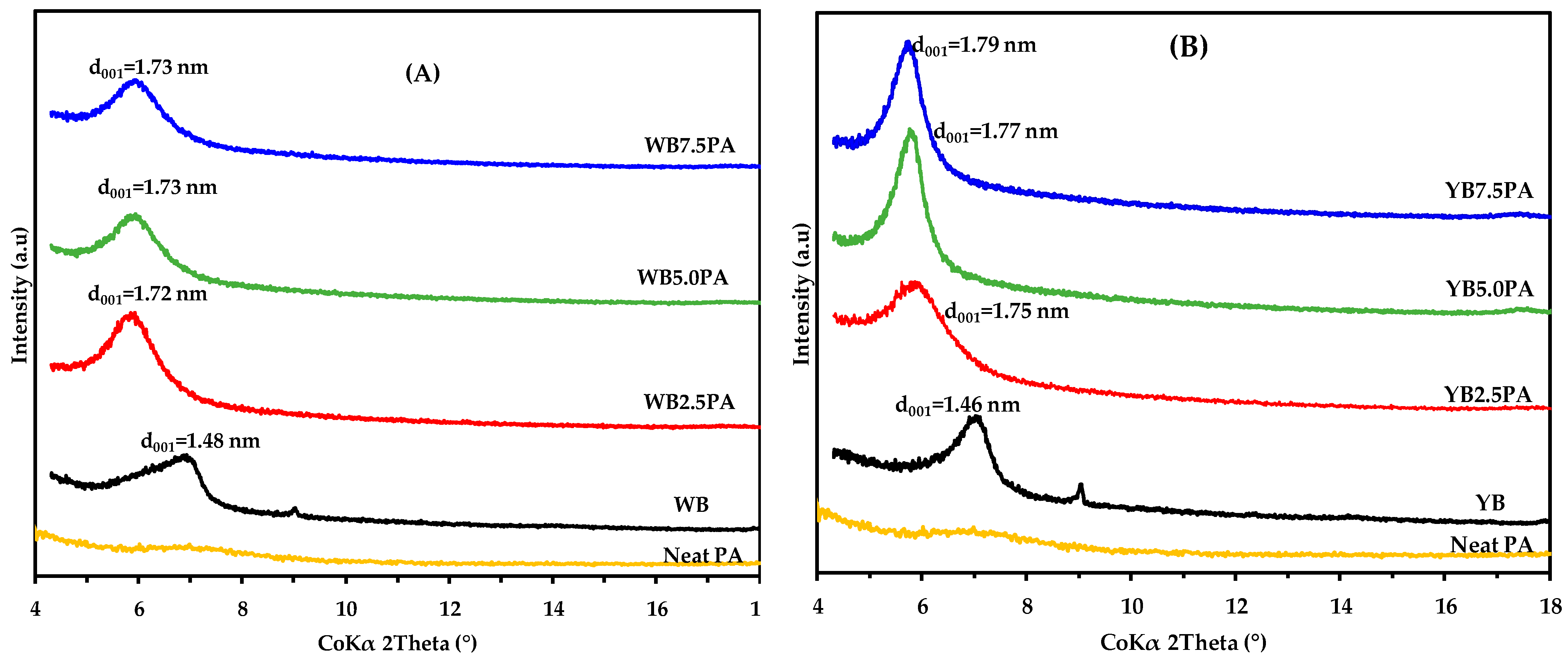
3.1. X-Ray Diffraction (XRD)
The XRD patterns of the neat PA, starting with the white and yellow bentonites (WB and YB), and polymer-based organoclays are shown in
Figure 1. As expected, the XRD pattern of neat dimer fatty acid-based polyamides displays a broad amorphous halo [
41], and the polymer-modified bentonite hybrids exhibit a characteristic (001) peak, indicating intercalation of polymer chains into the clay layers and structural organization absent in the neat polymer. From the figure, it is clear that clays exhibited d001 reflections corresponding to 1.48 nm for white bentonite and 1.46 nm for yellow bentonite, which is typical of clays containing an exchangeable cation of Na
+ and Ca
2+ [
14]. The presence of Ca
2+ ions in both bentonites reflects their natural calcium saturation, typical of Boane bentonites [
49,
50,
51], while the presence of Na
+ results from the modification process with Na
2CO
3 applied to the bentonites. The asymmetric broadening of the d
001 reflection in the white bentonite suggests reduced crystallinity, limited swelling capacity, and lower hydration power compared to the yellow bentonite [
18,
52].
