Abstract
It has been proven that the performance of fluid catalytic cracking (FCC), as the most important oil refining process for converting low-value heavy oils into high-value transportation fuels, light olefins, and feedstocks for petrochemicals, depends strongly on the quality of the feedstock. For this reason, characterization of feedstocks and their relationships to FCC performance are issues deserving special attention. This study systematically reviews various publications dealing with the influence of feedstock characteristics on FCC performance, with the aim of identifying the best characteristic descriptors allowing prediction of FCC feedstock cracking capability. These characteristics were obtained by mass spectrometry, SARA analysis, elemental analysis, and various empirical methods. This study also reviews published research dedicated to the catalytic cracking of biomass and waste oils, as well as blends of petroleum-derived feedstocks with sustainable oils, with the aim of searching for quantitative relationships allowing prediction of FCC performance during co-processing. Correlation analysis of the various FCC feed characteristics was carried out, and regression techniques were used to develop correlations predicting the conversion at maximum gasoline yield and that obtained under constant operating conditions. Artificial neural network (ANN) analysis and nonlinear regression techniques were applied to predict FCC conversion from feed characteristics at maximum gasoline yield, with the aim of distinguishing which technique provided the more accurate model. It was found that the correlation developed in this work based on the empirically determined aromatic carbon content according to the n-d-M method and the hydrogen content calculated via the Dhulesia correlation demonstrated highly accurate calculation of conversion at maximum gasoline yield (standard error of 1.3%) compared with that based on the gasoline precursor content determined by mass spectrometry (standard error of 1.5%). Using other data from 88 FCC feedstocks characterized by hydrogen content, saturates, aromatics, and polars contents to develop the ANN model and the nonlinear regression model, it was found that the ANN model demonstrated more accurate prediction of conversion at maximum gasoline yield, with a standard error of 1.4% versus 2.3% for the nonlinear regression model. During the co-processing of petroleum-derived feedstocks with sustainable oils, it was observed that FCC conversion and yields may obey the linear mixing rule or synergism, leading to higher yields of desirable products than those calculated according to the linear mixing rule. The exact reason for this observation has not yet been thoroughly investigated.
1. Introduction
Since the commissioning of the first industrial catalytic cracking (FCC) plant 83 years ago, the number of FCC units in the world has steadily increased. A review article by Vogt and Weckhuysen [1] mentioned that the FCC process was in operation in over 300 of the total 646 refineries worldwide as of early 2014, while in 2024, the number of FCC units operating in 825 refineries worldwide reached around 600 [2]. Global refinery fluid catalytic cracking unit (FCCU) capacity is expected to increase from 14,153 mbd in 2023 to 16,870 mbd in 2028 at an average annual growth rate (AAGR) of 3.5% [3]. FCC is considered the most important petroleum refining process for converting heavy oil into valuable products [4]. About 35–50% of the total gasoline produced worldwide comes from the FCC process [5]. In addition to its role as a major gasoline maker, the FCC process has been widely used and investigated as a producer of middle distillate fuels [6,7,8], a producer of ethylene and propylene [9,10,11], a producer of bio-fuels [12,13,14], and a processor of waste (scrap tires, plastics) [15,16,17]. The reason for the wide-spread application of FCC technology lies in its flexibility in processing a wide range of oils of quite different quality and producing various fuels and feedstocks for petrochemicals [18,19,20]. Vacuum gas oil is the major component of FCC feed [21,22,23], but atmospheric residues [24,25,26], gas oils from residue conversion processes such as coking, visbreaking, and hydrocracking [27,28,29], and deasphalted oils [30,31,32] are also processed in FCC units. The conversion at the point of maximum gasoline yield, or the so-called overcracking point, of FCC feedstocks of different qualities has been reported to vary between 50 and 85% [33,34,35,36]. A comparison of the influence of feedstock quality on the conversion level (35%) with that of the catalyst activity and reaction temperature showed that the catalyst activity can contribute about 10–12% and the reaction temperature about 10–12%, in the reaction temperature range 500–560 °C [37]. This comparison clearly indicates that feed quality is the single factor that has the greatest influence on the conversion level in an FCC unit. This may explain the reason for the special attention paid over the years to clarifying the basis of the different cracking behaviors of oils of various origins. In 1961, Reif et al. [38] were the first to relate the feedstock quality to product yields obtained during catalytic cracking of a wide variety of feedstocks in a pilot plant. They employed the n-d-M method [39] to characterize the catalytic cracking feeds by the contents of paraffinic, naphthenic, and aromatic carbons empirically calculated from the refractive index (n), density (d), molecular weight (M), and sulfur content of the cracked oils, and they developed polynomial correlations of yields. The second study devoted to the effect of catalytic cracking feed quality on yields was that by White [40]. In 1968, he used the mass spectrographic technique of Fitzgerald et al. [41] to characterize FCC feedstocks by their paraffin, naphthene, and aromatic contents to develop regression correlations for predicting product yields from various feedstock components including paraffins, naphthenes, and aromatics. Later, in 1971, Nace et al. [42] used silica gel separation and mass spectroscopy along with the n-d-M correlation method to characterize 16 catalytic cracking feedstocks with great variations in their composition. By combining mass spectrometric data with the n-d-M method, they were able to classify aromatic compounds by carbon atoms in the aromatic ring and substituent carbon groups attached to the aromatic rings [42]. They developed a kinetic model to quantitatively describe the conversions and gasoline selectivity. They established that feedstocks rich in paraffins and/or naphthenes had the largest rate constants for cracking and gasoline formation. They also found out that catalyst deactivation was augmented by the enhancement of aromatic concentration in the feeds. In 1971 [43] Voltz et al. determined that the kinetic rate constants for gas oil cracking and gasoline formation correlated with the aromatic/naphthenic ratio, which was quantified by mass spectrometric analysis of the 16 gas oils. The catalyst decay constant they found also correlated with the aromatic/naphthene ratio. Five years later, in 1976, Jacob et al. [44] developed a predictive kinetic model that involved lumped species consisting of paraffins, naphthenes, aromatic rings, and aromatic substituent groups in light and heavy fuel oil fractions. Their kinetic model included the effects of nitrogen poisoning, aromatic ring adsorption, and time-dependent catalyst decay. The rate constants for these lumped species were found to be invariant with respect to feedstock composition. The predictive capabilities of the model were confirmed for a wide range of feedstocks and process conditions. Later, over the years, in addition to the mass spectrometry used to characterize catalytic cracking feedstocks and the n-d-M method, other approaches have been applied.
Dhulesia [45] proposed empirical correlations to predict the contents of aromatic carbon and hydrogen of FCC feeds from five properties: specific gravity, refractive index, molecular weight, viscosity, and sulfur content. These correlations were utilized in the Total FCC unit model, which operated in conjuction with a nonlinear optimizer [45].
Riazi and Daubert [46] developed correlations to predict the content of paraffinic, naphthenic, and aromatic portions from the molecular weight, refractive index, and carbon-to-hydrogen ratio.
The n-d-M [39], Dhulesia [45], and Riazi and Daubert [46] methods were employed by Stratiev and Minkov [47] to characterize ten FCC feeds, which were cracked in a micro-activity unit on a commercial equilibrium catalyst at a temperature of 510 °C and catalyst-to-oil ratios between 2 and 6 wt./wt. Stratiev and Minkov [47] developed correlations to relate the FCC conversion at the point of maximum gasoline yield to the parameters characterizing the FCC feed’s aromatic carbon and hydrogen contents estimated by Dhulesia’s correlations [45], the paraffinic and naphthenic portions calculated by the correlations of Riazi and Daubert [46], and the paraffinic and naphthenic carbons computed by the n-d-M method. They found that the correlations used by Dhulesia [45] to estimate the FCC feed aromatic carbon and hydrogen contents were the most appropriate feedstock descriptors to access the potential of a feedstock to crack to gasoline.
Choudhary and Meier [48] also developed correlations to predict the contents of aromatic carbon and hydrogen in heavy oils from only two properties: specific gravity and T50 (temperature at which 50% of the heavy oil evaporates). There are no reports yet that relate FCC feed crackability to the aromatic carbon and hydrogen contents calculated using the Choudhary and Meier correlations.
Watson’s characterization factor (Kw) [49] has been also availed to characterize FCC feeds [50]. Stratiev et al. [50] established that the conversion of eleven distinct vacuum residue hydrocracker gas oils cracked in an advanced cracking equipment (ACE) laboratory FCC unit on a commercial equilibrium catalyst at a temperature of 527 °C and a catalyst-to-oil ratio of 7.5 wt./wt. strongly correlated with the Kw characterizing factor (correlation coefficient R of 0.99, average error of 1.7%).
Another FCC feed characterization parameter, the aromatic ring index (ARI) developed by Abutaqiya [51,52], was also found by Stratiev et al. [50] to affect FCC feed conversion.
Navarro et al. [33] determined that the US Bureau of Mines Correlation Index [53] best correlated with FCC feed hydrogen content (correlation coefficient R of 0.966 and standard error of 0.2%) and also negatively affected FCC conversion at the point of maximum gasoline yield. Navarro et al. [33] developed a correlation to predict FCC conversion at the point of maximum gasoline yield from FCC feed hydrogen content (R of 0.948 and standard error of 2%), based on cracking data from more than 100 types of FCC feeds (0.8654 ≤ specific gravity ≤ 1.025; 9.5 wt.% ≤ hydrogen content ≤ 14.25 wt.%) obtained in a laboratory ACE FCC unit using two different commercial catalysts. They observed that the range of maximum conversion variation was between 50.4 and 83.4 wt.% and that the majority of feedstocks used in FCC plants had maximum conversions above 70%, with lower values associated with secondary gas oils, such as coker and residue hydrocracker gas oils and heavy cycle oils [33].
Behera et al. [54,55] used nuclear magnetic resonance (NMR) methods to characterize FCC feedstocks. Unfortunately, they did not correlate the characteristics obtained from NMR analyses of FCC feedstock with their crackability.
In addition to empirical correlations for calculating FCC feed characterizing parameters [39,45,46,48,49,53] from physicochemical properties, an artificial neural network (ANN) model to predict the paraffin, naphthenic, and aromatic content of FCC feed from density, ASTM distillation temperatures, Conradson carbon residue (CCR), and sulfur and total nitrogen contents was proposed by Dasila et al. [56]. Twenty-eight FCC feed samples with densities between 0.8366 and 0.996 g/cm3 and aromatics between 16.5 and 76.5 wt.% analyzed by HPLC and mass spectroscopy were employed to develop two ANN models [56]. The better ANN model predicted gas oil compositions within ±10% of the experimental values. No attempt to link the ANN model-predicted composition of the feed with its crackability has been reported.
Gilbert et al. [57], examining 174 FCC feed samples, developed chemometric models of feed properties (specific gravity, aniline point, basic nitrogen, sulfur and Conradson carbon content, viscosity, and distillation) using both near-infrared (NIR) and NMR spectroscopy, which were used as input data for an FCC process simulator. It was shown the two spectroscopic methods for feed characterization produced comparable results and are viable choices for on-line FCC feed analyzers.
Fisher [34] and Hg et al. [35,36] used mass spectrometry to characterize 19 FCC feedstocks with widely varying properties (0.864 ≤ density at 15 °C ≤ 1.007 g/cm3) and correlated the content of so-called gasoline precursors (saturates plus mononuclear aromatics) with the conversion and yields at the point of maximum gasoline yield. Fisher’s data [34] showed that the gasoline precursor content of the feed could be used to predict maximum conversion with a correlation coefficient R of 0.982 and a standard error of 2.6%. The calculation of all types of carbon atoms associated with the gasoline precursor molecules showed a higher correlation coefficient R of 0.994 and a lower standard error of 1.5% when used to predict maximum conversion according to that data. However, Ng et al.’s [36] data did not show so precise a prediction of maximum conversion as Fisher’s data from gasoline precursor content for FCC feed (a correlation coefficient R of 0.847 and a standard error of 3.3%).
Bollas et al. [58] developed a feedstock characterization procedure in which the aromatic carbon (CA) from the n-d-M method, the average carbon number (NC), and the total nitrogen (NT) and sulfur contents were appropriately combined to predict the effect of feedstock quality on the conversion and coke yield of the fluid catalytic cracking process. They examined thirteen different feedstocks with a wide range of properties under real FCC conditions. The variation of FCC feed properties was as follows: 0.8713 ≤ specific gravity (SG) ≤ 1.0028; 1.4782 ≤ refractive index at 20 °C (RI) ≤ 1.5689; 283 g/mol ≤ molecular weight ≤ 438 g/mol; 0.18% ≤ sulfur content ≤ 2.98%; 0007% ≤ nitrogen content ≤ 0.178%; 85.0% ≤ carbon content ≤ 86.3%; 358 °C ≤ T50% ≤ 471 °C. They defined the term “crackability” as a relative assessment of the feed’s potential to enhance catalytic cracking compared with a reference FCC feed. The variation in FCC conversion under the studied conditions was between 47 and 82 wt.%, while of the variation in coke yield was between 3.8 and 11.0 wt.%. Bollas et al. [58] concluded that the aromaticity expressed by CA and size of the average hydrocarbon signified by NC were dominant factors affecting the crackability, whereas the total nitrogen content was confirmed to be the main catalyst inhibitor.
In addition to Reif et al. [38] and later, Nace et al. [42] and Jacob et al. [44], other researchers have used the n-d-M method to qualify various FCC feeds. Ancheyta [59,60], using the experimental information reported by Nace et al. [42], developed correlations to predict the gasoline formation and gasoline cracking kinetic constants as a function of the total sulfur (S) and nitrogen (N) contents, aromatic carbons (CA), and paraffinic (CP), /naphthenic (CN) carbons ratio determined by the n-d-M method. Ancheyta et al. [60] communicated that the accuracy of the correlation developed in [60] for predicting the kinetic constant of gasoline formation was higher than that developed by Voltz et al. [43] based on mass spectroscopic measurement of aromatic (A) and naphthenic (N) contents in FCC feeds. Therefore, one may conclude that the FCC feed characterization based on the empirical parameters, calculated using the n-d-M method, as shown in the study by Ancheyta et al. [59], seems reliable enough to substitute for the more sophisticated mass spectrometry analysis. Furthermore, Ancheyta et al. [60] developed correlations to predict the kinetic constants of gas and coke formation and catalyst deactivation.
Harding et al. [61,62] studied ten different FCC feedstocks that were characterized in terms of their distillation characteristics and content of saturates, aromatics, and polar compounds (SAPs). The feeds were cracked on a low-matrix rare earth ultrastable Y catalyst (REUSY) in a laboratory microactivity unit at 525 °C, 30 s residence time, and different catalyst-to-oil ratios. They compared the conversion and yields at a constant catalyst-to-oil ratio of 5 wt./wt. and found that the boiling range had almost no effect on the crackability, while the chemical composition expressed by the SAP contents had a strong effect on the crackability of the feedstock. They reported that the conversion of SAP fractions was as follows: saturates—93.8 wt.%, aromatics—53.6 wt.%, and polars—8.8 wt.%.
Xu et al. [31] characterized 24 FCC deasphalted oil feeds, availing liquid chromatography to obtain their contents of saturates, aromatics, and resins (SARs). The feeds were cracked in a laboratory on a confined fluid bed reactor using a commercial catalyst at a reaction temperature between 480 and 560 °C, catalyst-to-oil ratio between 4 and 10 wt./wt., and residence time between 1.4 and 5 s. They developed correlations to relate the yields of gasoline, diesel, light oil, and coke to the contents of saturates, aromatics, and resins. They found that saturates and aromatics contributed to the formation of gasoline, with the influence of saturates being twice as great as that of aromatics. The aromatics contributed predominantly to the formation of diesel, while the main contributors to coke formation were the resins. It was found that aromatic compounds contributed primarily to the formation of diesel fuel, while the main factor in the formation of coke was found to be resins.
It is evident from the discussed investigations that different studies have employed various methods to characterize FCC feeds, and that their crackability can be assessed in diverse ways. For example, the FCC feed can be characterized by using distinct empirical methods, n-d-M [39], aromatic carbon and hydrogen content calculated by the correlations described by Dhulesia [45] and Choudhary and Meier [48], saturate, naphthenic, and aromatic portions calculated by the correlations described by Riazi and Daubert [46], a Correlation Index [53], the Kw characterizing factor [49], or an aromatic ring index [51,52]. The FCC feed can be also characterized by availing liquid chromatography, so called SAP [63] or SAR [31] analysis, or by using high-performance liquid chromatography (HPLC) along with mass spectrometry (MS) [34,35,36]. Another method for characterizing FCC feedstocks, discussed in previous studies [64,65,66,67,68,69], uses non-aqueous ion-exchange liquid chromatography to fractionate the FCC feedstock into acidic, basic, and neutral types. In addition, the crackability of FCC feeds can be also examined in different ways. For example, Fisher [34], Ng et al. [35,36], Stratiev [37,47], and Navarro et al. [33] studied the crackability of different feedstocks at the point of maximum gasoline yield. Harding et al. [61,62] and Green et al. [64,65,66,67] investigated different crackability of feedstocks under constant operating conditions (same catalyst-to-oil ratio and same reaction temperature). On the other hand, Bollas et al. [58] and Xu et al. [31] examined the crackability of various feedstocks under different operating conditions. There is still no comprehensive study highlighting which FCC feed characterization method is most suitable for assessing feed crackability. For example, Ancheyta et al. [60] reported that the use of the n-d-M method to calculate the average paraffinic, naphthenic, and aromatic carbons provided a more accurate prediction of the kinetic constant for gasoline formation than aromatic and naphthenic content measured by mass spectrometry. Therefore, it can be concluded that the n-d-M method is superior to mass spectrometry. On the other hand, Green et al. [66] concluded in their study that average feed parameters, such as percentage of paraffinic, naphthenic, and aromatic carbons, are not sufficient to adequately model the crackability of FCC feedstock. It has also not yet been confirmed which FCC testing approach should be used to assess the influence of a specific feed characteristic on its crackability. Furthermore, the reviewed studies describe only the application of empirical correlations between the parameters characterizing the FCC feed and its crackability. No reports were found investigating the feasibility of applying the artificial neural network (ANN) modeling technique to relate the characteristics of the FCC feed to its crackability.
In addition to the processing of petroleum-derived feedstocks, interest in the conversion of biomass and waste-derived oils into FCC is continuously growing, in an attempt to reduce greenhouse gas emissions and mitigate global warming [70,71,72], as well as to find an environmentally friendly approach to utilizing waste tires and plastics. The use of renewable or bio-based feedstock sources for fuel production has the potential to reduce carbon footprint and greenhouse gas emissions and reduce dependence on non-renewable fossil fuel sources [73,74,75]. The use of existing FCC plants for the co-processing of vacuum gas oils and other heavy oils derived from petroleum with feedstocks from renewable sources offers advantages from both a technological and economic point of view, since it can employ the existing refining infrastructure and configuration, and little additional capital investment is needed [76,77,78]. Catalytic cracking of biobased feedstocks has been extensively studied over the past two decades [79]. A search in ScienceDirect with the keywords “biofuel” AND “catalytic cracking” [80] and “VGO” AND “catalytic cracking” [81], “resid” AND “catalytic cracking” [82] showed that the number of publications dedicated to catalytic cracking of biobased feedstocks has been increasing steadily since 2009, with a slope of 25, while for the same period (2009–2024), the slope for numbers of research publications regarding the catalytic cracking of VGO and residues was 6 times lower (4.1), as can be seen in Figure 1 [80,81,82]. The data in Figure 1 undoubtedly show that the interest of contemporary research in the field of FCC is primarily focused on the production of biofuels by FCC. Therefore, this study also investigates the influence of the properties of bio-oils and waste pyrolysis oils on their crackability.
