Utilization of Red Mud from Processing of Low-Quality Bauxites
Abstract
1. Introduction
2. Research Methodology
3. Discussion of Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lainer, A.I. Alumina Production—M: Metallurgy. 1978, p. 341. Available online: https://f.eruditor.link/file/552430/ (accessed on 14 May 2025).
- Ibragimov, A.T.; Budon, S.V. Development of Alumina Production Technology from Kazakhstan Bauxites; ENRC Annual Report and Accounts: Pavlodar, Kazahstan, 2010; p. 299. Available online: https://f.eruditor.link/file/2213812/ (accessed on 14 May 2025).
- Bai, Z.; Han, C.; Yuan, S.; Li, X. Separate recycling of iron and aluminum from iron-rich red mud by coal gangue reduction to realize solid waste utilization. Adv. Powder Tech. 2024, 35, 104506. [Google Scholar] [CrossRef]
- Bendaikha, W.; Larbi, S.; Ramdane, A. Mineralogical and chemical characterization of an oolitic iron ore, and sustainable phosphorus removal. J. South. Afr. Inst. Min. Metall. 2024, 124, 59–66. [Google Scholar] [CrossRef]
- Zhaobo, L.; Hongxu, L. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar]
- Kaussen, F.; Friedrich, B. Reductive smelting of red mud for iron recovery. Chem. Ing. Technik. 2015, 87, 1535–1542. [Google Scholar] [CrossRef]
- Alam, S.; Das, B.K.; Das, S.K. Dispersion and Sedimentation Characteristics of Red Mud. J. Hazard. Toxic Radioact. Waste 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Anton, A.; Rekasi, M.; Uzinger, N.; Széplábi, G.; Makó, A. Modelling the potential effects of the hungarian red mud disaster on soil properties. Water Air Soil Pollut. 2012, 223, 5175–5188. [Google Scholar] [CrossRef]
- Boily, R. Twenty cases of red hazard, an inventory of ecological problems caused by bauxite residue from alumina production. In Proceedings of the Conference Paper in Inforex, Larval, QC, Canada, 3 October 2012. [Google Scholar]
- Archambo, M.; Kawatra, S.K. Red Mud: Fundamentals and New Avenues for Utilization. Miner. Process. Extr. Metall. Rev. 2021, 42, 427–450. [Google Scholar] [CrossRef]
- Rao, M.; Zhuang, J.; Li, G.; Zeng, J.; Jiang, T. Iron recovery from red mud by reduction roasting and magnetic separation. In Light Metals 2013; The Minerals, Metals & Materials Series; Springer: Berlin/Heidelberg, Germany, 2016; pp. 125–130. [Google Scholar]
