Effects of Different Cooking Parameters on Various Quality Criteria, Lipid Oxidation, Mineral Composition, and Free Amino Acid Profile of Chicken Breast
Abstract
1. Introduction
2. Materials and Methods
2.1. Chicken Meat and Cooking Device
2.2. Chemicals
2.3. Cooking Process
2.4. Analyses
2.4.1. pH Value
2.4.2. TBARS Analysis
2.4.3. Water Content
2.4.4. Cooking Loss
2.4.5. Color Analysis
2.4.6. Free Amino Acid Screening by LC-MS/MS
Sample Preparation
2.4.7. Minaral Analysis
2.4.8. Statistical Analysis
3. Results
Evaluation of Physicochemical, Free Amino Acid Profile, and Mineral Content of Chicken Breast Meat by PCA Analysis
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Haščík, P.; Pavelková, A.; Tkáčová, J.; Čuboň, J.; Kačániová, M.; Habánová, M.; Mlyneková, E. The amino acid profile of broiler chicken meat after dietary administration of bee products and probiotics. Biologia 2020, 75, 1899–1908. [Google Scholar] [CrossRef]
- Savaş, A.; Ekiz, E.; Elbir, Z.; Savaş, B.D.; Proestos, C.; Elobeid, T.; Khan, M.R.; Oz, F. Advantageous effects of sumac usage in meatball preparation on various quality criteria and formation of heterocyclic aromatic amines. Seperations 2023, 10, 29. [Google Scholar] [CrossRef]
- Savas, A.; Oz, E.; Elbir, Z.; Oz, F. Effect of dry aging on quality parameters, protein profile and protein oxidation level of beef. Int. J. Food Sci. Technol. 2024, 59, 7598–7609. [Google Scholar] [CrossRef]
- Savaş, A.; Oz, E.; Oz, F. Is oven bag really advantageous in terms of heterocyclic aromatic amines and bisphenol-A? Chicken meat perspective. Food Chem. 2021, 355, 129646. [Google Scholar] [CrossRef]
- Ma, X.; Yu, M.; Liu, Z.; Deng, D.; Cui, Y.; Tian, Z.; Wang, G. Effect of amino acids and their derivatives on meat quality of finishing pigs. J. Food Sci. Technol. 2020, 57, 404–412. [Google Scholar] [CrossRef]
- Roobab, U.; Chen, B.R.; Madni, G.M.; Guo, S.M.; Zeng, X.A.; Abdi, G.; Aadil, R.M. Enhancing chicken breast meat quality through ultrasonication: Physicochemical, palatability, and amino acid profiles. Ultrason. Sonochem. 2024, 104, 106824. [Google Scholar] [CrossRef]
- Ekiz, E.; Savaş, A.; Aoudeh, E.; Elbir, Z.; Oz, E.; Proestos, C.; Ahmad, N.; Oz, F. Impact of cumin (Cuminum cyminum) incorporation on the generation of heterocyclic aromatic amines in meatballs. Seperations 2023, 10, 458. [Google Scholar] [CrossRef]
