Characterization of Antioxidant and Antimicrobial Activity, Phenolic Compound Profile, and VOCs of Agresto from Different Winegrape Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Raw Material
2.2.1. Grapes
2.2.2. Herbs
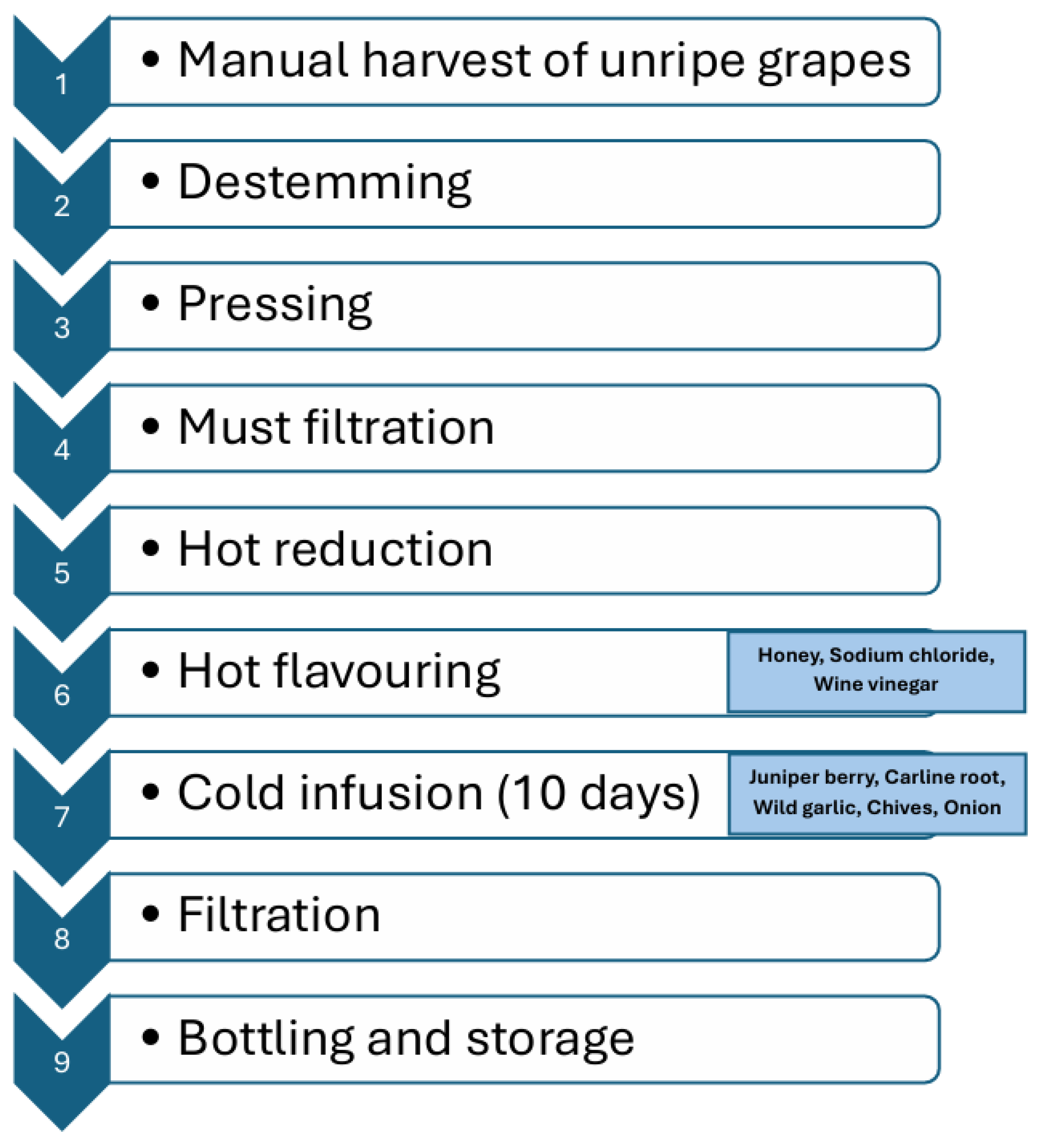
2.3. Production of Unripe Grape Juice
2.4. Grapes and Herbs Extraction
2.5. Determination of Phenolic Profile by UHPLC-ESI-MS/MS
2.6. Determination of the Total Phenolic Content (TPC) and Flavonoid and Flavonol Content
2.7. In Vitro Antioxidant Activity Assays
2.8. Determination of the Antimicrobial Activity
2.9. Analysis of Volatile Organic Compounds (VOCs) by GC-MS
2.10. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Compound Profile of Herbs and Grapes Juice Extracts
3.2. Phenolic Compound Profile of Agresto Samples
3.3. Total Phenolic Content (TPC) and Flavonoid and Flavonol Content
3.4. Antioxidant Activity
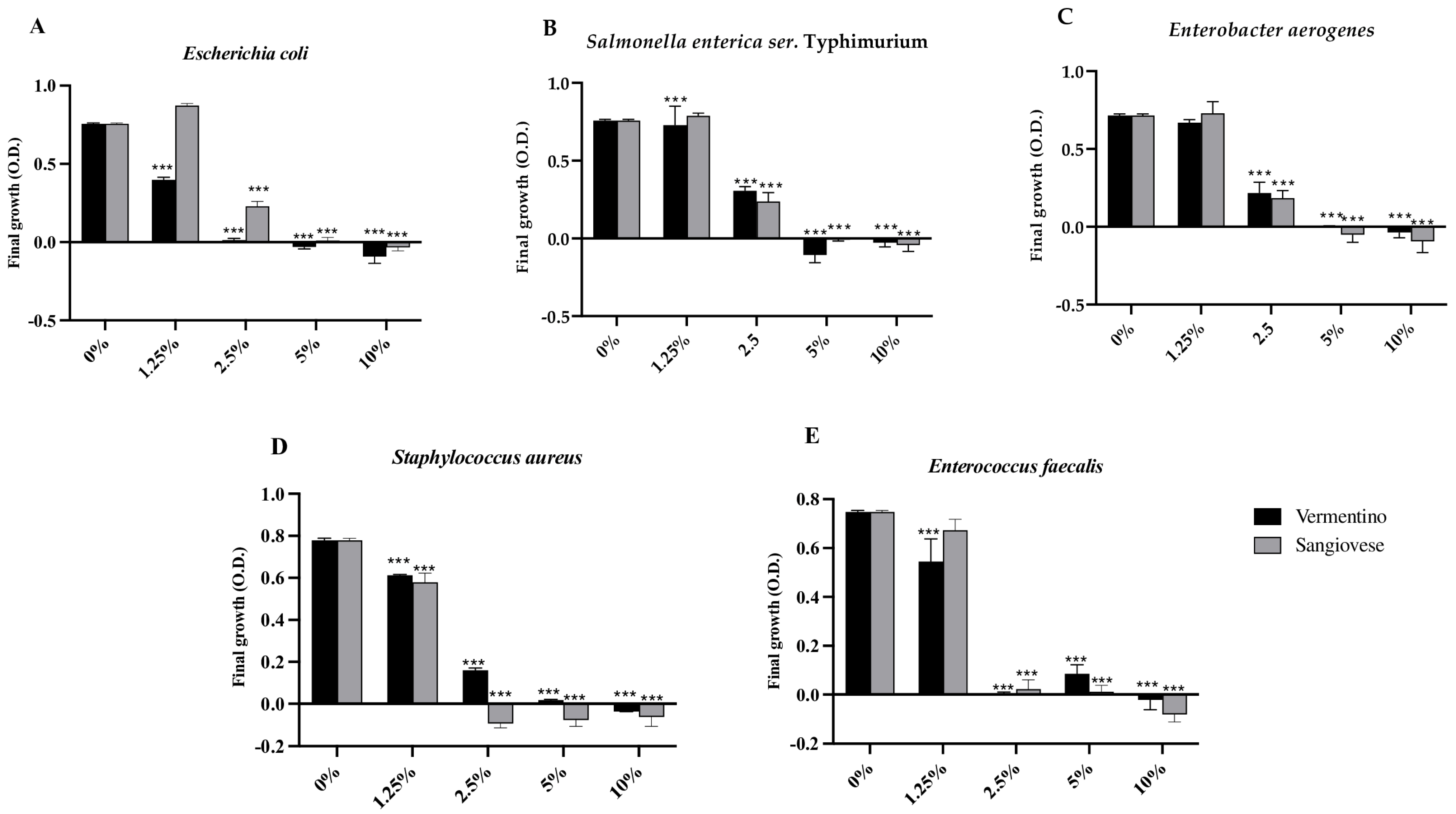
3.5. Antimicrobial Activity
3.6. Analysis of VOCs
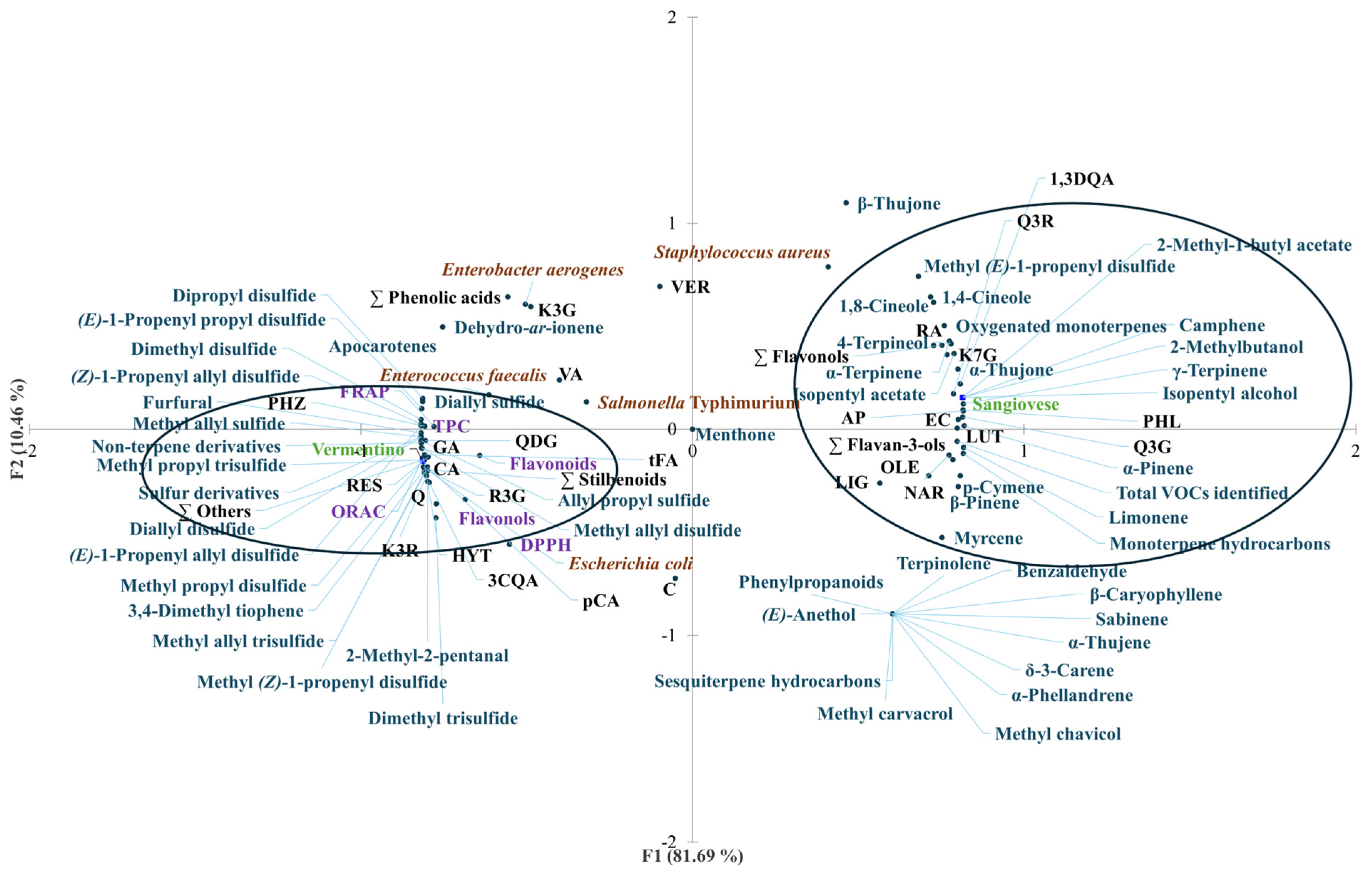
