CO2 Sequestration Potential Competitive with H2O and N2 in Abandoned Coal Mines Based on Molecular Modeling
Abstract
1. Introduction
2. Models and Methodology
2.1. Construction and Optimization of Models
2.2. Simulation and Analytical Methods
3. Results
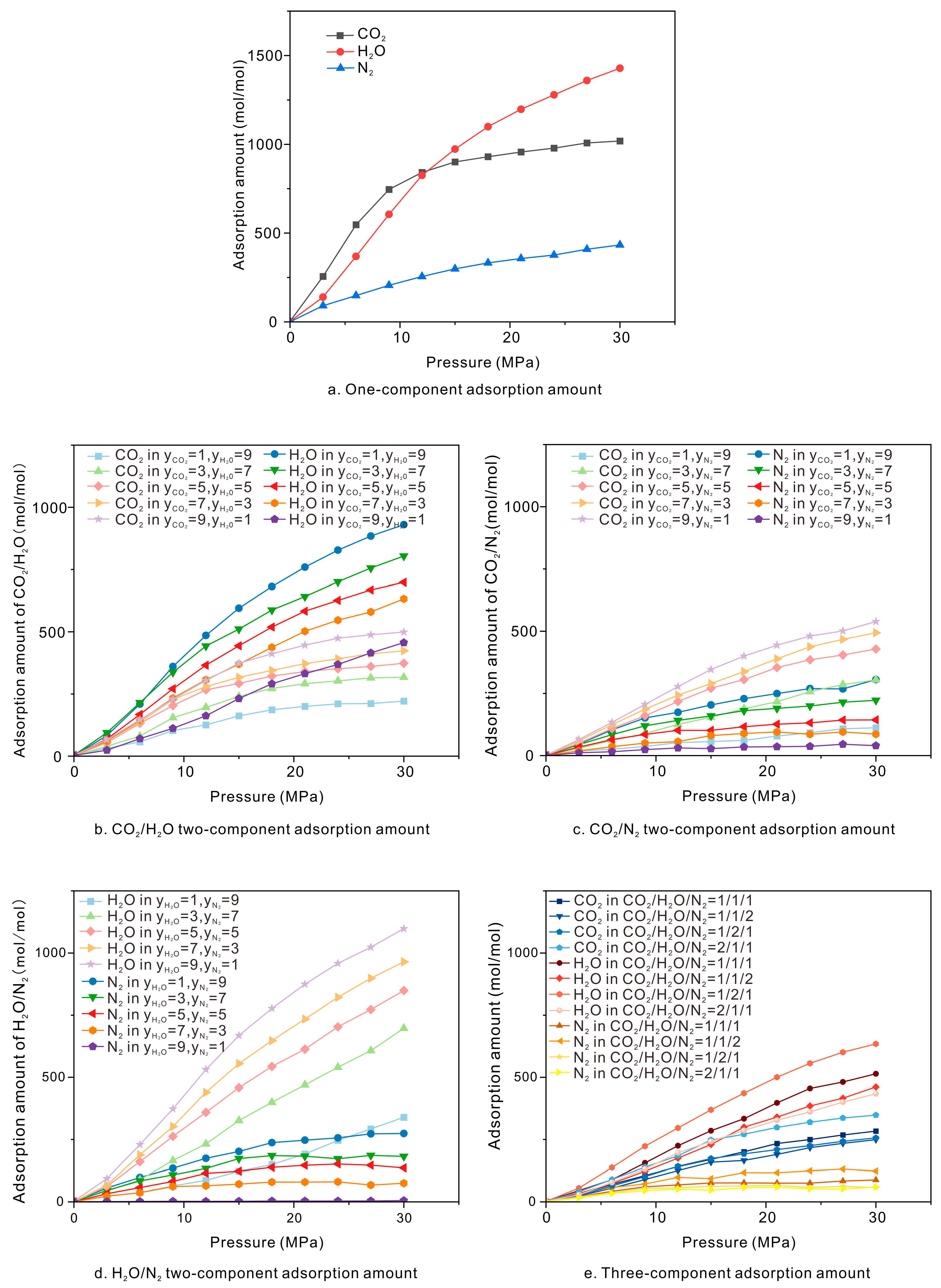
3.1. Adsorption Simulation Results
3.2. Isothermal Adsorption Capacity
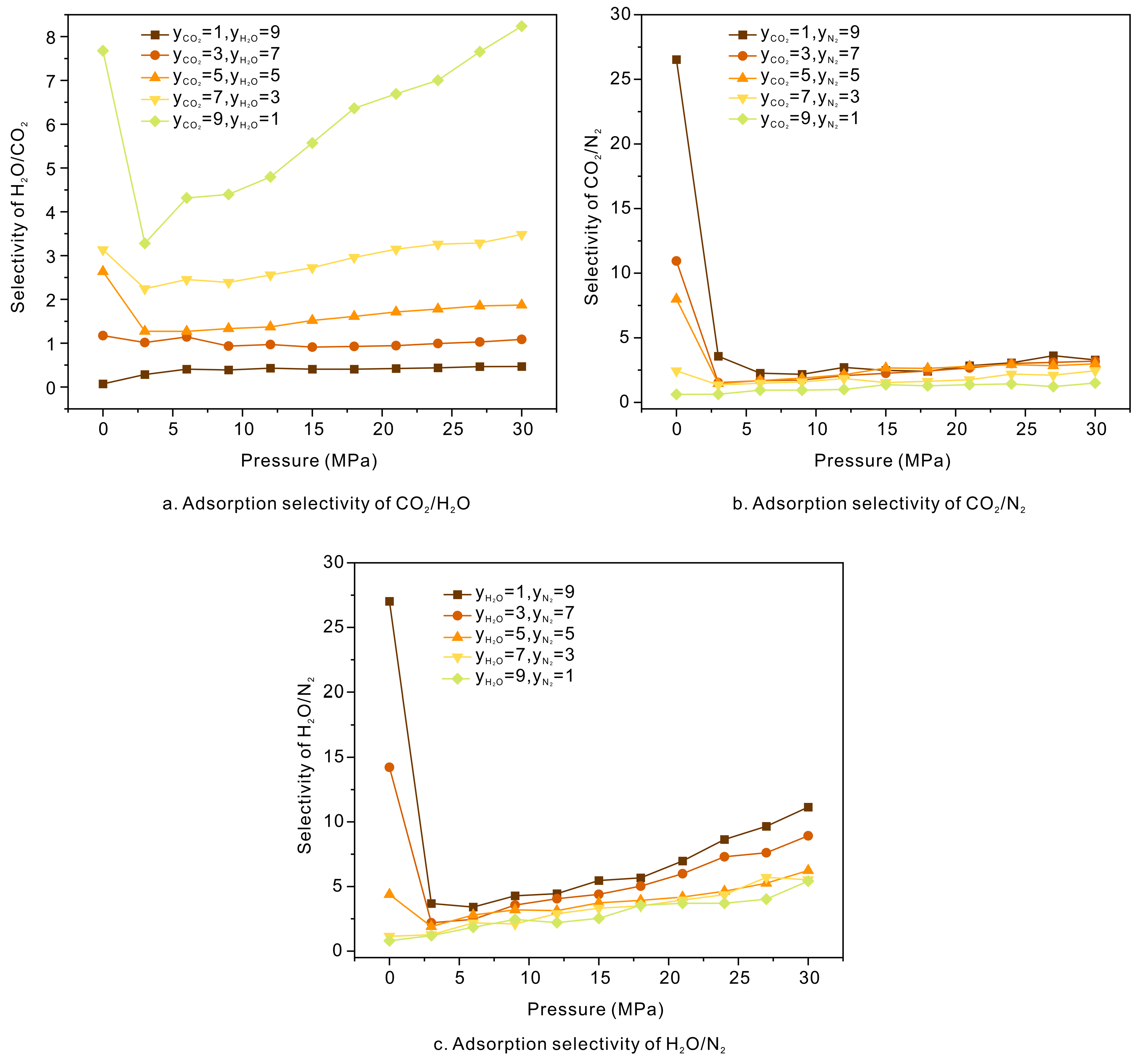
3.3. Competitive Adsorption
4. Discussion
