Abstract
Eco-friendly packaging for functional foods aims to reduce environmental impact while maintaining product integrity and ensuring consumer safety. Both the food industry and consumers must transition toward packaging solutions that are sustainable, biodegradable, and non-toxic. Among the key benefits of using environmentally friendly materials for functional food packaging are their sustainability, growing consumer preference, and regulatory compliance. Functional foods are products that offer health benefits beyond basic nutrition, such as enhancing immunity, improving digestion, or promoting overall well-being. This review emphasizes that the packaging of functional foods using an eco-friendly design approach is aligned with the Sustainable Development Goals and the consumers’ preferences. It included the definition and regulatory framework of functional foods, the bioactivity and health effects of bioactive compounds/microorganisms, packaging requirements and solutions for functional foods, as well as conventional and innovative analytical techniques for bioactive compound analysis. Eco-friendly packaging for functional foods is environmentally vital for waste reduction, socially crucial for meeting conscious demand, and economically sound for driving sustainable innovation and green markets.
1. Introduction
Functional foods have garnered significant attention due to their potential health benefits beyond basic nutrition. These products contain bioactive compounds or beneficial microorganisms that contribute to improved health and disease prevention [1]. Bioactive compounds encompass a wide range of naturally occurring substances with physiological effects on the human body [2]. Some of the most important categories include polyphenols, omega-3 PUFAs, phytosterols, functional peptides, and pro-, pre-, post-, and synbiotics. However, consumer acceptance remains a key factor in the success of functional foods. Understanding consumer preferences, perceptions, and trust in these products is essential for their widespread adoption [3].
To ensure the efficacy and safety of functional foods, it is imperative to implement comprehensive quality control measures [4]. These procedures primarily focus on the identification of bioactive compounds/microorganisms and the determination of their concentrations within the functional food matrix, as variations in their concentration or chemical/biological nature can compromise the food’s effectiveness and, in some cases, result in adverse effects on the consumer [5]. Given the substantial diversity of these bioactive compounds/microorganisms, a broad spectrum of analytical techniques is available for their quantification and characterization. Among the most widely employed are separative techniques, such as HPLC and GC, with various types of detectors [5]. Additionally, for living organisms, culture-based techniques are routinely used [6]. In addition to the conventional techniques used in the quality control of these foods, ongoing research is exploring the potential of novel analytical tools that may offer rapid, cost-effective, and environmentally sustainable alternatives, thereby advancing the field of functional food quality control.
To preserve the quality of food from production to consumption, packaging plays a crucial role. Particularly during distribution, storage, retail, and even final processing—such as cooking or preparation—the packed food is exposed to surrounding environmental conditions, including temperature, humidity, oxygen concentration, pH, solar radiation, and mechanical stress from handling. Then, in order to protect food integrity and quality, the appropriate packaging must be specifically tailored to meet their particular requirements [7,8]. This has a direct impact on food security by reducing food losses, thereby contributing to the sustainability of food production. In this context, the sustainability of the packaging itself is of fundamental importance.
Eco-friendly packaging is specifically designed to minimize environmental impact [9,10,11]. All aspects involved in its production and its effects on packaging performance must be carefully considered. One of the most critical factors is the choice of material, which must be safe for food contact and comply with current regulations. Moreover, it should ideally be biodegradable, compostable, recyclable, or derived from renewable sources. Although there are still significant challenges to widespread adoption, biodegradable materials are generally preferred; otherwise, the end-of-life disposal of packaging becomes a major environmental concern [12,13,14].
In addition, innovative packaging incorporates materials with improved gas barrier properties, as well as active and intelligent packaging systems. Active packaging technologies go beyond basic protective functions by interacting with the food to extend shelf life and even enhance product quality. Meanwhile, intelligent packaging provides real-time information about the food quality [15]. Combining eco-friendly materials with innovative packaging technologies is challenging, yet it holds great promise for improving sustainability. Edible active packaging with bioactive compounds is an example of research and real-life application of this combination [16,17,18,19]. Furthermore, the profile of functional food consumers is likely to align with environmentally friendly packaging solutions.
The integration of bioactive compounds, sustainable materials, and smart packaging technologies in functional foods not only enhances health benefits but also promotes environmental responsibility. The future of food packaging lies in the combination of innovation, consumer education, and sustainability efforts to foster an eco-friendlier food industry.
2. Functional Foods
2.1. Definition and Regulatory Framework
The concept of functional foods has been widely discussed in scientific literature; however, there is no universally accepted definition. Despite attempts to regulate these foods, a clear distinction between functional foods, nutraceuticals, and dietary supplements remains unresolved. Generally, functional foods contain ingredients that provide health benefits beyond basic nutrition.
The term “functional food” was first introduced in Japan in 1984, when the government established the category “Food for Specific Health Uses.” This initiative provided a legal framework for foods with proven physiological benefits. The United States followed in the 1990s, implementing regulations for health claims, although no formal definition for functional foods was provided. European countries adopted the concept over a decade later, with Regulation (EU) No. 1924/2006 addressing nutritional and health claims but not defining functional foods explicitly [20].
According to this, diverse definitions for functional foods have been proposed. The FAO defines them as foods containing compounds/microorganisms beyond basic nutrition that may be beneficial to health. Similarly, the Mayo Clinic describes them as foods with potentially positive health effects beyond fundamental nutrition. The Functional Food Center proposed a definition in 2018, describing them as natural or processed foods containing biologically active compounds in effective and non-toxic amounts, offering clinically proven health benefits [21].
A key issue in defining functional foods is their broad classification. Some definitions include commonly consumed healthy foods such as whole grains, nuts, and fish, leading to confusion. Moreover, the term “beyond basic nutrition” remains ambiguous, as many bioactive compounds contribute both to basic nutrition and additional health benefits.
The absence of a standardized definition has resulted in a fragmented regulatory landscape, particularly in the European Union. While multiple regulations apply to functional foods, a dedicated legal framework is lacking. This regulatory gap discourages investment in research and development, as companies fear operating in a legal grey area. Existing regulations, such as Regulation (EU) No. 1924/2006 and No. 353/2008, address health claims but impose strict criteria that are difficult to meet due to the complexity of food matrices and the challenge of demonstrating clear cause–effect relationships [20].
Functional foods influence physiological processes at the gastrointestinal level by enhancing biochemical parameters and improving neuronal functions. They contribute to immune system optimization, reducing susceptibility to infections and chronic diseases. Health claims for functional foods include strengthening immune system function, reducing oxidative stress and cardiovascular disease risk, managing blood glucose levels, supporting bone and skin health, and enhancing cognitive and nervous system function. Despite their potential benefits, demonstrating these effects is challenging. Many claims are rejected due to insufficient scientific evidence, reinforcing the need for standardized evaluation criteria.
Considering the current inconsistencies, a new definition has been suggested: functional foods are novel foods formulated to contain substances or live microorganisms with potential health benefits or disease-preventing properties at concentrations that are both safe and effective. This definition emphasizes that functional foods are distinct from conventional foods and include added bioactive compounds.
The concept of functional foods continues to evolve, but the lack of a universally accepted definition poses regulatory and market challenges. While these foods offer promising health benefits, standardization in definition and regulation is crucial for advancing their development. A harmonized framework would facilitate scientific validation, consumer trust, and industry growth, ensuring that functional foods fulfill their potential in promoting health and preventing disease.
2.2. Global Market and Consumer Acceptability
According to a report published by Markets and Markets TM, it is estimated that the global market of functional foods will grow at a compound annual growth rate of 6.8% from 2024 to 2029, reaching 165.8 billion dollars [22]. This report emphasizes that the notable growth of this market is primarily related to the desire of the population worldwide to consume healthier foods and the current lifestyle that favors the occurrence of some diseases. These are the main reasons that lead consumers to purchase functional foods. According to Baker et al. [23], the key characteristics of functional foods that most influence consumers’ purchasing decisions include health benefits, organoleptic properties, and price.
The health benefits of a functional food must be accurately reported on its label, as they are an essential factor in consumer acceptance. In this sense, accurately communicated health benefit information can enhance purchase intention [24]. Resistance to buying functional foods is typically linked to a lack of knowledge about their health benefits [25]. It is important to ensure that the information provided to consumers is accessible, such as using common names for bioactive compounds instead of scientific terms, to encourage the acceptance of functional foods [26,27]. The benefits of functional foods can be related to a reduction in both physiological and psychological diseases. However, some authors suggest that consumers tend to prefer functional foods that prevent or reduce the risk of physiological diseases, such as cancer, hypertension, or osteoporosis, over those that help alleviate fatigue or stress [28,29].
The organoleptic properties of functional foods play a crucial role in consumer acceptance, with flavor—encompassing both taste and aroma—being the primary determinant. Numerous statistical studies in the literature, based on consumer surveys from various countries, highlight the importance of functional foods being not only beneficial to health but also sensorially attractive [30,31,32,33,34,35].
Many consumers are willing to pay a premium for functional food, provided it is organoleptically appealing, safe, and reliable. This demands that the food industry adopt more efficient processing methods that produce equilibrated functional foods regarding their health benefits, sensory and safety properties, and cost (Figure 1).

Figure 1.
Equilibrated functional foods.
3. Bioactive Compounds/Microorganisms
3.1. Definition
As with the term “functional food,” there is still no consensus about the definition of “bioactive compound.” In scientific literature, different definitions have been proposed. In 1994, Kitts defined them as “extra nutritional” constituents naturally present in small quantities in the food matrix, produced upon either in vivo or industrial enzymatic digestion, the latter being a result of food-processing activities [36]. Another broader definition was introduced in 2006 by the Agriculture and Agri-Food Canada, defining bioactive compounds as “naturally occurring chemical compounds present in, or derived from a plant, animal, or marine source, that exert the desired health/wellness benefit” [37]. Additionally, at the 23rd Hohenheim Consensus Meeting in 2009, a group of specialized scientists arrived at an agreed definition of these bioactive substances: “essential and nonessential compounds (i.e., proteins vitamins or polyphenols) that occur in nature, are part of the food chain, and can be shown to have an effect on human health beyond the basic nutritional value of the product” [38]. The concept of “extra nutritional” or “beyond the basic nutritional value” suggests that nutrients, both micro- and macro-nutrients (i.e., proteins, lipids, carbohydrates, vitamins, and minerals), are excluded from this category. Nonetheless, recent research has revealed new physiological functions and preventive/therapeutic actions of specific groups of nutrients that have gained popularity as food ingredients intended to increase their biofunctionality. For instance, prebiotics are considered bioactive compounds, while most of them are classified as dietary fiber, a class of carbohydrate. This is the case with omega-3 PUFAs and phytosterols, which are included in the lipid classification.
“Bioactive microorganisms,” such as probiotics, fall outside the bioactive compounds category since they cannot be classified as compounds or substances. However, they are vastly used as food ingredients with the aim of exerting a positive effect on human health upon consumption. Therefore, they are included in the present review as bioactive ingredients for food formulations due to their gained popularity and wide acceptance in recent times.
In this context, the following bioactive compounds and microorganisms are considered in the present review:
- Polyphenols;
- Omega-3 PUFAs;
- Phytosterols;
- Pro-, pre-, post-, and symbiotics;
- Bioactive peptides.
3.2. Bioactivity and Health Effects of Bioactive Compounds
3.2.1. Polyphenols
Polyphenols are a group of numerous chemical compounds present in plants as secondary metabolites whose main action is linked to plant stress regulation. Chemically, they have a common structure of one or more aromatic rings with a single or multiple bound hydroxyl groups [39]. Most polyphenols in nature fall under the flavonoid category (flavonols, flavanols, flavones, flavanones, isoflavones, and anthocyanins), while the remaining phenolic compounds can be classified as non-flavonoid compounds (phenolic acids, hydroxycinnamic acids, lignans, stilbenes, and tannins) [40].
They are generally recognized for their antioxidant potential. During aerobic cellular metabolism, oxygen reactive molecules known as “free radicals” or ROS are generated. These molecules can cause permanent damage to cells, DNA, enzymes, and cell membranes [41]. This deleterious process can also occur due to exogenous ROS generated by smoking, UV radiation, and ionizing radiation. Cells have their own antioxidant defense system. Under normal physiological conditions, there is a balance between endogenous oxidants and antioxidants [42]. When this balance is disrupted, either by excessive generation of oxidants or a reduction in antioxidants, what is known as “oxidative stress” or “oxidative damage” occurs. Numerous studies have shown that oxidative stress is directly related to various degenerative diseases, such as cardiovascular diseases, cancer, diabetes, inflammatory disorders, and infectious illnesses [43]. Additionally, in 1956, Harman proposed the free radical theory of aging, which suggests that a dysfunction of the regulatory system would induce oxidative damage to biomolecules, which, in their accumulation, would be responsible for the aging process [44]. Like humans, and being aerobic organisms, plants also have mechanisms to control ROS levels and protect cells from oxidative stress. One of these mechanisms is the above-mentioned process of polyphenols’ synthesis.
In addition to their antioxidant action, various therapeutic effects have been attributed to polyphenols, such as cytotoxicity and anti-inflammatory, antihypertensive, and antidiabetic actions [20].
The antioxidant properties of polyphenols are responsible for their protective action; however, these same properties make them sensitive to light, heat, and oxygen. Thus, it is crucial to maintain conditions that minimize their degradation and ensure the claimed bioactivity throughout the entire production and distribution chain of functional foods.
3.2.2. Omega-3 PUFAs
Among omega-fatty acids, the omega-6 and omega-3 families are regarded as essential fatty acids since they are not synthesized by animals. The ratio of the most consumed omega-6 PUFA—linoleic acid (18:2ω-6)—to the omega-3 counterpart—α-linolenic acid (18:3ω-3)—in the typical Western diet is 5 to 15 [45]. The most studied metabolic product of linoleic acid is arachidonic acid (20:4ω-6), while for α-linolenic acid, there are two main fatty acids: EPA (20:5ω-3) and DHA (22:6ω-3). The metabolic pathways of linoleic acid and α-linolenic acid conversion to these long-chain fatty acids share the same enzymatic system, giving birth to a competition between linoleic acid and α-linolenic acid [25]. Given the imbalance in omega-3/omega-6 ratio consumption, the synthesis of EPA and DHA from α-linolenic acid is limited in most humans. And this feature accounts for the importance of dietary supplementation with omega-3 PUFAs. Their health effect is mostly attributed to their anti-inflammatory [46] and antioxidant action [47]. The preventive and therapeutic effects of omega-3 PUFAs on various diseases have been studied, i.e., cardiovascular diseases [48], mental health [49], and the immune system [50], among others. As mentioned, α-linolenic acid is found exclusively in plants, with green plant tissues, walnuts, and seeds of flax, chia, rapeseed, and soybean being good sources [51]. On the other hand, EPA and DHA are found in the oils of fish, particularly fatty fish [52], and in micro- and macro-algae [32,53]. The various double bonds found in omega-3 fatty acids make them prone to oxidation; therefore, packaging that provides an effective barrier against light and oxygen is highly advisable.
3.2.3. Phytosterols
Phytosterols are classified as lipids. They are sterols and stanols, which are present in plants. Each species has its own phytosterol profile, with more than 250 compounds recognized so far [54]. The most important pharmacological property of phytosterols is the potential to reduce total and low-density lipoprotein cholesterol levels, which is directly related to a lower risk of cardiovascular diseases [55]. Furthermore, they are also identified as molecules with the potential to modulate inflammation and the immune system and to exert antioxidant, anticancer, antiulcer, antibacterial, anti-obesity, antidiabetic, anti-microbial, and antifungal effects [54,55,56]. Moreover, evidence has attributed to phytosterols the potential to intervene in wound healing promotion and platelet aggregation inhibition. The richest sources of phytosterols in plants are unrefined oils from seeds and fruits [57,58]. In particular, the richest sources are rapeseed oil, wheat germ oil, and corn oil, while pistachio is the oily fruit containing the highest concentration of phytosterols [59]. Other good dietary sources of phytosterols are nuts, cereals, and legumes [56,60].
Phytosterols’ chemical structure includes a double bond, which is prone to oxidation when exposed to external factors such as heat, light, and oxygen [61].
3.2.4. Pro-, Pre-, Syn-, and Postbiotics
The worldwide widely accepted definition of probiotics is the one given in 2013 by an expert panel convened by the International Scientific Association for Probiotics and Prebiotics, which states that a probiotic is “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [62]. It is important to state that not all microorganisms present in foods can be called probiotics. This term is reserved to characterize strains with a scientifically demonstrated effect on health [63]. The most common probiotics include Lactobacillus spp., Bifidobacterium spp., Streptococcus spp., Enterococcus spp., and Saccharomyces boulardii [64]. Within the field of functional foods, the segment of probiotic foods became the one with the fastest-growing rate [65]. In recent years, there has been a growing interest in gut microbiota and the link between this specific microbiome and health. Research has indicated that various diseases, such as inflammatory, immune, and nervous system-related ones, are linked to an imbalance in the gut’s bacterial composition, known as dysbiosis. One of the factors influencing the population of these microorganisms is diet [66]. Dietary interventions may help modulate the composition of microbial communities, having a direct impact on health, helping to prevent and treat diseases [67,68]. Probiotics can be isolated from three main sources in nature: (1) humans and animals, (2) foods, and (3) the environment [69].
Prebiotics are defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [63]. Some types of soluble fiber, such as FOS, GOS, and inulin, cannot be digested by human enzymes in the small intestine, so they reach the colon and are metabolized by beneficial microbes, generating health-beneficial short-chain fatty acids [70,71]. In this manner, consumption of prebiotics also helps modulate gut microbiota. Among the sources of FOS, fruits, vegetables, and cereals like bananas, sugar beets, asparagus, garlic, onions, tomatoes, wheat, and barley can be mentioned. GOS are present mainly in human and cow milk, while inulin’s most common sources are globe artichoke, chicory, Jerusalem artichoke, and elecampane [72].
Related to these concepts of healthy microorganisms and substrates, another possible food ingredient is the group of “synbiotic” compounds. They are defined as “a mixture, comprising live microorganisms and substrate(s) selectively utilized by host microorganisms, that confers a health benefit on the host” [73]. Those substrates are usually prebiotics, although they are not limited to them.
Another term that has been recently incorporated into the biotics family is “postbiotic.” According to a panel of experts convened by the International Scientific Association for Probiotics and Prebiotics in 2019, a postbiotic is a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” [74]. In the food industry, fermentation is the most common source of postbiotics, such as bacterial lysates with cell surface proteins, bacterial enzymes and peptides, metabolites produced by bacteria such as teichoic acids, peptidoglycan-derived neuropeptides, polysaccharides, short-chain fatty acids, and lower organic acids, for instance, lactic acid [75,76].
3.2.5. Bioactive Peptides
Bioactive peptides, or biopeptides, are specific protein fractions that show a wide range of physiological actions when released from the protein by hydrolysis [77,78]. Protein breakdown can be achieved by enzymatic, chemical, or microbial methods [79]. The latter refers to microbial fermentation [80]; so, as mentioned above, in this type of process, the released peptides are categorized as postbiotics. Bioactive peptides have shown antihypertensive, antioxidant, antidiabetic, anti-inflammatory, antimicrobial, and anticoagulant effects [77,78,81], reducing the risk of non-communicable diseases and improving consumer health. Several foods of animal and plant origin are a source of biopeptides: milk, dairy products, nuts, cereal germ, soybeans, fermented products, eggs, marine organisms, edible insects, and meat proteins (e.g., beef, chicken, pork, and fish) [76,77,78,82,83,84].
A compilation of recent scientific studies on functional food products is shown in Table 1.

Table 1.
Summary of some recent publications on functional foods and their bioactive compounds/microorganisms.
3.3. Determination and Quantification of Bioactive Compounds/Microorganisms
The growing interest in functional foods has driven the implementation of various analytical techniques for both qualitative and quantitative analysis. Traditional analytical techniques have long served as the foundation for identifying and quantifying these compounds. However, advancements in analytical chemistry now provide a range of innovative alternatives, including improved sample preparation, advanced separation techniques, and the development of environmentally friendly methods. This knowledge allows evaluating the packaging performance to preserve bioactive compounds/microorganisms’ function. Moreover, in the case of active packaging, analytical assays are used to test the release kinetics of the active agents and their concentration within the food matrix. This section aims to provide an overview of recent advances in analytical tools and their contributions to the determination and quantification of bioactive compounds/microorganisms that ensure food functionality. Figure 2 summarizes the most employed analytical techniques for the qualitative and quantitative analysis of bioactive compounds and microorganisms.

Figure 2.
Analytical techniques for the qualitative and quantitative analysis of bioactive compounds and microorganisms.
3.3.1. Polyphenols
Polyphenols are bioactive compounds present in many foods, known for their antioxidant properties and health benefits. Accurate quantification of polyphenols is crucial for assessing the nutritional and functional quality of foods. Both the total amount of polyphenols and the identification of individual polyphenols present in functional foods are of interest. For this reason, various methods have been developed to evaluate total polyphenol content, as well as others that allow for their identification and quantification. Typically, the total amount of polyphenols is determined by the Folin–Ciocalteu method [138]. This method is based on a redox reaction between polyphenols and the Folin–Ciocalteu reagent, which produces an intense blue complex whose absorbance can be measured spectrophotometrically. Although the Folin–Ciocalteu method is useful for a quick and general estimate of polyphenol content, the spectrometric response is highly affected by non-phenolic interferences [139]. Thus, other spectroscopic techniques have emerged to provide valuable insights into polyphenol content, their structural properties, and concentrations within a given sample [140]. Among these techniques, infrared (IR) spectroscopy is a non-destructive, rapid, and simple analytical method used to estimate the total polyphenol content in food samples. When combined with chemometrics, IR becomes a powerful tool for extracting meaningful information from the spectral data obtained. In this regard, MIR spectroscopy was applied to evaluate the phenolic compounds present in moringa powder [141], wines [142], and different fruits [143], while NIR spectroscopy coupled to partial least square regression was successfully applied for predicting catechins in tea [144] and total polyphenols in vine tea through a smartphone-based portable NIR device [145]. The combination of MIR and NIR techniques constitutes a superior strategy, providing complementary information for the characterization and analysis of polyphenol compounds in complex food samples [146,147].
Electrochemical techniques have gained attention in the determination of polyphenols owing to their rapid and sensitive response. Voltammetry, including cyclic voltammetry, differential pulse voltammetry, and square wave voltammetry, provides different approaches for assessing total polyphenols in various food matrices [97]. However, electrochemical biosensors, which are constituted with a biorecognition element, establish specific interaction with the target polyphenols, adding selectivity to the inherent sensitivity of these systems [148]. The output signals correspond to changes in the properties of the electroactive species by producing a redox reaction that occurs on the electrode surface. Among recognition elements, enzymes exhibit high specificity of enzyme–substrate interactions, allowing the detection of low concentrations of the target compounds. Laccase and tyrosinase are the primary enzymes used in the construction of biosensors for monitoring polyphenols in functional foods. For instance, laccase was applied to the development of a biosensor used to determine quercetin in beverages [149] and gallic acid and catechin in agri-foods [139]. Also, tyrosinase was implemented as a bio-element for the quantification of chlorogenic acid in green coffee beans [150]. An interesting approach is the use of crude polyphenol oxidase extract, avoiding the need for separation and purification of the enzymes [151]. Although there are some examples of the use of these enzymes for the specific detection of certain polyphenols, in general, they are used for the selective detection of total polyphenolic content [152].
Similarly, cell-based biosensors exploit living cells as the recognition element [153]. In this case, ROS are generated due to intracellular oxidative stress and react with catalytic species on the surface of the electrode, leading to variations in electrochemical signals [154].
On the other hand, electrochemical nanosensors, which use nanoscale materials such as metal nanoparticles, quantum dots, polymer nanoparticles, or carbon nanomaterials as sensing materials, have also been exploited to selectively detect polyphenols in food samples [140]. Among sensing materials, gold nanoparticles combined with carbon nanomaterials have been widely applied to detect different phenolic acids [155,156,157,158], stilbenes [159,160,161], and flavonoids [162,163,164,165,166]. A more complex architecture of the nanosensor includes not only nanomaterials but also enzymes. Thus, nanobiosensors were developed and applied to assess the polyphenol content [167,168].
