1. Introduction
Biodegradable materials are vital in advancing sustainable industrial practices. They provide environmentally friendly alternatives to traditional synthetic materials. As industries face growing pressure to mitigate their ecological footprint, these materials offer an effective solution by reducing pollution and minimizing waste accumulation [
1].
Adopting natural biodegradable materials supports a circular economy, enabling resources to be efficiently used and reintegrated into natural cycles without harming ecosystems. In the packaging, agriculture, and healthcare sectors, biodegradable materials contribute to sustainability by lowering carbon emissions and reducing reliance on fossil fuels. Furthermore, many of these materials, including carrageenan and starch, originate from renewable sources, ensuring long-term feasibility [
2].
By integrating biodegradable materials into production processes, industries foster environmental conservation and meet the rising consumer demand for sustainable products. Investing in such solutions drives innovation while maintaining a delicate balance between economic growth and ecological responsibility.
Current innovation in sustainable packaging has led to the development of biodegradable starch, carrageenan, and blend films [
3,
4]. These eco-friendly materials are an alternative to conventional plastic packaging, contributing to persistent environmental pollution and growing landfill waste. Starch can be sourced from renewable resources like corn, bananas, potatoes, tapioca, and other starch-rich plants. Carrageenan is derived from marine red algae, such as
Hypnea musciformis,
Chondrus crispus,
Kappaphycus alvarezii, and
Eucheuma denticulatum, among many others [
5,
6]. Starch and carrageenan are two biopolymers that have gained significant attention for their potential in developing biodegradable films. These films are being explored as sustainable alternatives to synthetic plastics in food packaging and other applications since they break down naturally and safely upon disposal, significantly lowering the environmental impact of food packaging [
7,
8,
9]. Such films are increasingly being used in food packaging and wound healing applications, where their natural properties can be advantageous [
4,
10].
Although starch-based films offer numerous benefits, they also present certain unresolved challenges. One primary concern is their moisture sensitivity, which can compromise their structural integrity and performance. In humid conditions, these films tend to absorb moisture, resulting in swelling, degradation, and a reduction in barrier effectiveness [
11,
12,
13]. This limitation restricts their suitability for specific food packaging applications, especially for liquids or high-moisture-content products. Another drawback of starch-based films is their mechanical strength and durability, which often fall short compared to synthetic plastics. These films are generally more susceptible to tearing and exhibit lower impact resistance, making them less ideal for packaging items that require robust protection [
14,
15,
16].
Carrageenan has excellent film-forming capabilities, allowing for the creation of transparent and flexible films suitable for various applications, including food packaging and wound healing [
17]. In wound healing, carrageenan films help maintain a moist environment, which is conducive to healing, and they are non-toxic, reducing the risk of adverse reactions [
18]. Pure carrageenan films often exhibit poor mechanical strength and barrier properties, such as high water vapor permeability, which can limit their effectiveness in certain applications [
19]. The inherent hydrophilic nature of carrageenan can lead to issues with water resistance, making the films less suitable for applications requiring moisture barriers. As with starch-based films, carrageenan films often require modification through the incorporation of other compounds, such as starch, nanoparticles, or essential oils, to overcome their limitations [
17,
19].
While many natural biodegradable films present a sustainable alternative to traditional plastics, their limitations necessitate further technological development. Blends, additives, and advanced fabrication techniques can enhance their properties, making them more competitive with conventional materials. However, these modifications can introduce complexities and potential cost increases, which must be carefully managed to maintain the films’ environmental and economic benefits.
Starch and carrageenan-based biodegradable films exhibit distinct chemical properties that make them suitable for various applications. The chemical composition of these films is primarily influenced by the polysaccharides present in starch and carrageenan. Starch, a carbohydrate consisting of glucose units, contributes to the film’s structural integrity, while carrageenan, a sulfated polysaccharide derived from red algae, enhances the film’s flexibility and water resistance [
20,
21]. Starch and carrageenan films offer several advantages over other biodegradable films. One of the most significant advantages is their biodegradability, which reduces environmental pollution [
22].
The incorporation of bioactive components, such as antioxidants and antimicrobial agents, further enhances the functionality of starch and carrageenan films. These components improve the shelf life of packaged food and provide additional health benefits to consumers [
23]. Incorporating natural pigments like carotenoids and anthocyanins into films improves their functionality while introducing innovative, intelligent food packaging solutions. The addition of natural pigments to biodegradable films offers a sustainable approach to enhance visual appeal, provide functional properties, and serve as indicators of freshness or spoilage. The main benefits include providing diverse, appealing colors to biodegradable films, enhancing consumer acceptance, introducing antioxidant, antimicrobial, or UV-blocking properties, and extending food shelf life. Natural pigments also align with the “green” image of biodegradable films by using renewable, and often food-waste-derived, colorants. These pigments meet the growing consumer demand for natural ingredients and “clean label” products, free from synthetic dyes [
24,
25].
Anthocyanins, naturally found in fruits and vegetables, undergo color changes in response to variations in pH levels, offering a visual indicator of food freshness [
26,
27]. When integrated into films, these pigments serve as pH sensors, improving food safety by signaling potential spoilage or contamination caused by microbial activity or chemical reactions. The ability to visually detect pH shifts enables consumers and food handlers to take timely action, reducing food waste and ensuring that products remain safe for consumption. Many food items can benefit from such pH-sensitive films. For instance, fruits experience pH fluctuations as they ripen or deteriorate, allowing the film to indicate freshness. Additionally, meat and seafood are highly prone to bacterial spoilage, leading to their pH alterations, making the film’s color change an effective warning system to ensure only fresh, safe products reach consumers [
28,
29,
30].