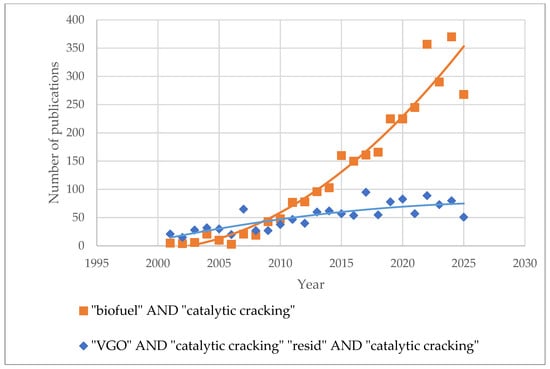
Figure 1.
Number of published articles for the period 2001–2025 with search words “biofuel” AND “catalytic cracking”, and “VGO” AND “catalytic cracking” “resid” AND “catalytic cracking” (Data taken from [81].
The findings of this section led the author to formulate the following tasks to be accomplished in this research:
- To evaluate which FCC feed characterization method (empirically calculated FCC feed parameters and measured mass spectrometry data) provides the highest accuracy for prediction of conversion at maximum gasoline yield using the data reported by Fisher [34];
- To evaluate which FCC feed characterization parameters, measured by NMR or calculated from empirical correlations and mass spectrometry data, provide the highest accuracy in predicting conversion and product yield at maximum gasoline yield, using data reported by Fisher [34] and Ng et al. [36];
- To evaluate which method, regression or artificial neural network, can provide more accurate prediction of conversion at maximum gasoline yield using the data provided by Fisher [34], Ng et al. [36], and Navarro et al. [33];
- To review FCC feed characterization by total and basic nitrogen contents and their relation to the feed crackability, and to develop a correlation between FCC conversion obtained under the same operating conditions and feed characteristics, such as the contents of basic nitrogen and aromatic carbon or hydrogen, using data from Ng et al. [35] and Ng and Rahimi [83];
- To review the use of liquid chromatography to fractionate the FCC feed into acid, base, and neutral types and the relation of these types to the feed crackability;
- To review the co-processing of bio-oils and pyrolysis oils from waste plastics and scrapped tires as part of the FCC process.
The aim of this study is to discuss the results of the implementation of the tasks formulated above.
This research is dedicated to chemical engineers working in the field of petroleum refining.
2. Materials and Methods
The experimental data reported by Fisher [34] for nine vacuum gas oils contain the necessary physicochemical properties to calculate FCC feed characterization parameters using empirical n-d-M methods [39], Riazi and Daubert [46], the Total method (Dhulesia correlations [45]) and Conoco–Philips (COP) (Choudhary and Meir correlations [48]) to predict aromatic carbon and hydrogen content, the KW characterization factor, and BMCI. Table S1 presents the physicochemical properties of the nine vacuum gas oils cracked in the research by Fisher [34]. Fisher [34] also reported the conversion values obtained from these nine vacuum gas oils at maximum gasoline yield. Thus, the ability to predict conversion at maximum gasoline yield using mass spectrometry-determined gasoline precursor (saturates plus mononuclear aromatics) content can be compared to that obtained by various empirical methods using regression analysis.
In their study, Fisher [34] and Ng et al. [35,36] presented characterization data determined by mass spectrometry (contents of gasoline precursors, saturates, aromatics, polar compounds, aromatic carbon and hydrogen contents, and physicochemical properties) for 19 FCC feedstocks, along with the conversion at the overcracking point, maximum gasoline yield, optimum LCO yield, coke yield, and coke on catalyst (wt.%). These data are summarized in Table 1 and were used in this study to develop correlations to predict maximum conversion, maximum gasoline yield, optimum LCO yield, coke yield, and coke on catalyst.

Table 1.
Data for 19 oils characterized by mass spectrometry and physico-chemical methods, cracked in MAT laboratory units, as reported in the works of Fisher [34] and Ng et al. [35,36].
To investigate the ability of artificial neural networks (ANNs) to model maximum FCC conversion from FCC feed characterization data, the data reported by Navarro et al. [33] and Stratiev [37] were combined with data from Table 1 for the contents of hydrogen, saturates, aromatics polars, along with maximum FCC conversion. These data are summarized in Table S2. In this way, data on the characterization and maximum conversion of 88 FCC feedstocks were collected, which can be considered sufficient for the purposes of modeling with artificial neural networks (ANNs).
To investigate the influence of total nitrogen, basic nitrogen, aromatic carbon, and hydrogen content on the crackability of FCC feedstocks, data reported by Ng et al. [35] and Ng and Rahimi [83] for the catalytic cracking of Canadian feedstocks of various origins [35] and Canadian unconventional feedstocks [83] were combined and are presented in Table 2.

Table 2.
Data of catalytic cracking performed in an FCC MAT unit at catalyst-to-oil ratio of 5 wt./wt., reaction temperature of 510 °C, and 72% catalyst microactivity (data taken from references [35,83]).
The ANN modeling was implemented using Matlab 2020 software. The training, validation, and test sets of the data employed by the ANN were divided as follows: 80% for training; 10% for testing; and 10% for validation. The architecture of the ANN used in this research to model the FCC maximum conversion consisted of six layers with the following structure: 64, 32, 16, 10, 8, and 1 neurons. The first layer contained 64 neurons, the second 32, the third 16, the fourth 10, the fifth 8, and the sixth (output) layer contained a single neuron. This architecture was selected to effectively process the input data of size 4×88. The number of neurons in each successive layer was approximately halved, starting from 64 neurons in the first layer. This configuration was considered optimal for capturing the essential features of the input data. Increasing the number of neurons leads to information redundancy and, consequently, an increase in the mean squared error (MSE), which negatively affects modeling performance [84].
The development of regression models in this study was carried out using CAS Maple 2024.2 Global Optimization Tool (method = diffevol). To search for the best parameter values in compact subsets in the parameter space, an optimization tool, the differential evolution (DE) algorithm [85], was used. The DE algorithm does not require differentiability or even continuity of the optimized function. Moreover, it is very applicable to construct some necessary bounds in the parameter space [85].
The n-d-M method was established by Van Nes and van Westen [86] and allies the structural group composition assigned by NMR in terms of paraffinic, naphthenic, and aromatic carbon content to three physical properties of the oil: refractive index (RI), density (d), and molecular weight (MW), and a chemical property: sulfur content (S). The n-d-M method does not provide direct composition of petroleum oils in terms of paraffins, naphthenes, and aromatics. It calculates the distribution of carbon in paraffins (CP), naphthenes (CN), and aromatics (CA) in oils. The following equations are used for estimation of CP, CN, and CA:
Equations (1)–(3) use variables a, v, w and CR, which are computed by Equations (4)–(7). Equations (4)–(7) are presented below:
According to ASTM D-3228 [39] the range within the n-d-M method valid for CP is between 32.3 and 68.6%; for CN, it is between 23.7 and 47.2%; and for CA, it is between 2.7 and 34.6%.
Riazi determined that the n-d-M method provided an inaccurate prediction of the composition of the petroleum fraction [87]. This finding was the reason for Riazi and Daubert to develop a set of correlations for molecular type analysis [46]. For petroleum oils with a molecular weight higher than 200 g/mol, which is valid for FCC feeds, the Riazi and Daubert correlations for calculating the contents of the paraffinic portion (P), naphthenic portion (N), and aromatic portion (A) from the refractive index at 20 °C (RI), molecular weight (MW), and carbon to hydrogen ratio are presented as Equations (8)–(11).
The range of variation in the paraffinic (P), naphthenic (N), and aromatic (A) portions is as follows: 10.2% ≤ P ≤ 81%; 13.3% ≤ N ≤ 63.9%; 0% ≤ N ≤ 44.3%. This is a wider range than obtained using the n-d-M method discussed above.
Dhulesia developed correlations for predicting the aromatic carbon (CA) and hydrogen (H) contents in FCC feedstocks [45]. These correlations were reported to be used in the Total model to simulate FCC unit performance, and for that reason are known as the Total method for characterization of FCC feeds [45]. Equations (12) and (13) present the Total method to compute CA and H from RI, MW, SG, and viscosity at 98.9 °C (VIS).
Using the Total method, the range of variation for CA is between 1.2 and 51.6%, and for H, it is between 9.6 and 14.6%.
Choudhary et al. [48] also established correlations for predicting aromatic carbon (CA) and hydrogen (H) contents from SG and T50% (in degrees Farenheit), known as Conoco–Philips (COP) correlations. Unlike the Total method, which was based on 33 oil samples, the COP method was developed based on 354 heavy oil samples [48]. Equations (14) and (15) state the COP correlations.
Using the COP method, the range of variation for CA is between 14.0 and 75.0%, and for H it is between 8.2 and 13.1%.
The Bureau of Mines Correlation Index (BMCI) was also used by Navarro et al. to assess its correlation with FCC feed crackability [33]. The BMCI value can be calculated from SG and T50%C (in degrees Celcius) via Equation (16) [53].
Another empirical method used in FCC studies to relate feed characterization to crackability is the KW-characterizing factor [50,83,88], which can be computed by employing Equation (17) [49,89].
The molecular weight of FCC feed can be calculated via Goossens’ correlation [90]. Goossens’ molecular weight correlation employs FCC feed density at 20 °C (d) and T50%C, as exemplified in Equation (18):
The empirical parameter aromatic ring index (ARI) developed by Abutaqiya [51] and Abutaqiya et al. [52], which is a function of molecular weight and refractive index and is used to estimate the average aromatic ring numbers in the average hydrocarbon structure of the FCC feed, has been employed as a FCC feed characterizing parameter [37]. ARI is estimated via Equations (19) and (20) [51,52].
The function of the refractive index can be estimated by Equation (20):
3. Results
3.1. Review on Methods for Fossil Based FCC Feed Characterization and Their Relations to Feed Crackability
3.1.1. Empirical Methods for FCC Feed Characterization and Their Relation to the Feed Crackability
Equations (1)–(20) were applied to the data in Table S1 to calculate the empirical parameters for characterizing the nine vacuum gas oils cracked by Fisher [34]. Table 3 summarizes the empirical characterization data for the nine vacuum gas oils.

Table 3.
Various empirical parameters calculated from the physicochemical properties of the nine vacuum gas oils from Table S1, along with conversion data at maximum gasoline yield point.
To assess the degree of relationship between the conversion at maximum gasoline yield and the various empirical parameters, a correlation matrix was created, as shown in Table 4.

Table 4.
Correlation matrix of empirical FCC feed parameters and conversion at maximum gasoline yield.
The data in Table 4 indicate that maximum conversion strongly negatively correlates with aromatic carbon content for all empirical methods (−0.98 ≤ R ≤ −0.99), and strongly positively correlates with hydrogen content (0.97 ≤ R ≤ 0.99). The combination of these two empirical parameters calculated through the Total correlations (Equations (12) and (13)) as exemplified in Equation (21) provides a prediction of maximum conversion with R = 0.991, and a standard error of 1.8% for the data in Table 3.
The COP correlations (Equations (14) and (15)) for calculating CA and H provide lower prediction accuracy for maximum conversion, demonstrating R = 0.979 and a standard error of 3.1 wt.%. A possible reason for the lower prediction accuracy for the maximum FCC conversion when using CA and H calculated from COP correlations may lie in the lower prediction accuracy of CA and H for the COP correlations. To test this assumption, four FCC feeds characterized in the work of Ng and Rahimi [83] were used, and their CA and H values calculated according to the Total and COP correlations were compared with the measured CA and H values. Figure 2 presents parity plots of CA and H calculated against the measured values; the Total correlations demonstrate their superiority over the COP correlations in terms of prediction accuracy. The greater accuracy of calculating CA and H through the Total correlations may be due to the greater number of FCC feed input characteristics (SG, RI, S, MW, and VIS) used to calculate CA and H, compared with the two FCC feed variables (SG and T50F) employed in the COP correlations.
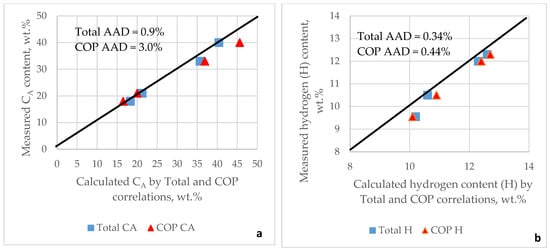
Figure 2.
Agreement between measured and calculated aromatic carbon content (a) and measured and calculated hydrogen content (b) according to Total and COP correlations. AAD = absolute average deviation.
The higher accuracy of the Total correlations for predicting aromatic and carbon content may explain why predicting conversion at maximum gasoline yield for the data reported in the Fisher study [34] gave a lower standard error (1.8%) than using the Conoco–Phillips correlations (standard error of 3.1%). The regression of the combined hydrogen content in the Total correlation and the aromatic carbon content predicted by the n-d-M method (Equations (1) and (4)) showed the highest accuracy in predicting maximum conversion, as shown in Equation (22).
Comparing the prediction accuracy of Equation (30) (standard error of 1.8%) with that of Equation (22) (standard error of 1.3%), it can be seen that Equation (22) outperformed Equation (21), which may imply that the aromatic carbon content calculated by the n-d-M method was a better prediction than that obtained via the Total correlation.
Examination via multiple regression revealed that the combination of aromatic portions calculated by the Riazi and Daubert method [46] and the hydrogen content according to the Total correlation using the data from Fisher’s study [34] showed a correlation coefficient R of 0.989 and a standard error of 2.2%. This may indicate that the aromatic content calculated using the correlations of the Riazi and Daubert method [46] is a worse predictor of FCC crackability than aromatic carbon calculated using the n-d-M method and the Total method. Equation (22) provided the highest accuracy in predicting the conversion at maximum gasoline yield. This was even higher than the Fischer conversion predicted using all types of carbon atoms associated with the gasoline precursor molecules, which was the highest based on mass spectrometry data alone.
3.1.2. Mass Spectrometry Characterization of FCC Feeds, Aromatic Carbon and Hydrogen Contents Measured by NMR, and Their Relation to Feed Crackability
To assess the relationships of the feed characterizing parameters with the maximum conversion, maximum gasoline yield, optimal LCO yield, coke yield, and coke on the catalyst, a correlation matrix was created for the data in Table 1, as presented in Table 5. It is evident from the data in Table 5 that among all the feed characterizing parameters, hydrogen content had the strongest correlation with maximum conversion (R = 0.935). Again, hydrogen content was the feed characterizing parameter that had the strongest correlation with maximum gasoline yield (R = 0.972). The optimum LCO yield had the strongest correlation with the KW characterizing parameter (R = −0.925). The coke yield had the strongest correlation with the polar content (R = 0.945). Coke on the catalyst also had the strongest correlation with the polar content (R = 0.905).

Table 5.
Correlation matrix of data from Table 1.
After regression of the feed characterizing parameters, together with maximum conversion, maximum gasoline yield, optimal LCO yield, coke yield, and coke on the catalyst, the following equations were developed:
Equations (23)–(27) provide a relatively good prediction of the conversion and product yields in the FCC process, meaning that the main characteristic parameters of the FCC feed are the contents of hydrogen, polars, and aromatic carbon. Another important point to notice is that the studies by Fisher [34] and Ng [36] employed catalysts with similar performance (Nova D with MAT activity of 70% in the research by Fisher [34], and Dimension 60 with MAT activity of 72% in the investigation by Ng et al. [36]). Although the reaction temperature at which Fisher [34] cracked the nine vacuum gas oils was 490 °C, and that employed by Ng et al. [36] to crack the ten FCC feeds was 510 °C, the good predictability of Equations (23)–(27) suggests that this difference is not crucial in the evaluation of FCC feeds. As Fisher’s work [34] did not contain data for CA and H measured like in the research by Ng et al. [36], these data were calculated using the Total correlations (Equations (12) and (13)).
Navarro et al. [33] found out that the hydrogen content of the FCC feedstock measured via NMR was the parameter that best correlated with maximum conversion, using Equation (24) for feedstocks whose hydrogen content ranged between 9.6 and 14.2 wt.% and whose maximum conversion was between 50.4 and 83.4 wt.%.
Applying Equation (28) to the data in Table 1, used to develop Equation (23), showed that the FCC maximum conversion was predicted with R = 0.948 and a standard error of 2.7 wt.%. This comparison is consistent with the conclusion of Navarro et al. [33] that hydrogen content is the best descriptor of FCC feed crackability.
Figure 3 shows the agreement between observed and ANN-predicted FCC maximum conversion for the 88 oils in Table S2.
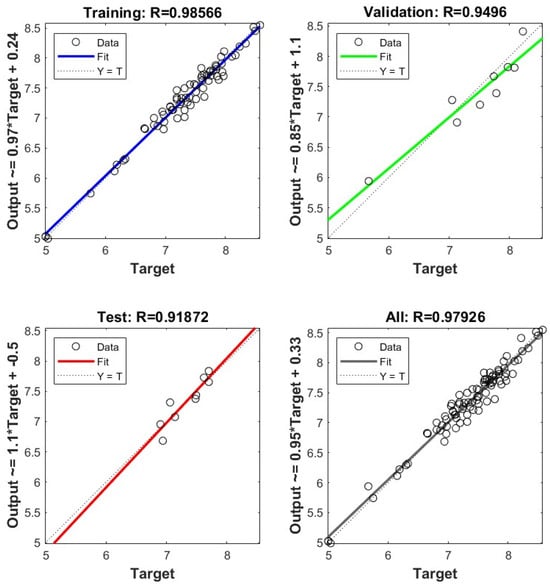
Figure 3.
ANN-predicted versus observed FCC maximum conversion for training, validation, testing, and overall data for 88 cracked oils.
The FCC maximum conversion estimated by the ANN model had R = 0.979 and a standard error of 1.4%, which was considerably better than the regression Equations (23) and (28). In this case, a mean squared error of 0.061531 was achieved at epoch 10, as shown in Figure 4.
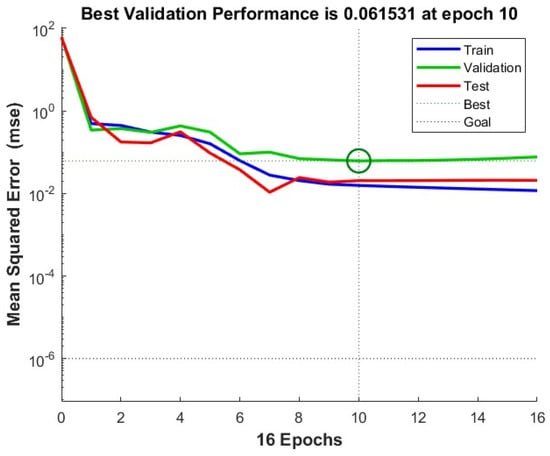
Figure 4.
Neural network training performance for prediction of FCC maximum conversion. The green circle indicates the point where the minimum mean squared error is obtained.
Equation (29) presents a new regression model that employs the data of contents of hydrogen, saturates, aromatics, and polars, used in the ANN modeling, to estimate the maximum FCC feed conversion:
where H = hydrogen content, wt.%; Saturates = saturate content, wt.%; Aromatics = aromatic content, wt.%; Polars = polar content, wt.%.
Comparing the correlation coefficient and standard error of the maximum FCC feed conversion prediction of the ANN model with the regression model in Equation (29), it is seen that the ANN model is superior to the regression model.
The FCC maximum conversion modeling results obtained in this study indicate that the FCC feed parameters of hydrogen content, saturates, aromatics, and polar contents, and the use of the ANN technique can provide the most accurate prediction of FCC maximum conversion.
