Process Optimization of Ultrasonic-Assisted Extraction and Resin Purification of Flavonoids from Eucommia ulmoides Leaves and Their Antioxidant Properties In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Optimization of Extraction Conditions of Total Flavonoids from Eucommia ulmoides Leaves
2.3. Adsorption and Desorption Tests
2.4. Flavonoids Purification Process
2.5. Determination of Total Flavonoid Content
2.6. Analysis of Antioxidant Activity of Total Flavonoids
2.6.1. Total Flavonoid Sample Preparation
2.6.2. DPPH Radical Scavenging Activity
2.6.3. Determination of Reducing Power
2.6.4. Hydroxyl Radical Scavenging Activity
2.6.5. Scavenging Activity on Superoxide Anion Radical
2.6.6. ABTS Radical Scavenging Activity
2.7. HPLC Analysis of the Purified Total Flavonoids
2.8. Statistical Analysis
3. Results and Analyses
3.1. Optimization of Ultrasonic-Assisted Extraction for Total Flavonoids
3.2. Purification of Total Flavonoids from Eucommia ulmoides Leaves Using Macroporous Resins
3.2.1. Resin Selection
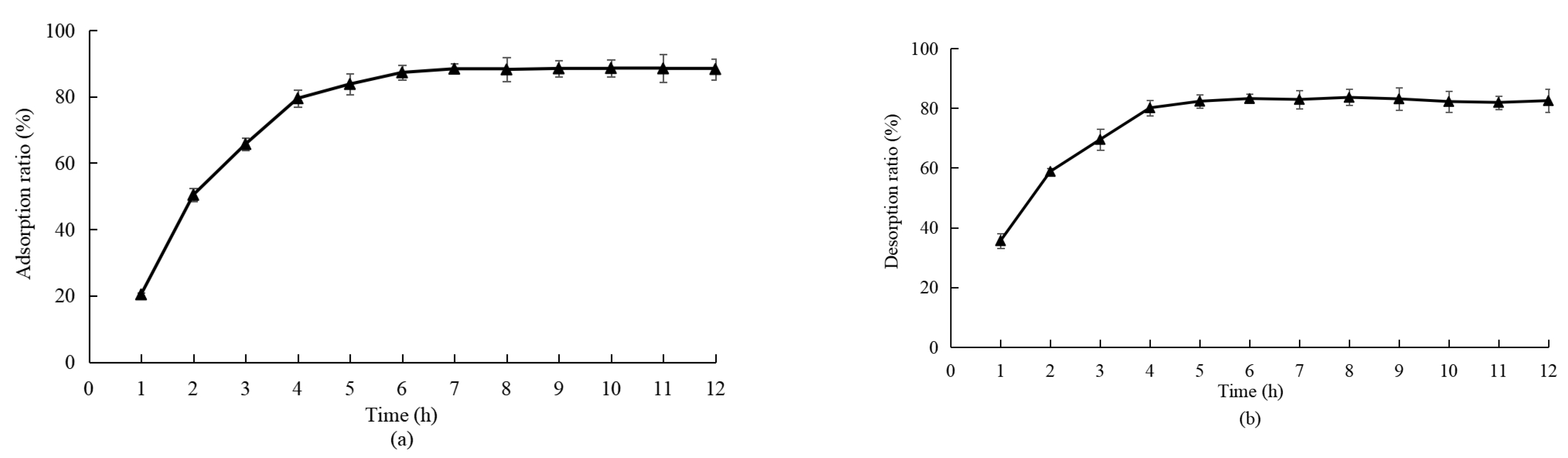
3.2.2. Adsorption and Desorption of XDA-8 Resin on Total Flavonoids
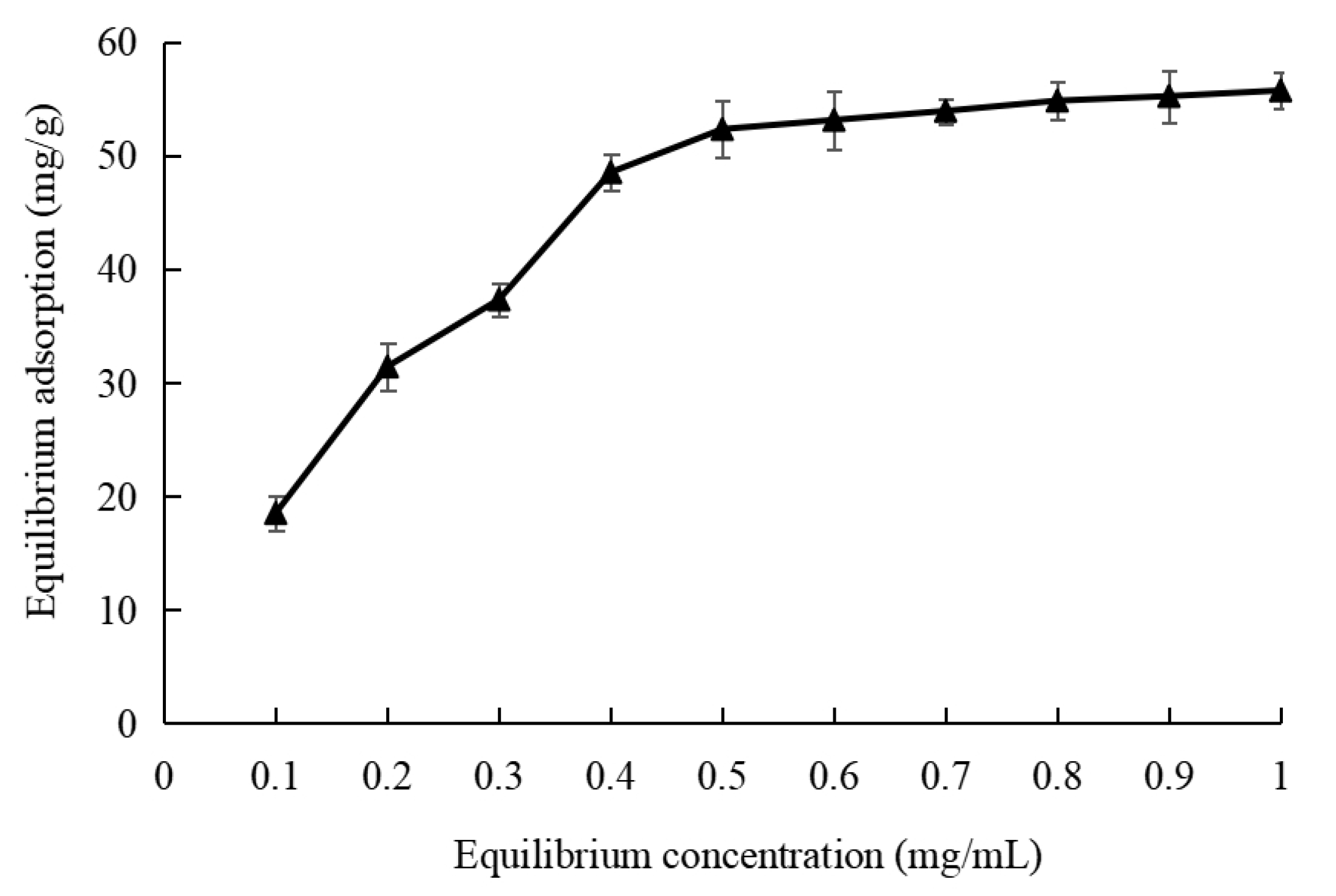
3.2.3. Adsorption Isotherms of XDA-8 Resin
3.3. Purification of Flavonoids Using XDA-8 Resin
3.3.1. Effect of Flow Rate on Adsorption Ratio
3.3.2. Effect of Solution pH on Adsorption Ratio
3.3.3. Effect of Sample Concentration on Adsorption Ratio
3.3.4. Effect of Desorption Solution on Desorption Ratio and Flavonoid Purity
3.3.5. Effect of Eluent Flow Rate on Desorption Ratio
3.4. Determination of Antioxidant Activities
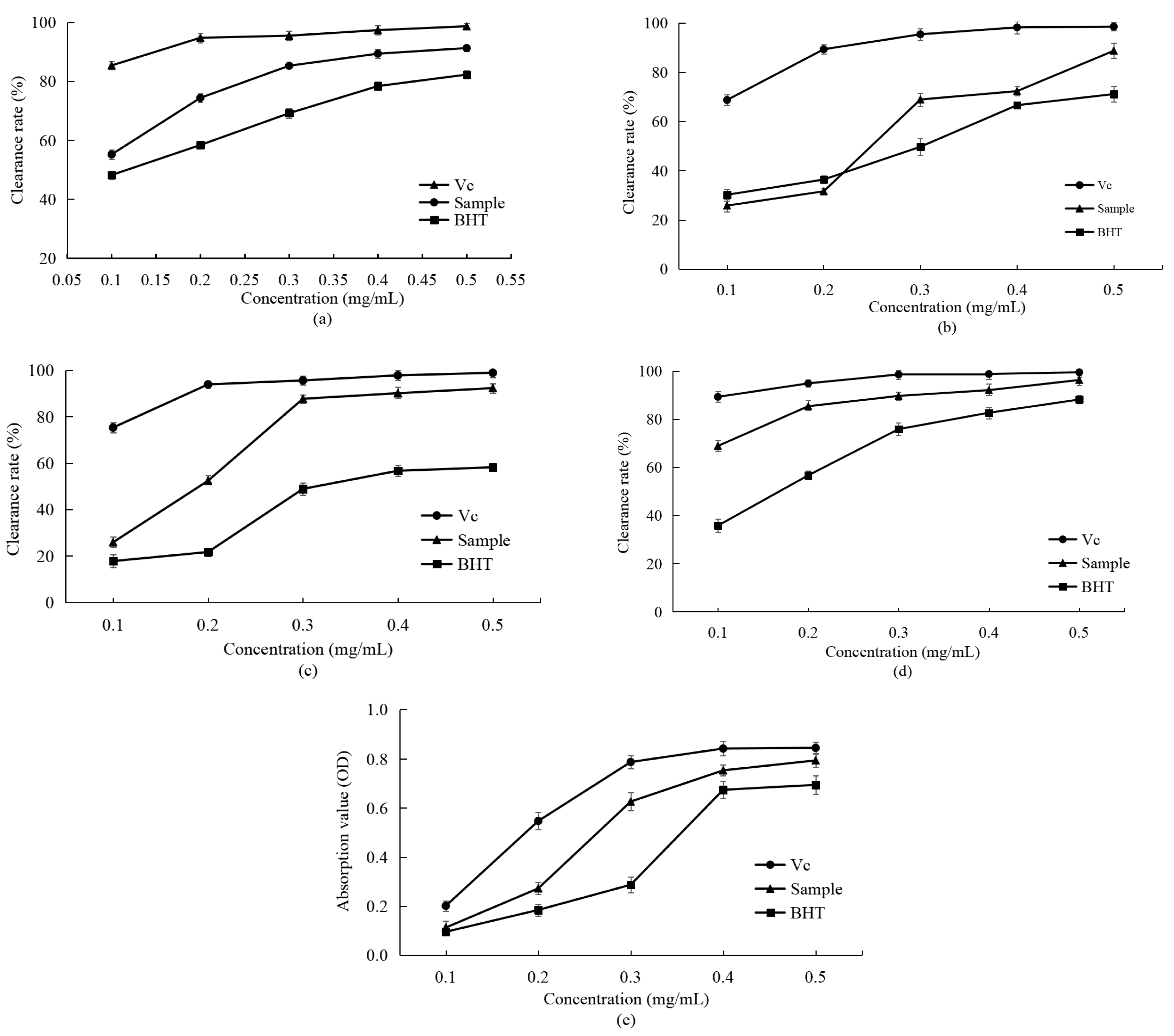
3.4.1. Determination of Scavenging Activity on DPPH Radical
3.4.2. Determination of Scavenging Activity on Hydroxyl Radicals
3.4.3. Determination of Scavenging Activity on Superoxide Radical
3.4.4. Determination of Scavenging Activity on ABTS Radicals
3.4.5. Determination of Reducing Power
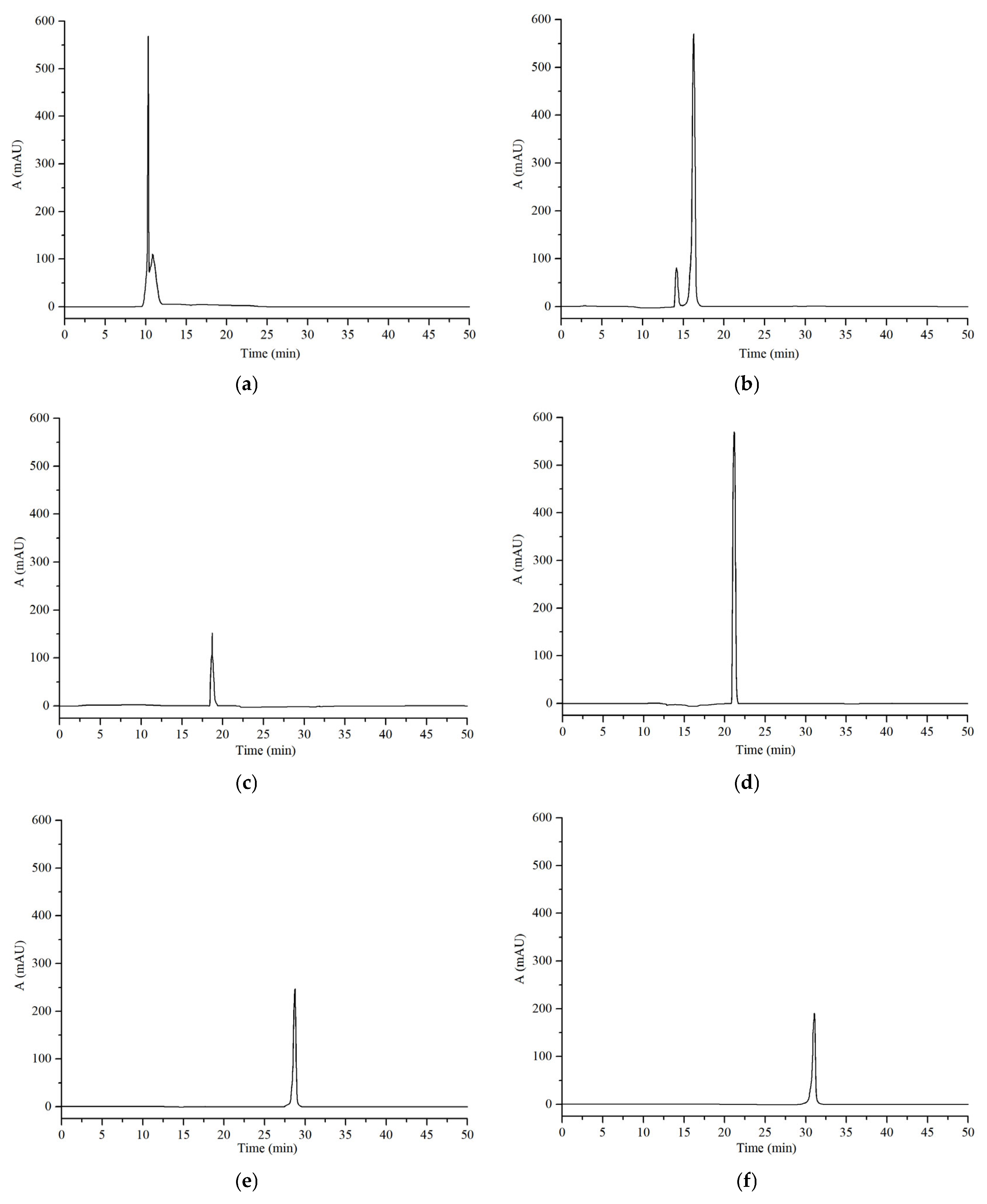
3.5. HPLC Analysis of the Purified Total Flavonoids
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, J.J.; Huang, L.Q.; Li, C.Y.; Zhou, X.Q.; He, J. Study on isolation and purification of Eucommia ulmoides leaves flavonoids by two-column method. Appl. Chem. Ind. 2023, 52, 2972–2975+2980. [Google Scholar] [CrossRef]