Carotenoids are not directly pH-sensitive; however, pH changes the oxidation rate of carotenoids, acting as an indirect factor for color change. While carotenoids don’t change color directly with pH, they can have an indirect effect on their perceived color in a matrix with acerola extract, primarily by influencing their stability and the stability of other compounds. Carotenoids are highly susceptible to oxidation due to their extensive system of conjugated double bonds. Oxidation breaks down the carotenoid molecule into smaller, colorless or less intensely colored fragments, leading to a loss of their intense colors. The rate of oxidative reactions can be significantly influenced by pH. Furthermore, in some systems, pH can affect the aggregation state of components, such as polysaccharides that might encapsulate or interact with carotenoids. Changes in these interactions or the matrix structure can alter how light is scattered or absorbed, potentially affecting the perceived color, even if the carotenoid molecules themselves did not change [
31,
32,
33].
Incorporating natural pigments into biodegradable starch-based films meets the increasing consumer preference for eco-friendly, safe, and naturally sourced food packaging. Since these pigments are non-toxic and obtained from renewable sources, they offer a healthier and more sustainable alternative to synthetic indicators [
27].
Grape pomace (Vitis labrusca), a wine production by-product, is an abundant source of anthocyanins, while the residue from acerola (Malpighia emarginata) processing contains high levels of carotenoids. Extracted from these materials, anthocyanins and carotenoids serve as natural antioxidants and colorants in foods and biodegradable packaging, enhancing nutritional value and visual appeal.
Due to the scale of global grape production, particularly in winemaking, the industry produces large quantities of grape pomace. Similarly, acerola processing, especially for juice and pulp, produces a large number of residues, including peels, seeds, and pulp. Contrary to grapes, acerola cultivation is concentrated in a few regions, with Brazil being the primary producer. Recognizing the potential of these by-products, the agroindustry is actively exploring ways to repurpose grape pomace and acerola residues into commercial applications, aligning with sustainability efforts to minimize waste and maximize resource utilization.
This study focused on developing pH-sensitive starch and carrageenan blend films enriched with acerola and grape pomace extracts. Various characteristics were assessed, including chemical group profiling, hydrophobicity, moisture content, physical structure, and the film’s effectiveness as a pH-sensitive indicator.
2. Materials and Methods
2.1. Materials
Commercial corn starch (Maizena brand, Garanhuns, Brazil) was bought in the supermarket. Amylose standard was bought from Merck (Rahway, NJ, USA). Vetec Química Fina (Rio de Janeiro, Brazil) provided glycerol. Carrageenan was extracted from Hypnea musciformis seaweed.
2.2. Extraction of Carrageenan
Specimens of the red marine macroalgae H. musciformis were obtained from an existing culture on the beach of Volta do Rio, municipality of Acaraú, Ceará State, Brazil, in long line ropes. The macroalgae, randomly chosen, were placed in plastic bags containing some seawater from the environment itself and quickly transported to the Aquaculture Biotechnology Laboratory of the Federal Institute of Education, Science, and Technology of Ceará—IFCE, Campus Acaraú, Ceará, Brazil, where they were properly cleaned, separating them from sediments and other materials.
The macroalgae were washed several times with water to remove excess salt and moisture. After cleaning, they were dried in a drying oven for 48 h at 60 °C. Then, they were cut into small pieces and weighed on a semi-analytical balance.
Prior to the extraction of the phycocolloids, the dried algal material was rehydrated and then treated with a mixture of 100% methanol and 100% acetone to eliminate the organosoluble fraction [
34]. Then, the samples were placed in a solution (150 mL g
−1) of NaOH (1 M) in a water bath at 85 °C for 3 h [
35]. The carrageenan solution was neutralized (pH 6–8) with HCl (0.3 M). After adding twice the volume of ethanol (96%), carrageenan precipitated, forming a whitish clot. After washing with ethanol (96%), this clot was dried in an oven at 60 °C for a period of 48 h [
35].
2.3. Extraction of Carotenoids and Anthocyanins
Anthocyanins were extracted from grape pomace waste. Carotenoids were extracted from acerola industrial waste. The samples were dried for 24 h in a circulating drying oven (Tecnal, TE-394/1, Piracicaba, Brazil) at 60 °C, then ground into flour using a coffee mill (Cuisinart, Stamford, CT, USA). A 3% (w/v) flour suspension was prepared in distilled water and subjected for 3 min to ultrasound treatment (500 W, Unique DES500, Indaiatuba, Brazil) with a 1.3 cm diameter tip working at full power. The extract was subsequently filtered using a qualitative filter paper.
2.4. Production of Intelligent Starch-Based Films
The films were produced using the casting technique [
36] with some modifications. An amount of 5 g of corn starch was dissolved in 100 mL of distilled water and heated to 95 °C under magnetic stirring for 30 min. Grape and acerola extracts were added to the film-forming solution for the pH-sensing films. Glycerol (25%
w/
w) was incorporated, and the mixture was maintained at 60–65 °C for 15 min. The dispersion was homogenized using an Ultra-Turrax (IKA model T25 Staufen im Breisgau, Germany) for 15 min at 10,000 rpm. A volume of 10 mL of the film-forming solution was then poured onto a Petri dish and left to dry at room temperature (25 °C) until complete solvent evaporation (24 h).
