Statistical Analysis of Temperature Sensors Applied to a Biological Material Transport System: Challenges, Discrepancies, and a Proposed Monitoring Methodology
Abstract
1. Introduction
- ✓
- The application of parametric (ANOVA, Tukey test) and non-parametric (Kruskal–Wallis, Levene’s, and Shapiro–Wilk statistical methods to assess data normality, identify differences between sensors, and demonstrate the need for individual calibration.
- ✓
- Statistical verification of significant variations between sensors of the same model and specification.
- ✓
- Experimental identification of the influence of systematic errors (bias) in critical applications.
- ✓
- Proposal of a standardized, practical, and replicable methodology for validating temperature sensors, based on periodic calibration, statistical verification, and use of redundant sensors, applicable in laboratory and field environments.
2. Experimental Analysis
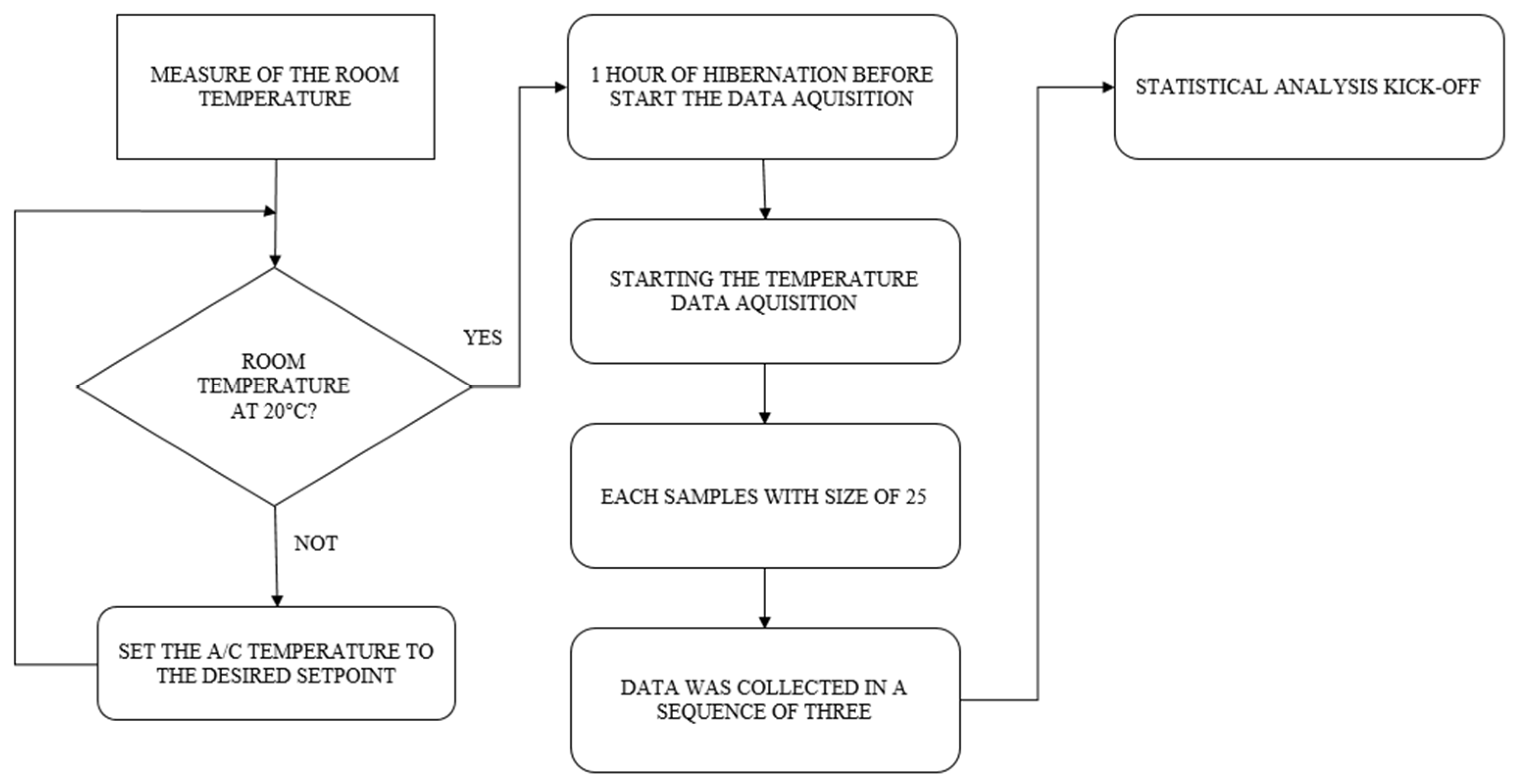
2.1. Description of the Experimental Setup
2.2. Experimental Procedure
3. Statistical Analysis
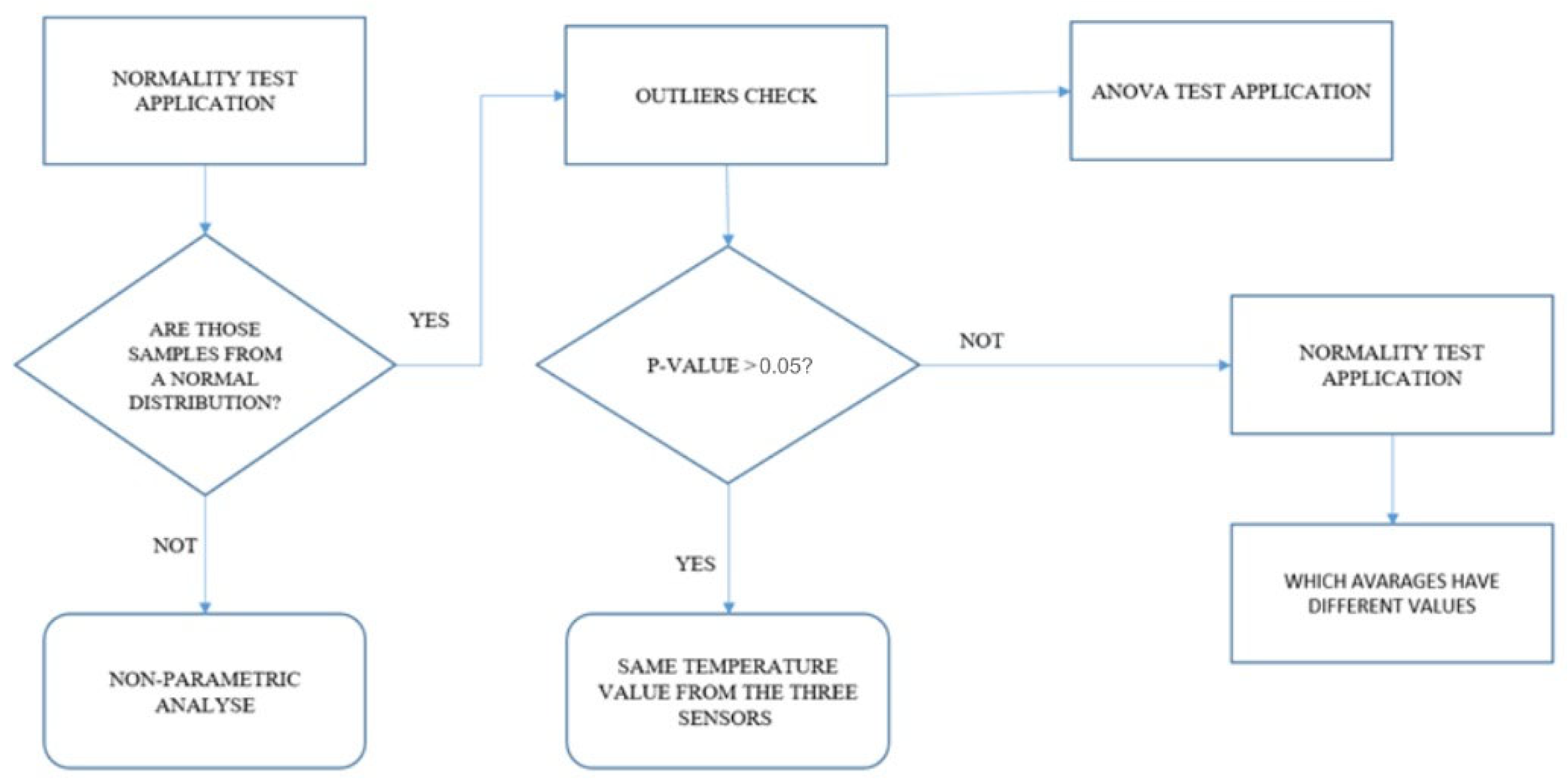
3.1. Parametric Analysis Procedure
- Rejecting a true null hypothesis (Type I error).