Among the analytical techniques used to assess polyphenol content in different functional foods, the identification and individual quantification of these compounds are undoubtedly performed by chromatographic techniques [140]. In addition, complex food matrices represent a potential source of error due to matrix effects and the presence of interferences. In this regard, chromatographic methods are of great interest not only for quantifying individual polyphenolic compounds but also for establishing correlations between the results and total polyphenol content [169]. Regarding this, polyphenol profile has been determined by gas chromatography and liquid chromatography associated with different detection techniques. Concerning liquid chromatography, HPLC with a photodiode array detector is widely used for the development of various methods aimed at identifying polyphenols in functional foods and determining their concentration [170,171,172,173,174]. Commonly, HPLC is coupled with MS to provide additional information about the structure and molecular mass, offering compositional fingerprints of the polyphenols present in foods. Another interesting advantage is that both targeted and untargeted determination of polyphenols is possible, allowing the screening and identification of unknown compounds [175]. In this sense, HPLC-MS and UHPLC-MS were applied to the determination of phenolic acids and flavonoids in apple pomace [176], wild and cultivated grapes [177], and tea-macerated wines [178], among others. As some polyphenols can be found at trace concentration levels, MS/MS offers a detailed examination of specific ions, leading to more accurate results. In this regard, LC-MS/MS has been used for the identification of polyphenols in prickly pear and in a gluten-free pasta made from rice and field bean flours enriched with dehydrated prickly pear fruit [179]. Also, the polyphenolic composition of raspberry leaf, a by-product of raspberry production, was assessed by using LC-MS/MS [180]. For some polyphenols that are unstable and highly reactive as o-quinone compounds, derivatization prior to their quantification by LC is necessary to guarantee their direct, sensitive, and reliable determination [181].
Regarding gas chromatography, the combination with GC-MS is the most widely employed technique for determining polyphenols in functional foods. However, GC-MS has certain limitations related to thermal stability and the possible interactions of the hydroxyl groups of the phenolic compounds and the silanol groups of the stationary phase. Thus, a derivatization step to reduce their polarity is frequently required [175,182,183].
In conclusion, the assessment of polyphenols in functional foods can be performed using spectroscopic, electrochemical, and chromatographic techniques, either for evaluating total polyphenol content or for identifying and quantifying them.
3.3.2. Omega-3 Fatty Acids
The omega-3 (n-3) PUFAs, such as ALA, EPA, and DHA, are widely recognized for their protective effects against various metabolic disorders, promoting human health [36]. Thus, the identification and quantification of omega-3 PUFAs, as well as the quality assurance of the functional foods containing them, are highly required. Consequently, different methods using diverse analytical techniques, such as GC, HPLC, and IR spectroscopy, have been developed.
GC has been extensively reported for separating and determining omega-3 PUFAs [184]. Before analysis, it is necessary to convert these compounds into fatty acid methyl esters to make them volatile enough for GC separation in order to obtain accurate results. Typically, GC is coupled to a flame ionization detector to determine the omega-3 PUFAs in fish [185,186,187], crabs [188], matcha tea [189], and seeds [190].
GC coupled to MS spectrometry has also become a widely used technique for the confirmation and quantification of omega-3 PUFAs using selective ion monitoring of their characteristic ion fragments. As a result, GC-MS spectrometry provides a highly sensitive determination of the target compounds, detailed information about the molecular structure, and reduced possible signal interferences [191]. For instance, the monitoring of the oxidation of different edible oils containing a high proportion of omega-3 PUFAs during simulated conditions of transport, storage, and consumption was possible by performing the acquisition of the mass spectra and the comparison with the NIST Mass Spectral Library (National Institute of Standards and Technology, Gaithersburg, MD, USA) [192].
Although GC-FID and GC-MS are considered standard techniques for analyzing omega-3 PUFAs, the extraction and derivatization procedures involved are often labor-intensive and time-consuming. Moreover, the high temperatures used during injections and separation can compromise the stability of the analytes. Thus, HPLC-MS is preferred since a derivatization step is avoided [193,194].
Molecular spectroscopic techniques offer straightforward, rapid, and reliable analytical solutions for studying functional foods rich in omega-3 PUFAs, as they can extract extensive information from molecular spectra. In this regard, NIR, MIR, and Raman spectroscopies, when coupled with multivariate data analysis, are commonly employed [195]. Alotaibi et al. [196] review different spectroscopic methods developed for the analysis of omega-3 PUFAs in cocoa butter. Also, to ensure the quality and authenticity of virgin olive oil, omega-3 PUFAs can be detected using NIR, MIR, and Raman techniques through fingerprinting and profiling studies [197]. In addition, NIR and MIR spectroscopy associated with appropriate chemometric multivariate calibration approaches have been effectively utilized to determine the fatty acid content in fish oil samples [154]. Indeed, a portable device based on NIR spectroscopy, combined with multivariate calibration, was used to determine omega-3 PUFAs and obtain the fatty acid profile of fish oils in the fish by-product industry in a rapid and non-destructive manner [198].
3.3.3. Phytosterols
Various foods containing phytosterols, such as oils, mayonnaise, and flours, have been developed. However, these compounds exhibit low solubility [199]. These bioactive molecules are found within complex matrices, including fruits, vegetables, nuts, seeds, cereals, and legumes. Regular consumption of these sterols has been shown to reduce total cholesterol levels, primarily by lowering low-density lipoprotein cholesterol, thereby helping to prevent cardiovascular diseases [200]. Additionally, these fat-soluble bioactive compounds are used in the development of functional foods due to their ability to prevent or reduce polymer formation (antipolymerizing effect) in lipids during heat treatments [201,202]. Hence, it is crucial to develop analytical methods that enable the identification and quantification of phytosterols. These methods involve a series of operations, from extracting these compounds from the sample to obtaining accurate results.
A wide variety of phytosterols from different plant matrices are currently known, with β-sitosterol, stigmasterol, and campesterol being the most prevalent [203]. Therefore, developing analytical strategies that ensure the efficient extraction of phytosterols is essential. The literature highlights saponification as a key step in the extraction of these bioactive molecules. Saponification enhances the extraction of phytosterols, as they are typically bound to sterols or triglycerides in vegetable oils and fats. Given the complexity of plant matrices, the saponification process—which involves organic solvents such as sodium hydroxide or potassium hydroxide (alkaline saponification)—not only facilitates the release of phytosterols but also improves the purification of these bioactive compound extracts [95,204].
Recently, numerous studies have highlighted significant efforts to optimize methods, particularly in the extraction, separation, and purification of phytosterols. This growth is closely linked to the expanding phytosterols market, driven by the increasing popularity of functional foods and beverages. The global market is estimated to reach approximately 364 billion dollars in 2024 and is projected to grow to 790 billion dollars by 2032 [205]. In the case of phytosterols, an increase of 8.1% in the compound annual growth rate is expected from the period between 2024 and 2032 [206]. To achieve both qualitative and quantitative determination of phytosterols in functional foods, separation techniques such as GC [164,207,208,209], HPLC [210], or SFC [211] are commonly used. It is important to note that many of the methods employing these chromatographic techniques require a derivatization step for phytosterols. For instance, in HPLC coupled to UV detection, derivatization is often necessary because these compounds generally lack chromophore groups and exhibit low sensitivity due to their lipophilic nature [212]. Additionally, other techniques, such as NIR spectrometry, can also be used [213,214].
It is important to note that, for foods containing phytosterols, proper controls are essential to ensure the functionality of these fat-soluble molecules [215]. Amaral et al. [216] evaluated the thermogravimetric profile of goat milk cream cheese and concluded that the process should be limited to temperatures of 110 °C in order to preserve the functionality of the phytosterols.
3.3.4. Pro-, Pre-, and Postbiotics
Three major groups of compounds responsible for the beneficial properties of functional foods are probiotics, prebiotics, and postbiotics. As mentioned in the previous section, probiotics are bacterial or fungal species that are part of our intestinal microbiota and are recognized for their positive effects on health [217]. These mainly include bacteria and yeasts such as lactic acid bacteria, Bifidobacterium spp., and Saccharomyces spp. [218].
The quality of a probiotic is determined by its safety, efficacy, and stability [6]. Two main objectives in efficiency evaluation are the precise identification of the probiotic and the quantification of viable bacteria per food dose. There are molecular and non-molecular methods for quality control of probiotic foods [219]. Molecular techniques have enabled significant advances in detecting and identifying probiotic species, facilitating their taxonomic characterization. The taxonomy of probiotic organisms is established based on their biochemical, morphological, and physiological characteristics using phenotypic and genomic approaches based on molecular techniques [218].
Several reviews have addressed these methodologies in depth, such as those by Ranjan et al. [218] and Georgieva et al. [219]. Georgieva et al. [219] present graphical schemes on the commonly used steps for identifying probiotics through molecular methods, while Ranjan et al. [218] thoroughly explore approaches based on ribosomal RNA analysis, whole genome sequencing, and proteomic profiling. Numerous methods and techniques are employed for this purpose, some of which are outlined next. Molecular methods used for probiotic characterization based on ribosomal RNA include, among others, 16S rRNA sequencing, molecular ribotyping, denaturing gradient gel electrophoresis, and temperature gradient gel electrophoresis. Genomic-based techniques encompass amplified fragment length polymorphism, randomly amplified polymorphic DNA, and real-time polymerase chain reaction. Protein-based approaches involve, among others, whole-cell protein profiling (particularly via polymerase chain reaction) and MS [218].
On the other hand, there are non-molecular methods. Among them, conventional culture-based techniques allow for detecting, enumerating, and identifying probiotic strains through growth in selective/differential culture media [220]. Additionally, fluorescence-based methods have proven to be effective for assessing bacterial viability. These techniques are capable of distinguishing between viable, damaged, stressed, and dead cells using fluorescent dyes, which can be detected through fluorescence microscopy, fluorometry, flow cytometry, or fluorescence in situ hybridization [219].
Vibrational techniques are gaining relevance in probiotic quality control. FT-IR has been used for the detection, discrimination, identification, and classification of probiotics, being capable of distinguishing between viable, damaged, and dead bacteria [219]. Papadopoulou et al. [221] used FT-IR to quantitatively estimate the microbiological content of yogurt and assess its sensory status. In addition, NIR spectroscopy has also begun to be applied in this field; in fact, Aguinaga Bósquez et al. [222] employed NIR to predict the number of colony-forming units in probiotic powders.
In conclusion, quality controls in probiotics are numerous and varied, as the goal is not only to quantify a chemical compound but to characterize and evaluate living organisms. Therefore, studies must be approached from multiple perspectives to ensure the efficacy, safety, and stability of probiotic-containing functional foods.
On the other hand, prebiotics are substrates, typically indigestible carbohydrates (by the mammalian host) found in food, which selectively stimulate the growth and metabolic activity of beneficial gut microbes. These compounds are primarily utilized by microorganisms that confer health benefits to the host [223]. Beyond being considered beneficial for humans, variations in the type and quantity of prebiotics can influence their effects, and excessive intake may be detrimental. Therefore, it is crucial to implement adequate quality control for these functional foods [5]. Many compounds can be classified as prebiotics. Most studies focus on non-digestible carbohydrates, such as FOS, inulin, GOS, MOS, and XOS. However, other compounds may also exhibit prebiotic properties, such as CLA, polyphenolic compounds, and PUFAs [4]. This section will focus specifically on non-digestible carbohydrates, as they are the most extensively studied. On the other hand, polyphenolic compounds and PUFAs are addressed in the sections dedicated to antioxidants and omega-3 PUFAs, respectively. Regarding oligosaccharides, the review article by Catenza et al. [5] presents the commonly employed methods and emerging trends for the determination of the oligosaccharides FOS, GOS, MOS, and XOS in functional foods. This review highlights separation techniques such as HPLC, GC, and CE, which are often coupled with various detection systems, including RID, ELSD, PAD, and MS detection. Similarly, Corradini et al. [224] conducted a comprehensive review of analytical methods used for the screening and identification of FOS and inulin in functional foods. The authors emphasize that the most widely employed technique for characterizing these molecules is HPAEC-PED, followed by MS, particularly MALDI-TOF-MS. Additionally, Iqbal et al. [225] provide an overview of the most commonly used techniques for quality control of functional foods containing GOS. The authors describe various analytical methods and highlight that the most frequently employed techniques for GOS assessment include HPLC, in conjunction with RID, pulsed PAD, mass spectrometry sensors, and fluorescence detection.
In addition to the commonly used chromatographic techniques and NMR, vibrational techniques such as NIR and MIR have also been explored. While chromatographic techniques remain the most widely applied, methods based on MIR and NIR are gaining increasing relevance [226,227,228]. These techniques are currently under investigation to fully assess their potential for quality control in functional foods, as they are particularly valued for their simplicity, minimal or no sample preparation requirements, environmental sustainability, and suitability for real-time and in-line analysis [229].
Another important group of bioactive compounds to highlight is postbiotics, which include teichoic acids, enzymes, antimicrobial peptides, and short-chain fatty acids [230]. These compounds result from the metabolic activity of probiotic microorganisms, typically after their interaction with prebiotic compounds. Consequently, postbiotics are the substances that remain once the bacteria that produced them are no longer alive or present [231]. The qualitative and quantitative determination, as well as the characterization of these compounds, requires a multidisciplinary approach and the use of various analytical techniques based on their chemical or functional properties. In this context, recent advances in omics sciences, particularly metabolomics, offer valuable tools for discovering potential postbiotics and understanding their interactions with the host organism. Progenesis MetaScope databases are used to identify molecular weights, while the HMDB is employed to retrieve potential biomarkers in untargeted metabolomics [232]. Additionally, MetaboAnalyst 5.0 [232] enables the evaluation of metabolite changes between samples and facilitates principal component analysis. Numerous studies report on the use of liquid and gas chromatography techniques for postbiotic analysis [233,234]. Targeted metabolomics, which focuses on the study of specific metabolites, also relies on chromatographic separation techniques [235,236]. In conclusion, based on the authors’ perspective, untargeted metabolomics is currently one of the most widely used tools for postbiotic analysis.
3.3.5. Bioactive Peptides
Bioactive peptides, produced during gastrointestinal digestion or food production (enzymatic hydrolysis or fermentation), are protein segments composed of short chains of amino acids [237]. Their participation is key in the development of functional foods due to their ability to modulate physiological functions and contribute to the prevention of diseases, as mentioned above. The literature reports the importance of peptide isolation, purification, and analysis processes to optimize their extraction and thus achieve adequate sample pretreatments [238]. By virtue of improving extraction efficiency and the use of sustainable and environmentally friendly technologies such as ultrasound-assisted extraction, microwave-assisted extraction, the application of pulsed electric fields, and high-pressure processing is proposed that allow the performance and selectivity of the extracted compounds be improved while reducing energy consumption and the use of chemical solvents [239,240,241]. The most commonly used analytical techniques to determine functional peptides include chromatographic, spectroscopic, and enzyme-linked immunosorbent methods, selected according to the type of peptide and the objectives of the study [242]. Guerra-Fajardo et al. [243] report that to detect peptide bioactivities, the combination of high-performance thin-layer chromatography with bioassays in effects-directed analysis is considered an emerging technology, complementary to the most common analytical methods such as capillary electrophoresis and HPLC. Della Cerra et al. [244] propose the use of ultra-high-performance nanoliquid chromatography coupled to a mass spectrometer to determine the peptide profile of blue buffalo milk cheese, with β-casein being the predominant one. It is concluded from the above that it is crucial to take into account that bioactive peptides must be isolated and extracted from natural sources, playing a key role in the purification, quantification, and verification of their structure. Therefore, the analytical techniques used in bioactive peptides that make up functional foods are considered fundamental tools to address the concerns associated with the safety, quality, and efficacy of these molecules.
Table 2 presents examples of studies in which different bioactive molecules or microorganisms were determined in functional foods.

Table 2.
Analytical techniques used for the determination of bioactive compounds/microorganisms in functional foods.
4. Food Packaging
Packaging is an item that contains and protects products, allowing them to be transported, portioned, stored, and communicated to consumers. Packaging specifically designed and manufactured to enclose an essential product for human survival, such as food, is of great significance. Food is, indeed, one of the most consumed, transported, and commercialized goods worldwide. One of the biggest and most ancient problems with food is that, like all organic products, it spoils and deteriorates over time, leading to significant losses and waste [257]. One of the ways to slow down this harmful process is by using appropriate packaging [7,8].
Conventional packaging aims to preserve food safety and increase food shelf life by protecting it from chemical, physical, and biological contamination. Packaging is designed to diminish, and even avoid, substance spills, attacks by microorganisms, and effects of environmental factors such as temperature, moisture, oxygen, light, and physical damage [258,259]. The selection of materials and the design of the packaging must be made according to the specific requirements of the food to be contained and its economic and manufacturing viability [260].
4.1. Innovative Packaging
Innovative and functional food packaging goes beyond these functions, evolving from a passive protective role to an active one. It seeks not only to maintain food quality and increase shelf life but also to improve it [261]. Food quality encompasses its organoleptic properties, including color, aroma, flavor, and texture, as well as microbiological and chemical safety, which are essential for maintaining its stability [262]. Additionally, food quality includes its nutritional value and the health benefits associated with the presence of bioactive compounds/microorganisms [263]. In pursuit of this objective, the concepts of active, intelligent, and smart packaging have emerged [12].
Active food packaging is a dynamic system where the packaging materials, the packaged food, and its surroundings interact, being modified accordingly to maintain or improve the conditions that affect food quality. Scavenging and emitting are the main interaction mechanisms of these active systems [264]. Scavenger is applied to absorb unwanted substances or those present in harmful quantities, such as moisture, ethylene, oxygen, carbon dioxide [265], odors, flavors, and toxins. To achieve this, specific scavenging materials are incorporated into the packaging, including mineral and metal particles, metallic acids, salts and oxides [266], organic acids, activated carbon, clays, zeolites, glucose oxidase, halloysite nanotubes [267], bio-based materials [268], and natural phenolic compounds [269], among others [270]. On the other hand, emitting is applied to deliver specific actives, such as antimicrobials, antioxidants, gases, and nutraceuticals and probiotics to a lesser extent, through different release and retention strategies [271,272,273]. Antimicrobials are among the most commonly used active ingredients in active packaging and include typical food preservatives such as natamycin and benzoic acid, natural compounds such as essential oils and vegetable extracts, enzymes, clays, mineral and metal particles, and many others. The antioxidant releasers are most often of natural origin, such as essential oils, polyphenols, polyunsaturated fatty acids, vitamins, lignins, and also nanoparticles of minerals such as selenium, among others [267,272,274,275]. Antimicrobials and antioxidants are often incorporated together into packaging, and many of them, such as essential oils, have both activities [271,276]. Regarding gas release, the gases of interest include carbon dioxide, ethanol, chlorine dioxide, and volatile compounds, which are either added inside an emitter system to the packaging or generated on-site [277]. In the work of Vilela et al. [278], commercial active agents are detailed and classified. There are certain limitations on the agents added to the packaging, as they must be food-safe and within permitted concentrations. Recently, the incorporation of metal and metal oxide nanoparticles, such as zinc, silver, copper, and titanium dioxide, into food packaging materials has gained attention due to their ability to provide multifunctional benefits. These include typical active packaging functionalities, such as antimicrobial, photocatalytic, and oxygen scavenging activities, along with enhancements in mechanical, optical, and barrier properties through the formation of nanocomposite materials [279,280,281,282]. However, potential health hazards and environmental impacts remain insufficiently understood and must be carefully considered [279,283]. Among these mechanisms, there are others, such as antimicrobial packaging covalently grafted on the surface and packaging loaded with light and UV radiation blockers [284,285,286,287].
Intelligent packaging materials are designed to detect changes in packaged food and report its condition. These packaging systems sense the state of the food and/or its environmental conditions and are capable of monitoring and even tracking, recording, and reporting this information. Knowing relevant data about package integrity, food freshness, ripeness, refrigeration maintenance, or contamination enhances food security, safety, and convenience, and food losses and foodborne illnesses are reduced. Sensors, indicators, and data carriers are the main applied technologies [288]. There are chemical, electrochemical, optical, bio-based, and even edible sensors. These are small electronic devices that must be powered and integrated into the packaging, which is still a challenge. Most indicators are colorimetric labels, which change color when chemically reacting with a compound, such as moisture or pH variation, or due to the effect of temperature. Sensitive dyes or pigments of natural origin, such as anthocyanins, curcumin, shikonin, alizarin, and betalain, or synthetic origin, such as methyl red, cresol red, bromocresol green, and bromocresol purple, are used [289,290,291,292,293]. Regarding data carrier devices, barcode labels and systems of RFID tags are the most widespread [281,294].
Smart packaging integrates both intelligent and active packaging technologies; however, the term is sometimes used interchangeably to refer to either one or the other [259]. The combined packaging systems allow the packaging to detect and respond to changes or needs of the food or surrounding environments to improve or restore their conditions with a synergic effect [232,295]. These packaging systems, whether combined or not, achieve remarkable customization of the packaging to suit the food. Furthermore, taking advantage of new developments in materials with improved mechanical and barrier properties can achieve the highest food preservation performance [296,297].
Regulatory requirements are essential for active and intelligent packaging, with the European Union providing a leading example. Framework Regulation (EC) No 1935/2004 sets out general safety requirements for all materials intended to come into contact with food. Regulation (EC) No. 450/2009 specifically governs the use and pre-market authorization of active and intelligent materials, requiring safety evaluations prior to their commercialization. Additionally, Regulation (EC) No 2023/2006 mandates compliance with GMP to ensure product safety and quality throughout the production process [298].
The widespread adoption of active and intelligent packaging technologies continues to face several critical challenges. One of the most significant barriers is the high initial investment cost associated with advanced materials and specialized infrastructure. Furthermore, the integration of these technologies into existing production lines often entails complex modifications, requiring technical expertise and workforce training.
Consumer acceptance remains another considerable challenge. Public concerns regarding cost and the perceived complexity of these systems contribute to skepticism. As such, transparent risk communication and consumer education are essential to foster trust and understanding [299]. From an environmental standpoint, while these technologies aim to improve sustainability by reducing food waste, components such as embedded electronics and batteries raise concerns about lifecycle impacts, recyclability, and resource sourcing [300].
4.2. Barrier Packaging Strategies
In food packaging, gas barrier properties—particularly against oxygen and water vapor—are among the most critical factors for preserving packed food [301,302]. Common industrial strategies for enhancing gas barrier properties of polymers include metal coatings and multilayer films. Metalized films, typically incorporating aluminum, have long been favored for their excellent barrier to gases and moisture. However, this technology still faces both technical and environmental challenges. Achieving defect-free and long-term stable coatings remains difficult. Plasma or corona pretreatments are often required to ensure good metal-to-substrate adhesion. These treatments can increase the rigidity of the polymer film, render it opaque, and make it incompatible with microwave use. Moreover, metalized films have limited recyclability due to the difficulty of separating thin metallic layers. As alternatives, transparent surface coatings such as Al2O3, SiOx, and DLC are being explored for their advantages in transparency and microwave compatibility. However, these coatings can exacerbate existing issues, including microcrack formation. Multilayer systems, on the other hand, combine polymers with complementary barrier, mechanical, and processing properties, enabling optimized performance. Coextrusion is the most widely used manufacturing technique due to its versatility, allowing independent adjustment of layer composition and thickness, as well as the inclusion of tie layers. Typical multilayer films are primarily composed of polyolefins (e.g., LDPE, PP, HDPE), PET, adhesives, and aluminum coatings and are produced by cast film or blown film coextrusion [302,303]. However, multilayer films also face technical and environmental concerns, such as incompatibility between layers, which may result in interfacial adhesion failures and delamination; challenges in controlling layer thickness and uniformity; and recycling difficulties due to the presence of immiscible polymers [301,303,304].
