Optimization of Spirulina platensis Incorporation in Coated Beef Meatballs: Impact on Quality Characteristics and Polycyclic Aromatic Hydrocarbon (PAH) Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coated Beef Meatball Production
2.3. Analyses
2.3.1. DPPH Analysis Performed on the Spirulina platensis Powder
2.3.2. Analyses of Coated Beef Meatballs
Proximate Composition Analysis
pH Value Determination
Texture Profile Evaluation
Color Characteristics Measurement
TBARS Analysis
Polycyclic Aromatic Hydrocarbons (PAHs) Analysis
- -
- Chemicals
- -
- Sample preparation
- -
- Chromatographic Conditions
- -
- Method validation
Sensory Evaluation
Statistical Analysis
2.4. Experimental Design and Optimization
3. Results and Discussion
3.1. DPPH Value of Spirulina platensis Powder
3.2. Proximate Composition and pH Value of Coated Meatballs
3.3. Texture Profile of Coated Meatballs
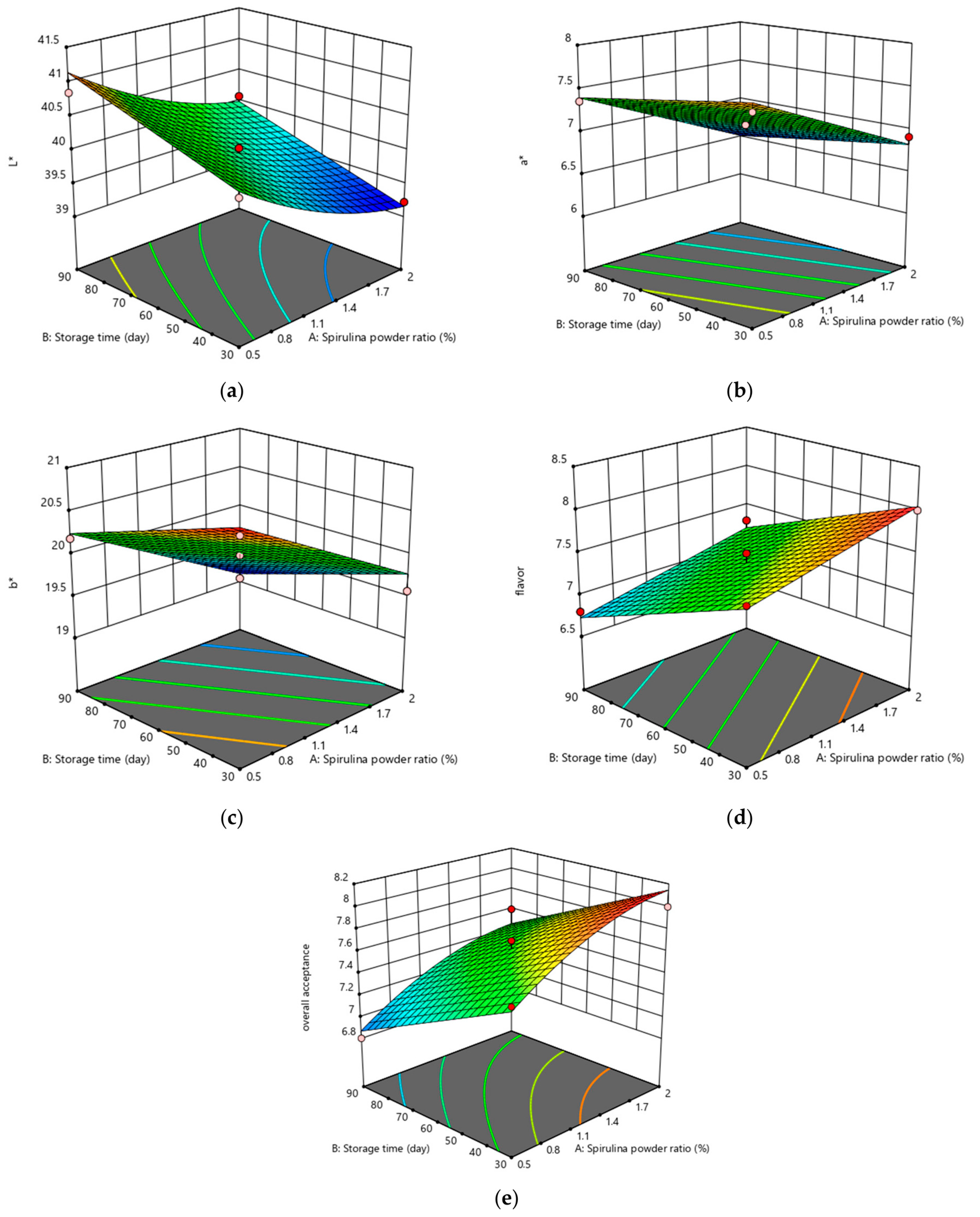
3.4. Color Characteristics of Coated Meatballs
3.5. TBARS Values of Coated Meatballs
3.6. PAH4 Levels of Coated Meatballs
3.7. Sensory Evaluation of Coated Meatballs
3.8. The Optimum S. platensis Powder Level and Storage Period
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Unsal, A. Beslenmenin önemi ve temel besin öğeleri [The Importance of Nutrition and Essential Nutrients]. Kırşehir Ahi Evran Üniversitesi Sağlık Bilim. Dergisi 2019, 2, 1–10. [Google Scholar]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Tavares, J.O.; Cotas, J.; Valado, A.; Pereira, L. Algae food products as a healthcare solution. Mar. Drugs 2023, 21, 578. [Google Scholar] [CrossRef]
- Adam, D. How far will global population rise? Researchers can’t agree. Nature 2021, 597, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Wu, J.Y.; Tso, R.; Teo, H.S.; Haldar, S. The utility of algae as sources of high value nutritional ingredients, particularly for alternative/complementary proteins to improve human health. Front. Nutr. 2023, 10, 1277343. [Google Scholar] [CrossRef]
- Clark, H.; Wu, H. The Sustainable Development Goals: 17 Goals to Transform Our World; United Nations: New York, NY, USA, 2016. [Google Scholar]
- Matos, Â.P.; Novelli, E.; Tribuzi, G. Algae as food and ingredient: From production to consumer acceptance. Front. Food Sci. Technol. 2023, 3, 1220050. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology; Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Ilter, I.; Akyıl, S.; Koç, M.; Kaymak-Ertekin, F. Alglerden elde edilen stabilize edici maddeler [Stabilizing agents derived from algae]. Akad. Gıda 2016, 14, 315–321. [Google Scholar]
- Kumkapu, M.; Yeşilçubuk, N.Ş. Sürdürülebilir gıda, gıda takviyesi ve gıda katkı maddesi üretiminde alglerin önemi [The Importance of Algae in Sustainable Food, Food Supplement, and Food Additive Production]. Akad. Gıda 2023, 21, 187–197. [Google Scholar] [CrossRef]
- Sirinyildiz, D.D.; Yorulmaz, A. Alternatif ve sürdürülebilir bir gıda kaynağı olarak algler [Algae as an Alternative and Sustainable Food Source]. Toros Univ. J. Food Nutr. Gastron. 2022, 1, 101–117. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Teng, S.Y.; Yew, G.Y.; Sukačová, K.; Show, P.L.; Máša, V.; Chang, J.S. Microalgae with artificial intelligence: A digitalized perspective on genetics, systems and products. Biotechnol. Adv. 2020, 44, 107631. [Google Scholar] [CrossRef]
- Ratnapuram, H.P.; Vutukuru, S.S.; Yadavalli, R. Mixotrophic transition induced lipid productivity in Chlorella pyrenoidosa under stress conditions for biodiesel production. Heliyon 2018, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vu, C.H.T.; Lee, H.G.; Chang, Y.K. Axenic cultures for microalgal biotechnology: Establishment, assessment, maintenance, and applications. Biotechnol. Adv. 2018, 36, 380–396. [Google Scholar] [CrossRef]
- Mutanda, T.; Naidoo, D.; Bwapwa, J.K.; Anandraj, A. Biotechnological applications of microalgal oleaginous compounds: Current trends on microalgal bioprocessing of products. Front. Energy Res. 2020, 8, 598803. [Google Scholar] [CrossRef]
- Nicoletti, M. Microalgae nutraceuticals. Foods 2016, 5, 54. [Google Scholar] [CrossRef]
- Ljubic, A.; Safafar, H.; Holdt, S.L.; Jacobsen, C. Biomass composition of Arthrospira platensis during cultivation on industrial process water and harvesting. J. Appl. Phycol. 2018, 30, 943–954. [Google Scholar] [CrossRef]
- De Marco Castro, E.; Shannon, E.; Abu-Ghannam, N. Effect of fermentation on enhancing the nutraceutical properties of Arthrospira platensis (Spirulina). Fermentation 2019, 5, 28. [Google Scholar] [CrossRef]
- Papalia, T.; Sidari, R.; Panuccio, M.R. Impact of different storage methods on bioactive compounds in Arthrospira platensis biomass. Molecules 2019, 24, 2810. [Google Scholar] [CrossRef]