- Accepting a false null hypothesis (Type II error).
3.2. Non-Parametric Analysis Procedure
4. Analysis and Discussion of Results
4.1. Case Study Description
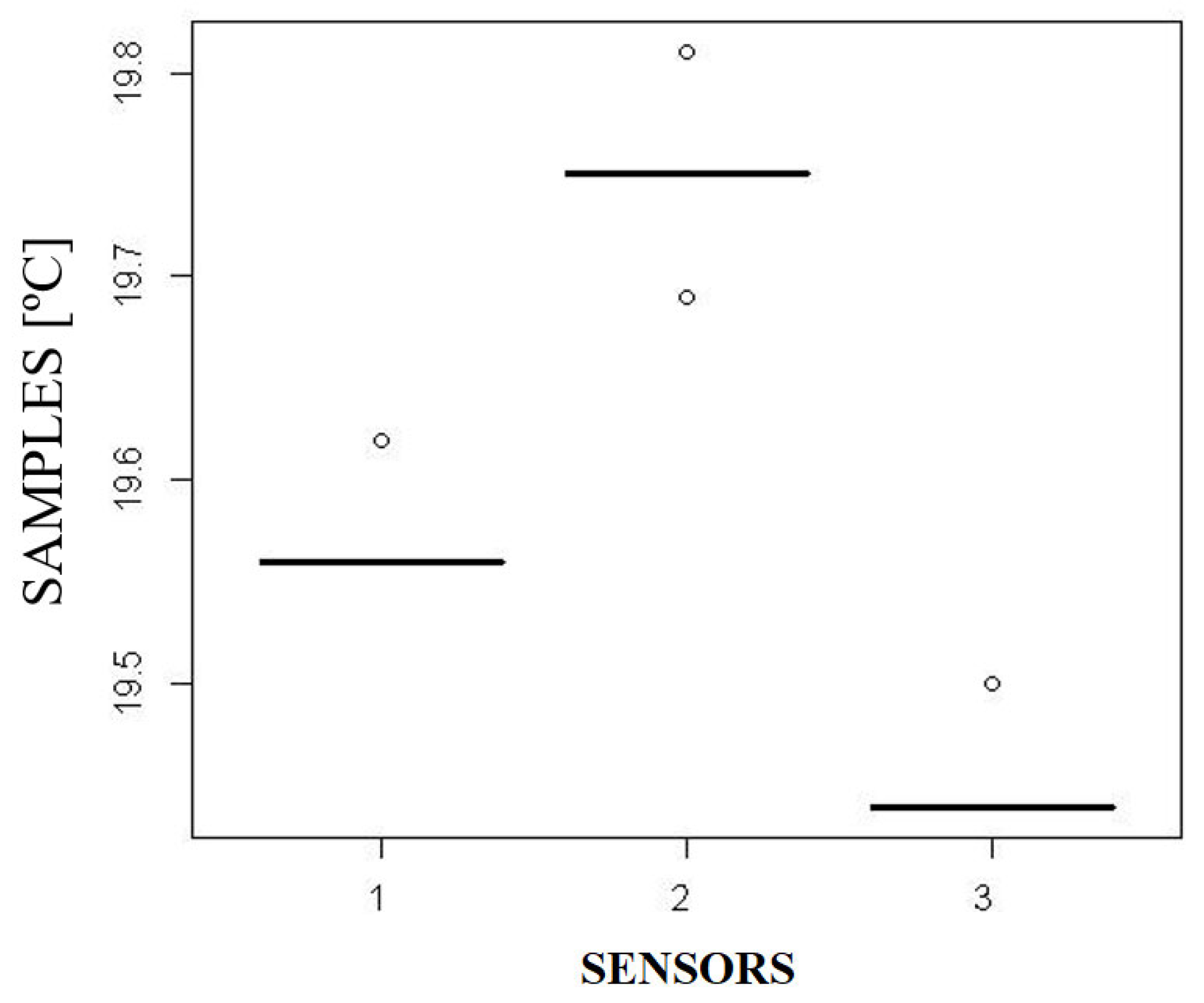
4.2. Measurement Results from the Three Temperature Sensors
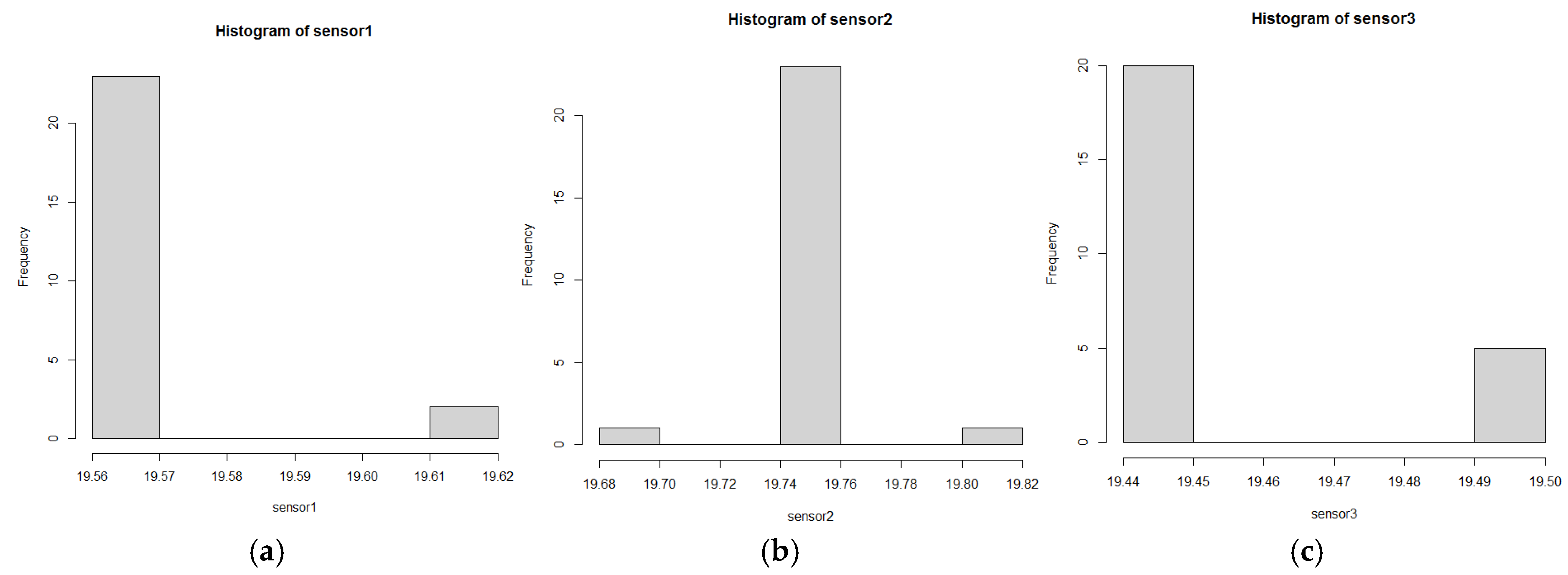
4.3. Parametric and Non-Parametric Statistical Study
4.4. Critical Considerations, Limitations and Challenges
- Difficulty in distinguishing systematic errors (e.g., manufacturing defects) from random errors (e.g., thermal noise).
- Limited generalizability, as only three sensors of a single model (DS18B20) were tested.
- Lack of metrological validation of the data acquisition system (Arduino UNO), which may affect measurement resolution.
5. Conclusions
- ✓
- Statistical tests (ANOVA, Tukey, Kruskal–Wallis, and Levene’s) showed that, being identical models from the same manufacturer, the three sensors showed statistically significant differences in readings (p-value < 0.05). This highlights the need for individual calibration and continuous monitoring to ensure data reliability.
- ✓
- The Shapiro–Wilk test indicated non-normal data distribution (p-value < 0.05), supporting the use of non-parametric methods such as Kruskal–Wallis for analyzing asymmetric data.
- ✓
- Levene’s test showed homogeneity of variances among sensors (p-value = 0.3303); however, differences in medians suggest systematic errors, likely due to the absence of prior calibration.
