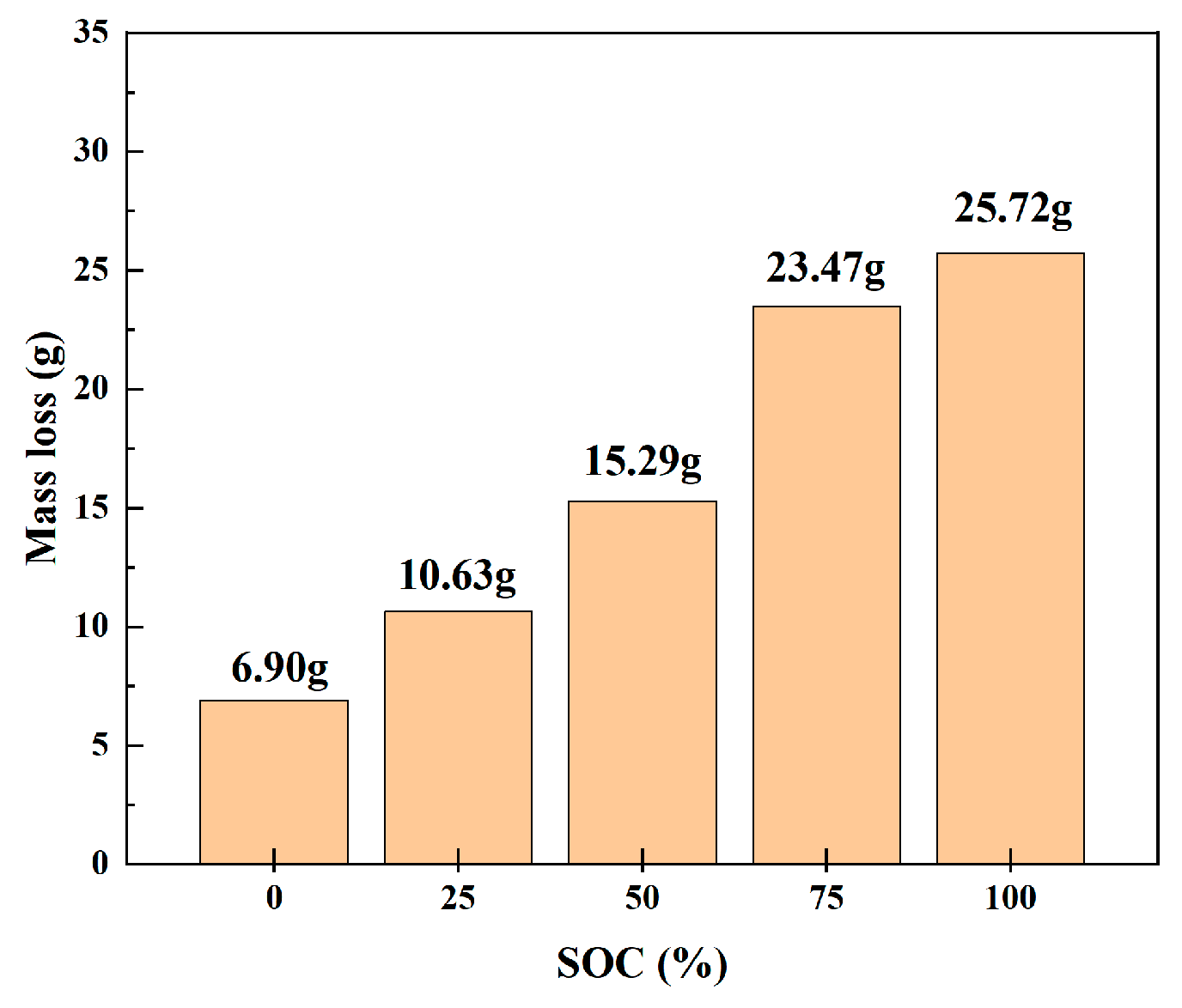Abstract
In this study, we systematically investigated the characteristic parameter evolution laws of thermal runaway with respect to 18,650 lithium-ion batteries (LIBs) under thermal abuse conditions at five state-of-charge (SOC) levels: 0%, 25%, 50%, 75%, and 100%. In our experiments, we combined infrared thermography, mass loss analysis, temperature monitoring, and gas composition detection to reveal the mechanisms by which SOC affects the trigger time, critical temperature, maximum temperature, mass loss, and gas release characteristics of thermal runaway. The results showed that as the SOC increases, the critical and maximum temperatures of thermal runaway increase notably. At a 100% SOC, the highest temperature on the positive electrode side reached 1082.1 °C, and the mass loss increased from 6.90 g at 0% SOC to 25.75 g at 100% SOC, demonstrating a salient positive correlation. Gas analysis indicated that under high-SOC conditions (75% and 100%), the proportion of flammable gases such as CO and CH4 produced during thermal runaway significantly increases, with the CO/CO2 ratio exceeding 1, indicating intensified incomplete combustion and a significant increase in fire risk. In addition, flammability limit analysis revealed that the lower explosive limit for gases is lower (17–21%) at a low SOC (0%) and a high SOC (100%), indicating greater explosion risks. We also found that the composition of gases released during thermal runaway varies substantially at different SOC levels, with CO, CO2, and CH4 accounting for over 90% of the total gas volume, while toxic gases, such as HF, although present in smaller proportions, pose noteworthy hazards. Unlike prior studies that relied on post hoc analysis, this work integrates real-time multi-parameter monitoring (temperature, gas composition, and mass loss) and quantitative explosion risk modeling (flammability limits via the L-C formula). This approach reveals the unique dynamic SOC-dependent mechanisms of thermal runaway initiation and gas hazards. This study provides theoretical support for the source tracing of thermal runaway fires and the development of preventive LIB safety technology and emphasizes the critical influence of the charge state on the thermal safety of batteries.
1. Introduction
As the global energy structure transitions and the “dual carbon” goals advance, lithium-ion batteries are increasingly becoming a core technology carrier in the new-energy sector due to their high energy density, long life cycles, and low self-discharge rates [1,2]. However, when thermal runaway occurs in lithium-ion batteries, it manifests as intense temperature increases, gas emission, and even explosions, posing a severe threat to user safety and property. According to incomplete statistics, the annual growth rate of fire accidents caused by thermal runaway in LIBs has exceeded 20% globally in recent years [3,4], highlighting the urgency of battery safety issues.
In essence, state of charge (SOC) is a key parameter representing the energy storage level of a battery. It directly affects the distribution of active materials within the cell, polarization effects, and side reaction pathways [5], thereby regulating the thermal runaway trigger threshold and energy release intensity. Researchers have mainly studied the thermal runaway phenomena pertaining to LIBs under different abuse conditions, analyzing the thermal runaway characteristics of LIBs under the influence of various triggering methods to explore common patterns of thermal runaway [6,7]. Li [8] experimentally investigated the external expansion force, voltage, and temperature behavior of batteries under different SOCs in thermal abuse scenarios. Liu [9] used a new experimental technique, copper segmented cell calorimetry (CSBC), to measure the energetics and kinetics of the thermally induced failure of 18,650-shaped LIBs containing three different cathodes. Li [10] reported an early-warning method for TR via online electrochemical impedance spectroscopy (EIS) monitoring. Mao [11] compared the thermal runaway and heat production characteristics of different cells with the same capacity under thermal abuse conditions. Based on the results of an overheating experiment, Zhou [12] conducted a detailed analysis of the temperature, heat generation, and TR propagation velocity inside a battery to reveal the TR triggering mechanism at the cell level.
Regarding electrical abuse, Lin [13], Ren [14], Ouyang [15], Zhou [16], Deng [17], and Wang [18] used experimental methods to study LIB overcharging thermal runaway phenomena under different cooling conditions as well as the impact of side-reaction heat on thermal runaway when charging LIBs at different rates. By studying reaction products, Yuan [19] and Zhang [20] explored the mechanism of overcharging thermal runaway and analyzed the impact of internal resistance changes on thermal runaway. In terms of mechanical abuse, based on a needle penetration test platform, scholars have investigated the effects and mechanisms of different needle penetration speeds [21], needle tip angles [22], fixture forms [23], and needle penetration positions [24,25] on LIBs, finding that the probability of triggering thermal runaway increases with higher needle penetration speeds and smaller fixture apertures, while the energy loss from short-circuit discharges caused by different needle penetration angles penetrating the cell is roughly consistent. The gaseous products generated during the LIB thermal runaway process are flammable and can lead to thermal spread issues. Therefore, Zhang [26], Huang [27], Gregory [28,29], Gerelt-Od [30], Zhang [31], Liu [32], and Diaz [33] used GC/MS, FTIR, and Raman spectroscopy combined with thermogravimetric analysis to assess characteristic parameters, such as the characteristic temperature, gas composition, and exothermic time of LIB thermal runaway. The aim of these studies was to investigate the chemical reaction mechanisms and hazards of thermal runaway at various stages in regard to LIBs, revealing that the types of gases produced by different cathode materials after thermal runaway are largely similar, and the gas composition increases as the SOC rises.
In summary, scholars all over the world have simulated thermal runaway in LIBs under various abuse conditions to reveal the mechanisms of this process. However, systematic studies on thermal runaway behavior at different SOC levels remain scarce. Therefore, by integrating previous research, we developed a self-built thermal runaway fault simulation platform using an 18,650-type LIB as the subject. We studied the characteristic parameter evolution laws of thermal runaway at five charge states (SOCs): 0%, 25%, 50%, 75%, and 100%. In this paper, the effects of SOC on the thermal runaway trigger time, critical temperature, and maximum temperature are explained; the recombination between mass loss and SOC is quantified; the composition, concentration, and flammability characteristics of gas release at different SOC levels are analyzed; and the explosion risk posed by the released gases is assessed. Unlike prior research, which primarily relied on post hoc analysis of thermal runaway events, our study incorporates real-time monitoring of temperature, mass loss, and gas composition during thermal runaway.
This approach allowed us to directly observe the dynamic evolution of thermal runaway parameters, providing insights into the mechanisms by which the SOC influences thermal runaway initiation, propagation, and gas release characteristics. The thermal runaway behavior of LIBs at different SOC levels was comprehensively evaluated through multi-dimensional parameter analysis, including with respect to trigger time, critical temperature, maximum temperature, mass loss, and gas release characteristics. Furthermore, we conducted a detailed analysis of the gas release safety boundary, including the flammability limits and toxicity of the released gases, aspects that have not been extensively explored in previous studies. Through this work, we aim to foster a more comprehensive understanding of the safety implications of different SOC levels in LIBs and provide valuable data for the development of improved battery safety management strategies.
2. Experimental Platform and Device
2.1. Charging and Discharging Cycle Equipment
We used Neware battery (EVE Energy Co., Ltd., Huizhou, China) charge–discharge cycle testing equipment (model BTS-5V12A) to run the batteries through constant-current, constant-voltage, constant-power, and constant-resistance charge–discharge cycles. The data acquisition frequency was 1 s. Parameters such as the current, voltage, and capacity of the batteries could be intuitively displayed during the charge and discharge process. The battery samples needed to undergo three charge–discharge cycles to activate them, stimulate internal resistance, and enhance stability.
2.2. Thermal Runaway Fault Simulation Platform
To observe the thermal runaway behavior of LIBs under abusive conditions, we independently constructed a thermal runaway fault simulation platform to simulate the thermal runaway of LIBs under heating conditions, as shown in Figure 1.