- Ding, W.; Xiao, J.-H.; Peng, Y.; Shen, S.-Y.; Chen, T. Iron Extraction from Red Mud using Roasting with Sodium Salt. Miner. Process. Extr. Metall. Rev. 2021, 42, 153–161. [Google Scholar] [CrossRef]
- Li, G.; Liu, M.; Rao, M.; Jiang, T.; Zhuang, J.; Zhang, Y. Stepwise extraction of valuable components from red mud based on reductive roasting with sodium salts. J. Hazard. Mater. 2014, 280, 774–780. [Google Scholar] [CrossRef]
- Ding, W.; Xiao, J.; Peng, Y.; Shen, S.; Chen, T.; Zou, K.; Wang, Z. A novel process for extraction of iron from a refractory red mud. Physicochem. Probl. Min. Process 2020, 56, 125–136. [Google Scholar] [CrossRef]
- Gateshki, M.; Petkov, V.; Pradhan, S.K.; Vogt, T. Structure of nanocrystalline MgFe2O4 from X-ray diffraction, Rietveld and atomic pair distribution function analysis. J. Appl. Crystallogr. 2005, 38, 772–779. [Google Scholar] [CrossRef]
- Foroughi, F.; Hassanzadeh-Tabrizi, S.A.; Bigham, A. In situ microemulsion synthesis of hydroxyapatite-MgFe2O4 nanocomposite. Mater. Sci. Eng. C 2016, 68, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, M.; Manimuthu, P.; Venkateswaran, C. Structural and Magnetic properties of MgFe2O4 Ceramic. In Proceedings of the 2nd International Conference on Optoelectronic Materials and Thin Films for Advanced Technology (OMTAT), Kochi, India, 3–5 January 2013; pp. 194–196. [Google Scholar]
- Chen, X.; Guo, Y.; Ding, S.; Zhang, H.; Xia, F.; Wang, J.; Zhou, M. Utilization of red mud in geopolymer-based pervious concrete with function of adsorption of heavy metal ions. J. Clean. Prod. 2019, 207, 789–800. [Google Scholar] [CrossRef]
- Ahmadi, H.; Khalaj, G.; Najafi, A.; Abbasi, S.M.; Safari, M. Metakaolin-red mud/carbon nanotubes geopolymer nanocomposite: Mechanical properties and structural studies. Mater. Res. Express 2022, 9, 025011. [Google Scholar] [CrossRef]
- Thibodeau, E.; Gheribi, A.E.; Jung, I.-H. A Structural Molar Volume Model for Oxide Melts Part III: Fe Oxide-Containing Melts. Metall. Mater. Trans. B 2016, 47, 1187–1202. [Google Scholar] [CrossRef]