- ✓
- A discrepancy of up to 0.37 °C was observed and statistically validated among the tested sensors. While this value is lower than system-level errors sometimes reported, it confirms a baseline of systematic error that, if uncalibrated, can contribute to larger, critical temperature deviations that could compromise the preservation of sensitive biological materials such as organs and vaccines.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima, O.F., Jr.; Novaes, A.G.; Santos, J.B., Jr.; Arias, J.A. Pharmaceutical cold chain and novel technological tools: A systematic review. Transportes 2021, 29, 67–85. [Google Scholar] [CrossRef]
- Maiorino, A.; Petruzziello, F.; Aprea, C. Refrigerated transport: State of the art, technical issues, innovations and challenges for sustainability. Energies 2021, 14, 7237. [Google Scholar] [CrossRef]
- Taira, K.; Waku, T.; Hagiwara, Y. Ice growth suppression in the solution flows of antifreeze protein and sodium chloride in a mini-channel. Processes 2021, 9, 306. [Google Scholar] [CrossRef]
- Elyounsi, A.; Kalashnikov, A.N. Evaluating Suitability of a DS18B20 Temperature Sensor for Use in an Accurate Air Temperature Distribution Measurement Network. Eng. Proc. 2021, 10, 56. [Google Scholar] [CrossRef]
- Koritsoglou, K.; Christou, V.; Ntritsos, G.; Tsoumanis, G.; Tsipouras, M.G.; Giannakeas, N.; Tzallas, A.T. Improving the accuracy of low-cost sensor measurements for freezer automation. Sensors 2020, 20, 6389. [Google Scholar] [CrossRef]
- EN 12830:2018; Temperature Recorders for the Transport, Storage and Distribution of Temperature-Sensitive Goods—Tests, Performance, Suitability. European Committee For Standardization (CEN): Brussels, Belgium, 2018.
- Siswoyo, A. Optimization of Temperature Sensor Selection for Incubators: Real—Time Accuracy Analysis of DHT22, LM35, and DS18B20 in Controlled Environment Simulations. Internet Things 2025, 5. [Google Scholar] [CrossRef]
- Nie, S.; Cheng, Y.; Dai, Y. Characteristic Analysis of DS18B20 Temperature Sensor in the High-voltage Transmission Lines’ Dynamic Capacity Increase. Energy Power Eng. 2013, 05, 557–560. [Google Scholar] [CrossRef]
- Roque, F.A.N.; Cleydson, A.; Tomás, A.; Peixoto da Costa, J.Â.; Ochoa, A. Development of a Numerical Heat Transfer Model via FEM for Transport and Storage of Biological Material and Vaccines Based on Peltier Effect. In Proceedings of the 27th Brazilian Congress of Thermal Sciences and Engineering, Florianópolis, Brazil, 4–8 December 2023; ABCM—UFSC: Florianópolis-SC, Brazil, 2023; pp. 1–10. [Google Scholar]
- Lima, A.C.; Alves, J.C.R.; Borga, A.L.; Ocampos, H.B.d.L.; Deboni, L.M.; Guterres, J.C.P.; Garcia, C.E. Análise da Temperatura Durante o Armazenamento e o Período de Isquemia Morna do Enxerto em Transplantes Renais. Braz. J. Transplant. 2023, 26, e0423. [Google Scholar] [CrossRef]
- Hien, D.N.; Thanh, N. Van Optimization of Cold Chain Logistics with Fuzzy MCDM Model. Processes 2022, 10, 947. [Google Scholar] [CrossRef]
- Lin, Y.T.; Permana, I.; Wang, F.; Chang, R.J. Improvement of heating and cooling performance for thermoelectric devices in medical storage application. Case Stud. Therm. Eng. 2023, 54, 104017. [Google Scholar] [CrossRef]
- Daou, R.A.Z.; Boutros, E.; Youssef, T.; Hayek, A.; Boercsoek, J.; Olmedo, J.J.S. Design and Implementation of a Safe Medical Box to Maintain Insulin Pens in Good Conditions. In Proceedings of the 2021 18th International Multi-Conference on Systems, Signals & Devices (SSD), Monastir, Tunisia, 22–25 March 2021; pp. 416–420. [Google Scholar] [CrossRef]