Figure 1.
Lithium-ion battery thermal runaway fault simulation platform.
Since thermal runaway in an LIB can cause the safety valve to open and spray internal substances or even lead to the explosion of the battery during experiments, the platform was externally enclosed with an iron shell to ensure personal safety. A glass observation window was placed at the front to enable easy observation of experimental phenomena, and infrared thermography was used to record the infrared temperature distribution. The internal part of the simulation platform was connected to a gas collection device and a Fourier gas analyzer, while sample holders were placed inside to prevent the safety valve from opening because of thermal runaway, which could cause gas to spray out and spread in all directions, leading to the detachment of the thermocouples attached to the battery. The custom parameters included a cylindrical heating rod with a length of 65 mm, a diameter of 18 mm, and a constant power of 200 W (Figure 2). This rod, along with a control switch and an operational red light, comprised the heating control system, which controlled the heating experiment through the control switch.
Figure 2.
Customized heating rods.
2.3. Data Acquisition System
A data acquisition system composed of thermocouples, electronic balances, and infrared thermal imaging cameras was established to collect data on the lithium battery samples and the characteristic parameters of thermal runaway. Using the TASI thermocouple produced by Telesis, temperature probes were placed at three specific locations on the LIB sample and connected to a computer to allow real-time monitoring of temperature changes in the experimental samples, yielding temperature data during the thermal runaway process under heating conditions. An electronic balance with the model number UW8200S (Shimadzu, Kyoto, Japan) from, with a precision of 0.1 g and a repeatability equal to 0.08 g, was used to measure the mass changes before and after the lithium batteries underwent thermal runaway. The FOTRIC Fei Foundation 323Q-L49 high-precision intelligent handheld infrared thermal imager (FOTRIC, Shanghai, China) was used to record the experimental process, and the corresponding thermal imaging videos were processed into images at one frame per second for analysis.
2.4. Fourier Gas Analyzer
A Fourier gas analyzer can detect gases, such as C2H2, C2H4, C2H6, C3H8, CH4, CO, CO2, H2S, HCl, HF, N2O, and NO (Figure 3). To collect the components and mass concentrations of the gaseous emissions triggered by the thermal abuse of LIBs, we employed a Fourier gas analyzer in conjunction with an independently developed thermal runaway fault simulation platform for LIBs. The specific steps followed for the real-time monitoring of the gases during the thermal runaway process of LIBs are as follows: First, we used a charge–discharge cycling instrument to charge and discharge the battery samples to different SOCs. We then placed the battery samples into the independently developed thermal runaway fault simulation platform for LIBs, secured the batteries in place with clamps, attached a gas collection hood to the battery samples, connected the gas collection hose to the Fourier gas analyzer, turned on the heating switch, and commenced the gas collection experiment as the LIBs underwent thermal runaway.

Figure 3.
Diagram of the Fourier gas analyzer used.
3. Experimentation
3.1. Samples
In this experiment, a commonly used cylindrical 18,650 LIB (as shown in Figure 4) was selected as the research subject. Its energy density, lifespan, and safety performance all surpass those of traditional lead–acid batteries. They can, for instance, enhance the range, lifespan, and safety of electric bicycles, making their operation more stable. The battery sample had a diameter of 18 mm and a length of 65 mm, with a nominal capacity of 3200 mAh. The battery’s charging cut-off voltage, rated voltage, and discharge cut-off voltage were 4.2 V, 3.6 V, and 2.5 V, respectively. The initial mass of the battery sample was ±46.0 g. The basic parameters of the battery sample are listed in Table 1.

Figure 4.
LIB samples: (a) packaged battery samples and (b) battery cell samples.

Table 1.
Basic parameters of the 18,650 LIB.
3.2. Experimental Procedure
The experiment first involved subjecting the battery to three charging and discharging cycles to obtain battery samples at 0%, 25%, 50%, 75%, and 100% SOC levels. Then, the following steps were sequentially executed: we fixed thermocouples to the positive electrode, central area, and negative side of the battery using high-temperature tape to ensure there were accurate temperature measurement points; we placed the samples into the fixture of the LIB thermal runaway failure simulation platform and closed the door to the glass chamber; we activated the heating system to monitor and record changes in temperature throughout the process, from initial heating to the onset of thermal runaway; when thermal runaway characteristics (such as a sudden temperature rise or a voltage drop) were detected, we immediately cut off the heating power to terminate external heat input; after the battery completed the thermal runaway reaction and the system stabilized, we halted the thermocouple data acquisition program to complete the single experimental cycle. This process simulated external thermal abuse conditions, allowing us to systematically explore the impact of different SOCs on battery thermal runaway behavior.
3.3. Test Conditions for Lithium Battery Thermal Runaway
The focus of the experiment was primarily monitoring the thermal runaway process of 18,650 LIBs and preparing samples for thermal runaway testing. We selected 18,650 LIBs for the experiment. Before their ultimate placement, the thermocouples were placed on the positive and negative sides of the 18,650 lithium-ion battery sample to measure surface temperature changes near the positive and negative electrodes before and after thermal runaway. The positions of the battery sample and the thermocouples are shown in Figure 5. The battery samples with different charge states were sequentially placed in the lithium-ion battery thermal runaway fault simulation platform to be heated; the thermal runaway experiment was conducted using this platform. During the experiment, real-time temperature data on the thermal runaway process in the battery sample were monitored. Once thermal runaway was complete, the power supply of the lithium-ion battery thermal runaway fault simulation platform was turned off, and the thermal runaway sample was allowed to return to room temperature before the remaining battery components were removed for later use.

Figure 5.
Schematic diagram of the placement of battery samples and the thermocouple layout position.
The experimental conditions for the 18,650 LIB samples are shown in Table 2. In this study, extensive experiments were conducted on thermal runaway behavior and sample preparation for lithium-ion batteries, as shown in Table 3. However, during the experiments, it was found that the repeatability of thermal runaway tests for lithium-ion batteries was low, with significant instability in the reactions. When the SOC was ≥75%, approximately 68% of the samples exploded during thermal runaway, causing the battery casing to completely rupture and internal materials to scatter, hampering the collection of thermal runaway samples. The effective sample acquisition rate was merely 32%. To ensure the experimental data were reliable, we conducted multiple repeated experiments on each charged-state battery sample, totaling 153 thermal abuse tests, ultimately yielding 67 sets of effective residual samples. By collecting thermal runaway samples and fragment residues, we were still able to obtain some characteristic components from the exploded battery samples. All the effective samples met the following criteria: they (1) retained no less than 50% of the original casing structure; (2) had an identifiable spatial distribution between positive and negative electrode groups; and (3) exhibited clear thermal runaway trigger feature points.

Table 2.
Experimental conditions of 18,650 LIB samples.

Table 3.
Statistics on the samples subjected to the thermal abuse test.
4. Results and Discussion
4.1. Thermal Image Analysis of Thermal Runaway
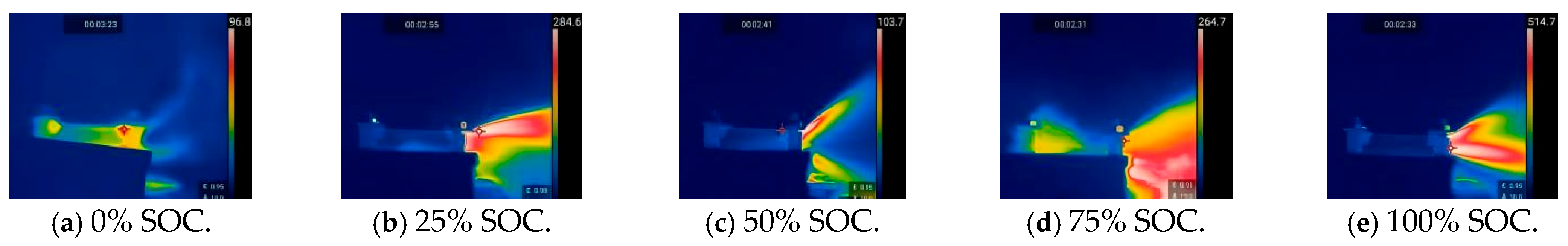
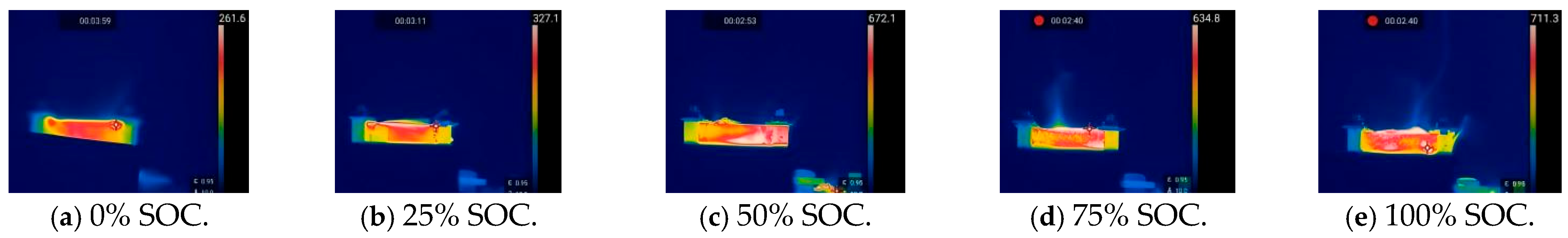
The original samples of 18,650 lithium-ion batteries and the thermal runaway process they underwent under heating conditions were monitored using infrared thermography, and the thermal images of the battery samples in five different charging states under heating abuse conditions were analyzed. Figure 6 and Figure 7 show the thermal images of the thermal runaway process in 18,650 LIBs under the influence of heating abuse.

Figure 6.
Thermal images of the eruption gas during thermal runaway in 18,650 LIBs under heating conditions. (A temperature scale bar is shown on the right side of the image, indicating the temperature range in degrees Celsius.).

Figure 7.
Thermal images of 18,650 LIBs after thermal runaway under heating conditions. (A temperature scale bar is shown on the right side of the image, indicating the temperature range in degrees Celsius.).
The infrared thermal images reveal the temperature distribution throughout the entire process, from when the 18,650 LIBs first overheat to when the safety valve opens and gas is released and to the final stage of thermal runaway. As shown in Figure 6, when the safety valve opened, it released the gas that had accumulated inside. During this gas release, an enormous amount of flammable gas mixed with the surrounding air, forming a combustible mixture that produced flames. Figure 7 clearly shows that heat was transferred throughout the battery after the gas was released. As the SOC increased, the internal temperature of the battery rose, because the higher the SOC, the more intense the chemical reactions inside the battery, releasing more thermal energy. If this thermal energy did not dissipate in time, it led to an increase in the internal temperature of the battery.
4.2. Thermal Runaway Mass Loss Analysis
We weighed the original 18,650 LIB samples and those that had undergone heating-induced thermal runaway by using an electronic balance. To diminish experimental errors, the mass was measured three times under each SOC, and the average value was calculated. Table 4 is Temperature variation range of thermal imaging in different charging states. Table 5 lists the changes in mass during thermal runaway for the 18,650 LIBs. As shown in Figure 8, the mass losses before and after thermal runaway for 18,650 LIBs were as follows: the average mass losses at 0%, 25%, 50%, 75%, and 100% SOC were 6.90, 10.65, 15.29, 23.47, and 25.75 g, respectively, indicating that the mass loss increased with an increase in charge levels. In a continuously warming environment, the internal separator of 18,650 lithium-ion batteries melts, causing multiple chemical reactions between the positive and negative electrode materials. The expeditious accumulation of internal heat leads to excessive internal pressure, prompting the safety valve to open and release electrolytes, gases, and internal substances. During this process, as the charge level increases, the mass of the melting separator, gas emission, and internal substance release also increase. Therefore, high charge states resulted in greater mass loss, leading to a corresponding decrease in the mass of the thermal runaway samples.