The basal spacing values of the polymer-modified samples increased from 1.48 nm to a maximum of 1.73 nm for white bentonite (
Figure 1A) and from 1.46 nm to a maximum of 1.79 nm for yellow bentonite (
Figure 1B) as the polymer content was varied from 2.5 wt.% to 7.5 wt.%. This increment behavior suggests that treatment with protonated dimer fatty acid-based polyamide chains affected the clays. From the configuration structure, the d
001 values of 1.73 nm and 1.77 nm observed for the two organo-modified clay samples (WB and YB) suggest the appearance of two layers of ions in the interlamellar region [
32,
53], which typically show a basal spacing d
001 of 1.70 nm.
No significant changes were observed in d
001 across the three polymer contents (2.5 wt.%, 5.0 wt.%, and 7.5 wt.%) for both WB and YB samples. However, the yellow bentonite sample has a slightly larger d
001 than that of the white bentonite sample, suggesting that the yellow bentonite gives a better response to polymer modification. The higher cristobalite (SiO
2) content in white bentonite (35.1 wt.%) compared to yellow bentonite (23.3 wt.%) [
50,
54] may explain the lower response of white bentonite to polymer treatment.
3.5. Screening of Factors for d001
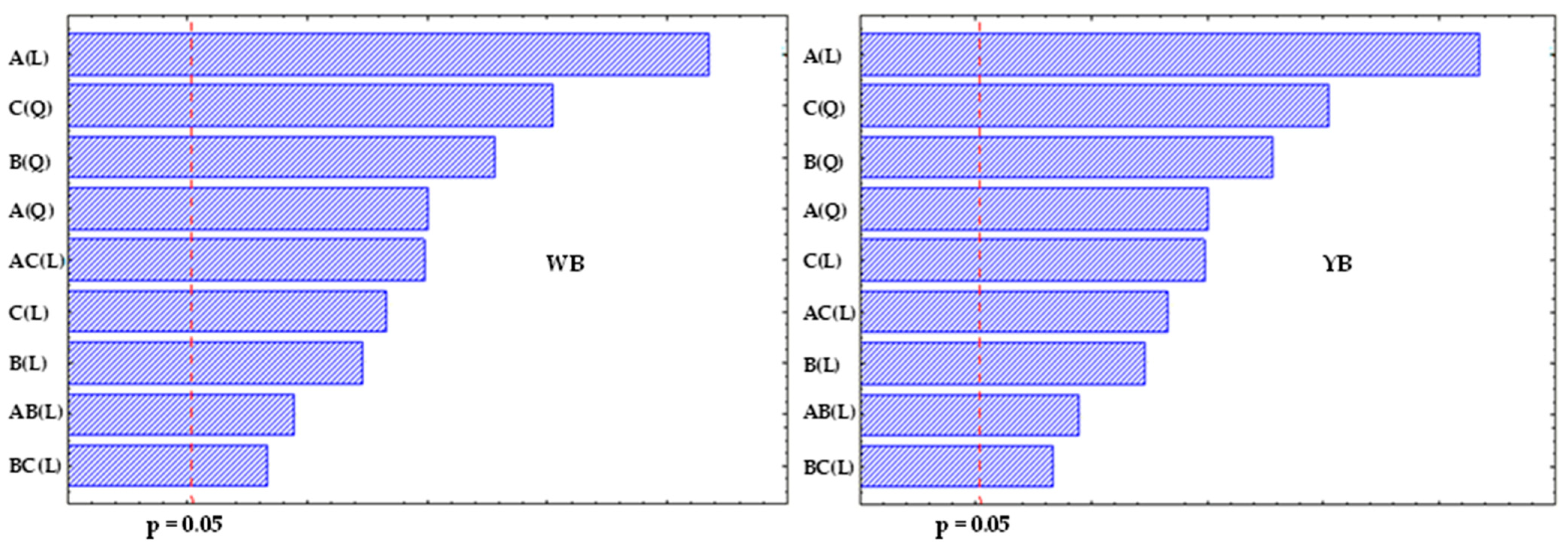
Figure 5 illustrates Pareto charts of the standardized effects at the
p = 0.05 level of significance for both WB and YB clay families, in which values above 0.05 indicate significant effects. The results show that all independent factor terms, whether linear and quadratic, and their interactions, significantly affect the extent of intercalation in polymer chains, with 95% confidence. The linear terms’ significance for the two clay families follows the order: temperature > stirring speed > contact time, while the quadratic terms’ significance is in the order: stirring speed > contact time > temperature. The significance of the combined or interaction effects appears in the following order: temperature/stirring speed > temperature/contact time > contact time/stirring speed.
Table 6 and
Table 7 show the ANOVA results for the interaction influences of the factors on d
001 for WB and YB, respectively. All factor effects, for both individual and interaction, were found to significantly influence the degree of intercalation in both clay types (WB and YB), as they all exhibited positive signs, consistent with the Pareto charts presented in the previous section.
Table 8 and
Table 9 present the estimated effects and coefficients of the factors for d
001 in WB and YB, respectively. As shown in the Pareto charts, temperature had the most significant impact on the degree of intercalation, followed by stirring speed and contact time.