- Ivanov, K.; Belovski, I.; Aleksandrov, A. Design, building and study of a small-size portable thermoelectric refrigerator for vaccines. In Proceedings of the 2021 17th Conference on Electrical Machines, Drives and Power Systems (ELMA), Sofia, Bulgaria, 1–4 July 2021. [Google Scholar] [CrossRef]
- Novais, J.W.Z.; Hillesheim, A.C.; Fank, N.C.; Siqueira Varella Oliveira, L.; Soares dos Santos Brito, N.; Dos Santos Oliveira Zangeski, D.; Pereira de Oliveira, B.B. Técnica de Calibração de Sensores Meteorológicos de Temperatura e Umidade Relativa do ar Utilizando um Sensor de Referência. Uniciências 2021, 24, 30–33. [Google Scholar] [CrossRef]
- Taspinar, Y.S.; Işik, P.D.H. Organ Transportation Thermoelectric Cooling System Design and Application. Int. J. Appl. Math. Electron. Comput. 2021, 9, 110–115. [Google Scholar] [CrossRef]
- Umchid, S.; Samae, P.; Sangkarak, S.; Wangkram, T. Design and Development of a Temperature Controlled Blood Bank Transport Cooler. In Proceedings of the 2019 12th Biomedical Engineering International Conference (BMEiCON), Ubon Ratchathani, Thailand, 19–22 November 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Abderezzak, B.; Dreepaul, R.K.; Busawon, K.; Chabane, D. Experimental characterization of a novel configuration of thermoelectric refrigerator with integrated finned heat pipes. Int. J. Refrig. 2021, 131, 157–167. [Google Scholar] [CrossRef]
- Ahmed, I.; Jena, A.K. Using non-parametric Kruskal-Wallis H test for assessing mean differences in the opinion of environmental sustainability. Int. J. Geogr. Geol. Environ. 2023, 5, 74–80. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Fan, Y.W.; Li, X.L.; Yang, J.G.; Zhang, X.R. Experimental Validation of a Novel CO2 Refrigeration System for Cold Storage: Achieving Energy Efficiency and Carbon Emission Reductions. Energies 2025, 18, 1129. [Google Scholar] [CrossRef]
- Ko, J.W.; Bae, K.J.; Kwon, O.K. Correlation development on heat and mass transfer of absorber tube for absorption chiller. Appl. Therm. Eng. 2025, 265, 125467. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Wang, L.; Hu, Y. Heat Transfer Enhancement of Diamond Rib Mounted in Periodic Merging Chambers of Micro Channel Heat Sink. Micromachines 2025, 16, 533. [Google Scholar] [CrossRef]
- Ammar, S.M.; Ramadan, Z.; Park, C.W. Performance evaluation of novel plate-type condenser for an absorption/adsorption refrigeration system: Experimental and CFD study. Case Stud. Therm. Eng. 2024, 56, 104260. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, L.; Hu, X.; Zhou, M. A Symmetry-Based Hybrid Model of Computational Fluid Dynamics and Machine Learning for Cold Storage Temperature Management. Symmetry 2025, 17, 539. [Google Scholar] [CrossRef]
- Nanda, A.; Mohapatra, D.B.B.; Mahapatra, A.P.K.; Mahapatra, A.P.K.; Mahapatra, A.P.K. Multiple comparison test by Tukey’s honestly significant difference (HSD): Do the confident level control type I error. Int. J. Stat. Appl. Math. 2021, 6, 59–65. [Google Scholar] [CrossRef]
- da Silva, J.P.; dos Santos, Y.R.P.; Bello, M.I.M.d.C. Aplicação da ANOVA e dos testes de Fisher e Tukey em dados de recalque de edifícios de múltiplos pavimentos. Rev. Principia—Divulg. Científica Tecnológica IFPB 2022, 59, 829. [Google Scholar] [CrossRef]
- Yulizar, D.; Soekirno, S.; Ananda, N.; Prabowo, M.A.; Perdana, I.F.P.; Aofany, D. Performance Analysis Comparison of DHT11, DHT22 and DS18B20 as Temperature Measurement; Atlantis Press International BV: Amsterdam, The Netherlands, 2023; Volume 1. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R-project.org (accessed on 2 May 2024).
