils 2002, 35, 359–368. [Google Scholar] [CrossRef]
- Lopez, B.R.; Palacios, O.A.; Bashan, Y.; Hernández-Sandoval, F.E.; de-Bashan, L.E. Riboflavin and lumichrome exuded by the bacterium Azospirillum brasilense promote growth and changes in metabolites in Chlorella sorokiniana under autotrophic conditions. Algal Res. 2019, 44, 101696. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
- Mcmillan, M.; Pereg, L. Evaluation of reference genes for gene expression analysis using quantitative RT-PCR in Azospirillum brasilense. PLoS ONE 2014, 9, e98162. [Google Scholar] [CrossRef]
- Palacios, O.A.; Gomez-Anduro, G.; Bashan, Y.; de-Bashan, L.E. Tryptophan, thiamine and indole-3-acetic acid exchange between Chlorella sorokiniana and the plant growth-promoting bacterium Azospirillum brasilense. FEMS Microbiol. Ecol. 2016, 92, fiw077. [Google Scholar] [CrossRef]
- Gonzales-Infante, E.J. Metabolismo de polihidroxibutirato en Azospirillum brasilense Cd durante la interacción Azospirillum brasilense con Chlorella sorokiniana. Master’s Thesis, Universidad Autónoma de Baja California Sur, La Paz, Mexico, 2020. Available online: https://biblio.uabcs.mx/tesis/tesis/te4530.pdf (accessed on 25 March 2024).
- Puente, M.L.; Maroniche, G.A.; Panepucci, M.; Sabio y Garcia, J.; Garcia, J.E.; Criado, M.V.; Molina, R.; Cassan, F. Localization and survival of Azospirillum brasilense Az39 in soybean leaves. Lett. Appl. Microbiol. 2021, 72, 626–633. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Contreras-Angulo, J.R.; Mata, T.M.; Cuellar-Bermudez, S.P.; Caetano, N.S.; Chandra, R.; Garcia-Perez, J.S.; Muylaert, K.; Parra-Saldivar, R. Symbiotic Co-Culture of Scenedesmus sp. and Azospirillum brasilense on N-Deficient media with biomass production for biofuels. Sustainability 2019, 11, 707. [Google Scholar] [CrossRef]
- Peng, H.; de-Bashan, L.E.; Higgins, B.T. Azospirillum brasilense reduces oxidative stress in the green microalgae Chlorella sorokiniana under different stressors. J. Biotechnol. 2021, 325, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.F. Vitamin formation from fatty acid precursors. In Biogenesis of Fatty Acids, Lipids and Membranes, Handbook of Hydrocarbon and Lipid Microbiology; Geiger, O., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 259–271. [Google Scholar] [CrossRef]
- Dahm, H.; Rózycki, H.; Strzelczyk, E.; Li, C.Y. Production of B-group vitamins by Azospirillum spp. grown in media of different pH at different temperatures. Zentralblatt Mikrobiol. 1993, 148, 195–203. [Google Scholar] [CrossRef]
- Tandon, P.; Jin, Q.; Huang, L. A promising approach to enhance microalgae productivity by exogenous supply of vitamins. Microb. Cell Fact. 2017, 16, 219. [Google Scholar] [CrossRef] [PubMed]
- Cassán, F.; Okon, Y.; Creus, C.M. Handbook for Azospirillum: Technical Issues and Protocols; Springer International Publishing: Cham, Switzerland, 2015; Volume 10. [Google Scholar] [CrossRef]
- Müller-Santos, M.; Koskimäki, J.J.; Silveira-Alves, L.P.; de Souza, E.M.; Jendrossek, D.; Pirttilä, A.M. The protective role of PHB and its degradation products against stress situations in bacteria. FEMS Microbiol. Rev. 2021, 45, fuaa058. [Google Scholar] [CrossRef]
- Orlova, M.V.; Tarlachkov, S.V.; Dubinina, G.A.; Belousova, E.V.; Tutukina, M.N.; Grabovich, M.Y. Genomic insights into metabolic versatility of a lithotrophic sulfur-oxidizing diazotrophic Alphaproteobacterium Azospirillum thiophilum. FEMS Microbiol. Ecol. 2016, 92, fiw199. [Google Scholar] [CrossRef][Green Version]
- Muñoz-Velasco, I.; Garcia-Ferris, C.; Hernandez-Morales, R.; Lazcano, A.; Pereto, J.; Becerra, A. Methanogenesis on early stages of life: Ancient but not primordial. Orig. Life Evol. Biosph. 2018, 48, 407–420. [Google Scholar] [CrossRef]