- Ramírez-Rodrigues, M.M.; Estrada-Beristain, C.; Metri-Ojeda, J.; Pérez-Alva, A.; Baigts-Allende, D.K. Spirulina platensis protein as sustainable ingredient for nutritional food products development. Sustainability 2021, 13, 6849. [Google Scholar] [CrossRef]
- Wu, H.-L.; Wang, G.-H.; Xiang, W.-Z.; Li, T.; He, H. Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef]
- Anvar, A.A.; Nowruzi, B. Bioactive properties of spirulina: A review. Microb. Bioact. 2021, 4, 134–142. [Google Scholar] [CrossRef]
- Manet, A. La Spiruline: Indications Thérapeutiques, Risques Sanitaires et Conseils à l’Officine. Master’s Thesis, Faculté de Médecine et Pharmacie—Université Grenoble Alpes, La Tronche, France, 2016. [Google Scholar]
- Bencheikh, A.; Mamache, W.; Gharzouli, A.; Kouachi, A.; Khadidja, H.; Daichi, M.B.; Rouag, N. Evaluation of the spirulina (Arthrospira platensis Gomont) antimicrobial activity. Turk. J. Agric. Food Sci. Technol. 2022, 10, 2051–2055. [Google Scholar] [CrossRef]
- Sotiroudis, T.G.; Sotiroudis, G.T. Health aspects of Spirulina (Arthrospira) microalga food supplement. J. Serbian Chem. Soc. 2013, 78, 395–405. [Google Scholar] [CrossRef]
- Gogna, S.; Kaur, J.; Sharma, K.; Prasad, R.; Singh, J.; Bhadariya, V.; Jarial, S. Spirulina—An edible cyanobacterium with potential therapeutic health benefits and toxicological consequences. J. Am. Nutr. Assoc. 2023, 42, 559–572. [Google Scholar] [CrossRef]
- Gromek, W.; Kołdej, N.; Kurowski, M.; Majsiak, E. Spirulina (Arthrospira platensis): Antiallergic agent or hidden allergen? A literature review. Foods 2024, 13, 1052. [Google Scholar] [CrossRef]
- Industry ARC. Spirulina Powder Market—Forecast (2022–2027). Available online: https://www.industryarc.com/Report/19629/spirulina-powder-market.html (accessed on 5 July 2023).
- Fantechi, T.; Contini, C.; Casini, L. Pasta goes green: Consumer preferences for spirulina-enriched pasta in Italy. Algal Res. 2023, 75, 103275. [Google Scholar] [CrossRef]
- Rahimah, S.; Sonjaya, M.F.; Andoyo, R.; Satya, A.; Nurhasanah, S.; Chrismadha, T. Physical and organoleptic characteristic of bread substituted with Spirulina platensis. In Biomass Conversion and Sustainable Biorefinery: Towards Circular Bioeconomy; Springer Nature: Singapore, 2024; pp. 295–306. [Google Scholar]
- Kanojia, S. Development of value-added cookies incorporated with Spirulina platensis for the mitigation of malnutrition in children. Agriculture 2022, 5, 102–108. [Google Scholar]
- El-Anany, A.M.; Althwab, S.A.; Alhomaid, R.M.; Ali, R.F.; Mousa, H.M. Effect of spirulina (Arthrospira platensis) powder addition on nutritional and sensory attributes of chicken mortadella. Ital. J. Food Sci. 2023, 35, 1–11. [Google Scholar] [CrossRef]
- Barkallah, M.; Ben Atitallah, A.; Hentati, F.; Dammak, M.; Hadrich, B.; Fendri, I.; Abdelkafi, S. Effect of Spirulina platensis biomass with high polysaccharides content on quality attributes of common carp (Cyprinus carpio) and common barbel (Barbus barbus) fish burgers. Appl. Sci. 2019, 9, 2197. [Google Scholar] [CrossRef]
- Luo, A.; Feng, J.; Hu, B.; Lv, J.; Liu, Q.; Nan, F.; Xie, S. Arthrospira (Spirulina) platensis extract improves oxidative stability and product quality of Chinese-style pork sausage. J. Appl. Phycol. 2018, 30, 1667–1677. [Google Scholar] [CrossRef]
- Marti-Quijal, F.J.; Zamuz, S.; Galvez, F.; Roohinejad, S.; Tiwari, B.K.; Gómez, B.; Lorenzo, J.M. Replacement of soy protein with other legumes or algae in turkey breast formulation: Changes in physicochemical and technological properties. J. Food Process. Preserv. 2018, 42, e13845. [Google Scholar] [CrossRef]
- Ozbay, G.; Semint, S.; Tuysuz, V. Et ve et ürünleri tarihi üzerine bir araştırma [A Research on the History of Meat and Meat Products]. MANAS Sos. Araştırmalar Derg. 2024, 13, 765–779. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef]
- Mehta, S.S.; Arroyave, W.D.; Lunn, R.M.; Park, Y.M.M.; Boyd, W.A.; Sandler, D.P. A prospective analysis of red and processed meat consumption and risk of colorectal cancer in women. Cancer Epidemiol. Biomark. Prev. 2020, 29, 141–150. [Google Scholar] [CrossRef]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef]
- Oz, E. Mutagenic and/or carcinogenic compounds in meat and meat products: Polycyclic aromatic hydrocarbons perspective. Theory Pract. Meat Process. 2022, 7, 282–287. [Google Scholar] [CrossRef]
- Aoudeh, E.; Oz, E.; Oz, F. Effect of beef patties fortification with black garlic on the polycyclic aromatic hydrocarbons (PAHs) content and toxic potency. Food Chem. 2023, 428, 136763. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Polycyclic aromatic hydrocarbons in food-Scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008, 724, 1–114. [Google Scholar]