- De Carvalho, A.M.X.; Éder, M.; da Silva Maia, M. Evaluation of normality, validity of mean tests and non-parametric options: Contributions to a necessary debate. Ciência E Natura 2023, 45, e9. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Rani Das, K. A Brief Review of Tests for Normality. Am. J. Theor. Appl. Stat. 2016, 5, 5. [Google Scholar] [CrossRef]
- Wani, G.A.; Nagaraj, V. Effect of Sustainable Infrastructure and Service Delivery on Sustainable Tourism: Application of Kruskal Wallis Test (Non-parametric). Int. J. Sustain. Transp. Technol. 2022, 5, 38–50. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 2014, 47, 583–621. [Google Scholar] [CrossRef]
- de Almeida, A.; Elian, S.; Nobre, J. Modificações e alternativas aos testes de Levene e de Brown e Forsythe para igualdade de variâncias e médias. Rev. Colomb. Estad. 2008, 31, 241–260. [Google Scholar]
- Trindade, D.d.B.; Teixeira, N.d.S.; Couto, L.A.; Coqueiro, J.S. Ferramenta estatística para análise de dados: Comandos do software R. Brazilian J. Sci. 2022, 1, 70–84. [Google Scholar] [CrossRef]
- Oliveira, J.E.F. Praticando a Estatística Não Paramétrica na Linguagem R, 1st ed.; de Autores, C., Ed.; Clube de Autores: São Paulo, Brazil, 2024; ISBN 9786500972924. [Google Scholar]
- Oliveira, J.E.F.; Oliveira, L.F. Praticando a Linguagem R. Um Caderninho de Bolso com Comandos Básicos Para Análise Estatística, 1st ed.; de Autores, C., Ed.; Clube de Autores: São Paulo, Brazil, 2023; Volume 1, ISBN 9786500893038. [Google Scholar]
- Mobaraki, B.; Seyedmilad, K.; Pascual, F.J.C.; Lozano-Galant, J.A. Application of Low-Cost Sensors for Accurate Ambient Temperature Monitoring. Buildings 2022, 12, 133–139. [Google Scholar] [CrossRef]
- Tanvir, E.M.; Komarova, T.; Comino, E.; Sumner, R.; Whitfield, K.M.; Shaw, P.N. Effects of storage conditions on the stability and distribution of clinical trace elements in whole blood and plasma: Application of ICP-MS. J. Trace Elem. Med. Biol. 2021, 68, 126804. [Google Scholar] [CrossRef]
- Peterson, M.E.; Daniel, R.M.; Danson, M.J.; Eisenthal, R. The dependence of enzyme activity on temperature: Determination and validation of parameters. Biochem. J. 2007, 402, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, Y.; Chang, Y.; Liang, X.; Zhang, H.; Lin, X.; Qing, K.; Zhou, X.; Luo, Z. The Effects of Ventilation, Humidity, and Temperature on Bacterial Growth and Bacterial Genera Distribution. Int. J. Environ. Res. Public Health 2022, 19, 5345. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Paredes, C.; Tájmel, D.; Rousk, J. Can moisture affect temperature dependences of microbial growth and respiration? Soil Biol. Biochem. 2021, 156, 108223. [Google Scholar] [CrossRef]
- Lloyd, J.; Lydon, P.; Ouhichi, R.; Zaffran, M. Reducing the loss of vaccines from accidental freezing in the cold chain: The experience of continuous temperature monitoring in Tunisia. Vaccine 2015, 33, 902–907. [Google Scholar] [CrossRef]
- Clénet, D. Accurate prediction of vaccine stability under real storage conditions and during temperature excursions. Eur. J. Pharm. Biopharm. 2018, 125, 76–84. [Google Scholar] [CrossRef]

| Equation Name | Equation | Variables | Number |
|---|---|---|---|
| ANOVA (Analysis of Variance) | (1) | ||
| Turkey’s Test | (2) | ||
| Critical value for comparison | the studentized range statistic | (3) | |