- Blackman, L. On the Formation of Fe2+ in the System MgO-Fe2O3-MgFe2O4 at High Temperatures. J. Am. Ceram. Soc. 1959, 42, 143–145. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Annersten, H.; Virgo, D. The temperature dependence of the cation distribution in magnesioferrite (MgFe2O4) from powder XRD structural refinements and Mössbauer spectroscopy. Am. Mineral. 1992, 77, 725–740. [Google Scholar]
- Nan, H.; Zhang, X.; Yang, J.; Jia, K.; Cao, Y.; Wang, C. Hydrothermal treatment of alkaline red mud and sewage sludge: Formation of a soil-like matrix. Environ. Technol. 2024, 45, 2012–2021. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhou, Y.; Zhang, J.; Mao, Q.; Yang, Y.; Huang, H.; Liu, Z.; Peng, Q.; Luo, L. Efects of red mud based passivator on the transformation of Cd fraction in acidic Cd-polluted paddy soil and Cd absorption in rice. Sci. Total Environ. 2018, 640–641, 736–745. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef]
- Bao, S.; Chen, B.; Zhang, Y.; Ren, L.; Xin, C.; Ding, W.; Yang, S.; Zhang, W. A comprehensive review on the ultrasound-enhanced leaching recovery of valuable metals: Applications, mechanisms and prospects. Ultrason. Sonochem. 2023, 98, 106525. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.I.; Mares, W.M.; Rogerson, M.; Stewart, D.I.; Burke, I.T. Alkaline residues and the environment: A review of impacts, management practices and opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorisation of rare-earthcontaining industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef]
- Abdulvaliev, R.A.; Surkova, T.Y.; Baltabekova, Z.; Yessimova, D.M.; Stachowicz, M.; Smailov, K.M.; Dossymbayeva, Z.D.; Ainur, B. Effect of Amino Acids on the Extraction of Copper from Sub-Conditional Raw Materials. Komplesnoe ispolzovanie mineral’nogo syr’a. Complex Use Miner. Resour. 2024, 335, 50–58. [Google Scholar]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite residue issues: III. Alkalinity and associated chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Tang, Z.-D.; Gao, P.; Han, Y.-X.; Guo, W. Fluidized bed roasting technology in iron ores dressing in China: A review on equipment development and application prospect. J. Min. Metall. Sect. B 2019, 55, 295–303. [Google Scholar] [CrossRef]