3.1.3. FCC Feed Characterization by Total and Basic Nitrogen Contents and Their Relation to Feed Crackability
As shown in the previous section, the maximum conversion, i.e., the overcracking point, of an FCC feed depends on the hydrogen and aromatic carbon content (Equations (22)–(24)). Meanwhile, the feed reactivity, coking tendency [58], and catalyst deactivation [91,92,93] depend strongly on the nitrogen content of the feedstock. Nitrogen poisons the active sites of the FCC zeolitic catalyst, reducing its activity and making it less selective towards valuable products [94,95,96]. About one-third of the nitrogen content in the FCC feed is represented by basic nitrogen compounds [97,98,99]. Total nitrogen can be measured according to the standard ASTM D 5762 [100], while basic nitrogen can be measured following the requirements of the standards ASTM D2896 [101] and UOP 269 [102]. Most of the basic nitrogen compounds are present as alkylpyridines or alkylquinolines, while non-basic nitrogen compounds are generally pyrroles, indols, carbazoles, or carboxamides [96,103,104]. The types of nitrogen species in the FCC feed depend on the processing conditions, notably whether the feed is hydrotreated before it is cracked [105,106,107]. The reaction severity and the type of catalyst in the hydrotreatment process can drastically change the distribution of nitrogen type [96,108]. Fu and Schaffer [96] calculated that the total nitrogen (or even basic nitrogen) concentration in the FCC feed was insufficient to predict the poisoning effect of nitrogen of the FCC feed. Ho et al. [103] developed a non-linear model for the poisoning effect of nitrogen species, showing that molecular weight and gas-phase basicity measured by proton affinity are the factors affecting the poisoning power of nitrogen species in FCC feed. The higher the molecular weight and proton affinity of the nitrogen components, the greater is their poisoning effect on the FCC catalyst. Research by Chen et al. [109] showed that 41% of nitrogen in the feedstock was converted to coke, 8% to ammonia, 2% to gasoline, 14% to light cycle oil, and 35% to slurry oil. The results of research reported by Qian et al. [110] and Behera et al. [111] firmly indicate that FCC feed nitrogen molecules are the primary precursors of coke.
Scherzer and McArthur [112] cracked four FCC feeds with nitrogen content varying between 0.3% (basic nitrogen of 0.094%) and 1.56% (basic nitrogen of 1.23%), on eight experimental FCC catalysts in a laboratory micro-activity (MAT) unit at 510 °C reaction temperature, a catalyst-to-oil ratio of 3.5 wt./wt, and a weight hourly space velocity (WHSV) of 14.5 h−1. They found that during constant conversion, the increase of FCC feed nitrogen content decreased the gasoline yield and enhanced the yields of coke, gas, and hydrogen. Increasing the zeolite content in the examined catalysts from 20 to 40% increased the conversion of the FCC feed with a nitrogen content of 0.75% from 58.4 to 83.6%, and the conversion of the FCC feed with a nitrogen content of 0.3% was enhanced from 80.4 to 88.8% when the zeolite content in the catalyst increased from 20 to 40%. The threefold higher conversion enhancement for a feed with a nitrogen content of 0.75% compared with a feed with a nitrogen content of 0.3% when the zeolite content in the catalyst was increased from 20 to 40% indicates that catalysts with higher zeolite content are more efficient for cracking FCC feed with a high nitrogen content. Scherzer and McArthur [112] also showed that catalysts containing more rare earth elements were less affected by the increase in the FCC feed’s nitrogen content, therefore making them more suitable for cracking FCC feedstocks with high nitrogen content.
Caeiro et al. [113] treated industrial FCC gas oil with 0.325% total nitrogen content and 0.1307 basic nitrogen content with a stoichiometric amount of H2SO4 (95%), which resulted in a reduction in total nitrogen content to 0.178% and basic nitrogen content to 0.0135%. It was found that the H2SO4 treatment affected only the basic nitrogen content. The original and H2SO4-treated gas oils were cracked on an industrial FCC equilibrium catalyst in a laboratory advanced cracking equipment (ACE) unit at a reactor temperature of 535 °C under atmospheric pressure, with the catalyst-to-oil ratio varying between 5 and 8 wt./wt. A tenfold reduction in the basic nitrogen content in the vacuum gas oil was associated with an increase in conversion from 61.2 to 69.4 wt.% at a catalyst-to-oil ratio of 5 wt./wt. and from 69.1 to 74.1 wt.% at a catalyst-to-oil ratio of 8 wt./wt. The higher difference in conversion at a lower catalyst-to-oil ratio was explained by the higher quantity of basic nitrogen components per mass of catalyst, and the inhibition was more pronounced. At higher catalyst-to-oil ratios, the influence of basic nitrogen components on crackability is less pronounced because their relative amount in the feed compared with the catalyst weight is lower. The results reported by Caeiro et al. [113] are consistent with those reported by Scherzer and McArthur [112], indicating that during constant conversion, an increase in the basic nitrogen content is associated with a decrease in gasoline yield and increases in coke and hydrogen yields.
Li et al. [114] investigated the inhibitory effect of different kinds and contents of basic nitrogen compounds on the catalytic cracking of hydrocarbons in coker gas oil. They treated Dagang coker gas oil with aqueous solutions of 0.1 and 0.5 mol/L HCl and reduced the basic nitrogen content from 0.1300% to 0.0805% and 0.0720%, respectively. As a result, FCC conversion increased from 72.4% for the original coker gas oil to 85.5% and 88.7% for the raffinate oils extracted with HCl with concentrations of 0.1 and 0.5 mol/L, respectively. The gasoline selectivity increased from 42.6% for the original coker gas oil to 44.5% and 45.0% for the raffinate oils, respectively. Li et al. [98] established that the structure of basic nitrogen compounds had a greater influence on the catalytic cracking of coker gas oil than the concentration of the basic nitrogen. The compositional and structural identification of basic nitrogen compounds via positive-ion electrospray Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) indicated that the basic nitrogen compounds in coker gas oil included N1, N2, NO, N2O1, and NS class species. The N1 class species centered at 9 < double bond equivalent (DBE) < 13 with a carbon number ranging from 20 to 24 was found to be the most abundant and was the dominant factor for the coker gas oil’s retarding performance. The authors found that basic nitrogen compounds could reversibly absorb onto the acid site, resulting in reductions in acid centers, or they could act as coke precursors due to their size and aromatic nature, which was partly responsible for the aggravating coker gas oil conversion.
Wang et al. [115] studied the coked catalysts obtained from the catalytic cracking of high-saturate-content Daqing vacuum gas oil (VGO) (83% saturates; density of 0.801 g/cm3) and Dagang coker gas oil (60.8% saturates; density of 0.9010 g/cm3), characterized by high contents of nitrogen and basic nitrogen compounds. Both gas oils were cracked on a commercial FCC catalyst with microactivity of 66% in a fixed fluidized-bed reactor at 510 °C reactor temperature and a catalyst-to-oil ratio that varied between 4.5 and 10.2 wt./wt at a constant WHSV of 15 h−1. At a catalyst-to-oil ratio of 6 wt./wt., the conversion of the Daqing VGO was 94.2 wt.%, while that of the Dagang coker gas oil was 20 wt.% lower (73.8 wt.%). Wang et al. [100] found that the coke formed during the catalytic cracking consisted of adsorption coke (Cad), dehydrogenation condensation coke (Cdh), and hydrogen transfer coke (Cht). Cad was formed by basic nitrogen compounds in feedstocks adsorbed on Lewis acid sites, which was one of the main factors for the greatly decreased catalyst activity, being responsible for 37 wt.% of the total coking content under conventional reaction conditions. Cdh was formed by the dehydrogenation condensation of polycyclic aromatic hydrocarbons and accounted for about 43 wt.% of the total coking content. The coking content of Cht was greatly determined by the degree of the secondary reaction. It was found that the coke selectivity could be reduced and the Cht yield could be controlled by simultaneously increasing the reaction temperature and shortening the reaction time.
Li et al. [116] selectively separated non-basic nitrogen species and condensed aromatics by solvent extraction from Dagang coker gas oil. They first removed the basic nitrogen components by extraction with HCl aqueous solution. Then, furfural was used to enrich non-basic nitrogen compounds and condensed aromatics from the basic-nitrogen-free oil. The effects of non-basic nitrogen compounds and condensed aromatics on the coker gas oil catalytic cracking were evaluated by comparing the FCC performance before and after solvent extraction in a fixed fluidized bed reactor over a commercial equilibrium catalyst at reactor temperature of 500 °C, at a catalyst-to-oil ratio of 6 wt./wt. and a WHSV of 20 h−1. The authors found that removal of basic nitrogen components improved the FCC conversion by 16.3 wt.%, while removal of non-basic nitrogen species and condensed aromatics enhanced conversion by only 1.3 wt.%. These results imply that non-basic nitrogen species and condensed aromatics have much lower impact on the coker gas oil reactivity compared with basic nitrogen species. However, non-basic nitrogen compounds and condensed aromatics have proved difficult to convert into smaller molecules, reducing the yields of gasoline and LPG, and increasing the yields of light cycle oil (LCO) and slurry oil (SLO). Non-basic nitrogen compounds with single N species were observed to bedominant in the Dagang coker gas oil, where they were identified as carbazoles, cycloalkyl-carbazoles, benzocarbazoles, and cycloalkyl-benzocarbazoles. Condensed aromatics were found to include three to four rings of large dynamic size, usually presented as chrysene, pyrene, and phenanthrene. These species deposit on the surface of catalysts and form a coke that prevents other hydrocarbons from reaching the acid centers.
Zhang et al. [117] investigated the catalytic cracking of Qilu coker gas oil (density 0.930 g/cm3; total nitrogen 0.66%, basic nitrogen 0.2178%) over a commercial FCC catalyst in a pilot-scale riser FCC plant under various conditions. They found that a high reaction temperature and short residence time could inhibit the adsorption of nitrogen compounds on the catalyst, decreasing their retardation effect. Increasing the catalyst-to-oil ratio maintained the average catalyst activity at a higher level, thus increasing the conversion of nitrogen-free hydrocarbons (mainly saturates), while nitrogen-containing species were concentrated in the heavy oil, thus increasing the feedstock conversion. The compositional and structural identification of nitrogen compounds in cracked heavy oils obtained using Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) revealed six nitrogen class species, N1, N2, N1O1, N1O2, N1S1, and N1O1S1. The N1 class nitrogen compounds were the dominant species, and the N1O1S1 species were generated during the FCC process. Most of the nitrogen-containing species included four or five rings with short alkyl side chains.
Wang et al. [118] investigated high-nitrogen-content coker gas oil (density 0.960 g/cm3; total nitrogen 0.6927%, basic nitrogen 0.2639%) and heavy cycle oil (density 0.9553 g/cm3; total nitrogen 0.1850%, basic nitrogen 0.1391%) (HCO) before and after solvent extraction with furfural. Solvent refinement removes refractory poly nuclear aromatics and heterocyclic compounds from oils, which represent 30% of the original oils under optimal operating conditions, and produces less-contaminated, hydrogen-rich coker gas oil and HCO materials. Analysis of the basic nitrogen species using GC-MS total ion chromatography showed that they consisted of between two and four rings, corresponding to quinoline-derived and acridine-derived compounds, respectively. In addition, benzoquinoline and benzoacridine compounds appeared to be the predominant nitrogen species, with side chains of zero to five and zero to three carbon atoms, respectively. No multiheteroatom nitrogen compounds were identified. The identified polynuclear aromatics comprised three or more aromatic rings, most of which had three or four other rings, as well as high degrees of condensation. In addition, phenanthrene, anthracene, pyrene, chrysene, fluoranthene, and phenylnaphthalene species, with side chains of zero to three carbon atoms, were predominant in the coker gas oil. The original and refined gas oils were cracked in a technical-scale pilot plant on a commercial Y zeolite-based equilibrium FCC catalyst at a riser outlet temperature of 500 °C, residence time of 3 s, and catalyst-to-oil ratio of 6 wt./wt. Removal of refractory polynuclear aromatics and heterocyclic compounds by furfural solvent extraction from the coker gas oil enhanced the conversion from 43.2 to 81.5 wt.%
Regarding the detrimental effects of nitrogen contaminants and refractory polynuclear aromatic compounds on the catalytic cracking of coker gas oils, the reader may refer to references [119,120,121,122,123,124]. Removal of these FCC-retarding contaminants, as discussed previously, can be accomplished either by solvent extraction or solid adsorption, as described in [93,125]. Modified natural chabazite was found to be able to remove 74% of the nitrogen from catalytic cracking of bitumen [125].
The characterization data of 14 feedstocks (aromatic carbon, hydrogen, total nitrogen, and elemental nitrogen content) as well as the conversion of the FCC feedstocks at a catalyst-to-oil ratio of 5 w/w, presented in Table 2, were regressed. The following expression, shown as Equation (30), was developed:
where FCC feed conversion = FCC feed conversion at catalyst-to-oil ratio of 5 wt./wt. and reactor temperature of 510 °C, wt.%; BN = basic nitrogen content, ppm.
Figure 5 presents a parity graph of measured FCC feed conversion values versus those estimated via Equation (30).
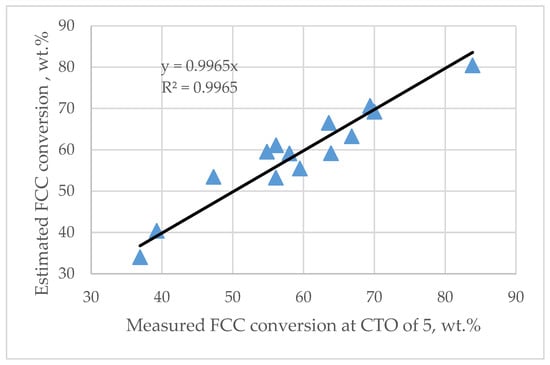
Figure 5.
Measured versus calculated by Equation (30) FCC feed conversion at a catalyst-to-oil ratio of 5 wt./wt. and reactor temperature of 510 °C.
Equation (30) indicates that the FCC feed reactivity depends on the contents of aromatic carbon and basic nitrogen. Therefore, these two feed parameters control the FCC feed conversion at a constant catalyst-to-oil ratio, a finding that is consistent with the results reported by Bollas et al. [58].
3.1.4. Liquid Chromatography for Fractionation of FCC Feed into Acidic, Basic, and Neutral Types and the Relationship to Feed Crackability
Another characterization technique employed to fractionate heavy oil into compound classes and determine their cracking behavior is non-aqueous ion-exchange liquid chromatography [64], which separates acid, base, and neutral types from the heavy oil. The cracking behavior of the acid and base fractions is assessed by comparing cracking tests of whole neutrals versus blends of acids/bases plus neutrals. Blending acid and base fractions with neutrals prior to cracking is a practical necessity because of their intractable physical properties (typically brittle solids at room temperature). Furthermore, catalytic cracking of pure acid or base fractions is unrealistic for any practical refining situation. In previous research, the mixing of the fractions was carried out via stirring and heating. Selected mixtures were checked for incomplete mixing, using hot filtration [64]. Cracking experiments were carried out in a microconfiied bed unit over a commercial equilibrium zeolite containing catalyst with microactivity of 73% at a reactor temperature of 521 °C and a catalyst-to-oil ratio of 8.5 wt./wt. [111]. Heavy oils (atmospheric residuals: >650 °F (343 °C)) obtained from Wilmington [64], Brass River [65], Maya [66], Lagomedio [67], and Merey [68] crude oils were fractionated by vacuum distillation of gas oil and vacuum residual fractions. The vacuum gas oils and vacuum residues were then separated into acid, base and neutral fractions. The contents of the neutral, acid and base fractions separated by non-aqueous ion exchange liquid chromatography from the vacuum gas oil and vacuum residue fractions from the aforementioned heavy oils are presented in Table 6. Table S3 summarizes the data on the elemental composition of vacuum gas oil and vacuum residue fractions, as well as the acid, base, and neutral fractions separated from the Wilmington, Brass River, Maya, Lagomedio, and Merey crude oils.

Table 6.
Distribution of acid, base and neutral fractions in heavy oils from Wilmington, Brass River, Maya, Lagomedio, and Merey crude oils (data taken from [64,65,66,67,68]).
The characterization data from Table 6 and Table S3 indicate that the heavy oils differed considerably not only in their contents of acid, base, and neutral fractions, but also in their chemical constitution. For example, vacuum gas oil from Brass River crude oil contained considerably lower levels of acidic, basic, polar-neutral, and sulfide species than vacuum gas oil from Wilmington crude oil (Table 6). The heteroatom contents in the acidic and basic fractions of the Brass River crude oil were generally lower than those in the corresponding Wilmington fraction (Table S3) [65]. The lower heteroatom levels in Brass River 343–538 °C acids and bases, together with their higher MCR values, suggest that they had higher average molecular weights than the corresponding Wilmington fractions [65]. On the other hand, the higher heteroatom content and generally higher MCR for the Wilmington > 538 °C acid/base types were probably a result of their greater multifunctionality and higher aromaticity compared with the corresponding Brass River fractions [65].
Catalytic cracking studies carried out on heavy oils derived from Wilmington, Brass River, Maya, Lagomedio, and Merey crude oils and their acid, base, and neutral fractions [64,65,66,67,68,69] have shown that the various polar fractions (acid or base) contribute to gasoline production at a level nearly equivalent to that of neutrals, to such an extent that they significantly suppress gasoline production and alter its composition. The gasoline composition was found to be affected by catalytic inhibition by acid/ base components, as well as by their direct contribution to the gasoline pool via participation in cracking reactions [67]. The direct contribution of polar fractions to gasoline production was significantly greater for the Brass River and Lagomedio acid/bases than for those from Wilmington or Maya [67]. Thus, the increased content of aromatics resulting from the lack of catalyst inhibition in the case of pure neutral cracking from Maya or Wilmington crude oil was offset for the Brass River and Lagomedio crude oils through the direct contribution of aromatics from the cracking acid/base types [67].
Data were analyzed from the catalytic cracking of 55 mixtures of neutral, acid, and base fractions derived from vacuum gas oil and vacuum residual fractions obtained from heavy oils from Wilmington, Brass River, Maya, Lagomedio, and Merey crude oils discussed in this series of six papers [64,65,66,67,68,69] over a nine-year period. The analysis revealed that eight feed features affected the product yield values. These feed characteristics were the atomic ratio of hydrogen divided by carbon plus sulfur (H/(C + S)), the fraction of the feed evaporated at the cracking temperature fcT, the effective metal content (Meff = Ni + V/4), basic nitrogen (NB, wt.%), amide-type nitrogen (NAm, wt.%), sulfur content (S, wt.%), hydrogen content (H, wt.%), and microcarbon residue content (MCR, wt.%) [67,68]. Using multiple linear regression analysis of these feed characteristics and product yields allowed the development of the following equations (Equations (31)─(35)) [67,68]:
H = hydrogen content, wt.%;
fcT = fraction of the feed boiling below the cracking temperature (521 °C).
MCR = microcarbon residue content, wt.%;
Meff = effective metal content = Ni + V/4, ppm;
S = sulfur content, wt.%;
NB = basic nitrogen content, wt.%;
NAm = amide-type nitrogen, wt.%.