- Elbir, Z.; Ekiz, E.; Aoudeh, E.; Oz, E.; Savaş, A.; Brennan, C.; Oz, F. Enhancing effect of chia seeds on heterocyclic amine generation in meatball. Int. J. Food Sci. Technol. 2023, 58, 2560–2572. [Google Scholar] [CrossRef]
- Beltran, E.; Pla, R.; Yuste, J.; Mor-Mur, M. Lipid oxidation of pressurized and cooked chicken: Role of sodium chloride and mechanical processing on TBARS and hexanal values. Meat Sci. 2003, 64, 19–25. [Google Scholar] [CrossRef]
- Babür, T.E.; Gürbüz, Ü. Geleneksel pişirme yöntemlerinin et kalitesine etkileri. J. Tour. Gastron. Stud. 2015, 3, 58–64. [Google Scholar]
- Kim, D.H.; Kim, K.W.; Kim, Y.H.; Kim, J.A.; Kim, J.; Moon, K.D. Nutritional composition of horsemeat compared to white meat (chicken and duck). Food Sci. Preserv. 2015, 22, 644–651. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.N.; Shin, J.A.; Chun, J.Y.; Lee, J.; Lee, K.T. Content of fat-soluble nutrients (cholesterol, retinol, and α-tocopherol) in different parts of poultry meats according to cooking method. J. Korean Soc. Food Sci. Nutr. 2015, 44, 234–241. [Google Scholar] [CrossRef]
- Kim, H.; Do, H.W.; Chung, H. A comparison of the essential amino acid content and the retention rate by chicken part according to different cooking methods. Korean J. Food Sci. Anim. Res. 2017, 37, 626. [Google Scholar] [CrossRef]
- Barbanti, D.; Pasquini, M. Influence of cooking conditions on cooking loss and tenderness of raw and marinated chicken breast meat. LWT-Food Sci. Technol. 2005, 38, 895–901. [Google Scholar] [CrossRef]
- Bi, J.; Lin, Z.; Li, Y.; Chen, F.; Liu, S.; Li, C. Effects of different cooking methods on volatile flavor compounds of chicken breast. J. Food Biochem. 2021, 45, e13770. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, B.; Oh, E.; Kim, Y.S.; Choi, Y.M. Combined effects of sous-vide cooking conditions on meat and sensory quality characteristics of chicken breast meat. Poult. Sci. 2020, 99, 3286–3291. [Google Scholar] [CrossRef]
- Kim, M.J.; Parvin, R.; Mushtaq, M.M.H.; Hwangbo, J.; Kim, J.H.; Na, J.C.; Choi, H.C. Influence of monochromatic light on quality traits, nutritional, fatty acid, and amino acid profiles of broiler chicken meat. Poult. Sci. 2013, 92, 2844–2852. [Google Scholar] [CrossRef]