- Chen, B.; Bao, S.; Zhang, Y.; Li, S. A high-efciency and sustainable leaching process of vanadium from shale in sulfuric acid systems enhanced by ultrasound. Sep. Purif. Technol. 2020, 240, 116624. [Google Scholar] [CrossRef]
- Ding, W.; Xiao, J.-H.; Peng, Y.; Shen, S.-Y.; Chen, T.; Zou, K.; Wang, Z. Extraction of scandium and iron from red mud. Miner. Process. Extr. Metall. Rev. 2022, 43, 61–68. [Google Scholar] [CrossRef]
- Liu, X.; Zou, Y.; Geng, R.; Li, B.; Zhu, T. Red mud recycling by Fe and Al recovery through the hydrometallurgy method: A collaborative strategy for aluminum and iron industry. Environ. Sci. Pollut. Res. Int. 2023, 30, 43377–43386. [Google Scholar] [CrossRef]
- Zhou, F. Progress in extraction and comprehensive utilization of valuable metals from red mud. China Met. Bull. 2019, 1, 274–275. [Google Scholar]
- Ding, Z.; Cheng, Y.; Jin, L.; Wang, W.; Yan, S. Study on the strength characteristics and micro-mechanism of modifed solidifed red mud. Front. Mater. 2024, 11, 1461198. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini-Leonardou, S.; Oustadakis, P. Red mud addition in the raw meal for the production of Portland 3cement clinker. J. Hazard. Mater. 2004, 116, 103–110. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fy ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sabitov, A.R.; Ibragimov, A.T.; Budon, S.V.; Medvedev, V.V.; Mikhailova, O.I. Method for Preparing a Suspension of Lime-Containing Additive for Hydrochemical Processing of Aluminosilicate Raw Materials. Patent 25937, 15 August 2012. [Google Scholar]
- Sabitov, A.R.; Markov, A.P.; Mikhailova, O.I.; Ambarnikova, G.A.; Budon, S.V.; Ibragimov, A.T. Combined Method of Bauxite Processing. Patent 19693, 15 March 2012. [Google Scholar]
- Eremin, N.I.; Grigorieva, G.D.; Kozlov, V.M. Development of technology for complex processing of bauxites. Izv. VUZov. Nonferrous Metallur 1975, 5, 166–168. Available online: https://www.sciencedirect.com/topics/earth-and-planetary-sciences/bauxite#:~:text=The%20principal%20uses%20of%20bauxite,cement%2C%20steel%2C%20and%20petroleum (accessed on 14 May 2025).