To address sustainability and recyclability concerns, new strategies are focus on enhancing the barrier properties of single-component films through polymer blending, nanocomposite formation, modification of crystalline structure, and combined approaches [303,305,306]. In polymer blends, high-barrier materials such as PE, PEN, PVA, and EVOH are dispersed within conventional polymer matrices like PE or PET. Depending on the processing method, materials, and conditions, the dispersed phase may adopt various morphologies—such as spherical particles, micro/nanofibers, or layered structures—which influence the resulting barrier properties. Common techniques include melt compounding and solution casting. There is a difficulty in that while polar dispersed phases improve oxygen barrier performance, they can reduce water vapor resistance. Key challenges in polymer blending involve achieving good interfacial compatibility, maintaining morphological stability, and ensuring uniform dispersion. Regarding polymer nanocomposites, the addition of impermeable nanoparticles to the polymer matrix introduces a “tortuous path” effect that slows gas molecule diffusion. Common nanoparticles include nanoclays, graphite, graphene, and inorganic fillers. Nanoparticle aspect ratio, interfacial adhesion, and dispersion quality significantly affect the performance of the nanocomposite. These fillers can also enhance mechanical properties and influence matrix crystallinity, further improving barrier function. Despite their potential, all of these strategies face technological scaling challenges, compatibility issues, and risks of polymer matrix degradation. Moreover, economic barriers remain due to the high costs of nanotechnology, and health and environmental impacts are still under investigation and require further study [304].
A recent combined strategy is the use of LbL assembly technology, a multilayer coating technique capable of forming nanocomposite materials through the incorporation of nanoscale fillers in a nanostructured arrangement. By employing non-vacuum techniques such as dip coating, spin coating, or spraying, alternating nanostructured thin layers are deposited. This architecture increases the tortuosity of diffusion paths, thereby enhancing the gas barrier properties of the material. Additional advantages of this approach include favorable mechanical performance, transparency, microwave compatibility, and—due to the extremely thin nature of the coatings—potential recyclability. However, its application remains largely at the laboratory scale [307].
Another recent strategy that enables substantial barrier enhancement at low nanofiller loadings is the fabrication of three-dimensional (3D) segregated network nanocomposites [305,308]. This approach increases the tortuosity of gas diffusion pathways by forming a continuous network of nanofillers within the polymer matrix through an efficient spatial arrangement that maximizes obstruction to gas permeation. The selection of suitable nanofillers is critical to the success of this method. Graphene nanoplatelets, for instance, are widely employed due to their high aspect ratio and intrinsic impermeability to gases [308,309,310]. The formation of segregated structures is based on the excluded volume theory, which predicts that nanofillers in a segregated system preferentially localize at the interfaces between polymer particles rather than being randomly dispersed. The fabrication process typically involves (1) dispersing nanofillers in a volatile solvent using ultrasonication or high-shear mixing; (2) adding polymer particles to the dispersion and stirring to facilitate coating of their surfaces; (3) solvent removal through drying; and (4) compression molding of the coated particles to form a consolidated composite. In addition to the advantage of low filler loading, this approach also preserves the optical clarity and mechanical flexibility of the final material. However, several challenges remain for the industrial scaling of this technology. Most notably, the commonly used method for nanofiller exfoliation—solvent blending assisted by ultrasonication—is often inefficient, costly, and environmentally burdensome due to solvent use and disposal concerns, which limits its sustainability. Moreover, compression molding temperature is a critical processing parameter that must be carefully optimized. It must be high enough to achieve sufficient interparticle cohesion without creating interfacial voids, but not so high as to significantly reduce the polymer’s viscosity, which could lead to unwanted diffusion of the nanofillers and the loss of network structure [305].
A comprehensive description of all existing strategies for enhancing the barrier properties of polymers is beyond the scope of this review. However, it is worth briefly highlighting some prominent developments in the field of barrier films for food packaging. Two notable areas are: (i) the development of bio-based materials with improved barrier performance, driven by increasing sustainability concerns; and (ii) the integration of barrier films into smart packaging systems, in line with current trends in food packaging. The first approach addresses a critical challenge, as bio-based polymers typically exhibit poor barrier properties [311]. Strategies to overcome these limitations are discussed in detail in Section 4.7. The second area involves the incorporation of functional nanomaterials—such as metals, metal oxides, graphene, and others—into polymer matrices, either through surface coating techniques [312] or nanocomposite formation [227,280,281,282,283]. These materials not only improve barrier and mechanical properties but also impart additional functionalities, such as antimicrobial activity or gas scavenging capacity. Moreover, barrier films also play a key role in smart packaging applications, where sensors are embedded to monitor the composition or concentration of specific gases, thereby providing real-time indicators of food quality, gas leakage, or package integrity [313,314]. For example, synthetic multilayer films have been developed to monitor the freshness of fish [315], while bilayer assemblies of bio-based polymers have been investigated to monitor and preserve seafood quality [316].
4.3. Modified Atmosphere Packaging
Thanks to the development of polymeric packaging materials with enhanced and tailored gas barrier properties, MAP, an advanced food packaging technology, can be widely used. This technology is applied to extend the shelf life and preserve the quality of perishable food products by altering the composition of the internal atmosphere within the package. This technology typically involves the reduction in O2 levels and the enrichment of CO2 and/or N2, in order to inhibit microbial growth, retard oxidative reactions, and slow down enzymatic activity. MAP systems can be broadly categorized into passive and active approaches. In passive MAP, the desired atmosphere is achieved naturally through the interaction between the respiration rate of the product (e.g., fresh produce) and the gas permeability of the packaging material over time. In contrast, active MAP involves the direct modification of the internal gas composition through vacuuming and gas flushing techniques or by incorporating functional additives such as oxygen scavengers, CO2 emitters, or moisture regulators, allowing for immediate atmosphere control [317]. Conventional gas mixtures vary depending on the food type: low O2/high CO2 atmospheres are commonly used for meat, fish, and dairy to suppress aerobic microbial growth [318], whereas high N2 environments are employed in dry or lipid-rich products such as nuts and snacks to prevent oxidation. For fresh fruits and vegetables, EMAP is often applied, balancing the product’s respiration with the packaging film’s gas transmission properties.
MAP offers significant advantages, including prolonged shelf life, preservation of sensory and nutritional attributes, and reduced dependence on chemical preservatives. However, challenges persist, such as the need for precise gas composition control, the high cost of specialized equipment and multilayer films, and limited recyclability due to polymer incompatibility in multilayer structures. Furthermore, insufficient oxygen control can lead to anaerobic microbial growth, particularly under temperature abuse conditions [319].
Advances in this technology are associated with the development of new materials with improved or controllable barrier properties, such as microporous MAP [320] or temperature-dependent permeability [321], intelligent MAP systems that allow monitoring of gas composition and product freshness [314], the combination with active packaging systems by the incorporation and release of antioxidants and antimicrobials [322], and the use of bio-based [323] or recyclable single-component barrier films. Figure 3 schematizes smart, barrier, and MAP packaging systems.

Figure 3.
Smart, barrier, and MAP packaging systems.
4.4. Effect of Industrial Food Processing on Packaging
Food processing techniques can significantly impact food products’ quality, including nutritional value and organoleptic properties, such as texture, flavor, and color. Subjecting foods to certain preservation processes, which are essential for controlling biochemical and microorganism-mediated degradation reactions and ensuring food safety, can seriously affect bioactive compound retention if not properly applied. The most popular traditional preservation techniques are heating, drying, and freezing [324], while more innovative methods based on irradiation, cold plasma, pulsed electric fields, pulsed light, microwaves, ultrasound, and pressure-assisted treatments, among others, have been developed in recent years [325,326]. Production lines can be designed in such a way that the preservation step comes either before or after the packaging one. In the former, and when food is intended to be stored at room temperature, the so-called “aseptic packaging” method is applied. Food and packaging materials are sterilized independently, and the filling takes place under aseptic conditions [327]. In the latter case, both food and package material are simultaneously exposed to the chosen preservation method, thus preventing undesirable downstream recontamination and packaging pre-sterilization. This is the so-called “in-pack” processing [314]. For both processing configurations, packaging materials are subjected to processes that can alter their structure and eventually their mechanical and barrier properties.
Conventional thermal processing operations are known to be aggressive in terms of food organoleptic and nutritional properties. Packaging materials used in these types of processes are expected to exhibit certain thermal stability. To date, advanced synthetic polymers have proven suitable, while sustainable packaging materials are being evaluated at the research level [328]. Thermal processing not only has the drawback of being aggressive to both food and packaging, but it is also a unit operation characterized by low energy efficiency and high-water consumption. Therefore, it is essential to develop a new generation of technologies capable of producing safe, high-quality foods with minimal environmental impact. The above-mentioned innovative techniques are examples of NTP. Some of them are also used to modify or functionalize packaging materials; thus, an enhancement of certain material properties could take place during the preservation process [326]. Nilsen-Nygaard et al. [326] have comprehensively reviewed the impact of NTP on bio-based and biodegradable—and also conventional—food packaging materials. Another work that addressed the effect of NTP on packaging materials—bio-based polymers, edible films, and nanomaterials—is the one authored by Gabric et al. [325]. Several material properties have been analyzed, and those that exhibited a greater tendency to undergo modifications were chemical migration, gas transmission rate (mainly oxygen, water vapor, and carbon dioxide), thermal stability, roughness, water contact angle, tensile/puncture strength, elongation at break, free volume fraction, relative crystallinity, digestibility (in the case of edible materials), viscosity, firmness, hydrophilicity, and glass transition temperature. The combination of NTP-packaging material should be carefully analyzed since every processing technique exerts a differential impact on each material property. Moreover, the inclusion of active compounds, biocomposites, and nanosized particles enhances the resistance of biomaterials to food processing technologies. Nonetheless, they contribute to higher complexity and cost while also reducing biodegradability [326].
4.5. Eco-Friendly Packaging
Terms such as eco-friendly packaging, sustainable packaging, green packaging, environmentally friendly packaging, and eco-designed packaging refer to packaging solutions strategically developed to minimize environmental impact throughout their lifecycle. This encompasses all aspects of packaging, including the materials used, the manufacturing process, its functionality, and its final disposal. It aims to reduce waste, energy consumption, and carbon footprint while maintaining functionality and product protection. Particularly, when discussing “sustainable packaging,” the economic and social impacts must also be considered [9,10,11].
Polymers are among the most widely used materials in packaging for all types of food due to their beneficial cost-performance ratio, great versatility, easy processability, and innocuity, among other advantages. However, there is an important concern about its negative effects on the environment [13]. In this context, the use of bio-based and biodegradable polymers is gaining ground over petroleum derivatives [259]. In fact, in recent years, a significant portion of research in the field of food packaging materials has concentrated on this type of material. Table 3 presents a compilation of reviews on biopolymers and food packaging published over the past five years. The design of active, intelligent, and/or smart packaging based on biomaterials can have a synergistic effect on sustainability. These packaging solutions help reduce food waste and lower energy costs associated with storage and distribution by enabling perishable foods, such as fruits, to be stored without refrigeration while also extending the shelf life of packaged products. These benefits are further complemented by the environmental advantages of bio-based and biodegradable materials [14].

Table 3.
Reviews related to green food packaging (in the last five years).
Packaging material is one of the aspects that is most taken into account with regard to eco-friendly design and to which consumers pay a lot of attention. Such materials can be made from renewable or recycled resources, and/or they can be recyclable, compostable, or biodegradable. Furthermore, they should not constitute hazardous materials at any stage of the packaging life cycle and should facilitate effective recovery through closed-loop systems. Among packaging materials, polymer-based materials stand out for their convenient cost/performance ratio and are the most widely used [338,339]. Polymeric packaging materials can be classified according to their origin as bio-based or petroleum-based and if they are biodegradable or non-biodegradable (Figure 4), according to ASTM D6400 (industrial compostability) or ISO 14855 standards [10].
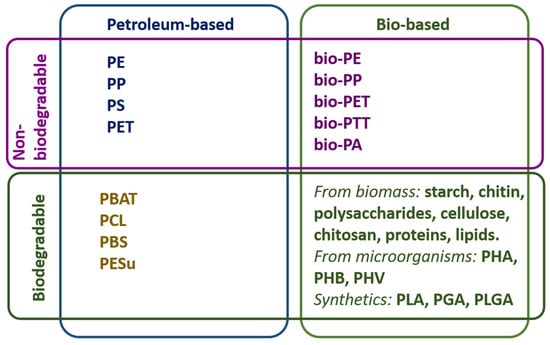
Figure 4.
Most common polymeric packaging materials.
The most conventional packaging materials are petroleum-based and non-biodegradable plastics, mainly polyolefins such as PE, PP, PS, and PET [338]. Although not preferred for eco-packaging, these materials, when used responsibly, can be recycled and may allow for some reuse. Given their excellent characteristics that make them suitable for packaging applications, such as lightweight, barrier properties, processability, and low cost, they are still difficult to replace.
The category of biodegradable petroleum-based polymers includes some aliphatic polyesters such as PBAT [340] and PCL [341], some aromatic-aliphatic copolyesters such as PBS [342,343,344] and PESu [345], and other copolymers [10]. Unlike conventional petroleum-based polymers (e.g., PE, PP, PET), these materials break down into CO2, water, and biomass through natural degradation processes. It is worth mentioning oxo-degradable plastics, which, although not biodegradable, contain pro-degradant additives that accelerate the degradation process. However, the environmental impact of these materials is highly controversial, since instead of mitigating environmental pollution, they could aggravate it by generating microplastics. For this reason, they have been banned in Europe since 2021 [346,347].
Regarding non-biodegradable and bio-based polymers, they are designed to be identical to conventional plastics, but they are made from biomass instead of fossil fuels. Mainly, bioethanol, obtained through biomass fermentation and hydrolysis, is used as a renewable resource. Examples of these are bio-PE, bio-PP, bio-PET, bio-PTT, bio-PA, and polyol-polyurethane. They can be recycled as conventional polymers [348].
Bio-based and biodegradable polymers are preferred for eco-packaging applications [349]. These are usually classified according to their production method and origin into three main groups. The group of polymers extracted from biomass, which are mainly obtained from plant-based agricultural waste through mechanical and chemical methods, are blended with other biopolymers and chemically modified. This group includes polysaccharides, which are some of the most used biomaterials for packaging, such as starch, cellulose, chitin, and chitosan; proteins, such as collagen, gelatin, casein, whey, soy protein, zein, and wheat gluten, widely used in pharmaceutical applications; and cross-linked lipids. The group of polymers extracted from microorganisms that are by-products of the vital cycle of some natural or genetically modified organisms, which use agricultural wastes as a medium for growth. The main polymers in this group are PHA, such as PHV and PHB, and bacterial cellulose. This group stands out in that it can be completely and efficiently decomposed by microorganisms. The last group corresponds to polymers synthesized from renewable bio-based monomers. The main one is PLA, a biopolyester thermoplastic synthesized from lactic acid monomers produced by fermentation of cornstarch or sugarcane. Another is PGA, which, when polymerized in combination with PLA monomers, produces PLGA, with a better degradation rate than PLA. These polymers are compostable but require industrial facilities for degradation [350,351,352].
However, the adoption of biopolymers in packaging applications remains limited due to several factors. Compared with conventional petroleum-based plastics, large-scale production is more complex, manufacturing costs are higher, and their overall performance is inferior. In general, biopolymers exhibit lower mechanical strength, thermal and moisture stability, and barrier properties [15]. Strategies to overcome these performance limitations include chemical and physical modifications [353,354], blending [355], plasticization [356], composite reinforcement [330,357], coatings and additives incorporation [358], and others [359]. However, comprehensively overcoming the production, cost, and property limitations of biopolymers remains a challenge that has yet to be fully resolved [352,360].
Determining the environmental impact and sustainability of bio-based packaging remains a subject of debate and ongoing research [361,362,363]. The current trend emphasizes conducting increasingly comprehensive life cycle analyses, incorporating a broader range of factors. Beyond assessing the resources used in packaging production—such as raw materials, energy, time, and labor—consideration is given to product shelf life, quality preservation, end-of-life disposal, and societal impact, including its influence on human well-being and implications for future generations [364,365,366]. In the specific case of biopolymers, concerns arise regarding limited biodegradability, ambiguities in end-of-life processing, and a lack of standardization, in addition to the previously mentioned limitations in cost, production, and technical performance [367]. Furthermore, in some instances, competition with food production emerges, as their raw materials are often sourced from edible crops or can alternatively serve as fertilizers or protective soil covers [361,368,369].
4.6. Packaging Requirements and Solutions for Functional Foods
Packaging must be tailored to the specific food type and its susceptibility to spoilage, as well as to logistical considerations, the availability and cost of packaging materials, design and manufacturing feasibility, consumer convenience, and sustainability. Food spoilage is a complex process driven by microbial growth, enzymatic activity, and chemical reactions, all of which are influenced by intrinsic and extrinsic factors. Intrinsic factors are related to the physicochemical properties of the food itself, including water activity, pH, oxidation-reduction potential, antimicrobial content, surface exposure, biological structure, and nutrient levels. Extrinsic factors, on the other hand, encompass environmental conditions such as temperature, humidity, light exposure, and oxygen availability [8]. Selecting the appropriate packaging requires a fundamental understanding of both intrinsic and extrinsic spoilage mechanisms specific to the packaged food [370].
Many functional foods include natural antioxidants or unsaturated fatty acids [350,371]. Packaging solutions must therefore be designed to limit oxygen availability while simultaneously maintaining their role in preventing microbial growth [372].
Encompassing all aspects of the packaging of functional food in a comprehensive review is challenging due to the extensive diversity of products and formats. To the best of our knowledge, only two book chapters, a review specifically on dairy products, and a few scattered papers focusing on particular types of functional foods exist, leaving several key issues unaddressed. In Deschênes’ chapter [373], a comprehensive approach is presented, analyzing the requirements of each group of functional foods and the existing packaging alternatives. Additionally, it briefly discusses the benefits of active packaging for this type of product and delves into the applications of nano-packaging. However, these data are outdated, as the chapter was published a decade ago. Chhikara et. al. [374] present an analysis of the potential for using biopolymers for packaging functional foods. It details the requirements for antimicrobial and antioxidant packaging and the packaging capabilities of biopolymers such as starch, PLA, cellulose, chitosan, proteins, carrageenan, and alginate. However, the discussion is presented in general terms, and it probably misses the opportunity to go into more detail about specific cases of packaging applied to functional foods. While the chapters provide an overview, most of the papers found focus on a specific type of functional food. Francis et al. [375] review the commercially available packaging alternatives for fermented products, such as yogurt, probiotic-rich milk, and kefir, most of which are based on polyolefins. The review also analyzes sustainability aspects, including recyclability and biodegradability, and provides an overview of the potential of innovative solutions, such as active packaging and nanotechnology. Table 4 resumes the papers from the last ten years that compare the performance of different technologies, formats, and materials used in commercially available packaging for preserving the bioactivity of packaged foods. The analyzed foods are primarily minimally or mildly processed fruits and, to a lesser extent, fortified foods. Packaging types vary depending on the product, with most being flexible petroleum-based polyolefin containers. No comparable studies based on biopolymers have been found, despite their increasing use in commercial packaging. The results indicate that bioactivity preservation of functional food—considering bioactivity, stability, shelf life, and overall quality—improves as gas barrier properties, particularly oxygen barrier properties, are enhanced and as oxygen exposure within the package is minimized through technologies such as MAP. While the use of antimicrobials is also beneficial, most studies emphasize the advantages of slowing oxidative degradation processes.

Table 4.
Summary of studies comparing different packaging solutions for the preservation of functional foods.
Table 5 presents proposed novel packaging solutions and packaging-food combinations aimed at improving the preservation of polyphenols in functional foods (in the last 5 years). Various packaging technologies were analyzed, primarily bio-based edible polymers, composites, and antimicrobial-releasing materials, as well as MAP technologies. These innovations were mainly applied to perishable foods such as fresh fruits and seafood (Table 5).

Table 5.
Novel food packaging solutions for preserving polyphenols (in the last 5 years).
Bioactive compounds extracted from foods are even more susceptible to spoilage than functional foods themselves, as they lack the protective matrix structure of the original food. Oxygen, moisture, pH, temperature, light, and humidity significantly impact the stability of polyphenols [402], omega-3 PUFAs [51], phytosterols [403], and probiotics [404]. These factors become particularly critical during storage, food processing, and ingestion. If the stability, survival, bioavailability, and functionality of the bioactive component are compromised, its associated health benefits are also lost.
Encapsulation technologies have been the most effective strategy for preserving bioactive properties. Encapsulation involves enclosing a target substance, either partially or completely, to shield it from external interactions while enabling controlled release under specific conditions. Capsules, which can be classified as macro-, micro-, or nano-capsules based on size, function as carriers for labile bioactive compounds. Encapsulation is widely applied in functional foods, either for direct enrichment and fortification or for integration into packaging materials. Biopolymers are the most commonly used coating materials due to their suitability for consumption. The most frequently employed encapsulation techniques include spray drying, freeze-drying, spray chilling, emulsification, liposome entrapment, and extrusion [333,350,403,404]. The available literature on this topic is too extensive to be covered in detail in this work. Table 6 summarizes the main biomaterials used for each type of bioactive compound or microorganism, referencing the most recent review studies, the majority of which were published in 2024.

Table 6.
Bioactive compounds/microorganisms protected by encapsulation techniques.
A simpler and more cost-effective approach to incorporating bioactive compounds/microorganisms into foods is through edible packaging. This type of packaging serves as an inner layer, primarily aimed at protecting the food product, maintaining its quality, and extending its shelf life. Depending on the application technique, edible packaging is categorized into edible films and coatings. Edible films are prepared separately, mainly using solvent casting, and are then applied to wrap the food. In contrast, edible coatings are formed directly on the food surface through spraying or dipping methods [413]. Active edible packaging incorporates functional compounds/microorganisms that provide additional properties. The bioactive compounds/microorganisms of functional foods contribute antioxidant and antimicrobial properties to the packaging while also offering health benefits to the consumer. Furthermore, they have potential applications in intelligent edible packaging, as they can serve as indicators of food status with high accuracy due to their direct contact with the product [414]. Similar to encapsulation technologies, edible packaging materials are bio-based, primarily composed of biopolymers. Table 7 summarizes recent review studies and the most commonly used polymeric matrices for each bioactive compound/microorganisms. Although this is a well-studied and highly relevant topic, significant challenges remain. One major concern is ensuring that the organoleptic properties of the food are not altered, which is a key objective. Additionally, the barrier properties of edible packaging are still somewhat inadequate, particularly when compared with conventional packaging solutions [415,416].

Table 7.
Active edible packaging with bioactive compounds/microorganisms (reviews of the last five years).
Natural bioactive compounds are widely used as active agents in the development of active packaging, primarily to enhance the preservation of packaged food, whether functional or not. However, in most cases, the potential additional health benefits associated with bioactive compounds from functional foods are not a primary consideration. These active packaging systems can be based on either edible or non-edible matrices, utilizing biopolymeric or synthetic materials.
To impart antioxidant and antimicrobial properties, various bioactive compounds have been incorporated into active packaging formulations. These include phenolic compounds [213], polyphenols [429], polyphenol-rich propolis extract [203], tea polyphenols [430], bioactive peptides [431,432], and essential oils (terpenoids) [433,434]. In addition to their antioxidant and antimicrobial roles, some bioactive compounds enhance packaging properties such as barrier performance, mechanical strength, and color stability. For instance, tea polyphenols have been incorporated to provide antioxidant, antibacterial, enhanced barrier properties, and intelligent discoloration functions [228,435]. Curcumin (a polyphenol) has been used to confer antioxidant, antimicrobial, freshness indicator (color change with pH), and improved mechanical, gas barrier, and UV barrier properties [436]. Peels and extracts from fruits and vegetables have also been incorporated to introduce antioxidant, antimicrobial, freshness indication, and oxygen scavenging functionalities while improving mechanical, gas barrier, and UV barrier properties [437,438]. Furthermore, flavonoids have been integrated into packaging to enhance UV barrier properties [439], while polyphenols, anthocyanins, phenolics, flavonoids, terpenes, carotenoids, and tocopherols have been used for their antimicrobial, antioxidant, and pH-sensitive sensor capabilities [437].