Table 4.
Temperature variation range of thermal imaging in different charging states.

Table 5.
Thermal runaway mass statistics of 18,650 LIBs.
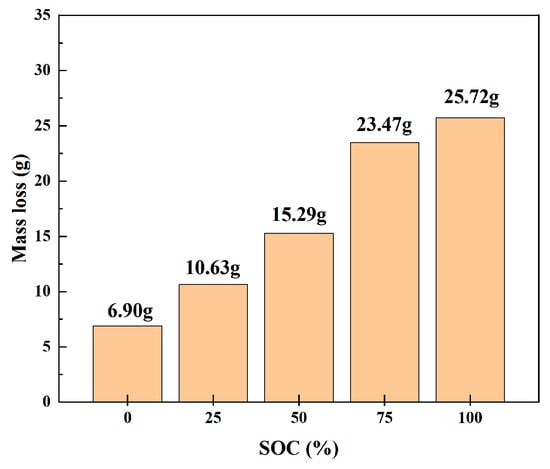
Figure 8.
Mass loss during thermal runaway in 18,650 LIBs.
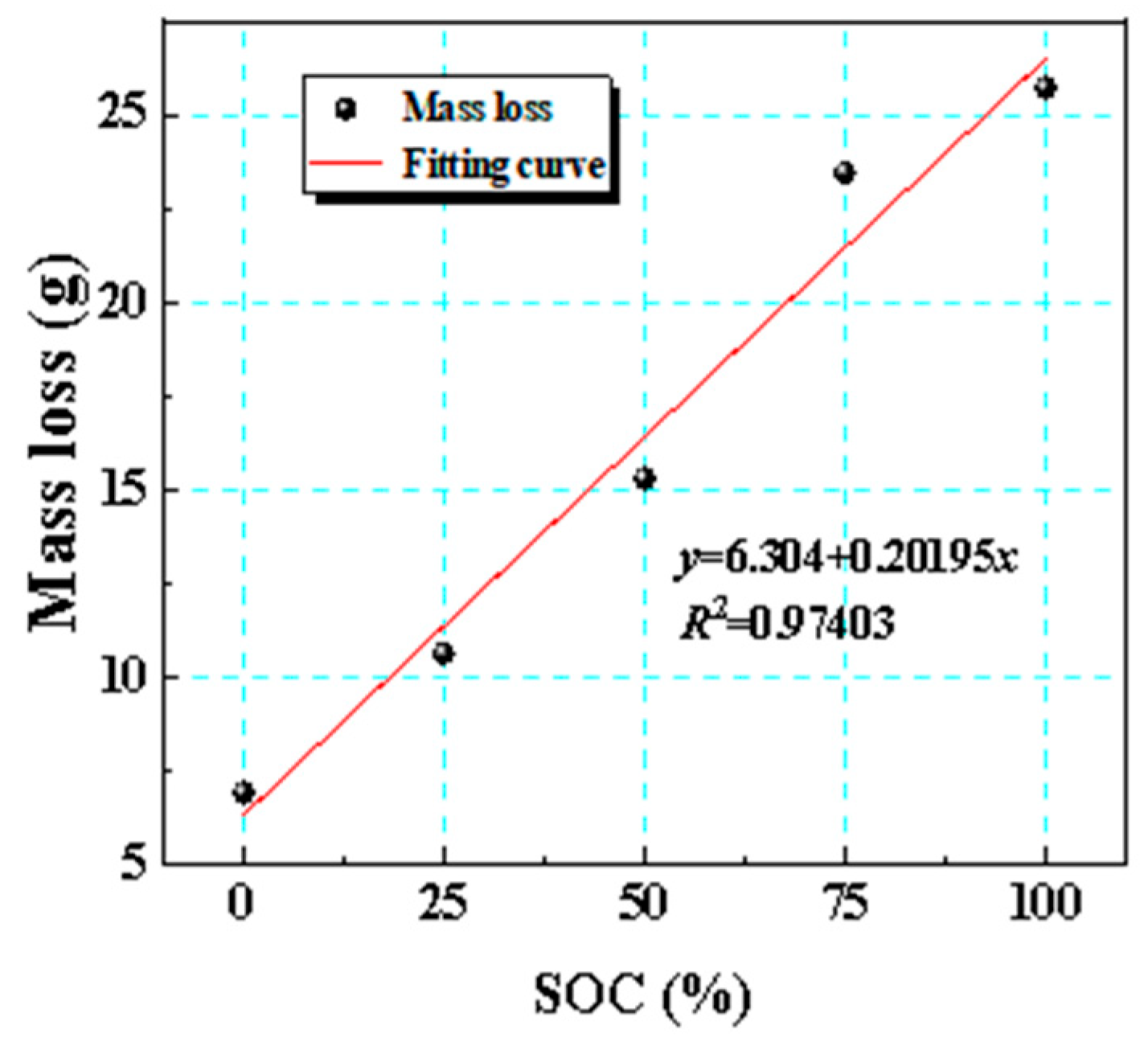
Figure 9 illustrates the fitted curve of the mass loss of the battery as it varies with its SOC. By conducting linear regression analysis on the mass loss data collected under five different SOCs, we clarified the relationship between SOC and mass loss. The analysis showed that as the SOC increased, mass loss exhibited a quantitatively distinct linear increase. This indicated that an elevated SOC substantially accelerated the mass loss process during thermal runaway, further emphasizing that the SOC was one of the key factors affecting the batteries’ thermal stability.
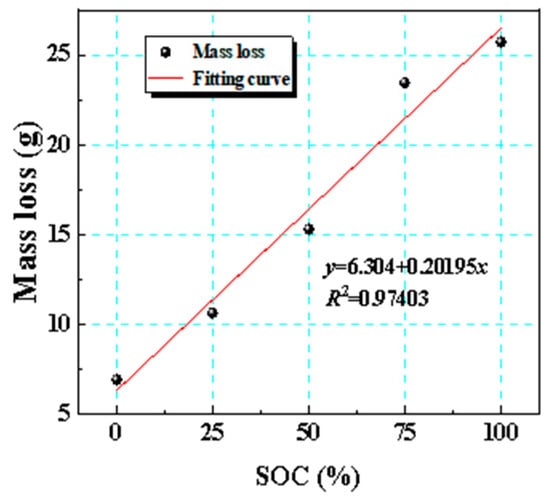
Figure 9.
Fitted curve of mass loss with respect to SOC variation.
4.3. Thermal Runaway Surface Temperature Analysis
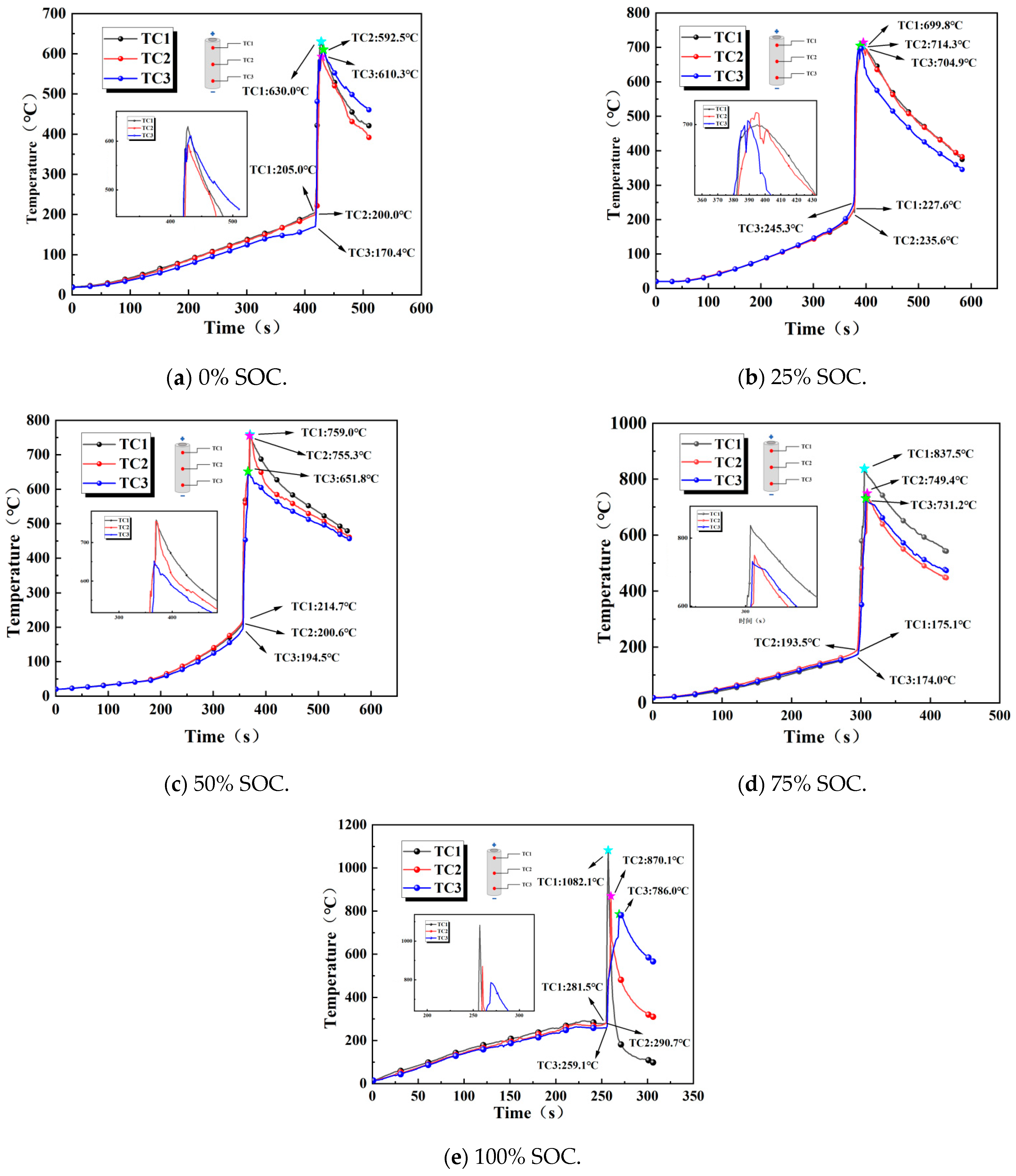
Comparing the surface temperature trends of the batteries during thermal runaway under different charge states revealed the impact of the charge state on the thermal runaway process. In this stage, we primarily measured the temperature alternations at three locations, namely, TC1 on the positive side, TC2 in the center, and TC3 on the negative side of 18,650 LIBs, during thermal runaway, as demonstrated in Figure 10.
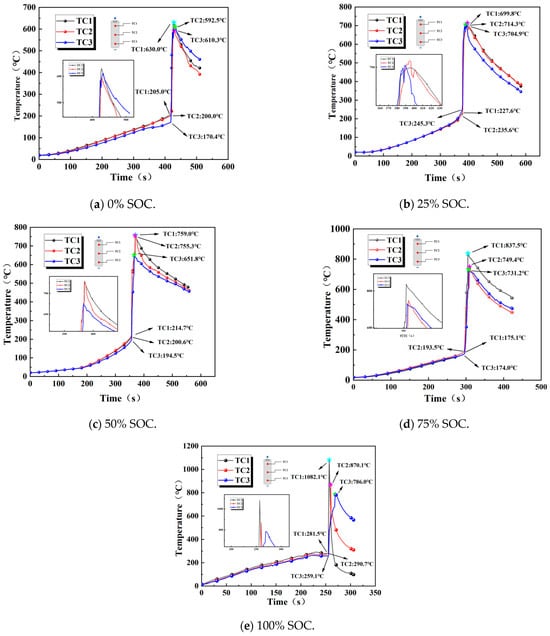
Figure 10.
Variation in the surface temperatures of 18,650 LIBs in different charge states.
When an external heating rod was applied to the 18,650 LIBs, their surface temperature gradually increased, and the internal separator began to melt, with chemical reactions becoming increasingly active. As the temperature continued to rise, most of the internal separator decomposed completely, and the adhesion between the positive and negative electrode materials intensified, increasing the energy of the chemical reaction. The safety valve was then triggered, and the internal temperature reached critical values at 418, 377, 356, 295, and 255 s, thus initiating thermal runaway in the batteries. The flammable smoke emitted mixed with the air and was ignited by the external high temperatures, a process accompanied by the emission of sparks. At this point, the surface temperature of the batteries rose sharply to a maximum, and as the high-temperature gas carried away most of the heat, the internal combustible material was consumed, causing the surface temperature of the batteries to gradually decrease.
Scrutinizing the temperature curve longitudinally revealed that due to the heating rod’s upward heat transfer path having a certain thickness during heating, the internal reactions within the battery were uneven. Therefore, there was a temperature gradient across the three measurement points on the battery, and the response speed was quantified by both the time until the critical temperature was reached (Table 6) and the initial heating rate (ΔT/Δt in 0–100 s). Spatial differences arise from uneven joule heating and material reactivity, with SOC modulating the heat source distribution [34] and with TC1 responding the fastest and TC3 the slowest. The temperature responses regarding thermal runaway in the LIBs under different SOC levels showed noteworthy spatial differences. At a 0% SOC, the temperature response was fastest at the cathode-side measurement point, TC1, and slowest at the center measurement point, TC2; at a 25% SOC, the central region (TC2) reached the critical temperature earliest (377 s), while the cathode side (TC1) exhibited the slowest initial heating rate (slope: 0.8 °C/s vs. TC3: 1.2 °C/s). This result suggests localized heat accumulation in low-SOC batteries, attributed to the insufficient active material and reaction energy in low-SOC batteries, leading to reduced heat generation rates and an uneven distribution, causing localized thermal fluctuations. At 0% and 50% SOCs, the temperature response followed a pattern consisting of TC1 (cathode side) > TC2 (center) > TC3 (anode side), primarily because the high SOC exacerbated polarization effects: charged transfer impedance in the cathode material increases dramatically in the high-deintercalation state, leading to prominent accumulation of Joule heat at the cathode–conductor interface. TC2 experienced a gradient temperature rise due to thermal conduction from both the cathode and anode sides, while the anode side (TC3), despite better heat dissipation conditions, had the slowest temperature response due to its low electrochemical activity and minimal heat generation. This pattern indicated that the SOC primarily influenced the evolution of the temperature field by regulating polarization and the distribution of heat sources.