- Huang, D.; Zhu, J.; Zhang, L.; Ge, X.; Ren, M.; Liang, H. Dietary supplementation with Eucommia ulmoides leaf extract improved the intestinal antioxidant capacity, immune response, and disease resistance against Streptococcus agalactiae in genetically im-proved farmed Tilapia (GIFT; Oreochromis niloticus). Antioxidants 2022, 9, 1800. [Google Scholar] [CrossRef] [PubMed]
- Leea, G.H.; Leea, H.Y.; Choia, M.K.; Choib, A.H.; Shinb, T.S.; Chae, H.J. Eucommia ulmoides leaf (EUL) extract enhances NO production in ox-LDL-treated human endothelial cells. Biomed. Pharmacother. 2018, 97, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Sun, Y.L.; Wang, J.M.; Li, W.B.; Liu, J.; Li, T.Y.; Liu, Z.Q. A review of “plant gold” Eucommia ulmoides Oliv.: A medici-nal and food homologous plant with economic value and prospect. Heliyon 2023, 10, e24851. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, Y.F.; Wang, Z.; Zhuang, W.B.; Wang, T.; Li, N.W. Comparative study on the spatiotemporal variation of four chemical components in different Eucommia ulmoides cultivars. Microchem. J. 2025, 208, 112583. [Google Scholar] [CrossRef]
- Ma, L.; Meng, N.; Liu, B.; Wang, C.; Chai, X.; Huang, S.; Yu, H.; Wang, Y. Quantitative analysis and stability study on iridoid glyco-sides from seed meal of Eucommia ulmoides oliver. Molecules 2022, 27, 5924. [Google Scholar] [CrossRef]
- Tang, F.R.; Zhang, Z.L.; Zuo, Y.M.; Chen, L.Y.; Luo, J.; Liu, R.H.; Li, Y.Y. Chemical Components of Flavonoids of Eucommiae Folium. Chin. J. Exp. Tradit. Med. Formulae 2014, 5, 90–92. [Google Scholar]
- Li, J. Research Progress in the Extraction Methods and Bioactivity of Flavonoids from Eucommia ulmoides leaves. Sci. Technol. Food Ind. 2019, 7, 346–350. [Google Scholar] [CrossRef]
- Fu, G.M.; Luo, Y.F.; Zhang, Y.N.; Wan, Y.; Chai, J.X.; Chen, Y.H.; Wu, X.F.; Chen, J.F. Using Macroporous Resin for Enriching and Purifying Total Flavonoids from the Crude Extract of Eucommia ulmoides Oliv. Leaves Food Sci. 2010, 14, 67–70. [Google Scholar]
- Yuan, F.J.; Sun, Y.H.; Hamulati, H.; Wang, X.T.; He, J.H.; Mao, Y. Effects of the Different Elutions of Hyssopus cuspidatus on Smooth Muscle Contraction of Isolated Tracheal in Guinea Pigs. China Pharm. 2017, 13, 1787–1790. [Google Scholar]