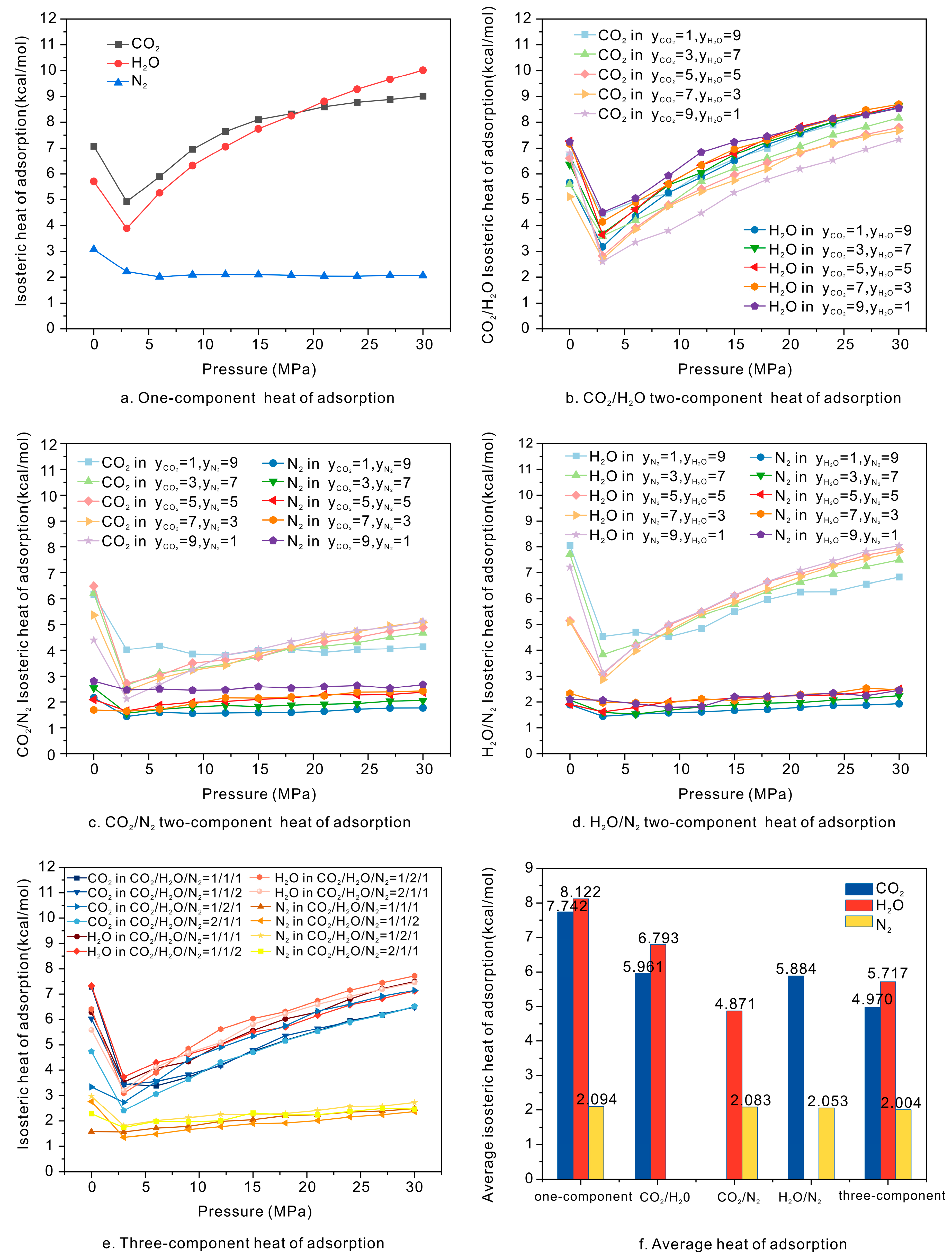
4.1. Adsorption Heat Influences
4.2. Interaction Energy of the System Influences
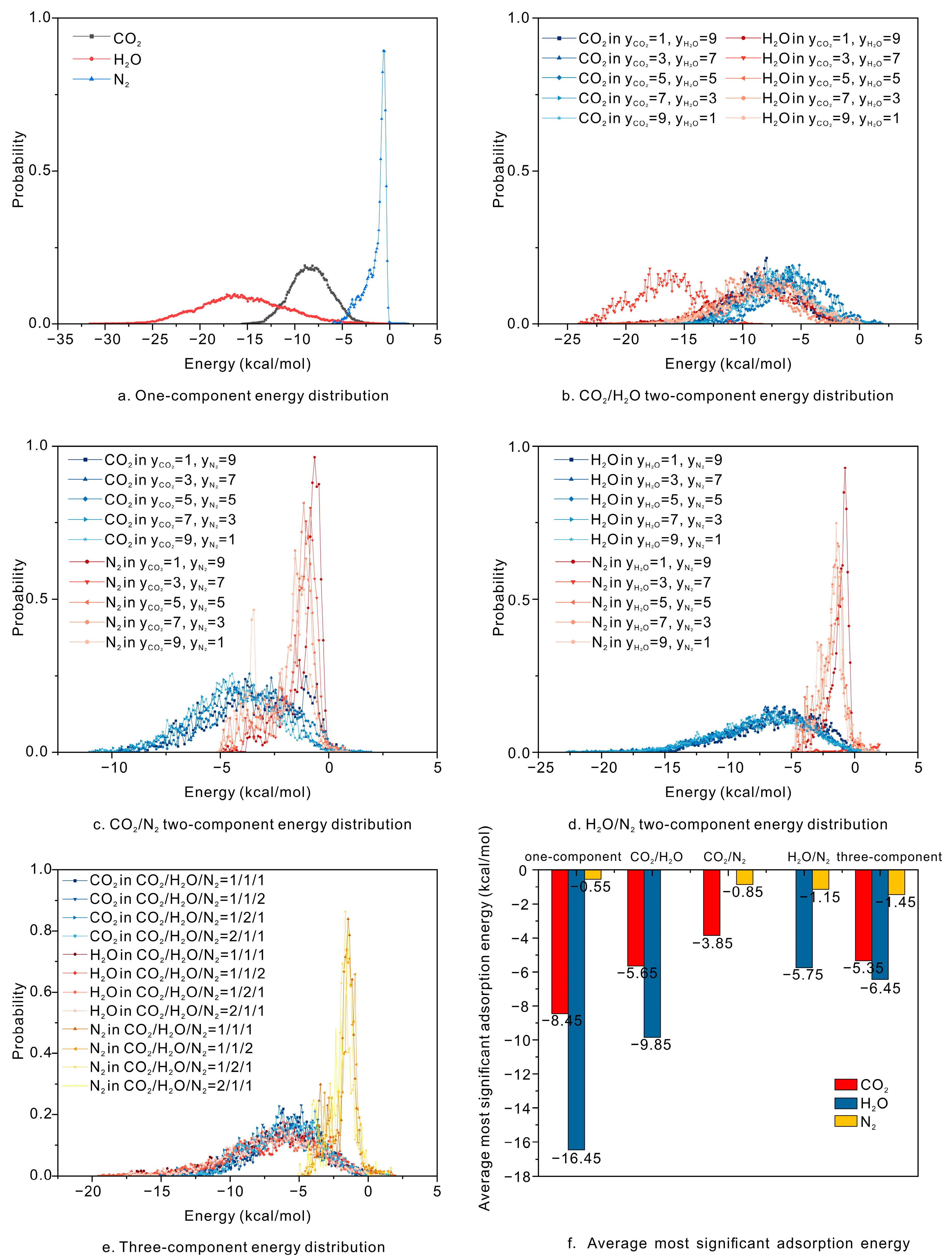
4.3. Energy Distribution of Different Gases Influences
4.4. Coal Mine Waste Gas Geologic Sequestration Practice
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The Paris Agreement. Available online: https://www.un.org/en/climatechange/paris-agreement (accessed on 1 June 2025).