4.7. Strategies to Overcome the Drawbacks of Using Bio-Based Polymers in Packaging of Functional Food
As previously discussed, one of the most critical performance requirements for packaging functional foods is gas barrier capacity, given that these products are particularly susceptible to oxidative deterioration. Unfortunately, despite their environmental benefits, bio-based polymers often face significant limitations that restrict their broad application in food packaging systems [311]. These drawbacks include inherently poor gas barrier properties, limited mechanical strength, high production costs, accelerated degradation under certain storage or processing conditions, and difficulties in preserving food sensory qualities, especially in edible coatings [374,440]. Compared with conventional synthetic polymers, bio-based alternatives tend to exhibit greater hydrophilicity, which contributes to higher water vapor permeability and reduced mechanical stability under humid conditions. Consequently, improving their barrier performance often requires more tailored and multifunctional strategies that maintain biodegradability and food-grade safety, thereby narrowing the scope of usable additives and solvents.
Multilayer film systems are commonly employed to address these limitations. These typically consist of a central barrier layer flanked by outer layers that provide additional functions such as mechanical support, moisture resistance, or flexibility. Assembly techniques for these multilayer systems include solvent casting, extrusion lamination, compression molding, and LbL deposition. To further enhance gas barrier properties, nanofillers such as nanoclays, cellulose nanocrystals, and graphene oxide are frequently incorporated into one or more layers to create nanocomposite structures. These additions improve barrier performance by increasing the tortuosity of diffusion paths and thereby reducing gas permeability [441,442,443]. Additional strategies include the use of carefully selected biopolymer blends [444], the incorporation of hydrophobic or bio-based nanofillers [445], plasticizers, and crosslinking agents, as well as chemical modifications aimed at reducing hydrophilicity. Techniques such as LbL assembly and electrospinning have been more extensively explored with bio-based polymers than with synthetic ones, as they are compatible with low-temperature processes and enable the formation of highly oriented or nanostructured barrier layers. Furthermore, because processing conditions strongly influence barrier performance, these parameters can be optimized to further enhance functionality [446,447,448].
5. Conclusions
The integration of eco-packaging in the functional food sector is closely aligned with the United Nations Sustainable Development Goals, particularly those related to responsible consumption, production, and climate action. Consumer behavior plays a critical role in the successful adoption of such innovations. Notably, individuals who seek out functional foods often exhibit a strong preference for health-conscious, natural, and environmentally responsible consumption patterns. This overlap underscores the strategic value of incorporating sustainable and innovative packaging solutions into functional food offerings, as it enhances consumer appeal while contributing to the development of a more sustainable and resilient food system.
Despite the evident synergies between functional food consumption and sustainable packaging, the scientific literature providing a comprehensive overview of packaging specifically tailored for functional foods remains scarce. This represents a critical knowledge gap, especially in contrast to the growing body of research in biomaterials and functional food development. In this context, systematic technological monitoring and interdisciplinary collaboration are essential to inform the design of packaging systems that effectively preserve the bioactivity, shelf life, and sensory attributes of functional foods. Moving forward, research efforts should prioritize the convergence of food science, materials engineering, and consumer behavior to develop tailored packaging solutions that support both product functionality and sustainability objectives.
Author Contributions
Conceptualization, A.L.G., N.G., C.P., M.F.R., C.C.A. and O.V.L.; investigation, A.L.G., N.G., C.P., M.F.R., C.C.A. and O.V.L.; writing—original draft preparation, A.L.G., N.G., C.P., M.F.R. and C.C.A.; writing—review and editing, A.L.G., N.G. and O.V.L.; supervision, A.L.G., N.G. and O.V.L.; project administration, O.V.L.; funding acquisition, O.V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Nacional del Sur (UNS) (grant number PGI TIR Resolution CSU-1116/23).
Data Availability Statement
All reported data are published, and the corresponding bibliographic sources are referenced.
Acknowledgments
The authors express their gratitude to Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AA | Arachidonic acid |
| AFLP | Amplified fragment length polymorphism |
| ALA | α-linolenic acid |
| Al2O3 | Aluminum oxide |
| bio-PA | Bio-polyamide |
| bio-PE | Bio-polyethylene |
| bio-PET | Bio-polyethylene-terephthalate |
| bio-PP | Bio-polypropylene |
| bio-PTT | Bio-polytrimethylene terephthalate |
| BOPP | Biaxially oriented polypropylene |
| CAGR | Compound Annual Growth Rate |
| CE | Capillary electrophoresis |
| CLA | Conjugated linoleic acid |
| CO2 | Carbon dioxide |
| DGGE | Denaturing gradient gel electrophoresis |
| DHA | Docosahexaenoic acid |
| DLC | Diamond-like carbon |
| DNA | Deoxyribonucleic acid |
| DPA | Docosapentaenoic acid |
| ELSD | Evaporative light scattering detection |
| EMAP | Equilibrium Modified Atmosphere Packaging |
| EPA | Eicosapentaenoic acid |
| EU | European Union |
| EVOH | Ethylene vinyl alcohol |
| FAO | Food and Agriculture Organization |
| FFC | Functional Food Center |
| FID | Flame ionization detector |
| FLE | Ficus leaf extract |
| FOS | Fructooligosaccharides |
| FOSHU | Food for Specific Health Uses |
| FT-IR | Fourier transform infrared spectroscopy |
| GC | Gas chromatography |
| GMP | Good Manufacturing Practices |
| GOS | Galactooligosaccharides |
| HMDB | Human Metabolome Database |
| HPAEC-PED | High-performance anion-exchange chromatography with pulsed electrochemical detection |
| HPE | Hypericum perforatum extract |
| HPLC | High-performance liquid chromatography |
| IR | Infrared spectroscopy |
| ISAPP | International Scientific Association for Probiotics and Prebiotics |
| KC | Natural kaolinite clay |
| LA | Linoleic acid |
| LC | Liquid chromatography |
| LDL | Low-density lipoprotein |
| LDPE | Low-density polyethylene |
| MALDI-TOF-MS | Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| MAP | Modified-atmosphere-packed |
| MIR | Mid-infrared spectroscopy |
| MOS | Milk oligosaccharides |
| MS | Mass spectrometry |
| N2 | Nitrogen |
| NIR | Near-infrared spectroscopy |
| NMR | Nuclear magnetic resonance |
| NTP | Non-thermal processing technologies |
| O2 | Oxygen |
| OBSM | Ocimum basilicum seed mucilage |
| OPP | Oriented polypropylene |
| PA | Polyamide |
| PAD | Pulsed amperometric detection |
| PBAT | Polybutylene adipate terephthalate |
| PBS | Polybutylene succinate |
| PCL | Polycaprolactone |
| PDA | Photodiode array detector |
| PGA | Polyglycolic acid |
| PE | Polyethylene |
| PESu | Polyethylene succinate |
| PET | Polyethylene terephthalate |
| PHA | Polyhydroxyalkonoates |
| PHB | Polyhydroxy-butyrate |
| PHV | Polyhydroxyvalerate |
| PLA | Polylactic acid |
| PLGA | Poly(lactide-co-glycolide) |
| PLSR | Partial least squares regression |
| pMAP | Passive modified atmosphere packaging |
| PP | Polypropylene |
| PS | Polystyrene |
| PTT | Poly(trimethylene terephthalate) |
| PUFAs | Polyunsaturated fatty acids |
| PVA | Polyvinyl alcohol |
| RAPD | Randomly amplified polymorphic |
| RFID | Radio frequency identification |
| RID | Refractive index detection |
| RNA | Ribonucleic acid |
| rRNA | Ribosomal ribonucleic acid |
| ROS | Reactive oxygen species |
| SDA | Stearidonic acid |
| SFC | Supercritical fluid chromatography |
| SiOx | Silicon oxide |
| TGGE | Temperature gradient gel electrophoresis |
| UHPLC | Ultra-high-performance liquid chromatography |
| UHPLC-QTOF-MS/MS | Ultra-high-performance liquid chromatography-triple/time-of-flight mass spectrometry |
| VP | Vacuum-packed |
| WE | Working electrode |
| XOS | Xylooligosaccharides |
References
- Vignesh, A.; Amal, T.C.; Sarvalingam, A.; Vasanth, K. A review on the influence of nutraceuticals and functional foods on health. Food Chem. Adv. 2024, 5, 100749. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent advances in health benefits of bioactive compounds from food wastes and by-products: Biochemical aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef] [PubMed]
- Ponte, L.G.S.; Ribeiro, S.F.; Pereira, J.C.V.; Antunes, A.E.C.; Bezerra, R.M.N.; da Cunha, D.T. Consumer Perceptions of Functional Foods: A Scoping Review Focusing on Non-Processed Foods. Food Rev. Int. 2025, 1–19. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Guimarães, J.F.; Coutinho, N.M.; Pimentel, T.C.; Ranadheera, C.S.; Santillo, A.; Albenzio, M.; Cruz, A.G.; Sant’Ana, A.S. The future of functional food: Emerging technologies application on prebiotics, probiotics and postbiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2560–2586. [Google Scholar] [CrossRef]
- Catenza, K.F.; Donkor, K.K. Recent approaches for the quantitative analysis of functional oligosaccharides used in the food industry: A review. Food Chem. 2021, 355, 129416. [Google Scholar] [CrossRef]
- Ahire, J.J.; Rohilla, A.; Kumar, V.; Tiwari, A. Quality management of probiotics: Ensuring safety and maximizing health benefits. Curr. Microbiol. 2024, 81, 1. [Google Scholar] [CrossRef]
- Boylston, T.D. Understanding and Measuring the Shelf Life of Food. J. Food Qual. 2005, 28, 403–404. [Google Scholar] [CrossRef]
- Karanth, S.; Feng, S.; Patra, D.; Pradhan, A.K. Linking microbial contamination to food spoilage and food waste: The role of smart packaging, spoilage risk assessments, and date labeling. Front. Microbiol. 2023, 14, 1198124. [Google Scholar] [CrossRef]
- Koch, J.; Frommeyer, B.; Schewe, G. Managing the transition to eco-friendly packaging—An investigation of consumers’ motives in online retail. J. Clean. Prod. 2022, 351, 131504. [Google Scholar] [CrossRef]
- Hussain, S.; Akhter, R.; Maktedar, S.S. Advancements in sustainable food packaging: From eco-friendly materials to innovative technologies. Sustain. Food Technol. 2024, 2, 1297–1364. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Parker, L.; Brennan, L.; Lockrey, S. A consumer definition of eco-friendly packaging. J. Clean. Prod. 2020, 252, 119792. [Google Scholar] [CrossRef]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and bio-based food packaging: A review on past and current design innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Kan, M.; Miller, S.A. Environmental impacts of plastic packaging of food products. Resour. Conserv. Recycl. 2022, 180, 106156. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable biopolymers for active packaging: Demand, development and directions. Sustain. Food Technol. 2023, 1, 50–72. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.K.I.; Maan, A.A.; Nazir, A.; Riaz, S.; Khan, M.U.; Sultan, M.; Munekata, P.E.S.; Lorenzo, J.M. Biodegradable active, intelligent, and smart packaging materials for food applications. Food Packag. Shelf Life 2022, 33, 100903. [Google Scholar] [CrossRef]
- D’Amico, V.; Cavaliere, M.; Ivone, M.; Lacassia, C.; Celano, G.; Vacca, M.; la Forgia, F.M.; Fontana, S.; De Angelis, M.; Denora, N.; et al. Microencapsulation of Probiotics for Enhanced Stability and Health Benefits in Dairy Functional Foods: A Focus on Pasta Filata Cheese. Pharmaceutics 2025, 17, 185. [Google Scholar] [CrossRef]
- Ramazanidoroh, F.; Hosseininezhad, M.; Shahrampour, D.; Wu, X. Edible packaging as a functional carrier of prebiotics, probiotics, and postbiotics to boost food safety, quality, and shelf life. Probiotics Antimicrob. Proteins 2024, 16, 1327–1347. [Google Scholar] [CrossRef]
- Jagtiani, E.; Adsare, S. Microencapulsation: Probiotics, Prebiotics, and Nutraceuticals. J. Nanotechnol. Nanomater. 2022, 3, 34–60. [Google Scholar]
- Neekhra, S.; Pandith, J.A.; Mir, N.A.; Manzoor, A.; Ahmad, S.; Ahmad, R.; Sheikh, R.A. Innovative approaches for microencapsulating bioactive compounds and probiotics: An updated review. J. Food Process. Preserv. 2022, 46, e16935. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Temple, N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef] [PubMed]
- Markets and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports (accessed on 27 March 2025).
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer acceptance toward functional foods: A scoping review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef] [PubMed]
- González-Díaz, C.; Vilaplana-Aparicio, M.J.; Iglesias-García, M. How is functional food advertising understood? An approximation in university students. Nutrients 2020, 12, 3312. [Google Scholar] [CrossRef]
- Ahn, B.I.; Bae, M.S.; Nayga, R.M., Jr. Information effects on consumers’ preferences and willingness to pay for a functional food product: The case of red ginseng concentrate. Asian Econ. J. 2016, 30, 197–219. [Google Scholar] [CrossRef]
- Ares, G.; Gimenez, A.; Gambaro, A. Consumer perceived healthiness and willingness to try functional milk desserts. Influence of ingredient, ingredient name and health claim. Food Qual. Prefer. 2009, 20, 50–56. [Google Scholar] [CrossRef]
- Marette, S.; Roosen, J.; Blanchemanche, S.; Feinblatt-Mélèze, E. Functional food, uncertainty and consumers’ choices: A lab experiment with enriched yoghurts for lowering cholesterol. Food Policy 2010, 35, 419–428. [Google Scholar] [CrossRef]
- Siegrist, M.; Stampfli, N.; Kastenholz, H. Consumers’ willingness to buy functional foods. The influence of carrier, benefit and trust. Appetite 2008, 51, 526–529. [Google Scholar] [CrossRef]
- Van Kleef, E.; Van Trijp, H.C.; Luning, P. Functional foods: Health claim-food product compatibility and the impact of health claim framing on consumer evaluation. Appetite 2005, 44, 299–308. [Google Scholar] [CrossRef]
- Bruschi, V.; Teuber, R.; Dolgopolova, I. Acceptance and willingness to pay for health-enhancing bakery products–Empirical evidence for young urban Russian consumers. Food Qual. Prefer. 2015, 46, 79–91. [Google Scholar] [CrossRef]
- Narayana, N.M.N.K.; Fernando, S.; Samaraweera, G.C. Awareness and attitude towards functional dairy products among consumers in western province of Sri Lanka. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 1308–1314. [Google Scholar] [CrossRef]
- Lyly, M.; Roininen, K.; Honkapää, K.; Poutanen, K.; Lähteenmäki, L. Factors influencing consumers’ willingness to use beverages and ready-to-eat frozen soups containing oat β-glucan in Finland, France and Sweden. Food Qual. Prefer. 2007, 18, 242–255. [Google Scholar] [CrossRef]
- Çakiroğlu, F.P.; Uçar, A. Consumer attitudes towards purchasing functional products. Age 2018, 18, 494. [Google Scholar]
- Michell, K.A.; Isweiri, H.; Newman, S.E.; Bunning, M.; Bellows, L.L.; Dinges, M.M.; Grabos, L.E.; Rao, S.; Foster, M.T.; Heuberger, A.L.; et al. Microgreens: Consumer sensory perception and acceptance of an emerging functional food crop. J. Food Sci. 2020, 85, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Gutkowska, K.; Czarnecki, J. Postawy konsumentów wobec innowacyjnej żywności z uwzględnieniem produktów funkcjonalnych-implikacje dla komunikacji marketingowej w zakresie oświadczeń żywieniowych i zdrowotnych. Mark. Inst. Nauk. I Badaw. 2020, 4, 107–129. [Google Scholar]
- Kitts, D.D. Bioactive substances in food: Identification and potential uses. Can. J. Physiol. Pharmacol. 1994, 72, 423–434. [Google Scholar] [CrossRef]
- Basu, S.K.; Thomas, J.E.; Acharya, S.N. Prospects for growth in global nutraceutical and functional food markets: A Canadian perspective. Aust. J. Basic Appl. Sci. 2007, 1, 637–649. [Google Scholar]
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Aluyor, E.O.; Oboh, I.O. Preservatives: Traditional preservatives-vegetable oils. Encycl. Food Microbiol. 2014, 3, 137–140. [Google Scholar]
- Wu, S.J.; Ng, L.T.; Lin, C.C. Antioxidant activities of some common ingredients of traditional Chinese medicine, Angelica sinensis, Lycium barbarum and Poria cocos. Phytother. Res. 2004, 18, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Kumar Deb, P.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism (s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation biology. J. Geront. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Kavyani, Z.; Musazadeh, V.; Fathi, S.; Faghfouri, A.H.; Dehghan, P.; Sarmadi, B. Efficacy of the omega-3 fatty acids supplementation on inflammatory biomarkers: An umbrella meta-analysis. Int. Immunopharmacol. 2022, 111, 109104. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef]
- Lange, K.W. Omega-3 fatty acids and mental health. Glob. Health J. 2020, 4, 18–30. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of omega-3 fatty acids on immune cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef]
- Patted, P.G.; Masareddy, R.S.; Patil, A.S.; Kanabargi, R.R.; Bhat, C.T. Omega-3 fatty acids: A comprehensive scientific review of their sources, functions and health benefits. Future J. Pharm. Sci. 2024, 10, 94–105. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.S. Omega− 3 polyunsaturated fatty acids (PUFAs): Emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits—A review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as sources of omega-3 polyunsaturated fatty acids: Biotechnological aspects. Algal Res. 2021, 58, 102410. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From preclinical evidence to potential clinical applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef] [PubMed]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef]
- Shahzad, N.; Khan, W.; Shadab; Ali, A.; Saluja, S.S.; Sharma, S.; Al-Allaf, F.A.; Abduljaleel, Z.; Ibrahim, I.A.A.; Abdel-Wahab, A.F.; et al. Phytosterols as a natural anticancer agent: Current status and future perspective. Biomed. Pharmacother. 2017, 88, 786–794. [Google Scholar] [CrossRef]
- Ostlund, R.E., Jr.; McGill, J.B.; Zeng, C.M.; Covey, D.F.; Stearns, J.; Stenson, W.F.; Spilburg, C.A. Gastrointestinal absorption and plasma kinetics of soy Δ5-phytosterols and phytostanols in humans. Am. J. Physiol. -Endocrinol. Metab. 2002, 282, E911–E916. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Santos-Hidalgo, A.B.; Saura-Calixto, F. Common sources and estimated intake of plant sterols in the Spanish diet. J. Agric. Food Chem. 2006, 54, 3462–3471. [Google Scholar] [CrossRef]
- Esche, R.; Müller, L.; Engel, K.H. Online LC-GC-based analysis of minor lipids in various tree nuts and peanuts. J. Agric. Food Chem. 2013, 61, 11636–11644. [Google Scholar] [CrossRef]
- Poli, A.; Marangoni, F.; Corsini, A.; Manzato, E.; Marrocco, W.; Martini, D.; Medea, G.; Visioli, F. Phytosterols, cholesterol control, and cardiovascular disease. Nutrients 2021, 13, 2810. [Google Scholar] [CrossRef]
- Barriuso, B.; Otaegui-Arrazola, A.; Menéndez-Carreño, M.; Astiasarán, I.; Ansorena, D. Sterols heating: Degradation and formation of their ring-structure polar oxidation products. Food Chem. 2012, 135, 706–712. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Berni Canani, R.; Flint, H.J.; Salminen, S.; et al. Activity of cecropin P1 and FA-LL-37 against urogenital microflora. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- International Scientific Association for Probiotics and Prebiotics. Available online: https://isappscience.org/for-scientists/resources/probiotics/ (accessed on 20 March 2025).