- Tarafdar, A.; Chawda, S.; Sinha, A. Health risk assessment from polycyclic aromatic hydrocarbons (PAHs) present in dietary components: A meta-analysis on a global scale. Polycycl. Aromat. Compd. 2018, 40, 1–12. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human dietary exposure to polycyclic aromatic hydrocarbons: A review of the scientific literature. Food Chem. Toxicol. 2015, 86, 144–153. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Y.; Wang, H.; Bai, Y.; Dai, C.; Li, C.; Zhou, G. The influence of natural antioxidants on polycyclic aromatic hydrocarbon formation in charcoal-grilled chicken wings. Food Control 2019, 98, 34–41. [Google Scholar] [CrossRef]
- Alomirah, H.; Al-Zenki, S.; Al-Hooti, S.; Zaghloul, S.; Sawaya, W.; Ahmed, N. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control 2011, 22, 2028–2035. [Google Scholar] [CrossRef]
- Racovita, R.C.; Secuianu, C.; Israel-Roming, F. Quantification and risk assessment of carcinogenic polycyclic aromatic hydrocarbons in retail smoked fish and smoked cheeses. Food Control 2021, 121, 107586. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O.; Ashaolu, T.J. Polycyclic aromatic hydrocarbons formation and mitigation in meat and meat products. Polycycl. Aromat. Compd. 2022, 42, 3401–3411. [Google Scholar] [CrossRef]
- European Union. European Commission Regulation (EU) No 1327/2014 of 12 December 2014 amending Regulation (EC) No 1881/2006 as regards maximum levels of polycyclic aromatic hydrocarbons (PAHs) in traditionally smoked meat and meat products and traditionally smoked fish and fishery products. Off. J. Eur. Union 2014, 358, 13–14. [Google Scholar]
- Wang, Z.; Ng, K.; Warner, R.D.; Stockmann, R.; Fang, Z. Reduction strategies for polycyclic aromatic hydrocarbons in processed foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Huang, X.; Tang, X.; Zhan, J.; Liu, S. The effects of different natural plant extracts on the formation of polycyclic aromatic hydrocarbons (PAHs) in roast duck. Foods 2022, 11, 2104. [Google Scholar] [CrossRef] [PubMed]
- El Badry, N. Effect of household cooking methods and some food additives on polycylic aromatic hydrocarbons (PAHs) formation in chicken meat. World Appl. Sci. J. 2010, 9, 963–974. [Google Scholar]
- Farhadian, A.; Jinap, S.; Faridah, A.; Zaidul, I.S.M. Effects of marinating on the formation of polycyclic aromatic hydrocarbons (benzo[a]pyrene, benzo[b]fluoranthene and fluoranthene) in grilled beef meat. Food Control 2012, 28, 420–442. [Google Scholar] [CrossRef]
- Tkacz, K.; Wiek, A.; Kubiak, M.S. Influence of marinades on the level of PAHs in grilled meat products. Ital. J. Food Sci. 2012, 24, 270–278. [Google Scholar]
- Karslıoğlu, B. Et ve et ürünlerinde polisiklik aromatik hidrokarbonların oluşum mekanizmaları ve azaltıcı yaklaşımlar [Formation Mechanisms and Reduction Approaches of Polycyclic Aromatic Hydrocarbons in Meat and Meat Products]. Gıda 2022, 47, 1032–1045. [Google Scholar]
- Wang, M.F.; Tadmor, Y.; Simon, J.E. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). Abstr. Pap. Am. Chem. Soc. 2003, 223, 42. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; AOAC Int.: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOCS. Official Method, Ba 4a-38, Nitrogen-ammonia-protein modified Kjedahl method. In Official Methods and Recommended Practices of the AOCS.; Firestone, D., Ed.; Am. Oil Chem. Soc.: Champaign, IL, USA, 1997. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; AOAC Int.: Washington, WA, USA, 2006. [Google Scholar]
- Kramer, A.; Twigg, B.A. Quality Control for the Food Industry, 3rd ed.; The Avi Publishing: Roslyn, NY, USA, 1984; Volume I. [Google Scholar]
- Witte, V.C.; Krause, G.F.; Bailey, M.E. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J. Food Sci. 1970, 35, 582–585. [Google Scholar] [CrossRef]
- Chung, S.Y.; Yettella, R.R.; Kim, J.S.; Kwon, K.; Kim, M.C.; Min, D.B. Effects of grilling and roasting on the levels of polycyclic aromatic hydrocarbons in beef and pork. Food Chem. 2011, 129, 1420–1426. [Google Scholar] [CrossRef]
- Kendirci, P.; Icier, F.; Kor, G.; Altug-Onogur, T. Influence of infrared final cooking on polycyclic aromatic hydrocarbon formation in ohmically pre-cooked beef meatballs. Meat Sci. 2014, 97, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Oz, E. The impact of fat content and charcoal types on quality and the development of carcinogenic polycyclic aromatic hydrocarbons and heterocyclic aromatic amines formation of barbecued fish. Int. J. Food Sci. Technol. 2021, 56, 954–964. [Google Scholar] [CrossRef]
- Altug Onogur, T.; Elmaci, Y. Gıdalarda Duyusal Değerlendirmeler [Sensory Evaluation in Foods]; Sidas Publications: Izmir, Türkiye, 2015; p. 135. [Google Scholar]
- ISO 6658:2017; Sensory Analysis—Methodology—General Guidance. International Organization for Standardization: Geneva, Switzerland, 2017.