2.5. Physical and Chemical Characterizations
2.5.1. Amylose Content
Amylose content determination followed the method presented by Hu et al. [
37]. Segments of film (0.1 g) were immersed in ethanol (1 mL) and NaOH solution (10 mL, 1 mol/L). The mixture was heated for 15 min in a water bath (70 °C), then cooled to ambient temperature. An aliquot of 5 mL was collected and mixed with water (46 mL), potassium iodide aqueous solution (2 mL, 0.2 g I
2 + 2 g KI/100 mL), and acetic acid aqueous solution (1 mL, 1 mol/L). After staining for 10 min, its absorbance was measured at 620 nm using a UV-Vis Spectrophotometer (Thermo Scientific model Evolution 201, Waltham, MA, USA).
2.5.2. Solubility and Moisture
A mass of 1 g of film was dried in a circulating drying oven (Tecnal, TE-394/1, Piracicaba, Brazil) at 60 °C for 24 h. The sample was weighed after drying to determine its moisture content.
For the solubility determination, the films weighed at the end of the moisture analysis were immersed in distilled water (15 mL) and shaken (Kasvi, Thermo Shaker K80-200, São José dos Pinhais, Brazil) at 300 rpm and ambient temperature for 24 h. Finally, they were dried at 60 °C for 24 h and weighed to determine the solubility.
2.5.3. Hydrophobicity
The hydrophobicity was evaluated by determining the contact angle, which was measured following the ASTM Standard D-5725-99 [
38]. In brief, a drop of water was placed on the surface of the films (2 × 2 cm) fixed on a glass support. When the drop touched the surface, a Nikon Pix Link camera captured the image. The contact angle measurement was based on the data collected from the image.
2.5.4. Chemical Groups and Molecular Structure
Fourier Transform Infrared Spectroscopy (FTIR) was used to evaluate the molecular structures of the starch and carrageenan-based films. An Agilent model Cary 630 (USA) equipped with an ATR measurement accessory was used for the measurements. IR spectra were obtained within the range of 4000 to 400 cm
−1. The absorbances at 995 cm
−1 and 1022 cm
−1 were used to determine the crystalline/amorphous ratio (CAR) and the short-range ordered structure [
39,
40].
2.5.5. Surface Morphology
Surface morphology of the films was evaluated by scanning electron microscopy (SEM) using a Quanta 450 FEG-FEI with an acceleration voltage of 20 kV and a magnification of 1000×.
2.5.6. Water Vapor Permeability (WVP)
The water vapor permeability of the films was evaluated by means of the E96-00 method [
41]. Permeation cells (24 mm in diameter) containing 1.5 mL of distilled water were used. The cells were maintained at 25 ± 2 °C in a vertical desiccator (Arsec DCV040) for 24 h. Measurements were done at an interval of 1 h. Eight replicates were carried out per film.
2.6. Color Change Capacity of Films as a Function of pH
The colorimetric pH response was measured using UV-Vis spectrophotometry. Film strips (20 × 2 mm) were subjected to different buffer solutions (pH 3, 4, 5, 6, 7, 8, 9, 10, and 10.6) for 5 min and then scanned at absorbances between 380 and 780 nm.
2.7. Statistical Analysis
Statistical analysis was performed using variance analysis using the Statistica® 14 software. Tukey’s test and t-test were applied at a 95% confidence level to detect significant differences.
3. Results
3.1. Chemical Group Profile
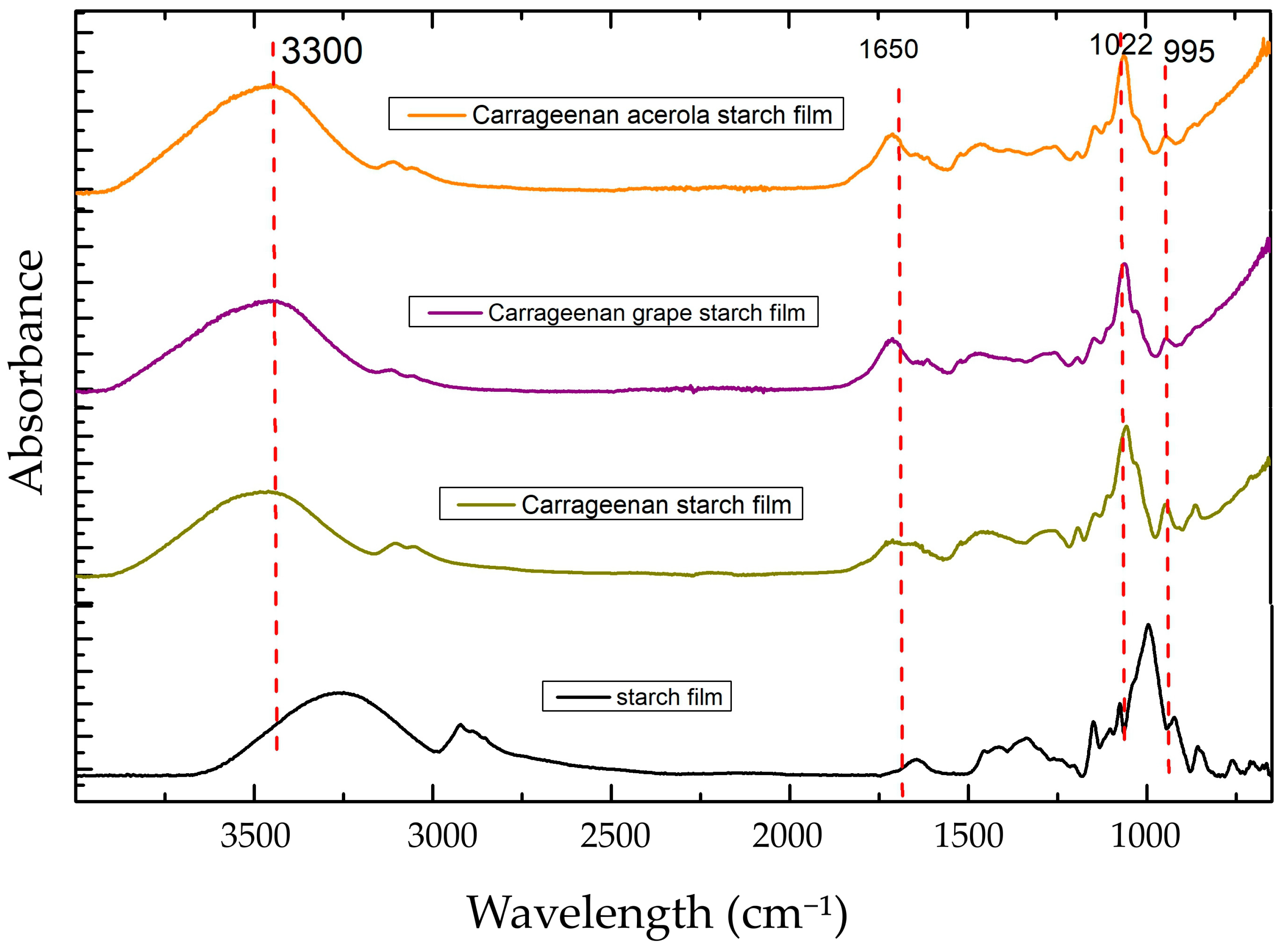
The functional groups and interactions between starch, carrageenan, and the extracts in the biodegradable films were evaluated by means of FTIR analyses (
Figure 1). No new characteristic bands appeared in the FTIR spectra due to the addition of the extracts. The insignificant change may be due to the addition of low concentrations of extracts (3%
v/
v) to the film solution. The analysis showed minor band shifts and intensity changes.
The starch-based film was characterized by O-H stretching observed at 3300 cm−1 and associated with hydroxyl groups, C-H stretching observed at 2900 cm−1 indicating the presence of aliphatic hydrocarbons, C=O stretching observed at 1650 cm−1 associated with carbonyl groups, and C-O stretching observed at 1100 cm−1 indicating the glycosidic bonds in starch molecules and glycerol used as plasticizer.