- Arkhipov, O.A.; Volkova, P.I.; Pavlov, F.N. Processing of red mud into cast iron, self-disintegrating alumina slag and cement. Nonferrous Metall. 1962. No 20. [Google Scholar]
- Gagarina, I.M.; Meshcheryakova, N.I.; Yakovlev, L.S. Production of partially metallized pellets from red mud. Ferr. Metallurgy. Bul. Inst. Chermetinformatsiya 1972. No 19. [Google Scholar]
- Utkov, V.A.; Leontiev, L.I.; Matyash, V.G.; Kiselev, V.A.; Nikolaev, S.A.; Petrov, S.I. Investigation of the reduction processes of pelletized red mud. In Investigation of New Processes and Apparatuses in the Production of Alumina and By-Products; VAMI: Leningrad, Russia, 1985. [Google Scholar]
- Tanutrov, I.N.; Sviridova, M.N.; Savenya, A.N. New technology for joint processing of technogenic waste. Metall. Non-Ferr. Met. 2013, 54, 21–26. [Google Scholar]
- Liu, X.; Han, Y.; He, F.; Gao, P.; Yuan, S. Characteristic, hazard and iron recovery technology of red mud—A critical review. J. Hazard. Mater. 2021, 420, 126542. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Han, Y.; Li, Y.; Gao, P. Review on coal-based reduction and magnetic separation for refractory iron-bearing resources. Int. J. Miner. Metall. Mater. 2022, 29, 2087–2105. [Google Scholar] [CrossRef]
- Chun, T.J.; Zhu, D.Q.; Pan, J.; He, Z. Preparation of metallic iron powder from red mud by sodium salt roasting and magnetic separation. Can. Metall. Q. 2014, 53, 183–189. [Google Scholar] [CrossRef]
- Zhu, D.; Chun, T.-J.; Pan, J.; He, Z. Recovery of iron from high-iron red mud by reduction roasting with added sodium salt. J. Iron Steel Res. Int. 2012, 19, 1–5. [Google Scholar] [CrossRef]
- Huang, Z.C.; Cai, L.-B.; Zhang, Y.; Yang, Y.-B. Reduction of iron oxides in red mud reinforced by Na2CO3 and CaF2. J. Cent. South Univ. (Sci. Technol.) 2010, 41, 838–844. [Google Scholar]
- Liu, W.; Yang, J.; Xiao, B. Application of Bayer red mud for iron recovery and building material production from aluminosilicate residues. J. Hazardous Mater. 2009, 161, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Anciferov, E.A.; Shchadov, I.M.; Yelkin, K.S.; Antsiferova, A.V.; Begunov, A.A.; Begunov, A.I. Method for Processing Alumina Production Sludges. Patent RU2441927, 10 February 2012. Bulletin No 14. [Google Scholar]