- Pham, N.T.M.; Nguyen, T.T.; Nguyen, D.K.; Vo, N.M.; Nguyen, M.T.T.; Ngo, L.H.T.; Tran, L.C. Optimization of the extraction protocol to uptake the flavonoid content from jamaican cherry (Muntingia calabura L.) leaves extract as a source for an antifun-gal bioproduct development. Biocatal. Agric. Biotechnol. 2025, 64, 103520. [Google Scholar] [CrossRef]
- Li, Y.M.; Zhang, Q.; Fang, Q.; Zhu, H.; Zong, X.L.; Gao, X.; Shi, Y.; Qin, K.M. A novel ultrasound-assisted enzyme extraction method of total flavonoids from Viticis Fructus and processed Viticis Fructus: Comparison of in vitro antioxidant activity. Ultrason. Sonochem. 2024, 110, 107045. [Google Scholar] [CrossRef]
- Yang, W.; Huang, G. Extraction methods and activities of natural glucans. Trends Food Sci. Technol. 2021, 112, 50–57. [Google Scholar] [CrossRef]
- Peng, Z.J.; Wang, Y.C.; Li, W.F.; Zhan, B.J.F.; Zhu, L.P.; Yang, D.P.; Li, G.; Zhang, L.X.; Zhao, Z.M. Ultrasonic-assisted extraction of flavonoids from Amomum villosum Lour. Using natural deep eutectic solvent: Process optimization, comparison, identification, and bioactivity. Ultrason. Sonochem. 2025, 116, 107304. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Jiang, D.Z.; Yu, D.P.; Zeng, M.; Liu, W.B.; Li, D.L.; Liu, K.Y. Optimization of ultrasonic-assisted extraction of total flavonoids from Oxalis corniculata by a hybrid response surface methodology-artificial neural network-genetic algorithm (RSM-ANN-GA) approach, coupled with an assessment of antioxidant activities. RSC Adv. 2024, 14, 39069–39080. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.W.; Yang, Z.M.; Huang, X.H.; Cai, J.Y.; Zhong, H.Y. Efficient and green ultrasonic-assisted extraction of flavonoids from Sophorae Tonkinensis Radix Et Rhizoma using deep eutectic solvent: Optimization and extraction mechanism. Ind. Crops Prod. 2025, 226, 120738. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization of heat and ultra-sound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, H.; Cui, L.; Hussain, H.; Nadolnik, L.; Zhang, Z.; Zhao, Y.; Qin, X.; Li, J.; Park, J.H.; et al. Ultrasonic-assisted extraction of flavonoids from peanut leave and stem using deep eutectic solvents and its molecular mechanism. Food Chem. 2024, 434, 137497. [Google Scholar] [CrossRef]
- Wang, T.T.; Liu, X.R.; Jia, J.; Zhang, L.L. Optimization of extraction process of total flavonoids from Eucommia ulmoides leaves by ultrasonic-assisted enzymatic method. Feed Res. 2022, 17, 69–74. [Google Scholar] [CrossRef]