The film produced with starch and carrageenan presented similar characteristics, with band shifts occurring mainly for O-H stretching and C-O stretching. A broadband was observed at 1225 cm−1, representing the S=O stretching that is characteristic of carrageenan. The low number of extracts incorporated into the films did not significantly change the FTIR spectra.
The molecular interactions between carrageenan and starch are primarily governed by hydrogen bonding, electrostatic interactions, and physical entanglements, which are influenced by the chemical structure of both starch and carrageenan.
Among these interactions, hydrogen bonding is the most significant molecular interaction in starch–carrageenan films. Carrageenan has hydroxyl groups that form hydrogen bonds with the starch’s hydroxyl groups [
20,
42]. Carrageenan contains sulfate groups that interact electrostatically with starch, further stabilizing the film structure, which can lead to enhanced barrier properties [
4,
21].
Table 1 presents the short-range ordered structure parameters of the starch and carrageenan-based films added with grape pomace and acerola residue extracts. Two parameters were presented, representing the ratios of the amorphous structure to the ordered carbohydrate structure in starch and carrageenan films (995 cm
−1/1022 cm
−1) and the degree of order in the films (1045 cm
−1/1022 cm
−1).
The FTIR measurement at 995 cm
−1/1022 cm
−1 is a critical tool for determining the short-range structure of starch-based films. This ratio indicates the conformational changes in the molecules, particularly in relation to their crystalline and amorphous regions. The 995 cm
−1 band is associated with the amorphous regions, while the 1022 cm
−1 band corresponds to the crystalline regions of starch. Analyzing the ratio of these two peaks makes it possible to infer the degree of crystallinity and the structural organization within the films, which are crucial for understanding their functional properties [
44].
Compared to starch-based films [
43], the introduction of carrageenan has decreased the 995/1022 ratio by 13%, indicating shifts in the molecular conformation of starch, such as during gelatinization or retrogradation processes. The decrease in the ratio indicated an increase in the crystalline region of the molecule in relation to the amorphous region.
Incorporating carrageenan into starch-based films significantly influences their crystallinity, affecting their mechanical and barrier properties. Carrageenan, a polysaccharide derived from seaweed, interacts with starch to modify the crystalline structure of the composite films. Depending on the specific formulation and processing conditions, this interaction can lead to either an increase or a decrease in crystallinity. The changes in crystallinity are crucial as they impact the film’s mechanical strength, flexibility, and permeability. In this case, adding carrageenan increased the film’s crystallinity.
Carrageenan has enhanced the crystallinity of the starch film by interacting with starch molecules, leading to a more ordered structure. Such phenomena may occur because carrageenan, as a polysaccharide, forms hydrogen bonds with starch, reinforcing its molecular alignment. This reinforcement can promote gel formation, which leads to a more structured and crystalline network. Furthermore, it can restrict the movement of starch chains, helping maintain a more stable crystalline phase generated during the retrogradation of starch. Reports on starch and carrageenan films have also demonstrated a reduced degradation rate and swelling degree, making them viable alternatives to conventional plastics [
4].
In a related study, FTIR analyses have indicated physical entanglements between cassava starch and κ-carrageenan, likely due to hydrogen bonding. This interaction contributed to the formation of a more rigid network, enhancing the mechanical properties and reducing the films’ hygroscopic nature [
21].