- Alfaig, E.; Angelovičova, M.; Kral, M.; Bučko, O. Effect of probiotics and thyme essential oil on the essential amino acid content of the broiler chicken meat. Acta Sci. Pol. Technol. Aliment. 2014, 13, 425–432. [Google Scholar] [CrossRef]
- Savaş, B.D.; Gürses, M.; Savaş, A.; Engin, T.; Karaoğlu, M.M.; Binici, H.İ. Effect of the addition of rosehip marmalade on the textural, rheological, microbiological, physicochemical and organic acid profile of kefir. Int. J. Gastron. Food Sci. 2025, 39, 101125. [Google Scholar] [CrossRef]
- Korkmaz, A.; Oz, F. Effect of the use of dry breadcrumb in meatball production on the formation of heterocyclic aromatic amines. Br. Food J. 2020, 122, 2105–2119. [Google Scholar] [CrossRef]
- Öz, E.; Ekiz, E.; Savaş, A.; Aoudeh, E.; El-Aty, A.A.; Öz, F. Impact of roasting level on fatty acid composition, oil and polycyclic aromatic hydrocarbon contents of various dried nuts. Turk. J. Agric. For. 2021, 45, 213–221. [Google Scholar] [CrossRef]
- Bulan, R.; Oz, F. Impact of tarragon usage on lipid oxidation and heterocyclic aromatic amine formation in meatball. Int. J. Food Sci. Technol. 2022, 57, 942–950. [Google Scholar] [CrossRef]
- Bingol, M.; Brennan, C.; Zeng, M.; Oz, F. Effect of the fortification with astaxanthin on the quality parameters and heterocyclic amines content of meatballs. Int. J. Food Sci. Technol. 2022, 57, 7653–7665. [Google Scholar] [CrossRef]
- Kilic, B.; Richards, M.P. Lipid oxidation in poultry döner kebab: Pro-oxidative and anti-oxidative factors. J. Food Sci. 2003, 68, 686–689. [Google Scholar] [CrossRef]
- Gökalp, H.Y.; Kaya, M.; Tülek, Y.; Zorba, Ö. Et ve Ürünlerinde Kalite Kontrolü ve Laboratuvar Uygulama Klavuzu; Baskı, V., Ed.; Atatürk Üniversitesi Yayın: Erzurum, Turkey, 2010. [Google Scholar]
- Antoine, F.R.; Wei, C.I.; Littell, R.C.; Marshall, M.R. HPLC method for analysis of free amino acids in fish using o-phthaldialdehyde precolumn derivatization. J. Agric. Food Chem. 1999, 47, 5100–5107. [Google Scholar] [CrossRef] [PubMed]
- Oz, E. Kas Tipinin Pastirmanin Proteolitik Değişimleri ve bazi Kalitatif Özelliklerine Etkisi. Ph.D. Thesis, Fen Bilimleri Enstitüsü, Atatürk Üniversitesi, Erzurum, Turkey, 2018. [Google Scholar]
- Binici, H.İ. Effects of Different Cooking Processes on the Phytochemical Profile and Mineral Content of Garlic (Allium sativum L.). J. Oleo Sci. 2025, 74, 89–95. [Google Scholar] [CrossRef]