- Kobus, Z.; Pecyna, A.; Buczaj, A.; Krzywicka, M.; Przywara, A.; Nadulski, R. Optimization of the Ultrasound-Assisted Extraction of Bioactive Compounds from Cannabis sativa L. Leaves and Inflorescences Using Response Surface Methodology. Appl. Sci. 2022, 12, 6747. [Google Scholar] [CrossRef]
- Agarwal, C.; Máthé, K.; Hofmann, T.; Csóka, L. Ultrasound-Assisted Extraction of Cannabinoids from Cannabis sativa L. Opti-mized by Response Surface Methodology. J. Food Sci. 2018, 83, 700–710. [Google Scholar] [CrossRef]
- Deng, Y.L.; Tang, S.; Wang, M.C.; Yang, P.P. Semi Bionic Extraction and Ultrasonic-Assisted Semi Bionic Extraction of Flavonoids from Eucommia ulmoides. J. Jishou Univ. (Nat. Sci. Ed.) 2017, 38, 66–70. [Google Scholar]
- Hao, J.; Wang, Z.; Jia, Y.; Sun, L.; Fu, Z.; Zhao, M.; Li, Y.; Yuan, N.; Cong, B.; Zhao, L.; et al. Optimization of ultrasonic-assisted extraction of flavonoids from Lactuca indica L. cv. Mengzao and their antioxidant properties. Front. Nutr. 2023, 10, 1065662. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Tram Anh, M.; Van Hung, P.; Thi Lan Phi, N. Optimized Conditions for Flavonoid Extraction from Pomelo Peel By-products under Enzyme- And Ultrasound-Assisted Extraction Using Response Surface Methodology. J. Food Qual. 2021, 1, 6666381. [Google Scholar] [CrossRef]
- Xu, X.M.; Zhang, D.D.; Liu, X.H.; Zheng, R.; Jiang, T.Q. Ultrasonic-Assisted Extraction and Antioxidant Evaluation of Resveratrol from Peanut Sprouts. Processes 2024, 12, 2295. [Google Scholar] [CrossRef]
- Xu, L.Y.; Zhao, Y.; Wang, Y.; Guo, R.X.; Wang, P.; Zhang, L.; Zhang, H.; Zhai, Y.X.; Liu, Y.N. Optimization of Ultra-sound-Assisted Extraction of Roselle Flavonoids by Deep Eutectic Solvent and Its Antioxidant Activity. Sci. Technol. Food Ind. 2024, 12, 1–19. [Google Scholar] [CrossRef]
- Irshad, M.; Zafaryab, M.; Singh, M.; Rizvi, M.M. Comparative Analysis of the Antioxidant Activity of Cassia fistula Extracts. Int. J. Med. Chem. 2012, 9, 157125. [Google Scholar] [CrossRef]
- Wang, Y.N.; Huang, S.; Yin, Y.Y.; Wei, H.L.; Tao, J. Extraction and study on antioxidant activity analysis of total flavonoids from hawthorn leaf health food. J. Food Saf. Qual. 2024, 15, 89–98. [Google Scholar] [CrossRef]