| Kruskal–Wallis test statistic | total number of observations : number of observations : rank of observation from group sum of ranks in group : the mean rank of group : mean of all ranks | (4) | |
| Levene’s Test | is the absolute deviation from group mean, is the mean of for group and is the overall mean of | (5) |
| N° of Samples | S1 | S2 | S3 | N° of Samples | S1 | S2 | S3 |
|---|---|---|---|---|---|---|---|
| 1 | 19.56 | 19.75 | 19.44 | 14 | 19.56 | 19.75 | 19.44 |
| 2 | 19.62 | 19.75 | 19.44 | 15 | 19.56 | 19.75 | 19.5 |
| 3 | 19.56 | 19.75 | 19.44 | 16 | 19.56 | 19.75 | 19.44 |
| 4 | 19.56 | 19.75 | 19.44 | 17 | 19.56 | 19.81 | 19.5 |
| 5 | 19.56 | 19.75 | 19.44 | 18 | 19.56 | 19.75 | 19.44 |
| 6 | 19.56 | 19.75 | 19.44 | 19 | 19.56 | 19.75 | 19.44 |
| 7 | 19.56 | 19.75 | 19.44 | 20 | 19.56 | 19.75 | 19.44 |
| 8 | 19.56 | 19.75 | 19.44 | 21 | 19.56 | 19.75 | 19.5 |
| 9 | 19.62 | 19.75 | 19.44 | 22 | 19.56 | 19.69 | 19.5 |
| 10 | 19.56 | 19.75 | 19.44 | 23 | 19.56 | 19.75 | 19.44 |
| 11 | 19.56 | 19.75 | 19.44 | 24 | 19.56 | 19.75 | 19.44 |
| 12 | 19.56 | 19.75 | 19.44 | 25 | 19.56 | 19.75 | 19.5 |
| 13 | 19.56 | 19.75 | 19.44 |
| Sensor | p-Value |
|---|---|
| 1 | 7.518 × 10−10 |
| 2 | 3.637 × 10−9 |
| 3 | 3.21 × 108 |
| Sensor 1 | Sensor 2 | Sensor 3 | |
|---|---|---|---|
| Minimum Value (°C) | 19.56 | 19.69 | 19.44 |
| Q1 (°C) | 19.56 | 19.75 | 19.44 |
| Median (°C) | 19.56 | 19.75 | 19.44 |
| Q3 (°C) | 19.56 | 19.75 | 19.44 |
| Maximum Value (°C) | 19.62 | 19.81 | 19.50 |
| Chi-Squared | Degree of Freedom | p-Value |
|---|---|---|
| 71.262 | 2 | 3.354 × 10−16 |
| Sensor 1 | Sensor 2 | |
|---|---|---|
| Sensor 2 | 1.5 × 10−11 | - |
| Sensor 3 | 3.8 × 10−11 | 3.8 × 10−11 |
| Degrees of Freedom | F-Value | Pr (>F) |
|---|---|---|
| 2 | 1.125 | 0.3303 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Albuquerque Neto, F.R.; de Oliveira, J.E.F.; Dourado da Silva, R.G.; Tomás, A.C.C.; Ochoa, A.A.V.; da Costa, J.Â.P.; de Souza, A.C.; Michima, P.S.A. Statistical Analysis of Temperature Sensors Applied to a Biological Material Transport System: Challenges, Discrepancies, and a Proposed Monitoring Methodology. Processes 2025, 13, 1904. https://doi.org/10.3390/pr13061904
de Albuquerque Neto FR, de Oliveira JEF, Dourado da Silva RG, Tomás ACC, Ochoa AAV, da Costa JÂP, de Souza AC, Michima PSA. Statistical Analysis of Temperature Sensors Applied to a Biological Material Transport System: Challenges, Discrepancies, and a Proposed Monitoring Methodology. Processes. 2025; 13(6):1904. https://doi.org/10.3390/pr13061904
Chicago/Turabian Stylede Albuquerque Neto, Felipe Roque, José Eduardo Ferreira de Oliveira, Rodrigo Gustavo Dourado da Silva, Andrezza Carolina Carneiro Tomás, Alvaro Antonio Villa Ochoa, José Ângelo Peixoto da Costa, Alisson Cocci de Souza, and Paula Suemy Arruda Michima. 2025. "Statistical Analysis of Temperature Sensors Applied to a Biological Material Transport System: Challenges, Discrepancies, and a Proposed Monitoring Methodology" Processes 13, no. 6: 1904. https://doi.org/10.3390/pr13061904
APA Stylede Albuquerque Neto, F. R., de Oliveira, J. E. F., Dourado da Silva, R. G., Tomás, A. C. C., Ochoa, A. A. V., da Costa, J. Â. P., de Souza, A. C., & Michima, P. S. A. (2025). Statistical Analysis of Temperature Sensors Applied to a Biological Material Transport System: Challenges, Discrepancies, and a Proposed Monitoring Methodology. Processes, 13(6), 1904. https://doi.org/10.3390/pr13061904