- Soltani, S.M.; Lahiri, A.; Bahzad, H.; Yan, Y. Sorption-enhanced steam methane reforming for combined CO2 capture and hydrogen production: A state-of-the-art review. Carbon Capture Sci. Technol. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Soltani, S.M. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Bachu, S. CO2 storage in geological media: Role, means, status and barriers to deployment. Prog. Energy Combust. Sci. 2008, 34, 254–273. [Google Scholar] [CrossRef]
- Lin, Z.; Kuang, Y.; Li, W.; Zheng, Y. Research status and prospects of CO2 geological sequestration technology from onshore to offshore: A review. Earth-Sci. Rev. 2024, 258, 104928. [Google Scholar] [CrossRef]
- Liu, Q.; Teng, F.; Nielsen, C.P.; Zhang, Y.; Wu, L. Large methane mitigation potential through prioritized closure of gas-rich coal mines. Nat. Clim. Change 2024, 14, 652–658. [Google Scholar] [CrossRef]
- Dang, Y.; Zhao, L.; Lu, X.; Xu, J.; Sang, P.; Guo, S.; Zhu, H.; Guo, W. Molecular simulation of CO2/CH4 adsorption in brown coal: Effect of oxygen-, nitrogen-, and sulfur-containing functional groups. Appl. Surf. Sci. 2017, 423, 33–42. [Google Scholar] [CrossRef]
- Majdinasab, A.; Zhang, Z.; Yuan, Q. Modelling of landfill gas generation: A review. Rev. Environ. Sci. Bio/Technol. 2017, 16, 361–380. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Anthony, E.J.; Jiang, X.; Duan, L.; Wu, Y.; Dong, W.; Zhao, C. Capturing CO2 in flue gas from fossil fuel-fired power plants using dry regenerable alkali metal-based sorbent. Prog. Energy Combust. Sci. 2013, 39, 515–534. [Google Scholar] [CrossRef]
- Azadi, M.; Northey, S.A.; Ali, S.H.; Edraki, M. Transparency on greenhouse gas emissions from mining to enable climate change mitigation. Nat. Geosci. 2020, 13, 100–104. [Google Scholar] [CrossRef]
- Wang, X.; Pan, J.; Wang, K.; Ge, T.; Wei, J.; Wu, W. Characterizing the shape, size, and distribution heterogeneity of pore-fractures in high rank coal based on X-ray CT image analysis and mercury intrusion porosimetry. Fuel 2020, 282, 118754. [Google Scholar] [CrossRef]
- Wang, T.; Deng, Z.; Hu, H.; Ding, R.; Tian, F.; Zhang, T.; Ma, Z.; Wang, D. Pore structure of deep coal of different ranks and its effect on coalbed methane adsorption. Int. J. Hydrogen Energy 2024, 59, 144–158. [Google Scholar] [CrossRef]
- Duan, X.; Hu, Z.; Shao, N.; Li, W.; Li, Y.; Chang, J.; Shen, R. Establishment of a new slip permeability model of gas flow in shale nanopores based on experimental and molecular dynamics simulations studies. J. Pet. Sci. Eng. 2020, 193, 107365. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Li, Z.; Chen, X.; Zhang, Q.; Nie, B.; Yang, T. Nanopore characteristics of coal and quantitative analysis of closed holes in coal. ACS Omega 2020, 5, 24639–24653. [Google Scholar] [CrossRef]
- Mou, P.; Pan, J.; Niu, Q.; Wang, Z.; Li, Y.; Song, D. Coal pores: Methods, types, and characteristics. Energy Fuels 2021, 35, 7467–7484. [Google Scholar] [CrossRef]
- Okolo, G.N.; Everson, R.C.; Neomagus, H.W.; Roberts, M.J.; Sakurovs, R. Comparing the porosity and surface areas of coal as measured by gas adsorption, mercury intrusion and SAXS techniques. Fuel 2015, 141, 293–304. [Google Scholar] [CrossRef]
- Dong, K.; Zhai, Z.; Guo, A. Effects of pore parameters and functional groups in coal on CO2/CH4 adsorption. ACS Omega 2021, 6, 32395–32407. [Google Scholar] [CrossRef]