3.7. Overall Rate of Results with Pearson’s Correlation and Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morsetto, P. Targets for a circular economy. Resour. Conserv. Recycl. 2020, 153, 104553. [Google Scholar] [CrossRef]
- Perra, M.; Cuena-Lombraña, A.; Bacchetta, G.; Manca, M.L.; Manconi, M.; Maroun, R.G.; Muntoni, A.; Tuberoso, C.I.G.; Gil, K.A.; De Gioannis, G. Combining Different Approaches for Grape Pomace Valorization: Polyphenols Extraction and Composting of the Exhausted Biomass. Sustainability 2022, 14, 10690. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S. Valorization of agro-industrial wastes for biorefinery process and circular bioeconomy: A critical review. Bioresour. Technol. 2022, 343, 126126. [Google Scholar] [CrossRef]
- Gnanavinthan, A. Bioactives in Fruit: Health Benefits and Functional Foods; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 1–18. [Google Scholar] [CrossRef]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem. 2021, 12, 100149. [Google Scholar] [CrossRef]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canalis, J.M.; Zamora, F. Use of unripe grapes harvested during cluster thinning as a method for reducing alcohol content and pH of wine. Aust. J. Grape Wine Res. 2011, 17, 230–238. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Evaluation of antibrowning and antioxidant activities in unripe grapes recovered during bunch thinning. Aust. J. Grape Wine Res. 2017, 23, 33–41. [Google Scholar] [CrossRef]
- Fia, G.; Bucalossi, G.; Proserpio, C.; Vincenzi, S. Unripe grapes: An overview of the composition, traditional and innovative applications, and extraction methods of a promising waste of viticulture. Aust. J. Grape Wine Res. 2021, 28, 1322–7130. [Google Scholar] [CrossRef]
- Karapinar, M.; Sengun, I.Y. Antimicrobial effect of koruk (unripe grape—Vitis vinifera) juice against Salmonella typhimurium on salad vegetables. Food Control 2007, 18, 702–706. [Google Scholar] [CrossRef]
- Nikfardjam, M.S.P. General and polyphenolic composition of unripe grape juice (verjus/verjuice) from various producers. Mitt. Klosterneuburg. 2008, 58, 28–31. [Google Scholar]