The addition of grape pomace and acerola extracts increased the 995/1022 ratio by 23% and 31%, respectively, compared to the starch and carrageenan film. The increase in the ratio indicated a decrease in the molecule’s crystalline region in relation to the amorphous region. The addition of the fruit extracts into the starch and carrageenan film has slightly shifted the vibration absorption peak of -OH, indicating that the extracts have affected the intermolecular force of starch. This phenomenon has also been reported by Wang et al. [
23], suggesting that starch, carrageenan, and the extracts interacted in a hydrogen bonding interaction. As the FTIR analysis indicates, the extracts can form hydrogen bonds with relevant functional groups in the film, reducing free hydrogen and forming hydrophilic bonds.
3.2. Amylose Content
Table 2 presents the amylose content of the starch–carrageenan films added with acerola and grape pomace extracts. The starch–carrageenan film presented an amylose content of 4.60%.
For comparison purposes, a typical starch-based film has approximately 22.6% amylose [
36,
43]. The incorporation of carrageenan tends to reduce these levels due to introducing a polysaccharide chain, rather than amylose or amylopectin. Further addition of acerola and grape pomace extracts to the starch solution slightly changed the amylose content of the films. These changes, however, can be attributed to the natural variations of the production process, rather than chemical changes caused by the introduction of the extracts.
Carrageenan forms good films due to its specific molecular structure and high molecular weight, allowing for strong intermolecular interactions and creating a continuous, cohesive matrix. Unlike starch, which relies on the linear amylose fraction for strong film formation, carrageenan’s film-forming ability stems from its highly branched and flexible polymeric chains. High molecular weight polymers are generally better film formers because they have more opportunities for entanglement and intermolecular interactions (like hydrogen bonding and van der Waals forces) over a larger chain length. This contributes to the mechanical strength and continuity of the film. Carrageenan is highly soluble in cold water, forming viscous solutions even at low concentrations. This high viscosity is crucial for film casting processes, as it allows for forming a uniform and continuous wet film layer.
3.3. Solubility and Moisture Content
Table 3 presents the moisture contents and solubility of the films. The starch–carrageenan film presented a solubility of 45.0%. Including acerola and grape pomace extract slightly increased the film’s solubility. A higher increase in solubility was observed in the film added with acerola extract. However, this increase was not statistically significant. The starch–carrageenan film presented a moisture content of 32.5%. The addition of the extracts slightly increased the moisture content of the films, with the addition of acerola extract presenting the greatest reduction.
Solubility and moisture content are vital in shaping polymer interactions, directly affecting the physicochemical and functional properties of materials throughout processing and storage [
48]. Several factors influence the solubility and moisture content of starch and carrageenan films, including the composition and interaction of the film components, the presence of plasticizers (such as glycerin), and the structural properties of the polysaccharides involved.
Pure starch and carrageenan films have very high solubility and are usually unsuitable for several applications. Adding glycerol as a plasticizer significantly changes the moisture content and solubility of the films. The hydrophilic nature of glycerol, with its higher number of hydroxyl groups (OH), increases the moisture content. Such an increase in the moisture content of the starch and carrageenan film is more evident than that of the starch film, where the moisture content increases from 20.6 to 32.5%. The increase in moisture content can be explained by the hydrophilic nature of the plasticizer that may result in a reorganization of the polysaccharide network, free volume, and an increase in segmental movements, which makes it easier for water molecules to diffuse and results in a greater moisture content of the film [
46].
The solubility of the starch and carrageenan film produced herein can be considered low, at 45.0%, when compared to other starch films and carrageenan films described in the literature [
45,
46,
47]. Usually, the ratio of carrageenan to starch significantly affects the solubility and moisture content of the films. Higher carrageenan content tends to increase water solubility and moisture absorption due to its hydrophilic nature and crystalline structure, which enhances water interaction. However, the amount of plasticizer used herein has compensated to maintain the film’s solubility at lower levels.
The addition of grape pomace and acerola residue extracts has slightly changed the film’s moisture content and solubility. The changes induced by introducing grape pomace extract were not considered statistically significant. In contrast, the introduction of acerola residue extract was considered statistically significant only regarding the moisture content. In a related study, Qin et al. [
49] found that adding anthocyanins in specific concentrations did not change the moisture of cassava starch films.
3.4. Hydrophobicity
A surface’s hydrophobicity or wettability can be evaluated by measuring its contact angle. This indicates how liquid droplets spread across different materials and reflects their degree of hydrophobicity [
50].
Table 4 presents the contact angle values of the films analyzed in this study and data from the literature. The starch and carrageenan film presented a low contact angle (52.9°) and can be considered hydrophilic. Films incorporating anthocyanins and carotenoids exhibited an increased contact angle.
The most common method for assessing a surface’s hydrophobicity or hydrophilicity is measuring its water contact angle. A surface is considered hydrophobic if its water contact angle exceeds 90°, while a hydrophilic surface has a contact angle below 90°. Based on these ranges, all the films produced herein are considered hydrophilic.
The hydrophobicity of starch and carrageenan films is influenced by several factors, including the chemical composition, modification techniques, and the incorporation of additives. Starch and carrageenan, both naturally hydrophilic, require modifications to enhance their hydrophobic properties for applications such as food packaging. These modifications can be achieved through various methods, including chemical, physical, and enzymatic processes, and the addition of hydrophobic agents.