- SPSS. Statistical Package, SPSS for Windows, version 26.0; SPSS Inc.: Chicago, IL, USA, 2019. [Google Scholar]
- Gabr, G.A.; El-Sayed, S.M.; Hikal, M.S. Antioxidant activities of phycocyanin: A bioactive compound from Spirulina platensis. J. Pharm. Res. Int. 2020, 32, 73–85. [Google Scholar] [CrossRef]
- Parniakov, O.; Toepfl, S.; Barba, F.J.; Granato, D.; Zamuz, S.; Galvez, F.; Lorenzo, J.M. Impact of the soy protein replacement by legumes and algae based proteins on the quality of chicken rotti. J. Food Sci. Technol. 2018, 55, 2552–2559. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Hamadi, M.; Douik, L.H.; Cherifi, Z.; Nazir, A. Physicochemical, functional, and nutraceutical properties of Spirulina and Chlorella biomass: A comparative study. Algal Res. 2024, 81, 103561. [Google Scholar] [CrossRef]
- Zaki, E.F. Quality characteristics of camel burger formulated with different levels of microalgae. J. Food Qual. Hazards Control 2023, 10, 92–102. [Google Scholar] [CrossRef]
- Hentati, F.; Barkallah, M.; Ben Atitallah, A.; Dammak, M.; Louati, I.; Pierre, G.; Abdelkafi, S. Quality characteristics and functional and antioxidant capacities of algae-fortified fish burgers prepared from common barbel (Barbus barbus). Biomed. Res. Int. 2019, 2019, 2907542. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Jeong, T.J.; Kim, H.W.; Hwang, K.E.; Sung, J.M.; Seo, D.H.; Kim, C.J. Combined effects of sea mustard and transglutaminase on the quality characteristics of reduced-salt frankfurters. J. Food Process. Preserv. 2017, 41, e12945. [Google Scholar] [CrossRef]
- Cortez-Vega, W.R.; Pizato, S.; Prentice, C. Quality of raw chicken breast stored at 5 °C and packaged under different modified atmospheres. J. Food Saf. 2012, 32, 268–276. [Google Scholar] [CrossRef]
- Hector, D.A.; Brew-Graves, C.; Hassen, N.; Ledward, D.A. Relationship between myosin denaturation and the colour of low-voltage-electrically-stimulated beef. Meat Sci. 1992, 31, 299–307. [Google Scholar] [CrossRef]
- López-López, I.; Cofrades, S.; Jiménez-Colmenero, F. Low-fat frankfurters enriched with n−3 PUFA and edible seaweed: Effects of olive oil and chilled storage on physicochemical, sensory and microbial characteristics. Meat Sci. 2009, 83, 148–154. [Google Scholar] [CrossRef]
- Zhou, C.; Li, J.; Tan, S. Effect of l-lysine on the physicochemical properties of pork sausage. Food Sci. Biotechnol. 2014, 23, 775–780. [Google Scholar] [CrossRef]
- Luo, A.; Feng, J.; Hu, B.; Lv, J.; Chen, C.Y.O.; Xie, S. Polysaccharides in Spirulina platensis improve antioxidant capacity of Chinese-style sausage. J. Food Sci. 2017, 82, 2591–2597. [Google Scholar] [CrossRef]
- Munsu, E.; Mohd Zaini, H.; Matanjun, P.; Ab Wahab, N.; Sulaiman, N.S.; Pindi, W. Physicochemical, sensory properties and lipid oxidation of chicken sausages supplemented with three types of seaweed. Appl. Sci. 2021, 11, 11347. [Google Scholar] [CrossRef]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef]
- Han, P.; Li, J.; Zhong, H.; Xie, J.; Zhang, P.; Lu, Q.; Zhou, W. Anti-oxidation properties and therapeutic potentials of spirulina. Algal Res. 2021, 55, 102240. [Google Scholar] [CrossRef]
- Hlima, H.B.; Smaoui, S.; Barkallah, M.; Elhadef, K.; Tounsi, L.; Michaud, P.; Abdelkafi, S. Sulfated exopolysaccharides from Porphyridium cruentum: A useful strategy to extend the shelf life of minced beef meat. Int. J. Biol. Macromol. 2021, 193, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Haiba, N.S.; Asaal, A.M.; El Massry, A.M.; Ismail, I.; Basahi, J.; Hassan, I.A. Effects of “doneness” level on PAH concentrations in charcoal-grilled beef and chicken: An Egyptian study case. Polycycl. Aromat. Compd. 2021, 41, 553–563. [Google Scholar] [CrossRef]
- Janoszka, B. HPLC-fluorescence analysis of polycyclic aromatic hydrocarbons (PAHs) in pork meat and its gravy fried without additives and in the presence of onion and garlic. Food Chem. 2011, 126, 1344–1353. [Google Scholar] [CrossRef]
- Kafouris, D.; Koukkidou, A.; Christou, E.; Hadjigeorgiou, M.; Yiannopoulos, S. Determination of polycyclic aromatic hydrocarbons in traditionally smoked meat products and charcoal grilled meat in Cyprus. Meat Sci. 2020, 164, 108088. [Google Scholar] [CrossRef]