- Malinich, E.A.; Bauer, C.E. Transcriptome analysis of Azospirillum brasilense vegetative and cyst states reveals large-scale alterations in metabolic and replicative gene expression. Microb. Genom. 2018, 4, e000200. [Google Scholar] [CrossRef]
- Su, A.; Yu, Q.; Luo, Y.; Yang, J.; Wang, E.; Yuan, H. Metabolic engineering of microorganisms for the production of multifunctional non-protein amino acids: γ-aminobutyric acid and δ-aminolevulinic acid. Microb. Biotechnol. 2021, 14, 2279–2290. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, S.; Li, B.; Li, J.; Wang, X.; Zhang, J.; Guan, C.; Ji, J. The combined use of a plant growth promoting Bacillus sp. strain and GABA promotes the growth of rice under salt stress by regulating antioxidant enzyme system, enhancing photosynthesis and improving soil enzyme activities. Microbiol. Res. 2023, 266, 127225. [Google Scholar] [CrossRef]
- Teng, C.S.; Xue, C.; Lin, J.Y.; Ng, I.S. Towards high-level protein, beta-carotene, and lutein production from Chlorella sorokiniana using aminobutyric acid and pseudo seawater. Biochem. Eng. J. 2022, 184, 108473. [Google Scholar] [CrossRef]
- Teng, C.S.; Ng, I.S. Optimization of 4-aminobutyric acid feeding strategy and clustered regularly interspaced short palindromic repeats activation for enhanced value-added chemicals in halophilic Chlorella sorokiniana. Bioresour. Technol. 2023, 387, 129599. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, X.; Zhong, D.B.; Yu, L.; Yu, X. γ-Aminobutyric acid (GABA) regulates lipid production and cadmium uptake by Monoraphidium sp. QLY-1 under cadmium stress. Bioresour. Technol. 2020, 297, 122500. [Google Scholar] [CrossRef]
- Planamente, S.; Mondy, S.; Hommais, F.; Vigouroux, A.; Moréra, S.; Faure, D. Structural basis for selective GABA binding in bacterial pathogens. Mol. Microbiol. 2012, 86, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, S.; Bell, T.A.S.; Dahlin, L.R.; Kunde, Y.; LaButti, K.; Louie, K.B.; Kuftin, A.; Treen, D.; Dilworth, D.; Mihaltcheva, S.; et al. A multi-omic characterization of temperature stress in a halotolerant Scenedesmus strain for algal biotechnology. Commun. Biol. 2021, 4, 333. [Google Scholar] [CrossRef]
- Planamente, S.; Moréra, S.; Faure, D. In planta fitness-cost of the Atu4232-regulon encoding for a selective GABA-binding sensor in agrobacterium. Commun. Integr. Biol. 2013, 6, e23692. [Google Scholar] [CrossRef] [PubMed]
- Silva-Dias, B.H.; Jung, S.H.; Castro-Oliveira, J.V.D.; Ryu, C.M. C4 bacterial volatiles improve plant health. Pathogens 2021, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Baz, L.; Abulfaraj, A.A.; Tashkandi, M.A.; Baeissa, H.M.; Refai, M.Y.; Barqawi, A.A.; Shami, A.; Abuauf, H.W.; Ashy, R.A.; Jalal, R.S. Predicted functional shifts due to type of soil microbiome and watering of two wild plants in western region of Saudi Arabia. Phyton 2022, 91, 2249. [Google Scholar] [CrossRef]
- Ganusova, E.E.; Vo, L.T.; Abraham, P.E.; O’Neal-Yoder, L.; Hettich, R.L.; Alexandre, G. The Azospirillum brasilense core chemotaxis proteins CheA1 and CheA4 link chemotaxis signaling with nitrogen metabolism. mSystems 2021, 6, e01354-20. [Google Scholar] [CrossRef]
- Ganusova, E.E.; Vo, L.T.; Mukherjee, T.; Alexandre, G. Multiple CheY proteins control surface associated lifestyles of Azospirillum brasilense. Front. Microbiol. 2021, 12, 664826. [Google Scholar] [CrossRef]
- Ganusova, E.E.; Rost, M.; Aksenova, A.; Abdulhussein, M.; Holden, A.; Alexandre, G. Azospirillum brasilense AerC and Tlp4b cytoplasmic chemoreceptors are promiscuous and interact with the two membrane-bound chemotaxis signaling clusters mediating chemotaxis responses. J. Bacteriol. 2023, 205, e00484-22. [Google Scholar] [CrossRef]
- Gumerov, V.M.; Ortega, D.R.; Adebali, O.; Ulrich, L.; Zhulin, I.B. MiST 3.0: An updated microbial signal transduction database with an emphasis on chemosensory systems. Nucleic Acids Res. 2020, 48, D459–D464. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).