The main effects of the linear terms for both clays displayed positive values, indicating that their presence in the process contributes to an increase in d001. However, the quadratic terms showed negative values, suggesting that, despite being statistically significant, they help in a reduction in d001. This implies that higher levels of these three factors lead to this reduction.
The interaction effects between temperature and stirring speed also showed negative values, indicating that, while both factors individually play a significant role in increasing d001, their combination leads to a decrease.
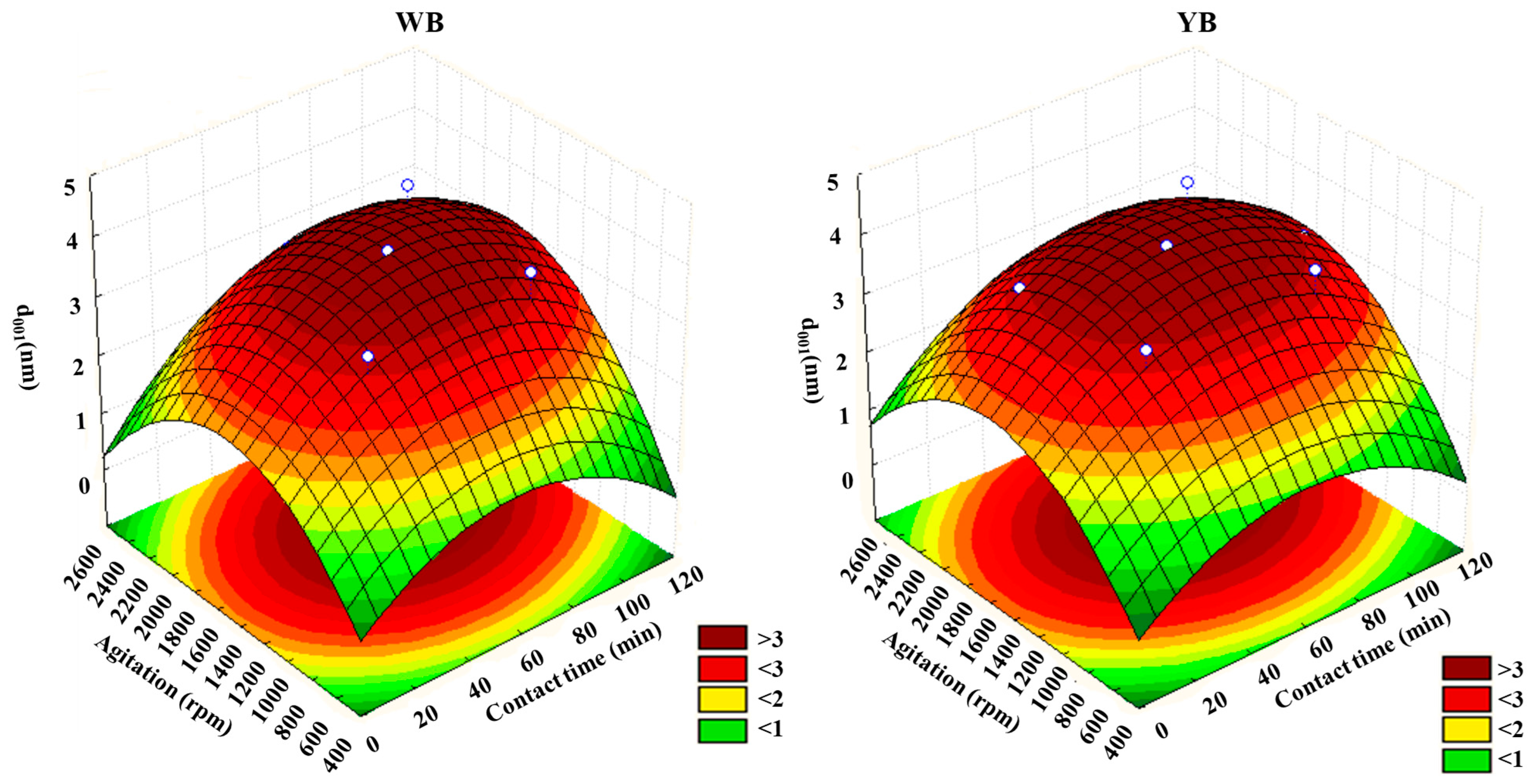
3.6. Optimization of the Organo-Modification Conditions
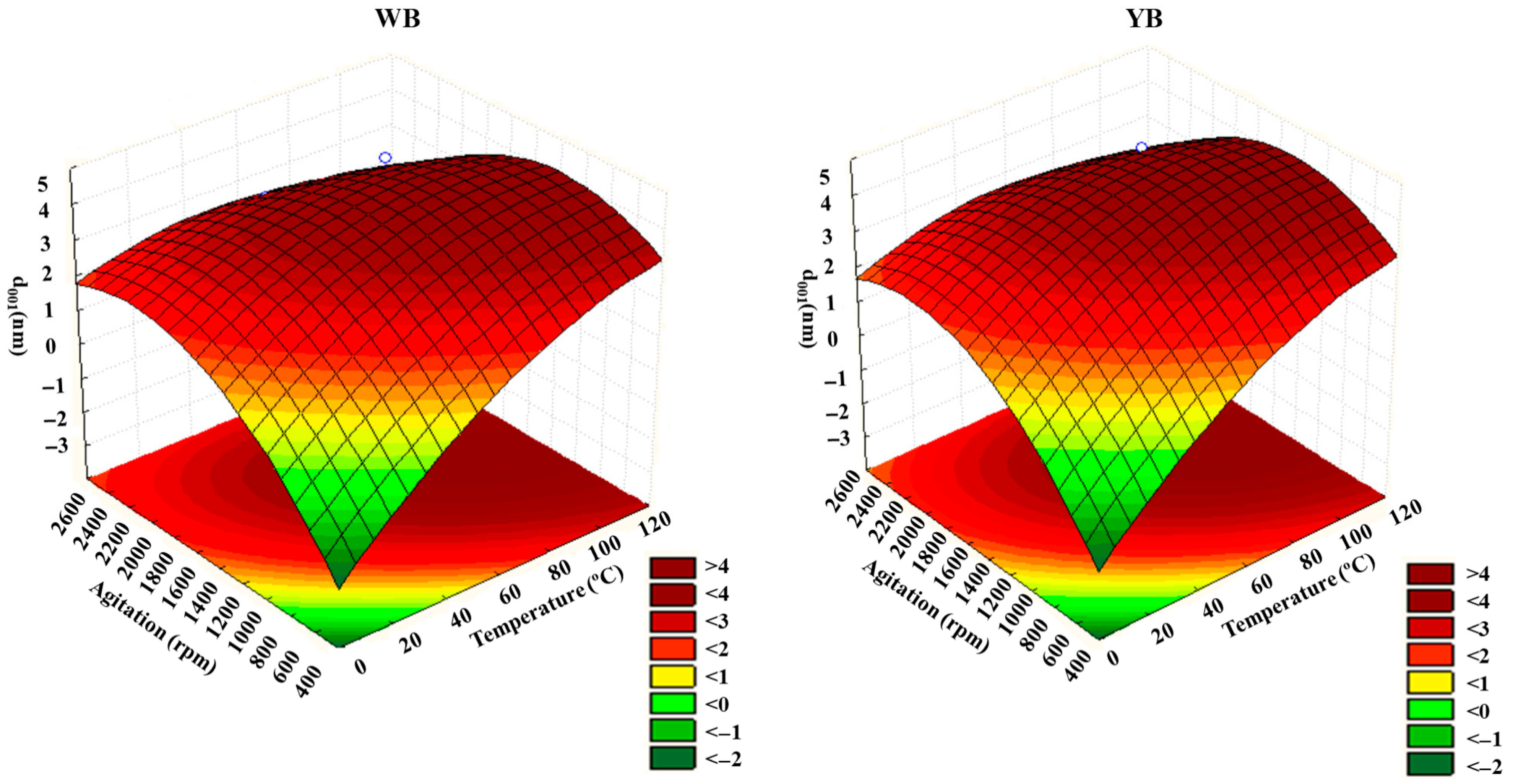
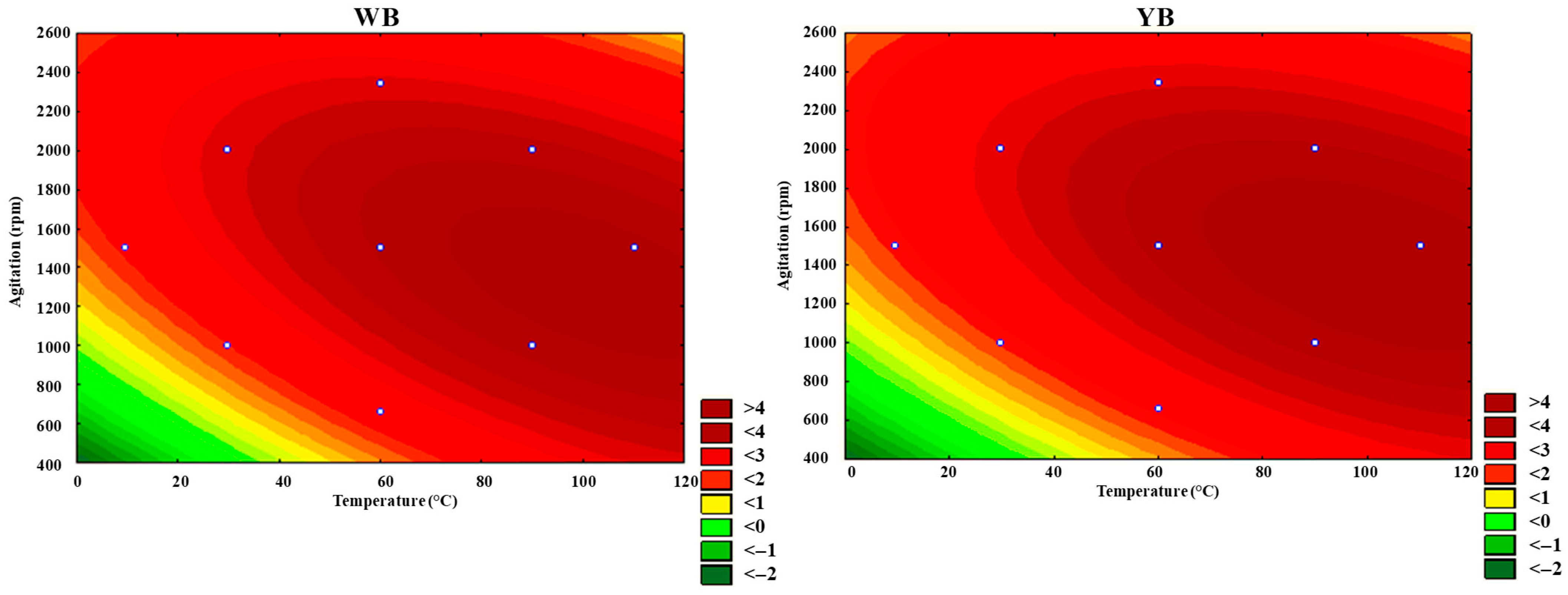
Figure 6 and
Figure 7 show the response surfaces and contour plots depicting the dependency of temperature and agitation on d
001 for the two clays, generated with Statsoft Statistica 8. The presence of curvatures indicates a significant and relevant interaction effect between temperature and agitation. This is consistent with the conclusions drawn from the evaluation of interaction effects using the Pareto charts (
Figure 5), as well as from the ANOVA and estimated effects tables (
Table 6,
Table 7,
Table 8 and
Table 9). As expected,
Figure 7 confirms that d
001 is influenced by both temperature and agitation speed, with the increase in d
001 being notably more sensitive to temperature rise than to changes in agitation speed. At very high agitation speeds, d