- Liu, Y.; Wilcox, J. Molecular simulation studies of CO2 adsorption by carbon model compounds for carbon capture and sequestration applications. Environ. Sci. Technol. 2013, 47, 95–101. [Google Scholar] [CrossRef]
- Liu, Y.; Wilcox, J. Molecular simulation of CO2 adsorption in micro-and mesoporous carbons with surface heterogeneity. Int. J. Coal Geol. 2012, 104, 83–95. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, B.; Lan, F. Competitive adsorption of CO2/N2/CH4 onto coal vitrinite macromolecular: Effects of electrostatic interactions and oxygen functionalities. Fuel 2019, 235, 23–38. [Google Scholar]
- Tan, B.; Cheng, G.; Fu, S.; Wang, H.; Li, Z.; Zhang, X. Molecular simulation for physisorption characteristics of O2 in low-rank coals. Energy 2022, 242, 122538. [Google Scholar] [CrossRef]
- Long, H.; Lin, H.; Yan, M.; Chang, P.; gang Li, S.; Bai, Y. Molecular simulation of the competitive adsorption characteristics of CH4, CO2, N2, and multicomponent gases in coal. Powder Technol. 2021, 385, 348–356. [Google Scholar] [CrossRef]
- Li, Z.; Bai, Y.; Yu, H.; Hu, H.; Wang, Y. Molecular simulation of thermodynamic properties of CH4 and CO2 adsorption under different moisture content and pore size conditions. Fuel 2023, 344, 127833. [Google Scholar] [CrossRef]
- Wu, S.; Jin, Z.; Deng, C. Molecular simulation of coal-fired plant flue gas competitive adsorption and diffusion on coal. Fuel 2019, 239, 87–96. [Google Scholar] [CrossRef]
- Chen, J.; Min, F.-f.; Liu, L.; Liu, C.; Lu, F. Experimental investigation and DFT calculation of different amine/ammonium salts adsorption on kaolinite. Appl. Surf. Sci. 2017, 419, 241–251. [Google Scholar] [CrossRef]
- Si, J.; Yang, X.; Li, L.; Yang, B.; Chen, J. Molecular simulation of competitive adsorption of CO2/N2/O2 gas in bituminous coal. Colloids Surf. A Physicochem. Eng. Asp. 2024, 693, 134068. [Google Scholar] [CrossRef]
- Li, Z.; Hu, H.; Wang, Y.; Gao, Y.; Yan, F.; Bai, Y.; Yu, H. Molecular simulation of CO2/N2 injection on CH4 adsorption and diffusion. Sci. Rep. 2024, 14, 20777. [Google Scholar] [CrossRef]
- Liu, X.-Q.; He, X.; Qiu, N.-X.; Yang, X.; Tian, Z.-Y.; Li, M.-J.; Xue, Y. Molecular simulation of CH4, CO2, H2O and N2 molecules adsorption on heterogeneous surface models of coal. Appl. Surf. Sci. 2016, 389, 894–905. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, H.; Guo, C.; Nie, R.; Liang, X. Technology, Numerical simulation of adsorption process of O2/H2O mixed gas in coal porous media. Int. J. Coal Sci. Technol. 2024, 11, 53. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, S.; Zhang, S.; Xi, Z.; Xin, D.; Jia, T.; Yang, X.; Zhang, K.; Li, J.; Wang, Z. Methane Adsorption Capacity of Deep Buried Coal Seam Based on Full-Scale Pore Structure. Nat. Resour. Res. 2025, 34, 1481–1505. [Google Scholar] [CrossRef]
- Sui, H.; Li, X.; Cai, J.; Deng, S.; Xu, E.; Xue, F.; Xie, H. Study on the mechanism of methane “solid–liquid–gas” conversion controlled by the evolution of coal micro-and nanopore structure. Sci. Rep. 2024, 14, 11473. [Google Scholar] [CrossRef] [PubMed]
- Esen, O.; Fişne, A. A comprehensive study on methane adsorption capacities and pore characteristics of coal seams: Implications for efficient coalbed methane development in the Soma Basin, Türkiye. Rock Mech. Rock Eng. 2024, 57, 6355–6375. [Google Scholar] [CrossRef]
- ISO 17246:2024; Coal and Coke—Proximate Analysis. ISO: Geneve, Switzerland, 2024. Available online: https://www.iso.org/standard/86978.html (accessed on 1 June 2025).