- Jiang, L.C.; Zhang, Y.J.; Jiang, D.Y.; Fu, D.Y.; Zhao, X.Y. Response surface optimization of ultrasonic-microwave synergistic extraction of flavonoids from Actinidia arguta stems and analysis of antioxidant activity. J. Sichuan Univ. (Nat. Sci. Ed.) 2024, 61, 1–9. [Google Scholar] [CrossRef]
- Xing, Y.H.; Yang, C.H.; Huang, Z.Z.; Peng, X.J. Establishment of HPLC characteristic map of fructus choerospondiatis and de-termination of four flavonoid components. Hubei Agric. Sci. 2025, 64, 144–155. [Google Scholar] [CrossRef]
- Yang, H.T.; Liu, J.H. Study on optimizing extraction technology of total flavonoids in Eucommia ulmoides olive leaves through orthogonal regression design. Sci. Technol. Food Ind. 2009, 30, 248–250. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing Total Phenolics, Total Flavonoids Contents and Antioxidant Activity of Moringa oleifera Leaf Extract by the Appropriate Extraction Method. Ind. Crop. Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Yang, F.Y.; Wan, L.; Chen, J.Y.; Shao, J.L.; Lan, S.S. Optimization of ultrasonic assisted ethanol extraction for total flavonoids from buckwheat hust. Food Mach. 2015, 31, 234–238. [Google Scholar] [CrossRef]
- Hou, X.L.; Wang, J. Optimization of Extraction Process of Flavonoids from Citrus Peel by the Response Surface Method and its Antioxidant Activity. Farm Prod. Process. 2024, 24, 47–51. [Google Scholar] [CrossRef]
- Song, M.L.; Tao, J.Y.; Yang, J.; Hu, T.J. Study on Separation and Purification of Total Flavonoids from Polygonum hydropiper Linn. by Macroporous Resin. Chin. J. Inf. TCM 2018, 25, 74–78. [Google Scholar] [CrossRef]
- Yan, H.J.; Han, Y.B. Study on Bioactivity Mechanism of Total Flavonoids and Total Polyphenols in Apple Cider Vinegar. China Condiment 2022, 47, 77–80. [Google Scholar] [CrossRef]
- Weng, Y.Y.; Wu, C.Y.; Xu, Y. Optimization of Decompressing Extraction Process and Purification by Macroporous Resin of Total Flavonoids from Kapok Bark and antioxidant activity of the Extract. Shanxi Chem. Ind. 2025, 231, 1–5. [Google Scholar] [CrossRef]
- Ma, T.T.; Sun, X.Y.; Tian, C.R.; Luo, J.Y.; Zheng, C.P.; Zhan, J.C. Enrichment and purification of polyphenol extract from Sphallerocarus gracilis stems and leaves and in vitro evaluation of DNA Damage-Protective activity and inhibitory effects of α-amylase and α-glucosidase. Molecules 2015, 20, 21442–21457. [Google Scholar] [CrossRef]