- Golubev, A.A.; Gudim Yu, A. Method for Pyrometallurgical Processing of Red Sludges. Patent RU2479648, 20 April 2013. [Google Scholar]
- Philippe, K.; Perry, D. Method and System for Processing Red Mud. Patent US0113925 A1, 19 May 2005. [Google Scholar]
- Balomnenos, E.; Kastritis, D.; Panias, D.; Paspaliaris, I.; Boufounos, D. The Enexal bauxite residue treatment process: Industrial scale pilot plant results. In Light Metals; TMS: Grantham, UK, 2014; pp. 143–147. [Google Scholar]
- Ning, G.; Zhang, B.; Liu, C.; Li, S.; Ye, Y.; Jiang, M. Large-Scale Consumption and Zero-Waste Recycling Method of Red Mud in Steel Making Process. Minerals 2018, 8, 102. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J. Sustain. Metall. 2016, 2, 28–37. [Google Scholar] [CrossRef]
- Kenjaliev, B.K.; Akhmadieva, N.K.; Abdulvaliev, R.A.; Gladyshev, S.V.; Omarova, S.A.; Manapova, A.I.; Zinovyeva, L.V. Method for Processing Red Sludge. Patent RK 33499, 7 March 2019. Bulletin No10. [Google Scholar]
- Ni, L.P.; Khalyapina, O.B.; Goldman, M.M.; Bocharova, G.V.; Ryskina, L.I. Atlas of Physicochemical Characteristics of Products from the Achinsk Alumina Plant; IMiO, Academy of Sciences Kaz SSR: Almaty, Kazahstan, 1978; p. 62. [Google Scholar]
- Abdulvaliev, R.A.; Gladyshev, S.V.; Pozmogov, V.A.; Akhmadieva, N.K.; Beisembekova, K.O. Pilot plant for testing Bayer-hydrogarnet technology for processing iron-rich bauxites. Komplesnoe ispolzovanie mineral’nogo syr’a Complex Use Miner. Resour. 2016, No 3. 8–14. [Google Scholar]
- Moenke, H. Mineralspektren; Akademie Verlag: Berlin, Germany, 1962; 394p. [Google Scholar]
- Nakamoto, K.K. Infrared Spectra of Inorganic and Coordination Compounds; Wiley: Moscow, Russia, 1966; Mir; 412p. [Google Scholar]
- Farmer, V.C. The Infrared Spectra of Minerals; MIneralogical Society: London, UK, 1974; 539p. [Google Scholar]
- Yurchenko, E.N.; Kustova, G.N.; Batsanov, S.S. Vibrational Spectra of Inorganic Compounds; Nauka: Novosibirsk, Russia, 1981; p. 145. [Google Scholar]
- Povarennykh, A.S.; Gevorkyan, S.V. Crystal Chemistry and Vibrational Spectra of Minerals; Scientific thought: Kyiv, Ukraine, 1980. [Google Scholar]