Table 6.
Critical temperature parameters of thermal runaway for 18,650 LIBs in different charging states.
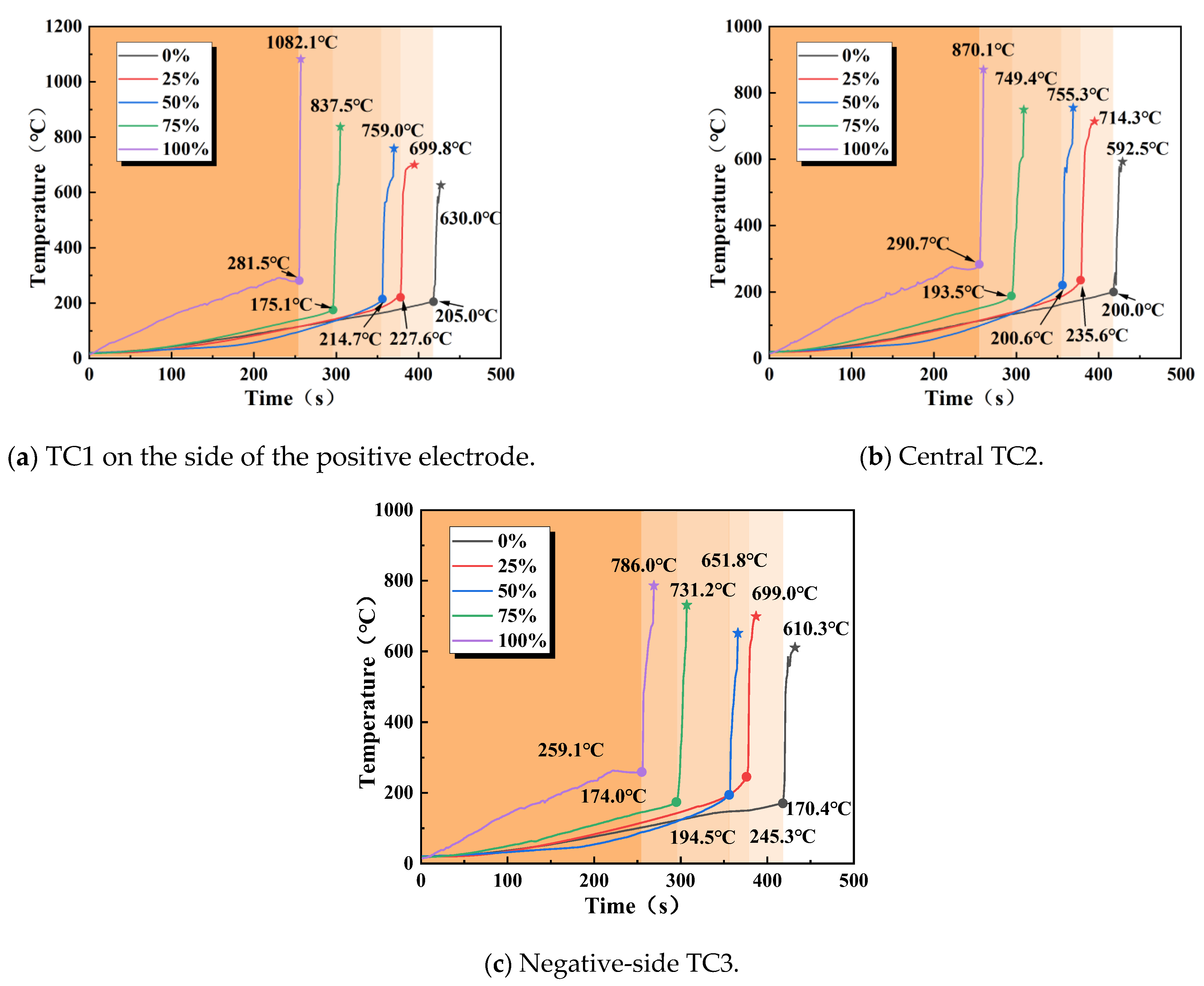
The dual influence of the SOC on the thermal runaway temperature characteristics of LIBs (as shown in Figure 11) was evident. One factor was the critical temperature Tc (critical temperature), and the other was the maximum surface temperature Tmax during thermal runaway. As shown in Table 6, the critical temperature (Tc) changed non-monotonically with an increasing SOC: as the SOC increased, the number of high-valence ions in the battery rose, leading to reduced internal stability and an increase in thermal runaway risk. At low SOCs (0% or 25%), the delay in triggering was due to the inert nature of active materials and low internal stress; in the tested case at a 50% SOC, the critical temperature was lower than that at a 25% SOC, though replication is needed to confirm the statistical significance of this finding. This change may be closely related to electrochemical reactions and charge conduction within the battery [34]. Conversely, at a high SOC (100%), the intense Joule heating caused by lithium depletion in the cathode resulted in the shortest triggering time; at a 75% SOC, the accumulated heat from charging and structural degradation generated more heat during thermal runaway, while the cooling capacity was relatively limited, causing prompt heat accumulation within the battery and the explosive threshold to be reached before the temperature rose to the corresponding level, leading to thermal runaway before the temperature reached the critical point.
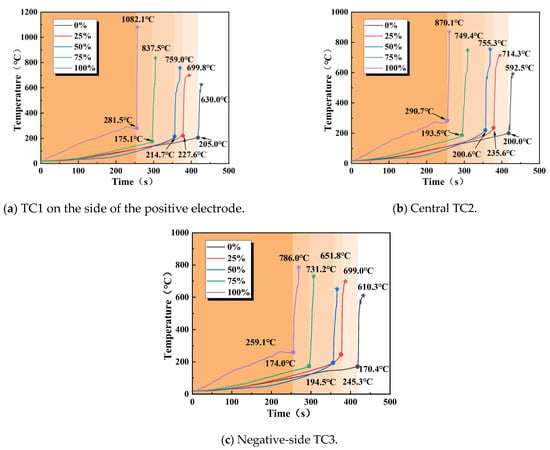
Figure 11.
Surface temperature variation of 18,650 LIBs at different locations under different SOCs.
The maximum temperature (Tmax) is noteworthily positively correlated with the SOC, and the temperature gradient on the positive electrode side increased as the SOC rose: as shown in Table 7, from a 0% to 100% SOC, the Tmax1 on the positive electrode side rose sharply from 630.0 to 1082.1 °C (an increase of 71.8%), far exceeding the temperature difference on the negative electrode side (the temperature difference between the positive and negative electrodes at a 100% SOC reached 296.1 °C). The underlying mechanism lies in how the SOC regulated the distribution of active lithium (a low SOC led to lithium depletion on the negative electrode, while a high SOC led to lithium depletion on the positive electrode) and the intensity of interfacial side reactions, driving thermal runaway from an “energy-limited” state at a low SOC to a “kinetics-dominated” state at a high SOC. At a 75% SOC, the sensitivity window for thermal runaway was shaped by lattice strain and lithiation synergy, while a fully charged state poses an extreme fire risk due to intense chain exothermic reactions.