- Shao, B.; Wang, S.; Li, T.; Chen, X.; Ma, Y. GCMC-MD prediction of adsorption and diffusion behavior of shale gas in nanopores. Fuel 2024, 377, 132696. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Yuan, C.; Tao, Q.; Zhao, Y.; Zhang, G.; Liu, J. Influence of temperature on adsorption selectivity: Coal-based activated carbon for CH4 enrichment from coal mine methane. Powder Technol. 2019, 347, 42–49. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, P.; Zhou, Q.; Meng, G.; Liu, W.; Xing, Y.; Xiao, X. Controls on Graphitization and Nanopore Characteristics of Organic Matter in Marine Overmature Shale. Nat. Resour. Res. 2025, 34, 1527–1556. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Luo, H.; Yang, T.; Tong, L.; Yuan, Y.; Yuan, C.; Chahine, R.; Xiao, J. Analytical and numerical estimations of isosteric heat of adsorption with application to hydrogen purification. Fuel 2025, 381, 133398. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Li, J. Computational study of adsorption and separation of CO2, CH4, and N2 by an rht-type metal–organic framework. Langmuir 2012, 28, 12122–12133. [Google Scholar] [CrossRef]
- Jia, J.; Xing, Y.; Li, B.; Wu, Y.; Wang, D. Molecular simulation study of adsorption-diffusion of CH4, CO2 and H2O in gas-fat coal. Sci. Rep. 2024, 14, 24131. [Google Scholar] [CrossRef]
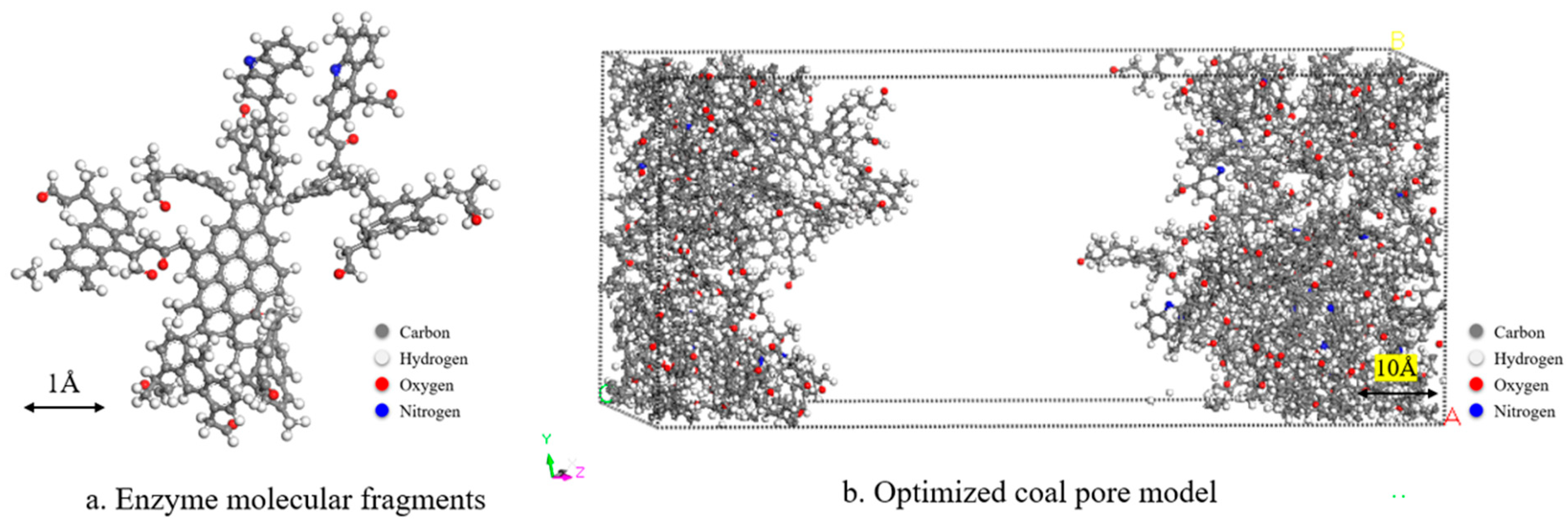



| Proximate Analysis | Maceral Composition Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Mad 1 | Ad 2 | Vdaf 3 | FCdaf 4 | Vitrinite | Inertinite | Liptinite | Mineral |
| 0.73% | 15.32% | 14.88% | 85.12% | 90.63% | 3.13% | 3.51% | 2.73% |
| Setting | Amorphous Cell Parameters | Setting | Sorption Parameters |
|---|---|---|---|
| Task | Packing | Task | Adsorption isotherm |
| Quality | Fine | Method | Metropols |
| Temperature | 353.15 K | Temperature | 353.15 K |
| Force field | COMPASS | Fugacity steps | 10 |
| Charges | Forcefield-assigned | Charges | Forcefield-assigned |