001 tends to decrease. These outcomes align with the data in
Table 8 and
Table 9, which confirm that d
001 is more responsive to changes in temperature. In both clays, temperature increases result in nearly a 1.2% rise in d
001 compared to an approximately 0.5% increase in agitation speed. This fact is further supported by the higher temperature coefficient, which is twice as large as that of the stirring speed factor.
From
Figure 7, it can be observed that optimal d
001 values can be obtained in the range of temperature of 60 to 120 °C and stirring speeds between 1000 and 2000 rpm. Under these conditions, d
001 reaches a maximum of around 4 nm.
The increased d
001 with increasing temperatures can be attributed essentially to three factors: (a) Molecular mobility: Higher temperatures raise the kinetic energy of both the polyamide chains and the clay particles, likely increasing the mobility and reactivity of the amine groups, enabling them to penetrate the interlayer spaces of bentonite clay more easily; (b) Swelling thermodynamics: Bentonite clay, a layered material, experiences swelling when interacting with water or organic solvents [
13,
14,
58]. High temperatures promote better swelling of the clay layers in the polymer solution, creating more space for polymer chains to intercalate; and (c) Reduced viscosity: Rising temperatures enhance the solubility of the polymer solution in acetic acid by disrupting hydrogen bonds, which leads to a reduction in viscosity. The lower viscosity enhances the diffusion of polyamide chains, facilitating their movement into the interlayer spaces of the bentonite.
The increase in intercalation degree with higher stirring speeds is primarily due to the following factors: (a) Enhanced mass transfer: Faster stirring improves the interaction between polyamide chains in the solution and the clay surface by disrupting stagnant layers around the clay particles. The polymer chains penetrate more effectively into the clay’s interlayer space, and (b) improved mixing and distribution: Agitation ensures the even distribution of polyamide chains throughout the mixture, preventing clay particle settling.
As previously mentioned, at very high stirring speeds, d
001 tends to decrease.
Figure 7 shows that above 2000 rpm, d
001 decreases, indicating that the increase in d
001 is affected at such high speeds. Although increasing stirring speed generally promotes intercalation,
Figure 7 shows that at speeds above 2000 rpm, d
001 begins to decrease. This suggests that excessively high shear forces generated at such speeds may disrupt the intercalated structure or cause mechanical degradation of the polymer chains, such as chain scission. A recent study by Morelly and co-workers [
59], combining theoretical analysis and experimental results, demonstrated that increasing the shear rate during the mixing of polymers and particles leads to polymer chain scission, resulting in reduced molecular weight and viscosity.