The introduction of the extracts increased the film’s hydrophobicity, but they could not increase the value of the contact angle at the point where the films become hydrophobic. The low contact angle observed herein will determine some limitations in the applicability of this material.
The film’s low amylose content has also contributed to its low hydrophobicity. Additionally, the interaction between starch and carrageenan, along with the retrogradation process, affects the structural properties of the films, further influencing their hydrophobicity. Different sources of starch and carrageenan may be required to improve this property because the amylose content and its association with amylopectin during retrogradation play a crucial role in determining the film’s hydrophobicity [
53]. Also, different types of carrageenan may interact differently with starch and other components, influencing the overall film properties [
54].
3.5. Water Vapor Permeability
Water vapor permeability measures a material’s effectiveness in blocking moisture transfer from the surrounding air into a package. High water resistance in packaging enhances food stability and prolongs shelf life [
55]. The starch–carrageenan film’s water vapor permeability was 3.64 g·mm/kPa·h·m
2 (
Table 5). The addition of plant extracts slightly increased the WVP.
Incorporating grape pomace and acerola residue extracts into the films enhanced their WVP, with a more pronounced effect observed in acerola-added films. The presence of sugars and soluble pectin in these extracts likely acted as plasticizers, weakening the intermolecular forces within the starch and carrageenan and expanding the system’s free volume. As a result, chain mobility increased, making water permeation through the structure more efficient [
56].
The barrier properties of starch and carrageenan films are critical for their use in food packaging. These films exhibit low water vapor permeability (WVP), which is essential for preventing moisture transfer between packaged food and the environment. The WVP of starch films and carrageenan films is comparable to some synthetic polymers [
57]. However, the starch and carrageenan films produced herein presented a water vapor permeability at least 131% and 468% higher, respectively, than the WVP reported in the literature for starch films and carrageenan films [
43,
52].
The barrier properties of starch–carrageenan films, such as water vapor permeability (WVP), are influenced by the molecular interactions between the polymers. Films with higher carrageenan content generally exhibit lower WVP due to the hydrophobic nature of carrageenan and the formation of a more compact film structure [
21]. Thus, the difference between the WVP of the carrageenan film and the blended starch and carrageenan film was much higher than the comparison with the starch film.
Related studies in the literature also showed that the incorporation of fruit extracts into starch and carrageenan films increases their water vapor permeability because the presence of the extract produces a less crystalline film, resulting in an increased WVP [
23].
Water vapor permeability (WVP) directly affects food shelf life. For items such as fresh produce, bread, or moist snacks, a low WVP is essential to prevent dehydration, weight loss, and changes in texture. This is equally important for dry foods like crackers, chips, or cereals, where a low WVP is crucial to keeping them from becoming soggy.
However, certain products like cheeses or fresh produce may require a moderate WVP to allow them to “breathe.” Additionally, some items need higher permeability for ripening processes while preventing excessive moisture build-up that could lead to spoilage. While low WVP is generally desirable for most conventional food packaging applications, the ideal WVP depends on the specific food and its preservation needs.
The WVP values of the films containing acerola and grape pomace extracts are considered high, so this should be addressed for cheeses, fresh produce, and fruits that continue to ripen. For other applications, these films might not be suitable unless the food is intended for rapid consumption or has very specific moisture exchange requirements.
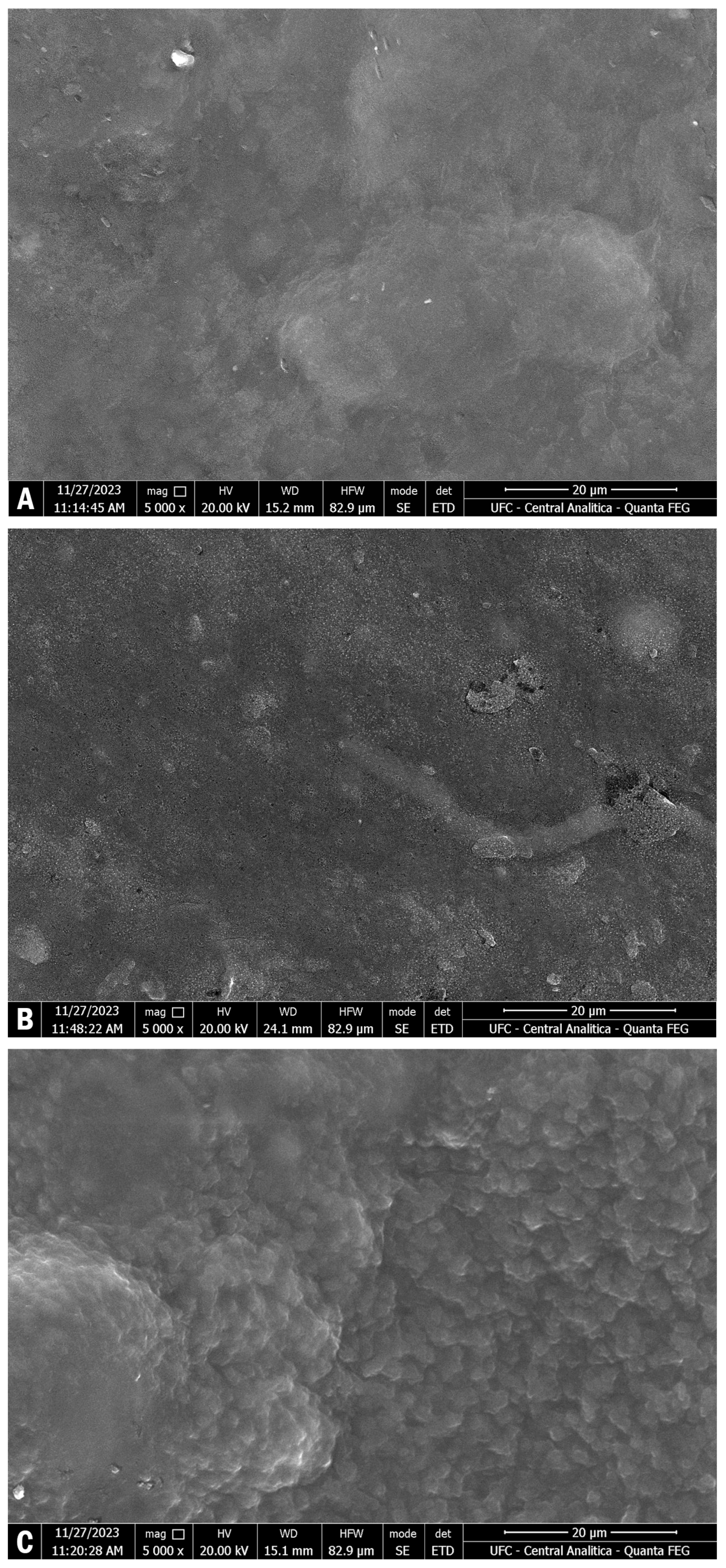
3.6. Surface Morphology
Figure 2 shows the films’ surface morphology. The starch and carrageenan film presented a more uniform surface morphology. The addition of the plant extracts incorporated sugar granules into the film, which presented some “lumps” on its surface.