| Electrostatic | Ewald | Quality | Customized |
| van der Waals | Atom-based | Equilibration steps | 10,000 |
| Production steps | 100,000 | ||
| Force field | COMPASS | ||
| Electrostatic | Atom-based | ||
| van der Waals | Atom-based |
| NO. | Adsorbate | NO. | Adsorbate | ||||
|---|---|---|---|---|---|---|---|
| Set Pressure (MPa) | Set Pressure (MPa) | ||||||
| CO2 | N2 | H2O | CO2 | N2 | H2O | ||
| 1 | 30 | / | / | 14 | 3 | / | 27 |
| 2 | / | 30 | / | 15 | 9 | / | 21 |
| 3 | / | / | 30 | 16 | 15 | / | 15 |
| 4 | 3 | 27 | / | 17 | 21 | / | 9 |
| 5 | 9 | 21 | / | 18 | 27 | / | 3 |
| 6 | 15 | 15 | / | 19 | 10 | 10 | 10 |
| 7 | 21 | 9 | / | 20 | 15 | 7.5 | 7.5 |
| 8 | 27 | 3 | / | 21 | 7.5 | 15 | 7.5 |
| 9 | / | 3 | 27 | 22 | 7.5 | 7.5 | 15 |
| 10 | / | 9 | 21 | ||||
| 11 | / | 15 | 15 | ||||
| 12 | / | 21 | 9 | ||||
| 13 | / | 27 | 3 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Li, Y.; Hu, Y.; Li, H.; Chen, B.; Zhang, Q.; Xu, Q.; Li, Y. CO2 Sequestration Potential Competitive with H2O and N2 in Abandoned Coal Mines Based on Molecular Modeling. Processes 2025, 13, 2123. https://doi.org/10.3390/pr13072123
Liu T, Li Y, Hu Y, Li H, Chen B, Zhang Q, Xu Q, Li Y. CO2 Sequestration Potential Competitive with H2O and N2 in Abandoned Coal Mines Based on Molecular Modeling. Processes. 2025; 13(7):2123. https://doi.org/10.3390/pr13072123
Chicago/Turabian StyleLiu, Tianyang, Yun Li, Yaxuan Hu, Hezhao Li, Binghe Chen, Qixu Zhang, Qiufeng Xu, and Yong Li. 2025. "CO2 Sequestration Potential Competitive with H2O and N2 in Abandoned Coal Mines Based on Molecular Modeling" Processes 13, no. 7: 2123. https://doi.org/10.3390/pr13072123
APA StyleLiu, T., Li, Y., Hu, Y., Li, H., Chen, B., Zhang, Q., Xu, Q., & Li, Y. (2025). CO2 Sequestration Potential Competitive with H2O and N2 in Abandoned Coal Mines Based on Molecular Modeling. Processes, 13(7), 2123. https://doi.org/10.3390/pr13072123