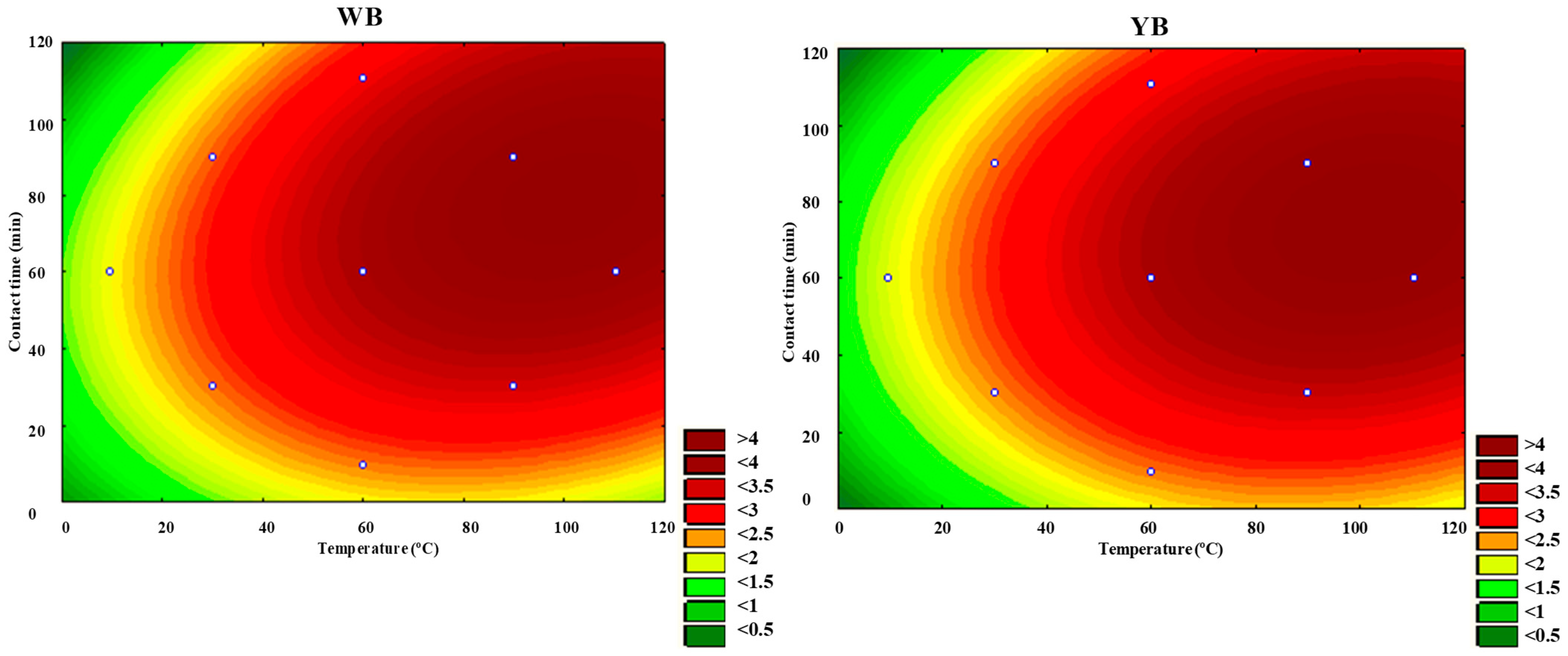
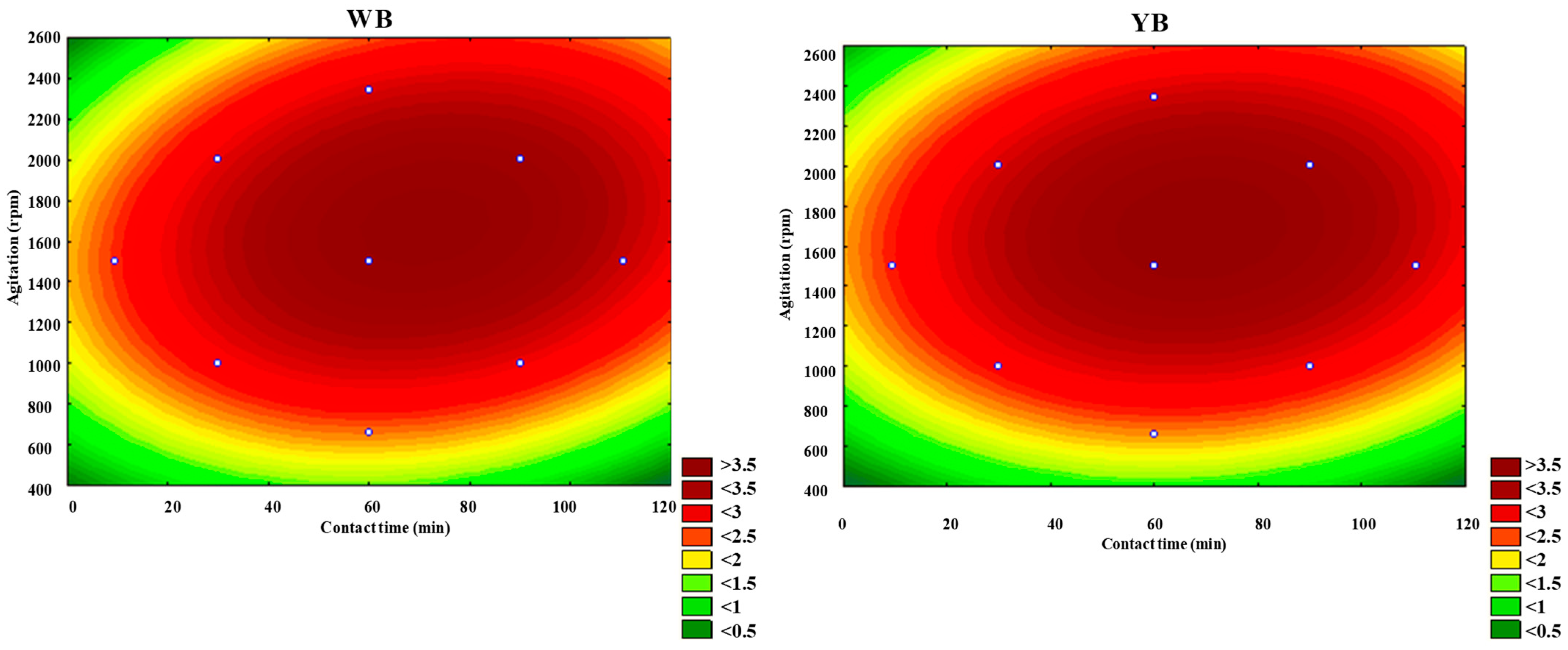
Figure 8 and
Figure 9 illustrate the response surfaces and contour plots that demonstrate how the temperature and contact time of the two clays influence d
001. The curvature of the response surfaces and contour lines indicates a significant interaction effect between these two factors, as evidenced by the data presented in
Table 6,
Table 7,
Table 8 and
Table 9. Optimal d
001 values were obtained at a temperature range of 60 to 120 °C and a contact time between 30 and 90 min. Under these conditions, d
001 also reaches a maximum of approximately 4 nm.
From
Table 8 and
Table 9, it is clear that simultaneous heating and contact time confers a positive effect on d
001. However, the increase in d
001 appears to be more sensitive to increasing temperature than contact time.
Contact time has a relatively minor impact on the organo-modification process compared to temperature and stirring speed. The time impact on the organo-modification process can be attributed to the saturation of intercalation and kinetic limitations, as explained in studies related to polymer crystallization and intercalation phenomena [
60,
61,
62]. Temperature and agitation possess a stronger impact on the degree of intercalation. This suggests that under suitable temperature and agitation conditions, the system reaches equilibrium relatively quickly. At this point, the availability of polymer chains to access the interlayer spaces seems to become a limiting factor, rather than the duration of diffusion. Therefore, extending the contact time is unlikely to further influence the intercalation process once the chains have already entered the interlayer spaces.
The sensitivity of d
001 to temperature increase may be linked to the low molecular mass of bio-based polyamides, influenced by its glass transition temperature (Tg). Below Tg, polymer chains are restricted in their movement, making the polymer rigid, while above Tg, the chains gain mobility, increasing flexibility [
63,
64]. This demonstrates that Tg is the boundary between low and high molecular mobility. Dimer fatty acid-based polyamides have a very low Tg, around 10 °C [
41,
42], which explains why any rise in temperature results in an increase in d
001. Additionally, the polymer being in solution may further enhance chain segment mobility. The presence of solvent molecules disrupts the polymer chains’ intermolecular interactions, reducing the energy barrier for segmental motion, hence, enhanced mobility of polymer chains, enabling them to move more freely at lower temperatures compared to the pure polymer. The solvent acts as a plasticizer by inserting itself between the polymer chains, effectively lowering the Tg by reducing the rigidity of the polymer structure [
65,
66,
67].
Figure 10 and
Figure 11 illustrate the response surfaces and contour plots that demonstrate how contact time and agitation of the two clays influence d
001. The curvature of the response surfaces and contour lines indicate a significant interaction effect between these two factors, as evidenced by the data presented in
Table 6,
Table 7,
Table 8 and
Table 9.