- Fredua-Agyeman, M.; Stapleton, P.; Basit, A.W.; Gaisford, S. Microcalorimetric evaluation of a multi-strain probiotic: Interspecies inhibition between probiotic strains. J. Funct. Foods 2017, 36, 357–361. [Google Scholar] [CrossRef]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on non-dairy probiotics and their use in non-dairy based products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef]
- Li, S.; Fan, S.; Ma, Y.; Xia, C.; Yan, Q. Influence of gender, age, and body mass index on the gut microbiota of individuals from South China. Front. Cell. Infect. Microbiol. 2024, 14, 1419884. [Google Scholar] [CrossRef]
- Salvucci, E. The human-microbiome superorganism and its modulation to restore health. Int. J. Food Sci. Nutr. 2019, 70, 781–795. [Google Scholar] [CrossRef]
- Ballan, R.; Battistini, C.; Xavier-Santos, D.; Saad, S.M.I. Interactions of probiotics and prebiotics with the gut microbiota. Prog. Mol. Biol. Transl. Sci. 2020, 171, 265–300. [Google Scholar]
- Parichat, P.; Pongsak, R. Probiotics: Sources, selection and health benefits. Res. J. Biotechnol. 2023, 18, 5. [Google Scholar]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Yan, Y.L.; Hu, Y.; Gänzle, M.G. Prebiotics, FODMAPs and dietary fiber—Conflicting concepts in development of functional food products? Curr. Opin. Food Sci. 2018, 20, 30–37. [Google Scholar] [CrossRef]
- Kherade, M.; Solanke, S.; Tawar, M.; Wankhede, S. Fructooligosaccharides: A comprehensive review. J. Ayurvedic Herb. Med. 2021, 7, 193–200. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzene, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, P.; Tomasik, P. Probiotics, non-dairy prebiotics and postbiotics in nutrition. Appl. Sci. 2020, 10, 1470. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, J.; Ling, Z. Short-chain fatty acids-producing probiotics: A novel source of psychobiotics. Crit. Rev. Food Sci. Nutr. 2022, 62, 7929–7959. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Zielińska, E.; Zieliński, D. Current trends of bioactive peptides—New sources and therapeutic effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Karami, Z.; Pateiro, M.; Lorenzo, J.M. A review on health-promoting, biological, and functional aspects of bioactive peptides in food applications. Biomolecules 2021, 11, 631. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel technologies for the production of bioactive peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Le, T.D.; Suttikhana, I.; Olatunji, O.J. The regulatory mechanisms of biopeptides in insulin and glucose uptake. J. Funct. Foods 2023, 104, 105552. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765. [Google Scholar] [CrossRef]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Gonzalez-de la Rosa, T.; Montserrat-de la Paz, S. Edible insects as a source of biopeptides and their role in immunonutrition. Food Funct. 2024, 15, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- González-Ortega, R.; Šturm, L.; Skrt, M.; Di Mattia, C.D.; Pittia, P.; Poklar Ulrih, N. Liposomal encapsulation of oleuropein and an olive leaf extract: Molecular interactions, antioxidant effects and applications in model food systems. Food Biophys. 2021, 16, 84–97. [Google Scholar] [CrossRef]
- Miedes, D.; Makran, M.; Barberá, R.; Cilla, A.; Alegría, A.; Garcia-Llatas, G. Elderly gastrointestinal conditions increase sterol bioaccessibility in a plant sterol-enriched beverage: Adaptation of the INFOGEST method. Food Funct. 2022, 13, 4478–4485. [Google Scholar] [CrossRef]
- Copado, C.N.; Ixtaina, V.Y.; Tomás, M.C. Enrichment of a fruit-based smoothie beverage with omega-3 fatty acids from microencapsulated chia seed oil. J. Sci. Food Agric. 2024, 104, 3352–3360. [Google Scholar] [CrossRef]
- Limbad, M.; Gutierrez-Maddox, N.; Hamid, N.; Kantono, K.; Liu, T.; Young, T. Microbial and chemical changes during fermentation of coconut water kefir beverage. Appl. Sci. 2023, 13, 7257. [Google Scholar] [CrossRef]
- Tang, B.; Wu, L.; Weng, M.; Chen, J.; Li, Y.; Lai, P. Effect of Hypsizygus marmoreus powder on cooking characteristics, color and texture of wheat noodles. Food Sci. Technol. 2022, 42, e00622. [Google Scholar] [CrossRef]
- Lara-Cervantes, T.D.J.; Carrillo-Inungaray, M.L.; Balderas-Hernández, V.E.; Aguilar-Zárate, P.; Veana, F. Valorization of Parmentiera aculeata juice in growth of probiotics in submerged culture and their postbiotic production: A first approach to healthy foods. Arch. Microbiol. 2022, 204, 679–687. [Google Scholar] [CrossRef]
- Poliński, S.; Topka, P.; Tańska, M.; Kowalska, S.; Czaplicki, S.; Szydłowska-Czerniak, A. Impact of bioactive compounds of plant leaf powders in white chocolate production: Changes in antioxidant properties during the technological processes. Antioxidants 2022, 11, 752. [Google Scholar] [CrossRef]
- Topka, P.; Rudzińska, M.; Poliński, S.; Szydłowska-Czerniak, A.; Tańska, M. Enhancing Antioxidant Activity and Nutritional Profile of Dark Chocolate Through Enrichment with Plant Sterols: A Study on Phytosterol Concentrations and Functional Properties. Foods 2024, 13, 3578. [Google Scholar] [CrossRef]
- Faccinetto-Beltrán, P.; Gómez-Fernández, A.R.; Orozco-Sánchez, N.E.; Pérez-Carrillo, E.; Marín-Obispo, L.M.; Hernández-Brenes, C.; Santacruz, A.; Jacobo-Velázquez, D.A. Physicochemical properties and sensory acceptability of a next-generation functional chocolate added with omega-3 polyunsaturated fatty acids and probiotics. Foods 2021, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Arapović, M.; Puljić, L.; Kajić, N.; Kartalović, B.; Habschied, K.; Mastanjević, K. New Insights in Prebiotic Utilization: A Systematic Review. Processes 2024, 12, 867. [Google Scholar] [CrossRef]
- Singh, S.; Gaur, S. Novel Formulations of Cinnamon-and Orange-Flavored Synbiotic Corn Chocolates with Enhanced Functional Properties and Probiotic Survival Rates. ACS Food Sci. Technol. 2025, 5, 1268–1279. [Google Scholar] [CrossRef]
- Akgül, A.; Sert, D.; Mercan, E. Effects of Bifidobacterium animalis and inulin addition on quality characteristics of synbiotic milk chocolate. J. Food Saf. Food Qual.-Arch. Leb. 2021, 72, 142–149. [Google Scholar] [CrossRef]
- Karimi, R. Viability of Lactobacillus paracasei, L. helveticus and Bifidobacterium lactis in sour cream and considering their effects on textural and sensorial properties of the product. FSCT 2024, 21, 76–85. [Google Scholar]
- Bellinazo, P.L.; Vitola, H.R.S.; Cruxen, C.E.D.S.; Braun, C.L.K.; Hackbart, H.C.D.S.; da Silva, W.P.; Fiorentini, Â.M. Probiotic butter: Viability of Lactobacillus casei strains and bixin antioxidant effect (Bixa orellana L.). J. Food Process. Preserv. 2019, 43, e14088. [Google Scholar] [CrossRef]
- Pereira, C.; Gomes, D.; Dias, S.; Santos, S.; Pires, A.; Viegas, J. Impact of Probiotic and Bioprotective Cultures on the Quality and Shelf Life of Butter and Buttermilk. Dairy 2024, 5, 625. [Google Scholar] [CrossRef]
- Jovanović, M.; Vojvodić, P.; Petrović, M.; Radić, D.; Mitić-Ćulafić, D.; Kostić, M.; Veljović, S. Yogurt fortified with GABA-producing strain and Ganoderma lucidum industrial waste. Czech J. Food Sci. 2022, 40, 456–464. [Google Scholar] [CrossRef]
- Chourasia, R.; Abedin, M.M.; Chiring Phukon, L.; Sahoo, D.; Singh, S.P.; Rai, A.K. Biotechnological approaches for the production of designer cheese with improved functionality. Compr. Rev. Food Sci. Food Saf. 2021, 20, 960–979. [Google Scholar] [CrossRef]
- Figueroa, L.E.; Brugnoni, L.I.; Staffolo, M.D.; Genovese, D.B. Development of a functional dulce de leche (milk jam) with prebiotic carbohydrates and Lacticaseibacillus rhamnosus GG. Int. Dairy J. 2024, 156, 105975. [Google Scholar] [CrossRef]
- Yang, J.; Ciftci, D.; Ciftci, O.N. Fortification of Milk with Omega-3 Fatty Acids Using Novel Bioactive-Carrier Hollow Solid Lipid Micro-and Nanoparticles for Improved Omega-3 Stability and Bioaccessibility. ACS Food Sci. Technol. 2024, 4, 813–820. [Google Scholar] [CrossRef]
- Khalil, R.A.; El-Sawah, T.H.; Alsulami, T.; Zaidalkilani, A.T.; Al-Farga, A.; Elkot, W.F. Development and characterization of functional low-fat frozen dairy dessert enhanced with dried lemongrass powder. Open Chem. 2024, 22, 20240081. [Google Scholar] [CrossRef]
- Sarwar, A.; Aziz, T.; Al-Dalali, S.; Zhang, J.; ud Din, J.; Chen, C.; Cao, Y.; Fatima, H.; Yang, Z. Characterization of synbiotic ice cream made with probiotic yeast Saccharomyces boulardii CNCM I-745 in combination with inulin. LWT 2021, 141, 110910. [Google Scholar] [CrossRef]
- Kainat, F.; Ali, M.; Akbar, A.; Masih, R.; Mehnaz, S.; Sadiq, M.B. Ultrasonic Extraction of Phenolic Compounds from Eggplant Peel and Formulation of Eggplant Peel Extract-Enriched Ice-Cream. J. Food Qual. 2023, 2023, 3267119. [Google Scholar] [CrossRef]
- Goh, A.S.E.; Ningtyas, D.W.; Bhandari, B.; Prakash, S. Investigating phytosterol as a potential functional component in milk through textural, flavour and oral perception study. LWT 2021, 141, 110873. [Google Scholar] [CrossRef]
- Pereira, J.O.; Soares, J.; Monteiro, M.J.; Amaro, A.; Gomes, A.; Pintado, M. Cereal bars functionalized through Bifidobacterium animalis subsp. lactis BB-12 and inulin incorporated in edible coatings of whey protein isolate or alginate. Food Funct. 2019, 10, 6892–6902. [Google Scholar] [CrossRef]
- Sarika, K.; Jayathilakan, K.; Lekshmi, R.G.K.; Priya, E.R.; Greeshma, S.S.; Rajkumar, A. Omega-3 enriched Granola bar: Formulation and Evaluation under different Storage Conditions. Fish. Technol. 2019, 56, 130–139. [Google Scholar]
- Spim, S.R.V.; Castanho, N.R.C.M.; Pistila, A.M.H.; Jozala, A.F.; Oliveira Júnior, J.M.; Grotto, D. Lentinula edodes mushroom as an ingredient to enhance the nutritional and functional properties of cereal bars. J. Food Sci. Technol. 2021, 58, 1349–1357. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. In vitro bioaccessibility of novel low-crystallinity phytosterol nanoparticles in non-fat and regular-fat foods. Food Res. Int. 2019, 123, 27–35. [Google Scholar] [CrossRef]
- Unno, K.; Furushima, D.; Hamamoto, S.; Iguchi, K.; Yamada, H.; Morita, A.; Pervin, M.; Nakamura, Y. Stress-reducing effect of cookies containing matcha green tea: Essential ratio among theanine, arginine, caffeine and epigallocatechin gallate. Heliyon 2019, 5, e01653. [Google Scholar] [CrossRef]
- Knežević-Jugović, Z.; Culetu, A.; Mijalković, J.; Duta, D.; Stefanović, A.; Šekuljica, N.; Đorđević, V.; Antov, M. Impact of different enzymatic processes on antioxidant, nutritional and functional properties of soy protein hydrolysates incorporated into novel cookies. Foods 2022, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biodi, N.; Tredici, M.R. Microalgae as functional ingredients in savory food products: Application to wheat crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef] [PubMed]
- Troncoso Recio, R. Development of Biscuits Enriched with Phytosterols and Fortified with Iron and Calcium Using Ccasein Hydrolysates as Carriers. Ph.D. Thesis, Universidad de Vigo, Spain, 2018. [Google Scholar]
- Meral, R.; Kına, E.; Ceylan, Z. Low-Calorie Cookies Enhanced with Fish Oil-Based Nano-ingredients for Health-Conscious Consumers. ACS Omega 2024, 9, 39159–39169. [Google Scholar] [CrossRef]
- Belmadani, N.; Kassous, W.; Keddar, K.; Amtout, L.; Hamed, D.; Douma-Bouthiba, Z.; Costache, V.; Gérard, P.; Ziar, H. Functional Cyperus esculentus L. Cookies Enriched with the Probiotic Strain Lacticaseibacillus rhamnosus SL42. Foods 2024, 13, 2541. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, J.; Tu, J.; Yu, L.; Niu, L. Matcha-fortified rice noodles: Characteristics of in vitro starch digestibility, antioxidant and eating quality. LWT 2021, 149, 111852. [Google Scholar] [CrossRef]
- Szydłowska-Tutaj, M.; Szymanowska, U.; Tutaj, K.; Domagała, D.; Złotek, U. The addition of reishi and lion’s mane mushroom powder to pasta influences the content of bioactive compounds and the antioxidant, potential anti-inflammatory, and anticancer properties of pasta. Antioxidants 2023, 12, 738. [Google Scholar] [CrossRef]
- Valdez-Meza, E.E.; Raymundo, A.; Figueroa-Salcido, O.G.; Ramírez-Torres, G.I.; Fradinho, P.; Oliveira, S.; de Souza, I.; Suárez-Jiménez, M.; Cárdenas-Torres, F.I.; Islas-Rubio, A.R.; et al. Pasta enrichment with an amaranth hydrolysate affects the overall acceptability while maintaining antihypertensive properties. Foods 2019, 8, 282. [Google Scholar] [CrossRef]
- Konuray, G.; Erginkaya, Z. Quality evaluation of probiotic pasta produced with Bacillus coagulans GBI-30. Innov. Food Sci. Emerg. Technol. 2020, 66, 102489. [Google Scholar] [CrossRef]
- González, A.; Bordón, M.G.; Bustos, M.C.; Córdova Salazar, K.L.; Ribotta, P.D.; Martínez, M.L. Study of the incorporation of native and microencapsulated chia seed oil on pasta properties. Int. J. Food Sci. Technol. 2021, 56, 233–241. [Google Scholar] [CrossRef]
- Garzon, R.; Skendi, A.; Lazo-Velez, M.A.; Papageorgiou, M.; Rosell, C.M. Interaction of dough acidity and microalga level on bread quality and antioxidant properties. Food Chem. 2021, 344, 128710. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Shojaee-Aliabadi, S.; Dadkhodazade, E.; Sheidaei, Z.; Abedi, A.S.; Mirmoghtadaie, L.; Hosseini, S.M. Comparison of properties of breads enriched with omega-3 oil encapsulated in β-glucan and Saccharomyces cerevisiae yeast cells. Appl. Food Biotechnol. 2019, 7, 11–20. [Google Scholar]
- Nartea, A.; Fanesi, B.; Pacetti, D.; Lenti, L.; Fiorini, D.; Lucci, P.; Frega, N.G.; Falcone, P.M. Cauliflower by-products as functional ingredient in bakery foods: Fortification of pizza with glucosinolates, carotenoids and phytosterols. Curr. Res. Food Sci. 2023, 6, 100437. [Google Scholar] [CrossRef] [PubMed]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A.; Zarzycki, P.; Blicharz-Kania, A. The Impact of Ganoderma lucidum (Curtis) P. Karst. Supplementation on the Technological, Chemical, and Quality Parameters of Wheat Bread. Foods 2024, 13, 3101. [Google Scholar] [CrossRef] [PubMed]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A.; Ivanišová, E. Lion’s Mane (Hericium erinaceus (Bull.) Pers.) as a functional component for wheat bread production: Influence on physicochemical, antioxidant, and sensory properties. Int. Agrophysics 2025, 39, 13–28. [Google Scholar] [CrossRef]
- Amorim, C.; Cardoso, B.B.; Silvério, S.C.; Silva, J.C.; Alves, J.I.; Pereira, M.A.; Moreira, R.; Rodrigues, L.R. Designing a functional rice muffin formulated with prebiotic oligosaccharides and sugar reduction. Food Biosci. 2021, 40, 100858. [Google Scholar] [CrossRef]
- Singu, B.D.; Bhushette, P.R.; Annapure, U.S. Thermo-tolerant Saccharomyces cerevisiae var. boulardii coated cornflakes as a potential probiotic vehicle. Food Biosci. 2020, 36, 100668. [Google Scholar] [CrossRef]
- Delgado-Nieblas, C.I.; Ahumada-Aguilar, J.A.; Agramón-Velázquez, S.; Zazueta-Morales, J.J.; Jacobo-Valenzuela, N.; Ruiz-Armenta, X.A.; Ahumada-Aguilar, J.A.; Carrillo-Lopez, A.; Barraza-Elenes, C. Physical, phytochemical and sensory characteristics of extruded high-fiber breakfast cereals prepared by combining carrot by-products with wheat and oat bran. Rev. Mex. Ing. Química. 2021, 20, Alim2441. [Google Scholar] [CrossRef]
- de Alencar, M.G.; de Quadros, C.P.; Luna, A.L.L.P.; Neto, A.F.; da Costa, M.M.; Queiroz, M.A.A.; de Carvalho, F.A.L.; da Silva Araújo, D.H.; Costa Gois, G.; dos Anjos Santos, V.L.; et al. Grape skin flour obtained from wine processing as an antioxidant in beef burgers. Meat Sci. 2022, 194, 108963. [Google Scholar] [CrossRef]
- Pratap, M.; Das, A.K.; Nanda, P.K.; Samiran, B.; Prasant, J.; Akshay, S.; Maity, B. Dragon fruit (Hylocereus undatus) peel as antioxidant dietary fibre on quality and lipid oxidation of chicken nuggets. J. Food Sci. Technol. 2020, 57, 1449–1461. [Google Scholar]
- Martínez, E.; Pardo, J.E.; Álvarez-Ortí, M.; Rabadán, A.; Pardo-Giménez, A.; Alvarruiz, A. Substitution of pork fat by emulsified seed oils in fresh deer sausage (‘Chorizo’) and its impact on the physical, nutritional, and sensory properties. Foods 2023, 12, 828. [Google Scholar] [CrossRef]
- Barbut, S.; Marangoni, A. Organogels use in meat processing–Effects of fat/oil type and heating rate. Meat Sci. 2019, 149, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, L.; Yang, Q. Partial replacement of nitrite with a novel probiotic Lactobacillus plantarum on nitrate, color, biogenic amines and gel properties of Chinese fermented sausages. Food Res. Int. 2020, 137, 109351. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Godoy, C.L.; Costa, L.M.; Guerra, C.A.; de Oliveira, V.S.; de Paula, B.P.; Lemos Junior, W.J.F.; da Silva Duarte, V.; Luchese, R.H.; Rossi Bautitz, I.; Guerra, A.F. Potentially postbiotic-containing preservative to extend the use-by date of raw chicken sausages and semifinished chicken products. Sustainability 2022, 14, 2646. [Google Scholar] [CrossRef]
- Lone, A.B.; Bhat, H.F.; Aït-Kaddour, A.; Hassoun, A.; Aadil, R.M.; Dar, B.N.; Bhat, Z.F. Cricket protein hydrolysates pre-processed with ultrasonication and microwave improved storage stability of goat meat emulsion. Innov. Food Sci. Emerg. Technol. 2023, 86, 103364. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar]
- Almeida, L.C.; Zeferino, J.F.; Branco, C.; Squillaci, G.; Morana, A.; Santos, R.; Ihalainen, P.; Sobhana, L.; Correia, J.P.; Viana, A.S. Polynorepinephrine and polydopamine-bacterial laccase coatings for phenolic amperometric biosensors. Bioelectrochemistry 2025, 161, 108826. [Google Scholar] [CrossRef]
- Patil, N.D.; Bains, A.; Sridhar, K.; Sharma, M.; Dhull, S.B.; Gosken, G.; Chawla, P.; Inbaraj, B.S. Recent advances in the analytical methods for quantitative determination of antioxidants in food matrices. Food Chem. 2024, 463, 141348. [Google Scholar] [CrossRef]
- Joshi, R.; Sathasivam, R.; Park, S.U.; Lee, H.; Kim, M.S.; Baek, I.; Cho, B.K. Application of Fourier transform infrared spectroscopy and multivariate analysis methods for the non-destructive evaluation of phenolics compounds in moringa powder. Agriculture 2021, 12, 10. [Google Scholar] [CrossRef]
- Scano, P. Characterization of the medium infrared spectra of polyphenols of red and white wines by integrating FT IR and UV–Vis spectral data. LWT 2021, 147, 111604. [Google Scholar] [CrossRef]
- Santos, Y.J.D.S.; Malegori, C.; Colnago, L.A.; Vanin, F.M. Application on infrared spectroscopy for the analysis of total phenolic compounds in fruits. Crit. Rev. Food Sci. Nutr. 2024, 64, 2906–2916. [Google Scholar] [CrossRef]
- Wu, Z.; Li, C.; Liu, H.; Lin, T.; Yi, L.; Ren, D.; Gu, Y.; Wang, S. Quantification of caffeine and catechins and evaluation of bitterness and astringency of Pu-erh ripen tea based on portable near-infrared spectroscopy. J. Food Compos. Anal. 2024, 125, 105793. [Google Scholar] [CrossRef]
- Hu, Y.; Sheng, W.; Adade, S.Y.S.S.; Wang, J.; Li, H.; Chen, Q. Comparison of machine learning and deep learning models for detecting quality components of vine tea using smartphone-based portable near-infrared device. Food Control 2025, 174, 111244. [Google Scholar] [CrossRef]
- Carvalho, D.G.; Ranzan, L.; Jacques, R.A.; Trierweiler, L.F.; Trierweiler, J.O. Analysis of total phenolic compounds and caffeine in teas using variable selection approach with two-dimensional fluorescence and infrared spectroscopy. Microchem. J. 2021, 169, 106570. [Google Scholar] [CrossRef]
- Carbas, B.; Machado, N.; Oppolzer, D.; Queiroz, M.; Brites, C.; Rosa, E.A.; Barros, A.I. Prediction of phytochemical composition, in vitro antioxidant activity and individual phenolic compounds of common beans using MIR and NIR spectroscopy. Food Bioprocess Technol. 2020, 13, 962–977. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized enzymes in biosensor applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef]
- Gomes, A.; Mattos, G.J.; Coldibeli, B.; Dekker, R.F.; Dekker, A.M.B.; Sartori, E.R. Covalent attachment of laccase to carboxymethyl-botryosphaeran in aqueous solution for the construction of a voltammetric biosensor to quantify quercetin. Bioelectrochemistry 2020, 135, 107543. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Tyrosinase-based biosensor—A new tool for chlorogenic acid detection in nutraceutical formulations. Materials 2022, 15, 3221. [Google Scholar] [CrossRef]
- Apetrei, R.M.; Cârâc, G.; Bahrim, G.; Camurlu, P. Utilization of enzyme extract self-encapsulated within polypyrrole in sensitive detection of catechol. Enzym. Microb. Technol. 2019, 128, 34–39. [Google Scholar] [CrossRef]
- Tarasov, A.; Stozhko, N.; Bukharinova, M.; Khamzina, E. Biosensors based on phenol oxidases (laccase, tyrosinase, and their mixture) for estimating the total phenolic index in food-related samples. Life 2023, 13, 291. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Li, J.; Liu, J.; Pi, F.; Ping, J. Recent advances of three-dimensional micro-environmental constructions on cell-based biosensors and perspectives in food safety. Biosens. Bioelectron. 2022, 216, 114601. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Fan, J.; Wang, Z.; Li, L. Detection of catechol using an electrochemical biosensor based on engineered Escherichia coli cells that surface-display laccase. Anal. Chim. Acta 2018, 1009, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Shahamirifard, S.A.; Ghaedi, M.; Razmi, Z.; Hajati, S. A simple ultrasensitive electrochemical sensor for simultaneous determination of gallic acid and uric acid in human urine and fruit juices based on zirconia-choline chloride-gold nanoparticles-modified carbon paste electrode. Biosens. Bioelectron. 2018, 114, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Bottari, D.; Pigani, L.; Zanardi, C.; Terzi, F.; Paţurcă, S.V.; Grigorescu, S.D.; Matei, C.; Lete, C.; Lupu, S. Electrochemical sensing of caffeic acid using gold nanoparticles embedded in poly (3, 4-ethylenedioxythiophene) layer by sinusoidal voltage procedure. Chemosensors 2019, 7, 65. [Google Scholar] [CrossRef]
- Della Pelle, F.; Rojas, D.; Silveri, F.; Ferraro, G.; Fratini, E.; Scroccarello, A.; Escarpa, A.; Compagnone, D. Class-selective voltammetric determination of hydroxycinnamic acids structural analogs using a WS 2/catechin-capped AuNPs/carbon black–based nanocomposite sensor. Microchim. Acta 2020, 187, 296. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, L.; Li, X.; He, J.; Huang, W.; Cai, Y.; Wang, J.; Tingting, C.; Du, Y.; Yao, Y. Au–nitrogen-doped graphene quantum dot composites as “on–off” nanosensors for sensitive photo-electrochemical detection of caffeic acid. Nanomaterials 2020, 10, 1972. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Zhang, J.; Li, Y.; Zhang, Y.; Li, Y.; Ye, B.C. Novel electrochemical sensing platform based on integration of molecularly imprinted polymer with Au@ Ag hollow nanoshell for determination of resveratrol. Talanta 2019, 196, 479–485. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Q.; Yang, J.; Chen, S.; Hu, F.; Hu, Y.; Lin, H. Synergistic effects of layer-by-layer films for highly selective and sensitive electrochemical detection of trans-resveratrol. Food Chem. 2021, 338, 127851. [Google Scholar] [CrossRef]
- Huang, S.; Yang, J.; Li, S.; Qin, Y.; Mo, Q.; Chen, L.; Li, X. Highly sensitive molecular imprinted voltammetric sensor for resveratrol assay in wine via polyaniline/gold nanoparticles signal enhancement and polyacrylamide recognition. J. Electroanal. Chem. 2021, 895, 115455. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.C.; Balram, D. Synthesis of amine-functionalized multi-walled carbon nanotube/3D rose flower-like zinc oxide nanocomposite for sensitive electrochemical detection of flavonoid morin. Anal. Chim. Acta 2020, 1095, 71–81. [Google Scholar] [CrossRef]
- Teker, T.; Aslanoglu, M. A novel voltammetric sensing platform based on carbon nanotubes-niobium nanoparticles for the determination of chlorogenic acid. Arab. J. Chem. 2020, 13, 5517–5525. [Google Scholar] [CrossRef]