The technique of separating FCC feedstocks into acid, base, and neutral fractions for the catalytic cracking of these mixtures has allowed further investigation of the influence of heteroatom species on the cracking behavior of all types of FCC feedstocks, from conventional gas oils with low levels of heteroatoms to residual feedstocks that often contain levels of heteroatom species that are orders of magnitude higher [67]. In contrast to Equation (30), which was developed based on data for 14 FCC feedstocks, some of which contained residual oils and others of which were unconventional (including coal–oil coprocessing products and a shale oil, Table 2, [83]) with higher heteroatom contents, Equations (31)–(35) indicate that more feedstock characteristics than both aromatic carbon (CA) and basic nitrogen (BN) contents are needed to adequately predict FCC product yields. The prevalence of non-carboxylic carbonyl-containing species such as nominally amide types in the 343–499 °C acid fraction of Maya vacuum gas oil necessitated the inclusion of the term for these species (NAm) in Equation (31). Infrared spectrophotometry was used to determine amide forms of nitrogen in the acid fractions. These findings are consistent with the conclusion reached by Fu and Schaffer [96] that the concentration of total nitrogen (or even basic nitrogen) alone is not sufficient to predict the poisoning effect of nitrogen in an FCC feed. Despite this agreement, the quantitative nonlinear correlation developed by Ho et al. [103] using the data from Fu and Schaffer [96], suggesting a greater inhibitory effect of the Wilmington base fraction >538 °C than the same fraction boiling in the vacuum gas oil range (343–538 °C) due to the higher molecular weight of the >538 °C fraction, was not confirmed in the study by Green et al. [64]. These results indicate the complexity of the interaction of heteroatom-containing species with the catalyst during the catalytic cracking process, and that the experimental methodology applied can influence the conclusions made. The experimental methodology applied and documented in a series of six papers over the period 1994–2003 [64,65,66,67,68,69] presents a comprehensive investigation of the impact of FCC feed on yields and product quality. FCC feeds were characterized in sufficient numbers and cracked on a commercial catalyst under conditions very close to those of commercial FCC units [126]. This research project revealed the complex interactions of the different components of FCC feeds and allowed them to be quantified via Equations (31)–(35). Using a larger number of FCC feed characteristics can lead to a more adequate prediction of product yields, as a more potential interactions between the diverse FCC feed components and the catalyst are taken into account. Given the complex nature of the FCC feedstock, Green et al. [66] concluded that characterizing the feedstock using average parameters such as percentages of paraffinic, naphthenic, and aromatic carbons was not sufficient to properly model yields and product quality in the FCC process.
3.2. Review on Co-Processing of Bio-Oils and Pyrolysis Oils from Waste Plastics and Scrapped Tires in the FCC Process
3.2.1. Co-Processing of Bio-Oils in the FCC Process
Several reviews summarizing the results of catalytic cracking studies on biobased feedstocks have appeared over the years, discussing various aspects of the application of catalytic cracking of biomass-derived oils [127,128,129,130,131,132,133,134,135,136,137,138,139]. Bio-oils subject to catalytic cracking can be classified in two main groups: oleaginous bio-oils and lignocellulosic biomass [129]. Oleaginous bio-oils are mainly composed of triglycerides, which can be extracted from animal fats, vegetable oils, and wastes [129]. Triglycerides consist of three fatty acid molecules attached to one of glycerol. Lignocellulose is made up of two carbohydrate polymers (cellulose and hemicellulose), and one aromatic polymer (lignin). Lignocellulosic biomass includes raw biomass (terrestrial plants), waste biomass (from agriculture and forestry), and energy crops (crops specially grown for the production of biofuels). The contents of lauric acid (C12:0), myristic acid (C14:0), palmitic acid (C16:0), palmitoleic acid (C16:1), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), linolenic acid (C18:3), arachidic acid (C20:0), eicosenoic acid (C20:1), behenic acid (C22:0), erucic acid (C22:1) in most common vegetable oils used as feedstock in the biofuel production are summarized in the work of Naji et al. [132].
In Table 7, the properties of oleaginous bio-oils and lignocellulosic bio-oils, taken from the literature [132,140,141,142,143,144,145,146,147,148,149], are compared with those of petroleum-based FCC feedstocks. The atomic H/C ratio correlates with aromaticity [150,151,152] and therefore should correlate with crackability, as indicated in Equations (22), (30) and (31). Data derived from petroleum-based FCC feedstocks suggest that oleaginous bio-oils should perform well as FCC feedstocks, because their H/C ratio is relatively high and varies in the range 1.73–2.11. The quality of lignocellulosic bio-oils fluctuates in a wider range (0.76 ≤ H/C ≤ 2.69) and generally shows poorer quality as an FCC feedstock compared with oleaginous bio-oils. These observations are consistent with the statement by Serrano et al. [129] that oleaginous bio-oils are more readily converted to liquid automotive fuels than lignocellulose-based bio-oils.

Table 7.
Comparison of properties of oleaginous bio-oils and lignocellulosic bio-oils with petroleum-based FCC feeds (data taken from [132,140,141,142,143,144,145,146,147,148,149]).
The reaction pathways for oleaginous bio-oils were found to occur in two diverse stages, i.e., (1) thermal cracking of the triglyceride that released fatty acid chains from the glycerin backbone; (2) catalytic cracking of the high molecular weight fatty acid molecules into smaller, more valuable products [153]. Higher-saturated free fatty acids are harder to crack than those that contain greater amounts of double bonds [154]. Using this rationale, Watkins et al. [140] explained why palm oil, which contained the least fatty acids with double bonds, showed the worst performance compared with soybean and rapeseed oils, which performed better during co-catalytic cracking with a composite residue (specific gravity 0.9076, sulfur 0.53%). The presence of unsaturated free fatty acids in oleaginous bio-oils resulted in reduced protolytic cracking of fatty acids compared with typical FCC feedstock, which in turn reduced hydrogen and propane yields when they were co-cracked with petroleum-based feedstocks. Consequently, the double bonds of each of the free fatty acid molecules, which were more reactive, were the initiation sites for the cracking reactions [140]. Co-catalytic cracking of petroleum-based FCC feedstock with 15% oleaginous bio-oils (soybean, rapeseed, and palm oils) produced more gasoline (between 1 and 2% more) than pure petroleum-based oil, and this gasoline was characterized by slightly lower research and motor octane numbers (RON and MON) [140]. The yield response following the addition of a bio-feed material into the FCC feed depends on the properties of the base FCC feed. When petroleum-based feed is characterized by high hydrogen and low aromatic carbon content, the addition of oleaginous bio-oil leads to the production of more coke and hydrogen and a low conversion level is registered for the same coke yield [140]. The oxygen from the fatty acids is converted primarily to water [153].
To better understand the differences in the behavior of vegetable oil during catalytic cracking compared with that of petroleum-based FCC feedstock, Brydon et al. [155] cracked 100% soybean oil and 100% vacuum gas oils in a Davison circulating riser (DCR) pilot plant under the same operating conditions. Table 8 summarizes the properties of both the 100% vacuum gas oil FCC feed and the 100% soybean oil, as well as the yields under the same operating conditions. It is evident from the data in Table 8 that the soybean oil produced more LCO, less gasoline, fewer C3s, and fewer C4s than VGO. The gasoline produced by cracking soybean oil was highly aromatic, consistent with the results reported by Dupain et al. [153] and Bielansky et al. [156]. Only trace amounts of oxygenates were found in the gasoline produced from 100% soybean oil.

Table 8.
Yields at same operating conditions for 100% petroleum-based FCC VGO feed and 100% soybean oil (Data taken from [155]).
When 100% soybean oil was cracked, CO and CO2 were detected in the product gas, representing a total of ~15% of the oxygen in the soybean oil, and~85% of the oxygen in the soybean oil reacted to water [140]. Brydon et al. [155] also observed that during the catalytic cracking of 100% soybean oil, the temperature profile of the riser was almost flat, with a temperature difference of only 5.6 °C between the bottom and top of the riser, compared with 44 °C when 100% VGO was cracked. According to the temperature drop across the riser, the heat for the cracking of soybean oil was only about 15% of the heat for the cracking of standard vacuum gas oil, which was consistent with the exothermic formation of carbon monoxide, carbon dioxide, and water from the oxygen present in the soybean oil. This heat behavior resulted in the soybean oil running at a significantly lower catalyst-to-oil ratio (6.7 wt./wt.) than VGO (9.3 wt./wt.) under the same conditions.
Lignocellulosic bio-oils are complex mixtures of water (up to 30 wt.%) and organic oxygenates (70–80 wt.%) which are obtained by thermal decomposition of lignocellulosic feed [130]. They contain highly reactive oxygen containing species, which are responsible for many deleterious properties such as instability, immiscibility with hydrocarbon fuels, low heating value, and corrosivity [130,158]. During catalytic cracking, lignocellulosic bio-oil is vaporized at medium temperatures and the vaporized bio-oil is in contact with a heterogeneous acidic catalyst [130]. The catalytic cracking chemistry of lignocellulosic based bio-oils proceeds involving conventional FCC reactions such as protolytic cracking (carbon–carbon bond cleavage), hydrogen transfer, and isomerization, in addition to deoxygenation reactions such as dehydration, decarboxylation, and decarbonylation [127]. Catalytic reactions remove oxygen from the vapors via formation of water, CO2, and CO, and the resulting oil is a less oxygenated mixture of aromatic hydrocarbons and less problematic oxygenates [130]. The dehydration reactions that take place on the catalyst acid sites lead to the formation of water and dehydrated compounds, which may include ketones, aldehydes, and alcohols. Decarboxylation and decarbonylation reactions result in the formation of CO2 and CO, respectively [127]. The instability of the bio-oil feedstock and its hydrogen-deficit nature are conducive to the formation of charring and coke, resulting in considerably lower oil yields, fast deactivation of the catalyst, and enhanced formation of solid byproducts [130]. The formation of char is a result of the polymerization of unstable components present in bio-oils, including aromatic hydrocarbons and phenols [127]. Furthermore, the obtained liquid product is only partially deoxygenated and still requires further upgrading [130]. Bio-oil cracking is associated with irreversible deactivation (dealumination) of the catalyst, primarily due to the high oxygen content in the bio-oil and, consequently, the high water/steam content in the reaction medium. Thus, catalytic cracking of 100% lignocellulosic bio-oil can be considered inappropriate.
Due to the lower atomic H/C ratio in lignocellulosic bio-oils, when cracked in mixtures with petroleum-based feedstocks, they exhibit lower conversion than conventional FCC feedstocks [127]. They yield more low-value dry gas than conventional FCC feedstocks. During co-processing, synergistic reactions occur that lead to reduced olefin formation but enhanced formation of aromatic and saturated compounds through hydrogen transfer. Co-processing of lignocellulose-based bio-oils also exhibited increased catalyst deactivation, although this was not as severe as for cracking of pure bio-oil feedstocks. The coke yield was found not to be a linear function of the bio-oil content of the co-processed feed mixture, suggesting the presence of an interaction between the bio-oil and the petroleum components/products. The product from co-processing lignocellulosic bio-oils and petroleum feedstock mixtures may contain oxygenated compounds such as phenols and alkylphenols. Oxygen in the feed blend is mainly converted to water, similar to the co-catalytic cracking of oleaginous bio-oils via dehydration reactions [127]. The effect of co-processing of pine-derived pyrolysis oil at 3% blended with 97% VGO on catalytic cracking performance was investigated by Bryden et al. [146] in a DCR pilot plant, and the data are presented in Table 9.

Table 9.
Yields under same operating conditions for 100% petroleum-based FCC VGO feed and a blend of 3 wt.% pine-based pyrolysis oil and 97 wt.% VGO (data taken from [155]).
The data in Table 9 indicate that co-feeding pyrolysis oil resulted in more coke, less gasoline, and production of CO and CO2 in the product gas. Only traces of oxygenates were found in the liquid product obtained from the cracking of the bio-oil-containing feedstock [155]. When cracking the pyrolysis oil, CO and CO2 were detected in the product gas, amounting to a total of ~22 percent of the oxygen in the pyrolysis oil, while ~78% of the oxygen in the pyrolysis oil reacted to water [155]. These results indicate that co-processing of lignocellulosic bio-oils, even at small additional quantities, can lead to significant changes in yield.
In general, co-processing of lignocellulosic bio-oils has been reported to be difficult due to the presence of highly reactive oxygen species that cause feedstock pumping problems, injector plugging, valve plugging, corrosion, and polymerization of blends in the feed tank at high temperatures (80 °C) and with long storage times [130,138,158]. An additional issue in cracking bio-oils is the occurrence of copper strip corrosion values from the resulting FCC products that exceed the specified limits for fuels because of the effect of residual oxygen in the liquid product, which is quite high in some cases [127,159].
Special care is needed to avoid exposing bio-oils to temperatures higher than 50 °C to sidestep from polymerization in the feed tanks and lines [130]. Separate feeding of VGO and raw bio-oil at different heights in the reactor allowed successful co-processing in a demonstration-scale unit without operational issues at 5/95, 10/90, and 20/80 bio-oil/VGO mass ratios, and without any prior upgrading [130,160,161]. The separate feed injection enabled the heating of the VGO up to 220 or 280 °C, as is usual in commercial FCC operations, while the bio-oil was not exposed to temperatures higher than 50 °C [162]. Additionally, injection near the bottom of the riser reactor allowed rapid exposure of the bio-oil to a high temperature (about 650 °C) for a short amount of time [153]. Pinho et al. [163] suggested that the resulting thermal shock was crucial for breaking down high-molecular-weight components and for the reduction of coke formation. Adequate atomization of the bio-oil into small droplets was also critical in order to inhibit rapid transformation into coke on the catalyst [163]. Pinho et al. [76] during co-processing 20% bio-oil with vacuum gas oil in the FCC revealed that most of the biogenic carbon was recovered as coke (40–54%). These researchers suggested that the renewable carbon recovery in the liquid effluent remained at approximately 30 wt.%, regardless of the percentage bio-oil inserted into the FCC [164]. While “green coke” may have some decarbonization benefits in a refinery [165], high yields of renewable carbon in the coke reduce the potential decarbonization of the liquid fuel products.
Accumulated experience of research into bio-oil co-processing was the foundation behind the commercialization of this process. Cepsa Gibraltar refinery performed a bio-feedstock co-processing trial in their FCC unit [166]. The selected vegetable oil had a density of 0.915 g/cm3, similar to the VGO quality typically processed at the unit, and its oxygen content was 11.5 wt.%. A commercial trial was conducted to increase non-fossil feed from 0 wt.% to 5 wt.% in a stepwise fashion. The trial was carried out under constant operating conditions that were similar to typical operation. The commercial trial indicated no operating issues. Increased production of sour water was noticed and was handled accordingly. The yield deltas were minor and within the typical variation often encountered with changes in feed quality or type. It was concluded that this vegetable oil was processable in the FCC unit with minimal impact and did not constrain the unit. Given this positive experience, the refinery team is considering further expanding the feedstock window with second generation biogenic oils, to further minimize the carbon intensity of the FCC products [166].
The characteristics of bio-oils that affect the conversion level in the FCC process are similar to those of petroleum-derived oils, as the catalytic cracking chemistry is the same. Basic nitrogen and aromaticity inhibit conversion and reduce gasoline production while increasing coke and hydrogen yields, as reported by Eschenbacher et al. [167]. The presence of saturated carboxylic acids in petroleum-derived oils, such as heavy oil from Wilmington crude, was reported by Green et al. [64] to increase the yield of light products, suggesting their cracking to lower-molecular-weight products in the FCC process. Therefore, the high content of saturated carboxylic acids in bio-oils (high H/C atomic ratio) is expected to contribute to a higher yield of light products during their cracking. In contrast to petroleum-derived oils, which do not contain unsaturated compounds, except the gas oils from thermal cracking-based processes such as coking and visbreaking, bio-oils may have high concentrations of unsaturated carboxylic acids. As reported by Watkins et al. [140], they are easier to crack and provide higher yields of gasoline and lower yields of coke. Watkins et al. [140] showed in their study that palm oil with an average of 1.1 double bonds per fatty acid chain exhibited the lowest conversion at constant coke yield and the lowest yield of gasoline compared with those obtained from soybean and rapeseed oils, which had higher average values of double bonds per fatty acid chain, when co-processed at 15 wt.% with vacuum gas oil in a pilot FCC unit.
The total number of studies in the reviewed literature [127,128,129,130,132,133,134,136,137,138] relating to the catalytic cracking of bio-oils exceeded 2000, which is an order of magnitude more than the articles devoted to the investigation of the catalytic cracking of various oils derived from petroleum listed in this review. This overwhelming research indicates the goal of human society to decarbonize the fuels used in internal combustion engine vehicles. Although the research on catalytic cracking of bio-oils exceeds that dedicated to the cracking of petroleum oils, it is not systematic enough, as reported in the studies by Fisher [34], Ng et al. [35,36], Nace et al. [42], Voltz et al. [43], Bollas et al. [58], and Green et al. [64,65,66,67,68,69], who studied the behavior of different petroleum-derived oils during catalytic cracking on the same catalyst and under the same operating conditions. The approach that they used allowed the development of correlations that relate the characteristics of petroleum-derived FCC feedstock to conversion and product yields. For this reason, it is difficult to derive quantitative relationships for the catalytic cracking of bio-oils similar to those for oils derived from petroleum, as described in the previous section (Equations (21)–(35)), which can be used to predict conversion and product yield in the catalytic cracking of bio-oils. Only the study by Eschenbacher et al. [167] paid attention to the effect of the basic nitrogen contained in fast pyrolysis oils on conversion during the co-processing of 20 wt.% bio-oil with 80 wt.% conventional fossil FCC feedstock. As is evident from Equations (30)–(32) in the previous section, basic nitrogen has a strong inhibitory effect on the conversion and formation of gasoline and promotes the formation of coke. Similar equations need to be developed for bio-oils to predict their behavior during catalytic cracking. As shown in Table 7, the atomic H/C ratio for lignocellulosic bio-oils can vary over a very wide range. Bio-oils such as those obtained from Taihu cyanonphyta, peanut shell, rice husk, pond algae oil, or spirulina oil [168] have H/C ratios around 2.0, meaning they have high contents of saturated oxygenates and may show good results in an FCC plant.
3.2.2. Co-Processing of Pyrolysis Oils from Waste Plastics and Scrapped Tires in the FCC Process
Population growth in the modern world is closely linked to the increased generation of different types of waste. Among these, the increase in plastic waste and scrap tires is becoming a threat to the environment [169,170]. Plastic production is expected to increase worldwide from 311 t/y in 2017 to 1800 t/y in 2050 [169]. Three billion tires are estimated to be produced annually, and waste tire generation is expected to reach 1200 t/y by the end of the 2030s [170]. Conversion of waste tires and plastics through pyrolysis allows mitigation of waste management problems involving scrapped tires and used plastics [171,172,173,174,175,176,177,178]. However, waste pyrolysis oils cannot be directly used as fuels or chemicals and require further refining to meet specific quality standards [178,179,180]. Fluid catalytic cracking, due to its flexibility to process feeds with variable quality, has been identified as one of the main refining processes that can treat waste pyrolysis oils [181,182,183,184,185,186,187]. Options for refining waste pyrolysis oils (WPO) in the FCC process have been investigated, including their use as 100% feedstock [83,182,184] or in blends consisting of 20% WPO with 80% conventional vacuum gas oils [176,177,178]. Waste plastics have also been co-processed in FCC, dissolved in conventional VGO at levels of 5 wt.% and 10 wt.% [188,189].
Considering that the properties of FCC feedstock have a profound effect on the process, the performance characteristics of various waste pyrolysis oils, taken from references [181,182,185,186,190,191,192,193,194,195,196,197], are summarized in Table 10. The data in Table 10 indicate that the quality of waste pyrolysis oils as feedstock for FCC varies over a wide range. For example, the atomic H/C ratio oscillates between 1.11 and 1.52 for scrap tire pyrolysis oil (STPO) and between 1.3 and 2.13 for waste plastic pyrolysis oil (WPPO), suggesting that WPPO should exhibit higher conversion than STPO in FCC. The nitrogen levels in WPPO and STPO are relatively high, reaching 1.3%, suggesting significant suppression of cracking reactions. Unfortunately, no data were found to report how much of this nitrogen is basic nitrogen, since basic nitrogen suppresses cracking reactions, as shown in Equations (30) and (32)–(35). Comparing the properties of petroleum-derived cracking feedstocks (Table 7) with those of waste pyrolysis oils (Table 10), it can be seen that the H/C ratio in STPO is low, and STPO can therefore be considered a difficult-to-crack feedstock. However, WPPO exhibits a high H/C ratio, suggesting that it is a good feedstock for cracking. The content of olefins in the waste pyrolysis oils is high, and the bromine number is high, suggesting a tendency for feedstock pumping problems, injector plugging, valve plugging, and polymerization of blends in the feed tank at high temperatures (80 °C) across long storage times. To avoid these problems, the same strategy of separate feeding of waste pyrolysis oils and conventional petroleum oils as proposed in references [130,160,161] for biomass pyrolysis oils, should be applied when waste pyrolysis oils are co-processed in an FCC plant.