Table 7.
Maximum temperature parameters of thermal runaway for 18,650 LIBs in different charging states.
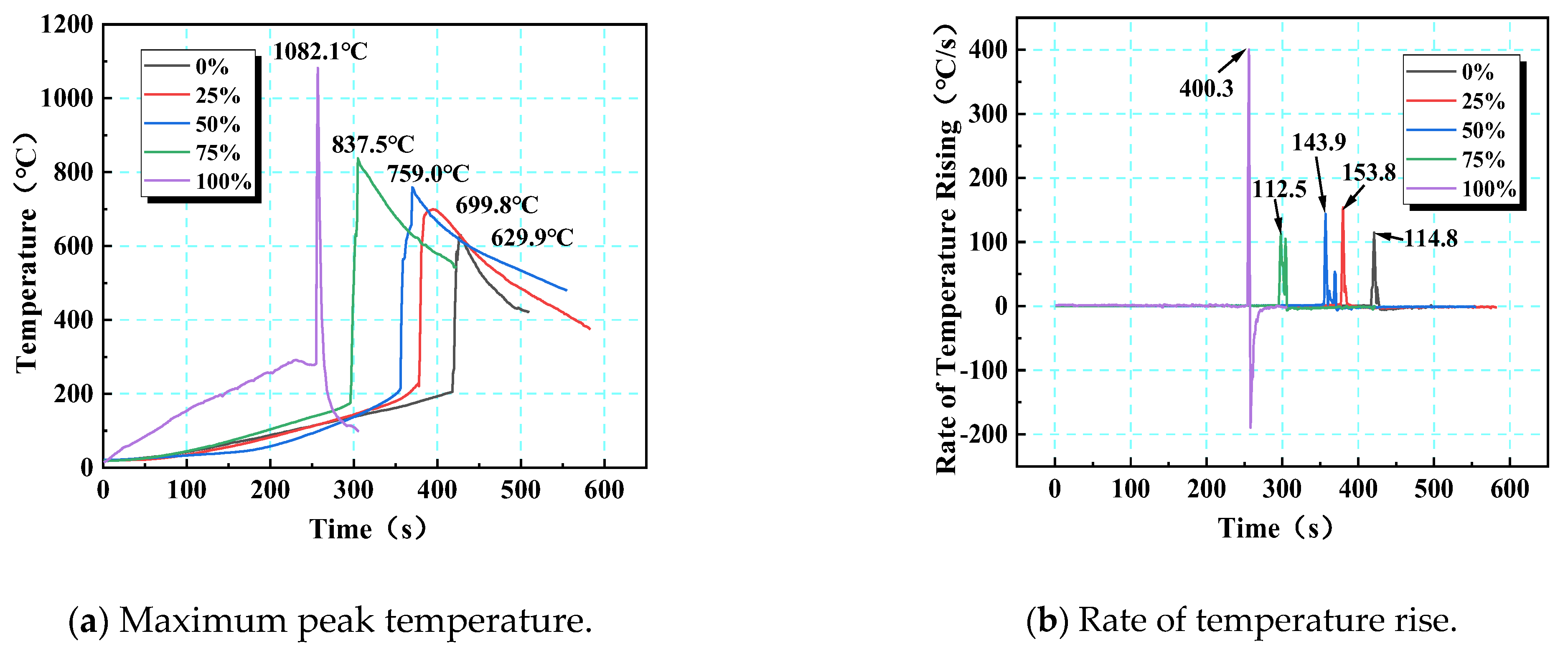
The instantaneous intensity of thermal runaway in LIBs can be quantitatively characterized according to the rate of temperature rise. Based on constant-power heating experiments, the temperature rise rate curves for different SOC levels show salient differences (Figure 12): the maximum temperature rise rates at 0%, 25%, 50%, 75%, and 100% SOC were 114.8, 153.8, 143.8, 112.5, and 400.3 °C/s, respectively. Before the safety valve opened, the temperature rise rate was mainly governed by external heating, showing a gentle trend; after the critical point was reached, internal chain reactions triggered a sudden increase in the temperature rise rate. The high-SOC (≥50%) batteries had substantially accelerated reaction kinetics due to chemical potential accumulation (such as a 3.5-fold increase in energy density at a 100% SOC), with the peak temperature rise rate increasing by 248% compared with that at a 0% SOC. Notably, at 50% and 75% SOCs, the temperature rise curve exhibits secondary peaks due to gas emission disturbances, while at a 100% SOC, the temperature rise rate becomes negative (−85 °C/s) after reaching the peak temperature (1082.1 °C), indicating that the high-SOC batteries exhibited both intense and short-term self-limiting characteristics during thermal runaway. Notably, at a 75% SOC, the critical temperature (175.1 °C) was lower than that at a 50% SOC (214.7 °C), possibly because of accelerated structural degradation at intermediately high SOCs enhancing thermal sensitivity. However, the 100% SOC exhibited the highest temperature extremes and the most intense reaction kinetics. This pattern revealed that SOC regulates the intensity of thermal runaway transient events and the mode of energy release through the competition between chemical energy storage and reaction pathways.
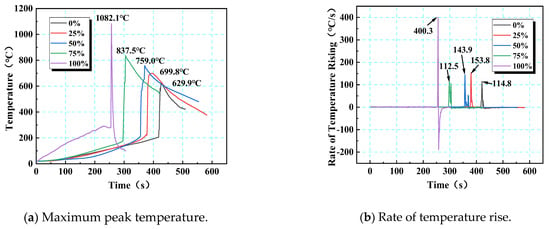
Figure 12.
Time–temperature and rate of time–temperature rise variation curves of the maximum peak temperature at different SOCs.
4.4. Analysis of Gas Release Characteristics of Thermal Runaway
4.4.1. Real-Time Gas Composition Analysis of Thermal Runaway
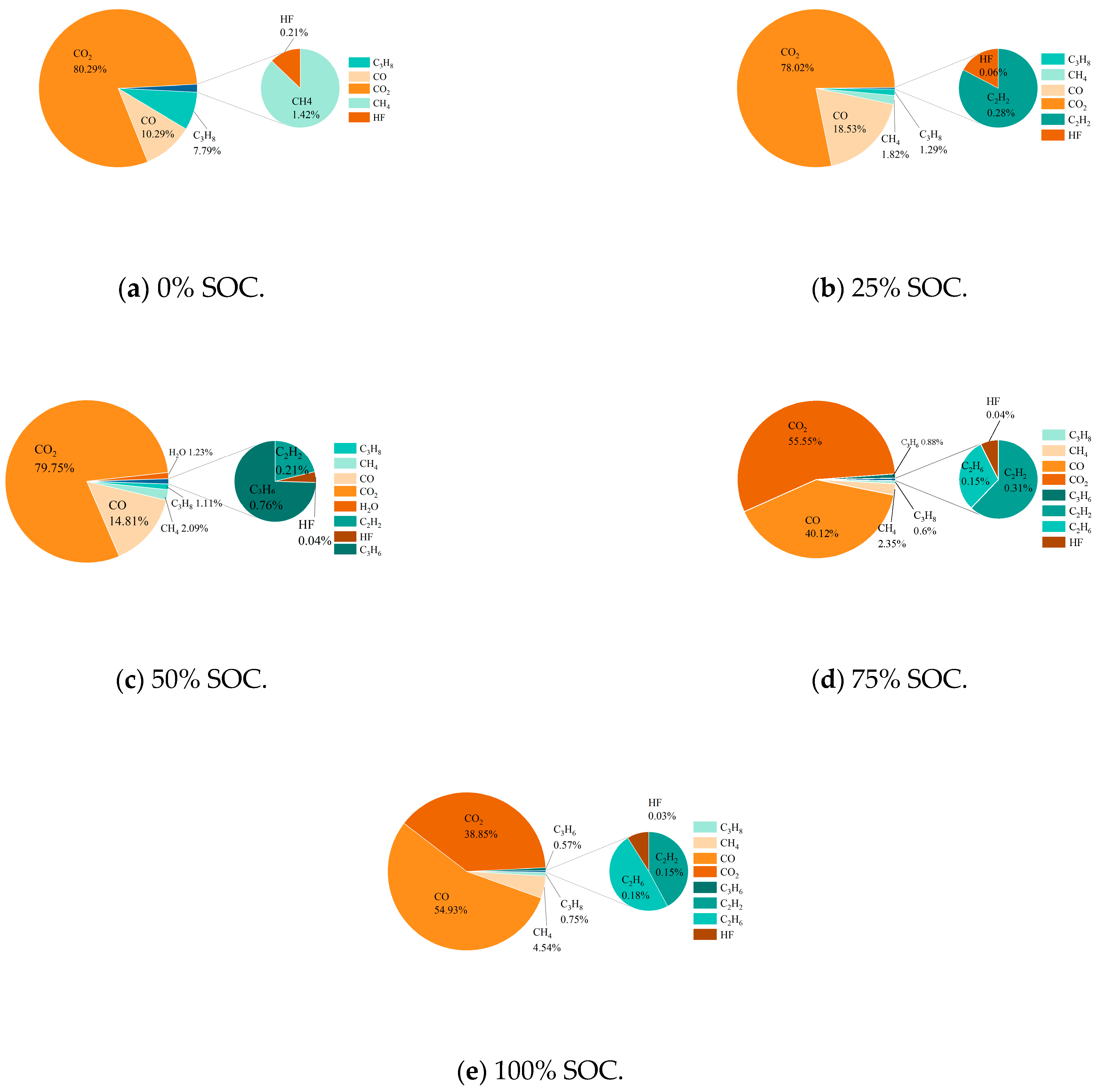
The gas generation behavior of thermal runaway in LIBs directly affects their safety. When the internal pressure exceeds the safety valve threshold, gas is released. An explosion may be triggered if the concentration reaches the explosive limit and the gas comes into contact with a high-temperature ignition source. The experiments showed (Table 7) that at a low SOC (0% and 25%), the number of different types of gases produced increased as the SOC rose because of specific temperature thresholds required for side reactions, whereas at a high SOC (75% and 100%), the intensity of reactions increased, with both the positive and negative electrodes being deeply involved in the reaction, leading to similar gas components and a noteworthy increase in hydrocarbons (C2−C3) (for example, the volume fraction of C3H8 reached 12.7% at 100% SOC). This finding indicates that a high SOC drives the reaction pathway toward long-chain hydrocarbon formation [35,36]. This pattern revealed that SOC regulates the degree of material decomposition and thermodynamic competition, thus controlling the toxicity of the gas generated and the risk level regarding explosions.
Figure 13 shows that the gases released during thermal runaway in the 18,650 LIBs were primarily CO2 (accounting for the highest proportion), CO, and CH4, with trace amounts of HF and CxHy. In practice, CO2 originates from the decomposition of the SEI film, oxidation of the electrolyte, and combustion processes, while hydrocarbons (such as C2H6 and C3H8) are mainly generated from the reaction between lithium at the negative electrode and organic solvents in the electrolyte. Some of these gases are consumed by combustion with oxygen during the eruption, while the rest are emitted as smoke [37]. As the SOC increases, the proportion of CO2 decreases, and the dominant reactions shift towards SEI decomposition and electrolyte pyrolysis. Here, the quantity of CO increases due to enhanced reduction reactions initiated by CO2, and the quantity of CH4 substantially increases because of an increase in the number of active reduction reactions at a high SOC. Although HF was present in very low proportions (0.03% to 0.21%), it is highly toxic, and it originates from oxidation byproducts of the electrode/electrolyte. At a high SOC, the flammability of gas components (CH4/hydrocarbons) and toxicity (CO/HF) increase synergistically, exacerbating the risk of explosion and health hazards. The generation mechanism is as follows:
LiPF6→LiF + PF5
PF5 + H2O→POF3 + 2HF
LiPF6 + H2O→LiF + POF3 + 2HF
POF3 + H2O→POF2(OH) + HF
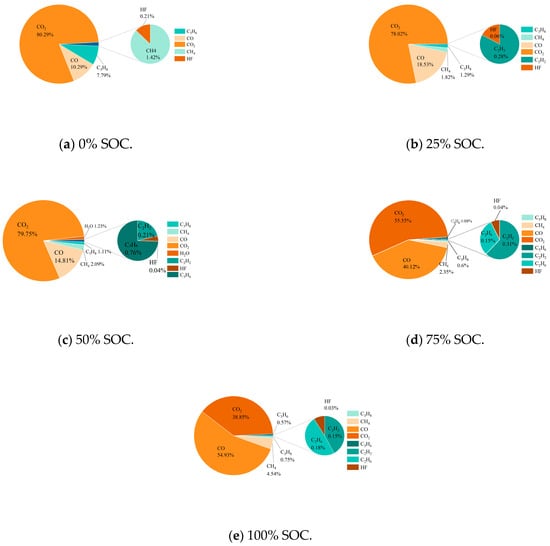
Figure 13.
Proportion of gas produced in real time during thermal runaway in 18,650 LIBs in different charging states.
HF accounts for a relatively low proportion of the total amount of gas produced, mainly because its generation is limited by competitive pathways of electrolyte/electrode decomposition reactions and it is partially consumed during release through safety valves or shell cracks. While toxic gases, such as HF, are present in smaller proportions (ranging from 0.03% to 0.21% across different SOC levels), their toxicological significance cannot be overlooked. Specifically, HF is a highly toxic gas known to cause severe respiratory and ocular irritation, and long-term exposure can lead to more serious health effects, including fluorosis and bone damage. According to the occupational exposure limits (OELs) set by various health and safety agencies, the maximum allowable concentration (MAC) for HF is typically around 2 mg/m3. In this study, although the absolute concentrations of HF detected were relatively low, they could still approach or exceed these limits under certain conditions, particularly in confined spaces or during prolonged exposure. Therefore, the presence of HF, even in small quantities, poses a substantial health risk that warrants further attention and mitigation measures in terms of battery safety design and emergency response protocols.
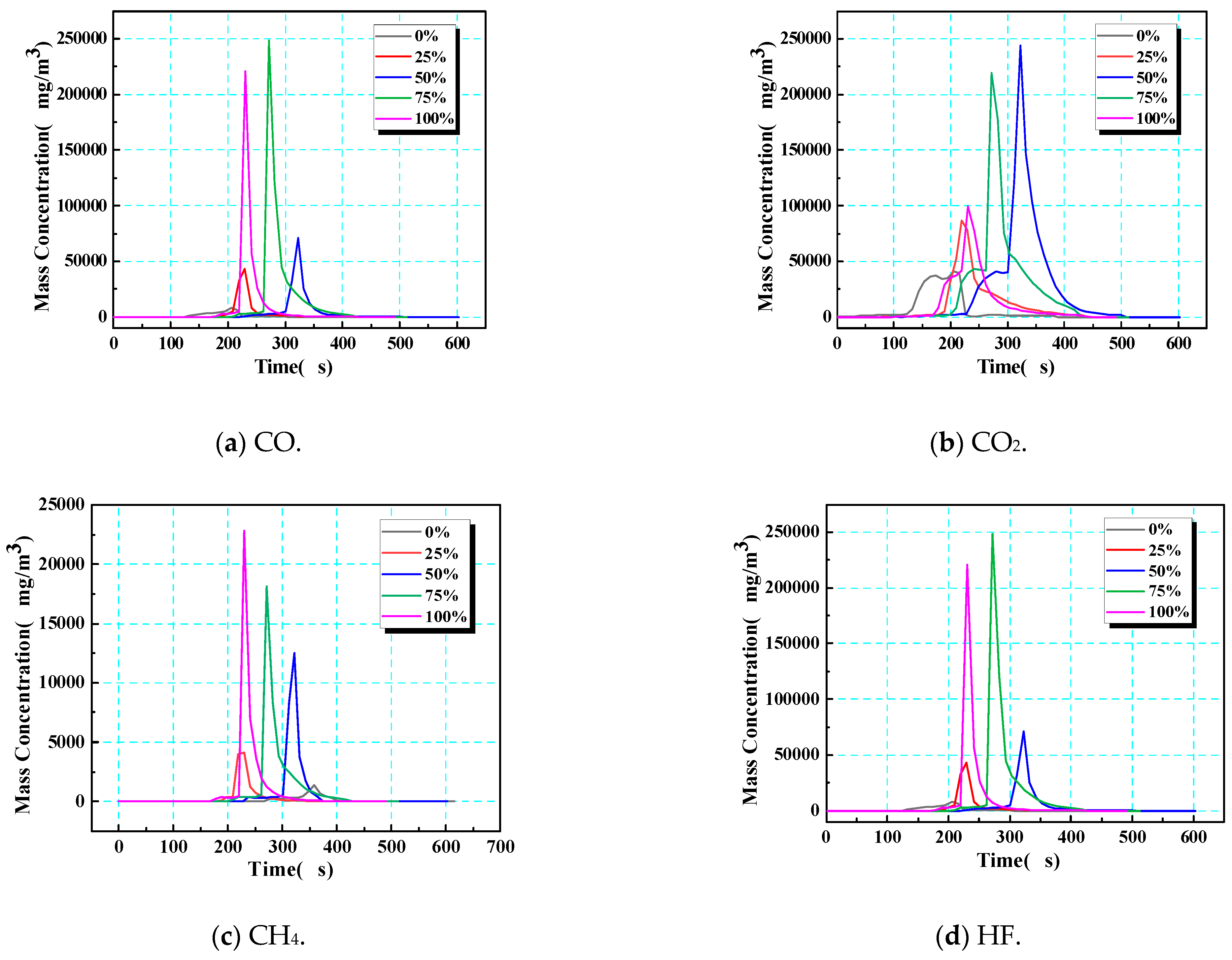
In the gas produced by thermal runaway in 18,650 LIBs, CO, CO2, and CH4 account for more than 90% of the total, while HF accounts for a low proportion, although it should be borne in mind that this gas is highly toxic/corrosive. The dynamic release of combustible/toxic gas and the duration of this hazard can be evaluated by analyzing the change in mass concentration over time (Figure 14). Notably, the gas eruption observed in Figure 14 corresponds to safety valve opening—a prerequisite for thermal runaway but not its onset. At low SOCs (0% and 25%), electrolyte decomposition produces gas gradually, causing early valve opening (e.g., 320 s at a 0% SOC) yet requiring prolonged heating to accumulate enough heat to reach the critical temperature (418 s). Conversely, at a 100% SOC, swift pressure buildup triggers valve opening at nearly the same time as thermal runaway is triggered (ΔT < 2 s), explaining the apparent time reversal in Figure 14.
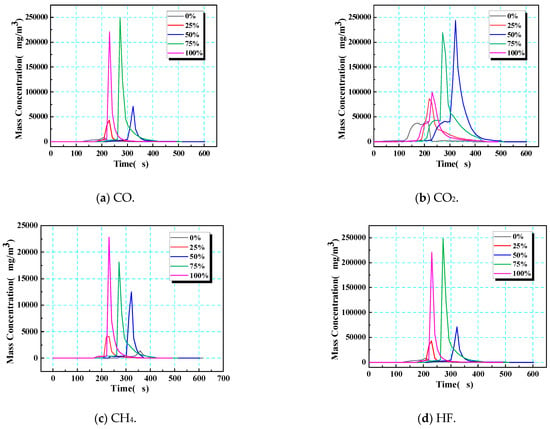
Figure 14.
Time–mass concentration of gases after the opening of the safety valve (corresponding to the initiation of gas eruption) during thermal runaway.
This SOC-dependent decoupling between gas release and thermal runaway ignition stems from two competing effects: (1) at a low SOC, higher SEI stability delays exothermic reactions despite early venting; (2) at a high SOC, metastable electrode materials accelerate chain reactions post-venting, minimizing the delay. Under different SOC conditions, the gas generation behavior of 18,650 LIBs showed regular differences: at a low SOC (0%), the total amount of gas produced was the lowest, and the release times of CO, CO2, and HF were the shortest; at medium SOCs (25% or 50%), total gas production gradually increased, and the CH4 concentration continued to rise with an increasing SOC; at high SOCs (75% or 100%), due to the change in the decomposition mechanism of electrolytes and electrode materials, the yields of CO2 and HF distinctly increased, revealing an evolutionary trend towards higher toxicity and corrosion under high-SOC conditions.