- Tang, J.; Huang, R.; Zheng, S.; Jiang, S.; Yu, H.; Li, Z.; Wang, J. A sensitive and selective electrochemical sensor based on graphene quantum dots/gold nanoparticles nanocomposite modified electrode for the determination of luteolin in peanut hulls. Microchem. J. 2019, 145, 899–907. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, P.; Wang, C.; Yang, P.; Xie, Y.; Fei, J. Ultra-sensitive amperometric determination of quercetin by using a glassy carbon electrode modified with a nanocomposite prepared from aminated graphene quantum dots, thiolated β-cyclodextrin and gold nanoparticles. Microchim. Acta 2020, 187, 130. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Hu, S.; Chen, X.; Zhang, G.; Liu, G.; Liu, W. Au@ Pt nanoparticles embedded in n-doped graphene as sensor for determination of catechin. Int. J. Electrochem. Sci. 2020, 15, 6778–6789. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef]
- Zrinski, I.; Pungjunun, K.; Martinez, S.; Zavašnik, J.; Stanković, D.; Kalcher, K.; Mehmeti, E. Evaluation of phenolic antioxidant capacity in beverages based on laccase immobilized on screen-printed carbon electrode modified with graphene nanoplatelets and gold nanoparticles. Microchem. J. 2020, 152, 104282. [Google Scholar] [CrossRef]
- Vidal-Casanella, O.; Moreno-Merchan, J.; Granados, M.; Nuñez, O.; Saurina, J.; Sentellas, S. Total polyphenol content in food samples and nutraceuticals: Antioxidant indices versus high performance liquid chromatography. Antioxidants 2022, 11, 324. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, F.; Sun, M.; Tang, Z.; Wu, Y.; Lyu, J.; Khan, K.S.; Yu, J. Development of a high-performance liquid chromatography method for simultaneous quantification of sixteen polyphenols and application to tomato. J. Chromatogr. A 2024, 1733, 465254. [Google Scholar] [CrossRef]
- Rodríguez, I.F.; Cattaneo, F.; Zech, X.V.; Svavh, E.; Pérez, M.J.; Zampini, I.C.; Isla, M.I. Aloja and Añapa, two traditional beverages obtained from prosopis alba pods: Nutritional and functional characterization. Food Biosci. 2020, 35, 100546. [Google Scholar] [CrossRef]
- Huang, X.; Wang, N.; Ma, Y.; Liu, X.; Guo, H.; Song, L.; Zhao, Q.; Hai, D.; Cheng, Y.; Bai, G.; et al. Flaxseed polyphenols: Effects of varieties on its composition and antioxidant capacity. Food Chem. X 2024, 23, 101597. [Google Scholar] [CrossRef]
- Jakabová, S.; Árvay, J.; Šnirc, M.; Lakatošová, J.; Ondejčíková, A.; Golian, J. HPLC-DAD method for simultaneous determination of gallic acid, catechins, and methylxanthines and its application in quantitative analysis and origin identification of green tea. Heliyon 2024, 10, e35819. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Anaguano, M.; Molina, M.; Tupuna-Yerovi, D.S.; Ruales, J. Characterization and quantification of bioactive compounds and antioxidant activity in three different varieties of mango (Mangifera indica L.) peel from the Ecuadorian region using HPLC-UV/VIS and UPLC-PDA. NFS J. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Temerdashev, A.; Atapattu, S.N.; Pamunuwa, G.K. Determination and identification of polyphenols in wine using mass spectrometry techniques. J. Chromatogr. Open 2024, 6, 100175. [Google Scholar] [CrossRef]
- Orozco-Flores, L.A.; Salas, E.; Rocha-Gutiérrez, B.; Peralta-Pérez, M.D.R.; González-Sánchez, G.; Ballinas-Casarrubias, L. Determination of polyphenolic profile of apple pomace (Malus domestica golden delicious variety) by HPLC–MS. ACS Omega 2023, 9, 196–203. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Pikula, K.; Manakov, Y.; Ercisli, S.; Derbush, I.; Kislin, E.; Seryodkin, I.; Savitov, A.; Kalenik, T.; et al. LC-MS/MS screening of phenolic compounds in wild and cultivated grapes Vitis amurensis Rupr. Molecules 2021, 26, 3650. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, P.; Ma, W.; Zeng, X.A.; Fang, Z. Physicochemical properties, antioxidant activities and comprehensive phenolic profiles of tea-macerated Chardonnay wine and model wine. Food Chem. 2024, 436, 137748. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Matwijczuk, A.; Dib, A.; Markut-Miotła, E. Opuntia fruits as food enriching ingredient, the first step towards new functional food products. Molecules 2020, 25, 916. [Google Scholar] [CrossRef]
- Alkhudaydi, H.M.S.; Muriuki, E.N.; Spencer, J.P. Determination of the Polyphenol Composition of Raspberry Leaf Using LC-MS/MS. Molecules 2025, 30, 970. [Google Scholar] [CrossRef]
- Sun, Y.; Geng, Y.; Ma, L. Determination of o-quinones in foods by a derivative strategy combined with UHPLC-MS/MS. Food Chem. 2024, 453, 139638. [Google Scholar] [CrossRef]
- Masek, A.; Latos-Brozio, M.; Kałużna-Czaplińska, J.; Rosiak, A.; Chrzescijanska, E. Antioxidant properties of green coffee extract. Forests 2020, 11, 557. [Google Scholar] [CrossRef]
- Siejak, P.; Smułek, W.; Nowak-Karnowska, J.; Dembska, A.; Neunert, G.; Polewski, K. Bird cherry (Prunus padus) fruit extracts inhibit lipid peroxidation in PC liposomes: Spectroscopic, HPLC, and GC–MS studies. Appl. Sci. 2022, 12, 7820. [Google Scholar] [CrossRef]
- Rohman, A.; Irnawati Windarsih, A.; Riswanto, F.D.O.; Indrayanto, G.; Fadzillah, N.A.; Riyanto, S.; Bakar, N.K.A. Application of chromatographic and spectroscopic-based methods for analysis of Omega-3 (Ω-3 Fas) and Omega-6 (Ω-6 Fas) fatty acids in marine natural products. Molecules 2023, 28, 5524. [Google Scholar] [CrossRef] [PubMed]
- Matos, Â.P.; Matos, A.C.; Moecke, E.H.S. Polyunsaturated fatty acids and nutritional quality of five freshwater fish species cultivated in the western region of Santa Catarina, Brazil. Braz. J. Food Technol. 2019, 22, e2018193. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; González-Barriga, V.; Romero, J.; Rojas, R.; López-Arana, S. Quantification and distribution of omega-3 fatty acids in South Pacific fish and shellfish species. Foods 2020, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Alinafiah, S.; Azlan, A.; Ismail, A.; Mahmud Ab Rashid, N.K. Method development and validation for omega-3 fatty acids (DHA and EPA) in fish using gas chromatography with flame ionization detection (GC-FID). Molecules 2021, 26, 6592. [Google Scholar] [CrossRef]
- Rangasamy, E.; Muthu, V.L.; Dhanabalan, K.; Muniyandi, M. Determination of proximate composition on some edible crabs with special reference to nutritional aspects collected from coastal waters of Rameshwaram, Tamil Nadu. Food Chem. Adv. 2024, 4, 100686. [Google Scholar] [CrossRef]
- Kika, J.; Jakubczyk, K.; Ligenza, A.; Maciejewska-Markiewicz, D.; Szymczykowska, K.; Janda-Milczarek, K. Matcha green tea: Chemical composition, phenolic acids, caffeine and fatty acid profile. Foods 2024, 13, 1167. [Google Scholar] [CrossRef]
- . Van Nieuwenhove, C.P.; Moyano, A.; Castro-Gómez, P.; Fontecha, J.; Sáez, G.; Zárate, G.; Pizarro, P.L. Comparative study of pomegranate and jacaranda seeds as functional components for the conjugated linolenic acid enrichment of yogurt. LWT 2019, 111, 401–407. [Google Scholar] [CrossRef]
- Mikołajczak, N.; Sobiechowska, D.A.; Tańska, M. Edible flowers as a new source of natural antioxidants for oxidative protection of cold-pressed oils rich in omega-3 fatty acids. Food Res. Int. 2020, 134, 109216. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Scolaro, B.; Milne, G.L.; Castro, I.A. Oxidation products from omega-3 and omega-6 fatty acids during a simulated shelf life of edible oils. LWT 2019, 101, 113–122. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Xiang, H.; Sun-Waterhouse, D.; Zhao, Y.; Chen, S.; Li, L.; Wang, Y. UHPLC-Q-Exactive Orbitrap MS/MS-based untargeted lipidomics reveals molecular mechanisms and metabolic pathways of lipid changes during golden pomfret (Trachinotus ovatus) fermentation. Food Chem. 2022, 396, 133676. [Google Scholar] [CrossRef]
- Cui, J.; Cao, J.; Zeng, S.; Ge, J.; Li, P.; Li, C. Comprehensive evaluation of lipidomics profiles in golden threadfin bream (Nemipterus virgatus) and its by-products using UHPLC-Q-exactive Orbitrap-MS. LWT 2022, 165, 11369. [Google Scholar] [CrossRef]
- Irnawati, I.; Windarsih, A.; Fadzillah, N.A.; Azmi, A.A.; Kusbandari, A.; Rohman, A. The use of spectroscopic methods in combination with multivariate data analysis for determination of omega fatty acids: A review. J. Appl. Pharm. Sci. 2024, 14, 045–053. [Google Scholar] [CrossRef]
- Alotaibi, R.F.; AlTilasi, H.H.; Al-Mutairi, A.M.; Alharbi, H.S. Chromatographic and spectroscopic methods for the detection of cocoa butter in cocoa and its derivatives: A review. Heliyon 2024, 10, e31467. [Google Scholar] [CrossRef] [PubMed]
- Chaji, S.; Bajoub, A.; Cravotto, C.; Voss, M.; Tabasso, S.; Hanine, H.; Cravotto, G. Metabolomics in action: Towards producing authentic virgin olive oil rich in bioactive compounds and with distinctive organoleptic features. LWT 2024, 191, 115681. [Google Scholar] [CrossRef]
- Nieto-Ortega, S.; Olabarrieta, I.; Saitua, E.; Arana, G.; Foti, G.; Melado-Herreros, Á. Improvement of oil valorization extracted from fish by-products using a handheld near infrared spectrometer coupled with chemometrics. Foods 2022, 11, 1092. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Y.; Song, X.; Wang, Y.; Liu, S.; Ren, F.; Kong, L.; Zhang, H. Enhancing water solubility of phytosterols through Co-amorphization with food-grade coformers. Curr. Res. Food Sci. 2025, 10, 100984. [Google Scholar] [CrossRef]
- Gies, M.; Servent, A.; Borel, P.; Dhuique-Mayer, C. Phytosterol vehicles used in a functional product modify carotenoid/cholesterol bioaccessibility and uptake by Caco-2 cells. J. Funct. Foods 2020, 68, 103920. [Google Scholar] [CrossRef]
- Lin, L.Y.; Chen, C.W.; Chen, H.C.; Chen, T.L.; Yang, K.M. Developing the procedure-enhanced model of ginger-infused sesame oil based on its flavor and functional properties. Food Chem. X 2024, 21, 101227. [Google Scholar] [CrossRef]
- Guo, Q.; Li, T.; Qu, Y.; Wang, X.; Liu, L.; Liu, H.; Wang, Q. Action of phytosterols on thermally induced trans fatty acids in peanut oil. Food Chem. 2021, 344, 128637. [Google Scholar] [CrossRef]
- Oliva, E.; Palmieri, S.; Della Valle, F.; Eugelio, F.; Fanti, F.; Ciccola, A.; Sergi, M.; Del Carlo, M.; Compagnone, D. Versatile and reliable extraction of phytosterols employing sonochemical synthesized molecularly imprinted polymer. J. Chromatogr. Open 2024, 6, 100174. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Yang, R.; Mao, J.; Jiang, J.; Wang, X.; Zhang, W.; Zhang, Q.; Li, P. Simultaneous determination of tocopherols, carotenoids and phytosterols in edible vegetable oil by ultrasound-assisted saponification, LLE and LC-MS/MS. Food Chem. 2019, 289, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Food Supplements. Available online: https://www.fortunebusinessinsights.com/es/functional-foods-market-102269 (accessed on 30 March 2025).
- Global Market Insights. Available online: https://www.gminsights.com/es/industry-analysis/phytosterols-market (accessed on 29 March 2025).
- Trovato, E.; Arena, K.; La Tella, R.; Rigano, F.; Vinci, R.L.; Dugo, P.; Mondello, L.; Guarnaccia, P. Hemp seed-based food products as functional foods: A comprehensive characterization of secondary metabolites using liquid and gas chromatography methods. J. Food Compos. Anal. 2023, 117, 105151. [Google Scholar] [CrossRef]
- Obranović, M.; Balbino, S.; Repajić, M.; Robić, K.; Ritoša, E.; Dragović-Uzelac, V. Wild nettle (Urtica dioica L.) root: Composition of phytosterols and pentacyclic triterpenes upon habitat diversity. Food Chem. Adv. 2023, 2, 100262. [Google Scholar] [CrossRef]
- Sun, S.; Wang, S.; Li, S.; Wei, L.; Wang, X.; Wang, Y.; Li, B.; Hu, Y.; Wang, L. New insights on the analysis of phytosterols in pollen and anther wall of tree peony (Paeonia ostii ‘Fengdan’) revealed by GC-MS/MS. Anal. Chim. Acta 2022, 1212, 339891. [Google Scholar] [CrossRef]
- Akhtar, N.; Khadim, A.; Siddiqui, A.J.; Musharraf, S.G. Simultaneous quantification and validation of triterpenoids and phytosterols in plant extracts, foods, and nutraceuticals using HPLC-DAD. J. Food Compos. Anal. 2025, 142, 107418. [Google Scholar] [CrossRef]
- Dienaitė, L.; Baranauskienė, R.; Venskutonis, P.R. Lipophilic extracts isolated from European cranberry bush (Viburnum opulus) and sea buckthorn (Hippophae rhamnoides) berry pomace by supercritical CO2–Promising bioactive ingredients for foods and nutraceuticals. Food Chem. 2021, 348, 129047. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Caprioli, G.; Ricciutelli, M.; Cortese, M.; Alesi, A.; Vittori, S.; Sagratini, G. Development of an innovative phytosterol derivatization method to improve the HPLC-DAD analysis and the ESI-MS detection of plant sterols/stanols. Food Res. Int. 2020, 131, 108998. [Google Scholar] [CrossRef]
- dos Santos Caramês, E.T.; Baqueta, M.R.; Pierna, J.A.F.; Pallone, J.A.L.; Baeten, V. Advanced chemometric discrimination of intact organic and conventional brown rice kernels: Comparing NIR benchtop, hand-held NIR and NIR hyperspectral imaging. J. Food Compos. Anal. 2025, 139, 107120. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Xia, Z.; Wang, Y.; Wu, Y.; Gong, Z. Rapid determination of phytosterols by NIRS and chemometric methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 336–341. [Google Scholar] [CrossRef]
- Sun, P.; Xia, B.; Ni, Z.J.; Wang, Y.; Elam, E.; Thakur, K.; Ma, Y.L.; Wei, Z.J. Characterization of functional chocolate formulated using oleogels derived from β-sitosterol with γ-oryzanol/lecithin/stearic acid. Food Chem. 2021, 360, 130017. [Google Scholar] [CrossRef]
- Amaral, J.B.S.; Grisi, C.V.B.; Vieira, E.A.; Ferreira, P.S.; Rodrigues, C.G.; Diniz, N.C.M.; Pinheiro Fernandes Vieira, P.; Albuquerque dos Santos, N.; Correia Gonçalves, M.; Mattos Braga, A.L.; et al. Light cream cheese spread of goat milk enriched with phytosterols: Physicochemical, rheological, and microbiological characterization. LWT 2022, 157, 113103. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in medicine: A long debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Arora, J.; Chauhan, A.; Basniwal, R.K.; Kumari, A.; Rajput, V.D.; Prazdnova, E.V.; Ghosh, A.; Mukerjee, N.; Mandzhieva, S.S.; et al. Advances in characterization of probiotics and challenges in industrial application. Biotechnol. Genet. Eng. Rev. 2024, 40, 3226–3269. [Google Scholar] [CrossRef]
- Georgieva, M.; Andonova, L.; Peikova, L.; Zlatkov, A. Probiotics–Health benefits, classification, quality assurance and quality control–Review. Pharmacia 2014, 61, 22–31. [Google Scholar]
- Boyte, M.E.; Benkowski, A.; Pane, M.; Shehata, H.R. Probiotic and postbiotic analytical methods: A perspective of available enumeration techniques. Front. Microbiol. 2023, 14, 1304621. [Google Scholar] [CrossRef]
- Papadopoulou, O.S.; Argyri, A.A.; Kounani, V.; Tassou, C.C.; Chorianopoulos, N. Use of Fourier transform infrared spectroscopy for monitoring the shelf life and safety of yogurts supplemented with a Lactobacillus plantarum strain with probiotic potential. Front. Microbiol. 2021, 12, 678356. [Google Scholar] [CrossRef]
- Aguinaga Bósquez, J.P.; Oǧuz, E.; Cebeci, A.; Majadi, M.; Kiskó, G.; Gillay, Z.; Kovacs, Z. Characterization and viability prediction of commercial probiotic supplements under temperature and concentration conditioning factors by NIR spectroscopy. Fermentation 2022, 8, 66. [Google Scholar] [CrossRef]
- Peng, M.; Tabashsum, Z.; Anderson, M.; Truong, A.; Houser, A.K.; Padilla, J.; Akmel, A.; Bhatti, J.; Rahaman, S.O.; Biswas, D. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1908–1933. [Google Scholar] [CrossRef]
- Corradini, C.; Lantano, C.; Cavazza, A. Innovative analytical tools to characterize prebiotic carbohydrates of functional food interest. Anal. Bioanal. Chem. 2013, 405, 4591–4605. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Riaz, T.; Mahmood, S.; Liaqat, H.; Mushtaq, A.; Khan, S.; Amin, S.; Qi, X. Recent advances in the production, analysis, and application of galacto-oligosaccharides. Food Rev. Int. 2023, 39, 5814–5843. [Google Scholar] [CrossRef]
- Amanah, H.Z.; Tunny, S.S.; Masithoh, R.E.; Choung, M.G.; Kim, K.H.; Kim, M.S.; Baek, I.; Lee, W.; Cho, B.K. Nondestructive prediction of isoflavones and oligosaccharides in intact soybean seed using Fourier transform near-infrared (FT-NIR) and Fourier transform infrared (FT-IR) spectroscopic techniques. Foods 2022, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lei, T.; Li, G.; Liu, S.; Chu, X.; Hao, D.; Xiao, G.; Khan, A.A.; Ul Haq, T.; Sameeh, M.Y.; et al. Rapid detection of micronutrient components in infant formula milk powder using near-infrared spectroscopy. Front. Nutr. 2023, 10, 1273374. [Google Scholar] [CrossRef] [PubMed]
- Rico-Rodriguez, F.; Strani, L.; Grassi, S.; Lancheros, R.; Serrato, J.C.; Casiraghi, E. Study of Galactooligosaccharides production from dairy waste by FTIR and chemometrics as Process Analytical Technology. Food Bioprod. Process. 2021, 126, 113–120. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Baker, G.A.; Bwambok, D.K.; Bhawawet, N.; Elzey, B.; Siraj, N.; Macchi, S.; Pollard, D.A.; Perez, R.L.; Duncan, A.V.; et al. Molecular (Raman, NIR, and FTIR) spectroscopy and multivariate analysis in consumable products analysis1. Appl. Spectrosc. Rev. 2020, 55, 647–723. [Google Scholar] [CrossRef]
- Ramasamy, T.; Chellapandian, N.; Dharumadurai, D. Separation, characterization and identification of postbiotics from probiotic microbes. In Postbiotics; Academic Press: New York, NY, USA, 2025; pp. 119–129. [Google Scholar]
- Gervasoni, L.F.; Gervasoni, K.; de Oliveira Silva, K.; Mendes, M.E.F.; Maddela, N.R.; Prasad, R.; Winkelstroter, L.K. Postbiotics in active food packaging: The contribution of cellulose nanocomposites. Sustain. Chem. Pharm. 2023, 36, 101280. [Google Scholar] [CrossRef]
- MetaboAnalyst 6.0. Available online: https://www.metaboanalyst.ca/ (accessed on 25 March 2025).
- Lopez, C.M.; Rocchetti, G.; Fontana, A.; Lucini, L.; Rebecchi, A. Metabolomics and gene-metabolite networks reveal the potential of Leuconostoc and Weissella strains as starter cultures in the manufacturing of bread without baker’s yeast. Food Res. Int. 2022, 162, 112023. [Google Scholar] [CrossRef]
- Hsu, P.H.; Lin, Y.J.; Hwang, P.A. Fermentation-induced metabolic changes and bioactive metabolites in Laminaria japonica fermented by Bacillus subtilis. LWT 2025, 216, 117357. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, M.; Yan, Y.; Lu, J.; Sheng, J.; Gui, M.; Ma, X. Comprehensive characterisation of bioactive compounds in Boletus edulis as functional foods to improve muscle atrophy; through whole plant targeted metabolomics, network pharmacology, in vivo and in vitro experiments, molecular docking and molecular dynamics analysis. J. Ethnopharmacol. 2025, 346, 119685. [Google Scholar]
- Kim, S.H.; Singh, D.; Son, S.Y.; Lee, S.; Suh, D.H.; Lee, N.R.; Park, G.-S.; Kang, J.; Lee, C.H. Characterization and temporal dynamics of the intra-and extracellular environments of Lactiplantibacillus plantarum using multi-platform metabolomics. LWT 2023, 175, 114376. [Google Scholar] [CrossRef]
- Bhuva, B.; Gawai, K.M.; Singh, B.P.; Sarkar, P.; Hassan, M.Z.; Kovaleva, E.G.; Hati, S. Production, Characterization and Bio-functional properties of multi-functional peptides from fermented plant-based foods: A Review. Food Biosci. 2025, 64, 105877. [Google Scholar] [CrossRef]
- Chavapradit, C.; Visessanguan, W.; Panjanapongchai, S.; Anal, A.K. Techno-Functional Properties and Potential Applications of Peptides from Agro-Industrial Residues. J. Renew. Mater. 2025, 13, 553–582. [Google Scholar] [CrossRef]
- White, B.L.; Sanders, T.H.; Davis, J.P. Potential ACE-inhibitory activity and nanoLC-MS/MS sequencing of peptides derived from aflatoxin contaminated peanut meal. LWT-Food Sci. Technol. 2014, 56, 537–542. [Google Scholar] [CrossRef]
- Kogiso, K. Assessment of functional components in sika deer and wild boar meats with improvement in processing and flavor and a novel analytical prediction method. Appl. Food Res. 2023, 3, 100343. [Google Scholar] [CrossRef]
- Karki, S.; Prathumpai, W.; Anal, A.K. Microwave-assisted protein extraction from foxtail millet: Optimization, structural characterization, techno-functional properties, and bioactivity of peptides. Int. J. Biol. Macromol. 2025, 293, 139312. [Google Scholar] [CrossRef]
- Kijewska, M.; Zawadzka, M.; Śleziak, M.; Stefanowicz, P. Microwave-assisted solid-phase synthesis of lactosylated peptides for food analytical application. Food Chem. 2024, 433, 137367. [Google Scholar] [CrossRef]
- Guerra-Fajardo, L.D.; Pavón-Pérez, J.; Vallejos-Almirall, A.; Jorquera-Pereira, D. Advances in analytical techniques coupled to in vitro bioassays in the search for new peptides with functional activity in effect-directed analysis. Food Chem. 2022, 397, 133784. [Google Scholar] [CrossRef]
- Della Cerra, F.; Garro, G.; Cozzolino, R.; De Pascale, S.; Esposito, M.; Picariello, G.; Caira, S.; Scaloni, A.; Marino, F.; Addeo, F. Proteolysis and Volatile Compounds in Mediterranean Buffalo Milk Blue Cheese: Quality Determinants and Functional Peptides. J. Agric. Food Res. 2025, 21, 101923. [Google Scholar] [CrossRef]
- Bordiga, M.; Montella, R.; Travaglia, F.; Arlorio, M.; Coïsson, J.D. Characterization of polyphenolic and oligosaccharidic fractions extracted from grape seeds followed by the evaluation of prebiotic activity related to oligosaccharides. Int. J. Food Sci. Technol. 2019, 54, 1283–1291. [Google Scholar] [CrossRef]
- Alyassin, M.; Campbell, G.M.; O’Neill, H.M.; Bedford, M.R. Simultaneous determination of cereal monosaccharides, xylo-and arabinoxylo-oligosaccharides and uronic acids using HPAEC-PAD. Food Chem. 2020, 315, 126221. [Google Scholar] [CrossRef]
- Başaran, U.; Akkbik, M.; Mut, H.; Gülümser, E.; Çopur Doğrusöz, M.; Koçoğlu, S. High-performance liquid chromatography with refractive index detection for the determination of inulin in chicory roots. Anal. Lett. 2018, 51, 83–95. [Google Scholar] [CrossRef]
- Pico, J.; Vidal, N.P.; Widjaja, L.; Falardeau, L.; Albino, L.; Martinez, M.M. Development and assessment of GC/MS and HPAEC/PAD methodologies for the quantification of α-galacto-oligosaccharides (GOS) in dry beans (Phaseolus vulgaris). Food Chem. 2021, 349, 129151. [Google Scholar] [CrossRef] [PubMed]
- Savych, A.; Marchyshyn, S.; Kozyr, H.; Yarema, N. Determination of inulin in the herbal mixtures by GC-MS method. Pharmacia 2021, 68, 181–187. [Google Scholar] [CrossRef]
- Hao, Q.; Nan, T.; Zhou, L.; Kang, L.; Guo, L.; Yu, Y. Rapid simultaneous quantification of fructooligosaccharides in Morinda officianalis by ultra-high performance liquid chromatography. J. Sep. Sci. 2019, 42, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Lima, M.; Nunes, P.C.; de Lourdes de Araújo Silva, B.; da Silva Padilha, C.V.; do Bonfim, T.H.F.; Stamford, T.L.M.; da Silva Vasconcelos, M.A.; de Souza Aquino, J. Determining 1-kestose, nystose and raffinose oligosaccharides in grape juices and wines using HPLC: Method validation and characterization of products from Northeast Brazil. J. Food Sci. Technol. 2019, 56, 4575–4584. [Google Scholar] [CrossRef]
- Wu, T.; Guo, S.; Liu, K.; Kwok, L.Y.; Wang, J.; Zhang, H. Exploring the postbiotic potential of multi-strain pasteurized fermented milk: A metabolomics study. LWT 2024, 209, 116802. [Google Scholar] [CrossRef]
- Lingua, M.S.; Gies, M.; Descalzo, A.M.; Servent, A.; Páez, R.B.; Baroni, M.V.; Blajman, J.E.; Dhuique-Mayer, C. Impact of storage on the functional characteristics of a fermented cereal product with probiotic potential, containing fruits and phytosterols. Food Chem. 2022, 370, 130993. [Google Scholar] [CrossRef]
- Yao, G.; Wang, X.; Yang, M.; Chen, F.; Ling, Y.; Liu, T.; Xing, S.; Yao, M.; Zhang, F. Co-immobilization of bi-lipases on magnetic nanoparticles as an efficient catalyst for synthesis of functional oil rich in diacylglycerols, phytosterol esters and α-linolenic acid. LWT 2020, 129, 109522. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Cui, R.; Zhang, C.; Xu, M.; Liu, W.; Xu, L.; Song, L. Effect of screw pressing temperature on apricot (Prunus armeniaca L.) kernels oil quality properties and apricot kernels protein isolate functional properties. LWT 2024, 213, 117002. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Zadeh, A.N.; Tayefe, M.; Zolqadri, R.; Ramezani, A.; Mazloomi, N.; Sarabandi, K. Biological conversion of red garlic (Allium sativum L) protein to bioactive peptides: ACE-DPP-IV inhibitory, and Techno-functional features. Appl. Food Res. 2024, 4, 100551. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/publications/fao-flagship-publications/the-state-of-food-security-and-nutrition-in-the-world/en (accessed on 25 March 2025).