Table 10.
Properties of waste plastic-pyrolysis oil (WPPO) and scrapped-tire pyrolysis oil (STOP) obtained from different raw materials and various pyrolysis process conditions, taken from references [181,182,185,186,190,191,192,193,194,195,196,197].
Catalytic cracking of 100% STPO, 100% VGO, and an 80% VGO/20% STPO mixture was carried out on the same catalyst and under the same operating conditions, in the same laboratory FCC plant [182,187]. Data about properties of both 100% STPO, 100% VGO are presented in Table 11. It is evident from the data in Table 11 that STPO has a lower H/C ratio but also a lower nitrogen content compared with conventional VGO; these two characteristics affect FCC conversion in opposite directions. It is therefore difficult to properly assess how these opposing directions would affect the conversion of STPO compared with that of VGO. In addition, STPO contains 7.1 wt.% olefins, in contrast to VGO, which contains no olefins, which, as discussed in the study by Watkins et al. [140], has a positive effect on FCC conversion. Thus, as a result of the combined positive effect on conversion of the lower nitrogen content and higher olefin content of STPO, as well as the slight difference between the H/C ratios of STPO and VGO, higher conversion of STPO than VGO can be expected during catalytic cracking. The catalytic cracking data of 100% STPO, 100% VGO, and the mixture of 80% VGO/20% STPO (see Table 12) confirm that STPO is more reactive than VGO in the FCC process. The right-hand column in Table 12 presents the conversion values calculated using the linear mixing rule for the individual STPO and VGO conversions and their contents in the mixture.

Table 11.
Properties of conventional VGO and STPO (data taken from references [182,187]).

Table 12.
Conversions of 100% STPO, 100% VGO, and 80% VGO/20% STPO at three different reaction temperatures (data taken from references [182,187]).
An additional comparison was made with data for the catalytic cracking of straight-run hydrotreated vacuum gas oil (SRHTVGO), H-Oil VGO, and a 50/50 blend of these obtained from an FCC laboratory ACE unit at a reaction temperature of 527 °C, varying catalyst-to-oil ratios between 3.5 and 7.5 wt./wt.%, and 30 s catalyst contact time. Properties of the SRHTVGO and H-Oil VGO are given in Table 13. Table 14 summarizes the data of the cracking experiments with SRHTVGO, H-Oil VGO and 50%SRHTVGO/50%H-Oil VGO.

Table 13.
Properties of conventional straight-run hydrotreated VGO and H-Oil VGO.

Table 14.
Conversions of SRHTVGO, H-Oil VGO, and their 50/50 mixture.
The data in Table 14 again confirm the validity of the linear mixing rule. These experimental cracking results show that mixtures of conventional petroleum-derived FCC feedstocks with waste pyrolysis oils obey the same rules applied to conventional FCC feedstocks.
4. Discussion
FCC feedstocks may include atmospheric and vacuum gas oils, coker gas oils, hydrocracked vacuum gas oil residue, atmospheric residue, deasphalted oils, and other heavy oils. They contain a wide range of hydrocarbon molecules with varying carbon chain lengths and structures, as well as some non-hydrocarbon molecules containing sulfur, nitrogen, and oxygen. The exact number of species in the FCC feedstock is difficult to determine precisely because it is a mixture and the specific components can vary depending on the source oil and refining process. However, it is deemed that they may number around 100,000 [198]. These species can be grouped in the following families: paraffins and cycloparaffins, which are saturates, aromatic hydrocarbons with different numbers of aromatic rings, and non-hydrocarbon compounds containing sulfur, nitrogen, and oxygen, which can be classified as polars or resins, and asphaltenes [33]. FCC feedstocks, which contain gas oils derived from thermal conversion processes like coking and visbreaking, often contain olefins [199]. It was found that the reactivity of different hydrocarbon classes during catalytic cracking increased in the following order: paraffins ˂ naphthenes ˂ alkyl-benzenes ˂ naphtheno-aromatics. The poly-nuclear aromatic hydrocarbons were determined to be the least reactive individual hydrocarbons [200]. Compared with paraffins, olefins are easier to crack, due to the presence of a double bond, which makes them more prone to bond cleavage and the formation of smaller molecules [201]. Regarding the reactivity of the group fractions (saturates, aromatics, polars (resins), and asphaltenes), it was established that the reactivity decreased in the following order: saturates ˃ aromatics ˃ resins ˃ asphaltenes [202]. Navarro et al. [33] showed that hydrogen content correlated with the contents of saturates and aromatics, and they logically found that the conversion at the point of maximum gasoline yield could be predicted by the hydrogen content of the FCC feeds (see Equation (28)). Based on this finding, one may conclude that hydrogen content appears to be the best descriptor of crackability of the FCC feedstock. On the other hand, Bollas et al. [62] showed that the aromatic carbon content together with the nitrogen content could be used to predict the conversion and yield of coke in the FCC process.
Indeed, with regard to the parameters characterizing FCC feed, the aromatic carbon and hydrogen contents showed the best correlation with conversion at maximum gasoline yield, according to the data from Fisher [34] (Equation (22)). The combination of CA calculated by the n-d-M method [39] (Equations (1) and (4)) and H calculated by the Dhulesia correlation [45] (Equation (13)), demonstrated the highest accuracy for predicting the conversion at maximum gasoline yield, which was even higher than that based on mass spectrometric analysis data.
Regarding the data provided by Fisher [34] and Ng [35,36] (Table 1), combining data from feed analysis obtained via mass spectrometry, together with data from CA and H determined by NMR and empirical parameters such as KW, one can see that the contents of hydrogen, aromatic carbon, and polars and the KW value are sufficient to model the conversion and yields at maximum gasoline point. Hydrogen positively affects conversion and gasoline yield, while it negatively influences LCO and coke yields. Polars inhibit gasoline and LCO formation and promote coke production. Surprisingly, polar compounds have a positive effect on conversion, which can be attributed to their promoting effect on coke yield, which also contributes to conversion. Aromatic carbon suppresses gasoline formation, and higher KW reduces LCO production. Although the negative impact of CA on gasoline yield is understandable, as aromatics are difficult to crack [202], the lack of influence on conversion is not consistent with the data reported by Bollas et al. [58]. Most likely, the combination of hydrogen and polar compounds outweighs the influence of CA on conversion, as observed by Bollas et al., who did not measure the content of polar compounds in the cracked feedstocks in their study [58].
Artificial neural network (ANN) modeling techniques have been reported to be superior to regression techniques [203,204]. However, there are also cases where regression correlations have been reported to perform better than machine learning methods [205]. In this work, both an artificial neural network (ANN)-based approach and a regression approach were tested for predicting conversion at maximum gasoline yield for 88 FCC feedstocks that were characterized by four parameters (hydrogen content, saturates, aromatics, and polar contents; data in Table S2). As noted in Section 3.1.2, the ANN model demonstrated a standard error of 1.4%, compared with 2.3% for the regression model, proving its superiority.
It is worth noting here that total nitrogen content or basic nitrogen content does not affect FCC conversion at maximum gasoline yield, although it is known from many studies that basic nitrogen inhibits catalytic cracking [28,93,96,113,116,117,123]. FCC operating conditions, under which more easily crackable feeds (containing less nitrogen) achieve maximum gasoline yield, must be milder than those applied to more refractory feeds that contain more nitrogen and aromatics, a fact reported in the works of Stratiev [38] and Caeiro et al. [113]. Under these different operating conditions applied to diverse feedstocks, where all hydrocarbons that can be cracked to gasoline have already been cracked and all inhibitory effects have been overcome, the detrimental effect of nitrogen on the catalyst cannot manifest itself. For this reason, the influence of nitrogen (basic nitrogen) on the crackability of the feedstock can be determined when different feedstocks are cracked under the same operating conditions. The correlation developed in this work (Equation (30)) based on data from Ng et al. [35] and Ng and Rahimi [83] (data in Table 2) obtained under constant FCC operating conditions shows that both aromatic carbon and basic nitrogen inhibit FCC conversion. Therefore, it can be concluded that the conversion at maximum gasoline yield quantifies the components that can be cracked to gasoline under optimal conditions, which are different for various feedstocks. While the conversion that can be achieved in an FCC plant would be better determined by conducting experiments under the same operating conditions, it is understandable that different commercial plants may have diverse constraints, including air blower limitations, wet gas compressor capacity, maximum achievable regenerator temperature, etc. Then, the feeds crack better under realistic FCC conditions, and the yields and conversion are interpolated at the critical operating parameter boundary value. Nevertheless, it is also helpful to obtain the conversion at maximum gasoline yield to assess the FCC potential that can be utilized from the feedstock.
The most comprehensive studies on the influence of the quality of petroleum-derived feedstocks on the conversion rate and product yields in FCC are those by Navarro et al. [33] (over 100 feedstocks) and Green et al. [64,65,66,67,68,69] (55 feedstocks), who applied mass spectrometry and NMR to characterize their feedstocks. Navarro et al. [33] investigated the crackability of the feedstock in FCC at maximum gasoline yield, while Green et al. [64,65,66,67,68,69] investigated the crackability of the feedstock under constant operating conditions. Navarro et al. [33] found that the hydrogen content, determined by NMR, was the single parameter that best correlated with conversion and yields at maximum gasoline yield. Green et al. [64,65,66,67,68,69] found that the best FCC feed descriptors were the atomic ratio of hydrogen divided by carbon plus sulfur (H/(C + S)), the fraction of the feed evaporated at the cracking temperature fcT, the effective metal content (Meff = Ni + V/4), basic nitrogen (NB, wt.%), amide-type nitrogen (NAm, wt.%), sulfur content (S, wt.%), hydrogen content (H, wt.%), and microcarbon residue content (MCR, wt.%). The atomic H/C ratio strongly correlates with the aromatic carbon content, as reported in [150,152], which again confirms that the aromatic carbon content has a profound effect on FCC feed reactivity. The observed effect of basic nitrogen on FCC feed reactivity, as quantified by Equation (30), is substantiated by Equations (31)─(35). However, Equations (31)─(35) show that the metal content and amide-type nitrogen content also affect the FCC feedstock conversion and product yields. Characterization of FCC feed crackability according to conversion at maximum gasoline yield reflects the influence of the hydrocarbon composition of the FCC feedstock on the conversion. This can be very well modelled using the hydrogen, saturates, aromatics, and polar contents and the ANN technique, providing an indication of the reactivity of the FCC feedstock under variable operating conditions. The reactivity of the FCC feedstock, as shown in Equation (30), depends on the aromatic carbon and basic nitrogen contents, which means that these two parameters for characterizing FCC feed can describe its reactivity.
Zeolite-containing catalysts are typically employed in petroleum refining for catalytic cracking [206] and have also been successfully applied in the catalytic cracking of bio-oils [207,208,209]. These catalysts, known for their acid-base catalysis, are effective in breaking down the complex molecules of bio-oil into more desirable, lighter hydrocarbons [210,211,212]. Raw bio-oil is a rather complex mixture containing substantial amount of water (see the data in Table 7) as well as components including oxygen (acids, alcohols, aldehydes, esters, ethers, ketones, phenols, guaiacol, syringol, sugars, furans), nitrogen, sulfur, metals, and aromatic compounds [213]. The bio-oil contains ketones, furans, phenols, acids, esters, hydrocarbons, and other substances, which undergo a series of reactions including dehydration, cracking, decarboxylation, decarbonylation, and others [211]. Bio-oil cracking studies have shown that vegetable oils are much easier to crack than oils produced by biomass pyrolysis, because they have a higher atomic H/C ratio, lower oxygen content, lower water content, and lower levels of highly reactive oxygen species that cause injector plugging, valve sticking, corrosion, and polymerization, which are difficult to overcome in existing FCC plants. Although catalytic cracking of individual 100% soybean oils and 100% FCC VGO at constant operating conditions (Table 8) demonstrated that soybean oil produced less gasoline, when soybean oil and VGO were cracked together at 15% soybean oil in the blend, a synergistic effect was observed, resulting in the production of 2.2 wt.% more gasoline than when cracking 100% VGO [140]. Another interesting observation is that for 100% VGO, the overcracking point was registered at conversion of 76.7 wt.%, while for the 85% VGO/15% soybean oil feed blend, the overcracking point was at 73.8 wt.% conversion [140]. The presence of double-bonded species in soybean oil, in addition to increasing the reactivity of the mixture 85% VGO/15% soybean oil compared to that of 100% VGO [140], is likely to have accelerated the secondary cracking reactions of gasoline to lighter products. These data clearly show that synergism exists during the catalytic cracking of petroleum-derived VGOs and vegetable oils, resulting in higher gasoline yields. It should be noted that the yield response to the addition of bio-oil to FCC feed depends on both the properties of the base FCC feed and the properties of the bio-oil. While data [140] show that adding up to 15% soybean, rapeseed, and palm oil to VGO (properties shown in Table 8 and Table 9) led to increases in conversion and gasoline yield, adding only 3% pyrolysis oil derived from pine to the same VGO led to a decrease in gasoline yield by 1.6 wt.% (Table 9). Dupain et al. [153] observed that the addition of rapeseed oil to a hydrowax feed yielded less gasoline and C4 minus products, and higher amounts of LCO, slurry, and coke [153].
During the catalytic cracking of pyrolysis oils derived from biomass and waste materials, a lack of synergism or antagonism may be observed, as reported in [212], following the linear mixing rule, as discussed in Section 3.2.2. The presence of synergism was reported by Ibarra et al. [77]. In some studies on bio-oil co-processing, Ibarra et al. [212] reported that when up to 15–20 wt.% bio-oil was added to the feed blend, FCC product yields were the same as those from VGO cracking alone, with almost negligible oxygenate yields. However, increasing the bio-oil content to 35 wt.% resulted in a decrease in gasoline yield of 3–4 wt.%, as reported by Ibarra et al. [212]. Mastry et al. [14] communicated that co-processing 10% of five pyrolysis oils derived from municipal solid waste and biogenic material (olive stones/pits) with standard VGO in a bench-scale reactor using a commercial FCC catalyst based on ultrastable Y zeolite showed similar yield patterns to vacuum gas oil. In another study by Ibarra et al. [77], investigating the catalytic cracking of a mixture of 80% VGO/20% bio-oil, the bio-oil was obtained by fast pyrolysis of black poplar sawdust, and an interaction between the hydrocarbons in the VGO and the oxygenates in the bio-oil was observed. This interaction resulted in increased production of LPG and gasoline (higher yields than expected in the absence of synergy) and reduced formation of CO2 and CO. It was found that co-feeding decreased catalytic deactivation and promoted catalytic cracking reactions instead of thermal decarboxylation and decarbonylation reactions. This observed synergism was attributed by Ibarra et al. [77] to the competitive adsorption of hydrocarbons and oxygenates in the feed mixture and the role of H2O in the selectivity of the cracking reactions. They highlighted the fact that increasing the water concentration contributed to inhibiting the undesirable decarbonylation and decarboxylation reactions of oxygenates and reducing coke formation and therefore, catalyst deactivation.
The catalytic cracking of pyrolysis oils derived from waste plastic and scrapped tires provides a good perspective for utilization of the continually increasing amount of waste generated by modern human society. The research indicates that pyrolysis oils from waste plastics appear to be easier to crack in existing FCC units than scrapped-tire pyrolysis oils, because they have a higher H/C ratio and higher olefin content. The higher olefin content in the waste pyrolysis oils can make them more reactive than conventional vacuum gas oils. The conversion of oil blends, regardless of their origin from fossil fuel or waste materials, appears to involve a linear combination of the conversion of the individual oils multiplied by their content in the blend, a fact that is not always observed during catalytic cracking of blends of petroleum-derived oils and bio-oils from different sources.
Despite the much larger number of studies devoted to the catalytic cracking of pyrolysis oils derived from waste biomass, plastics, and scrapped tires compared with the use of fossil feedstocks for FCC, there is still a lack of systematicity in catalytic cracking studies involving various pyrolysis oils for the development of predictive equations such as those developed for fossil feedstocks for FCC.
5. Conclusions
It has been found that FCC feedstocks derived from petroleum are converted at maximum gasoline yield between 50 and 85 wt.%. The majority of FCC feedstocks are converted at greater than 70 wt.% at the overcracking point, with lower values associated with secondary gas oils such as coker, vacuum residue hydrocrackers, and heavy cycle oils. Over the years, various methods have emerged for the characterization of FCC feedstocks, from sophisticated high-performance liquid chromatography with mass spectrometry to liquid chromatography SARA analysis, as well as empirical methods. In this work, it was found that the empirically determined aromatic carbon content by the n-d-M method and the hydrogen content calculated by the Dhulesia correlation (Total method) provided a more accurate correlation for predicting FCC conversion at maximum gasoline yield than the correlation developed based on mass spectrometric analysis data reported in Fisher’s work for nine different vacuum gas oils. Using a larger database of nineteen FCC feeds characterized by mass spectrometry, together with aromatic and hydrogen contents data determined by NMR and empirical parameters such as KW, it was found that the hydrogen, aromatic carbon, and polar compound contents and the KW value were sufficient to model the conversion and yields at the maximum gasoline yield point. The artificial neural network (ANN) method for modeling FCC conversion at maximum gasoline yield for 88 FCC feedstocks proved its superiority over nonlinear regression empirical correlation using FCC feedstock data for hydrogen, saturates, aromatics, and polar compounds. While the conversion at maximum gasoline yield appears to be unaffected by nitrogen content (total or basic) due to the optimized operating conditions in the FCC plant to ensure complete conversion of gasoline-generating hydrocarbons, the conversion at constant operating conditions shows dependence on aromatic carbon and basic nitrogen contents. The most complete characterization of a petroleum-derived FCC feedstock was found to include determination of the atomic ratio of hydrogen divided by carbon plus sulfur (H/(C + S)), the fraction of the feedstock vaporized at the cracking temperature fcT, the effective metal content (Meff = Ni + V/4), basic nitrogen (NB, wt.%), amide nitrogen (NAm, wt.%), sulfur content (S, wt.%), hydrogen content (H, wt.%), and microcarbon residue content (MCR, wt.%).
Despite the large number of published studies dedicated to the catalytic cracking of bio-oil, the co-processing of bio-oil and petroleum-derived feedstocks differs. In some cases, product yields obtained by co-processing mixtures containing between 10 and 20% bio-oils and waste derived oils are reported to be similar to those obtained from petroleum-derived feedstocks. In other cases, they have been reported to be higher than those calculated based on the cracking yields of the individual feed components, suggesting the presence of synergism. There are also cases showing decreasing yields of valuable products. Sustainable oil co-processing and processing of different petroleum-derived feeds have also been observed to obey the linear mixing rule, suggesting a lack of synergism. The explanation for these various behaviors during co-processing is ascribed to the different properties of the various petroleum feedstocks and the diverse bio-oils. However, no studies have been found that report the existence of quantitative relationships between the properties of feedstock derived from petroleum and those from bio-oil and the yields obtained during co-processing.