- Allen, C.D.; Fletcher, D.L.; Northcutt, J.K.; Russell, S.M. The relationship of broiler breast color to meat quality and shelf-life. Poult. Sci. 1998, 77, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Alugwu, S.U.; Okonkwo, T.M.; Ngadi, M.O. Effect of Cooking on Physicochemical and Microstructural Properties of Chicken Breast Meat. Eur. J. Nutr. Food Saf. 2022, 14, 43–62. [Google Scholar] [CrossRef]
- Nyam, K.L.; Goh, K.M.; Chan, S.Q.; Tan, C.P.; Cheong, L.Z. Effect of sous vide cooking parameters on physicochemical properties and free amino acids profile of chicken breast meat. J. Food Compos. Anal. 2023, 115, 105010. [Google Scholar] [CrossRef]
- Girard, P.J. Technology of Meat and Meat Products; Ellis Horwood: Hemel Hempstead, UK, 1992; pp. 32–83. [Google Scholar]
- Rabeler, F.; Feyissa, A.H. Kinetic modeling of texture and color changes during thermal treatment of chicken breast meat. Food Bioprocess Technol. 2018, 11, 1495–1504. [Google Scholar] [CrossRef]
- Sanwo, K.A.; Adegoke, A.V.; Akinola, O.S.; Njoku, C.P.; Okolo, S.O.; Oladipo, N.A.; Oladejo, A.S. Meat quality characteristics of improved indigenous chickens (FUNAAB-ALPHA) fed turmeric (Curcuma longa) or clove (Syzygium aromaticum) as feed additives. J. Agric. Environ. Sci. 2019, 19, 102–112. [Google Scholar] [CrossRef]
- Kilic, S.; Oz, E.; Oz, F. Effect of turmeric on the reduction of heterocyclic aromatic amines and quality of chicken meatballs. Food Control 2021, 128, 108189. [Google Scholar] [CrossRef]
- Rodriguez-Estrada, M.T.; Penazzi, G.; Caboni, M.F.; Bertacco, G.; Lercker, G. Effect of different cooking methods on some lipid and protein components of hamburgers. Meat Sci. 1997, 45, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Broncano, J.M.; Petrón, M.J.; Parra, V.; Timón, M.L. Effect of different cooking methods on lipid oxidation and formation of free cholesterol oxidation products (COPs) in Latissimus dorsi muscle of Iberian pigs. Meat Sci. 2009, 83, 431–437. [Google Scholar] [CrossRef]
- Wideman, N.; O’bryan, C.A.; Crandall, P.G. Factors affecting poultry meat colour and consumer preferences—A review. World’s Poult. Sci. J. 2016, 72, 353–366. [Google Scholar] [CrossRef]