4.4.2. Analysis of the Gas Explosion Limit in Thermal Runaway
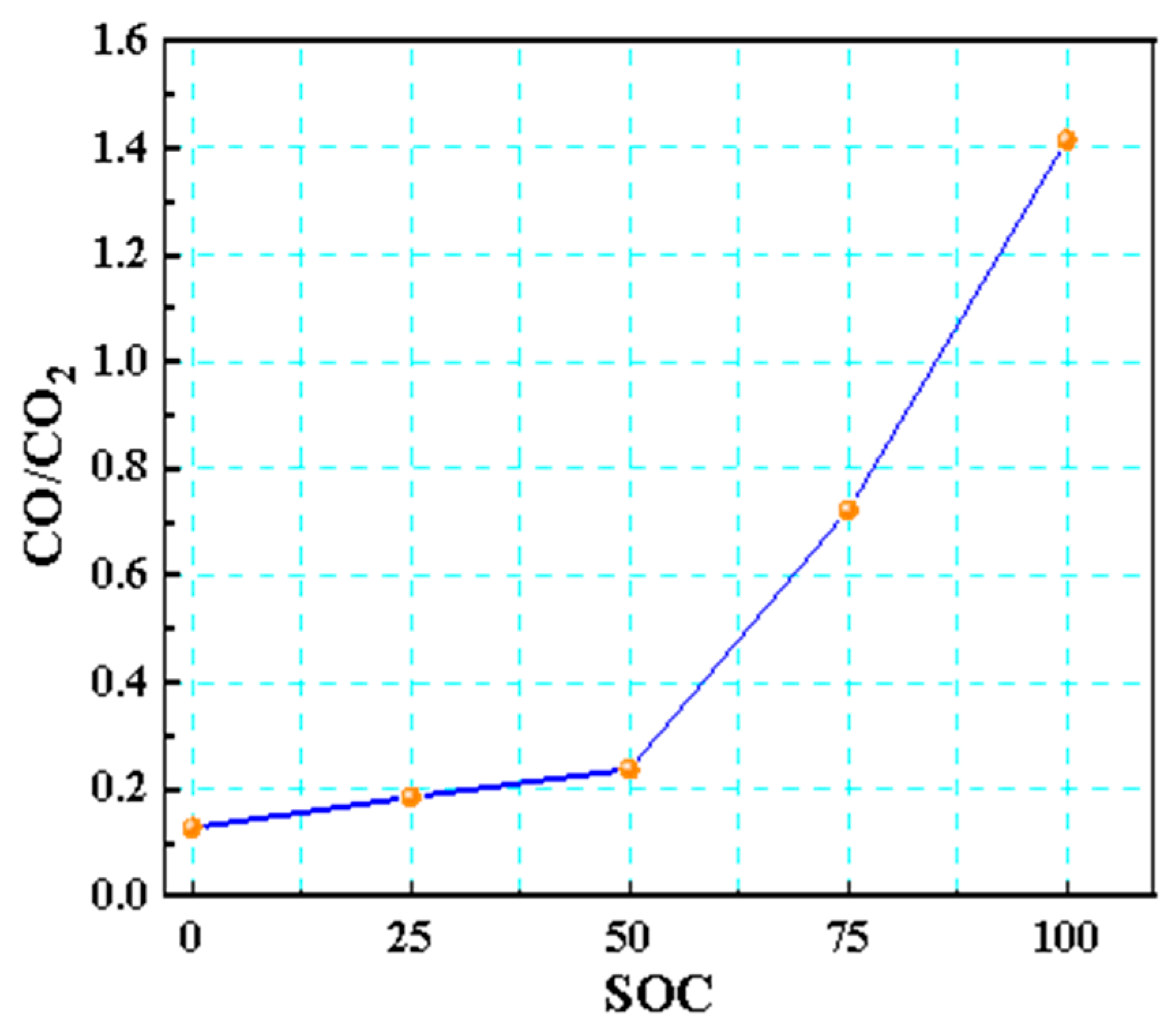
The CO/CO2 ratio is a key indicator for assessing the risk of thermal runaway fires in LIBs (Figure 15). As the SOC increased, this ratio rose markedly, with 50% SOC being the critical threshold. At a 100% SOC, the ratio exceeded 1, indicating that intense incomplete combustion predominates at high SOCs, leading to a surge in the release of toxic gases, such as CO and HF, presenting an extreme [35] level of fire risk. This pattern revealed that a high SOC suppressed complete oxidation pathways, exacerbating the release of toxic carbon-containing gases and raising the risk of combustion and explosion.
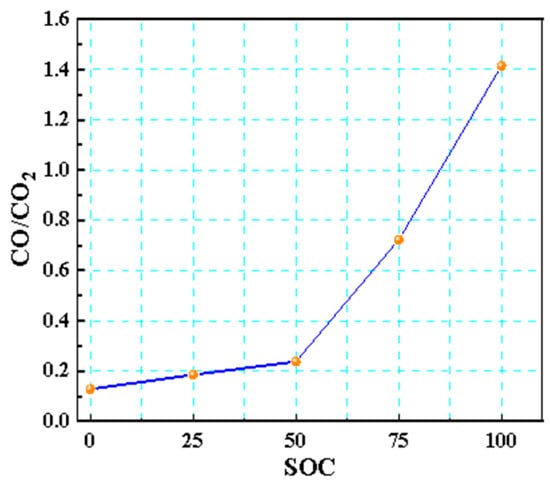
Figure 15.
CO/CO2 ratio of 18,650 LIBs in different charging states.
The fire risk posed by thermal runaway in LIBs is primarily attributable to flammable gases (CO, CH4, and CxHy). To quantify this risk, we employed the L-C formula combined with the paired elimination principle: the inert gas CO2 was paired with the flammable components to form a “composite” flammable gas. The explosive limit range of the mixed gas was calculated using the multi-component explosion limit formula (Equation (5)). Equation (5) is illustrated below:
In Equation (5), Lm is the explosion limit of combustible gas, as a %; L1, L2...Ln is the explosion limit of each gas component in the mixed gas, as a %; and V1, V2...Vn is the volume proportion of each component in the mixed gas, as a %.
Simply substitute the upper and lower explosion limit (LEL) values of each flammable gas component into the formula to solve for the combustible upper and lower limit ranges of the mixed gas. In our assessment, we focused on the LEL and the width of the concentration range; the lower the LEL, the more readily the gas ignites; the wider the concentration range, the higher the level of combustion flexibility, and the potential risk multiplies accordingly [38,39]. This method systematically revealed the regulatory mechanism of the SOC with respect to the sensitivity of mixed-gas combustion and explosion.
To illustrate the application of the multi-component explosion limit equation (Equation (5)), we provide a specific calculation example. Suppose we have a gas mixture consisting of CO, CH4, and C3H8 with the following volume proportions and LELs:
CO: 30%, LEL = 12.5 vol%;
CH4: 50%, LEL = 5.0 vol%;
C3H8: 20%, LEL = 2.1 vol%.
Using Equation (5), the LEL of the mixture (Lm) can be calculated as follows:
This calculation demonstrates how the LELs of individual gases can be integrated to determine the LEL of the gas mixture. In the above calculation, the gas concentrations were normalized based on volume proportions. This approach is commonly used in explosion limit calculations for gas mixtures, as it directly relates to the volume of gas present in the mixture. Alternatively, mass-based fractions could also be used, but in this study, we focused on volume proportions due to their direct relevance to the explosive properties of gas mixtures.
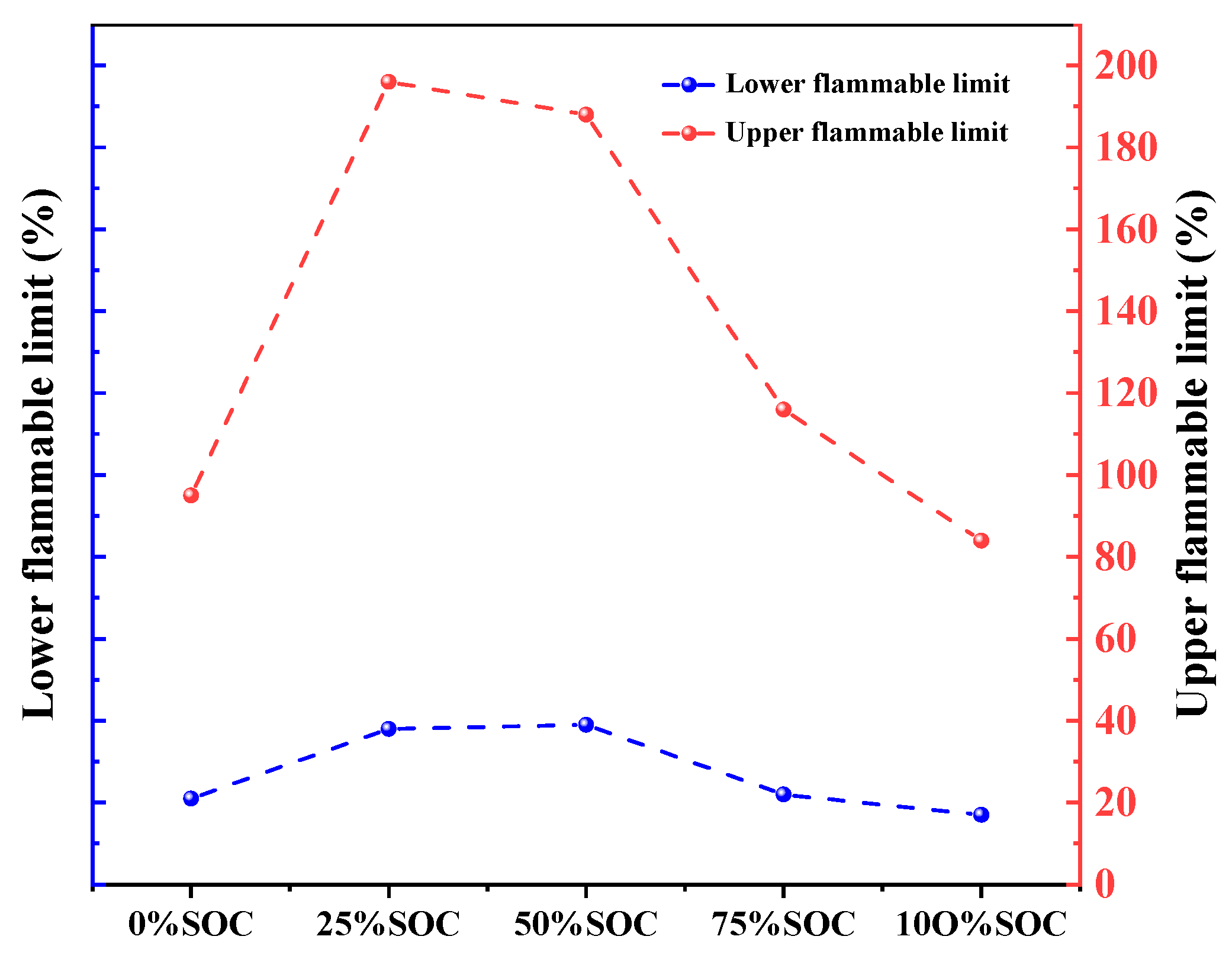
Table 8 is combustion limit range of 18,650 LIBs in different SOCs. Figure 16 and Table 9 show that the explosive limit of flammable gases from thermal runaway in 18,650 lithium-ion batteries evolves nonlinearly with the SOC: at a 0% SOC, the explosive limit ranges from 21% to 95%, increasing to 38% to 196% and 39% to 188% at 25% and 50% SOCs, respectively; this range drops sharply to 17% to 84% at a 75% SOC and returns to 38% to 196% at a 100% SOC. Low/high SOCs (0%, 75%, and 100%) had lower limits (≤38%) and narrower ranges (Δ ≤ 74%), rendering the gases more prone to ignition but with limited diffusion; and medium SOCs (25% and 50%) have higher lower limits (≥38%) and wider ranges (Δ ≥ 158%), requiring a higher ignition energy but with an expanded potential for explosion. This pattern revealed that the SOC can dynamically balance the risk of immediate ignition and continuous diffusion by adjusting the gas composition ratio.