Quantitative correlations still need to be developed to relate the quality of petroleum-derived feedstocks to that of sustainable oils, to predict yields during co-processing in FCC. Such correlations can be very useful for refiners to adequately simulate the performance of their FCC plants during co-processing of diverse petroleum-derived feedstocks with different bio-oils.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13072169/s1, Table S1: Physicochemical properties of nine vacuum gas oils cracked in a laboratory MAT unit in the research by Fisher [34]; Table S2: Data of contents of hydrogen, saturates, aromatics, and polars together with the FCC maximum conversions of 88 oils; Table S3: Data of elemental composition of vacuum gas oil and vacuum residue fractions and separated thereof acid, base, and neutral fractions from Wilmington, Brass River, Maya, Lagomedio, and Merey crude oils (data taken from references [64,65,66,67,68,69]).
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the author.
Acknowledgments
Author Dicho Stratiev would like to thank Ivelina Shishkova from the research laboratory in LUKOIL Neftohim Burgas, Sotir Sotirov from State University “Assen Zlatarov”, Burgas, and Svetoslav Nenov from the University of Chemical Technologies and Metallurgy, Sofia, for the fruitful discussion during the preparation of the manuscript.
Conflicts of Interest
Author Dicho Stratiev was employed by LUKOIL Neftohim Burgas. The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.statista.com/statistics/1445314/number-of-oil-refineries-worldwide/ (accessed on 11 April 2025).
- GlobalNewsWire. Global Refinery Fluid Catalytic Cracking Units (FCCU) Industry Outlook 2024−2028: Capacity and Capital Expenditure Outlook with Details of All Operating and Planned Fluid Catalytic Cracking Units. May 2024. Available online: www.globenewswire.com/news-release/2024/07/10/2911231/28124/en/Global-Refinery-Fluid-Catalytic-Cracking-Units-FCCU-Industry-Outlook-2024-2028-Capacity-and-Capital-Expenditure-Outlook-with-Details-of-All-Operating-and-Planned-Fluid-Catalytic-Cr.html (accessed on 25 March 2025).
- Liu, J.; Chen, H.; Pi, Z.; Liu, Y.; Sun, H.; Shen, B. Molecular-Level-Process Model with Feedback of the Heat Effects on a Complex Reaction Network in a Fluidized Catalytic Cracking Process. Ind. Eng. Chem. Res. 2017, 56, 3568–3577. [Google Scholar] [CrossRef]
- Pathak, A.; Rana, M.S.; Marafi, M.; Kothari, R.; Gupta, P.; Tyagi, V.V. Waste petroleum fluid catalytic cracking catalysts as a raw material for synthesizing valuable zeolites: A critical overview on potential, applications, and challenges. SM&T 2023, 38, e00733. [Google Scholar] [CrossRef]
- Corma, A.; Sauvanaud, L. Chapter 4 Increasing LCO yield and quality in the FCC: Cracking pathways analysis. In Fluid Catalytic Cracking VII: Materials. Methods and Process Innovations; Elsevier B.V.: Amsterdam, The Netherlands, 2007; pp. 41–54. [Google Scholar]
- Corma, A.; Martínez, C.; Sauvanaud, L. New materials as FCC active matrix components for maximizing diesel (light cycle oil, LCO) and minimizing its aromatic content. Catal. Today 2007, 127, 3–16. [Google Scholar] [CrossRef]
- Gilbert, W.R.; Morgado, E., Jr.; de Abreu, M.A.S.; de la Puente, G.; Passamonti, F.; Sedran, U. A novel fluid catalytic cracking approach for producing low aromatic LCO. FPT 2011, 92, 2235–2240. [Google Scholar]
- Shirzad, P.; Kantor, I. Acosta-López, J.G.; Muñoz, J.L.; de Lasa, H. Unravelling Vacuum Gas Oil Catalytic Cracking: The Influence of the Catalyst-to-Oil Ratio on FCC Catalyst Performance. Catalysts 2025, 15, 170. [Google Scholar]
- Wang, Y.; Zhang, J.; Guo, X.; Chen, B.; Sun, Q.; Liu, A.; Sun, C.; Chen, G.; Yang, L. Experiments and modeling for recovery of hydrogen and ethylene from fluid catalytic cracking (FCC) dry gas utilizing hydrate formation. Fuel 2017, 209, 473–489. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, S.; Li, C.; Chen, X.; Yang, C. Residue catalytic cracking process for maximum ethylene and propylene production. Ind. Eng. Chem. Res. 2013, 52, 14366–14375. [Google Scholar] [CrossRef]
- Kakku, S.; Naidu, S.; Anand, G.; Chakinala, A.G.; Joshi, J.; Thota, C.; Maity, P.; Sharma, A. Co-processing of organic fraction from groundnut shell biocrude with VGO in FCC unit to produce petrochemical products. Renew. Energy 2024, 224, 120182. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, X.; Fang, Y. Coprocessing of cashew nut shell liquid and phenol model compounds with VGO in a pilot-scale FCC riser. Energy 2024, 307, 132764. [Google Scholar] [CrossRef]
- Mastry, M.C.; Dorazio, L.; Fu, J.C.; Gómez, J.P.; Sedano, S.; Ail, S.S.; Castaldi, M.J.; Yilmaz, B. Processing renewable and waste based feedstocks with fluid catalytic cracking: Impact on catalytic performance and considerations for improved catalyst design. Front. Chem. 2023, 11, 1067488. [Google Scholar] [CrossRef] [PubMed]
- Palos, R.; Rodríguez, E.; Gutierrez, A.; Bilbao, J.; Arandes, J.M. Cracking of plastic pyrolysis oil over FCC equilibrium catalysts to produce fuels: Kinetic modeling. Fuel 2022, 316, 123341. [Google Scholar] [CrossRef]
- Kittel, H.; Frasko, F.; Psenicka, M. Coprocessing of low concentration waste plastics and scrapped tires pyrolysis oil in the FCC Advanced Cracking Evaluation laboratory unit. J. Energy Inst. 2024, 113, 101500. [Google Scholar] [CrossRef]
- Tran, X.T.; Kim, E.S.; Mun, D.H.; Jung, T.; Shin, J.; Kang, N.Y.; Park, Y.-K.; Kim, D.K. Catalytic Cracking of Crude Waste Plastic Pyrolysis Oil for Enhanced Light Olefin Production in a Pilot-Scale Circulating Fluidized Bed Reactor. ACS Sustain. Chem. Eng. 2024, 12, 12493–12503. [Google Scholar] [CrossRef]
- Thangaraj, B.; Lee, Y.-K. Review on recent development in catalytic cracking of waste polyolefins: Effect of zeolite-based catalysts and reaction parameters. Fuel 2025, 380, 133220. [Google Scholar] [CrossRef]
- Xu, Y.; Zuo, Y.; Yang, W.; Shu, X.; Chen, W.; Zheng, A. Targeted Catalytic Cracking to Olefins (TCO): Reaction Mechanism, Production Scheme, and Process Perspectives. Engineering 2023, 30, 100–109. [Google Scholar] [CrossRef]
- Mafat, I.H.; Sharma, S.K.; Surya, D.V.; Rao, C.S.; Maity, U.; Barupal, A.; Jasra, R. Development of machine learning model for the prediction of selectivity to light olefins from catalytic cracking of hydrocarbons. Fuel 2025, 381, 133682. [Google Scholar] [CrossRef]
- Mizuno, T.; Yamazaki, H.; Takamiya, Y.; Hasegawa, H.; Tanaka, C.; Mitsui, T. Effects of the FCC catalyst binder type on propylene production during catalytic cracking of VGO. Appl. Catal. A Gen. 2023, 661, 119214. [Google Scholar] [CrossRef]
- García, J.R.; Fals, J.; Dietta, L.E.; Sedran, U. VGO from shale oil. FCC processability and co-processing with conventional VGO. Fuel 2022, 328, 125327. [Google Scholar] [CrossRef]
- Fals, J.; García, J.R.; Falco, M.; Sedran, U. Performance of equilibrium FCC catalysts in the conversion of the SARA Fractions in VGO. Energy Fuels. 2020, 34, 16512–16521. [Google Scholar] [CrossRef]
- Adanenche, D.E.; Aliyu, A.; Atta, A.Y.; El-Yakubu, B.J. Residue fluid catalytic cracking: A review on the mitigation strategies of metal poisoning of RFCC catalyst using metal passivators/traps. Fuel 2023, 343, 127894. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; He, L.; Jia, C.Q.; Yao, Q.; Sun, M.; Ma, X. Anti-deactivation of zeolite catalysts for residue fluid catalytic cracking. Appl. Catal. A Gen. 2023, 657, 119159. [Google Scholar] [CrossRef]
- Walker, P.; Peterman, R. RFCC units set new standard for propylene production. PTQ 2012, Q4, 1–11. [Google Scholar]
- Li, Z.; Wang, G.; Liu, Y.; Wang, H.; Liang, Y.; Xu, C.; Gao, J. Catalytic cracking constraints analysis and divisional fluid catalytic cracking process for coker gas oil. Energy Fuels. 2012, 26, 2281–2291. [Google Scholar] [CrossRef]
- Stratiev, D.; Minkov, D. Investigation of the influence of basic nitrogen compounds on yield distribution in fluid catalytic cracking. Bulg. Chem.Com. 1998, 30, 536–542. [Google Scholar]
- Stratiev, D.; Shishkova, I.; Yankov, V.; Yordanov, D.; Tankov, I. Fluid catalytic cracking of H-Oil derived heavy oils. Oxid. Commun. 2020, 43, 289–301. [Google Scholar]
- Pope, A.E.; Ng, S.H. Evaluation of deasphalted heavy oil residues as catalytic cracking feed using a riser kinetic model. Fuel 1990, 69, 539–546. [Google Scholar] [CrossRef]
- Xu, C.; Gao, J.; Zhao, S.; Lin, S. Correlation between feedstock SARA components and FCC product yields. Fuel 2005, 84, 669–674. [Google Scholar] [CrossRef]
- Ng, S.H. Nonconventional Residuum Upgrading by Solvent Deasphalting and Fluid Catalytic Cracking. Energy Fuels 1997, 11, 1127–1136. [Google Scholar] [CrossRef]
- Navarro, U.; Ni, M.; Orlicki, D. Understanding the Potential for FCC Feed to Generate Valuable Products and How This Knowledge can Benefit Refinery Operation. Available online: www.digitalrefining.com/article/1001054 (accessed on 25 March 2025).
- Fisher, L.P. Effect of feedstock variability on catalytic cracking yields. Appl. Catal. 1990, 65, 189–210. [Google Scholar] [CrossRef]
- Ng, S.H.; Wang, J.; Fairbridge, C.; Zhu, Y.; Yang, L.; Ding, F.; Yui, S. Study of Canadian FCC feeds from various origins and treatments. 1. Ranking of feedstocks based on feed quality and product distribution. Energy Fuels 2004, 8, 160–171. [Google Scholar] [CrossRef]
- Ng, S.H.; Wang, J.; Fairbridge, C.; Zhu, Y.; Yang, L.; Ding, F.; Yui, S. Study of Canadian FCC feeds from various origins and treatments. 2. Some specific cracking characteristics and comparisons of product yields and qualities between a riser reactor and a MAT unit. Energy Fuels 2004, 18, 172–187. [Google Scholar] [CrossRef]
- Stratiev, D. Catalytic cracking of non-hydrotreated, hydrotreated and sulfuric acid treated vacuum gas oils. Processes 2025, 13, 1351. [Google Scholar] [CrossRef]
- Reif, H.E.; Kress, R.F.; Smith, J.S. How feeds affect cat cracker yields. Petrol. Refiner 1961, 40, 237–244. [Google Scholar]
- ASTM D3238-17a; Standard Test Method for Calculation of Carbon Distribution and Structural Group Analysis of Petroleum Oils by the n-d-M Method. ASTM International: West Conshohocken, PA, USA, 2017.
- White, P.J. How cracker feed influences yield. Hydrocarbon Process. 1968, 47, 103–108. [Google Scholar]
- Fitzgerald, M.E.; Moirano, J.L.; Morgan, H.; Cirillo, V.A. Characterization of Gas Oil Stocks: An Integrated Analysis. Appl. Spectrosc. 1970, 24, 106–114. [Google Scholar] [CrossRef]
- Nace, D.M.; Voltz, S.E.; Weekman, V.W., Jr. Application of a Kinetic Model for Catalytic Cracking. Effects of Charge Stocks. Ind. Eng. Chem. Process Des. Develop. 1971, 10, 530–538. [Google Scholar] [CrossRef]
- Voltz, S.E.; Nace, D.M.; Weekman, V.W., Jr. Application of a Kinetic Model for Catalytic Cracking. Some Correlations of Rate Constants. Ind. Eng. Chem. Process Des. Develop. 1971, 10, 538–541. [Google Scholar] [CrossRef]
- Jacob, S.M.; Gross, B.; Voltz, S.E.; Weekman, V.W., Jr. A lumping and reaction scheme for catalytic cracking. AIChE J. 1976, 22, 701–713. [Google Scholar] [CrossRef]
- Dhulesia, H. New Correlations Predict FCC Feed Characterizing Parameters. Oil Gas J. 1986, 84, 51–54. [Google Scholar]
- Riazi, M.R.; Daubert, T.E. Prediction of molecular-type analysis of petroleum fractions and coal liquids. Ind. Eng. Chem. Process. Dev. 1986, 25, 1009–1015. [Google Scholar] [CrossRef]
- Stratiev, D.; Minkov, D. Prediction of FCC yields from feedstock quality characterized by empirical methods. OGEM 2000, 1, 27–32. [Google Scholar]
- Choudhary, T.V.; Meier, P.F. Characterization of heavy petroleum feedstocks. FPT 2008, 89, 697–703. [Google Scholar]
- Gharagheizi, F.; Fazeli, A. Prediction of the Watson characterization factor of hydrocarbon components from molecular properties. QSAR Comb. Sci. 2008, 227, 758–767. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Ivanov, M.; Dinkov, R.; Georgiev, B.; Argirov, G.; Atanassova, V.; Vassilev, P.; Atanassov, K.; Yordanov, D.; et al. Catalytic cracking of diverse vacuum residue hydrocracking gas oils. Chem. Eng. Technol. 2021, 44, 997–1008. [Google Scholar] [CrossRef]
- Abutaqiya, M. Advances in Thermodynamic Modeling of Nonpolar Hydrocarbons and Asphaltene Precipitation in Crude Oils. Ph.D. Thesis, Rice University, Houston, TX, USA, 2019. [Google Scholar]
- Abutaqiya, M.I.L.; AlHammadi, A.A.; Sisco, C.J.; Vargas, F.M. Aromatic Ring Index (ARI): A Characterization Factor for Nonpolar Hydrocarbons from Molecular Weight and Refractive Index. Energy Fuels 2021, 35, 1113–1119. [Google Scholar] [CrossRef]
- Speight, J.G. Bureau of Mines Correlation Index. In Rules of Thumb for Petroleum Engineers; Scrivener Publishing LLC.: Austin, TX, USA, 2017. [Google Scholar]
- Behera, B.; Ray, S.S.; Singh, I.D. Structural characterization of FCC feeds from Indian refineries by NMR spectroscopy. Fuel 2008, 87, 2322–2333. [Google Scholar] [CrossRef]
- Behera, B.; Ray, S.S.; Singh, I.D. Chapter 12 NMR studies of FCC feeds, catalysts and coke. Stud. Surf. Sci. Catal. 2007, 166, 163–200. [Google Scholar] [CrossRef]
- Dasila, P.K.; Choudhury, I.R.; Saraf, D.N.; Kagdiyal, V.; Rajagopal, S.; Chopra, S.J. Estimation of FCC feed composition from routinely measured lab properties through ANN model. FPT 2014, 125, 155–162. [Google Scholar]
- Gilbert, W.R.; Fiavio, S.; de Lima, F.G.; Bueno, A.F. Comparison of NIR and NMR spectra chemometrics for FCC feed online characterization. Stud. Surf. Sci. Catal. 2004, 149, 203–215. [Google Scholar]
- Bollas, G.M.; Vasalos, I.A.; Lappas, A.A.; Iatridis, D.K.; Tsioni, G.K. Bulk molecular characterization approach for the simulation of FCC feedstocks. Ind. Eng. Chem. Res. 2004, 43, 3270–3281. [Google Scholar] [CrossRef]
- Ancheyta, J.J. Kinetic Study for Gas Oil Catalytic Cracking Reactions. Ph.D. Thesis, Universidad Autónoma Metropolitana (UAM-I), Mexico City, Mexico, 1998. (In Spanish). [Google Scholar]
- Ancheyta-Juárez, J.; López-Isunza, F.; Aguilar-Rodríguez, E. Correlations for predicting the effect of feedstock properties on catalytic cracking kinetic parameters. Ind. Eng. Chem. Res. 1998, 37, 4637–4640. [Google Scholar] [CrossRef]
- Harding, R.H.; Zhao, X.; Qian, K.; Rajagopalan, K.; Cheng, W.C. The fluid catalytic cracking selectivities of gas oil boiling point and hydrocarbon fractions. Prep. Am. Chem. Soc. Div. Pet. Chem. 1995, 40, 2561–2569. [Google Scholar]
- Harding, R.H.; Zhao, X.; Qian, K.; Rajagopalan, K.; Cheng, W.C. The fluid catalytic cracking selectivities of gas oil boiling point and hydrocarbon fractions. Ind. Eng. Chem. Res. 1996, 35, 2561–2569. [Google Scholar] [CrossRef]
- ASTM D2007-19; Standard Test Method for Characteristic Groups in Rubber Extender and Processing Oils and Other Petroleum-Derived Oils by the Clay-Gel Absorption Chromatographic Method. ASTM International: West Conshohocken, PA, USA, 2008.
- Green, J.B.; Zagula, E.J.; Reynolds, J.W.; Wandke, H.H.; Young, L.L.; Chew, H. Relating Feedstock Composition to Product Slate and Composition in Catalytic Cracking. 1. Bench Scale Experiments with Liquid Chromatographic Fractions from Wilmington, CA, >650 °F Resid? Energy Fuels 1994, 8, 856–867. [Google Scholar] [CrossRef]
- Green, J.B.; Zagula, E.J.; Reynolds, J.W.; Young, L.L.; Chew, H.; McWilliams, T.B.; Grigsby, R.D. Relating Feedstock Composition to Product Slate and Composition in Catalytic Cracking. 2. Feedstocks Derived from Brass River, a High-Quality Nigerian Crude. Energy Fuels 1996, 10, 450–462. [Google Scholar] [CrossRef]
- Green, J.B.; Zagula, E.J.; Reynolds, J.W.; Young, L.L.; Chew, H.; McWilliams, T.B.; Green, J.A. Relating Feedstock Composition to Product Slate and Composition in Catalytic Cracking. 3. Feedstocks Derived from Maya, a Mexican Crude. Energy Fuels 1997, 11, 46–60. [Google Scholar] [CrossRef]
- Green, J.B.; Zagula, E.J.; Grigsby, R.D.; J Reynolds, J.W.; Young, L.L.; McWilliams, T.B.; Green, J.A.; Chew, H. Relating Feedstock Composition to Product Slate and Composition in Catalytic Cracking. 5. Feedstocks Derived from Lagomedio, a Venezuelan Crude. Energy Fuels 1999, 13, 655–666. [Google Scholar] [CrossRef]
- Sheppard, C.M.; Al-Alloush, S.S.; Green, J.B.; Zagula, E.J.; Young, L.L.; Wisecarver, K.D. Relating Feedstock Composition to Product Slate and Composition in Catalytic Cracking. 6. Feedstocks Derived from Merey, a Venezuelan Crude. Energy Fuels 2003, 17, 1360–1366. [Google Scholar] [CrossRef]
- Sheppard, C.M.; Green, J.B.; Vanderveen, J.W. Relating Feedstock Composition to Product Slate and Composition in Catalytic Cracking. 4. An Extended Pendant-Core Model for Gasoline Composition. Energy Fuels 1998, 12, 320–328. [Google Scholar] [CrossRef]
- Rose, S.K.; Richels, R.; Blanford, G.; Rutherford, T. The Paris Agreement and next steps in limiting global warming. Clim. Chang. 2017, 142, 255–270. [Google Scholar] [CrossRef]
- Khodeir, L. An FCC Sustainability Game Changer. November 2022. Available online: https://www.digitalrefining.com/article/1002982/an-fcc-sustainability-game-changer-ert (accessed on 26 June 2025).