- Hayoglu, I.; Basyigit, B.; Hayoglu, G.; Atasoy, A.F. Cream zahter: A functional food some chemical and sensory properties. Curr. Res. Nutr. Food Sci. 2016, 4, 32–36. [Google Scholar] [CrossRef]
- Hayoglu, I.; Kola, O.; Kaya, C.; Özer, S.; Turkoglu, H. Chemical and sensory properties of verjuice, a traditional Turkish non-fermented beverage from Kabarcik and Yediveren grapes. J. Food Process. Preserv. 2009, 33, 252–263. [Google Scholar] [CrossRef]
- Öncül, N.; Karabıyıklı, Ş. Survival of foodborne pathogens in unripe grape products. LWT 2016, 74, 168–175. [Google Scholar] [CrossRef]
- Öncül, N.; Karabıyıklı, Ş. Factors affecting the quality attributes of unripe grape functional foods products. J. Food Biochem. 2015, 39, 689–695. [Google Scholar] [CrossRef]
- Scalabrelli, G.; Toffanin, A.; Frassinetti, S.; Visconti, A. Agresto: A natural product from unripe grape. In Proceedings of the 4th International Conference and Exhibition on Food Processing & Technology, London, UK, 10–12 August 2015; Available online: https://hdl.handle.net/20.500.14243/304426 (accessed on 3 March 2025).