- Duedahl-Olesen, L.; Ionas, A.C. Formation and mitigation of PAHs in barbecued meat—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3553–3568. [Google Scholar] [CrossRef]
- Ozlu, T.; Bayram, B. Spirulina mikroalginin besinsel özellikleri ve sağlık üzerine potansiyel etkileri [Nutritional Properties and Potential Health Effects of Spirulina Microalgae]. Akad. Gıda 2022, 20, 296–304. [Google Scholar] [CrossRef]
- Zejnelhoxha, S. Formation of Polycyclic Aromatic Hydrocarbons in Cooked Meat and the Possibility of Reducing Their Formation by Vitamin E. Ph.D. Thesis, Università degli Studi del Molise, Campobasso, Italy, 2023. [Google Scholar]
- Aljabery, R.N.; Auda, M.A.; Al-Rekabi, H.Y. Apoptotic effects of phenolic extract from Spirulina platensis on esophagus cancer cell line SK-GT-4. Oncol. Radiother. 2023, 17, 580–587. [Google Scholar]
- Wang, X.; Han, Y.; Cao, Y.; Ni, Y.; Wang, D.; Luan, Y. The formation, stabilization mechanism, and environmental impacts of persistent free radicals in soil humic substances. Agronomy 2025, 15, 602. [Google Scholar] [CrossRef]
- Bulanda, S.; Janoszka, B. Polycyclic aromatic hydrocarbons (PAHs) in roasted pork meat and the effect of dried fruits on PAH content. Int. J. Environ. Res. Public Health 2023, 20, 4922. [Google Scholar] [CrossRef]
- Mohammed, H.O.; O’Grady, M.N.; O’Sullivan, M.G.; Hamill, R.M.; Kilcawley, K.N.; Kerry, J.P. Acceptable inclusion levels for selected brown and red Irish seaweed species in pork sausages. Foods 2022, 11, 1522. [Google Scholar] [CrossRef]
- Sellimi, S.; Ksouda, G.; Benslima, A.; Nasri, R.; Rinaudo, M.; Nasri, M.; Haiji, M. Enhancing colour and oxidative stabilities of reduced-nitrite turkey meat sausages during refrigerated storage using fucoxanthin purified from the Tunisian seaweed Cystoseira barbata. Food Chem. Toxicol. 2017, 107, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Widati, A.S.; Rosyidi, D.; Radiati, L.E.; Nursyam, H. The effect of seaweed (Eucheuma cottonii) flour addition on physicochemical and sensory characteristics of an Indonesian-style beef meatball. Int. J. Food Stud. 2021, 10, 112–120. [Google Scholar] [CrossRef]
- Capan, B.; Yildiz Turp, G. Development of Gluten-Free Coated Chicken Liver, Examination of the Effects of Spices and Cooking Methods on Product Quality Characteristics and Heterocyclic Aromatic Amine (HCA) Compounds. Appl. Sci. 2025, 15, 5295. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N. Enhancement of the phytochemical and fibre content of beef patties with Himanthalia elongata seaweed. Int. J. Food Sci. Technol. 2013, 48, 2239–2249. [Google Scholar] [CrossRef]
- Ovissipour, M.; Abedian Kenari, A.; Motamedzadegan, A.; Nazari, R.M. Optimization of enzymatic hydrolysis of visceral waste proteins of yellowfin tuna (Thunnus albacares). Food Bioprocess Technol. 2012, 5, 696–705. [Google Scholar] [CrossRef]
- Karazhiyan, H.; Razavi, S.M.; Phillips, G.O.; Fang, Y.; Al-Assaf, S.; Nishinari, K.; Farhoosh, R. Rheological properties of Lepidium sativum seed extract as a function of concentration, temperature and time. Food Hydrocoll. 2009, 23, 2062–2068. [Google Scholar] [CrossRef]
- Samavati, V. Polysaccharide extraction from Abelmoschus esculentus: Optimization by response surface methodology. Carbohydr. Polym. 2013, 95, 588–597. [Google Scholar] [CrossRef]
| Sample Group | Beef (%) | Sunflower Oil (%) | Salt (%) | Garlic Powder and Spice Mix (%) | S. platensis Powder (%) |
|---|---|---|---|---|---|
| C | 89.6 | 3.5 | 1.5 | 5.4 | 0 |
| S0.2 | 89.4 | 3.5 | 1.5 | 5.4 | 0.2 |
| S0.5 | 89.1 | 3.5 | 1.5 | 5.4 | 0.5 |
| S1.25 | 88.35 | 3.5 | 1.5 | 5.4 | 1.25 |
| S2 | 87.6 | 3.5 | 1.5 | 5.4 | 2 |
| S2.3 | 87.3 | 3.5 | 1.5 | 5.4 | 2.3 |
| Run | Factor 1 S. platensis Powder Ratio (%) | Factor 2 Storage Period (Days) |
|---|---|---|
| 1 | 0.5 | 30 |
| 2 | 2 | 30 |
| 3 | 0.5 | 90 |
| 4 | 2 | 90 |
| 5 | 0.2 | 60 |
| 6 | 2.3 | 60 |
| 7 | 1.25 | 60 |
| 8 | 1.25 | 18 |
| 9 | 1.25 | 102 |
| 10 | 1.25 | 60 |
| 11 | 1.25 | 60 |
| Sample | Moisture (%) | Fat (%) | Ash (%) | Protein (%) | pH |