- Avery, C.; Ketjen, J.S. FCC Pathways to Co-Processing. November 2021. Available online: https://www.digitalrefining.com/article/1002664/fcc-pathways-to-co-processing (accessed on 26 June 2025).
- Allende, A.; Bortolaia, V.; Bover-Cid, S.; Dohmen, W.; Guillier, L.; Herman, L.; Jacxsens, L.; Nauta, M.; Mughini-Gras, L.; Ottoson, J.; et al. Evaluation of a fluidised catalytic cracking co-processing method for the production of renewable fuels using Category 3 animal fat and used cooking oils. EFSA J. 2025, 23, e9337. [Google Scholar] [PubMed]
- Gunawan, M.L.; Novita, T.H.; Aprialdi, F.; Aulia, D.; Nanda, A.S.F.; CRasrendra, C.B.; Addarojah, Z.; Mujahidin, D.; Kadja, G.T.M. Palm-oil transformation into green and clean biofuels: Recent advances in the zeolite-based catalytic technologies. Bioresour. Technol. Rep. 2023, 23, 101546. [Google Scholar]
- Doronin, V.P.; Potapenko, O.V.; Lipin, P.V.; Sorokina, T.P. Catalytic cracking of vegetable oils and vacuum gas oil. Fuel 2013, 106, 757–765. [Google Scholar] [CrossRef]
- Pinho, A.R.; de Almeida, M.B.B.; Rochedo, P.R.R. Renewable-carbon recovery in the co-processing of vacuum gas oil and bio-oil in the FCC process–Where does the renewable carbon go? FPT 2022, 229, 107176. [Google Scholar]
- Ibarra, A.; Rodríguez, E.; Sedran, U.; Arandes, J.M.; Bilbao, J. Synergy in the Cracking of a Blend of Bio-oil and Vacuum Gasoil under Fluid Catalytic Cracking Conditions. Ind. Eng. Chem. Res. 2016, 55, 1872–1880. [Google Scholar] [CrossRef]
- Strohm, J.; Rainer, D.; Oyola-Rivera, O.; Avery, C. FCC Co-Processing of Biogenic and Recyclable Feedstocks: Part I. Catalysis. 2024. Available online: https://ptqmagazines.digitalrefining.com/view/70232828/25/ (accessed on 26 June 2025).
- Marinho, L.H.N.; Aragão, F.V.; Chiroli, D.M.G.; Zola, F.C.; Tebcherani, S.M. A systematic review of fusel oil as a renewable biofuel: Challenges, opportunities, and circular economy integration. Fuel 2025, 402, 135924. [Google Scholar] [CrossRef]
- Available online: https://www.sciencedirect.com/search?qs=%E2%80%9Cbiofuel%E2%80%9D%20AND%20%E2%80%9Ccatalytic%20cracking%E2%80%9D%20s (accessed on 26 June 2025).
- Available online: https://www.sciencedirect.com/search?qs=%E2%80%9CVGO%E2%80%9D%20AND%20%E2%80%9Ccatalytic%20cracking%E2%80%9D (accessed on 26 June 2025).
- Available online: https://www.sciencedirect.com/search?qs=%E2%80%9Cresid%E2%80%9D%20AND%20%E2%80%9Ccatalytic%20cracking%E2%80%9D (accessed on 26 June 2025).
- Ng, S.H.; Rahimi, P.M. Catalytic Cracking of Canadian Nonconventional Feedstocks. 1. Cracking Characteristics of Gas Oils Derived from Coprocessing Distillate and Shale Oil. Energy Fuels 1991, 5, 595–601. [Google Scholar] [CrossRef]
- Sotirov, S.; Sotirova, E.; Dinkov, R.; Stratiev, D.; Shiskova, I.; Kolev, I.; Argirov, G.; Georgiev, G.; Bureva, V.; Atanassov, K.; et al. Heavy Fuel Oil Quality Dependence on Blend Composition, Hydrocracker Conversion, and Petroleum Basket. Fuels 2025, 6, 43. [Google Scholar] [CrossRef]
- Shiskova, I.; Stratiev, D.; Sotirov, S.; Sotirova, E.; Dinkov, R.; Kolev, I.; Stratiev, D.D.; Nenov, S.; Ribagin, S.; Atanassov, K.; et al. Predicting Petroleum SARA Composition from Density, Sulfur Content, Flash Point, and Simulated Distillation Data Using Regression and Artificial Neural Network Techniques. Processes 2024, 12, 1755. [Google Scholar] [CrossRef]
- Van Nes, K.; Van Westen, H.A. Aspects of the Constitution of Mineral Oils; Elsevier: New York, NY, USA, 1951. [Google Scholar]
- Riazi, M.R. Prediction of Thermophysical Properties of Petroleum Fractions. Department of Chemical Engineering. Ph.D. Thesis, The Pennsylvania State University, University Park, TX, USA, 1979. [Google Scholar]
- Guzmán, G.N.; Cruz, F.L.M.; Suárez, J.P.O. Prediction of the FCC feedstocks crackability. CT&F—Cienc. Tecnol. Y Futuro 2009, 3, 125–142. [Google Scholar]
- Najafi-Marghmaleki, A.; Barati-Harooni, A.; Tatar, A.; Mohebbi, A.; Mohammadi, A.H. On the prediction of Watson characterization factor of hydrocarbons. J. Mol. Liq. 2017, 231, 419–429. [Google Scholar] [CrossRef]
- Goossens, A.G. Prediction of Molecular Weight of Petroleum Fractions. Ind. Eng. Chem. Res. 1996, 35, 985–988. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Bilbao, J. Deactivation kinetic models for the fluid catalytic cracking (FCC). A review. J. Chem. Eng. 2025, 514, 162856. [Google Scholar] [CrossRef]
- Corma, A.; Fornos, V.; Month, J.B.; Orchilli, A.V. Catalytic Cracking of Alkanes on Large Pore, High SiO2/Al2O3 Zeolites in the Presence of Basic Nitrogen Compounds. Influence of Catalyst Structure and Composition in the Activity and Selectivity. Ind. Eng. Chem. Res. 1987, 26, 882–886. [Google Scholar] [CrossRef]
- Prado, G.H.C.; Rao, Y.; de Klerk, A. Nitrogen Removal from Oil: A Review. Energy Fuels 2017, 31, 14–36. [Google Scholar] [CrossRef]
- Chung, H.; Kolbush, S.; de la Fuente, E.; Christensen, P. FCC feed preparation for improved quality. PTQ 1997, Q2. Available online: www.digitalrefining.com/article/1000162 (accessed on 22 March 2025).
- Bai, P.; Etim, U.J.; Yan, Z.; Mintova, S.; Zhang, Z.; Zhong, Z.; Gao, X. Fluid catalytic cracking technology: Current status and recent discoveries on catalyst contamination. Catal. Rev. 2019, 61, 333–405. [Google Scholar] [CrossRef]
- Fu, C.M.; Schaffer, A.M. Effect of nitrogen compounds on cracking catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 68–75. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Veli, A.; Nikolova, R.; Stratiev, D.D.; Mitkova, M.; Yordanov, D. Fluid catalytic cracking and thermal cracking of vacuum gas oils. Effect of feedstock properties on conversion and yields. OGEM 2017, 43, 84–89. [Google Scholar]
- Ovalles, C.; Rogel, E.; Hurt, M.; Duma, V.; Morazan, H.; Hench, K.; Moir, M.E. Nitrogen Speciation: Application to Reactivity of Feeds to Hydroprocessing and Catalyst Deactivation. In Proceedings of the ACS Symposium Series Volume 1320; Chemistry Solutions to Challenges in the Petroleum Industry: Washington, DC, USA, 2019; Chapter 10; pp. 261–280. [Google Scholar]
- Zhu, X.C.; Shi, Q.A.; Zhang, Y.H.; Pan, N.; Xu, C.M.; Chung, K.H.; Zhao, S. Characterization of nitrogen compounds in Coker heavy gas oil and its subfractions by liquid chromatographic separation followed by Fourier transform ion cyclotron resonance mass spectrometry. Energy Fuel. 2011, 25, 281–287. [Google Scholar] [CrossRef]
- ASTM D5762-18a; Standard Test Method for Nitrogen in Liquid Hydrocarbons, Petroleum and Petroleum Products by Boat-Inlet Chemiluminescence. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D2896-21; Standard Test Method for Base Number of Petroleum Products by Potentiometric Perchloric Acid Titration. ASTM International: West Conshohocken, PA, USA, 2021.
- UOP 269-10; Nitrogen Bases in Hydrocarbons by Potentiometric Titration. Available online: https://www.scribd.com/document/634465312/Untitled (accessed on 1 July 2025).
- Ho, T.C.; Katritzky, A.R.; Cato, S.J. Effect of nitrogen compounds on cracking catalysts. Ind. Eng. Chem. Res. 1992, 31, 1589–1597. [Google Scholar] [CrossRef]
- Wei, Q.; Wen, S.; Tao, X.; Zhang, T.; Zhou, Y.; Chung, K.; Xu, C. Hydrodenitrogenation of basic and non-basic nitrogen-containing compounds in coker gas oil. Fuel Process. Technol. 2015, 129, 76–84. [Google Scholar] [CrossRef]
- Zeuthen, P.; Knudsen, K.G.; Whitehurst, D.D. Organic nitrogen compounds in gas oil blends, their hydrotreated products and the importance to hydrotreatment. Catal. Today 2001, 65, 307–314. [Google Scholar] [CrossRef]
- Zeuthen, P.; Schmidt, M.T. Benefits of Cat Feed Hydrotreating and the Impact of Feed Nitrogen on Catalyst Stability. In Proceedings of the NPRA Annual Meeting 2010, Phoenix, AZ, USA, 21–23 March 2010. AM-10-167. [Google Scholar]
- Wiwel, P.; Hinnemann, B.; Hidalgo-Vivas, A.; Zeuthen, P.; Petersen, B.O.; Duus, J. Characterization and identification of the most refractory nitrogen compounds in hydroprocessed vacuum gas oil. Ind. Eng. Chem. Res. 2010, 49, 3184–3193. [Google Scholar] [CrossRef]
- Deng, Z.-H.; Guo, S.-Y.; Nie, X.-P.; Cai, X.-H.; Jia, Y.-Z.; Han, W.; Dai, L.-S. Insight into the evolution of refractory basic and neutral nitrogen compounds during residue hydrotreating process. Pet. Sci. 2025, 22, 1787–1801. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Kim, G.; Peters, A.; Zhao, X.; Rajagopalan, K.; Ziebarth, M.; Pereira, C. Environmental Fluid Catalytic Cracking Technology. Catal Rev. 1998, 40, 39–79. [Google Scholar] [CrossRef]
- Qian, K.; Tomczak, D.C.; Rakiewicz, E.F.; Harding, R.H.; George Yaluris, G.; Cheng, W.-C.; Zhao, X.; Peters, A.W. Coke Formation in the Fluid Catalytic Cracking Process by Combined Analytical Techniques. Energy Fuels 1997, 11, 596–601. [Google Scholar] [CrossRef]
- Behera, B.; Gupta, P.; Ray, S.S. Structure and composition of hard coke deposited on industrial fluid catalytic cracking catalysts by solid state 13C nuclear magnetic resonance. Appl. Catal. A-Gen. 2013, 466, 123–130. [Google Scholar] [CrossRef]
- Scherzer, J.; McArthur, D.P. Catalytic cracking of high-nitrogen petroleum feedstocks: Effect of catalyst composition and properties. Ind. Eng. Chem. Res. 1988, 27, 1571–1576. [Google Scholar] [CrossRef]
- Caeiro, G.; Costa, A.F.; Cerqueira, H.S.; Magnoux, P.; Lopes, J.M.; Matias, P.; Ribeiro, F.R. Nitrogen poisoning effect on the catalytic cracking of gasoil. Appl. Catal. A Gen. 2007, 320, 8–15. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Shi, Q.; Xu, C.; Gao, J. Retardation effect of basic nitrogen compounds on hydrocarbons catalytic cracking in coker gas oil and their structural identification. Ind. Eng. Chem. Res. 2011, 50, 4123–4132. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Liu, Y.D.; Gao, J.; Xu, C.; Lan, X.; Ning, G.; Liang, Y. FCC-catalyst coking: Sources and estimation of their contribution during coker gas oil cracking process. Ind. Eng. Chem. Res. 2012, 51, 2247–2256. [Google Scholar] [CrossRef]
- Li, Z.; Gao, J.; Wang, G.; Shi, Q.; Xu, C. Influence of nonbasic nitrogen compounds and condensed aromatics on coker gas oil catalytic cracking and their characterization. Ind. Eng. Chem. Res. 2011, 50, 9415–9424. [Google Scholar] [CrossRef]
- Zhang, J.; Shan, H.; Chen, X.; Liu, W.; Yang, C. Synergistic process for high nitrogen content feedstocks catalytic cracking: A case study of controlling the reactions of nitrogen compounds in situ. Ind. Eng. Chem. Res. 2014, 53, 5718–5727. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Huang, H.; Lan, X.; Xu, C.; Gao, J. Synergistic Process for Coker Gas Oil and Heavy Cycle Oil Conversion for Maximum Light Production. Ind. Eng. Chem. Res. 2010, 49, 11260–11268. [Google Scholar] [CrossRef]
- Zhang, J.H.; Shan, H.H.; Yang, C.H.; Chen, X.B.; Li, C.Y. Catalytic cracking of coker gas oil at high reaction temperature and catalyst to oil ratio. Adv. Mater. Res. 2013, 724–725, 1112–1115. [Google Scholar] [CrossRef]
- Zhang, J.; Shan, H.; Chen, X.; Liu, W.; Chen, X.; Li, C.; Yang, C. Synergistic Process for Coker Gas Oil Catalytic Cracking and Gasoline Reformation. Energy Fuels 2013, 27, 654–665. [Google Scholar] [CrossRef]
- Sheng, Q.; Wang, G.; Liu, Y.; Husein, M.M.; Gao, C.; Shi, Q.; Gao, J. Combined hydrotreating and fluid catalytic cracking processing for the conversion of inferior coker gas oil: Effect on nitrogen compounds and condensed aromatics. Energy Fuels 2018, 32, 4979–4987. [Google Scholar] [CrossRef]
- Zhang, J.; Shan, H.; Chen, X.; Liu, W.; Yang, C. Fluid catalytic cracking study of coker gas oil. Effects of processing parameters on sulfur and nitrogen distributions. Energy Fuels 2014, 28, 1362–1371. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Li, S.; Feng, X.; Shan, H.; Yang, C. Structure and Composition Changes of Nitrogen Compounds during the Catalytic Cracking Process and Their Deactivating Effect on Catalysts. Energy Fuels 2017, 31, 3659–3668. [Google Scholar] [CrossRef]
- Sheng, Q.; Wang, G.; Liu, Y.; Husein, M.M.; Gao, C.; Gao, J. Pilot-scale evaluation of hydrotreating inferior coker gas oil prior to its fluid catalytic cracking. Fuel 2018, 226, 27–34. [Google Scholar] [CrossRef]
- Bian, J.; Kuznicki, S.M.; McCaffrey, W.C.; Koenig, A.; Lin, C.C.H. Chabazite-clay composite for bitumen upgrading. Chin. J. Catal. 2008, 29, 1084–1088. [Google Scholar] [CrossRef]
- Stratiev, D.; Ivanov, M.; Chavdarov, I.; Argirov, G.; Strovegli, G. Revamping Fluid Catalytic Cracking Unit, and Optimizing Catalyst to Process Heavier Feeds. Appl. Sci. 2023, 13, 2017. [Google Scholar] [CrossRef]
- Al-Sabawi, M.; Chen, J.; Ng, S. Fluid Catalytic Cracking of Biomass-Derived Oils and Their Blends with Petroleum Feedstocks: A Review. Energy Fuels 2012, 26, 5355–5372. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Serrano, D.P.; Melero, J.A.; Morales, G.; Iglesias, J.; Patricia Pizarro, P. Progress in the design of zeolite catalysts for biomass conversion into biofuels and bio-based chemicals. Catal. Rev. 2018, 60, 1–70. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Lappas, A.A. Co-processing bio-oil in the refinery for drop-in biofuels via fluid catalytic cracking. Wires Energy Environ. 2018, 7, e281. [Google Scholar] [CrossRef]
- Liu, N.R.; Rahman, M.M.; Sarker, M.; Chai, M.; Li, C.; Cai, J. A review on the catalytic pyrolysis of biomass for the bio-oil production with ZSM-5: Focus on structure. Fuel Process. Technol. 2020, 199, 106301. [Google Scholar]
- Naji, S.Z.; Ching Tye, C.T.; Abd, A.A. State of the art of vegetable oil transformation into biofuels using catalytic cracking technology: Recent trends and future perspectives. Process Biochem. 2021, 109, 148–168. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Mohamed, A.R.; Farzad Ismail, F.; Lee, K.T.; Amini, G. Upgrading biomass-derived pyrolysis bio-oil to bio-jet fuel through catalytic cracking and hydrodeoxygenation: A review of recent progress. Energy Convers. Manag. 2022, 268, 115956. [Google Scholar] [CrossRef]
- Chaihad, N.; Karnjanakom, S.; Abudula, A.; Guan, G. Zeolite-based cracking catalysts for bio-oil upgrading: A critical review. Resour. Chem. Mater. 2022, 1, 167–183. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: A review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef]
- Zhao, C.; Hong, C.; Hu, J.; Xing, Y.; Ling, W.; Zhang, B.; Wang, Y.; Feng, L. Upgrading technologies and catalytic mechanisms for heteroatomic compounds from bio-oil—A review. Fuel 2023, 333, 126388. [Google Scholar] [CrossRef]
- Jindal, M.; Negi, A.; Palla, V.C.S.; Krishna, B.B.; Thallada, B. Catalytic interventions in bio-oil production from lignocellulosic biomass and Co-processing with petroleum refinery fractions: A review. Biomass Bioenergy 2024, 183, 107119. [Google Scholar] [CrossRef]
- Liaqat, S.; Sun, Z.; Zeng, Y.; Maeda, N.; Liu, J. Technical challenges and corrosion research progress in bio-crude co-processing. J. Chem. Eng. 2024, 499, 155981. [Google Scholar] [CrossRef]
- Shirzad, P.; Kantor, I. Toward sustainable propylene: A comparison of current and future production pathways. Renew. Sustain. Energy Transit. 2025, 7, 100099. [Google Scholar] [CrossRef]
- Watkins, B.; Olsen, C.; Sutovich, K. New Opportunities for Co-Processing Renewable Feeds in Refinery Processes. Catalagram 2008, 103, 1–13. [Google Scholar]
- Carmona, H.D.P.; Alfaro, O.d.l.T.; Alayón, A.B.; Vázquez, M.A.R.; Hernándezd, J.J.M. Co-processing of straight run gas oil with used cooking oil and animal fats. Fuel 2019, 254, 115583. [Google Scholar] [CrossRef]
- Horácek, J.; Kubicka, D. Bio-oil hydrotreating over conventional CoMo & NiMo catalysts: The role of reaction conditions and additives. Fuel 2017, 198, 49–57. [Google Scholar]
- Xiu, S.; Shahbazi, A. Bio-oil production an dupgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- El-Sawy, M.S.; Hanafi, S.A.; Ashour, F.; Aboul-Fotouh, T.M. Co-hydroprocessing and hydrocracking of alternative feed mixture (vacuum gas oil/waste lubricating oil/waste cooking oil) with the aim of producing high quality fuels. Fuel 2020, 269, 117437. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Sharma, D.K. Characterization of liquid products obtained from co-cracking of petroleum vacuum residue with coal and biomass. J. Anal. Appl. Pyrolysis 2008, 81, 37–44. [Google Scholar] [CrossRef]
- Xing, T.; Alvarez-Majmutov, A.; Gieleciak, R.; Chen, J. Co-hydroprocessing HTL Biocrude from Waste Biomass with Bitumen-Derived Vacuum Gas Oil. Energy Fuels 2019, 33, 11135–11144. [Google Scholar] [CrossRef]
- Wang, H.; Meyer, P.A.; Santosa, D.M.; Zhu, C.; Olarte, M.V.; Jones, S.B.; Zacher, A.H. Performance and techno-economic evaluations of co-processing residual heavy fraction in bio-oil hydrotreating. J. Environ. Chem. Eng. 2021, 365, 357–364. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A.; Kikhtyanin, O.; Kubicka, D. Refinery co-processing of renewable feeds. Prog Energ Combust. 2018, 68, 29–64. [Google Scholar] [CrossRef]
- Fernández, A.; Mena, A.; Rivas, C.; Bescansa, M.; González, R. Defossilizing the FCC Unit via Co-processing of Biogenic Feedstocks: From Laboratory to Commercial Scale. Catalagram 2023, 128, 9–19. [Google Scholar]
- Zhang, C.-Y.; Wu, Q.; Wang, Y.-D.; Fan, J.-T.; Zhu, Z.-Z. Study on the Differences of Chemical Structures and Pyrolysis Characteristics between the Jurassic and Carboniferous Coking Coals. ACS Omega 2022, 7, 6768–6777. [Google Scholar] [CrossRef]
- Satin, C.; Doerr, S.H.; Merino, A.; Bucheli, T.D.; Bryant, R.; Ascough, P.; Gao, X.; Masiello, C.A. Carbon sequestration potential and physiochemical properties differ between wildfire charcoals and slow-pyrolysis biochars. Sci. Rep. 2017, 7, 11233. [Google Scholar]
- Maroto-Valer, M.M.; Love, G.D.; Snape, C.E. Relationship between carbon aromaticities and H/C ratios for bituminous coals. Fuel 1994, 73, 1926–1928. [Google Scholar] [CrossRef]
- Dupain, X.; Daniel, J.; Costa, D.J.; Schaverien, C.J.; Makkee, M.; Moulijn, J.A. Cracking of a rapeseed vegetable oil under realistic FCC conditions. Appl. Catal. B Environ. 2007, 72, 44–61. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Habib, E.T., Jr.; Rajagopalan, K.; Roberie, T.G.; Wormsbecher, R.F.; Ziebarth, M.S. Handbook of Heterogeneous Catalysis, 2nd ed.; Ertl, G., Knoezinger, H., Schueth, F., Weitkamp, J., Eds.; Wiley-VCH: Weinheim, Germany, 2008; Chapter 13.5. [Google Scholar]
- Bryden, K.; Weatherbee, G.; Habib, E.T., Jr. Flexible Pilot Plant Technology for Evaluation of Unconventional Feedstocks and Processes. Catalagram 2013, 113, 3–21. [Google Scholar]
- Bielansky, P.; Weinert, A.; Schönberger, C.; Reichhold, A. Catalytic Conversion of Vegetable Oils in a Continuous FCC Pilot Plant. Fuel Process. Technol. 2011, 92, 2305–2311. [Google Scholar] [CrossRef]
- ASTM D2887-22e1; Standard Test Method for Boiling Range Distribution of Petroleum Fractions by Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2022.