- Vasile, C.; Sivertsvik, M. (Eds.) Food Packaging: Materials and Technologies; MDPI: Basel, Switzerland, 2019. [Google Scholar]
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. Food packaging materials with special reference to biopolymers-properties and applications. Chem. Afr. 2023, 6, 117–144. [Google Scholar] [CrossRef]
- Alamri, M.S.; Qasem, A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food packaging’s materials: A food safety perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef] [PubMed]
- Karwowska, M.; Stadnik, J.; Stasiak, D.M.; Wójciak, K.; Lorenzo, J.M. Strategies to improve the nutritional value of meat products: Incorporation of bioactive compounds, reduction or elimination of harmful components and alternative technologies. Int. J. Food Sci. Technol. 2021, 56, 6142–6156. [Google Scholar] [CrossRef]
- González-López, M.E.; Calva-Estrada, S.D.J.; Gradilla-Hernández, M.S.; Barajas-Álvarez, P. Current trends in biopolymers for food packaging: A review. Front. Sustain. Food Syst. 2023, 7, 1225371. [Google Scholar] [CrossRef]
- Dixit, V.; Joseph Kamal, S.W.; Bajrang Chole, P.; Dayal, D.; Chaubey, K.K.; Pal, A.K.; Xavier, J.; Manjunath, B.T.; Bachheti, R.K. Functional foods: Exploring the health benefits of bioactive compounds from plant and animal sources. J. Food Qual. 2023, 2023, 5546753. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Hakim, L.; Gaikwad, K.K. Active packaging materials. Curr. Food Sci. Technol. Rep. 2023, 1, 123–132. [Google Scholar] [CrossRef]
- Lee, D.S.; Wang, H.J.; Jaisan, C.; An, D.S. Active food packaging to control carbon dioxide. Packag. Technol. Sci. 2022, 35, 213–227. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Vasiljevic, Z.Z.; Auger, S.; Vidic, J. Metal oxide nanoparticles for safe active and intelligent food packaging. Trends Food Sci. Technol. 2021, 116, 655–668. [Google Scholar] [CrossRef]
- Kumar, L.; Deshmukh, R.K.; Hakim, L.; Gaikwad, K.K. Halloysite nanotube as a functional material for active food packaging application: A review. Food Bioprocess Technol. 2024, 17, 33–46. [Google Scholar] [CrossRef]
- Jain, P.; Kumar, L.; Singh, S.; Gaikwad, K.K. Catechu (Senegalia catechu) based oxygen scavenger for active food packaging: A sustainable alternative. Sustain. Chem. Pharm. 2024, 37, 101350. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, Y.S. Novel natural phenolic compound-based oxygen scavenging system for active packaging applications. J. Food Meas. Charact. 2016, 10, 533–538. [Google Scholar] [CrossRef]
- Alves, J.; Gaspar, P.D.; Lima, T.M.; Silva, P.D. What is the role of active packaging in the future of food sustainability? A systematic review. J. Sci. Food Agric. 2023, 103, 1004–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, H.; Rhim, J.W.; Cao, J.; Jiang, W. Effective strategies of sustained release and retention enhancement of essential oils in active food packaging films/coatings. Food Chem. 2022, 367, 130671. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Baican, M. Progresses in food packaging, food quality, and safety—Controlled-release antioxidant and/or antimicrobial packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef] [PubMed]
- Kuai, L.; Liu, F.; Chiou, B.S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled release of antioxidants from active food packaging: A review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Petka-Poniatowska, K. Antimicrobial compounds in food packaging. Int. J. Mol. Sci. 2023, 24, 2457. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Gaikwad, K.K. Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Convers. Biorefinery 2024, 14, 4419–4440. [Google Scholar] [CrossRef]
- Lajqi, V. A review on active food packing as innovation strategies for the future. Int. J. Bus. Technol. 2021, 9, 1–8. [Google Scholar]
- Zaitoon, A.; Luo, X.; Lim, L.T. Triggered and controlled release of active gaseous/volatile compounds for active packaging applications of agri-food products: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 541–579. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Lamsaf, H.; Sebastian, C.V.; Cerqueira, M.A.; Pastrana, L.; Teixeira, J.A. Active packaging systems based on metal and metal oxide nanoparticles. In Nanotechnology-Enhanced Food Packaging; Wiley Online Library: Hoboken, NJ, USA, 2022; pp. 143–181. [Google Scholar]
- Joshi, N.C.; Negi, P.B.; Gururani, P. A review on metal/metal oxide nanoparticles in food processing and packaging. Food Sci. Biotechnol. 2024, 33, 1307–1322. [Google Scholar] [CrossRef]
- Ganesh, A.; Rajan, R.; Simon, S.M.; Thankachan, S. An overview on metal oxide incorporated bionanocomposites and their potential applications. Nano-Struct. Nano-Objects 2024, 38, 101126. [Google Scholar] [CrossRef]
- Terzioğlu, P. Metal/Metal Oxide Nanomaterials in Biodegradable Food Packaging. In Functional Nanomaterials and Nanocomposites for Biodegradable Food Packaging; Springer Nature: Singapore, 2025; pp. 75–107. [Google Scholar]
- Stuparu-Cretu, M.; Braniste, G.; Necula, G.A.; Stanciu, S.; Stoica, D.; Stoica, M. Metal oxide nanoparticles in food packaging and their influence on human health. Foods 2023, 12, 1882. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, Y.; Deng, Y. Latest advances in active materials for food packaging and their application. Foods 2023, 12, 4055. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A review on active packaging for quality and safety of foods: Current trends, applications, prospects and challenges. Food Packag. Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Azevedo, A.G.; Barros, C.; Miranda, S.; Machado, A.V.; Castro, O.; Silva, B.; Saraiva, M.; Sanches Silva, A.; Pastrana, L.; Sousa Carneiro, O.; et al. Active flexible films for food packaging: A review. Polymers 2022, 14, 2442. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable active packaging with controlled release: Principles, progress, and prospects. ACS Food Sci. Technol. 2022, 2, 1166–1183. [Google Scholar] [CrossRef]
- Azeredo, H.M.; Correa, D.S. Smart choices: Mechanisms of intelligent food packaging. Curr. Res. Food Sci. 2021, 4, 932–936. [Google Scholar] [CrossRef]
- Luo, X.; Zaitoon, A.; Lim, L.T. A review on colorimetric indicators for monitoring product freshness in intelligent food packaging: Indicator dyes, preparation methods, and applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2489–2519. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Ezati, P.; Rhim, J.W. Recent advances in intelligent food packaging applications using natural food colorants. ACS Food Sci. Technol. 2021, 1, 124–138. [Google Scholar] [CrossRef]
- Almasi, H.; Forghani, S.; Moradi, M. Recent advances on intelligent food freshness indicators; an update on natural colorants and methods of preparation. Food Packag. Shelf Life 2022, 32, 100839. [Google Scholar] [CrossRef]
- Pirsa, S.; Sani, I.K.; Mirtalebi, S.S. Nano-biocomposite based color sensors: Investigation of structure, function, and applications in intelligent food packaging. Food Packag. Shelf Life 2022, 31, 100789. [Google Scholar] [CrossRef]
- Echegaray, N.; Guzel, N.; Kumar, M.; Guzel, M.; Hassoun, A.; Lorenzo, J.M. Recent advancements in natural colorants and their application as coloring in food and in intelligent food packaging. Food Chem. 2023, 404, 134453. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xu, H.; McClements, D.J.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent advances in intelligent food packaging materials: Principles, preparation and applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in smart packaging concepts for food: An extensive review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef]
- Jamwal, V.; Mittal, A. Recent progresses in nanocomposite films for food-packaging applications: Synthesis strategies, technological advancements, potential risks and challenges. Food Rev. Int. 2024, 40, 3634–3665. [Google Scholar] [CrossRef]
- Kushwaha, S.P.; Hasan, S.M.; Ved, A.; Kumar, P.; Singh, K.; Shukla, K.S.; Kumar, A.; Shoaib, A. Nanotechnology in the Fabrication of Improved, Active and Smart Packaging Materials. In Nanotechnology in Food Packaging; Springer Nature: Cham, Switzerland, 2025; pp. 89–114. [Google Scholar]
- Food Contact Materials. Legislation. Available online: https://food.ec.europa.eu/food-safety/chemical-safety/food-contact-materials/legislation_en (accessed on 25 March 2025).
- Mkhari, T.; Adeyemi, J.O.; Fawole, O.A. Recent Advances in the Fabrication of Intelligent Packaging for Food Preservation: A Review. Processes 2025, 13, 539. [Google Scholar] [CrossRef]
- Palazzo, M.; Vollero, A.; Siano, A. Intelligent packaging in the transition from linear to circular economy: Driving research in practice. J. Clean. Prod. 2023, 388, 135984. [Google Scholar] [CrossRef]
- Alias, A.R.; Wan, M.K.; Sarbon, N.M. Emerging materials and technologies of multi-layer film for food packaging application: A review. Food Control 2022, 136, 108875. [Google Scholar] [CrossRef]
- Dziadowiec, D.; Matykiewicz, D.; Szostak, M.; Andrzejewski, J. Overview of the cast polyolefin film extrusion technology for multi-layer packaging applications. Materials 2023, 16, 1071. [Google Scholar] [CrossRef]
- Viacava, G.E.; Ansorena, M.R.; Marcovich, N.E. Multilayered films for food packaging. In Nanostructured Materials for Food Packaging Applications; Elsevier: New York, NY, USA, 2024; pp. 447–475. [Google Scholar]
- Huang, H.D.; Ren, P.G.; Zhong, G.J.; Olah, A.; Li, Z.M.; Baer, E.; Zhu, L. Promising strategies and new opportunities for high barrier polymer packaging films. Prog. Polym. Sci. 2023, 144, 101722. [Google Scholar] [CrossRef]
- Rovera, C.; Ghaani, M.; Farris, S. Nano-inspired oxygen barrier coatings for food packaging applications: An overview. Trends Food Sci. Technol. 2020, 97, 210–220. [Google Scholar] [CrossRef]
- Zabihzadeh Khajavi, M.; Ebrahimi, A.; Yousefi, M.; Ahmadi, S.; Farhoodi, M.; Mirza Alizadeh, A.; Taslikh, M. Strategies for producing improved oxygen barrier materials appropriate for the food packaging sector. Food Eng. Rev. 2020, 12, 346–363. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.; Kim, D.; Ju, B.K. Layer-by-layer assembled nano-composite multilayer gas barrier film manufactured with stretchable substrate. Appl. Sci. 2021, 11, 5794. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Zhu, Y.; Zheng, C. Recent advances in the construction and properties of carbon-based nanofillers/polymer composites with segregated network structures. Mater. Today Commun. 2023, 36, 106773. [Google Scholar] [CrossRef]
- Mandal, S.; Roy, D.; Mukhopadhyay, K.; Dwivedi, M.; Joshi, M. Mechanistic insight into the role of the aspect ratio of nanofillers in the gas barrier properties of polymer nanocomposite thin films. RSC Appl. Interfaces 2024, 1, 977–991. [Google Scholar] [CrossRef]
- Ashfaq, J.; Channa, I.A.; Memon, A.G.; Chandio, I.A.; Chandio, A.D.; Shar, M.A.; Alsalhi, M.S.; Devanesan, S. Enhancement of thermal and gas barrier properties of graphene-based nanocomposite films. ACS Omega 2023, 8, 41054–41063. [Google Scholar] [CrossRef]
- Yan, M.R.; Hsieh, S.; Ricacho, N. Innovative food packaging, food quality and safety, and consumer perspectives. Processes 2022, 10, 747. [Google Scholar] [CrossRef]
- Tyagi, P.; Salem, K.S.; Hubbe, M.A.; Pal, L. Advances in barrier coatings and film technologies for achieving sustainable packaging of food products—A review. Trends Food Sci. Technol. 2021, 115, 461–485. [Google Scholar] [CrossRef]
- Sari, A.N.A.; Warsiki, E.; Kartika, I.A. Innovation of oxygen indicator for packaging leak detector: A review. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 749, p. 012009. [Google Scholar]
- Heo, W.; Lim, S. A Review on Gas Indicators and Sensors for Smart Food Packaging. Foods 2024, 13, 3047. [Google Scholar] [CrossRef]
- Ibrahim, S.; Fahmy, H.; Salah, S. Application of interactive and intelligent packaging for fresh fish shelf-life monitoring. Front. Nutr. 2021, 8, 677884. [Google Scholar] [CrossRef]
- Li, S.; Hu, X.; Zhang, S.; Zhao, J.; Wang, R.; Wang, L.; Wang, X.; Yuan, Y.; Yue, T.; Cai, R.; et al. A versatile bilayer smart packaging based on konjac glucomannan/alginate for maintaining and monitoring seafood freshness. Carbohydr. Polym. 2024, 340, 122244. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Devgan, K.; Kumar, A.; Kaur, P.; Mahajan, P. Active and passive modified atmosphere packaging: Recent advances. In Nonthermal Food Engineering Operations; Wiley: Hoboken, NJ, USA, 2024; pp. 225–259. [Google Scholar] [CrossRef]
- Xu, C.C.; Lu, M.Y.; Li, R.; Liu, D.K.; Guo, C.X. Super-atmospheric oxygen modified atmosphere package of whole and fresh-cut fruits and vegetables. Food Bioprocess Technol. 2024, 17, 2499–2518. [Google Scholar] [CrossRef]
- Czerwiński, K.; Rydzkowski, T.; Wróblewska-Krepsztul, J.; Thakur, V.K. Towards impact of modified atmosphere packaging (MAP) on shelf-life of polymer-film-packed food products: Challenges and sustainable developments. Coatings 2021, 11, 1504. [Google Scholar] [CrossRef]
- Qu, P.; Zhang, M.; Fan, K.; Guo, Z. Microporous modified atmosphere packaging to extend shelf life of fresh foods: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 51–65. [Google Scholar] [CrossRef]
- Kim, D.; Thanakkasaranee, S.; Lee, K.; Sadeghi, K.; Seo, J. Smart packaging with temperature-dependent gas permeability maintains the quality of cherry tomatoes. Food Biosci. 2021, 41, 100997. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Y.; Fang, Z.; Wang, Z.C.; Nan, Y.; Shi, H.; Zhang, H.; Song, W.; Gu, H. Modified atmosphere packaging and plant extracts synergistically enhance the preservation of meat: A review. Food Control 2024, 164, 110622. [Google Scholar] [CrossRef]
- Paulsen, E.; Barrios, S.; Bogdanoff, N.; Leandro, G.C.; Valencia, G.A. Recent progress in modified atmosphere packaging and biopolymeric films and coatings for fresh strawberry shelf-life extension. Packag. Technol. Sci. 2024, 37, 619–640. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food preservation techniques and nanotechnology for increased shelf life of fruits, vegetables, beverages and spices: A review. Environ. Chem. Lett. 2021, 19, 1715–1735. [Google Scholar] [CrossRef]
- Gabrić, D.; Kurek, M.; Ščetar, M.; Brnčić, M.; Galić, K. Effect of non-thermal food processing techniques on selected packaging materials. Polymers 2022, 14, 5069. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Anderson, N.M.; Benyathiar, P.; Mishra, D.K. Aseptic processing and packaging. In Food Safety Engineering; Springer: Berlin/Heidelberg, Germany, 2020; pp. 661–692. [Google Scholar]
- Bhunia, K.; Tang, J.; Sablani, S.S. Microwave-based sustainable in-container thermal pasteurization and sterilization technologies for foods. Sustain. Food Technol. 2024, 2, 926–944. [Google Scholar] [CrossRef]
- Stoica, M.; Bichescu, C.I.; Crețu, C.M.; Dragomir, M.; Ivan, A.S.; Podaru, G.M.; Stoica, D.; Stuparu-Crețu, M. Review of Bio-Based Biodegradable Polymers: Smart Solutions for Sustainable Food Packaging. Foods 2024, 13, 3027. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Panda, J.; Mishra, A.K.; Nath, P.C.; Nayak, P.K.; Mahapatra, U.; Sharma, M.; Chopra, H.; Mohanta, Y.K.; Sridhar, K. Recent advances in sustainable biopolymer-based nanocomposites for smart food packaging: A review. Int. J. Biol. Macromol. 2024, 279, 135583. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, A.P.; de Albuquerque, T.L. Innovations in food packaging: From bio-based materials to smart packaging systems. Processes 2024, 12, 2085. [Google Scholar] [CrossRef]
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-based sustainable food packaging materials: Challenges, solutions, and applications. Foods 2023, 12, 2422. [Google Scholar] [CrossRef]
- Maurizzi, E.; Bigi, F.; Quartieri, A.; De Leo, R.; Volpelli, L.A.; Pulvirenti, A. The green era of food packaging: General considerations and new trends. Polymers 2022, 14, 4257. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Peerzada, J.G.; Ojha, N.; Jaabir, M.M.; Lakshmi, B.; Hannah, S.; Chidambaram, R.; Sinclair, B.J.; Krishna, K.; Muthuramalingam, P.; Mossa, A.T. Advancements in eco-friendly food packaging through nanocomposites: A review. Polym. Bull. 2024, 81, 5753–5792. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U. Novel biopolymer-based sustainable composites for food packaging applications: A narrative review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.H. Advances in functional biopolymer-based nanocomposites for active food packaging applications. Polymers 2021, 13, 4198. [Google Scholar] [CrossRef]
- Ibrahim, I.D.; Hamam, Y.; Sadiku, E.R.; Ndambuki, J.M.; Kupolati, W.K.; Jamiru, T.; Eze, A.A.; Snyman, J. Need for sustainable packaging: An overview. Polymers 2022, 14, 4430. [Google Scholar] [CrossRef] [PubMed]
- Stark, N.M.; Matuana, L.M. Trends in sustainable biobased packaging materials: A mini review. Mater. Today Sustain. 2021, 15, 100084. [Google Scholar] [CrossRef]
- Ng, W.L.; Chua, C.K.; Shen, Y.F. Print me an organ! Why we are not there yet. Prog. Polym. Sci. 2019, 97, 101145. [Google Scholar] [CrossRef]
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone (PCL) based polymer and composites. Phys. Sci. Rev. 2023, 8, 4391–4414. [Google Scholar] [CrossRef]
- Peñas, M.I.; Pérez-Camargo, R.A.; Hernández, R.; Müller, A.J. A review on current strategies for the modulation of thermomechanical, barrier, and biodegradation properties of poly (butylene succinate) (PBS) and its random copolymers. Polymers 2022, 14, 1025. [Google Scholar] [CrossRef]
- Kim, J.; Yun, H.; Won, S.; Lee, D.; Baek, S.; Heo, G.; Park, S.; Jin, H.-J.; Kwak, H.W. Comparative degradation behavior of polybutylene succinate (PBS), used PBS, and PBS/Polyhydroxyalkanoates (PHA) blend fibers in compost and marine–sediment interfaces. Sustain. Mater. Technol. 2024, 41, e01065. [Google Scholar] [CrossRef]
- Muranaka, Y.; Koike, T.; Osuga, T.; Maki, T. Degradation behavior of polybutylene succinate with fillers. Polymer Degradation and Stability 2025, 111266. [Google Scholar] [CrossRef]
- Chrysafi, I.; Ainali, N.M.; Xanthopoulou, E.; Zamboulis, A.; Bikiaris, D.N. Thermal degradation mechanism and decomposition kinetic studies of poly (ethylene succinate)/hemp fiber composites. J. Compos. Sci. 2023, 7, 216. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Dahab, I.; Ragab, E.M.; Abdelsalam, O.A.; Mustafa, A. Bio-and oxo-degradable plastics: Insights on facts and challenges. Polym. Adv. Technol. 2021, 32, 1981–1996. [Google Scholar] [CrossRef]
- Heimowska, A. Environmental Degradation of Oxo-Biodegradable Polyethylene Bags. Water 2023, 15, 4059. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bhoi, P.R. An overview of non-biodegradable bioplastics. J. Clean. Prod. 2021, 294, 126218. [Google Scholar] [CrossRef]
- Sinha, S. An overview of biopolymer-derived packaging material. Polym. Renew. Resour. 2024, 15, 193–209. [Google Scholar] [CrossRef]
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Recent developments in smart food packaging focused on biobased and biodegradable polymers. Front. Sustain. Food Syst. 2021, 5, 630393. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Okolie, O.; Kumar, A.; Edwards, C.; Lawton, L.A.; Oke, A.; McDonald, S.; Takur, V.K.; Njuguna, J. Bio-based sustainable polymers and materials: From processing to biodegradation. J. Compos. Sci. 2023, 7, 213. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 2020, 7, 100028. [Google Scholar] [CrossRef]
- Ganie, S.A.; Ali, A.; Mir, T.A.; Li, Q. Physical and chemical modification of biopolymers and biocomposites. In Advanced Green Materials; Woodhead Publishing: Cornwall, UK, 2021; pp. 359–377. [Google Scholar]
- Fredi, G.; Dorigato, A. Compatibilization of biopolymer blends: A review. Adv. Ind. Eng. Polym. Res. 2024, 7, 373–404. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, X. Principle of biopolymer plasticization. In Processing and Development of Polysaccharide-Based Biopolymers for Packaging Applications; Elsevier: New York, NY, USA, 2020; pp. 1–19. [Google Scholar]
- Taherimehr, M.; YousefniaPasha, H.; Tabatabaeekoloor, R.; Pesaranhajiabbas, E. Trends and challenges of biopolymer-based nanocomposites in food packaging. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5321–5344. [Google Scholar] [CrossRef]
- Mathew, S.S.; Jaiswal, A.K.; Jaiswal, S. A comprehensive review on hydrophobic modification of biopolymer composites for food packaging applications. Food Packag. Shelf Life 2025, 48, 101464. [Google Scholar] [CrossRef]