- Ferraro, V.; Cruz, I.B.; Jorge, R.F.; Pintaclo, M.E.; Castro, M.E. Effects of physical parameters onto adsorption of the borderline amino acids glycine, lysine, taurine, and tryptophan upon amberlite XAD16 resin. J. Chem. Eng. Data 2013, 58, 707–717. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, S.X.; Zhu, J.; Li, B.H.; Li, R.C.; Hou, H.L.; Liu, K.H.; Li, J.F. Extraction, Purification Technology of Flavonoids from Chestnut Flowers and Comparison of Antioxidant Activity Before and After Purification. Food Sci. Technol. 2025, 50, 207–216. [Google Scholar] [CrossRef]
- Wang, P.F.; Sheng, Z.L.; Han, Q.; Zhao, Y.L.; Cheng, G.D.; Li, Y.H. Enrichment and purification of total flavonoids from Flos Populi extracts with macroporous resins and evaluation of antioxidant activities in vitro. J. Chromatogr. B 2014, 945, 68–74. [Google Scholar] [CrossRef]
- Xi, L.H.; Mu, T.H.; Sun, H.N. Preparative purification of polyphenols from sweet potato (Iponoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef]
- Feng, C.W.; Kang, S.H.; Lu, L.N.; Li, J.; Yang, X.J.; Zhang, L.B. Purification of Total Flavonoids from Eucalyptus smith Leaf with Macroporous Resin and Analysis of Its Antioxidant and Nitrosation Inhibitory Activities. Sci. Technol. Food Ind. 2025, 4, 1–11. [Google Scholar] [CrossRef]
- Yan, J.W.; Zeng, H.; Chen, W.J.; Zheng, S.; Luo, J.L.; Jiang, H.F.; Yang, B.R.; Farag, M.A.; Lou, H.Q.; Song, L.L.; et al. Effects of tree age on flavonoids and antioxidant activity in Torreya grandis nuts via integrated metabolome and transcriptome analyses. Food Front. 2023, 4, 358–367. [Google Scholar] [CrossRef]
- Akbar, A.; Soekamto, N.H.; Firdaus; Bahrun. Total Phenolics and Flavonoids Level of n-Hexane, Ethyl Acetate and Methanol Extracts of Sargassum sp. along with Their Antioxidant Activity by DPPH Method. AIP Conf. Proc. 2022, 2638, 060009. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Yin, Q.; Dong, Z.; Zheng, L.; Qian, Y.; Sun, Y.; Chen, Z.; Zhai, K. Extraction, Identification, and Antioxidant Activity of Flavonoids from Hylotelephium spectabile (Boreau) H. Ohba. Foods 2024, 13, 2652. [Google Scholar] [CrossRef]
- Tao, Y.P.; Zhang, H.W.; Wang, Y. Revealing and predicting the relationship between the molecular structure and antioxidant activity of flavonoids. LWT 2023, 174, 114433. [Google Scholar] [CrossRef]