- Li, S.; Tang, Y.; Fan, Q.; Li, Z.; Zhang, X.; Wang, J.; Li, Q. When quantum dots meet blue phase liquid crystal elastomers: Visualized full-color and mechanically-switchable circularly polarized luminescence. Light Sci. Appl. 2024, 13, 140. [Google Scholar] [CrossRef]
- Ergezer, H. Değişik Yöntemlerle Marine Edilmiş Kanatlı Etlerinin Kimyasal, Mikrobiyolojik, Tekstürel Ve Duyusal Özellikleri. Master’s Thesis, Pamukkale Üniversitesi Fen Bilimleri Enstitüsü, Denizli, Turkey, 2005. [Google Scholar]
- Okpala, C.O.R.; Juchniewicz, S.; Leicht, K.; Korzeniowska, M.; Guiné, R.P. Antioxidant, organoleptic and physicochemical changes in different marinated oven-grilled chicken breast meat. Foods 2022, 11, 3951. [Google Scholar] [CrossRef]
- Öztürk, Ş. Production of low sugar orange marmalade using rebaudioside a and ıts sensory properties. Acad. Food 2023, 21, 57–69. [Google Scholar] [CrossRef]
- Oz, E.; Kabil, E.; Kaya, M. The effects of curing agents on the proteolysis and lipid oxidation of pastırma produced by the traditional method. J. Food Sci. Technol. 2021, 58, 2806–2814. [Google Scholar] [CrossRef]
- Muthulakshmi, M.; Chandirasekaran, V.; Kalaikannan, A.; Jagadeeswaran, A.; Selvaraju, G.; Muthukumar, M.; Irshad, A. Effect of cooking methods on nutritional quality of chicken meat. Pharma Innov. J. 2021, 10, 1064–1070. [Google Scholar]
- Yang, J.; Qin, K.; Wang, Q.; Li, S.; Zhu, Y.; Yang, X. Comparative analysis of amino acids and volatile compounds as markers in Lueyang black-boned chicken and Arbor Acres broilers. Poult. Sci. 2025, 104, 104897. [Google Scholar] [CrossRef] [PubMed]
- Boz, M.A.; Oz, F.; Yamak, U.S.; Sarica, M.; Cilavdaroglu, E. The carcass traits, carcass nutrient composition, amino acid, fatty acid, and cholesterol contents of local Turkish goose varieties reared in an extensive production system. Poult. Sci. 2019, 98, 3067–3080. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, L.; Huang, Z.; Guo, N.; An, H.; Zhan, X.; Abddollahi, M.; Kovacs, Z. Dynamics of free amino acids in beef during thermal processing and mathematical modeling of doneness correlation. Food Mater. Res. 2024, 4, e036. [Google Scholar] [CrossRef]
- Tasoniero, G.; Cullere, M.; Cecchinato, M.; Puolanne, E.; Dalle Zotte, A. Technological quality, mineral profile, and sensory attributes of broiler chicken breasts affected by White Striping and Wooden Breast myopathies. Poult. Sci. 2016, 95, 2707–2714. [Google Scholar] [CrossRef]