|---|---|---|---|---|---|
| C | 55.92 ± 0.24 f | 32.59 ± 0.25 a | 1.68 ± 0.01 a | 17.46 ± 0.13 a | 5.63 ± 0.01 a |
| S0.2 | 54.66 ± 0.22 e | 33.32 ± 0.1 b | 1.71 ± 0.01 ab | 18.32 ± 0.12 b | 5.71 ± 0.01 b |
| S0.5 | 52.46 ± 0.24 d | 33.78 ± 0.11 b | 1.75 ± 0.01 b | 19.23 ± 0.04 c | 5.82 ± 0.01 c |
| S1.25 | 50.64 ± 0.14 c | 35.16 ± 0.11 c | 1.85 ± 0.01 c | 24.00 ± 0.09 d | 5.92 ± 0.01 d |
| S2 | 48.37 ± 0.19 b | 37.22 ± 0.24 d | 1.95 ± 0.01 d | 27.35 ± 0.25 e | 5.99 ± 0.01 e |
| S2.3 | 47.06 ± 0.18 a | 37.88 ± 0.42 e | 2.00 ± 0.03 d | 28.78 ± 0.09 f | 6.10 ± 0.01 f |
| Sample | Hardness (N) | Springiness (mm) | Cohesiveness | Gumminess (N) | Chewiness (Nxmm) | Resilience |
|---|---|---|---|---|---|---|
| C | 17.17 ± 0.66 a | 0.17 ± 0.02 a | 0.22 ± 0.01 | 3.47 ± 0.27 b | 1.42 ± 0.11 c | 0.04 ± 0.01 |
| S0.2 | 17.64 ± 0.75 ab | 0.22 ± 0.03 ab | 0.23 ± 0.01 | 3.20 ± 0.32 b | 1.32 ± 0.09 c | 0.05 ± 0.01 |
| S0.5 | 17.84 ± 0.71 ab | 0.25 ± 0.02 bc | 0.23 ± 0.01 | 2.33 ± 0.21 a | 0.97 ± 0.08 b | 0.06 ± 0.02 |
| S1.25 | 18.53 ± 0.46 ab | 0.27 ± 0.02 bc | 0.26 ± 0.01 | 2.23 ± 0.32 a | 0.51 ± 0.10 a | 0.07 ± 0.01 |
| S2 | 22.55 ± 0.36 bc | 0.31 ± 0.02 cd | 0.27 ± 0.02 | 1.88 ± 0.10 a | 0.47 ± 0.09 a | 0.07 ± 0.01 |
| S2.3 | 27.00 ± 0.43 c | 0.37 ± 0.02 d | 0.28 ± 0.02 | 1.86 ± 0.16 a | 0.32 ± 0.08 a | 0.08 ± 0.01 |
| Storage Period (Day) | C | S0.2 | S0.5 | S1.25 | S2 | S2.3 | |
|---|---|---|---|---|---|---|---|
| L* | 0 | 40.58 ± 0.63 d,A | 40.01 ± 0.31 cd,A | 39.70 ± 0.26 cd,A | 38.88 ± 0.17 bc,A | 37.73 ± 0.61 ab,A | 37.25 ± 0.56 a,A |
| 18 | 40.89 ± 0.25 c,AB | 40.43 ± 0.24 c,AB | 39.99 ± 0.24 bc,A | 39.30 ± 0.50 b,AB | 38.25 ± 0.24 a,AB | 37.94 ± 0.49 a,AB | |
| 30 | 41.10 ± 0.37 b,AB | 40.90 ± 0.53 b,ABC | 40.22 ± 0.27 ab,AB | 39.88 ± 0.76 ab,AB | 39.22 ± 0.82 ab,BC | 38.49 ± 1.29 a,ABC | |
| 60 | 41.51 ± 0.30 c,AB | 41.38 ± 0.1 c,BC | 40.39 ± 0.44 b,AB | 40.03 ± 0.36 ab,AB | 39.73 ± 0.14 ab,C | 39.39 ± 0.09 a,BC | |
| 90 | 41.82 ± 0.24 c,B | 41.60 ± 0.43 c,C | 40.85 ± 0.16 b,B | 40.49 ± 0.23 ab,B | 40.08 ± 0.26 ab,C | 39.85 ± 0.15 a,BC | |
| 102 | 42.00 ± 0.31 d,B | 41.52 ± 0.16 cd,C | 41.06 ± 0.21 bc,B | 40.60 ± 0.11 ab,B | 40.12 ± 0.17 a,C | 40.04 ± 0.19 a,C | |
| a* | 0 | 8.93 ± 0.13 c,C | 8.41 ± 0.19 bc,C | 8.16 ± 0.14 bc,C | 7.77 ± 0.26 ab | 7.26 ± 0.35 a | 7.01 ± 0.38 a |
| 18 | 8.73 ± 0.15 c,BC | 8.19 ± 0.22 c,BC | 7.96 ± 0.06 bc,BC | 7.30 ± 0.39 ab | 6.98 ± 0.25 a | 6.80 ± 0.37 a | |
| 30 | 8.29 ± 0.32 c,AB | 7.99 ± 0.18 bc,AB | 7.61 ± 0.09 abc,AB | 7.20 ± 0.31 ab | 6.91 ± 0.46 a | 6.67 ± 0.47 a | |
| 60 | 8.02 ± 0.21 c,A | 7.91 ± 0.31 c,A | 7.46 ± 0.28 bc,A | 7.07 ± 0.36 ab | 6.72 ± 0.16 ab | 6.46 ± 0.12 a | |
| 90 | 7.82 ± 0.12 d,A | 7.73 ± 0.16 d,A | 7.36 ± 0.11 cd,A | 6.96 ± 0.31 bc | 6.60 ± 0.23 ab | 6.32 ± 0.18 a | |
| 102 | 7.71 ± 0.14 d,A | 7.71 ± 0.26 d,A | 7.26 ± 0.06 cd,A | 6.83 ± 0.20 bc | 6.54 ± 0.08 ab | 6.24 ± 0.20 a | |
| b* | 0 | 22.04 ± 0.24 d,D | 21.78 ± 0.37 cd,B | 21.42 ± 0.12 bcd,C | 21.01 ± 0.33 abc,C | 20.73 ± 0.34 ab,B | 20.24 ± 0.15 a,C |
| 18 | 21.89 ± 0.13 c,CD | 21.31 ± 0.31 c,AB | 21.09 ± 0.18 bc,C | 20.83 ± 0.37 abc,BC | 20.16 ± 0.44 ab,AB | 19.87 ± 0.54 a,BC | |
| 30 | 21.55 ± 0.25 d,BCD | 21.00 ± 0.25 d,AB | 20.83 ± 0.32 cd,BC | 20.13 ± 0.17 bc,AB | 19.75 ± 0.34 ab,A | 19.30 ± 0.24 a,AB | |
| 60 | 21.33 ± 0.06 d,ABC | 20.93 ± 0.37 cd,AB | 20.34 ± 0.30 bc,AB | 20.0 ± 0.25 b,A | 19.57 ± 0.1 ab,A | 19.16 ± 0.27 a,AB | |
| 90 | 20.99 ± 0.19 e,AB | 20.52 ± 0.24 de,A | 20.19 ± 0.18 cd,AB | 19.65 ± 0.21 bc,A | 19.41 ± 0.08 ab,A | 18.98 ± 0.22 a,AB | |
| 102 | 20.72 ± 0.31 e,A | 20.49 ± 0.17 de,A | 20.02 ± 0.21 cd,A | 19.53 ± 0.06 bc,A | 19.30 ± 0.13 ab,A | 18.87 ± 0.20 a,A |
| Sample | TBARS (mg Malonaldehyde/kg) | |||||
|---|---|---|---|---|---|---|
| Storage Time (Days) | ||||||
| 0 | 18 | 30 | 60 | 90 | 102 | |
| C | 0.20 ± 0.05 e,A | 0.25 ± 0.01 f,B | 0.30 ± 0.01 f,C | 0.36 ± 0.01 f,D | 0.50 ± 0.07 f,E | 0.66 ± 0.01 f,F |
| S0.2 | 0.18 ± 0.02 d,A | 0.22 ± 0.01 e,B | 0.23 ± 0.02 e,C | 0.27 ± 0.01 e,D | 0.35 ± 0.03 e,E | 0.50 ± 0.07 e,F |
| S0.5 | 0.18 ± 0.02 d,A | 0.19 ± 0.05 d,B | 0.21 ± 0.01 d,C | 0.25 ± 0.01 d,D | 0.30 ± 0.01 d,E | 0.41 ± 0.01 d,F |