- Vasile Simone, G.; Montevecchi, G.; Masino, F.; Matrella, V.; Imazio, S.A.; Antonelli, A.; Bignami, C. Ampelographic and chemical characterization of Reggio Emilia and Modena (northern Italy) grapes for two traditional seasonings: ‘saba’ and ‘agresto’. J. Sci. Food Agric. 2013, 93, 3502–3511. [Google Scholar] [CrossRef]
- Flamini, R. Recent Applications of Mass Spectrometry in the Study of Grape and Wine Polyphenols. Int. Sch. Res. Notices 2013, 813563, 45. [Google Scholar] [CrossRef]
- Adams, D. Phenolics and ripening in grape berries. Am. J. Enol. Vitic. 2006, 57, 249–256. [Google Scholar] [CrossRef]
- Brossaud, F.; Cheynier, V.; Noble, A.C. Bitterness and astringency of grape and wine polyphenols. Aust. J. Grape Wine Res. 2001, 7, 33–39. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Fernández-Fernández, J.I. Phenolic Compounds and Color Stability of Red Wines: Effect of Skin Maceration Time. Am. J. Enol. Vitic. 2001, 52, 266–270. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Vilela, A. Phenolic Compounds and Antioxidant Activity in Grape Juices: A Chemical and Sensory View. Beverages 2018, 4, 22. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Aziz, I.; Rasheed, M.; Gul, B.; Khan, M.A. Effect of Extraction Solvents on Polyphenols and Antioxidant Activity of Medicinal Halophytes. Pak. J. Bot. 2016, 48, 621–627. [Google Scholar]
- Raffaelli, A.; Saba, A. Ion Scanning or Ion Trapping: Why Not Both? Mass Spectrom. Rev. 2021, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Oxidants and Antioxidants Part A; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Souid, A.; Gabriele, M.; Longo, V.; Pucci, L.; Bellani, L.; Smaoui, A.; Abdelly, C.; Hamed, K.B. Salt tolerance of the halophyte Limonium delicatulum is more associated with antioxidant enzyme activities than phenolic compounds. Funct. Plant Biol. 2016, 43, 607–619. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef]
- Pozzo, L.; Grande, T.; Raffaelli, A.; Longo, L.; Weidner, S.; Amarowicz, R.; Karamać, M. Characterization of Antioxidant and Antimicrobial Activity and Phenolic Compound Profile of Extracts from Defatted Seeds of Different Vitis Species. Molecules 2023, 28, 4924. [Google Scholar] [CrossRef]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed Cake as a Tool for the Improvement of Nutraceutical and Sensorial Features of Sourdough Bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Curioni, A.; Bakalinsky, A.T.; Marangon, M.; Pasini, G.; Vincenzi, S. Chemical and sensory analysis of verjuice: An acidic food ingredient obtained from unripe grape berries. Inn. Food Sci. Emerg. Technol. 2017, 44, 9–14. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Magli, M.; Marangon, M.; Curioni, A.; Pasini, G.; Vincenzi, S. Use of verjuice as an acidic salad seasoning ingredient: Evaluation by consumers’ liking and Check-All-That-Apply. Eur. Food Res. Technol. 