- Khan, A.A.; Randhawa, M.A.; Carne, A.; Mohamed Ahmed, I.A.; Barr, D.; Reid, M.; Bekhit, A.E.D.A. Quality and nutritional minerals in chicken breast muscle treated with low and high pulsed electric fields. Food BioProcess Technol. 2018, 11, 122–131. [Google Scholar] [CrossRef]
- Anıl, N.; Doğruer, Y.; Gürbüz, Ü.; Keleş, A.; Kayaardı, S. Tavuk Sucuğu Üretim Teknolojisi 1: Kimyasal Mikrobiyolojik ve Organoleptik Kalitesi Üzerinde Araştırmalar. Eurasian J. Vet. Sci. 1995, 11, 83–94. [Google Scholar]
- Ali, M.; Lee, S.Y.; Park, J.Y.; Jung, S.; Jo, C.; Nam, K.C. Comparison of functional compounds and micronutrients of chicken breast meat by breeds. Food Sci. Anim. Res. 2019, 39, 632. [Google Scholar] [CrossRef]
- Kavcı, Z.; Ozan, M.; Buzdağlı, Y.; Savaş, A.; Uçar, H. Investigation of the effect of nitrate and L-arginine intake on aerobic, anaerobic performance, balance, agility, and recovery in elite taekwondo athletes. J. Int. Soc. Sports Nutr. 2025, 22, 2445609. [Google Scholar] [CrossRef]
- Binici, H.İ.; Savaş, A.; Erim, B. Processing-induced modifications in bioactive compounds of black garlic: A comparative analysis. Ital. J. Food Sci. 2025, 37, 432–440. [Google Scholar] [CrossRef]
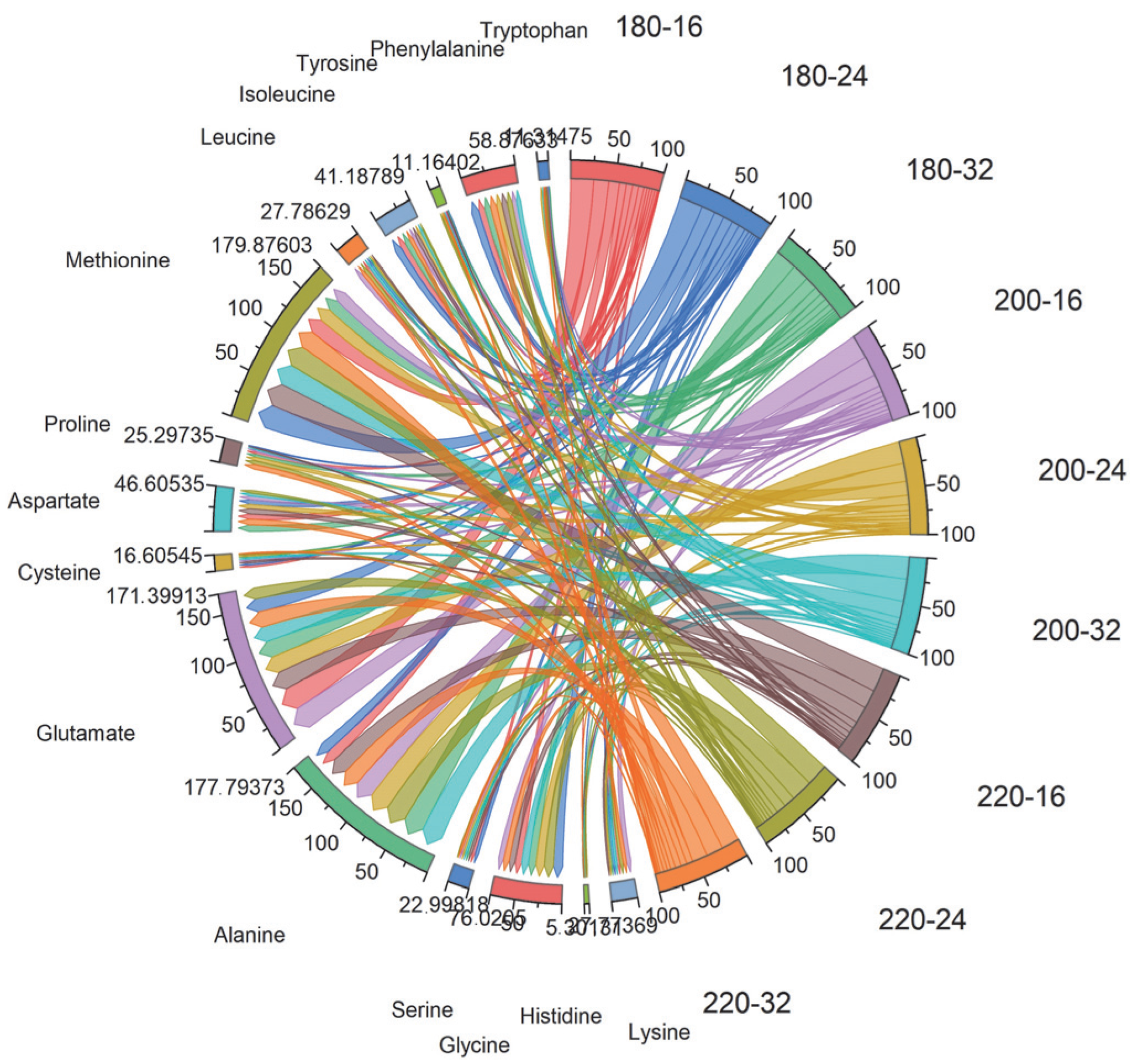
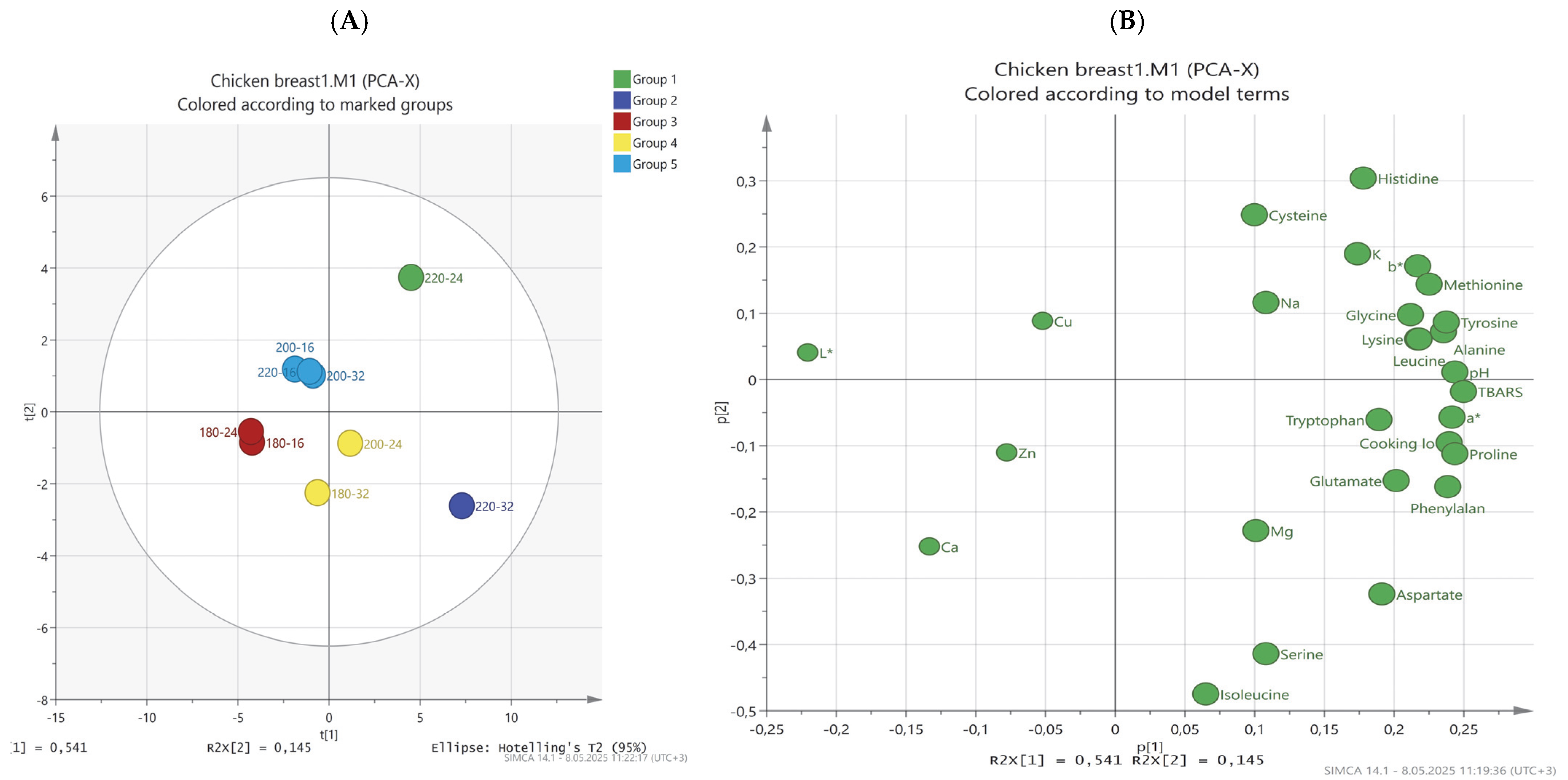
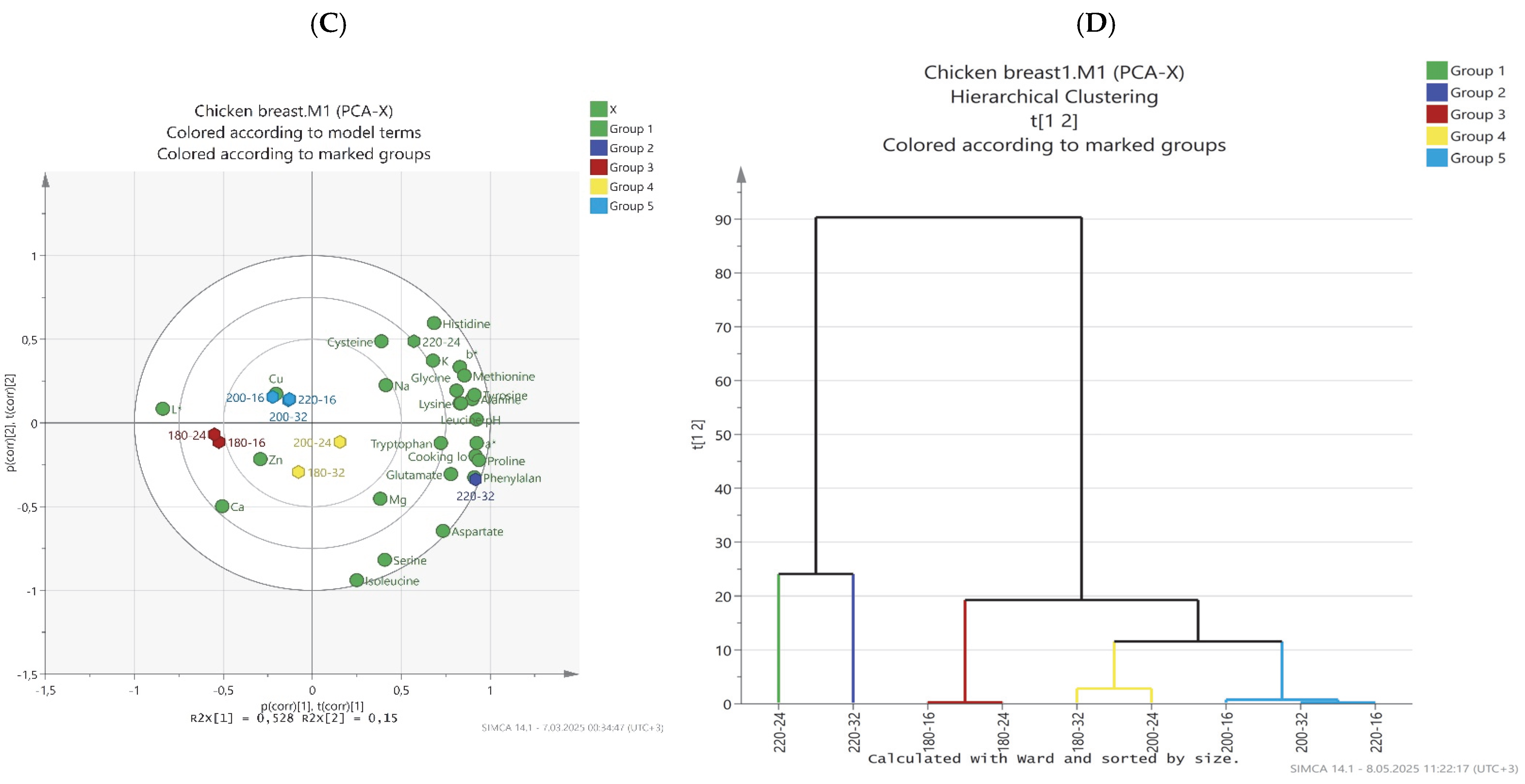
| Cooking Temperature | pH | Cooking Loss (%) | TBARS (mg MDA kg−1) |
|---|---|---|---|
| 180 °C | 6.26 ± 0.02 c | 34.25 ± 1.96 b | 0.24 ± 0.02 b |
| 200 °C | 6.27 ± 0.02 b | 34.65 ± 0.84 b | 0.25 ± 0.01 ab |
| 220 °C | 6.35 ± 0.05 a | 36.85 ± 1.61 a | 0.29 ± 0.08 a |
| Sign. | ** | ** | * |
| Cooking Time | |||
| 16 min | 6.26 ± 0.02 c | 33.91 ± 1.31 c | 0.23 ± 0.02 a |
| 24 min | 6.29 ± 0.05 b | 35.27 ± 1.56 b | 0.26 ± 0.03 a |
| 32 min | 6.33 ± 0.06 a | 36.56 ± 1.86 a | 0.28 ± 0.06 b |
| Sign. | ** | ** | * |
| Interactions | |||
| CT × CT | |||
| Sign. | ** | ns | ns |
| Raw materials (uncooked) | 6.11 ± 0.01, | 75.74 ± 0.65 | 0.19 ± 0.02 |
| Cooking Temperature | Lightness (L*) | Redness (a*) | Yellowness (b*) | Chroma (C*) | Hue Angle (H°) |
|---|---|---|---|---|---|
| 180 °C | 65.14 ± 6.86 a | 6.02 ± 3.62 b | 22.57 ± 2.38 c | 23.64 ± 1.95 c | 75.00 ± 9.56 a |
| 200 °C | 62.89 ± 5.76 a | 6.99 ± 2.41 b | 26.74 ± 2.74 b | 27.71 ± 3.07 b | 75.54 ± 3.99 a |
| 220 °C | 54.55 ± 5.22 b | 13.32 ± 3.22 a | 39.21 ± 2.94 a | 41.48 ± 3.51 a | 71.37 ± 3.48 a |