| S1.25 | 0.13 ± 0.01 c,A | 0.14 ± 0.07 c,AB | 0.15 ± 0.05 c,B | 0.17 ± 0.02 c,C | 0.21 ± 0.05 c,D | 0.27 ± 0.01 c,E |
| S2 | 0.10 ± 0.03 b,A | 0.10 ± 0.02 b,A | 0.10 ± 0.03 b,A | 0.11 ± 0.02 b,A | 0.14 ± 0.07 b,B | 0.20 ± 0.03 b,C |
| S2.3 | 0.07 ± 0.06 a,A | 0.08 ± 0.06 a,A | 0.08 ± 0.06 a,A | 0.09 ± 0.04 a,A | 0.11 ± 0.04 a,B | 0.15 ± 0.02 a,C |
| PAH Compound | R2 | LOD (ng/g) | LOQ (ng/g) | Recovery Value (%) |
|---|---|---|---|---|
| BaA | 0.9988 | 0.0001 | 0.0003 | 97.7 |
| BaP | 1.0000 | 0.0054 | 0.0181 | 97.1 |
| BbF | 0.9999 | 0.4083 | 1.3610 | 95.9 |
| Chry | 1.0000 | 0.0649 | 0.2164 | 94.2 |
| Sample | BaA | BaP | BbF | Chry | ΣPAH4 |
|---|---|---|---|---|---|
| C | 3.39 ± 0.4 c | 1.00 ± 0.03 c | 0.35 ± 0.01 c | 3.15 ± 0.22 b | 7.89 f |
| S0.2 | 3.18 ± 0.31 c | 0.99 ± 0.03 c | 0.32 ± 0.01 bc | 3.15 ± 0.2 b | 7.64 e |
| S0.5 | 3.06 ± 0.23 c | 0.94 ± 0.04 bc | 0.31 ± 0.01 b | 3.03 ± 0.17 b | 7.34 d |
| S1.25 | 2.66 ± 0.11 bc | 0.84 ± 0.04 ab | 0.29 ± 0.02 b | 2.82 ± 0.02 a | 6.61 c |
| S2 | 2.17 ± 0.1 ab | 0.76 ± 0.04 a | 0.21 ± 0.01 a | 2.36 ± 0.11 a | 5.50 b |
| S2.3 | 1.85 ± 0.06 a | 0.72 ± 0.04 a | 0.19 ± 0.01 a | 2.01 ± 0.06 a | 4.77 a |
| Sample | |||||||
|---|---|---|---|---|---|---|---|
| Storage (Days) | C | S0.2 | S0.5 | S1.25 | S2 | S2.3 | |
| Appearance | 0 | 8.60 ± 0.16 B | 8.60 ± 0.16 C | 8.50 ± 0.17 C | 8.40 ± 0.22 B | 8.30 ± 0.15 B | 8.20 ± 0.07 B |
| 18 | 8.50 ± 0.17 B | 8.50 ± 0.17 C | 8.40 ± 0.16 C | 8.30 ± 0.20 B | 8.20 ± 0.13 B | 8.10 ± 0.10 B | |
| 30 | 8.30 ± 0.20 B | 8.30 ± 0.20 C | 8.20 ± 0.29 C | 8.10 ± 0.35 B | 8.00 ± 0.15 B | 8.00 ± 0.21 B | |
| 60 | 8.10 ± 0.21 B | 8.00 ± 0.15 C | 8.00 ± 0.15 BC | 8.00 ± 0.26 B | 7.90 ± 0.18 B | 7.90 ± 0.17 AB | |
| 90 | 7.30 ± 0.30 A | 7.30 ± 0.26 B | 7.40 ± 0.27 AB | 7.80 ± 0.43 B | 7.80 ± 0.42 B | 7.80 ± 0.43 AB | |
| 102 | 6.70 ± 0.21 A | 6.70 ± 0.21 A | 6.80 ± 0.29 A | 7.00 ± 0.30 A | 7.20 ± 0.29 A | 7.30 ± 0.40 A | |
| Color | 0 | 8.50 ± 0.17 C | 8.40 ± 0.16 C | 8.40 ± 0.16 B | 8.30 ± 0.21 B | 8.20 ± 0.25 | 8.20 ± 0.24 |
| 18 | 8.40 ± 0.16 C | 8.30 ± 0.21 C | 8.20 ± 0.36 B | 8.10 ± 0.31 B | 8.10 ± 0.28 | 8.00 ± 0.21 | |
| 30 | 8.20 ± 0.25 C | 8.20 ± 0.20 BC | 8.10 ± 0.23 B | 8.00 ± 0.26 B | 8.00 ± 0.33 | 8.00 ± 0.26 | |
| 60 | 7.90 ± 0.18 BC | 7.90 ± 0.28 BC | 7.90 ± 0.31 B | 7.80 ± 0.21 B | 7.80 ± 0.23 | 7.80 ± 0.28 | |
| 90 | 7.50 ± 0.17 B | 7.60 ± 0.22 B | 7.60 ± 0.26 B | 7.80 ± 0.13 A | 7.80 ± 0.13 | 7.80 ± 0.36 | |
| 102 | 6.70 ± 0.26 A | 6.70 ± 0.25 A | 6.80 ± 0.26 A | 7.10 ± 0.23 A | 7.50 ± 0.22 | 7.50 ± 0.31 | |
| Texture | 0 | 8.50 ± 0.17 C | 8.60 ± 0.16 D | 8.60 ± 0.16 C | 8.70 ± 0.15 C | 8.80 ± 0.13 | 8.90 ± 0.10 |
| 18 | 8.30 ± 0.26 C | 8.40 ± 0.22 CD | 8.50 ± 0.17 C | 8.60 ± 0.16 C | 8.70 ± 0.15 | 8.80 ± 0.13 | |
| 30 | 8.00 ± 0.21 a,C | 8.20 ± 0.20 ab,CD | 8.30 ± 0.26 ab,C | 8.50 ± 0.22 ab,C | 8.60 ± 0.16 ab | 8.70 ± 0.21 b | |
| 60 | 7.70 ± 0.21 a,BC | 7.80 ± 0.33 ab,BC | 8.00 ± 0.30 abc,BC | 8.30 ± 0.15 abc,BC | 8.50 ± 0.17 bc | 8.60 ± 0.16 c | |
| 90 | 7.20 ± 0.33 a,B | 7.30 ± 0.15 a,B | 7.40 ± 0.31 a,B | 7.90 ± 0.18 ab,B | 8.20 ± 0.20 b | 8.30 ± 0.26 b | |
| 102 | 6.40 ± 0.34 a,A | 6.50 ± 0.27 a,A | 6.70 ± 0.21 a,A | 7.00 ± 0.21 ab,A | 7.20 ± 0.55 b | 7.30 ± 0.34 b | |
| Flavor | 0 | 8.50 ± 0.17 C | 8.50 ± 0.16 C | 8.60 ± 0.16 C | 8.70 ± 0.15 C | 8.70 ± 0.15 | 8.90 ± 0.10 |
| 18 | 8.30 ± 0.21 C | 8.30 ± 0.18 C | 8.40 ± 0.16 C | 8.60 ± 0.16 BC | 8.70 ± 0.15 | 8.80 ± 0.13 | |
| 30 | 8.10 ± 0.23 C | 8.10 ± 0.23 C | 8.10 ± 0.27 BC | 8.40 ± 0.22 BC | 8.60 ± 0.16 | 8.70 ± 0.15 | |
| 60 | 7.80 ± 0.25 a,BC | 7.80 ± 0.24 a,BC | 8 ± 0.26 ab,BC | 8.30 ± 0.26 ab,BC | 8.50 ± 0.17 ab | 8.60 ± 0.16 b | |
| 90 | 7.20 ± 0.20 a,AB | 7.30 ± 0.21 a,AB | 7.40 ± 0.22 a,AB | 8.00 ± 0.15 b,B | 8.30 ± 0.15 b | 8.40 ± 0.22 b | |
| 102 | 6.50 ± 0.45 a,A | 6.60 ± 0.40 a,A | 6.70 ± 0.45 a,A | 7.00 ± 0.21 b,A | 7.20 ± 0.2 b | 7.30 ± 0.26 b | |
| Overall Acceptance | 0 | 8.60 ± 0.16 D | 8.60 ± 0.17 C | 8.70 ± 0.15 C | 8.70 ± 0.16 C | 8.70 ± 0.12 B | 8.80 ± 0.13 B |
| 18 | 8.40 ± 0.22 CD | 8.40 ± 0.16 C | 8.60 ± 0.16 C | 8.60 ± 0.15 C | 8.70 ± 0.16 B | 8.70 ± 0.15 B | |