Although the combination of agitation and contact time contributes to an increase in d
001, the effect is relatively modest compared to other factor combinations, such as temperature with agitation speed or temperature with contact time. This is consistent with the results in
Table 8 and
Table 9, which demonstrate that, in absolute terms, the combination of agitation speed and contact time has a smaller impact on the increase in d
001. Optimal d
001 values can be achieved with stirring speeds between 1200 and 2000 rpm and contact times ranging from 50 to 90 min.
Table 10 presents the stationary points for the maximum values of d
001 for both WB and YB clays. On average, the maximum d
001 is achieved at approximately 105 °C, with a contact time of 75 min and a stirring speed of 1400 rpm. These values are within the range of the independent variables under investigation. Under these conditions, d
001 for both clays reaches a maximum of about 4 nm. However, the analysis of the response surface and contour plots suggests that this maximum can also be attained at 90 °C, with a stirring speed of 1500 rpm and a contact time of 60 min.
Based on the analysis shown in
Table 8 and
Table 9, it is evident that temperature, stirring speed, and contact time impact the degree of polymer chain intercalation into clay galleries. XRD analysis demonstrated that the d
001 spacing increased from 1.73 nm to 3.79 nm for WB and from 1.77 nm to 3.83 nm for YB, as the organo-modification temperature, contact time, and stirring speed were adjusted from 30 to 90 °C, 30 to 90 min, and 1000 to 2000 rpm, respectively. Similar
d-spacings were reported for Boane’s white organo-bentonites [
32]. These were organo-bentonite samples containing quaternary ammonium surfactants that were purified and modified with soda ash. The
d-spacings of 1.73 and 1.77 nm to approximately 4 nm observed in the two clays indicate the presence of lateral bilayer, pseudotrimolecular layer, and paraffin-type bilayer structures [
18,
32,
53].
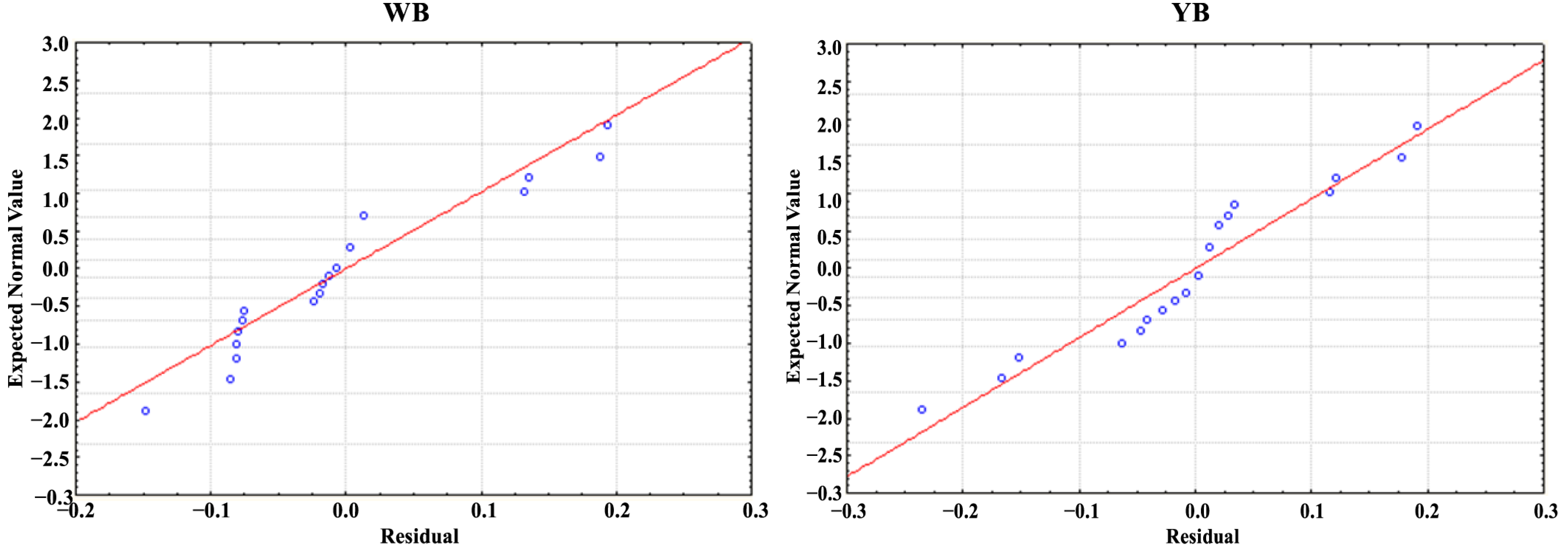
Plots of the standardized residuals with normal probability are commonly used to evaluate how well the fitted model represents the real system.
Figure 12 displays the graphs for d
001, where most of the data points align with the straight line or predicted data, suggesting that the errors are attributed to a normal distribution for many responses.