The interaction between starch and carrageenan affects the surface morphology of the films. A decrease in crystallinity often results in smoother surfaces, which can improve the film’s appearance and tactile properties [
58].
The two films incorporated with the fruit extracts presented a granular, rough appearance, denoting the presence of carotenoids and anthocyanins in the starch and carrageenan films. When well-integrated into the film matrix, natural pigments tend to promote a better compatibility between the film components, while the presence of small cracks does not weaken their mechanical properties [
55]. Xiao et al. [
59] have also confirmed the porous appearance of films incorporated with anthocyanins, with the formation of a greater wrinkled appearance.
The addition of anthocyanins from grape pomace and carotenoids from acerola extract resulted in a more heterogeneous and irregular surface morphology of starch films. This is due to the aggregation of anthocyanin particles within the film matrix, which becomes more pronounced with higher concentrations of anthocyanins and carotenoid [
60,
61].
3.7. Color Sensing Property
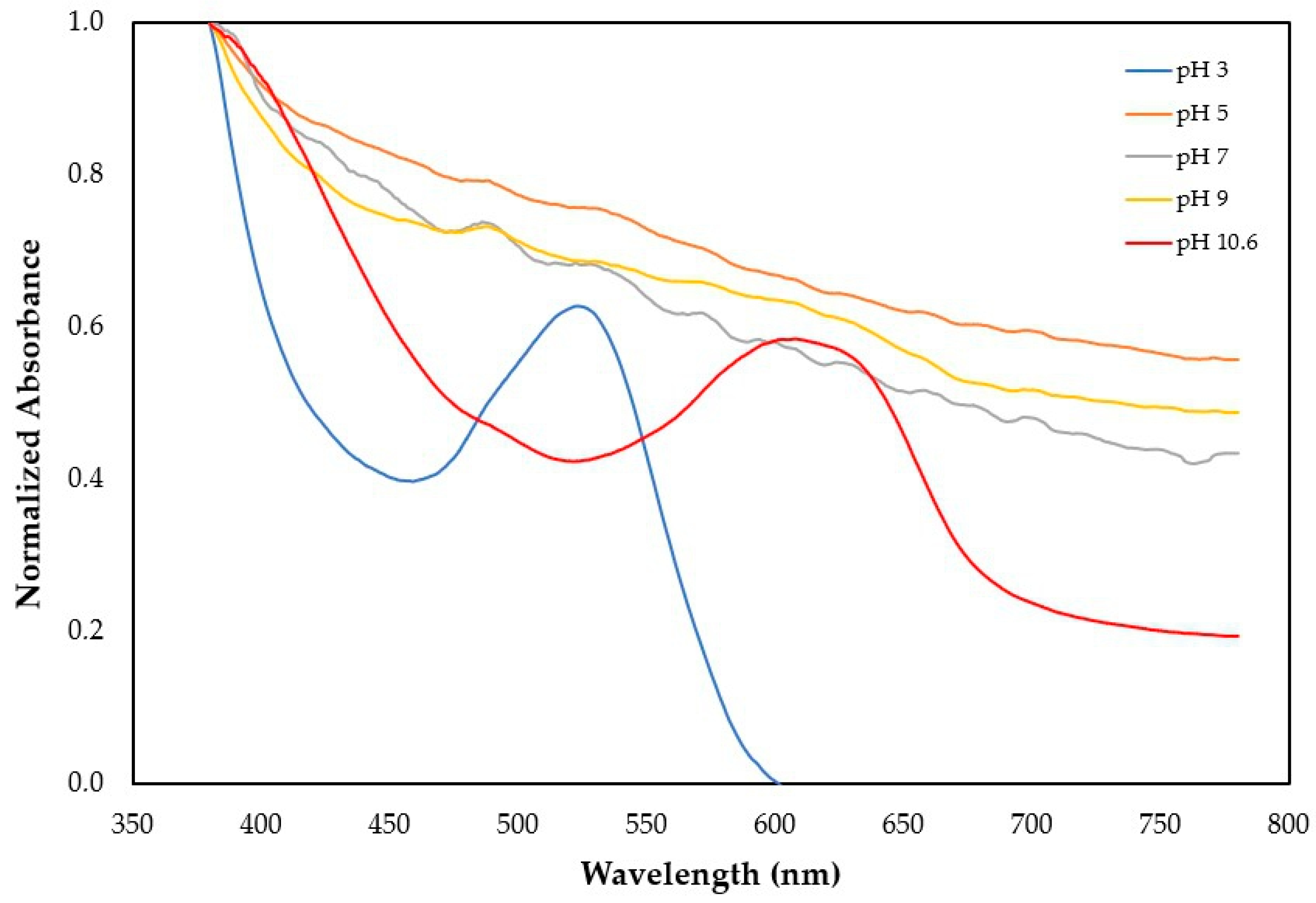
Figure 3 and
Figure 4 present the visible wavelength spectra for the starch and carrageenan films added with grape pomace and acerola residue extracts.