| Sign. | ** | ** | ** | ** | ns |
| Cooking Time | |||||
| 16 min | 63.90 ± 5.11 a | 6.93 ± 2.92 b | 27.96 ± 6.41 b | 28.84 ± 6.91 b | 76.57 ± 2.72 a |
| 24 min | 63.89 ± 6.76 a | 7.88 ± 4.11 b | 31.28 ± 8.26 a | 32.35 ± 8.91 a | 76.67 ± 4.64 a |
| 32 min | 54.81 ± 6.63 b | 11.53 ± 5.11 a | 29.27 ± 8.65 ab | 31.65 ± 9.36 a | 68.67 ± 7.39 b |
| Sign. | ** | ** | ** | * | ** |
| Interactions | |||||
| CT × CT | |||||
| Sign. | ns | * | ns | ns | * |
| FAA (mg 100 g−1) | Cooking Temperature | Cooking Time | CT × CT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 180 °C | 200 °C | 220 °C | Sign. | 16 min | 24 min | 32 min | Sign. | Sign. | |
| Lysine | 9.93 ± 1.03 c | 16.52 ± 4.97 b | 23.85 ± 12.37 a | ** | 13.67 ± 7.13 c | 16.67 ± 9.18 b | 19.96 ± 11.42 a | ** | ** |
| Histidine | 1.71 ± 0.14 c | 3.19 ± 0.52 b | 4.87 ± 3.19 a | ** | 2.26 ± 0.98 c | 4.68 ± 3.24 a | 2.83 ± 1.01 b | ** | ** |
| Glycine | 35.03 ± 9.05 c | 43.12 ± 15.48 b | 56.18 ± 21.42 a | ** | 27.66 ± 2.35 c | 58.64 ± 20.07 a | 48.02 ± 8.43 b | ** | ** |
| Serine | 12.29 ± 0.85 a | 12.20 ± 4.64 a | 12.92 ± 4.29 a | ns | 11.12 ± 1.83 b | 11.74 ± 3.94 b | 14.55 ± 3.84 a | * | * |
| Alanine | 66.29 ± 43.14 c | 119.45 ± 22.71 b | 141.11 ± 46.87 a | ** | 71.09 ± 24.53 c | 120.27 ± 65.41 b | 135.49 ± 21.92 a | ** | ** |
| Glutamate | 76.64 ± 21.88 c | 104.39 ± 16.40 b | 110.62 ± 29.83 a | ** | 94.33 ± 13.44 b | 87.21 ± 30.89 c | 110.12 ± 30.49 a | ** | ** |
| Cysteine | 8.38 ± 0.52 b | 9.31 ± 0.49 a | 8.99 ± 0.67 a | ** | 8.81 ± 0.52 a | 9.07 ± 0.97 a | 8.82 ± 0.44 a | ns | * |
| Aspartate | 24.70 ± 10.96 b | 22.46 ± 7.26 c | 34.87 ± 16.81 a | ** | 21.35 ± 3.22 c | 23.19 ± 7.34 b | 37.49 ± 17.72 a | ** | ** |
| Proline | 9.83 ± 5.47 c | 13.36 ± 8.95 b | 27.94 ± 18.54 a | ** | 7.85 ± 2.16 c | 19.96 ± 12.66 b | 23.31 ± 18.64 a | ** | ** |
| Methionine | 70.71 ± 11.63 c | 89.06 ± 27.57 b | 156.67 ± 35.89 a | ** | 74.81 ± 28.01 b | 119.89 ± 48.39 a | 121.74 ± 45.58 a | ** | ** |
| Leucine | 9.92 ± 0.91 c | 16.51 ± 5.37 b | 23.90 ± 12.41 a | ** | 13.67 ± 7.29 c | 16.67 ± 9.29 b | 19.99 ± 11.47 a | ** | ** |
| Isoleucine | 26.29 ± 4.18 a | 18.57 ± 3.74 c | 21.64 ± 11.32 b | ** | 18.94 ± 2.98 b | 19.95 ± 6.11 b | 27.63 ± 9.83 a | ** | ** |
| Tyrosine | 3.66 ± 0.85 c | 7.27 ± 3.03 b | 10.14 ± 4.55 a | ** | 4.05 ± 0.95 c | 9.36 ± 4.96 a | 7.65 ± 3.56 b | ** | ** |