- Xu, X.L.; Dai, G.Q.; Wei, H.F.; Yu, J. Optimization of Aqueous Two-phase Extraction Technology of Total Flavonoids from Lonicera japonica Thunb. Leaves and Its Antioxidant Activity. Sci. Technol. Food Ind. 2023, 44, 242–248. [Google Scholar] [CrossRef]
- Yoshimi, S.; Risako, N.; Naru, K. Resveratrol analogues like piceatannol are potent antioxidants as quantitatively demonstrated through the high scavenging ability against reactive oxygen species and methyl radical. Bioorg. Med. Chem. Lett. 2017, 23, 5203–5206. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, C.; Deng, J.; Xia, T.; Zhang, X.; Xue, S.; Song, M.K.; Olatunji, O.J. Schisandrin B ameliorates adjuvant-induced arthritis in rats via modulation of inflammatory mediators, oxidative stress, and HIF-1α/VEGF pathway. J. Pharm. Pharmacol. 2024, 76, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron complexes of flavonoids-antioxidant capacity and beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef] [PubMed]



| Run | A/Ethanol Concentration | B/Ultrasonic Power | C/Solid-to-Liquid Ratio | D/Ultrasonic Time | Response (mg/g Plant Material) | ||
|---|---|---|---|---|---|---|---|
| 1 | 1 (50%) | 1 (200 W) | 1 (1:25 g/mL) | 1 (20 min) | 152.4 | 151.3 | 151.9 |
| 2 | 1 | 2 (250 W) | 2 (1:30 g/mL) | 2 (25 min) | 161.5 | 162.3 | 163.4 |
| 3 | 1 | 3 (300 W) | 3 (1:35 g/mL) | 3 (30 min) | 123.5 | 123.5 | 124.1 |
| 4 | 2 (60%) | 1 | 2 | 3 | 159.8 | 159.9 | 159.1 |
| 5 | 2 | 2 | 3 | 1 | 166.3 | 165.7 | 166.8 |
| 6 | 2 | 3 | 1 | 2 | 126.5 | 128.1 | 126.7 |
| 7 | 3 (70%) | 1 | 3 | 2 | 163.3 | 163.3 | 162.9 |
| 8 | 3 | 2 | 1 | 3 | 163.6 | 163.2 | 162.9 |
| 9 | 3 | 3 | 2 | 1 | 127.5 | 126.9 | 127.9 |
| K1 | 438.0 | 474.6 | 442.2 | 445.6 | |||
| K2 | 453.0 | 491.9 | 449.4 | 452.7 | |||
| K3 | 453.8 | 378.2 | 453.1 | 446.5 | |||
| k1 | 146.0 | 158.2 | 147.4 | 148.5 | |||
| k2 | 151.0 | 164.0 | 149.8 | 150.9 | |||
| k3 | 151.3 | 126.1 | 151.0 | 148.8 | |||
| R | 5.3 | 37.9 | 3.6 | 2.4 | |||
| Source of Variation | Sum of Squares (SS) | DF | Mean Square (MS) | F | Significance (p) | |
|---|---|---|---|---|---|---|
| A | 159.167 | 2 | 79.584 | 237.432 | p < 0.001 | ** |
| B | 7503.736 | 2 | 3751.868 | 11,193.419 | p < 0.001 | ** |
| C | 61.850 | 2 | 30.925 | 92.262 | p < 0.001 | ** |
| D | 29.654 | 2 | 14.827 | 44.235 | p < 0.001 | ** |
| Error E | 6.033 | 18 | 0.335 |