| 30 | 8.20 ± 0.20 CD | 8.30 ± 0.21 C | 8.30 ± 0.26 C | 8.40 ± 0.22 BC | 8.60 ± 0.16 B | 8.70 ± 0.15 B | |
| 60 | 7.90 ± 0.18 a,C | 7.90 ± 0.28 a,BC | 8.10 ± 0.23 ab,BC | 8.30 ± 0.21 ab,BC | 8.50 ± 0.17 ab,B | 8.60 ± 0.16 b,B | |
| 90 | 7.20 ± 0.20 a,B | 7.30 ± 0.21 ab,B | 7.40 ± 0.27 bc,AB | 7.90 ± 0.10 bc,B | 8.30 ± 0.15 c,B | 8.40 ± 0.27 c,B | |
| 102 | 6.50 ± 0.31 a,A | 6.60 ± 0.37 a,A | 6.80 ± 0.44 bc,A | 7.00 ± 0.26 bc,A | 7.20 ± 0.25 c,A | 7.30 ± 0.30 c,A | |
| Source | df | L* p-Value | a* p-Value | b* p-Value | Flavor p-Value | Overall Acceptance p-Value |
|---|---|---|---|---|---|---|
| Mean vs. total | 1 | |||||
| Linear vs. mean | 2 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 2FI vs. linear | 1 | 0.6236 | 0.7705 | 0.2245 | 0.7418 | 0.1724 |
| Quadratic vs. 2FI | 2 | 0.0682 | 0.4273 | 0.4760 | 0.1306 | 0.0136 |
| Cubic vs. quadratic | 2 | 0.0370 | 0.0160 | 0.0635 | 0.6539 | 0.3223 |
| Residual | 5 | |||||
| Total | 13 | |||||
| Lack-of-fit test | ||||||
| Linear | 6 | 0.0468 | 0.1057 | 0.1173 | 0.1048 | 0.8665 |
| 2FI | 5 | 0.0371 | 0.0826 | 0.1235 | 0.0823 | 0.8807 |
| Quadratic | 3 | 0.0757 | 0.0628 | 0.0906 | 0.9083 | 0.9012 |
| Cubic | 1 | 0.3478 | 0.8602 | 0.2516 | 0.0418 | 0.9185 |
| Pure error | 4 |
| Source | df | L* | df | a* | df | b* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |||||||
| Model | 3 | 53.28 | <0.0001 | Significant | 2 | 108.33 | <0.0001 | Significant | 2 | 45.74 | <0.0001 | Significant |
| A—S. platensis powder ratio (%) | 1 | 98.65 | <0.0001 | 1 | 193.23 | <0.0001 | 1 | 68.27 | <0.0001 | |||
| B—Storage time (day) | 1 | 52.23 | <0.0001 | 1 | 23.43 | 0.0007 | 1 | 23.20 | <0.0001 | |||
| Residual | 9 | 10 | 10 | |||||||||
| Lack of fit | 5 | 3.46 | 0.1263 | Not significant | 6 | 3.87 | 0.1057 | Not significant | 6 | 3.61 | 0.1173 | Not significant |
| Pure error | 4 | 4 | 4 | |||||||||
| Cor total | 12 | 12 | 12 | |||||||||
| R2 | 0.9467 | 0.9559 | 0.9015 | |||||||||
| Adj-R2 | 0.9289 | 0.9471 | 0.8817 | |||||||||
| C.V. (%) | 0.4056 | 1.25 | 0.9710 | |||||||||
| Source | df | Flavor | Df | Overall Acceptance | ||||
|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | |||||
| Model | 2 | 46.57 | <0.0001 | Significant | 3 | 49.49 | <0.0001 | Significant |
| A—S. platensis powder ratio (%) | 1 | 22.61 | 0.0008 | 1 | 58.44 | <0.0001 | ||
| B—Storage time (day) | 1 | 70.52 | <0.0001 | 1 | 81.51 | <0.0001 | ||
| Residual | 10 | 9 | ||||||
| Lack of fit | 6 | 3.89 | 0.1048 | Not significant | 5 | 2.10 | 0.2461 | Not significant |
| Pure error | 4 | 4 | ||||||
| Cor total | 12 | 12 | ||||||
| R2 | 0.9030 | 0.9428 | ||||||
| Adj-R2 | 0.8836 | 0.9238 | ||||||
| C.V. (%) | 1.90 | 1.43 | ||||||
| Response | Predicted Value | Mean Experimental Value | Mean Difference | p-Value |
|---|---|---|---|---|
| L* value | 39.96 | 39.97 ± 0.11 | 0.005 | 0.923 |
| a* value | 7.11 | 7.13 ± 0.03 | 0.023 | 0.209 |
| b* value | 20.01 | 19.98 ± 0.13 | −0.300 | 0.644 |
| Flavor | 7.39 | 7.38 ± 0.08 | −0.005 | 0.893 |
| Overall Acceptance | 7.63 | 7.64 ± 0.11 | 0.012 | 0.824 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elikucuk, Y.; Yildiz Turp, G. Optimization of Spirulina platensis Incorporation in Coated Beef Meatballs: Impact on Quality Characteristics and Polycyclic Aromatic Hydrocarbon (PAH) Formation. Processes 2025, 13, 2031. https://doi.org/10.3390/pr13072031
Elikucuk Y, Yildiz Turp G. Optimization of Spirulina platensis Incorporation in Coated Beef Meatballs: Impact on Quality Characteristics and Polycyclic Aromatic Hydrocarbon (PAH) Formation. Processes. 2025; 13(7):2031. https://doi.org/10.3390/pr13072031
Chicago/Turabian StyleElikucuk, Yagmur, and Gulen Yildiz Turp. 2025. "Optimization of Spirulina platensis Incorporation in Coated Beef Meatballs: Impact on Quality Characteristics and Polycyclic Aromatic Hydrocarbon (PAH) Formation" Processes 13, no. 7: 2031. https://doi.org/10.3390/pr13072031
APA StyleElikucuk, Y., & Yildiz Turp, G. (2025). Optimization of Spirulina platensis Incorporation in Coated Beef Meatballs: Impact on Quality Characteristics and Polycyclic Aromatic Hydrocarbon (PAH) Formation. Processes, 13(7), 2031. https://doi.org/10.3390/pr13072031