Table 6 summarizes the spectra information, presenting the normalized absorbance at four distinct color reflectances.
The film with grape pomace extract was mainly characterized by a high absorbance at 400 and 500 nm and insignificant absorbances at 600 and 700 nm, resulting in the reflectance of its red-purple color. The increase in pH changes the characteristic color of the film. At a pH between 5 and 7, the absorbance of all wavelengths increases, changing the proportion between the wavelengths. While the red-purple reflectance increases, the cyan and the blue-green tones increase more significantly, resulting in a fading of the red color towards a paler palette. The change from neutral pH (7.0) to basic pH (9.0) was not accompanied by a significant color change. However, the increase of pH to more basic conditions (pH 10.6) significantly changed the color of the films. At this pH, the absorbance of the red and blue tones decreased considerably, and the film reflected a greenish color. In practical terms, at acidic pH (3.0), the film has a strong purple color, which becomes paler at neutral pH, and then starts to become green at basic pH (10.6).
Figure 5 shows the films’ colors after 5 min of contact with three different pH solutions (3.0, 7.0, and 10.6).
Choi et al. [
62] obtained similar results in starch films incorporated with anthocyanins. The film started with red, moving to pink until reaching violet. Then, as the pH increased, the film turned green. This particular property of anthocyanins has been the most used in the development of smart packaging films for monitoring food freshness based on pH indicators.
The film with acerola residue extract was mainly characterized by a high absorbance at 400 nm, mid-range absorbances at 500 and 600 nm, and low absorbance at 700 nm, resulting in the reflectance of its orange color, which resulted from the combination of the predominance of the red and yellow tones. The increase in pH did not significantly change the characteristic color of the film, as occurred with the film incorporated with grape pomace extract. At pH 5.0, the absorbance of the red and blue tones (500 to 700 nm) increased, intensifying the film’s color. At neutral pH (7.0), the blue and cyan tones started to fade, giving way to a more yellowish characteristic. In practical terms, at acidic pH (3.0), the film has an orange color, which becomes paler at pH 5.0 and then starts to become less orange and more yellow at neutral and basic pHs.
Carotenoids are susceptible to oxidation due to their extensive system of conjugated double bonds. Oxidation breaks down the carotenoid molecule into smaller, colorless, or less intensely colored fragments, leading to a loss of their intense colors. Given the loss of color presented by the films with acerola extract, changes in pH have probably influenced the rate of oxidative reactions.
Migration and leaching of anthocyanins and carotenoids from the film to the food is usually a concern. This study has focused on the development of the films and their physical and chemical characterization. No study on migration has been conducted. However, studies on producing biodegradable films with extracts containing anthocyanins and carotenoids affirm that some degree of migration of these extracts from the film to the food occurs. Such migration is usually caused by the solubility of these extracts in water and, therefore, in moist foods [
61,
63].
3.8. Environmental Implications
Beyond the scope of novel film development, this research holds a significant promise for addressing broader environmental concerns and unlocking diverse applications, particularly through its utilization of grape pomace and acerola residue and the potential for sustainable processing.
An important environmental benefit of this research lies in its focus on utilizing acerola and grape residues. Annually, significant quantities of fruit processing by-products are generated, often as landfill waste, contributing to greenhouse gas emissions and pollution. The use of acerola and grape residues aligns directly with the principles of the circular economy and waste valorization by demonstrating the potential of these residues as a source for film-forming materials and natural pigments (like anthocyanins and carotenoids). It transforms a low-value waste stream into a higher-value product, reducing waste generation, lessening the burden on landfills, and potentially creating new revenue streams for the agricultural sector.
The development of biodegradable films from natural sources like starch and carrageenan, reinforced or colored with natural pigments, contributes to reducing reliance on conventional petroleum-based plastics. This lessens the demand for fossil fuels, decreases the carbon footprint associated with plastic production, and mitigates the persistent problem of plastic pollution in ecosystems.
While water-based extraction of carotenoids may have faced challenges, the ongoing exploration of green extraction methods, such as ultrasound-assisted techniques, is important. Adopting such a method minimizes the use of hazardous organic solvents, reduces energy consumption, and prevents the discharge of toxic effluents, further enhancing the environmental profile of the entire production chain. This commitment to green chemistry principles ensures that the development of sustainable materials does not come at the cost of polluting extraction processes.
These biodegradable films can extend the shelf life of packaged food products by tackling the problem of barrier properties and offering features such as pH-sensitive indication. Reducing food spoilage directly translates to a decrease in global food waste, which has substantial environmental implications related to water, land, and energy consumption and greenhouse gas emissions from decomposing food.