- Solenko, L.P.; Ni, T.V.; Daulbaev, E.U. X-Ray Patterns, IR Spectra and Heating Curves of Main Compounds in Alumina Production; Atlas, IMiO, AAS Kaz SSR: Almaty, Kazakhstan, 1976; p. 81. [Google Scholar]
- Kuznetsova, T.V.; Talaber, I. Aluminous Cement; Kuznetsova, T.V., Ed.; Stroyizdat: Moscow, Russia, 1988; p. 272. [Google Scholar]
- Kenzhaliyev, B.; Koizhanova, A.; Fischer, D.; Magomedov, D.; Yerdenova, M.; Smailov, K.; Abdyldayev, N. Study of efficiency of organic activator application to process difficult-to-beneficiate polymetallic ridder ores. Transit. Met. Chem. 2025. [Google Scholar] [CrossRef]
- Ni, L.P.; Khalyapina, O.B. Physicor—Chemical Properties of Raw Materials and Products of Alumina Production; Publishing House Science of the Kazakh SSR: Almaty, Kazakhstan, 1978; p. 247. [Google Scholar]
- GOST 4832-95; Cast Iron. Technical Specifications. IPC Publishing Standards: Minsk, Russia, 2000.
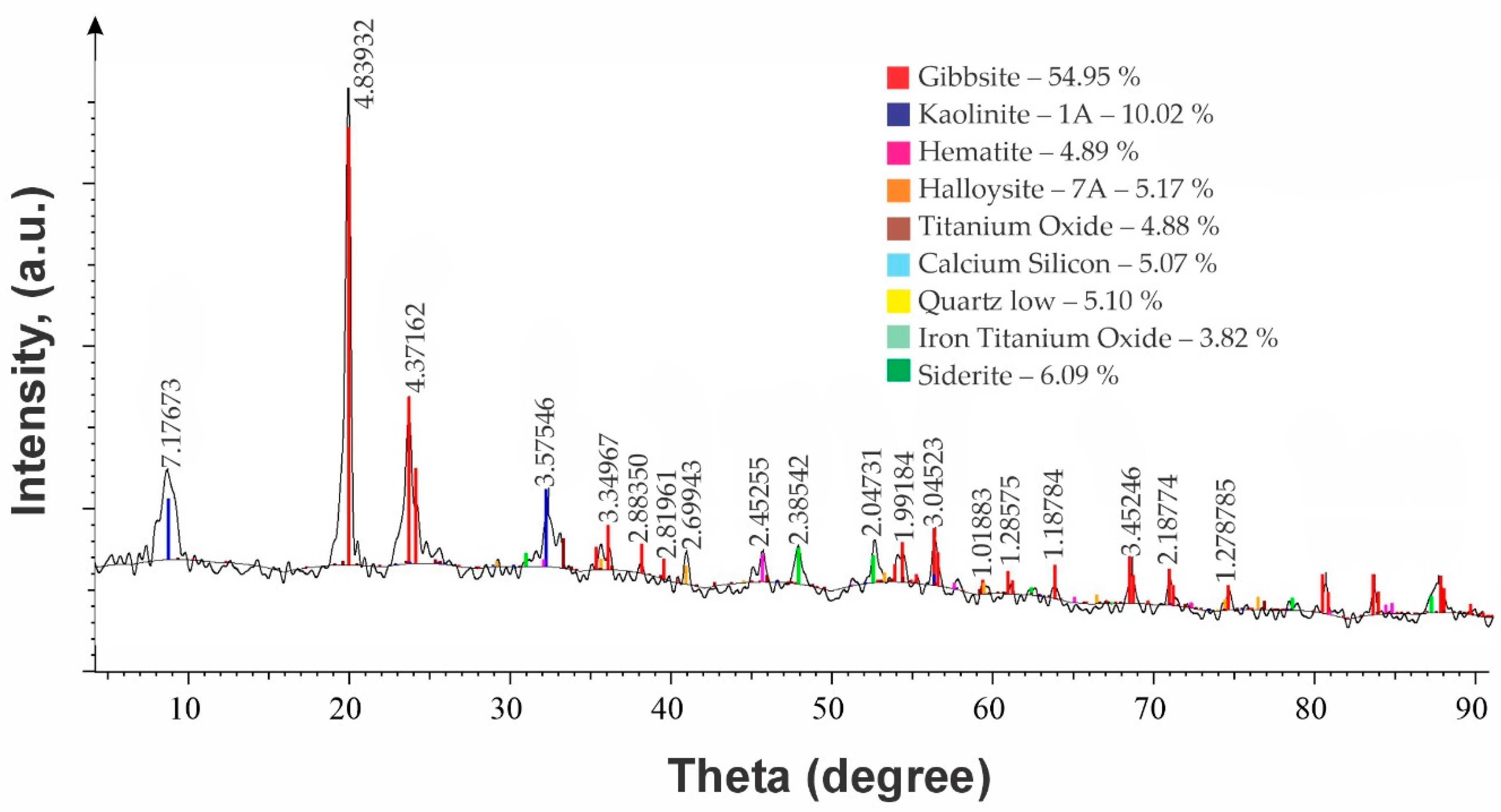
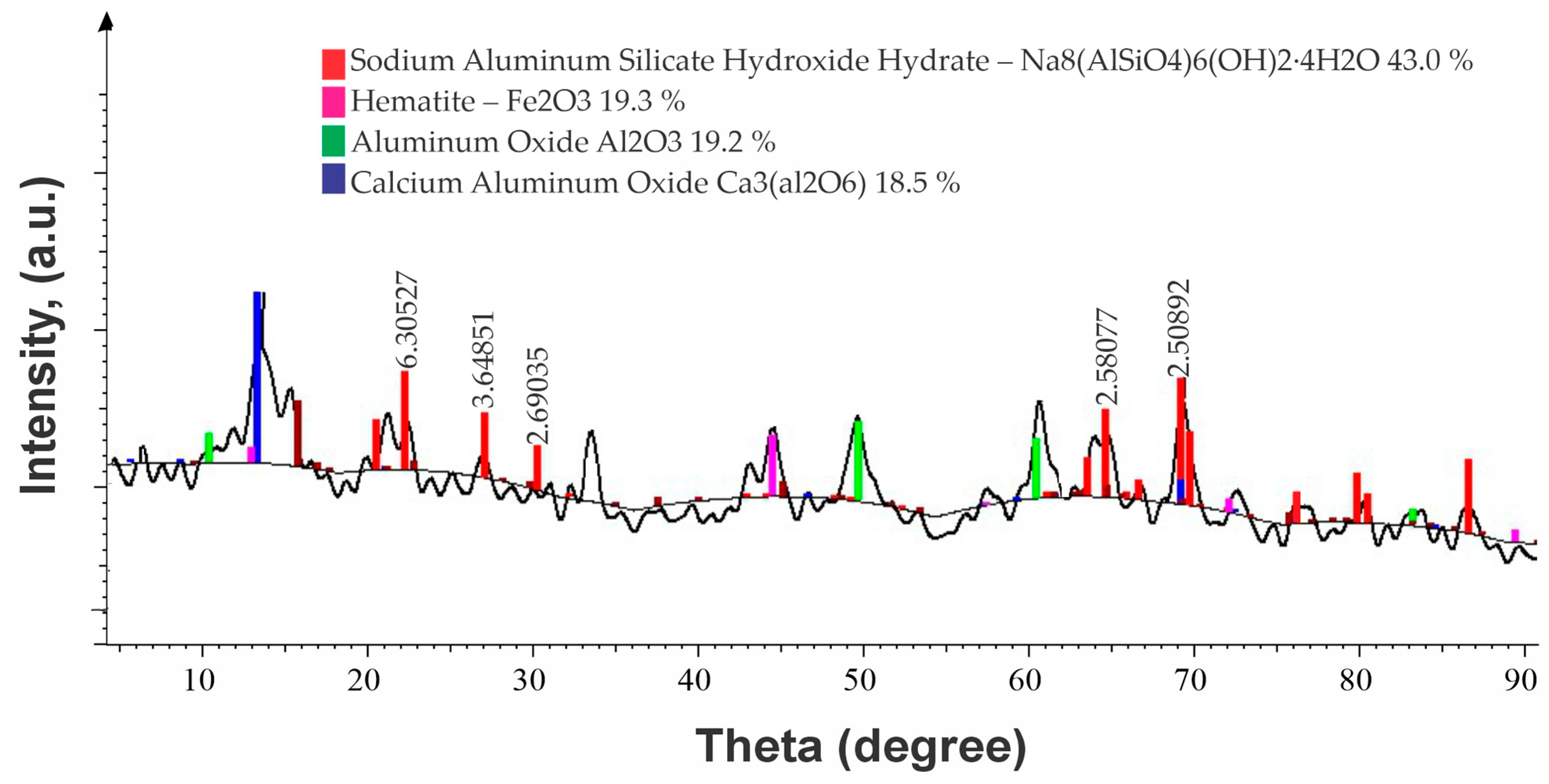
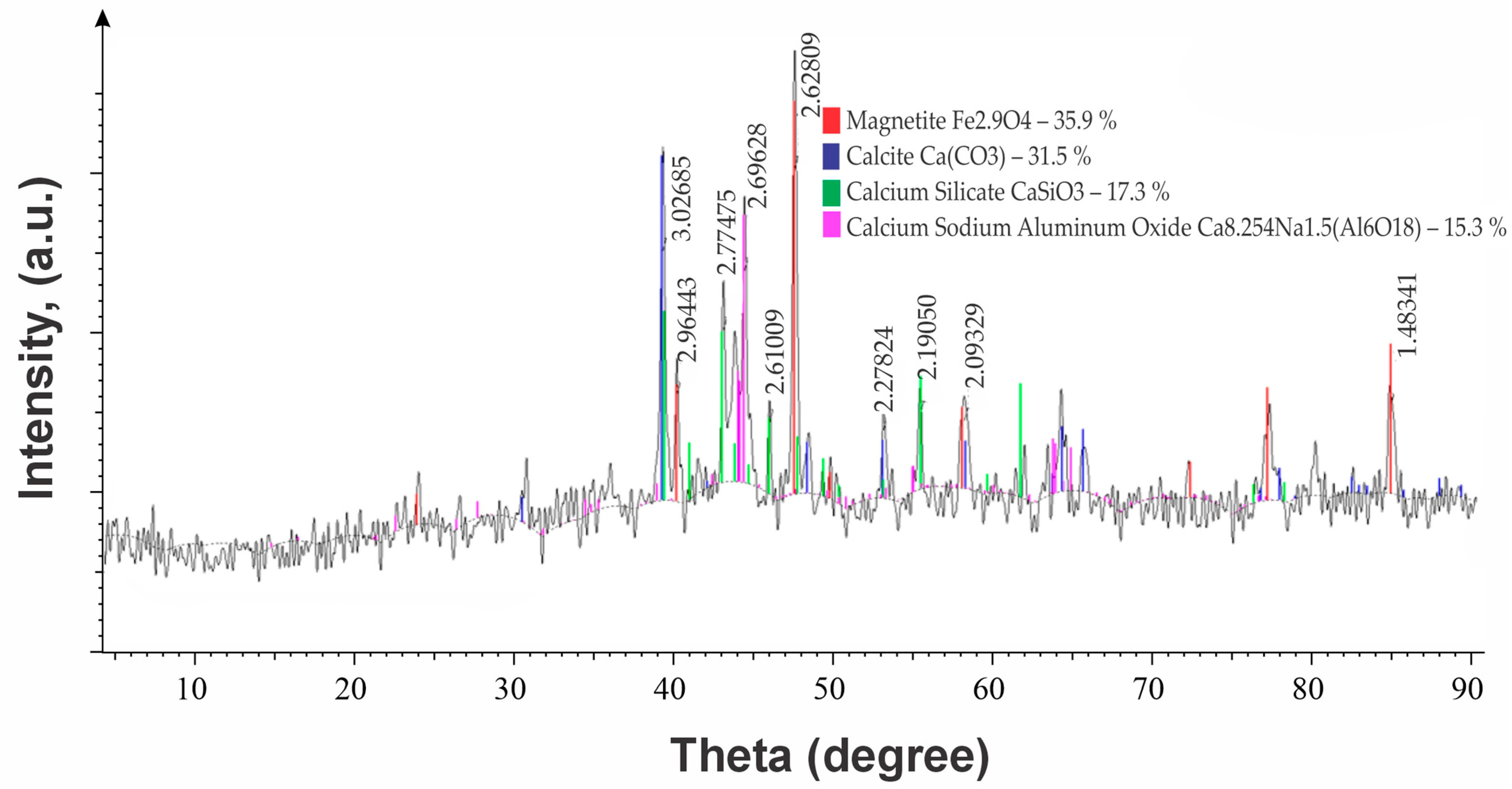
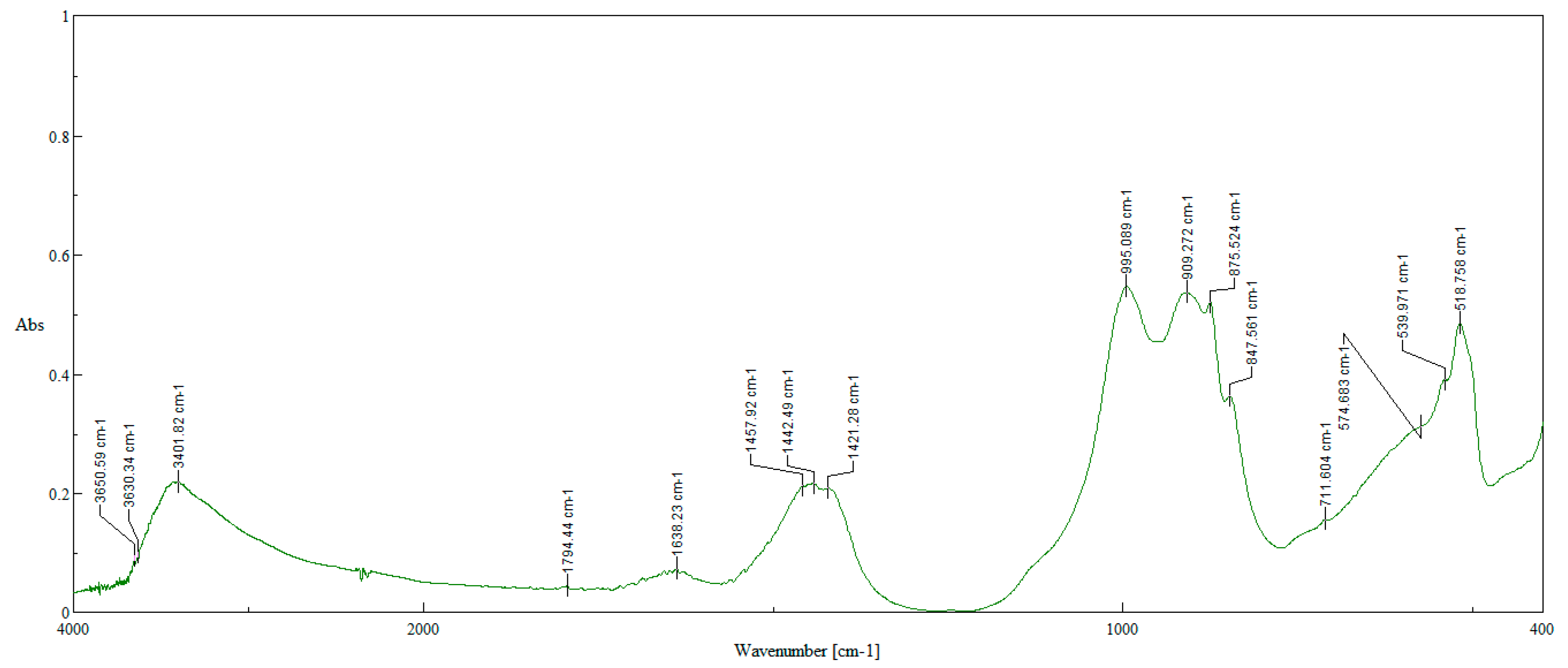




| No | Name | Content, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Na2O | Al2O3 | SiO2 | Fe2O3 | CaO | TiO2 | ∑ox.REE | o.p | ||
| 1 | Bauxite | 0.22 | 42.0 | 11.5 | 19.5 | 1.08 | 2.05 | 0.071 | 23.57 |
| 2 | RM | 15.0 | 19.47 | 11.19 | 26.95 | 1.49 | 2.82 | 0.0849 | 22.99 |
| 3 | WS | 0.798 | 6.94 | 15.6 | 20.4 | 35.64 | 5.93 | 0.0727 | 14.58 |
| Name | Phase Content | |
|---|---|---|
| X-Ray Phase Analysis | IR Spectroscopy Analysis | |
| Magnetite Fe3O4 | + | + |
| Iron(III) oxide α-Fe2O3 | – | + |
| Calcite CaCO3 | + | + |
| Calcium silicate CaSiO3 | + | – |
| Larnite 2CaO·SiO2 | – | + |
| Calcium hydroxide Ca (OH)2 | – | + |
| Sodium–calcium aluminate Ca8.25Na1.5(Al6O18) | + | – |
| Monocalcium aluminate hydrate CaAl2O4·12H2O | – | + |
| Sodium calcium silicate Na2O·CaO·SiO2 | – | + |
| Perovskite CaTiO3 | – | + |
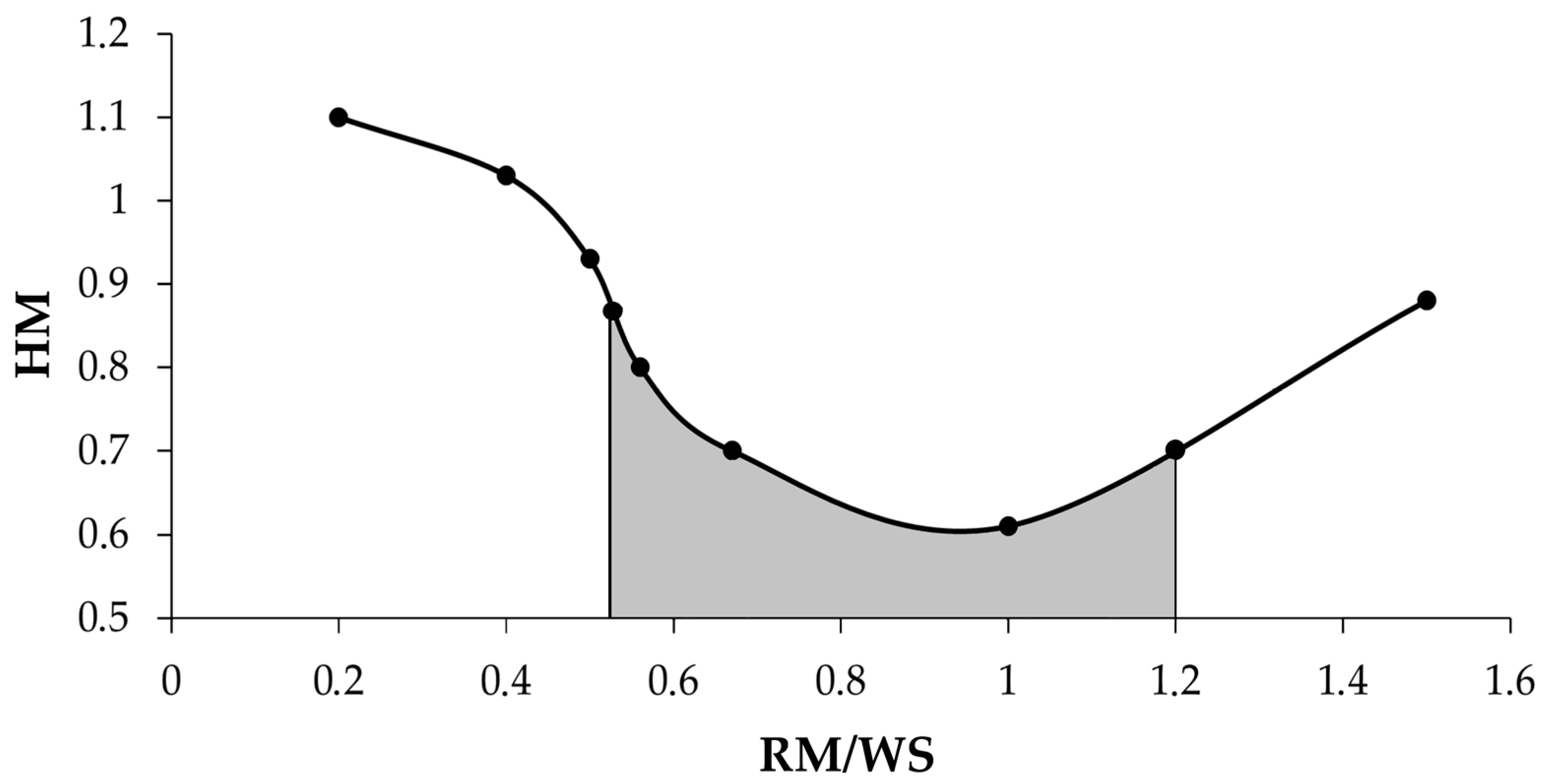
| No | Ratio RM/WS | HM | Slag Composition, % | Slag Yield | Ratio CaO/SiO2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na2O | Al2O3 | SiO2 | CaO | Fe2O3 | TiO2 | |||||