2018, 244, 2117–2125. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Gomes Reis, M.; Maggs, R.; Hort, J. Understanding consumer acceptability of verjuice, its potential applications and sensory and chemical drivers of liking. Food Res. Int. 2024, 188, 114480. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Sengun, I.Y. Inactivation effect of marination liquids prepared with koruk juice and dried koruk pomace on Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes inoculated on meat. Int. J. Food Microbiol. 2019, 304, 32–38. [Google Scholar] [CrossRef]
- Tinello, F.; Mihaylova, D.; Lante, A. Effect of dipping pre-treatment with unripe grape juice on dried “Golden Delicious” apple slices. Food Bioproc. Technol. 2018, 11, 2275–2285. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Goztepe, E.; Ozturk, B. Efficiency of marination liquids prepared with koruk (Vitis vinifera L.) on safety and some quality attributes of poultry meat. LWT Food Sci. Technol. 2019, 113, 108317. [Google Scholar] [CrossRef]

| Compound Name | Acronym | Vermentino | Sangiovese | p-Value |
|---|---|---|---|---|
| Gallic acid | GA | 809.82 ± 9.85 | 329.57 ± 17.58 | <0.0001 |
| 3-O-Caffeoylquinic acid (Chlorogenic acid) | 3CQA | 779.27 ± 30.70 | 439.96 ± 40.32 | <0.001 |
| Rosmarinic acid | RA | 0.32 ± 0.08 | 0.59 ± 0.07 | <0.05 |
| Caffeic acid | CA | 809.96 ± 9.96 | 329.57 ± 29.46 | <0.0001 |
| Vanillic acid | VA | 163.50 ± 34.10 | 139.93 ± 19.97 | n.s. |
| p-Coumaric acid | pCA | 1922.67 ± 204.64 | 639.05 ± 19.77 | <0.001 |
| trans-Ferulic acid | tFA | 1297.36 ± 105.26 | 350.64 ± 17.33 | <0.001 |
| 1,3-Dicaffeoylquinic acid (Cynarin) | 1,3DQA | n.d. | 0.46 ± 0.03 | <0.0001 |
| ∑ Phenolic acids | 5782.89 | 2229.78 | ||
| Quercetin | Q | 7.77 ± 0.17 | 5.00 ± 0.61 | <0.01 |
| Quercetin 3-O-glucoside | Q3G | 42.34 ± 2.11 | 76.40 ± 3.10 | <0.0001 |
| Quercetin 3-O-rutinoside (Rutin) | Q3R | 112.01 ± 17.41 | 196.61 ± 5.97 | <0.01 |
| Quercetin 3,4-O-diglucoside | QDG | 31.18 ± 1.02 | 23.02 ± 1.04 | <0.01 |
| Kaempferol 7-O-glucoside | K7G | 365.82 ± 23.62 | 536.84 ± 36.56 | <0.01 |
| Kaempferol 3-O-glucoside | K3G | 504.06 ± 11.09 | 483.35 ± 25.46 | n.s. |
| Kaempferol 3-O-rutinoside | K3R | 65.82 ± 2.99 | 23.44 ± 3.44 | <0.0001 |
| ∑ Flavonols | 1129.00 | 1344.66 | ||
| (+)-Catechin | C | 13.11 ± 0.59 | 12.98 ± 0.73 | n.s. |
| (−)-Epicatechin | EC | 9.59 ± 0.65 b | 13.52 ± 0.50 a | n.s. |
| ∑ Flavan-3-ols | 22.70 | 26.50 | ||
| Resveratrol 3-O-glucoside (Piceid) | R3G | 17.99 ± 1.74 | 7.08 ± 0.34 | <0.001 |
| Resveratrol | RES | 1.13 ± 0.20 | n.d. | <0.001 |
| ∑ Stilbenoids | 19.12 | 7.08 | ||
| Hydroxytyrosol | HYT | 323.46 ± 19.34 | 48.45 ± 2.64 | <0.0001 |
| Verbascoside | VER | 3.66 ± 0.33 | 3.61 ± 0.62 | n.s. |