Table 8.
Combustion limit range of 18,650 LIBs in different SOCs.
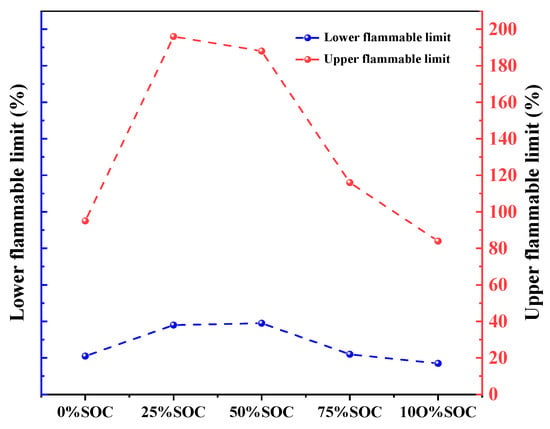
Figure 16.
Line graph of the flammability limit range of 18,650 LIBs in different charging states.

Table 9.
Types of gases detected in real-time monitoring of thermal runaway in 18,650 LIBs in different charging states.
5. Conclusions
Based on the 18,650 LIB thermal runaway experimental platform, we drew the following conclusions through multi-dimensional parameter analysis:
- Temperature response and gas eruption: After the safety valve was opened, the accumulated gas erupted, forming a combustible mixture and igniting, and the higher the SOC, the more intense the temperature distribution of the infrared thermal image, indicating that the charged state had a notable impact on the thermal runaway temperature distribution of 18,650 LIBs. Quantifying gas explosion risks via flammability limit calculations revealed lower explosive limits (17–21%) at extreme SOCs. Spatial thermal evolution ascertained through synchronized multi-point monitoring showed high-SOC-induced cathode-side dominance.
- Law of mass loss: Mass loss after thermal runaway was strongly positively correlated with the SOC (6.90–25.75 g corresponds to a 0–100% SOC), indicating that the SOC is the key factor affecting mass loss during thermal runaway in 18,650 LIBs.
- Reaction intensity classification: Batteries with high SOCs (≥50%) generally exhibited elevated critical and maximum temperatures, though the 75% SOC LIB showed a slight deviation in critical temperature (Table 5). The rate of temperature rise accelerated markedly at a 100% SOC, triggering chain exothermic reactions.
- Gas generation characteristics and fire risk: CO, CO2, and CH4 accounted for over 90% of the gases generated, with incomplete combustion being prominent at a 100% SOC, making this SOC the most hazardous for fires; the lower limit of flammability was low (gas readily ignited but with a narrow concentration window) at low/high SOCs, while the flammability range was wide at a 25–50% SOC, indicating that gas hazards were relatively low during thermal runaway but the potential for combustion remained high. Therefore, the SOC influences the intensity of energy release, gas toxicity, and fire dynamics by regulating the distribution and reaction pathways of active materials.
Author Contributions
J.Z.: supervision, validation, investigation, and funding acquisition. K.X.: conceptualization, data curation, and writing—original draft. X.J.: resources, writing—review and editing, and project administration. X.S.: formal analysis and funding acquisition. C.-M.S.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Key Research and Development Program of Shaanxi (Program No. 2023-GHZD-29).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Xiangrong Sun was employed by the company Xi’an Urban Rural Water Utilities Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Guo, F.; Wu, X.-W.; Liu, L.-L.; Ye, J.-L.; Wang, T.; Fu, L.-J.; Wu, Y.-P. Prediction of remaining useful life and state of health of lithium batteries based on time series feature and Savitzky-Golay filter combined with gated recurrent unit neural network. Energy 2023, 270, 126880. [Google Scholar] [CrossRef]
- Ren, W.-H.; Ding, C.-F.; Fu, X.-W.; Huang, Y. Advanced gel polymer electrolytes for safe and durable lithium metal batteries: Challenges, strategies, and perspectives. Energy Storage Mater. 2021, 34, 515–535. [Google Scholar] [CrossRef]
- Liu, Z.J.; Han, K.-H.; Zhang, Q.; Li, M.-H. Thermal safety focus and early warning of lithium-ion batteries: A systematic review. Energy Storage 2025, 115, 115944. [Google Scholar] [CrossRef]
- Shan, T.-X.; Zhang, P.C.; Wang, Z.P.; Zhu, X.-Q. Insights into extreme thermal runaway scenarios of lithium-ion batteries fire and explosion: A critical review. Energy Storage 2024, 88, 111532. [Google Scholar] [CrossRef]
- Li, P.-C.; Ju, S.-X.; Bai, S.-X.; Zhao, H.; Zhang, H.-Y. State of charge estimation for lithium-ion batteries based on physics-embedded neural network. Power Sources 2025, 640, 236785. [Google Scholar] [CrossRef]
- Kim, J.; Bae, D.; Park, C.; Park, H. Pre-detection of thermal runaway in Li-ion 18,650 batteries via temperature and voltage: The importance of temperature measurement location. Appl. Therm. Eng. 2025, 269, 125991. [Google Scholar] [CrossRef]
- Feng, X.-N.; Zheng, S.-Q.; Ren, D.-S.; He, X.-M.; Wang, L.; Cui, H.; Liu, X.; Jin, C.-Y.; Zhang, F.-S.; Xu, C.-S.; et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl. Energy 2019, 246, 53–64. [Google Scholar] [CrossRef]
- Li, K.-J.; Chen, L.; Gao, X.-L.; Lu, Y.; Wang, D.-P.; Zhang, W.-X.; Wu, W.-X.; Han, X.-B.; Cao, Y.-C.; Wen, J.-Y.; et al. Implementing expansion force-based early warning in LiFePO4 batteries with various states of charge under thermal abuse scenarios. Appl. Energy 2024, 362, 122998. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.-B.; Stoliarov, S.-I.; Denlinger, M.; Masias, A.; Snyder, K. Heat release during thermally-induced failure of a lithium-ion battery: Impact of cathode composition. Fire Saf. J. 2016, 85, 10–22. [Google Scholar] [CrossRef]
- Li, Y.-X.; Jiang, L.-H.; Zhang, N.-J.; Wei, Z.; Mei, W.-X.; Duan, Q.-L.; Sun, J.-H.; Wang, Q.-S. Early warning method for thermal runaway of lithium-ion batteries under thermal abuse condition based on online electrochemical impedance monitoring. Energy Chem. 2024, 92, 74–86. [Google Scholar] [CrossRef]
- Mao, N.; Gadkari, S.; Wang, Z.-R.; Zhang, T.; Bai, J.-L.; Cai, Q. A comparative analysis of lithium-ion batteries with different cathodes under overheating and nail penetration conditions. Energy 2023, 278, 128027. [Google Scholar] [CrossRef]
- Zhou, Z.-Z.; Li, M.-Y.; Zhou, X.-D.; Li, L.; Ju, X.-Y.; Yang, L.-Z. Investigating thermal runaway triggering mechanism of the prismatic lithium iron phosphate battery under thermal abuse. Renew. Energy 2024, 220, 119674. [Google Scholar] [CrossRef]
- Lin, C.-J.; Mao, J.-B.; Yang, J.; Qi, C.; Zhang, Y.-M.; Dan, S.; Liu, X. Early warning for thermal runaway of Li [Ni0.5Co0.2Mn0.3] O2 and LiFePO4 batteries under external heating, penetration and overcharging conditions. Energy Storage 2025, 109, 115085. [Google Scholar] [CrossRef]
- Ren, D.-S.; Feng, X.-N.; Lu, L.-G.; He, X.-M.; Ouyang, M.-G. Overcharge behaviors and failure mechanism of lithium-ion batteries under different test conditions. Appl. Energy 2019, 250, 323–332. [Google Scholar] [CrossRef]
- Ouyang, D.-X.; Liu, J.-H.; Chen, M.-Y.; Wang, J. Investigation into the fire hazards of lithium-ion batteries under overcharging. Appl. Sci. 2017, 7, 1314. [Google Scholar] [CrossRef]
- Zhou, G.; Lu, H.-H.; Zhang, Q.; Yang, S.-Q.; Liu, Y.; Niu, C.-X.; Kong, Y.; Huang, Q.; Wei, Z.-K. Experimental study on thermal runaway and flame eruption characteristics of NCM523 lithium-ion battery induced by the coupling stimulations of overcharge-penetration. PSEP 2024, 191, 131–145. [Google Scholar] [CrossRef]
- Deng, J.; Chen, B.-H.; Lu, J.-Z.; Zhou, T.-N.; Wu, C.-P. Thermal runaway and combustion characteristics, risk and hazard evaluation of lithium-iron phosphate battery under different thermal runaway triggering modes. Appl. Energy 2024, 368, 123451. [Google Scholar]
- Wang, C.-J.; Zhu, Y.-L.; Gao, F.; Bu, X.-Y.; Chen, H.-S.; Quan, T.; Xu, Y.-B.; Jiao, Q.-J. Internal short circuit and thermal runaway evolution mechanism of fresh and retired lithium-ion batteries with LiFePO4 cathode during overcharge. Appl. Energy 2022, 328, 120224. [Google Scholar] [CrossRef]
- Yuan, Q.-F.; Zhao, F.-G.; Wang, W.-D.; Zhao, Y.-M.; Liang, Z.-Y.; Yan, D.-L. Overcharge failure investigation of lithium-ion batteries. Electrochim. Acta 2015, 178, 682–688. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Yang, X.-Q.; Zhang, G.-Q.; Huang, Q.-Q.; Xiao, C.-R.; Yang, C. Investigation on the root cause of the decreased performances in the overcharged lithium iron phosphate battery. Int. J. Energy Res. 2018, 42, 2448–2455. [Google Scholar] [CrossRef]
- Parasumanna, A.-B.-K.; Karle, U.-S.; Saraf, M.-R. Material characterization and analysis on the effect of vibration and nail penetration on lithium-ion battery. WEVJ 2019, 10, 69. [Google Scholar] [CrossRef]
- Li, A.-X.; Cui, Y.; Wang, G.-H.; He, X.-H.; Guo, Z.-L.; Wang, J.; Mao, Y.-Z. Pyrolysis pretreatment for recycling spent LiFePO4 batteries in argon gas: Kinetic behaviors and reaction mechanism. J. Environ. Chem. Eng 2025, 13, 117019. [Google Scholar] [CrossRef]
- Li, Y.; Du, J.-H.; Yang, S.-Z.; Zhang, M.-Q.; Tu, R.; Zhang, R.-C.; Bi, K. Study on thermal runaway of cylindrical lithium iron phosphate battery under needle prick. ESST 2019, 8, 559–566. [Google Scholar]
- Abaza, A.; Ferrari, S.; Wong, H.-K.; Lyness, C.; Moore, A.; Weaving, J.-L.; Blanco-Martin, M.; Dashwood, R.; Bhagat, R. Experimental study of internal and external short circuits of commercial automotive pouch lithium-ion cells. Energy Storage 2018, 16, 211–217. [Google Scholar] [CrossRef]
- Feng, X.-N.; Ouyang, M.-G.; Liu, X.; Lu, L.-G.; Xia, Y.; He, X.-M. Thermal runaway mechanism of lithium-ion battery for electric vehicles: A Review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Zhang, Y.; Ping, P.; Dai, X.-Y.; Li, C.-T.; Li, Z.; Zhuo, P.; Tang, L.; Kong, D.-D.; Yin, X.K. Failure mechanism and thermal runaway behavior of lithium-ion battery induced by arc faults. RSER 2025, 207, 114914. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Zhao, Y.-Q.; Bai, W.; Cao, Y.; Xu, W.-F.; Shen, X.-Q.; Wang, Z.-R. Study on the influence of high rate charge and discharge on thermal runaway behavior of lithium-ion battery. PSEP 2024, 191, 1483–1494. [Google Scholar] [CrossRef]
- Gachot, G.; Grugeon, S.; Eshetu, G.-G.; Mathiron, D.; Ribière, P.; Armand, M.; Laruelle, S. Thermal behaviour of the lithiated-graphite/electrolyte interface through GC/ MS analysis. Electrochimica. Acta 2012, 83, 402–409. [Google Scholar] [CrossRef]
- Gachot, G.; Ribière, P.; Mathiron, D.; Grugeon, S.; Armand, M.; Leriche, J.-B.; Pilard, S.; Laruelle, S. Gas chromatography and mass spectrometry as a suitable tool for the Li-ion battery electrolyte degradation mechanisms study. Anal. Chem. 2011, 83, 478–485. [Google Scholar] [CrossRef]
- Gerelt-Od, B.; Kim, J.; Shin, E.; Kang, H.; Kim, N.; Jo, C.; Son, H.; Yoon, S. In situ raman investigation of resting thermal effects on gas emission in charged commercial 18,650 lithium-ion batteries. Ind. Eng. Chem. 2021, 96, 339–344. [Google Scholar] [CrossRef]
- Zhang, Q.-S.; Qu, Y.-R.; Hao, Z.-L.; Liu, T.-T.; Chen, D. In situ analysis of thermal runaway gas in ternary lithium ion battery. High Volt. Tech. 2022, 48, 2817–2825. [Google Scholar]
- Liu, W.; Zhao, F.-S.; Yin, S.-F.; Ma, T.-Z.; Qing, J. Analysis of solid combustion products to establish a theoretical model of the causes of thermal runaway of ternary lithium-ion battery overcharge and heating. Fire Sci. 2023, 41, 3–15. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Weyhe, R.; Friedrich, B. Gas generation measurement and evaluation during mechanical processing and thermal treatment of spent Li-ion batteries. Waste Manag. 2019, 84, 102–111. [Google Scholar] [CrossRef]
- Gilaki, M.; Sahraei, E. Modeling state-of-charge dependent mechanical response of lithium-ion batteries with volume expansion. Energy Rep. 2024, 12, 3607–3619. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Li, F.-Z.; Yu, L.; Wang, Y.-H.; Wang, K.; Chang, C.-Y.; Li, M.-H.; Hao, W.-H.; Qian, X.-M. Research on the explosive characteristics and suppression mechanisms of gas generation during thermal runaway of batteries in a charged state. Chem. Eng. J. 2025, 505, 159699. [Google Scholar] [CrossRef]
- Zhang, Q.-S.; Qu, Y.-R. Study on gas toxicity of thermal runaway in recycled ternary lithium ion battery. Beihang Univ. 2024, 50, 1761–1769. [Google Scholar]
- Wang, Z.; Yin, B.; Zhao, Q.-J.; An, W.-G.; Shi, B.-B.; Jiang, L.-Y. Study on thermal runaway propagation characteristics of lithium iron phosphate battery pack under different SOCs. ELEC 2022, 12, 200. [Google Scholar]
- Wang, Y.; Ren, D.-S.; Feng, X.-N.; Wang, L.; Ouyang, M.-G. Thermal runaway modeling of large format high-nickel/silicon-graphite lithium-ion batteries based on reaction sequence and kinetics. Appl. Energy 2022, 306, 117943. [Google Scholar] [CrossRef]
- Said, A.-O.; Lee, C.; Stoliarov, S.-I.; Marshall, A.W. Comprehensive analysis of dynamics and hazards associated with cascading failure in 18,650 lithium-ion cell arrays. Appl. Energy 2019, 248, 415–428. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).