- Dutta, D.; Sit, N. A comprehensive review on types and properties of biopolymers as sustainable bio-based alternatives for packaging. Food Biomacromolecules 2024, 1, 58–87. [Google Scholar] [CrossRef]
- Kumar, R.; Lalnundiki, V.; Shelare, S.D.; Abhishek, G.J.; Sharma, S.; Sharma, D.; Kumar, A.; Abbas, M. An investigation of the environmental implications of bioplastics: Recent advancements on the development of environmentally friendly bioplastics solutions. Environ. Res. 2024, 244, 117707. [Google Scholar] [CrossRef] [PubMed]
- Mülhaupt, R. Green polymer chemistry and bio-based plastics: Dreams and reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Akpoghelie, P.O.; Joshua, O.A.; Isoje, E.F.; Igbuku, U.A.; Essaghah, A.E.A. Comparative analysis of the environmental impact of biopolymer-based and conventional plastic packaging in food engineering applications. Al-Mustaqbal J. Sustain. Eng. Sci. 2024, 2, 4. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Jan-Georg, R.; Langer, R.; Giovanni, T. Bioplastics for a circular economy. Nat. Reviews. Mater. 2022, 7, 117–137. [Google Scholar]
- Kogje, M.; Satdive, A.; Mestry, S.; Mhaske, S.T. Biopolymers: A comprehensive review of sustainability, environmental impact, and lifecycle analysis. Iran. Polym. J. 2025, 1–44. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Monje Martínez, B.; Alonso, R.; Agostinis, L.; et al. Bio-based packaging: Materials, modifications, industrial applications and sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Alias, N.H.; Abdullah, N.; Othman, N.H.; Marpani, F.; Zainol, M.M.; Shayuti, M.S.M. Sustainability challenges and future perspectives of biopolymer. In Biopolymers: Recent Updates, Challenges and Opportunities; Springer International Publishing: Cham, Switzerland, 2022; pp. 373–389. [Google Scholar]
- Poletto, M. (Ed.) Composites from Renewable and Sustainable Materials; BoD–Books on Demand: Norderstedt, Germany, 2016. [Google Scholar]
- Wellenreuther, C.; Wolf, A.; Zander, N. Cost competitiveness of sustainable bioplastic feedstocks—A Monte Carlo analysis for polylactic acid. Clean. Eng. Technol. 2022, 6, 100411. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Makia, R.S.; Joshua, O.A.; Akpoghelie, P.O.; Gaaz, T.S.; Jikah, A.N.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; et al. A review on food spoilage mechanisms, food borne diseases and commercial aspects of food preservation and processing. Food Chem. Adv. 2024, 5, 100852. [Google Scholar] [CrossRef]
- Su, Q.; Zhao, X.; Zhang, X.; Wang, Y.; Zeng, Z.; Cui, H.; Wang, C. Nano functional food: Opportunities, development, and future perspectives. Int. J. Mol. Sci. 2022, 24, 234. [Google Scholar] [CrossRef]
- Panou, A.; Karabagias, I.K. Composition, Properties, and Beneficial Effects of Functional Beverages on Human Health. Beverages 2025, 11, 40. [Google Scholar] [CrossRef]
- Deschênes, L. Packaging Functional Foods: From Basic Requirements to Nano Perspectives. In Functional Food Ingredients and Nutraceuticals; Shi, J., Ed.; CRC Press: Boulder, CO, USA, 2015. [Google Scholar]
- Chhikara, S.; Kumar, D. Edible coating and edible film as food packaging material: A review. J. Packag. Technol. Res. 2022, 6, 1–10. [Google Scholar] [CrossRef]
- Francis, D.V.; Dahiya, D.; Gokhale, T.; Nigam, P.S. Sustainable packaging materials for fermented probiotic dairy or non-dairy food and beverage products: Challenges and innovations. AIMS Microbiol. 2024, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Do, Y.V.; Le, Q.N.T.; Nghia, N.H.; Vu, N.D.; Tran, N.T.Y.; Bay, N.T.; Tran, T.T.; Bach, L.G.; Dao, T.P. Assessment of the changes in product characteristics, total ascorbic acid, total flavonoid content, total polyphenol content and antioxidant activity of dried soursop fruit tea (Annona muricata L.) during product storage. Food Sci. Nutr. 2024, 12, 2679–2691. [Google Scholar] [CrossRef]
- Li, J.; Xu, B.; Yu, H.; Ma, Q.; Zhao, D.; Dou, X.; Liu, L. Impact of packaging film colour on phytosterol photooxidation in safflower seed oil. LWT 2015, 224, 117818. [Google Scholar] [CrossRef]
- Hasanshahi, M.; ZamaniBahramabadi, E.; Nazoori, F. Preservation of fresh pistachio fruit by some packaging types in cold storage. J. Food Meas. Charact. 2023, 17, 6566–6576. [Google Scholar] [CrossRef]
- Rizzo, V.; Dattilo, S.; Barbagallo, S.; Puglisi, C.; Muratore, G. Packaging effects on highly nutritional value beverage obtained by a mix of typical sicilian fruits in accelerated storage. Food Packag. Shelf Life 2023, 38, 101138. [Google Scholar] [CrossRef]
- Yan, H.; Li, W.; Chen, H.; Liao, Q.; Xia, M.; Wu, D.; Liu, C.; Chen, J.; Zou, L.; Peng, L.; et al. Effects of storage temperature, packaging material and wash treatment on quality and shelf life of Tartary buckwheat microgreens. Foods 2022, 11, 3630. [Google Scholar] [CrossRef]
- Dantas, F.B.H.; Alvim, I.D.; Miguel, A.M.R.D.O.; Alves, R.M.V.; Marangoni Júnior, L. Influence of different packaging materials on the stability of Omega-3-Enriched milk powder during storage. J. Packag. Technol. Res. 2022, 6, 225–233. [Google Scholar] [CrossRef]
- Yenipazar, H.; Şahin-Yeşilçubuk, N. Effect of packaging and encapsulation on the oxidative and sensory stability of omega-3 supplements. Food Sci. Nutr. 2023, 11, 1426–1440. [Google Scholar] [CrossRef]
- Ebrahimi Monfared, K.; Gharachorloo, M.; Jafarpour, A.; Varvani, J. Effect of storage and packaging conditions on physicochemical and bioactivity of matcha-enriched muesli containing probiotic bacteria. J. Food Process. Preserv. 2022, 46, e16878. [Google Scholar] [CrossRef]
- Nazoori, F.; ZamaniBahramabadi, E.; Hosseinipoor, B.; Mirdehghan, S.H. Shelf life of fresh in-hull pistachio in perforated polyethylene packaging. J. Food Meas. Charact. 2021, 15, 5528–5536. [Google Scholar] [CrossRef]
- Thakur, A.; Thakur, N.S.; Gautam, S. Effect of packaging on phenols, flavonoids and antioxidant characteristics of mechanical cabinet dried wild pomegranate (Punica granatum L.) arils. J. Appl. Nat. Sci. 2021, 13, 101. [Google Scholar] [CrossRef]
- Kurek, M.A.; Wyrwisz, J.; Karp, S.; Wierzbicka, A. Effect of modified atmosphere packaging on the quality of wheat bread fortified with soy flour and oat fibre. J. Food Meas. Charact. 2019, 13, 1864–1872. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Linus Opara, U. Functional properties of pomegranate fruit parts: Influence of packaging systems and storage time. J. Food Meas. Charact. 2017, 11, 2233–2246. [Google Scholar] [CrossRef]
- Smith, E.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Storage stability of egg sticks fortified with omega-3 fatty acids. J. Sci. Food Agric. 2018, 98, 3452–3461. [Google Scholar] [CrossRef]
- Kumar, A.; Hussain, S.A.; Raju, P.N.; Singh, A.K.; Singh, R.R.B. Packaging material type affects the quality characteristics of Aloe-probiotic lassi during storage. Food Biosci. 2017, 19, 34–41. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.; Barreira, J.C.; Vinha, A.F.; Alves, R.C.; Rocha, A.; Oliveira, M.B.P. How functional foods endure throughout the shelf storage? Effects of packing materials and formulation on the quality parameters and bioactivity of smoothies. LWT 2016, 65, 70–78. [Google Scholar] [CrossRef]
- Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Ježek, D.; Dragović-Uzelac, V. Impact of packaging material and storage conditions on polyphenol stability, colour and sensory characteristics of freeze-dried sour cherry (prunus cerasus var. Marasca). J. Food Sci. Technol. 2016, 53, 1247–1258. [Google Scholar] [CrossRef]
- Liu, K.; Dong, H.; Peng, J.; Liao, W.; Yang, X.; He, Q. Design of equilibrium modified atmosphere packaging for postharvest cabbages preservation based on introducing available active sites into film materials as gas transport channels. Food Res. Int. 2024, 177, 113900. [Google Scholar] [CrossRef]
- Faradonbeh, M.Z.; Barzegar, H.; Hojjati, M.; Behbahani, B.A.; Taki, M. Active packaging coating based on Ocimum basilicum seed mucilage and Hypericum perforatum extract: Preparation, characterization, application and modeling the preservation of ostrich meat. Appl. Food Res. 2024, 4, 100524. [Google Scholar] [CrossRef]
- He, M.; Pan, J.; Hong, M.; Shen, Y.; Zhang, H.; Jiang, Y.; Gong, L. Fabrication of antimicrobial packaging based on polyaminopropyl biguanide incorporated pectin/polyvinyl alcohol films for fruit preservation. Food Chem. 2024, 457, 140106. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.T.; Qian, J.; Yin, C. Equilibrium modified atmosphere packaging on postharvest quality and antioxidant activity of strawberry. Int. J. Food Sci. Technol. 2022, 57, 7125–7134. [Google Scholar] [CrossRef]
- Sun, T.; Yi, W.; Wang, Y.; Cheng, P.; Dong, T.; Yun, X. Application of poly (L-lactic acid)-based films for equilibrium modified atmosphere packaging of “Kyoho” grapes and its favorable protection for anthocyanins. Food Chem. 2024, 452, 139573. [Google Scholar] [CrossRef]
- El Mouzahim, M.; Eddarai, E.M.; Eladaoui, S.; Guenbour, A.; Bellaouchou, A.; Zarrouk, A.; Boussen, R. Food packaging composite film based on chitosan, natural kaolinite clay, and Ficus. carica leaves extract for fresh-cut apple slices preservation. Int. J. Biol. Macromol. 2023, 233, 123430. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, M.; Wang, Y.; Zhao, P.; Wang, K.; Wang, Y.; Wang, X.; Wang, J. Evaluation of film packaging containing mesoporous nanosilica and oregano essential oil for postharvest preservation of mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2023, 198, 112263. [Google Scholar] [CrossRef]
- Wu, Q.; Li, C.; Zhang, D.; Tian, Q.; Tao, X.; Luo, Z.; Fu, X.; Zhang, Y. Nitrogen modified atmosphere packaging maintains the bioactive compounds and antioxidant capacity of postharvest fresh edible peanuts. Postharvest Biol. Technol. 2022, 190, 111957. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Sellamuthu, P.S.; Emmanuel, R.S. Use of modified atmosphere packaging combined with essential oils for prolonging post-harvest shelf life of mango (cv. Banganapalli and cv. Totapuri). LWT 2021, 148, 111662. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.; Sallam, K.I.; Malak, N.M. Improvement of the microbial quality, antioxidant activity, phenolic and flavonoid contents, and shelf life of smoked herring (Clupea harengus) during frozen storage by using chitosan edible coating. Food Control 2021, 130, 108317. [Google Scholar] [CrossRef]
- Bińkowska, W.; Szpicer, A.; Stelmasiak, A.; Wojtasik-Kalinowska, I.; Półtorak, A. Microencapsulation of Polyphenols and Their Application in Food Technology. Appl. Sci. 2024, 14, 11954. [Google Scholar] [CrossRef]
- Pavani, M.; Singha, P.; Dash, D.R.; Asaithambi, N.; Singh, S.K. Novel encapsulation approaches for phytosterols and their importance in food products: A review. J. Food Process Eng. 2022, 45, e14041. [Google Scholar] [CrossRef]
- Vijayaram, S.; Sinha, R.; Faggio, C.; Ringø, E.; Chou, C.C. Biopolymer encapsulation for improved probiotic delivery: Advancements and challenges. AIMS Microbiol. 2024, 10, 986. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaete, C.; Avendaño-Godoy, J.; Escobar-Avello, D.; Campos-Requena, V.H.; Rogel-Castillo, C.; Estevinho, L.M.; Martorell, M.; Sharifi-Rad, J.; Calina, D. Revolutionizing fruit juice: Exploring encapsulation techniques for bioactive compounds and their impact on nutrition, flavour and shelf life. Food Prod. Process. Nutr. 2024, 6, 8. [Google Scholar] [CrossRef]
- Homroy, S.; Chopra, R.; Singh, P.K.; Dhiman, A.; Chand, M.; Talwar, B. Role of encapsulation on the bioavailability of omega-3 fatty acids. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13272. [Google Scholar] [CrossRef]
- Bazzaz, S.; Abbasi, A.; Ghotbabad, A.G.; Pourjafar, H.; Hosseini, H. Novel encapsulation approaches in the functional food industry: With a focus on probiotic cells and bioactive compounds. Probiotics Antimicrob. Proteins 2024, 17, 1132–1170. [Google Scholar] [CrossRef]
- Tolve, R.; Cela, N.; Condelli, N.; Di Cairano, M.; Caruso, M.C.; Galgano, F. Microencapsulation as a tool for the formulation of functional foods: The phytosterols’ case study. Foods 2020, 9, 470. [Google Scholar] [CrossRef]
- Gheorghita, R.; Anchidin-Norocel, L.; Filip, R.; Dimian, M.; Covasa, M. Applications of biopolymers for drugs and probiotics delivery. Polymers 2021, 13, 2729. [Google Scholar] [CrossRef]
- Sultana, M.; Chan, E.S.; Pushpamalar, J.; Choo, W.S. Advances in extrusion-dripping encapsulation of probiotics and omega-3 rich oils. Trends Food Sci. Technol. 2022, 123, 69–86. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano-Zaragoza, M.L. Encapsulation of bioactive peptides: A strategy to improve the stability, protect the nutraceutical bioactivity and support their food applications. RSC Adv. 2022, 12, 6449–6458. [Google Scholar] [CrossRef]
- Najafian, L. A review of bioactive peptides as functional food ingredients: Mechanisms of action and their applications in active packaging and food quality improvement. Food Funct. 2023, 14, 5835–5857. [Google Scholar] [CrossRef]
- Ceylan, H.G.; Atasoy, A.F. New bioactive edible packing systems: Synbiotic edible films/coatings as carries of probiotics and prebiotics. Food Bioprocess Technol. 2023, 16, 1413–1428. [Google Scholar] [CrossRef]
- Trajkovska Petkoska, A.; Daniloski, D.; Kumar, N.; Pratibha; Broach, A.T. Active edible packaging: A sustainable way to deliver functional bioactive compounds and nutraceuticals. In Sustainable Packaging; Springer: Berlin, Germany, 2021; pp. 225–264. [Google Scholar]
- Kirtil, E.; Aydogdu, A.; Svitova, T.; Radke, C.J. Assessment of the performance of several novel approaches to improve physical properties of guar gum based biopolymer films. Food Packag. Shelf Life 2021, 29, 100687. [Google Scholar] [CrossRef]
- Jeevahan, J.J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Joseph, G.B.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Ma, M.; Gu, M.; Zhang, S.; Yuan, Y. Effect of tea polyphenols on chitosan packaging for food preservation: Physicochemical properties, bioactivity, and nutrition. Int. J. Biol. Macromol. 2024, 259, 129267. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2106–2145. [Google Scholar] [CrossRef]
- Messinese, E.; Pitirollo, O.; Grimaldi, M.; Milanese, D.; Sciancalepore, C.; Cavazza, A. By-products as sustainable source of bioactive compounds for potential application in the field of food and new materials for packaging development. Food Bioprocess Technol. 2024, 17, 606–627. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, N.; Upadhyay, A.; Pratibha; Anurag, R.K. Edible packaging from fruit processing waste: A comprehensive review. Food Rev. Int. 2023, 39, 2075–2106. [Google Scholar] [CrossRef]
- Pedreiro, S.; Figueirinha, A.; Silva, A.S.; Ramos, F. Bioactive edible films and coatings based in gums and starch: Phenolic enrichment and foods application. Coatings 2021, 11, 1393. [Google Scholar] [CrossRef]
- Hasl, K. Karakterizacija Filmova od Alginata i Kitozana s Prirodnim Antioksidansima iz Ekstrakta Ružmarina za Pakiranje Hrane. Ph.D. Thesis, Laboratory for Food Packaging, Department of Food Engineering, Faculty of Food Technology and Biotechnology, University of Zagreb, Zagreb, Croatia, 2022. [Google Scholar]
- Yerramathi, B.B.; Muniraj, B.A.; Kola, M.; Konidala, K.K.; Arthala, P.K.; Sharma, T.S.K. Alginate biopolymeric structures: Versatile carriers for bioactive compounds in functional foods and nutraceutical formulations: A review. Int. J. Biol. Macromol. 2023, 253, 127067. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, X.; Ding, W.; Remón, J.; Xin, M.; Sun, T.; Wang, T.; Yu, L.; Wang, J. Fabrication, performance, and potential environmental impacts of polysaccharide-based food packaging materials incorporated with phytochemicals: A review. Int. J. Biol. Macromol. 2023, 249, 125922. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kim, J.Y.; Lee, Y.S. Phenolic compounds in active packaging and edible films/coatings: Natural bioactive molecules and novel packaging ingredients. Molecules 2022, 27, 7513. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Palanisamy, C.P.; Srinivasan, G.P.; Panagal, M.; Kumar, S.S.D.; Mironescu, M. A comprehensive review on starch-based sustainable edible films loaded with bioactive components for food packaging. Int. J. Biol. Macromol. 2024, 274, 133332. [Google Scholar] [CrossRef]
- Vlčko, T.; Golian, J.; Fikselová, M.; Rybnikár, S. Current overview in the field of application of edible coatings/films (meat products examples). J. Microbiol. Biotechnol. Food Sci. 2022, 12, e9281. [Google Scholar] [CrossRef]
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef]
- Dai, J.; Sameen, D.E.; Zeng, Y.; Li, S.; Qin, W.; Liu, Y. An overview of tea polyphenols as bioactive agents for food packaging applications. LWT 2022, 167, 113845. [Google Scholar] [CrossRef]
- Pandey, S.; Sharma, K.; Gundabala, V. Antimicrobial bio-inspired active packaging materials for shelf life and safety development: A review. Food Biosci. 2022, 48, 101730. [Google Scholar] [CrossRef]
- Liu, Y.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W. Antimicrobial peptides and their application in food packaging. Trends Food Sci. Technol. 2021, 112, 471–483. [Google Scholar] [CrossRef]
- Devi, L.S.; Das, B.; Dutta, D.; Kumar, S. Essential oils as functional agents in biopolymer-based sustainable food packaging system: A review. Sustain. Chem. Pharm. 2024, 39, 101563. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J.; Yang, W.; Wan, J.B. Biopolymer food packaging films incorporated with essential oils. J. Agric. Food Chem. 2023, 71, 1325–1347. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.W.; Cao, J.; Jiang, W. Tea polyphenols (TP): A promising natural additive for the manufacture of multifunctional active food packaging films. Crit. Rev. Food Sci. Nutr. 2022, 63, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.W. Curcumin and its uses in active and smart food packaging applications-a comprehensive review. Food Chem. 2022, 375, 131885. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, S.; Jafarzadeh, S.; Qazanfarzadeh, Z.; Wijekoon, J.O.; Nafchi, A.M. Sustainable strategies for using natural extracts in smart food packaging. Int. J. Biol. Macromol. 2024, 267, 131537. [Google Scholar] [CrossRef] [PubMed]
- Bayram, B.; Ozkan, G.; Kostka, T.; Capanoglu, E.; Esatbeyoglu, T. Valorization and application of fruit and vegetable wastes and by-products for food packaging materials. Molecules 2021, 26, 4031. [Google Scholar] [CrossRef]
- Chandran, G.U.; Kumar, A.A.; Menon, S.K.; Sambhudevan, S.; Shankar, B. The potential role of flavonoids in cellulose-based biopolymeric food packaging materials for UV radiation protection. Cellulose 2024, 31, 4733–4773. [Google Scholar] [CrossRef]
- Juikar, S.K.; Warkar, S.G. Biopolymers for packaging applications: An overview. Packag. Technol. Sci. 2023, 36, 229–251. [Google Scholar] [CrossRef]
- La Fuente Arias, C.I.; Kubo, M.T.K.N.; Tadini, C.C.; Augusto, P.E.D. Bio-based multilayer films: A review of the principal methods of production and challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 2260–2276. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A review of multilayer and composite films and coatings for active biodegradable packaging. npj Sci. Food 2022, 6, 18–34. [Google Scholar] [CrossRef]
- Macnamara, J.F., Jr.; Rubino, M.; Daum, M.; Kathuria, A.; Auras, R. Biodegradable and transparent water and oxygen barrier multilayer film. ACS Appl. Polym. Mater. 2024, 6, 10865–10874. [Google Scholar] [CrossRef]
- Liu, S.; Li, L.; Li, B.; Zhu, J.; Li, X. Size effect of carnauba wax nanoparticles on water vapor and oxygen barrier properties of starch-based film. Carbohydr. Polym. 2022, 296, 119935. [Google Scholar] [CrossRef]
- Edo, G.I.; Mafe, A.N.; Ali, A.; Akpoghelie, P.O.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; Zainulabdeen, K.; Owheruo, J.O.; Essaghah, A.E.A.; et al. Advancing sustainable food packaging: The role of green nanomaterials in enhancing barrier properties. Food Eng. Rev. 2025, 1–35. [Google Scholar] [CrossRef]
- Shah, Y.A.; Bhatia, S.; Al-Harrasi, A.; Tarahi, M.; Almasi, H.; Chawla, R.; Ali, A.M.M. Insights into recent innovations in barrier resistance of edible films for food packaging applications. Int. J. Biol. Macromol. 2024, 271, 132354. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Yue, S.; Zhang, T.; Wang, S.; Han, D.; Huang, S.; Xiao, M.; Meng, Y. Recent progress of biodegradable polymer package materials: Nanotechnology improving both oxygen and water vapor barrier performance. Nanomaterials 2024, 14, 338. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).