| Oleuropein | OLE | 18.03 ± 0.54 | 19.47 ± 0.57 | <0.05 |
| Ligstroside | LIG | 2.28 ± 0.33 | 2.72 ± 0.29 | n.s. |
| Phloridzin | PHZ | 45.26 ± 0.48 | 39.05 ± 1.04 | <0.001 |
| Phloretin | PHL | 42.05 ± 2.44 | 85.24 ± 2.98 | <0.0001 |
| Apigenin | AP | 0.06 ± 0.03 | 0.85 ± 0.05 | <0.001 |
| Luteolin | LUT | 0.28 ± 0.05 | 0.55 ± 0.04 | <0.01 |
| Naringenin | NAR | 6.17 ± 0.49 | 9.16 ± 0.94 | <0.01 |
| ∑ Others | 441.25 | 209.09 |
| Grapevine Species | TPC (mg GAE/mL) | Flavonoids (mg CE/mL) | Flavonols (mg QE/mL) |
|---|---|---|---|
| Vermentino | 1.31 ± 0.03 | 0.10 ± 0.03 | 0.21 ± 0.05 |
| Sangiovese | 0.76 ± 0.03 | 0.06 ± 0.00 | 0.10 ± 0.03 |
| p-value | <0.0001 | n.s. | <0.05 |
| Grapevine Species | FRAP (mg TE/mL) | DPPH Assay (mg TE/mL) | ORAC (mg TE/mL) |
|---|---|---|---|
| Vermentino | 4.11 ± 0.16 | 3.64 ± 0.00 | 41.01 ± 0.50 |
| Sangiovese | 2.78 ± 0.37 | 3.51 ± 0.11 | 26.76 ± 0.17 |
| p-value | <0.05 | n.s. | <0.0001 |
| Compound | Vermentino | Sangiovese | p-Value |
|---|---|---|---|
| Methyl allyl sulfide | 3.70 ± 0.10 | 0.55 ± 0.25 | <0.0001 |
| Isopentyl alcohol (syn. 3-methylbutanol) | n.d. | 0.20 ± 0.00 | <0.0001 |
| 2-Methylbutanol | n.d. | 0.30 ± 0.00 | <0.0001 |
| Dimethyl disulfide | 5.60 ± 0.10 | 1.90 ± 0.50 | <0.001 |
| 2-Methyl-2-pentanal | 1.10 ± 0.10 | 0.25 ± 0.05 | <0.001 |
| Furfural | 5.59 ± 0.10 | 1.65 ± 0.35 | <0.0001 |
| Diallyl sulfide | 2.20 ± 0.10 | 0.35 ± 0.35 | <0.001 |
| Allyl propyl sulfide | 4.03 ± 0.09 | 0.25 ± 0.25 | <0.0001 |
| Isopentyl acetate | n.d. | 0.35 ± 0.05 | <0.001 |
| 2-Methyl-1-butyl acetate | n.d. | 0.40 ± 0.00 | <0.0001 |
| 3,4-Dimethyl tiophene | 2.47 ± 0.11 | 0.85 ± 0.05 | <0.0001 |
| Methyl allyl disulfide | 9.40 ± 0.10 | 3.75 ± 0.25 | <0.0001 |
| α-Thujene | n.d. | 0.10 ± 0.10 | n.s. |
| Methyl propyl disulfide | 13.67 ± 0.08 | n.d. | <0.0001 |
| Methyl (E)-1-propenyl disulfide | n.d. | 3.25 ± 1.45 | <0.05 |
| α-Pinene | n.d. | 1.65 ± 0.15 | <0.0001 |
| Methyl (Z)-1-propenyl disulfide | 14.04 ± 0.11 | 4.35 ± 0.35 | <0.0001 |
| Camphene | n.d. | 0.37 ± 0.06 | <0.0001 |
| Benzaldehyde | n.d. | 0.15 ± 0.15 | n.s. |
| Dimethyl trisulfide | 2.71 ± 0.10 | 1.60 ± 0.20 | <0.01 |
| Sabinene | n.d. | 0.25 ± 0.25 | n.s. |
| β-Pinene | n.d. | 0.85 ± 0.25 | <0.01 |
| Myrcene | n.d. | 1.40 ± 0.70 | <0.05 |
| α-Phellandrene | n.d. | 0.10 ± 0.10 | n.s. |
| δ-3-Carene | n.d. | 0.30 ± 0.30 | n.s. |
| 1,4-Cineole | 0.61 ± 0.12 | 4.97 ± 1.55 | <0.01 |
| α-Terpinene | 0.29 ± 0.09 | 1.40 ± 0.20 | <0.01 |
| p-Cymene | 0.59 ± 0.12 | 9.20 ± 2.20 | <0.01 |
| Limonene | 3.10 ± 0.11 | 30.6 ± 4.40 | <0.001 |
| 1,8-Cineole | 0.30 ± 0.10 | 4.65 ± 1.45 | <0.01 |
| γ-Terpinene | 1.10 ± 0.09 | 7.95 ± 0.15 | <0.0001 |
| Diallyl disulfide | 3.41 ± 0.10 | 0.70 ± 0.20 | <0.0001 |
| Terpinolene | n.d. | 0.55 ± 0.55 | n.s. |