| 1 | 0.2:1 | 1.1 | 0.21 | 5.9 | 23.1 | 36.5 | 20.9 | 5.77 | 100 | 1.58 |
| 2 | 0.4:1 | 1.03 | 0.28 | 6.0 | 23.0 | 36.34 | 20.8 | 5.8 | 100 | 1.58 |
| 3 | 0.5:1 | 0.93 | 0.3 | 6.2 | 23.2 | 36.65 | 20.75 | 5.85 | 100 | 1.58 |
| 4 | 0.56:1 | 0.8 | 5.14 | 11.5 | 23.5 | 27.7 | 0.23 | 6.98 | 57.3 | 1.34 |
| 5 | 0.67:1 | 0.75 | 5.57 | 11.97 | 22.8 | 25.6 | 0.26 | 7.79 | 56.4 | 1.28 |
| 6 | 1:1 | 0.61 | 5.4 | 11.6 | 19.55 | 18.6 | 0.24 | 6.72 | 59.3 | 1.13 |
| 7 | 1.5:1 | 0.88 | 7.17 | 14.7 | 20.8 | 22.35 | 0.31 | 7.03 | 47.0 | 0.96 |
| 8 | 100% RM + 30% CaO | 0.55 | 12.4 | 14.6 | 14.34 | 22.35 | 6.35 | 5.07 | 76.7 | 1.5 |
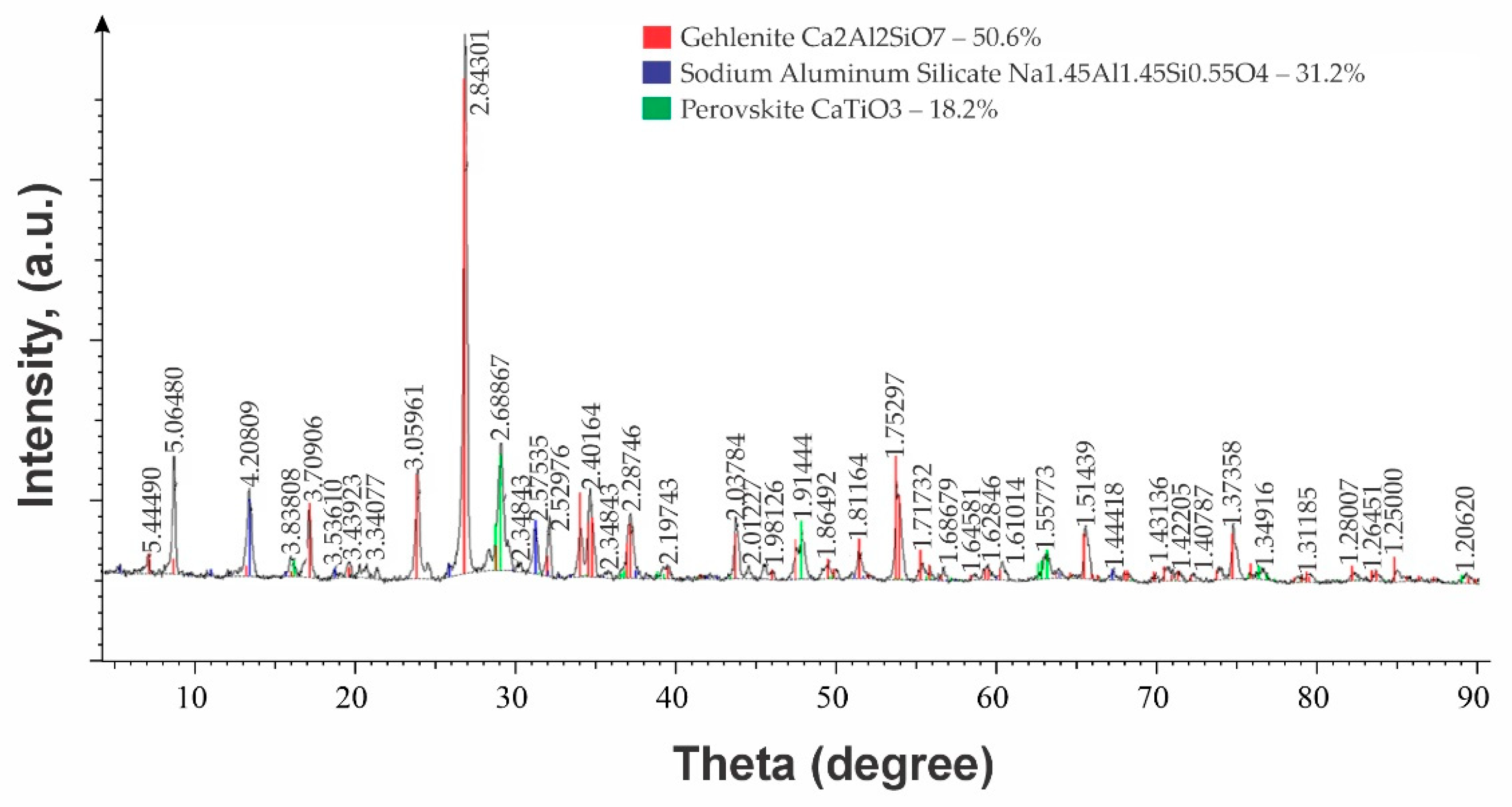
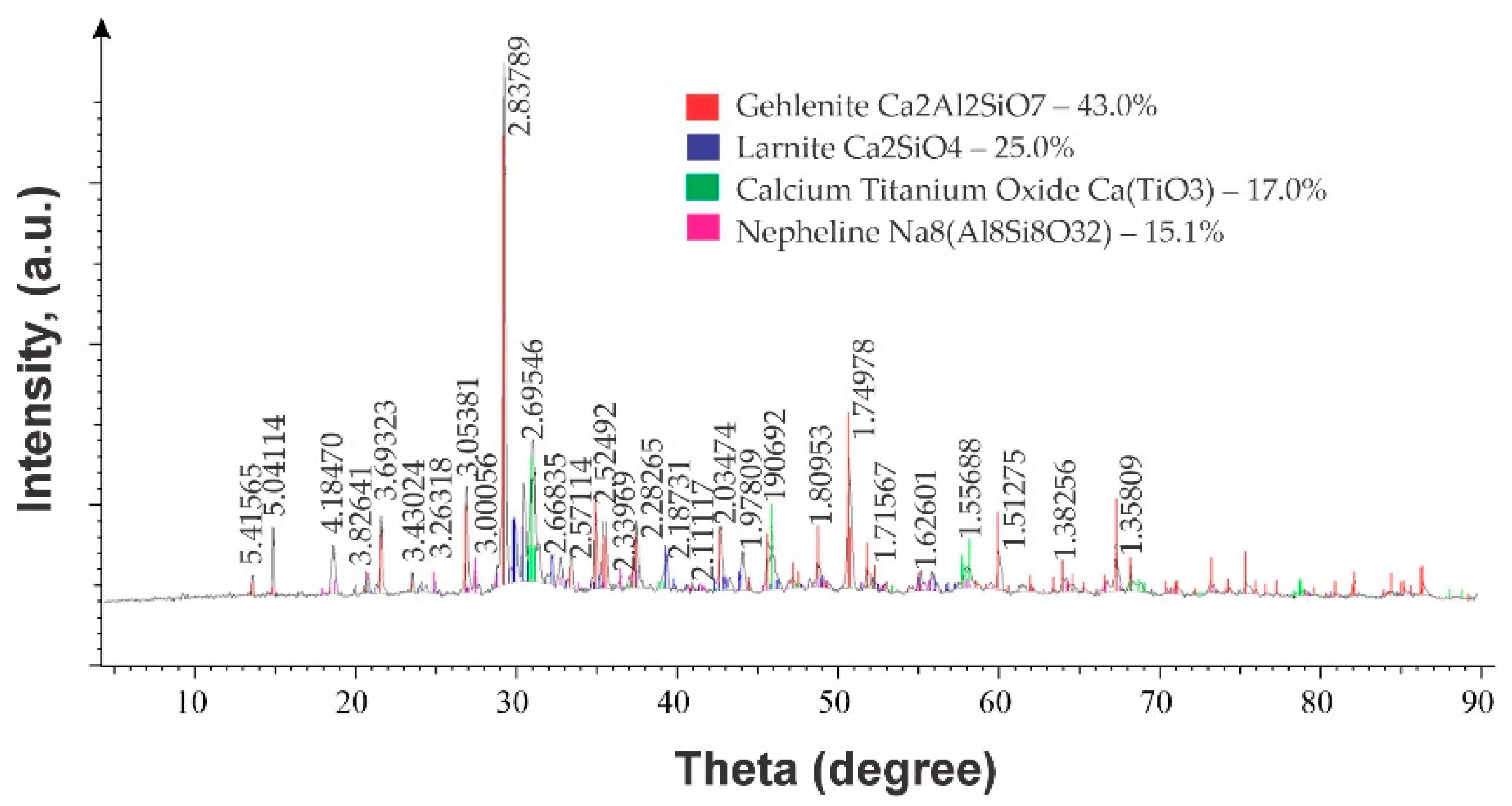
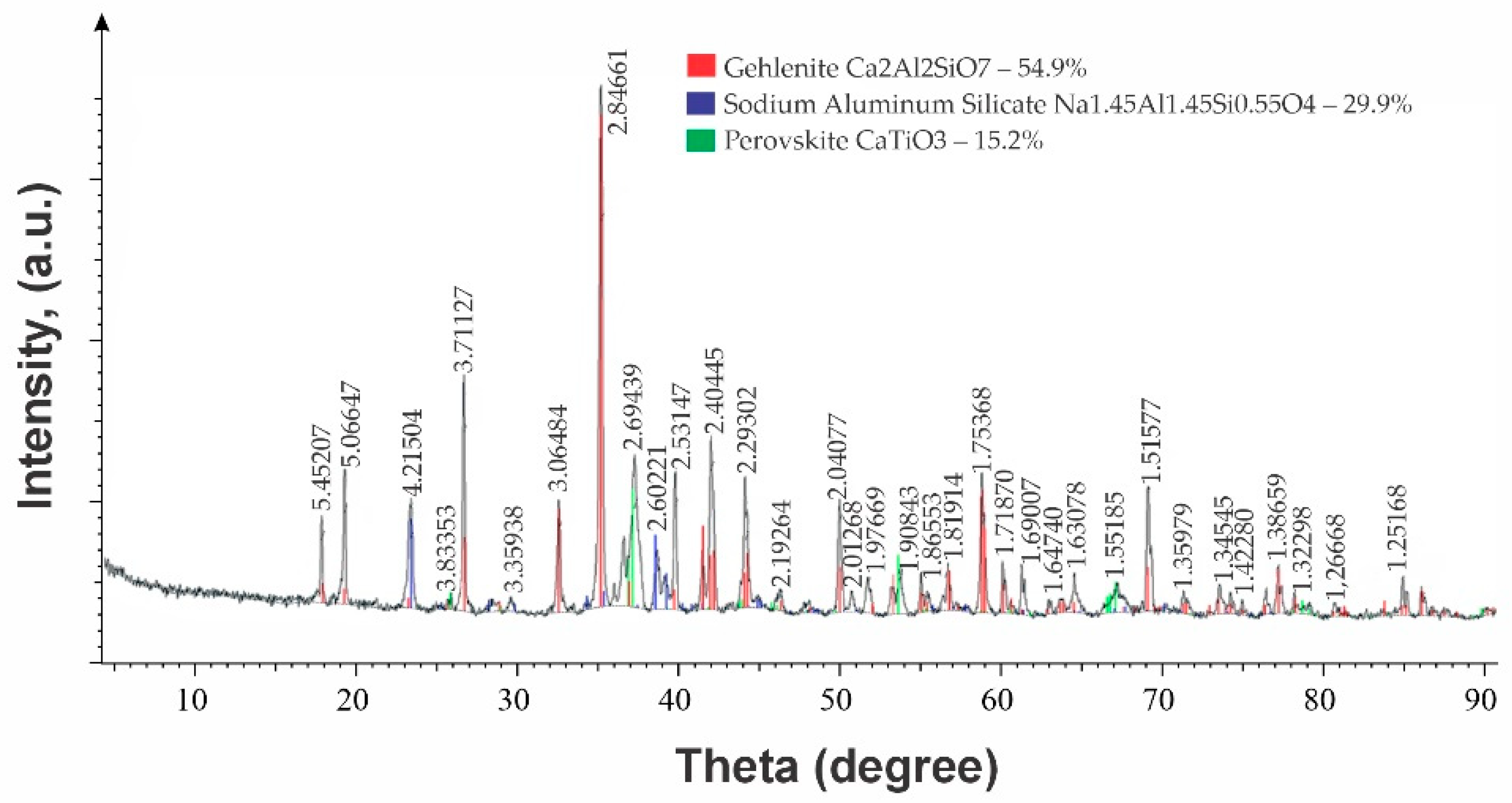
| Name | Phase Content, % | |
|---|---|---|
| Pig Iron Slag (HM 0.8) | Charge Slag (HM 1.03) | |
| Hedenbergite Ca2Al2SiO7 | 50.6 | 43.0 |
| Larnite Ca2SiO4 | - | 25.0 |
| Sodium aluminosilicate Na2Al2SiO6 | 31.2 | 17.0 |
| Nepheline NaAlSiO4 | – | 15.1 |
| Perovskite CaTiO3 | 18,2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladyshev, S.; Akhmadiyeva, N.; Abdulvaliyev, R.; Imangaliyeva, L.; Smailov, K.; Abikak, Y.; Kasymzhanova, A.; Amanzholova, L. Utilization of Red Mud from Processing of Low-Quality Bauxites. Processes 2025, 13, 1958. https://doi.org/10.3390/pr13071958
Gladyshev S, Akhmadiyeva N, Abdulvaliyev R, Imangaliyeva L, Smailov K, Abikak Y, Kasymzhanova A, Amanzholova L. Utilization of Red Mud from Processing of Low-Quality Bauxites. Processes. 2025; 13(7):1958. https://doi.org/10.3390/pr13071958
Chicago/Turabian StyleGladyshev, Sergey, Nazym Akhmadiyeva, Rinat Abdulvaliyev, Leila Imangaliyeva, Kenzhegali Smailov, Yerkezhan Abikak, Asya Kasymzhanova, and Leila Amanzholova. 2025. "Utilization of Red Mud from Processing of Low-Quality Bauxites" Processes 13, no. 7: 1958. https://doi.org/10.3390/pr13071958
APA StyleGladyshev, S., Akhmadiyeva, N., Abdulvaliyev, R., Imangaliyeva, L., Smailov, K., Abikak, Y., Kasymzhanova, A., & Amanzholova, L. (2025). Utilization of Red Mud from Processing of Low-Quality Bauxites. Processes, 13(7), 1958. https://doi.org/10.3390/pr13071958