| Phenylalanine | 29.41 ± 5.79 b | 29.03 ± 9.22 b | 44.41 ± 15.08 a | ** | 24.46 ± 2.49 c | 37.61 ± 7.93 b | 40.78 ± 16.78 a | ** | ** |
| Tryptophan | 3.67 ± 0.63 c | 8.76 ± 4.59 a | 8.25 ± 3.14 b | ** | 4.37 ± 0.38 c | 8.68 ± 5.12 a | 7.63 ± 3.35 b | ** | ** |
| Cooking Temperature | Ca | Mg | Cu | Zn | Na | K |
|---|---|---|---|---|---|---|
| 180 °C | 1455.63 ± 227.16 a | 208.17 ± 21.39 b | 1.89 ± 0.71 a | 69.43 ± 19.19 a | 1116.88 ± 109.39 c | 1326.42 ± 147.23 c |
| 200 °C | 795.92 ± 189.55 b | 221.34 ± 20.87 a | 1.91 ± 0.15 a | 46.38 ± 12.28 b | 1216.61 ± 15.77 b | 1354.29 ± 114.95 b |
| 220 °C | 431.32 ± 144.07 c | 199.07 ± 25.51 c | 1.47 ± 0.47 b | 43.35 ± 11.75 b | 1254.34 ± 46.01 a | 1700.46 ± 96.97 a |
| Sign. | ** | ** | * | ** | ** | ** |
| Cooking Time | ||||||
| 16 min | 1069.19 ± 484.71 a | 187.51 ± 16.76 c | 1.52 ± 0.56 a | 57.11 ± 26.54 a | 1173.15 ± 64.95 b | 1498.33 ± 136.07 a |
| 24 min | 954.37 ± 466.61 b | 213.91 ± 24.56 b | 1.88 ± 0.15 a | 47.45 ± 7.96 b | 1160.40 ± 132.62 c | 1447.17 ± 320.34 b |
| 32 min | 659.29 ± 401.92 c | 226.34 ± 7.93 a | 1.88 ± 0.66 a | 54.72 ± 16.66 ab | 1247.99 ± 33.11 a | 1445.99 ± 169.11 b |
| Sign. | ** | ** | ns | * | ** | ** |
| Interactions | ||||||
| CT × CT | ||||||
| Sign. | ** | ** | * | ** | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savaş, A. Effects of Different Cooking Parameters on Various Quality Criteria, Lipid Oxidation, Mineral Composition, and Free Amino Acid Profile of Chicken Breast. Processes 2025, 13, 1602. https://doi.org/10.3390/pr13051602
Savaş A. Effects of Different Cooking Parameters on Various Quality Criteria, Lipid Oxidation, Mineral Composition, and Free Amino Acid Profile of Chicken Breast. Processes. 2025; 13(5):1602. https://doi.org/10.3390/pr13051602
Chicago/Turabian StyleSavaş, Adem. 2025. "Effects of Different Cooking Parameters on Various Quality Criteria, Lipid Oxidation, Mineral Composition, and Free Amino Acid Profile of Chicken Breast" Processes 13, no. 5: 1602. https://doi.org/10.3390/pr13051602
APA StyleSavaş, A. (2025). Effects of Different Cooking Parameters on Various Quality Criteria, Lipid Oxidation, Mineral Composition, and Free Amino Acid Profile of Chicken Breast. Processes, 13(5), 1602. https://doi.org/10.3390/pr13051602