| (Z)-1-Propenyl allyl disulfide | 6.61 ± 0.10 | 0.70 ± 0.70 | <0.001 |
| (E)-1-Propenyl allyl disulfide | 3.89 ± 0.10 | 0.40 ± 0.40 | <0.001 |
| α-Thujone | n.d. | 0.55 ± 0.05 | <0.0001 |
| Dipropyl disulfide | 3.30 ± 0.08 | 0.55 ± 0.55 | <0.01 |
| (E)-1-Propenyl propyl disulfide | 4.20 ± 0.11 | 0.60 ± 0.60 | <0.001 |
| β-Thujone | n.d. | 0.20 ± 0.20 | n.s. |
| Methyl allyl trisulfide | 2.22 ± 0.11 | 0.95 ± 0.06 | <0.0001 |
| Methyl propyl trisulfide | 1.70 ± 0.10 | n.d. | <0.0001 |
| 4-Terpineol | 0.49 ± 0.12 | 8.50 ± 1.40 | <0.001 |
| Methyl chavicol (syn. estragol) | n.d. | 0.45 ± 0.45 | n.s. |
| Methyl carvacrol | n.d. | 0.25 ± 0.25 | n.s. |
| (E)-Anethol | n.d. | 0.35 ± 0.35 | n.s. |
| Dehydro-ar-ionene | 1.10 ± 0.10 | 0.35 ± 0.35 | <0.05 |
| β-Caryophyllene | n.d. | 0.37 ± 0.35 | n.s. |
| Monoterpene hydrocarbons | 5.08 ± 0.17 | 54.65 ± 8.95 | <0.001 |
| Oxygenated monoterpenes | 1.39 ± 0.08 | 19.20 ± 4.30 | <0.01 |
| Sesquiterpene hydrocarbons | n.d. | 0.35 ± 0.35 | n.s. |
| Oxygenated sesquiterpenes | n.d. | n.d. | |
| Apocarotenes | 1.10 ± 0.10 | 0.35 ± 0.35 | <0.05 |
| Phenylpropanoids | n.d. | 0.80 ± 0.80 | n.s. |
| Sulfur derivatives | 83.10 ± 0.20 | 20.75 ± 4.85 | <0.0001 |
| Non-terpene derivatives | 6.70 ± 0.00 | 3.30 ± 0.20 | <0.0001 |
| Total identified | 97.37 ± 0.39 | 99.30 ± 0.30 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozzo, L.; Raffaelli, A.; Grande, T.; Frassinetti, S.; Longo, V.; Venturi, F.; Sanmartin, C.; Ferroni, G.; Flamini, G.; Toffanin, A. Characterization of Antioxidant and Antimicrobial Activity, Phenolic Compound Profile, and VOCs of Agresto from Different Winegrape Varieties. Processes 2025, 13, 2174. https://doi.org/10.3390/pr13072174
Pozzo L, Raffaelli A, Grande T, Frassinetti S, Longo V, Venturi F, Sanmartin C, Ferroni G, Flamini G, Toffanin A. Characterization of Antioxidant and Antimicrobial Activity, Phenolic Compound Profile, and VOCs of Agresto from Different Winegrape Varieties. Processes. 2025; 13(7):2174. https://doi.org/10.3390/pr13072174
Chicago/Turabian StylePozzo, Luisa, Andrea Raffaelli, Teresa Grande, Stefania Frassinetti, Vincenzo Longo, Francesca Venturi, Chiara Sanmartin, Giuseppe Ferroni, Guido Flamini, and Annita Toffanin. 2025. "Characterization of Antioxidant and Antimicrobial Activity, Phenolic Compound Profile, and VOCs of Agresto from Different Winegrape Varieties" Processes 13, no. 7: 2174. https://doi.org/10.3390/pr13072174
APA StylePozzo, L., Raffaelli, A., Grande, T., Frassinetti, S., Longo, V., Venturi, F., Sanmartin, C., Ferroni, G., Flamini, G., & Toffanin, A. (2025). Characterization of Antioxidant and Antimicrobial Activity, Phenolic Compound Profile, and VOCs of Agresto from Different Winegrape Varieties. Processes, 13(7), 2174. https://doi.org/10.3390/pr13072174