| Resin Name | Mass Concentration Before Adsorption (mg/mL) | Mass Concentration After Adsorption (mg/mL) | Mass Concentration After Desorption (mg/mL) | Adsorption Ratio (%) | Desorption Ratio (%) |
|---|---|---|---|---|---|
| LX-38 | 3.0 | 0.8 | 1.6 | 73.3 ± 1.5 d | 72.7 ± 2.5 c |
| LX-60 | 3.0 | 1.0 | 1.4 | 66.7 ± 2.9 e | 70.0 ± 2.6 c |
| LS-46 | 3.0 | 0.7 | 1.6 | 76.7 ± 1.3 c | 69.6 ± 1.2 d |
| LS-306 | 3.0 | 0.9 | 1.7 | 70.0 ± 3.2 e | 81.0 ± 2.1 b |
| XDA-8 | 3.0 | 0.4 | 2.3 | 86.7 ± 1.2 a | 88.5 ± 1.3 a |
| AB-8 | 3.0 | 0.9 | 1.8 | 70.0 ± 2.1 b | 85.7 ± 1.5 b |
| D101 | 3.0 | 0.80 | 1.5 | 73.3 ± 1.7 d | 68.2 ± 2.2 d |
| Compound | Chlorogenic Acid | Rutin | Isoquercetin | Kaempferol-3-O-Rutinoside | Quercetin 3-Rhamnoside | Hyperoside | Quercetin |
|---|---|---|---|---|---|---|---|
| Regression equation | Y = 36,380X + 8317.5 | Y = 85,221X − 118,356 | Y = 75,336X − 63,523 | Y = 86,233X − 58,116 | Y = 652,523X − 18,697 | Y = 6256.8X + 1698.5 | Y = 7989.6X − 1 213.3 |
| Correlation coefficient | R2 = 0.9995 | R2 = 0.9993 | R2 = 0.9995 | R2 = 0.9998 | R2 = 0.9996 | R2 = 0.9999 | R2 = 0.9992 |
| Content (mg/g extract) | 269.536 ± 0.069 | 25.217 ± 0.035 | 67.023 ± 0.087 | 38.988 ± 0.056 | 19.659 ± 0.033 | 5.609 ± 0.026 | 12.338 ± 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Tang, L.; Wang, J. Process Optimization of Ultrasonic-Assisted Extraction and Resin Purification of Flavonoids from Eucommia ulmoides Leaves and Their Antioxidant Properties In Vitro. Processes 2025, 13, 1905. https://doi.org/10.3390/pr13061905
Li J, Tang L, Wang J. Process Optimization of Ultrasonic-Assisted Extraction and Resin Purification of Flavonoids from Eucommia ulmoides Leaves and Their Antioxidant Properties In Vitro. Processes. 2025; 13(6):1905. https://doi.org/10.3390/pr13061905
Chicago/Turabian StyleLi, Jia, Lingling Tang, and Jungang Wang. 2025. "Process Optimization of Ultrasonic-Assisted Extraction and Resin Purification of Flavonoids from Eucommia ulmoides Leaves and Their Antioxidant Properties In Vitro" Processes 13, no. 6: 1905. https://doi.org/10.3390/pr13061905
APA StyleLi, J., Tang, L., & Wang, J. (2025). Process Optimization of Ultrasonic-Assisted Extraction and Resin Purification of Flavonoids from Eucommia ulmoides Leaves and Their Antioxidant Properties In Vitro. Processes, 13(6), 1905. https://doi.org/10.3390/pr13061905