- Brady, M.P.; Keiser, J.R.; Leonard, D.N.; Zacher, A.H.; Bryden, K.J.; Weatherbee, G.D. Corrosion of stainless steels in the riser during co-processing of bio-oils in a fluid catalytic cracking pilot plant. Fuel Process. Technol. 2017, 159, 187–199. [Google Scholar] [CrossRef]
- Tian, H.; Li, C.; Yang, C.; Shan, H. Alternative Processing Technology for Converting Vegetable Oils and Animal Fats to Clean Fuels and Light Olefins. Chin. J. Chem. Eng. 2008, 16, 394–400. [Google Scholar] [CrossRef]
- Almeida, M.; Pinho, A. Co-processing of fast pyrolysis oil in FCC for transportation fuels. In Proceedings of the Empyro Symposium, Hengelo, The Netherlands, 21 May 2015. [Google Scholar]
- de Rezende Pinho, A.; de Almeida, M.B.B.; Mendes, F.L.; Ximenes, V.L. Production of lignocellulosic gasoline using fast pyrolysis of biomass and a conventional refining scheme. Pure Appl. Chem. 2014, 86, 859–865. [Google Scholar] [CrossRef]
- Lindfors, C.; Elliott, D.C.; Prins, W.; Oasmaa, A.; Lehtonen, J. Co-processing of Biocrudes in Oil Refineries. Energy Fuels 2023, 37, 799–804. [Google Scholar] [CrossRef]
- de Rezende Pinho, A.; de Almeida, M.B.B.; Mendes, F.L.; Ximenes, V.L.; Casavechia, L.C. Co-processing raw bio-oil and gasoil in an FCC unit. Fuel Process. Technol. 2015, 131, 159–166. [Google Scholar] [CrossRef]
- van Dyk, S.; Su, J.; Saddler, J. Update on Drop-In Biofuel and Co-Processing Commercialization. IEA Bioenergy: Task 39. 2024 IEA Bioenergy. Available online: https://www.ieabioenergy.com/wp-content/uploads/2024/09/IEA-Bioenergy-Task-39-drop-in-biofuels-and-co-processing-report-June-2024.pdf (accessed on 2 June 2025).
- Su, J.; Cao, L.; Lee, G.; Tyler, J.; Ringsred, A.; Rensing, M.; van Dyk, S.; O’Connor, D.; Pinchuk, R.; Saddler, J. Challenges in determining the renewable content of the final fuels after co-processing biogenic feedstocks in the fluid catalytic cracker (FCC) of a commercial oil refinery. Fuel 2021, 294, 120526. [Google Scholar] [CrossRef]
- Pérez-Guevara, E.; Velasco, C.P.; Pérez, J.A.M.; Liñeiro, J.T.; Albert Chavarria Miro, A.C.; Bescansa, M.; González, R.; Brandt, S.; Franken, J. Decarbonize the FCCU through Maximizing Low Carbon Propylene. Catalagram 2024, 129, 50–57. [Google Scholar]
- Eschenbacher, A.; Myrstad, T.; Bech, N.; Hang Dao Thi, H.D.; Auersvald, M.; Van Geem, K.M.; Jensen, A.D. Fluid catalytic co-processing of bio-oils with petroleum intermediates: Comparison of vapour phase low pressure hydrotreating and catalytic cracking as pretreatment. Fuel 2021, 302, 121198. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Khoaele, K.K.; Gbadeyan, O.J.; Chunilall, V.; Sithole, B. The Devastation ofWaste Plastic on the Environment and Remediation Processes: A Critical Review. Sustainability 2023, 15, 5233. [Google Scholar] [CrossRef]
- Guo, S.; Gu, M.; Zhang, X.; Chen, J.; Yao, H. Generation and utilization of waste tires pyrolysis carbon: A critical review. Fuel 2025, 400, 135740. [Google Scholar] [CrossRef]
- Dewang, Y.; Sharma, V.; Singla, Y.K. A critical review of waste tire pyrolysis for diesel engines: Technologies, challenges, and future prospects. SM&T 2025, 43, e01291. [Google Scholar]
- Alzahrani, N.; Nahil, M.A.; Williams, P.T. Co-pyrolysis of waste plastics and tires: Influence of interaction on product oil and gas composition. J. Energy Inst. 2025, 118, 101908. [Google Scholar] [CrossRef]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Review and Design Overview of Plastic Waste-to-Pyrolysis Oil Conversion with Implications on the Energy Transition. J. Energy 2023, 2023, 1821129. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Chang, S.H. Plastic waste as pyrolysis feedstock for plastic oil production: A review. Sci. Total Environ. 2023, 877, 162719. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, H.; Ali, H.M.; Khalid, U. Pyrolysis of waste plastics for alternative fuel: A review of key factors. RSC Sustain. 2025, 3, 208–218. [Google Scholar] [CrossRef]
- Pšenicka, M.; Roudová, A.; Vráblík, A.; Cerný, R. Pyrolysis Oils from Used Tires and Plastic Waste: A Comparison of a Co-Processing with Atmospheric Gas Oil. Energies 2022, 15, 7745. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Lopez, G.; Artetxe, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Waste tyre valorization by catalytic pyrolysis—A review. Renew. Sustain. Energy Rev. 2020, 129, 109932. [Google Scholar] [CrossRef]
- Kaplitz, A.S.; Marshall, S.; Bhakta, N.; Morshed, S.; Borny, J.-F.; Schug, K.A. Discrimination of plastic waste pyrolysis oil feedstocks using supercritical fluid chromatography. J. Chromatogr. A 2024, 1720, 464804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, C.; Tao, Y.; Chen, M.; Xiao, R. Catalytic cracking of model compounds of bio-oil: Characteristics and mechanism research on guaiacol and acetic acid. FPT 2022, 238, 107512. [Google Scholar] [CrossRef]
- Palos, R.; Gutiérrez, A.; Vela, F.J.; Olazar, M.; Arandes, J.M.; Bilbao, J. Waste Refinery: The Valorization of Waste Plastics and End-of-Life Tires in Refinery Units. A Review. Energy Fuels 2021, 35, 3529–3557. [Google Scholar] [CrossRef]
- Rodríguez, E.; Palos, R.; Gutiérrez, A.; Arandes, J.M.; Bilbao, J. Production of Non-Conventional Fuels by Catalytic Cracking of Scrap Tires Pyrolysis Oil. Ind. Eng. Chem. Res. 2019, 58, 5158–5167. [Google Scholar] [CrossRef]
- Tran, X.T.; Mun, D.H.; Shin, J.; Kang, N.Y.; Park, D.S.; YPark, Y.-K.; Choi, J.; Kim, D.K. Maximizing light olefin production via one-pot catalytic cracking of crude waste plastic pyrolysis oil. Fuel 2024, 361, 130703. [Google Scholar] [CrossRef]
- Rodríguez, E.; Gutiérrez, A.; Palos, R.; Azkoiti, M.J.; Arandes, J.M.; Bilbao., J. Cracking of scrap tires pyrolysis oil in a fluidized bed reactor under catalytic cracking unit conditions. Effects of operating conditions. Energy Fuels 2019, 33, 3133–3143. [Google Scholar] [CrossRef]
- Rodríguez, E.; Gutiérrez, A.; Palos, R.; Vela, F.J.; Arandes, J.M.; Bilbao, J. Fuel production by cracking of polyolefins pyrolysis waxes under fluid catalytic cracking (FCC) operating conditions. Waste Manag. 2019, 93, 162–172. [Google Scholar] [CrossRef]
- Rodríguez, E.; Izaddoust, S.; Valecillos, J.; Bilbao, J.; Arandes, J.M.; Castaño, P.; Epelde, E.; Elordi, G. Lessening coke formation and boosting gasoline yield by incorporating scrap tire pyrolysis oil in the cracking conditions of an FCC unit. Energy Convers. Manag. 2020, 224, 113327. [Google Scholar] [CrossRef]
- Rodríguez, E.; Palos, R.; Gutiérrez, A.; Arandes, J.M.; Bilbao, J. Scrap tires pyrolysis oil as a co-feeding stream on the catalytic cracking of vacuum gasoil under fluid catalytic cracking conditions. Waste Manag. 2020, 105, 18–26. [Google Scholar] [CrossRef]
- Rodríguez, E.; Gutiérrez, A.; Palos, R.; Vela, F.J.; Azkoiti, M.J.; Arandes, J.M.; Bilbao, J. Co-Cracking of High-Density Polyethylene (HDPE) and Vacuum Gasoil (VGO) under Refinery Conditions. Chem. Eng. J. 2020, 382, 122602. [Google Scholar] [CrossRef]
- García, F.J.O.; Juarez, E.M. Polyethylene waste co-processing in fluid catalytic cracking plants. Clean Eng Technol. 2024, 19, 100734. [Google Scholar] [CrossRef]
- Wang, B.; Song, Y.-B.; Wang, F.; Fan, Y.-C.; Cheng, N.; Duan, P.-G. Comparative study on properties of waste tyre pyrolysis oil and its distillates obtained by molecular distillation. JAAP 2025, 188, 107046. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. Pyrolysis of plastic waste for liquid fuel production as prospective energy resource. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012001. [Google Scholar] [CrossRef]
- Toraman, H.E.; Dijkmans, T.; Djokic, M.R.; Van Geem, K.M.; Marin, G.B. Detailed compositional characterization of plastic waste pyrolysis oilby comprehensive two-dimensional gas-chromatography coupled tomultiple detectors. J. Chromatogr. A 2014, 1359, 237–246. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Janus, R.; Rządzik, B.; Lewandowski, M.; Budzyń, S. Pyrolysis Oil from Scrap Tires as a Source of Fuel Components: Manufacturing, Fractionation, and Characterization. Energy Fuels 2020, 34, 5917–5928. [Google Scholar] [CrossRef]
- Pyshyev, S.; Korchak, B.; Miroshnichenko, D.; Lebedev, V.; Yasinska, A.; Lypko, Y. Obtaining New Materials from Liquid Pyrolysis Products of Used Tires for Waste Valorization. Sustainability 2025, 17, 3919. [Google Scholar] [CrossRef]
- Auersvald, M.; Krupka, V.; Vachkov, E.L.; Straka, P. Determination of olefins in pyrolysis oils from waste plastics and tires –Comparability of titration and chromatographic methods. Fuel 2025, 393, 134921. [Google Scholar] [CrossRef]
- Debek, C.; Walendziewski, J. Hydrorefining of oil from pyrolysis of whole tyres for passenger cars and vans. Fuel 2015, 159, 659–665. [Google Scholar] [CrossRef]
- Erkmen, B.; Ozdogan, A.; Ezdesir, A.; Celik, G. Can Pyrolysis Oil Be Used as a Feedstock to Close the Gap in the Circular Economy of Polyolefins? Polymers 2023, 15, 859. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, B.K.; Pant, K.K.; Gupta, S.K.; Saraf, D.N.; Choudhury, I.R.; Sau, I.R. A carbon-number lump based model for simulation of industrial hydrotreaters: Vacuum gas oil (VGO). J. Chem. Eng. 2019, 358, 504–519. [Google Scholar] [CrossRef]
- Wang, F.; Yu, Y.; Biney, B.W.; Zhang, Z.; Liu, H.; Chen, K.; Wang, Z.; Guo, A. Relationship between olefins and coking propensity of heavy residual oil derived from vacuum residue thermal cracking products. Fuel 2023, 331, 125737. [Google Scholar] [CrossRef]
- Tankov, I.; Stratiev, D.; Shishkova, I.; Dinkov, R.; Sharafutdinov, I.; Nikolova, R.; Veli, A.; Mitkova, M.; Yordanov, D.; Rudnev, N.; et al. Reactivity of heavy oils in catalytic and thermal cracking. Part I: Reactivity and stability of individual hydrocarbons. Oxid. Commun. 2017, 40, 1178–1190. [Google Scholar]
- Longstaff, D.C. Naphtha Cracking Kinetics and Process Chemistry on Y and ZSM5 Type Catalysts. Energy Fuels 2019, 33, 2445–2452. [Google Scholar] [CrossRef]
- Tankov, I.; Stratiev, D.; Shishkova, I.; Dinkov, R.; Sharafutdinov, I.; Nikolova, R.; Veli, A.; Mitkova, M.; Yordanov, D.; Rudnev, N.; et al. Reactivity of heavy oils in catalytic and thermal cracking. Part II: SARA fractions and heavy oils. Oxid. Commun. 2017, 40, 1191–1208. [Google Scholar]
- Bahonar, E.; Chahardowli, M.; Ghalenoei, Y.; Simjoo, M. New correlations to predict oil viscosity using data mining techniques. J. Pet. Sci. Eng. 2022, 208, 109736. [Google Scholar] [CrossRef]
- Hadavimoghaddam, F.; Ostadhassan, M.; Heidaryan, E.; Sadri, M.A.; Chapanova, I.; Popov, E.; Cheremisin, A.; Rafieepour, S. Prediction of Dead Oil Viscosity: Machine Learning vs. Classical Correlations. Energies 2021, 14, 930. [Google Scholar] [CrossRef]
- Sinha, U.; Dindoruk, B.; Soliman, M. Machine learning augmented dead oil viscosity model for all oil types. J. Pet. Sci. Eng. 2020, 195, 107603. [Google Scholar] [CrossRef]
- Wei, L.; Wang, H.; Dong, Q.; Li, Y.; Xiang, H. A Review on the Research Progress of Zeolite Catalysts for Heavy Oil Cracking. Catalysts 2025, 15, 401. [Google Scholar] [CrossRef]
- Temidayo, T.P.; Bukola, K.T.; Honesty, A.B. Application of Zeolite in the Catalytic Cracking of Waste Vegetable Oil for the Production of highly Volatile Liquid Fuel. BJMAS Eng. Technol. 2024, 5, 22–39. [Google Scholar]
- Valle, B.; Palos, R.; Bilbao, J.; Gayubo, A.G. Role of zeolite properties in bio-oil deoxygenation and hydrocarbons production by catalytic cracking. FPT 2022, 227, 107130. [Google Scholar] [CrossRef]
- Guvenc, C.; Alan, E.; Degirmencioglu, P.; Ozcan, M.C.; Karaman, B.P.; Oktar, N. Catalytic upgrading of bio-oil model mixtures in the presence of microporous HZSM-5 and γ-Al2O3 based Ni, Ta and Zr catalysts. Fuel 2023, 350, 128870. [Google Scholar] [CrossRef]
- Chaihad, N.; Situmorang, Y.A.; Anniwaer, A.; Kurnia, I.; Karnjanakom, S.; Kasai, Y.; Abudula, A.; Reubroycharoen, P.; Guan, G. Preparation of various hierarchical HZSM-5 based catalysts for in-situ fast upgrading of bio-oil. Renew. Energy 2021, 169, 283–292. [Google Scholar] [CrossRef]
- Xu, J.; Wen, Y.; Li, D.; Zhang, S.; Han, Z.; Hu, H.; Jin, L. Catalytic upgrading of biomass pyrolysis volatiles over Y zeolites modified with different metal oxides. Fuel 2024, 371, 131936. [Google Scholar] [CrossRef]
- Ibarra, A.; Hita, I.; Azkoiti, M.J.; Arandes, J.M.; Bilbao, J. Catalytic cracking of raw bio-oil under FCC unit conditions over different zeolite-based catalysts. J. Ind. Eng. Chem. 2019, 78, 372–382. [Google Scholar] [CrossRef]
- Nanda, S.; Pattnaik, F.; Borugadda, V.B.; Dalai, A.K.; Kozinski, J.A.; Naik, S. Catalytic and Noncatalytic Upgrading of Bio-Oil to Synthetic Fuels: An Introductory Review. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1379, pp. 1–28. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).